Abstract
Background
Studies have reported seasonal variations regarding the incidence and the short-term mortality of pulmonary embolism (PE). The aim of this study was to identify sex-specific and age-related differences in seasonal patterns regarding hospitalisations and mortality of PE patients.
Methods
We analysed the impact of seasons on incidence and in-hospital mortality of male and female hospitalised PE patients in Germany (2005–2015) based on the German nationwide inpatient sample.
Results
The German nationwide inpatient sample comprised 885 806 hospitalisations due to PE (2005–2015). Seasonal variations of both incidence (p=0.021) and in-hospital mortality (p<0.001) were of significant magnitude. Quarterly annual incidence (25.5 versus 23.7 of 100 000 citizens per year, p=0.021) and in-hospital mortality (17.0% versus 16.7%, p=0.008) were higher in winter than in summer. Risk of in-hospital mortality in winter was slightly higher (OR 1.03 (95% CI 1.01–1.06), p=0.015) compared to summer, independently of sex, age and comorbidities. Additionally, we observed sex-specific differences during seasons: the highest number of hospitalisations of PE patients of both sexes was during winter, whereas the nadir of male patients was in spring and that of female patients was in summer. Both sexes showed a maximum of in-hospital mortality in spring. Seasonal variation regarding incidence and mortality was pronounced in older patients.
Conclusion
Incidence and the in-hospital mortality of PE patients showed a significant seasonal variation with sex-specific differences. Although it has to be hypothesised that the seasonal variation of PE is multifactorially dependent, variation in each season was not explained by seasonal differences regarding age, sex and the prevalence of important comorbidities.
Short abstract
Incidence and mortality of PE patients shows a seasonal variation with sex-specific differences. Seasonal variation of PE is caused multifactorially but not primarily explained by age, sex or comorbidities. https://bit.ly/2XWxULT
Introduction
A growing body of evidence suggest that the onset and the mortality rate of several major cardiovascular events is influenced by seasonal patterns [1, 2]. Previous studies reported seasonal trends regarding incidence and/or mortality in acute myocardial infarction (MI) [3–6], sudden cardiac death [5, 7], aortic dissection [8] and stroke [9]. For MI, studies revealed that both, the incidence [3, 10, 11] as well as the short-term mortality [3, 4] of an acute MI event were higher in cold winter months than in warm summer months. In addition, the role of temperature has also been explored [3, 10, 12].
For the life-threatening cardiovascular event of an acute pulmonary embolism (PE) the existence of seasonal variability has been debated for years and results on sex-specific differences regarding these variations are sparse [2]. Published studies investigating seasonal variations of incidence and mortality of PE showed inconsistent results [1, 2, 13–23]. While some studies identified seasonal variations [1, 2, 13, 15, 16, 18–21, 23], others did not [14, 17].
Thus, the objectives of the present study were 1) to investigate seasonal variation of incidence and in-hospital mortality of patients with an acute PE; 2) identify seasonal differences regarding age, sex, comorbidities, risk stratification markers and usage of reperfusion treatments; and 3) to detect sex-specific and age-related differences regarding seasonal variations in the large German nationwide inpatient sample.
Methods
Data source
The German Research Data Center (RDC) of the Federal Statistical Office and the Statistical Offices of the federal states (source: RDC of the Federal Statistical Office and the Statistical Offices of the Federal States, DRG Statistics 2005–2015, own calculations) in Wiesbaden (Germany) analysed the data on our behalf and provided aggregated statistic results on the basis of SPSS syntaxes (SPSS® software, version 20.0, SPSS Inc., Chicago, IL, USA), which we had supplied to the RDC.
Diagnoses, procedural codes, and definitions
Diagnoses were coded according to the International Classification of Diseases and Related Health Problems in its 10th Revision with German Modification (ICD-10-GM) and diagnostic, surgical or interventional procedures were coded according to the German Procedure Classification (OPS, surgery, diagnostic and procedures codes; Operationen und Prozedurenschlüssel).
Hospitalised patients diagnosed with PE (ICD code I26) between 2005 and 2015 were included in this analysis. PE patients were stratified according to seasons and patients admitted in the seasons summer and winter were compared. Seasons were defined as: winter included the months December to February, spring included March to May, summer included June to August and autumn included September to November.
Monthly mean temperature in Germany was obtained by the German meteorological service (Deutscher Wetterdienst).
Study outcome
The primary study outcome was all-cause in-hospital death.
Ethical aspects
As this study did not involve direct access by the investigators to data of individual patients, approval by an ethics committee and informed consent were not required, in accordance with German law.
Statistical methods
Annual total numbers of hospitalisations for acute PE and incidence of acute PE were calculated. In addition, in-hospital mortality rate was computed.
Descriptive statistics for relevant baseline comparisons and outcomes of hospitalisations of PE patients in the different seasons were presented and hospitalisations of the winter and summer seasons (presumed maximum and nadir of temperature difference and incidence) were compared. We tested the continuous variables using the Mann–Whitney U-test and categorical variables with chi-squared test or Fisher's exact test, as appropriate.
Seasonal variation was additionally tested by ANOVA to compare the PE cases in the different seasons. If it was significant (i.e. p<0.05), then the Tukey post hoc test was used for post hoc comparison of multiple means to determine which seasons were significantly different.
Univariate and multivariate logistic regression models were analysed to investigate the impact of season (winter versus summer season) on in-hospital mortality, cardiopulmonary resuscitation (CPR), the presentation with haemodynamically instability (defined as shock and/or CPR), right ventricular (RV) dysfunction, tachycardia, syncope, bleeding events and reperfusion treatments. The results were presented as odds ratios (ORs) with corresponding 95% confidence intervals (CIs). Multivariate logistic regression models included the following parameters for adjustment:
Adjustment I: age, sex, active cancer (ICD codes C00-C97), heart failure (ICD code I50), chronic obstructive pulmonary disease (COPD, ICD code J44), essential arterial hypertension (ICD-code I10), acute and chronic kidney disease (ICD-codes N17-N19), diabetes mellitus (ICD-codes E10-E14), obesity, and coronary artery disease (ICD-code I25).
Adjustment II: for sex-specific analysis adjustment I was used without the variable sex.
Adjustment III: variables of adjustment I with further variables: RV dysfunction (ICD-code I26.0), tachycardia (ICD-code I47), syncope (ICD-code R55) and haemodynamically unstable PE (PE with additional shock or CPR).
Adjustment IV: for additional sex-specific analysis adjustment III was used without the variable sex.
The software SPSS® (versions 20.0 and 23.0; SPSS Inc., Chicago, IL, USA) was used for computerised analysis. P-values of <0.05 (two-sided) were considered to be statistically significant.
Results
Total numbers of pulmonary embolism and mortality rate (2005–2015)
During a time period of 11 years (from 2005 to 2015), the German nationwide sample included 885 806 hospitalised patients with PE. Of those, 147 234 (16.6%) died during in-hospital stay.
Patient characteristics stratified by seasons
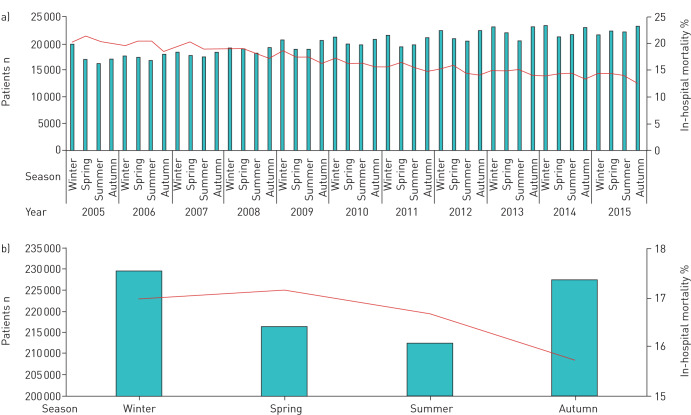
The patient characteristics stratified by seasons revealed a higher quarterly annual incidence of PE during winter compared to the summer (25.5 versus 23.7 hospitalisations per 100 000 population, p=0.021). In parallel, highest mortality and CPR rates were found in winter and spring and lowest in summer and autumn (table 1, figures 1 and 2). Patients hospitalised in winter were more often older than 70 years and of female sex in comparison to patients hospitalised in summer. Additionally, patients hospitalised in the summer were more often obese, presented less with diabetes mellitus and suffered more often from heart failure and cancer in comparison to patients hospitalised in winter (table 1).
TABLE 1.
Patient characteristics of 885 806 hospitalisations with acute pulmonary embolism (PE) stratified by seasons
| Winter | Spring | Summer | Autumn | p-value for difference between summer and winter | |
| Temperature | |||||
| Median temperature# in Germany | 1.1 (−0.5–3.6) | 8.7 (5.7–12.4) | 17.2 (16.1–18.2) | 9.1 (6.8–12.8) | <0.001 |
| Incidence, total numbers and length of stay | |||||
| 3-month incidence of PE per 100 000 citizens | 25.5 | 24.1 | 23.7 | 25.3 | 0.021 |
| Number of PE events | 229 379 (25.9%) | 216 420 (24.4%) | 212 595 (24.0%) | 227 412 (25.7%) | 0.021 |
| In-hospital stay days | 10 (6–16) | 10 (6–17) | 10 (6–16) | 10 (6–16) | 0.001 |
| Patients' characteristics and comorbidities | |||||
| Age at event years | 72.0 (61.0–80.0) | 72.0 (61.0–80.0) | 72.0 (60.0–80.0) | 72.0 (60.0–80.0) | <0.001 |
| Age >70 years | 124 578 (54.3%) | 117 899 (54.5%) | 114 166 (53.7%) | 122 950 (54.1%) | <0.001 |
| Female sex (n=885 770) | 124 150 (54.1%) | 117 827 (54.4%) | 113 473 (53.4%) | 123 428 (54.3%) | <0.001 |
| Obesity¶ | 21 224 (9.3%) | 20 936 (9.7%) | 21 162 (10.0%) | 21 961 (9.7%) | <0.001 |
| Diabetes mellitus | 42 018 (18.3%) | 40 781 (18.8%) | 40 720 (19.2%) | 42 233 (18.6%) | <0.001 |
| Hyperlipidaemia | 25 882 (11.3%) | 24 649 (11.4%) | 24 472 (11.5%) | 27 035 (11.9%) | 0.017 |
| Essential arterial hypertension | 96 860 (42.2%) | 91 899 (42.5%) | 89 806 (42.2%) | 97 545 (42.9%) | 0.916 |
| Coronary artery disease | 31 183 (13.6%) | 30 406 (14.0%) | 29 801 (14.0%) | 31 448 (13.8%) | <0.001 |
| Heart failure | 47 476 (20.7%) | 46 496 (21.5%) | 45 372 (21.3%) | 47 783 (21.0%) | <0.001 |
| Atrial fibrillation/flutter | 35 256 (15.4%) | 34 420 (15.9%) | 33 288 (15.7%) | 34 647 (15.2%) | 0.008 |
| Cancer | 43 596 (19.0%) | 43 227 (20.0%) | 44 066 (20.7%) | 45 344 (19.9%) | <0.001 |
| COPD | 24 313 (10.6%) | 23 513 (10.9%) | 21 756 (10.2%) | 23 076 (10.1%) | <0.001 |
| Stroke | 6099 (2.7%) | 5887 (2.7%) | 5921 (2.8%) | 5909 (2.6%) | 0.010 |
| Acute and chronic kidney disease | 44 862 (19.6%) | 43 408 (20.1%) | 43 633 (20.5%) | 45 340 (19.9%) | <0.001 |
| Bleeding events during hospitalisation | |||||
| Gastro-intestinal bleeding | 3127 (1.4%) | 2939 (1.4%) | 2961 (1.4%) | 3120 (1.4%) | 0.400 |
| Subarachnoid bleeding | 305 (0.1%) | 318 (0.1%) | 354 (0.2%) | 341 (0.1%) | 0.004 |
| Intracerebral bleeding | 1274 (0.6%) | 1273 (0.6%) | 1265 (0.6%) | 1248 (0.5%) | 0.082 |
| Transfusion of erythrocyte concentrates | 25 428 (11.1%) | 25 681 (11.9%) | 26 432 (12.4%) | 26 286 (11.6%) | <0.001 |
| Reperfusion treatments | |||||
| Surgical embolectomy | 351 (0.2%) | 322 (0.1%) | 346 (0.2%) | 375 (0.2%) | 0.415 |
| Systemic thrombolysis | 9791 (4.3%) | 8858 (4.1%) | 8812 (4.1%) | 9456 (4.2%) | 0.041 |
| Risk stratification and deep vein thrombosis | |||||
| Deep vein thrombosis or thrombophlebitis | 83 757 (36.5%) | 76 093 (35.2%) | 75 469 (35.5%) | 82 669 (36.4%) | <0.001 |
| Tachycardia | 4099 (1.8%) | 3947 (1.8%) | 4038 (1.9%) | 4211 (1.9%) | 0.005 |
| Syncope | 5459 (2.4%) | 5057 (2.3%) | 5006 (2.4%) | 5356 (2.4%) | 0.582 |
| Haemodynamic instability | 20 549 (9.0%) | 19 478 (9.0%) | 19 032 (9.0%) | 19 584 (8.6%) | 0.942 |
| Shock | 7848 (3.4%) | 7668 (3.5%) | 7582 (3.6%) | 7841 (3.4%) | 0.009 |
| Primary outcome | |||||
| CPR | 15 949 (7.0%) | 15 002 (6.9%) | 14 486 (6.8%) | 15 082 (6.6%) | 0.068 |
| In-hospital death | 38 921 (17.0%) | 37 115 (17.1%) | 35 439 (16.7%) | 35 759 (15.7%) | 0.008 |
Data are presented as n (%) or median (interquartile range), unless otherwise stated. CPR: cardiopulmonary resuscitation. #: Median temperature in the different seasons during the observational timeframe (2005–2015); ¶: Obesity was defined according to the World Health Organization (body mass index ≥30 kg·m−2). Bold text indicates p<0.05.
FIGURE 1.
a) Absolute numbers of pulmonary embolism (PE) (bars) and in-hospital mortality rate (line) stratified for seasons in the years 2005–2015. b) Absolute numbers of PE (bars) and in-hospital mortality rate (line) stratified by seasons (cumulative 2005–2015).
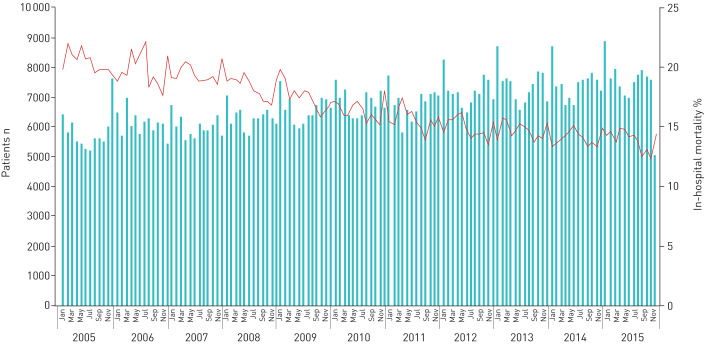
FIGURE 2.
Time trend of absolute numbers of pulmonary embolism (PE) (bars) and in-hospital mortality rate (line) stratified for months from 2005–2015.
Seasonal variation of PE incidence
The ANOVA confirmed that the seasonal variation regarding incidence of PE was of substantial magnitude (p=0.021) (table 1). PE incidence differed between winter and summer, but not between other seasons (figure 1). Regarding in-hospital mortality, the summer season was significantly different from winter, spring and autumn. In addition, seasonal variations between autumn and winter as well as between autumn and spring were significant (figure 1, table 2). In the logistic regression analysis, incidence of PE in winter was higher than in summer months (OR 1.85 (95% CI 1.09–3.13), p=0.022).
TABLE 2.
Results of the significance level computed with the Tukey post hoc test (ANOVA analysis) regarding in-hospital mortality differences between the different seasons
| All patients | Winter | Spring | Summer | Autumn | ||||
| Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Winter | −0.002 (−0.005–0.002) | 0.363 | 0.003 (0.000–0.006) | 0.039 | 0.012 (0.010–0.015) | <0.001 | ||
| Spring | 0.002 (−0.002–0.005) | 0.363 | 0.005 (0.002–0.008) | 0.0001 | 0.014 (0.011–0.017) | <0.001 | ||
| Summer | −0003 (−0.006– −0.000) | 0.039 | −0.005 (−0.008–0.002) | 0.0001 | 0.009 (0.007–0.012) | <0.001 | ||
| Autumn | −0.012 (−0.015– −0.010) | <0.001 | −0.014 (−0.017– −0.011) | <0.001 | −0.009 (−0.012– −0.007) | <0.001 | ||
| Male patients | Winter | Spring | Summer | Autumn | ||||
| Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Winter | −0.06 (−0.010 to −0.002) | 0.003 | 0.002 (−0.002 to 0.007) | 0.483 | 0.008 (0.004 to 0.012) | <0.001 | ||
| Spring | 0.006 (0.002 to 0.010) | 0.003 | 0.008 (0.004 to 0.012) | <0.001 | 0.014 (0.010 to 0.018) | <0.001 | ||
| Summer | −0.002 (−0.007 to 0.002) | 0.483 | −0.008 (−0.012 to −0.004) | <0.001 | 0.006 (0.002 to 0.010) | 0.002 | ||
| Autumn | −0.008 (−0.012 to −0.004) | <0.001 | −0.014 (−0.018 to −0.010) | <0.001 | −0.006 (−0.010 to −0.002) | 0.002 | ||
| Female patients | Winter | Spring | Summer | Autumn | ||||
| Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Winter | 0.001 (−0.002–0.005) | 0.760 | 0.003 (−0.001–0.007) | 0.106 | 0.160 (0.012–0.020) | <0.001 | ||
| Spring | −0.001 (−0.005–0.002) | 0.760 | 0.002 (−0.002–0.006) | 0.577 | 0.015 (0.011–0.018) | <0.001 | ||
| Summer | −0.003 (−0.007–0.005) | 0.106 | −0.002 (−0.006–0.002) | 0.577 | 0.013 (0.009–0.017) | <0.001 | ||
| Autumn | −0.016 (−0.020– −0.012) | <0.001 | −0.015 (−0.018– −0.011) | <0.001 | −0.013 (−0.017– −0.009) | <0.001 | ||
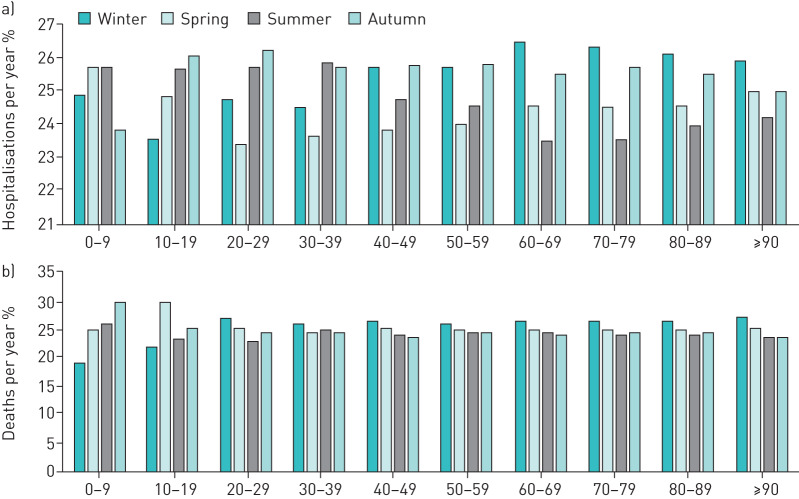
Age-dependent analysis demonstrated that a winter peak and summer nadir was identified in patients aged 60 years and older, but not in younger PE patients (figure 3a).
FIGURE 3.
a) Seasonal proportion of numbers of pulmonary embolism (PE) (cumulative 2005–2015) in the different age-decades (sum of seasonal percentages in each age-decade is 100%). b) Seasonal proportion of deaths (cumulative 2005–2015) in the different age-decades (sum of seasonal percentages in each age-decade is 100%).
Seasonal differences in risk stratification of PE
Rates of PE with haemodynamic instability and syncope were comparable between different seasons (table 1). In contrast, tachycardia was more often found in the summer season. The logistic regressions revealed that RV dysfunction occurred more often in the winter months than in the summer independently of age, sex and comorbidities (table 3).
TABLE 3.
Impact of winter season on different clinical parameters and outcomes in comparison to summer season
| All PE patients (sex-unspecific analyses) | Univariate analysis OR (95% CI) | p-value | Multivariate analysis# OR (95% CI) | p-value |
| Reperfusion treatment | ||||
| Surgical pulmonary embolectomy | 0.94 (0.81–1.09) | 0.415 | 1.00 (0.86–1.16) | 0.950 |
| Systemic thrombolysis | 1.03 (1.00–1.06) | 0.041 | 1.04 (1.01–1.07) | 0.008 |
| Risk stratification | ||||
| Haemodynamically unstable PE | 1.01 (0.98–1.02) | 0.942 | 1.01 (0.99–1.03) | 0.238 |
| Right ventricular dysfunction | 1.04 (1.03–1.06) | <0.001 | 1.04 (1.03–1.06) | <0.001 |
| Tachycardia | 0.94 (0.90–0.98) | 0.005 | 0.96 (0.91–1.00) | 0.041 |
| Syncope | 1.01 (0.97–1.05) | 0.582 | 1.00 (0.96–1.04) | 0.955 |
| In-hospital outcomes | ||||
| CPR | 1.02 (1.00–1.05) | 0.068 | 1.03 (1.01–1.05) | 0.017 |
| In-hospital death | 1.02 (1.01–1.04) | 0.008 | 1.04 (1.02–1.05) | <0.001 |
| Intra-hospital bleeding events | ||||
| Gastro-intestinal bleeding | 0.98 (0.93–1.03) | 0.400 | 0.97 (0.95–1.05) | 0.869 |
| Subarachnoid bleeding | 0.80 (0.69–0.93) | 0.004 | 0.80 (0.69–0.93) | 0.005 |
| Intracerebral bleeding | 0.93 (0.86–1.01) | 0.082 | 0.93 (0.86–1.01) | 0.073 |
| Men | Univariate OR (95% CI) | p-value | Multivariate¶ OR (95% CI) | p-value |
| Reperfusion treatment | ||||
| Surgical pulmonary embolectomy | 0.94 (0.77–1.15) | 0.524 | 1.00 (0.82–1.22) | 0.973 |
| Systemic thrombolysis | 1.06 (1.01–1.10) | 0.011 | 1.07 (1.03–1.12) | 0.002 |
| Risk stratification | ||||
| Haemodynamically unstable PE | 0.99 (0.96–1.02) | 0.424 | 1.00 (0.97–1.03) | 0.936 |
| Right ventricular dysfunction | 1.05 (1.03–1.07) | <0.001 | 1.05 (1.03–1.07) | <0.001 |
| Tachycardia | 0.93 (0.88–1.00) | 0.036 | 0.95 (0.89–1.02) | 0.145 |
| Syncope | 1.00 (0.95–1.07) | 0.901 | 0.99 (0.94–1.05) | 0.821 |
| In-hospital outcomes | ||||
| CPR | 1.00 (0.97–1.04) | 0.803 | 1.01 (0.98–1.05) | 0.483 |
| In-hospital death | 1.02 (0.99–1.04) | 0.154 | 1.03 (1.01–1.06) | 0.015 |
| Intra-hospital bleeding events | ||||
| Gastro-intestinal bleeding | 0.95 (0.88–1.02) | 0.185 | 0.97 (0.90–1.04) | 0.406 |
| Subarachnoid bleeding | 0.94 (0.75–1.17) | 0.565 | 0.94 (0.75–1.19) | 0.619 |
| Intracerebral bleeding | 1.02 (0.91–1.13) | 0.785 | 1.01 (0.91–1.13) | 0.829 |
| Women | Univariate OR (95% CI) | p-value | Multivariate¶ OR (95% CI) | p-value |
| Reperfusion treatment | ||||
| Surgical pulmonary embolectomy | 0.95 (0.76–1.19) | 0.647 | 1.00 (0.80–1.24) | 0.978 |
| Systemic thrombolysis | 1.01 (0.97–1.05) | 0.666 | 1.01 (0.97–1.06) | 0.522 |
| Risk stratification | ||||
| Haemodynamically unstable PE | 1.01 (0.99–1.04) | 0.332 | 1.02 (0.99–1.05) | 0.117 |
| Right ventricular dysfunction | 1.04 (1.02–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 |
| Tachycardia | 0.95 (0.89–1.00) | 0.068 | 0.96 (0.90–1.02) | 0.153 |
| Syncope | 1.01 (0.96–1.07) | 0.592 | 1.01 (0.96–1.06) | 0.812 |
| In-hospital outcomes | ||||
| CPR | 1.04 (1.01–1.08) | 0.016 | 1.05 (1.01–1.08) | 0.008 |
| In-hospital death | 1.03 (1.00–1.05) | 0.025 | 1.04 (1.02–1.06) | 0.001 |
| Intra-hospital bleeding events | ||||
| Gastro-intestinal bleeding | 1.00 (0.94–1.08) | 0.918 | 1.02 (0.95–1.09) | 0.566 |
| Subarachnoid bleeding | 0.70 (0.57–0.86) | 0.001 | 0.70 (0.57–0.86) | 0.001 |
| Intracerebral bleeding | 0.85 (0.76–0.96) | 0.006 | 0.85 (0.76–0.95) | 0.004 |
PE: pulmonary embolism; CPR: cardiopulmonary resuscitation. #: Adjustment I; ¶: Adjustment II.
Seasonal differences in reperfusion treatments
While surgical embolectomy was performed in all seasons in similar frequency, systemic thrombolysis was more often used in winter (table 1). The logistic regression analysis confirmed a higher use of the reperfusion treatment systemic thrombolysis in the winter months independently of age, sex and comorbidities (table 3).
Seasonal variation of PE patients' in-hospital mortality rate
Similarly, seasonal variation regarding PE patients' in-hospital mortality was also of substantial magnitude (p<0.001) (table 3). The regression models confirmed that winter was associated with an increased risk of mortality (OR 1.04 (95% CI 1.02–1.05), p<0.001) independently of age, sex and comorbidities (adjustment I) in comparison to the summer (table 3). This result remains stable after further adjustment for risk stratification markers (adjustment III) (OR 1.03 (95% CI 1.01–1.05), p=0.001).
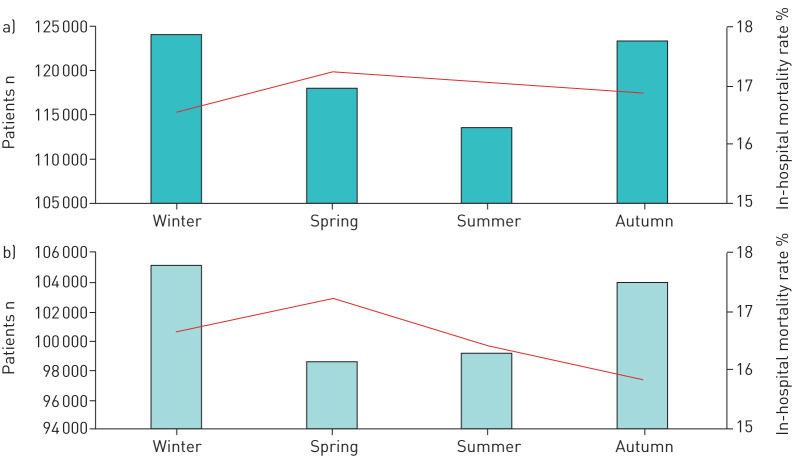
Winter was related to higher mortality in male and female independently of age and comorbidities (adjustment II) (male: OR 1.03 (95% CI 1.01–1.06), p=0.015; female: OR 1.04 (95% CI 1.02–1.06), p=0.001) (table 3) in comparison to summer season. These results remained significant after further adjustment for risk stratification markers (adjustment IV) (men: OR 1.03 (95% CI 1.00–1.06), p=0.048; women: OR 1.04 (95% CI 1.01–1.06), p=0.010). However, we detected some sex-specific differences regarding seasonal variation of incidence and in-hospital mortality (figure 3). In males and females, the highest quarterly annual incidence of PE was observed during winter, whereas the lowest hospitalisation rate of male patients was found in spring and of female patients in summer. Both sexes showed a maximum of in-hospital mortality in the spring (figure 4).
FIGURE 4.
Absolute numbers of PE events (bars) and in-hospital mortality rate (line) stratified by seasons in a) female and b) male patients (cumulative 2005–2015).
Age-dependent analysis showed, that PE patients in their third decade and older revealed a winter peak in the in-hospital mortality (figure 3b).
Discussion
PE is a major cause of morbidity and mortality worldwide [13, 24, 25]. Seasonal variation for incidence and mortality of PE is still debated [2]. Only a limited number of studies investigated seasonal variations of incidence and mortality in PE patients and the results were not consistent [1, 2, 13–23]. The German nationwide inpatient sample enabled us to investigate the seasonal variations regarding incidence and in-hospital mortality of PE in more than 885 000 patients. In addition, and of importance we could identify differences in age, sex, comorbidities, risk stratification markers and usage of reperfusion treatments, which might explain, even in part, the seasonal variations.
Key findings of our study are summarised as follows:
-
1)
Incidence and in-hospital mortality rate of hospitalised PE patients showed a significant seasonal variation.
-
2)
Highest incidence of PE was during winter and lowest in summer.
-
3)
Highest in-hospital mortality rate of PE was observed in winter and spring, whereas in-hospital mortality was lowest in summer and autumn.
-
4)
Seasonal variation regarding the in-hospital mortality rate revealed some sex-specific differences, but differences between summer and winter were identified in both sexes independently of age, comorbidities and risk stratification markers.
-
5)
Seasonal variation regarding PE incidence and mortality was pronounced in older patients.
Germany is located in the temperate climate zone of western Europe with annual mean temperatures between 8 and 9°C. The weather is characterised by four distinct seasons with a striking contrast between winter and summer: lowest mean temperatures were measured in January (winter, approximately −0.5 to 0.5°C) and highest in July (summer, approximately 17–18°C). The median cumulative seasonal temperatures in the timeframe between 2005 and 2015 are shown in table 1.
We identified in this German inpatient sample a significant seasonal variation regarding incidence and in-hospital mortality.
Seasonal difference regarding the incidence of acute PE
In accordance with most studies about seasonal variation in PE [2, 15, 19–22], the highest PE incidence in Germany was observed in the winter months. A winter peak was also demonstrated in three large studies with respectively more than 160 000 patients analysing nationwide samples of England, France and Spain [2, 19, 20]. In contrast, smaller studies showed peaks of incidence in autumn [23] and spring [18]. In addition, Stein et al. [17] published the largest study with 2 457 000 PE cases of the National Hospital Discharge Survey of the United States of America without a confirmation of a seasonal variation regarding quarterly annual PE incidence. This country-specific difference between smaller countries such as Germany and large countries such as the United States of America might be driven by climate diversity. While Germany has only the temperate climate of mid-western Europe, the United States of America comprise different climate zones.
Furthermore, studies suggested an age-dependent impact on seasonal variation of PE incidence with an increase in older individuals, especially in those older than 50 years [16, 19]. These results were confirmed by our study, showing the typically peak regarding the incidence in winter and the nadir in summer for patients in the 7th life-decade and older.
Seasonal differences regarding in-hospital mortality rate
In Germany, the highest in-hospital mortality rate of acute PE was observed in winter and spring, whereas a lowest in-hospital rate was found during summer and autumn. Remarkably, seasonal variation regarding the in-hospital mortality rate revealed some sex-specific differences, but the differences between summer and winter were identified in both sexes independently of age, comorbidities and risk stratification markers. Only a few studies have investigated a seasonal variation of short-term mortality, with inconsistent results [13, 14, 18, 19]. While two studies reported a winter peak [14, 19], one study could not confirm any seasonal variation [18] and another one described two peaks in spring and autumn [13]. The largest study investigating seasonal variation of in-hospital death investigated 599 432 PE patients and demonstrated an increase of 25% of mortality in winter compared to summer [19]. Although our study results are in accordance with the findings of Olie et al. [19], we detected only a 9% increase of in-hospital mortality in the winter season in comparison to summer. Interestingly, seasonal variation was identified for all patients of the third life-decade and older.
Although potential aetiologies for seasonal patterns are not well understood [3, 11], a number of pathophysiological factors might contribute to the seasonal variation regarding incidence and in-hospital mortality of patients with acute PE [22, 26]. As mentioned for Germany above, most countries with seasonal variation show a significant seasonal change in ambient temperature [22, 23]. Thus, most authors suggest that the temperature is an important factor causing seasonal pattering of cardiovascular mortality [1, 13, 22, 26]. Consecutively, studies demonstrated that cold weather with 1°C decrease in air temperature was associated with an excess of deaths for up to a month [1, 21, 22]. In addition, the wind-chill temperature should also be taken in account as it influences short-term mortality [1, 21, 22, 27]. Environmental temperature has an influence on the onset of acute PE, which is most likely multifactorial [1, 22, 26]. It has been proposed that temperature and the resulting variation in biological factors mediate seasonal differences [11, 21]. Pathophysiologically, even mild surface cooling of the skin and the tissues beneath the skin including the vessels produces an increased blood viscosity and changes in coagulation [1, 2, 21–23, 26]. Hypercoagulable states could be promoted by elevated fibrinogen levels, which increase in colder months [1, 19, 21–23]. Moreover, studies confirmed a correlation between air pressure and humidity with the occurrence of PE [22, 28]. Physical activity is dramatically reduced due to colder temperatures especially in older individuals, which favours prothrombotic changes [1, 2, 15, 19, 22, 23]. Respiratory infections such as bronchitis, pneumonia and acute exacerbation of COPD are more prevalent in the winter months and accompanied by inflammatory states, which could contribute to development of venous thromboembolism [15, 19, 29–32]. In summary, it has to be hypothesised that changes in coagulation system, inflammation/infections, comorbidities as well as peripheral vasoconstrictions with reduction of blood flow in the legs increase the risk of deep vein thrombosis and PE in the winter seasons [15, 22, 26]. Another explanation focuses on air pollution [21, 26]. Air pollution seems to have an effect on pulmonary diseases, the coagulation system and on platelet function [26, 31, 33, 34]. Air pollutants are elevated predominantly in the colder season of winter and might therefore contribute to the seasonal variation and the higher incidence of acute cardiovascular events such as deep vein thrombosis and PE [19, 26, 32, 33, 35, 36].
In studies about seasonal variation in MI patients, it was presumed that the higher mortality rate during the winter was attributed to a higher number of comorbidities [3, 11] as well as an increased age of the MI patients during winter months [4]. Our study confirmed a higher proportion of PE patients aged >70 years during the winter in comparison to summer season, but not an accumulation of important comorbidities in the winter season, which is in accordance with the study of Manfredini et al. [1]. However, in our multivariate logistic regression analyses, the independence of seasonal variation of in-hospital mortality regarding age, sex and important comorbidities was demonstrated. Thus, we could reject the presumed explanations of seasonal mortality variation with respect to age and the investigated comorbidities.
Nagarajan et al. [37] identified some seasonal treatment variations in MI patients admitted for acute MI in summer and winter seasons. Patients admitted in summer revealed a significant shorter door to balloon time than in the winter [37]. In contrast, our study results revealed no lower reperfusion treatment rates in PE patients admitted in the winter.
Interestingly, our study results identified a higher rate of RV dysfunction in the winter season. RV dysfunction and signs of haemodynamic compromise are important risk stratification tools in acute PE and associated with an increased mortality [24, 38]. Although the cause of the higher rate of RV dysfunction in the winter seasons remains unclear, this finding might explain in part the higher mortality rate of PE in the winter months compared to the summer. Colder weather is further accompanied by an increase in sympathetic tone, heart rate, blood pressure, atrial spasm, myocardial oxygen consumption, haematocrit, granulocyte count, cortisol level, serum cholesterol and triglyceride levels, red blood cell and platelet count, plasma β-thromboglobulin, platelet factor 4, activated factor VII and plasma fibrinogen, whereas anti-thrombin III decreases with colder temperatures [3, 6, 10, 11]. Therefore, the higher rate of RV dysfunction might be attributed especially to the elevated myocardial oxygen consumption in the winter season accompanied with an increased risk of cardiac adaptations (RV dysfunction).
In accordance with the study of Zöller et al. [16], we found a weak sex-specific difference regarding the seasonal variation in PE patients. We detected especially differences in the seasonal variation of the in-hospital mortality of male and female PE patients. In male patients, seasonal differences between spring and summer as well as spring and winter were demonstrated, whereas female patients did not have these seasonal differences between spring and the other seasons (table 3). An explanation for this finding was not obvious.
Adequate management of public health as well as healthcare service planning requires reliable information about temporal/seasonal variation regarding hospitalisations and adverse events due to common diseases [39]. Thus, our results regarding seasonal variation are important to allow prospective healthcare planning and management [39]. Seasonal trends regarding heart and lung diseases should not be underestimated, because these diseases have a high prevalence in Germany and the world. The adequate planning of healthcare resources is of particular interest to avoid the negative effects of a shortage of resources.
Limitations
There are certain limitations of our study that require consideration. First, study results were based on ICD discharge codes, which might be subject to underreporting or miscoding. Second, detailed baseline data such as concomitant medications, cardiac troponin plasma concentrations, and echocardiographic parameters were not available. Therefore, the focus of our study was on the clear end-point of in-hospital death.
Conclusions
The incidence and in-hospital mortality rate of hospitalised PE patients showed seasonal variation. Although it has to be hypothesised that the seasonal variation of PE is multifactorially induced, seasonal variation was not explained by seasonal differences regarding age, sex and important comorbidities. In addition, sex-specific differences of seasonal variation regarding PE were observed.
Acknowledgements
We thank the Federal Statistical Office of Germany (Statistisches Bundesamt, DEStatis) for providing the data and the kind permission to publish these data/results.
Footnotes
Data availability: We have no permission to share the data of the nationwide inpatient sample.
Conflict of interest: K. Keller reports that this study was supported by the German Federal Ministry of Education and Research (BMBF 01EO1503) via an Institutional Grant for the Center for Thrombosis and Hemostasis. The authors are responsible for the contents of this publication.
Conflict of interest: L. Hobohm reports lecture honoraria from MSD outside the submitted work, and that this study was supported by the German Federal Ministry of Education and Research (BMBF 01EO1503) via an Institutional Grant for the Center for Thrombosis and Hemostasis. The authors are responsible for the contents of this publication.
Conflict of interest: T. Münzel reports that this study was supported by the German Federal Ministry of Education and Research (BMBF 01EO1503) via an Institutional Grant for the Center for Thrombosis and Hemostasis. The authors are responsible for the contents of this publication. T. Münzel is principal investigator of the DZHK (German Center for Cardiovascular Research), Partner Site Rhine-Main, Mainz, Germany.
Conflict of interest: S.V. Konstantinides reports consultancy and lecture honoraria from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, MSD, Pfizer and Bristol-Myers Squibb, and institutional grants from Actelion, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Pfizer and Bristol-Myers Squibb, all outside the submitted work; and that this study was supported by the German Federal Ministry of Education and Research (BMBF 01EO1503) via an Institutional Grant for the Center for Thrombosis and Hemostasis. The authors are responsible for the contents of this publication.
Conflict of interest: M. Lankeit reports consultancy and lecture honoraria from Actelion, Bayer, Daiichi-Sankyo, MSD, Pfizer and Bristol-Myers Squibb, and research funding from BRAHMS and Thermo Fisher scientific, all outside the submitted work; and that this study was supported by the German Federal Ministry of Education and Research (BMBF 01EO1503) via an Institutional Grant for the Center for Thrombosis and Hemostasis. The authors are responsible for the contents of this publication.
Support statement: This study was supported by the German Federal Ministry of Education and Research (BMBF 01EO1503). The authors are responsible for the contents of this publication. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Manfredini R, Gallerani M, Boari B, et al. Seasonal variation in onset of pulmonary embolism is independent of patients’ underlying risk comorbid conditions. Clin Appl Thromb Hemost 2004; 10: 39–43. doi: 10.1177/107602960401000106 [DOI] [PubMed] [Google Scholar]
- 2.Guijarro R, Trujillo-Santos J, Bernal-Lopez MR, et al. Trend and seasonality in hospitalizations for pulmonary embolism: a time-series analysis. J Thromb Haemost 2015; 13: 23–30. doi: 10.1111/jth.12772 [DOI] [PubMed] [Google Scholar]
- 3.Spencer FA, Goldberg RJ, Becker RC, et al. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol 1998; 31: 1226–1233. doi: 10.1016/S0735-1097(98)00098-9 [DOI] [PubMed] [Google Scholar]
- 4.Sheth T, Nair C, Muller J, et al. Increased winter mortality from acute myocardial infarction and stroke: the effect of age. J Am Coll Cardiol 1999; 33: 1916–1919. doi: 10.1016/S0735-1097(99)00137-0 [DOI] [PubMed] [Google Scholar]
- 5.Gerber Y, Jacobsen SJ, Killian JM, et al. Seasonality and daily weather conditions in relation to myocardial infarction and sudden cardiac death in Olmsted County, Minnesota, 1979 to 2002. J Am Coll Cardiol 2006; 48: 287–292. doi: 10.1016/j.jacc.2006.02.065 [DOI] [PubMed] [Google Scholar]
- 6.Ornato JP, Peberdy MA, Chandra NC, et al. Seasonal pattern of acute myocardial infarction in the National Registry of Myocardial Infarction. J Am Coll Cardiol 1996; 28: 1684–1688. doi: 10.1016/S0735-1097(96)00411-1 [DOI] [PubMed] [Google Scholar]
- 7.Chung FP, Li HR, Chong E, et al. Seasonal variation in the frequency of sudden cardiac death and ventricular tachyarrhythmia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy: the effect of meteorological factors. Heart Rhythm 2013; 10: 1859–1866. doi: 10.1016/j.hrthm.2013.09.069 [DOI] [PubMed] [Google Scholar]
- 8.Kumar N, Pandey A, Venkatraman A, et al. Seasonality in acute aortic dissection related hospitalizations and mortality in the United States: a nationwide analysis from 2004–2011. Int J Cardiol 2015; 179: 321–322. doi: 10.1016/j.ijcard.2014.11.088 [DOI] [PubMed] [Google Scholar]
- 9.Turin TC, Kita Y, Murakami Y, et al. Higher stroke incidence in the spring season regardless of conventional risk factors: Takashima Stroke Registry, Japan, 1988–2001. Stroke 2008; 39: 745–752. doi: 10.1161/STROKEAHA.107.495929 [DOI] [PubMed] [Google Scholar]
- 10.Marchant B, Ranjadayalan K, Stevenson R, et al. Circadian and seasonal factors in the pathogenesis of acute myocardial infarction: the influence of environmental temperature. Br Heart J 1993; 69: 385–387. doi: 10.1136/hrt.69.5.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rumana N, Kita Y, Turin TC, et al. Seasonal pattern of incidence and case fatality of acute myocardial infarction in a Japanese population (from the Takashima AMI Registry, 1988 to 2003). Am J Cardiol 2008; 102: 1307–1311. doi: 10.1016/j.amjcard.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 12.Danet S, Richard F, Montaye M, et al. Unhealthy effects of atmospheric temperature and pressure on the occurrence of myocardial infarction and coronary deaths. A 10-year survey: the Lille-World Health Organization MONICA project (Monitoring trends and determinants in cardiovascular disease). Circulation 1999; 100: E1–E7. doi: 10.1161/01.CIR.100.1.e1 [DOI] [PubMed] [Google Scholar]
- 13.Green J, Edwards C. Seasonal variation in the necropsy incidence of massive pulmonary embolism. J Clin Pathol 1994; 47: 58–60. doi: 10.1136/jcp.47.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chau KY, Yuen ST, Ng TH, et al. An autopsy study of pulmonary thromboembolism in Hong Kong Chinese. Pathology 1991; 23: 181–184. doi: 10.3109/00313029109063562 [DOI] [PubMed] [Google Scholar]
- 15.Boulay F, Berthier F, Schoukroun G, et al. Seasonal variations in hospital admission for deep vein thrombosis and pulmonary embolism: analysis of discharge data. BMJ 2001; 323: 601–602. doi: 10.1136/bmj.323.7313.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoller B, Li X, Ohlsson H, et al. Age-and sex-specific seasonal variation of venous thromboembolism in patients with and without family history: a nationwide family study in Sweden. Thromb Haemost 2013; 110: 1164–1171. doi: 10.1160/TH13-04-0320 [DOI] [PubMed] [Google Scholar]
- 17.Stein PD, Kayali F, Olson RE. Analysis of occurrence of venous thromboembolic disease in the four seasons. Am J Cardiol 2004; 93: 511–513. doi: 10.1016/j.amjcard.2003.10.061 [DOI] [PubMed] [Google Scholar]
- 18.Kosacka U, Kiluk IE, Milewski R, et al. Variation in the incidence of pulmonary embolism and related mortality depending on the season and day of the week. Pol Arch Med Wewn 2015; 125: 92–94. [DOI] [PubMed] [Google Scholar]
- 19.Olie V, Bonaldi C. Pulmonary embolism: Does the seasonal effect depend on age? A 12-year nationwide analysis of hospitalization and mortality. Thromb Res 2017; 150: 96–100. doi: 10.1016/j.thromres.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 20.Aylin P, Bottle A, Kirkwood G, et al. Trends in hospital admissions for pulmonary embolism in England: 1996/7 to 2005/6. Clin Med (Lond) 2008; 8: 388–392. doi: 10.7861/clinmedicine.8-4-388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallerani M, Boari B, Smolensky MH, et al. Seasonal variation in occurrence of pulmonary embolism: analysis of the database of the Emilia-Romagna region, Italy. Chronobiol Int 2007; 24: 143–160. doi: 10.1080/07420520601139755 [DOI] [PubMed] [Google Scholar]
- 22.Dentali F, Manfredini R, Ageno W. Seasonal variability of venous thromboembolism. Curr Opin Pulm Med 2009; 15: 403–407. doi: 10.1097/MCP.0b013e32832d867a [DOI] [PubMed] [Google Scholar]
- 23.Jang MJ, Kim HJ, Bang SM, et al. Seasonal variation in the occurrence of venous thromboembolism: a report from the Korean Venous Thromboembolism Working Party. Thromb Res 2012; 130: e199–e202. doi: 10.1016/j.thromres.2012.07.019 [DOI] [PubMed] [Google Scholar]
- 24.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35: 3033–3069. doi: 10.1093/eurheartj/ehu243 [DOI] [PubMed] [Google Scholar]
- 25.Keller K, Hobohm L, Ebner M, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J 2019; 41: 522–529. doi: 10.1093/eurheartj/ehz236 [DOI] [PubMed] [Google Scholar]
- 26.Dentali F, Ageno W, Rancan E, et al. Seasonal and monthly variability in the incidence of venous thromboembolism. A systematic review and a meta-analysis of the literature. Thromb Haemost 2011; 106: 439–447. doi: 10.1160/TH11-02-0116 [DOI] [PubMed] [Google Scholar]
- 27.Kunst AE, Groenhof F, Mackenbach JP. The association between two windchill indices and daily mortality variation in The Netherlands. Am J Public Health 1994; 84: 1738–1742. doi: 10.2105/AJPH.84.11.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oztuna F, Ozsu S, Topbas M, et al. Meteorological parameters and seasonal variations in pulmonary thromboembolism. Am J Emerg Med 2008; 26: 1035–1041. doi: 10.1016/j.ajem.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 29.Borvik T, Braekkan SK, Enga K, et al. COPD and risk of venous thromboembolism and mortality in a general population. Eur Respir J 2016; 47: 473–481. doi: 10.1183/13993003.00402-2015 [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro DD, Lijfering WM, Van Hylckama Vlieg A, et al. Pneumonia and risk of venous thrombosis: results from the MEGA study. J Thromb Haemost 2012; 10: 1179–1182. doi: 10.1111/j.1538-7836.2012.04732.x [DOI] [PubMed] [Google Scholar]
- 31.Kaluzna-Oleksy M, Aunan K, Rao-Skirbekk S, et al. Impact of climate and air pollution on acute coronary syndromes: an update from the European Society of Cardiology Congress 2017. Scand Cardiovasc J 2018; 52: 1–3. doi: 10.1080/14017431.2017.1405069 [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro DD, Bucciarelli P, Braekkan SK, et al. Seasonal variation of venous thrombosis: a consecutive case series within studies from Leiden, Milan and Tromso. J Thromb Haemost 2012; 10: 1704–1707. doi: 10.1111/j.1538-7836.2012.04811.x [DOI] [PubMed] [Google Scholar]
- 33.Baccarelli A, Martinelli I, Zanobetti A, et al. Exposure to particulate air pollution and risk of deep vein thrombosis. Arch Intern Med 2008; 168: 920–927. doi: 10.1001/archinte.168.9.920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baccarelli A, Zanobetti A, Martinelli I, et al. Effects of exposure to air pollution on blood coagulation. J Thromb Haemost 2007; 5: 252–260. doi: 10.1111/j.1538-7836.2007.02300.x [DOI] [PubMed] [Google Scholar]
- 35.Baccarelli A, Martinelli I, Pegoraro V, et al. Living near major traffic roads and risk of deep vein thrombosis. Circulation 2009; 119: 3118–3124. doi: 10.1161/CIRCULATIONAHA.108.836163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dales RE, Cakmak S, Vidal CB. Air pollution and hospitalization for venous thromboembolic disease in Chile. J Thromb Haemost 2010; 8: 669–674. doi: 10.1111/j.1538-7836.2010.03760.x [DOI] [PubMed] [Google Scholar]
- 37.Nagarajan V, Fonarow GC, Ju C, et al. Seasonal and circadian variations of acute myocardial infarction: Findings from the Get with the Guidelines-Coronary Artery Disease (GWTG-CAD) program. Am Heart J 2017; 189: 85–93. doi: 10.1016/j.ahj.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 38.Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, et al. Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J 1997; 134: 479–487. doi: 10.1016/S0002-8703(97)70085-1 [DOI] [PubMed] [Google Scholar]
- 39.Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018; 391: 572–580. doi: 10.1016/S0140-6736(17)32520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]