Abstract
CD8 T cells contribute to effective clearance of mouse adenovirus type 1 (MAV-1) and to virus-induced pulmonary inflammation. We characterized effects of a CD8 T cell effector, TNF, on MAV-1 pathogenesis. TNF inhibited MAV-1 replication in vitro. TNF deficiency or immunoneutralization had no effect on lung viral loads or viral gene expression in mice infected intranasally with MAV-1. Absence of TNF delayed virus-induced weight loss and reduced histological evidence of pulmonary inflammation, although concentrations of proinflammatory cytokines and chemokines in bronchoalveolar lavage fluid (BALF) were not significantly affected. BALF concentrations of IL-10 were greater in TNF-deficient mice compared to controls. Our data indicate that TNF is not essential for control of viral replication in vivo, but virus-induced TNF contributes to some aspects of immunopathology and disease. Redundant CD8 T cell effectors and other aspects of immune function are sufficient for antiviral and proinflammatory responses to acute MAV-1 respiratory infection.
1. Introduction
Human adenoviruses (HAdVs) are common causes of infections. HAdVs cause a variety of illnesses, including forms of respiratory, cardiac, eye, and gastrointestinal disease (Wold and Isom, 2013). To overcome the barrier created by the strict species-specificity of the adenoviruses, which precludes extensive studies of HAdV pathogenesis, we use mouse adenovirus type 1 (MAV-1) to study the pathogenesis of an adenovirus in its natural host. Following intranasal (i.n.) inoculation, MAV-1 replicates in lungs and disseminates to other target organs such as heart, liver, spleen, and brain (Chandrasekaran et al., 2019; Kajon et al., 1998; Weinberg et al., 2007; Weinberg et al., 2005). The expression of interferon (IFN)-γ, tumor necrosis factor (TNF), and other proinflammatory cytokines and chemokines increases in the lungs during acute infection (McCarthy et al., 2015b; Molloy et al., 2017; Procario et al., 2012). Acute MAV-1 respiratory infection is also associated with pulmonary inflammation characterized by the influx of immune cells, including CD4 and CD8 T cells, into lungs, heart, and brain (McCarthy et al., 2015b; McCarthy et al., 2014; Procario et al., 2012; Weinberg et al., 2007).
CD8 T cells are required for control of many viral infections, but CD8 T cells may also contribute to bystander tissue damage. CD8 T cell responses to HAdV have been previously described, and patients with defects in cellular immune function are prone to severe HAdV disease (Kojaoghlanian et al., 2003; Walls et al., 2003). CD8 T cells are not required for control of MAV-1 replication in the lungs at the peak of acute respiratory infection, although they are essential for efficient clearance of actively replicating MAV-1 from the lungs at later time points as acute infection resolves (Molloy et al., 2017). However, CD8 T cells are not sufficient to completely clear virus; MAV-1 DNA persists in the lungs of infected mice without detectable viral gene expression even in the presence of functional CD8 T cells (Molloy et al., 2017). CD8 T cells also exert a proinflammatory effect, with CD8 T cell deficiency or depletion protecting from virus-induced airway inflammation and weight loss during acute infection (Molloy et al., 2017). Specific mechanisms underlying contributions of CD8 T cells on MAV-1 pathogenesis in the lungs are not yet fully understood. The effects of CD8 T cells on MAV-1 respiratory infection are not mediated by IFN-γ or perforin (Pfn) (McCarthy et al., 2015a; McCarthy et al., 2015b; Molloy et al., 2017). Fas deficiency decreases MAV-1-induced production of inflammatory cytokines in the airways but has no effect on MAV-1 replication in the lungs or virus clearance from the lungs (Adkins et al., 2018).
TNF is produced by CD8 T cells, macrophages, and other cell types in response to a wide variety of infections. TNF signaling induces diverse cellular responses, including apoptosis as well as the regulation of many innate and acquired immune responses (Rahman and McFadden, 2006). TNF inhibits replication of viruses such as vesicular stomatitis virus, encephalomyocarditis virus, herpes simplex virus, influenza, and hepatitis B virus (Biermer et al., 2003; Mestan et al., 1986; Seo and Webster, 2002). TNF deficiency or blockade increases the efficiency of HAdV vector-mediated transgene delivery and delays vector clearance in mice (Abougergi et al., 2005; Benihoud et al., 2007; Kafrouni et al., 2003; Minter et al., 2000). In this study, we used a combination of in vitro and in vivo approaches to determine the extent to which TNF exerts antiviral and proinflammatory effects during acute MAV-1 respiratory infection.
2. Results
2.1. Effects of TNF on MAV-1 replication in vitro
To determine whether TNF directly inhibited MAV-1 replication, we infected 3T12 cells with a recombinant green fluorescent protein (GFP)-expressing MAV-1 in the presence of recombinant TNF and measured accumulating fluorescence as a surrogate for viral replication. In the absence of TNF, increasing fluorescence above background was detected in infected wells by 48 hours post infection (hpi) and continued to increase through 72 hpi (Figure 1A). We detected statistically significant dose-dependent decreases in fluorescence in cells exposed to TNF at 48 and 72 hpi. Decreased fluorescence correlated with decreased expression of late viral genes, measured using reverse transcriptase quantitative real-time PCR (RT-qPCR) to quantify MAV-1 tripartite leader (TPL) mRNA levels (Figure 1B). MAV-1 infection itself decreased cell viability slightly, but TNF had minimal additional effect on the viability of infected or mock-infected cells (Figure 1C). These data suggest that TNF exerts an antiviral effect on MAV-1 in a manner that is not directly related to TNF-mediated cytotoxicity.
Figure 1. Effects of TNF on MAV-1 replication in vitro.
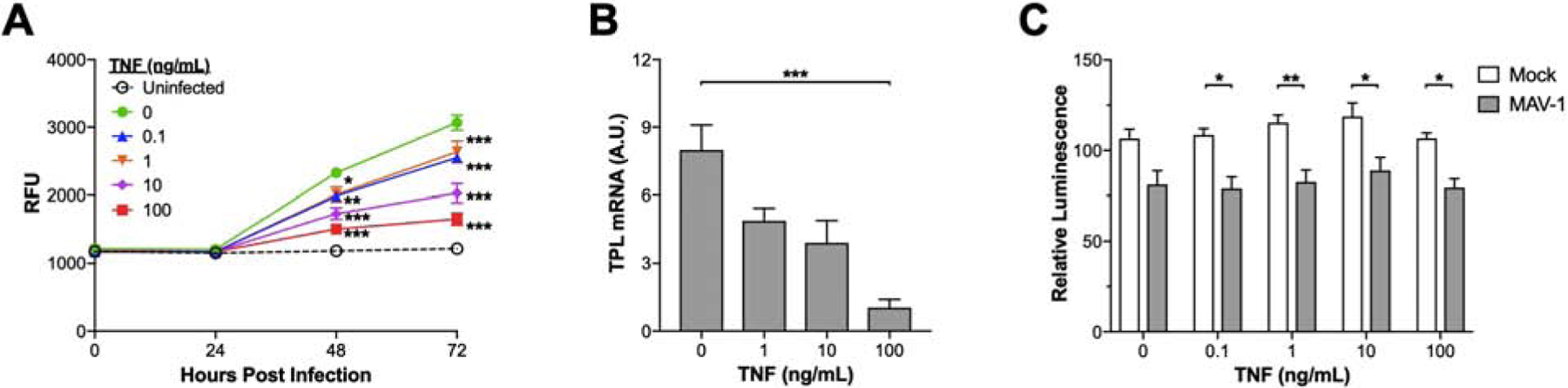
(A) Mouse 3T12 fibroblasts were infected (MOI=1) with MAV 1.pIXeGFP, a recombinant GFP-expressing MAV-1, in the presence of the indicated concentrations of TNF or vehicle control (PBS). Accumulating fluorescence expressed as relative fluorescence units (RFU) was used as an indicator of viral replication (mean ± S.E.M., n=6 per condition combined from two identical experiments). The bottom horizontal dashed line represents background fluorescence in wells with uninfected cells. Statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests; *P<0.05 and ***P<0.001 compared to vehicle at a given time point. (B) Mouse 3T12 fibroblasts were infected with MAV-1 (MOI=1) in the presence of the indicated concentrations of TNF or vehicle control. RT-qPCR was used to quantify MAV-1 TPL mRNA levels at 72 hpi. Data are shown in arbitrary units standardized to GAPDH (mean ± S.E.M., n=6 per condition combined from two identical experiments). Statistical comparisons were made using one-way ANOVA followed by Kruskal-Wallis tests; ***P<0.001 compared to vehicle. (C) Mouse 3T12 fibroblasts were infected with MAV-1 (MOI=1) or mock-infected with conditioned media in the presence of the indicated concentrations of TNF or vehicle control. Cytotoxicity was assessed at 48 hpi using a luminescent cell viability assay. Data are expressed as the percentage of signal in wells with uninfected, untreated cells (mean ± S.E.M., n=9 per condition combined from three identical experiments). Statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests; *P<0.05 and **P<0.01 comparing mock to infected at a given concentration. There were no statistically significant differences between concentrations within a condition (mock or infected).
2.2. Effects of TNF deficiency on virus-induced disease
Adult C57BL/6 (B6) mice survive infection following i.n. inoculation, even in the absence of CD8 T cells, IFN-γ, Pfn, or Fas (Adkins et al., 2018; Molloy et al., 2017). Mice are protected from MAV-1-induced weight loss by CD8 T cell depletion or deficiency (Molloy et al., 2017); Pfn deficiency attenuates virus-induced weight loss to a lesser degree, but IFN-γ and Fas deficiency do not protect against weight loss (Adkins et al., 2018; Molloy et al., 2017). We infected B6 and TNF-deficient (TNF−/−) mice with MAV-1 and monitored survival and body weight until 14 dpi. All B6 and TNF−/− mice survived (data not shown). As we have previously demonstrated, B6 mice lost weight by 7 dpi, continued to lose weight until 8 dpi, and then recovered to baseline weight by 12 dpi (Figure 2A). Weight loss was delayed in TNF−/− mice, whose weights were significantly greater than B6 mice at 7 dpi. However, weights of B6 and TNF−/− mice were similar at 8 dpi, and subsequent weight gain did not differ between B6 and TNF−/− mice. Thus, although TNF makes some contribution to weight loss, its absence is not sufficient to account for the complete protection from weight loss afforded by CD8 T cell deficiency.
Figure 2. Effects of TNF deficiency on MAV-1-induced weight loss and viral replication in vivo.
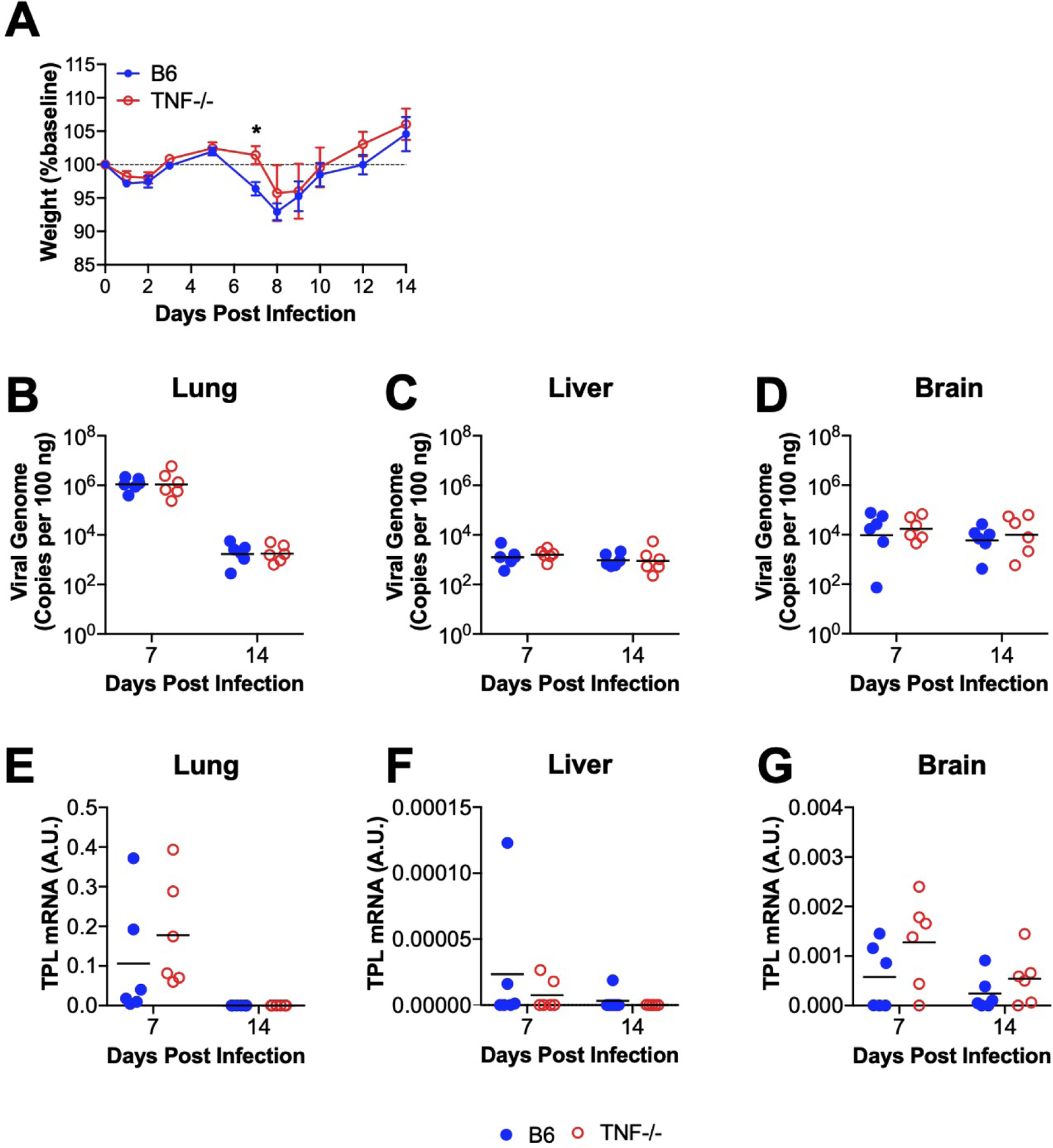
B6 and TNF−/− mice were infected intranasally with MAV-1. (A) Body weights were measured at the indicated time points. Body weights are expressed as the percentage of starting weight (mean ± S.E.M.; n=6–12 mice per group; data are combined from two independent experiments). (B-D) qPCR was used to quantify MAV-1 genome copies in organs at the indicated time points. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA. (E–G) RT-qPCR was used to quantify MAV-1 TPL mRNA levels in organs. In B-G, data are shown in arbitrary units standardized to GAPDH. Individual circles represent values for individual mice, and horizontal bars represent geometric means (B-D) or means (E-G) for each group (n=5–6 mice per group; data are combined from two independent experiments). Statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests. *P<0.05
2.3. Effects of TNF deficiency on MAV-1 replication
Our initial data indicated that TNF inhibits MAV-1 replication in vitro. To determine whether TNF production in the setting of acute MAV-1 infection is necessary for control of viral replication in vivo, we infected TNF−/− mice and B6 controls with MAV-1 and used qPCR to measure DNA viral loads in the lungs (Figure 2B). There were no differences in lung viral loads between TNF−/− and B6 mice at 7 dpi, when peak viral loads are typically detected, or at 14 dpi, when viral loads have decreased to levels at which they persist following resolution of acute infection (Molloy et al., 2017). Viral loads were low and did not significantly differ between TNF−/− and B6 mice at either time point in other target organs, including liver (Figure 2C) and brain (Figure 2D). As an additional measure of viral replication, we quantified MAV-1 late transcription using RT-qPCR to assess TPL mRNA levels in TNF−/− mice and B6 mice. We detected no differences in TPL mRNA levels in lung, liver, or brain at 7 or 14 dpi in(Figure 2E–G). These data suggest that TNF is not essential for the previously demonstrated effects of CD8 T cells on clearance of MAV-1 from the lungs.
2.4. Effects of TNF deficiency on MAV-1-induced pulmonary inflammation
CD8 T cell deficiency does not affect histological evidence of pulmonary inflammation, but the production of proinflammatory cytokines in the airways is diminished in CD8 T cell-deficient mice infected with MAV-1 (Molloy et al., 2017). We observed histological evidence of pulmonary inflammation in both B6 and TNF−/− mice at 7 dpi that was less pronounced in TNF−/− mice (Figure 3A). The amount of inflammation had decreased and was qualitatively similar in B6 and TNF−/− mice by 14 dpi (data not shown). Lung pathology scores were significantly lower in TNF−/− mice compared to B6 mice at 7 dpi (Figure 3B). Corresponding to the qualitative histological findings, lung pathology scores had decreased and were similar in B6 and TNF−/− mice at 14 dpi. To further characterize contributions of TNF to MAV-1-induced pulmonary inflammation, we used ELISA to measure concentrations of cytokines and chemokines in bronchoalveolar lavage fluid (BALF, Figure 3C–H) that are increased during acute MAV-1 respiratory infection but to a lesser degree in the absence of CD8 T cells or Fas (Adkins et al., 2018; Molloy et al., 2017). There were no statistically significant differences between B6 and TNF−/− mice in BALF concentrations of IFN-γ, IL-1β, CXCL1, CCL2, and CCL5 at 7 or 14 dpi. IL-10 concentrations were significantly greater in BALF from TNF−/− compared to B6 mice at 7 dpi, but concentrations had decreased and were similar in B6 and TNF−/− mice by 14 dpi (Figure 3E).
Figure 3. Effects of TNF deficiency on MAV-1-induced pulmonary inflammation.
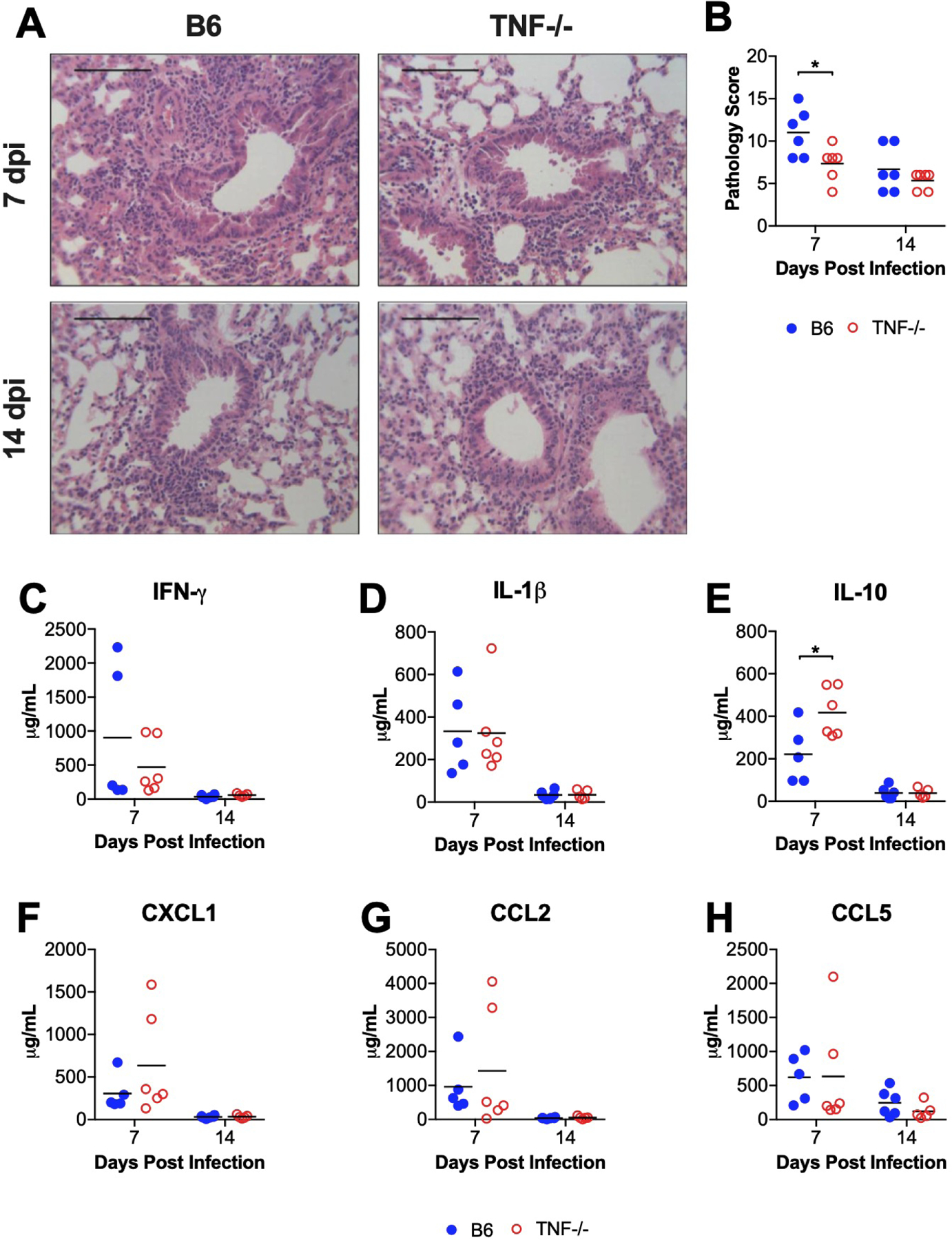
B6 and TNF−/− mice were infected intranasally with MAV-1. Lungs were harvested at the indicated time points and hematoxylin-and-eosin-stained sections were prepared from paraffin-embedded specimens. (A) Representative images are shown from infected B6 and TNF−/− mice. Scale bars, 100 μm. (B) Pathology index scores were generated to quantify cellular inflammation in the lungs of mock-infected and infected mice. (C–H) ELISA was used to quantify concentrations of the indicated cytokines and chemokines in BALF. For B–H, individual circles represent values for individual mice, and horizontal bars represent means for each group. n=5–6 mice per group combined from two independent experiments at each time point. Statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests. *P<0.05.
2.5. Effects of TNF immunoneutralization on MAV-1 replication and virus-induced inflammation
We used antibody-mediated immunoneutralization of TNF in B6 mice to confirm our findings in TNF−/− mice at 7 dpi. Infected mice treated with nonspecific IgG lost a significant amount of weight compared to uninfected controls by 7 dpi (Figure 4A). Infected mice treated with anti-TNF antibody also lost weight, but the magnitude of weight loss was significantly less than that of infected control mice. TNF immunoneutralization had no statistically significant effect on lung viral loads (Figure 4B). TPL mRNA levels were somewhat higher in lungs of mice treated with anti-TNF antibody compared to controls, but the difference was not statistically significant (Figure 4C). TNF immunoneutralization had no statistically significant effect on BALF concentrations of IFN-γ, IL-1β, IL-10, CXCL1, CCL2, or CCL5 (Figure 4D–I). Collectively, these results in TNF-immunoneutralized mice are largely consistent with our findings in TNF−/− mice.
Figure 4. Effects of TNF immunoneutralization on MAV-1 replication and virus-induced pulmonary inflammation.
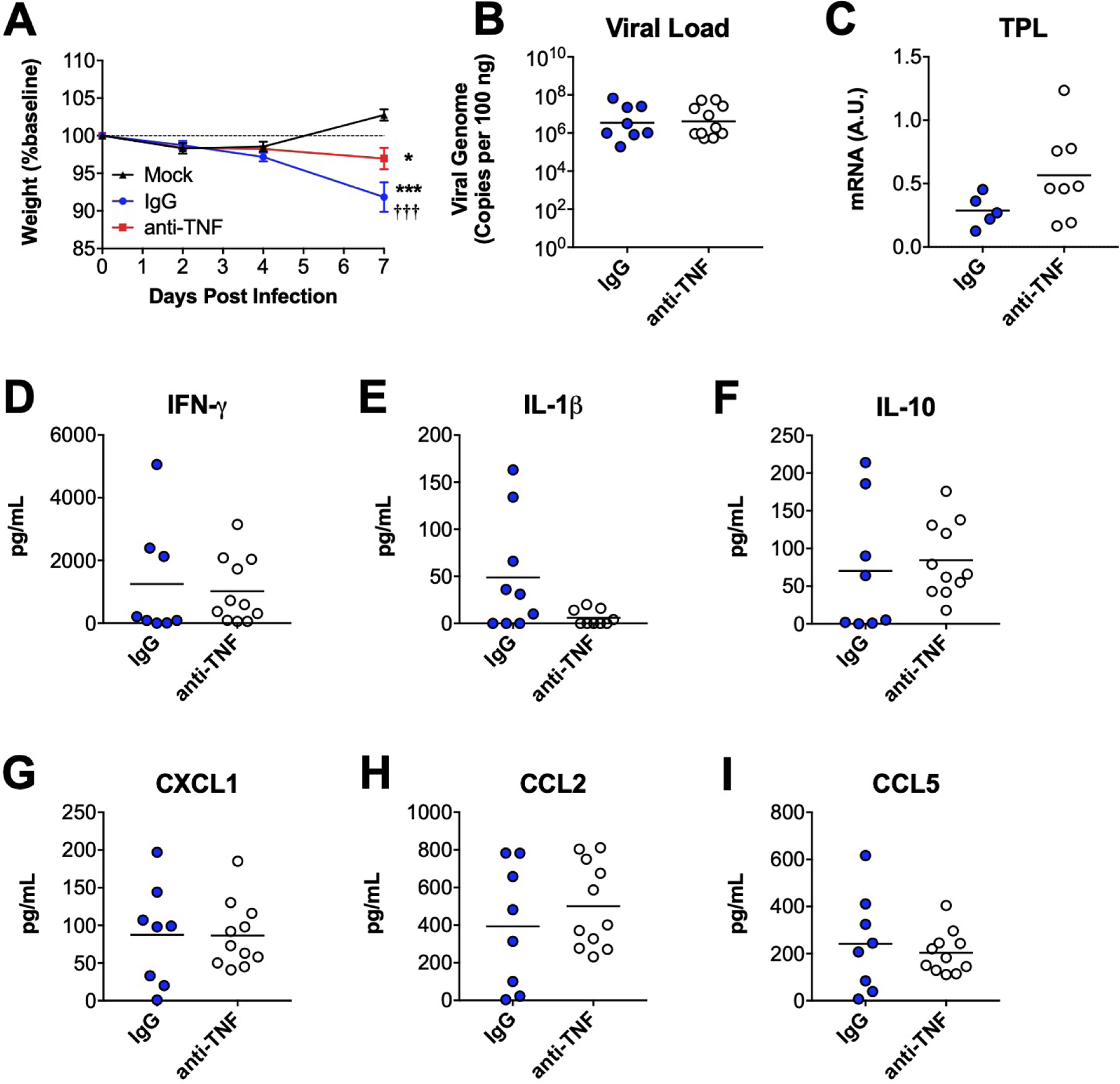
Following intranasal infection with MAV-1, B6 mice were treated with anti-TNF antibody. Infected control mice received a nonspecific IgG control antibody. Lungs and BALF were harvested at 7 dpi. (A) Body weights were measured at the indicated time points. Body weights are expressed as the percentage of starting weight (mean ± S.E.M.; n=8–11 mice per group, except n=3 for mock-infected controls; data are combined from three independent experiments). (B) qPCR was used to quantify MAV-1 genome copies in lungs. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA. (C) RT-qPCR was used to quantify MAV-1 TPL mRNA levels in lungs. In B–G, data are shown in arbitrary units standardized to GAPDH. (D-I) ELISA was used to quantify concentrations of the indicated cytokines and chemokines in BALF. In B–I, individual circles represent values for individual mice, and horizontal bars represent means (or geometric mean in B) for each group. n=5–11 per group combined from three independent experiments. In A, statistical comparisons were made using two-way ANOVA followed by Bonferroni’s multiple comparison tests. In B-I, statistical comparisons were made using Mann-Whitney test. *P<0.05 and ***P<0.001
3. Methods and materials
3.1. Mice
Male C57BL/6J (B6) and B6.129S-Tnftm1Gkl/J (TNF−/−) mice were obtained from the Jackson Laboratory. All mice were maintained under specific pathogen-free conditions and provided with food and water ad libitum. All experiments were approved by the University of Michigan Institutional Animal Care and Use Committee.
3.2. Virus and cells
MAV-1 was grown and passaged in mouse 3T6 fibroblasts, and titers of viral stocks were determined by plaque assay on 3T6 cells (Cauthen et al., 2007). MAV-1.pIXeGFP, a recombinant GFP-expressing MAV-1 (Ashley et al., 2017; Tirumuru et al., 2016), was kindly provided by Kathy Spindler (University of Michigan). MAV-1.pIXeGFP was also grown, passaged, and titered in 3T6 fibroblasts.
3.3. In vitro infection
Mouse 3T12 fibroblasts (ATCC CCL-164) were grown in Dulbecco’s Modified Eagle Medium (Gibco) containing 5% heat-inactivated calf serum and 1% penicillin and streptomycin (Gibco). Cells were infected with MAV-1 at a multiplicity of infection (MOI) of 1 plaque-forming units (pfu)/cell. Infected cells were incubated for 48 h in media supplemented with recombinant mouse TNF-α (BioLegend) or an equivalent volume of sterile phosphate-buffered saline (PBS). RNA was isolated from infected cells using TRIzol (Invitrogen).
3.4. In vitro infection with recombinant MAV-1 expressing GFP
Mouse 3T12 fibroblasts cells were plated in clear-bottom, opaque-walled 96-well plates (Greiner Bio-One) at 5 × 104 cells/well and incubated overnight. Cells were then infected with MAV 1.pIXeGFP at an MOI of 1 pfu/cell for 60 min at 37°C. Negative control wells included uninfected cells. Infected cells were then incubated for 72 h at 37°C in indicator-free Dulbeco’s Modified Eagle Medium (Gibco) containing 5% heat-inactivated calf serum and 1% penicillin and streptomycin (Gibco) and supplemented with recombinant mouse TNF or an equivalent volume of sterile PBS. Fluorescence was measured immediately after infection and then at 24 h intervals using a Synergy HTX Multi-Mode plate reader with Gen5 software (BioTek Instruments, Inc.).
3.5. Cell viability assays
Mouse 3T12 fibroblasts were plated in 96-well plates at 5 × 104 cells/well and incubated overnight. Cells were infected with MAV-1 at an MOI of 1 pfu/cell or mock-infected with an equivalent volume of conditioned media. Cells were and then incubated for 48 h with media supplemented with recombinant TNF or sterile PBS. Wells containing uninfected cells incubated in media alone served as an additional control. Cell viability was assessed using the CellTiter-Glo Luminescent Cell Viability Assay (Promega) according to the manufacturer’s directions. Luminescence was measured using a Synergy HTX Multi-Mode plate reader with Gen5 software.
3.6. Mouse infections
Mice were infected at 6 to 8 weeks of age. Male and female mice were used in all experiments. Mice were anesthetized with ketamine and xylazine, then infected i.n with 105 pfu of MAV-1 in 40 μl of sterile PBS. In some experiments, control mice were mock-infected i.n. with conditioned medium at an equivalent dilution in sterile PBS. Mice were weighed on the day of infection and then intermittently throughout the course of the experiment. Mice were euthanized by ketamine/xylazine overdose at the indicated time points. Organs were harvested, snap-frozen in dry ice and stored at −80°C.
3.7. TNF immunoneutralization
An Armenian hamster antibody recognizing mouse TNF-α (clone TN3–19.12, BioXCell) was administered intraperitoneally at 100 μg/dose. Control mice received equivalent amounts of nonspecific IgG (Armenian Hamster IgG isotype control, BioXCell). Antibody was administered on days −1, 0, 2, 4, and 6 relative to infection (day 0).
3.8. Isolation of DNA and RNA from organs
Portions of organs were homogenized using sterile glass beads in a Mini-Beadbeater (Biospec Products) for 30 s in 1 ml of TRIzol. RNA and DNA were then isolated from homogenates according to the manufacturer’s protocol.
3.9. Analysis of viral gene expression and DNA viral loads
Expression of the MAV-1 tripartite leader (TPL) was quantified using reverse transcriptase quantitative real-time PCR (RT-qPCR) as previously described (Molloy et al., 2017). MAV-1 viral loads were measured in organs using quantitative real-time polymerase chain reaction (qPCR) as previously described (McCarthy et al., 2015a; Procario et al., 2012).
3.10. Measurement of cytokine concentrations in bronchoalveolar lavage fluid
BALF was obtained by lavaging lungs three times with the same aliquot of 1 ml sterile PBS containing protease inhibitor (complete, Mini, EDTA-free tablets; Roche Applied Science). Cytokine concentrations were determined by ELISA (Duoset Kits, R&D Systems) according to the manufacturer’s directions. ELISA was performed by the University of Michigan Cancer Center Immunology Core.
3.11. Histology
Lungs were harvested from mice and fixed in 10% formalin. Prior to fixation, lungs were inflated with PBS via the trachea to maintain lung architecture. After fixation, organs were embedded in paraffin, and 5 μm sections were obtained for histopathology. Sections were stained with hematoxylin and eosin to evaluate cellular infiltration. To quantify cellular inflammation in the lungs, slides were examined in a blinded fashion to determine a pathology index as previous described (Procario et al., 2012).
Sectioning and staining were performed by the Michigan Medicine Rogel Cancer Tissue and Molecular Pathology Shared Resource. Digital images were obtained with an EC3 digital imaging system (Leica Microsystems) using Leica Acquisition Software (Leica Microsystems). Any adjustments to brightness and contrast in digital images were applied equally to all experimental and control images.
3.12. Statistics
Statistical analysis was performed using Prism 7 (GraphPad Software, Inc.). Viral loads were log-transformed for statistical analysis. Differences between two groups at a single time point were analyzed using the Mann-Whitney rank sum test. Differences between groups at multiple time points were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison tests. P values of <0.05 were considered statistically significant.
4. Discussion
CD8 T cell responses have the potential to limit viral replication and facilitate viral clearance as acute infection resolves, but CD8 T cell effector mechanisms that are responsible for those functions are also likely to contribute to pathology. CD8 T cells are essential for efficient control of viral replication in the lungs during the resolution of acute MAV-1 respiratory infection, although they are not sufficient to completely clear MAV-1, a persistent virus. CD8 T cells also contribute to aspects of immunopathology including virus-induced weight loss and the production of proinflammatory cytokines in the airways (Molloy et al., 2017). The effects of CD8 T cells on MAV-1 clearance from the lungs are not dependent on IFN-γ, Pfn, or Fas, and effects on virus-induced inflammation are only partially related to Fas (Adkins et al., 2018; Molloy et al., 2017). In this study, we tested the hypothesis that TNF, a different effector mechanism involved in CD8 T cell function, inhibits MAV-1 replication and contributes to virus-induced inflammation in the lungs during acute MAV-1 respiratory infection. Although TNF inhibited MAV-1 replication in vitro, we found that TNF deficiency had minimal effect on MAV-1 pathogenesis in vivo.
TNF inhibits replication of a diverse group of viruses, with examples including Kaposi’s sarcoma-associated herpesvirus (Park et al., 2019), varicella-zoster virus (Como et al., 2018), hepatitis C virus (Laidlaw et al., 2017; Lee et al., 2015; Wang et al., 2016), hepatitis B virus (Biermer et al., 2003; Park et al., 2016; Phillips et al., 2010; Puro and Schneider, 2007), porcine reproductive and respiratory syndrome virus (Li et al., 2016), vesicular stomatitis virus, encephalomyocarditis virus, herpes simplex virus (Mestan et al., 1986), and influenza (DeBerge et al., 2013). The effect of TNF on viral replication is not universal, though. For instance, TNF has no effect on dengue virus replication in monocyte-derived macrophages, and infection leads to TNF unresponsiveness (Wati et al., 2007). Our data indicate that TNF inhibits MAV-1 replication in vitro. It therefore seems unlikely that MAV-1 encodes a protein that interferes with TNF signaling in a manner similar to HAdV early region 3 (E3) 10.4K/14.5K complex-mediated downregulation of tumor necrosis factor receptor 1 (Chin and Horwitz, 2005, 2006; Friedman and Horwitz, 2002). Indeed, there are substantial differences between HAdV and MAV-1 E3 regions (Beard et al., 1990; Raviprakash et al., 1989).
In vivo, HAdV-based vector transgene delivery is increased and clearance of HAdV-based vectors is delayed in mouse models of TNF deficiency or blockade (Abougergi et al., 2005; Benihoud et al., 2007; Kafrouni et al., 2003; Minter et al., 2000). Although CD8 T cell deficiency has a similar effect on MAV-1 clearance from the lungs, our results indicate that TNF deficiency or immunoneutralization did not impair control of MAV-1 replication or clearance of virus from lungs or other organs. Likewise, isolated deficiency of IFN-γ Pfn, or Fas did not affect MAV-1 replication or clearance (McCarthy et al., 2015b; Molloy et al., 2017). These results are similar to findings in other models. For instance, both CD4 and CD8 T cells contribute to the clearance of pneumonia virus of mice replication and immunopathology (Frey et al., 2008), Similar to our findings with MAV-1, those effects are independent of IFN-γ, perforin, and TNF. Overlapping function and redundancy of multiple CD8 T cell effector mechanisms, which have been demonstrated for other viruses, including influenza (Topham et al., 1997) and murine gammaherpesvirus 68 (Tsai et al., 2011), are likely to maintain effective control of MAV-1 replication in the absence of TNF. It is possible that the anti-TNF antibody that we used in vivo did not completely neutralize TNF function. However, biologically relevant effects have been observed in other studies using both lower and higher doses of the antibody (Brasseit et al., 2016; Gopinath et al., 2014). In addition, the statistically significant effect of immunoneutralization on virus-induced weight loss was similar to the effect of TNF deficiency, providing evidence for TNF neutralization.
CD8 T cells exert a proinflammatory effect during acute MAV-1 respiratory infection (Molloy et al., 2017), similar to effects in mouse models of infection with RSV and influenza infection (DeBerge et al., 2013; Hussell et al., 2001; Peper and Van Campen, 1995; Xu et al., 2004). Fas/FasL interactions contribute to CD8 T cell-mediated airway inflammation during MAV-1 infection (Adkins et al., 2018). TNF serves as a proinflammatory mediator in mouse models of respiratory infection with RSV and influenza (DeBerge et al., 2013; Hussell et al., 2001; Peper and Van Campen, 1995; Xu et al., 2004). In this study, TNF deficiency decreased histological evidence of pulmonary inflammation, although we did not detect significant decreases in cytokine and chemokine concentrations in the airways. Effects of TNF deficiency on host factors not measured in our study are likely to have contributed to decreased recruitment of inflammatory cells to lungs of TNF−/− mice infected with MAV-1.
TNF is a proinflammatory cytokine, but it also exerts an immunoregulatory influence by promoting contraction of CD8 T cell effector functions as acute viral infection resolves (reviewed in Wortzman et al., 2013). We did not evaluate virus-specific CD8 T cell function in this study. However, the absence of such a regulatory effect on CD8 T cell function in TNF−/− mice infected with MAV-1 may have mitigated any loss of direct antiviral and proinflammatory effects mediated by TNF. Crosstalk between TNF and the immunoregulatory cytokine IL-10 has been described, with TNF immunoneutralization or blockade enhancing IL-10 responses (Evans et al., 2014; Roberts et al., 2017; Sato et al., 2003). We detected increased concentrations of IL-10 in BALF of infected TNF−/− mice compared to B6 controls, consistent with those effects. This differs from our previous findings in CD8 T cell-deficient mice, in which BALF IL-10 concentrations were lower in the absence of CD8 T cells (Molloy et al., 2017). It is plausible that increased IL-10 production in TNF−/− mice briefly decreased the magnitude of MAV-1-induced weight loss and histological evidence of pulmonary inflammation at 7 dpi. However, our data indicate that effects of TNF, either direct or indirect, are not essential for weight loss induced by MAV-1.
In summary, the results of this study indicate that TNF is capable of inhibiting MAV-1 replication in vitro, but the antiviral activity of TNF is not essential for control of MAV-1 replication or clearance of MAV-1 in infected mice. TNF makes contributions to disease in MAV-1-infected mice, including initial weight loss and histological evidence of pulmonary inflammation. However, many of the proinflammatory effects of CD8 T cells that are detected during acute MAV-1 respiratory infection, including the production of proinflammatory cytokines and chemokines in the airways (Molloy et al., 2017), remain intact in the absence of TNF. These results are similar to our findings in studies using MAV-1 infection of mice deficient in IFN-γ, Pfn, and Fas (Adkins et al., 2018; Molloy et al., 2017), in which isolated deficiency of a single host factor does not fully recapitulate the effects of CD8 T cell deficiency or depletion. It is therefore likely that the functional redundancy of multiple CD8 T cell effector mechanisms that has been reported in models using other viruses, such as influenza (Topham et al., 1997) and murine gammaherpesvirus 68 (Tsai et al., 2011), provides multiple layers of overlapping activity during acute MAV-1 respiratory infection.
Research Highlights.
TNF inhibits MAV-1 replication in vitro.
TNF deficiency does not impair control of MAV-1 replication in the lungs.
TNF deficiency delays virus-induced weight loss.
TNF reduces histological evidence of pulmonary inflammation.
TNF deficiency does not affect proinflammatory cytokine production in airways.
Acknowledgements
We thank Kathy Spindler for helpful review of the manuscript. Expert technical assistance from Joel Whitfield in the University of Michigan Cancer Center Immunology Core is greatly appreciated. This research was supported by NIH R21 AI142073 and by a University of Michigan Department of Pediatrics Charles Woodson Pilot Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Declarations of interest: none
References
- Abougergi MS, Gidner SJ, Spady DK, Miller BC, Thiele DL, 2005. Fas and TNFR1, but not cytolytic granule-dependent mechanisms, mediate clearance of murine liver adenoviral infection. Hepatology 41, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins LJ, Molloy CT, Weinberg JB, 2018. Fas activity mediates airway inflammation during mouse adenovirus type 1 respiratory infection. Virology 521, 129–137. [DOI] [PubMed] [Google Scholar]
- Ashley SL, Pretto CD, Stier MT, Kadiyala P, Castro-Jorge L, Hsu TH, Doherty R, Carnahan KE, Castro MG, Lowenstein PR, Spindler KR, 2017. Matrix Metalloproteinase Activity in Infections by an Encephalitic Virus, Mouse Adenovirus Type 1. J. Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard CW, Ball AO, Wooley EH, Spindler KR, 1990. Transcription mapping of mouse adenovirus type 1 early region 3. Virology 175, 81–90. [DOI] [PubMed] [Google Scholar]
- Benihoud K, Esselin S, Descamps D, Jullienne B, Salone B, Bobe P, Bonardelle D, Connault E, Opolon P, Saggio I, Perricaudet M, 2007. Respective roles of TNF-alpha and IL-6 in the immune response-elicited by adenovirus-mediated gene transfer in mice. Gene Ther. 14, 533–544. [DOI] [PubMed] [Google Scholar]
- Biermer M, Puro R, Schneider RJ, 2003. Tumor necrosis factor alpha inhibition of hepatitis B virus replication involves disruption of capsid Integrity through activation of NF-kappaB. J. Virol 77, 4033–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasseit J, Althaus-Steiner E, Faderl M, Dickgreber N, Saurer L, Genitsch V, Dolowschiak T, Li H, Finke D, Hardt WD, McCoy KD, Macpherson AJ, Corazza N, Noti M, Mueller C, 2016. CD4 T cells are required for both development and maintenance of disease in a new mouse model of reversible colitis. Mucosal Immunol. 9, 689–701. [DOI] [PubMed] [Google Scholar]
- Cauthen AN, Welton AR, Spindler KR, 2007. Construction of mouse adenovirus type 1 mutants. Methods Mol. Med 130, 41–59. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran A, Adkins LJ, Seltzer HM, Pant K, Tryban ST, Molloy CT, Weinberg JB, 2019. Age-Dependent Effects of Immunoproteasome Deficiency on Mouse Adenovirus Type 1 Pathogenesis. J. Virol 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YR, Horwitz MS, 2005. Mechanism for removal of tumor necrosis factor receptor 1 from the cell surface by the adenovirus RIDalpha/beta complex. J. Virol 79, 13606–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YR, Horwitz MS, 2006. Adenovirus RID complex enhances degradation of internalized tumour necrosis factor receptor 1 without affecting its rate of endocytosis. J. Gen. Virol 87, 3161–3167. [DOI] [PubMed] [Google Scholar]
- Como CN, Pearce CM, Cohrs RJ, Baird NL, 2018. Interleukin-6 and type 1 interferons inhibit varicella zoster virus replication in human neurons. Virology 522, 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerge MP, Ely KH, Cheng GS, Enelow RI, 2013. ADAM17-mediated processing of TNF-alpha expressed by antiviral effector CD8+ T cells is required for severe T-cell-mediated lung injury. PLoS One 8, e79340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HG, Roostalu U, Walter GJ, Gullick NJ, Frederiksen KS, Roberts CA, Sumner J, Baeten DL, Gerwien JG, Cope AP, Geissmann F, Kirkham BW, Taams LS, 2014. TNF-alpha blockade induces IL-10 expression in human CD4+ T cells. Nat Commun 5, 3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Krempl CD, Schmitt-Graff A, Ehl S, 2008. Role of T cells in virus control and disease after infection with pneumonia virus of mice. J. Virol 82, 11619–11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Horwitz MS, 2002. Inhibition of tumor necrosis factor alpha-induced NF-kappa B activation by the adenovirus E3–10.4/14.5K complex. J. Virol 76, 5515–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath S, Lichtman JS, Bouley DM, Elias JE, Monack DM, 2014. Role of disease-associated tolerance in infectious superspreaders. Proc. Natl. Acad. Sci. U. S. A 111, 15780–15785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T, Pennycook A, Openshaw PJ, 2001. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol 31, 2566–2573. [DOI] [PubMed] [Google Scholar]
- Kafrouni MI, Brown GR, Thiele DL, 2003. The role of TNF-TNFR2 interactions in generation of CTL responses and clearance of hepatic adenovirus infection. J. Leukoc. Biol 74, 564–571. [DOI] [PubMed] [Google Scholar]
- Kajon AE, Brown CC, Spindler KR, 1998. Distribution of mouse adenovirus type 1 in intraperitoneally and intranasally infected adult outbred mice. J. Virol 72, 1219–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojaoghlanian T, Flomenberg P, Horwitz MS, 2003. The impact of adenovirus infection on the immunocompromised host. Rev. Med. Virol 13, 155–171. [DOI] [PubMed] [Google Scholar]
- Laidlaw SM, Marukian S, Gilmore RH, Cashman SB, Nechyporuk-Zloy V, Rice CM, Dustin LB, 2017. Tumor Necrosis Factor Inhibits Spread of Hepatitis C Virus Among Liver Cells, Independent From Interferons. Gastroenterology 153, 566–578 e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Tian Y, Chan ST, Kim JY, Cho C, Ou JH, 2015. TNF-alpha Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism. PLoS Pathog. 11, e1004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Guo L, Gu W, Luo X, Zhang J, Xu Y, Tian Z, Feng L, Wang Y, 2016. Production of porcine TNFalpha by ADAM17-mediated cleavage negatively regulates porcine reproductive and respiratory syndrome virus infection. Immunol. Res 64, 711–720. [DOI] [PubMed] [Google Scholar]
- McCarthy MK, Procario MC, Twisselmann N, Wilkinson JE, Archambeau AJ, Michele DE, Day SM, Weinberg JB, 2015a. Proinflammatory effects of interferon gamma in mouse adenovirus 1 myocarditis. J. Virol 89, 468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MK, Procario MC, Wilke CA, Moore BB, Weinberg JB, 2015b. Prostaglandin E2 production and T cell function in mouse adenovirus type 1 infection following allogeneic bone marrow transplantation. PLoS One 10, e0139235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MK, Zhu L, Procario MC, Weinberg JB, 2014. IL-17 contributes to neutrophil recruitment but not to control of viral replication during acute mouse adenovirus type 1 respiratory infection. Virology 456–457, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestan J, Digel W, Mittnacht S, Hillen H, Blohm D, Moller A, Jacobsen H, Kirchner H, 1986. Antiviral effects of recombinant tumour necrosis factor in vitro. Nature 323, 816–819. [DOI] [PubMed] [Google Scholar]
- Minter RM, Rectenwald JE, Fukuzuka K, Tannahill CL, La Face D, Tsai V, Ahmed I, Hutchins E, Moyer R, Copeland EM 3rd, Moldawer LL, 2000. TNF-alpha receptor signaling and IL-10 gene therapy regulate the innate and humoral immune responses to recombinant adenovirus in the lung. J. Immunol 164, 443–451. [DOI] [PubMed] [Google Scholar]
- Molloy CT, Andonian JS, Seltzer HM, Procario MC, Watson ME Jr., Weinberg JB, 2017. Contributions of CD8 T cells to the pathogenesis of mouse adenovirus type 1 respiratory infection. Virology 507, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Cho H, Roh SW, Kim SJ, Myoung J, 2019. Cell Type-Specific Interferon-gamma-mediated Antagonism of KSHV Lytic Replication. Sci. Rep 9, 2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YK, Park ES, Kim DH, Ahn SH, Park SH, Lee AR, Park S, Kang HS, Lee JH, Kim JM, Lee SK, Lim KH, Isorce N, Tong S, Zoulim F, Kim KH, 2016. Cleaved c-FLIP mediates the antiviral effect of TNF-alpha against hepatitis B virus by dysregulating hepatocyte nuclear factors. J. Hepatol 64, 268–277. [DOI] [PubMed] [Google Scholar]
- Peper RL, Van Campen H, 1995. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb. Pathog 19, 175–183. [DOI] [PubMed] [Google Scholar]
- Phillips S, Chokshi S, Riva A, Evans A, Williams R, Naoumov NV, 2010. CD8(+) T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J. Immunol 184, 287–295. [DOI] [PubMed] [Google Scholar]
- Procario MC, Levine RE, McCarthy MK, Kim E, Zhu L, Chang CH, Hershenson MB, Weinberg JB, 2012. Susceptibility to acute mouse adenovirus type 1 respiratory infection and establishment of protective immunity in neonatal mice. J. Virol 86, 4194–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro R, Schneider RJ, 2007. Tumor necrosis factor activates a conserved innate antiviral response to hepatitis B virus that destabilizes nucleocapsids and reduces nuclear viral DNA. J. Virol 81, 7351–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, McFadden G, 2006. Modulation of tumor necrosis factor by microbial pathogens. PLoS Pathog. 2, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviprakash KS, Grunhaus A, el Kholy MA, Horwitz MS, 1989. The mouse adenovirus type 1 contains an unusual E3 region. J. Virol 63, 5455–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CA, Durham LE, Fleskens V, Evans HG, Taams LS, 2017. TNF Blockade Maintains an IL-10(+) Phenotype in Human Effector CD4(+) and CD8(+) T Cells. Front. Immunol 8, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TA, Keelan JA, Mitchell MD, 2003. Critical paracrine interactions between TNF-alpha and IL-10 regulate lipopolysaccharide-stimulated human choriodecidual cytokine and prostaglandin E2 production. J. Immunol 170, 158–166. [DOI] [PubMed] [Google Scholar]
- Seo SH, Webster RG, 2002. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J. Virol 76, 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirumuru N, Pretto CD, Castro Jorge LA, Spindler KR, 2016. Mouse Adenovirus Type 1 Early Region 1A Effects on the Blood-Brain Barrier. mSphere 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham DJ, Tripp RA, Doherty PC, 1997. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol 159, 5197–5200. [PubMed] [Google Scholar]
- Tsai CY, Hu Z, Zhang W, Usherwood EJ, 2011. Strain-dependent requirement for IFN-gamma for respiratory control and immunotherapy in murine gammaherpesvirus infection. Viral Immunol. 24, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls T, Shankar AG, Shingadia D, 2003. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. Lancet Infect. Dis 3, 79–86. [DOI] [PubMed] [Google Scholar]
- Wang W, Xu L, Brandsma JH, Wang Y, Hakim MS, Zhou X, Yin Y, Fuhler GM, van der Laan LJ, van der Woude CJ, Sprengers D, Metselaar HJ, Smits R, Poot RA, Peppelenbosch MP, Pan Q, 2016. Convergent Transcription of Interferon-stimulated Genes by TNF-alpha and IFN-alpha Augments Antiviral Activity against HCV and HEV. Sci. Rep 6, 25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wati S, Li P, Burrell CJ, Carr JM, 2007. Dengue virus (DV) replication in monocyte-derived macrophages is not affected by tumor necrosis factor alpha (TNF-alpha), and DV infection induces altered responsiveness to TNF-alpha stimulation. J. Virol 81, 10161–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Jensen DR, Gralinski LE, Lake AR, Stempfle GS, Spindler KR, 2007. Contributions of E1A to mouse adenovirus type 1 pathogenesis following intranasal inoculation. Virology 357, 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Stempfle GS, Wilkinson JE, Younger JG, Spindler KR, 2005. Acute respiratory infection with mouse adenovirus type 1. Virology 340, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold WSM, Isom MG, 2013. Adenoviruses, in: Knipe DM, Howley PM (Eds.), Fields Virology, 6 ed. Lippincott, Williams & Wilkins, Philadelphia, pp. 1732–1767. [Google Scholar]
- Wortzman ME, Clouthier DL, McPherson AJ, Lin GH, Watts TH, 2013. The contextual role of TNFR family members in CD8(+) T-cell control of viral infections. Immunol. Rev 255, 125–148. [DOI] [PubMed] [Google Scholar]
- Xu L, Yoon H, Zhao MQ, Liu J, Ramana CV, Enelow RI, 2004. Cutting edge: pulmonary immunopathology mediated by antigen-specific expression of TNF-alpha by antiviral CD8+ T cells. J. Immunol 173, 721–725. [DOI] [PubMed] [Google Scholar]


