Abstract
The efficacy and possible role of epidermal growth factor receptor tyrosine kinase inhibitors in treating early-stage non-small-cell lung cancer have yet to be established. Therefore, we aimed to explore the efficacy and safety of icotinib in completely resected EGFR-mutant stage II–IIIA lung adenocarcinoma patients who underwent standard chemotherapy. This is a randomised, double-blinded, placebo-controlled, multicentre, Phase III trial. A total of 124 patients aged 18–75 years who qualified the inclusion criteria were recruited. These patients were randomised (1:1) to receive either icotinib (125 mg 3 times per day) or placebo (the same dosage and frequency) for 36 months, followed by a further 36 months of observational window. The primary endpoint is disease-free survival (DFS), while the secondary endpoints are overall survival, 3-year and 5-year DFS, safety and tolerability of the medication, and health-related quality-of-life. Analyses will be conducted in a full analysis set and a per-protocol set as well. To our knowledge, the present study is the first randomised, double-blinded, placebo-controlled, multicenter trial designed to explore efficacy and safety of icotonib in this population. The results obtained in the near future may provide potential guidance in clinical practice.
Trial Registration: This trial was registered on www.ClinicalTrail.gov as NCT02125240.
Keywords: EGFR mutation, non-small-cell lung cancer, lung adenocarcinoma, icotinib, adjuvant chemotherapy
Introduction
In China and all around the world, lung cancer remains the most common cause of death in cancer patients.1 Of all lung cancers, non-small cell lung cancer (NSCLC), as a subclass, accounts for about 80–85% of the total incidence.2 Adenocarcinoma is currently the predominant histological subtype of NSCLC.3,4 The reported average 5-year survival rate varies in the literature, but is estimated at approximately 15–30%.5,6 So far, surgical resection is the main stream treatment for NSCLC patients at early stages (I–IIIA).6,7 However, 40–60% of these patients die within 5 years after surgical resection due to relapse.8 Therefore, an urgent need of establishing an appropriate adjuvant therapy is calling for these patients.
Emerging data from meta-analyses have shown improved survival from adjuvant chemotherapy of a platinum-based regimen.6,9-13 In 2013, the guideline issued by the National Comprehensive Cancer Network (NCCN) recommended postoperative adjuvant chemotherapy in stage IB-IIIA NSCLC patients who underwent complete resection (R0).14 However, haematological toxicity has become a critical issue in platinum-based adjuvant regimens.9,15,16 This also leads to delay, dose reduction, and incompletion of the treatment.11,15,16
In recent years, epidermal growth factor receptor (EGFR) has drawn researchers’ interest ever since its role was discovered in tumorigenesis.17–20 Mutations in the EGFR gene are detected in approximately 10–40% of the general population, with a higher prevalence in Asian populations than in Caucasian.21–24 The most common mutations are the exon 19 deletion and exon 21 L858R (a leucine to arginine amino acid substitution at 858), accounting for nearly 90% of all EGFR mutations.25,26 The treatment of NSCLC has dramatically improved since the discovery that EGFR mutations respond to EGFR tyrosine kinase inhibitors (EGFR-TKIs).27–29 So far, studies have shown EGFR-TKIs are associated with higher response rates and longer progression-free survival (PFS) compared to platinum doublet regimens.21,27,30-34
Icotonib, a first-generation EGFR-TKI, has shown some unique advantages over gefitinib in previous studies. The high specificity and selectivity for EGFR were demonstrated in a preclinical kinase profiling study.35 Safety profiles reported only 3 cases of interstitial lung disease (ILD), while the most common adverse drug reactions (ADRs) were rashes and diarrhoea.36–38 The clinical benefit of icotinib was further reported in a Phase II study.39 Our previous phase III non-inferiority trial40 demonstrated that icotinib was non-inferior to a standard treatment of gefitinib with regard to PFS. Moreover, patients who were given icotinib experienced fewer ADRs than those given gefitinib.
We then initiated this randomised, placebo-controlled, double-blinded trial to explore the efficacy and safety of icotinib in EGFR-mutant stage II–IIIA lung adenocarcinoma patients.
Methods
Study Design
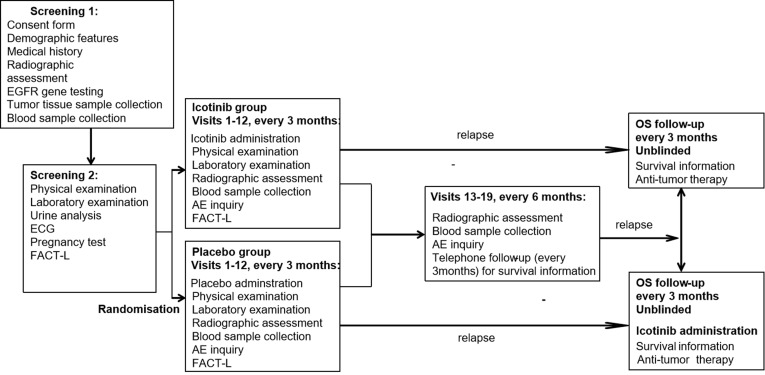
The present study is a randomised, placebo-controlled, double-blinded, phase III clinical trial conducted at 13 medical centres in China. The study process is presented in Figure 1 as a flow chart and in Table 1. Eligible patients are randomly assigned to receive icotinib or placebo with both patients and investigators being blinded. Patients stay in the intervention period for 36 months or until relapse, death, or unacceptable toxic effect(s) occurs. Those who complete the intervention period and those who have to suspend intervention due to intolerance of toxicity will be followed up for a further 36 months without being administered of the study medication until relapse or death occurs. Patients who experience disease progression/relapse will be unblinded, followed up for OS, and provided the study medication if they were assigned to the placebo group during the intervention period. Following the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1, radiographic assessments are conducted every 3 months during intervention and every 6 months afterwards to evaluate tumour status. These assessments include brain computed tomography (CT) or magnetic resonance imaging (MRI) whichever is appropriate, bone scan, bone X-ray (only if bone scan shows abnormality at a new site), contrast-enhanced chest and abdominal CT including bilateral adrenal glands, and pelvic sonography or CT (if necessary).
Figure 1.
Study flow-chart.
Abbreviations: ECG, electrocardiography. AE, adverse event. FACT-L, Functional Assessment of Cancer Therapy-Lung.
Table 1.
Overall Schedule of Enrolment, Interventions, and Assessment, in Accordance with the SPIRIT Statement
| TIMEPOINT | Enrolment | Allocation | Post-Allocation | Close-Out | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -t1 (−28 to −7 d) | -t2 (−7 to −1 d) | 0 | t1 (3 m) | t2 (6 m) | t3 (9 m) | … | t12 (36 m) | t13 (42 m) | t14 (48 m) | t15 (54 m) | … | t19 (72 m) | |
| ENROLMENT: | X | ||||||||||||
| Informed consent | X | ||||||||||||
| Demographic features | X | ||||||||||||
| Medical history | X | ||||||||||||
| Allocation | X | ||||||||||||
| INTERVENTIONS: | |||||||||||||
| Icotinib group | X | X | X | X | X | ||||||||
| Placebo group | Y | Y | Y | Y | Y | ||||||||
| ASSESSMENTS: | |||||||||||||
| Vital signs | X | X | X | X | X | X | |||||||
| EGFR gene test | X | ||||||||||||
| Tumour tissue sample collection | X | ||||||||||||
| Performance status | X | X | X | X | X | X | |||||||
| Complete blood count | X | X | X | X | X | X | |||||||
| Blood biochemistry | X | X | X | X | X | X | |||||||
| Carcinoembryonic antigen | X | X | X | X | X | X | |||||||
| Blood Biomarkers | X | X | X | X | X | X | X | X | X | X | X | ||
| Urine analysis | X | ||||||||||||
| ECG (potentially left ventricular ejection fraction) | X | ||||||||||||
| Health-related quality of life | X | X | X | X | X | X | |||||||
| Pregnancy test | X | ||||||||||||
| Radiographic assessment | X | X | X | X | X | X | X | X | X | X | X | ||
| Adverse event | X | X | X | X | X | X | X | X | X | X | |||
| Anti-tumour therapy | X | X | X | X | X | ||||||||
Screening and Eligibility Criteria
At the first screening visit, which is planned 7–28 days before randomisation, patients are invited to medical centres for information collection and examinations listed below: (1) signed consent form; (2) demographic information; (3) medical history (especially lung cancer history and related treatment/medication); (4) radiographic assessment; (5) concomitant medication(s); (6) EGFR gene test results; (7) blood sample collection; and (8) tumour tissue sample collection.
At the second screening visit, which is scheduled within 7 days before randomisation, patients are invited for collection of further measurements: (1) height, weight, body temperature, blood pressure, heart rate, respiratory rate and performance status (PS) assessed using the Eastern Cooperative Oncology Group (ECOG) method; (2) complete blood count; (3) blood biochemistry including total bilirubin (TB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, total protein (TP), alkaline phosphatase (ALP), blood urea nitrogen (BUN), serum creatinine, electrolytes (sodium, potassium, chloride and calcium) and carcinoembryonic antigen (CEA); (4) urine analysis; (5) electrocardiography (ECG), and left ventricular ejection fractions (LVEF) if ECG indicates any abnormality; (6) pregnancy test if needed; and (7) health-related-quality-of-life (HRQoL) evaluated by Functional Assessment of Cancer Therapy-Lung (FACT-L).
The clinical data at the screening are collected to determine if patients meet the following inclusion criteria:
Having undergone surgical excision, with the tumour being completely resected (R0) and pathologically confirmed to be lung adenocarcinoma, stage II–IIIA.
Having an EGFR mutation of deletion in exon 19 or L858R in exon 21.
Having received four cycles of standard platinum doublet adjuvant chemotherapy prior to the present study, which included vinorelbine, gemcitabine, docetaxel, paclitaxel, or pemetrexed combined with cisplatin or carboplatin. (The dosage of platinum in the first cycle of the chemotherapy should be either 75 mg/m2 ± 10% for cisplatin or area under curve (AUC) = 5 ± 10% for carboplatin.)
Aged ≥18 years but ≤75 years.
Scored 0–1 for PS according to the ECOG scale.
Able to commence the trial 4–8 weeks after the last dose of adjuvant chemotherapy.
Having a life expectancy >6 months.
Laboratory results meeting the following requirements:
(1) Complete blood count: absolute neutrophil count (ANC) ≥ 1.5 × 109/L, platelets ≥ 100 × 109/L, haemoglobin ≥ 9 g/dl.
(2) Liver: TB ≤ 2 times the upper limit of normal range, AST and ALT ≤ 2.5 times the upper limit of normal range.
(3) Kidney: serum creatinine ≤ 1.5 times the upper limit of normal range or creatinine clearance ≥ 60 mL/min.
(4) No malabsorption or other gastrointestinal disorders that affect drug absorption.
9. Female tested negative for pregnancy within 7 days before starting the treatment. Men should either use contraception throughout the study until 3 months after the last dose of treatment or have gone through surgical sterilization.
10. Having understood the study protocol and provided written informed consent.
Patients are excluded if they meet the criteria listed below:
Having been diagnosed with or treated for malignancies other than lung adenocarcinoma within 5 years prior to randomisation. This does not include excised cutaneous basal cell carcinoma or other carcinoma in situ.
Having been treated with systematic anti-tumour therapies other than postoperative adjuvant chemotherapy, which mainly refers to chemotherapy and targeted therapies (including but not limited to monoclonal antibodies, small molecule TKIs, etc.).
Having received radiotherapy prior to the study. This does not include the cases of N2 patients receiving postoperative adjuvant radiotherapy.
Having any evidence of systemic illnesses as listed below:
(1) previous history of ILD, drug-induced ILD, radiation pneumonitis requiring hormone therapy, or any active ILD with clinical evidence.
(2) being diagnosed with ILD by CT at screening.
(3) emerging evidence suggesting a serious or uncontrollable systemic disease by assessments.
(4) any unstable systemic disease (eg, active infection, high-risk hypertension, unstable angina, congestive heart failure, etc.).
(5) previous history of a definite neurological or mental disorder, including epilepsy and dementia.
5. Emerging evidence of significant clinical abnormalities or abnormal laboratory findings that make the patient unsuitable for participating in the study.
6. Being allergic to icotinib or any of its excipients.
7. Women who are pregnant, lactating, or are willing to be pregnant.
8. Confirmation of an EGFR exon 20 T790M mutation.
9. Unwilling to complete the trial or to follow the protocol throughout the study (due to regulatory or other reasons).
10. Previous or concomitant therapy and medication including any of those listed below:
(1) anticonvulsants or antipsychotics;
(2) unapproved drugs or other experimental drugs used within 30 days prior to the study;
(3) continued adjuvant therapy not included in the present study within 2 weeks prior to the randomisation.
Randomisation
A computer-supported Interactive Web-based Randomisation System (IWRS) is employed for randomisation. The system follows Pocock and Simon minimization, which is a dynamic allocation method. When an eligible patient is successfully recruited, an investigator will log onto the randomisation system and enter the detailed information of the patient. The system will then randomly assign the subject (1:1) to either the icotinib or placebo group depending on the patient’s stratification factors balance. These factors include disease stage (stage II/IIIA), site of mutation (19/21), ECOG PS (0/1), and smoking (yes/no). Clinicians and patients are blinded to treatment allocation. Patients are expected to commence the intervention within 7 days after randomisation.
Administration of Icotinib and Placebo
A dose of 125 mg Icotinib is orally administered 3 times per day to achieve a total daily amount of 375 mg in the icotinib group. A placebo that is identical in colour, size and packaging (one tablet each time, three times per day) is orally administered by patients in the placebo group. The intervention lasts continuously for 36 months, or until relapse, death or intolerant toxicity occurs. The dosage of icotinib is adjusted according to the severity of the toxic reaction by the investigator(s) during the intervention. Suspension of icotonib may be needed if ILD (established by imaging studies or clinically) or any Grade 3 or worse adverse event(s) (AE), according to National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4, occurs. The treatment is resumed when the diagnosis of ILD is excluded and AEs are improved to Grade 1 or better. The maximum duration for suspension is 14 days; otherwise, the patient is withdrawn from the study.
Data Collection
During the intervention period, patients are invited to visit the centres every 3 months in order to complete 12 visits in total (Visit 1–12). At each visit, clinical data are obtained including: (1) height, weight, body temperature, blood pressure, heart rate, respiratory rate, and ECOG PS; (2) complete blood count (haemoglobin, white blood cell count, ANC, and platelet count); (3) blood biochemistry (TB, AST, ALT, albumin, TP, ALP, BUN, serum creatinine, electrolytes (sodium, potassium, chloride and calcium), and CEA); (4) 8 mL full blood for biomarkers; (5) FACT-L evaluation; (6) radiographic assessments (according to RECIST aforementioned); and (7) concomitant medication and any AE(s) since the previous visit.
After the intervention period, patients are followed-up and invited to re-visit the centre every 6 months in order to complete 6 visits (Visit 13–19). Clinical data are obtained including: (1) 8 mL full blood for biomarkers; (2) radiographic assessments (according to RECIST 1.1 aforementioned); (3) concomitant medication and any AE(s) since the previous visit; and (4) anti-tumour therapy since the last visit on telephone follow-up (every 3 months).
Response Measures
The duration of DFS in each intervention group is the primary study endpoint. DFS is defined as the time between randomisation and the first observed relapse, or the time between randomisation and death if a patient dies due to other causes before relapse even occurs. Relapse is confirmed by evidence of any new lesion using imaging studies. In some special cases, unambiguous progression of the lesion is also recognised as evidence of relapse.
The secondary endpoints of this study include:
Duration of OS in each intervention group. OS is defined as the time between randomisation and death due to any cause.
Three-year and 5-year DFS rate and 5-year OS rate in each group.
Safety and tolerability assessed by NCI-CTCAE 4.0.
HRQoL assessed by FACT-L.
Safety Evaluation
All patients included in the intervention are validated for safety evaluation. Patients’ physical examination results, vital signs, AEs, and laboratory abnormalities will be summarised. All AEs will be reported and graded according to NCI-CTCAE 4.0.
AE is defined as any unforeseen medical condition or worsening of an existing medical condition that occurs during or after the intervention, not necessarily related to the study medication. Unforeseen medical conditions may be symptoms (eg, nausea, chest pain), physical signs (eg, tachycardia, enlarged liver) or abnormal findings (eg, laboratory results, ECG). Unpredictable adverse medical conditions could happen at any stage, including screening and washout, or even when patients are not yet introduced to the intervention. Usual AEs may include: (1) AEs that occur during the use of medication(s) by professionals; (2) AEs caused by overdosage (whether intentionally or unintentionally); (3) drug abuse-induced AEs; (4) AEs caused by withdrawal reaction; and (5) AEs unrelated to the study medication. All AEs must be completely recorded in the patients’ case report form (CRF) with original data’s support. Each incident should be described in detail, including starting and ending dates, the severity, the relationship with the study medication under test, the assessments taken, and the outcome of the event. A patient who experienced AE should be followed up after the initial report until the patient’s condition stabilises no matter at which stage of the study, unless the investigator(s) decides that the AE cannot be alleviated due to the patient’s own condition or patient’s loss to follow-up.
A serious AE (SAE) is defined as an event occurring at any stage of the study that leads to one or more of the following: (1) death; (2) immediate life-threatening condition; (3) need of hospitalisation or extended stay of hospitalisation; (4) persistent or significant disability/loss of function; (5) congenital malformations or birth defects; and (6) injury to patients. Once a SAE occurs, it should be reported to the hospital ethics committee or the centre ethics committee within 24 hours. Investigators must address SAEs with all possible treatments. Medication(s) used in a SAE case will be recorded in the patient’s CRF. Any patient who experiences a SAE will be followed up until the last day of the patient’s involvement in the study, or until the investigator(s) decides that the patient’s condition is stabilised. If ILD is clinically suspected, a lung biopsy is required to confirm the diagnosis (unless the patient refuses). If a patient dies of an unknown reason (except an accidental death), the investigator should advise an autopsy to clarify the cause of death unless the patient’s family refuses.
Withdrawal
Patients may choose to withdraw from the study at any stage. The main reasons for withdrawal may be: (1) voluntary withdrawal (this will not affect any future treatment for the patients); (2) an AE or a SAE leading to a safety concern; (3) poor compliance with the study protocol; (4) death; (5) loss to follow-up; and (6) any other reasons for which the investigator(s) believes the patient should be removed from the study.
When withdrawing from the study, a patient should be further examined and treated if any Grade 3 or worse laboratory results are noticed, until the results are improved to Grade 1 or better, unless the investigator(s) determines the laboratory results cannot be improved based on the patient’s condition. After withdrawal from the study, a patient should be followed up for all existing or newly developed AEs that occur within 30 days of the last dose of the study medication, and reported all new AEs and SAEs that occur within this time frame until alleviated. Any patient who withdraws from the study (except due to relapse and withdrawal of informed consent) should be followed-up every 3 months for collection of information related to relapse and survival duration.
Planned Statistical Analyses
The DFS of the gefitinib and control groups in the ADJUVANT study41 for EGFR-mutant stage II–IIIA (N1-N2) NSCLC patients were 28.7 and 18.0 months, respectively (HR: 0.60, P=0.005). Based on this result, and also considering that the patients included in the present study have received standard adjuvant chemotherapy and the scheduled administration of icotinib is planned for longer term, the median DFS durations for the icotinib and placebo groups are expected to be approximately 40 and 22 months, respectively. Therefore, in order to detect a statistically significant difference with a level of 0.05 and a power of 80%, at least 56 patients are needed in each group. Furthermore, considering a 10% dropout rate, we expect at least 62 patients (total 124 patients) to be recruited for each intervention group.
Three statistical data sets will be used in the analyses: (1) a full analysis set (FAS): including all patients who are randomised and given the study medication for at least one dose before closure of the study; (2) per-protocol set (PPS): including patients in the FAS who follow the protocol throughout the intervention without using any contraindicated medication before closure of the study; and (3) safety set (SS): including all patients who are randomised and included in the intervention to receive at least one dosage of the study medication. The efficacy of the study medication will be explored in both the FAS and PPS, while its safety will be evaluated in the SS.
Baseline data, including demographic features, tumour characteristics, medical history, previous anti-tumour therapy, concomitant medication, and vital signs will be reported as well. For continuous variables, mean, standard deviation (SD), median, minimum, and maximum values will be used to describe the data. For categorical variables, frequency and percentage will be used. t-test, Wilcoxon rank sum test, chi-square test, and Fisher’s exact test will be used for comparison of the baseline data where appropriate.
Natural log transformed data will be used for DFS according to its distribution. Kaplan–Meier survival curves will be used for estimating DFS and OS. A Cox hazard proportional model will be used to explore differences between groups. Models adjusted for necessary covariates will also be performed. Moreover, the longitudinal changes in the HRQoL will be compared between the two groups using analysis of covariance. All patients will be evaluated for toxicity. A Fisher’s exact test will be used for comparison of Grade 3 AE incident proportions between groups. All tests will be two-sided, and the significant level set at 0.05.
Discussion
EGFR-TKIs have become the first-line treatment for EGFR-mutant advanced NSCLC. However, whether EGFR-TKIs are effective as adjuvant chemotherapy in EGFR-mutant early-stage NSCLC has yet to be established. Supporting results were reported by the RADIANT,42 SELECT,43–45 and NCT0243097446 studies. The first two trials assessed adjuvant erlotinib in postoperative (R0) early-stage (IB-IIIA) NSCLC with or without previous chemotherapy in unselected and EGFR-mutant patients, respectively. The results from these two studies indicated that erlotinib improved DFS in EGFR-mutant early-stage patients as adjuvant therapy compared to that in genotype control. However, the significance in the RADIANT trial was weak due to the hierarchical testing procedure.42 The NCT02430974 study46 included 41 patients with resected EGFR-mutated NSCLC stage IB-IIIA and compared the DFS of two intervention groups treated with either platinum-based doublet chemotherapy followed by icotinib or platinum-based chemotherapy only. The researchers found no significant differences in DFS at 12, 18, and 24 months between the two groups. However, the DFS at the 24-month time point was 90.5% for the chemotherapy followed by icotinib group vs 66.7% for the chemotherapy only group (p=0.066), showing a potential improvement in treatment efficacy with the addition of icotinib. The use of EGFR-TKIs as adjuvant therapy was also supported by another two retrospective analyses.47,48 Moreover, the recently published ADJUVANT/CTONG1104 study41 in a Chinese population observed significant benefits in DFS from adjuvant genifinib in completely resected (R0) stage II–IIIA EGFR-mutant NSCLC compared to vinorelbine plus cisplatin. Controversially, the Southwestern Oncology Group (SWOG) S0023 trial49 and the NCIC CTG BR1950 study reported opposing findings. These two trails assessed adjuvant gefitinib in inoperable stage III and completely resected stage IB-IIIA NSCLC patients, respectively. Both studies reported no improvement in survival with gefitinib treatment compared to placebo. However, due to the premature closure of BR19 and also the low percentage of EGFR-mutant patients in the study population, a definitive conclusion regarding the efficacy of adjuvant gefitinib could not be simply drawn.50
There are strengths to this study in design when comparing with previous ones. Firstly, the stage of the target population was carefully selected in the present study. In previous studies: the SELECT study43,44 recruited stage IA-IIIA NSCLC patients; the BR19 study,50 the RADIANT study,42 and the NCT02430974 study46 all recruited stage IB-IIIA NSCLC patients; the ADJUVANT study enrolled stage II–IIIA NSCLC patients. It has been reported that stage I NSCLC patients have a 5-year survival rate of 47–80% and a low relapse rate.51 Without including these patients, the future results of the current study, which only focuses on stage II–IIIA patients, will provide powerful and convincing evidence for treatment in early-stage NSCLC patients.
Secondly, in order to observe a better therapeutic effect from icotinib in early-stage NSCLC patients, the current study only includes patients who have gone through surgical resection and standard chemotherapy. In the ADJUVANT study, the investigators were aiming to decide if EGFR-TKI can be alternative to chemotherapy in the adjuvant setting.41 Thus, the patients in the gefitinib arm had not received standard chemotherapy. The aims of the SELECT study,43–45 the BR19 study,50 and the RADIANT study42 were to explore the efficacy of EGFR-TKI in NSCLC patients after complete surgical resection with or without adjuvant chemotherapy.
Thirdly, with administration of icotinib, which was reported to be less likely to lead to ADRs,36–38 the current study aims to include a longer treatment period of 3 years. The BR19 study achieved a median treatment time of 4.8 months (range: 1 day to 25 months) for gefitinib;50 the RADIANT study achieved a median treatment time of 11.9 months for erlotinib;42 and the SELECT trial resulted in that 69% patients received erlotinib treatment for at least 22 months with 2-year DFS being 90% (median follow-up: 3 years).44 The median treatment duration for gefitinib in the ADJUVANT study was 21.9 months. In the NCT02430974 study,46 which has been the only study to use the same medication used in our present study, the patients were only given icotinib for 4–8 months. Using icotinib for 3 years in the present study may improve the tolerability of treatment among patients, leading to a longer treatment period and possibly longer DFS.
Additionally, there are several ongoing studies that share similarities with the present study. The NCT01929200 study,52 a randomised, open-label trial, was designed to evaluate the efficacy of icotinib as adjuvant therapy in EGFR-mutant stage II–IIIA NSCLC patients. Patients are receiving either 1-year or 2-year treatment of icotinib. The primary outcome is recurrence-free survival while the secondary outcome is OS. The ICTAN study53 aimed to examine the efficacy of administering icotinib following chemotherapy compared to chemotherapy only in treating resected EGFR-mutant stage IIA-IIIA NSCLC patients. The patients are given icotinib for either 6 or 12 months, and the primary outcome is DFS. The NCT02272127 study54 aimed to observe and compare the efficacy and safety of chemotherapy plus icotinib in treating patients with resected EGFR-mutant stage IB-IIIA NSCLC. This study is single arm and its primary outcome is DFS. The EVIDENCE study55 was designed to evaluate the efficacy of icotinib as adjuvant therapy in treating resected EGFR-mutant stage II–IIIA NSCLC patients. Patients received icotinib for 2 years in this study.
To our knowledge, the present study is the first randomised, double-blinded, placebo-controlled, multicenter trial designed to explore efficacy and safety of icotonib in EGFR-mutant stage II–IIIA lung adenocarcinoma patients, who have gone through surgical resection and standard platinum-based doublet chemotherapy. The results obtained in future may be meaningful for guiding clinical practice.
Trial Status
The present study was initiated on 20th March 2014 and was registered on ClinicalTrials.gov as NCT02125240. The current protocol version is 2.0, revised on 4th July 2017. The recruitment started on June 3rd 2014 and was completed on 15th November 2018 . The expected date of completion of the study is December 2021.
Funding Statement
This work is supported by the Beijing Municipal Science and Technology Commission Major Project (D141100000214005).
Data Sharing Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of National Cancer Centre/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College on 20th March 2014 (Approval N0.14-001/775). The study is conducted according to guidelines from the Declaration of Helsinki and The International Conference on Harmonisation and Good Clinical Practice guidelines. (ICH-GCP). The trial was registered on www.ClinicalTrail.gov as NCT02125240. Written informed consent is obtained from all participants. An ethics committee or institutional review board approved the final protocol at every study site.
Author Contributions
Yuan-Kai Shi was the leading principal investigator of this study, who contributed to the concept and design of the study. Yu-Tao Liu contributed to the study design. Yu-Tao Liu and Yuan-Kai Shi drafted the manuscript. Yu-Tao Liu, Xue-Zhi Hao, De-Ruo Liu, Gang Cheng, Shu-Cai Zhang, Wen-Hua Xiao, Yi Hu, Jun-Feng Liu, Ming He, Cui-Min Ding, Li Zhang, Jun Wang, Hui Li, Gui-Lan Dong, Xiu-Yi Zhi, Jian Li, Yuankai Shi contributed to data analysis, interpretation of data and revising the article. All authours gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Navada S, Lai P, Schwartz A, Kalemkerian G. Temporal trends in small cell lung cancer: analysis of the national Surveillance, Epidemiology, and End-Results (SEER) database. J Clin Oncol. 2006;24:7082. doi: 10.1200/jco.2006.24.18_suppl.7082 [DOI] [Google Scholar]
- 3.Kaisermann MC, Trajman A, Madi K. Evolving features of lung adenocarcinoma in Rio de Janeiro, Brazil. Oncol Rep. 2001;8:189–281. doi: 10.3892/or.8.1.189 [DOI] [PubMed] [Google Scholar]
- 4.Roggli VL, Vollmer RT, Greenberg SD, McGavran MH, Spjut HJ, Yesner R. Lung cancer heterogeneity: a blinded and randomized study of 100 consecutive cases. Hum Pathol. 1985;16:569–579. doi: 10.1016/S0046-8177(85)80106-4 [DOI] [PubMed] [Google Scholar]
- 5.Visbal AL, Williams BA, Nichols FC, et al. Gender differences in non–small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004;78:209–215. doi: 10.1016/j.athoracsur.2003.11.021 [DOI] [PubMed] [Google Scholar]
- 6.Group NM-aC. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267–1277. doi: 10.1016/S0140-6736(10)60059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi: 10.4065/83.5.584 [DOI] [PMC free article] [PubMed]
- 8.Fry WA, Phillips JL, Menck HR. Ten‐year survey of lung cancer treatment and survival in hospitals in the United States. Cancer. 1999;86:1867–1876. doi: [DOI] [PubMed] [Google Scholar]
- 9.Douillard J-Y, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X [DOI] [PubMed] [Google Scholar]
- 10.Waller D, Peake M, Stephens R, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004;26:173–182. doi: 10.1016/j.ejcts.2004.03.041 [DOI] [PubMed] [Google Scholar]
- 11.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non–small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623 [DOI] [PubMed] [Google Scholar]
- 12.Group IALCTC. Cisplatin-based adjuvant chemotherapy in patients with completely resected non–small-cell lung cancer. N Engl J Med. 2004;2004:351–360. [DOI] [PubMed] [Google Scholar]
- 13.Strauss GM, Herndon JE, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non–small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2013;10:1236–1271. [Google Scholar]
- 15.Pignon J-P, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030 [DOI] [PubMed] [Google Scholar]
- 16.Alam N, Shepherd FA, Winton T, et al. Compliance with post-operative adjuvant chemotherapy in non-small cell lung cancer: an analysis of National Cancer Institute of Canada and intergroup trial JBR. 10 and a review of the literature. Lung Cancer. 2005;47:385–394. doi: 10.1016/j.lungcan.2004.08.016 [DOI] [PubMed] [Google Scholar]
- 17.Fujino S, Enokibori T, Tezuka N, et al. A comparison of epidermal growth factor receptor levels and other prognostic parameters in non-small cell lung cancer. Eur J Cancer. 1996;32:2070–2074. doi: 10.1016/S0959-8049(96)00243-2 [DOI] [PubMed] [Google Scholar]
- 18.Moscatello DK, Holgado-Madruga M, Godwin AK, et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55:5536–5539. [PubMed] [Google Scholar]
- 19.Rusch V, Klimstra D, Venkatraman E, Pisters P, Langenfeld J, Dmitrovsky E. Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor alpha is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res. 1997;3:515–522. [PubMed] [Google Scholar]
- 20.Nicholson R, Gee J, Harper M. EGFR and cancer prognosis. Eur J Cancer. 2001;37:9–15. doi: 10.1016/S0959-8049(01)00231-3 [DOI] [PubMed] [Google Scholar]
- 21.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised Phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 22.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055 [DOI] [PubMed] [Google Scholar]
- 23.Yang P-C, Shi Y, Au JS-K, et al. Molecular Epidemiological Prospective Study of EGFRMutations from Asian Patients (Pts) with Advanced Lung Adenocarcinoma (PIONEER). American Society of Clinical Oncology; 2012. [Google Scholar]
- 24.Hirsch FR, Bunn JPA. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009;10:432–433. doi: 10.1016/S1470-2045(09)70110-X [DOI] [PubMed] [Google Scholar]
- 25.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- 26.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 27.Sequist LV, Yang JC-H, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 28.Wu Y-L, Zhou C, Hu C-P, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 29.Yang JC-H, Wu Y-L, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 30.Mok TS, Wu Y-L, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 31.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 32.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 33.Zhou C, Wu Y-L, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 34.Han J-Y, Park K, Kim S-W, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–1128. doi: 10.1200/JCO.2011.36.8456 [DOI] [PubMed] [Google Scholar]
- 35.Tan F, Shen X, Wang D, et al. Icotinib (BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. Lung Cancer. 2012;76:177–182. doi: 10.1016/j.lungcan.2011.10.023 [DOI] [PubMed] [Google Scholar]
- 36.Zhao Q, Shentu J, Xu N, et al. Phase I study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine kinase inhibitor, in patients with advanced NSCLC and other solid tumors. Lung Cancer. 2011;73:195–202. doi: 10.1016/j.lungcan.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 37.Wang H-P, Zhang L, Wang Y-X, et al. Phase I trial of icotinib, a novel epidermal growth factor receptor tyrosine kinase inhibitor, in Chinese patients with non-small cell lung cancer. Chin Med J. 2011;124:1933. [PubMed] [Google Scholar]
- 38.Hu X, Han B, Gu A, et al. A single-arm, multicenter, safety-monitoring, phase IV study of icotinib in treating advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2014;86:207–212. doi: 10.1016/j.lungcan.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 39.Tan F-L, Zhang L, Zhao Q, et al. Pharmacology and clinical evaluation of icotinib hydrochloride. Chin J New Drugs. 2009;18:1–4. [Google Scholar]
- 40.Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14:953–961. doi: 10.1016/S1470-2045(13)70355-3 [DOI] [PubMed] [Google Scholar]
- 41.Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2017;19:139–148. [DOI] [PubMed] [Google Scholar]
- 42.Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non–small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol. 2015;33:4007–4014. doi: 10.1200/JCO.2015.61.8918 [DOI] [PubMed] [Google Scholar]
- 43.Pennell N, Neal J, Govindan R, et al. The SELECT trial: a multicenter phase II trial of adjuvant erlotinib (E) in patients with resected, early-stage non-small cell lung cancer (NSCLC) and confirmed mutations in the epidermal growth factor receptor (EGFR). J Clin Oncol. 2011;29:TPS209. doi: 10.1200/jco.2011.29.15_suppl.tps209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pennell NA, Neal JW, Chaft JE, et al. SELECT: A Multicenter Phase II Trial of Adjuvant Erlotinib in Resected Early-Stage EGFR Mutation-Positive NSCLC. American Society of Clinical Oncology; 2014. [Google Scholar]
- 45.Neal JW, Pennell NA, Govindan R, et al. The SELECT Study: A Multicenter Phase II Trial of Adjuvant Erlotinib in Resected Epidermal Growth Factor Receptor (EGFR) Mutation-Positive Non-Small Cell Lung Cancer (NSCLC). American Society of Clinical Oncology; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng S, Wang Y, Cai K, et al. Randomized adjuvant chemotherapy of EGFR-mutated non-small cell lung cancer patients with or without icotinib consolidation therapy. PLoS One. 2015;10:e0140794. doi: 10.1371/journal.pone.0140794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Angelo SP, Janjigian YY, Ahye N, et al. Distinct clinical course of EGFR-mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol. 2012;7:1815–1822. doi: 10.1097/JTO.0b013e31826bb7b2 [DOI] [PubMed] [Google Scholar]
- 48.Janjigian Y, Park B, Kris M, et al. Impact on disease-free survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor epidermal growth factor receptor (EGFR) mutations. J Clin Oncol. 2009;27:7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non–small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–2456. doi: 10.1200/JCO.2007.14.4824 [DOI] [PubMed] [Google Scholar]
- 50.Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non–small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31:3320–3326. doi: 10.1200/JCO.2013.51.1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsim S, O’Dowd C, Milroy R, Davidson S. Staging of non-small cell lung cancer (NSCLC): a review. Respir Med. 2010;104:1767–1774. doi: 10.1016/j.rmed.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 52.ClinicalTrials.gov. Icotinib as adjuvant therapy in treating non-small-cell lung cancer patients with positive EGFR mutation. Available from: https://clinicaltrials.gov. Accessed May8, 2020.
- 53.ClinicalTrials.gov. Icotinib following chemotherapy versus chemotherapy as adjuvant therapy in stage IIA-IIIA NSCLC with EGFR mutation (ICTAN). Available from: https://clinicaltrials.gov. Accessed May8, 2020.
- 54.ClinicalTrials.gov. Study of chemotherapy plus icotinib to treat egfr mutation-positive non-small-cell lung cancer. Available from: https://clinicaltrials.gov. Accessed May8, 2020.
- 55.ClinicalTrials.gov. Icotinib as adjuvant therapy compared with standard chemotherapy in stage II-IIIA NSCLC with EGFR-mutation (EVIDENCE). Available from: https://clinicaltrials.gov. Accessed May8, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- ClinicalTrials.gov. Icotinib as adjuvant therapy in treating non-small-cell lung cancer patients with positive EGFR mutation. Available from: https://clinicaltrials.gov. Accessed May8, 2020.
- ClinicalTrials.gov. Icotinib following chemotherapy versus chemotherapy as adjuvant therapy in stage IIA-IIIA NSCLC with EGFR mutation (ICTAN). Available from: https://clinicaltrials.gov. Accessed May8, 2020.
- ClinicalTrials.gov. Study of chemotherapy plus icotinib to treat egfr mutation-positive non-small-cell lung cancer. Available from: https://clinicaltrials.gov. Accessed May8, 2020.
- ClinicalTrials.gov. Icotinib as adjuvant therapy compared with standard chemotherapy in stage II-IIIA NSCLC with EGFR-mutation (EVIDENCE). Available from: https://clinicaltrials.gov. Accessed May8, 2020.