Dear Editor
The pandemic of coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) represents the greatest global public health crisis that occurred during the last decades. Among hospitalized patients, up to 25% will develop acute respiratory failure and the acute respiratory distress syndrome (ARDS) and require intensive care unit (ICU) admission. The median duration between onset of symptoms and ICU admission ranges from 7 to 12 days [1], suggesting a gradual deterioration in the majority of cases. Although the clinical characteristics of patients requiring ICU admission have now been well described [2–4], their viro-immunological features are still unknown. Whether a higher titer of SARS-CoV-2-specific antibodies may reduce viral RNA load in upper respiratory samples and eventually mitigate the course of infection in patients admitted in the ICU has not been established. We examined the relationship between SARS-CoV-2 viral loads collected from nasopharyngeal swabs on ICU admission, concomitant SARS-CoV-2-specific IgA and IgG antibody titers, and day-28 mortality.
This is a prospective monocenter study, which included all patients diagnosed with RT-PCR-confirmed SARS-CoV-2 infection consecutively admitted in the medical ICU at Henri Mondor Hospital, Créteil, France, between March 8, 2020, and March 26, 2020. The study has received the approbation of an institutional review board (Comité de Protection des Personnes Ile de France II; reference number: 3675-NI). Informed consent was obtained from all patients or their relatives. Nasopharyngeal swabs and sera were collected from patients within 48 h of ICU admission. The cycle threshold values of RT-PCR were used as indicators of the viral load of SARS-CoV-2 RNA in nasopharyngeal specimens, with lower cycle threshold values corresponding to higher viral load. IgA and IgG antibodies against SARS-CoV-2 spike protein subunit 1 (S1) were quantified in patients’ serum using ELISAs (Euroimmun Medizinische Labordiagnostika, Lübeck, Germany) and expressed in arbitrary units (AU). The primary clinical outcome was day-28 mortality.
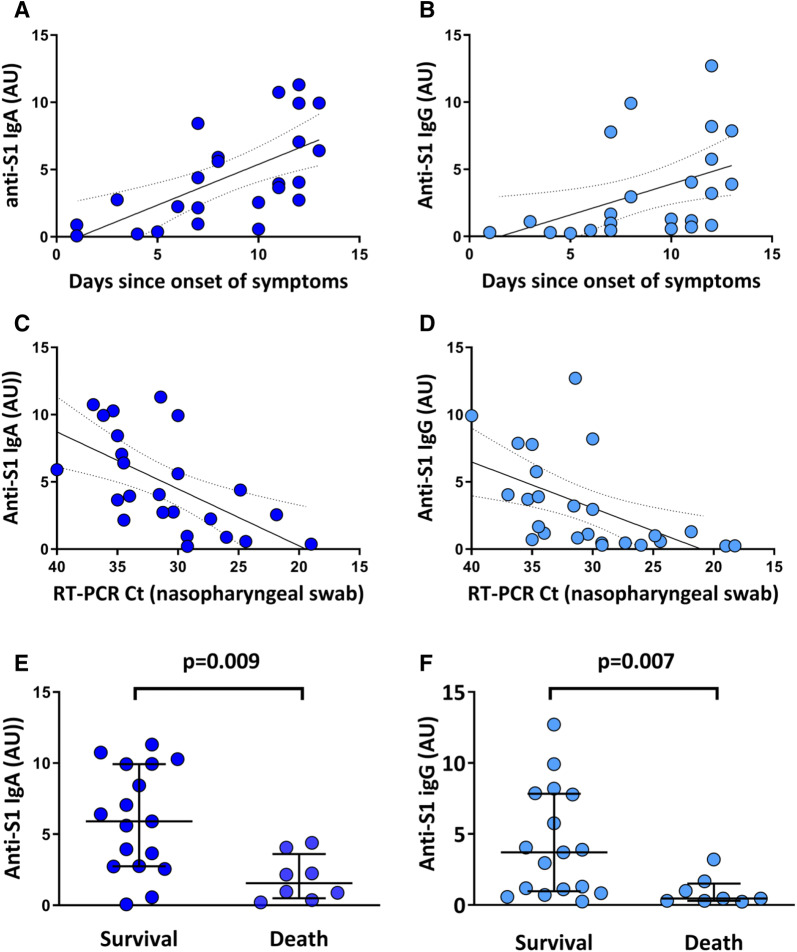
Twenty-five patients [mean age 60 ± 14 years; males 80% (n = 20/25)] were admitted in the ICU for severe SARS-CoV-2 infection during the study period. The median time elapsed between the first symptoms and ICU admission was 9 days [6–12]. Invasive mechanical ventilation was required in 96% (n = 24/25) of patients during ICU stay, and the mortality at day-28 of ICU admission was 32% (n = 8/25) (Supplemental Table 1). There was a significant correlation between the time elapsed between the first symptoms and ICU admission and the titer of both anti-S1 IgA (Spearman’s r = 0.70; p < 0.001) and anti-S1 IgG (r = 0.68; p < 0.001) measured in sera obtained upon ICU admission (Fig. 1a, b). No correlation was found between the absolute counts of peripheral B or T lymphocytes and the titers of anti-S1 IgA (r = 0.002, p = 0.99 and r = 0.10, p = 0.624, respectively) or IgG (r = 0.12, p = 0.56, and r = 0.13, p = 0.53, respectively). There was also no correlation between peripheral B or T lymphocyte counts and viral loads (r = 0.34, p = 0.093, and r = 0.24, p = 0.247, respectively). We observed an inverse correlation between the viral load obtained from nasopharyngeal swabs and the serum level of anti-S1 IgA (r = 0.69; p < 0.001) or IgG (r = 0.72; p < 0.0001) (Fig. 1c, d). Finally, we explored the relationship between IgA/G titers measured upon ICU admission and day-28 mortality. As shown in Fig. 1e, f, patients who were still alive at day-28 displayed significantly higher titers of anti-S1 IgA or IgG upon admission than those who had died at day-28. Additionally, the serum titer of anti-S1 IgA was a protective factor of day-28 mortality, even after adjusting for SOFA (adjusted odds ratio (aOR) = 0.45 [0.21–0.98]; p = 0.045) or age (aOR = 0.56 [0.33-0.95]; p = 0.032) (Supplemental Table 2), two important determinants of outcome during severe SARS-CoV-2 infections.
Fig. 1.
a Correlation between the number of days since onset of symptoms and intensive care unit (ICU) admission and the serum titer of anti-S1 IgA, expressed in arbitrary units (AU) (Spearman’s r = 0.70; p < 0.001; r2 = 0.40); b correlation between the number of days since onset of symptoms of SARS-CoV-2 infection and ICU admission and the serum titer of anti-S1 IgG (AU) (Spearman’s r = 0.68; p < 0.001, r2 = 0.22); c correlation between the serum titer of anti-S1 IgA (AU) and the SARS-CoV-2 viral load measured in nasopharyngeal swabs (expressed in cycle threshold value, Ct) (Spearman’s r = 0.69; p < 0.001; r2 = 0.35); d correlation between the serum titer of anti-S1 IgG (AU) and the SARS-CoV-2 viral load measured in nasopharyngeal swabs (expressed in Ct) (Spearman’s r = 0.72; p < 0.0001; r2 = 0.29); note that the x-axis of c, d is inverted so as to reflect that the RT-PCR Ct is inversely correlated with RNA viral load; e comparisons of serum anti-S1 IgA titers obtained upon ICU admission between patients who were alive at day-28 of ICU admission and patients who died; f comparisons of serum anti-S1 IgG titers obtained upon ICU admission between patients who were alive at day 28 of ICU admission and patients who died; continuous lines represent the lines of best fit of the linear regressions and the dotted lines show their 95% confidence intervals; horizontal lines represent the median value; p values displayed in e, f come from the Mann–Whitney test
Our study certainly has some limitations, related to its monocenter design as well as to the small number of patients included, limiting our ability to adjust the observed relationship between anti-S1 IgA/G titers and day-28 mortality for potential confounders. Yet, our results suggest that the absence of early humoral response in a subset of severe patients is associated with a higher viral load in the upper respiratory tract upon admission and might have a deleterious impact on survival.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr Inès Bendib, Thiziri Sadaoui, Thomas Frapart, and Simon Rivoal for helping collect clinical data, Audrey Riou, Alexandre Soulier, and Asma Beldi-Ferchiou for performing laboratory analyses, and Prof. Marie-Hélène Delfau-Larue for critically reviewing the manuscript.
Funding
None.
Compliance with ethical standards
Conflicts of interest
SF, SH, AMD, and NDP have no conflict of interest to disclose. JMP has received research grants from Abbott, Abbvie, and Gilead; he has served as an advisor for Abbott, Abbvie, Gilead, Merck, and Siemens Healthcare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Slim Fourati and Sophie Hue have contributed equally to this work.
References
- 1.Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, Shrestha BR, Arabi YM, Ng J, Gomersall CD, Nishimura M, Koh Y, Du B. Intensive care management of coronavirus disease 2020 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.