Summary
A Disintegrin And Metalloproteinase with ThromboSpondin motif (ADAMTS)‐5 was identified in 1999 as one of the enzymes responsible for cleaving aggrecan, the major proteoglycan in articular cartilage. Studies in vitro, ex vivo and in vivo have validated ADAMTS‐5 as a target in osteoarthritis (OA), a disease characterized by extensive degradation of aggrecan. For this reason, it attracted the interest of many research groups aiming to develop a therapeutic treatment for OA patients. However, ADAMTS‐5 proteoglycanase activity is not only involved in the dysregulated aggrecan proteolysis, which occurs in OA, but also in the physiological turnover of other related proteoglycans. In particular, versican, a major ADAMTS‐5 substrate, plays an important structural role in heart and blood vessels and its proteolytic processing by ADAMTS‐5 must be tightly regulated. On the occasion of the 20th anniversary of the discovery of ADAMTS‐5, this review looks at the evidence for its detrimental role in OA, as well as its physiological turnover of cardiovascular proteoglycans. Moreover, the other potential functions of this enzyme are highlighted. Finally, challenges and emerging trends in ADAMTS‐5 research are discussed.
Keywords: ADAMTS, aggrecan, cardiovascular disease, osteoarthritis, proteoglycans, versican
1. INTRODUCTION
Twenty years ago, a team of scientists at the pharmaceutical company DuPont unveiled the identity of the elusive enzymes responsible for cleaving aggrecan, a major proteoglycan in articular cartilage.1, 2 This ‘aggrecanase activity’ has been the subject of intensive research efforts during the past two decades. By mid‐80s, it became apparent from post‐traumatic animal models of osteoarthritis (OA) that aggrecan degradation is one of the major changes affecting extracellular matrix (ECM) function.3, 4 Later, cartilage proteoglycan fragments were detected in the synovial fluid of patients with knee injury.5 In 1992, Sandy et al6 identified a major cleavage event, occurring at the Glu392↓Ala393 bond, was responsible for releasing aggrecan fragments in the synovial fluid of OA patients. This initiated a worldwide hunt for the ‘aggrecanase’ that ended only 7 years later. Aggrecanase activity was found to be a distinct enzymatic feature of two members of A Disintegrin and Metalloproteinase with ThromboSpondin motifs (ADAMTS) family of metalloproteinases, aggrecanase‐1 (ADAMTS‐4) and aggrecanase‐2 (ADAMTS‐5, which was originally named ADAMTS‐11).1, 2, 7 Since then, multiple lines of evidence have established ADAMTS‐5 as a major aggrecanase in humans and mice. Therapeutic treatment of degenerative joint diseases appeared to be around the corner. However, 20 years of frantic investigations have revealed a complexity in ADAMTS‐5 biology that has made the development of a therapeutic ADAMTS‐5 inhibitor more difficult to achieve. The child was a difficult one. Here on the occasion of its 20th birthday, I will attempt to summarize the teenage years of ADAMTS‐5 and our current knowledge of its place in the ECM field.
2. THE FIRST DECADE: ADAMTS‐5 AND OA
2.1. Setting the stage: OA, cartilage destruction and aggrecan
OA is the most common joint disorder, affecting a significant proportion of the human population.8 The pain related to this pathology is the leading cause of impaired mobility in the elderly population in the USA.9 A major hallmark of OA is the destruction of articular cartilage.10 The function of articular cartilage is to absorb the stress created within the joint during movement and distribute it to the underlying bone. Articular cartilage is composed of chondrocytes embedded in an ECM that is rich in type II collagen fibrils and aggrecan. The composition of human articular cartilage is 65%‐80% water, 10%‐30% collagen, 10%‐15% proteoglycans and <2% chondrocytes.11 Collagen is the main structural component of cartilage and provides tensile strength to the tissue whereas aggrecan provides flexibility, viscoelasticity and compressibility. These functions are mediated by the negatively charged glycosaminoglycans (GAG) chains attached to its core which ultimately are responsible for the high osmotic pressure in cartilage (3.4‐3.6 atmospheres).12 GAGs attached to aggrecan protein core are chondroitin sulphate (CS) and keratan sulphate (KS) chains, which cluster into so‐called CS‐1 and CS‐2 domains and a KS‐rich region (Figure 1).13, 14 To give an idea of the predominance in mass of the GAGs over the protein core, the latter only corresponds to ~10%‐15% of the weight of the molecule (220 kDa) and bears as many as 50‐100 CS chains and up to 60 KS chains.15, 16, 17 The osmotic pressure in cartilage is mainly due to the negative charges on aggrecan GAGs, which attract counter ions, such as Na+, and, consequently, due to the Donnan effect, induces water into the tissue.12 The protein core of aggrecan comprises globular domains at the N‐ and C‐terminus (named G1 and G3 respectively), similar to other large aggregating proteoglycans, but additionally contains an internal G2 domain (Figure 1). The region between G2 and G3 contains the GAG‐attachment sites, whereas the region between G1 and G2 (called interglobular domain, IGD) comprises 150 amino acids. The aggrecan G1 domain interacts with link protein, a glycoprotein and hyaluronan (HA), an anionic, nonsulphated GAG, to form larger molecular weight aggregates. These contain up to 100 aggrecan molecules per molecule of HA.18
Figure 1.
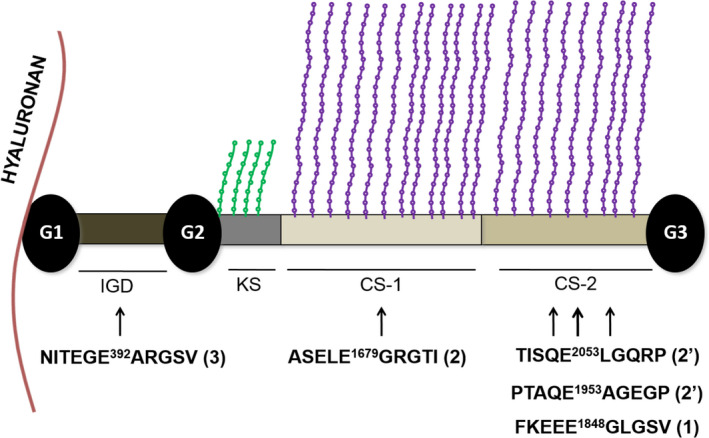
Schematic of aggrecan. KS chains are shown as green strings, CS chains as violet strings. Major ADAMTS‐5 cleavage sites are reported. Sequences are for human aggrecan (UniProt ID: P16112). In brackets the preferential order of cleavage is reported36.Cleavages at Glu2053↓Leu2054 and Glu1953↓Ala1954 (indicated by 2') follow the primary cleavage at Glu1848↓Gly1849 and represent C‐terminal processing events occurring in parallel to cleavage at Glu1679↓ Gly1680.
Earlier studies have shown that aggrecan, collagen and HA constantly undergo turnover in healthy cartilage.19, 20 Under physiological conditions, cartilage homeostasis is maintained by a balance between the synthesis and degradation of aggrecan and collagen, but in OA and other joint disorders this equilibrium shifts towards catabolism. Proteases involved in the catabolism of collagen and aggrecan are secreted in response to inductive stimuli (such as inflammatory cytokines, mechanical stress and injury) by both chondrocytes and synoviocytes.10 Synoviocytes comprise cells such as fibroblasts and macrophages which are present in the synovium, the thin membrane around the joint that secretes the lubricating synovial fluid.
Aggrecan degradation represents an early stage of cartilage destruction, preceding degradation of the major fibrillar type II collagen. In bovine articular cartilage explants stimulated with interleukin‐1 (IL‐1), collagen release could be detected only after 14 days of culture, when most of the aggrecan had already been depleted from the ECM.21 Moreover, whereas aggrecan degradation can be compensated for by new synthesis and is completely reversible upon anabolic stimuli, collagen degradation is an irreversible process.22, 23 Remarkably, a collagenase‐selective inhibitor (Ro‐32‐3555/Trocade/Cipemastat), developed by Roche, did not prevent progression of joint damage in patients with rheumatoid arthritis24 despite the favourable preclinical data25 and pharmacokinetics.26 Since it has been shown that collagen fibrils interact with the KS domain of multiple aggrecan molecules,27 it has been proposed that aggrecan protects the collagen fibrillar network from proteolytic attack by collagenases due to steric and charge hindrance by the long negatively charged GAG chains attached to its core.28 To support this, live explants that were depleted of aggrecan GAGs by pretreatment with chondroitinase ABC showed release of collagen in as little as 24 hours after IL‐1 stimulation.29 Cleavage of aggrecan within the IGD domain would then release the protective C‐terminal portion of the molecule, thus exposing the collagen fibril to collagenolytic activity. Aggrecan fragments bearing the GAG‐rich domains are in fact detected in the synovial fluid of both normal and OA patients, following diffusion from the cartilage.30, 31 A major cleavage site in the human aggrecan IGD was identified as the Glu373↓Ala374 bond.6 This corresponds to the Glu392↓Ala393 bond in the modern nomenclature (UniProt ID: P16112 for human aggrecan). The reason for the 19‐residue discrepancy between the classic nomenclature and the UniProt database numbering is that the former starts from the natural N‐terminus of protein (first identified as 20VEVS in porcine aggrecan, corresponding to 20VETS in human).32 In this review, I will follow the UniProt numbering so that researchers not familiar with the field can easily track the cleavage sites.
Cleavage within the IGD releases most of the molecule from its anchorage to HA and results in loss of mechanical properties. On the other hand, cleavage sites towards the C‐terminus of the CS‐rich domains leave most of the molecule still functional within the tissue33 and for this reason may represent homeostatic turnover of aggrecan.34, 35 Although members of other families of proteases such as matrix metalloproteinases (MMPs) and cysteine proteases such as cathepsins can cleave aggrecan at different cleavage sites in the IGD and CS‐rich domains (reviewed in 36), the ‘aggrecanase’ cleavage site at Glu392↓Ala393 was shown to play a fundamental role in cartilage degradation. In mouse models of arthritis, cleavage fragments generated by aggrecanases at this site appear before those generated by MMPs in the IGD (cleavage at Asn360↓Phe361), but they disappear with the progression of cartilage damage.37 Perhaps the most compelling evidence comes from mice bearing a mutated ‘aggrecanase’ site (393ALG → 393NVYS) that makes them resistant to aggrecanase activity in the IGD, without affecting other cleavage sites.38 These mice are protected from aggrecan loss and cartilage erosion in surgical and inflammatory models of OA and show increased cartilage repair.38 Importantly, they do not show cartilage or skeletal anomalies.
2.2. ADAMTS‐5 as a target in OA
In 1999, the two enzymes responsible for cleavage at the Glu392↓Ala393 bond were purified from bovine cartilage tissues.1, 2 It was found that their N‐terminal peptide sequences shared significant homology with a murine enzyme described two years before as the founding member of the ADAMTS family of metalloproteinases, ADAMTS‐1.39 For this reason, the two aggrecanases were named ADAMTS‐4 (aggrecanase‐1) and ADAMTS‐5 (aggrecanase‐2) respectively.1, 2, 7 (The gene cloned in the original report and named ADAMTS‐112 was found to match with an EST previously named ADAMTS‐57). The three proteases were found to be secreted extracellular proteins and share a similar domain organization, consisting of a signal peptide, a prodomain, a metalloproteinase catalytic domain, followed by non‐catalytic ancillary domains such as a disintegrin‐like domain, a thrombospondin‐type I motif domain, a cysteine‐rich, domain, a spacer domain, and a various number of thrombospondin‐type I motifs in the C‐terminus (2 for ADAMTS‐1, 1 for ADAMTS‐5 and none for ADAMTS‐4). The metalloproteinase domain contains the sequence H EIGHLLGLSHD, in which the three underlined histidine residues coordinate a zinc atom and the glutamate residue (in bold) exerts a catalytic role. This motif is followed C‐terminally by a conserved methionine residue which constitutes a tight turn (‘Met‐turn’) acting as a constraint in the topology of active site.40 The C‐terminal ancillary domains are essential for catalytic activity against native substrates such as aggrecan, since they contain residues (called exosites) that mediate recognition and binding of substrates.41 Later, other ADAMTS family members such as ADAMTS‐1,42 ADAMTS‐8,43 ADAMTS‐9, ADAMTS‐16 and ADAMTS‐1844 were shown to cleave at the ‘aggrecanase site’ Glu392↓Ala393, although this was achieved using non‐physiological enzyme to substrate ratios (up to 1:0.3 for ADAMTS‐843). With the notable exceptions of ADAMTS‐4 and ADAMTS‐9, the expression of all other aggrecanases has been found to increase either in the synovium or in the cartilage of OA patients as compared to controls (for a review, see ref [45]). So what made ADAMTS‐5 the favoured target for OA therapies? Four lines of evidence support this conclusion.
2.2.1. ADAMTS‐5 is the most potent aggrecanase in vitro
Semi‐quantitative studies using Western blot analysis have established that purified human recombinant ADAMTS‐5 is a ~30‐fold more potent aggrecanase at the Glu392↓Ala393 bond than ADAMTS‐4. Moreover, it is a ~20‐fold more potent in the CS2 domain (Glu1679↓Gly1680 bond, Figure 1).46 To give an idea of the extreme potency of this enzyme, as little as 2 pmol/L or 1 nmol/L of active ADAMTS‐5 is sufficient to show robust aggrecan cleavage within the CS‐2 (Glu1953↓Ala1954 bond) and the aggrecanase site, respectively, following 2‐hour digestion at 37°C.41, 47 It is important to highlight that the 1000‐fold figure for the higher aggrecanase activity of ADAMTS‐5 compared with ADAMTS‐4,48 still sometimes found in the literature, was determined using batches of ADAMTS‐4 contaminated with heparin, which has been shown to inhibit aggrecanase activity.46 By comparison, approximately 300 nmol/L ADAMTS‐1 is required to generate cleavage at the Glu392↓Ala393 bond (concentration calculated from ref. [42]), suggesting a ~150 000‐fold lower activity than ADAMTS‐5. Cofactors such as fibulin‐1 have been shown to enhance the activity of both ADAMTS‐1 and ADAMTS‐5.49, 50 However, while it is possible that cofactors may enhance the activity of one or more aggrecanases in vivo, it is very unlikely that they will change the relative potency of these enzymes.
2.2.2. ADAMTS‐5 ablation or inhibition protects mice from joint degeneration
The importance of ADAMTS‐5 in facilitating the progression of cartilage loss has been outlined by in vivo models of OA (Table 1). In these models, specific Adamts knockout mice are challenged either mechanically, by knee surgery (destabilization of medial meniscus, DMM), or by injection of methylated bovine serum albumin into the joint (antigen‐induced arthritis, AIA) and their phenotype is compared with that of wild‐type littermates.
Table 1.
Phenotypes of Adamts5 knockout mice
| Organ/tissue | Model | Phenotype compared to wild type (penetrance) | References |
|---|---|---|---|
| Adipose tissue | HFD | Increased brown adipose tissue | [121, 131] |
| Adipose tissue | Cold exposure | Reduced WAT mass, increased BAT mass, enhanced insulin sensitivity | [121, 131] |
| Aorta/Heart | – | Myxomatous cardiac valve (100%) | [95] |
| Bicuspid pulmonary and aortic valves (10%‐20%) | [102] | ||
| Bicuspid and tricuspid aortic valves (100%) | [103] | ||
| HFD | Increased LV wall thickness and volume | [117] | |
| Cartilage | DMM | Reduced cartilage degradation | [53] |
| Cartilage | DMM | Reduced cartilage degradation and mechanical allodynia | [59] |
| Cartilage | AIA | Reduced cartilage degradation | [54] |
| AIA | Reduced cartilage degradation | [120] | |
| Cartilage | TTR | Reduced cartilage degradation and fibrosis | [60] |
| CNS | SCI | Reduced versican cleavage | [164] |
| Immune system | Influenza virus infection | Delayed virus clearance, reduced T‐cell infiltration, increased weight loss | [140] |
| Limb | – | Syndactyly (44%) | [50] |
| Liver | HFD | Reduced liver weight and hepatic triglyceride accumulation | [121, 124] |
| Liver | MCD | Reduced steatosis and fibrosis | [121] |
| Skeletal muscle | – | Delayed myotube formation | [166] |
| Skin | Wound healing | Delayed contraction | [122] |
| Tendon | – | Reduced maximum tensile strength | [62] |
| Tendon | TTR | Reduced maximum tensile strength | [61] |
Data for combinatorial knockout models are not shown.
Abbreviations: AIA, antigen‐induced arthritis; BAT, brown adipose tissue; CNS, central nervous system; DMM, destabilization of medial meniscus; HFD, high‐fat diet; LV, left ventricle; MCD, methionine‐/choline‐deficient diet; SCI, spinal cord injury; TTR, TGF‐β injection and enforced uphill treadmill running; WAT, white adipose tissue.
Initially, the phenotype of Adamts4 and Adamts1 knockout mice was analysed. These mice did not exhibit any significant protection from cartilage aggrecan loss in the DMM or the AIA models, respectively,51, 52 suggesting that the contribution of these proteases to joint pathology in mice is negligible. Mechanistically, the articular cartilage from these mice showed significant cleavage of aggrecan at the at the Glu392↓Ala393 bond.51, 52 On the other hand, mice with a targeted deletion of the catalytic domain of ADAMTS‐5 were protected from cartilage loss both in the DMM53 and AIA54 models, with minimal cleavage of aggrecan at the Glu392↓Ala393 bond. Moreover, in these mice a significant cleavage in the CS‐2 domain was observed, suggesting an involvement of ADAMTS‐5 in pathological aggrecan proteolysis rather than physiological turnover.54, 55 Adamts5 knockout mice also show a significant reduction in the thickness of the subchondral plate and less epiphyseal trabecular bone following DMM compared with wild‐type mice,56 suggesting that the protective effects of ADAMTS‐5 ablation are extended to the bone, where ADAMTS‐5 is also normally expressed.57, 58 Importantly, Adamts5 knockout mice do not develop mechanically induced pain sensitization (allodynia), a major cause of distress in OA patients, up to 8 weeks post‐DMM.59 In an OA model induced by the combination of transforming growth factor (TGF)‐β injection and enforced uphill treadmill running (TTR), Adamts5 knockout mice were protected from joint fibrosis and cartilage erosion and showed increased aggrecan deposition,60 but their tendons showed a reduced maximum tensile stress as a result of disrupted collagen organization and may be more susceptible to rupture.61, 62
Adamts4/Adamts5 double knockout mice were also protected from developing OA and physiologically normal.63 In the absence of both ADAMTS‐4 and ADAMTS‐5, aggrecan cleavage still occurs in the CS‐2 domain,64 an activity probably involved in the homeostatic turnover of the molecule.
Another approach to investigate the role of ADAMTS‐5 in vivo is by analysing the effect of small interfering RNAs (siRNA). An advantage is that in this case the effect can be tissue‐limited and short‐lived, therefore mimicking more closely therapeutic administration. Intra‐articular injection of Adamts5 siRNA was protective in DMM models and the combined injection of an MMP‐13 (collagenase‐3) siRNA showed a synergistic protective effect on cartilage integrity.65, 66
The role of ADAMTS‐5 in the pathology of murine OA was further corroborated by studies which employed monoclonal antibodies (mAbs). Systemic treatment with anti‐ADAMTS‐5 mAbs either before or after DMM protected mice from OA progression67 and mechanical allodynia,68 thus phenocopying the findings from Adamts5 knockout mice. Similarly, an anti‐ADAMTS‐5 mAb was found to be protective in a murine model of spontaneous OA.69 Importantly, an anti‐ADAMTS‐4 mAb was not effective as a prophylactic treatment.67
Collectively, these data point to a pivotal role exerted in vivo by ADAMTS‐5 in mouse cartilage breakdown during the progression of OA. However, ADAMTS‐5 seems to exert a role also in bone and tendon biology and a future ADAMTS‐5‐based OA therapy should take also this into consideration.
2.2.3. Inhibition of ADAMTS‐5 in human chondrocytes significantly reduces aggrecan degradation
The role of aggrecanases in human OA has been extensively investigated using either isolated chondrocytes or cartilage explants. In these models, it is important to take into account the role of inflammation in OA. Chondrocytes as well as macrophages and mononuclear cells in the synovial membrane of OA joints exhibit an activated phenotype and play a pivotal role in cartilage degradation by releasing pro‐inflammatory cytokines such as IL‐1β and tumour necrosis factor‐α, whose levels are found increased in OA patients.70, 71 For this reason, cytokines are often added in chondrocytes/explants cultures. However, whereas the expression of ADAMTS4 is upregulated by pro‐inflammatory cytokines, that of ADAMTS5 is constitutive in OA synovium and cartilage (for a review, see ref. [72]). Under these inflammatory conditions, human normal cartilage explants and primary chondrocytes have been subjected to siRNA‐mediated knockdown of ADAMTS4 or ADAMTS5, which showed that both treatments were equally effective in decreasing aggrecan cleavage.73 The same effects were observed following siRNA‐mediated knockdown of either gene in cartilage of OA patients, but an siRNA against ADAMTS1 failed to inhibit aggrecan degradation in either normal or OA cartilage.73 Similar results were observed with inhibitory mAbs. In human OA explants, anti‐ADAMTS‐5 mAbs were more effective than anti‐ADAMTS‐4 mAbs in inhibiting aggrecan degradation under unstimulated conditions, whereas in the presence of cytokines the two mAbs showed similar efficacy.67 A different anti‐ADAMTS‐5 mAb, 2D3, effectively inhibited aggrecan degradation both in healthy human chondrocytes47 and human OA cartilage explants74 under non‐inflammatory conditions.
Taken together, these results suggest that ADAMTS‐5 plays a major role in human OA pathology under non‐inflammatory conditions, whereas in the presence of cytokines both ADAMTS‐5 and ADAMTS‐4 contribute to aggrecan degradation.
2.2.4. Impairment of ADAMTS‐5 endocytosis significantly increases aggrecan degradation
In light of all the evidence for an important role of ADAMTS‐5 in OA, it was puzzling to find that ADAMTS‐5 mRNA levels do not correlate with aggrecan degradation.75, 76, 77 This suggests that ADAMTS‐5 dysregulation in OA may principally result from altered post‐translational regulation. In fact, it has been found that ADAMTS‐5 half‐life is regulated by endocytosis through the low‐density lipoprotein receptor‐related protein‐1 (LRP‐1) receptor.78 Interestingly, LRP‐1 protein levels are severely reduced in OA cartilage and this has been attributed to an increase in the shedding of its ectodomain by proteases such as ADAM‐17 and MMP‐14.74, 78 To test the effect of an impairment in ADAMTS‐5 endocytosis on aggrecan degradation by human chondrocytes, we developed a mAb, 1B7, able to specifically bind to ADAMTS‐5 and inhibit its endocytosis with no effect on its aggrecanase activity.79 Addition of 1B7 to normal chondrocytes under unstimulated conditions increased the levels of endogenous ADAMTS‐5 and produced a significant increase in aggrecan degradation, mimicking the effect of impaired ADAMTS‐5 uptake by LRP‐1. This activity could be specifically inhibited by another anti‐ADAMTS‐5 mAb, 2D3, suggesting that the observed aggrecanase activity was ADAMTS‐5‐dependent.79 These results strongly link aggrecan degradation with a dysregulated post‐translational regulation of ADAMTS‐5 levels in cartilage.
2.3. Side effects of anti‐ADAMTS‐5 mAbs
Currently, OA treatment is limited to steroidal and nonsteroidal anti‐inflammatory drugs which provide symptomatic relief for pain and inflammation.80 Physical activity is one of the most widely prescribed non‐pharmacological therapies for OA management.81 So there is an unmet demand for drugs able to reverse, halt or slow disease progression and improve the quality of life in OA patients. The body of work described in the previous section strongly advanced ADAMTS‐5 as a viable target for such disease‐modifying osteoarthritic drugs. In the previous section, mAbs were introduced as a tool to investigate the potential of an anti‐ADAMTS‐5 therapeutic therapy. In Cynomolgus, a primate which spontaneously develops OA‐like symptoms,82 an anti‐ADAMTS‐5 mAb, GSK2394002, was indeed able to decrease the levels of circulating aggrecan fragments upon systemic administration.67 However, this study also exposed cardiovascular side effects associated with ADAMTS‐5 inhibition, ranging from increased mean arterial pressure to subendocardial haemorrhage.83 Such cardiovascular effects were not due to cross‐reaction of the mAb67 and pose significant challenges for clinical development since the OA population may be already affected by cardiovascular disease. In addition, many individuals who are strong candidates for medical treatment but not surgery are young athletes with post‐traumatic OA, and it would be wise to be wary of long‐term impact in these patients. On the other hand, this disappointing outcome prompted a reassessment of ADAMTS‐5 function in the cardiovasculature.
3. THE SECOND DECADE: ADAMTS‐5 AND CARDIOVASCULAR DISEASE
3.1. ADAMTS‐5 and versican in the development of cardiovascular system
In large blood vessels and cardiac valves, versican is the main proteoglycan, although recently also aggrecan has been detected in the aorta.84, 85, 86, 87, 88, 89 Both proteoglycans regulate viscoelasticity and stiffness of large vessels.88, 90, 91 Genetic deletion of versican is lethal in mice due to cardiac anomalies,92 and versican processing is necessary for the remodelling of the cardiac outlet, which will develop into the future aortic and pulmonary arteries of the mature heart.93 Versican is present in 5 isoforms (V0‐V4) arising from alternative splicing of two large exons encoding GAG‐attachment domains, named αGAG and βGAG.91 In adult mice, ADAMTS‐5 has been shown to be co‐expressed with versican in cardiac valves and aorta.94, 95, 96 ADAMTS‐5 cleaves versican V1 at Glu441↓Ala442 in the βGAG region41, 97, 98 and shares this ability with other cardiovascular proteoglycanases such as ADAMTS‐1, ADAMTS‐4,99 ADAMTS‐9,100 and ADAMTS‐15.101 Perhaps because of this apparent redundancy, researchers in the field desisted from questioning the first reports about the absence of developmental phenotypes in Adamts5 knockout mice53, 54 (Table 1). In fact, later studies demonstrated that Adamts5 knockout mice show severe anomalies in the pulmonary valve cusps due to decreased versican cleavage and subsequent versican accumulation.95, 102 The pulmonary valves of these mice also exhibit reduced cleavage of aortic aggrecan at the Glu392↓Ala393 site, whereas cleavage in the CS‐2 domain still proceeds through other aggrecanases. Mice with a mutant cleavage site at Glu392↓Ala393 also show aortic anomalies, although not as severe as those in Adamts5 knockout mice,103 suggesting that ADAMTS‐5 activity at other sites (or, alternatively, on other substrates) may compensate for the abrogation of cleavage in the aggrecan IGD. On the other hand, mice over‐expressing ADAMTS‐4 and ADAMTS‐5 show sparse trabeculation in the heart due to excessive degradation of versican.104
These data suggest that ADAMTS‐5 proteoglycanase activity is required to maintain adequate levels of proteoglycans such as aggrecan and versican, not only during heart development but also in large blood vessels.
3.2. Aneurysms
Increased proteoglycan levels have been detected in ascending aortas from patients with thoracic aortic aneurysm and dissections (TAAD) and mice with Marfan syndrome, particularly in dissected and ruptured aortas.87, 89 The increased proteoglycan content may then lead to tissue swelling and, consequently, alterations in blood flow and peripheral organ perfusion. Aggrecan was found to be increased in TAAD aortas, where the expression of ADAMTS5 was found to be downregulated.87 By contrast, expression of ADAMTS1 and ADAMTS4 was found to be normal.87 Similarly, a transcriptome analysis of aortas with dissections found significant downregulation of ADAMTS‐5 relative to controls.105 However, a limitation of measuring mRNA levels is that they may not adequately reflect ADAMTS‐5 protein levels, since its regulation is mainly post‐translational, for example through LRP‐1 endocytosis78 (see Section 2.2.4). Therefore, measuring ADAMTS‐5 protein levels in tissues/biological fluids poses a significant challenge. Moreover, there may be differences between human disease and mouse models. When infused with angiotensin II, which induces aortic degeneration, Adamts5 knockout mice show increased aortic dilation with accumulation of versican and reduced versican cleavage.85 However, mice with ADAMTS‐1 haploinsufficiency also show a predisposition to TAAD in the same model.106
3.3. Atherosclerosis
Proteoglycans also play a primary role in the deposition of low‐density lipoproteins (LDL) in the arterial wall and in the aetiology of atherosclerosis. These LDLs reach the arterial wall when the integrity of the endothelium is compromised.107 Already in the mid‐70s, complexes between LDL and proteoglycans were isolated from human atherosclerotic lesions.107, 108, 109 The affinity of these complexes is relatively high (association constants values for versican and biglycan of 23 and 170 nmol/L respectively)110 and is mediated by an ionic interaction between the negatively charged GAGs on the proteoglycans and positively charged residues in apolipoprotein B.111 The result of this interaction is an increased internalization of LDLs by vascular smooth muscle cells (VSMCs) and macrophages compared with GAG‐free LDL.112, 113 This causes intracellular accumulation of cholesterol, chosteryl‐esters, triglycerides and phospholipids.114 By regulating proteoglycan levels, proteoglycanases may reduce LDL uptake by VSMCs and macrophages. Recombinant ADAMTS‐5 has been shown to reduce the LDL‐binding ability of biglycan and releases LDLs from human aortic lesions.96 The aortas of Adamts5 knockout mice show increased levels of both biglycan and versican and no ADAMTS‐generated versican fragments.96 Importantly, ApolipoproteinE (ApoE) knockout mice, which spontaneously develop atherosclerotic lesions, also show accumulation of biglycan and versican which was associated with a marked reduction in ADAMTS‐5 expression at the protein level.96 In addition to ADAMTS‐5, human VSMCs and macrophages express ADAMTS‐1 and ADAMTS‐4.86, 115, 116 Why do these proteoglycanases not compensate for the absence of ADAMTS‐5? A possible answer may come from our in vitro kinetic analysis of versican cleavage (Glu441↓Ala442 bond), using purified enzymes and proteoglycans.41 This revealed that ADAMTS‐5 is a 20‐fold more potent versicanase than ADAMTS‐4, whereas ADAMTS‐1 versicanase activity is negligible, at least in the absence of cofactors.41 Importantly, adult Adamts5 knockout mice have been recently reported to show reduced versican cleavage in the cardiac ECM, but this was not associated with any significant impairment of cardiac function.117 Only when kept on a high‐fat diet (HFD), Adamts5 knockout mice show subtle cardiac anomalies such as an increased diastolic posterior wall thickness and left ventricle volume, and increased diastolic and systolic blood pressure,117 suggesting that the pathological consequences of Adamts5 knockout may be exacerbated by a pro‐atherosclerotic diet. In contrast, ADAMTS‐4 seems to exert a detrimental role in atherosclerosis, since Adamts4/ApoE double knockout mice exhibit enhanced plaque stability.118
Taken together, these data point to an important role exerted by ADAMTS‐5 in the cardiovasculature where it is necessary to maintain adequate levels of proteoglycans. Due to its much higher versicanase41 and aggrecanase activity,46 a reduction in ADAMTS‐5 levels/activity is only partially compensated by both ADAMTS‐1 and ADAMTS‐4. Any therapy aiming at targeting ADAMTS‐5 must take into account this cardiovascular function.
4. OTHER ROLES OF ADAMTS‐5
Analysis of Adamts5 knockout mice has uncovered a range of unexpected functions and phenotypes (Table 1; Figure 2). However, caution must be taken in extrapolating mouse models to human diseases. Not only do pathophysiologies differ between human and mice, but also the specific mouse strain used to investigate ADAMTS‐5 ablation may affect the observed phenotypes. To date, two different Adamts5 knockout strains have been developed. To investigate the effect of ADAMTS‐5 ablation on aggrecan cleavage, Pfizer, in collaboration with Lexicon Genetics, generated so‐called Adamts5 P mice (Adamts5 tm1.1Lex),59, 60, 61, 117, 118, 119, 120 whereas for investigating versican cleavage, Adamts5 J mice (Adamts5 tm1Dgen) were generated by Deltagen Inc (now commercially available through the Jackson Laboratory, Stock No: 005771).50, 94, 95, 102, 103 Whereas the Adamts5 J mice show an out‐of‐frame deletion of exon 2 (coding for the catalytic site),94 the Adamts5 P mice show an in‐frame deletion of exon 2.119 The Adamts5 P mice are similar to the Adamts5 knockout mice originally reported in 2005.53, 54 Both Adamts5 P and Adamts5 J mice do not show expression of the enzyme at the protein level.119 Although phenotypes shown by these mice are similar, qualitative and quantitative differences were observed in several models.119, 120, 121, 122, 123 Importantly, no conditional knockout has been reported so far. In Section 2 and 3, I discussed the involvement of ADAMTS‐5 in OA and cardiovascular diseases respectively. In the following sections, the involvement of ADAMTS‐5 in other biological/pathological processes will be discussed. Whenever discrepancies emerged between the two aforementioned Adamts5 knockout models, these are explicitly stated; otherwise, I refer to the references for the description of the particular knockout model investigated.
Figure 2.
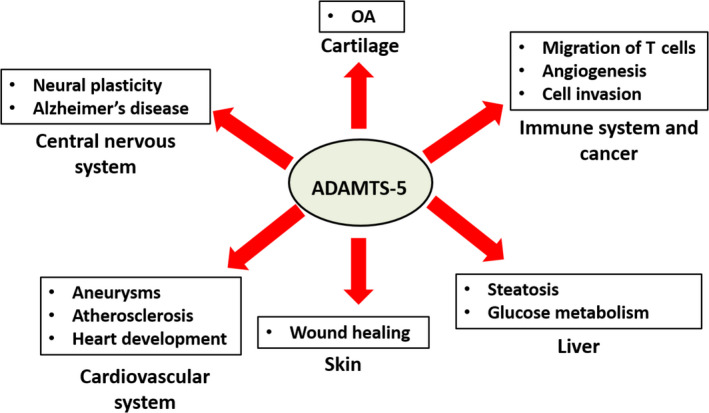
Physiological and pathological functions of ADAMTS‐5. For discussion, see text
4.1. Wound healing
Adamts5 P knockout mice show delayed contraction in an excisional wound healing model, a phenotype associated with accumulation in the dermal layer of cell aggregates and fibroblasts surrounded by a pericellular ECM enriched in full‐length aggrecan.122 These changes in the ECM composition result in an altered signalling of TGF‐β1, a key regulator of multiple processes in wound healing. In contrast, Adamts4 knockout mice exhibit a normal wound healing response.122 On the other hand, using Adamts5 J mice knockout mice, Hattori et al123 observed normal proliferation and migration by isolated dermal fibroblasts, but increased contractility, associated with accumulation of uncleaved pericellular versican and upregulation of α‐smooth muscle actin as well as increased canonical TGF‐β signalling. Although contradictory to some extent in their conclusions, these studies showed that loss of ADAMTS‐5 in dermal fibroblasts results in an abnormal ECM enriched in aggrecan and versican, suggesting a crucial role of ADAMTS‐5 in regulating proteoglycan levels in the skin.
4.2. Liver disease, obesity and metabolic disorders
As discussed in Section 3, lipoprotein retention increases in Adamts5 knockout mice due to accumulation of uncleaved proteoglycans such as versican and biglycan.96 Both versican and heparan sulphate proteoglycans such syndecan‐1, an ADAMTS‐5 substrate (Table 2),124 also regulate lipid uptake in the liver.117, 124, 125, 126 Surprisingly, when Adamts5 knockout mice are subjected to HFD to induce obesity, their plasma lipid levels are higher than wild type.117,124 Their total body weight does not differ from wild‐type mice, but their liver weight and hepatic lipid accumulation are significantly reduced.117, 124 Additionally, Adamts5 knockout mice are protected from steatohepatitis in a methionine‐/choline‐deficient diet (MCD) model.121, 124 Although cleavage of versican remains unaffected probably due to compensation from other ADAMTS proteoglycanases, Adamts5 knockout mice show a reduced expression of versican at the mRNA level when subjected to HFD.117, 124 On the other hand, Adamts5 knockout mice show both reduced cleavage and increased expression of its substrate syndecan‐1.124 More studies are necessary to ascertain the mechanism behind the hepatic phenotype of Adamts5 knockout mice.
Table 2.
List of ADAMTS‐5 substrates
| Substrate | UniProt ID | Cleavage site (P1↓P1′) | References |
|---|---|---|---|
| ADAMTS‐5 | Q9UNA0 | Glu753↓Gly754 | [44]a |
| Aggrecan | P16112‐1 | Glu392↓Ala393 | [1, 2]a |
| Glu1679↓Gly1680 | [48]a | ||
| Glu1848↓Gly1849 | [182]a | ||
| Glu1953↓Ala1954 | [47]a | ||
| Glu2053↓Leu2054 | [182]a | ||
| α2M | P01023 | Met713↓Gly714 | [183]a |
| Biglycan | P21810 | Asn186↓Cys187 |
[48] |
| Brevican | Q96GW7‐1 | Glu400↓Ser401 | [152]a |
| CILP‐1 | O75339 | Glu591↓Val592 | [185]a |
| Val592↓Val593 | [185]a | ||
| Ser607↓Phe608 | [185]a | ||
| Trp699↓Ser700 | [185]a | ||
| Glu717↓Asn718 | [185]a | ||
| Asn718↓Gln719 | [185]a | ||
| Gln719↓Arg720 | [185]a | ||
| Asn722↓Lys723 | [185]a | ||
| CILP‐2 | Q8IUL8 | Thr810↓Ala811 | [185]a |
| Ala811↓Thr812 | [185]a | ||
| Leu813↓Gly814 | [185]a | ||
| Ala830↓Thr831 | [185]a | ||
| Val832↓Gly833 | [185]a | ||
| Clusterin | P10909 | Asn43↓Lys44 | [185]a |
| Cm‐tf | P02787‐1 | Cys58↓Val59 | [48]a |
| Cys213↓Leu214 | [48]a | ||
| Ser409↓Leu410 | [48]a | ||
| Cys542↓Leu543 | [48]a | ||
| Collagen type II α1 | P02458 | Ala902↓Gly903 | [185]a |
| Pro922↓Ser923 | [185]a | ||
| Ser923↓Gly924 | [185]a | ||
| Gly1053↓Val1054 | [185]a | ||
| Pro1078↓Ala1079 | [185]a | ||
| Collagen type III α1 | P02461 | Gln374↓Gly375 | [185]a |
| Asn389↓Gly390 | [185]a | ||
| Decorin | P07585 | Lys74↓Val75 |
[48] [186] |
| Leu85↓Leu86 |
[48] [186] |
||
| Fibromodulin | P13605 | Tyr63↓Ala64 | [186]a |
| Fibronectin | P02751 | Phe2113↓Val2114 |
[48] |
| Pro2135↓Ser2136 |
[48] |
||
| Inter‐α‐inhibitor HC2 | P19823 | Arg359↓Asn360 | [187]a |
| Thr366↓Lys367 | [187]a | ||
| Tyr490↓Asn491 | [187]a | ||
| Asn491↓Gln492 | [187]a | ||
| Leu681↓Ala682 | [187]a | ||
| Prolargin | P51888 | His340↓Asp341 | [185]a |
| His351↓Leu352 | [185]a | ||
| Reelin | ‐ | [165] | |
| Syndecan‐1 | ‐ | [124] | |
| Tenascin | P24821 | Cys64↓Ser65 | [185]a |
| Ser70↓Ala71 | [185]a | ||
| Glu89↓His90 | [185]a | ||
| Versican V1 | P13611‐2 | Glu441↓Ala442 | [41, 97, 98] |
Glutamic acid is over‐represented in P1 position.
Abbreviations: α2M, α2‐macroglobulin; CILP, cartilage intermediate layer protein; Cm‐Tf, carboxymethylated transferrin.
R i Cleavage sites identified by mass‐spectrometry/Edman sequencing. Note that for aggrecan, the cleavage sites reported by the original references were for the bovine homologue (UniProt ID: P13608).
To support a role of ADAMTS‐5 in obesity, Adamts5 expression is upregulated in mice on HFD.127, 128 Moreover, mice knockout for Timp3 (tissue inhibitor of metalloproteinase‐3), the most potent endogenous inhibitor of ADAMTS‐4 and ADAMTS‐5,129 are more susceptible to develop hepatic steatosis when kept on HFD.130
ADAMTS‐5 may be also involved in glucose metabolism. Adamts5 knockout mice have more interscapular brown adipose tissue, and cold exposure induces in these mice a more pronounced browning of white adipose tissue compared to wild type as a result of an enhanced glucose metabolism.121, 131 Adipose‐derived stromal cells from Adamts5 knockout mice show an increased uptake of glucose, a precursor for the synthesis of CS chains, compared with wild‐type mice, resulting in an increased expression of aggrecan.119 Therefore, one function of ADAMTS‐5 may be to depress glucose metabolism. Intriguingly, injection of leptin, a hormone that downregulates lipid accumulation by adipocytes, increases ADAMTS‐5 expression in rats.132
These data suggest that ADAMTS‐5 may be involved in metabolic disorders, although the specific mechanisms have not been fully elucidated.
4.3. Inflammation and cancer
The N‐terminal versican fragment generated by ADAMTS‐5 cleavage, called versikine, has been shown to exert a pro‐apoptotic function.50 Versikine has been shown to accumulate in regions of blood vessels undergoing atrophy and cell death as well as in tumour vasculature.99, 133, 134 Versikine acts as a damage associated molecular pattern by triggering Toll‐like receptor‐2 signalling and activation of the NF‐kB pathway and thus supporting T‐cell activation though the release of pro‐inflammatory cytokines such as IL‐6 and IL‐12 and interferon.135, 136, 137, 138 Versikine has been associated with a higher frequency of activated CD8+ T cells in myeloma and colorectal cancer.135, 139 To corroborate these findings, decreased versikine levels were associated with decreased numbers of total CD4+ and CD8+ T cells in the spleen and lung of Adamts5 knockout mice following infection with influenza virus, resulting in delayed virus clearance and higher weight loss compared with wild‐type mice.140 These results suggest that ADAMTS‐5 versicanase activity contributes to migration of T cells which may have implications in other pathological conditions such as cancer.
High ADAMTS5 expression in colorectal cancer patients indeed correlated with higher levels of lymphatic invasion and lymph node metastasis but no differences in survival rate were observed.141 The ADAMTS5 promoter has also been found to be hyper‐methylated in colorectal cancer142 and T‐cell acute lymphoblastic leukaemia.143 Moreover, ADAMTS5 expression has been found to be downregulated in breast cancer,144 prostate cancer,145 head and neck squamous cell carcinoma,146, 147 and hepatocellular carcinoma.148 ADAMTS‐5 has also been shown to display an anti‐tumorigenic activity which is independent of its catalytic activity. The central thrombospondin‐type I motif of ADAMTS‐5 has been suggested to act as an anti‐angiogenic and pro‐apoptotic peptide.149, 150, 151 Suppression of angiogenesis is mediated by downregulation of pro‐angiogenic factors,150 although the mechanism by which the central thrombospondin‐type I modulates transcriptions of these genes is not known. On the other hand, a pro‐tumorigenic, catalytic‐dependent role has been suggested for ADAMTS‐5 in glioblastoma, where its expression is greatly increased.152 By cleaving brevican, a proteoglycan specific to the central nervous system, ADAMTS‐5 may contribute to the invasiveness of glioblastoma cells.153, 154 ADAMTS‐5 is also highly expressed in laryngeal squamous cell carcinoma,155 where it may contribute to the severe decrease in aggrecan content observed in late stage cancers.156
4.4. Neural plasticity
Proteoglycans inhibit neural plasticity under physiological and pathological conditions, and this is mainly due to their GAG groups which restrict the formation of new neuronal nets.157 Among the neuronal proteoglycans, versican and brevican are strongly upregulated immediately after spinal cord injury (SCI),158, 159, 160 whereas aggrecan is downregulated.161 Expression of Adamts5 is also upregulated in a SCI model.162 Recombinant ADAMTS‐5 has been shown to promote neurite outgrowth in vitro by cleaving proteoglycans.163 Following SCI, Adamts5 knockout mice show severely reduced versican cleavage but significant cleavage of other proteoglycans such as aggrecan and brevican suggests compensation from other ADAMTS family members, such as ADAMTS‐1 and ADAMTS‐4.164
ADAMTS‐5 also cleaves the N‐terminal region of reelin, a secreted glycoprotein that is mainly expressed in the brain, where it is essential for proper neurodevelopment and synaptic plasticity.165 This proteolytic processing results in the degradation of the N‐terminal reelin fragment, a region that is prone to aggregate and, therefore, plays a role in Alzheimer's disease.165 AD mice show decreased expression of Adamts5 in the hippocampus; therefore, decreased processing of the reelin N‐terminal fragment by ADAMTS‐5 may be responsible for accelerated reelin aggregation in the hippocampus of AD mice.165
4.5. Muscle maturation and intervertebral disc disease
Although Adamts5 knockout mice do not show apparent myopathy or deficit in gait and mobility,53, 54 they show delayed muscle maturation during postnatal growth as a result of decreased versican cleavage.166
Genome‐wide association studies have identified ADAMTS5 as a risk locus for degenerative intervertebral disc disease.167 To support a role for ADAMTS‐5 in this pathology, injection of anADAMTS‐5 siRNA suppressed invertebral disc degeneration in the rabbit anular needle‐puncture model.168 So far, Adamts5 knockout mice have not been investigated in relation to intervertebral disc disease.
5. CONCLUSIONS AND PERSPECTIVE
5.1. Anti‐ADAMTS‐5 therapy: the jury is still out
Inhibition of metalloproteinases has a long and troubled history. In the 90s, the failure of broad spectrum MMP inhibitors in cancer clinical trials came as a shock.169 The major causes of this failure were attributed to a lack of specificity of these inhibitors and incomplete knowledge of the role which the targeted enzymes exert in the disease, especially a naivety in assuming that endogenous proteases are destructive agents only active in cancer.170 The first problem was addressed by the development of inhibitory mAbs which can be used to ‘post‐translationally knock down’ the activity of a certain protease.171 However, a lot still needs to be done to uncover the multiple biological processes orchestrated by metalloproteinases. The history of ADAMTS‐5 research shows this in a dramatic way. In few years, ADAMTS‐5 turned from an OA target to a cardiovascular anti‐target while yet other unpredicted biological roles were uncovered by analysing the phenotypes of Adamts5 knockout mice (Table 1; Figure 2).
Notwithstanding the adverse side effects observed in Cynomolgus upon administration of GSK2394002,67, 83 pharmaceutical companies are still actively pursuing ADAMTS‐5 as a therapeutic target for OA. After all, it may well have been that those effects were drug‐related rather than target‐related. In this regard, it will be interesting to compare the effects of this anti‐ADAMTS‐5 mAb with those exhibited by other mAbs or small molecule inhibitors.
Agg‐523, Wyeth's ADAMTS‐4/ADAMTS‐5 small molecule inhibitor, was investigated in phase I clinical trials in patients with mild to moderate and severe knee OA (NCT00454298 and NCT00427687), but no results are currently available. Another orally available small molecule inhibitor of ADAMTS‐5 developed by Galapagos NV, GLPG1972, with a modest (5‐fold) selectivity over ADAMTS‐4, is protective against surgically induced OA in mice and rats.172, 173 GLPG1972 has been tested in a phase 1 trial (ID: NCT02612246) and shown to be well tolerated in healthy male subjects with doses up to 1050 mg/d.174 The absence of adverse effects is remarkable and suggests differences in target engagement compared with GSK2394002. A phase 2 clinical trial (ID: NCT03595618) comparing its effects with placebo is currently underway (results expected December 2020).
EMD Serono has developed an anti‐ADAMTS‐5 nanobody (M6495, Ablynx) able to block OA progression in mice following DMM175 and reduce circulating levels of aggrecanase‐generated aggrecan fragments in Cynomolgus monkeys.176 Nanobodies are single domain mAbs whose antigen binding site is composed only of one heavy chain, rather than one light and one heavy chain; by being much smaller than conventional mAbs, nanobodies have higher tissue penetration in vivo.177 M6945 is a bifunctional nanobody binding to ADAMTS‐5 metalloproteinase/disintegrin domains and serum albumin to extend its in vivo half‐life.178 Phase 1 clinical trials (ID: NCT03583346 and NCT03224702) after subcutaneous administration were completed but results have yet to be posted on https://clinicaltrials.gov.
Whatever will be the outcome of these clinical trials, there are a few problems that ADAMTS‐5 targeting therapies should address in a clinically relevant context. For example, administration of ADAMTS‐5 inhibitors may require a careful follow‐up in OA patients, as they may make them more susceptible to adverse cardiovascular effects such as aortic aneurysms, myxomatous valves, atherosclerosis and influenza infection.96, 140 Given the administration of ADAMTS‐5 inhibitors to an aged patient population with multiple co‐morbidities, it is important to fully understand their effects beyond cartilage degradation alone. Moreover, an important application of ADAMTS‐5 therapy may be on young athletes with post‐traumatic OA, who may better respond to pharmacological treatment. Intra‐articular injections may circumvent potential systemic side effects, although this is not a preferable route of administration due to the discomfort for the patients and the necessity to be administered by expert physicians.
Substrate‐specific inhibitors have been hypothesized as a way to limit the side effects of protease inhibitors by restricting their actions to a specific substrate or set of substrates,171 but in the case of ADAMTS‐5 this approach may be unsuccessful for two reasons. First, in the ADAMTS‐5 spacer domain, the substrate‐binding sites (exosites) are shared between aggrecan and versican.41 Second, aggrecan has been recently shown to be expressed not only in cartilage but also in the blood vessels,84, 85, 86, 87, 88, 89, 105 where it exerts similar functions to versican.
In the end, only the outcome of clinical trials and, eventually, their follow‐up will tell if ADAMTS‐5 represents a viable target for OA therapy.
5.2. The next twenty years
Scientifically, many questions about ADAMTS‐5 biology still need an answer. Other potential biological roles of ADAMTS‐5 discussed in Section 4 should be thoroughly investigated and assessed in relevant mouse models.
In recent years, the number of ADAMTS‐5 substrates has increased (Table 2). However, this number is still relatively small, compared to other enzymes, and, with the exception of proteoglycans, most of these substrates are cleaved at a slow rate, suggesting that ADAMTS‐5 has a quite strict substrate specificity. Future degradomic studies can help to define its substrate repertoire and therefore clarify if some elusive aspects of its biology are due to unknown substrates or unknown non‐proteolytic functions. Moreover, researchers should assess the biological activity of ADAMTS‐5‐generated cleavage products. In Section 4.3, I discussed the pro‐apoptotic activity of versikine, the N‐terminal versican cleavage fragment generated by ADAMTS cleavage at Glu441↓Ala442.50 This is not the only example, as a 32‐amino acid aggrecan fragment (32‐mer) generated by the combined proteolytic activity of aggrecanases (cleavage at Glu392↓Ala393) and MMPs (cleavage at Asn360↓Phe361) is endowed with cytokine‐like activities.179 The 32‐mer excites nociceptive neurons in chondrocytes through Toll‐Like receptor 2179 and may be at least partially responsible for pain‐related OA. Mice bearing a mutated MMP cleavage site in aggrecan which, for this reason, cannot produce the 32‐mer aggrecan fragments are protected from increased pain sensitivity (hyperalgesia) although they still show severe cartilage degradation due to ADAMTS‐5 activity at Glu392↓Ala392.38 The generation of such matrix‐derived cryptic cytokines (matrikines) may be a more general strategy which ADAMTS‐5 employs to fulfil its biological functions.
Finally, much work still needs to be done on the biochemical characterization of ADAMTS‐5. Although it is known that the spacer and cysteine‐rich domains contain exosites important for proteoglycanase activity,41, 46, 48 the three‐dimensional structure of these domains has not been reported. The only spacer/cysteine‐rich domains structures reported so far for any ADAMTS family member are those of ADAMTS‐13, whose proteolytic activity, domain composition and regulation are completely different.180, 181 Solving these structures may help to clarify the way that ADAMTS‐5 interacts with proteoglycans and at the same time inform the rational design/optimization of more selective small molecule inhibitors.
In conclusion, after twenty years the field of ADAMTS‐5 research has developed considerably and reached a complexity that was unimaginable when it was first discovered. The complicated teenager is getting more complicated, but, hopefully, more manageable.
CONFLICT OF INTEREST
The author declares no conflicts of interest.
ACKNOWLEDGEMENTS
S.S is supported by the British Heart Foundation (PG/18/15/33566). He wishes to thank Dr Josefin Ahnström and Dr Rens de Groot (Imperial College London) and Dr Suneel S. Apte (Cleveland Clinic) for their critical reading of the manuscript.
Santamaria S. ADAMTS‐5: A difficult teenager turning 20. Int J Exp Path. 2020;101:4–20. 10.1111/iep.12344
Footnotes
In contrast with active‐site titrations for ADAMTS‐5, Rodriguez‐Manzaneque et al42 used optical density of purified ADAMTS‐1 to estimate concentrations. However, generally the difference between the two concentrations is no more than 2/10‐fold for ADAMTS‐5 (my unpublished results), making the relative difference between ADAMTS‐5 and ADAMTS‐1 still impressive.
REFERENCES
- 1. Abbaszade I, Liu RQ, Yang F et al Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999;274:23443‐23450. [DOI] [PubMed] [Google Scholar]
- 2. Tortorella MD, Burn TC, Pratta MA et al Purification and cloning of aggrecanase‐1: a member of the ADAMTS family of proteins. Science. 1999;284:1664‐1666. [DOI] [PubMed] [Google Scholar]
- 3. Carney SL, Billingham ME, Muir H, Sandy JD. Demonstration of increased proteoglycan turnover in cartilage explants from dogs with experimental osteoarthritis. J Orthop Res. 1984;2:201‐206. [DOI] [PubMed] [Google Scholar]
- 4. Carney SL, Billingham ME, Muir H, Sandy JD. Structure of newly synthesised (35S)‐proteoglycans and (35S)‐proteoglycan turnover products of cartilage explant cultures from dogs with experimental osteoarthritis. J Orthop Res. 1985;3:140‐147. [DOI] [PubMed] [Google Scholar]
- 5. Lohmander LS, Dahlberg L, Ryd L, Heinegård D. Increased levels of proteoglycan fragments in knee joint fluid after injury. Arthritis Rheum. 1989;32:1434‐1442. [DOI] [PubMed] [Google Scholar]
- 6. Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373‐Ala 374 bond of the interglobular domain. J Clin Invest. 1992;89:1512‐1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hurskainen TL, Hirohata S, Seldin MF, Apte SS. ADAM‐TS5, ADAM‐TS6, and ADAM‐TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM‐TS family. J Biol Chem. 1999;274:25555‐25563. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Helmick CG, Felson DT, Lawrence RC et al Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008;58:15‐25. [DOI] [PubMed] [Google Scholar]
- 10. Heinegård D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7:50‐56. [DOI] [PubMed] [Google Scholar]
- 11. Hunziker EB, Schenk RB. Structural organization of proteoglycans in cartilage In: Wight T, Mecham R, eds. Biology of Proteoglycans. New York: Academic Press; 1987:155‐185. [Google Scholar]
- 12. Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975;12:233‐248. [DOI] [PubMed] [Google Scholar]
- 13. Doege KJ, Sasaki M, Kimura T, Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan: human‐specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991;266:894‐902. [PubMed] [Google Scholar]
- 14. Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12:19‐32. [DOI] [PubMed] [Google Scholar]
- 15. Eyring EJ, Yang JT. Conformation of protein‐polysaccharide complex from bovine nasal septum. J Biol Chem. 1968;243:1306‐1311. [PubMed] [Google Scholar]
- 16. Luscombe M, Phelps CF. The composition and physicochemical properties of bovine nasal‐septa protein‐polysaccharide complex. Biochem J. 1967;102:110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pasternack SG, Veis A, Breen M. Solvent‐dependent changes in proteoglycan subunit conformation in aqueous guanidine hydrochloride solutions. J Biol Chem. 1974;249:2206‐2211. [PubMed] [Google Scholar]
- 18. Hardingham TE, Fosang AJ, Dudhia J. Aggrecan, thechondroitin sulfate/keratan sulfate proteoglycan from cartilage In: Kuettner KE, Schleyerbach R, Peyton JG, Hascall VC, eds. Articular Cartilage and Osteoarthritis. New York: Raven Press; 1992:5‐20. [Google Scholar]
- 19. Fell HB, Jubb RW. The effect of synovial tissue on the breakdown of articular cartilage in organ culture. Arthritis Rheum. 1977;20:1359‐1371. [DOI] [PubMed] [Google Scholar]
- 20. Morales TI, Hascall VC. Correlated metabolism of proteoglycans and hyaluronic acid in bovine cartilage organ cultures. J Biol Chem. 1988;263:3632‐3638. [PubMed] [Google Scholar]
- 21. Billinghurst RC, Wu W, Ionescu M et al Comparison of the degradation of type II collagen and proteoglycan in nasal and articular cartilages induced by interleukin‐1 and the selective inhibition of type II collagen cleavage by collagenase. Arthritis Rheum. 2000;43:664‐672. [DOI] [PubMed] [Google Scholar]
- 22. Lorenzo P, Bayliss MT, Heinegård D. Altered patterns and synthesis of extracellular matrix macromolecules in early osteoarthritis. Matrix Biol. 2004;23:381‐391. [DOI] [PubMed] [Google Scholar]
- 23. Karsdal MA, Madsen SH, Christiansen C, Henriksen K, Fosang AJ, Sondergaard BC. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther. 2008;10:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Close DR. Matrix metalloproteinase inhibitors in rheumatic diseases. Ann Rheum Dis. 2001;60(Suppl. 3):62‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis EJ, Bishop J, Bottomley KM et al Ro 32–3555, an orally active collagenase inhibitor, prevents cartilage breakdown in vitro and in vivo. Br J Pharmacol. 1997;121:540‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brewster M, Lewis JE, Wilson KL, Greenham AK, Bottomley KMK. Ro 32–3555, an orally active collagenase selective inhibitor prevents structural damage in the STR/ORT mouse model of osteoarthritis. Arthritis Rheum. 1998;41:1639‐1644. [DOI] [PubMed] [Google Scholar]
- 27. Hedlund H, Hedbom E, Heinegård D et al Association of the aggrecan keratan sulfate‐rich region with collagen in bovine articular cartilage. J Biol Chem. 1999;274:5777‐5781. [DOI] [PubMed] [Google Scholar]
- 28. Pratta MA, Yao W, Decicco C et al Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278:45539‐45545. [DOI] [PubMed] [Google Scholar]
- 29. Flannery CR, Lark MW, Sandy JD. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992;267:1008‐1014. [PubMed] [Google Scholar]
- 30. Lohmander LS, Neame PJ, Sandy JD. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993;36:1214‐1222. [DOI] [PubMed] [Google Scholar]
- 31. Sandy JD, Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C‐terminal processing in vivo. Biochem J. 2001;358:615‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barry FP, Gaw JU, Young CN, Neame PJ. Hyaluronan binding region of aggrecan from pig laryngeal cartilage. Amino acid sequence, analysis of N‐linked oligosaccharides and location of the keratan sulphate. Biochem J. 1992;286:761‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patwari P, Kurz B, Sandy JD, Grodzinsky AJ. Mannosamine inhibits aggrecanase‐mediated changes in the physical properties and biochemical composition of articular cartilage. Arch Biochem Biophys. 2000;374:79‐85. [DOI] [PubMed] [Google Scholar]
- 34. Buckwalter JA, Rosenberg LC. Electron microscopic studies of cartilage proteoglycans. Direct evidence for the variable length of the chondroitin sulfate‐rich region of proteoglycan subunit core protein. J Biol Chem. 1982;257:9830‐9839. [PubMed] [Google Scholar]
- 35. Paulsson M, Mörgelin M, Wiedemann H et al Extended and globular protein domains in cartilage proteoglycans. Biochem. J. 1987;245:763‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santamaria S, Yamamoto K. Analysis of aggrecanase activity using neoepitope antibodies. Methods Mol Biol. 2020;2043:125‐136. [DOI] [PubMed] [Google Scholar]
- 37. Van Meurs JBJ, Van Lent PLEM, Holthuysen AEM, Singer II, Bayne EK, Van Den Berg WB. Kinetics of aggrecanase‐ and metalloproteinase‐induced neoepitopes in various stages of cartilagedestruction in murine arthritis. Arthritis Rheum. 1999;42:1128‐1139. [DOI] [PubMed] [Google Scholar]
- 38. Little CB, Meeker CT, Golub SB et al Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. J Clin Invest. 2007;117:1627‐1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuno K, Kanada N, Nakashima E et al Molecular cloning of a gene encoding a new type of metalloproteinase‐disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 1997;272:556‐562. [DOI] [PubMed] [Google Scholar]
- 40. Bode W, Gomis‐Rüth FX, Stöcker W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc‐binding environments (HEXXHXXGXXH and Met‐turn) and topologies and should be grouped into a common family, the ‘metzincins’. FEBS Lett. 1993;331:134‐140. [DOI] [PubMed] [Google Scholar]
- 41. Santamaria S, Yamamoto K, Teraz‐Orosz A et al Exosites in hypervariable loops of ADAMTS spacer domains control substrate recognition and proteolysis. Sci Rep. 2019;9:10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carlos Rodríguez‐Manzaneque J, Westling J, Thai S‐M et al ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293:501‐508. [DOI] [PubMed] [Google Scholar]
- 43. Collins‐Racie LA, Flannery CR, Zeng W et al ADAMTS‐8 exhibits aggrecanase activity and is expressed in human articular cartilage. Matrix Biol. 2004;23:219‐230. [DOI] [PubMed] [Google Scholar]
- 44. Zeng W, Corcoran C, Collins‐Racie LA, LaVallie ER, Morris EA, Flannery CR. Glycosaminoglycan‐binding properties and aggrecanase activities of truncated ADAMTSs: comparative analyses with ADAMTS‐5, ‐9, ‐16 and ‐18. Biochim Biophys Acta. 2006;1760:517‐524. [DOI] [PubMed] [Google Scholar]
- 45. Yang CY, Chanalaris A, Troeberg L. ADAMTS and ADAM metalloproteinases in osteoarthritis—looking beyond the 'usual suspects'. Osteoarthritis Cartilage. 2017;25:1000‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fushimi K, Troeberg L, Nakamura H, Lim NH, Nagase H. Functional differences of the catalytic and non‐catalytic domains in human ADAMTS‐4 and ADAMTS‐5 in aggrecanolytic activity. J Biol Chem. 2008;283:6706‐6716. [DOI] [PubMed] [Google Scholar]
- 47. Santamaria S, Yamamoto K, Botkjaer K et al Antibody‐based exosite inhibitors of ADAMTS‐5 (aggrecanase‐2). Biochem J. 2015;471:391‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gendron C, Kashiwagi M, Lim NH et al Proteolytic activities of human ADAMTS‐5. Comparative studies with ADAMTS‐4. J Biol Chem. 2007;282:18294‐18306. [DOI] [PubMed] [Google Scholar]
- 49. Lee NV, Rodriguez‐Manzaneque JC, Thai S‐M et al Fibulin‐1 acts as a cofactor for the matrix metalloprotease ADAMTS‐1. J Biol Chem. 2005;280:34796‐34804. [DOI] [PubMed] [Google Scholar]
- 50. McCulloch DR, Nelson CM, Dixon LJ et al ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell. 2009;17:687‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Glasson SS, Askew R, Sheppard B et al Characterization of and osteoarthritis susceptibility in ADAMTS‐4‐knockout mice. Arthritis Rheum. 2004;50:2547‐2558. [DOI] [PubMed] [Google Scholar]
- 52. Little CB, Mittaz L, Belluoccio D et al ADAMTS‐1‐knockout mice do not exhibit abnormalities in aggrecan turnover in vitro or in vivo. Arthritis Rheum. 2005;52:1461‐1472. [DOI] [PubMed] [Google Scholar]
- 53. Glasson SS, Askew R, Sheppard B et al Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644‐648. [DOI] [PubMed] [Google Scholar]
- 54. Stanton H, Rogerson FM, East CJ et al ADAMTS‐5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648‐652. [DOI] [PubMed] [Google Scholar]
- 55. East CJ, Stanton H, Golub SB et al ADAMTS‐5 deficiency blocks aggrecanase cleavage in the interglobular domain but not in the chondroitin sulfate‐rich region of aggrecan. J Biol Chem. 2007;282:8632‐8640. [DOI] [PubMed] [Google Scholar]
- 56. Botter SM, Glasson SS, Hopkins B et al ADAMTS5‐/‐ mice have less subchondral bone changes after induction of osteoarthritis through surgical instability: implications for a link between cartilage and subchondral bone changes. Osteoarthritis Cartilage. 2009;17:636‐645. [DOI] [PubMed] [Google Scholar]
- 57. Nakamura M, Sone S, Takahashi I, Mizoguchi I, Echigo S, Sasano Y. Expression of versican and ADAMTS1, 4, and 5 during bone development in the rat mandible and hind limb. J Histochem Cytochem. 2005;53:1553‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lind T, McKie N, Wendel M, Racey SN, Birch MA. The hyalectan degrading ADAMTS‐1 enzyme is expressed by osteoblasts and up‐regulated at regions of new bone formation. Bone. 2005;36:408‐417. [DOI] [PubMed] [Google Scholar]
- 59. Malfait AM, Ritchie J, Gil AS et al ADAMTS‐5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis Cartilage. 2010;18:572‐580. [DOI] [PubMed] [Google Scholar]
- 60. Li J, Anemaet W, Diaz MA et al Knockout of ADAMTS5 does not eliminate cartilage aggrecanase activity but abrogates joint fibrosis and promotes cartilage aggrecan deposition in murine osteoarthritis models. J Orthop Res. 2011;29:516‐522. [DOI] [PubMed] [Google Scholar]
- 61. Bell R, Li J, Shewman EF et al ADAMTS5 is required for biomechanically‐stimulated healing of murine tendinopathy. J Orthop Res. 2013;31:1540‐1548. [DOI] [PubMed] [Google Scholar]
- 62. Wang VM, Bell RM, Thakore R et al Murine tendon function is adversely affected by aggrecan accumulation due to the knockout of ADAMTS5. J Orthop Res. 2012;30:620‐626. [DOI] [PubMed] [Google Scholar]
- 63. Majumdar MK, Askew R, Schelling S et al Double‐knockout of ADAMTS‐4 and ADAMTS‐5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56:3670‐3674. [DOI] [PubMed] [Google Scholar]
- 64. Rogerson FM, Stanton H, East CJ et al Evidence of a novel aggrecan‐degrading activity in cartilage: studies of mice deficient in both ADAMTS‐4 and ADAMTS‐5. Arthritis Rheum. 2008;58:1664‐1673. [DOI] [PubMed] [Google Scholar]
- 65. Chu X, You H, Yuan X, Zhao W, Li W, Guo X. Protective effect of lentivirus‐mediated siRNA targeting ADAMTS‐5 on cartilage degradation in a rat model of osteoarthritis. Int J Mol Med. 2013;31:1222‐1228. [DOI] [PubMed] [Google Scholar]
- 66. Hoshi H, Akagi R, Yamaguchi S et al Effect of inhibiting MMP13 and ADAMTS5 by intra‐articular injection of small interfering RNA in a surgically induced osteoarthritis model of mice. Cell Tissue Res. 2017;368:379‐387. [DOI] [PubMed] [Google Scholar]
- 67. Larkin J, Lohr TA, Elefante L et al Translational development of an ADAMTS‐5 antibody for osteoarthritis disease modification. Osteoarthritis Cartilage. 2015;23:1254‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Miller RE, Tran PB, Ishihara S, Larkin J, Malfait AM. Therapeutic effects of an anti‐ADAMTS‐5 antibody on joint damage and mechanical allodynia in a murine model of osteoarthritis. Osteoarthritis Cartilage. 2016;24:299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chiusaroli R, Visentini M, Galimberti C et al Targeting of ADAMTS‐5’s ancillary domain with the recombinant mAb CRB0017 ameliorates disease progression in a spontaneous murine model of osteoarthritis. Osteoarthritis Cartilage. 2013;21:1807‐1810. [DOI] [PubMed] [Google Scholar]
- 70. Blom AB, van Lent PL, Libregts S et al Crucial role of macrophages in matrix metalloproteinases‐mediates cartilage destruction during experimental osteoarthritis. Arthritis Rheum. 2007;56:147‐157. [DOI] [PubMed] [Google Scholar]
- 71. Fernandes JC, Martel‐Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237‐246. [PubMed] [Google Scholar]
- 72. Bondeson J, Wainwright S, Hughes C, Caterson B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin Exp Rheumatol. 2008;26:139‐145. [PubMed] [Google Scholar]
- 73. Song R‐H, D. Tortorella M, Malfait A‐M et al Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS‐4 and ADAMTS‐5. Arthritis Rheum. 2007;56:575‐585. [DOI] [PubMed] [Google Scholar]
- 74. Yamamoto K, Santamaria S, Botkjaer KA et al Inhibition of shedding of low‐density lipoprotein receptor‐related protein 1 reverses cartilage matrix degradation in osteoarthritis. Arthritis Rheumatol. 2017;69:1246‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648‐2657. [DOI] [PubMed] [Google Scholar]
- 76. Kevorkian L, Young DA, Darrah C et al Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50:131‐141. [DOI] [PubMed] [Google Scholar]
- 77. Naito S, Shiomi T, Okada A et al Expression of ADAMTS4 (aggrecanase‐1) in human osteoarthritic cartilage. Pathol Int. 2007;57:703‐711. [DOI] [PubMed] [Google Scholar]
- 78. Yamamoto K, Troeberg L, Scilabra SD et al LRP‐1‐mediated endocytosis regulates extracellular activity of ADAMTS5 in articular cartilage. FASEB J. 2013;27:511‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Santamaria S, Fedorov O, McCafferty J et al Development of a monoclonal anti‐ADAMTS‐5 antibody that specifically blocks the interaction with LRP1. MAbs. 2017;9:595‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Alcaraz MJ, Guillén MI, Ferrándiz ML. Emerging therapeutic agents in osteoarthritis. Biochem Pharmacol. 2019;165:4‐16. [DOI] [PubMed] [Google Scholar]
- 81. Tanaka R, Ozawa J, Kito N, Moriyama H. Effects of exercise therapy on walking ability in individuals with knee osteoarthritis: a systematic review and meta‐analysis of randomised controlled trials. Clin Rehabil. 2016;30:36‐52. [DOI] [PubMed] [Google Scholar]
- 82. Carlson CS, Loeser RF, Jayo MJ, Weaver DS, Adams MR, Jerome CP. Osteoarthritis in cynomolgus macaques: a primate model of naturally occurring disease. J Orthop Res. 1994;12:331‐339. [DOI] [PubMed] [Google Scholar]
- 83. Larkin J, Lohr T, Elefante L et al The highs and lows of translational drug development: antibody mediated inhibition of ADAMTS‐5 for osteoarthritis disease modification. Osteoarthritis Cartilage. 2014;22:S883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Talusan P, Bedri S, Yang S et al Analysis of intimal proteoglycans in atherosclerosis‐prone and atherosclerosis‐resistant human arteries by mass spectrometry. Mol Cell Proteomics. 2005;4:1350‐1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fava M, Barallobre‐Barreiro J, Mayr U et al Role of ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs)‐5 in aortic dilatation and extracellular matrix remodeling. Arterioscler Thromb Vasc Biol. 2018;38:1537‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Suna G, Wojakowski W, Lynch M et al Extracellular matrix proteomics reveals interplay of aggrecan and aggrecanases in vascular remodeling of stented coronary arteries. Circulation. 2018;137:166‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cikach FS, Koch CD, Mead TJ et al Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight. 2018;3:e97167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yasmin , Maskari RA, McEniery CM et al The matrix proteins aggrecan and fibulin‐1 play a key role in determining aortic stiffness. Sci Rep. 2018;8:8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yin X, Wanga S, Fellows AL et al Glycoproteomic analysis of the aortic extracellular matrix in Marfan patients. Arterioscler Thromb Vasc Biol. 2019;39:1859‐1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Armentano RL, Graf Sebastián, Barra JG et al Carotid wall viscosity increase is related to intima‐media thickening in hypertensive patients. Hypertension. 1998;31:534‐539. [DOI] [PubMed] [Google Scholar]
- 91. Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res. 2004;94:1158‐1167. [DOI] [PubMed] [Google Scholar]
- 92. Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the HDF mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56‐66. [DOI] [PubMed] [Google Scholar]
- 93. Kern CB, Norris RA, Thompson RP et al Versican proteolysis mediates myocardial regression during outflow tract development. Dev. Dyn. 2007;236:671‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. McCulloch DR, Goff CL, Bhatt S, Dixon LJ, Sandy JD, Apte SS. Adamts5, the gene encoding a proteoglycan‐degrading metalloprotease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr Patterns. 2009;9:314‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dupuis LE, McCulloch DR, McGarity JD et al Altered versican cleavage in ADAMTS5 deficient mice; a novel etiology of myxomatous valve disease. Dev Biol. 2011;357:152‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Didangelos A, Mayr U, Monaco C, Mayr M. Novel role of ADAMTS‐5 protein in proteoglycan turnover and lipoprotein retention in atherosclerosis. J Biol Chem. 2012;287:19341‐19345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Longpré JM, McCulloch DR, Koo BH et al Characterization of proADAMTS5 processing by proprotein convertases. Int J Biochem Cell Biol. 2009;41:1116‐1126. [DOI] [PubMed] [Google Scholar]
- 98. Foulcer SJ, Nelson CM, Quintero MV et al Determinants of versican‐V1 proteoglycan processing by the metalloproteinase ADAMTS5. J Biol Chem. 2014;289:27859‐27873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sandy J, Westling J, Kenagy R et al Versican V1 proteolysis in human aorta in vivo occurs at the Glu441‐Ala442 bond, a site that is cleaved by recombinant ADAMTS‐1 and ADAMTS‐4. J Biol Chem. 2001;276:13372‐13378. [DOI] [PubMed] [Google Scholar]
- 100. Somerville RPT, Longpre JM, Jungers KA et al Characterization of ADAMTS‐9 and ADAMTS‐20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON‐1. J Biol Chem. 2003;278:9503‐9513. [DOI] [PubMed] [Google Scholar]
- 101. Dancevic CM, Fraser FW, Smith AD et al Biosynthesis and expression of a disintegrin‐like and metalloproteinase domain with thrombospondin‐1 repeats‐15: a novel versican‐cleaving proteoglycanase. J Biol Chem. 2013;288:37267‐37276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dupuis LE, Osinska H, Weinstein MB, Hinton RB, Kern CB. Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves. J Mol Cell Cardiol. 2013;60:50‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dupuis LE, Nelson EL, Hozik B et al Adamts5−/− mice exhibit altered acan (Aggrecan) proteolytic profiles that correlate with ascending aortic anomalies. Arterioscler Thromb Vasc Biol. 2019;39:2067‐2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhou Z, Rawnsley DR, Goddard L et al The cerebral cavernous malformation pathway controls cardiac development via regulation of endocardial MEKK3 signaling and KLF expression. Dev Cell. 2015;32:168‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kimura N, Futamura K, Arakawa M et al Gene expression profiling of acute type A aortic dissection combined with in vitro assessment. Eur J Cardiothorac Surg. 2017;52:810‐817. [DOI] [PubMed] [Google Scholar]
- 106. Oller J, Méndez‐Barbero N, Ruiz EJ et al Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat Med. 2017;23:200‐212. [DOI] [PubMed] [Google Scholar]
- 107. Srinivasan SR, Dolan P, Radhakrishnamurthy B et al Lipoprotein‐acid mucoporysaccharide complexes of human atherosclerotic lesions. Biochim Biophys Acta. 1975;388:58‐70. [DOI] [PubMed] [Google Scholar]
- 108. Srinivasan SR, Dolan P, Radhakrishnamurthy B, Berenson GS. Isolation of lipoprotein‐acid mucopolysaccharide complexes from fatty streaks of human aortas. Atherosclerosis. 1972;16:95‐104. [DOI] [PubMed] [Google Scholar]
- 109. Hollander W. Unified concept on the role of acid mucopolysaccharides and connective tissue proteins in the accumulation of lipids, lipoproteins, and calcium in the atherosclerotic plaques. Exp Mol Pathol. 1976;25:106‐120. [DOI] [PubMed] [Google Scholar]
- 110. Olin KL, Potter‐Perigo S, Barrett PHR, Wight TN, Chait A. Lipoprotein lipase enhances the binding of native and oxidized low density lipoproteins to versican and biglycan synthesized by cultured arterial smooth muscle cells. J Biol Chem. 1999;274:34629‐34636. [DOI] [PubMed] [Google Scholar]
- 111. Borén J, Olin K, Lee I, Chait A, Wight TN, Innerarity TL. Identification of the principal proteoglycan‐binding site in LDL. A single‐point mutation in apo‐B100 severely affects proteoglycan interaction without affecting LDL receptor binding. J Clin Invest. 1998;101:2658‐2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hurt‐Camejo E, Camejo G, Rosengren B et al Effect of arterial proteoglycans and glycosaminoglycans on low density lipoprotein oxidation and its uptake by human macrophages and arterial smooth muscle cells. Arterioscler Thromb. 1992;12:569‐583. [DOI] [PubMed] [Google Scholar]
- 113. Llorente‐Cortés V, Otero‐Viñas M, Hurt‐Camejo E, Martínez‐González José, Badimon L. Human coronary smooth muscle cells internalize versican‐modified LDL through LDL receptor related protein and LDL receptors. Arterioscler Thromb Vasc Biol. 2002;22:387‐393. [DOI] [PubMed] [Google Scholar]
- 114. Hurt E, Camejo G. Effect of arterial proteoglycans on the interaction of LDL with human monocyte‐derived macrophages. Atherosclerosis. 1987;67:115‐126. [DOI] [PubMed] [Google Scholar]
- 115. Jönsson‐Rylander A‐C, Nilsson T, Fritsche‐Danielson R et al Role of ADAMTS‐1 in atherosclerosis: remodeling of carotid artery, immunohistochemistry, and proteolysis of Versican. Arterioscler Thromb Vasc Biol. 2005;25:180‐185. [DOI] [PubMed] [Google Scholar]
- 116. Wågsäter D, Björk H, Zhu C et al ADAMTS‐4 and ‐8 are inflammatory regulated enzymes expressed in macrophage‐rich areas of human atherosclerotic plaques. Atherosclerosis. 2008;196:514‐522. [DOI] [PubMed] [Google Scholar]
- 117. Hemmeryckx B, Carai P, Lijnen R. ADAMTS5 deficiency in mice does not affect cardiac function. Cell Biol Int. 2019;43:593‐604. [DOI] [PubMed] [Google Scholar]
- 118. Kumar S, Chen M, Li Y et al Loss of ADAMTS4 reduces high fat diet‐induced atherosclerosis and enhances plaque stability in ApoE−/− mice. Sci Rep. 2016;6:31130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gorski DJ, Xiao W, Li J et al Deletion of ADAMTS5 does not affect aggrecan or versican degradation but promotes glucose uptake and proteoglycan synthesis in murine adipose derived stromal cells. Matrix Biol. 2015;47:66‐84. [DOI] [PubMed] [Google Scholar]
- 120. Kosasih HJ, Last K, Rogerson FM et al A disintegrin and metalloproteinase with thrombospondin motifs‐5 (ADAMTS‐5) forms catalytically active oligomers. J Biol Chem. 2016;291:3197‐3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bauters D, Bedossa P, Lijnen HR, Hemmeryckx B. Functional role of ADAMTS5 in adiposity and metabolic health. PLoS ONE. 2018;13:e0190595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Velasco J, Li J, DiPietro L et al ADAMTS5 ablation blocks murine dermal repair through CD44‐mediated aggrecan accumulation and a switch in TGFb1 signaling from ALK5 to ALK1. J Biol Chem. 2011;286:26016‐26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hattori N, Carrino DA, Lauer ME et al Pericellular versican regulates the fibroblast‐myofibroblast transition: a role for ADAMTS5 protease‐mediated proteolysis. J Biol Chem. 2011;286:34298‐34310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bauters D, Spincemaille P, Geys L et al ADAMTS5 deficiency protects against non‐alcoholic steatohepatitis in obesity. Liver Int. 2016;36:1848‐1859. [DOI] [PubMed] [Google Scholar]
- 125. MacArthur JM, Bishop JR, Stanford KI et al Liver heparan sulfate proteoglycans mediate clearance of triglyceride‐rich lipoproteins independently of LDL receptor family members. J Clin Invest. 2007;117:153‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Stanford KI, Bishop JR, Foley EM et al Syndecan‐1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride‐rich lipoproteins in mice. J Clin Invest. 2009;119:3236‐3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Voros G, Sandy JD, Collen D, Lijnen HR. Expression of aggrecan(ases) during murine preadipocyte differentiation and adipose tissue development. Biochim Biophys Acta. 2006;1760:1837‐1844. [DOI] [PubMed] [Google Scholar]
- 128. Voros G, Maquoi E, Collen D, Lijnen HR. Differential expression of plasminogen activator inhibitor‐1, tumor necrosis factor‐alpha, TNF‐alpha converting enzyme and ADAMTS family members in murine fat territories. Biochim Biophys Acta. 2003;1625:36‐42. [DOI] [PubMed] [Google Scholar]
- 129. Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP‐3 is a potent inhibitor of aggrecanase 1 (ADAM‐TS4) and aggrecanase 2 (ADAM‐TS5). J Biol Chem. 2001;276:12501‐12504. [DOI] [PubMed] [Google Scholar]
- 130. Menghini R, Menini S, Amoruso R et al Tissue inhibitor of metalloproteinase 3 deficiency causes hepatic steatosis and adipose tissue inflammation in mice. Gastroenterology. 2009;136:663‐672.e4. [DOI] [PubMed] [Google Scholar]
- 131. Bauters D, Cobbaut M, Geys L, Van Lint J, Hemmeryckx B, Lijnen HR. Loss of ADAMTS5 enhances brown adipose tissue mass and promotes browning of white adipose tissue via CREB signaling. Mol Metab. 2017;6:715‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bao J‐P, Chen W‐P, Feng J, Hu P‐F, Shi Z‐L, Wu L‐D. Leptin plays a catabolic role on articular cartilage. Mol Biol Rep. 2010;37:3265‐3272. [DOI] [PubMed] [Google Scholar]
- 133. Kenagy RD, Fischer JW, Lara S, Sandy JD, Clowes AW, Wight TN. Accumulation and loss of extracellular matrix during shear stress‐mediated intimal growth and regression in baboon vascular grafts. J Histochem Cytochem. 2005;53:131‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Asano K, Nelson CM, Nandadasa S et al Stromal versican regulates tumor growth by promoting angiogenesis. Sci Rep. 2017;7:17225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hope C, Foulcer S, Jagodinsky J et al Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood. 2016;128:680‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gorter A, Zijlmans HJ, van Gent H, Trimbos JB, Fleuren GJ, Jordanova ES. Versican expression is associated with tumor‐infiltrating CD8‐positive T cells and infiltration depth in cervical cancer. Mod Pathol. 2010;23:1605‐1615. [DOI] [PubMed] [Google Scholar]
- 137. Wang W, Xu G‐L, Jia W‐D et al Ligation of TLR2 by versican: a link between inflammation and metastasis. Arch Med Res. 2009;40:321‐323. [DOI] [PubMed] [Google Scholar]
- 138. Kim S, Takahashi H, Lin W‐W et al Carcinoma‐produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hope C, Emmerich PB, Papadas A et al Versican‐derived matrikines regulate Batf3‐dendritic cell differentiation and promote T cell infiltration in colorectal cancer. J Immunol. 2017;199:1933‐1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. McMahon M, Ye S, Izzard L et al ADAMTS5 is a critical regulator of virus‐specific T cell immunity. PLoS Biol. 2016;14:e1002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Haraguchi N, Ohara N, Koseki J et al High expression of ADAMTS5 is a potent marker for lymphatic invasion and lymph node metastasis in colorectal cancer. Mol Clin Oncol. 2017;6:130‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kim Y‐H, Lee HC, Kim S‐Y et al Epigenomic analysis of aberrantly methylated genes in colorectal cancer identifies genes commonly affected by epigenetic alterations. Ann Surg Oncol. 2011;18:2338‐2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Roman‐Gomez J, Jimenez‐Velasco A, Agirre X, Prosper F, Heiniger A, Torres A. Lack of CpG island methylator phenotype defines a clinical subtype of T‐cell acute lymphoblastic leukemia associated with good prognosis. J Clin Oncol. 2005;23:7043‐7049. [DOI] [PubMed] [Google Scholar]
- 144. Porter S, Scott SD, Sassoon EM et al Dysregulated expression of adamalysin‐thrombospondin genes in human breast carcinoma. Clin. Cancer Res. 2004;10:2429‐2440. [DOI] [PubMed] [Google Scholar]
- 145. Cross NA, Chandrasekharan S, Jokonya N et al The expression and regulation of ADAMTS‐1, ‐4, ‐5, ‐9, and ‐15, and TIMP‐3 by TGFbeta1 in prostate cells: relevance to the accumulation of versican. Prostate. 2005;63:269‐275. [DOI] [PubMed] [Google Scholar]
- 146. Demircan K, Gunduz E, Gunduz M et al Increased mRNA expression of ADAMTS metalloproteinases in metastatic foci of head and neck cancer. Head Neck. 2009;31:793‐801. [DOI] [PubMed] [Google Scholar]
- 147. Stokes A, Joutsa J, Ala‐aho R et al Expression profiles and clinical correlations of degradome components in the tumor microenvironment of head and neck squamous cell carcinoma. Clin Cancer Res. 2010;16:2022‐2035. [DOI] [PubMed] [Google Scholar]
- 148. Li C, Xiong Y, Yang X et al Lost expression of ADAMTS5 protein associates with progression and poor prognosis of hepatocellular carcinoma. Drug Des Dev Ther. 2015;9:1773‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sharghi‐Namini S, Fan H, Sulochana KN et al The first but not the second thrombospondin type 1 repeat of ADAMTS5 functions as an angiogenesis inhibitor. Biochem Biophys Res Commun. 2008;371:215‐219. [DOI] [PubMed] [Google Scholar]
- 150. Kumar S, Sharghi‐Namini S, Rao N, Ge R. ADAMTS5 functions as an anti‐angiogenic and anti‐tumorigenic protein independent of its proteoglycanase activity. Am J Pathol. 2012;181:1056‐1068. [DOI] [PubMed] [Google Scholar]
- 151. Renganathan B, Durairaj V, Kirman D, Esubonteng P, Ang S, Ge R. Recombinant TSR1 of ADAMTS5 suppresses melanoma growth in mice via an anti‐angiogenic mechanism. Cancers (Basel). 2018;10:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nakada M, Miyamori H, Kita D et al Human glioblastomas overexpress ADAMTS‐5 that degrades brevican. Acta Neuropathol. 2005;110:239‐246. [DOI] [PubMed] [Google Scholar]
- 153. Held‐Feindt J, Paredes EB, Blömer U et al Matrix‐degrading proteases ADAMTS4 and ADAMTS5 (disintegrins and metalloproteinases with thrombospondin motifs 4 and 5) are expressed in human glioblastomas. Int J Cancer. 2006;118:55‐61. [DOI] [PubMed] [Google Scholar]
- 154. Viapiano MS, Hockfield S, Matthews RT. BEHAB/brevican requires ADAMTS mediated proteolytic cleavage to promote glioma invasion. J Neurooncol. 2008;88:261‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Filou S, Stylianou M, Triantaphyllidou IE et al Expression and distribution of aggrecanases in human larynx: ADAMTS‐5/aggrecanase‐2 is the main aggrecanase in laryngeal carcinoma. Biochimie. 2013;95:725‐734. [DOI] [PubMed] [Google Scholar]
- 156. Skandalis SS, Theocharis AD, Theocharis DA et al Matrix proteoglycans are markedly affected in advanced laryngeal squamous cell carcinoma. Biochim Biophys Acta. 2004;1689:152‐161. [DOI] [PubMed] [Google Scholar]
- 157. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146‐156. [DOI] [PubMed] [Google Scholar]
- 158. Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399‐411. [DOI] [PubMed] [Google Scholar]
- 159. Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin‐C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res. 2003;71:427‐444. [DOI] [PubMed] [Google Scholar]
- 160. Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci. 2002;22:2225‐2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lemons ML, Sandy JD, Anderson DK, Howland DR. Intact aggrecan and fragments generated by both aggrecanase and metalloproteinase‐like activities are present in the developing and adult rat spinal cord and their relative abundance is altered by injury. J Neurosci. 2001;21:4772‐4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Demircan K, Yonezawa T, Takigawa T et al ADAMTS1, ADAMTS5, ADAMTS9 and aggrecanase‐generated proteoglycan fragments are induced following spinal cord injury in mouse. Neurosci Lett. 2013;544:25‐30. [DOI] [PubMed] [Google Scholar]
- 163. Hamel MG, Ajmo JM, Leonardo CC, Zuo F, Sandy JD, Gottschall PE. Multimodal signaling by the ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs) promotes neurite extension. Exp Neurol. 2008;210:428‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Demircan K, Topcu V, Takigawa T et al ADAMTS4 and ADAMTS5 knockout mice are protected from versican but not aggrecan or brevican proteolysis during spinal cord injury. Biomed Res Int. 2014;2014:693746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Krstic D, Rodriguez M, Knuesel I. Regulated proteolytic processing of Reelin through interplay of tissue plasminogen activator (tPA), ADAMTS‐4, ADAMTS‐5, and their modulators. PLoS ONE. 2012;7:e47793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Stupka N, Kintakas C, White JD et al Versican processing by a disintegrin‐like and metalloproteinase domain with thrombospondin‐1 repeats proteinases‐5 and ‐15 facilitates myoblast fusion. J Biol Chem. 2013;288:1907‐1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Rajasekaran S, Kanna RM, Senthil N et al Genetic susceptibility of lumbar degenerative disc disease in young Indian adults. Eur Spine J. 2015;24:1969‐1975. [DOI] [PubMed] [Google Scholar]
- 168. Seki S, Asanuma‐Abe Y, Masuda K et al Effect of small interference RNA (siRNA) for ADAMTS5 on intervertebral disc degeneration in the rabbit anular needle‐puncture model. Arthritis Res Ther. 2009;11:R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387‐2392. [DOI] [PubMed] [Google Scholar]
- 170. Shay G, Lynch CC, Fingleton B. Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44–46:200‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Santamaria S, de Groot R. Monoclonal antibodies against metzincin targets. Br J Pharmacol. 2019;176:52‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Clement‐Lacroix P, Little C, Meurisse S et al GLPG1972: a potent, selective, orally available ADAMTS‐5 inhibitor for the treatment of OA. Osteoarthritis Cartilage. 2017;25:S58‐S59. [Google Scholar]
- 173. Clement‐Lacroix P, Meurisse S, Lepescheux L et al ADAMTS‐5 inhibition with the potent and highly selective inhibitor GLPG1972 results in strong disease modifying OA drug effects in the rat meniscectomy model. Osteoarthritis Cartilage. 2018;26:S26. [Google Scholar]
- 174. van der Aar EM, Desrivot J, Fagard L et al ADAMTS‐5 inhibitor GLPG1972, a potential new treatment in osteoarthritis, shows favorable safety, pharmacokinetics and pharmacodynamics in healthy subjects. Osteoarthritis Cartilage. 2018;26:S310. [Google Scholar]
- 175. Brenneis C, Serruys B, Van Belle T et al Structural and symptomatic benefit of a half‐live extended, systemically applied anti‐ADAMTS‐5 inhibitor (M6495). Osteoarthritis Cartilage. 2018;26:S299‐S300. [Google Scholar]
- 176. Pereira J, Ottevaere I, Serruys B et al Pharmacokinetic an pharmacodynamic modelling of the novel anti‐ADAMTS‐5 nanobody M6495 using the neoepitope ARGS as a biomarker. Osteoarthritis Cartilage. 2018;26:S176. [Google Scholar]
- 177. Wesolowski J, Alzogaray V, Reyelt J et al Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med Microbiol Immunol. 2009;198:157‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Werkmann D, Buyse M‐A, Dejager L et al In vitro characterization of the ADAMTS‐5 specific nanobody® M6495. Osteoarthritis Cartilage. 2018;26:S178. [Google Scholar]
- 179. Miller RE, Ishihara S, Tran PB et al An aggrecan fragment drives osteoarthritis pain through Toll‐like receptor 2. JCI Insight. 2018;3:e95704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Akiyama M, Takeda S, Kokame K, Takagi J, Miyata T. Crystal structures of the noncatalytic domains of ADAMTS13 reveal multiple discontinuous exosites for von Willebrand factor. Proc Natl Acad Sci USA. 2009;106:19274‐19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Petri A, Kim HJ, Xu Y et al Crystal structure and substrate‐induced activation of ADAMTS13. Nat Commun. 2019;10:3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Tortorella MD, Liu R‐Q, Burn T, Newton RC, Arner E. Characterization of human aggrecanase 2 (ADAMTS5): substrate specificity studies and comparison with aggrecanase 1 (ADAM‐TS4). Matrix Biol. 2002;21:499‐511. [DOI] [PubMed] [Google Scholar]
- 183. Tortorella MD, Arner EC, Hills R et al Alpha2‐macroglobulin is a novel substrate for ADAMTS‐4 and ADAMTS‐5 and represents an endogenous inhibitor of these enzymes. J Biol Chem. 2004;279:17554‐17561. [DOI] [PubMed] [Google Scholar]
- 184. Melching LI, Fisher WD, Lee ER, Mort JS, Roughley PJ. The cleavage of biglycan by aggrecanases. Osteoarthritis Cartilage. 2006;14:1147‐1154. [DOI] [PubMed] [Google Scholar]
- 185. Zhen EY, Brittain IJ, Laska DA et al Characterization of metalloprotease cleavage products of human articular cartilage. Arthritis Rheum. 2008;58:2420‐2431. [DOI] [PubMed] [Google Scholar]
- 186. Kashiwagi M, Enghild JJ, Gendron C et al Altered proteolytic activities of ADAMTS‐4 expressed by C‐terminal processing. J Biol Chem. 2004;279:10109‐10119. [DOI] [PubMed] [Google Scholar]
- 187. Scavenius C, Poulsen EC, Thøgersen IB et al Matrix‐degrading protease ADAMTS‐5 cleaves inter‐α‐inhibitor and releases active heavy chain 2 in synovial fluids from arthritic patients. J Biol Chem. 2019;294:15495‐15504. [DOI] [PMC free article] [PubMed] [Google Scholar]


