Abstract
Tissue-specific immune regulation is an important component of the immune response relevant to many areas of immunology. The focus of this study is on tissue-specific mechanisms that contribute to autoimmune uveitis. Precise gene regulation is necessary for the proper expression of an inflammatory or regulatory response. This precision gene regulation can be accomplished by microRNA at the level of the mRNA transcript. miR-155, in particular, has a complicated role in the immune response with positive and negative inflammatory effects. In this work, we identify a decrease in miR-155 in suppressor macrophages and further examine how tissue-specific production of miR-155 impacts experimental autoimmune uveitis. Importantly, we show that eliminating miR-155 expression by the target tissue before initiation reduces disease severity, but elimination of miR-155 after the onset of inflammation does not alter the course of disease. Additionally, expression of miR-155 by the target tissue before initiation is necessary for the induction of regulatory immunity that protects from further autoimmune disease, but not after the onset of inflammation. In summary, we find a MC5r-dependent decrease in miR-155 in postexperimental autoimmune uveitis APC, miR-155 production by the target tissue is necessary for the initiation of autoimmune uveitis, and may have a role in establishing protective regulatory immunity.
Keywords: Autoimmunity, Experimental autoimmune uveitis, Macrophages, miR-155
Introduction
Small noncoding RNA or microRNA (miRNA) have been identified as regulatory elements that allow for an additional level of gene regulation by binding the complementary mRNA to either stabilize the transcript or aid in the degradation of the transcript [1, 2]. Roles for miRNA were initially discovered in cancer biology [3] and more recently, in physiological development and function, and in immunology [1, 4–9]. In particular, roles for miR-155 in cancer and immunology have been demonstrated [6, 10–14]. Interestingly, the immunological role of miR-155 can vary depending on the cell type. For example, expression of miR-155 in macrophages promotes an inflammatory state [15, 16]. In contrast, miR-155 has a role in the induction of Tregs to suppress inflammation [17–19]. As such, it is of interest to determine the roles of miR-155 in the initiation and after the onset of autoimmune disease.
Experimental autoimmune uveitis (EAU) is a widely used neuroinflammatory mouse model in which the uveoretina is the target of an inflammatory immune response [20]. This is clinically relevant because uveitis is a leading cause of blindness, with 25.6–122 new cases per 100 000 a year, and a prevalence of 69–623 cases per 100 000 [21–24]. While corticosteroids are an effective anti-inflammatory treatment that works well for acute uveitis, the myriad of undesirable side-effects make them unsuitable as a long-term treatment option [25–27]. Therefore, chronic uveitis patients are transitioned to immunosuppressive medications with the goal of sustained remission [28–33]. Unfortunately, not all the immunosuppressive medications are effective and recalcitrant uveitis cause patients to fail multiple treatment regimens. As such, a better understanding of the immunobiology of autoimmune uveitis is necessary to develop additional immunosuppressive treatment options for the treatment of autoimmune uveitis. The most widely used model of human autoimmune uveitis, EAU, is used to study the immunobiology of autoimmune uveitis. EAU can be divided into different phases, the onset of EAU is 2–4 weeks after immunization, followed by a chronic phase for 1–2 months, and resolution spontaneously occurs without relapse at 2–3 months after immunization [20, 34, 35]. When EAU resolves (post-EAU), regulatory immunity emerges in the spleen [36] and provides resistance to EAU during reimmunization and when adoptively transferred to mice that are immunized for EAU [36–39].
We have previously demonstrated that the melanocortin 5 receptor (MC5r), a receptor for the potent immunosuppressive neuropeptide, α-melanocyte stimulating hormone (α-MSH), is necessary for the emergence of suppressor macrophages in the spleen of post-EAU mice [37, 38, 40]. These post-EAU MC5r-dependent macrophages are important because they are necessary to activate post-EAU regulatory immunity. In addition to expression on macrophages, MC5r is expressed in retinal pigmented epithelial cells (RPE) [41]. Therefore, we asked if there is a role for miR-155 in MC5r-stimulated macrophages and the role that tissue-specific RPE production of miR-155 has on EAU. In this report, we show that miR-155 is downregulated in suppressor macrophages, and has a role in the initiation of EAU, but not in the progression of EAU. We further demonstrate that miR-155 has a role in the induction of post-EAU regulatory immunity during the initiation of EAU, but not after the onset of EAU.
Results
miRNA expression in α-MSH-treated macrophages
We have previously demonstrated that stimulation of MC5r on macrophages with the immunosuppressive neuropeptide, α-MSH, results in a suppressor macrophage that can activate regulatory T cells [39, 40, 42]. Therefore, to identify miRNA that may have an inflammatory role in macrophages, we asked what miRNAs were significantly downregulated in a suppressor macrophage population when treated with α-MSH. The top 10 miRNAs we focused on are shown in Table 1. These miRNA were first selected based on the condition that FDRq should be less than 0.25, p ≤ 0.01, and downregulated with α-MSH treatment. Because it has been demonstrated that the post-EAU suppressor APC expresses PD-L1, CD73, and CD39 [38, 40], we determined the first list of miRNA that target PD-L1, CD73, or CD39 miRNA shown in Table 2. We next focused on miRNA with a fold change of two or greater, leaving six miRNAs to focus on. While miR-17, miR-106, miR-222 were downregulated greater than twofold, miR-155 has been previously shown to be involved in the pathogenesis EAU [12, 43–45] and it is predicted to interact with CD39 and PD-L1 mRNA (Table 2). We, therefore, decided to focus on the role of miR-155 in EAU.
Table 1.
Top 10 downregulated transcripts from miRNA microarray
| Transcript ID | Fold change | p | Ly-6G α-MSH 1 | Ly-6G α-MSH 2 | Ly-6G α-MSH 3 | Ly-6G untreated 1 | Ly-6G untreated 2 | Ly-6G untreated 3 |
|---|---|---|---|---|---|---|---|---|
| mmu-miR-17–3p | −5.5 | 1.30 × 10−4 | 1.7 | 2.3 | 2.2 | 3.9 | 4.4 | 5.1 |
| mmu-miR-106b-5p | −4.1 | 2.50 × 10−5 | 3.9 | 3.6 | 4.1 | 5.9 | 5.6 | 6.3 |
| mmu-miR-222–3p | −4.1 | 2.60 × 10−3 | 1.5 | 2.5 | 1.6 | 3.6 | 3.2 | 4.7 |
| mmu-miR-106a-5p | −3.7 | 1.60 × 10−3 | 3.2 | 2.8 | 2.2 | 4.7 | 3.9 | 5.2 |
| mmu-miR-17–5p | −2.9 | 2.30 × 10−5 | 5.3 | 5.3 | 5.5 | 7 | 6.7 | 7.1 |
| mmu-miR-155–5p | −2 | 7.00 × 10−2 | 4.7 | 5.8 | 2.9 | 6 | 5.7 | 5.8 |
| mmu-miR-296–3p | −1.6 | 1.50 × 10−2 | 1.7 | 2.2 | 1.4 | 2.7 | 2.5 | 2.3 |
| mmu-miR-485–3p | −1.6 | 8.80 × 10−2 | 2.6 | 1.6 | 2.4 | 2.7 | 3.4 | 2.5 |
| mmu-miR-16–5p | −1.5 | 2.10 × 10−2 | 8.1 | 8.3 | 7.9 | 8.7 | 8.3 | 9.1 |
| mmu-miR-181a-5p | −1.5 | 5.00 × 10−3 | 6.8 | 6.6 | 6.6 | 7.1 | 7.2 | 7.4 |
The fold change and p value are shown for each miRNA, FDRq values are less than 0.25 for all of the miRNA transcripts shown.
Table 2.
Predicted mRNA target transcripts of selected miRNA
| Transcript ID | Fold change | Predicted target: PD-L1 | Predicted PD-L1 Alignment | PD-L1 alignment score | Predicted target: CD39 | Predicted CD39 alignment | CD39 alignment score | Predicted target: CD73 | Predicted CD73 alignment | CD73 alignment score |
|---|---|---|---|---|---|---|---|---|---|---|
| mmu-miR-17–3p | −5.5 | X | ||||||||
| mmu-miR-106b-5p | −4.1 | X | :|| |:| ||::||||||| | 153 | ||||||
| mmu-miR-222–3p | −4.1 | X | | |:||||||||| | 158 | ||||||
| mmu-miR-106a-5p | −3.7 | X | :|| |:| || |:||||||| | 157 | ||||||
| mmu-miR-17–5p | −2.9 | X | ||||||||
| mmu-miR-155–5p | −2.0 | X | || |||::| |||||||| | 156 | X | ||| |: || ||||| || | 123 | |||
| mmu-miR-296–3p | −1.6 | X | ||||||| | 140 | X | |||| ||||| || | 121 | |||
| mmu-miR-485–3p | −1.6 | X | | |:| |: |||||||| | 152 | X | |||::|:: |||||||||| | 124 | |||
| mmu-miR-16–5p | −1.5 | X | :||| |:|||| |||| | 125 | X | || | |:: ||||||| | 141 | |||
| mmu-miR-181a-5p | −1.5 | X | |||: |::||||||| || | 125 | X | |||||||| | 145 |
The predicted target transcripts were indicated on www.microrna.org, fold change and p value are shown for each miRNA, FDRq values are less than 0.25 for all of the miRNA transcripts shown. The predicted alignment is shown, the line indicates complimentary base pairing and the alignment score is shown in the next column.
miR-155 Expression in post-EAU macrophages
We have previously demonstrated that deletion of the MC5r does not change the course of EAU [37, 40], and the severity of disease is not significantly different compared to MC5r-sufficient mice (Supporting Information Fig. 1), but is required for the emergence of a post-EAU suppressor macrophage [37, 40]. Therefore, we next determined the miR-155 expression in post-EAU suppressor macrophages from the spleen of WT and MC5r(−/−) mice to confirm the in vitro expression is similar with in vivo expression. The post-EAU suppressor macrophage is dependent on expression of MC5r and has been previously identified as CD11b+F4/80+Ly-6CloLy-6G+ [38], so we sorted the spleen of post-EAU mice and unimmunized age-matched mice. The fold change in post-EAU suppressor macrophages from the spleen relative to the same population from the spleen of unimmunized was determined (Fig. 1). We observed a greater than threefold downregulation of miR-155 in WT mice and a greater than twofold upregulation of miR-155 in MC5r(−/−) mice. These observations confirm the microarray miRNA analysis and demonstrate a role for miR-155 in macrophages during EAU.
Figure 1.

Expression of miR-155 in post-EAU CD11b+ F4/80+ Ly-6G+ Ly-6Clo cells. Spleen cells were collected from post-EAU C57BL/6J and post-EAU MC5r( −/−) mice and sorted for CD11b+ F4/80+ Ly-6G+ Ly-6Clo cells. Total RNA was collected and miR-155 was measured by RT-PCR, normalized to housekeeping genes, and the fold regulation of post-EAU to unimmunized was calculated as shown. Results shown are obtained from three independent experiments consisting of one to three mice per experiment. Statistical significance is designated by * when p ≤ 0.05 determined by nonparametric Mann-Whitney U test.
Effect of miR-155 production by retinal pigmented epithelium on EAU
It has been demonstrated that miR-155 functions to promote EAU using a global knock-out mouse [12]. Because microRNAs have an important role in the retina and specifically the RPE [46, 47], we asked if tissue-specific expression of miR-155 is necessary for EAU. A doxycycline inducible RPE-specific Cre (RPE/rtTA) expressing mouse that has been extensively characterized in multiple models [48–50] was crossed with a floxed miR-155 (miR-155fl/fl) mouse to create an inducible RPE-specific miR-155 knock-out. Specific excision of miR-155 in the RPE was confirmed (Supporting Information Fig. 2). Mice carrying the doxycycline inducible RPE-Cre and floxed miR-155 (RPE/rtTA; miR-155fl/fl) were fed doxycycline containing chow for 1 week prior to immunization for EAU. Control mice were also put on the same feeding schedule but were WT mice. The course of EAU in RPE/rtTA; miR-155fl/fl doxycycline-fed mice showed significantly earlier resolution (Fig. 2A). The severity of disease was also determined with the maximum EAU score for each mouse over the entire course of disease, and was significantly reduced in the RPE/rtTA; miR-155fl/fl doxycycline-fed mice compared to doxycycline-fed WT mice (Fig. 2B). EAU was not significantly different in miR-155fl/fl mice not fed doxycycline compared with WT doxycycline-fed mice (Supporting Information Fig. 3). These observations show that a RPE-specific deletion of miR-155 provides resistance to EAU.
Figure 2.
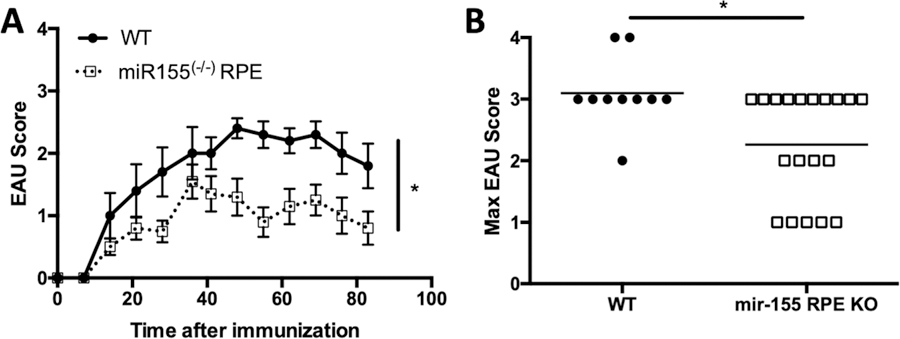
Effect of eliminating miR-155 production by RPE before EAU induction. Mice with flox sites at the miR-155 locus and a doxycycline inducible RPE-specific Cre (RPE/rtTA; mir-155fl/fl) were fed doxycycline before immunization for EAU. Mice were monitored for clinical signs of retinal inflammation one to two times per week. The course of EAU with the average scores ± SEM is shown (A). The closed circle and solid line represent the scores of WT mice (n = 10) and the open square and dashed line represent scores of RPE/rtTA; mir-155fl/fl mice (n = 10). The highest clinical score for each mouse over the entire course of disease was also determined and is shown with the line representing the mean for each group (B). Each experiment consisted of four to five mice per group, and was repeated three times. Statistical significance is designated by * when p ≤ 0.05 determined by two-way ANOVA for the course of disease and nonparametric Mann-Whitney U test for the maximum EAU scores.
Role of miR-155 production by RPE during EAU
We next asked if miR-155 production by the RPE was important for the progression of EAU. The RPE/rtTA;miR-155fl/fl mice were immunized for EAU and were given doxycycline between the onset and peak of retinal inflammation (day 28 after immunization). We observed no significant change in the course of disease or the severity of EAU (Fig. 3). Importantly, there was also no delay in the resolution of EAU. These observations demonstrate that miR-155 production by the RPE is not necessary for the progression or resolution of EAU.
Figure 3.
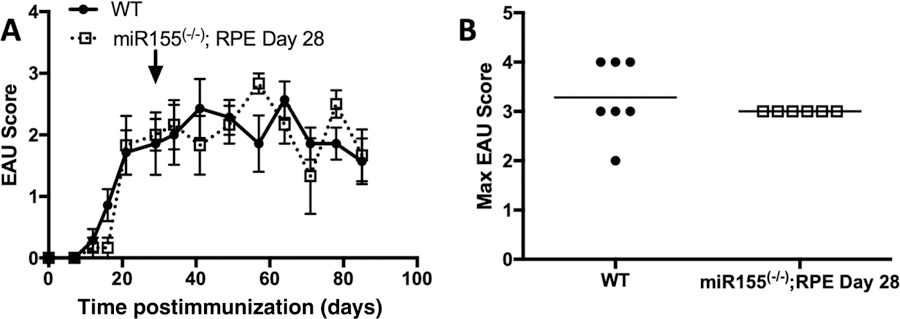
Effect of eliminating miR-155 production by RPE between the onset and peak of EAU. Mice with flox sites at the miR-155 locus and a doxycycline inducible RPE-specific Cre (RPE/rtTA; mir-155fl/fl) were fed doxycycline at the day 28 of EAU (indicated by arrow). Mice were monitored for clinical signs of retinal inflammation one to two times per week. The course of EAU with the average scores ± SEM is shown (A). The closed circle and solid line represent the scores of WT mice (n = 7) and the open square and dashed line represent scores of RPE/rtTA; mir-155fl/fl mice (n = 6). The highest clinical score for each mouse over the entire course of disease was also determined and is shown with the line representing the mean for each group (B). Each experiment consisted of three to five mice per group, and was repeated two times. Statistical significance is designated by * when p ≤ 0.05 determined by two-way ANOVA for the course of disease and nonparametric Mann-Whitney U test for the maximum EAU scores.
Effect of miR-155 production by RPE on the induction of post-EAU regulatory immunity
The role of miR-155 is generally proinflammatory, but the role in Treg cells is more ambiguous with evidence that it is involved in Treg development, but not in function [17, 18]. Because post-EAU Treg cells are found in the spleen of EAU-recovered mice, we asked if miR-155 production by the RPE is necessary for emergence of post-EAU Treg cells in the spleen. The spleen from post-EAU RPE/rtTA;miR-155fl/fl doxycycline-fed mice was collected and splenocytes were restimulated with interphotoreceptor retinoid binding protein (IRBP) in vitro, as we have done before [37–40]. The restimulated splenocytes were transferred to recipient mice immunized for EAU and the fundus was monitored for signs of inflammation. We compared the EAU scores of mice that received splenocytes from mice 90 days after immunization with CFA without IRBP with EAU mice that received no transfer of cells and found no significant difference (Supporting Information Fig. 4A). While the course of disease appears lower and the maximum severity trends lower, the differences compared to EAU mice that did not receive an adoptive transfer were not statistically significant (Fig. 4). These observations demonstrate that miR-155 production by the RPE at the initiation of EAU is necessary to promote the generation of post-EAU regulatory immunity in the spleen.
Figure 4.
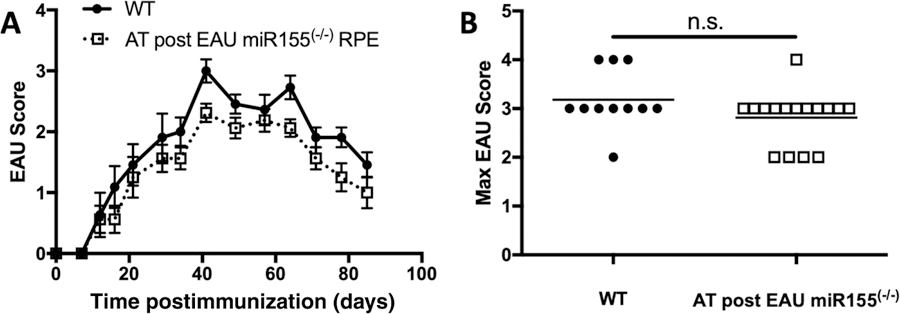
Effect of eliminating miR-155 production by RPE before EAU initiation on induction of post-EAU regulatory immunity. Spleen cells were collected from post-EAU mice with flox sites at the miR-155 locus and a doxycycline inducible RPE-specific Cre (RPE/rtTA; mir-155fl/fl) that were fed doxycycline before EAU. Spleen cells were reactivated with IRBP and transferred to recipient mice immunized for EAU. Recipient mice were monitored for clinical signs of retinal inflammation one to two times per week. The course of EAU with the average scores ± SEM is shown (A). The closed circle and solid line represent the scores of mice that received no adoptive transfer (n = 10) and the open square and dashed line represent scores of recipient mice (n = 16). The highest clinical score for each mouse over the entire course of disease was also determined and is shown with the line representing the mean for each group (B). Each experiment consisted of four to seven mice per group, and was repeated three to four times. No statistical significance was observed as determined by two-way ANOVA for the course of disease and nonparametric Mann-Whitney U test for the maximum EAU scores.
Temporal effect of miR-155 production by RPE on the induction of post-EAU regulatory immunity
Since the role of miR-155 has been demonstrated to be involved in the induction of Treg cells [17, 18], we asked if production of miR-155 by the RPE after the onset of EAU has an impact on the generation of regulatory immunity in the spleen of mice that have recovered from EAU. This is of particular interest because it has been demonstrated in the past that resolution of EAU is independent from the generation of post-EAU Tregs in the spleen [36]. RPE/rtTA;miR-155fl/fl mice were given doxycycline at day 28 of EAU and monitored until resolution. At resolution of EAU, the spleen from the EAU-recovered RPE/rtTA;miR-155fl/fl mice were collected, reactivated in vitro, and transferred to recipient mice immunized for EAU. Mice that received post-EAU RPE/rtTA;miR-155fl/fl, spleen cells had significantly lower EAU scores and accelerated resolution of disease compared to mice that did not receive an adoptive transfer (Fig. 5) and was also significantly lower compared to EAU mice that received a transfer of cells from splenocytes from mice 90 days after immunization with CFA without IRBP (Supporting Information Fig. 4B). This demonstrates that miR-155 production by the RPE is not necessary for the generation of post-EAU regulatory immunity once EAU has started.
Figure 5.

Effect of eliminating miR-155 production by RPE between the onset and peak of EAU on induction of post-EAU regulatory immunity. Spleen cells were collected from post-EAU mice with flox sites at the miR-155 locus and a doxycycline inducible RPE-specific Cre (RPE/rtTA; mir-155fl/fl) that were fed doxycycline at day 28 of EAU as in Figure 3. Spleen cells were reactivated with IRBP and transferred to recipient mice immunized for EAU. Recipient mice were monitored for clinical signs of retinal inflammation one to two times per week. The course of EAU with the average scores ± SEM is shown (A). The closed circle and solid line represent the scores of mice that received no adoptive transfer (n = 11) and the open square and dashed line represent scores of recipient mice (n = 10). The highest clinical score for each mouse over the entire course of disease was also determined and is shown with the line representing the mean for each group (B). Each experiment consisted of two to five mice per group, and was repeated three times. Statistical significance is designated by * when p ≤ 0.05 determined by two-way ANOVA for the course of disease and nonparametric Mann-Whitney U test for the maximum EAU scores.
Discussion
Our observations that deletion of miR-155 in the RPE impacts the course of EAU indicates that production by the RPE is necessary for the initiation of tissue-specific inflammatory disease. Because miR-155 in macrophages is proinflammatory, a potential explanation is that the miR-155 produced by the RPE at the initiation of EAU is promoting the inflammation by promoting an inflammatory macrophage. However, because eliminating miR-155 in the RPE after the onset of inflammation had no impact on inflammation and because the progression of an inflammatory disease involves additional mechanisms that extend past antigen presentation by macrophages, these observations demonstrate that miR-155 is not necessary for progression of disease from initiation of inflammation to the chronic phase.
There are conflicting reports regarding the role of miR-155 in uveitis. In the rat model of autoimmune anterior uveitis, miR-155 decreases in the iris and ciliary body at the peak of disease, but increases in leukocytes at the peak [45]. In contrast, STAT3 activates miR-155 to promote EAU by activating Th17 cells [12]. In human studies, upregulation of miR-155 has been observed in PBMCs from Behcet’s disease (BD) patients [44] and in BD patients with active disease [10]. However, an earlier report showed a decrease in miR-155 in PBMCs from active BD patients [43]. The immunological role of miR-155 in T cells is the expansion of Th1, Th17, Th2, and Tregs [6, 11, 17, 18, 51], and functions in macrophages and DCs to promote an inflammatory state [7, 16]. As such our observations demonstrate an inflammatory role for miR-155, and additionally show that the tissue-specific expression of miR-155 is also timing specific in the progression of autoimmune disease.
We further show that the generation of systemic regulatory immunity that emerges following the resolution of EAU does not emerge if miR-155 is not produced by the RPE at the initiation of disease. However, emergence of this regulatory immunity does not depend on miR-155 production after the onset of disease. This is important because the absence of this regulatory immunity may contribute to chronic, relapsing autoimmune disease. However, it should be noted that the lack of post-EAU regulatory immunity when the miR-155 production by the RPE was blocked before the initiation of inflammation may be due to the absence of severe EAU and not necessarily because of a specific absence of miR-155 production by the RPE.
The observation that miR-155 production by the RPE is also interesting because it demonstrates the ability of the tissue to modulate the immune response. Furthermore, this tissue-specific modulation of the immune response occurs through production of miR-155 by the RPE. This could be relevant to other retinal diseases with an inflammatory component such as diabetic retinopathy and age-related macular degeneration. As such, retinal neovascularization is attenuated by miR-155 through the PI3K/Akt pathway [52]. Therefore, targeting miR-155 could have potential therapeutic benefit to these other diseases, as well.
It should be noted that there are potential drawbacks to the EAU model. Because it is a posterior autoimmune model, it does not represent that majority of uveitis cases. Infectious uveitis accounts for the majority of cases with geography as a factor [53]. Among autoimmune uveitis cases, anterior idiopathic uveitis accounts for the majority of cases [53]. While there are some susceptibility loci, such as HLA-B27, HLA-B51, and HLA-B5 [54–56], there is also likely another environmental trigger as well. While autoimmune uveitis can manifest with the eye as the only target, it can be the target in addition to other organs such as the joints in ankylosing spondylitis, the gut in colitis, and brain as in MS [57, 58]. Because not all autoimmune uveitis patients respond to the same therapy, even if it is the same type, this suggests that there are multiple etiologies. This is supported with our observation that not all the mice are fully protected from EAU. The EAU model is limited in that it is posterior uveitis whereas clinical uveitis includes anterior, intermediate, scleritis, and conjunctival involvement. However, the most devastating type of uveitis is posterior uveitis. While these observations indicate that miR-155 has a role in the initiation of EAU, it is likely that it is only part of a larger pathway that is not completely understood. Additional factors that are involved in the initiation of EAU include the induction of Th1 and Th17 cells that are specific for retinal antigen [59], and this response is mediated through induction of other factors such as TNF-α and IL-6. Others have reported that miR-155 promotes EAU by augmenting the Th17 response through STAT3 induction [12]. However, because these observations indicate that miR-155 production by the RPE is involved in the initiation of EAU, neutralizing miR-155 with a complementary ssDNA or RNA could be used as a localized therapeutic if a sufficient delivery system is designed to deliver it directly to the eye. Phase 1 clinical trials are underway to test cobomarsen (MRG-106), a miR-155 inhibitor, that is sponsored by miRagen Therapeutics, Inc. (Boulder, CO) in patients diagnosed with lymphoma or leukemia (ClinicalTrials.gov Identifier: NCT02580552).
Materials and methods
Mice
The University of Oklahoma Health Sciences Center Institutional Animal Care and Use Committee (OUHSC IACUC) approved of all mouse procedures described in this study and were carried out in accordance with the relevant guidelines approved by the OUHSC IACUC. C57BL/6J mice and miR-155fl/fl mice with a floxed exon 2 that encodes the hairpin region of the miRNA (026700) were purchased from Jackson Laboratories. Mice containing a doxycycline inducible RPE-specific (human 2.9-kb VMD2 promoter associated with Best disease) Cre (RPE/rtTA) was a generous gift from Dr. Yun-Zheng Le. Cre expression in these mice was demonstrated to be specific to the RPE in multiple models [48–50]. We further confirmed that excision of miR-155 was specific for the RPE (Supporting Information Fig. 2). MC5r(−/−) mice on a C57BL/6J background were obtained from Roger D. Cone (Oregon Health Sciences, Portland, Oregon).
RNA assays
In vitro cultured spleen cells with and without α-MSH were enriched for CD11b+ cells and then sorted for Ly-6G+ Ly-6Clo cells into Trizol. Sorted cells were then sent to the Boston University Clinical and Translational Science Institute where the RNA was extracted and analyzed using Affymetrix GeneChip miRNA 4.0 arrays. All samples had similar quality metrics, including mean Relative Log Expression and percent Present calls (%P). All arrays were normalized together using Affymetrix Expression Console (version 1.3.0.187) using RMA normalization and Detection Above BackGround (DABG). Normalization was performed only across mouse and control probe sets. The expression values are log2-transformed by default. Each gene was also assigned a Present or Absent call in each sample, denoting whether its expression was significantly higher than that of a collection of negative control probes.
Post-EAU spleen cells were sorted as described above from WT and MC5r(−/−) mice. The sorted samples were sent to Anna Trivett at the National Cancer Institute for quantification of miR-155. The miR-155 primers from Qiagen were used (MS00001701 Mm miR-155 1 miScript primer) and SNORD-68, 73, 95 were used as housekeeping genes for normalization.
Experimental autoimmune uveoretinitis
EAU was induced in mice as previously described [39]. Briefly, CFA was emulsified with 5 mg/mL desiccated Myobacterium tuberculosis (Difco Laboratories, Detroit, MI) and 2 mg/mL IRBP (peptides 1–20) (Genscript, Piscataway, NJ) was used to immunize mice for EAU. Mice received a volume of 100 μL of the emulsion injected subcutaneously at two sites in the lower back followed by an intraperitoneal injection of 0.3 μg pertussis toxin. Retinal inflammation during the course of EAU was evaluated every 3–4 days by fundus examination using a slit lamp microscope. Before examining the retina, the iris was dilated with 1% tropicamide, and the cornea was flattened with a glass coverslip to examine the retina. The clinical signs of observable infiltration and vasculitis in the retina were scored on a 5-point scale, as previously described [60]. Both eyes were scored and the higher score was used to represent the mouse for that day, the average score for the group of mice was then calculated. When maximum scores were calculated, the maximum score for each mouse over the entire course of disease is shown.
Doxycycline administration
Doxycycline containing chow was purchased from Bio-Serv (Flemington, NJ). The doxycycline concentration was 200 mg/kg and mice were fed doxycycline for 1 week, then switched back to the regular chow for the remainder of the experiment.
In vitro α-MSH treatment
The spleens from mice that recovered from EAU (day 85–90 after immunization) or unimmunized mice were collected into 5% FBS in RPMI supplemented with 10 μg/mL Gentamycin (Sigma, St. Louis, MI), 10 mM HEPES, 1 mM sodium pyruvate (BioWhittaker, Basel, Switzerland), nonessential amino acids 0.2% (BioWhit-taker). Spleen cells were made into a single cell suspension that was subsequently depleted of RBCs with RBC lysis buffer (Sigma, St Louis, MO). The RBC-free spleen cells were then separated to obtain adherent APC that were then cultured in serum-free media (SFM) with 1 ng/mL α-MSH for 48 h at 37°C and 5% CO2. SFM consisted of RPMI-1640 with 1% ITS+1 solution (Sigma) and 0.1% BSA (Sigma). The cultured APC were then stained and sorted to obtain a pure population of suppressor APC.
Adoptive transfer experiments
Spleen cells from post-EAU mice were collected, made into a single cell suspension, RBC depleted, and reactivated in vitro in SFM with 50 μg/mL IRBP 48 h at 37°C and 5% CO2. Following the reactivation, cells were collected and 1 × 106 cells were adoptively transferred to recipient mice intravenously at the time of immunization for EAU.
Cell sorting
Mouse spleen cells were washed with PBS with 1% BSA (staining buffer), blocked with mouse IgG in staining buffer, then stained with conjugated antibodies. Antibodies used were anti-CD11b (clone M1/70, Biolegend, San Diego, CA), anti-Ly-6C (clone HK1.4, Biolegend), and anti-Ly-6G (clone 1A8, Biolegend). Cells were enriched using a mouse CD11b positive selection kit (StemCell Technologies, Vancouver, BC, Canada), then sorted in the BU Flow Cytometry Core.
Statistics
Statistical significance between EAU scores was determined using nonparametric Mann-Whitney U test between groups of mice. Two-way ANOVA was also used to assess significant differences in the course of disease between the groups of treated EAU mice. Statistical significance was determined when p ≤ 0.05.
Supplementary Material
Acknowledgments:
We would like to thank the Oklahoma Medical Research Foundation Core facility. This work was supported by National Institutes of Health/National Eye Institute grants EY021725, EY024951 (DJL), and in part by an unrestricted Research to Prevent Blindness grant (New York, NY). We would like to thank Andrew W. Taylor and the Boston University Clinical Translational Sciences Institute. Dr. Yun-Zheng Le generously provided the RPE/rtTA mice used to generate the RPE/rtTA; miR-155fl/fl strain.
Abbreviations:
- α-MSH
α-melanocyte stimulating hormone
- BD
Behcet’s disease
- EAU
experimental autoimmune uveitis
- IRBP
interphotoreceptor retinoid binding protein
- MiRNA
microRNA
- MC5r
melanocortin 5 receptor
- RPE
retinal pigmented epithelial cells
- SFM
serum-free media
Footnotes
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Conflict of Interest: The authors declare no commercial or financial conflict of interest.
References
- 1.Catalanotto C, Cogoni C and Zardo G, MicroRNA in control of gene expression: an overview of nuclear functions. Int. J. Mol. Sci 2016. 17: pii: E1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valinezhad Orang A, Safaralizadeh R and Kazemzadeh-Bavili M, Mechanisms of miRNA-mediated gene regulation from common down-regulation to mRNA-specific upregulation. Int. J. Genomics 2014. 2014: 970607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berabez N, Durand S and Gabut M, Post-transcriptional regulations of cancer stem cell homeostasis. Curr. Opin. Oncol 2019. 31: 100–107. [DOI] [PubMed] [Google Scholar]
- 4.Singh RP, Massachi I, Manickavel S, Singh S, Rao NP, Hasan S, Mc Curdy DK et al. , The role of miRNA in inflammation and autoimmunity. Autoimmun. Rev 2013. 12: 1160–1165. [DOI] [PubMed] [Google Scholar]
- 5.Qu Z, Li W and Fu B, MicroRNAs in autoimmune diseases. Biomed Res Int 2014. 2014: 527895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME et al. , MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T-cell development. Immunity 2010. 33: 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garo LP and Murugaiyan G, Contribution of microRNAs to autoimmune diseases. Cell. Mol. Life Sci 2016. 73: 2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung J, Miscianinov V, Sluimer JC, Newby DE and Baker AH, Targeting non-coding RNA in vascular biology and disease. Front. Physiol 2018. 9: 1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner AMJ, MicroRNAs and the neural crest: from induction to differentiation. Mech. Dev 2018. 154: 98–106. [DOI] [PubMed] [Google Scholar]
- 10.Na SY, Park MJ, Park S and Lee ES, MicroRNA-155 regulates the Th17 immune response by targeting Ets-1 in Behcet’s disease. Clin. Exp. Rheumatol 2016. 34: S56–S63. [PubMed] [Google Scholar]
- 11.Leng RX, Pan HF, Qin WZ, Chen GM and Ye DQ, Role of microRNA-155 in autoimmunity. Cytokine Growth Factor Rev 2011. 22: 141–147. [DOI] [PubMed] [Google Scholar]
- 12.Escobar T, Yu CR, Muljo SA and Egwuagu CE, STAT3 activates miR-155 in Th17 cells and acts in concert to promote experimental autoimmune uveitis. Invest. Ophthalmol. Vis. Sci 2013. 54: 4017–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alivernini S, Gremese E, McSharry C, Tolusso B, Ferraccioli G, McInnes IB and Kurowska-Stolarska M, MicroRNA-155-at the critical interface of innate and adaptive immunity in arthritis. Front. Immunol 2017. 8: 1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaille JJ, Awad H, Fortman EC, Efanov AA and Tili E, miR-155 expression in anti-tumor immunity: the higher the better? Genes Chromosomes Cancer 2019. 58: 208–218. [DOI] [PubMed] [Google Scholar]
- 15.Eigsti RL, Sudan B, Wilson ME and Graff JW, Regulation of activation-associated microRNA accumulation rates during monocyte-to-macrophage differentiation. J. Biol. Chem 2014. 289: 28433–28447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Essandoh K, Li Y, Huo J and Fan GC, Mirna-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock 2016. 46: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohlhaas S, Garden OA, Scudamore C, Turner M, Okkenhaug K and Vigorito E, Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J. Immunol 2009. 182: 2578–2582. [DOI] [PubMed] [Google Scholar]
- 18.Spoerl D, Duroux-Richard I, Louis-Plence P and Jorgensen C, The role of miR-155 in regulatory T cells and rheumatoid arthritis. Clin. Immunol 2013. 148: 56–65. [DOI] [PubMed] [Google Scholar]
- 19.Shu Y, Hu Q, Long H, Chang C, Lu Q and Xiao R, Epigenetic variability of CD4+ CD25+ Tregs contributes to the pathogenesis of autoimmune diseases. Clin. Rev. Allergy Immunol 2017. 52: 260–272. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Qian H, Horai R, Chan CC and Caspi RR, Mouse models of experimental autoimmune uveitis: comparative analysis of adjuvant-induced vs spontaneous models of uveitis. Curr. Mol. Med 2015. 15: 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darrell RW, Wagener HP and Kurland LT, Epidemiology of uveitis. Incidence and prevalence in a small urban community. Arch. Ophthalmol 1962. 68: 502–514. [DOI] [PubMed] [Google Scholar]
- 22.Hwang DK, Chou YJ, Pu CY and Chou P, Epidemiology of uveitis among the Chinese population in Taiwan: a population-based study. Ophthalmology 2012. 119: 2371–2376. [DOI] [PubMed] [Google Scholar]
- 23.Suhler EB, Lloyd MJ, Choi D, Rosenbaum JT and Austin DF, Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am. J. Ophthalmol 2008. 146: 890–896. [DOI] [PubMed] [Google Scholar]
- 24.Reeves SW, Sloan FA, Lee PP and Jaffe GJ, Uveitis in the elderly: epidemiological data from the National Long-term Care Survey Medicare Cohort. Ophthalmology 2006. 113: 307. [DOI] [PubMed] [Google Scholar]
- 25.Lerman MA and Rabinovich CE, The future is now: biologics for non-infectious pediatric anterior uveitis. Paediatr. Drugs 2015. 17: 283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heiligenhaus A, Michels H, Schumacher C, Kopp I, Neudorf U, Niehues T, Baus H et al. , Evidence-based, interdisciplinary guidelines for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Rheumatol. Int 2012. 32: 1121–1133. [DOI] [PubMed] [Google Scholar]
- 27.Lee FF and Foster CS, Pharmacotherapy of uveitis. Expert Opin. Pharmacother 2010. 11: 1135–1146. [DOI] [PubMed] [Google Scholar]
- 28.Foster CS, Kothari S, Anesi SD, Vitale AT, Chu D, Metzinger JL and Ceron O, The Ocular Immunology and Uveitis Foundation preferred practice patterns of uveitis management. Surv. Ophthalmol 2016. 61: 1–17. [DOI] [PubMed] [Google Scholar]
- 29.Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, Louie JS, Nussenblatt RB et al. , Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am. J. Ophthalmol 2000. 130: 492–513. [DOI] [PubMed] [Google Scholar]
- 30.Kruh J and Foster CS, The philosophy of treatment of uveitis: past, present and future. Dev. Ophthalmol 2012. 51: 1–6. [DOI] [PubMed] [Google Scholar]
- 31.Maleki A, Meese H, Sahawneh H and Foster CS, Progress in the understanding and utilization of biologic response modifiers in the treatment of uveitis. Expert Rev. Clin. Immunol 2016. 12: 775–786. [DOI] [PubMed] [Google Scholar]
- 32.Siddique SS, Shah R, Suelves AM and Foster CS, Road to remission: a comprehensive review of therapy in uveitis. Expert Opin. Investig. Drugs 2011. 20: 1497–1515. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen QD, Callanan D, Dugel P, Godfrey DG, Goldstein DA and Wilensky JT, Treating chronic noninfectious posterior segment uveitis: the impact of cumulative damage. Proceedings of an expert panel roundtable discussion. Retina 2006. Suppl: 1–16. [DOI] [PubMed]
- 34.Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA, Lando Z and Nussenblatt RB, A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J. Immunol 1988. 140: 1490–1495. [PubMed] [Google Scholar]
- 35.Caspi RR, Silver PB, Luger D, Tang J, Cortes LM, Pennesi G, Mattapallil MJ and Chan CC, Mouse models of experimental autoimmune uveitis. Ophthalmic Res 2008. 40: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitaichi N, Namba K and Taylor AW, Inducible immune regulation following autoimmune disease in the immune-privileged eye. J. Leukoc. Biol 2005. 77: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee DJ and Taylor AW, Following EAU recovery there is an associated MC5r-dependent APC induction of regulatory immunity in the spleen. Invest. Ophthalmol. Vis. Sci 2011. 52: 8862–8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee DJ and Taylor AW, Both MC5r and A2Ar are required for protective regulatory immunity in the spleen of post-experimental autoimmune uveitis in mice. J. Immunol 2013. 191: 4103–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee DJ and Taylor AW, Recovery from experimental autoimmune uveitis promotes induction of antiuveitic inducible Tregs. J. Leukoc. Biol 2015. 97: 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee DJ, Preble J, Lee S, Foster CS and Taylor AW, MC5r and A2Ar deficiencies during experimental autoimmune uveitis identifies distinct t cell polarization programs and a biphasic regulatory response. Sci. Rep 2016. 6: 37790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor AW, Kitaichi N and Biros D, Melanocortin 5 receptor and ocular immunity. Cell. Mol. Biol. (Noisy-le-grand) 2006. 52: 53–59. [PubMed] [Google Scholar]
- 42.Kawanaka N and Taylor AW, Localized retinal neuropeptide regulation of macrophage and microglial cell functionality. J. Neuroimmunol 2011. 232: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q, Xiao X, Wang C, Zhang X, Li F, Zhou Y, Kijlstra A and Yang P, Decreased microRNA-155 expression in ocular Behcet’s disease but not in Vogt Koyanagi Harada syndrome. Invest. Ophthalmol. Vis. Sci 2012. 53: 5665–5674. [DOI] [PubMed] [Google Scholar]
- 44.Kolahi S, Farajzadeh MJ, Alipour S, Abhari A, Farhadi J, Bahavarnia N, Malek Mahdavi A et al. , Determination of mir-155 and mir-146a expression rates and its association with expression level of TNF-alpha and CTLA4 genes in patients with Behcet’s disease. Immunol. Lett 2018. 204: 55–59. [DOI] [PubMed] [Google Scholar]
- 45.Hsu YR, Chang SW, Lin YC and Yang CH, Expression of microRNAs in the eyes of lewis rats with experimental autoimmune anterior uveitis. Mediators Inflamm 2015. 2015: 457835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohana R, Weiman-Kelman B, Raviv S, Tamm ER, Pasmanik-Chor M, Rinon A, Netanely D et al. , MicroRNAs are essential for differentiation of the retinal pigmented epithelium and maturation of adjacent photoreceptors. Development 2015. 142: 2487–2498. [DOI] [PubMed] [Google Scholar]
- 47.Sundermeier TR and Palczewski K, The impact of microRNA gene regulation on the survival and function of mature cell types in the eye. FASEB J 2016. 30: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chucair-Elliott AJ, Elliott MH, Wang J, Moiseyev GP, Ma JX, Politi LE, Rotstein NP et al. , Leukemia inhibitory factor coordinates the down-regulation of the visual cycle in the retina and retinal-pigmented epithelium. J. Biol. Chem 2012. 287: 24092–24102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sethna S, Chamakkala T, Gu X, Thompson TC, Cao G, Elliott MH and Finnemann SC, Regulation of phagolysosomal digestion by caveolin-1 of the retinal pigment epithelium is essential for vision. J. Biol. Chem 2016. 291: 6494–6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le YZ, Zheng W, Rao PC, Zheng L, Anderson RE, Esumi N, Zack DJ and Zhu M, Inducible expression of cre recombinase in the retinal pigmented epithelium. Invest. Ophthalmol. Vis. Sci 2008. 49: 1248–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Testa U, Pelosi E, Castelli G and Labbaye C, miR-146 and miR-155: two key modulators of immune response and tumor development. Non-coding RNA 2017. 3: pii: E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhuang Z, Xiao Q, Hu H, Tian SY, Lu ZJ, Zhang TZ and Bai YL, Down-regulation of microRNA-155 attenuates retinal neovascularization via the PI3K/Akt pathway. Mol. Vis 2015. 21: 1173–1184. [PMC free article] [PubMed] [Google Scholar]
- 53.Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C and Androudi S, A focus on the epidemiology of uveitis. Ocul. Immunol. Inflamm 2018: 26: 1–15. [DOI] [PubMed] [Google Scholar]
- 54.Suhler EB, Martin TM and Rosenbaum JT, HLA-B27-associated uveitis: overview and current perspectives. Curr. Opin. Ophthalmol 2003. 14: 378–383. [DOI] [PubMed] [Google Scholar]
- 55.Zhao B, Chen W, Jiang R, Zhang R, Wang Y, Wang L, Gordon L and Chen L, Expression profile of IL-1 family cytokines in aqueous humor and sera of patients with HLA-B27 associated anterior uveitis and idiopathic anterior uveitis. Exp. Eye Res 2015. 138: 80–86. [DOI] [PubMed] [Google Scholar]
- 56.Shenavandeh S, Jahanshahi KA, Aflaki E and Tavassoli A, Frequency of HLA-B5, HLA-B51 and HLA-B27 in patients with idiopathic uveitis and Behcet’s disease: a case-control study. Reumatologia 2018. 56: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barisani-Asenbauer T, Maca SM, Mejdoubi L, Emminger W, Machold K and Auer H, Uveitis: a rare disease often associated with systemic diseases and infections—a systematic review of 2619 patients. Orphanet J Rare Dis 2012. 7: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cooper GS, Bynum ML and Somers EC, Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J. Autoimmun 2009. 33: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caspi RR, A look at autoimmunity and inflammation in the eye. J. Clin. Invest 2010. 120: 3073–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Namba K, Kitaichi N, Nishida T and Taylor AW, Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2. J. Leukoc. Biol 2002. 72: 946–952. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


