Abstract
This report presents a case of endotracheal metastasis in which elective veno–venous extracorporeal membrane oxygenation (VV ECMO) was used to undergo tracheal laser-surgery prior to establishment of a definitive airway.
Specifically, we describe the respiratory and airway management in an adult patient from the preclinical phase throughout elective preoperative ECMO implantation to postoperative ECMO weaning and decannulation in the Intensive Care Unit.
This case report lends further supports to the idea that the extracorporeal membrane oxygenation could be electively used to provide safe environment for surgery in situations where the standard maneuvers of sustaining adequate gas exchange are anticipated to fail.
Keywords: Preemptive extracorporeal membrane oxygenation, ECMO, Tracheal obstruction, Airway management
1. Introduction
Tracheal obstructions represent a common cause of a difficult airway. Symptoms vary depending on the extent of the airway blockade (partial, near complete or complete obstruction). Such a situation could appear either as an acute or urgent problem or as an elective situation with a known or an anticipated anatomical difficulty [1]. In a clinical situation surgeons and anesthesiologists typically work closely together in achieving a definitive airway [2]. On the other hand, the loss of respiratory muscle tone and the following collapse of airway tissues is a regular effect during induction of general anesthesia [3]. This effect is commonly being overcome by applying a positive pressure bag valve mask ventilation and positive end–expiratory pressure (PEEP) during mechanical ventilation. However, the induction of anesthesia could result in complete airway blockage in patients with near complete tracheal obstruction and the application of the aforementioned techniques may not be successful. Clear airway algorithms and effective communication in the operating room are essential for adequate management of such patients.
The anesthesiology guidelines for the critical management of unanticipated airway difficulty are being regularly updated and provide straightforward and clearly described algorithms [[4], [5], [6], [7]]. Nonetheless, the conventional strategies in the elective management of an anticipated difficult tracheal intubation primarily recommend techniques for insuring ventilation when maintaining spontaneous breathing (for review see, e.g. Ref. [8,9]). These techniques commonly include awake tracheal intubation or awake tracheostomy [[10], [11], [12], [13]]. Similarly, the airway–management techniques typically used by the otolaryngologist–head and neck surgeon include direct or rigid endoscopic laryngoscopy with intubation using an Eschmann stylet, “awake” fiberoptic nasotracheal intubation, awake tracheotomy, and emergency cricothyrotomy [1]. Indeed, lesions above the level of the larynx could be managed with awake fiberoptic intubation, tracheotomy or jet ventilation with a tracheal catheter [14]. However, even if a tracheal intubation or tracheostomy are successfully performed, ventilation may be impossible in patients with near complete tracheal obstruction below the larynx [15]. A different airway–management approach is required in such a situation in order for the surgery to be safely performed. The Extracorporeal Life Support (ECLS) techniques could provide this different approach.
ECLS techniques are “a set of therapies that focus on oxygenation, carbon dioxide removal, cardiac support, or a combination thereof. Extracorporeal membrane oxygenation (ECMO) is one ECLS entity used for temporary support of patients with respiratory and/or cardiac failure” [16]. The ECMO comprises a pump driven circuit drawing venous blood from the body. The blood flows temporary through an artificial lung and restores the oxygenated and decarboxylated blood to the patient. There are two common modes of ECMO; veno-arterial (VA ECMO) and veno-venous (VV ECMO) [16,17]. In VV ECMO the oxygenated blood is returned to the venous system and thus it aims to supplement respiratory failure, whereas in VA ECMO the oxygenated blood is returned to the arterial system and therefore provides both circulatory and respiratory support [18]. Efficiency of oxygenation and decarboxylation are determined by the blood flow and the sweep gas flow through the oxygenator membrane [19]. Today the VV ECMO is an established therapeutic option that serves as a rescue therapy in ARDS patients who do not respond to conventional therapy [[20], [21], [22]]. Presently, an ECMO is being used in other domains outside the ICU, such as in the advanced general thoracic surgery [15], in order to facilitate the performance of otherwise impossible airway surgical procedures in both adults and pediatric patients [[23], [24], [25]].
This report presents a case of severe tracheal occlusion by a metastasis from advanced colon cancer in which an elective veno-venous extracorporeal membrane oxygenation (VV ECMO) was successfully used to provide a safe environment for surgery. Specifically, we describe the airway management of the patient from the preclinical situation through the preoperative elective ECMO implantation and the induction of anesthesia in the Operation Room to the postoperative ECMO explantation in the ICU.
2. Case presentation
A 61-year-old female presents to emergency medical service via emergency call for an acute episode of shortness of breath. Upon arrival the patient was found to have dyspnea. The upper airway was unobstructed, the patient was alert (Glasgow Coma Scale score of 15). She stated to have had episodes of breathing difficulty and cough over the past two weeks and reported to have chronic obstructive lung disease (COPD) and colorectal cancer with lung metastasis. In the initial physical examination coarse crackles were heard by auscultation. Her oxygen saturation (SpO2) on room air was 89%. The pulse was regular, the skin color and capillary refill were normal. Sinus tachycardia was recognized on ECG with a heart rate (HR) of 130 bpm. The blood pressure (BP) on arrival obtained an initial reading of 210/110 mmHg. The immediate management of the patient consisted of titrated oxygen supplementation and inhalation of 1mg of Epinephrin via a non-rebreather mask (NRB). The patient's breathing pattern improved immediately. SpO2 increased to 95%, HR dropped to 90bpm. The BP was lowered to 170/100 mmHg by administration of 10 mg Urapidil.
The patient was then transported to the emergency department (ED) for follow-up evaluation of increasing stridor over the past two weeks turning into dyspnea for several hours. Her arterial blood gas read on admission was partial pressure of oxygen of 130 mmHg and partial pressure of carbon dioxide 48 mmHg, pH 7.35. The patient's past medical history also revealed a complex history of colorectal cancer involving neoadjuvant radio–chemotherapy and subsequent lower anterior resection of the rectum in 2017. Nevertheless, she had a good quality of life over the last year.
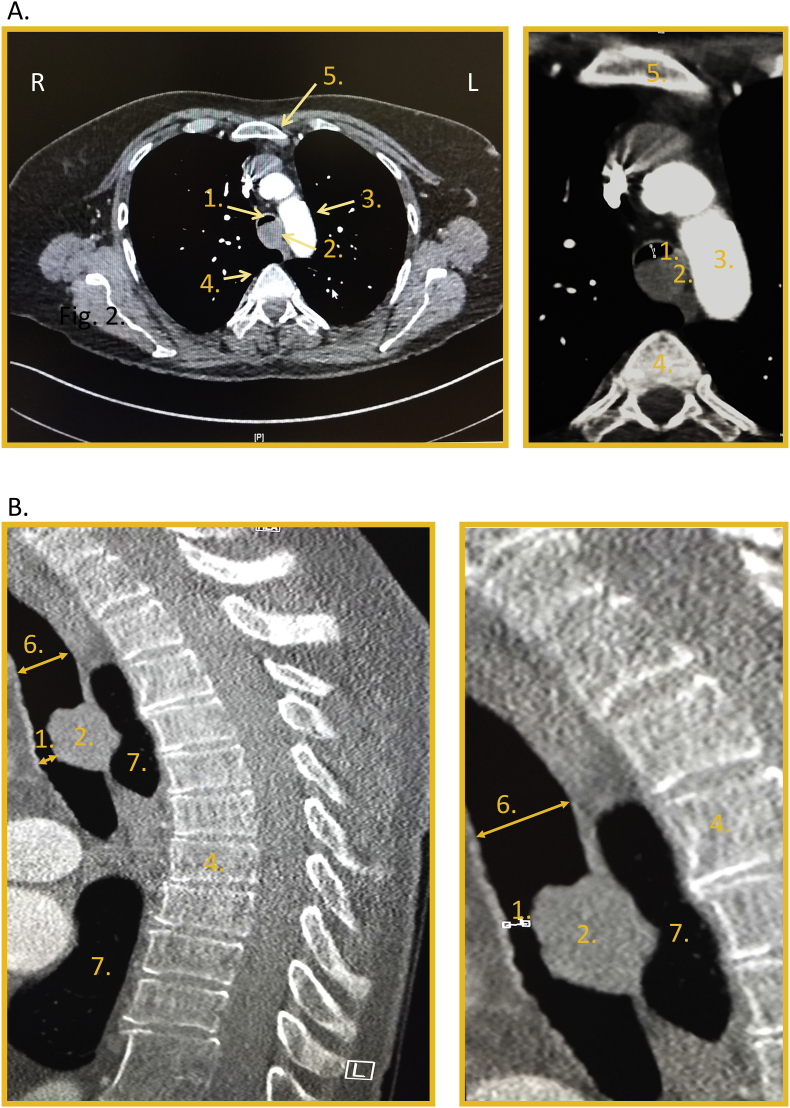
The immediately performed computed tomographic (CT) view of the chest revealed a severe occlusion of the tracheal lumen at the level of the aortic arch with a high density and homogeneous content (see Fig. 1). After she received a single dose of 50mg Prednisolone and an oral application of 10mg Paracodeine, she showed a sufficient spontaneous breathing and no need for assisted ventilation. The patient was therefore directly referred to stepdown unit for further bronchoscopic evaluation. Nevertheless, she presented another episode of dyspnea and the bronchoscopy was deferred. The patient was admitted to the ICU where her breathing could further be improved after another course of inhaled antiobstructive medication.
Fig. 1.
CT-Scans of the thorax: A. Axial view and B. Sagittal view showing less than 5 mm residual tracheal lumen (1.) due to an endotracheal metastasis (2.) on the level of aortic arch (3.). 4. and 5. denote the vertebra and the sternum, respectively. 6. and 7. show the tracheal lumen above the obstruction and the lungs parenchyma, respectively.
A multidisciplinary team consulting, including intensivists, pulmonologist, the anesthesia team and ENT surgeon was conducted at the bedside in the ICU with particular attention on the airway management. All front-of-neck airway techniques were excluded as reasonable possibilities due to the location of the obstruction. Supraglottic airway tools or tracheal intubation were also excluded because of the profound placement of the tumor i.e., at the level of aortic arch. Moreover, the small residual tracheal lumen and the possibility of bleeding complications could predispose the patient to difficult bag mask ventilation. Therefore, the in-house ECMO-Team was contacted in order to contemplate the option of preoperatively instituting of VV ECMO in awake state in the ICU in order to secure the airway in controlled condition. After thorough discussion, an individualized management strategy consisting of institution of a VV ECMO circuit under local anesthesia with the patient awake in the ICU was developed. This strategy also included the use of high ECMO blood flow, in order to prevent the need for continued anticoagulation therapy for surgery and thus reduce the chance of endotracheal bleeding complications. Once the ECMO circuit runs the patient would be brought to the OR and anesthesia would be induced. In order to avoid bleeding, the ENT surgeon would perform CO2-laser assisted debulking of the mass, reexpand the airway and obtain samples for pathologic assessment. After surgery, the patient would be further managed in the ICU.
For the ECMO cannulation, the patient was lightly sedated with 5 mg Midazolam i.v. and 3 mg Oxycodon i.v. The oxygen saturation was 100% under 6 l/min via NRB. The right groin and the right side of the neck were prepared and infiltrated with 0,25%-Bupivacainhydrochloride. After sonographic identification of the right femoral and the right internal jugular vein, a multibore–venous drainage cannula (23F diameter, 38 cm length) and a single port–reinfusion cannula (21F, 23 cm length) were uneventfully placed in the vessels by a Seldinger technique. The circuit was established under continuous wetting with a balanced crystalloid fluid with 10IE/ml of unfractionated Heparin. Once, the circuit was started, an ECMO blood flow of 4.5l/min was established (by patient's bodyweight of 152 lbs (69kg)), in order to avoid the need for systemic anticoagulation. The ECMO gas flow was adjusted to normal pH values. The patient was awake and responsive and showed a normal respiratory pattern throughout the entire procedure.
Once the ECMO flows were established the awake patient was brought to the OR. She was placed in supine position on the operation table. A Remifentanil–Propofol–Rocuronium sequence was then used for induction and maintaining of anesthesia. The glottis and the cranial trachea were easily visualized using a Kleinsasser operating laryngoscope through which placement of an endotracheal MLT-tube (size 5.0) was uneventfully performed by the ENT surgeon (above the occlusion). The tube was removed for the surgery, the tumor was subtotally resected using a CO2-laser under microscopic sight and a Dumon tracheal stent (14 mm) was placed. SpO2 remained 100% during the several minutes of apnea while performing the surgery.
After completion of the surgery the patient was taken to the intensive care unit still sedated and intubated. The postoperative management strategy of the ICU team consisted of establishment of spontaneous breathing and early extubation prior to the ECMO weaning. Early after discontinuation of sedation the patient developed spontaneous breathing and could be put on an assisted breathing mode and then uneventfully extubated. With the patient still fully awake and spontaneously breathing without dyspnea or wheezing, and no surgical complications, the ECMO blood flow was reduced to 1 L/min and the gas flow was turned off. Subsequently, her breathing pattern and blood gases remained stable and thus the ECMO was successfully removed after 19 hours total runtime. After removal of the ECMO the patient was kept in the ICU for one more day to account for complications but fortunately the further course was uneventful.
On the next day the patient was transferred in a general good condition to a regular internal medicine ward for a COPD-therapy adjustment and further evaluation of her tumor disease. The histopathologic examination of the resection specimens revealed a moderately differentiated adenocarcinoma (G2), histomorphologically reconcilable with the earlier tissue-pathological outcome from the colorectal tumor. A check bronchoscopy 4 months after the surgery revealed no postoperative or postintubation tracheal stenosis.
3. Discussion and conclusions
This case report presents the airway management in a case of a severe tracheal occlusion from the preclinical setting trough the OR to the ICU. The report emphasizes on the use of the perioperative ECMO support for providing a safe environment for surgery in a patient at high risk of complete airway obstruction. The approach used in this patient was based on the broad clinical practice with extracorporeal life support devices from our institution (e.g., see Refs. [[26], [27], [28], [29], [30], [31], [32]]). A variety of Extracorporeal Life Support Techniques and other forms of Cardiopulmonary Bypass have already been used for perioperative support in adult patients with different airway obstructions. For instance, this systemic review on the use of extracorporeal life support in anticipated difficult airway identified 45 cases published between 1976 and 2017 [33]. All 45 patients from the analyzed clinical cases were reported to survive to hospital discharge without significant complications [33]. However, very few studies reported the elective use of both forms of ECMO (VV and VA) in patients with anticipated difficult airway [24,[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]]. Indeed, a very recent study reported the perioperative use of VV ECMO in a case of severe extrinsic airway obstruction of the trachea [38]. However, unlike in our case the nature of the obstruction allowed the ECMO initiation under general anesthesia and the removal of the mass via median sternotomy. The VV ECMO is instituted using femoral or jugular venous cannulation that could also be applied with local anesthesia, whereas the standard cardiopulmonary bypass usually requires transthoracic cannulation under general anesthesia. The cardiopulmonary bypass takes not only over the function of the lungs but also over the heart. The extracorporeal membrane oxygenation, and especially the veno-venous form, is therefore the easily accessible and applicable technique that could be performed outside of the operation room and from non-surgeons as well. Thereby, the VV ECMO was the technique of choice for the presented patient.
One could argue, that the surgery presented in this report would theoretically allow the use of jet ventilation with a rapid flow of compressed gas delivered at a supraphysiologic frequency to maintain sufficient oxygenation, while carbon dioxide removal being maintained through passive expiration [47]. Jet ventilation catheters are thinner than the standard endotracheal tubes and could maintain a sufficient gas exchange by providing small tidal volumes delivered with a high frequency. This approach was however denied in the presented case considering the following aspects: high-frequency jet ventilation could generate a rapid increase of the airway pressure because of a gas trapping due to an inadequate expiratory passageway, which could cause barotrauma or block the surgical area in both upper and lower tracheal obstructions [48]. Moreover, it has been suggested that the high-frequency jet ventilation could cause a barotrauma independently, whether applied above or below the tracheal stenosis when the small residual tracheal lumen is minor [48]. Therefore, the jet ventilation should be applied with intubation and control of driving pressure and end–expiratory–tracheal pressure. Finally, the chance of intratracheal bleeding during surgery excluded the possibility to use an open airway system such as jet ventilation as an option to insure sufficient gas exchange.
Another important aspect in the management of the presented case is that the patient was kept intubated and ventilated until transferred to the ICU. Although the tracheal extubation does not receive the same attention as the intubation in the airway management guidelines, it also represents a high‐risk phase of anesthesia [49] that can be challenging even when performed in the controlled setting of an intensive care unit [50]. The most common incidence of hypoxic brain damage and perianesthetic death take place during tracheal intubation and extubation [7,49]. In line with this notion, the guidelines on airway management of the German Society of Anesthesiology and Intensive Care Medicine stated, that “the extubation can be as equally critical as the intubation, especially after difficult airway management” [9]. Moreover, the Difficult Airway Society of Britain and Ireland developed guidelines for tracheal extubation and post‐extubation care emphasizing the importance of planning and preparation [49]. The authors distinguish between "lowrisk" and "atrisk" extubations; pre‐existing airway difficulties, perioperative airway deterioration and restricted airway access are proposed as factors defining "at‐risk" extubation [49]. So far, the airway guidelines don't consider the perioperative ECMO support as a technique to provide sufficient tissue oxygenation and there are no recommendations on how to proceed to extubation during or after ECMO-therapy. The case of the patient presented here was determined as "at‐risk" of extubation and hence the strategy of the ICU team was first to enable spontaneous breathing and complete weaning from mechanical ventilation and then to proceed to ECMO-weaning with the patient fully awake and extubated. Thereby, a safe extubation could be achieved in a controlled situation with the ECMO continuing in the ICU, and thus eliminating the risk to solve airway complication compounded by time pressure.
Nevertheless, the benefits of protective perioperative ECMO in non-ARDS patients have to be weighed against the risk of ECMO. Concerning the complications associated with the use of veno-venous extracorporeal membrane oxygenation, a recent systematic review and meta-analysis assessed the hospital mortality and complication rates associated with VV ECMO from the latest literature [51]. Twelve studies representing a population of 1042 patients with refractory ARDS and VV ECMO were included into analysis. The outcomes of the analyzed studies were highly dependent on the age of the patient, the year of study realization, and the days of mechanical ventilation. Accordingly, the authors reported that complications are commonly present in VV ECMO in ARDS patients during the treatment course, however with a limited impact on the overall patient outcome accounting for 7% of fatal cases [51]. Despite of the fact that bleeding complications are frequent, its attributable mortality in veno-venous ECMO is minimal. Overall the authors stated, that the ECMO-complications’ impact on mortality is limited [51]. Given that, a significantly lower risk of mortality associated complications should be expected in case of elective perioperative VV ECMO support in patients with no respiratory failure, no systemic anticoagulation and less than 48 hours expected time of mechanical ventilation.
Surgical resections of tracheal tumors cannot be performed safely without providing adequate ventilation to the patient. However, ventilation may be impossible in patients with lower, more distal tracheal occlusions. Most of the national guidelines for management of anticipated airway difficulties recommend methods that maintain spontaneous respiration while attempting to secure a definitive airway. The elective use of extracorporeal membrane oxygenation in patients with severe tracheal obstruction below the level of the larynx has not been recommended so far. In such patients ECMO could ensure an adequate gas exchange facilitating the two most critical phases of anesthesia; the tracheal intubation and extubation. On the other hand, it minimizes the influence of the endotracheal tube and therefore it comforts the surgeon enabling an optimal surgical exposure [52]. In the postoperative phase, the ECMO provides the opportunity for ultra-protective mechanical ventilation and early extubation [22] which could facilitate wound healing and reduce the risk of developing a tracheal stenosis due to growing scar tissue in the trachea induced by prolonged intubation or tracheostomy [[53], [54], [55]].
In conclusion, this case report lends further support to the notion, that the extracorporeal membrane oxygenation should be incorporated in the airway guidelines at least as a complementary approach, that could provide a safe environment for surgery in situations in which the standard maneuvers of sustaining adequate gas exchange are anticipated to fail.
Author's contributions
All authors were directly involved in the treatment of the patient (ICU treatment, ECMO cannulation, surgery). IC condensed the case data and drafted and revised the manuscript. LOH and OM supervised the data analysis and helped drafting and revising the manuscript. AM revised the manuscript. All authors read and approved the final version of the manuscript.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Declaration of competing interest and funding
IC and LOH received noncash benefits for advanced trainings from CSL Behring. ACM has no conflicts of interest to report. OM received honoraria for lectures during workshops on hemodynamic monitoring, supported by Pulsion (Maquet Critical Care) and for 2 lectures during industrial sessions at national congresses (HillRom, Hepawash) We acknowledge support by the Open Access Publication Funds of the Göttingen University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101130.
Contributor Information
I. Chakalov, Email: ivan.chakalov@med.uni-goettingen.de, chakalov.ivan@gmail.com.
L.O. Harnisch, Email: lars-olav.harnisch@med.uni-goettingen.de.
A.C. Meyer, Email: alexander.meyer@med.uni-goettingen.de.
O. Moerer, Email: omoerer@med.uni-goettingen.de.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Goldstein B.J., Goldenberg D. The difficult airway: implications for the otolaryngologist–head and neck surgeon. Oper. Tech. Otolaryngol. Neck Surg. 2007 Jun 1;18(2) https://linkinghub.elsevier.com/retrieve/pii/S1043181007000358 [cited 2019 May 27] 72–6. Available from. [Google Scholar]
- 2.Bruijstens L., Titulaer I., Scheffer G.J., Steegers M., van den Hoogen F. Emergency front-of-neck airway by ENT surgeons and residents: a Dutch national survey. Laryngoscope Investig. Otolaryngol. 2018 Oct;3(5) doi: 10.1002/lio2.183. http://www.ncbi.nlm.nih.gov/pubmed/30410989 [cited 2019 May 26] 356–63. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedenstierna G. Alveolar collapse and closure of airways: regular effects of anaesthesia. Clin. Physiol. Funct. Imag. 2003 May 1;23(3) doi: 10.1046/j.1475-097X.2003.00483.x. [cited 2019 Jun 19] 123–9. Available from. [DOI] [PubMed] [Google Scholar]
- 4.Bagheri S.C., Bell B., Khan B. Difficult airway society 2015 guidelines for management of unanticipated difficult intubation in adults: need to be revisited? Br. J. Anaesth. 2016;115(4) doi: 10.1093/bja/aew278. https://bjanaesthesia.org/article/S0007-0912(17)30175-7/pdf [cited 2019 Feb 2] 529–72. Available from. [DOI] [PubMed] [Google Scholar]
- 5.Frerk C., Mitchell V.S., Mcnarry A.F., Mendonca C., Bhagrath R., Patel A. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults †. Br. J. Anaesth. 2015;115(6) doi: 10.1093/bja/aev371. http://www.anzca.edu.au [cited 2019 Feb 2] 827–75. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagberg C., Gabel J.C., Connis R. Difficult Airway Society 2015 guidelines for the management of unanticipated difficult intubation in adults: not just another algorithm. 2015. http://www.anzca.edu.au/resources/ [cited 2019 Feb 2]; Available from. [DOI] [PubMed]
- 7.Henderson J.J., Popat M.T., Latto I.P., Pearce A.C. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004 Jul 1;59(7) doi: 10.1111/j.1365-2044.2004.03831.x. [cited 2019 Apr 28] 675–94. Available from. [DOI] [PubMed] [Google Scholar]
- 8.Apfelbaum J.L., Hagberg C.A., Caplan R.A., Blitt C.D., Connis R.T., Nickinovich D.G. Practice guidelines for management of the difficult airway. Anesthesiology. 2013 Feb 1;118(2) doi: 10.1097/ALN.0b013e31827773b2. http://insights.ovid.com/crossref?an=00000542-201302000-00012 [cited 2019 Feb 2] 251–70. Available from. [DOI] [PubMed] [Google Scholar]
- 9.Piepho T., Cavus E., Noppens R., Byhahn C., Dörges V., Zwissler B. S1 guidelines on airway management. Anaesthesist. 2015 Dec 4;64(S1) doi: 10.1007/s00101-015-0087-6. http://link.springer.com/10.1007/s00101-015-0109-4 [cited 2019 Apr 24] 27–40. Available from. [DOI] [PubMed] [Google Scholar]
- 10.Ho A.M.H., Chung D.C., To E.W.H., Karmakar M.K. Total airway obstruction during local anesthesia in a non-sedated patient with a compromised airway. Can. J. Anaesth. 2004 Oct;51(8) doi: 10.1007/BF03018461. http://link.springer.com/10.1007/BF03018461 [cited 2019 Apr 21] 838–41. Available from. [DOI] [PubMed] [Google Scholar]
- 11.McGuire G., el-Beheiry H. Complete upper airway obstruction during awake fibreoptic intubation in patients with unstable cervical spine fractures. Can. J. Anaesth. 1999 Feb;46(2) doi: 10.1007/BF03012553. http://link.springer.com/10.1007/BF03012553 [cited 2019 Apr 21] 176–8. Available from. [DOI] [PubMed] [Google Scholar]
- 12.Shaw I.C., Welchew E.A., Harrison B.J., Michael S. Complete airway obstruction during awake fibreoptic intubation. Anaesthesia. 1997 Jun;52(6) doi: 10.1111/j.1365-2044.1997.155-az0155.x. http://www.ncbi.nlm.nih.gov/pubmed/9203888 [cited 2019 Apr 21] 582–5. Available from. [DOI] [PubMed] [Google Scholar]
- 13.White A.N.J., Wong D.T., Goldstein C.L., Wong J. Cervical spine overflexion in a halo orthosis contributes to complete upper airway obstruction during awake bronchoscopic intubation: a case report. Can. J. Anesth. 2015;62(3) doi: 10.1007/s12630-014-0282-y. http://www.ncbi.nlm.nih.gov/pubmed/25467754 Mar 3 [cited 2019 Apr 21] 289–93. Available from. [DOI] [PubMed] [Google Scholar]
- 14.McRae K. Principles and Practice of Anesthesia for Thoracic Surgery [Internet] Springer International Publishing; Cham: 2019. Tracheal resection and reconstruction.http://link.springer.com/10.1007/978-3-030-00859-8_13 [cited 2019 Apr 22]. pp. 231–48. Available from. [Google Scholar]
- 15.McRae K., de Perrot M. Principles and indications of extracorporeal life support in general thoracic surgery. J. Thorac. Dis. 2018 Apr;10(Suppl 8) doi: 10.21037/jtd.2018.03.116. http://www.ncbi.nlm.nih.gov/pubmed/29744220 [cited 2019 Apr 22] S931–46. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broman L.M., Taccone F.S., Lorusso R., Malfertheiner M.V., Pappalardo F., Di Nardo M. The ELSO Maastricht Treaty for ECLS Nomenclature: abbreviations for cannulation configuration in extracorporeal life support - a position paper of the Extracorporeal Life Support Organization. Crit. Care. 2019 Dec 8;23(1):36. doi: 10.1186/s13054-019-2334-8. https://ccforum.biomedcentral.com/articles/10.1186/s13054-019-2334-8 [cited 2020 Jun 1] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad S.A., Broman L.M., Taccone F.S., Lorusso R., Malfertheiner M.V., Pappalardo F. American Journal of Respiratory and Critical Care Medicine. vol. 198. American Thoracic Society; 2018. The extracorporeal life support organization Maastricht treaty for nomenclature in extracorporeal life support a position paper of the extracorporeal life support organization; pp. 447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez G., Vuylsteke A., Fficm F. Extracorporeal membrane oxygenation in adults. https://pdfs.semanticscholar.org/9767/90e437e9c4a3cd6571173a1a9fc23abfc7b2.pdf [cited 2019 Feb 2]; Available from.
- 19.Moerer O., Vasques F., Duscio E., Cipulli F., Romitti F., Gattinoni L. Extracorporeal gas exchange. Crit. Care Clin. 2018 Jul 1;34(3) doi: 10.1016/j.ccc.2018.03.011. http://www.ncbi.nlm.nih.gov/pubmed/29907273 [cited 2019 Jun 19] 413–22. Available from. [DOI] [PubMed] [Google Scholar]
- 20.Hemmila M.R., Rowe S.A., Boules T.N., Miskulin J., McGillicuddy J.W., Schuerer D.J. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann. Surg. 2004 Oct;240(4v):595–605. doi: 10.1097/01.sla.0000141159.90676.2d. http://www.ncbi.nlm.nih.gov/pubmed/15383787 [cited 2019 Apr 22] discussion 605-7. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brogan T.V., Thiagarajan R.R., Rycus P.T., Bartlett R.H., Bratton S.L. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intens. Care Med. 2009 Dec 22;35(12) doi: 10.1007/s00134-009-1661-7. http://link.springer.com/10.1007/s00134-009-1661-7 [cited 2019 Apr 22] 2105–14. Available from. [DOI] [PubMed] [Google Scholar]
- 22.Fichtner F., Moerer O., Weber-Carstens S., Nothacker M., Kaisers U., Laudi S. Clinical guideline for treating acute respiratory insufficiency with invasive ventilation and extracorporeal membrane oxygenation: evidence-based recommendations for choosing modes and setting parameters of mechanical ventilation. Respiration. 2019 Oct 1;98(4) doi: 10.1159/000502157. http://www.ncbi.nlm.nih.gov/pubmed/31505511 [cited 2020 Feb 8] 357–72. Available from. [DOI] [PubMed] [Google Scholar]
- 23.Hoetzenecker K., Klepetko W., Keshavjee S., Cypel M. Extracorporeal support in airway surgery. J Thorac Dis. 2017;9(7):2108. doi: 10.21037/jtd.2017.06.17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5542968/ [cited 2019 Apr 22] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim C.W., Kim D.H., Son B.S., Cho J.S., Kim Y.D., I H. The feasibility of extracorporeal membrane oxygenation in the variant airway problems. Ann. Thorac. Cardiovasc. Surg. 2015;21(6):517. doi: 10.5761/atcs.oa.15-00073. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4905028/ [cited 2019 Apr 22] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monaco F., Belletti A., Bove T., Landoni G., Zangrillo A. Extracorporeal membrane oxygenation: beyond cardiac surgery and intensive care unit: unconventional uses and future perspectives. J. Cardiothorac. Vasc. Anesth. 2018 Aug 1;32(4) doi: 10.1053/j.jvca.2018.03.031. https://www.sciencedirect.com/science/article/pii/S1053077018302234 [cited 2019 Apr 22] 1955–70. Available from. [DOI] [PubMed] [Google Scholar]
- 26.Gattinoni L., Tonetti T., Quintel M. How best to set the ventilator on extracorporeal membrane lung oxygenation. Curr. Opin. Crit. Care. 2017 Feb;23(1):66–72. doi: 10.1097/MCC.0000000000000376. http://www.ncbi.nlm.nih.gov/pubmed/27898437 [cited 2019 May 4] Available from. [DOI] [PubMed] [Google Scholar]
- 27.Harnisch L.O., Moerer O. Sequential use of extracorporeal devices to avoid mechanical ventilation in a patient with complicated pulmonary fibrosis. J. Artif. Organs. 2017 Dec 1;20(4) doi: 10.1007/s10047-017-0983-4. http://www.ncbi.nlm.nih.gov/pubmed/28864998 [cited 2019 May 4] 365–70. Available from. [DOI] [PubMed] [Google Scholar]
- 28.Moerer O., Harnisch L.-O., Barwing J., Heise D., Heuer J.F., Quintel M. Minimal-flow ECCO2R in patients needing CRRT does not facilitate lung-protective ventilation. J. Artif. Organs. 2019 Mar 3;22(1):68–76. doi: 10.1007/s10047-018-1068-8. http://link.springer.com/10.1007/s10047-018-1068-8 [cited 2019 May 4] Available from. [DOI] [PubMed] [Google Scholar]
- 29.Heuer J.F., Mirschel M., Bleckmann A., Quintel M., Moerer O. Interhospital transport of ARDS patients on extracorporeal membrane oxygenation. J. Artif. Organs. 2019 Mar 18;22(1):53–60. doi: 10.1007/s10047-018-1065-y. http://www.ncbi.nlm.nih.gov/pubmed/30121790 [cited 2019 May 4] Available from. [DOI] [PubMed] [Google Scholar]
- 30.Mielck F., Quintel M. Extracorporeal membrane oxygenation. Curr. Opin. Crit. Care. 2005 Feb;11(1):87–93. doi: 10.1097/00075198-200502000-00014. http://www.ncbi.nlm.nih.gov/pubmed/15659951 [cited 2019 May 4] Available from. [DOI] [PubMed] [Google Scholar]
- 31.Moerer O., Harnisch L.-O., Herrmann P., Zippel C., Quintel M. Patient-Ventilator interaction during noninvasive ventilation in simulated COPD. Respir. Care. 2016 Jan 1;61(1):15–22. doi: 10.4187/respcare.04141. http://rc.rcjournal.com/cgi/doi/10.4187/respcare.04141 [cited 2019 May 3] Available from. [DOI] [PubMed] [Google Scholar]
- 32.Moerer O., Tonetti T., Quintel M. Rescue therapies for acute respiratory distress syndrome. Curr. Opin. Crit. Care. 2017 Feb;23(1) doi: 10.1097/MCC.0000000000000374. http://www.ncbi.nlm.nih.gov/pubmed/27898438 [cited 2019 May 4] 52–9. Available from. [DOI] [PubMed] [Google Scholar]
- 33.Malpas G., Hung O., Gilchrist A., Wong C., Kent B., Hirsch G.M. The use of extracorporeal membrane oxygenation in the anticipated difficult airway: a case report and systematic review. Can. J. Anesth. 2018 Jun 1;65(6) doi: 10.1007/s12630-018-1099-x. http://link.springer.com/10.1007/s12630-018-1099-x [cited 2019 Feb 2] 685–97. Available from. [DOI] [PubMed] [Google Scholar]
- 34.Fung R.K.F., Stellios J., Bannon P.G., Ananda A., Forrest P. Elective use of veno-venous extracorporeal membrane oxygenation and high-flow nasal oxygen for resection of subtotal malignant distal airway obstruction. Anaesth. Intensive Care. 2017 Jan 1;45(1):88–91. doi: 10.1177/0310057X1704500113. [DOI] [PubMed] [Google Scholar]
- 35.Giovacchini C., Blough B.…LC-CEA undefined. Primary endobronchial schwannoma successfully removed via rigid bronchoscopy on veno-venous extracorporeal membrane oxygenation (VV-ECMO) support. atsjournals.org. 2017. https://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A5616 [cited 2020 Feb 13]; Available from.
- 36.Natt B., Knepler J., Kazui T., Mosier J.M. The use of extracorporeal membrane oxygenation in the bronchoscopic management of critical upper airway obstruction. J. Bronchol. Interv. Pulmonol. 2017 Jan 1;24(1) doi: 10.1097/LBR.0000000000000347. http://www.ncbi.nlm.nih.gov/pubmed/27984394 [cited 2020 Feb 13] e12–4. Available from. [DOI] [PubMed] [Google Scholar]
- 37.Tian F., Li W., Liang X., Wang X., Zhou Y., Yan X. Case Report Application of extracorporeal membrane oxygenation (ECMO) in tracheal tumor resection [Internet] Int. J. Clin. Exp. Med. 2017;10 www.ijcem.com/ [cited 2020 Feb 13]. Available from. [Google Scholar]
- 38.Raza H.A., Nokes B.T., Jaroszewski D., Garrett A., Sista R., Ross J. VV-ECMO for surgical cure of a critical central airway obstruction. Respir. Med. Case Rep. 2019 Jan 1:28. doi: 10.1016/j.rmcr.2019.100890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onozawa H., Tanaka T., Takinami M., Kagaya S., Tanifuji Y. Anesthetic management using extracorporeal circulation of a patient with severe tracheal stenosis by thyroid cancer. Masui. 1999 Jun;48(6) http://www.ncbi.nlm.nih.gov/pubmed/10402824 [cited 2019 Jun 16] 658–61. Available from. [PubMed] [Google Scholar]
- 40.Shiraishi T., Shirakusa T., Hiratsuka M., Yamamoto S., Iwasaki A., Kawahara K. Stenting for critical airway stenosis under percutaneous cardiopulmonary support. Jpn. J. Thorac. Cardiovasc. Surg. 2004;52(12):592–596. doi: 10.1007/s11748-004-0032-0. [DOI] [PubMed] [Google Scholar]
- 41.Shao Y., Shen M., Ding Z., Liang Y., Zhang S. Extracorporeal membrane oxygenation-assisted resection of goiter causing severe extrinsic airway compression. Ann. Thorac. Surg. 2009 Aug;88(2) doi: 10.1016/j.athoracsur.2008.12.073. http://www.ncbi.nlm.nih.gov/pubmed/19632436 [cited 2020 Feb 13] 659–61. Available from. [DOI] [PubMed] [Google Scholar]
- 42.Jeon H.K., So Y.K., Yang J.H., Jeong H.S. Extracorporeal oxygenation support for curative surgery in a patient with papillary thyroid carcinoma invading the trachea. J. Laryngol. Otol. 2009 Jul;123(7) doi: 10.1017/S0022215108003216. http://www.ncbi.nlm.nih.gov/pubmed/18808728 [cited 2020 Feb 13] 807–10. Available from. [DOI] [PubMed] [Google Scholar]
- 43.Gourdin M., Dransart C., Delaunois L., Louagie Y.A.G., Gruslin A., Dubois P. Use of venovenous extracorporeal membrane oxygenation under regional anesthesia for a high-risk rigid bronchoscopy. J Cardiothorac. Vasc. Anesth. 2012 Jun;26(3) doi: 10.1053/j.jvca.2011.02.013. http://www.ncbi.nlm.nih.gov/pubmed/21546268 [cited 2020 Feb 13] 465–7. Available from. [DOI] [PubMed] [Google Scholar]
- 44.Hong Y., Jo K.-W., Lyu J., Huh J.W., Hong S.B., Jung S.-H. Use of venovenous extracorporeal membrane oxygenation in central airway obstruction to facilitate interventions leading to definitive airway security. J. Crit. Care. 2013 Oct;28(5) doi: 10.1016/j.jcrc.2013.05.020. http://www.ncbi.nlm.nih.gov/pubmed/23845793 [cited 2020 Feb 13] 669–74. Available from. [DOI] [PubMed] [Google Scholar]
- 45.Liou J.-Y., Chow L.-H., Chan K.-H., Tsou M.-Y. Successful anesthetic management of a patient with thyroid carcinoma invading the trachea with tracheal obstruction, scheduled for total thyroidectomy. J. Chin. Med. Assoc. 2014 Sep 1;77(9) doi: 10.1016/j.jcma.2014.06.006. http://www.ncbi.nlm.nih.gov/pubmed/25150647 [cited 2020 Feb 13] 496–9. Available from. [DOI] [PubMed] [Google Scholar]
- 46.Dunkman W.J., Nicoara A., Schroder J., Wahidi M.M., El Manafi A., Bonadonna D. Elective venovenous extracorporeal membrane oxygenation for resection of endotracheal tumor: a case report. 2017 Aug 15. http://www.ncbi.nlm.nih.gov/pubmed/28542046 [cited 2020 Feb 13], Available from, 9(4):97-100. [DOI] [PubMed]
- 47.Putz L., Mayné A., Dincq A.-S. Jet ventilation during rigid bronchoscopy in adults: a focused review. Biomed. Res. Int. 2016 Oct 26;2016:1–6. doi: 10.1155/2016/4234861. https://www.hindawi.com/journals/bmri/2016/4234861/ [cited 2019 Apr 10] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buczkowski P.W., Fombon F.N., Lin E.S., Russell W.C., Thompson J.P. Air entrainment during high-frequency jet ventilation in a model of upper tracheal stenosis †. Br. J. Anaesth. 2007 Dec;99(6) doi: 10.1093/bja/aem312. http://www.ncbi.nlm.nih.gov/pubmed/17977861 [cited 2019 Jun 15] 891–7. Available from. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell V., Dravid R., Patel A., Swampillai C., Higgs A., Higgs A. Difficult airway society guidelines for the management of tracheal extubation. Anaesthesia. 2012 Mar 1;67(3) doi: 10.1111/j.1365-2044.2012.07075.x. [cited 2019 Apr 28] 318–40. Available from. [DOI] [PubMed] [Google Scholar]
- 50.Sturgess D.J., Greenland K.B., Senthuran S., Ajvadi F.A., van Zundert A., Irwin M.G. Tracheal extubation of the adult intensive care patient with a predicted difficult airway - a narrative review. Anaesthesia. 2017 Feb 1;72(2) doi: 10.1111/anae.13668. [cited 2019 May 4] 248–61. Available from. [DOI] [PubMed] [Google Scholar]
- 51.Vaquer S., de Haro C., Peruga P., Oliva J.C., Artigas A. Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann. Intens. Care. 2017 Dec;7(1):51. doi: 10.1186/s13613-017-0275-4. http://www.ncbi.nlm.nih.gov/pubmed/28500585 [cited 2019 Aug 10] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rinieri P., Peillon C., Bessou J.-P., Veber B., Falcoz P.-E., Melki J. National review of use of extracorporeal membrane oxygenation as respiratory support in thoracic surgery excluding lung transplantation. Eur. J. Cardiothorac. Surg. 2015 Jan 1;47(1):87–94. doi: 10.1093/ejcts/ezu127. https://academic.oup.com/ejcts/article-lookup/doi/10.1093/ejcts/ezu127 [cited 2019 Feb 2] Available from. [DOI] [PubMed] [Google Scholar]
- 53.Esteller-Moré E., Ibañez J., Matiñó E., Ademà J.M., Nolla M., Quer I.M. Prognostic factors in laryngotracheal injury following intubation and/or tracheotomy in ICU patients. Eur. Arch. Oto-Rhino-Laryngol. 2005 Nov;262(11) doi: 10.1007/s00405-005-0929-y. http://www.ncbi.nlm.nih.gov/pubmed/16258758 [cited 2020 Feb 8] 880–3. Available from. [DOI] [PubMed] [Google Scholar]
- 54.Papla B., Dyduch G., Frasik W., Olechnowicz H. Post-intubation tracheal stenosis--morphological-clinical investigations. Pol. J. Pathol. 2003;54(4) http://www.ncbi.nlm.nih.gov/pubmed/14998295 [cited 2020 Feb 8] 261–6. Available from. [PubMed] [Google Scholar]
- 55.Farzanegan R., Farzanegan B., Zangi M., Eraghi M.G., Noorbakhsh S., Tabarestani N.D. Incidence rate of post-intubation tracheal stenosis in patients admitted to five intensive care units in Iran. Iran. Red Crescent Med. J. 2016 Sep 1;18(9) doi: 10.5812/ircmj.37574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.