Abstract
Background
The aim of this study was to investigate the performance and predictive value of hypocalcemia in severe COVID-19 patients.
Methods
We retrospectively investigated the clinical and laboratory characteristics of severe COVID-19 patients. 107 patients were divided into hypocalcemia group and normal serum calcium group. The clinical and laboratory data were compared between two groups. The discriminative power of hypocalcemia regarding poor outcome were evaluated by receiver operating curves (ROC) analyses.
Results
Sixty seven patients (62.6%) had hypocalcemia. In hypocalcemia group, leukocytes, c-reactive protein (CRP), procalcitonin (PCT), Interleukin 6 (IL-6), and D-dimer levels was higher, while lymphocytes and albumin (ALB) levels was lower. No significant difference was identified in gender, age, signs and symptoms, comorbidities and other laboratory indicators. Serum calcium levels were negatively correlated with leukocytes, CRP, PCT, IL-6 and D-dimer, while positively correlated with lymphocytes and ALB. Patients with hypocalcemia more commonly presented poor outcome (47.8% (32/67) vs 25% (10/40), p = 0.02). Median serum calcium levels were significantly lower in the patients with poor outcome (2.01(1.97–2.05) vs 2.10(2.03–2.20), p < 0.001), and it could predict the prognosis with an area under the ROC curve (AUC) of 0.73(95% confidence interval (CI) 0.63–0.83, p < 0.001).
Conclusions
Hypocalcemia commonly occurred in severe COVID-19 patients and it was associated with poor outcome.
Keywords: COVID-19, Hypocalcemia, Prognosis, Clinical characteristics
Introduction
In December 2019, a number of patients with pneumonia of unknown cause appeared in Wuhan, Hubei province, China [1]. Subsequently, the Chinese Center for Disease Control and Prevention (CDC) confirmed that the pneumonia was caused by a novel coronavirus, which named the 2019 novel coronavirus (2019-nCoV), and Corona Virus Disease 19 (COVID-19) indicates infections complicated with pneumonia [2]. Full-genome sequencing and phylogenic analysis indicated that the sequence of 2019-nCoV was similar to the coronavirus responsible for severe acute respiratory syndrome (SARS-CoV), so the virus was also named SARS-Cov-2 [3]. Studies have shown that the disease could induce the clinical symptoms including fever, cough, fatigue, myalgia, dyspnea, and it could even cause acute respiratory distress syndrome (ARDS) [4], [5], [6]. In addition, clinical evidences have suggested that this virus is transmissible from person to person [7]. Currently, COVID-19 outbreak worldwide and there is no effective therapies or vaccines, available treatments are only supportive and symptomatic. The origins and the mechanism of this virus still need to be further investigated.
Although several studies have described the clinical characteristics of patients infected with 2019-nCoV [4], [5], [6], hypocalcemia in COVID-19 patients has not been reported yet. Hypocalcemia is a common phenomenon among critically ill patients, its prevalence ranges from 15% to 88% in adults [8], [9]. In addition, hypocalcemia is associated with disease severity and increased mortality [10], [11]. Previous studies confirmed hypocalcemia was a laboratory abnormality in several types of viral infections, such as severe acute respiratory syndrome (SARS), avian influenza H7N9 and Ebola virus disease (EVD) [12], [13], [14]. In this study, we analyzed the clinical and laboratory data of severe COVID-19 patients to reveal the correlation between serum calcium levels and COVID-19.
Materials and methods
Patients and data collection
We included the patients with diagnosis of severe COVID-19 admitted in Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology during the period from February 9 to February 15, 2020. COVID-19 was diagnosed according to the World Health Organization interim guidance criteria. Severe COVID-19 was defined according to the diagnostic and treatment guideline criteria issued by Chinese National Health Committee (Version 3-5): respiratory distress with respiratory frequency ≥ 30/min, or pulse oximeter oxygen saturation ≤93% at rest, or artery partial pressure of oxygen(PaO2)/inspired oxygen fraction(FiO2) ≤ 300 mmHg. Exclusion criteria were patients who with parathyroid disease, bone disease, chronic liver and kidney dysfunction, malignant tumor, and who received calcium or vitamin D treatment. Clinical characteristics (i.e, comorbidities, signs and symptoms) and laboratory findings of each patient were obtained from electronic medical records. The clinical outcome was monitored up to February 29, 2020, the final date of follow-up. The poor outcome was defined when at least one of the following criteria was present: the need for mechanical ventilation, intensive care unit (ICU) admission or died of any cause during admission. According to the corrected serum calcium level within 24 h of admission, the patients were divided into two group, the normal serum calcium group (corrected serum calcium 2.15–2.50 mmol/L) and the hypocalcemia group (corrected serum calcium < 2.15 mmol/L). Corrected serum calcium calculator formula: corrected calcium = 0.02 × (40-albumin(g/L)) + serum calcium. The study was approved by the Ethics Committee of Tongji hospital of Tongji Medical College, Huazhong University of Science and Technology, China. Informed consent was obtained from each patient.
Laboratory testing
A nose swab and/or throat swab specimens were collected for the SARS-CoV-2 viral nucleic acid detection using real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR) assay. The viral nucleic acid testing for all patients were performed by the clinical laboratory from Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, in Wuhan. Laboratory tests were conducted with 24 h of admission. Laboratory results, including leukocyte counts, lymphocyte counts, hemoglobin, platelets, c-reactive protein (CRP), procalcitonin (PCT), Interleukin 6 (IL-6), alanine transaminase (ALT), aspartate transaminase (AST), albumin (ALB), lactate dehydrogenase (LDH), bilirubin, creatinine, serum electrolytes (including potassium, sodium, chloride and calcium), prothrombin time (PT), activated partial thromboplastin time (APTT), prothrombin activity (PTA) and D-dimer concentrations were collected for each patient. All medical laboratory data were generated by the clinical laboratory of Tongji hospital of Tongji Medical College, Huazhong University of Science and Technology, in Wuhan.
Statistical analysis
All data were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Means for continuous variables were compared using independent group t tests or Mann–Whitney U test. Proportions for categorical variables were compared using the chi-square test or Fisher exact test. Spearman correlation test was used for calculation of correlation between different factors. Univariate and multivariate logistic regression analysis were adopted to identify risk factors of poor outcome. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the prognosis capability of serum calcium on COVID-19 patients. The tests with p value < 0.05 was considered statistically significant.
Results
Baseline characteristics of the severe COVID-19 patients
A total of 107 patients with severe COVID-19 were included in this study. The majority (63.6%, 68/107) of patients was the elderly, and the median age was 68 years, ranging from 31 to 86 years old. Among them, 55 patients (51.40%) were female. The most common symptoms at illness onset were fever (80, 74.8%), cough (56, 52.3%), fatigue (47, 43.9%), gastrointestinal symptoms (19, 17.8%) and myalgia (13, 12.1%). Only three patients had shortness of breath and two cases had chest tightness. 73 (68.2%) patients had a history of chronic diseases, including hypertension (37.4%), diabetes mellitus (18.7%), coronary heart disease (CHD) (10.3%), respiratory system disease (including chronic obstructive pulmonary disease (COPD) and tuberculosis) (10.3%) and cerebrovascular diseases (including cerebral infarction and cerebral hemorrhage) (9.3%) (Table 1 ).
Table 1.
Comparison of the parameters between hypocalcemia group and normal calcium group.
| Variables | Median (IQR) |
|||
|---|---|---|---|---|
| Total (N = 107) |
Hypocalcemia (N = 67) |
Normal calcium (N = 40) |
p Value | |
| Age (y) | 68(61–76) | 68(62–75) | 67(56–77) | 0.388 |
| Sex | ||||
| Male | 52(49%) | 36(54%) | 16(40%) | 0.169 |
| Female | 55(51%) | 31(46%) | 24(60%) | |
| Comorbidities | ||||
| Hypertension | 40(37%) | 28(42%) | 12(30%) | 0.223 |
| Diabetes mellitus | 20(19%) | 11(16%) | 9(23%) | 0.435 |
| Coronary heart disease | 11(10%) | 6(9%) | 5(13%) | 0.561 |
| Respiratory system diseases | 11(10%) | 6(9%) | 5(13%) | 0.561 |
| Cerebrovascular diseases | 10(9%) | 8(12%) | 2(5%) | 0.235 |
| Signs and symptoms | ||||
| Fever | 80(75%) | 52(78%) | 28(70%) | 0.380 |
| Cough | 56(52%) | 37(55%) | 19(48%) | 0.439 |
| Fatigue | 47(44%) | 34(51%) | 13(33%) | 0.066 |
| Gastrointestinal symptoms | 19(18%) | 10(15%) | 9(23%) | 0.321 |
| Myalgia | 13(12%) | 8(12%) | 5(13%) | 0.932 |
| Shortness of breath | 3(3%) | 2(3%) | 1(3%) | 0.884 |
| Chest tightness | 2(2%) | 1(1%) | 1(3%) | 0.711 |
| Outcomes | ||||
| Favorable outcome | 65(61%) | 35(52%) | 30(75%) | 0.020 |
| Poor outcome | 42(39%) | 32(48%) | 10(25%) | |
| Leukocytes (×109/L) | 5.9(4.5–8.6) | 6.4(4.8–8.9) | 5.2(4.0–7.4) | 0.013 |
| Lymphocytes (×109/L) | 1.0(0.7–1.3) | 0.9(0.6–1.2) | 1.1(0.8–1.6) | 0.010 |
| Hemoglobin (g/L) | 124(114–137) | 124(114–138) | 125(111–136) | 0.792 |
| Platelets (×109/L) | 220(150–300) | 211(137–268) | 238(191–319) | 0.058 |
| ALT (U/L) | 23(15–41) | 25(17–44) | 20(11–36) | 0.070 |
| AST (U/L) | 28(20–45) | 30(21–51) | 27(17–40) | 0.122 |
| Albumin (ALB) (g/L) | 34(31–36) | 32(29–34) | 35(34–38) | <0.001 |
| LDH (U/L) | 298(233–435) | 310(242–490) | 287(207–332) | 0.063 |
| Bilirubin (umol/L) | 8.7(6.4–12.8) | 9.2(6.3–14.3) | 7.9(6.4–11.9) | 0.241 |
| Creatinine (umol/L) | 68(58–84) | 71(58–96) | 67(57–80) | 0.424 |
| CRP (mg/L) | 46.9(13.5–105.7) | 62.2(18.7–114.7) | 31.1(7.2–71.8) | 0.032 |
| Procalcitonin (PCT) (ng/ml) | 0.09(0.06–0.17) | 0.10(0.06–0.19) | 0.06(0.05–0.14) | 0.014 |
| Interleukin 6 (IL-6) (pg/ml) | 20.8(6.2–49.3) | 24.3(10.9–57.8) | 9.9(3.8–36.9) | 0.007 |
| Potassium (mmol/L) | 3.95(3.56–4.34) | 3.94(3.55–4.35) | 3.97(3.60–4.29) | 0.918 |
| Sodium (mmol/L) | 138.5(135.3–140.4) | 138.4(134.6–139.9) | 139.1(136.2–141.1) | 0.126 |
| Chloride (mmol/L) | 99.7(96.7–102.7) | 100.2(96.4–102.7) | 99.5(97.2–102.7) | 0.964 |
| Calcium (mmol/L) | 2.09(2.00–2.19) | 2.01(1.97–2.05) | 2.21(2.19–2.25) | <0.001 |
| PT (s) | 13.9(13.3–14.9) | 14.0(13.3–15.4) | 13.8(13.3–14.3) | 0.069 |
| APTT (s) | 38.3(35.2–42.3) | 38.6(34.7–43.0) | 38.0(35.3–41.4) | 0.716 |
| PTA (%) | 89(78–96) | 87(73–96) | 90(83–96) | 0.068 |
| D-dimer (μg/ml) | 1.14(0.56–2.75) | 1.47(0.60–8.56) | 0.84(0.45–1.79) | 0.012 |
Note: IQR, interquartile range; ALT, alanine transaminase; AST, aspartate transaminase; LDH, lactate dehydrogenase; CRP, c-reactive protein; PT, prothrombin time; APTT, activated partial thromboplastin time; PTA, prothrombin activity.
Association between hypocalcemia and clinical characteristics
The incidence of hypocalcemia was 62.6% (67/107) in all the enrolled patients, and 3 patients had severe hypocalcemia (Ca2+ < 1.9 mmol/L). There was no significant difference in age, gender, signs and symptoms, and comorbidities between the hypocalcemia group and the normal serum calcium group. Compared to the normal serum calcium group, higher leukocytes, as well as higher levels of CRP, PCT, IL-6 and D-dimer, while lower lymphocytes, lower ALB level were found in the hypocalcemia group. No significant difference was identified for other laboratory indicators, such as hemoglobin, platelets, ALT, AST, LDH, bilirubin, creatinine, potassium, sodium, chloride, PT, APTT and PTA (Table 1).
Correlation between serum calcium and other indicators
The level of serum calcium was positively correlated with lymphocyte counts and ALB level. Negative correlation was found between the serum calcium levels and leukocytes counts, the levels of CRP, PCT, IL-6 and D-dimer (Table 2 ).
Table 2.
Correlation between serum calcium and other indicators.
| Variables | Spearman value | p Value |
|---|---|---|
| Leukocytes | −0.201 | 0.037 |
| Lymphocytes | 0.292 | 0.002 |
| Albumin (ALB) | 0.487 | <0.001 |
| C-reactive protein (CRP) | −0.326 | 0.001 |
| Procalcitonin (PCT) | −0.350 | <0.001 |
| Interleukin 6 (IL-6) | −0.353 | 0.001 |
| D-dimer | −0.354 | <0.001 |
The prognosis of severe COVID-19 patients
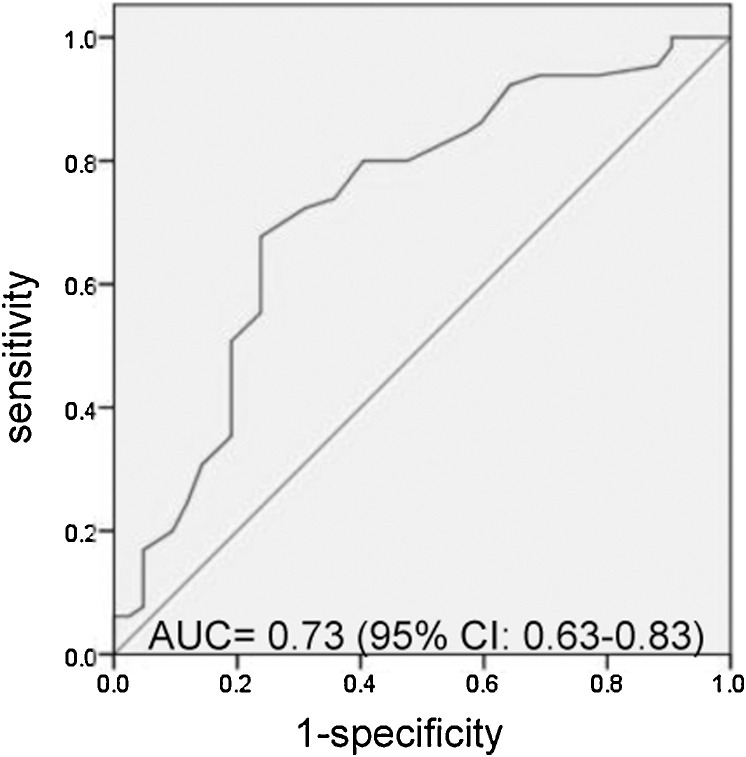
Up to February 29, severe COVID-19 patients with hypocalcemia had a poor outcome (47.8% (32/67) vs 25% (10/40), p = 0.02). The proportions of patients using different drugs between favorable outcome and poor outcome were not significantly different (Table 3 ). Median serum calcium levels were significantly lower in the subgroup of patients with poor outcome (2.01(1.97–2.05) vs 2.10(2.03–2.20), p < 0.001). Furthermore, the multivariate analysis indicated that age, serum calcium, CRP and IL-6 were risk factors for poor outcome of severe COVID-19 patients (Table 4 ). Finally, we analyzed the prognostic performance of hypocalcemia to predict the development of poor outcome. Area under the ROC curve was 0.73 (95% CI: 0.63–0.83), p < 0.001 (Fig. 1 ).
Table 3.
Treatments of severe COVID-19 patients.
| Treatment | Total (N = 107) |
Favorable outcome (N = 65) |
Poor outcome (N = 42) |
p Value |
|---|---|---|---|---|
| Antibiotic treatment | 99(92.5%) | 59(90.8%) | 40(95.2%) | 0.393 |
| Antiviral treatment | 105(98.1%) | 63(96.9%) | 42(100%) | 0.253 |
| Traditional Chinese medicine | 32(29.9%) | 22(33.8%) | 10(23.8%) | 0.268 |
| Hormone therapy | 24(22.4%) | 11(16.9%) | 13(31.0%) | 0.089 |
| Immunoglobulin therapy | 6(5.6%) | 2(3.1%) | 4(9.5%) | 0.159 |
Table 4.
Logistic analysis results of risk factors for poor outcome.
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p Value | OR | 95%CI | p Value | |
| Age (≥68 y vs <68 y) | 3.000 | 1.333–6.751 | 0.008 | 2.577 | 1.040–6.383 | 0.041 |
| Sex (male vs female) | 1.631 | 0.736–3.537 | 0.233 | |||
| Hypertension | 1.242 | 0.559–2.760 | 0.595 | |||
| Diabetes | 1.719 | 0.646–4.574 | 0.278 | |||
| Calcium (<2.15 mmol/L vs ≥2.15 mmol/L) | 4.121 | 1.658–10.247 | 0.002 | 2.962 | 1.085–8.090 | 0.034 |
| CRP (≥50 mg/L vs <50 mg/L) | 8.887 | 3.572–22.064 | <0.001 | 6.685 | 2.584–17.298 | <0.001 |
| PCT (≥0.1 ng/ml vs <0.1 ng/ml) | 3.522 | 1.514–9.753 | 0.031 | 2.752 | 0.890–8.633 | 0.079 |
| IL-6 (≥10 pg/ml vs <10 pg/ml) | 9.797 | 3.136–30.61 | 0.001 | 7.228 | 2.222–23.514 | 0.006 |
| D-dimer (≥0.05ug/ml vs <0.05ug/ml) | 8.261 | 1.811–37.672 | 0.007 | 5.619 | 1.043–30.283 | 0.054 |
Notes: OR, Odds ratio; CI, confidence interval; CRP, c-reactive protein; PCT, procalcitonin; IL-6, interleukin 6.
Fig. 1.
Receiver operating characteristic (ROC) curve analysis. AUC, area under the ROC curve; CI, confidence interval.
Discussion
To our knowledge, this report is the first to address frequency of hypocalcemia in severe COVID-19 patients. In our study, we found that 62.6% of the severe COVID-19 patients had hypocalcemia, and 3 cases had severe hypocalcemia (Ca < 1.9 mmol/L). Moreover, hypocalcemia predicted a worse prognosis of severe COVID-19 patients. In addition, 63.6% of the patients were older than 65 years, median age of all patients was 68 years old, which suggested aged people are susceptible to severe COVID-19.
Hypocalcemia is a common laboratory abnormality in viral infection and pneumonia [15]. The cause of hypocalcemia in COVID-19 patients with severe status is not clear, several mechanisms may be suggested for this. The majority of patients in our study were elderly, with poor nutritional status. Chronic malnutrition will lead to vitamin D deficiency, result in hypocalcemia [16]. Moreover, it can affect the intestinal absorption of calcium, lead to inadequate intake, and thus result in a negative calcium balance [17]. Moreover, calcium is predominantly bound to albumin in the plasma, and a decrease in serum albumin will cause hypocalcemia. In addition, hypoxia of tissue and organ induce the cell membrane damage, result in calcium influx. Finally, the pro-inflammatory cytokines in COVID-19 patients inhibited parathyroid hormone (PTH) secretion, impaired response to PTH, thus cause the imbalance of calcium [18].
Other abnormal laboratory results in severe COVID-19 patients included lymphopenia, hypoalbuminemia, and elevation of inflammatory mediators (CRP, PCT and IL-6) and D-dimer concentrations. These laboratory abnormalities are similar to previously published articles [4], [5], [6]. Increased level of serum CRP, PCT, IL-6 and D-dimer indicated 2019-nCoV infection might induced sustain inflammatory response and coagulation disorder. Viral infection induces a series of the physiological reaction of the host, including immunity responses. Cytokines are actively involved in the process, such as TNF-α, IL-1, IL-6. However, the rapidly and massively release of the inflammatory cytokines, can contribute to tissue destruction and organ failure, which known as a “cytokine storm” [19]. The phenomenon has been previously observed in patients with SARS, MERS and EVD [20], [21]. Recent studies confirmed that cytokine storm also occurred in patients with 2019-nCoV infection, especially in severe and critical ill cases, caused acute respiratory distress syndrome (ARDS) and multiple organ disorder syndrome (MODS), and even led to death [22]. According to our data, CRP, PCT, IL-6 and D-dimer concentration were significantly higher in COVID-19 patients with hypocalcemia, and hypocalcemia was positively correlated with these indicators, which may represent patients with hypocalcemia have a greater inflammatory response.
Hypocalcemia is harmful to health. The classic symptoms of hypocalcemia are neuromuscular excitability in the form of muscle twitching, spasms, tingling, and numbness. Once severe hypocalcemia is not corrected in time, it will result in severe neuroendocrine and cardiovascular complications, thus increasing mortality [18]. In 1982, Chernow et al. demonstrated that hypocalcemia was associated with prolonged ICU stay and increased mortality [23]. Recently, studies have also reported that hypocalcemia is an indicator of disease severity and fatality [9], [11]. In our study, compare to the normal serum calcium group, the outcome of severe COVID-19 patients was worse in hypocalcemia group. Moreover, we performed ROC analysis to evaluate the predictive value of serum calcium. High AUC reflected the discriminative power of serum calcium when predicting poor outcome of severe COVID-19 patients. It is worthwhile to pay more attention to the occurrence of hypocalcemia in severe COVID-19 patients. In addition, studies have shown that other factors were associated with the outcome of COVID-19 patients, including age, the history of chronic diseases, the level of inflammatory mediators and treatment protocols [24], [25]. In our study, we found that higher age, elevated levels of CRP and IL-6 were associated with poor outcome of severe COVID-19 patients.
There were a few limitations for this study. One of the potential limitations of our study is that our data comes from a single center study, and the sample size was relatively small, we believe that larger studies are needed to confirm our findings. Secondly, there are no data that treating hypocalcemia can improve prognosis in hypocalcemic individuals, so continued observations would provide more information about potential risk factors of poor outcome. Due to some patients had a high leukocyte counts, we cannot rule out the possibility of bacterial co-infection in severe COVID-19 patients. To sum up, we consider that our observations should be externally validated.
Conclusion
The COVID-19 is spreading globally. Our study is the first to focus on hypocalcemia of severe COVID-19 patients. In our study, we found that almost two-thirds of severe COVID-19 patients had hypocalcemia at time of admission. Patients who presented with hypocalcemia were more severely ill on admission, and had worse outcomes.
Contributors’ statement
Jingmei Liu and Dean Tian designed the work. Jingmei Liu, Jingwen Wu and Jin Gong collected the data. Jingwen Wu, Jingmei Liu and Ping Han contributed to the statistical analysis. All authors contributed to draft the article and final approval of the version to be submitted.
Funding
This study is supported by the National Natural Science Foundation of China (NO: 81800547).
Competing interests
None declared.
Ethical approval
The study was approved by the Ethics Committee of Tongji hospital of Tongji Medical College, Huazhong University of Science and Technology, China.
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zivin J.R., Gooley T., Zager R.A., Ryan M.J. Hypocalcemia: a pervasive metabolic abnormality in the critically ill. Am J Kidney Dis. 2001;37:689–698. doi: 10.1016/s0272-6386(01)80116-5. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z., Xu X., Ni H., Deng H. Predictive value of ionized calcium in critically ill patients: an analysis of a large clinical database MIMIC II. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095204. e95204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly A., Levine M.A. Hypocalcemia in the critically ill patient. J Intensive Care Med. 2013;28:166–177. doi: 10.1177/0885066611411543. [DOI] [PubMed] [Google Scholar]
- 11.Egi M., Kim I., Nichol A. Ionized calcium concentration and outcome in critical illness. Crit Care Med. 2011;39:314–321. doi: 10.1097/CCM.0b013e3181ffe23e. [DOI] [PubMed] [Google Scholar]
- 12.Booth C.M., Matukas L.M., Tomlinson G.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J., Zhao Y., Chen Y. Laboratory findings in patients with avian-origin influenza A (H7N9) virus infections. J Med Virol. 2014;86:895–898. doi: 10.1002/jmv.23780. [DOI] [PubMed] [Google Scholar]
- 14.Uyeki T.M., Mehta A.K., Davey R.T., Jr. Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med. 2016;374:636–646. doi: 10.1056/NEJMoa1504874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sankaran R.T., Mattana J., Pollack S. Laboratory abnormalities in patients with bacterial pneumonia. Chest. 1997;111:595–600. doi: 10.1378/chest.111.3.595. [DOI] [PubMed] [Google Scholar]
- 16.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 17.Ni Bhraonain S., Lawton L.D. Chronic malnutrition may in fact be an acute emergency. J Emerg Med. 2013;44:72–74. doi: 10.1016/j.jemermed.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 18.Fong J., Khan A. Hypocalcemia: updates in diagnosis and management for primary care. Can Fam Physician. 2012;58:158–162. [PMC free article] [PubMed] [Google Scholar]
- 19.Tisoncik J.R., Korth M.J., Simmons C.P. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younan P., Iampietro M., Nishida A. Ebola virus binding to Tim-1 on T lymphocytes induces a cytokine storm. mBio. 2017:8. doi: 10.1128/mBio.00845-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tetro J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22:72–73. doi: 10.1016/j.micinf.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chernow B., Zaloga G., McFadden E. Hypocalcemia in critically ill patients. Crit Care Med. 1982;10:848–851. doi: 10.1097/00003246-198212000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W., Tao Z.W., Wang L. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]