Abstract
Methotrexate (MTX) is the most important drug used in the treatment of several kinds of cancers, such as colon cancer. However, this drug can cause a reduction in the target tissue bioavailability. It is administered orally and absorbed quickly. This study aimed to produce an anti-colon cancer prodrug based on MTX via loading it into a biopolymer compound. Chitosan (CS) was extracted from scales of local fish by utilizing a previously published protocol. The MTX was then transformed to Methotrexate – imidazole and loaded into CS to prepare Chitosan - Methotrexate (CS-MTX) conjugates as colon cancer prodrugs. Fourier-transform infrared (FTIR), UV-visible spectroscopy, and 1H-NMR were used to analyse the structure of the prepared compounds. The prepared compounds were also tested for hemolytic activity. Chemical stability was studied using 0.2 M from the different buffer types with a pH of 1.2 and 7.4 over different periods about 240 min and kept in an incubator at 37 °C. The loading percentage was measured by hydrolysing the amide bond in basic media followed by the measurement of the absorbency at 273 nm. Three types of cancer cells, MCF-7, MDA-MB-231, and MDA-MB-453, were used to test the anticancer effects of CS-MTX by using tetrazolium bromide (MTT) assay. The results indicated that the viability of human breast cancer cell lines decreased because of the use of CS-MTX. This study also showed that CS-MTX was less toxic than the original drug. Therefore, it may be measured for additional biological analyses and medical applications. The results presented here showed that the new compound is remarkably stable in comparison with MTX and has longer half-life (t ½). Therefore, the CS-MTX has promising strategies through minimising the side effects of anti-colon tumour drugs.
Keywords: Methotrexate, Chitosan, Cancer, Drug release, Chitosan - Methotrexate, MTT assay, Chemistry, Food science, Agricultural science, Biological sciences, Health sciences
Methotrexate, Chitosan, Cancer, Drug release, Chitosan - Methotrexate, MTT assay; Chemistry; Food science; Agricultural science; Biological sciences; Health sciences.
1. Introduction
Cancer is the foremost cause of global death. The worldwide cancer burden has risen to 18.1 million cases and 9.6 million deaths in 2018. One in five males and one in six females will develop cancer during their lifetime, and one out of eight males and one out of eleven females would die due to cancer disease. Carcinoma forms in different kinds of tissues and organs, for instance, prostate, lung, colon, and breast (International Agency for Research on Cancer (IARC) 2018; Sharma et al., 2013). MTX is antimetabolic operator that blocks the metabolism of folic acid. The clinical practice for the medication as antineoplastic began in 1948, after the discovery of its aberrant consequences on DNA synthesis (Benedek, 2010). Oral administration causes sedate retention at the beginning of the alimentary canal because of the physical and chemical features of the directed medication. Many significant limitations and disadvantages of the drug were observed when directed at a particular site of ingestion or at specific part of the alimentary canal as in the colon. Therefore, it is important to explain the specific details of the movement of the medication to reach a particular place of treatment (Pinto, 2010). Many procedures were used to prepare a new drug system which can reach colon particularly. The system depends on enzyme, pH and time (Chourasia and Jain, 2003; Journal Russian, 2017; Sinha and Kumria, 2001). Recently, the prodrug was prepared through bonding the colon-drug with the transporter covalently. These prodrugs often sedate physicochemical properties to expand fixation at the activity site, work for a longer period, and reduce harmfulness and unfortunate symptoms. Furthermore, these prodrugs appear to be non-toxic, stable in a variety of pH levels (in the stomach and in the digestive tract), and biologically degradable and compatible. Besides, they could stay as a small molecule by loading the drug on little size compounds such as amino acid or sugar. However, they could be a macromolecule by loading it into biopolymers (Hoste et al., 2004; Jung et al., 2006; Shah et al., 2017; Varshosaz et al., 2009; Zou et al., 2005).
CS is a natural base polymer, formed by alkaline deacetylation of Chitin, which supports the exoskeleton of crustaceous (crabs, shrimps, and so forth). After cellulose, CS is the greatest abundant natural materials in nature. CS possesses a unique position because of its bounty and slight adjustment. It also has unusual properties such as biodegradability, biocompatibility, non-dangerous antimicrobial, hydrophilicity and mucoadhesion, although it is anti-cholesterolemic. These properties have made CS beneficial in medicine, horticultural help, staple stabilizer, biocatalyst limitation, and biological field (Elieh-Ali-Komi and Hamblin, 2016).
In this study, the MTX (nitroimidazole derivative) drug was selected due to its wide range of use, predominantly, for chemotherapy as an anti-folate chemotherapeutic. The mechanism of the drug action blocks the synthesis of the DNA in the cancer cells (Cronstein and Aune, 2020; Niemelä et al., 2020). In addition, MTX can be a therapy for Protozoa and Amoebiasis infections, as anti-bacteria and anti-parasites (Elieh-Ali-Komi and Hamblin, 2016; Niemelä et al., 2020). When bacteria and parasite arrive at the colon, they cause ulceration and then bleeding. Consequently, MTX can be released into the colon to inhibit the growth of the organism, while the free MTX is absorbed directly after oral administration. Although only a small amount (<10%) of the medication arrives at the colon; severe side effects are recorded (Knowles et al., 1994; Lamp et al., 1999; Lau et al., 1992; Schreiber and Spernal, 1997). Here, the main point is to make a drug for colon from MTX utilising a polymeric prodrug strategy. Thus, local fish scales were collected and treated chemically to extract the CS according to a previously reported method (Kumari and Rath, 2014). The MTX was then transformed to Methotrexate – imidazole and loaded into CS to prepare CS-MTX conjugates as colon cancer prodrugs. Spectroscopic analysis was used to prove the structure of the prepared compounds. The compound was also tested for hemolytic activity and chemical stability. The calculation of the percentages of the drug content was carried out. Finally, In vitro cytotoxicity test using MTT was done for the synthesised compounds utilizing cell cancer lines MDA-MB-231, MCF-7 and MDA-MB-453.
2. Results and discussion
2.1. Chemistry
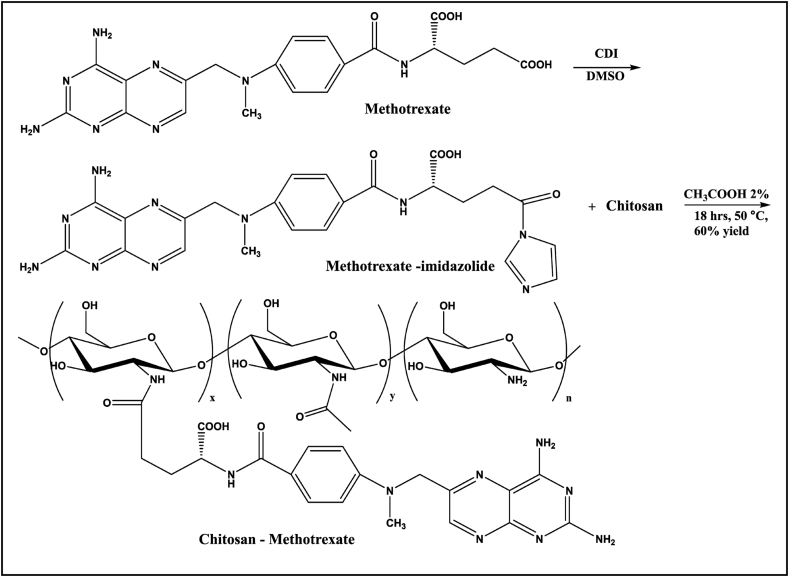
In this study, the CS-MTX conjugate was prepared as a future colon cancer drug derived from MTX. The CS was extracted from fish scales; as explained in Figure S1 illustrated in Supplementary data (SD). Figure S2 in SD shows the I.R chart of CS which proved their structure. In the I.R spectroscopy, the peaks range from 3423 to3000 cm−1 related to the ν N-H in NH2 group. All the other peaks were identical with those reported (Liebert and Heinze, 2005). The synthesis of the CS-MTX conjugate was accomplished, as presented in Scheme 1. The process started with the combination of the Methotrexate – imidazole. The preparation of MTX-imidazole was done in situ by adding equivalent amounts of MTX and imidazole to the reaction mixture. A catalytic amount of N, N-carbonyldiimidazole (CDI) was also added. In the mixture, CS was dissolved in 2% glacial acetic acid (GAA), followed by the addition of a few drops of triethylamine (TEA) as a catalyst (Liebert and Heinze, 2005). Aqueous acetic acid was chosen because of the insolubility of CS in most other organic solvents. The CS-MTX conjugate was obtained after stirring the reaction mixture for 18 h. The prodrug was purified by exhaustive dialysis to obtain the final product whose purity was confirmed by TLC, which showed single spots. The formation of the conjugate structure was spectroscopically and analytically established. Different mole amounts of both MTX and CS were used in a similar environment.
Scheme 1.
Preparation of CS-MTX conjugate.
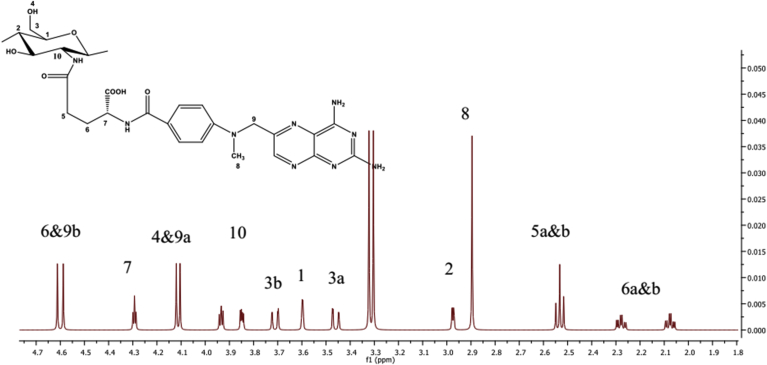
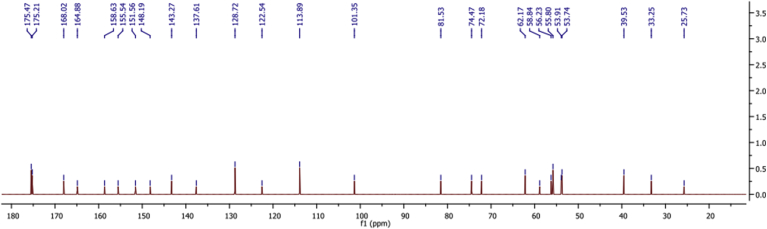
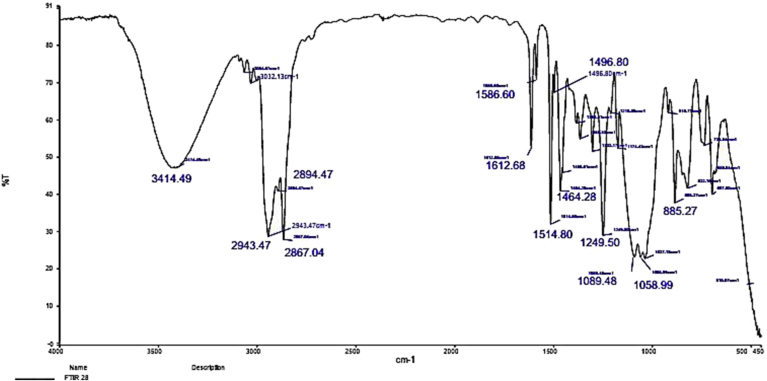
The structure of CS-MTX conjugate was confirmed by (1H and 13C) NMR and FTIR spectroscopy. 1H NMR chart (Figure 1) showed a downfield signal at δ 6.02 corresponding to the proton attached to the anomeric carbon of the sugar. The signal at δ 3.60 due to the proton was attached to C1. The methylene group C5 displayed signals at δ 2.53 and C6 gave signals at δ 2.08 and 2.28 because the two protons were in different environments. The 13C NMR (Figure 2) spectrum's carbonyls signals were δ 173.2 and δ 173.4. The anomeric carbon signalled δ 101.5. The IR chart (Figure 3) of the conjugate showed the characteristic vibrations of CS and MTX. Peaks were at 3064 related to the N-H and 3032–2834 cm−1 related to C-H groups. Signals related to C-O vibration in the COOH group were at 1660, 1720 cm−1. Signals ranged between 1499–1183 cm−1 corresponding to the vibration of the (C-C, C-N, and C-O). A new peak at 1700 cm−1 was shown and this peak confirmed the amide bond between MTX and CS. This bond was susceptible to hydrolysis by amidase enzyme or acid hydrolysis, causing the drug release.
Figure 1.
Apart from the proton NMR chart of the CS-MTX.
Figure 2.
The carbon NMR chart of the CS-MTX.
Figure 3.
FT-IR chart of the CS-MTX.
2.2. Stability studies
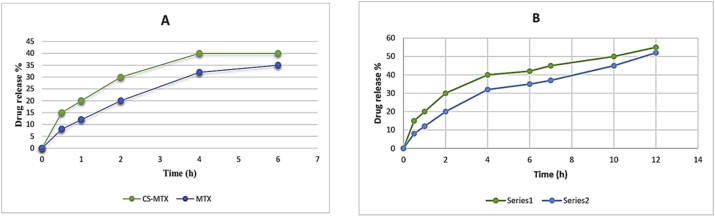
The consistency of the newly synthesised prodrug in different pH ranges, required during the transition in the gastrointestinal tract, was examined by the stability assay. Thus, a stability test was done in vitro to investigate the synthesised compound stability in both acidic (pH 1.2) and basic (pH 7.4) media. Different physiologically relevant pH levels were set satisfying the World Health Organization report and the relative reference (Gupta et al., 2009; Jingou et al., 2011; Report, 2015). The results were shown in Figure 5 A and B. The test of the stability was carried out over 2 and 6 h periods in pH 1.2 and over 2, 6 and 12 h periods in in the pH 7.4.
Figure 5.
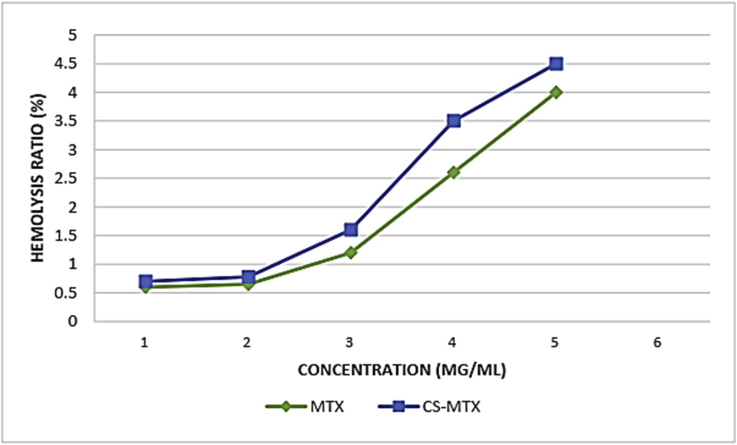
The level of red platelet hemolysis treated with CS-MTX.
However, the transit period in the small intestin and in the stomach were 4 and 2 h, respectively. The examples of the prepared compound indicated 3–5% release in an acidic buffer and 3–4% in a basic buffer.
The t ½ of the CS-MTX was studied. The CS-MTX conjugate and free MTX aqueous solutions were taken. The maximum wavelength λmax and the UV spectra were measured. In the literature, two types of kinetics are commonly used and compared: the pseudo-first (K1) and –second (K2) order rate laws. In the majority of kinetics studies, from the experimental values of log concentration in relation to time and from plotting the diagram, the observed rate of hydrolysis was measured. The plot was a straight line. From the incline, the order of the hydrolysis was calculated. As can be seen in Figure S5 in SD, it was then followed by ‘pseudo-first-order kinetics’. All the data and equations were illustrated in Scheme S1, Table S1 and Figure S5 in SD (Dandik and Aksoy, 1992; Simonin, 2016). The hydrolysis processes were carried out over different periods, approximately 0–240 min at 15 min intervals. Two buffers were used, phosphate and KCl/HCl at pH levels of 7.4 and 1.2, respectively. These two buffers were used to dissolve 15 μg/mL from the synthesised molecule which was stored in incubation at 37 °C.
Depending on the law of “pseudo-first-order kinetic”, the t ½ was measured for the prepared compound in both acidic and basic media and it was found to be 4.52 and 16.01 respectively. These results indicated a notable rise in the t1/2 of CS-MTX in comparison to the free drug in the acidic condition and a slight increase in the basic condition (Paradis et al., 1992) (see Figure 4).
Figure 4.
(A) The stability of CS-MTX conjugate and free MTX in acidic condition. (B) the stability of CS-MTX conjugate and free MTX in basic condition. Every value was communicated as mean ± SD and repeated three times.
2.3. The percentage of drug content
The UV-visible spectroscopy was used to calculate the proportion of MTX/CS. The principle of this experiment depended on the hydrolyses of the amide link between MTX and CS in primary conditions.
The results were improved, as expected, by using a higher amount of both CS and raising the amount of MTX in the conjugates. In the CS-MTX (1:1), the contents of MTX were 0.60 w% and yield of 65%. However, in the case of CS-MTX (1:2), the contents were 0.72 w% and yield of 70%. All reaction conditions were done at 50 °C for 20 h.
2.4. Hemolytic activity
The percentage of the hemolysis caused by the CS-MTX conjugates was studied with different concentrations. The result can be seen in Figure 5 and the percentage of the hemolysis raised depending on the rising level of CS-MTXs. The result indicated that the hemolysis in the CS-MTXs was less than 4.5% meeting the international standard which is less than 5% (Ali et al., 2019).
2.5. In-vitro cytotoxicity
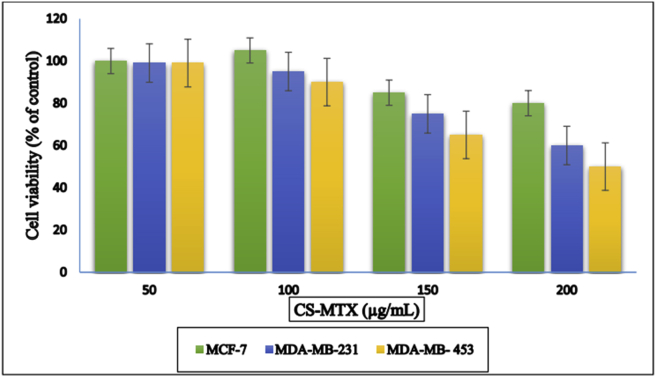
Three models of human breast cancer cell lines (MDA-MB-231, MCF-7 and MDA-MB-453) were used in this test. MTT analyses entailed that CS-MTX conjugates minimized the viability of the cell lines in a dose-dependent manner. CS-MTX displayed a variable cytotoxicity effect on all the used cell lines. The results showed the values of IC50 over 24 h were 363.5 ± 31.2, 198.8 ± 20.4, and 163.4 ± 10.8 μg of CS-MTX/mL for MCF-7, MDA-MB-231, and MDA-MB-453, respectively. However, the cell MDA-MB-453 was more precise in the CS-MTX usage than the other cell lines used as in Figure 6.
Figure 6.
MTT viability assay of the cell lines (different concentrations) after reacted with 200 μg/mL for 24 h in DMSO as a solvent and control. The final presentation of each solution was equal to 0.5%. Information data were communicated as mean ± SD (n = 3), P < 0.01 compared with control.
The suggested mechanism for reducing the cytotoxicity of CS-MTX could be due to the weak interaction between the CS derivatives and the cell wall. This interaction decreased because of the flexibility of the molecule (Huang et al., 2004). Also, the amine group on the MTX molecule and the nitrogen atom inside the ring may be another suggested mechanism to decrease the cytotoxicity (Mao et al., 2005). Furthermore, the type of cells could be another factor in reducing the compound cytotoxicity (Sajomsang et al., 2013).
3. Experimental
3.1. Materials and methods
Methotrexate, 1,1–carbonyldiimidedazole (CDI), 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide (MTT), sodium chlorite, chloroacetic acid, potassium hydrogen carbonate, dimethylsulfoxide (DMSO), sodium hydroxide, hydrochloric acid, and dialysis bags (Mw = 14,000) were purchased from Sigma-Aldrich Chemicals. The cell lines' MCF-7, MDA-MB-231, and MDA-MB-453, were supplied from the Institute of Biochemistry and Cell Biology, China. Cells were grown in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) medium supplemented with 15% (FBS) fetal bovine serum at 37 °C in a humid atmosphere with 5% carbon dioxide and 95% air. Chitin and Chitosan were extracted from fish scales collected from a market in Kirkuk City-Iraq. Reported methods were used in the extraction presses (Kumari and Rath, 2014). The solution of NaOH, 40 g/mol, 0.5 N and HCL, 36.5 g/mol, 1% solution were prepared for demineralisation and deproteinization receptivity. Perkin Elmer FT-IR 4200 spectrometer was used to carry out the IR spectrum. Bruker Avance (500) spectrometer was used to record the 1H and 13C NMR spectra. Perkin Elmer UV- Visible Spectrophotometer was used to record the UV- Visible samples.
3.2. Synthesis of CS-MTX
In a round bottom flask that contains CS (0.27 g, 2.00 mmol, dissolved in 2 % GAA), CDI (0.70 g, 4.00 mmol) and MTX (11.8 mmol, dissolved in 80 mL of acetonitrile) were gradually added. The mixture was continuously stirred for 6 h. At the end of stirring, TEA (500 μL) was added and the reaction mixture was stirred for 18 h in a water bath at 50 °C. The reaction was monitored by TLC. To precipitate the product, the reaction mixture was transferred to another flask, and 100 mL ethanol was put in. The desired compound was filtered and washed several times by acetone to remove any unreacted materials. Then the residue was dried in a vacuum and the reaction yield was 60%.
3.3. The stability studies
The stability of the prepared prodrug was verified in pH 7.4 and incubated at 37 °C (Yang et al., 2016). The test initiated by dissolving 15 mg from CS-MTX conjugate in 3 mL of the buffer solution phosphate-buffered saline (PBS). The mixture was kept in a dialysis bag. Then, 150 mL of the buffer was added and kept in a water bath at 37 °C. UV absorption was measured at different time intervals for the release medium at 273 nm. For every sample, a new medium was added, followed by measuring the release analysis three times, and the average was then calculated. The weight percentage for the deliverance MTX (% w/w) at the release time was measured. The t ½ of the of CS-MTX and the λmax was calculated by recording UV spectra for the solutions. Plotting the diagram of concentration logarithm and time resulted in a straight line. From that, the hydrolysis was pursued the pseudo-first-order kinetic (Conover, 1998). From the slope of the straight line, the constant rate was calculated. Hydrolysis degree experiments were also conducted by the addition of 20 mg from the compound dissolved in phosphate and KCl/HCl buffers (0.2 M) and kept in a water bath at 37 °C. The study was done over different periods from 0 to 240 min. The t ½ was calculated using the law of pseudo-first-order kinetic.
3.4. Drug content calculation
Hydrolysis of the amide bond in the CS-MTX was used to calculate the drug contents. The calibration curve was plotted for the MTX solution by preparing a set of standard solutions (MTX in 1N NaOH, 1–5 mg/mL), and the absorbance was measured at 243 nm (Li et al., 2014). A concentration of CS-MTX (1 mg/mL) solution was set at 1N NaOH, and the absorbance was measured at 243 nm. The MTX amount in the conjugates was compared with the standard to calculate the percentage of the MTX. To measure the percentage of drug loading, the below equation was used:
| (1) |
3.5. Hemolytic activity
This test was performed according to the reported method (Gul et al., 2017). The experiment was conducted using a red blood cell (RBC) obtained from white rabbits. 3 mL from the blood was taken and centrifuged for 20 min at 4000 rpm, followed by the addition of 0.9 % NaCl solution. Negative and positive controls were prepared by the addition of 1 mL RBC, 5 mL normal saline, 1 mL distilled water and 1 mL RBC successively. A series of solutions from both MTX and the prepared compound was obtained by the addition of 6,2,0.6,0.3,0.7 g from both compounds to tubes followed by, 2 mL normal saline and 1 mL RBC. The resultant solutions were kept in a water bath at 37 °C for 2 h. Each tube was centrifuged for 20 min at 4000 rpm. UV absorbance was measured at 541 nm weave length. To calculate the percentage of hemolysis, the below equation was used:
| (2) |
3.6. In-vitro cytotoxicity
The CS-MTX conjugates and the free drug MTX were used for the cytotoxicity study on MCF-7, MDA-MB-231, and MDA-MB-453 cells and the effect was measured by MTT assay (Kumar and Gopinath, 2015). A plate with 96-well was used with a range of concentrations (1–10 M) of CS-MTX and MTX. These concentrations were added before coating the plate by approximately 3 × 103 cells then kept over different periods (12, 24 and 48) h. In the last period, fresh media were added after removing present media and (DMEM/F12) supplemented with 15 μL of (MTT, concentration-500 μg/mL). Then cells were raised at37 °C for 3–4 h. The dark-blue formazan crystals were dissolved in DMSO, and their absorptivity was measured at 574 nm for each well by Cytation 3 multimode plate reader, Biotek, USA. The procured absorbance esteems were illustrated as practical rate cells referring to untreated control cells by the accompanying equation:
| (3) |
4. Conclusion
The synthesized CS-MTX conjugate is a probable human colon cancer prodrug. The synthesised compound was sufficiently stable at acidic conditions, pH 1.2. Remarkably, the prepared prodrug presented a higher t1/2 value of 4.52 in acidic conditions and 16.01 in the basic media compared to the original drug. The prepared CS-MTX conjugate showed significant results in the MTT assay. The viability of the three cancer cell lines MCF-7, MDA-MB-231, and MDA-MB-453 in a dose-dependent manner was reduced, as compared to the free drug. The IC50 values over 24 h were 363.5 ± 31.2, 198.8 ± 20.4, and 163.4 ± 10.8 μg of CS-MTX/mL. According to these outcomes, CS-MTX conjugate as prodrug may have possible applications for a colon cancer medication. Additional assays to estimate more biologic activities of the synthesised prodrug are underway.
Declarations
Author contribution statement
M.O. Mohammed: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
H.M.M. Alkubaisi: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
N.Q.Haj: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
MOM wishes to thank the department of biology, college of science, Kirkuk University, Iraq, for their help in carrying out the biology test.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supporting Information-last_V3
References
- Ali Sameh S. Pharmaceutical potential of a novel Chitosan derivative Schiff base with special reference to antibacterial, anti-biofilm, antioxidant, anti-inflammatory, hemocompatibility and cytotoxic activities. Pharmaceut. Res. 2019;36(1):5. doi: 10.1007/s11095-018-2535-x. [DOI] [PubMed] [Google Scholar]
- Benedek T.G. Methotrexate: from its introduction to non-oncologic therapeutics to anti-TNF-α. Clin. Exp. Rheumatol. 2010;28(5 SUPPL. 61) [PubMed] [Google Scholar]
- Chourasia M.K., Jain S.K. Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Pharmaceut. Sci. 2003;6(1):33–66. [PubMed] [Google Scholar]
- Conover Wheeler. Buffer solutions: the basics (Beynon, R. J.; Easterby, J. S.) J. Chem. Educ. 1998;75(2):153. [Google Scholar]
- Cronstein Bruce N., Aune Thomas M. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020;16(3):145–154. doi: 10.1038/s41584-020-0373-9. [DOI] [PubMed] [Google Scholar]
- Dandik L., Aksoy H.A. The kinetics of hydrolysis of Nigella Sativa (Black Cumin) seed oil catalyzed by native lipase in ground seed. JAOCS (J. Am. Oil Chem. Soc.) 1992;69(12):1239–1241. [Google Scholar]
- Elieh-Ali-Komi Daniel, Hamblin Michael R. Chitin and Chitosan: production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016;4(3):411–427. [PMC free article] [PubMed] [Google Scholar]
- Gul Samreen. Synthesis, antimicrobial evaluation and hemolytic activity of 2-[[5-alkyl/aralkyl substituted-1,3,4-oxadiazol-2-Yl]Thio]-N-[4-(4-Morpholinyl)Phenyl]Acetamide derivatives. J. Saudi Chem. Soc. 2017;21:S425–S433. [Google Scholar]
- Gupta Deepak, Gupta Sheeba Varghese, Lee Kyung-Dall, Amidon Gordon L. Chemical and enzymatic stability of amino acid prodrugs containing methoxy, ethoxy and propylene glycol linkers. Mol. Pharm. 2009;6(5):1604–1611. doi: 10.1021/mp900084v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoste K., De Winne K., Schacht E. Polymeric prodrugs. Int. J. Pharm. 2004;277(1–2):119–131. doi: 10.1016/j.ijpharm.2003.07.016. [DOI] [PubMed] [Google Scholar]
- Huang Min, Khor Eugene, Lim Lee Yong. Uptake and cytotoxicity of Chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharmaceut. Res. 2004;21(2):344–353. doi: 10.1023/b:pham.0000016249.52831.a5. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) World Health Organization; 2018. Latest Global Cancer Data, 2018. (September): 13–15. [Google Scholar]
- Jingou Ji. Preparation, characterization of hydrophilic and hydrophobic drug in combine loaded Chitosan/Cyclodextrin nanoparticles and in vitro release study. Colloids Surf. B Biointerfaces. 2011;83(1):103–107. doi: 10.1016/j.colsurfb.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Journal, Russian Colon targeted drug delivery systems-A review. Curr. Drug Deliv. 2017;5:186–198. doi: 10.2174/156720108784911712. June 2011. [DOI] [PubMed] [Google Scholar]
- Jung Yunjin. Evaluation of 5-aminosalicyltaurine as a colon-specific prodrug of 5-aminosalicylic acid for treatment of experimental colitis. Eur. J. Pharmaceut. Sci. 2006;28(1–2):26–33. doi: 10.1016/j.ejps.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Knowles S., Choudhury T., Shear N.H. Metronidazole hypersensitivity. Ann. Pharmacother. 1994;28(3):325–326. doi: 10.1177/106002809402800305. [DOI] [PubMed] [Google Scholar]
- Kumar S. Uday, Gopinath P. Controlled delivery of BPEI-niclosamide complexes by PEO nanofibers and evaluation of its anti-neoplastic potentials. Colloids Surf. B Biointerfaces. 2015;131:170–181. doi: 10.1016/j.colsurfb.2015.04.063. [DOI] [PubMed] [Google Scholar]
- Kumari Suneeta, Rath Pradip Kumar. Extraction and characterization of Chitin and Chitosan from (Labeo Rohit) fish scales. Proc. Mater. Sci. 2014;6(Icmpc):482–489. [Google Scholar]
- Lamp Kenneth C., Freeman Collin D., Klutman Neil E., Melinda K Lacy. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin. Pharmacokinet. 1999;36(5):353–373. doi: 10.2165/00003088-199936050-00004. [DOI] [PubMed] [Google Scholar]
- Lau Alan H. Clinical pharmacokinetics of metronidazole and other nitroimidazole anti-infectives. Clin. Pharmacokinet. 1992;23(5):328–364. doi: 10.2165/00003088-199223050-00002. [DOI] [PubMed] [Google Scholar]
- Li Dan. Synthesis and in vitro evaluation of methotrexate conjugated O,N-Carboxymethyl Chitosan via peptidyl spacers. J. Nanoparticle Res. 2014;16(9) [Google Scholar]
- Liebert Tim F., Heinze Thomas. Tailored cellulose esters: synthesis and structure determination. Biomacromolecules. 2005;6(1):333–340. doi: 10.1021/bm049532o. [DOI] [PubMed] [Google Scholar]
- Mao Shirui. Synthesis, characterization and cytotoxicity of poly(Ethylene glycol)-graft-trimethyl Chitosan block Copolymers. Biomaterials. 2005;26(32):6343–6356. doi: 10.1016/j.biomaterials.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Niemelä E. Nanoparticles carrying fingolimod and methotrexate enables targeted induction of apoptosis and immobilization of invasive thyroid cancer. Eur. J. Pharm. Biopharm. 2020;148:1–9. doi: 10.1016/j.ejpb.2019.12.015. [DOI] [PubMed] [Google Scholar]
- Paradis D. Comparative study of pharmacokinetics and serum bactericidal activities of Cefpirome, Ceftazidime, Ceftriaxone, imipenem, and Ciprofloxacin. Antimicrob. Agents Chemother. 1992;36(10):2085–2092. doi: 10.1128/aac.36.10.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto João F. Site-specific drug delivery systems within the gastro-intestinal tract: from the mouth to the colon. Int. J. Pharm. 2010;395(1–2):44–52. doi: 10.1016/j.ijpharm.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Report Fortieth. World Health Organization Technical Report Series (992): 1–210. 2015. WHO expert Committee on specifications for pharmaceutical preparations. Forty-Ninth Report. [PubMed] [Google Scholar]
- Sajomsang Warayuth. Effects of molecular weight and pyridinium moiety on water-soluble Chitosan derivatives for mediated gene delivery. Carbohydr. Polym. 2013;91(2):508–517. doi: 10.1016/j.carbpol.2012.08.053. [DOI] [PubMed] [Google Scholar]
- Schreiber W., Spernal J. Metronidazole-induced psychotic disorder. Am. J. Psychiatr. 1997;154(8):1170–1171. doi: 10.1176/ajp.154.8.1170b. [DOI] [PubMed] [Google Scholar]
- Shah Kamal. Prodrugs of NSAIDs: a review. Open Med. Chem. J. 2017;11(1):146–195. doi: 10.2174/1874104501711010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Rajiv. Design, synthesis and ex vivo evaluation of colon-specific azo based prodrugs of anticancer agents. Bioorg. Med. Chem. Lett. 2013;23(19):5332–5338. doi: 10.1016/j.bmcl.2013.07.059. [DOI] [PubMed] [Google Scholar]
- Simonin Jean-Pierre. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016;300:254–263. [Google Scholar]
- Sinha V.R., Kumria Rachna. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001;224(1–2):19–38. doi: 10.1016/s0378-5173(01)00720-7. [DOI] [PubMed] [Google Scholar]
- Varshosaz Jaleh. Synthesis and evaluation of dextran-budesonide conjugates as colon specific prodrugs for treatment of ulcerative colitis. Int. J. Pharm. 2009;365(1–2):69–76. doi: 10.1016/j.ijpharm.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Yang Huihui. Carboxymethyl Chitosan-mediated synthesis of hyaluronic acid-targeted graphene oxide for cancer drug delivery. Carbohydr. Polym. 2016;135:72–78. doi: 10.1016/j.carbpol.2015.08.058. [DOI] [PubMed] [Google Scholar]
- Zou Meijuan. Synthesis and properties of polysaccharide prodrugs of 5-aminosalicylic acid as potential colon-specific delivery systems. Eur. J. Pharm. Biopharm. 2005;59(1):155–160. doi: 10.1016/j.ejpb.2004.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information-last_V3