Dear Editor,
Since the first cases in November 2019, the spread of SARS-CoV-2 infections has placed unprecedented strain on healthcare. The intensive care unit (ICU) is of particular concern as large numbers of patients with severe respiratory complications mean that in some areas, ICUs have been completely overwhelmed [1].
Understanding determinants of ICU outcome is crucial both for surge planning and shared decision making. Whilst a number of risk scores have been published [2], they do not specifically look at this population. Furthermore, ICU availability, admission policy and structure vary across Europe [3] as do demographics and government policy. Thus, it is likely that ICU outcomes could also vary significantly by region motivating an individualised modelling approach. UK mortality has been particularly high, and we sought to urgently identify predictors of mortality in patients admitted to the ICU with COVID-19.
We obtained de-identified COVID-19 Hospitalisation in England Surveillance System (CHESS) data from Public Health England (PHE) for the period from 8th February (data collection start) to 22nd May 2020 (5062 ICU cases—1547 deaths, 1618 discharges from 94 NHS trusts across England). Mean APACHE-II score for each site from ICU national audit data for COVID-19 patients over a similar period was used to attempt to correct for case-severity/as a proxy for admission policy since a variety of presentation severity indicators may also drive outcome [4].
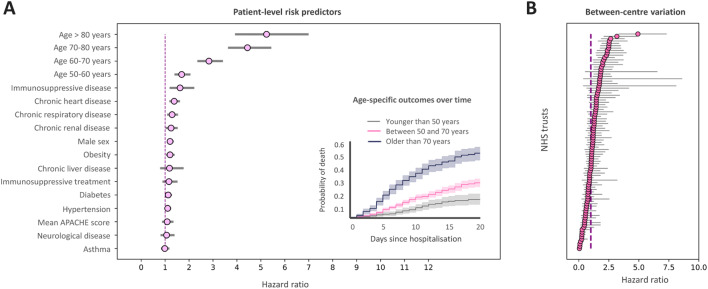
We used a Cox proportional hazards mixed-effects model for mortality, with NHS trust as the random effect. The estimated coefficients for each predictor are shown in Fig. 1. The proportional hazards assumption was not violated (p = 0.061) (Supplementary material).
Fig. 1.
a Fixed effects estimates for CHESS predictors and mean APACHE-II score for each site from national audit data. The strongest patient-factor predictors are older age, immunosuppressive disease and chronic heart/renal disease. The inset shows mortality probability over time for young, intermediate and older age groups. b Random effects showing between centres variation showing a hazard ratio variation between sites from 0 to over + 4 which is comparable in magnitude to the strongest CHESS predictor (older age) showing that between-centres variation is an appreciable determinant of outcome. 95% CIs shown
Immunosuppressive disease, chronic cardiorespiratory/renal disease and age were key predictors. Compared with these fixed effects, the magnitude of the between-centre variation (hazard ratio between 0 to over + 4) is comparable to the strongest fixed effects predictor. The cause of such between-centre variation is unclear and may have a variety of residual case-mix or structural explanations. In particular, ICU demand varies both regionally and locally and we may hypothesize that high levels of strain or constraints on surge capacity could be actionable determinants, although we do not have data to examine this. Such considerations are important to understand as they may influence optimal configuration or transfer considerations locally.
Analysis limitations include possible incomplete ascertainment (particularly before ~ 15th March), potential lead time bias from earlier deaths in the elderly group (although a sensitivity analysis excluding patients with < 7 days follow-up yielded qualitatively similar results—Supplement Figure S1) and an inability to track patient transfers. Nevertheless, the magnitude of the random effects is striking. This motivates urgent comparative effectiveness research to characterise between-centre differences to inform surge best-practice in both in England and elsewhere.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge PHE for providing us access to the CHESS dataset and the intensive care national audit and research centre for providing aggregate APACHE-II data, and also thank Anees Pari and Geraldine Linehan for their support.
Author contributions
AE and MvdS conceived of and supervised the study. ZQ and AMA coded the statistical models. All authors contributed to the manuscript.
Funding
Not externally funded.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Availability of data and materials statement
Source data controlled by PHE. Under the terms of our data sharing agreement, the authors are not able to re-share the source data but will entertain requests for scientific collaboration.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynants L, Van Calster B, Bonten MMJ, Collins GS, Debray TPA, De Vos M, Haller MC, Heinze G, Moons KGM, Riley RD, Schuit E, Smits LJM, Snell KIE, Steyerberg EW, Wallisch C, van Smeden M. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020 doi: 10.1136/Bmj.M1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhodes A, Ferdinande P, Flaatten H, Guidet B, Metnitz PG, Moreno RP. The variability of critical care bed numbers in Europe. Intensive Care Med. 2012;38:1647–1653. doi: 10.1007/s00134-012-2627-8. [DOI] [PubMed] [Google Scholar]
- 4.Wang K, Zhang Z, Yu M, Tao Y, Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data controlled by PHE. Under the terms of our data sharing agreement, the authors are not able to re-share the source data but will entertain requests for scientific collaboration.