Abstract
To better understand the synergistic antibacterial activity between piperacillin and Lavandula angustifolia essential oil (LEO) against multidrug-resistant Escherichia coli, we performed microarray transcriptomic analysis of LEO when used alone and in combination with piperacillin against the non-treated control. In total, 90 genes were differentially expressed after the combination of LEO and piperacillin treatment. Among the up-regulated genes, nfsB, nemA, fruA, nfsB, nemA are known to control microbial metabolism and nitrotoluene degradation, which were observed only in the LEO–piperacillin combinatory treatment. Four candidate genes from the microarray result, srIA, srID, waaR and nfsB, were validated by qRT-PCR as these genes showed differential expression consistently in the two methods. Biochemical pathway analysis showed that there was upregulation of genes involved in several biological processes including fructose and mannose metabolism, phosphotransferase system (PTS), lipopolysaccharide biosynthesis and nitrotoluene degradation. Genes involved in microbial metabolism in diverse environments were found both up- and down-regulated in LEO–piperacillin combinatory treatment. Our study provides new information concerning the transcriptional changes that occur during the LEO and piperacillin interaction against the multidrug-resistant bacteria and contributes to unravel the mechanisms underlying this synergism.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02304-3) contains supplementary material, which is available to authorized users.
Keywords: Transcriptome analysis, Escherichia coli, Lavandula angustifolia, Piperacillin
Introduction
Antibiotic resistance among bacteria belonging to the family Enterobacteriaceae such as Escherichia coli has had many countries highlighted it as a major concern. In 2017, a list of priority pathogens has been published by the World Health Organization (WHO) and new antibiotics are urgently needed to combat multidrug-resistant E. coli (WHO 2014). Since the use of any single type of antibiotic alone may increase the chance of resistance to it, some researchers have proposed to combine antibiotic with other chemical compounds to either strike multiple targets in the bacteria genome or enhance the potency of the antibiotic by weakening pathogen ability to develop resistance (Yap et al. 2013, 2014; Moo et al. 2019). The term “synergy” implies that the combined effect of two agents when used in a combinatory therapy is greater than the sum of individual effects. According to Odds (2003), synergy can be quantified and expressed in fractional inhibitory concentration (FIC). The results in between synergy and antagonism can be defined as additive or indifferent by determining FIC index (FICI).
Piperacillin, the beta-lactam antibiotic, is known to have a broad spectrum of antibacterial activity against Gram-positive, Gram-negative aerobic and anaerobic bacteria as well as many pathogens that produce beta-lactamase (Holmes et al. 1984). A study by Walsh (2000) showed that piperacillin affects the cell membrane of bacteria by acting on different targets such as transpeptidases and transglycosylases (also termed penicillin-binding proteins or PBPs). However, the inappropriate use of piperacillin has exerted an enormous selective pressure on microorganisms, which leads to resistance across multiple microbial species (Shah and Ryzner, 2013). While the search for novel antibiotic continues, the use of natural alternative remedies is increasingly popular to hinder antibiotic resistance, for example through synergistic effect of essential oils and conventional antibiotics (Wolska et al. 2012; Mancini et al. 2014; Elshafie et al. 2015).
Lavandula angustifolia, commonly known as English lavender, is a member of the genus Lavandula L. in the mint family of flowering plants, Lamiaceae. It is native to the Mediterranean region such as France, Italy and Spain (Prusinowska and Śmigielski 2014; GBIF 2017) but later it has spread to European countries that includes Germany, Sweden, Belgium, just to name a few (GBIF 2017). This evergreen perennial species is famous for its volatile compounds of plant extract, namely essential oil. Essential oil is stored in the secretory cells, cavities or glandular trichomes which can be extracted and blossom for many beneficial uses (Mahizan et al. 2019). The essential oil of lavender (LEO) is composed predominantly of monoterpenes and sesquiterpenes, of which linalool (9.3–68.8%) and linalyl acetate (1.2–59.4%) are its abundant constituents (Prusinowska and Śmigielski 2014; De Rapper et al. 2016; Soković et al. 2010). However, LEO may also vary qualitatively and quantitatively in their composition, depending on the plant geographic locations, genotypes, propagation, environmental conditions and morphological characteristics (Prusinowska and Śmigielski 2014).
The essential oil of Lavandula angustifolia has been known to exhibit a broad spectrum of biological activities. These include antibacterial, antifungal, antioxidant, antimutagenic, anti-inflammatory and analgesic properties (Dapkevicius et al. 1998; Hajhashemi et al. 2003; D’auria et al. 2005; Evandri et al. 2005; Fabio et al. 2007; Hanamanthagouda et al. 2010). With that, current early findings open up the possibility that synergistic combinations, specifically of LEO and piperacillin, its corresponding antibiotics, may play a highly promising role in resistance modifying activities against multidrug resistant E. coli. As such, synergistic potential with a FICI of 0.26 has been observed when 0.5% v/v of LEO was used in combination with 128 µg/mL of piperacillin against E. coli strain J53 R1, a mutant derivative of E. coli K-12 strain (Yap et al. 2013, 2014). It was found to reduce the minimum inhibitory concentration (MIC) of piperacillin from 1024 to 128 µg/mL when used in combination with LEO.
The antimicrobial-specific mechanism of LEO synergistic effect with piperacillin remains elusive and is the focus of this study. Transcriptional changes in response to various combination of LEO and piperacillin can potentially inform on key genes that underlie synergism between essential oil and antibiotic. A comprehensive understanding on how LEO works may reveal genes in biological pathway worthy as drug targets to combat the emergence of multidrug-resistant Enterobacteriaceae.
Method and materials
Materials
The essential oil of Lavandula angustifolia was purchased from Aroma Trading Ltd. (Milton Keynes, UK). Piperacillin was purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA) and dissolved in its solvent to make a concentrated 10 mg/mL stock solution. All procedures were carried out as described in the Clinical and Laboratory Standards Institute (CLSI) M100-S21.
Bacteria culture
Escherichia coli J53 R1 strain was cultured in cation-adjusted Mueller Hinton Broth (MHB) supplemented with 0.5% (v/v) Tween 80 for emulsification of LEO, as established in our previous work (Yap et al. 2013). E. coli J53 R1 was subjected to four different treatment conditions, which are untreated (control), LEO-treated, piperacillin-treated and LEO with piperacillin-treated as previously described in Yap et al. (2014). Each treatment was prepared in triplicate. Samples were placed in a shaking incubator at 200 revolutions per minute (rpm) at a controlled temperature of 37 °C.
RNA isolation
RNA was extracted using a commercial column based RNA Purification Kit (RNeasy; Qiagen, Valencia, CA) following manufacturer’s instructions. RNA quality was determined by the A260/A280 ratio with NanoDrop Spectrophotometer (Thermo Scientific, Wilmington, MA) and capillary electrophoresis on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) for the assessment of the integrity of the RNA samples. RNA Integrity Number (RIN) provides a quantitative value for RNA integrity for standardisation of quality interpretation.
Microarray and gene expression
Microarray analysis was performed using Agilent’s E. coli Microarray Kit (Agilent Microarray ID: AMADID 060524). Fluorescence labelling of cDNA and hybridisations were performed as per Agilent One-Colour Microarray-Based Prokaryote Analysis Protocol, version 1.4. Briefly, RNA samples were labelled with Cyanine-3 dye to produce labelled cDNA. Labelled cDNA yields and specific activity were assessed using NanoDrop. All samples passed the minimum recommended thresholds prior to microarray hybridisation. All hybridisations were carried out for 17 h at 65 °C and 6 RPM. The slides were then washed with wash buffer and scanned using Agilent SureScan scanner. Probe features information were extracted from the scanned slides using Agilent Feature Extraction software (version 10.7.3).
Gene expression analysis
Microarray data from all samples were imported into Partek Genomics Suite v6.6 (PGS; Partek Inc., St Louis MO, USA) for gene expression analysis. The median of probes’ signal intensities were calculated for probes of each gene. Other metadata associated with each sample were added in to facilitate analysis. The distribution of samples signal intensities was assessed using boxplots. Quantile normalisation was applied to reduce variation detected in the samples signal intensities and then transformed into log2 scale. The quality of the transformed normalized data was checked again with boxplots and principle component analysis (PCA). The PCA plot identified two inconsistent samples (i.e. 1 sample from control and another from piperacillin treatment). The mean signal intensity of each sample was subtracted from the mean signal intensities of all 12 samples for each gene. The data were then subjected to unsupervised hierarchical clustering to identify groups of samples, which also confirmed the two inconsistent samples. These inconsistent samples were removed and the entire microarray data analysis was repeated from the beginning to the clustering step. The heat map from unsupervised hierarchical clustering of the remaining 10 samples is shown in Fig. 1. Detection of gene expression differences among sample conditions of the remaining samples was done using One-way Analysis of Variance (ANOVA) followed by Benjamini-Hochberg (BH) correction for multiple tests on comparisons of interest, Lavender vs. Control, Piperacillin vs. Control and Lavender plus Piperacillin vs. Control. The data were then exported for further visualisation and manipulation in Spotfire DecisionSite for Functional Genomics (TIBCO Spotfire Boston MA, USA).
Fig. 1.
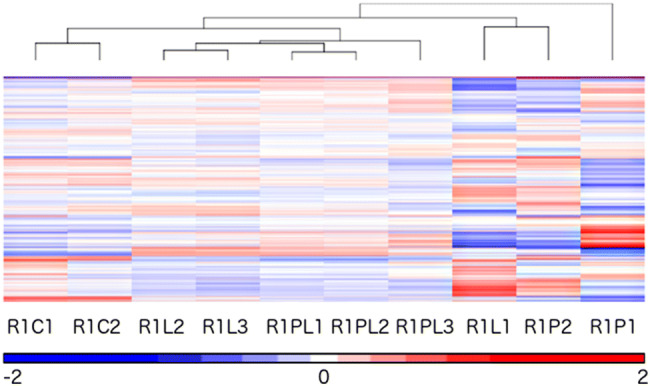
Unsupervised hierarchical clustering with heat map of 10 samples from E. coli J53 R1 strain under four different conditions: control, piperacillin alone, LEO alone and combination of piperacillin and LEO. Outliers have been removed and clustering was based on Euclidean dissimilarity and complete linkage method. All samples are prefixed with ‘R1’ and have a suffix of either ‘C’, ‘L’, ‘P’ or ‘PL’, followed by a number. ‘C’ denotes control, ‘L’ denotes lavender treatment, ‘P’ denotes piperacillin treatment, and ‘PL’ denotes combination of piperacillin and lavender treatment. The number at the end of each sample label denotes replicates. The clustering clearly shows samples of the same experimental condition grouped together with the exception of a sample in lavender treatment, i.e. ‘R1L1′. Differentially expressed genes are shown on the heat map as rows and the colour red denotes up-regulation and blue denotes down-regulation
Identification of differentially expressed genes
Differentially expressed genes (DEGs) were ranked and selected using the combination of non-stringent p value cutoff and fold change ranking, as recommended by the Microarray Quality Control (MAQC) Consortium. A few combinations of criteria have been used to determine DEG and for this work, genes that satisfied the conditions of BH-adjusted p value < 0.05 and log2 fold change (logFC) > 2 was identified as up-regulated genes. Genes that satisfied conditions of BH-adjusted p value < 0.05 and logFC < − 2 were identified as down-regulated genes. Volcano plots of ANOVA-BH results were plotted for each comparison, which showed DEGs.
Pathway enrichment analysis
To study biological pathways that might be affected by our treatments, the DEGs identified were subjected to pathway enrichment analysis. We have used the Kyoto Encyclopedia of Gene and Genomes (KEGG) pathways implemented in the Database for Annotation, Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov/) to determine pathways that were potentially enriched (Huang et al. 2009). In order to take advantage of the annotations for E. coli K-12 MG1655 in DAVID, each DEG was converted to UniRef100 ID before uploading for functional annotation analysis. A BH-adjusted p value < 0.1 and only pathways that contain more than two of our DEG genes were used as the parameters to select significantly enriched KEGG pathway.
Total RNA isolation and quantitative real-time PCR analysis
To further validate the DEGs found from microarray analysis, four genes among the DEGs were selected for fluorescent quantitative validation. Total RNA was isolated from no treatment (control), LEO treatment, piperacillin treatment and LEO with piperacillin treatment using RNeasy Mini Kit (Qiagen, USA) following the manufacturer’s protocol. Primers for four candidate genes (nfsB, srIA, srID and war) were designed using the NCBI Primer Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) with the parameters set to create a product of 70–300 base pair (bp) at the expected loci. Primers were synthesized by Integrated DNA Technologies (Coralville, USA) and dissolved in Milli-Q. Primer working stocks contained both forward and reverse primers at a concentration of 10 µM. A quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed using a Bio-Rad CFX Connect 96 (Hercules, CA, USA). The thermal cycling conditions were as follows: 10 s(s) at 98 °C, followed by 40 cycles of 1 s at 98 °C, 5 s at 60 °C and 30 s at 72 °C, completed the action with 5 min at 72 °C. Five biological replicates with three technical replicates each were assayed for each sample. The 2-ΔΔCT method was employed to analyse the relative changes in expression of the specific genes.
Results and discussion
Essential oils have been widely used since early times and are recognized for their therapeutic benefits. Numerous studies have shown the application of essential oils in medicinal and therapeutics used such as aromatherapy, antibacterial, antifungal and others (Yang et al. 2018). Lavender (Lavandula angustifolia), one of the most commonly used essential oils has been known to have therapeutic use when traced back to as early as Roman and Greek times (Eveleigh, 1994). A number of studies have shown the use of lavender in antimicrobial applications (Jianu et al. 2013). There is now substantial evidence that Lavandula species have the potential to improve treatment of bacterial infections which showed resistance to conventional antimicrobial agents. Nelson (1997) showed that at the concentration of less than 1% of lavender essential oil could inhibit the growth of microorganisms such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis. Previous study from Yap et al. (2013) indicated the synergetic effect of LEO and its potential to hinder bacterial resistance to piperacillin in E. coli J53 R1.
To further understand the mode of the synergistic effect of LEO and piperacillin, the profiling for the synergistic effects of LEO and piperacillin against multidrug resistant E. coli was carried out with Agilent’s E. coli Microarray Kit. Data were obtained in triplicate which E. coli J53 R1 was subjected to four different treatment conditions, which are untreated (control), LEO-treated, piperacillin-treated and LEO plus piperacillin-treated as previously described in Yap et al. (2014). The hierarchical clustering of gene expression heat map of E. coli J53 R1 strain treated with different conditions is showed in the dendrogram together with the heat map (Fig. 1). The gene expressions are marked using different intensities of blue (downregulated genes), white (are not differentially expressed) and red (upregulated genes). From the result, replicates from same experimental condition grouped together with the exception of a sample in lavender treatment, i.e. ‘R1L1’. While this may have an impact on uncovering differentially expressed genes in the lavender group, all candidate genes from the microarray result were validated with qPCR and hence, the interpretation of our results is still valid.
There is a broad range of changes in gene expression when the E. coli strain was subjected to piperacillin alone or in combination with LEO as shown in the microarray analysis results. The volcano plots of fold change (FC) of each gene and their statistical significance for comparisons between lavender against (vs.) control, piperacillin vs. control and lavender plus piperacillin vs. control (Fig. 2) were created to visualise the distribution of genes. Genes that were expressed at more than (>) 2 absolute linear FC less than (<) − 2 or > + 2 were considered as differentially expressed genes (DEGs). A total of 306 DEGs were identified across the three comparisons: 57 genes in lavender vs. control, 159 genes in piperacillin vs. control and 90 genes in lavender plus piperacillin vs. control (Table 1). The top 16 down- and up-regulated genes across all comparisons were ranked and shortlisted in Table 2. Ranking was based on statistical significance (AbsLinFC < 2, BHP-value < 0.05). Besides its statistical significance (AbsLinFC > 2, BHP-value < 0.05), the list (with top 20 genes) has been uploaded to DAVID Bioinformatics for functional annotation, and the results have shown that these 16 genes were identified to be associated with known biological mechanisms. Therefore, these were candidate genes for qPCR validation. Among these genes, four genes, srIA and srID, from LEO treatment, waaR from piperacillin and nfsB from LEO-piperacillin, were selected for validation. Figure 3 showed the expression patterns of the selected four genes verified by qPCR. The results showed a high consistency between the results generated by microarray and qRT-PCR.
Fig. 2.
Visualization of differentially expressed genes in E. coli J53 R1 strain using volcano plots for three different comparisons of experimental conditions. a Lavender treatment against control, b piperacillin treatment against control and c lavender and piperacillin treatment against control. The y-axis is the log10 Benjamini–Hochberg corrected p value, whereas the x-axis is log2 fold change (FC). Blue colour indicates downregulation, whereas red indicates upregulation, with increasing intensity of the colours to indicate more departure from mean gene expression
Table 1.
The number of differentially expressed genes identified in each comparison from microarray analysis
| Comparison | Number of differentially expressed genes |
|---|---|
| Lavender vs. control | 57 |
| Piperacillin vs. control | 159 |
| Lavender + piperacillin vs. control | 90 |
Table 2.
Top 16 differentially expressed genes across all samples
| No. | Gene ID | Gene symbol | Description | Frequency of occurrence | Gene expression changes |
|---|---|---|---|---|---|
| 1 | P20966 | fruA | Fused fructose-specific PTS enzymes: IIB component/IIC components | 5 | Up-regulation |
| 2 | P05706 | srlB | Glucitol/sorbitol-specific enzyme IIA component of PTS | 5 | Up-regulation |
| 3 | P56580 | srlE | Glucitol/sorbitol-specific enzyme IIB component of PTS | 5 | Up-regulation |
| 4 | P56579 | srlA | Glucitol/sorbitol-specific enzyme IIC component of PTS | 5 | Up-regulation |
| 5 | P05707 | srlD | Sorbitol-6-phosphate dehydrogenase | 3 | Up-regulation |
| 6 | P27128 | waaO | UDP-d-galactose:(glucosyl)lipopolysaccharide-alpha-1,3-d-galactosyltransferase | 1 | Up-regulation |
| 7 | P25740 | waaG | UDP-glucose:(heptosyl)lipopolysaccharide alpha-1,3-glucosyltransferase; lipopolysaccharide core biosynthesis protein; lipopolysaccharide glucosyltransferase I | 1 | Up-regulation |
| 8 | P25741 | waaP | Kinase that phosphorylates core heptose of lipopolysaccharide | 1 | Up-regulation |
| 9 | P27129 | waaR | Lipopolysaccharide 1,2-glucosyltransferase; UDP-glucose:(glucosyl)LPS alpha-1,2-glucosyltransferase | 1 | Up-regulation |
| 10 | P27127 | waaB | Lipopolysaccharide 1,6-galactosyltransferase; UDP-d-galactose:(glucosyl)lipopolysaccharide-1,6-d-galactosyltransferase | 1 | Up-regulation |
| 11 | P25553 | aldA | Aldehyde dehydrogenase A, NAD-linked | 1 | Down-regulation |
| 12 | P77258 | nemA | Chromate reductase, quinone reductase, FMN-linked; N-ethylmaleimide reductase; old yellow enzyme | 2 | Up-regulation |
| 13 | P38489 | nfsB | Dihydropteridine reductase, NAD(P)H-dependent, oxygen-insensitive | 2 | Up-regulation |
| 14 | P19926 | agp | Glucose-1-phosphatase/inositol phosphatise | 1 | Down-regulation |
| 15 | P11349 | narH | Nitrate reductase 1, beta (Fe-S) subunit | 1 | Down-regulation |
| 16 | P11350 | narI | Nitrate reductase 1, gamma (cytochrome b(NR)) subunit | 1 | Down-regulation |
Fig. 3.
Expression patterns of srIA, srID, waaR and nfsB genes from RT-qPCR in a treatment condition versus control. There are four treatments: grey colour indicates control, pink indicates lavender, orange indicates piperacillin and yellow indicates combination of piperacillin and lavender. The error bars indicate standard error mean
Pathway enrichment analyses using DAVID were done to investigate particular pathways affected by transcriptional changes associated with different treatments (Huang et al. 2009). Selected DEGs from each comparison were mapped to KEGG (Kyoto Encyclopaedia of Genes and Genomes) database and the pathways enriched with the DEGs were studied. The analysis revealed significantly altered biological pathways as determined by the p value of less than 0.1 in the KEGG pathways. The altered biological pathways were fructose and mannose metabolism (Fig. S1), phosphotransferase system (PTS) (Fig. S2), lipopolysaccharide biosynthesis (Fig. S3), microbial metabolism in diverse environments and nitrotoluene degradation (Fig. S4). It was revealed that five pathways, namely fructose and mannose metabolism, phosphotransferase system (PTS), lipopolysaccharide biosynthesis, microbial metabolism in diverse environments and nitrotoluene degradation were affected by the up-regulated DEGs and one, namely microbial metabolism, in diverse environments pathway was affected by the down-regulated DEGs (Table 3). Remarkably, LEO in combinatory treatment was more likely to upregulate genes in another two metabolic pathways: microbial metabolism and nitrotoluene degradation, as compared to single treatment. While others demonstrated no gene down-regulation, the combinatory treatment suggested a synergistic effect by down-regulating genes aldA, agp, narH and narI in microbial metabolism of E. coli. Aldehyde dehydrogenase, encoded by gene aldA, is the vital key for the evolved E. coli K-12 strains to survive and even replicate under harsh conditions (Mancini et al. 2014).
Table 3.
KEGG pathway analysis of differentially expressed genes (DEGs)
| Comparison | Pathways | Fold enrichment | p value | Gene symbol | ||
|---|---|---|---|---|---|---|
| Up-regulated DEGs | ||||||
| Lavender vs. control | Metabolism | |||||
| Fructose and mannose metabolism | 16.40625 | 1.04E−04 | srlE, srlD, fruA, srlB, srlA | |||
| Environmental information processing | ||||||
| Phosphotransferase system (PTS) | 12.20930233 | 0.002686688 | srlE, fruA, srlB, srlA | |||
| Piperacillin vs. control | Metabolism | |||||
| Fructose and mannose metabolism | 6.057692308 | 0.023702249 | srlE, srlD, srlB, srlA | |||
| Lipopolysaccharide biosynthesis | 9.770471464 | 0.00116543 | waaP, waaG, waaB, waaR, waaO | |||
| Lavender + piperacillin vs. control | Metabolism | |||||
| Fructose and mannose metabolism | 9.84375 | 0.001051296 | srlE, srlD, fruA, srlB, srlA | |||
| Microbial metabolism in diverse environments | 2.635983264 | 0.01673227 | fruA, nfsB, nemA | |||
| Nitrotoluene degradation | 22.5 | 0.081599022 | nfsB, nemA | |||
| Environmental information processing | ||||||
| Phosphotransferase system (PTS) | 7.325581395 | 0.01354449 | srlE, fruA, srlB, srlA | |||
| Down-regulated DEGs | ||||||
| Lavender + piperacillin vs. control | Metabolism | |||||
| Microbial metabolism in diverse environments | 2.635983264 | 0.01673227 | aldA, agp, narH, narI | |||
Other combinations of essential oil and antibiotic have also shown synergistic effect on antimicrobial activity, e.g. cinnamon bark and meropenem (Yang et al. 2019). A proteomic study on the effect of cinnamon bark essential oil on Klebsiella pneumoniae, a nosocomial pathogen, showed the essential oil is associated with 1.62-fold lower UDP-4-amino-4-deoxy-l-arabinose-oxoglutarate aminotransferase protein abundance (Yang et al. 2019). arnB encodes for UDP-4-amino-4-deoxy-l-arabinose-oxoglutarate aminotransferase and this gene plays a role in lipopolysaccharide (LPS) biosynthesis. Our piperacillin treatment has also revealed the pathway LPS biosynthesis as being significant (Table 3) and a gene, waaR, from this pathway was validated by our qPCR experiment.
In both the cinnamon bark and our piperacillin experiments, genes from the LPS biosynthesis pathway have lower expression but interestingly, different genes have been uncovered. LPS is a highly acylated saccharolipid found on the outer layer of the outer membrane of Gram-negative bacteria (Zhang et al. 2013). Both K. pneumonia and E. coli are Gram-negative bacteria. Given the role LPS plays in the maintenance of membrane integrity and preventing passive diffusion of hydrophobic solutes (Zhang et al. 2013) such as antibiotics, genes in LPS biosynthesis might be attractive drug targets or their gene expression are useful biomarkers to evaluate potential antimicrobial effect of other essential oils. Lavender oil is also known to act synergistically with meropenem against K. pneumoniae by disrupting its outer membrane (Yang et al. 2020). However, no significant LPS pathway has been found in the lavender or the combination of lavender and piperacillin treatments on multidrug-resistant E. coli.
Conclusion
The present study reveals that the fructose and mannose metabolism, phosphotransferase system (PTS), lipopolysaccharide biosynthesis and nitrotoluene degradation were affected by the up-regulated DEGs, and microbial metabolism in diverse environment pathway was affected by the down-regulated DEGs. In combinatory treatment of LEO and piperacillin, genes in microbial metabolism and nitrotoluene degradation were more likely to upregulate, as compared to single treatment. However, the combinatory treatment elicits synergistic effect by down-regulating genes aldA, agp, narH and narI in microbial metabolism of E. coli, where aldehyde dehydrogenase which is encoded by gene aldA, is the vital key for the evolved E. coli K-12 strains to survive and even replicate under harsh conditions. This study has suggested combinatory treatment of essential oil and antibiotic is a promising approach to reversal of antibiotic resistance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was funded by the Higher Colleges of Technology Interdisciplinary Research Grant (113118). We gratefully acknowledge Dr. George A. Jacoby for his kind gift of bacterial strains in this study. We also thank Ms. Nur Atiqah Azhar for her technical assistance and constructive suggestions on the bioinformatics analysis.
Author contributions
E-VN drafted the manuscript, P-JL, S-KY, C-LM, WYL, PS-XY, S-HEL and K-SL edited the draft. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Contributor Information
Pey-Jiun Lai, Email: pey_jiunlai@hotmail.com.
Ee-Von Ng, Email: Ng.EeVon@student.imu.edu.my.
Shun-Kai Yang, Email: kaichan992@gmail.com.
Chew-Li Moo, Email: gs51135@student.upm.edu.my.
Wai Yee Low, Email: wai.low@adelaide.edu.au.
Polly Soo-Xi Yap, Email: polly_yapsooxi@yahoo.com.
Swee-Hua Erin Lim, Email: lerin@hct.ac.ae.
Kok-Song Lai, Email: lkoksong@hct.ac.ae.
References
- Catalogue of life. https://www.gbif.org/dataset/7ddf754f-d193-4cc9-b351-99906754a03b. Cited 27 Feb 2018
- Dapkevicius A, Venskutonis R, Van Beek TA, Linssen JPH. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J Sci Food Agric. 1998;77:140–146. doi: 10.1002/(SICI)1097-0010(199805)77:1<140::AID-JSFA18>3.0.CO;2-K. [DOI] [Google Scholar]
- Dauria FD, Tecca M, Strippoli V, Salvatore G, Battinelli L, Mazzanti G. Antifungal activity of Lavandula angustifolia essential oil against Candida albicans yeast and mycelial form. Med Mycol. 2005;43:391–396. doi: 10.1080/13693780400004810. [DOI] [PubMed] [Google Scholar]
- De Rapper S, Viljoen A, Van Vuuren S. The in vitro antimicrobial effects of Lavandula angustifolia essential oil in combination with conventional antimicrobial agents. Evid Based Complement Altern Med. 2016;2016:1–9. doi: 10.1155/2016/2752739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshafie HS, Mancini E, Sakr S, De Martino L, Mattia CA, De Feo V, Camele I. Antifungal activity of some constituents of Origanum vulgare L. essential oil against postharvest disease of peach fruit. J Med Food. 2015;18:929–934. doi: 10.1089/jmf.2014.0167. [DOI] [PubMed] [Google Scholar]
- Evandri MG, Battinelli L, Daniele C, Mastrangelo S, Bolle P, Mazzanti G. The antimutagenic activity of Lavandula angustifolia (lavender) essential oil in the bacterial reverse mutation assay. Food Chem Toxicol. 2005;43:1381–1387. doi: 10.1016/j.fct.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Eveleigh T. Lavender. Sydney: Lorenz Books; 1994. [Google Scholar]
- Fabio A, Cermelli C, Fabio G, Nicoletti P, Quaglio P. Screening of the antibacterial effects of a variety of essential oils on microorganisms responsible for respiratory infections. Phytother Res. 2007;21:374–377. doi: 10.1002/ptr.1968. [DOI] [PubMed] [Google Scholar]
- Hajhashemi V, Ghannadi A, Sharif B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J Ethnopharmacol. 2003;89:67–71. doi: 10.1016/S0378-8741(03)00234-4. [DOI] [PubMed] [Google Scholar]
- Hanamanthagouda MA, Kakkalameli SB, Naik PM, Nafella P, Seetharamareddy HR, Murthy HN. Essential oils of Lavandula bipinnata and their antimicrobial activities. Food Chem. 2010;118:836–839. doi: 10.1016/j.foodchem.2009.05.032. [DOI] [Google Scholar]
- Holmes B, Richards DM, Brogden RN, Heel RC. Piperacillin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1984;28(5):375–425. doi: 10.2165/00003495-198428050-00002. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Jianu C, Pop G, Gruia AT, Horhat FG. Chemical composition and antimicrobial activity of essential oils of lavender (Lavandula angustifolia) and lavandin (Lavandula × intermedia) grown in Western Romania. Int J Agric Biol. 2013;15:772–776. [Google Scholar]
- Mahizan NA, Yang SK, Moo CL, Song AAL, Chong CM, Chong CW, Abushelaibi A, Lim SHE, Lai KS. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules. 2019;24:2631. doi: 10.3390/molecules24142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini E, Camele I, Elshafie HS, De Martino L, Pellegrino C, Grulova D, De Feo V. Chemical composition and biological activity of the essential oil of Origanum vulgare ssp. hirtum from different areas in the Southern Apennines (Italy) Chem Biodivers. 2014;11:639–651. doi: 10.1002/cbdv.201300326. [DOI] [PubMed] [Google Scholar]
- Moo CL, Yang SK, Yusoff K, Ajat M, Thomas W, Abushelaibi A, Lim SHE, Lai KS. Mechanisms of antimicrobial resistance (AMR) and alternative approaches to overcome AMR. Curr Drug Discov Technol. 2019 doi: 10.2174/1570163816666190304122219. [DOI] [PubMed] [Google Scholar]
- Nelson RRS. In-vitro activities of five plant essential oils against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. J Antimicrob Chemother. 1997;40(2):305–306. doi: 10.1093/jac/40.2.305. [DOI] [PubMed] [Google Scholar]
- Odds FC. Synergy, antagonism, and what the checquerboard puts between then. J Antimicrob Chemother. 2003;52(1):1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- Prusinowska R, Śmigielski KB. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L.). A review. Herba Polonica. 2014 doi: 10.2478/hepo-2014-0010. [DOI] [Google Scholar]
- Shah PJ, Ryzner L. Evaluating the appropriate use of piperacillin/tazobactam in a community health system: a retrospective chart review. P T. 2013;38(8):462–483. [PMC free article] [PubMed] [Google Scholar]
- Soković M, Glamočlija J, Marin PD, Brkic D, van Griensven LJ. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15(11):7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775–781. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- Wolska KI, Grześ K, Kurek A. A Synergy between novel antimicrobials and conventional antibiotics or bacteriocins. Pol J Microbiol. 2012;61(2):95–104. doi: 10.33073/pjm-2012-012. [DOI] [PubMed] [Google Scholar]
- World Health Organization Antimicrobial resistance. Glob Rep Surveill. 2014;61(3):12–28. doi: 10.1007/s13312-014-0374-3. [DOI] [Google Scholar]
- Yang SK, Low LY, Yap PSX, Yusoff K, Mai CW, Lai KS, Lim SH. Plant-derived antimicrobials: insights into mitigation of antimicrobial resistance. Rec Nat Prod. 2018;12:295–316. doi: 10.25135/rnp.41.17.09.058. [DOI] [Google Scholar]
- Yang SK, Yusoff K, Ajat M, Thomas W, Abushelaibi A, Akseer R, Lim SHE, Lai KS. Disruption of KPC-producing Klebsiella pneumoniae membrane via induction of oxidative stress by cinnamon bark (Cinnamomum verum J. Presl) essential oil. PLoS ONE. 2019;14:e0214326. doi: 10.1371/journal.pone.0214326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SK, Yusoff K, Thomas W, Akseer R, Sultan Alhosani M, Abushelaibi A, Lim SHE, Lai KS. Lavender essential oil induces oxidative stress which modifies the bacterial membrane permeability of carbapenemase producing Klebsiella pneumoniae. Sci Rep. 2020;10:1–14. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap PSX, Krishnan T, Yiap BC, Hu CP, Chan KG, Lim SH. Membrane disruption and anti-quorum sensing effects of synergistic interaction between Lavandula angustifolia (lavender oil) in combination with antibiotic against plasmid-conferred multi-drug-resistant Escherichia coli. J Appl Microbiol. 2014;116(5):1119–1128. doi: 10.1111/jam.12444. [DOI] [PubMed] [Google Scholar]
- Yap PSX, Lim SHE, Hu CP, Yiap BC. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine. 2013;20(8–9):710–713. doi: 10.1016/j.phymed.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Zhang G, Meredith TC, Kahne D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr Opin Microbiol. 2013;16:779–785. doi: 10.1016/j.mib.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




