In these interesting times, attention is being paid to borders, which regulate traffic into and out of our nations. Evolution long ago crafted a solution to a similar dilemma in the animal body, with epithelial tissues serving as barriers and regulating traffic. The skin, the lining of the gut, and the airways of the lungs separate one from the outside world. However, just as international borders need to open to allow import and export of goods, so the lining of one’s gut needs to organize itself to take up nutrients. To accomplish this task, epithelia are polarized, localizing particular proteins on their apical (top) or basolateral (bottom) surfaces. For example, glucose importers are positioned apically, facing the gut lumen, while glucose exporters are basal, delivering nutrients to the blood. Cell–cell junctions are localized at the interface of these two domains, and multiprotein apical and basolateral polarity complexes act in a mutually antagonistic way to establish and maintain polarity (reviewed in ref. 1). Two decades ago the Scribble (Scrib) module was identified as a major basolateral polarity regulator, but how it functions mechanistically remained in question (reviewed in ref. 2). In PNAS, Khoury and Bilder (3) provide important insights into the regulation and function of Scrib and its collaborators, Discs Large (Dlg) and Lethal Giant Larvae (Lgl), revealing how they regulate the key apical polarity determinant atypical protein kinase C (aPKC).
Scrib, Dlg, and Lgl were originally identified in the fruit fly Drosophila. Dlg and Lgl were initially identified as tumor suppressor genes, because disrupting their roles in epithelial polarity leads to larval tissue overgrowth (4, 5). In 2000 Bilder and Perrimon (6) identified Scribble as an important regulator of polarity and went on to show it acts together with or in parallel to Dlg and Lgl in both epithelial polarity and growth regulation (7). Meanwhile other laboratories using Caenorhabditis elegans and Drosophila identified parallel roles for apical polarity modules—one of these, the Par complex, includes the kinase aPKC, which phosphorylates basolateral and junction proteins to restrict them from the apical domain. However, how the Scrib module antagonized aPKC remained a mystery.
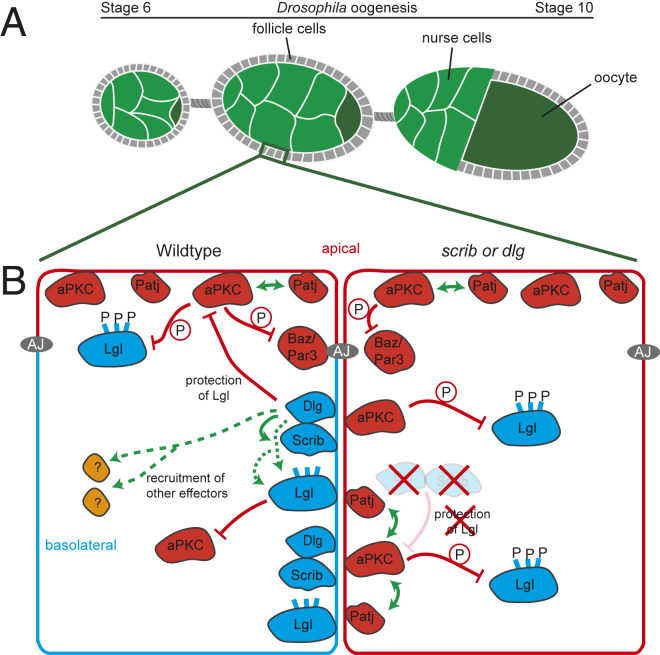
The main goal of Khoury and Bilder (3) was to define the molecular function of Scrib module proteins in basolateral domain regulation, using Drosophila follicle cells as a model (Fig. 1A). Previous work revealed that all three proteins in the Scrib module are important for the localization of the others (7). Khoury and Bilder (3) explored this further, using both immunolocalization in fixed tissue and dynamic analysis with fluorescence recovery after photobleaching (FRAP) assays. These reveal that in follicle cells a linear localization hierarchy exists, with Dlg on top, regulating Scrib localization, which then regulates the localization of Lgl (Fig. 1B, Left). However, a missing Scrib module protein cannot be bypassed by overexpressing other Scrib module proteins, and constitutive tethering of Scrib or Lgl to the cell cortex could not rescue polarity defects in dlg mutants. Thus, in addition to its role in the localization hierarchy, Dlg must have other roles in polarity, suggesting that Dlg and Scrib act in in parallel.
Fig. 1.
Scrib module proteins define the basolateral domain of epithelial cells. (A) Scheme of a Drosophila ovariole. The germline cells of each egg chamber are surrounded by an epithelium of follicle cells (gray) with their apical sides pointing to the nurse cells (green) and oocyte (dark green). (B) Scheme illustrating how wild-type polarity is defined by mutual antagonism between apical (red) and basolateral (blue) proteins. (B, Left) Wild type. aPKC phosphorylates and restricts Baz/Par3 from moving apically from adherens junctions, prevents apical localization of Lgl via phosphorylation, and recruits the apical determinant Patj. Basolateral Lgl inhibits aPKC activity. Cortical localization of the Scrib module involves a linear hierarchy with Dlg recruiting Scrib. Dlg and Scrib work together to protect basolateral Lgl from phosphorylation by aPKC, by inhibiting aPKC movement into the basolateral domain. Dlg and Scrib are also responsible for the recruitment of unknown basolateral effectors which help to define the basolateral domain. (B, Right) scrib or dlg mutants. Cells which are mutant for dlg and/or scrib lack the protection of Lgl. Apical aPKC spreads into the basolateral domain, phosphorylating basolateral Lgl and displacing it into the cytoplasm. Basolateral aPKC can then recruit other apical determinants like Patj, leading to a respecification of the basolateral domain into an apical domain.
They next explored how Dlg recruits Scrib to the cortex (3). They first rule out an essential role for Dlg in regulating posttranslational modification of Scrib, as Scrib did not show changes in palmitoylation in dlg mutants. They also rule out an essential role for the phospholipids PIP2 and PIP3 in Scrib cortical recruitment. Studies in mammals and in other Drosophila tissues led to the model that Dlg regulates Scrib localization by physical binding, with Dlg’s guanylate kinase (GUK) domain directly or indirectly binding to Scrib’s PDZ domains (8–10). However, Khoury and Bilder (3) rule out essential roles for these domains in follicle cells as Dlg or Scrib mutants lacking these domains do not show polarity defects. Instead, they find that Scrib recruitment and polarity function depend on the SH3 domain of Dlg and the N-terminal LRR domain of Scrib.
The mutually inhibitory relationship between the Scrib module protein Lgl and the apical polarity protein aPKC is well established. Lgl is removed from the apical cortex by phosphorylation by aPKC, and Lgl in turn can inhibit aPKC from entering the basolateral domain (Fig. 1B, Left). Khoury and Bilder (3) thus examined how Scrib and Dlg fit into the mutual antagonism picture. They first rule out roles for aPKC in regulating Dlg and Scrib localization—either in cells expressing constitutively active aPKC or in lgl mutants, basolateral expansion of aPKC does not displace Scrib and Dlg. Importantly, the presence of Dlg and Scrib in these situations also does not prevent basolateral expansion of aPKC. They next examined whether Dlg and Scrib act to directly inhibit aPKC activity. Their data suggest this is not the case, because when they targeted aPKC to the basolateral domain alongside Scrib/Dlg, it retained biological activity, as it was still able to recruit the apical protein PatJ. Given this, how do Scrib and Dlg protect Lgl from aPKC? Khoury and Bilder (3) support the idea that Dlg and Scrib stabilize and protect basolateral Lgl from aPKC, rather than directly recruiting it basolaterally (Fig. 1B, Left and Right). FRAP assays revealed Lgl is more mobile in dlg or scrib mutants—this is consistent with either direct recruitment or failure to antagonize aPKC. Interestingly, mutants of Lgl that cannot be phosphorylated by aPKC localize to the cortex even in dlg and scrib mutants, and codepleting aPKC in dlg mutants restores cortical Lgl, suggesting Dlg and Scrib are not essential for recruitment but play a protective role, antagonizing the basal spread of aPKC.
Khoury and Bilder (3) end by examining the sufficiency of the basolateral proteins to define a membrane as basolateral. Although phosphodeficient Lgl mutants can localize to the apical cortex and inhibit aPKC, they are not able to fully redefine this region as basolateral, as Scrib is not recruited to this region. Khoury and Bilder (3) then tested whether the proteins of the Scrib module are sufficient to define the basolateral domain by tethering them to the whole cell circumference via recruitment by a membrane-bound GFP nanobody or addition of a myristoylation tag. Even when all Scrib module proteins were simultaneously targeted to the apical domain, they colocalized there with aPKC and thus failed to redefine that membrane as basolateral. Thus, Scrib module proteins are necessary but not sufficient for the definition of the basolateral domain. Considered together, the work of Khoury and Bilder (3) reveals the complex functions of each of the members of the Scrib module, providing insights concerning regulation within the module and also insights into their broader roles in polarity formation and maintenance.
These insights have been amplified by parallel work from the laboratory of Morais-de-Sá and coworkers (11). Using highly complementary multidisciplinary approaches, they also explored the roles of the Scrib module in polarity. Like Khoury and Bilder (3), their data reveal that Lgl is a highly dynamic component of the basolateral domain, much more so than Scrib or Dlg. They find that aPKC plays an important role in restricting Lgl from the apical cortex, but it does not normally regulate Lgl dynamics on the basolateral membrane. However, if aPKC is repositioned basolaterally, as it is in scrib or dlg mutants, it can expel Lgl from the membrane (Fig. 1B, Right). This led to additional insights into the roles of Scrib and Dlg—rather than directly regulating Lgl, they act to prevent aPKC from moving basolaterally. Morais-de-Sá and coworkers (11) end by using a clever approach in which they test protein interactions in vivo. Their data suggest that while Scrib and Dlg form a complex, Lgl is not a part of this. Instead, the lipid PIP2 helps retain Lgl at the basolateral membrane. This also revealed that the Scrib LRR domain is required for binding Dlg, complementing the work of Khoury and Bilder (3).
Two other recent papers have further expanded the roles of the Scrib module in polarity. In the standard view, the apical and basolateral polarity modules primarily act after initial polarity establishment, elaborating and maintaining the initial domains set up by positioning the cell–cell adherens junctions at the apical–basolateral domain boundary. However, recent work revealed a critical role of Scrib and Dlg from the onset of polarity establishment. Scrib and Dlg act in a multifaceted way to ensure correct positioning of both Bazooka/Par3 and the cadherin–catenin complex as polarity is established in Drosophila (12). Another puzzle from the literature was that although Scrib plays an essential role in apical–basal polarity in Drosophila, knockout suggested its functions in mammals were more subtle and tissue specific (reviewed in ref. 2). Recent work from the laboratory of Troyanovsky and coworkers (13) helped resolve this dilemma. Mammals have three members of the LAP family: Scrib, Erbin, and Lano. Using triple knockouts in cultured mammalian epithelial cells, they found that that the three LAP proteins act in parallel to maintain adherens junction integrity, the tight junction epithelial barrier, and the localization of both apical and basolateral polarity proteins. Thus Scrib and its family members play conserved roles in both polarity establishment and maintenance.
These exciting data significantly advance our understanding of the molecular underpinnings of apical–basal polarity and set the foundation for further advances. We still do not understand the role of Lgl. Its yeast relatives Sro7/Sro77 regulate polarized exocytosis, in part via interactions with Rab GTPases (e.g., ref. 14), but how this plays into Lgl’s polarity function(s) is unclear. We also need to define how Scrib and Dlg antagonize aPKC. One possibility is that they act as decoy substrates, as both are heavily phosphorylated. They also may form transient complexes with Lgl that inhibit aPKC phosphorylation basolaterally. Further complexity is added by the fact that mislocalizing all three Scrib module proteins apically is not sufficient to redefine this region as basolateral. More broadly, we need to define how universal are the roles of different polarity modules. Work in Drosophila has revealed many cases where a polarity regulator that is essential in one tissue is dispensable in another. A recent example was provided by analysis of the Drosophila adult midgut, where “key players” like aPKC, Crumbs, Bazooka/Par3, and the Scrib module are not essential for polarity (15). We suspect that this reflects the importance of polarity establishment and maintenance, leading to the evolution of a very robust mechanism ensuring this. Strikingly, early work in the follicle cell epithelium studied here found overlapping and partially redundant roles for apical, junctional, and basal polarity cues (16). Solving puzzles like this will provide further insights into this core tissue property and also may help us understand how it is abrogated during cancer progression.
Acknowledgments
Research in our laboratory is supported by National Institutes of Health Grant R35 GM118096.
Footnotes
The authors declare no competing interest.
See companion article, “Distinct activities of Scrib module proteins organize epithelial polarity,” 10.1073/pnas.1918462117.
References
- 1.Campanale J. P., Sun T. Y., Montell D. J., Development and dynamics of cell polarity at a glance. J. Cell Sci. 130, 1201–1207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonello T. T., Peifer M., Scribble: A master scaffold in polarity, adhesion, synaptogenesis, and proliferation. J. Cell Biol. 218, 742–756 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoury M. J., Bilder D., Distinct activities of Scrib module proteins organize epithelial polarity. Proc. Natl. Acad. Sci. U.S.A. 117, 11531–11540 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods D. F., Bryant P. J., The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 66, 451–464 (1991). [DOI] [PubMed] [Google Scholar]
- 5.Gateff E., Schneiderman H. A., Developmental capacities of benign and malignant neoplasms of Drosophila. Wilhelm Roux Arch. Entwickl. Mech. Org. 176, 23–65 (1974). [DOI] [PubMed] [Google Scholar]
- 6.Bilder D., Perrimon N., Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403, 676–680 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Bilder D., Li M., Perrimon N., Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113–116 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Mathew D., et al. , Recruitment of scribble to the synaptic scaffolding complex requires GUK-holder, a novel DLG binding protein. Curr. Biol. 12, 531–539 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian Y., Prehoda K. E., Interdomain interactions in the tumor suppressor discs large regulate binding to the synaptic protein GukHolder. J. Biol. Chem. 281, 35757–35763 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caria S., et al. , Drosophila melanogaster Guk-holder interacts with the Scribbled PDZ1 domain and regulates epithelial development with Scribbled and Discs Large. J. Biol. Chem. 293, 4519–4531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventura G., Moreira S., Barros-Carvalho A., Osswald M., Morais-de-Sá E., Lgl cortical dynamics are independent of binding to the Scrib-Dlg complex but require Dlg-dependent restriction of aPKC. bioRxiv: 10.1101/867929 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Bonello T. T., Choi W., Peifer M., Scribble and Discs-large direct initial assembly and positioning of adherens junctions during the establishment of apical-basal polarity. Development 146, dev180976 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J., Troyanovsky R. B., Indra I., Mitchell B. J., Troyanovsky S. M., Scribble, Erbin, and Lano redundantly regulate epithelial polarity and apical adhesion complex. J. Cell Biol. 218, 2277–2293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi G., Watson K., Kennedy W., Brennwald P., The tomosyn homologue, Sro7, is a direct effector of the Rab GTPase, Sec4, in post-Golgi vesicle tethering. Mol. Biol. Cell 29, 1476–1486 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J., Sayadian A. C., Lowe N., Lovegrove H. E., St. Johnston D., An alternative mode of epithelial polarity in the Drosophila midgut. PLoS Biol. 16, e3000041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanentzapf G., Smith C., McGlade J., Tepass U., Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J. Cell Biol. 151, 891–904 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]