Abstract
Introduction
Hormones may be one possible mechanism underlying sex differences in dementia incidence. We examined whether presumed differential prenatal hormone milieu is related to dementia risk by comparing dementia rates in same‐ and opposite‐sex dizygotic twin pairs in male and female twins.
Methods
The sample comprised 43,254 individuals from dizygotic twin pairs aged 60 and older from the Swedish Twin Registry. Survival analyses were conducted separately for females and males.
Results
Female twins from opposite‐sex pairs had significantly lower dementia risk than female twins from same‐sex pairs, but the differences emerged only after age 70 (hazard ratio = 0.64, P = 0.004). Results were not explained by postnatal risk factors for dementia, and no interaction between twin type and apolipoprotein E (APOE) ε4 was found. Male twins from same‐sex versus opposite‐sex pairs did not differ significantly.
Discussion
The results suggest that relatively masculine prenatal hormone milieus correlate with lower dementia risk in females.
Keywords: apolipoprotein E4, dementia, sex differences, testosterone, twin study
Sex differences in dementia prevalence are well documented. 1 , 2 Although findings are inconsistent, evidence has been found for higher incidence rates of dementia in women than men in both European and U.S. samples. 3 , 4 , 5 Sex differences in dementia rates generally emerge after age 80, 6 , 7 with some studies suggesting divergence in the late 80s 3 or even after age 90. 8
Mechanisms underlying sex differences in dementia risk remain unknown. Sex steroid hormones might contribute to sex differences in dementia rates via both activational effects (ie, transient actions of sex hormones in adulthood) and organizational effects (ie, permanent or persisting changes in brain structure and function). 9 , 10 , 11 Sex differences in dementia due to activational effects suggest sex‐specific declines in sex hormones with aging, 12 while the possible role of organizational effects have rarely been investigated. 9 Prenatal hormone milieu has been suggested to show organizational effects during critical prenatal and early postnatal periods as it mediates sexual differentiation of the brain, which occurs during critical prenatal and early postnatal periods. 13
Prenatal hormone milieu contributes to permanent sexually dimorphic characteristics in animal models. For example, female rodents with a higher level of perinatal testosterone exposure exhibited masculinized characteristics in adulthood, such as greater food intake and aggression, when compared to control group populations. 14 , 15 , 16 In humans, females with congenital adrenal hyperplasia, a condition that produces high levels of adrenal androgens from early in gestation, displayed increased male‐typical and decreased female‐typical behaviors. 17 It, thus, seems possible that differences in prenatal hormone milieu between males and females leads to persistent sexual differentiation that may affect sex differences in dementia rates.
Effects of prenatal hormone milieu on dementia risk can be inferred by comparing twins from same‐sex and opposite‐sex pairs. As observed in rodent and swine studies, 16 , 18 females with adjacent male fetuses are exposed to elevated concentrations of testosterone prenatally, 19 leading to more masculinized traits compared to females with adjacent female fetuses only. Also, males with adjacent female fetuses show demasculinized traits compared to males with adjacent male fetuses. 20 , 21 In human studies, due to ethical constraints, prenatal measures of hormone concentration are generally not available.
In human studies of same‐sex and opposite‐sex twin pairs, effects of prenatal hormone milieu have been inferred for cognitive abilities that are presumed to favor either males or females. For example, males and females with a female co‐twin exhibited larger vocabularies than their counterparts with a male co‐twin. 22 , 23 Females with a male co‐twin outperformed those with a female co‐twin on the Mental Rotation Test. 24 , 25 , 26 No effects were found, however, for a variety of non‐verbal and verbal cognitive tests. 27 Postnatal factors, like different developmental experiences of being raised with an opposite‐ versus same‐sex sibling, also might account for differences between twins from same‐sex and opposite‐sex pairs. A recent study separated prenatal from postnatal factors and found that females with male co‐twins experienced worse educational and labor‐market outcomes and were less likely to marry and have children. 28 The differences remained among a subset of women whose male co‐twins died very early in life, supporting the interpretation that prenatal hormone milieu, rather than postnatal socialization resulting from having a male sibling, explained observed differences. Most twin studies of prenatal testosterone with cognitive outcomes have not included middle‐aged and older adults, and to date no study has examined same‐sex versus opposite‐sex twin pairs with respect to dementia risk.
HIGHLIGHTS
Females from opposite‐sex twin pairs had lower dementia risk than females from same‐sex dizygotic twin pairs.
The effect of twin type (opposite‐ vs same‐sex) on dementia risk in females was not evident until age 70.
Twin type predicted dementia risk in females after controlling for postnatal risk factors.
Interactions between twin type and apolipoprotein E (APOE) ε4 were not found on dementia risk for men or women.
One possible inference is that a relatively masculine prenatal hormone milieu may lower dementia risks in females.
RESEARCH IN CONTEXT
Systematic review: As mechanisms for sex differences in dementia risk remain unresolved, with possible prenatal hormone differences not heretofore considered, the authors reviewed the literature on sex differences in dementia risk and on the twin testosterone transfer hypothesis using traditional sources (eg, PubMed, Google Scholar).
Interpretation: We showed that women from opposite‐sex twin pairs had lower dementia risk compared to women from same‐sex dizygotic twin pairs. One possible inference is that a relatively masculine prenatal hormone milieu correlates with lower dementia risk, suggesting that prenatal hormonal environment may be relevant to greater risk in women compared to men.
Future directions: Mechanisms linking female prenatal sex hormones to dementia risk should be considered and whether apolipoprotein E (APOE) ε genotype moderates effects of prenatal hormonal exposure and dementia risk, a question that should be addressed in future studies.
Apolipoprotein E (APOE) ε4 is the most significant genetic risk factor for late‐life dementia 29 , 30 and might be a more potent risk factor for women than men. 31 , 32 Women with one APOE ε4 allele have up to a four‐fold increased risk compared to those with APOE ε3, whereas in men, only those with two APOE ε4 alleles demonstrated increased risk. 31 , 33 Excess risk associated with APOE ε4 alleles might be age dependent, as women with one APOE ε4 allele showed a higher AD risk than men only at younger ages. 32 Moreover, in studies earlier in life, infants and children who were APOE ε4 carriers displayed significantly thinner cortices. 34 , 35 Child APOE ε4 carriers with a family history of AD also exhibited impaired cognitive performance. 36 , 37 However, how differences in prenatal hormonal milieu might contribute to the female bias of APOE ε4 remains unknown. We, thus, tested whether hypothesized prenatal hormonal milieu moderated effects of APOE ε4 on dementia risk.
In the current study, we compared risk of dementia for female and male members of same‐sex and opposite‐sex dizygotic twin pairs. We asked two research questions. First, are there differences in dementia risk between women in same‐sex and in opposite‐sex twin pairs, and if so, can the difference be explained by postnatal risk factors? Lower dementia risk in female and male twins with male co‐twins, compared to those with female co‐twins, would lend support for the hypothesis that a relatively more masculine, compared to feminine, hormone milieu in utero may lower dementia risk, adjusting for postnatal risk factors. Finally, does the interaction between twin type and APOE ε4 predict dementia risk?
1. METHOD
1.1. Participants
The sample was drawn from the same‐sex and opposite‐sex dizygotic (DZ) twins participating in the Swedish Twin Registry (STR). 38 A flow diagram for the sample is displayed in Figure 1.
FIGURE 1.
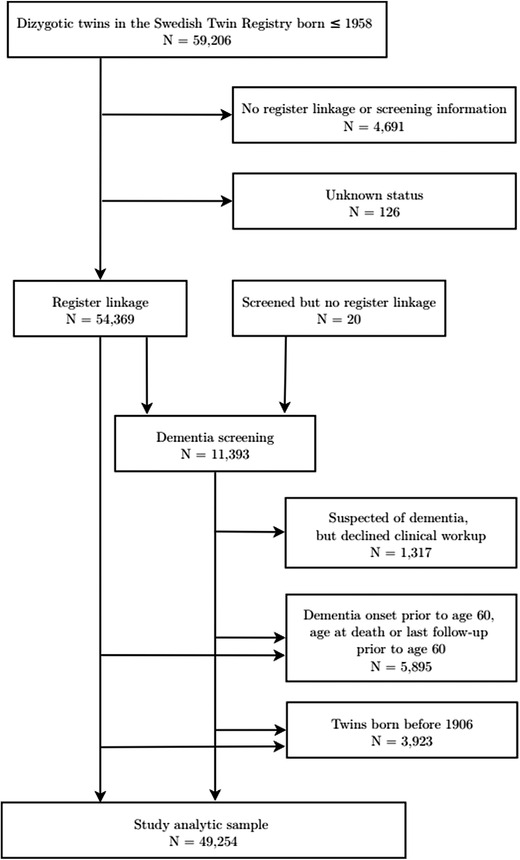
Selection of analytic sample in the Swedish Twin Registry
The data used in the current study include twins born between 1906 and 1957 who had information on dementia status from national registers or from clinical dementia diagnoses. For those with dementia, only those who had age at onset for dementia equal to or older than age 60 were included in the analytic sample. Individuals without a diagnosis of dementia were included if their age at death or the last follow‐up was equal to or older than age 60. The analytic sample included 43,254 individual twins, including 13,348 female same‐sex twins (SS females), 11,710 male same‐sex twins (SS males), 9,274 female twins from opposite‐sex pairs (OS females), and 8,922 male twins from opposite‐sex pairs (OS males). Table 1 presents the total analytic sample and subsamples by sex and twin type (same‐ vs opposite‐sex).
TABLE 1.
Demographic information for the female and male dizygotic twin samples and the subsamples tested in the study
| Number of individual twins | Birth year mean and range | Proportion of sample with dementia | Average age at onset and SD | Average censored age and SD | |
|---|---|---|---|---|---|
| Female SS twins | |||||
| Entire sample | 13,348 | 1933 (1906, 1957) | 14.5% | 77.56 (6.81) | 75.08 (9.37) |
| Sample with APOE ε4 data | 2,827 | 1935 (1906, 1956) | 16.3% | 78.75 (6.79) | 76.45 (9.09) |
| Female OS twins | |||||
| Entire sample | 9,274 | 1938 (1906, 1956) | 11.5% | 76.37 (6.69) | 73.77 (8.89) |
| Sample with APOE ε4 data | 2,278 | 1938 (1908, 1956) | 11.5% | 76.64 (6.75) | 75.52 (8.56) |
| Male SS twins | |||||
| Entire sample | 11,710 | 1934 (1906, 1956) | 8.9% | 75.80 (6.84) | 73.21 (8.34) |
| Sample with APOE ε4 data | 2,142 | 1936 (1906, 1956) | 12.7% | 77.03 (7.16) | 76.27 (8.17) |
| Male OS twins | |||||
| Entire sample | 8,922 | 1938 (1906, 1957) | 9.3% | 75.39 (6.40) | 73.36 (8.40) |
| Sample with APOE ε4 data | 2,230 | 1937 (1907, 1956) | 11.4% | 76.79 (6.07) | 75.82 (7.88) |
Abbreviations: APOE, apolipoprotein E; OS = opposite‐sex; SD, standard deviation; SS = same‐sex.
APOE ε4 allele data were available for only a subset of the sample and included 5,105 individual female DZ twins and 4,372 individual DZ male twins. Female and male twin subsamples with APOE ε4 allele data differed from subsamples without APOE ε4 allele data on all demographic variables (ie, year of birth, dementia diagnosis, age at onset, time in study, and source of dementia diagnosis; Table S.1 in Supplement).
1.2. Measures
Dementiainformation was available from either clinical diagnosis by the research team during the studies or one of the Swedish National Registers. Clinical diagnoses were based on in‐person dementia diagnostic clinical workup. Individuals were cognitively screened and, if flagged for the possibility of cognitive impairment, followed up in‐person. Final dementia diagnoses were established in multidisciplinary consensus conferences using information from the in‐person workup and medical records according to Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III‐R or DSM‐IV criteria for dementia. For all individuals not cognitively screened and cases that occurred after last follow‐up, dementia status was determined using International Classification of Disease codes for dementia in the Swedish National Patient Register (NPR), Outpatient Register (OPR), or Cause of Death Register (CDR). In addition, dementia medication according to the Anatomical Therapeutic Chemical classification was tracked in the Prescribed Drug Register (PDR) from 2005 onward and used as a proxy for dementia diagnosis. The subsample with clinical diagnosis information differed on all demographic and key variables from the subsample with registry information only (see Supplementary Table S.2). We, thus, performed sensitivity analyses using only cases with clinical diagnosis information.
For individuals diagnosed with dementia through clinical workup, age at onset was estimated from the proxy interview at the in‐person assessment and medical records. For cases identified through registers, only age at first record of dementia was available. Previous work has shown a lag time of 4 to 5.5 years between age at onset ascertained at clinical workup and first record of dementia in the NPR or OPR. 39 , 40 An update based on the most recent record linkage (Karlsson, personal communication) shows that the mean number of years between age at onset through clinical assessment and first registered record of dementia among those with both clinical and registry determined dementia was 5 years in the NPR, OPR, and PDR, and 9 years for dementia registered in the CDR. These corrections were applied in the present analyses. For cases identified through CDR, random error was added to each twin's age at onset to model actual variability in age at onset.
Individual twins had number of APOE ε4 alleles either directly genotyped or imputed (sample sizes presented in Supplementary Table S.3). Imputed values were based on the Illumina OmniExpress genotyping array. Imputation was based on the 1000 Genomes Project phase 1 version 3 data. 41
1.3. Statistical analysis
The key design feature is comparing SS females to OS females, and SS males to OS males (twin type). In all analyses, birth year (centered at 1957) and the interaction between birth year and twin type were included as covariates to test moderation by age/cohort. Family was included as a random effect to account for dependency between same‐sex pairs. The total number of families was 16,623 for females and 15,546 for males.
First, we conducted logistic regression analyses to compare dementia risk between same‐sex and opposite‐sex individuals. Given that sex differences in longevity could result in different dementia rates, ordinary least squares linear regressions were estimated to examine whether female and male individuals from same‐sex pairs and opposite‐sex pairs differed in longevity. All analyses were performed within sex.
Next, differences in dementia risk by twin type were tested using survival analysis. We first examined Kaplan‐Meier estimates of the survivor function to examine the conditional probability that individuals were free from dementia per year beyond age 60 by twin type. Individuals who did not develop dementia were censored at age of death or age at last follow‐up. Ties were handled using the Efron method. 42 Survival curves were estimated within female and male groups. We then used Cox proportional hazard regression to estimate effects of twin type on dementia risk. 42 In Cox regression, the logarithm of the hazard function is the conditional probability of dementia given that the twin survived up to a specific moment in time, that is, instantaneous risk of dementia. Traditional assumptions of Cox regression were made: (1) log hazard functions are identical across groups and (2) log hazard functions are equidistant at every possible moment between groups. Partial maximum likelihood was used to estimate all models. All models were fit in the survival package (version 3.1‐8) in R 3.5.1. 43
We next entered education, exercise, vascular risk, and postnatal hormone exposure into Cox regression models to estimate whether twin type remained significant, adjusting for effects of postnatal risk factors. (Information about postnatal covariates is presented in the supplemental material, including descriptive statistics). Not all twins had complete records for the postnatal risk factors, so we imputed missing records using multivariate imputation by chained equations in the mice package (version 3.7.0). Variables with missing values were imputed using separate multivariate models. 44 As data were missing by design (ie, covariates were not collected for part of the sample), we assumed missing data were missing at random. Even under conditions of missing not at random, multiple imputation leads to reduction in bias of population estimates. 45 We imputed 25 data sets, which were then analyzed using Cox regression with TYPE = IMPUTATION in Mplus 8.2. 46 Pooled estimates are reported.
Finally, Cox regression models were fit to the twin subsample with APOE ε4 information. In these models, we included main effects of twin type, APOE ε4 allele status, and their interaction on dementia risk.
2. RESULTS
Logistic regression results show that opposite‐sex female twins had significantly lower dementia odds than same‐sex females (odds ratio [OR] = 0.37, .95 confidence interval [CI]: [0.25, 0.54], P < 0.001). Opposite‐sex male twins also displayed significantly lower dementia odds than same‐sex males (OR = 0.47, .95 CI: [0.31, 0.70], P < 0.001). For twins who were deceased, linear regression results indicate that females from opposite‐sex pairs had shorter longevity than their same‐sex counterparts while males from same‐sex pairs had shorter longevity than their opposite‐sex counterparts (b = −0.83, P < 0.001 for females; b = 0.64, P = 0.001 for males). The observed difference, however, was negligible (Cohen's d = 0.09, M = 79.75 in OS females, M = 80.57 in SS females; Cohen's d = 0.07, M = 77.64 in OS males, M = 77.00 in SS males).
Figures 2 and 3 display the Kaplan‐Meier survival curves for female and male twins, respectively. Survival curves significantly diverged in female twins in the mid‐80s with OS twins surviving longer than SS twins whereas the overlapping 95% confidence bands in the male twins suggest no significant difference in survival rates.
FIGURE 2.
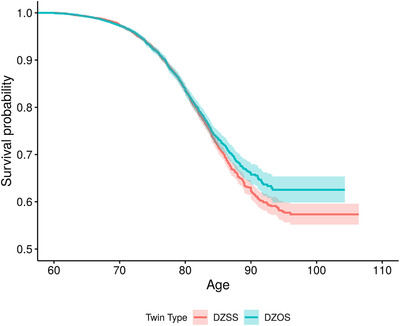
Survival curves for female with same‐sex co‐twins (red line) and female with opposite‐sex co‐twins (blue line) tested at age 60 and beyond. DZOS = dizygotic opposite‐sex twins; DZSS = dizygotic same‐sex twins. Shaded region around each curve is the 95% confidence band
FIGURE 3.
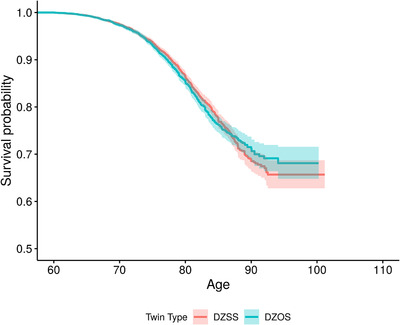
Survival curves for male with same‐sex co‐twins (red line) and male with opposite‐sex co‐twins (blue line) tested at age 60 and beyond. DZOS = dizygotic opposite‐sex twins; DZSS = dizygotic same‐sex twins. Shaded region around each curve is the 95% confidence band
Cox regression model results suggest that dementia risk of female twins from opposite‐sex pairs did not significantly differ from risk of females from same‐sex pairs (hazard ratio [HR] = 0.83, .95 CI = [0.65, 1.06], P = 0.141). Similarly, dementia risk in male twins did not differ by twin type (HR = 0.96, .95 CI = [0.74, 1.25], P = 0.751).
For females, birth year nearly significantly moderated effect of twin type on dementia risk (P = .075). A plot of dementia risk HRs by birth year for same‐sex and opposite‐sex female twins (see Supplementary Figure S.1) reveals that same‐sex female twins have much greater risk of dementia than opposite‐sex twins in groups born in earlier birth years than in later birth years. We, thus, performed post‐hoc Cox regression analyses in which dementia diagnoses before age 70 or before age 80 were left‐censored. With left censoring before age 70, opposite‐sex females had significantly lower risk of dementia than same‐sex females (HR = 0.64, .95 CI = [0.48, 0.87], P = 0.004). Left censoring before age 80 showed an even greater reduction in dementia risk in opposite‐sex female twins (HR = 0.38, .95 CI = [0.21, 0.68], P = 0.001). Complementary analyses in male twins revealed non‐significant effects of twin type.
To test for postnatal explanations, we adjusted the Cox regression for effects of education, exercise, postnatal hormone exposure, and vascular risk. Summary of the pooled estimates are presented in Table 2. (Hierarchical Cox regression model results are presented in Supplementary Tables S.5–S.7). Effects of twin type were not markedly reduced after adjusting for postnatal risk factors of dementia, regardless of left‐censoring age.
TABLE 2.
Log odds and standard errors of the effects of twin type and postnatal covariates on dementia risk in female twins
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Testing from age 60 | |||
| Twin type | −0.19 (0.13) | −0.15 (0.13) | |
| Education | −0.04 (0.01) | −0.04 (0.01) | |
| Exercise | −0.002 (0.08) | 0.00 (0.08) | |
| Vascular risk | 0.03 (0.02) | 0.03 (0.02) | |
| Postnatal hormone exposure | 0.01 (0.002) | 0.01 (0.002) | |
| Testing from age 70 | |||
| Twin type | −0.44 (0.15) | −0.40 (0.15) | |
| Education | −0.04 (0.01) | −0.04 (0.01) | |
| Exercise | 0.01 (0.08) | 0.02 (0.08) | |
| Vascular risk | 0.04 (0.02) | 0.04 (0.02) | |
| Postnatal hormone exposure | 0.01 (0.002) | 0.01 (0.002) | |
| Testing from age 80 | |||
| Twin type | −0.96 (0.29) | −0.94 (0.30) | |
| Education | −0.06 (0.01) | −0.06 (0.01) | |
| Exercise | 0.05 (0.12) | 0.05 (0.12) | |
| Vascular risk | 0.02 (0.03) | 0.02 (0.03) | |
| Postnatal hormone exposure | 0.002 (0.003) | 0.002 (0.003) | |
Note. Bolded values indicate P < 0.05. Parameter estimates are pooled estimates across 25 imputed data sets. Birth year and the interaction between birth year and twin type were statistically adjusted for in all models.
For the subsample of twins with information on APOE ε4 alleles, effects of twin type, APOE ε4, and their interactions on dementia were similar in both female and male twins (see Supplementary Table S.8). The effect of the number of APOE ε4 alleles was significant. Those without APOE ε4 alleles were least likely to be diagnosed with dementia. The interaction effect between twin type and APOE ε4 was not statistically significant.
3. DISCUSSION
The current study examined prenatal hormone milieu inferred from the sex of one's co‐twin, APOE ε4, and their interaction, with findings having implications for possible mechanisms underlying sex differences in dementia risk. Opposite‐sex female twins displayed lower dementia rates compared to same‐sex female twins. Effect of twin type on dementia risk was moderated by age in female twins, as statistically significant excess dementia risk was observed above 70, but not below age 70. These differences by twin type were not explained by postnatal hormone exposure or other postnatal risk factors for dementia nor were they moderated by number of APOE ε4 alleles. Finally, we only observed main effects of APOE ε4 alleles on dementia risk, with no differences by sex, consistent with recent research. 32 The results provide partial support for the hypothesis that prenatal exposure to a relatively masculine milieu might lower dementia risk in female twins, but with the effect emerging only after age 70, that is, in older birth years where risk is higher in general. For males, although differences in dementia risk were found between OS male twins and SS male twins with simple logistic regression, the findings were not supported in Cox regression models. Taken together we conclude that our results do not confirm a difference between men from OS pairs compared to men from SS pairs.
The current findings provide speculative support for organizational effects of hormones on dementia risk, as in utero hormones were not directly assessed. The twin testosterone transfer (TTT) hypothesis has suggested the possibility of hormonal transfer between fetuses from the same pregnancy, 19 , 47 leading to masculinization of female twins with male co‐twins. However, the observed differences in dementia risk by twin type may also be affected by postnatal risk factors. In the current study, after controlling for postnatal factors (education, exercise, vascular risk, and postnatal hormonal exposure) that might contribute to the discrepancy in dementia risk by twin type, the effects of twin type on dementia risk remained significant in female twins older than age 70, indicating dementia risk associated with twin type was not explained by postnatal factors. Thus, results in the current study provide provisional evidence for the protective effects of a relatively masculine prenatal hormone environment on dementia, suggesting that sex differences in prenatal hormone environment may be relevant to women's increased risk of dementia compared to men's.
Prenatal hormone milieu may influence dementia risk through several possible pathways. For example, different sex steroid hormone concentrations during the prenatal period may have an impact on sexual differentiation of the brain, which yields permanent structural and functional differences between adult male and female brains. 9 , 48 These differences, in turn, may heighten risk of dementia in women. This possibility is consistent with prior findings on the effects of sexual differentiation on neuropathology in Alzheimer transgenic mice. Specifically, Alzheimer‐related pathology in 3xTg‐AD mice was reduced by masculinization of females and increased by feminization of males. 49 While the mechanism is unknown, our finding that lower dementia risk for females from OS twin pairs compared to SS twin pairs emerged only after age 70 or 80 is consistent with the observation that greater risk for dementia in women than in men generally emerges only after age 80. 3 , 6
Finding more pronounced effects for females than for males was expected. Most reviews of TTT have found stronger support for predictions about women than about men. 21 , 47 In explanation, it has been suggested that, because male fetuses are already exposed to high levels of testosterone, the effect of co‐gestation may be more pronounced for females than for males. 47 Available literature does not support a consistent prediction. Greater brain volumes, which would be protective for dementia, 50 have been reported for both male and female 9‐year‐olds with a male co‐twin compared to children with a female co‐twin. 51 However, this result was not replicated in a sample aged 30 years, on average. 51 On the other hand, males from OS twin pairs have been found to have higher vocabulary scores than males from SS twin pairs. 22 , 23 Both linguistic ability and educational attainment have been related to lower dementia risk. 50 In the present study, there was no difference in education between OS and SS male twin pairs, and no data for vocabulary.
Finally, number of APOE ε4 alleles exerted significant effects on dementia risk similarly in both female and male twins. Most but not all previous studies have indicated more substantial effects of APOE ε4 on dementia risk in women compared to men, 31 , 33 and a linear effect in females for one versus two alleles, which was not seen here. Interaction effects between the number of APOE ε4 alleles and twin type were non‐significant, contrary to hypothesis.
There are several limitations to the current study. First, information about APOE ε4 alleles was only available in a subset of twins, resulting in greater possibility of Type II error. Second, due to limited sample sizes of twins with different types of dementia, especially in the subset of twins with APOE ε4 alleles measured, we did not conduct analyses of different types of dementia. Third, longevity was found to be higher in same‐sex females compared to opposite‐sex females. However, the small differences observed between twin types do not appear to be meaningful. Finally, direct measures of prenatal hormone environment were unavailable. Thus, factors other than prenatal hormone environment may account for differences in dementia risk that emerged by twin type.
In sum, the current study suggests possible effects of prenatal hormone environment on dementia risk, which may contribute to sex differences in dementia risk. Further research is needed, however, to support the supposition that prenatal hormone environment and APOE ε4 allele status interact to increase women's dementia risk.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
The current research is supported in part by NIH Grant nos. RF1 AG058068 and R01 AG060470 and Alzheimer's Association grant AARF‐17‐505302. Dr. Jing Luo is now at Department of Medical Social Sciences, Feinberg School of Medicine, Northwestern University.
Luo J, Beam CR, Karlsson IK, Pike CJ, Reynolds CA, Gatz M. Dementia Risk in Women Higher in Same‐Sex than Opposite‐Sex Twins. Alzheimer's Dement. 2020;12:e12049 10.1002/dad2.12049
REFERENCES
- 1. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010‐2050) estimated using the 2010 census. Neurology. 2013;80(19):1778‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matthews FE, Stephan BCM, Robinson L, et al. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun. 2016;7(1):11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M. Differences between women and men in incidence rates of dementia and Alzheimer's disease. J Alzheimer's Disease. 2018;64(4):1077‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corrada MM, Brookmeyer R, Paganini‐Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: The 90+ study. Ann Neurol. 2010;67(1):114‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MMB. Incidence of dementia: Does gender make a difference? Neurobiol Aging. 2001;22(4):575‐580. [DOI] [PubMed] [Google Scholar]
- 6. Chêne G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid‐adult life. Alzheimers Dement. 2015;11(3):310‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tom SE, Hubbard RA, Crane PK, et al. Characterization of dementia and Alzheimer's disease in an older population: Updated incidence and life expectancy with and without dementia. Am J Public Health. 2015;105(2):408‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and alzheimer's disease: Incidence data from the kungsholmen project, stockholm. Neurology. 1997;48(1):132‐138. [DOI] [PubMed] [Google Scholar]
- 9. Pike CJ. Sex and the development of Alzheimer's disease. J Neurosci Res. 2017;95(1‐2):671‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: A reanalysis. Horm Behav. 1985;19(4):469‐498. [DOI] [PubMed] [Google Scholar]
- 11. McEwen BS, Milner TA. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. 2017;95(1‐2):24‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hardy J. The amyloid hypothesis for alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110(4):1129‐1134. [DOI] [PubMed] [Google Scholar]
- 13. Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci, 2004;7(10):1034‐1039. [DOI] [PubMed] [Google Scholar]
- 14. Donohoe TP, Stevens R. Effects of ovariectomy, estrogen treatment and CI‐628 on food intake and body weight in female rats treated neonatally with gonadal hormones. Physiol Behav. 1983;31(3):325‐329. [DOI] [PubMed] [Google Scholar]
- 15. Madrid JA, Lopez‐Bote C, Martín E. Effect of neonatal androgenization on the circadian rhythm of feeding behavior in rats. Physiol Behav. 1993;53(2):329‐335. [DOI] [PubMed] [Google Scholar]
- 16. Ryan BC, Vandenbergh JG. Intrauterine position effects. Neurosci Biobehav Rev. 2002;26(6):665‐678. [DOI] [PubMed] [Google Scholar]
- 17. Hines M. Gender development and the human brain. Annu Rev Neurosci. 2011;34:69‐88. [DOI] [PubMed] [Google Scholar]
- 18. Vom Saal FS. Sexual differentiation in litter‐bearing mammals: Influence of sex of adjacent fetuses in utero. J Anim Sci. 1989;67(7):1824‐1840. [DOI] [PubMed] [Google Scholar]
- 19. Miller EM. Prenatal sex hormone transfer: A reason to study opposite‐sex twins. Pers Individ Dif. 1994;17(4):511‐529. [Google Scholar]
- 20. Cohen‐Bendahan C, van dB, Berenbaum SA. Prenatal sex hormone effects on child and adult sex‐typed behavior: Methods and findings. Neurosci Biobehav Rev. 2005;29(2):353‐384. [DOI] [PubMed] [Google Scholar]
- 21. Ahrenfeldt L, Petersen I, Johnson W, Christensen K. Academic performance of opposite‐sex and same‐sex twins in adolescence: A Danish national cohort study. Horm Behav. 2015;69:123‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galsworthy MJ, Dionne G, Dale PS, Plomin R. Sex differences in early verbal and non‐verbal cognitive development. Dev Sci. 2000;3(2):206‐215. [Google Scholar]
- 23. Van Hulle CA, Goldsmith HH, Lemery KS. Genetic, environmental, and gender effects on individual differences in toddler expressive language. J Speech Lang Hear Res. 2004;47(4):904‐912. [DOI] [PubMed] [Google Scholar]
- 24. Cole‐Harding S, Morstad AL Wilson JR. Spatial ability in members of opposite‐sex twin pairs. Behav Genet. 1988;18:710. [Google Scholar]
- 25. Heil M, Kavšek M, Rolke B, Beste C, Jansen P. Mental rotation in female fraternal twins: evidence for intra‐uterine hormone transfer? Biol Psychol. 2011;86(1):90‐93. [DOI] [PubMed] [Google Scholar]
- 26. Vuoksimaa E, Kaprio J, Kremen WS, et al. Having a male co‐twin masculinizes mental rotation performance in females. Psychol Sci. 2010;21(8):1069‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toivainen T, Papageorgiou KA, Tosto MG, Kovas Y. Sex differences in non‐verbal and verbal abilities in childhood and adolescence. Intelligence. 2017;64, 81‐88. [Google Scholar]
- 28. Bütikofer A, Figlio DN, Karbownik K, Kuzawa CW, Salvanes KG. Evidence that prenatal testosterone transfer from male twins reduces the fertility and socioeconomic success of their female co‐twins. Proc Natl Acad Sci. 2019;116(14) 6749‐6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: High‐avidity binding to beta‐amyloid and increased frequency of type 4 allele in late‐onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1993;90(5):1977‐1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta‐analysis. JAMA. 1997;278(16):1349‐1356. [PubMed] [Google Scholar]
- 32. Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta‐analysis. JAMA Neurol. 2017;74(10):1178‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bretsky PM, Buckwalter JG, Seeman TE, et al. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13(4):216‐221. [DOI] [PubMed] [Google Scholar]
- 34. Dean DC III, Jerskey BA, Chen K, et al. Brain differences in infants at differential genetic risk for late‐onset Alzheimer disease: a cross‐sectional imaging study. JAMA Neurol, 2014;71(1):11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaw P, Lerch JP, Pruessner JC, et al. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: An observational study. Lancet Neurol. 2007;6(6):494‐500. [DOI] [PubMed] [Google Scholar]
- 36. Bloss CS, Delis DC, Salmon DP, Bondi MW. Decreased cognition in children with risk factors for Alzheimer's disease. Biol Psychiatry. 2008;64(10):904‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reynolds CA, Smolen A, Corley RP, et al. APOE effects on cognition from childhood to adolescence. Neurobiol Aging. 2019;84:239.e1‐239.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Magnusson P, Almqvist C, Rahman I, et al. The Swedish Twin Registry: Establishment of a biobank and other recent developments. Twin Res Hum Genet. 2013;16(1):317‐329. [DOI] [PubMed] [Google Scholar]
- 39. Jin Y‐P, Gatz M, Johansson B, Pedersen NL. Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurology. 2004;63(4):739‐741. [DOI] [PubMed] [Google Scholar]
- 40. Rizzuto D, Feldman AL, Karlsson IK, Dahl Aslan AK, Gatz M, Pedersen NL. Detection of dementia cases in two Swedish Health Registers: A validation study. J Alzheimer's Dis. 2018;61(4):1301‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Radmanesh F, Devan WJ, Anderson CD, Rosand J, Falcone GJ. Accuracy of imputation to infer unobserved APOE epsilon alleles in genome‐wide genotyping data. Eur J Hum Genet. 2014;22(10):1239‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- 43. Therneau T. A Package for Survival Analysis in S. version 2.38, https://CRAN.R‐project.org/package = survival; 2015.
- 44. van Buuren S, Groothuis‐Oudshoorn K. Mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1‐67. [Google Scholar]
- 45. Enders CK. Applied missing data analysis. New York: Guilford Press; 2010. [Google Scholar]
- 46. Muthén LK, Muthén BO. Mplus user's guide. Los Angeles, CA: Muthén & Muthén; 1998‐2017. [Google Scholar]
- 47. Tapp AL, Maybery MT, Whitehouse AJO. Evaluating the twin testosterone transfer hypothesis: A review of the empirical evidence. Horm Behav. 2011;60(5):713‐722. [DOI] [PubMed] [Google Scholar]
- 48. Juraska JM, Sisk CL, DonCarlos LL. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm Behav. 2013;64(2):203‐210. [DOI] [PubMed] [Google Scholar]
- 49. Carroll J, Rosario E, Kreimer S, et al. Sex differences in β‐amyloid accumulation in 3xTg‐AD mice: Role of neonatal sex steroid hormone exposure. Brain Res. 2010;1366:233‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borenstein A, Mortimer J. Alzheimer's disease: Life course perspectives on risk reduction. Amsterdam, Boston: Academic Press; 2016. [Google Scholar]
- 51. Peper JS, Brouwer RM, van Baal GCM, et al. Does having a twin brother make for a bigger brain? Eur J Endocrinol. 2009;160(5):739‐746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


