Abstract
BACKGROUND:
Cognitive and emotional disturbances are common serious issues in patients with traumatic brain injury (TBI). However, predictors associated with neuropsychological functions were not consistent.
OBJECTIVE:
To investigate factors affecting cognition and emotion in patients with TBI, we evaluated executive function, memory, and emotion based on injury severity and lesion location.
METHODS:
Neuropsychological outcomes of 80 TBI patients were evaluated via Wisconsin Card Sorting Test (WCST), Color Trail Test (CTT), Controlled Oral Word Association Test (COWAT), Everyday Memory Questionnaire (EMQ), Geriatric Depression Scale (GDS), State-Trait Anxiety Inventory (STAI), and Agitated Behavior Scale (ABS). WCST, CTT, and COWAT assessed executive function; EMQ assessed everyday memory; and GDS, STAI, and ABS assessed emotion. Patients were categorized according to lateralization of lesion and existence of frontal lobe injury.
RESULTS:
Patients with longer duration of loss of consciousness (LOC) showed more severe deficits in everyday memory and agitated behaviors. The frontal lesion group showed poorer performance in executive function and higher agitation than the non-frontal lesion group. Patients with bilateral frontal lesion showed greater deficits in executive function and were more depressed than unilateral frontal lesion groups. Especially in those unilateral frontal lesion groups, right side frontal lesion group was worse on executive function than left side frontal lesion group.
CONCLUSIONS:
Duration of LOC and lesion location are main parameters affecting executive function, everyday memory, and emotion in neuropsychological outcomes following TBI, suggesting that these parameters need to be considered for cognitive rehabilitation interventions.
Keywords: Traumatic brain injury, executive function, memory, emotion
1. Introduction
Cognitive and emotional disturbances are common serious issues in patients with traumatic brain injury (TBI) (Dikmen et al., 2009; Hammond, Hart, Bushnik, Corrigan, & Sasser, 2004), which exert a negative impact on their quality of life and rehabilitation process (Hesdorffer, Rauch, & Tamminga, 2009; Rogers & Read, 2007). Neuropsychological dysfunction following TBI can be influenced by severity of injury, lesion site, duration after brain injury, intelligence, educational level, age, drug usage, and socioeconomic factors such as family and financial state (Ponsford et al., 2000; Rosenthal, Christensen, & Ross, 1998).
Cognitive functions, which are affected by TBI, include attention, memory, information processing speed, perception, judgment, language, and executive function (Carney et al., 1999; Cicerone et al., 2000). Emotional and behavioral disturbances can be derived from either direct brain injury or secondary psychological responses. The following conditions are frequently observed in TBI: agitation, impulsivity, restlessness, emotional instability, apathy, unwillingness, depression, anxiety, stress sensitivity, and denial (Jorge et al., 2004; Prigatano, 1992). It has been known that deficits in executive function and memory are easily noticed in TBI due to the vulnerability of brain areas such as frontal lobe and temporal lobe (Carlozzi, Grech, & Tulsky, 2013), and these are relatively more difficult to treat than other cognitive impairments (Sohlberg & Mateer, 1989).
Neuropsychological functions in TBI can be improved, depending on the time after brain injury (Ashman et al., 2004; Dikmen, Machamer, Powell, & Temkin, 2003; Hammond et al., 2004; Salmond, Menon, Chatfield, Pickard, & Sahakian, 2006; Senathi-Raja, Ponsford, & Schonberger, 2010; Whelan-Goodinson, Ponsford, Schonberger, & Johnston, 2010). In particular, age or educational level before brain injury has been considered to be associated with reserved capacity and vulnerability of brain against cognitive deficits after TBI, indicating that these variables need to be controlled in this study (Scheibel et al., 2009; Sole-Padulles et al., 2009). However, previous studies have shown that some predictors were not consistent in the properties of subject groups and study designs, while related variables were not controlled (Ciurli, Formisano, Bivona, Cantagallo, & Angelelli, 2011; Glascher et al., 2009; Whelan-Goodinson et al., 2010).
Emotional issues, shown after brain injury, can impair the quality of patient’s life beyond cognitive deficits or physiological disorders. However, the significance of such issues might have been overlooked in previous studies (Binder, Kelly, Villanueva, & Winslow, 2003; Rogers & Read, 2007). Studies on predictive variables related to emotional disorders are even more complex (Horner, Selassie, Lineberry, Ferguson, & Labbate, 2008; Whelan-Goodinson et al., 2010).
Therefore, the purpose of this study was to establish predictors that are associated with neuropsychological outcomes by analyzing neurobehavioral assessments and investigating the effects of TBI on executive function, memory, and emotions such as depression, anxiety, and agitation by controlling the effect of other extraneous variables.
2. Methods
2.1. Subjects
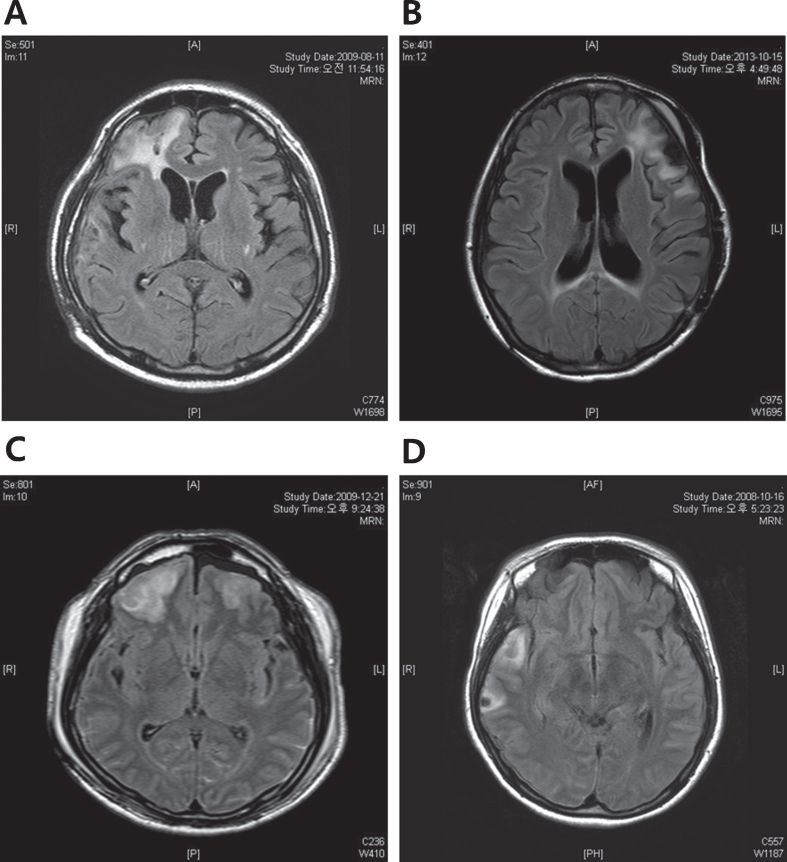
A total of 80 patients (62 males and 18 females) with TBI were recruited (Table 1). Neuropsychological assessment was performed on patients aged between 17 and 63 via Wisconsin Card Sorting Test (WCST), Color Trail Test (CTT), Controlled Oral Word Association Test (COWAT), Everyday Memory Questionnaire (EMQ), Geriatric Depression Scale (GDS), State-Trait Anxiety Inventory (STAI), and Agitated Behavior Scale (ABS). The ranges of period for loss of consciousness (LOC) and time after brain injury were 1–120 days and 3–36 months, respectively. Patients were categorized into having left side (n = 19), right side (n = 16), and bilateral (n = 45) lesions. In addition, there were 49 patients with frontal lobe lesion. Of these, 14 patients had only frontal lobe lesion, and 35 patients had lesions on both frontal and non-frontal lobes, while 31 patients had no frontal lesion. Among patients with frontal lesion, 11 patients had lesions on the left side while 15 had lesions on the right side, and 23 patients had bilateral lesions. The representative MRI images of each TBI group were shown in Fig. 1.
Table 1.
Demographic characteristics of subjects (n = 80)
| Variables | Values |
| Age at assessment (years) | 36.26±13.86 |
| Education (years) | 14.24±3.15 |
| Period of LOC (days) | 16.90±21.22 |
| Time after brain injury (months) | 11.20±10.12 |
| Gender | |
| Male | 62 (77.5%) |
| Female | 18 (22.5%) |
| Site of lesion | |
| LHL | 19 (23.8%) |
| RHL | 16 (20.0%) |
| BDL | 45 (56.3%) |
| FL | 14 (17.5%) |
| F-NFL | 35 (43.8%) |
| NFL | 31 (38.7%) |
| LFL | 11 (22.4%) |
| RFL | 15 (30.6%) |
| BFL | 23 (46.9%) |
Values are mean±standard deviation. LOC, loss of consciousness; LHL, left hemisphere lesion; RHL, right hemisphere lesion; BDL, bilateral or diffuse lesion; FL, frontal lobe lesion; F-NFL, frontal-non-frontal lobe lesion; NFL, non-frontal lobe lesion; LFL, left frontal lobe lesion; RFL, right frontal lobe lesion; BFL, bilateral frontal lobe lesion.
Fig. 1.
The representative MRI images of each TBI group. (A) Right frontal injury (B) Left frontal injury (C) Bilateral frontal injury (D) Non-frontal injury, Right temporal injury. MRI, Magnetic Resonance Imaging; TBI, Traumatic brain injury.
The study was approved by the Ethics Committee, and participants signed informed consent prior to the study. The Institutional Review Board of Severance Hospital, Yonsei University Health System approved this procedure as well as the entire study (no. 4-2016-0398).
2.2. Measures
2.2.1. Wisconsin Card Sorting Test
WCST is one of the representative measures of prefrontal executive function, requiring mental flexibility and problem solving ability. It was performed by the standardized procedures proposed by Heaton and percentage scores were used for dependent data to control the differences in a number of trials administered (Heaton RK, 1993). In this study, we analyzed four indices (i.e., percent errors, percent perseverative errors, percent conceptual level responses, and number of categories completed) because previous studies reported that these scores are decreased in TBI patients compared to those of healthy control (Haut et al., 1996).
2.2.2. Color Trail Test
CTT measures visual attention, visual scanning, and graphomotor skills, while recording the information-processing speed as well as motor-hand coordination. Although this test originated from Trail Making Test (TMT), it was modified in this study to eliminate the difference arising from cultural diversity. Reaction time of CTT2 was analyzed as CTT2 covers alternative patterns of orders, and to evaluate the system of the frontal lobe (D’Elia, 1996). TBI patients tend to exhibit poor performance in their ability to smoothly change cognitive frames (Kim, 2003). The reaction time was limited to less than 5 minutes, as it has been suggested not to last the task for more than 4 to 5 minutes in order to help lower the influence from extreme scores.
2.2.3. Controlled Oral Word Association Test
COWAT is the measurement of word fluency and idea generation as a proper index of divergent thinking by frontal lobe (Milner, 1984). Within one minute, participants are required to generate as many words as they can that belong to a specific category and begin with a specific letter. The total number of words generated is recorded for two categories and three letters. Test-retest reliability of COWAT-Korean version has been found to be 0.56 to 0.62 in an adult sample population (Kang, 2000).
2.2.4. Everyday Memory Questionnaire
EMQ is direct data on memory defects and retrospective evaluation of memory failure shown in everyday life in a week, by averaging reports from patients and their families (Sunderland, 1983). This test was translated in Korean and administrated. There were 35 questions in five memory categories including language, reading and writing, face and place, behaviors, and new learning ability. The total score range was 0 to 140.
2.2.5. Geriatric Depression Scale
GDS is a self-reported, True or False type depression scale (Yesavage et al., 1982). Although GDS was developed for the elderly, it is more useful for brain injury patients with impaired cognition. The test consists of 30 questions, with the total scores ranging from 0 to 30. The cut-off point of this test was reported as 18 (sensitivity 65.6%, specificity 64.9%) in standardized studies in Korea(Jung, 1997).
2.2.6. State-Trait Anxiety Inventory
In this study, STAI state anxiety scale which has been widely used to evaluate anxiety level caused by stress factors such as surgeries, treatments, and examinations was used (Hahn, 2000). The inventory consisted of 20 questions, and total score range was 20 to 80.
2.2.7. Agitated Behavior Scale
ABS is used to evaluate TBI patients’ behaviors by measuring them through 14 questions with a four-point scale (1 to 4 point) which includes distraction, impulsivity, noncooperation, aggression, restlessness, repetitive behaviors, and mood swings. The score range was from 14 to 56. This scale that has been suggested to offer a reliable measurement (Cronbach’s α=0.84 to 0.92) of behavioral disturbance in TBI patients (Corrigan, 1989).
2.3. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS v.15). Age, education level, duration of LOC and time after brain injury effects on executive function, everyday memory and emotional function were analyzed by Pearson’s correlation coefficients. Additionally, age, education level, duration of LOC and time after brain injury effects on brain lesion properties were analyzed by ANOVA. Effects of brain lesion properties on multiple variables associated with executive function, memory and emotion were analyzed by MANCOVA and MANOVA. To find significant group differences on each variable, ANCOVA and ANOVA were additionally utilized in this study.
3. Results
3.1. Correlations between patient characteristics
Detailed information about subjects is listed in Table 1 and correlations patient characteristics (age, education level, duration of LOC and time after injury) and neuropsychological functions were analyzed using Pearson’s correlation coefficients listed in Table 2. Age was negatively correlated with percent conceptual level responses and the number of completed categories of WCST (r=–0.36, p < 0.001; r=–0.43, p < 0.001, respectively), while positively correlated with CTT2, percent error and percent perseverative errors of WCST (r = 0.36, p < 0.001; r = 0.32, p < 0.01; r = 0.26, p < 0.05, respectively). Age was also positively correlated with GDS (r = 0.24, p < 0.05). Education level was positively correlated with COWAT, percent conceptual level responses and the number of completed categories of WCST (r = 0.29, p < 0.01; r = 0.28, p < 0.05; r = 0.27, p < 0.05, respectively), while negatively correlated with percent errors of WCST, ABS and GDS (r=–0.27, p < 0.05; r=–0.34, p < 0.01; r=–0.26, p < 0.05, respectively). The duration of LOC showed a significantly positive correlation with EMQ and ABS (r = 0.41, p < 0.001; r = 0.32, p < 0.01, respectively). The duration of LOC significantly affected everyday memory and agitated behaviors. A longer period of LOC showed more severe everyday memory deficits and agitated behaviors. However, the duration of LOC did not have an effect on WCST, GDS, and STAI.
Table 2.
Factors affecting neuropsychological functions
| Age | Education | Period of LOC | Time after brain injury | |
| WCST | ||||
| % errors | 0.32** | –0.27* | 0.18 | –0.13 |
| % perseverative errors | 0.26* | –0.19 | 0.08 | –0.02 |
| % conceptual level responses | –0.36*** | 0.28* | –0.19 | 0.10 |
| No. of completed categories | –0.43*** | 0.27* | –0.16 | 0.06 |
| CTT2 | 0.36*** | –0.23 | 0.19 | –0.11 |
| COWAT | –0.21 | 0.29** | –0.14 | 0.19 |
| EMQ | –0.03 | –0.14 | 0.41*** | 0.09 |
| GDS | 0.24* | –0.26* | 0.14 | 0.03 |
| STAI | 0.17 | –0.10 | 0.11 | 0.05 |
| ABS | 0.09 | –0.34** | 0.32** | 0.04 |
*p < 0.05, **p < 0.01, ***p < 0.001. WCST, Wisconsin Card Sorting Test; CTT2, Color Trail Test 2; COWAT, Controlled Oral Word Association Test; EMQ, Everyday Memory Questionnaire; GDS, Geriatric Depression Scale; STAI, State-Trait Anxiety Inventory; ABS, Agitated Behavior Scale.
3.2. Comparison of neuropsychological functions by lesion lateralization
The results of comparison of characteristics of each group in regard to lesion lateralization showed no difference between groups in the education level, the duration of LOC and time after injury while age was significantly different between groups (F = 3.25, p < 0.05) in Table 3. Bilateral or diffuse damaged group were significantly older than the group with unilateral lesion groups.
Table 3.
Factors associated with lateralization of lesion
| LHL | RHL | BDL | F | |
| (n = 19) | (n = 16) | (n = 45) | ||
| Age | 29.42±6.96 | 37.56±15.51 | 38.69±13.85 | 3.25* |
| Education level | 15.47±2.27 | 13.81±3.73 | 13.87±3.17 | 1.97 |
| Period of LOC | 13.05±16.59 | 10.56±12.89 | 20.78±24.55 | 1.81 |
| Time after brain injury | 11.21±12.50 | 10.56±10.07 | 11.41±9.23 | 0.41 |
*p < 0.05. LHL, Left Hemisphere Lesion; RHL, Right Hemisphere Lesion; BDL, Bilateral or Diffuse lesion.
To examine any difference in neuropsychological functions depending upon the lateralization of brain lesion when age was controlled, MANCOVA analysis was performed shown in Table 4. Even though the types of lateralization of brain lesion had no significant impact on executive function and everyday memory in general (Wilks’ Lambda 0.73, F = 1.65, p = 0.07), emotional functioning was significantly affected (Wilks’ Lambda 0.80, F = 2.87, p < 0.05). According to the results of ANCOVA in Table 5, lateralization of brain lesion showed significant differences between groups in GDS (F = 8.36, p < 0.001) and STAI (F = 4.70, p < 0.01). In results of post hoc test, TBI groups with unilateral lesion were not different in depression and anxiety parameters, while bilateral lesion group was more depressed compared to unilateral lesion group and more anxious than left-sided lesion group. Therefore, bilateral lesion group might be more vulnerable in terms of emotional dysfunction than any other lesion group after severe TBI.
Table 4.
Multiple analysis of covariances with covariance for neuropsychological functions by lateralization of lesion
| Independent variables | Dependent variables | Wilks’ Lambda(F) | univariate F | df | η2 |
| Lateralization of lesion | % errors | 0.73(1.65) | 1.62 | 2/75 | 0.04 |
| % perseverative errors | 2.68 | 2/75 | 0.07 | ||
| % conceptual level responses | 2.10 | 2/75 | 0.05 | ||
| No. of categories completed | 2.06 | 2/75 | 0.05 | ||
| CTT2 | 0.19 | 2/75 | 0.01 | ||
| COWAT | 1.01 | 2/75 | 0.03 | ||
| EMQ | 3.21* | 2/75 | 0.08 | ||
| GDS | 0.80(2.87)* | 8.36*** | 2/75 | 0.18 | |
| STAI | 4.70** | 2/75 | 0.11 | ||
| ABS | 1.86 | 2/75 | 0.05 |
*p < 0.05, **p < 0.01, ***p < 0.001 Covariates: Age.
Table 5.
Mean comparison of neuropsychological functions by lateralization of lesion
| LHL | RHL | BDL | F | |
| (n = 19) | (n = 16) | (n = 45) | ||
| WCST | ||||
| % errors | 32.00±14.29 | 42.87±18.91 | 45.29±21.21 | 1.62 |
| % perseverative errors | 17.74±7.60 | 20.56±10.47 | 27.78±17.95 | 2.68 |
| % conceptual level responses | 60.47±19.05 | 44.69±26.01 | 41.13±27.63 | 2.10 |
| No. of completed categories | 4.58±1.71 | 2.81±2.32 | 2.82±2.50 | 2.06 |
| CTT2 | 174.84±88.74 | 192.25±79.80 | 206.96±90.05 | 0.19 |
| COWAT | 38.68±27.64 | 42.94±21.47 | 33.58±20.18 | 1.01 |
| EMQ | 52.79±39.37 | 42.63±34.75 | 67.33±39.65 | 3.21 |
| GDS | 7.58±5.01 | 10.69±8.96 | 15.96±8.06 | 8.36*** |
| STAI | 39.58±9.89 | 45.69±15.02 | 49.91±13.02 | 4.70** |
| ABS | 6.95±8.13 | 10.38±8.59 | 11.93±8.39 | 1.86 |
**p < 0.01, ***p < 0.001 Covariates: Age. LHL, Left Hemisphere Lesion; RHL, Right Hemisphere Lesion; BDL, Bilateral or Diffuse lesion; WCST, Wisconsin Card Sorting Test; CTT2, Color Trail Test 2; COWAT, Controlled Oral Word Association Test; EMQ, Everyday Memory Questionnaire; GDS, Geriatric Depression Scale; STAI, State-Trait Anxiety Inventory; ABS, Agitated Behavior Scale.
3.3. Comparison of neuropsychological functions by frontal lobe damage
In the results of analysis of properties of subgroups divided according to the existence of frontal lobe lesion, age (F = 4.70, p < 0.05), education level (F = 3.07, p < 0.05), duration of LOC (F = 8.55, p < 0.001) were different between groups in Table 6. Frontal lobe lesion group was significantly older than the other groups, and non-frontal lesion group was the youngest among three groups. The education level was higher in the non-frontal lesion group compared to the two frontal lobe lesion groups while the duration of LOC was significantly longer in the frontal-non-frontal lesion group compared to the other groups.
Table 6.
Factors associated with existence of frontal lobe lesion
| FL | F-NFL | NFL | F | |
| (n = 14) | (n = 35) | (n = 31) | ||
| Age | 41.71±12.44 | 39.03±15.30 | 30.68±10.87 | 4.70* |
| Education level | 14.71±2.79 | 13.29±3.46 | 15.10±2.69 | 3.07* |
| Period of LOC | 8.29±8.30 | 27.09±27.80 | 9.29±8.00 | 8.55*** |
| Time after brain injury | 5.37±4.69 | 12.42±10.41 | 11.36±10.63 | 2.15 |
*p < 0.05, ***p < 0.001. FL, frontal lesion; F-NFL, frontal-non-frontal lesion; NFL, non-frontal lesion.
To investigate whether frontal lobe lesion affects neuropsychological functions when controlling the effects of other extraneous variables including age, education level and duration of LOC, MANCOVA was performed shown in Table 7. According to the results, whether the frontal lobe is damaged or not significantly affected on the executive functions (Wilks’ Lambda 0.69, F = 1.97, p < 0.05) and emotional functioning was significantly affected (Wilks’ Lambda 0.79, F = 2.99, p < 0.01).
Table 7.
Multiple analysis of covariances with covariance for neuropsychological functions depended on existence of frontal lobe lesion
| Independent variables | Dependent variables | Wilks’ Lambda(F) | univariate F | df | η2 |
| With or Without | % errors | 0.69(1.97*) | 10.08*** | 2/74 | 0.23 |
| Frontal lesion | % perseverative errors | 5.90** | 2/74 | 0.14 | |
| % conceptual level responses | 9.25*** | 2/74 | 0.20 | ||
| No. of categories completed | 9.73*** | 2/74 | 0.21 | ||
| CTT2 | 1.56 | 2/74 | 0.04 | ||
| COWAT | 0.96 | 2/74 | 0.03 | ||
| EMQ | 1.72 | 2/74 | 0.04 | ||
| GDS | 0.79(2.99**) | 0.58 | 2/74 | 0.02 | |
| STAI | 0.94 | 2/74 | 0.03 | ||
| ABS | 9.03*** | 2/74 | 0.20 |
*p < 0.05, **p < 0.01, ***p < 0.001 Covariates: Age, Education, period of LOC.
According to the results of ANCOVA, the indexes of WCST showed frontal lobe lesion group significantly performed worse than non-frontal lobe lesion group on percent errors (F = 10.89, p < 0.001), percent perseverative errors (F = 6.11, p < 0.01), percent conceptual level responses (F = 9.23, p < 0.001), and number of completed categories (F = 9.53, p < 0.001) as shown in Table 8. Although no significant difference in everyday memory was found, patients with frontal lobe lesion showed a significant difference in ABS was observed (F = 8.80, p < 0.001) and more severe agitated behavior compared to non-frontal lobe lesion group shown in Table 8. In results of post hoc comparison, frontal lobe lesion group and frontal-non-frontal lobe lesion group significantly performed worse than non-frontal lobe lesion group on the four indexes of WCST measuring executive function. However, no significant differences in everyday memory were found between patients with or without frontal lobe lesion. Moreover, frontal lobe lesion group and frontal-non-frontal lobe lesion group showed more severe agitated behavior problems than non-frontal lobe lesion group.
Table 8.
Mean comparison of neuropsychological functions depended on existence of frontal lobe lesion
| FL | F-NFL | NFL | F | |
| (n = 14) | (n = 35) | (n = 31) | ||
| WCST | ||||
| % errors | 53.00±19.79 | 49.97±17.96 | 27.13±12.10 | 10.89*** |
| % perseverative errors | 31.86±20.65 | 28.34±13.79 | 15.42±9.33 | 6.11** |
| % conceptual level responses | 32.07±26.56 | 35.49±23.88 | 65.29±16.98 | 9.23*** |
| No. of completed categories | 2.00±2.32 | 2.17±2.20 | 5.00±1.43 | 9.53*** |
| CTT2 | 213.14±93.73 | 227.54±77.15 | 153.65±81.04 | 1.49 |
| COWAT | 30.57±20.56 | 32.40±21.85 | 44.23±22.39 | 0.91 |
| EMQ | 50.07±44.07 | 74.86±34.36 | 44.97±37.38 | 1.76 |
| GDS | 12.07±9.47 | 15.37±7.47 | 10.52±8.34 | 0.57 |
| STAI | 44.43±13.42 | 50.26±13.26 | 43.48±12.78 | 0.89 |
| ABS | 11.57±9.51 | 14.94±8.30 | 4.84±4.11 | 8.80*** |
**p < 0.01, ***p < 0.001 Covariates: Age, Education, period of LOC. FL, frontal lesion; F-NFL, frontal-non-frontal lesion; NFL, non-frontal lesion; WCST, Wisconsin Card Sorting Test; CTT2, Color Trail Test 2; COWAT, Controlled Oral Word Association Test; EMQ, Everyday Memory Questionnaire; GDS, Geriatric Depression Scale; STAI, State-Trait Anxiety Inventory; ABS, Agitated Behavior Scale.
3.4. Comparison of neuropsychological functions by lateralization of frontal lobe damage
The comparison between groups with unilateral frontal lobe lesion and bilateral frontal lobe lesion showed that there were no differences in age, education level, duration of LOC, time after injury between the groups in Table 9.
Table 9.
Factors associated with lateralization of frontal lobe lesion
| LFL | RFL | BFL | F | |
| (n = 11) | (n = 15) | (n = 23) | ||
| Age | 38.00±13.22 | 38.73±14.70 | 41.35±15.31 | 0.25 |
| Education level | 14.64±2.91 | 14.40±3.29 | 12.78±3.41 | 1.70 |
| Period of LOC | 14.27±21.11 | 24.93±27.58 | 23.17±25.93 | 0.63 |
| Time after brain injury | 7.81±10.20 | 11.85±10.07 | 10.91±10.10 | 0.54 |
*p < 0.05, ***p < 0.001. FL, frontal lesion; F-NFL, frontal- non-frontal lesion; NFL, non-frontal lesion.
To test if the lateralization of frontal lobe damage affects neuropsychological functions, MANOVA was performed shown in Table 10. The lateralization of the frontal lobe damage significantly influenced executive function and everyday memory in general (Wilks’ Lambda 0.43, F = 2.97, p < 0.001). Additionally, the lateralization of frontal lobe damage significantly affected emotion in general (Wilks’ Lambda 0.72, F = 2.08, p < 0.05).
Table 10.
Multiple analysis of covariances for neuropsychological functions by lateralization of frontal lobe lesion
| Independent variables | Dependent variables | Wilks’ Lambda(F) | univariate F | df | η2 |
| Lateralization of frontal | % errors | 0.43(2.97***) | 12.03*** | 2/46 | 0.34 |
| lobe lesion | % perseverative errors | 10.05*** | 2/46 | 0.30 | |
| % conceptual level responses | 13.40*** | 2/46 | 0.37 | ||
| number of categories completed | 8.85*** | 2/46 | 0.28 | ||
| CTT2 | 3.56* | 2/46 | 0.13 | ||
| COWAT | 4.03* | 2/46 | 0.15 | ||
| EMQ | 4.12* | 2/46 | 0.15 | ||
| GDS | 0.72(2.08*) | 4.86** | 2/46 | 0.18 | |
| STAI | 0.72 | 2/46 | 0.03 | ||
| ABS | 0.85 | 2/46 | 0.04 |
*p < 0.05, **p < 0.01, ***p < 0.001.
For the executive functions, conceptual level (F = 13.40, p < 0.001), percent error (F = 12.03, p < 0.001), percent perseverative error (F = 10.05, p < 0.001), number of categories completed (F = 8.85, p < 0.001) of WCST showed significant differences between the groups. And there were no significant differences in anxiety and agitation while depression (F = 4.86, p < 0.01) was significantly different between groups shown in Table 11. In results of post hoc, bilateral frontal lobe lesion group showed significantly higher percent error, and higher percent perseverative error and lower conceptual level of WCST compared to the groups with unilateral frontal lobe lesion. The number of categories completed was not significantly different between right frontal lobe lesion and bilateral frontal lobe lesion groups; yet these groups were significantly lower than left frontal lobe lesion group. Additionally, the depression of bilateral frontal lobe lesion group was severe than the unilateral frontal lobe lesion group.
Table 11.
Mean comparison of neuropsychological functions by lateralization of frontal lobe lesion
| LFL | RFL | BFL | F | |
| (n = 11) | (n = 15) | (n = 23) | ||
| WCST | ||||
| % errors | 33.45±18.37 | 48.73±17.04 | 60.52±11.98 | 12.03*** |
| % perseverative errors | 18.09±9.34 | 23.87±10.60 | 38.30±16.51 | 10.05*** |
| % conceptual level responses | 59.00±23.88 | 36.53±24.24 | 21.48±13.80 | 13.40*** |
| No. of completed categories | 4.09±2.07 | 2.20±2.24 | 1.13±1.60 | 8.85*** |
| CTT2 | 199.64±90.37 | 192.93±80.75 | 254.70±68.44 | 3.56 |
| COWAT | 34.64±27.29 | 42.33±22.39 | 23.74±13.57 | 4.03 |
| EMQ | 57.00±42.32 | 51.60±31.27 | 83.48±36.35 | 4.12 |
| GDS | 11.00±6.13 | 11.47±8.59 | 18.00±7.42 | 4.86** |
| STAI | 42.27±15.30 | 45.87±11.15 | 51.00±14.00 | 0.72 |
| ABS | 11.00±8.94 | 15.20±8.44 | 14.61±8.81 | 0.85 |
**p < 0.01, ***p < 0.001. LFL, left frontal lesion; RFL, right frontal lesion; BFL, bilateral frontal lesion; WCST, Wisconsin Card Sorting Test; CTT2, Color Trail Test 2; COWAT, Controlled Oral Word Association Test; EMQ, Everyday Memory Questionnaire; GDS, Geriatric Depression Scale; STAI, State-Trait Anxiety Inventory; ABS, Agitated Behavior Scale.
4. Discussion
In this study, we found that the duration of LOC and lesion location are main parameters that affect executive function, memory, and emotion in neuropsychological outcomes following TBI. It has been generally accepted that the severity of brain injury induces various degrees of neuropsychological impairment after TBI (Fisher, Ledbetter, Cohen, Marmor, & Tulsky, 2000; Halldorsson et al., 2008). As patients had longer periods of LOC, their cognitive and emotional problems were more serious. In this study, the duration of LOC was a main parameter for agitated behavior in severe TBI patients, while no significant associations were noted for anxiety and depression. LOC had a strong prognostic value, especially for everyday memory and agitated behaviors, which is consistent with the results of previous studies: there are many studies showing that mild TBI patients exhibit dysfunction in early phase of memory formation such as difficulties in encoding strategies, and moderate TBI patients have difficulties in long-term memory related to storage; therefore, having severe head injury may induce more serious memory impairment (Alexander, Stuss, & Fansabedian, 2003; Norton, Malloy, & Salloway, 2001). It has been shown that agitation is more common in the acute phase when neurological state of brain is unstable, and further emotional disturbances due to organic factors are more frequently shown in the patients who have serious brain injury (Levy et al., 2005). In a recent study (Ciurli et al., 2010), severe TBI patients frequently showed that various emotional problems, such as apathy and disinhibition, were correlated with severity of brain injury, which was in agreement with our results.
Although lateralization of brain lesion did not affect executive function, everyday memory, agitated behaviors, group differences in anxiety and depression were observed in this study. Bilateral lesion group was significantly more depressive compared to both left and right lesion groups and showed significantly higher anxiety level than left lesion group. Moreover, bilateral lesion group showed lower performances on executive function and everyday memory with more agitated behaviors than the other groups. Therefore, bilateral lesion group might be more vulnerable to cognition, especially emotional dysfunction, than any other lesion groups after severe TBI. Results of previous studies regarding emotional problems in TBI by lateralization of lesion were inconsistent and mixed, and emotional characteristics of bilateral lesion groups were not sufficiently analyzed (Gazzaniga, 2002; Grafman et al., 1996; Robinson, 1999; Zillmer, 2001).
Frontal lobe deficit had a significant impact on executive function indicated by performances on WCST, showing worse performance on four indices of WCST and exhibiting agitated behaviors more often compared to the group without frontal damage. In contrast, no significant difference in trail making, word fluency, everyday memory, depression, and anxiety tests was found between frontal and non-frontal lesion groups. Previous study on this subject showed that TBI patients with frontal damage also exhibited executive dysfunction (Lindsay Wilson, 1990; Wallesch, Curio, Galazky, Jost, & Synowitz, 2001; Wallesch, Curio, Kutz, et al., 2001). Particularly, a series of investigations have shown that poor performance on WCST in patients with frontal lesion compared to patients with non-frontal lesion (Stuss & Levine, 2002; Stuss et al., 2000; Wallesch, Curio, Galazky, et al., 2001). On the other hand, there were studies suggesting that frontal lobe lesion in TBI was significantly associated with emotional restlessness, agitation, and impulsive behaviors (Ciurli et al., 2011; Lequerica et al., 2007).
Bilateral frontal lesion group scored significantly worse than unilateral frontal lesion groups on WCST. Compared to unilateral frontal group, they lacked the ability to respond against external feedback and the insight on accurate conceptual classification, while having severe preservation due to internal rigidity. On the other hand, left side frontal lesion group showed a significantly better performance in percent error, percent conceptual level responses, and completed categories compared to right side frontal and bilateral frontal lesion groups. Performance of right side frontal lesion group was somewhat intermediate in general. Bilateral frontal lesion group was also more depressive than unilateral frontal lesion group. However, lateralization of frontal lesion had no significant impact on visual tracking, word fluency, everyday memory, anxiety, and agitation. It has been suggested that the brain activity of left hemisphere increases after TBI (Scheibel et al., 2009), and if it is explained as a compensation mechanism of brain dysfunction, left frontal lesion patients may have relatively better performances on executive function tasks compared to right frontal lesion patients. However, there were a few and inconsistent previous studies exist on depression after TBI (Jorge et al., 2004; Kim, 1991; Lee, 1990; Robinson & Szetela, 1981).
Nonetheless, using the duration of LOC and lesion location, this study may provide meaningful information for not only setting the framework for predicting cognitive and emotional dysfunctions and functional recovery after severe TBI, but also for identifying the patients who are exposed to high risk of suffering from serious neuropsychological impairments. Using these parameters in clinical setting could help determine the direction of intervention.
5. Conclusions
Duration of LOC and lesion location are main parameters affecting executive function, everyday memory, and emotion in neuropsychological outcomes following TBI, suggesting that these parameters need to be considered for cognitive rehabilitation interventions.
Conflict of interest
None to report.
References
- Alexander, M. P., Stuss, D. T., & Fansabedian, N. (2003). California Verbal Learning Test: performance by patients with focal frontal and non-frontal lesions. Brain, 126(Pt 6), 1493–1503. [DOI] [PubMed] [Google Scholar]
- Ashman, T. A., Spielman, L. A., Hibbard, M. R., Silver, J. M., Chandna, T., & Gordon, W. A. (2004). Psychiatric challenges in the first 6 years after traumatic brain injury: cross-sequential analyses of Axis I disorders. Arch Phys Med Rehabil, 85(4 Suppl 2), S36–S42. [DOI] [PubMed] [Google Scholar]
- Binder, L. M., Kelly, M. P., Villanueva, M. R., & Winslow, M. M. (2003). Motivation and neuropsychological test performance following mild head injury. J Clin Exp Neuropsychol, 25(3), 420–430. doi: 10.1076/jcen.25.3.420.13806. [DOI] [PubMed] [Google Scholar]
- Carlozzi, N. E., Grech, J., & Tulsky, D. S. (2013). Memory functioning in individuals with traumatic brain injury: an examination of the Wechsler Memory Scale-Fourth Edition (WMS-IV). J Clin Exp Neuropsychol, 35(9), 906–914. doi: 10.1080/13803395.2013.833178. [DOI] [PubMed] [Google Scholar]
- Carney, N., Chesnut, R. M., Maynard, H., Mann, N. C., Patterson, P., & Helfand, M. (1999). Effect of cognitive rehabilitation on outcomes for persons with traumatic brain injury: A systematic review. J Head Trauma Rehabil, 14(3), 277–307. [DOI] [PubMed] [Google Scholar]
- Cicerone, K. D., Dahlberg, C., Kalmar, K., Langenbahn, D. M., Malec, J. F., Bergquist, T. F., & Morse, P. A. (2000). Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil, 81(12), 1596–1615. doi: 10.1053/apmr.2000.19240. [DOI] [PubMed] [Google Scholar]
- Ciurli, P., Bivona, U., Barba, C., Onder, G., Silvestro, D., Azicnuda, E., & Formisano, R. (2010). Metacognitive unawareness correlates with executive function impairment after severe traumatic brain injury. J Int Neuropsychol Soc, 16(2), 360–368. doi: 10.1017/S135561770999141X. [DOI] [PubMed] [Google Scholar]
- Ciurli, P., Formisano, R., Bivona, U., Cantagallo, A., & Angelelli, P. (2011). Neuropsychiatric disorders in persons with severe traumatic brain injury: prevalence, phenomenology, and relationship with demographic, clinical, and functional features. J Head Trauma Rehabil, 26(2), 116–126. doi: 10.1097/HTR.0b013e3181dedd0e. [DOI] [PubMed] [Google Scholar]
- Corrigan, J. D. (1989). Development of a scale for assessment of agitation following traumatic brain injury. J Clin Exp Neuropsychol, 11(2), 261–277. doi: 10.1080/01688638908400888. [DOI] [PubMed] [Google Scholar]
- D’Elia, L. F., Satz, P., Uchiyama, C. L., & White, T. (1996). Color Trails Test. Professional manual. Odessa, FL: Psychological Assessment Resources.
- Dikmen, S. S., Corrigan, J. D., Levin, H. S., Machamer, J., Stiers, W., & Weisskopf, M. G. (2009). Cognitive outcome following traumatic brain injury. J Head Trauma Rehabil, 24(6), 430–438. doi: 10.1097/HTR.0b013e3181c133e9. [DOI] [PubMed] [Google Scholar]
- Dikmen, S. S., Machamer, J. E., Powell, J. M., & Temkin, N. R. (2003). Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch Phys Med Rehabil, 84(10), 1449–1457. [DOI] [PubMed] [Google Scholar]
- Fisher, D. C., Ledbetter, M. F., Cohen, N. J., Marmor, D., & Tulsky, D. S. (2000). WAIS-III and WMS-III profiles of mildly to severely brain-injured patients. Appl Neuropsychol, 7(3), 126–132. doi: 10.1207/S15324826AN0703_2. [DOI] [PubMed] [Google Scholar]
- Gazzaniga, M. S., Ivry, R. B., & Mangun, G. R. (2002). Cognitive Neuroscience: the biology of the mind New York: W. W. Norton & Co. [Google Scholar]
- Glascher, J., Tranel, D., Paul, L. K., Rudrauf, D., Rorden, C., Hornaday, A., & Adolphs, R. (2009). Lesion mapping of cognitive abilities linked to intelligence. Neuron, 61(5), 681–691. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman, J., Schwab, K., Warden, D., Pridgen, A., Brown, H. R., & Salazar, A. M. (1996). Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology, 46(5), 1231–1238. [DOI] [PubMed] [Google Scholar]
- Hahn, D. W., LEE, C. H., Chon, K. G., & Spielberger, C. D. (2000). Korean version State-Trait Anxiety Inventory for Adults Seoul: Hakjisa. [Google Scholar]
- Halldorsson, J. G., Flekkoy, K. M., Arnkelsson, G. B., Tomasson, K., Gudmundsson, K. R., & Arnarson, E. O. (2008). The prognostic value of injury severity, location of event, and age at injury in pediatric traumatic head injuries. Neuropsychiatr Dis Treat, 4(2), 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, F. M., Hart, T., Bushnik, T., Corrigan, J. D., & Sasser, H. (2004). Change and predictors of change in communication, cognition, and social function between 1 and 5 years after traumatic brain injury. J Head Trauma Rehabil, 19(4), 314–328. [DOI] [PubMed] [Google Scholar]
- Haut, M. W., Cahill, J., Cutlip, W. D., Stevenson, J. M., Makela, E. H., & Bloomfield, S. M. (1996). On the nature of Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res, 65(1), 15–22. [DOI] [PubMed] [Google Scholar]
- Heaton, R. K., C. G, Talley, J. L., Kay G.G., & Curtiss G. (1993). Wisconsin Card Sorting Test manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources.
- Hesdorffer, D. C., Rauch, S. L., & Tamminga, C. A. (2009). Long-term psychiatric outcomes following traumatic brain injury: a review of the literature. J Head Trauma Rehabil, 24(6), 452–459. doi: 10.1097/HTR.0b013e3181c133fd. [DOI] [PubMed] [Google Scholar]
- Horner, M. D., Selassie, A. W., Lineberry, L., Ferguson, P. L., & Labbate, L. A. (2008). Predictors of psychological symptoms 1 year after traumatic brain injury: a population-based, epidemiological study. J Head Trauma Rehabil, 23(2), 74–83. doi: 10.1097/01.HTR.0000314526.01006.c8. [DOI] [PubMed] [Google Scholar]
- Jorge, R. E., Robinson, R. G., Moser, D., Tateno, A., Crespo-Facorro, B., & Arndt, S. (2004). Major depression following traumatic brain injury. Arch Gen Psychiatry, 61(1), 42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- Jung, I. K., Kwak, D. I., Shin, D. K., Lee, H. S., & Kim, J. Y. (1997). A reliability and validity study of geriatric depression scale. Journal of Korean Neuropsychiatric Association, 36(1), 103–112. [Google Scholar]
- Kang, Y., Jin, J., Na, D., Lee, J., & Park, J. (2000). A normative study of the Korean version of Controlled Oral Word Association Test (COWAT) in the elderly.
- Kim, J. S., Chon, S.K., & Hwang, I.S. (1991). Relationship between psychiatric symptoms and lesion site of brain in patients with hesd trauma. Journal of Korean Neuropsychiatric Association, 30(6), 996–1003. [Google Scholar]
- Kim, M. K., Hyun, M. H., & Han, S. I. (2003). The Performance of Trail Making B Test of the Organic Patients and Alcoholics. The Korean Journal of Clinical Psychology, 22(2), 463–473. [Google Scholar]
- Lee, J. Y., Yum, T. H., & Jang, H. I. (1990). Cognitive and emotional Disturbances in patients with frontal and temporal lobe damages. Journal of the Korean Neuropsychiatric Association, 29(5), 1059–1074. [Google Scholar]
- Lequerica, A. H., Rapport, L. J., Loeher, K., Axelrod, B. N., Vangel, S. J. Jr, & Hanks, R. A. (2007). Agitation in acquired brain injury: impact on acute rehabilitation therapies. J Head Trauma Rehabil, 22(3), 177–183. doi: 10.1097/01.HTR.0000271118.96780.bc. [DOI] [PubMed] [Google Scholar]
- Levy, M., Berson, A., Cook, T., Bollegala, N., Seto, E., Tursanski, S., & Bhalerao, S. (2005). Treatment of agitation following traumatic brain injury: a review of the literature. NeuroRehabilitation, 20(4), 279–306. [PubMed] [Google Scholar]
- Lindsay Wilson, J. T. (1990). The relationship between neuropsychological function and brain damage detected by neuroimaging after closed head injury. Brain Injury, 4(4), 349–363. [DOI] [PubMed] [Google Scholar]
- Milner, B., & Petrides, M. (1984). Behavioural effects of frontal-lobe lesions in man. Trends in Neurosciences, 7(11), 403–407. [Google Scholar]
- Norton, L. E., Malloy, P. F., & Salloway, S. (2001). The impact of behavioral symptoms on activities of daily living in patients with dementia. Am J Geriatr Psychiatry, 9(1), 41–48. [PubMed] [Google Scholar]
- Ponsford, J., Willmott, C., Rothwell, A., Cameron, P., Kelly, A. M., Nelms, R., & Ng, K. (2000). Factors influencing outcome following mild traumatic brain injury in adults. J Int Neuropsychol Soc, 6(5), 568–579. [DOI] [PubMed] [Google Scholar]
- Prigatano, G. P. (1992). Personality disturbances associated with traumatic brain injury. J Consult Clin Psychol, 60(3), 360–368. [DOI] [PubMed] [Google Scholar]
- Robinson, R. G., Murata, Y., & Shimoda, K. (1999). Dimensions of social impairment and their effect on depression and recovery following stroke. International Psychogeriatrics, 11(4), 375–384. [DOI] [PubMed] [Google Scholar]
- Robinson, R. G., & Szetela, B. (1981). Mood change following left hemispheric brain injury. Ann Neurol, 9(5), 447–453. doi: 10.1002/ana.410090506. [DOI] [PubMed] [Google Scholar]
- Rogers, J. M., & Read, C. A. (2007). Psychiatric comorbidity following traumatic brain injury. Brain Inj, 21(13-14), 1321–1333. doi: 10.1080/02699050701765700. [DOI] [PubMed] [Google Scholar]
- Rosenthal, M., Christensen, B. K., & Ross, T. P. (1998). Depression following traumatic brain injury. Arch Phys Med Rehabil, 79(1), 90–103. [DOI] [PubMed] [Google Scholar]
- Salmond, C. H., Menon, D. K., Chatfield, D. A., Pickard, J. D., & Sahakian, B. J. (2006). Changes over time in cognitive and structural profiles of head injury survivors. Neuropsychologia, 44(10), 1995–1998. doi: 10.1016/j.neuropsychologia.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Scheibel, R. S., Newsome, M. R., Troyanskaya, M., Steinberg, J. L., Goldstein, F. C., Mao, H., & Levin, H. S. (2009). Effects of severity of traumatic brain injury and brain reserve on cognitive-control related brain activation. J Neurotrauma, 26(9), 1447–1461. doi: 10.1089/neu.2008.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senathi-Raja, D., Ponsford, J., & Schonberger, M. (2010). The association of age and time postinjury with long-term emotional outcome following traumatic brain injury. J Head Trauma Rehabil, 25(5), 330–338. doi: 10.1097/HTR.0b013e3181ccc893. [DOI] [PubMed] [Google Scholar]
- Sohlberg, M. M., & Mateer, C. A. (1989). Training use of compensatory memory books: a three stage behavioral approach. J Clin Exp Neuropsychol, 11(6), 871–891. doi: 10.1080/01688638908400941. [DOI] [PubMed] [Google Scholar]
- Sole-Padulles, C., Bartres-Faz, D., Junque, C., Vendrell, P., Rami, L., Clemente, I. C., & Molinuevo, J. L. (2009). Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging, 30(7), 1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Stuss, D. T., & Levine, B. (2002). Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol, 53, 401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Stuss, D. T., Levine, B., Alexander, M. P., Hong, J., Palumbo, C., Hamer, L., & Izukawa, D. (2000). Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia, 38(4), 388–402. [DOI] [PubMed] [Google Scholar]
- Sunderland, A., Harris, J.E., & Baddeley, A.D. (1983). Do laboratory tests predict everyday memory: a neuropsychological study. Jornal of verbal learning & verbal behavior, 22(3), 341–357. [Google Scholar]
- Wallesch, C. W., Curio, N., Galazky, I., Jost, S., & Synowitz, H. (2001). The neuropsychology of blunt head injury in the early postacute stage: effects of focal lesions and diffuse axonal injury. J Neurotrauma, 18(1), 11–20. doi: 10.1089/089771501750055730. [DOI] [PubMed] [Google Scholar]
- Wallesch, C. W., Curio, N., Kutz, S., Jost, S., Bartels, C., & Synowitz, H. (2001). Outcome after mild-to-moderate blunt head injury: effects of focal lesions and diffuse axonal injury. Brain Inj, 15(5), 401–412. doi: 10.1080/02699050010005959. [DOI] [PubMed] [Google Scholar]
- Whelan-Goodinson, R., Ponsford, J. L., Schonberger, M., & Johnston, L. (2010). Predictors of psychiatric disorders following traumatic brain injury. J Head Trauma Rehabil, 25(5), 320–329. doi: 10.1097/HTR.0b013e3181c8f8e7. [DOI] [PubMed] [Google Scholar]
- Yesavage, J. A., Brink, T. L., Rose, T. L., Lum, O., Huang, V., Adey, M., & Leirer, V. O. (1982). Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res, 17(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Zillmer, E., & Spiers, M. (2001). Principles of Neuropsychology. Belmont: Wadsworth, 321-355.