Abstract
Neuro-immune alterations in the peripheral and central nervous system play a role in the pathophysiology of chronic pain in general, and members of the non-coding RNA (ncRNA) family, specifically the short, 22 nucleotide microRNAs (miRNAs) and the long non-coding RNAs (lncRNAs) act as master switches orchestrating both immune as well as neuronal processes. Several chronic disorders reveal unique ncRNA expression signatures, which recently generated big hopes for new perspectives for the development of diagnostic applications. lncRNAs may offer perspectives as candidates indicative of neuropathic pain in liquid biopsies. Numerous studies have provided novel mechanistic insight into the role of miRNAs in the molecular sequelae involved in the pathogenesis of neuropathic pain along the entire pain pathway. Specific processes within neurons, immune cells, and glia as the cellular components of the neuropathic pain triad and the communication paths between them are controlled by specific miRNAs. Therefore, nucleotide sequences mimicking or antagonizing miRNA actions can provide novel therapeutic strategies for pain treatment, provided their human homologues serve the same or similar functions. Increasing evidence also sheds light on the function of lncRNAs, which converge so far mainly on purinergic signalling pathways both in neurons and glia, and possibly even other ncRNA species that have not been explored so far.
Keywords: lncRNA, microRNA, neuroimmune interations, neuropathic pain, non-coding RNA
Introduction
Human neuropathic pain disorders are difficult to diagnose and treat due to their diversity, which even increases with the development of chronic pain. The most frequent neuropathic pain disorder is diabetic painful neuropathy (DPN), which occurs as a common complication of diabetes mellitus [1,2]. Although good control of blood glucose levels can reduce the incidence of DPN mainly in Type I diabetes, more than half of the patients still develop DPN for which only symptomatic therapy of low to moderate efficacy is available to date [3]. Neuroinflammatory signatures have been identified as critical components of DPN but its complex pathogenesis is still incompletely understood [3,4]. Pathological neuro-immune communication has, likewise, been associated with other painful neuropathies such as neuropathic pain occurring in up to 50% of patients experiencing traumatic nerve injury as a consequence of accidents, warfare or surgical procedures [5–7]. Also the neurogenic complex regional pain syndrome (CRPS), an enigmatic complication of bone fracture or tissue injury, is associated with neuro-inflammatory deficits [8]. In the majority of patients symptoms largely resolve, however in 30% of cases the pain persists or even intensifies [9]. The beneficial effect of glucocorticosteroids in acute CRPS points towards pathophysiological mechanisms associated with neuro-immune dysfunction [9–11].
Such neuro-immune alterations in the peripheral and central nervous system play a role in the pathophysiology of chronic pain in general, and members of the non-coding RNA (ncRNA) family, specifically the short, 22 nucleotide microRNAs (miRNAs) as regulators of gene expression act as master switches orchestrating both immune and neuronal processes. The long non-coding RNAs (lncRNAs) can regulate gene expression but when containing multiple miRNA-binding elements can serve as endogenous sponges neutralizing these miRNAs. Several chronic disorders reveal unique miRNA and lncRNA expression signatures, which recently generated big hopes for new perspectives for the development of diagnostic applications. ncRNAs modulating both neuronal and immune processes further promise therapeutic potential for diseases with a neuro-immune component [12,13]. Specifically, ncRNAs may regulate neuro-immune communication signals in the pain pathway by controlling macromolecular complexes in neurons, glia and immune cells. Understanding the concerted function of miRNA and lncRNAs in the regulation of nociceptive transduction and action potential generation, the synaptic transmission in the spinal dorsal horn and brain, the intercellular communication between neurons and non-neuronal cells, such as microglia, and the endogenous inhibitory control circuits, and defining their importance in the brain circuitries connected to cognitive, emotional and behavioural components involved in pain will shed new light on the so far enigmatic pathophysiology of neuropathic pain disorders. This review will focus on miRNAs and lncRNAs, as a large amount of literature suggests important and partially opposing roles for these ncRNAs in the establishment and chronification of neuropathic pain.
microRNA
Generation of miRNA
Pain conditions have been associated with deregulated miRNA expression from primary afferent nociceptors to brain areas associated with emotional components of pain perception [14–19]. Unique signatures of ncRNAs are associated with altered innate immune signalling and secreted miRNAs are even considered a new form of neuro-immune communication, and control immune cell activity as well as neuron function [13,20–22]. Thus, ncRNAs may act as essential modulators of processes for the establishment and maintenance of neuropathic pain.
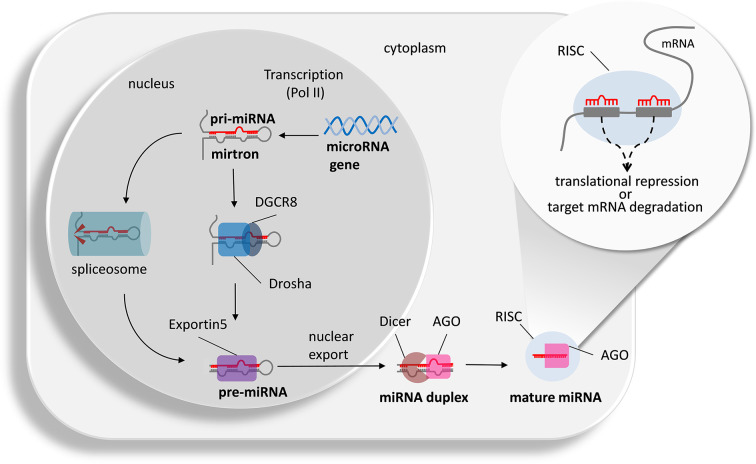
Generation of mature miRNAs takes place within two distinct cellular compartments (Figure 1): In the nucleus, miRNAs are transcribed from DNA sequences by polymerase II as pri-microRNAs, processed by the RNAse-III enzyme Drosha and its auxiliary protein DGCR8 (Pasha) into 5′ capped and poly-adenylated pre-miRNAs, which can be several kilobases long, and may comprise one (monocistronic) or several (polycistronic) miRNA precursors [23]. Pre-miRNA sequences form characteristic hairpin loop structures of ∼70 nucleotides with a two-nucleotide overhang at the 3′ end and 3′ hydroxyl and 5′ phosphate groups [23–25]. Alternatively, short introns forming a hairpin-like structure called mirtrons can be spliced in a Drosha-independent manner to be further processed into a pre-miRNA [26]. These recently discovered features illustrate unexpected flexibility and highlight how alternative RNA processing can encode multiple functions by individual transcripts. Pre-miRNAs are shuttled from the nucleus into the cytosol by Exportin-5 transporter molecules where the RNase-III enzyme Dicer cleaves them towards biologically active mature duplex single-stranded miRNAs (∼18–25 nucleotides) [23]. Both single-stranded miRNAs derived from the 5´ arm (-5p) and 3´ arm (-3p) of the precursor can become integrated with Argonaute (Ago) proteins to form the RNA-Induced Silencing Complex (RISC, see Figure 1; [27]). Depending on the degree of homology, the miRNA induces translational repression (incomplete match) or target mRNA degradation (full match, not in mammals). More than 50% of mammalian miRNAs are located within host genes [28]. Especially those intragenic miRNAs exhibiting a high degree of conservation between species appear to be coordinately regulated and expressed with their host genes, either with synergistic or antagonistic correlation patterns [29,30]. For example, a number of interleukin-6 (IL-6) regulated miRNAs are up-regulated in rodent models of neuropathic pain and distinct functions have been described [31]. In contrast to intragenic miRNAs, the regulatory elements for extragenic miRNA are still largely enigmatic.
Figure 1. Classical and alternative mechanisms of mature miRNA generation and their action.
The microRNA gene is transcribed into a primary microRNA (pri-microRNA) or mirtron by polymerase II (Pol II). Drosha together with the accessory protein DGCR8 cleaves the pri-miRNA into a pre-miRNA. Alternatively, the pre-miRNA can arise from alternative splicing of the mirtron. Pre-miRNA binds to Exportin5 for nuclear export. Within the cytoplasm, Dicer and Argonaute 2 (AGO) cleave off the hairpin structure to generate a duplex miRNA. The two strands separate and within the RNA silencing complex (RISC) and in the presence of AGO the mature miRNA hybridizes with complementary seed sequences (8 nucleotides length) of possible target RNA strands (adapted from [32,33]). Depending on the degree of homology, the miRNA induces translational repression (incomplete match) or target mRNA degradation (full match, not in mammals) is induced.
Potential prospect of miRNA patterns emerging as possible signatures for pain disorders
miRNA in body fluids
In addition to their intracellular location and function, miRNAs are detectable in extracellular vesicles, such as exosomes, which are released, for example, by glia cells to cargo messages to other cell types in the central nervous system. Therefore, exosomes are anticipated to represent a specific mode of intercellular communication (for review see [34,35]). In addition, they are detectable in body fluids, such as cerebrospinal fluid (CSF), blood plasma or saliva, where they can be exploited for diagnostic purposes as so called ‘liquid biopsies’. Extracellular miRNAs are emerging as important communication units not only for mental disorders but also in the pain pathway offering the advantage of long distance messaging [36,37]. Several studies propose individual miRNAs or miRNA signatures for pathological pain disorders, such as CRPS, diabetic neuropathic pain or fibromyalgia. However, the assays available for these first studies assessing miRNAs in human body fluids cover only a small number of miRNAs and the marginal overlap for the same disease when assessed at different locations dampens the great hopes in the possible value of liquid biopsies for clinical use in pain diagnostics (see Table 1).
Table 1. Deregulated miRNAs identified in liquid biopsies of patients with pain disorders.
| Disease | Detection method | Sample type | # up-regulated | # down-regulated | miRNA deregulated in minimum two studies on disease | Reference |
|---|---|---|---|---|---|---|
| CRPS | qPCR array | Blood | 4 | 14 | RNU48; miR-15a; miR-21; mir-25; miR-29c; miR-34a; mir-126; miR-212; mir-320B; miR-337; miR-367; miR-576; miR-645; mir-939; miR-1276; miR-1303 | [38] |
| CRPS | qPCR array | Serum-derived exosomes | 62 | 70 | [39] | |
| CRPS responders versus non-responders | qPCR array | Blood | 1 | 32 | [40] | |
| CRPS responders versus non-responders | qPCR array | Serum-derived exosomes | 1 | 8 | [41] | |
| Diabetic neuropathy | qPCR array | Serum | 1 | 63 | let-7d; let-7e; miR-28; miR-92a; miR-106a; miR-130a; miR-139; miR-150; mir-210; miR-342; miR-425; mir-486; miR-574 | [42] |
| Diabetic neuropathy with versus without critical limb ischaemia | microarray | Plasma | 7 | 4 | [43] | |
| Diabetic neuropathy with versus without critical limb ischaemia | microarray | Plasma | 6 | 5 | [44] | |
| Diabetic neuropathy | qPCR array | Serum | 21 | 0 | [45] |
In total, about 6000–7000 microRNA sequences have been identified, more than 2000 in humans [46]. In order to retrieve all known and possible currently unknown human miRNAs together with other ncRNAs and mRNAs, RNA sequencing (RNASeq) technologies represent the state of the art methodology for unbiased assessment of differentially expressed miRNA in patient and control cohorts [47,48]. For miRNA quantification in tissue, different experimental settings and also tissues, such as dorsal root ganglia (DRG), spinal cord or brain, require the use of appropriate reference genes and reliability is significantly higher if three different reference genes are used [49]. However, due to profound technical improvements, only unbiased RNASeq provides sufficient reliability and specificity for the discovery of disease specific miRNA patterns.
Nonetheless, for blood components, as well as nerve biopsies, a number of differentially regulated miRNAs has been identified and their target genes validated with a possible prospect to better understand disease pathophysiology (Table 2). Two up-regulated miRNAs (miR-124 and miR-155) target the histone deacetylase SIRT1, a structurally important promoter of axonal elongation, neurite outgrowth, and dendritic branching. SIRT1 also plays a role in memory formation by modulating synaptic plasticity and has protective roles in several neurodegenerative diseases [50]. The down-regulation of SIRT1 by miRNAs could thus be causally involved in neuropathic pain generation or in the exacerbation of the immune response [51]. Likewise, the increased IL-6 and VEGF expression resulting from decreased activity of miR-338-5p and miR-939, as well as miR-34a and miR-101 targeting Corticotropin releasing hormone receptor 1 (CRHR1) and Karyopherin beta 1 (KPNB1) are relevant candidates for the inflammatory component of neuropathic pain disorders [41,52,53].
Table 2. Deregulated miRNA in human samples including validated target genes.
| miRNA | Regulation | Target | Gene description | Validation | Pain disorder | Tissue | Reference |
|---|---|---|---|---|---|---|---|
| miR-124a | ↑ | SIRT1 | Sirtuin 1 | LucA | Neuropathic pain patients | CD4+ T cells | [51] |
| miR-132-3p | ↑ | GRIA1 | Glutamate ionotropic receptor AMPA type subunit 1 | LucA | Neuropathic pain patients | WBC / sural nerve | [54] |
| miR-155 | ↑ | SIRT1 | Sirtuin 1 | LucA | Neuropathic pain patients | CD4+ T cells | [51] |
| miR-199a-3p | ↑ | SERPINE2 | Serpin family E member 2 | LucA | Diabetes type II | Plasma | [55] |
| miR-455-3p | ↑ | TUBB3 | Tubulin beta 3 class III | mimic + immunofluorescence | HIV-induced polyneuropathy | Plasma | [56] |
| miR-34a | ↓ | XIST; YY1 | X inactive specific transcript; YY1 transcription factor | LucA | CRPS | Blood | [57] |
| miR-34a | ↓ | CRHR1 | Corticotropin releasing hormone receptor 1 | LucA | CRPS | Blood | [53] |
| miR-101 | ↓ | KPNB1 | Karyopherin subunit beta 1 | LucA | Neuropathic pain patients | Plasma / sural nerve | [58] |
| miR-338-5p | ↓ | IL6 | Interleukin 6 | LucA | CRPS | Plasma | [41] |
| miR-939 | ↓ | VEGFA | Vascular endothelial growth factor A | LucA | CRPS | Plasma | [52] |
miRNA tissue expression in rodent models
Similar to human studies, differentially expressed miRNAs are extensively explored in preclinical neuropathic pain models [31,59–64]. In several of the routinely used models, specific miRNAs are up- or down-regulated all along the pain pathway and described in a number of recent publications and reviews [59,60,62,64–71]. The following section takes a more mechanistic approach and provides an overview of miRNAs deregulated in tissues in relevant preclinical pain models for which a relevant target gene and insight into neuropathic pain mechanisms have been validated (see Table 3).
Table 3. Look-up table of deregulated miRNA and target genes related to neuropathic human pain disorders or preclinical models of neuropathic pain. For ethical reasons, bilateral CCI was excluded as a model.
| miRNA | Regulation | Target | Gene description | Validation | Pain model | Tissue | Species | Reference |
|---|---|---|---|---|---|---|---|---|
| miR‐15b | ↑ | Bace1 | Beta-secretase 1 | LucA | Oxaliplatin‐induced peripheral neuropathy | DRG | Rat | [72] |
| miR-18a | ↑ | Kcna1; Kcnd3 | Potassium voltage-gated channel subfamily A member 1; Potassium voltage-gated channel subfamily D member 3 | LucA | SNL | DRG | Rat | [73] |
| miR-19a | ↑ | Kcna4; Kcnc4; Kcnq5; Scn1b | Potassium voltage-gated channel subfamily A member 4; Potassium voltage-gated channel subfamily C member 4; Potassium voltage-gated channel subfamily Q member 5; Sodium voltage-gated channel beta subunit 1 | LucA | SNL | DRG | Rat | [73] |
| miR-19b | ↑ | Kcna4; Kcnc4; Kcnq5; Scn1b | Potassium voltage-gated channel subfamily A member 4; Potassium voltage-gated channel subfamily C member 4; Potassium voltage-gated channel subfamily Q member 5; Sodium voltage-gated channel beta subunit 1 | LucA | SNL | DRG | Rat | [74] |
| miR-32-5p | ↑ | Dusp5 | Dual specificity phosphatase 5 | LucA | SNL | Spinal cord / microglia | Rat | [75] |
| miR-92a | ↑ | Kcnc4; Dpp10 | Potassium voltage-gated channel subfamily C member 4; Dipeptidyl peptidase like 10 | LucA | SNL | DRG | Rat | [73] |
| miR-124a | ↑ | SIRT1 | Sirtuin 1 | LucA | Neuropathic pain patients | CD4+ T cells | Human | [51] |
| miR-132-3p | ↑ | GRIA1 | Glutamate ionotropic receptor AMPA type subunit 1 | LucA | Neuropathic pain patients | WBC / sural nerve | Human | [54] |
| miR-132-3p | ↑ | Gria1 | Glutamate ionotropic receptor AMPA type subunit 1 | LucA | SNI | Sural nerve / spinal cord / DRG | Rat | [54] |
| miR-146a-5p | ↑ | Traf6 | TNF receptor-associated factor 6 | LucA | SNL | Spinal astrocytes | Mouse | [76] |
| miR-155 | ↑ | Socs1 | Suppressor of cytokine signalling 1 | LucA | CCI | Spinal cord / microglia | Rat | [77] |
| miR-155 | ↑ | SIRT1 | Sirtuin 1 | LucA | Neuropathic pain patients | CD4+ T cells | Human | [51] |
| miR-183-5p | ↑ | Cldn1 | Claudin 1 | mimic + WB | Perineural injection of sciatic nerve with recombinant tissue plasminogen activator | Sciatic nerve | Rat | [78] |
| miR-195 | ↑ | Ptch1 | Patched 1 | LucA | Infraorbital nerve CCI | Brain stem | Rat | [79] |
| miR-195 | ↑ | Atg14 | Autophagy related 14 | LucA | SNL | Spinal cord / microglia | Rat | [80] |
| miR-199a-3p | ↑ | SERPINE2 | Serpin family E member 2 | LucA | Diabetes type II | Plasma | Human | [55] |
| miR-218 | ↑ | Socs3 | Suppressor of cytokine signalling 3 | LucA | CCI | Spinal cord / microglia | Rat | [81] |
| miR-221 | ↑ | Socs1 | Suppressor of cytokine signalling 1 | LucA | CCI | Spinal cord / microglia | Rat | [82] |
| miR-449a | ↑ | Pparg | Peroxisome proliferator-activated receptor gamma | LucA | SCI | Spinal cord | Rat | [83] |
| miR-455-3p | ↑ | TUBB3 | Tubulin beta 3 class III | mimic + immunofluorescence | HIV-induced polyneuropathy | Plasma | Human | [56] |
| miR-500 | ↑ | Gad1 | Glutamate decarboxylase 1 | LucA | Paclitaxel-induced neuropathic pain | Spinal dorsal horn | Rat | [84] |
| miR-7a | ↓ | Scn2b | Sodium voltage-gated channel beta subunit 2 | LucA | SNL | DRG | Rat | [85] |
| miR-7a | ↓ | Nefl | Neurofilament light | LucA | SNL | DRG | Rat | [86] |
| miR-9 | ↓ | Foxp1 | Forkhead box P1 | mimic + WB | Sciatic nerve crush | DRG | Mouse | [87] |
| miR-19a | ↓ | Mecp2 | Methyl CpG binding protein 2 | LucA | SNI | DRG | Mouse | [88] |
| miR-20b-5p | ↓ | Akt3 | AKT serine/threonine kinase 3 | LucA | CCI | Spinal cord | Rat | [89] |
| miR-21-5p | ↓ | Timp3; Ccl1 | TIMP metallopeptidase inhibitor 3; C-C motif chemokine ligand 1 | LucA | CCI | Spinal cord | Rat | [90] |
| miR-23a-3p | ↓ | Cxcr4 | Chemokine (C-X-C motif) receptor 4 | LucA | SNL | Spinal cord | Mouse | [91] |
| miR-23b | ↓ | Nox4 | NADPH oxidase 4 | LucA | Traumatic SCI (neuropathic pain) | Spinal cord | Mouse | [92] |
| miR-26a-5p | ↓ | Mapk6 | Mitogen-activated protein kinase 6 | LucA | CCI | Spinal cord | Rat | [93] |
| miR-30b | ↓ | Scn9a | Sodium voltage-gated channel alpha subunit 9 | LucA | SNI | DRG | rat | [94] |
| miR-30b | ↓ | Scn3a | Sodium voltage-gated channel alpha subunit 3 | LucA | SNL | DRG | Rat | [95] |
| miR-34a | ↓ | Scn2b; Vamp2 | Sodium voltage-gated channel beta subunit 2; Vesicle-associated membrane protein 2 | LucA | CCI | DRG | Rat | [96] |
| miR-34a | ↓ | XIST; YY1 | X inactive specific transcript; YY1 transcription factor | LucA | CRPS | Blood | Human | [57] |
| miR-34a | ↓ | CRHR1 | Corticotropin releasing hormone receptor 1 | LucA | CRPS | Blood | Human | [53] |
| miR-34c | ↓ | Nlrp3 | NLR family, pyrin domain containing 3 | LucA | CCI | Spinal cord | Mouse | [97] |
| miR-96 | ↓ | Scn3a | Sodium voltage-gated channel alpha subunit 3 | mimic + WB | CCI | DRG | Rat | [98] |
| miR-101 | ↓ | KPNB1 | Karyopherin subunit beta 1 | LucA | Neuropathic pain patients | Plasma / sural nerve | Human | [58] |
| miR-132 | ↓ | Mecp2 | Methyl CpG binding protein 2 | LucA | SNI | DRG | Mouse | [88] |
| miR-141 | ↓ | Hmgb1 | High mobility group box 1 | LucA | CCI | DRG | Rat | [99] |
| miR–142–3p | ↓ | Hmgb1 | High mobility group box 1 | LucA | SNL | DRG | Mouse | [100] |
| miR-145 | ↓ | Akt3 | AKT serine/threonine kinase 3 | LucA | CCI | DRG | Rat | [101] |
| miR-145 | ↓ | Rreb1 | Ras responsive element binding protein 1 | LucA | CCI | Spinal cord | Rat | [102] |
| miR-182-5p | ↓ | Ephb1 | Eph receptor B1 | LucA | CCI | Spinal cord | Mouse | [103] |
| miR-183 | ↓ | Mtor | Mechanistic target of rapamycin kinase | LucA | CCI | Spinal cord | Rat | [104] |
| miR-183 | ↓ | Scn3a; Bdnf | Sodium voltage-gated channel alpha subunit 3; Brain-derived neurotrophic factor | mimic + qPCR | SNL | DRG | Rat | [105] |
| miR-183-5p | ↓ | Kcnk2 | Potassium two pore domain channel subfamily K member 2 (Trek1) | LucA | CCI | DRG | Rat | [106] |
| miR-186-5p | ↓ | Cxcl13 | Chemokine (C-X-C motif) ligand 13 | LucA | SNL | Spinal cord | Mouse | [107] |
| miR-190a-5p | ↓ | Slc17a6 | Solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter), member 6 (Vglut2) | LucA | Diabethic neuropathy | Spinal cord | Mouse | [108] |
| miR-200b | ↓ | Zeb1 | Zinc finger E-box binding homeobox 1 | LucA | CCI | Spinal cord / microglia | Rat | [109] |
| miR-206 | ↓ | Bdnf | Brain-derived neurotrophic factor | LucA | CCI | DRG | Rat | [110] |
| miR-206-3p | ↓ | Hdac4 | Histone deacetylase 4 | LucA | CCI | DRG | Rat | [111] |
| miR-301 | ↓ | Mecp2 | Methyl CpG binding protein 2 | LucA | SNI | DRG | Mouse | [88] |
| miR-338-5p | ↓ | IL6 | Interleukin 6 | LucA | CRPS | Plasma | Human | [41] |
| miR-362-3p | ↓ | Pax2 | Paired box 2 | LucA | SCI | Spinal cord | Rat | [112] |
| miR-429 | ↓ | Zeb1 | Zinc finger E-box binding homeobox 1 | LucA | CCI | Spinal cord/microglia | Rat | [109] |
| miR–449a | ↓ | Trpa1; Kcnma1 | Transient receptor potential cation channel, subfamily A, member 1; Potassium large conductance calcium-activated channel, subfamily M, alpha member 1 | mimic + qPCR | SNI | DRG | Mouse | [113] |
| miR-539 | ↓ | Grin2b | Glutamate ionotropic receptor NMDA type subunit 2B | mimic + WB | CCI | ACC | Rat | [114] |
| miR-939 | ↓ | VEGFA | Vascular endothelial growth factor A | LucA | CRPS | Plasma | Human | [52] |
Abbreviations: CCI, chronic constriction injury; CRPS, chronic regional pain syndrome; SCI, spinal cord injury; SNI, spared nerve injury; SNL, sciatic nerve ligation.
Mechanisms of miRNA action
Intracellular miRNAs suppress gene expression through tightly regulated steps within the microprocessor complex. For this, the miRNA within the ‘activated’ RNA-induced silencing complex (RISC) attaches to the 3′-untranslated region (UTR) of a given target mRNA primarily through its heptameric 5′ seed region (positions 2–8). One given mRNA harbouring one or several miRNA-binding sites in its 3′UTR can be regulated by various miRNAs. miRNA binding to the target gene’s miRNA recognition element (MRE) with full complementarity (which is rare in mammals) leads to destabilization and degradation (see also Figure 1); if the complementarity is incomplete, binding induces translational repression [115]. Protein expression may be down-regulated, although mRNA levels may remain unaltered in case of incomplete complementarity. miRNAs regularly target a multitude of target genes at the same time and thus may regulate entire signalling networks within one cell. In addition to their regulated target mRNAs, miRNAs hybridize with pseudogenes or circular RNAs (circRNAs) acting as endogenous miRNA neutralizing sponges, which inhibit or limit intracellular miRNA effects [116]. An unconventional role of extracellular miRNAs for rapid excitation of nociceptor neurons has been discovered recently: miRNA-let-7b induces rapid inward currents and excitation of nociceptors. These responses require the GUUGUGU motif, only occur in neurons co-expressing TLR7 and TRPA1, and are abolished in mice lacking Tlr7 or Trpa1. Thus, extracellular miRNAs may in addition to their regulatory function act as aptamers with a role as pain mediators via activating TLR7/TRPA1 in nociceptor neurons [117].
Since the first publication on analgesic miR-124 effects, miRNA regulation has attracted increasing attention in the pain field. However, the increasing number of reports raises the problem how expression patterns and mechanisms can be interpreted with a more global perspective. In order to warrant the highest degree of stringency, only miRNAs are addressed in this review based on publications validating miRNA expression together with direct regulation of target genes related to pain processing (Table 3).
miRNAs deregulated in the peripheral nerve
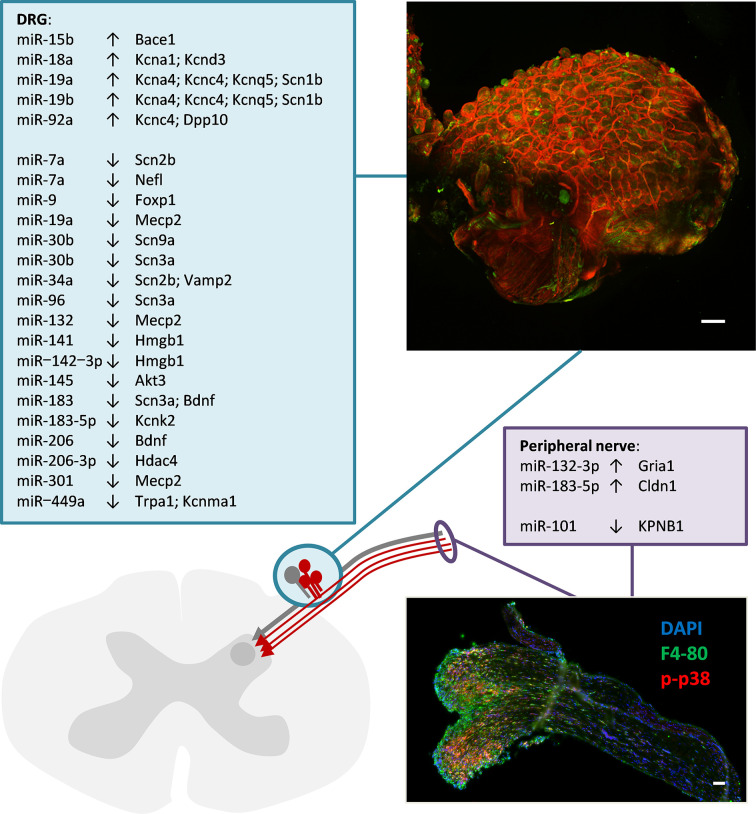
Only few studies address miRNAs in peripheral nerve tissue where axons, Schwann cells, connective tissue, cells of the vasculature and resident as well as transient immune cells can be possible sources (Figure 2). Up-regulation of miR-132-3p may target the ionotropic glutamate receptor AMPA type subunit 1 (Gria1) [54]. This has been identified as a microglial gene potentially linked to the maintenance of neuropathic pain [118]. Another up-regulated miRNA, miR-183-5p, targets Claudin 1, a tight junction protein that maintains the blood brain/blood nerve barrier and is down-regulated after nerve injury [78,119]. Along these lines, down-regulation of miR-101 by targeting Importin beta 1 (also known as Karyopherin beta 1, KPNB1) may release a brake on importin expression and augment the accessibility of axons to exosomal cargo as a mechanisms to foster nerve regeneration but possibly also for proalgesic factors [58,120].
Figure 2. Up- or down-regulated miRNAs in peripheral nerve or sensory ganglia and their target genes that are associated with neuropathic pain.
Akt3: AKT serine/threonine kinase 3; Bace1: Beta-secretase 1; Bdnf: Brain-derived neurotrophic factor; Dpp10: Dipeptidyl peptidase like 10; Foxp1: Forkhead box P1; Hdac4: Histone deacetylase 4; Hmgb1: High mobility group box 1; Kcna1: Potassium voltage-gated channel subfamily A member 1; Kcna4: Potassium voltage-gated channel subfamily A member 4; Kcnd3: Potassium voltage-gated channel subfamily D member 3; Kcnk2: Potassium two pore domain channel subfamily K member 2 (Trek1); Kcnma1: Potassium large conductance calcium-activated channel, subfamily M, alpha member 1; Kcnc4: Potassium voltage-gated channel subfamily C member 4; Kcnq5: Potassium voltage-gated channel subfamily Q member 5; Mecp2: Methyl CpG binding protein 2; Nefl: Neurofilament light; Scn1b: Sodium voltage-gated channel beta subunit 1; Scn2b: Sodium voltage-gated channel beta subunit 2; Scn3a: Sodium voltage-gated channel alpha subunit 3; Scn9a: Sodium voltage-gated channel alpha subunit 9; Trpa1: Transient receptor potential cation channel, subfamily A, member 1; Vamp2: Vesicle-associated membrane protein 2. Scale bar: 100 µm (micrographs were kindly provided by M. Langeslag)
miRNAs deregulated in the dorsal root and trigeminal ganglia
miRNA expression in the dorsal root or trigeminal ganglia (Figure 2 andTable 3) can be deregulated in various cell types, such as neurons, Schwann cells, resident or invading immune cells or even the vasculature. The most intensely studied sources are peptidergic and non-peptidergic primary afferent nociceptors. Conditional deletion of the miRNA-maturation enzyme Dicer exclusively in neurons expressing the nociceptor specific sodium channel Nav1.8 critically affects neuronal excitability [121] and increasing evidence suggests that several miRNAs directly or indirectly modulate neuron function (Table 3). Particular miRNAs are deregulated in peripheral neurons after nerve injury giving rise to deregulation of miRNA targeted ion channel and metabotropic receptor transcripts that presumably causes nociceptor dysfunction [117,121–123].
The most intensely investigated miRNA in the DRG is presently miR-21 that is expressed in neurons and up-regulated in several neuropathic pain models. Both intrathecal delivery of a miR-21-5p antagomir and conditional deletion of miR-21 in sensory neurons reduce neuropathic hypersensitivity [36]. As a new mechanism of action miR-21 cargo from neurons to immune cells via exosomes has been introduced recently: following capsaicin activation, miR-21-5p containing exosomes are released from cultured DRG and phagocytosed by macrophages in which the resulting increase in miR-21-5p levels promotes a pro-inflammatory phenotype. Both up-regulation and release of miR-21 contribute to sensory neuron-macrophage communication after damage to the peripheral nerve [36]. Since intrathecal miR-21 injection induces pain hypersensitivity in wild-type mice but not in mice with a global deletion of toll-like receptor 8 (Tlr8−/−), the TLR8 receptor appears to act as a downstream effector of miR-21 to maintain neuropathic pain; however, a direct targeting of the Tlr8 gene has not been validated yet [124].
miR-18, miR-19a, miR-19b as well as mir-92 are also up-regulated in neuropathic pain models and in turn down-regulate potassium channels including Kcna1, Kcna4, Kcnc4, Kcnd3 and Kcnq5 [73]. The suppression of potassium channels in general increases neuronal excitability and this may be a relevant mechanism causing nociceptor excitation and sensitization [125,126]. In line with the alterations towards hyperexcitability, several down-regulated miRNAs targeting voltage-gated sodium channels may further promote neuronal excitability by releasing the breaks on sodium channel expression: two alpha subunits of voltage-gated sodium channels, Scn3a giving rise to Nav1.3 and Scn9a giving rise to pain-related Nav1.7, are targeted by the down-regulated miRNAs miR-30b, miR-96 and miR-183, which probably contributes to up-regulation of the ion channel alpha subunits in neuropathic pain models [94,95,98,105]. In addition, down-regulated miR-7a and miR-34a targeting sodium voltage-gated channel beta subunit 2 (Scn2b) may be involved in improved trafficking of already formed alpha subunits [85,96]. Furthermore, transducer channels, such as TRPA1, can occur as a consequence of miR–449a down-regulation [113]. The only potential change counteracting these proalgesic alterations refers to the down-regulated miR-183-5p that targets potassium channel Trek1, a two pore domain potassium channel subfamily member 2 (Kcnk2), which as a leak current keeps the membrane potential hyperpolarized [106]. The up-regulation of this channel may counteract the brake set on potassium channel expression by the miR-17-92 cluster.
Alterations in enzymatic activity, inflammatory signalling pathways and epigenetic regulators are amongst the mechanisms targeted by deregulated miRNAs in DRG. For example, miR-15b up-regulation targets Beta-secretase 1 that may be involved in neuroprotective processes, whereas miR-145 down-regulation appears to release the break on Akt3 that can be targeted by miR-15a or miR-20b-5p mimics to relieve neuropathic pain [72,89,101,127]. Down-regulation of miR-183 and miR-206 contributes to the up-regulation of brain-derived neurotrophic factor (Bdnf) that is required for regenerative processes but also is an important pain modulator [105,110,128,129] (for review see [130]). Finally, several down-regulated miRNAs (miR-19a, miR-132, miR-301) appear to affect epigenetic regulatory pathways, such as Mecp2 or HDAC4 (via miR-206-3p), and dependent processes that are relevant contributors fueling pathological functions in the spinal dorsal horn but may also be relevant for dysfunction of primary afferent nociceptors [88,111,131–135].
miRNAs deregulated in the spinal cord
miR-124 was the first miRNA for which an analgesic action at spinal cord level was demonstrated and correlated with a shift in the M1/M2 microglial marker ratio towards an anti-inflammatory phenotype [136]. The functional consequences of miR-103 regulation of Cav1.2 calcium channels and intrinsic excitability of spinal projection neurons have also been demonstrated [123]. More evidence supporting miRNA analgesic effects emerge from mice intrathecally receiving miR-124 or miR-103, which are reported to prevent and treat persistent inflammatory and neuropathic pain [123,136]. Despite the fact that these miRNA treatments reduce signatures of synaptic modification, neuroinflammation and microglial response, the full extent and the mechanisms of the analgesic effect are not fully understood to date [34,123,136,137]. However, several miRNAs have been found deregulated in rodent pain models together, mainly in microglia, with validated downstream targets with a potential relevance to pain signalling (Figure 3).
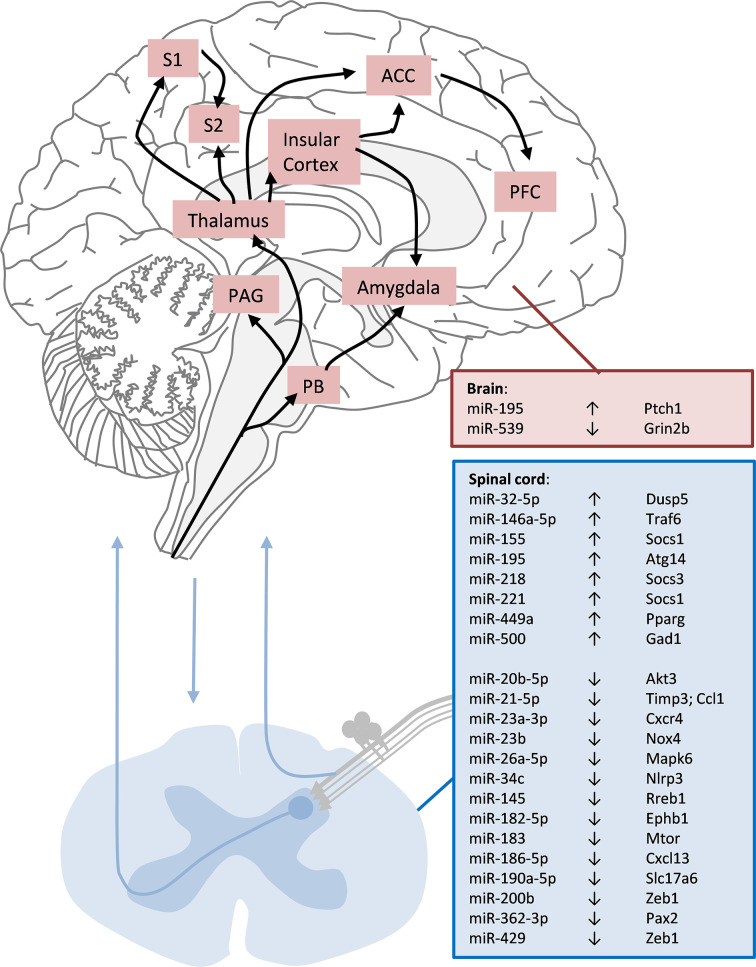
Figure 3. Deregulated miRNA in spinal cord and brain with the respective associated target genes.
Although specific brain areas are involved in different aspects of processing pain perception, miRNA expression so far has not been addressed specifically within these areas under neuropathic pain conditions. Akt3: AKT serine/threonine kinase 3; Atg14: Autophagy related 14; Dusp5: Dual specificity phosphatase; Ccl1: C-C motif chemokine ligand 1; Cxcl13: Chemokine (C-X-C motif) ligand 13; Cxcr4: Chemokine (C-X-C motif) receptor 4; Ephb1: Eph receptor B1; Gad1: Glutamate decarboxylase 1; Grin2b: Glutamate ionotropic receptor NMDA type subunit 2B; Mapk6: Mitogen-activated protein kinase 6; Mtor: Mechanistic target of rapamycin kinase; Nlrp3: NLR family, pyrin domain containing 3; Nox4: NADPH oxidase 4; Pax2: Paired box 2; Pparg: Peroxisome proliferator-activated receptor gamma; Ptch1: Patched 1; Rreb1: Ras responsive element binding protein 1; Slc17a6: Solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter); Socs1: Suppressor of cytokine signalling 1; Socs3: Suppressor of cytokine signalling 3; Timp3: TIMP metallopeptidase inhibitor 3; Traf6: TNF receptor-associated factor 6; Zeb1: Zinc finger E-box binding homeobox 1
Deregulated miRNAs in the spinal cord (Figure 3) can emerge from various sources, such as neurons, microglia, astroglia or even the vasculature. The most intensely investigated sources are microglia that react to maintained nociceptive input to the spinal cord with proliferation and a change in phenotype and activity (microgliosis). In microglia, a number of up-regulated miRNA (miR-155, miR-218 and miR-221) can inhibit the expression of suppressors of cytokine signalling, such as Socs1, and may promote inflammatory signatures, such as microgliosis [77,81,82]. A novel regulator of microglia, Dual specificity phosphatase 5 (Dusp5) is regulated by miR-32-5p, and this is involved in the regulation of neuroinflammatory processes, such as cytokine release in the spinal cord [75].
At the same time several miRNAs, such as miR-200b and miR-429, are down-regulated in microglia after nerve injury, and Zeb1 (Zinc finger E-box binding homeobox 1) has been identified as a target of these two miRNAs [109]. Zeb family members are essential for the developing nociceptors in the DRG [138]. ZEB1 overexpression regulates the microglia response after ischemic stroke and in turn inhibits the production of astrocytic CXCL1 which leads to a decline in neutrophil infiltration, thereby reducing CNS inflammation. This suggests involvement of miR-200b and miR-429 suppression in the resolution of neurological injury [139]. In contrast, miR-21-5p targeting the chemokine Ccl1 [90], miR-23a-3p targeting Cxcr4 [91], miR-34 targeting Nlrp3 (NLR family, pyrin domain containing 3) [97], and miR-186-5p targeting Cxcl13 [107] are down-regulated leading to increased expression of inflammatory mediators and an augmentation of inflammatory processes in the spinal cord. Other down-regulated miRNAs (miR-145, miR-183) at the same time may release the suppression of key components affecting cell morphology and microglia function via transcription factor Rreb1 [102,140] or mTOR [104,141] together with miR-195 up-regulation, which suppresses Autophagy related 14 (Atg14 [80]). These microRNAs appear to orchestrate spinal neuroinflammation related to neuropathic pain. Another negative-feedback regulator of the astrocyte-mediated inflammatory response to injury is miR-146a, which is up-regulated in astrocytes following nerve injury, and targets TNF receptor-associated factor 6 (Traf6) [76,142]. Persistent up-regulation of Traf6 in spinal cord astrocytes in the late phase after nerve injury maintains neuropathic pain by integrating TNF-α and IL-1β signaling and activating the JNK/CCL2 pathway and increased miR-146a expression can set a brake to the neuroinflammatory component maintained by Traf6 [76,143].
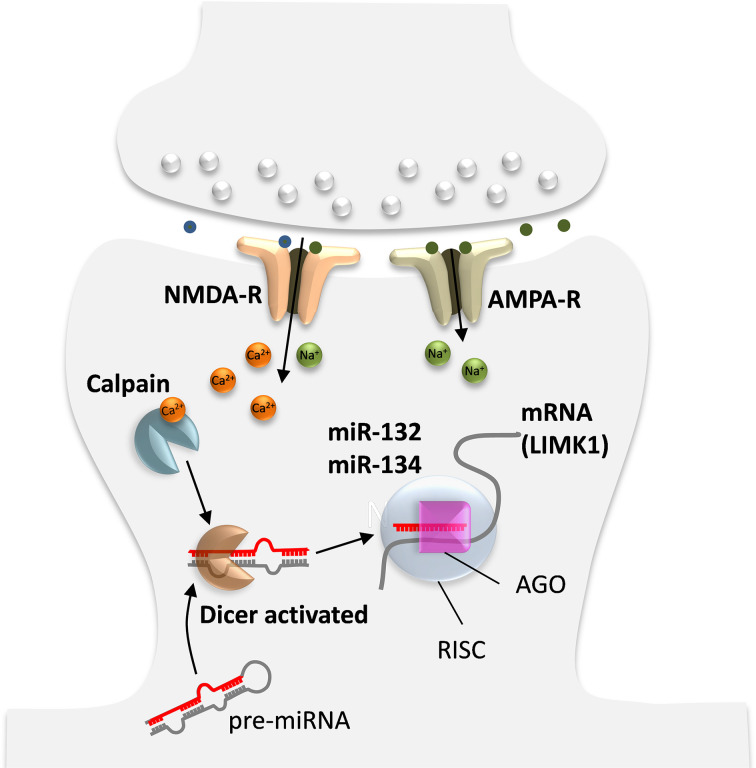
In addition, miRNAs are generated and act within neurons, and there are several possible mechanisms for activity-dependent miRNA regulation. First, upon strong synaptic input, Ca2+ influx through NMDA receptors activates the Ca2+-dependent enzyme calpain that can liberate Dicer from postsynaptic densities and stimulate Dicer RNAse III activity to facilitate processing of pre-miRNAs into mature miRNAs (Figure 4) [144]. Second, increased intracellular Ca2+ can induce de novo miRNA transcription. Third, although less is known about the mechanism of miRNA degradation, another possible mechanism controlling mature miRNA expression may be the activity-dependent degradation of the RISC component MOV10 by the proteasome. However, it is still not sufficiently clear whether MOV10 degradation promotes the disassembly of RISC and thus augments the turnover of miRNAs (for review see [145]).
Figure 4. Activity dependent generation of miRNA in neurons affecting synapse specific protein synthesis.
Adapted from [145].
Of particular interest and importance in the spinal dorsal horn (SDH) are up-regulated miRNAs targeting inhibitory ion channels, such as GABA or K+ channels. In the chronic constriction injury (CCI) model, miR-182-5p is down-regulated and targets Ephb1 [103]. This is of particular interest, since ephrinB–EphB receptor signalling plays a critical role in induction and maintenance of neuropathic pain by regulating neural excitability and synaptic plasticity in the DRG and the SDH [146]. Up-regulation of miR-449a via targeting peroxisome proliferator-activated receptor gamma (Pparg) and reduced Pparg expression can aggravate the increased neuroexcitability and neuroexcitotoxicity associated with neuropathic pain [83,147]. Increased excitatory activity in SDH may be further fueled by the release of glutamate transporter vGluT2 (Slc17a6, Solute carrier family 17 member 6) expression and augmentation by down-regulated miR-190a-5p [108,148–150]. At the same time, glutamate decarboxylase 1 (Gad1) regulates GABA synthesis and transport [151], and reduced expression of Gad1 impairs the function of GABAergic synapses, which appears to be induced by up-regulation of miR-500 [84]. The transcription factor Paired box 2 (Pax2) is necessary for the GABAergic differentiation [151] and a loss of SDH GABAergic interneurons causes reduced GABAergic tone that contributes to neuropathic pain [152]. Therefore, the down-regulation of miR-362-3p targeting Pax2 may rather be a compensatory change to restore GABAergic signaling in the SDH [112]. These processes are further enhanced by the down-regulation of three miRNAs (miR-20b-5p, miR-23 and miR-26a-5p) leading to the increased expression of the kinases Akt3, Nox4 and Mapk6 whose relevance for neuropathic pain is well accepted [89,92,93]. Together, miRNAs are emerging as major controllers of neuro-immune processes in the SDH by switching neurons as well as non-neuronal cells into proalgesic modes of action and promoting the development and maintenance of signatures aggravating neuropathic pain at spinal cord level.
miRNAs deregulated in the brain
miRNAs act at the neuro-immune interface which controls neuronal plasticity and memory but also are linked to the etiology of anxiety and mood disorders [12,13,34]. Deficits in the interaction of immune cells and neurons together with cognitive and emotional alterations in patients with neuropathic or neurogenic pain syndromes are hypothesized to converge on ncRNA deregulated mechanisms along the entire neuraxis, and alterations in ncRNAs expression may account for the variation of susceptibility to certain types of pain or even for the responsiveness to analgesics and development of opioid tolerance [153].
As in the spinal cord, neuronal and non-neuronal cells contribute to potential disease specific miRNA patterns in brain regions that are relevant for the processing of painful stimuli. Brain specific miRNAs are emerging as regulators of cognition, neuronal plasticity and memory, affecting synapse structure and function, and specific miRNAs not only control cognition and emotional processes but also neuro-immune communication in the brain [13,154]. In general, happiness, anxiety and depression seem to depend on miRNA expression levels, and specific miRNAs are deregulated in depression, anxiety, and preclinical models of psychological stress. Moreover psychoactive agents including antidepressants and mood stabilizers utilize miRNAs as downstream effectors [12]. In the brain, AMPA-mediated synaptic transmission is reduced by neuronal over-expression of miR-125b and increased by miR-132 due to differential regulation of glutamate NR2A and NR2B receptor mRNA [155]. Other glutamate receptor subunits in the brain are regulated by dopamine through miR-181a, which has been associated with the pain system [156]. In particular, miR-132 is a highly interesting brain specific miRNA, since it is up-regulated by BDNF and other growth factors in cortical neurons resulting in an increased expression of synaptic glutamate receptors NR2A, NR2B and AMPA GluR1 [157,158]. In the hippocampus, miR-132 targets acetylcholinesterase and this is relevant for stress-induced cognitive deficits. In the amygdala miR-34 is associated with the repression of stress-induced anxiety [159,160]. In neuropathic pain, maladaptive responses of the nucleus accumbens have been associated with deregulated miRNAs [66]. Endogenous pain control systems including GABAergic and opioidergic synaptic signals are down-regulated by miRNAs, such as miR-134 or miR-181a, with some of them linking miRNAs like let-7 or miR-339 to opioid tolerance [161–164]. Despite the increasing number of reports, only few studies validate the deregulated miRNAs acting on specific target genes in the brain in neuropathic pain models (Figure 3). Down-regulation of miR-539 can release the brake on the expression of Grin2b (Glutamate ionotropic receptor NMDA type subunit 2B) and enhance the formation of pain memory in the anterior cingulate cortex [114] and also in the brain stem, up-regulation of miR-195 and its target gene patched 1 (Ptch1) has been reported [79]. Altogether, although relevant roles for miRNA regulated processes in the pathogenesis of neuropathic pain can be anticipated also for relevant brain areas this field of pain research is still in its infancy.
Long ncRNAs
Synthesis and function
Long ncRNAs (lncRNAs) of more than 200 nucleotides are the second most studied group of ncRNAs. Currently 172,216 human and 131,697 mouse transcripts are annotated in the systematic database NONCODEv5 [165], whereas the curated knowledgebase LncBook documents 270,044 human lncRNAs [166]. Depending on their genomic location, lncRNA sequences can be intronic, natural antisense transcripts (NATs), sense, extragenic, enhancer, promoter and bidirectional, and can have a linear or circular structure (circRNAs [167,168]). lncRNA expression patterns highly depend on cell and tissue type as well as on developmental or disease states [169]. In general, lncRNA genes resemble protein coding genes, as their transcription follows similar rules [169,170]. lncRNAs can be capped at their 5′ end, polyadenylated at their 3′ end, alternatively adenylated or not, and can undergo alternative splicing [167,171]. Besides similar transcriptional mechanisms to protein coding genes, lncRNA biogenesis processes can include cleavage by ribonuclease P to form triple helical structures and the formation of circular structures [168]. Their localization in the nucleus, cytoplasm, mitochondria, ribosomes, extracellular membranes and exosomes defines and controls their functions in cellular processes, such as transcriptional, post-transcriptional and epigenetic regulation [169,170,172]. lncRNAs can act as decoys, competing endogenous RNAs (ceRNAs), guides, scaffolds and signals [167,168,173]. In the nucleus, lncRNAs can affect transcription via epigenetic mechanisms and chromatin remodeling as well as by stabilizing mRNA and changing mRNA splicing [173]. As decoys, lncRNAs can bind to proteins, such as transcription factors or RNA-binding proteins, making them unavailable to perform their actions, which can result in transcriptional inhibition and mRNA degradation [167]. Furthermore, lncRNAs can act as competing endogenous RNA (ceRNA) sponging miRNAs and making them unavailable for interaction with their target genes. In contrast to miRNAs, lncRNAs interact with single targets to up- or down-regulate expression levels. Due to the various mechanisms of actions and the high number of lncRNAs, their precise functions and interconnections are not fully unveiled. Their implications in pain conditions have been addressed mostly in animal models, providing a firm indication on the importance of lncRNAs and circRNAs in the establishment and development of pain; however, the precise mechanisms are not fully elucidated.
lncRNAs as possible signatures for pain disorders
lncRNAs are deregulated in diabetic peripheral neuropathy and CRPS [57,137,174–176], and similar to miRNAs, they can be detected in liquid biopsies (Table 4). The most studied lncRNA in DNP is NONRATT021972 which is up-regulated in the serum and correlates with higher neuropathic pain scores, indicating its potential as a biomarker [137,177]. Another up-regulated ncRNA in Type 2 diabetes mellitus and DNP serum is uc.48+ that is involved in purinergic receptor-mediated responses [74,178]. Amongst 1327 deregulated lncRNAs in the PBMCs of female patients with DNP, MALAT1, H19, PVT1 and MIR143HG could potentially qualify as biomarkers [174]. In female CRPS patients, XIST appears as a promising indicator of poor responsiveness to ketamine [57]. XIST acts as a ceRNA on miR-34a, thus enhancing expression levels of the transcription factor YY1 promoting XIST expression in an autoregulatory loop [57]. CircRNAs are much less studied in patients with neuropathic pain and currently there is only one study showing that circHIPK3 expression positively correlates with the severity of neuropathic pain in diabetic patients [176].
Table 4. Deregulated lncRNAs identified in liquid biopsies of patients with pain disorders.
| Disease | Detection method | Sample type | Up-regulation | Down-regulation | Deregulated lncRNA or circRNA | Reference |
|---|---|---|---|---|---|---|
| Diabetic neuropathy | microarray | PBMCs | 256 | 1071 | MALAT1, H19, PVT1, MIR143HG | [174] |
| Diabetic neuropathy | RT-qPCR | Serum | 1 | na | NONRATT021972 | [137] |
| Diabetic neuropathy | RT-qPCR | Serum | 1 | na | NONRATT021972 | [177] |
| Diabetic neuropathy | RT-qPCR | Serum | 1 | na | uc.48+ | [179] |
| Diabetic neuropathy | RT-qPCR | Serum | 1 | na | uc.48+ | [178] |
| Diabetic neuropathy | RT-qPCR | Serum | 1 | na | circHIPK3 | [176] |
| CRPS in female patients | RT-qPCR | Whole blood | 1 | na | XIST | [57] |
lncRNA tissue expression in animal models
lncRNAs and circRNAs are differentially expressed in animal pain models in sciatic nerve, DRG and spinal cord [180–185]. Most of these studies combine lncRNA and mRNA expression analyses including complex bioinformatics to generate networks of potential functional interactions [180,186,187]; however, only few have been explored using a more mechanistic approach (Table 5). Differential expression of lncRNAs upon peripheral nerve injuries is time-dependent and varies between strains and species [182,183,185]. LncRNAs play important roles in neuropathic pain processes not only in neurons, but also in non-neuronal cells, such as Schwann cells, satellite glial cells, macrophages and microglia [74,83,185,188–191].
Table 5. Deregulated lncRNA and target genes related to neuropathic human pain disorders or preclinical models of neuropathic pain. For ethical reasons, bilateral CCI was excluded as a model.
| lncRNA | Regulation | Target | Gene description | Validation | Pain model | Tissue | Species | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Competing endogenous RNA | |||||||||
| TUSC7 | ↓ | miR-449a | microRNA 449a | RIP | SCI | Spinal cord | Rat | lncRNA–miRNA interaction | [83] |
| XIST | ↑ | miR-34a | microRNA 34a | LucA | CRPS; CFA-induced inflammation | Whole blood | Human; mouse | lncRNA–miRNA interaction | [57] |
| circHIPK3 | ↑ | miR-124 | microRNA 124 | LucA/RNA pull down | DNP | Serum; DRG | Human;rat | lncRNA–miRNA interaction | [176] |
| Upregulation of target gene | |||||||||
| BC168687 | ↑ | Trpv1 | Transient receptor potential vanilloid type 1 | siRNA/WB, immunostaining | DPN | DRG | Rat | Not specified | [192] |
| BC168687 | ↑ | P2rx7 | Purinergic receptor P2X 7 | siRNA/RT-qPCR, WB, immunostaining | DNP | Satellite glial cells cultures | Rat | Not specified | [191] |
| BC168687 | ↑ | P2rx7 | Purinergic receptor P2X 7 | siRNA/WB, immunostaining | DPN | DRG | Rat | Not specified | [193] |
| MRAK009713 | ↑ | P2rx3 | Purinergic receptor P2X 3 | siRNA/WB, RIP, immunostaining | CCI | DRG | Rat | lncRNA–protein interaction | [194] |
| NONRATT021972 | ↑ | P2rx3 | Purinergic receptor P2X 3 | siRNA/WB, immunostaining | DPN | DRG | Rat | Not specified | [177] |
| NONRATT021972 | ↑ | P2rx7 | Purinergic receptor P2X 7 | siRNA/WB, immunostaining | DPN | DRG | Rat | lncRNA–mRNA prediction | [195] |
| PKIA-AS1 | ↑ | CDK6 | Cyclin dependent kinase 6 | LucA; siRNA/WB; RIP | SNL | Spinal cord | Rat | Enhancement of promotor activity | [196] |
| uc.48+ | ↑ | P2rx3 | Purinergic receptor P2X 3 | siRNA/WB, immunostaining | DPN | DRG | Rat | Not specified | [178] |
| uc.48+ | ↑ | P2rx7 | Purinergic receptor P2X 7 | siRNA/WB, immunostaining; RIP | CCI-ION | Trigeminal ganglia | Rat | lncRNA–protein interaction | [197] |
| Down-regulation of target gene | |||||||||
| Kcna2-NAT | ↑ | Kcna2 | Potassium voltage-gated channel subfamily A member 2 | LucA; overexpression, WB | SNL, CCI, sciatic nerve axotomy | DRG | Rat | lncRNA–mRNA interaction | [198] |
| Egr2-NAT | ↑ | Egr2 | Early growth response 2 | Overexpression; anti-GapMers; ChIP/WB; RIP/WB | Sciatic nerve injury | DRG: sciatic nerve | Mouse | Chromatin remodelling complex recruitment | [199] |
Abbreviations: CCI, chronic constriction injury; CCI-ION, chronic constriction injury of the infraorbital nerve; CFA, Complete Freund’s adjuvant; CRPS, chronic regional pain syndrome; DPN, diabetic peripheral neuropathy; DRG, dorsal root ganglia; LucA, luciferase assay; RIP, RNA immunoprecipitation; SCI, spinal cord injury; SNL, sciatic nerve ligation; WB, Western blot.
lncRNAs deregulated in the peripheral nerve
Differential expression of lncRNAs and circRNAs in peripheral nerves in neuropathic pain models have not been addressed in detail. Deregulated lncRNAs are reported in the sciatic nerve after a sciatic nerve crush [185]. This model is used to explore nerve regeneration and numerous differentially expressed lncRNAs were associated with inflammatory and immune responses, which could possibly be relevant for neuropathic pain [185]. Particularly, NONMMUG014387 enhances the proliferation of cultured mouse Schwann cells, presumably via cis-upregulation of collagene triple helix repeat containing 1 (Cthrc1) [185], whereas the antisense to the Egr2 promoter ncRNA (Egr2-antisense-RNA) down-regulates Egr2 and promotes demyelination upon peripheral nerve injury by an epigenetic mechanism, in which a chromatin remodeling complex is assembled at the Egr2 promoter [199].
lncRNAs deregulated in the dorsal root and trigeminal ganglia
Differentially expressed lncRNAs and their functions are intensely studied in rodent neuropathic pain models, whereas circRNAs have not been addressed yet [200,201]. lncRNAs down-regulate voltage-gated channels and one of the first lncRNAs found to be implicated in pain is the endogenous voltage-gated potassium channel (Kv) Kcna2-antisense-RNA (NAT), which upon nerve injury is transcriptionally induced by myeloid zinc finger protein 1 (MZF1) and specifically targets and down-regulates Kcna2, resulting in reduced potassium currents and increased DRG excitability [202]. Other up-regulated lncRNAs, such as BC168687 [192,193], MRAK009713 [194], NONRATT021972 [177,178,195] and uc.48+ [178,179], contribute to mechanical hypersensitivity via cell-type specific interactions and induction of different purinergic receptors known for their pain modulating properties. For example, NONRATT021972 and uc.48+ up-regulate the ionotropic purinoreceptor P2X3 in small-medium DRG neurons but P2X7 in satellite glia cells [177–179,195]. BC168687 up-regulates TRPV1 and MRAK009713 promotes the up-regulation of P2X3 receptor in DRG neurons [192,194]. Additionally, uc.48+ inhibition alleviates mechanical hypersensitivity in a rat model for trigeminal neuralgia, by inhibiting the expression of P2X7 receptor in glial cells within the trigeminal ganglia [203]. In addition, uc.48+ appears to exert inhibitory effects on neuroregeneration since inhibition of uc.127 enhances the outgrowth of DRG neurons in vitro [204].
lncRNAs deregulated in the spinal cord and brain
Most of the studies focus on whole spinal cord tissues and therefore do not distinguish between alterations occurring in neuronal and non-neuronal cells [183,184,205–208]. Nevertheless, the identification of deregulated lncRNAs and circRNAs and the computational construction of interaction-networks between lncRNAs/circRNAs–miRNAs–mRNAs provide directions for future studies and potential therapeutic targets. Interestingly, not only SNI-induced mechanical hyperalgesia but also the differential expression of lncRNAs is alleviated by the tetracycline antibiotic minocycline [205]. Few lncRNAs have been functionally investigated at the spinal cord level. PK1A-AS1 overexpression enhances pain behaviors by demethylating the promoter region and promoting the expression of CDK6, an important component in neuroinflammation and neuropathic pain, whereas PK1A-AS1 down-regulation exerts analgesic effects [196]. Of the lncRNAs that are down-regulated after peripheral nerve injury, TUSC7 may be of particular mechanistic relevance, as its up-regulation inhibits the activation of microglia by targeting miR-449a and increasing the expression of the miR-499a target gene PPAR-γ [83]. The only circRNA that has been functionally related to DPN is circHIPK3 which is up-regulated in diabetic rats, acts as a miR-124 sponge and inhibition of circHIPK3 has analgesic and anti-inflammatory effects [176]. In contrast with the emerging role of lncRNAs in the spinal cord, their importance in pain processing brain areas has so far not been mechanistically addressed.
Synopsis
The field of ncRNAs is quickly expanding in the recent years and numerous studies have addressed and associated differentially expressed ncRNAs with neuropathic pain disorders in humans and their corresponding preclinical models. Against initial expectations, common miRNA candidates indicative of neuropathic pain in liquid biopsies have not emerged so far but lncRNAs may offer better perspectives. Whereas the suitability of miRNAs as clinically applicable biomarkers for pain disorders is still disputable, numerous studies have provided novel mechanistic insight into the role of miRNAs in the molecular sequelae involved in the pathogenesis of neuropathic pain along the entire pain pathway. Specific processes within neurons, immune cells, glia as the cellular components of the neuropathic pain triad and the communication paths between them are controlled by specific miRNAs in immune cells, neurons or glia. Therefore, nucleotide sequences mimicking or antagonizing miRNA actions can provide novel therapeutic strategies for pain treatment, provided their human homologues serve the same or at least similar functions. Similar clinical applications can be expected for tools targeting lncRNAs, which converge so far mainly on purinergic signaling pathways both in neurons and glia, and possibly even for other ncRNA species that have not been explored so far.
Abbreviations
- CRPS
complex regional pain syndrome
- DPN
diabetic painful neuropathy
- lncRNA
long non-coding RNA
- miRNA
microRNA
- ncRNA
non-coding RNA
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Austrian Science Fund (FWF) [grant number P30809 (to K.K.)], and DK-SPIN W1206-06, P253450 and the European Commission [grant number 602133] (to M.K.).
References
- 1.Sadosky A., McDermott A.M., Brandenburg N.A. and Strauss M. (2008) A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract.: Off. J. World Inst. Pain 8, 45–56 10.1111/j.1533-2500.2007.00164.x [DOI] [PubMed] [Google Scholar]
- 2.Sommer C. (2003) Painful neuropathies. Curr. Opin. Neurol. 16, 623–628 10.1097/00019052-200310000-00009 [DOI] [PubMed] [Google Scholar]
- 3.Vincent A.M., Callaghan B.C., Smith A.L. and Feldman E.L. (2011) Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat. Rev. Neurol. 7, 573–583 10.1038/nrneurol.2011.137 [DOI] [PubMed] [Google Scholar]
- 4.Pabreja K., Dua K., Sharma S., Padi S.S. and Kulkarni S.K. (2011) Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur. J. Pharmacol. 661, 15–21 10.1016/j.ejphar.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 5.Birch R., Misra P., Stewart M.P., Eardley W.G., Ramasamy A., Brown K. et al. (2012) Nerve injuries sustained during warfare: part I–Epidemiology. J. Bone Joint Sur. Br. Vol. 94, 523–528 10.1302/0301-620X.94B4.28483 [DOI] [PubMed] [Google Scholar]
- 6.Ciaramitaro P., Mondelli M., Logullo F., Grimaldi S., Battiston B., Sard A. et al. (2010) Traumatic peripheral nerve injuries: epidemiological findings, neuropathic pain and quality of life in 158 patients. J. Peripher. Nerv. Syst.: JPNS 15, 120–127 10.1111/j.1529-8027.2010.00260.x [DOI] [PubMed] [Google Scholar]
- 7.Myers R.R., Campana W.M. and Shubayev V.I. (2006) The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov. Today 11, 8–20 10.1016/S1359-6446(05)03637-8 [DOI] [PubMed] [Google Scholar]
- 8.Parkitny L., McAuley J.H., Di Pietro F., Stanton T.R., O'Connell N.E., Marinus J. et al. (2013) Inflammation in complex regional pain syndrome: a systematic review and meta-analysis. Neurology 80, 106–117 10.1212/WNL.0b013e31827b1aa1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marinus J., Moseley G.L., Birklein F., Baron R., Maihofner C., Kingery W.S. et al. (2011) Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 10, 637–648 10.1016/S1474-4422(11)70106-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer S.G., Zuurmond W.W., Birklein F., Loer S.A. and Perez R.S. (2010) Anti-inflammatory treatment of Complex Regional Pain Syndrome. Pain 151, 251–256 10.1016/j.pain.2010.07.020 [DOI] [PubMed] [Google Scholar]
- 11.Uceyler N., Eberle T., Rolke R., Birklein F. and Sommer C. (2007) Differential expression patterns of cytokines in complex regional pain syndrome. Pain 132, 195–205 10.1016/j.pain.2007.07.031 [DOI] [PubMed] [Google Scholar]
- 12.O'Connor R.M., Dinan T.G. and Cryan J.F. (2012) Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol. Psychiatry 17, 359–376 10.1038/mp.2011.162 [DOI] [PubMed] [Google Scholar]
- 13.Soreq H. and Wolf Y. (2011) NeurimmiRs: microRNAs in the neuroimmune interface. Trends Mol. Med. 17, 548–555 10.1016/j.molmed.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 14.Barbash S. and Soreq H. (2012) Threshold-independent meta-analysis of Alzheimer's disease transcriptomes shows progressive changes in hippocampal functions, epigenetics and microRNA regulation. Current Alzheimer Res. 9, 425–435 10.2174/156720512800492512 [DOI] [PubMed] [Google Scholar]
- 15.Baron R., Forster M. and Binder A. (2012) Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol. 11, 999–1005 10.1016/S1474-4422(12)70189-8 [DOI] [PubMed] [Google Scholar]
- 16.Beggs S., Liu X.J., Kwan C. and Salter M.W. (2010) Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood-brain barrier. Mol. Pain 6, 74 10.1186/1744-8069-6-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beggs S., Trang T. and Salter M.W. (2012) P2X4R+ microglia drive neuropathic pain. Nat. Neurosci. 15, 1068–1073 10.1038/nn.3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berta T., Poirot O., Pertin M., Ji R.R., Kellenberger S. and Decosterd I. (2008) Transcriptional and functional profiles of voltage-gated Na(+) channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Mol. Cell. Neurosci. 37, 196–208 10.1016/j.mcn.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 19.Bierhaus A., Fleming T., Stoyanov S., Leffler A., Babes A., Neacsu C. et al. (2012) Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat. Med. 18, 926–933 10.1038/nm.2750 [DOI] [PubMed] [Google Scholar]
- 20.Goebel A. (2011) Complex regional pain syndrome in adults. Rheumatology (Oxford, England) 50, 1739–1750 10.1093/rheumatology/ker202 [DOI] [PubMed] [Google Scholar]
- 21.Mutso A.A., Radzicki D., Baliki M.N., Huang L., Banisadr G., Centeno M.V. et al. (2012) Abnormalities in hippocampal functioning with persistent pain. J. Neurosci. 32, 5747–5756 10.1523/JNEUROSCI.0587-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardoso A.L., Guedes J.R. and de Lima M.C. (2016) Role of microRNAs in the regulation of innate immune cells under neuroinflammatory conditions. Curr. Opin. Pharmacol. 26, 1–9 10.1016/j.coph.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 23.Lee Y., Jeon K., Lee J.T., Kim S. and Kim V.N. (2002) MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 21, 4663–4670 10.1093/emboj/cdf476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han J., Lee Y., Yeom K.H., Kim Y.K., Jin H. and Kim V.N. (2004) The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 18, 3016–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J. et al. (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419 10.1038/nature01957 [DOI] [PubMed] [Google Scholar]
- 26.Melamed Z., Levy A., Ashwal-Fluss R., Lev-Maor G., Mekahel K., Atias N. et al. (2013) Alternative splicing regulates biogenesis of miRNAs located across exon-intron junctions. Mol. Cell 50, 869–881 10.1016/j.molcel.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 27.Alexiou P., Vergoulis T., Gleditzsch M., Prekas G., Dalamagas T., Megraw M. et al. (2010) miRGen 2.0: a database of microRNA genomic information and regulation. Nucleic Acids Res. 38, D137–D141 10.1093/nar/gkp888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeidler M., Hüttenhofer A., Kress M. and Kummer K.K. Intragenic microRNAs autoregulate their host genes in both direct and indirect ways - a cross-species analysis. [DOI] [PMC free article] [PubMed]
- 29.Godnic I., Zorc M., Jevsinek Skok D., Calin G.A., Horvat S., Dovc P. et al. (2013) Genome-wide and species-wide in silico screening for intragenic MicroRNAs in human, mouse and chicken. PLoS ONE 8, e65165 10.1371/journal.pone.0065165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franca G.S., Vibranovski M.D. and Galante P.A. (2016) Host gene constraints and genomic context impact the expression and evolution of human microRNAs. Nat. Commun. 7, 11438 10.1038/ncomms11438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hori N., Narita M., Yamashita A., Horiuchi H., Hamada Y., Kondo T. et al. (2016) Changes in the expression of IL-6-Mediated MicroRNAs in the dorsal root ganglion under neuropathic pain in mice. Synapse (New York, N.Y.). 70, 317–324 10.1002/syn.21902 [DOI] [PubMed] [Google Scholar]
- 32.Ha M. and Kim V.N. (2014) Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell biol. 15, 509–524 10.1038/nrm3838 [DOI] [PubMed] [Google Scholar]
- 33.Treiber T., Treiber N. and Meister G. (2019) Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 20, 5–20 10.1038/s41580-018-0059-1 [DOI] [PubMed] [Google Scholar]
- 34.Brites D. and Fernandes A. (2015) Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front. Cell. Neurosci. 9, 476 10.3389/fncel.2015.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao P., Benito E. and Fischer A. (2013) MicroRNAs as biomarkers for CNS disease. Front. Mol. Neurosci. 6, 39 10.3389/fnmol.2013.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simeoli R., Montague K., Jones H.R., Castaldi L., Chambers D., Kelleher J.H. et al. (2017) Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 8, 1778 10.1038/s41467-017-01841-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajit S.K. (2012) Circulating microRNAs as biomarkers, therapeutic targets, and signaling molecules. Sensors (Basel, Switzerland). 12, 3359–3369 10.3390/s120303359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orlova I.A., Alexander G.M., Qureshi R.A., Sacan A., Graziano A., Barrett J.E. et al. (2011) MicroRNA modulation in complex regional pain syndrome. J. Transl. Med. 9, 195 10.1186/1479-5876-9-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald M.K., Tian Y., Qureshi R.A., Gormley M., Ertel A., Gao R. et al. (2014) Functional significance of macrophage-derived exosomes in inflammation and pain. Pain 155, 1527–1539 10.1016/j.pain.2014.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douglas S.R., Shenoda B.B., Qureshi R.A., Sacan A., Alexander G.M., Perreault M. et al. (2015) Analgesic Response to Intravenous Ketamine Is Linked to a Circulating microRNA Signature in Female Patients With Complex Regional Pain Syndrome. J. Pain: Off. J. Am. Pain Soc. 16, 814–824 10.1016/j.jpain.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 41.Ramanathan S., Douglas S.R., Alexander G.M., Shenoda B.B., Barrett J.E., Aradillas E. et al. (2019) Exosome microRNA signatures in patients with complex regional pain syndrome undergoing plasma exchange. J. Transl. Med. 17, 81 10.1186/s12967-019-1833-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasquier J., Ramachandran V., Abu-Qaoud M.R., Thomas B., Benurwar M.J., Chidiac O. et al. (2018) Differentially expressed circulating microRNAs in the development of acute diabetic Charcot foot. Epigenomics 10, 1267–1278 10.2217/epi-2018-0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng B., Li J.Y., Li X.C., Wang X.F., Wang Z.J., Liu J. et al. (2018) MiR-323b-5p acts as a novel diagnostic biomarker for critical limb ischemia in type 2 diabetic patients. Sci. Rep. 8, 15080 10.1038/s41598-018-33310-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J.Y., Cheng B., Wang X.F., Wang Z.J., Zhang H.M., Liu S.F. et al. (2018) Circulating MicroRNA-4739 May Be a Potential Biomarker of Critical Limb Ischemia in Patients with Diabetes. Biomed. Res. Int. 2018, 4232794 10.1155/2018/4232794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos-Bezerra D.P., Santos A.S., Guimaraes G.C., Admoni S.N., Perez R.V., Machado C.G. et al. (2019) Micro-RNAs 518d-3p and 618 Are Upregulated in Individuals With Type 1 Diabetes With Multiple Microvascular Complications. Front. Endocrinol. 10, 385 10.3389/fendo.2019.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alles J., Fehlmann T., Fischer U., Backes C., Galata V., Minet M. et al. (2019) An estimate of the total number of true human miRNAs. Nucleic Acids Res. 47, 3353–3364 10.1093/nar/gkz097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Git A., Dvinge H., Salmon-Divon M., Osborne M., Kutter C., Hadfield J. et al. (2010) Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA (New York, N.Y.). 16, 991–1006 10.1261/rna.1947110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hitzemann R., Bottomly D., Darakjian P., Walter N., Iancu O., Searles R. et al. (2013) Genes, behavior and next-generation RNA sequencing. Genes Brain Behavior 12, 1–12 10.1111/gbb.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalpachidou T., Kummer K.K., Mitric M. and Kress M. (2019) Tissue Specific Reference Genes for MicroRNA Expression Analysis in a Mouse Model of Peripheral Nerve Injury. Front. Mol. Neurosci. 12, 283 10.3389/fnmol.2019.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herskovits A.Z. and Guarente L. (2014) SIRT1 in neurodevelopment and brain senescence. Neuron 81, 471–483 10.1016/j.neuron.2014.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heyn J., Luchting B., Hinske L.C., Hubner M., Azad S.C. and Kreth S. (2016) miR-124a and miR-155 enhance differentiation of regulatory T cells in patients with neuropathic pain. J. Neuroinflammation 13, 248 10.1186/s12974-016-0712-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDonald M.K., Ramanathan S., Touati A., Zhou Y., Thanawala R.U., Alexander G.M. et al. (2016) Regulation of proinflammatory genes by the circulating microRNA hsa-miR-939. Sci. Rep. 6, 30976 10.1038/srep30976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shenoda B.B., Alexander G.M. and Ajit S.K. (2016) Hsa-miR-34a mediated repression of corticotrophin releasing hormone receptor 1 regulates pro-opiomelanocortin expression in patients with complex regional pain syndrome. J. Transl. Med. 14, 64 10.1186/s12967-016-0820-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leinders M., Uceyler N., Pritchard R.A., Sommer C. and Sorkin L.S. (2016) Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp. Neurol. 283, 276–286 10.1016/j.expneurol.2016.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y.B., Wu Q., Liu J., Fan Y.Z., Yu K.F. and Cai Y. (2017) miR199a3p is involved in the pathogenesis and progression of diabetic neuropathy through downregulation of SerpinE2. Mol. Med. Rep. 16, 2417–2424 10.3892/mmr.2017.6874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asahchop E.L., Branton W.G., Krishnan A., Chen P.A., Yang D., Kong L. et al. (2018) HIV-associated sensory polyneuropathy and neuronal injury are associated with miRNA-455-3p induction. JCI Insight. 3, e122450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shenoda B.B., Tian Y., Alexander G.M., Aradillas-Lopez E., Schwartzman R.J. and Ajit S.K. (2018) miR-34a-mediated regulation of XIST in female cells under inflammation. J. Pain Res. 11, 935–945 10.2147/JPR.S159458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J.C., Xue D.F., Wang X.Q., Ai D.B. and Qin P.J. (2019) MiR-101 relates to chronic peripheral neuropathic pain through targeting KPNB1 and regulating NF-kappaB signaling. Kaohsiung J. Med. Sci. 35, 139–145 10.1002/kjm2.12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aldrich B.T., Frakes E.P., Kasuya J., Hammond D.L. and Kitamoto T. (2009) Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience 164, 711–723 10.1016/j.neuroscience.2009.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bai G., Ambalavanar R., Wei D. and Dessem D. (2007) Downregulation of selective microRNAs in trigeminal ganglion neurons following inflammatory muscle pain. Mol. Pain 3, 15 10.1186/1744-8069-3-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bali K.K., Hackenberg M., Lubin A., Kuner R. and Devor M. (2014) Sources of individual variability: miRNAs that predispose to neuropathic pain identified using genome-wide sequencing. Mol. Pain 10, 22 10.1186/1744-8069-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kusuda R., Cadetti F., Ravanelli M.I., Sousa T.A., Zanon S., De Lucca F.L. et al. (2011) Differential expression of microRNAs in mouse pain models. Mol. Pain 7, 17 10.1186/1744-8069-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norcini M., Sideris A., Martin Hernandez L.A., Zhang J., Blanck T.J. and Recio-Pinto E. (2014) An approach to identify microRNAs involved in neuropathic pain following a peripheral nerve injury. Front. Neurosci. 8, 266 10.3389/fnins.2014.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.von Schack D., Agostino M.J., Murray B.S., Li Y., Reddy P.S., Chen J. et al. (2011) Dynamic changes in the microRNA expression profile reveal multiple regulatory mechanisms in the spinal nerve ligation model of neuropathic pain. PLoS ONE 6, e17670 10.1371/journal.pone.0017670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poh K.W., Yeo J.F. and Ong W.Y. (2011) MicroRNA changes in the mouse prefrontal cortex after inflammatory pain. Eur. J. Pain 15, 801.e801–812.e801 [DOI] [PubMed] [Google Scholar]
- 66.Imai S., Saeki M., Yanase M., Horiuchi H., Abe M., Narita M. et al. (2011) Change in microRNAs associated with neuronal adaptive responses in the nucleus accumbens under neuropathic pain. J. Neurosci. 31, 15294–15299 10.1523/JNEUROSCI.0921-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao S., Yuan J., Zhang D., Wen S., Wang J., Li Y. et al. (2019) Transcriptome Changes In Dorsal Spinal Cord Of Rats With Neuropathic Pain. J. Pain Res. 12, 3013–3023 10.2147/JPR.S219084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malcangio M. (2019) Role of the immune system in neuropathic pain. Scand. J. Pain20, 33-37 10.1515/sjpain-2019-0138 [DOI] [PubMed] [Google Scholar]
- 69.Dai D., Wang J., Jiang Y., Yuan L., Lu Y., Zhang A. et al. (2019) Small RNA sequencing reveals microRNAs related to neuropathic pain in rats. Brazi. J. Med. Biol. Res. = Revista Brasileira de Pesquisas Medicas e Biologicas 52, e8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li H., Wan H.Q., Zhao H.J., Luan S.X. and Zhang C.G. (2019) Identification of candidate genes and miRNAs associated with neuropathic pain induced by spared nerve injury. Int. J. Mol. Med. 44, 1205–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sommer C., Leinders M. and Uceyler N. (2018) Inflammation in the pathophysiology of neuropathic pain. Pain 159, 595–602 10.1097/j.pain.0000000000001122 [DOI] [PubMed] [Google Scholar]
- 72.Ito N., Sakai A., Miyake N., Maruyama M., Iwasaki H., Miyake K. et al. (2017) miR-15b mediates oxaliplatin-induced chronic neuropathic pain through BACE1 down-regulation. Br. J. Pharmacol. 174, 386–395 10.1111/bph.13698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakai A., Saitow F., Maruyama M., Miyake N., Miyake K., Shimada T. et al. (2017) MicroRNA cluster miR-17-92 regulates multiple functionally related voltage-gated potassium channels in chronic neuropathic pain. Nat. Commun. 8, 16079 10.1038/ncomms16079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu H., Wen F., Jiang M., Liu Q. and Nie Y. (2018) LncRNA uc.48+ is involved in the diabetic immune and inflammatory responses mediated by P2X7 receptor in RAW264.7 macrophages. Int. J. Mol. Med. 42, 1152–1160 [DOI] [PubMed] [Google Scholar]
- 75.Yan T., Zhang F., Sun C., Sun J., Wang Y., Xu X. et al. (2018) miR-32-5p-mediated Dusp5 downregulation contributes to neuropathic pain. Biochem. Biophys. Res. Commun. 495, 506–511 10.1016/j.bbrc.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 76.Lu Y., Cao D.L., Jiang B.C., Yang T. and Gao Y.J. (2015) MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord. Brain Behav. Immun. 49, 119–129 10.1016/j.bbi.2015.04.018 [DOI] [PubMed] [Google Scholar]
- 77.Tan Y., Yang J., Xiang K., Tan Q. and Guo Q. (2015) Suppression of microRNA-155 attenuates neuropathic pain by regulating SOCS1 signalling pathway. Neurochem. Res. 40, 550–560 10.1007/s11064-014-1500-2 [DOI] [PubMed] [Google Scholar]
- 78.Yang S., Krug S.M., Heitmann J., Hu L., Reinhold A.K., Sauer S. et al. (2016) Analgesic drug delivery via recombinant tissue plasminogen activator and microRNA-183-triggered opening of the blood-nerve barrier. Biomaterials 82, 20–33 10.1016/j.biomaterials.2015.11.053 [DOI] [PubMed] [Google Scholar]
- 79.Wang X., Wang H., Zhang T., He M., Liang H., Wang H. et al. (2019) Inhibition of MicroRNA-195 Alleviates Neuropathic Pain by Targeting Patched1 and Inhibiting SHH Signaling Pathway Activation. Neurochem. Res. 44, 1690–1702 10.1007/s11064-019-02797-2 [DOI] [PubMed] [Google Scholar]
- 80.Shi G., Shi J., Liu K., Liu N., Wang Y., Fu Z. et al. (2013) Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia 61, 504–512 10.1002/glia.22451 [DOI] [PubMed] [Google Scholar]
- 81.Li L. and Zhao G. (2016) Downregulation of microRNA-218 relieves neuropathic pain by regulating suppressor of cytokine signaling 3. Int. J. Mol. Med. 37, 851–858 10.3892/ijmm.2016.2455 [DOI] [PubMed] [Google Scholar]
- 82.Xia L., Zhang Y. and Dong T. (2016) Inhibition of MicroRNA-221 Alleviates Neuropathic Pain Through Targeting Suppressor of Cytokine Signaling 1. J. Mol. Neurosci. 59, 411–420 10.1007/s12031-016-0748-1 [DOI] [PubMed] [Google Scholar]
- 83.Yu Y., Zhu M., Zhao Y., Xu M. and Qiu M. (2018) Overexpression of TUSC7 inhibits the inflammation caused by microglia activation via regulating miR-449a/PPAR-gamma. Biochem. Biophys. Res. Commun. 503, 1020–1026 10.1016/j.bbrc.2018.06.111 [DOI] [PubMed] [Google Scholar]
- 84.Huang Z.Z., Wei J.Y., Ou-Yang H.D., Li D., Xu T., Wu S.L. et al. (2016) mir-500-Mediated GAD67 Downregulation Contributes to Neuropathic Pain. J. Neurosci. 36, 6321–6331 10.1523/JNEUROSCI.0646-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sakai A., Saitow F., Miyake N., Miyake K., Shimada T. and Suzuki H. (2013) miR-7a alleviates the maintenance of neuropathic pain through regulation of neuronal excitability. Brain 136, 2738–2750 10.1093/brain/awt191 [DOI] [PubMed] [Google Scholar]
- 86.Yang F.R., Chen J., Yi H., Peng L.Y., Hu X.L. and Guo Q.L. (2019) MicroRNA-7a ameliorates neuropathic pain in a rat model of spinal nerve ligation via the neurofilament light polypeptide-dependent signal transducer and activator of transcription signaling pathway. Mol. Pain 15, 1744806919842464 10.1177/1744806919842464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang J., Hu Y., Zhang B., Shi Y., Zhang J., Wu X. et al. (2017) MicroRNA-9 regulates mammalian axon regeneration in peripheral nerve injury. Mol. Pain 13, 1744806917711612 10.1177/1744806917711612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manners M.T., Tian Y., Zhou Z. and Ajit S.K. (2015) MicroRNAs downregulated in neuropathic pain regulate MeCP2 and BDNF related to pain sensitivity. FEBS Open Bio. 5, 733–740 10.1016/j.fob.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.You H., Zhang L., Chen Z., Liu W., Wang H. and He H. (2019) MiR-20b-5p relieves neuropathic pain by targeting Akt3 in a chronic constriction injury rat model. Synapse (New York, N.Y.) 73, e22125 10.1002/syn.22125 [DOI] [PubMed] [Google Scholar]
- 90.Zhong L., Xiao W., Wang F., Liu J. and Zhi L.J. (2019) miR-21-5p inhibits neuropathic pain development via directly targeting C-C motif ligand 1 and tissue inhibitor of metalloproteinase-3. J. Cell. Biochem. 120, 16614–16623 10.1002/jcb.28920 [DOI] [PubMed] [Google Scholar]
- 91.Pan Z., Shan Q., Gu P., Wang X.M., Tai L.W., Sun M. et al. (2018) miRNA-23a/CXCR4 regulates neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome axis. J. Neuroinflammation 15, 29 10.1186/s12974-018-1073-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Im Y.B., Jee M.K., Choi J.I., Cho H.T., Kwon O.H. and Kang S.K. (2012) Molecular targeting of NOX4 for neuropathic pain after traumatic injury of the spinal cord. Cell Death Dis. 3, e426 10.1038/cddis.2012.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y., Su Z., Liu H.L., Li L., Wei M., Ge D.J. et al. (2018) Effects of miR-26a-5p on neuropathic pain development by targeting MAPK6 in in CCI rat models. Biomed. Pharmacother. 107, 644–649 10.1016/j.biopha.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 94.Shao J., Cao J., Wang J., Ren X., Su S., Li M. et al. (2016) MicroRNA-30b regulates expression of the sodium channel Nav1.7 in nerve injury-induced neuropathic pain in the rat. Mol. Pain 12, 1-13 10.1177/1744806916671523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su S., Shao J., Zhao Q., Ren X., Cai W., Li L. et al. (2017) MiR-30b Attenuates Neuropathic Pain by Regulating Voltage-Gated Sodium Channel Nav1.3 in Rats. Front. Mol. Neurosci. 10, 126 10.3389/fnmol.2017.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brandenburger T., Johannsen L., Prassek V., Kuebart A., Raile J., Wohlfromm S. et al. (2019) MiR-34a is differentially expressed in dorsal root ganglia in a rat model of chronic neuropathic pain. Neurosci. Lett. 708, 134365 10.1016/j.neulet.2019.134365 [DOI] [PubMed] [Google Scholar]
- 97.Xu L., Wang Q., Jiang W., Yu S. and Zhang S. (2019) MiR-34c Ameliorates Neuropathic Pain by Targeting NLRP3 in a Mouse Model of Chronic Constriction Injury. Neuroscience 399, 125–134 10.1016/j.neuroscience.2018.12.030 [DOI] [PubMed] [Google Scholar]
- 98.Chen H.P., Zhou W., Kang L.M., Yan H., Zhang L., Xu B.H. et al. (2014) Intrathecal miR-96 inhibits Nav1.3 expression and alleviates neuropathic pain in rat following chronic construction injury. Neurochem. Res. 39, 76–83 10.1007/s11064-013-1192-z [DOI] [PubMed] [Google Scholar]
- 99.Zhang J., Zhang H. and Zi T. (2015) Overexpression of microRNA-141 relieves chronic constriction injury-induced neuropathic pain via targeting high-mobility group box 1. Int. J. Mol. Med. 36, 1433–1439 10.3892/ijmm.2015.2342 [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y., Mou J., Cao L., Zhen S., Huang H. and Bao H. (2018) MicroRNA-142-3p relieves neuropathic pain by targeting high mobility group box 1. Int. J. Mol. Med. 41, 501–510 [DOI] [PubMed] [Google Scholar]
- 101.Shi J., Jiang K. and Li Z. (2018) MiR-145 ameliorates neuropathic pain via inhibiting inflammatory responses and mTOR signaling pathway by targeting Akt3 in a rat model. Neurosci. Res. 134, 10–17 10.1016/j.neures.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 102.Pang X., Tang Y. and Zhang D. (2016) Role of miR-145 in chronic constriction injury in rats. Exp. Ther. Med. 12, 4121–4127 10.3892/etm.2016.3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou X., Zhang C., Zhang C., Peng Y., Wang Y. and Xu H. (2017) MicroRNA-182-5p Regulates Nerve Injury-induced Nociceptive Hypersensitivity by Targeting Ephrin Type-b Receptor 1. Anesthesiology 126, 967–977 10.1097/ALN.0000000000001588 [DOI] [PubMed] [Google Scholar]
- 104.Xie X., Ma L., Xi K., Zhang W. and Fan D. (2017) MicroRNA-183 Suppresses Neuropathic Pain and Expression of AMPA Receptors by Targeting mTOR/VEGF Signaling Pathway. Cell. Physiol. Biochem. 41, 181–192 10.1159/000455987 [DOI] [PubMed] [Google Scholar]
- 105.Lin C.R., Chen K.H., Yang C.H., Huang H.W. and Sheen-Chen S.M. (2014) Intrathecal miR-183 delivery suppresses mechanical allodynia in mononeuropathic rats. Eur. J. Neurosci. 39, 1682–1689 10.1111/ejn.12522 [DOI] [PubMed] [Google Scholar]
- 106.Shi D.N., Yuan Y.T., Ye D., Kang L.M., Wen J. and Chen H.P. (2018) MiR-183-5p Alleviates Chronic Constriction Injury-Induced Neuropathic Pain Through Inhibition of TREK-1. Neurochem. Res. 43, 1143–1149 10.1007/s11064-018-2529-4 [DOI] [PubMed] [Google Scholar]
- 107.Jiang B.C., Cao D.L., Zhang X., Zhang Z.J., He L.N., Li C.H. et al. (2016) CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J. Clin. Invest. 126, 745–761 10.1172/JCI81950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang D., Yang Q., Wei X., Liu Y., Ma D., Li J. et al. (2017) The role of miR-190a-5p contributes to diabetic neuropathic pain via targeting SLC17A6. J. Pain Res. 10, 2395–2403 10.2147/JPR.S133755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yan X.T., Zhao Y., Cheng X.L., He X.H., Wang Y., Zheng W.Z. et al. (2018) Inhibition of miR-200b/miR-429 contributes to neuropathic pain development through targeting zinc finger E box binding protein-1. J. Cell. Physiol. 233, 4815–4824 10.1002/jcp.26284 [DOI] [PubMed] [Google Scholar]
- 110.Sun W., Zhang L. and Li R. (2017) Overexpression of miR-206 ameliorates chronic constriction injury-induced neuropathic pain in rats via the MEK/ERK pathway by targeting brain-derived neurotrophic factor. Neurosci. Lett. 646, 68–74 10.1016/j.neulet.2016.12.047 [DOI] [PubMed] [Google Scholar]
- 111.Wen J., He T., Qi F. and Chen H. (2019) MiR-206-3p alleviates chronic constriction injury-induced neuropathic pain through targeting HDAC4. Exp. Anim. 68, 213–220 10.1538/expanim.18-0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu Y., Liu Q., Zhang M., Yan Y., Yu H. and Ge L. (2019) MicroRNA-362-3p attenuates motor deficit following spinal cord injury via targeting paired box gene 2. J. Integr. Neurosci. 18, 57–64 [DOI] [PubMed] [Google Scholar]
- 113.Lu S., Ma S., Wang Y., Huang T., Zhu Z. and Zhao G. (2017) Mus musculus-microRNA-449a ameliorates neuropathic pain by decreasing the level of KCNMA1 and TRPA1, and increasing the level of TPTE. Mol. Med. Rep. 16, 353–360 10.3892/mmr.2017.6559 [DOI] [PubMed] [Google Scholar]
- 114.Ding M., Shen W. and Hu Y. (2017) The Role of miR-539 in the Anterior Cingulate Cortex in Chronic Neuropathic Pain. Pain Med. 18, 2433–2442 10.1093/pm/pnx004 [DOI] [PubMed] [Google Scholar]
- 115.Goldie B.J. and Cairns M.J. (2012) Post-transcriptional trafficking and regulation of neuronal gene expression. Mol. Neurobiol. 45, 99–108 10.1007/s12035-011-8222-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K. et al. (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 10.1038/nature11993 [DOI] [PubMed] [Google Scholar]
- 117.Park C.K., Xu Z.Z., Berta T., Han Q., Chen G., Liu X.J. et al. (2014) Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 82, 47–54 10.1016/j.neuron.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jeong H., Na Y.J., Lee K., Kim Y.H., Lee Y., Kang M. et al. (2016) High-resolution transcriptome analysis reveals neuropathic pain gene-expression signatures in spinal microglia after nerve injury. Pain 157, 964–976 10.1097/j.pain.0000000000000470 [DOI] [PubMed] [Google Scholar]
- 119.Reinhold A.K., Schwabe J., Lux T.J., Salvador E. and Rittner H.L. (2018) Quantitative and Microstructural Changes of the Blood-Nerve Barrier in Peripheral Neuropathy. Front. Neurosci. 12, 936 10.3389/fnins.2018.00936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hervera A., De Virgiliis F., Palmisano I., Zhou L., Tantardini E., Kong G. et al. (2018) Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 20, 307–319 10.1038/s41556-018-0039-x [DOI] [PubMed] [Google Scholar]
- 121.Zhao J., Lee M.C., Momin A., Cendan C.M., Shepherd S.T., Baker M.D. et al. (2010) Small RNAs control sodium channel expression, nociceptor excitability, and pain thresholds. J. Neurosci. 30, 10860–10871 10.1523/JNEUROSCI.1980-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li X., Gibson G., Kim J.S., Kroin J., Xu S., van Wijnen A.J. et al. (2011) MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis. Gene 480, 34–41 10.1016/j.gene.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Favereaux A., Thoumine O., Bouali-Benazzouz R., Roques V., Papon M.A., Salam S.A. et al. (2011) Bidirectional integrative regulation of Cav1.2 calcium channel by microRNA miR-103: role in pain. EMBO J. 30, 3830–3841 10.1038/emboj.2011.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Z.J., Guo J.S., Li S.S., Wu X.B., Cao D.L., Jiang B.C. et al. (2018) TLR8 and its endogenous ligand miR-21 contribute to neuropathic pain in murine DRG. J. Exp. Med. 215, 3019–3037 10.1084/jem.20180800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Langeslag M., Malsch P., Welling A. and Kress M. (2014) Reduced excitability of gp130-deficient nociceptors is associated with increased voltage-gated potassium currents and Kcna4 channel upregulation. Pflugers Arch. 466, 2153–2165 10.1007/s00424-014-1443-0 [DOI] [PubMed] [Google Scholar]
- 126.Namer B., Orstavik K., Schmidt R., Mair N., Kleggetveit I.P., Zeidler M. et al. (2017) Changes in Ionic Conductance Signature of Nociceptive Neurons Underlying Fabry Disease Phenotype. Front. Neurol. 8, 335 10.3389/fneur.2017.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cai L., Liu X., Guo Q., Huang Q., Zhang Q. and Cao Z. (2019) MiR-15a attenuates peripheral nerve injury-induced neuropathic pain by targeting AKT3 to regulate autophagy. Genes Genomics 42, 77–85 [DOI] [PubMed] [Google Scholar]
- 128.Merighi A., Salio C., Ghirri A., Lossi L., Ferrini F., Betelli C. et al. (2008) BDNF as a pain modulator. Prog. Neurobiol. 85, 297–317 10.1016/j.pneurobio.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 129.Thompson S.W., Bennett D.L., Kerr B.J., Bradbury E.J. and McMahon S.B. (1999) Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc. Natl. Acad. Sci. U.S.A. 96, 7714–7718 10.1073/pnas.96.14.7714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McMahon S.B. and Priestley J.V. (1995) Peripheral neuropathies and neurotrophic factors: animal models and clinical perspectives. Curr. Opin. Neurobiol. 5, 616–624 10.1016/0959-4388(95)80067-0 [DOI] [PubMed] [Google Scholar]
- 131.Geranton S.M., Morenilla-Palao C. and Hunt S.P. (2007) A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J. Neurosci. 27, 6163–6173 10.1523/JNEUROSCI.1306-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin Y.T., Ro L.S., Wang H.L. and Chen J.C. (2011) Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: an in vivo and in vitro study. J. Neuroinflammation 8, 126 10.1186/1742-2094-8-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Crow M., Khovanov N., Kelleher J.H., Sharma S., Grant A.D., Bogdanov Y. et al. (2015) HDAC4 is required for inflammation-associated thermal hypersensitivity. FASEB J. 29, 3370–3378 10.1096/fj.14-264440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bai G., Wei D., Zou S., Ren K. and Dubner R. (2010) Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol. Pain 6, 51 10.1186/1744-8069-6-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin T.B., Hsieh M.C., Lai C.Y., Cheng J.K., Chau Y.P., Ruan T. et al. (2015) Modulation of Nerve Injury-induced HDAC4 Cytoplasmic Retention Contributes to Neuropathic Pain in Rats. Anesthesiology 123, 199–212 10.1097/ALN.0000000000000663 [DOI] [PubMed] [Google Scholar]
- 136.Willemen H.L., Huo X.J., Mao-Ying Q.L., Zijlstra J., Heijnen C.J. and Kavelaars A. (2012) MicroRNA-124 as a novel treatment for persistent hyperalgesia. J. Neuroinflammation 9, 143 10.1186/1742-2094-9-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu W., Zhao G.Q., Cao R.J., Zhu Z.H. and Li K. (2017) LncRNA NONRATT021972 Was Associated with Neuropathic Pain Scoring in Patients with Type 2 Diabetes. Behav. Neurol. 2017, 2941297 10.1155/2017/2941297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ohayon D., Venteo S., Sonrier C., Lafon P.A., Garces A., Valmier J. et al. (2015) Zeb family members and boundary cap cells underlie developmental plasticity of sensory nociceptive neurons. Dev. Cell 33, 343–350 10.1016/j.devcel.2015.03.021 [DOI] [PubMed] [Google Scholar]
- 139.Li D., Lang W., Zhou C., Wu C., Zhang F., Liu Q. et al. (2018) Upregulation of Microglial ZEB1 Ameliorates Brain Damage after Acute Ischemic Stroke. Cell Rep. 22, 3574–3586 10.1016/j.celrep.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 140.Farley J.E., Burdett T.C., Barria R., Neukomm L.J., Kenna K.P., Landers J.E. et al. (2018) Transcription factor Pebbled/RREB1 regulates injury-induced axon degeneration. Proc. Natl. Acad. Sci. U.S.A. 115, 1358–1363 10.1073/pnas.1715837115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hu Y., Mai W., Chen L., Cao K., Zhang B., Zhang Z. et al. (2019) mTOR-mediated metabolic reprogramming shapes distinct microglia functions in response to lipopolysaccharide and ATP. Glia 68, 1031–1045 [DOI] [PubMed] [Google Scholar]
- 142.Iyer A., Zurolo E., Prabowo A., Fluiter K., Spliet W.G., van Rijen P.C. et al. (2012) MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PLoS ONE 7, e44789 10.1371/journal.pone.0044789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu Y., Jiang B.C., Cao D.L., Zhang Z.J., Zhang X., Ji R.R. et al. (2014) TRAF6 upregulation in spinal astrocytes maintains neuropathic pain by integrating TNF-alpha and IL-1beta signaling. Pain 155, 2618–2629 10.1016/j.pain.2014.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lugli G., Larson J., Martone M.E., Jones Y. and Smalheiser N.R. (2005) Dicer and eIF2c are enriched at postsynaptic densities in adult mouse brain and are modified by neuronal activity in a calpain-dependent manner. J. Neurochem. 94, 896–905 10.1111/j.1471-4159.2005.03224.x [DOI] [PubMed] [Google Scholar]
- 145.Sim S.E., Bakes J. and Kaang B.K. (2014) Neuronal activity-dependent regulation of MicroRNAs. Mol. Cells 37, 511–517 10.14348/molcells.2014.0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Song X.J., Zheng J.H., Cao J.L., Liu W.T., Song X.S. and Huang Z.J. (2008) EphrinB-EphB receptor signaling contributes to neuropathic pain by regulating neural excitability and spinal synaptic plasticity in rats. Pain 139, 168–180 10.1016/j.pain.2008.03.019 [DOI] [PubMed] [Google Scholar]
- 147.Hung T.Y., Chu F.L., Wu D.C., Wu S.N. and Huang C.W. (2019) The Protective Role of Peroxisome Proliferator-Activated Receptor-Gamma in Seizure and Neuronal Excitotoxicity. Mol. Neurobiol. 56, 5497–5506 10.1007/s12035-018-1457-2 [DOI] [PubMed] [Google Scholar]
- 148.Wang L., Chen S.R., Ma H., Chen H., Hittelman W.N. and Pan H.L. (2018) Regulating nociceptive transmission by VGluT2-expressing spinal dorsal horn neurons. J. Neurochem. 147, 526–540 10.1111/jnc.14588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scherrer G., Low S.A., Wang X., Zhang J., Yamanaka H., Urban R. et al. (2010) VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc. Natl. Acad. Sci. U.S.A. 107, 22296–22301 10.1073/pnas.1013413108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Y., Abdel Samad O., Zhang L., Duan B., Tong Q., Lopes C. et al. (2010) VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 68, 543–556 10.1016/j.neuron.2010.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cheng L., Arata A., Mizuguchi R., Qian Y., Karunaratne A., Gray P.A. et al. (2004) Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat. Neurosci. 7, 510–517 10.1038/nn1221 [DOI] [PubMed] [Google Scholar]
- 152.Meisner J.G., Marsh A.D. and Marsh D.R. (2010) Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J. Neurotrauma 27, 729–737 10.1089/neu.2009.1166 [DOI] [PubMed] [Google Scholar]
- 153.Parsons M.J., Grimm C.H., Paya-Cano J.L., Sugden K., Nietfeld W., Lehrach H. et al. (2008) Using hippocampal microRNA expression differences between mouse inbred strains to characterise miRNA function. Mammalian Genome: Off. J. Int. Mammalian Genome Soc. 19, 552–560 10.1007/s00335-008-9116-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bredy T.W., Lin Q., Wei W., Baker-Andresen D. and Mattick J.S. (2011) MicroRNA regulation of neural plasticity and memory. Neurobiol. Learn. Mem. 96, 89–94 10.1016/j.nlm.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 155.Edbauer D., Neilson J.R., Foster K.A., Wang C.F., Seeburg D.P., Batterton M.N. et al. (2010) Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65, 373–384 10.1016/j.neuron.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saba R., Storchel P.H., Aksoy-Aksel A., Kepura F., Lippi G., Plant T.D. et al. (2012) Dopamine-regulated microRNA MiR-181a controls GluA2 surface expression in hippocampal neurons. Mol. Cell. Biol. 32, 619–632 10.1128/MCB.05896-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Numakawa T., Yamamoto N., Chiba S., Richards M., Ooshima Y., Kishi S. et al. (2011) Growth factors stimulate expression of neuronal and glial miR-132. Neurosci. Lett. 505, 242–247 10.1016/j.neulet.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 158.Kawashima H., Numakawa T., Kumamaru E., Adachi N., Mizuno H., Ninomiya M. et al. (2010) Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience 165, 1301–1311 10.1016/j.neuroscience.2009.11.057 [DOI] [PubMed] [Google Scholar]
- 159.Haramati S., Navon I., Issler O., Ezra-Nevo G., Gil S., Zwang R. et al. (2011) MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J. Neurosci. 31, 14191–14203 10.1523/JNEUROSCI.1673-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shaltiel G., Hanan M., Wolf Y., Barbash S., Kovalev E., Shoham S. et al. (2013) Hippocampal microRNA-132 mediates stress-inducible cognitive deficits through its acetylcholinesterase target. Brain Struct. Funct. 218, 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ni J., Gao Y., Gong S., Guo S., Hisamitsu T. and Jiang X. (2013) Regulation of mu-opioid type 1 receptors by microRNA134 in dorsal root ganglion neurons following peripheral inflammation. Eur. J. Pain (London, England) 17, 313–323 10.1002/j.1532-2149.2012.00197.x [DOI] [PubMed] [Google Scholar]
- 162.Sengupta J.N., Pochiraju S., Kannampalli P., Bruckert M., Addya S., Yadav P. et al. (2013) MicroRNA-mediated GABA Aalpha-1 receptor subunit down-regulation in adult spinal cord following neonatal cystitis-induced chronic visceral pain in rats. Pain 154, 59–70 10.1016/j.pain.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.He Y., Yang C., Kirkmire C.M. and Wang Z.J. (2010) Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J. Neurosci. 30, 10251–10258 10.1523/JNEUROSCI.2419-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu Q., Hwang C.K., Zheng H., Wagley Y., Lin H.Y., Kim D.K. et al. (2013) MicroRNA 339 down-regulates mu-opioid receptor at the post-transcriptional level in response to opioid treatment. FASEB J. 27, 522–535 10.1096/fj.12-213439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fang S., Zhang L., Guo J., Niu Y., Wu Y., Li H. et al. (2018) NONCODEV5: a comprehensive annotation database for long non-coding RNAs. Nucleic Acids Res. 46, D308–D314 10.1093/nar/gkx1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ma L., Cao J., Liu L., Du Q., Li Z., Zou D. et al. (2019) LncBook: a curated knowledgebase of human long non-coding RNAs. Nucleic Acids Res. 47, D128–D134 10.1093/nar/gky960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fernandes J.C.R., Acuña S.M., Aoki J.I., Floeter-Winter L.M. and Muxel S.M. (2019) Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dahariya S., Paddibhatla I., Kumar S., Raghuwanshi S., Pallepati A. and Gutti R.K. (2019) Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 112, 82–92 10.1016/j.molimm.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 169.Quinn J.J. and Chang H.Y. (2016) Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 17, 47–62 10.1038/nrg.2015.10 [DOI] [PubMed] [Google Scholar]
- 170.Yang J.X., Rastetter R.H. and Wilhelm D. (2016) Non-coding RNAs: An Introduction. Adv. Exp. Med. Biol. 886, 13–32 10.1007/978-94-017-7417-8_2 [DOI] [PubMed] [Google Scholar]
- 171.Jarroux J., Morillon A. and Pinskaya M. (2017) History, Discovery, and Classification of lncRNAs. Adv. Exp. Med. Biol. 1008, 1–46 10.1007/978-981-10-5203-3_1 [DOI] [PubMed] [Google Scholar]
- 172.Carlevaro-Fita J. and Johnson R. (2019) Global Positioning System: Understanding Long Noncoding RNAs through Subcellular Localization. Mol. Cell 73, 869–883 10.1016/j.molcel.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 173.Schmitz S.U., Grote P. and Herrmann B.G. (2016) Mechanisms of long noncoding RNA function in development and disease. Cell. Mol. Life Sci. 73, 2491–2509 10.1007/s00018-016-2174-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Luo L., Ji L.D., Cai J.J., Feng M., Zhou M., Hu S.P. et al. (2018) Microarray Analysis of Long Noncoding RNAs in Female Diabetic Peripheral Neuropathy Patients. Cell. Physiol. Biochem. 46, 1209–1217 10.1159/000489071 [DOI] [PubMed] [Google Scholar]
- 175.Suwal A., Hao J.L., Liu X.F., Zhou D.D., Pant O.P., Gao Y. et al. (2019) NONRATT021972 long-noncoding RNA: A promising lncRNA in diabetes-related diseases. Int. J. Med. Sci. 16, 902–908 10.7150/ijms.34200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang L., Luo T., Bao Z., Li Y. and Bu W. (2018) Intrathecal circHIPK3 shRNA alleviates neuropathic pain in diabetic rats. Biochem. Biophys. Res. Commun. 505, 644–650 10.1016/j.bbrc.2018.09.158 [DOI] [PubMed] [Google Scholar]
- 177.Peng H., Zou L., Xie J., Wu H., Wu B., Zhu G. et al. (2017) lncRNA NONRATT021972 siRNA Decreases Diabetic Neuropathic Pain Mediated by the P2X3 Receptor in Dorsal Root Ganglia. Mol. Neurobiol. 54, 511–523 10.1007/s12035-015-9632-1 [DOI] [PubMed] [Google Scholar]
- 178.Wang S., Xu H., Zou L., Xie J., Wu H., Wu B. et al. (2016) LncRNA uc.48+ is involved in diabetic neuropathic pain mediated by the P2X3 receptor in the dorsal root ganglia. Purinergic Signal. 12, 139–148 10.1007/s11302-015-9488-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu B., Zhang C., Zou L., Ma Y., Huang K., Lv Q. et al. (2016) LncRNA uc.48+ siRNA improved diabetic sympathetic neuropathy in type 2 diabetic rats mediated by P2X7 receptor in SCG. Autonomic Neurosci.: Basic Clin. 197, 14–18 [DOI] [PubMed] [Google Scholar]
- 180.Yu B., Zhou S., Hu W., Qian T., Gao R., Ding G. et al. (2013) Altered long noncoding RNA expressions in dorsal root ganglion after rat sciatic nerve injury. Neurosci. Lett. 534, 117–122 10.1016/j.neulet.2012.12.014 [DOI] [PubMed] [Google Scholar]
- 181.Mao P., Li C.R., Zhang S.Z., Zhang Y., Liu B.T. and Fan B.F. (2018) Transcriptomic differential lncRNA expression is involved in neuropathic pain in rat dorsal root ganglion after spared sciatic nerve injury. Brazil. J. Med. Biol. Res. = Revista Brasileira de Pesquisas Medicas e Biologicas 51, e7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Baskozos G., Dawes J.M., Austin J.S., Antunes-Martins A., McDermott L., Clark A.J. et al. (2019) Comprehensive analysis of long noncoding RNA expression in dorsal root ganglion reveals cell-type specificity and dysregulation after nerve injury. Pain 160, 463–485 10.1097/j.pain.0000000000001416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhou J., Fan Y. and Chen H. (2017) Analyses of long non-coding RNA and mRNA profiles in the spinal cord of rats using RNA sequencing during the progression of neuropathic pain in an SNI model. RNA Biol. 14, 1810–1826 10.1080/15476286.2017.1371400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jiang B.C., Sun W.X., He L.N., Cao D.L., Zhang Z.J. and Gao Y.J. (2015) Identification of lncRNA expression profile in the spinal cord of mice following spinal nerve ligation-induced neuropathic pain. Mol. Pain 11, 43 10.1186/s12990-015-0047-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pan B., Zhou H.X., Liu Y., Yan J.Y., Wang Y., Yao X. et al. (2017) Time-dependent differential expression of long non-coding RNAs following peripheral nerve injury. Int. J. Mol. Med. 39, 1381–1392 10.3892/ijmm.2017.2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Raju H.B., Englander Z., Capobianco E., Tsinoremas N.F. and Lerch J.K. (2014) Identification of potential therapeutic targets in a model of neuropathic pain. Front. Genet. 5, 131 10.3389/fgene.2014.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang W.-T., Sun Y.-M., Huang W., He B., Zhao Y.-N. and Chen Y.-Q. (2016) Genome-wide Long Non-coding RNA Analysis Identified Circulating LncRNAs as Novel Non-invasive Diagnostic Biomarkers for Gynecological Disease. Sci. Rep. 6, 23343 10.1038/srep23343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li Y., Li Q., Wang C., Li S. and Yu L. (2019) Long Noncoding RNA Expression Profile in BV2 Microglial Cells Exposed to Lipopolysaccharide. Biomed. Res. Int. 2019, 5387407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xu S., Ao J., Gu H., Wang X., Xie C., Meng D. et al. (2017) IL-22 Impedes the Proliferation of Schwann cells: Transcriptome Sequencing and Bioinformatics Analysis. Mol. Neurobiol. 54, 2395–2405 10.1007/s12035-016-9699-3 [DOI] [PubMed] [Google Scholar]
- 190.Iwasaki H., Sakai A., Maruyama M., Ito T., Sakamoto A. and Suzuki H. (2019) Increased H19 Long Non-coding RNA Expression in Schwann Cells in Peripheral Neuropathic Pain. J. Nippon Med. Sch. 86, 215–221 10.1272/jnms.JNMS.2018_86-402 [DOI] [PubMed] [Google Scholar]
- 191.Liu C.L., Deng Z.Y., Du E.R. and Xu C.S. (2018) Long noncoding RNA BC168687 small interfering RNA reduces high glucose and high free fatty acidinduced expression of P2X7 receptors in satellite glial cells. Mol. Med. Rep. 17, 5851–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu C., Li C., Deng Z., Du E. and Xu C. (2018) Long Non-coding RNA BC168687 is Involved in TRPV1-mediated Diabetic Neuropathic Pain in Rats. Neuroscience 374, 214–222 10.1016/j.neuroscience.2018.01.049 [DOI] [PubMed] [Google Scholar]
- 193.Liu C., Tao J., Wu H., Yang Y., Chen Q., Deng Z. et al. (2017) Effects of LncRNA BC168687 siRNA on Diabetic Neuropathic Pain Mediated by P2X7 Receptor on SGCs in DRG of Rats. Biomed. Res. Int. 2017, 7831251 10.1155/2017/7831251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li G., Jiang H., Zheng C., Zhu G., Xu Y., Sheng X. et al. (2017) Long noncoding RNA MRAK009713 is a novel regulator of neuropathic pain in rats. Pain 158, 2042–2052 10.1097/j.pain.0000000000001013 [DOI] [PubMed] [Google Scholar]
- 195.Liu S., Zou L., Xie J., Xie W., Wen S., Xie Q. et al. (2016) LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia. Mol. Brain 9, 44 10.1186/s13041-016-0226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hu J.Z., Rong Z.J., Li M., Li P., Jiang L.Y., Luo Z.X. et al. (2019) Silencing of lncRNA PKIA-AS1 Attenuates Spinal Nerve Ligation-Induced Neuropathic Pain Through Epigenetic Downregulation of CDK6 Expression. Front. Cell. Neurosci. 13, 50 10.3389/fncel.2019.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xiong W., Tan M., Tong Z., Yin C., He L., Liu L. et al. (2019) Effects of long non-coding RNA uc.48+ on pain transmission in trigeminal neuralgia. Brain Res. Bull. 147, 92–100 10.1016/j.brainresbull.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 198.Zhao X., Tang Z., Zhang H., Atianjoh F.E., Zhao J.Y., Liang L. et al. (2013) A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat. Neurosci. 16, 1024–1031 10.1038/nn.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Martinez-Moreno M., O'Shea T.M., Zepecki J.P., Olaru A., Ness J.K., Langer R. et al. (2017) Regulation of Peripheral Myelination through Transcriptional Buffering of Egr2 by an Antisense Long Non-coding RNA. Cell Rep. 20, 1950–1963 10.1016/j.celrep.2017.07.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu S., Marie Lutz B., Miao X., Liang L., Mo K., Chang Y.J. et al. (2016) Dorsal root ganglion transcriptome analysis following peripheral nerve injury in mice. Mol. Pain. 12, 1-14 10.1177/1744806916629048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Guo G., Ren S., Kang Y., Liu Y., Duscher D., Machens H.G. et al. (2019) Microarray analyses of lncRNAs and mRNAs expression profiling associated with diabetic peripheral neuropathy in rats. J. Cell. Biochem. 120, 15347–15359 10.1002/jcb.28802 [DOI] [PubMed] [Google Scholar]
- 202.Zhao X., Tang Z., Zhang H., Atianjoh F.E., Zhao J.-Y., Liang L. et al. (2013) A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat. Neurosci. 16, 1024 10.1038/nn.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xiong W., Tan M., Tong Z., Yin C., He L., Liu L. et al. (2019) Effects of long non-coding RNA uc.48+ on pain transmission in trigeminal neuralgia. Brain Res. Bull. 147, 92–100 10.1016/j.brainresbull.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 204.Yao C., Wang J., Zhang H., Zhou S., Qian T., Ding F. et al. (2015) Long non-coding RNA uc.217 regulates neurite outgrowth in dorsal root ganglion neurons following peripheral nerve injury. Eur. J. Neurosci. 42, 1718–1725 10.1111/ejn.12966 [DOI] [PubMed] [Google Scholar]
- 205.Liu Z., Liang Y., Wang H., Lu Z., Chen J., Huang Q. et al. (2017) LncRNA expression in the spinal cord modulated by minocycline in a mouse model of spared nerve injury. J. Pain Res. 10, 2503–2514 10.2147/JPR.S147055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhou J., Xiong Q., Chen H., Yang C. and Fan Y. (2017) Identification of the Spinal Expression Profile of Non-coding RNAs Involved in Neuropathic Pain Following Spared Nerve Injury by Sequence Analysis. Front. Mol. Neurosci. 10, 91 10.3389/fnmol.2017.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cao S., Deng W., Li Y., Qin B., Zhang L., Yu S. et al. (2017) Chronic constriction injury of sciatic nerve changes circular RNA expression in rat spinal dorsal horn. J. Pain Res. 10, 1687–1696 10.2147/JPR.S139592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Du H., Liu Z., Tan X., Ma Y. and Gong Q. (2019) Identification of the Genome-wide Expression Patterns of Long Non-coding RNAs and mRNAs in Mice with Streptozotocin-induced Diabetic Neuropathic Pain. Neuroscience 402, 90–103 10.1016/j.neuroscience.2018.12.040 [DOI] [PubMed] [Google Scholar]