Abstract
Aim:
To compare the prevalence and trends of antipsychotic drug use during pregnancy between countries across four continents.
Methods:
Individually linked health data in Denmark (2000–2012), Finland (2005–2014), Iceland (2004–2017), Norway (2005–2015), Sweden (2006–2015), Germany (2006–2015), Australia (New South Wales, 2004–2012), Hong Kong (2001–2015), UK (2006–2016), and the US (Medicaid, 2000–2013, and IBM MarketScan, 2012–2015) were used. Using a uniformed approach, we estimated the prevalence of antipsychotic use as the proportion of pregnancies where a woman filled at least one antipsychotic prescription within three months before pregnancy until birth. For the Nordic countries, data were meta-analyzed to investigate maternal characteristics associated with the use of antipsychotics.
Results:
We included 8,394,343 pregnancies. Typical antipsychotic use was highest in the UK (4.4%) whereas atypical antipsychotic use was highest in the US Medicaid (1.5%). Atypical antipsychotic use increased over time in most populations, reaching 2% in Australia (2012) and US Medicaid (2013). In most countries, prochlorperazine was the most commonly used typical antipsychotic and quetiapine the most commonly used atypical antipsychotic. Use of antipsychotics decreased across the trimesters of pregnancy in all populations except Finland. Antipsychotic use was elevated among smokers and those with parity ≥4 in the Nordic countries.
Conclusion:
Antipsychotic use during pregnancy varied considerably between populations, partly explained by varying use of the typical antipsychotic prochlorperazine, which is often used for nausea and vomiting in early pregnancy. Increasing usage of atypical antipsychotics among pregnant women reflects the pattern that was previously reported for the general population.
Keywords: antipsychotics, pregnancy, pharmacological treatment, epidemiology, drug utilization study
1. Introduction
Antipsychotic drugs are often prescribed as the standard of care for schizophrenia, other psychotic disorders and bipolar disorder. They are also prescribed, but to a lesser degree, for depression, anxiety, insomnia, autism, as well as for nausea and vomiting in early pregnancy (Halfdanarson et al., 2017; Minami et al., 2018; Toh et al., 2013). The mechanism of action and indications differ to a varying degree between typical (first generation) antipsychotics and the more recently introduced atypical (second generation) antipsychotics. In general, atypical antipsychotics have a stronger serotonin receptor antagonism, and are used to treat mood disorders to a larger extent.
Discontinuation of antipsychotic treatment during pregnancy may increase the risk of recurrence of mental disorders including bipolar disorder (Viguera et al., 2007) and psychosis (Tosato et al., 2017). On the other hand, potential risks associated with antipsychotic use during pregnancy include metabolic disturbances, abnormal fetal growth (Boden et al., 2012b), preterm birth (Lin et al., 2010), as well as congenital anomalies (Huybrechts et al., 2016). However, findings to date are not consistent and some increased risks for adverse outcomes may be illness-rather than drug-related (Boden et al., 2012a). Thus, women treated with antipsychotics and their clinicians, are faced with the complex challenge of balancing the benefits and potential risks of antipsychotic drug treatment during pregnancy.
Since the introduction of the first antipsychotic, chlorpromazine, in the 1950s, various antipsychotics have been developed, and studies have found increasing use of antipsychotics in the general population in recent years (Halfdanarson et al., 2017; Olfson et al., 2012). At the same time, a widening of both on- and off-label antipsychotic indications has been observed (Halfdanarson et al., 2017; Hojlund et al., 2019). However, little is known about the worldwide patterns of antipsychotic use among pregnant women.
To enable international comparisons and to inform future studies investigating the benefits and risks associated with antipsychotic use in pregnancy, our aim was to describe antipsychotic drug use during pregnancy and the three months before by type of antipsychotic, trends in prevalence and the characteristics of users in ten countries: Australia, Denmark, Finland, Germany, Hong Kong, Iceland, Norway, Sweden, the United Kingdom (UK), and the United States (US).
2. Methods
2.1. Study population
The study included pregnancies from 11 populations in 10 countries with pregnancies ending in live births or stillbirths. The full population is included in the Nordic countries data registries, while the datasets from the other countries are selected samples. However, the German and UK data sources are considered representative of their respective populations, and the databases from Hong Kong, Australia, and the two US databases combined, include the majority of the women giving birth in their respective regions. The data sources are described in Panel 1 and below.
Panel 1.
Study populations and data source characteristics
| Country and years of coverage | Data sources and study populations | Pregnancies included | Drug information available |
|---|---|---|---|
| Australia, New South Wales (NSW) 2004–2012 | a) NSW Perinatal Data Collection (state-wide birth register) b) Pharmaceutical Benefits Scheme (national claims data) Publicly insured |
All pregnancies resulting in live birth or stillbirth from 20 weeks of gestation or birthweight of at least 400 grams Only pregnancies among women who were concessional beneficiaries (eligible for increased subsidy for prescription drugs) were included |
All dispensed, subsidised prescription drugs in outpatient care and private hospitals |
|
Denmark 2000–2012 |
a) Medical Birth Register b) National Prescription Registry (National health registers) Publicly insured |
All pregnancies resulting in live birth or stillbirth from 22 weeks of gestation | All dispensed prescription drugs in outpatient care |
| Finland 2005–2014 | a) Medical Birth Register b) Register of Reimbursed Drug Purchases and Register of Medical Special Reimbursements (National health registers) Publicly insured |
All pregnancies resulting in live birth or stillbirth from 22 weeks of gestation | All dispensed, reimbursed prescription drugs in outpatient care |
| Germany 2006–2015 | German Pharmacoepidemiological Research Database (GePaRD) (Healthcare claims database) Publicly insured | All pregnancies resulting in live birth or stillbirth (>500 gram) | All dispensed, reimbursed prescription drugs in outpatient care |
| Hong Kong 2001–2015 | Clinical Data Analysis and Reporting System (CDARS) | All pregnancies in public hospitals resulting in live birth or stillbirth are directly identified in the database. | All dispensed prescription drugs in public in-and outpatient care |
| Iceland 2004–2017 | a) Medical Birth Register b) National Medicine Registry (National health registers) Publicly insured |
All pregnancies resulting in live birth or stillbirth from 22 weeks of gestation | All dispensed prescription drugs in outpatient care |
| Norway 2005–2015 | a) Medical Birth Registry of Norway b) Norwegian Prescription Database (National health registers) Publicly insured |
All pregnancies resulting in a live birth or stillbirth from 12 weeks of gestation | All dispensed prescription drugs in outpatient care |
| Sweden 2006–2015 | a) Medical Birth Register b) Prescribed Drug Register (National health registers) Publicly insured |
All pregnancies resulting in a live birth or stillbirth from 22 weeks of gestation | All dispensed prescription drugs in outpatient care |
| UK 2001–2015 | The Health Improvement Network (THIN) database (Primary care database) Publicly insured | All pregnancies identified based on the recorded birth date, the last menstrual period and the estimated birth dates | All drugs prescribed in general practice |
| US MarketScan, privately-insured 2012–2015 | IBM MarketScan® Commercial Claims and Encounters (MarketScan) database (Healthcare claims database) Privately insured | All pregnancies in women continuously enrolled in their health plan from before pregnancy until birth, identified with an ICD-9-based algorithm to identify live births and stillbirths | All dispensed, reimbursed prescription drugs in outpatient care |
| US MAX, publicly-insured 2000–2013 | Medicaid Analytic eXtract (MAX) database (Healthcare claims database) Publicly insured | All pregnancies in women continuously enrolled in a state Medicaid program from before pregnancy until birth, identified | All dispensed, reimbursed prescription drugs in outpatient care |
From the Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden) we used population-based birth and dispensed prescription drug registers which were individually-linked using the civil personal registration number, uniquely assigned to each resident at birth or immigration (Furu et al., 2010; Langhoff-Roos et al., 2014).
From New South Wales (NSW), the most populous state in Australia, we used population-based birth data and dispensed pharmaceutical claims data which were probabilistically linked using identifiers including name, address, and date of birth (Tran et al., 2017). The study population was restricted to pregnancies among concessional beneficiaries, eligible for reduced co-payments due to low income, chronic illness, or disability, representing 20.3% of births in NSW, 2006–2012, for whom complete dispensing data are recorded.
From Germany, we used the German Pharmacoepidemiological Research Database (GePaRD) which is based on claims data from four statutory health insurance providers, currently including information on about 25 million persons from all geographical regions of Germany, representative of all persons with a statutory health insurance in Germany, which is about 90% of the population. We identified pregnancies from this database using an algorithm based on diagnostic and health care codes (Wentzell et al., 2018).
From Hong Kong, we used the pregnancy cohort nested in the electronic health records of the Clinical Data Analysis and Reporting System (CDARS), which covers health care services available to all residents in Hong Kong (Lao et al., 2017). CDARS contains deterministic linkage of the records of all in-patient, out-patient, and emergency room admissions in hospital ambulatory clinics, drug prescription and dispensing, through a unique patient identification number (Man et al., 2017).
From the UK, we used data from The Health Improvement Network (THIN), a large primary care database that includes longitudinal clinical and prescribing records from general practice and includes data from about 6% of the UK population. Over 98% of the UK population is registered with a general practitioner, and the register is broadly representative of the UK population (Petersen et al., 2017).
From the US, we included a pregnancy cohort nested within the Medicaid Analytic eXtract (MAX) database which includes inpatient and outpatient claims, as well as outpatient prescriptions dispensed for publicly-insured individuals from 46 US states and the District of Columbia (Palmsten et al., 2013). We also included a pregnancy cohort nested within the IBM MarketScan© Commercial Claims and Encounters Database, which includes similar healthcare claims from privately-insured individuals from all regions of the US (MacDonald et al., 2019).
2.2. Drug exposure
Antipsychotic drugs were defined using the World Health Organization Anatomical Therapeutic Chemical (ATC) classification codes starting with N05A. Lithium (N05AN01) was excluded because it has a different mechanism of action. Prochlorperazine (N05AB04) was not captured in the data from Australia and Finland and was not approved in Germany and Hong Kong. Typical and atypical antipsychotic drugs were classified according to Supplementary Table 1.
Use of Antipsychotics any time during the pregnancy period was defined by at least one filled prescription for an antipsychotic drug from 90 days before the first day of the last menstrual period (LMP) until birth. We also classified use according to trimester including the three-month pre-pregnancy period (up to 90 days before LMP), first trimester (T1=0–97 days of gestation), second trimester (T2=98–202 days of gestation), and third trimester (T3=203 days of gestation to birth). The trimester definitions used in the Finnish data were as follows: T1=0–84, T2=85–182, and T3=183 days of gestation to birth.
2.3. Data analysis
The prevalence of antipsychotic use (any, typical, atypical) was calculated as the proportion of pregnancies in each population where the woman had filled at least one prescription for an antipsychotic drug from 90 days before the first day of LMP and throughout the whole pregnancy period. We described prevalence by maternal age category and by trimester. To assess the relative change in use of antipsychotics across calendar years, we calculated the prevalence ratios with 95% confidence intervals (CI) between the first and last year of available data for each population by antipsychotic class, with the first year as the reference. In addition, linear time trends in prevalence were calculated using linear regression models. The resulting linear regression estimate (β) can be interpreted as the average percentage point change in prevalence per year.
Further, among each population we identified the five most commonly dispensed antipsychotics in the first and last year of available data. As prochlorperazine is almost exclusively used as an antiemetic during pregnancy (Fiaschi et al., 2019), we performed sub-analyses excluding users of prochlorperazine from the estimated prevalence of typical antipsychotics. We also analyzed the prevalence and trends of prochlorperazine use separately.
For the Nordic countries, where data sources are similar, we further present the use of antipsychotics by women’s parity, smoking status, and cohabitation. To this end, we meta-analyzed the prevalence estimated from each Nordic country by weighting each population by the inverse of the variance of the prevalence in the population (Barendregt et al., 2013).
2.4. Ethical approvals
The study was approved by the following country specific institutional review boards. Australia: The NSW Population and Health Services Research Ethics Committee (2012/06/397) and the Australian Institute of Health and Welfare Ethics Committee (2012/2/22).
Denmark: The Data Protection Agency (Record No. 2013-41-2569).
Finland: The Finnish Institute for Health and Welfare (THL/1551/6.02.00/2018), The Social Insurance Institution of Finland (Kela 148/52/2018) and Statistics Finland (TK-53-1870-18).
Germany: Studies based on GePaRD are exempt from institutional review board review.
Hong Kong: The institutional review board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW15-619)
Iceland: The National Bioethics Committee (VSN-18-123).
Norway: The Norwegian Data Inspectorate and the Regional Committee for Medical and Health Research Ethics (REC-South East).
Sweden: The regional ethics review board in Stockholm, Sweden (N 2015/1826-31/2).
UK: The Health Improvement Network Scientific Review Committee (18THIN072).
US: The institutional review board of Brigham and Women’s Hospital for the Medicaid data and Harvard TH Chan School of Public Health for the MarketScan data.
In the remaining participating countries, according to their respective regulations, no ethical approval was necessary for this study.
3. Results
The study included 8,394,343 pregnancies. Table 1 shows the prevalence of antipsychotic use in pregnancy by population, maternal age, and antipsychotic class. The overall prevalence of antipsychotic use during pregnancy ranged from 0.28% in Germany to 4.64% in the UK. The use of typical and atypical antipsychotics was lowest in Germany (0.12%) and Denmark (0.16%), respectively. The use of typical antipsychotics was highest in the UK (4.42%), whereas the use of atypical antipsychotics was highest in the US Max population (1.53%). Young women (≤24 years) had the highest use of typical antipsychotics in six of the eleven populations (Denmark, Germany, Iceland, Norway, Sweden, and US MarketScan) and of atypical antipsychotics in eight populations (Denmark, Finland, Germany, Hong Kong, Iceland, Norway, Sweden, and US MarketScan).
Table 1.
Antipsychotic drug use during the pregnancy period by country and maternal age.
| Pregnancies with at least 1 filled prescription of: | ||||
|---|---|---|---|---|
| Total number of pregnancies | Any antipsychotic | Typical antipsychotic | Atypical antipsychotic | |
| N | N (%) | N (%) | N (%) | |
| NSW, Australia 2004–2012 | ||||
| All ages | 148,462 | 2,355 (1.59) | 497 (0.33) | 2,020 (1.36) |
| ≤ 24 years | 50,573 | 635 (1.26) | 110 (0.22) | 559 (1.11) |
| 25–34 years | 73,399 | 1,195 (1.63) | 269 (0.37) | 1,017 (1.39) |
| ≥35 years | 24,480 | 523 (2.14) | 119 (0.49) | 443 (1.81) |
| Denmark 2000–2012 | ||||
| All ages | 813,360 | 2,858 (0.35) | 1,844 (0.23) | 1,269 (0.16) |
| ≤24 years | 87,014 | 458 (0.53) | 307 (0.35) | 211 (0.24) |
| 25–34 years | 485,356 | 1,240 (0.26) | 864 (0.18) | 487 (0.10) |
| ≥35 years | 125,804 | 565 (0.45) | 382 (0.30) | 218 (0.17) |
| Finland 2005–2014 | ||||
| All ages | 584,139 | 4,374 (0.75) | 977 (0.17) | 3,741 (0.64) |
| ≤24 years | 103,690 | 1,114 (1.07) | 166 (0.16) | 1,014 (0.98) |
| 25–34 years | 370,232 | 2,372 (0.64) | 549 (0.15) | 2,021 (0.55) |
| ≥35 years | 110,217 | 888 (0.81) | 262 (0.24) | 706 (0.64) |
| Germany 2006–2015 | ||||
| All ages | 999,105 | 2,842 (0.28) | 1,193 (0.12) | 1,912 (0.19) |
| ≤24 years | 80,050 | 369 (0.46) | 165 (0.21) | 241 (0.30) |
| 25–34 years | 616,444 | 1,442 (0.23) | 613 (0.10) | 960 (0.16) |
| ≥35 years | 302,611 | 1,031 (0.34) | 415 (0.14) | 711 (0.23) |
| Hong Kong 2001–2015 | ||||
| All ages | 416,494 | 1,408 (0.34) | 910 (0.22) | 705 (0.17) |
| ≤24 years | 43,205 | 187 (0.43) | 113 (0.26) | 110 (0.25) |
| 25–34 years | 269,014 | 744 (0.28) | 490 (0.18) | 357 (0.13) |
| ≥35 years | 104,274 | 477 (0.46) | 307 (0.29) | 238 (0.23) |
| Iceland 2004–2017 | ||||
| All ages | 60,477 | 881 (1.46) | 504 (0.83) | 435 (0.55) |
| ≤ 24 years | 10,738 | 239 (2.23) | 110 (1.02) | 145 (1.05) |
| 25–34 years | 37,237 | 487 (1.31) | 304 (0.82) | 216 (0.41) |
| ≥35 years | 12,502 | 155 (1.24) | 90 (0.72) | 32 (0.41) |
| Norway 2005–2015 | ||||
| All ages | 645,459 | 7,492 (1.16) | 6,162 (0.95) | 1,539 (0.24) |
| ≤ 24 years | 103,305 | 1,560 (1.51) | 1,236 (1.20) | 378 (0.37) |
| 25–34 years | 418,034 | 4,472 (1.07) | 3,772 (0.90) | 809 (0.19) |
| ≥35 years | 124,120 | 1,460 (1.18) | 1,154 (0.93) | 352 (0.28) |
| Sweden 2006–2015 | ||||
| All ages | 1,028,732 | 3,929 (0.38) | 2,079 (0.20) | 2,097 (0.20) |
| ≤ 24 years | 148,042 | 731 (0.49) | 367 (0.25) | 423 (0.29) |
| 25–34 years | 654,477 | 2,249 (0.34) | 1,243 (0.19) | 1,136 (0.17) |
| ≥35 years | 226,190 | 949 (0.42) | 538 (0.24) | 469 (0.21) |
| UK 2006–2016 | ||||
| All ages | 767,251 | 35,577 (4.64) | 33,884 (4.42) | 2,115 (0.28) |
| ≤ 24 years | 232,391 | 8,427 (3.63) | 8,093 (3.48) | 431 (0.19) |
| 25–34 years | 374,185 | 20,053 (5.36) | 19,187 (5.13) | 1,096 (0.29) |
| ≥35 years | 160,675 | 7,097 (4.42) | 6,604 (4.11) | 588 (0.37) |
| US, MarketScan 2012–2015 | ||||
| All ages | 859,505 | 6,761 (0.79) | 3,371 (0.39) | 3,514 (0.41) |
| ≤ 24 years | 134,218 | 1,905 (1.42) | 688 (0.51) | 1,261 (0.94) |
| 25–34 years | 532,887 | 3,485 (0.65) | 2,007 (0.38) | 1,534 (0.29) |
| ≥35 years | 192,400 | 1,371 (0.71) | 676 (0.35) | 719 (0.37) |
| US, MAX 2000–2013 | ||||
| All ages | 2,071,359 | 66,820 (3.23) | 37,200 (1.80) | 31,712 (1.53) |
| ≤ 24 years | 1,180,493 | 34,530 (2.93) | 19,626 (1.66) | 15,741 (1.33) |
| 25–34 years | 752,111 | 27,241 (3.62) | 15,151 (2.01) | 13,109 (1.74) |
| ≥35 years | 138,755 | 5,049 (3.64) | 2,423 (1.75) | 2,862 (2.06) |
Note: The pregnancy period is defined as 90 days before the date of the last menstrual period to the date of birth.
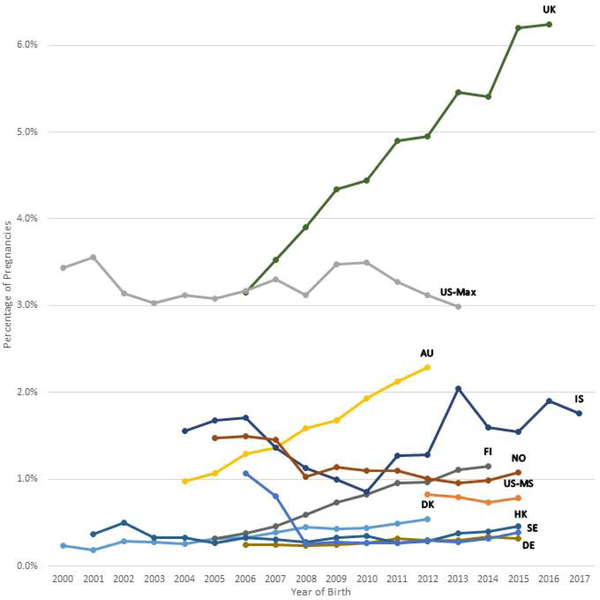
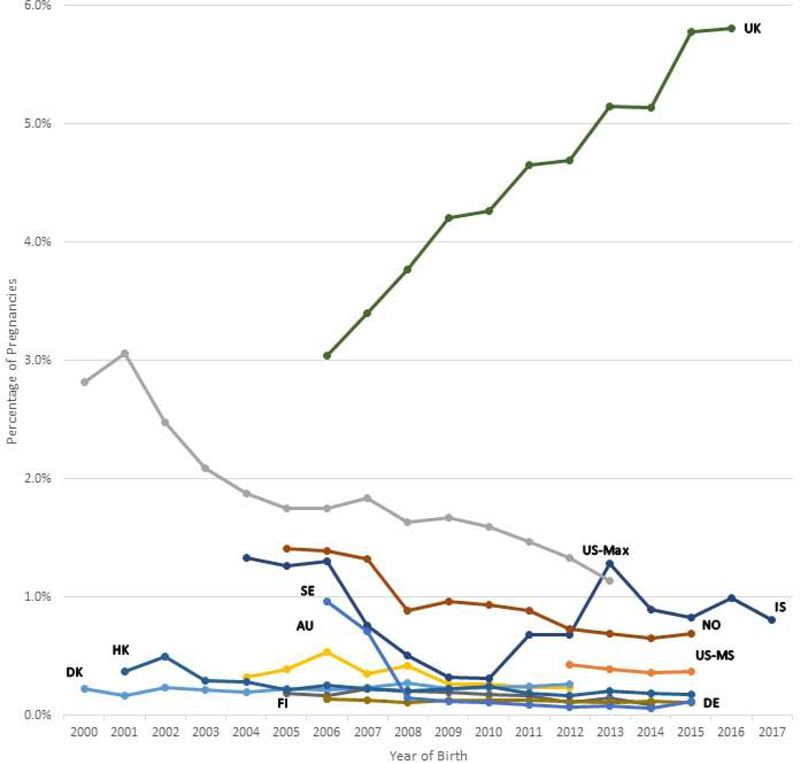
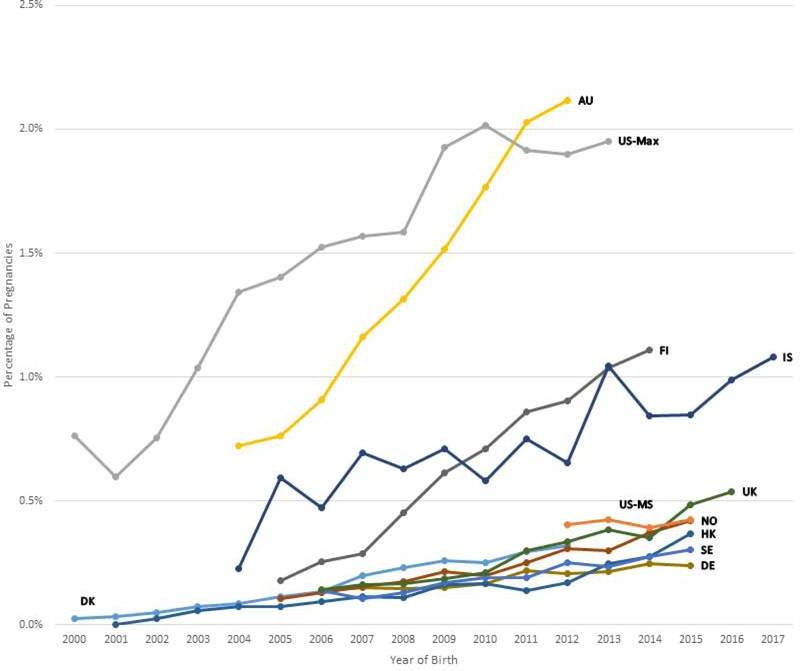
Figures 1a, 1b, and 1c show the trends in antipsychotic use in pregnancy by calendar year and population and Supplementary Table 2 shows the accompanying prevalence ratios and CIs. When comparing the first and last year of available data, overall antipsychotic use increased in six populations (Australia, Denmark, Finland, Germany, Iceland, and UK), with the largest increase in Finland (3.63-fold from 2005 to 2014) and Australia (2.34-fold from 2004 to 2012) (Suppl. Table 2). Overall antipsychotic use decreased in three populations (Norway, Sweden, and US Max).
Figure 1a-c.
Trends in antipsychotic drug use during the pregnancy period by population per year. The pregnancy period is defined as 90 days before the date of the last menstrual period to the date of birth.
Figure 1a Any antipsychotic drug use by population
Figure 1b Typical antipsychotic drug use by population
Figure 1c Atypical antipsychotic drug use by population
Abbreviations: AU = New South Wales, Australia; DK = Denmark; FI = Finland; DE = Germany; HK = Hong Kong; IS = Iceland; NO = Norway; SE = Sweden; GB = United Kingdom; US-MS = US MarketScan; US-MAX = US MAX
The y-axis scales for each country are different, and the trends in antipsychotic drug use should be interpreted accordingly.
The prevalence of typical antipsychotic use increased in the UK, was stable in three populations (Australia, Denmark, Iceland), and decreased in the other seven populations (Suppl. Table 2). Atypical antipsychotic use increased in all populations except in Iceland and US MarketScan (Figure 1c: Suppl. Table 2).
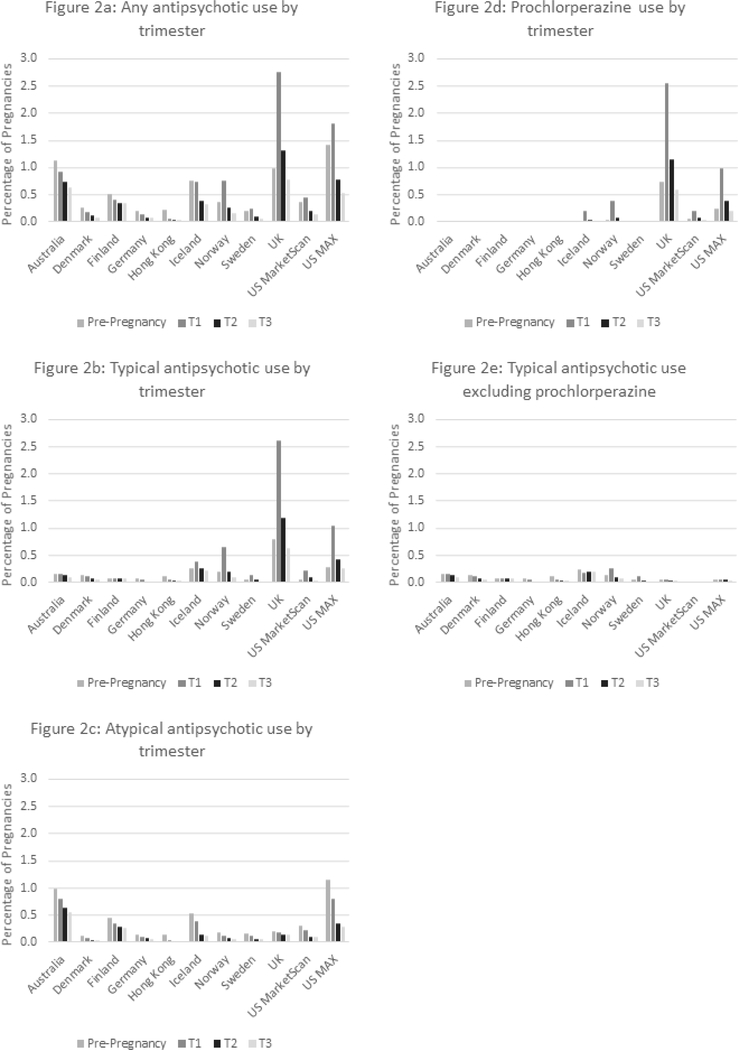
Figures 2a to 2e present the prevalence of antipsychotic drug use in the pre-pregnancy period and by trimester in each population. The overall use of antipsychotics was highest in the pre-pregnancy period in six populations and in the first trimester in the remaining five populations (Figure 2a). For typical antipsychotics, a slightly higher use in the pre-pregnancy period was found in four populations (Australia, Denmark, Germany, and Hong Kong), whereas the use was markedly higher in the first trimester in six populations (Iceland, Norway, Sweden, UK, US MarketScan, and US MAX) (Figure 2b). The use of typical antipsychotics declined from the first to the third trimester in all populations except for Finland, where prochlorperazine was not captured (Fig. 2b). For atypical antipsychotics, the use was highest 90 days before pregnancy in all populations, and thereafter decreased throughout pregnancy.
Figure 2: Antipsychotic drug use by trimester and population country.
Figure 2a-e Prevalence of antipsychotic drug use during the pregnancy period by trimester and population. The pregnancy period is defined as 90 days before the date of the last menstrual period to the date of birth.
Prochlorperazine was approved and captured in seven populations (Denmark, Iceland, Norway, Sweden, UK, US MarketScan, and US MAX). In these, its use decreased over time except in UK where its use nearly doubled (Suppl. Table 2, Suppl. Fig 1a). When the prochlorperazine users were excluded, a decreasing trend for typical antipsychotics was seen in six out of the eleven populations (Finland, Germany, Hong Kong, Norway, Sweden, and US MarketScan; Suppl. Table 2, Suppl. Figure 1b). Prochlorperazine use accounted for a large proportion of the use of typical antipsychotics in five populations (Iceland, Norway, UK, US MarketScan, and US MAX) (Fig. 2b and 2e), but the pattern of declining use over the trimesters remained after excluding the prochlorperazine users (Fig 2e).
Table 2 presents the five most commonly dispensed antipsychotics in the pregnancy period in the first and last year of available data by population. Atypical antipsychotics dominated in the most recent year in all populations, except that prochlorperazine continued to be the most commonly used antipsychotic in Norway, UK, and US. In the most recent year, quetiapine was the most commonly used atypical antipsychotic drug in all populations, followed by olanzapine in six populations and by aripiprazole in five populations. The proportion of pregnancies exposed to atypical antipsychotics increased markedly over time in all populations with quetiapine reaching 1.35% in Australia and 0.94% in Finland at the end of the study period.
Table 2.
Five most commonly dispensed antipsychotic drugs during the pregnancy period in the first and last year of available data by population.
| Rank | Antipsychotic | % of all APa users | % of all pregnancies | Rank | Antipsychotic | % of all APa users | % of all pregnancies |
|---|---|---|---|---|---|---|---|
| Australia | 2004 | 2012 | |||||
| 1 | Olanzapine | 36.94 | 0.36 | 1 | Quetiapine | 59.02 | 1.35 |
| 2 | Risperidone | 20.38 | 0.20 | 2 | Olanzapine | 24.59 | 0.56 |
| 3 | Quetiapine | 17.83 | 0.17 | 3 | Risperidone | 9.84 | 0.22 |
| 4 | Chlorpromazine | 13.38 | 0.13 | 4 | Aripiprazole | 5.46 | 0.12 |
| 5 | Haloperidol | 10.19 | 0.10 | 5 | Chlorpromazine | 3.83 | 0.09 |
| Denmark | 2000 | 2012 | |||||
| 1 | Flupentixol | 18.47 | 0.04 | 1 | Quetiapine | 39.87 | 0.21 |
| 2 | Zuclopenthixol | 17.83 | 0.04 | 2 | Chlorprothixene | 24.12 | 0.13 |
| 3 | Levomepromazine | 15.29 | 0.04 | 3 | Perphenazine | 17.04 | 0.09 |
| 4 | Chlorprothixene | 10.83 | 0.03 | 4 | Aripiprazole | 8.04 | 0.04 |
| 5 | Perphenazine | 9.55 | 0.02 | 5 | Olanzapine | 7.40 | 0.04 |
| Finland | 2005 | 2014 | |||||
| 1 | Quetiapine | 23.89 | 0.08 | 1 | Quetiapine | 81.37 | 0.94 |
| 2 | Olanzapine | 18.89 | 0.06 | 2 | Olanzapine | 9.92 | 0.11 |
| 3 | Perphenazine | 16.67 | 0.05 | 3 | Aripiprazole | 6.41 | 0.07 |
| 4 | Risperidone | 11.67 | 0.04 | 4 | Risperidone | 4.43 | 0.05 |
| 5 | Chlorprothixene | 8.89 | 0.03 | 5 | Perphenazine | 3.05 | 0.04 |
| Germany | 2006 | 2015 | |||||
| 1 | Olanzapine | 18.89 | 0.05 | 1 | Quetiapine | 47.86 | 0.15 |
| 2 | Fluspirilene | 17.97 | 0.04 | 2 | Aripiprazole | 12.03 | 0.04 |
| 3 | Quetiapine | 10.14 | 0.03 | 3 | Pipamperone | 9.36 | 0.03 |
| 4 | Perazine | 9.22 | 0.02 | 4 | Olanzapine | 8.56 | 0.03 |
| 5 | Risperidone | 9.22 | 0.02 | 5 | Risperidone | 8.02 | 0.03 |
| Hong Kong | 2001 | 2015 | |||||
| 1 | Haloperidol | 25.00 | 0.09 | 1 | Quetiapine | 38.20 | 0.17 |
| 2 | Chlorpromazine | 50.00 | 0.18 | 2 | Haloperidol | 20.22 | 0.09 |
| 3 | Trifluoperazine | 25.00 | 0.09 | 3 | Olanzapine | 16.85 | 0.08 |
| 4 | Thiridazine | 25.00 | 0.09 | 4 | Risperidone | 16.85 | 0.08 |
| 5 | - | - | - | 5 | Trifluoperazine | 16.29 | 0.07 |
| Iceland | 2004 | 2017 | |||||
| 1 | Prochlorperazine | 70.97 | 1.10 | 1 | Quetiapine | 50.00 | 0.88 |
| 2 | Quetiapine | 8.06 | 0.13 | 2 | Perphenazine | 32.86 | 0.58 |
| 3 | Chlorpromazine | 8.06 | 0.13 | 3 | Olanzapine | 11.43 | 0.20 |
| 4 | Levomepromazine | 4.84 | 0.08 | 4 | Levomepromazine | 7.14 | 0.13 |
| 5 | Risperidone | 3.23 | 0.05 | 5 | Flupentixol | 4.29 | 0.08 |
| Norway | 2005 | 2015 | |||||
| 1 | Prochlorperazine | 48.97 | 0.72 | 1 | Prochlorperazine | 41.53 | 0.45 |
| 2 | Chlorpromazine | 19.15 | 0.28 | 2 | Quetiapine | 27.32 | 0.29 |
| 3 | Levomepromazine | 13.82 | 0.20 | 3 | Levomepromazine | 10.86 | 0.12 |
| 4 | Dixyrazine | 8.97 | 0.13 | 4 | Olanzapine | 7.51 | 0.08 |
| 5 | Chlorprothixene | 5.21 | 0.08 | 5 | Chlorprothixene | 5.43 | 0.06 |
| Sweden | 2006 | 2015 | |||||
| 1 | Dixyrazine | 72.52 | 0.77 | 1 | Quetiapine | 45.06 | 0.18 |
| 2 | Prochlorperazine | 9.54 | 0.02 | 2 | Olanzapine | 22.99 | 0.09 |
| 3 | Olanzapine | 6.30 | 0.01 | 3 | Aripiprazole | 12.87 | 0.05 |
| 4 | Risperidone | 4.01 | 0.01 | 4 | Prochlorperazine | 10.80 | 0.04 |
| 5 | Levomepromazine | 3.05 | 0.01 | 5 | Levomepromazine | 8.74 | 0.03 |
| UK | 2006 | 2016 | |||||
| 1 | Prochlorperazine | 92.88 | 2.93 | 1 | Prochlorperazine | 91.39 | 5.71 |
| 2 | Olanzapine | 1.86 | 0.06 | 2 | Quetiapine | 5.68 | 0.35 |
| 3 | Chlorpromazine | 1.82 | 0.06 | 3 | Aripiprazole | 1.69 | 0.11 |
| 4 | Quetiapine | 1.61 | 0.05 | 4 | Olanzapine | 1.48 | 0.09 |
| 5 | Flupentixol | 1.40 | 0.04 | 5 | Chlorpromazine | 0.91 | 0.06 |
| US MarketScan | 2012 | 2015 | |||||
| 1 | Prochlorperazine | 48.66 | 0.40 | 1 | Prochlorperazine | 44.91 | 0.35 |
| 2 | Aripiprazole | 20.49 | 0.17 | 2 | Quetiapine | 22.66 | 0.18 |
| 3 | Quetiapine | 19.90 | 0.16 | 3 | Aripiprazole | 17.36 | 0.14 |
| 4 | Risperidone | 5.60 | 0.05 | 4 | Lurasidone | 6.44 | 0.05 |
| 5 | Olanzapine | 3.27 | 0.03 | 5 | Risperidone | 5.93 | 0.05 |
| US MAX | 2000 | 2013 | |||||
| 1 | Prochlorperazine | 80.93 | 2.88 | 1 | Prochlorperazine | 37.89 | 1.18 |
| 2 | Olanzapine | 7.97 | 0.28 | 2 | Quetiapine | 23.70 | 0.74 |
| 3 | Risperidone | 6.19 | 0.22 | 3 | Aripiprazole | 20.93 | 0.65 |
| 4 | Quetiapine | 3.65 | 0.13 | 4 | Risperidone | 11.71 | 0.37 |
| 5 | Haloperidol | 1.86 | 0.07 | 5 | Olanzapine | 4.67 | 0.15 |
Note: The pregnancy period is defined as 90 days before the date of the last menstrual period to the date of birth.
Annotation: Antipsychotic names in light gray = typical antipsychotic; white = atypical antipsychotic; dark gray = typical antipsychotic usually used as an antiemetic.
AP = Antipsychotic
Table 3 presents the prevalence of antipsychotic use among pregnant women in the Nordic countries by demographic and pregnancy-related characteristics. Antipsychotic use was more prevalent among women with higher parity, reaching 0.92% for any antipsychotic among women with parity of four or more. Furthermore, the prevalence of antipsychotic use was 1.46% in smokers versus 0.43% among non-smokers during pregnancy, and both typical and atypical antipsychotic use was higher in smokers.
Table 3.
Pooled prevalence of antipsychotic drug use during the pregnancy period in the Nordic countries by maternal and pregnancy characteristics.
| Any antipsychotic | Typical antipsychotic | Atypical antipsychotic | |||||
|---|---|---|---|---|---|---|---|
| Country (weight %) | Total | N | Prevalence (95% CI)a | N | Prevalence (95% CI)a | N | Prevalence (95% CI)a |
| Pooled (100) | 3,132,167 | 19,534 | 0.58 (0.58, 0.59) | 11,566 | 0.32 (0.31, 0.33) | 9,081 | 0.27 (0.26, 0.27) |
| Denmark (26.0) | 813,360 | 2,858 | 0.35 (0.34, 0.36) | 1,844 | 0.23 (0.22, 0.24) | 1,269 | 0.16 (0.15, 0.16) |
| Iceland (1.9) | 60,477 | 881 | 1.46 (1.36, 1.55) | 504 | 0.83 (0.76, 0.91) | 435 | 0.72 (0.65, 0.79) |
| Finland (18.7) | 584,139 | 4,374 | 0.75 (0.73, 0.77) | 977 | 0.17 (0.16, 0.18) | 3,741 | 0.64 (0.62, 0.66) |
| Norway (20.6) | 645,459 | 7,492 | 1.16 (1.13, 1.19) | 6,162 | 0.95 (0.93, 0.98) | 1,539 | 0.24 (0.23, 0.25) |
| Sweden (32.8) | 1,028,732 | 3,929 | 0.38 (0.37, 0.39) | 2,079 | 0.20 (0.19, 0.21) | 2,097 | 0.20 (0.20, 0.21) |
| Parity | |||||||
| 1 | 1,299,079 | 7,580 | 0.55 (0.54, 0.56) | 4,497 | 0.30 (0.29, 0.31) | 3,583 | 0.27 (0.26, 0.28) |
| 2 | 1,004,582 | 5,177 | 0.47 (0.45, 0.48) | 3,600 | 0.34 (0.33, 0.35) | 1,782 | 0.16 (0.16, 0.17) |
| 3 | 381,385 | 2,553 | 0.62 (0.60, 0.65) | 1,781 | 0.41 (0.39, 0.43) | 895 | 0.22 (0.21, 0.24) |
| ≥4 | 169,324 | 1,604 | 0.92 (0.87, 0.96) | 1,053 | 0.57 (0.53, 0.60) | 658 | 0.38 (0.36, 0.41) |
| missing | 277,797 | 2,620 | 0.93 (0.90, 0.97) | 635 | 0.21 (0.19, 0.23) | 2,163 | 0.21 (0.19, 0.23) |
| Smokingb | |||||||
| No | 2,551,171 | 11,892 | 0.43 (0.42, 0.44) | 7,411 | 0.24 (0.24, 0.25) | 4,953 | 0.18 (0.18, 0.19) |
| Yes | 341,726 | 5,123 | 1.46 (1.42, 1.50) | 2,448 | 0.68 (0.65, 0.71) | 3,198 | 0.86 (0.83, 0.89) |
| Missing | 178,793 | 1,638 | 0.88 (0.84, 0.92) | 1,203 | 0.62 (0.58, 0.65) | 495 | 0.27 (0.24, 0.29) |
| Cohabitationb | |||||||
| Cohabiting | 1,876,289 | 8,653 | 0.49 (0.48, 0.50) | 3,930 | 0.20 (0.20, 0.21) | 5,368 | 0.33 (0.32, 0.34) |
| Not cohabiting | 516,888 | 2,721 | 0.50 (0.48, 0.52) | 1,172 | 0.39 (0.38, 0.41) | 1,750 | 0.65 (0.63, 0.66) |
| Missing | 93,531 | 668 | 0.70 (0.65, 0.75) | 302 | 0.36 (0.32, 0.39) | 424 | 1.06 (1.03, 1.08) |
| Multi-fetal Pregnancy | |||||||
| No | 3,077,982 | 19,231 | 0.58 (0.58, 0.59) | 11,392 | 0.34 (0.33, 0.35) | 8,939 | 0.27 (0.26, 0.27) |
| Yes | 53,052 | 302 | 0.54 (0.48, 0.60) | 174 | 0.31 (0.26, 0.36) | 142 | 0.23 (0.19, 0.27) |
| Year of Birth | |||||||
| 2000–2004d | 323,511 | 852 | 0.26 (0.24, 0.27) | 712 | 0.21 (0.20, 0.23) | 179 | 0.23 (0.19, 0.27) |
| 2005–2009 | 1,285,897 | 8,695 | 0.64 (0.62, 0.65) | 6,457 | 0.44 (0.43, 0.45) | 2,730 | 0.20 (0.20, 0.21) |
| 2010–2017 | 1,522,759 | 9,987 | 0.61 (0.59, 0.62) | 4,397 | 0.23 (0.22, 0.24) | 6,172 | 0.37 (0.36, 0.38) |
Note: The pregnancy period is defined as 90 days before the date of the last menstrual period to the date of birth.
Prevalence per 100 pregnancies weighted by population and 95% confidence intervals (CI)
Data on smoking available for Denmark, Finland, Norway, Sweden; Data on cohabitation available for Denmark, Iceland, Finland, Sweden; For Denmark ‘Cohabiting’ reflects marital status
Only Denmark and Iceland included births occurring in 2000–2004
4. Discussion
4.1. Key results
In our study of over eight million pregnancies with data from 2000 to 2017 in ten countries (eleven populations), applying a uniform approach for data analysis, the use of antipsychotics during the pregnancy period varied considerably between countries. The highest prevalence of typical antipsychotics was observed in the UK (4.42%, driven by the use of prochlorperazine) and of atypical antipsychotics in the US Max population (1.53%). In most populations, the use of typical antipsychotics decreased or was stable, whereas atypical antipsychotic use increased over time. Use of antipsychotics decreased with each trimester of pregnancy in most populations.
4.2. Interpretation & comparison with other studies
Factors which may explain differences in antipsychotic use between the countries include varying clinical practices reflecting different guidelines (Graham et al., 2018), pricing policies and reimbursement practices which may influence physicians’ prescribing patterns. There may also be differences in what proportion of the actual antipsychotic medication is distributed from outpatient pharmacies as opposed to directly from psychiatric or other clinics. Furthermore, the prevalence of mental disorders may differ between settings and countries. Within the US, there was a notably higher antipsychotic use among the publicly-insured (MAX) than the privately-insured (MarketScan) women. This may be because the publicly-insured US MAX population includes women with low economic resources, in whom psychiatric disorders are more prevalent (Kasper, 1986). This interpretation should also be applied to the Australian estimates, It may also be partly due to our inclusion of prochlorperazine, as lower rates reported previously from US MAX did not include that medication (Park et al., 2017). Perceptions and attitudes among the mentally ill and care providers regarding the value of antipsychotics (Morrison et al., 2015; Velligan et al., 2009) may also differ. Finally, some classification differences may apply. For example, in Australia, prochlorperazine is classified as an antiemetic (ATC A04AD) instead of as an antipsychotic and was therefore not included in the study data.
Previous reports regarding patterns of antipsychotic drug use among pregnant women come from a number of country-specific studies. A study of data from 11 different private health plans from 2001 to 2007 in the US, found a stable prevalence of 0.09% for typical antipsychotics but an increasing trend from 0.33% to 0.82% for atypical antipsychotics (Toh et al., 2013). Similar patterns were reported in both a Tennessee Medicaid study (Epstein et al., 2013), and a previous Medicaid MAX study covering 2001 to 2010, which partly overlap with our study (Park et al., 2017). Also spanning different time periods, data from some of the other data sources included in this study have also been reported in country-specific studies previously. Thus, in THIN data (UK) from 1995 to 2007, 0.29% of women were prescribed antipsychotics in the six months before they became pregnant and 0.19% of women after the first six weeks of pregnancy, with an overall time trend of increasing use of atypical antipsychotics whereas that of typical antipsychotics decreased (Petersen et al., 2014). In Hong Kong, from 2004 to 2014, the prevalence of antipsychotic use in pregnancy increased from 0.18% to 0.27% (Lao et al., 2017). Our data from the UK and Hong Kong cover more recent years, and for the US we include a broader population, yet these trends have persisted. In Denmark, a prevalence of antipsychotic use of 0.20% was reported among pregnant women from 1997 to 2012 (Ingstrup et al., 2018) and in Norway, 1% of pregnant women used antipsychotics (including lithium) from 2005 to 2015 (Engeland et al., 2018)Time trends were not reported in these studies, but our analyses of data for similar time periods found increasing use of atypical antipsychotics also in Denmark and Norway. [22][23][24][25, 26]
During the study period, new atypical antipsychotics were marketed and indications were expanded, which together with off-label use (Alexander et al., 2011; Maher and Theodore, 2012) and removal of some older typical antipsychotics (e.g. dixyrazine) from the market in certain countries, may explain the increase in use of atypical antipsychotics in our study populations. Atypical antipsychotics have increasingly been recommended as treatment for bipolar disorder and as add-on treatment for unipolar depression, especially with quetiapine, olanzapine, and aripiprazole (Kennedy et al., 2016). Further, atypical antipsychotics may be preferred because of safety concerns regarding antiepileptics as mood stabilizers in women with bipolar disorder during pregnancy (Petersen et al., 2017). Quetiapine was the most commonly dispensed atypical antipsychotic in all countries, possibly partly due to off-label use for indications such as insomnia (McKean and Monasterio, 2012), with a similar pattern of increasing use found for aripiprazole. Our findings for pregnant women mirror the trend of increasing use of atypical antipsychotics in the general population worldwide (Halfdanarson et al., 2017).
For typical antipsychotics, use was clearly most common in the first trimester, especially in countries where prochlorperazine use was captured. Prochlorperazine is almost exclusively used as an antiemetic (Fiaschi et al., 2019), and nausea and vomiting is usually most pronounced in the first trimester (Louik et al., 2006). Our results further suggest that many women did not continue to refill their antipsychotic prescriptions, or physicians stopped prescribing, during the second and third trimester. This corroborates findings for antipsychotics in the UK in both the CPRD and THIN databases (Margulis et al., 2014; Petersen et al., 2014), Sweden in 2007 (Stephansson et al., 2011), and in the Sentinel system in the US (Illoh et al., 2018). Even after removing the women who were prescribed prochlorperazine, the pattern of decreased use remained as the pregnancies progressed. A similar pattern has also been observed for antidepressants (Illoh et al., 2018; Stephansson et al., 2011; Zoega et al., 2015). Discontinuation of psychotropic medication during pregnancy is common due to concerns that fetal exposure to these medication may have harmful effects for the child (Einarson et al., 2001), although the data regarding antipsychotics are not yet conclusive (Huybrechts et al., 2016). Some women who filled antipsychotic prescriptions in the first trimester may not yet have been aware that they were pregnant, and the pregnancy may have been unintended (Finer and Zolna, 2016). It could be speculated that stopping the use of antipsychotics during the latter part of pregnancy may decrease the risk of delayed neural development and pregnancy complications, including gestational diabetes. On the other hand, there is a high risk of relapse for those who discontinue medication for schizophrenia (Lin et al., 2010) and bipolar disorder (Viguera et al., 2007; Yonkers et al., 2004), and untreated psychiatric illness may confer health risks both for the mother and unborn child, as well as for the child after birth (Boden et al., 2012a; Gentile, 2017).
In the Nordic countries we found that pregnant women with four or more previous deliveries had the highest antipsychotic use, which was not explained by age; there was an inverse association between antipsychotic use and age. Pregnant women who smoked during pregnancy had a higher prevalence of typical and atypical antipsychotic use than non-smoking women, similar to findings reported for SSRIs and SNRIs (Zoega et al., 2015). This was expected since the rate of smoking is much higher among individuals with mental disorders (de Leon and Diaz, 2005; Jimenez-Solem et al., 2013). The finding may imply that women with mental disorders have a different pattern of risk factors of adverse outcomes, pointing to the need to control for such factors in future studies evaluating outcomes in relation to antipsychotic medication during pregnancy.
5.4. Limitations
Limitations that are inherent in the observational design include circumstances that may have led to overestimation because the analyses were based on prescriptions or dispensing of antipsychotic medication for which the adherence to treatment is not known. Underestimation of antipsychotic use may also have occurred since antipsychotic medication dispensed directly to the women by hospitals or other clinics were not captured, or because they were not reimbursed antipsychotics; the latter was the case for prochlorperazine in Australia and Finland. The underlying indication for the prescribed antipsychotics was not available in the study data. Further, the databases differ in their set up and collection of data, with the Nordic countries providing data for the whole population, whereas the data from the non-Nordic countries were selected samples to varying degrees but are still considered representative of their country’s pregnant population (Panel 1). For Finland, the first trimester was shorter than for the other countries, which may have affected the proportion of use during T1; however, this is not expected to affect the overall conclusions of the study which are not related to the investigation of outcomes during a specific exposure period. Finally, a limitation of our study is that countries had different time periods of data availability for antipsychotic use during pregnancy, but we consider it unlikely that the main patterns and trends identified in this study would change in the countries with fewer years of follow-up.
4.5. Conclusion
In summary, this study found that the prevalence of antipsychotic drug use varied between populations, partly driven by variations in the capture of prochlorperazine mainly used for nausea in early pregnancy. The use of antipsychotics was highest pre-pregnancy and at the beginning of the pregnancy. Most countries showed an increasing trend for use of atypical antipsychotics. This reflects the pattern in the general population, and demonstrates the worldwide uptake of newer antipsychotic medication, also in pregnant women.
Supplementary Material
Acknowledgements
Pär Karlsson for statistical assistance. The German statutory health insurance providers that provided data for the study in GePaRD, namely the AOK Bremen/Bremerhaven, the DAK-Gesundheit, and Die Techniker (TK). The NSW Ministry of Health, the Australian Government Department of Health and Ageing and the Department of Human Services provided data, and the Centre for Health Record Linkage (CHeReL) and the Australian Institute of Health and Welfare for conducting the linkage of Australian data.
Role of the funding source
This study was funded in part by NordForsk as part of the Nordic Pregnancy Drug Safety Studies (NorPreSS) project (Project No: 83539), the Research Council of Norway as part of the International Pregnancy Drug Safety Studies (InPreSS) (Project No: 273366 and Project No: 262700), and the National Institute of Child Health and Human Development (R21 HD092879). Linkage of the Australian data was supported by an Australian National Health and Medical Research Council project grant (No: 1028543). GB was supported by Region Stockholm (clinical postdoctoral appointment) and by the Swedish Society of Medicine. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
JR, CC, LP, and GB, are employees of the Centre for Pharmacopidemiology which receives funding from pharmaceutical companies and regulatory authorities for drug safety/utilization studies, unrelated to the submitted work. BTB has participated as an investigator on grants to the Brigham and Women’s Hospital from Pfizer, GSK, Lilly, Baxalta, and Pacira, not related to the topic of the submitted work. SH-D has participated as investigator in projects funded by Pfizer, GSK, and Lilly; and consulted for Boehringer-Ingelheim, Roche and UCB as a methods advisor for pregnancy studies. KFH has participated as an investigator on grants to the Brigham and Women’s Hospital from Boehringer Ingelheim, Pfizer, Lilly and GSK, not related to the topic of the submitted work.
Footnotes
Appendix A. Supplementary data
Conflict of interest
The other authors declare no personal conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS, 2011. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol. Drug. Saf 20(2), 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T, 2013. Meta-analysis of prevalence. J. Epidemiol. Community Health 67(11), 974–978. [DOI] [PubMed] [Google Scholar]
- Boden R, Lundgren M, Brandt L, Reutfors J, Andersen M, Kieler H, 2012a. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ 345, e7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden R, Lundgren M, Brandt L, Reutfors J, Kieler H, 2012b. Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Arch. Gen. Psychiatry 69(7), 715–721. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ, 2005. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res 76(2–3), 135–157. [DOI] [PubMed] [Google Scholar]
- Einarson A, Selby P, Koren G, 2001. Abrupt discontinuation of psychotropic drugs during pregnancy: fear of teratogenic risk and impact of counselling. J. Psychiatry Neurosci 26(1), 44–48. [PMC free article] [PubMed] [Google Scholar]
- Engeland A, Bjorge T, Klungsoyr K, Hjellvik V, Skurtveit S, Furu K, 2018. Trends in prescription drug use during pregnancy and postpartum in Norway, 2005 to 2015. Pharmacoepidemiol. Drug Saf 27(9), 995–1004. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Bobo WV, Shelton RC, Arbogast PG, Morrow JA, Wang W, Chandrasekhar R, Cooper WO, 2013. Increasing use of atypical antipsychotics and anticonvulsants during pregnancy. Pharmacoepidemiol. Drug Saf 22(7), 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaschi L, Nelson-Piercy C, Deb S, King R, Tata LJ, 2019. Clinical management of nausea and vomiting in pregnancy and hyperemesis gravidarum across primary and secondary care: a population-based study. BJOG 126(10):1201–1211. [DOI] [PubMed] [Google Scholar]
- Finer LB, Zolna MR, 2016. Declines in Unintended Pregnancy in the United States, 2008–2011. N. Engl. J. Med 374(9), 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sorensen HT, 2010. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin. Pharmacol. Toxicol 106(2), 86–94. [DOI] [PubMed] [Google Scholar]
- Gentile S, 2017. Untreated depression during pregnancy: Short- and long-term effects in offspring. A systematic review. Neuroscience 342, 154–166. [DOI] [PubMed] [Google Scholar]
- Graham RK, Tavella G, Parker GB, 2018. Is there consensus across international evidence-based guidelines for the psychotropic drug management of bipolar disorder during the perinatal period? J. Affect. Disord 228, 216–221. [DOI] [PubMed] [Google Scholar]
- Halfdanarson O, Zoega H, Aagaard L, Bernardo M, Brandt L, Fuste AC, Furu K, Garuoliene K, Hoffmann F, Huybrechts KF, Kalverdijk LJ, Kawakami K, Kieler H, Kinoshita T, Litchfield M, Lopez SC, Machado-Alba JE, Machado-Duque ME, Mahesri M, Nishtala PS, Pearson SA, Reutfors J, Saastamoinen LK, Sato I, Schuiling-Veninga CCM, Shyu YC, Skurtveit S, Verdoux H, Wang LJ, Yahni CZ, Bachmann CJ, 2017. International trends in antipsychotic use: A study in 16 countries, 2005–2014. Eur. Neuropsychopharmacol 27(10), 1064–1076. [DOI] [PubMed] [Google Scholar]
- Hojlund M, Pottegard A, Johnsen E, Kroken RA, Reutfors J, Munk-Jorgensen P, Correll CU, 2019. Trends in utilization and dosing of antipsychotic drugs in Scandinavia: Comparison of 2006 and 2016. Br. J. Clin. Pharmacol 85(7):1598–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts KF, Hernandez-Diaz S, Patorno E, Desai RJ, Mogun H, Dejene SZ, Cohen JM, Panchaud A, Cohen L, Bateman BT, 2016. Antipsychotic Use in Pregnancy and the Risk for Congenital Malformations. JAMA psychiatry 73(9), 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illoh OA, Toh S, Andrade SE, Hampp C, Sahin L, Gelperin K, Taylor L, Bird ST, 2018. Utilization of drugs with pregnancy exposure registries during pregnancy. Pharmacoepidemiol. Drug Saf 27(6), 604–611. [DOI] [PubMed] [Google Scholar]
- Ingstrup KG, Liu X, Gasse C, Debost JP, Munk-Olsen T, 2018. Prescription drug use in pregnancy and variations according to prior psychiatric history. Pharmacoepidemiol. Drug Saf 27(1), 105–113. [DOI] [PubMed] [Google Scholar]
- Jimenez-Solem E, Andersen JT, Petersen M, Broedbaek K, Andersen NL, Torp-Pedersen C, Poulsen HE, 2013. Prevalence of antidepressant use during pregnancy in Denmark, a nation-wide cohort study. PloS One 8(4), e63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper JD, 1986. Health status and utilization: differences by Medicaid coverage and income. Health Care Financ. Rev 7(4), 1–17. [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Muller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R, Group CDW, 2016. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Can. J. Psychiatry 61(9), 540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhoff-Roos J, Krebs L, Klungsoyr K, Bjarnadottir RI, Kallen K, Tapper AM, Jakobsson M, Bordahl PE, Lindqvist PG, Gottvall K, Colmorn LB, Gissler M, 2014. The Nordic medical birth registers--a potential goldmine for clinical research. Acta Obstet. Gynecol. Scand 93(2), 132–137. [DOI] [PubMed] [Google Scholar]
- Lao KSJ, Tam AWY, Wong ICK, Besag FMC, Man KKC, Chui CSL, Chan EW, 2017. Prescribing trends and indications of antipsychotic medication in Hong Kong from 2004 to 2014: General and vulnerable patient groups. Pharmacoepidemiol. Drug Saf 26(11), 1387–1394. [DOI] [PubMed] [Google Scholar]
- Lin HC, Chen IJ, Chen YH, Lee HC, Wu FJ, 2010. Maternal schizophrenia and pregnancy outcome: does the use of antipsychotics make a difference? Schizophr. Res 116(1), 55–60. [DOI] [PubMed] [Google Scholar]
- Louik C, Hernandez-Diaz S, Werler MM, Mitchell AA, 2006. Nausea and vomiting in pregnancy: maternal characteristics and risk factors. Paediatr. Perinat. Epidemiol 20(4), 270–278. [DOI] [PubMed] [Google Scholar]
- MacDonald SC, Cohen JM, Panchaud A, McElrath TF, Huybrechts KF, Hernandez-Diaz S, 2019. Identifying pregnancies in insurance claims data: Methods and application to retinoid teratogenic surveillance. Pharmacoepidemiol. Drug Saf 28(9), 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher AR, Theodore G, 2012. Summary of the comparative effectiveness review on off-label use of atypical antipsychotics. J. Manag. Care Pharm 18(5 Suppl B), S1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, Lau WCY, Schuemie MJ, Sturkenboom M, Wong ICK, 2017. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ 357, j2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis AV, Kang EM, Hammad TA, 2014. Patterns of prescription of antidepressants and antipsychotics across and within pregnancies in a population-based UK cohort. Matern. Child Health J 18(7), 1742–1752. [DOI] [PubMed] [Google Scholar]
- McKean A, Monasterio E, 2012. Off-label use of atypical antipsychotics: cause for concern? CNS Drugs 26(5), 383–390. [DOI] [PubMed] [Google Scholar]
- Minami F, Zohar J, Suzuki T, Koizumi T, Mimura M, Yagi G, Uchida H, 2018. Discrepancies Between Nomenclature and Indications of Psychotropics. Pharmacopsychiatry [DOI] [PubMed] [Google Scholar]
- Morrison P, Meehan T, Stomski NJ, 2015. Australian case managers’ views about the impact of antipsychotic medication on mental health consumers. Int. J. Ment. Health Nurs 24(6), 547–553. [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu SM, Wang S, Correll CU, 2012. National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Arch. Gen. Psychiatry 69(12), 1247–1256. [DOI] [PubMed] [Google Scholar]
- Palmsten K, Huybrechts KF, Mogun H, Kowal MK, Williams PL, Michels KB, Setoguchi S, Hernandez-Diaz S, 2013. Harnessing the Medicaid Analytic eXtract (MAX) to Evaluate Medications in Pregnancy: Design Considerations. PloS One 8(6), e67405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Huybrechts KF, Cohen JM, Bateman BT, Desai RJ, Patorno E, Mogun H, Cohen LS, Hernandez-Diaz S, 2017. Antipsychotic Medication Use Among Publicly Insured Pregnant Women in the United States. Psychiatr. Serv 68(11), 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen I, Collings SL, McCrea RL, Nazareth I, Osborn DP, Cowen PJ, Sammon CJ, 2017. Antiepileptic drugs prescribed in pregnancy and prevalence of major congenital malformations: comparative prevalence studies. Clin. Epidemiol 9, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen I, McCrea RL, Osborn DJ, Evans S, Pinfold V, Cowen PJ, Gilbert R, Nazareth I, 2014. Discontinuation of antipsychotic medication in pregnancy: a cohort study. Schizophr. Res 159(1), 218–225. [DOI] [PubMed] [Google Scholar]
- Stephansson O, Granath F, Svensson T, Haglund B, Ekbom A, Kieler H, 2011. Drug use during pregnancy in Sweden - assessed by the Prescribed Drug Register and the Medical Birth Register. Clin. Epidemiol 3, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh S, Li Q, Cheetham TC, Cooper WO, Davis RL, Dublin S, Hammad TA, Li DK, Pawloski PA, Pinheiro SP, Raebel MA, Scott PE, Smith DH, Bobo WV, Lawrence JM, Dashevsky I, Haffenreffer K, Avalos LA, Andrade SE, 2013. Prevalence and trends in the use of antipsychotic medications during pregnancy in the U.S., 2001–2007: a population-based study of 585,615 deliveries. Arch. Womens Ment. Health 16(2), 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato S, Albert U, Tomassi S, Iasevoli F, Carmassi C, Ferrari S, Nanni MG, Nivoli A, Volpe U, Atti AR, Fiorillo A, 2017. A Systematized Review of Atypical Antipsychotics in Pregnant Women: Balancing Between Risks of Untreated Illness and Risks of Drug-Related Adverse Effects. J. Clin. Psychiatry 78(5), e477–e489. [DOI] [PubMed] [Google Scholar]
- Tran DT, Havard A, Jorm LR, 2017. Data cleaning and management protocols for linked perinatal research data: a good practice example from the Smoking MUMS (Maternal Use of Medications and Safety) Study. BMC Med. Res. Methodol 17(1), 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Weiden PJ, Sajatovic M, Scott J, Carpenter D, Ross R, Docherty JP, Expert Consensus Panel on Adherence Problems in, S., Persistent Mental, I., 2009. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J. Clin. Psychiatry 70 Suppl 4, 1–46. [PubMed] [Google Scholar]
- Viguera AC, Whitfield T, Baldessarini RJ, Newport DJ, Stowe Z, Reminick A, Zurick A, Cohen LS, 2007. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am. J. Psychiatry 164(12), 1817–1824. [DOI] [PubMed] [Google Scholar]
- Wentzell N, Schink T, Haug U, Ulrich S, Niemeyer M, Mikolajczyk R, 2018. Optimizing an algorithm for the identification and classification of pregnancy outcomes in German claims data. Pharmacoepidemiol. Drug Saf 27(9), 1005–1010. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stowe Z, Leibenluft E, Cohen L, Miller L, Manber R, Viguera A, Suppes T, Altshuler L, 2004. Management of bipolar disorder during pregnancy and the postpartum period. Am. J. Psychiatry 161(4), 608–620. [DOI] [PubMed] [Google Scholar]
- Zoega H, Kieler H, Norgaard M, Furu K, Valdimarsdottir U, Brandt L, Haglund B, 2015. Use of SSRI and SNRI Antidepressants during Pregnancy: A Population-Based Study from Denmark, Iceland, Norway and Sweden. PloS One 10(12), e0144474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.