Abstract
A growing body of evidence supports that aerobic exercise can decrease the risk of future cognitive impairment and Alzheimer’s disease (AD). There is a pressing need to rigorously determine whether cognitively normal yet at-risk individuals stand to benefit from the protective effects of exercise. The present study will test the feasibility of an aerobic exercise intervention in such a population and inform the design of a larger-scale randomized, controlled trial examining the effect of aerobic exercise on biomarkers of AD in late-middle-aged, at-risk individuals. This was a single-site, 1 : 1 block-randomized, parallel, two-arm trial. Cognitively normal participants aged 45–80 with documentation of familial and genetic AD risk factors were randomly assigned to one of two interventions. The Usual Physical Activity group was provided educational materials about exercise. The Enhanced Physical Activity intervention delivered 26 weeks of individualized and supervised aerobic exercise. Exercise duration and intensity were incrementally increased to 150 min/week and 70–80% of heart rate reserve, respectively. Retention and adherence were measured to assess study feasibility. In addition, pre- and post- intervention differences between the two arms were evaluated for cardiorespiratory fitness, physical activity, brain glucose metabolism, cerebral structure, vascular health, memory, executive function, and mood. Data from randomized controlled trials of exercise training are needed to identify the proper exercise prescription for reducing accumulation of AD biomarkers in cognitively normal individuals. The current trial will contribute to filling that gap while informing the design of large-scale trials.
Keywords: Cardiorespiratory fitness, exercise, magnetic resonance imaging, physical activity, positron emission tomography, preclinical Alzheimer’s disease
INTRODUCTION
Recent forecasting suggests that the already burdensome prevalence of preclinical and clinical Alzheimer’s disease (AD) will nearly triple by the year 2060 [1]. Lowering the prevalence of AD would substantially diminish the human and economic burden associated with the disease [2, 3]. Unfortunately, studies of pharmacological agents have a failure rate of over 99% [4], and approved drugs that may slow cognitive decline are not curative [5]. A growing consensus in the field is that null trials may result from failure to implement disease-modifying therapy during the earliest stages of disease [6]. Thus, there is an urgent need for non-pharmacologic interventions in asymptomatic yet at-risk individuals that prevent the development and manifestation of AD symptomology.
Physical activity (PA) has been suggested as one of the foremost modifiable risk factors for AD [7]. Exercise constitutes a subset of PA that is structured, intentional, repetitive, and in the case of aerobic exercise, has the specific goal of increasing or maintaining cardiorespiratory fitness (CRF) [8]. Aerobic exercise training is a promising non-pharmacologic intervention that may stand to reduce the prevalence of AD. While national guidelines have established the recommended amount and type of exercise for general health in older adults [9], these guidelines have not been tested to determine whether they are relevant to markers of AD.
Evidence from our laboratory indicates that cognitively normal individuals who meet national PA guidelines exhibit less age-related decrement in cerebral glucose uptake, amyloid deposition, hippocampal volume, and memory function than their inactive peers [10]. However, the mechanisms by which PA and exercise influence brain health remain largely unknown. Epidemiological [11], cross-sectional [12, 13], and longitudinal [14] evidence supports that CRF is positively associated with cognition and negatively related to AD biomarker accumulation in middle age. Furthermore, evidence supports that CRF attenuates the influence of amyloid and genetic risk factors on AD-relevant outcomes [15, 16]. Preliminary randomized controlled trials (RCTs) in adults with normal cognition [17] and with mild cognitive impairment [18] indicate that aerobic exercise training can impart benefit on measures related to AD. Even when exercise training is reported to not improve cognition in individuals in the early stages of AD, increased CRF is positively related to brain health [19]. Further RCTs, however, are needed to definitively test the effect of aerobic exercise training and CRF on markers of AD risk.
Exercise interventions implemented in later disease stages [20–22] demonstrate less benefit than those implemented in the early stages of AD [18, 19, 23]; it may be important to halt the disease process earlier in life because there is little evidence that exercise training can reverse AD biomarker accumulation. Moreover, cognitively normal adults may respond to aerobic exercise interventions with greater CRF improvement than older, cognitively impaired individuals [19]. Family history of AD and carriage of the apolipoprotein E4 (APOE4) allele confer specific risk for developing AD [24, 25]. Collectively, these factors make late-middle-aged, cognitively normal, yet at-risk individuals an excellent population in which to study the effects of aerobic exercise interventions on AD-relevant outcomes. This is particularly salient because targeting disease prevention toward individuals with specific risk factors may have a substantial impact on curbing the prevalence of AD [26]. Although findings from existing RCTs of aerobic exercise training with AD-relevant outcomes are promising, the study participants either lacked specific AD risk [27] or were already experiencing cognitive decline [18], the exercise prescriptions have had a wide range of intensities [19, 27], and none have accounted for PA in which participants engage outside of the study intervention. Whether aerobic exercise training will modulate outcomes relevant to AD in an at-risk population remains unknown. Importantly, the feasibility of conducting an aerobic exercise intervention among late-middle-aged individuals with specific risk factors for AD, a population including individuals holding full-time jobs while concurrently serving as caregivers, is a crucial knowledge gap.
Therefore, the present study was conducted to determine the feasibility and acceptability of an aerobic exercise intervention in asymptomatic, late-middle-aged adults at risk for AD in order to support the design of a larger RCT. The primary outcomes were feasibility and acceptability. The hypothesis was that more than 90% of enrolled participants would complete at least 80% of the scheduled exercise training sessions. The study was also designed to test secondary outcomes of brain glucose metabolism because this is a novel outcome among exercise interventions conducted in at-risk individuals. Other secondary outcomes were cerebral blood flow, cerebrovascular health, hippocampal volume, memory/executive function, and mood. An exploratory aim was to determine the biological (neurotrophic and CRF) and behavioral (extra-study PA) mechanisms underpinning changes in brain glucose metabolism and other secondary outcomes.
By studying these aims in a pre-clinical cohort and targeting brain glucose metabolism (a novel outcome among this population), we address a critical knowledge gap: can an intervention be designed to lessen the burden of disease markers in the brains of individuals with specific risk for AD?
METHODS
Design overview
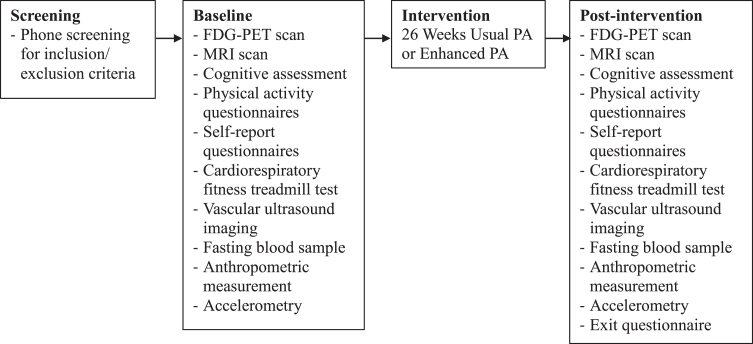
aeRobic Exercise And Cognitive Health (REACH) was a single-site, 1 : 1 block-randomized, parallel, two-arm trial conduced at the University of Wisconsin-Madison. The study was registered on ClinicalTrials.gov under the identifier NCT02384993. Although the trial start date is listed as January 2015, this date reflects when organizational and recruitment efforts began; the first participant was enrolled on May 1, 2015 and the last participant completed the study on July 21, 2016. REACH tested the effects of a 26-week aerobic exercise training intervention in late-middle-aged adults with documented parental history of AD, with disease marker outcome measures assessed pre- and post- intervention. Study visits consisted of one eligibility screening (performed via phone), a baseline visit, exercise training sessions three times per week, monthly check-ins via phone or in-person, and a post-intervention visit. The schedule of study procedures is presented in Fig. 1.
Fig. 1.
Study schedule. Progression of study procedures from screening through post-intervention assessment. FDG-PET, 18F fluorodeoxyglucose positron emission tomography; MRI, magnetic resonance imaging; PA, physical activity.
Participants
Participants were recruited from the Wisconsin Registry for Alzheimer’s Prevention (WRAP) or the Wisconsin Alzheimer’s Disease Research Center (WADRC) at the University of Wisconsin – Madison. WADRC participants were members of the Investigating Memory in People At risk, Causes and Treatments (IMPACT) cohort. WRAP and IMPACT are longitudinal cohorts of approximately 1,500 and 520 cognitively healthy adults, respectively, between the ages of 40 and 65 at study entry. The demographic composition of WRAP is 88% white, mean baseline age of 54 years, 71% female, 73% with parental history of AD, 40% carrying at least one APOE ɛ4 allele, and a mean educational attainment of 16 years of schooling in WRAP. IMPACT stood at 79% white, 57 years of age, 66% female, 55% with parental history of AD, 38% carrying at least one APOE ɛ4 allele, and a mean of 16 years of schooling. Further details about the WRAP cohort have been previously published [28]. Parental history of AD was determined by either neuropathological confirmation, diagnosis by a physician, review of medical records, or by evaluation using the Dementia Questionnaire [29]. While individuals with either positive or negative parental history of AD were considered for REACH, those with positive history were targeted and the final sample consisted entirely of participants with positive parental history of AD.
Eligibility criteria
Inclusion and exclusion criteria were designed to establish a sample at risk for AD and for whom the intervention would be novel and safe. Table 1 details these eligibility criteria.
Table 1.
Inclusion and exclusion criteria
| Inclusion Criteria | – 45–80 years old |
| – Documented positive/negative family history of AD | |
| – Physically inactive (<150 min/week moderate intensity assessed by TAPA [30]) | |
| – Physician consent to participate | |
| – Willing and able to complete aerobic exercise intervention and all assessments | |
| – English fluency for cognitive testing purposes | |
| Exclusion Criteria | – Pregnancy |
| – Diabetes | |
| – Diagnosis of significant neurological disease (including mild cognitive impairment or AD) or significant head trauma followed by persistent neurologic deficits | |
| – Known structural brain abnormalities | |
| – Contraindication to MRI (e.g., pacemaker, ferrous metal implant) | |
| – Contraindications to exercise testing (e.g., recent myocardial infarction) | |
| – Finding from exercise testing prohibiting safe participation in aerobic exercise intervention (e.g., 3rd degree AV block) | |
| – Psychiatric condition (e.g., major depression, bipolar disorder) per DSM-IV criteria | |
| – History of alcohol or substance abuse per DSM-IV criteria | |
| – Significant medical condition affecting cognition or other outcome measures | |
| – Severe untreated hypertension | |
| – Use of: antipsychotic medications (e.g., non-SSRI antidepressants, neuroleptics); cardiac glycoside medications; investigational agents | |
| – Cancer in the previous five years (except skin cancer) |
Recruitment
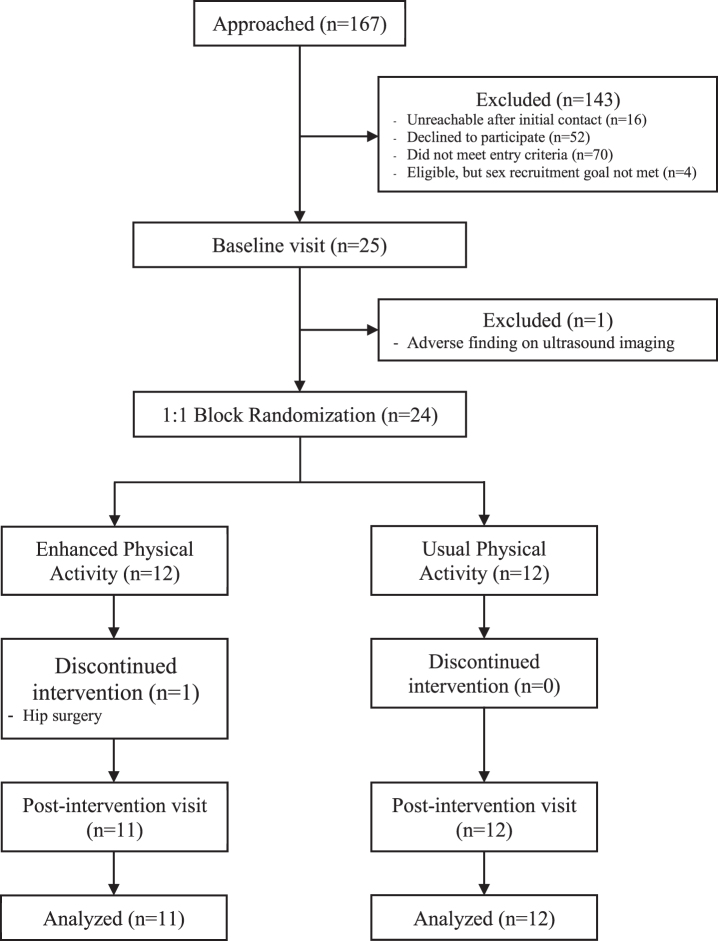
Recruitment efforts for this study were facilitated by the fact that participants were drawn from two existing cohorts (WRAP and WADRC IMPACT) that together contained approximately 270 potentially eligible participants prior to the start of the study, making recruitment cost low and return on investment high. Study coordinators queried the WRAP and WADRC IMPACT databases for individuals who were cognitively normal, aged between 45 and 80, and had positive or negative parental history of AD (though only individuals with positive parental history were ultimately included in the sample). Individuals meeting these initial criteria received a mailing containing an invitation letter and a flyer providing a brief description of the study. The letter encouraged the recipient to contact a study coordinator by phone if interested. A coordinator also contacted recipients via phone to follow up on the mailing. During the follow-up phone call, the coordinator presented details about the study. If the recipient was interested in participating, the study coordinator obtained verbal consent to proceed with a phone screening. The screening interview was done to more fully determine study eligibility. The interviewer collected information regarding inclusion and exclusion criteria (see Eligibility Criteria; [30]). Those participants who were deemed eligible were scheduled for their baseline visit. Individuals who did not meet study criteria were excluded. There was a goal to recruit equal numbers of males and females into each arm of the study, and thus further individuals were excluded when the limit of number of females had been met but more males needed to be recruited. Figure 2 details the enrollment flow of the study.
Fig. 2.
Flowchart of study design and recruitment. The final sample of participants whose data is to be analyzed was obtained after a thorough screening process; one participant had to discontinue the intervention after randomization.
Randomization and blinding
Participants were randomized to one of the two study groups: Usual Physical Activity (Usual PA), or Enhanced PA. Group allocation was achieved using a computer generated, 1 : 1 block randomization scheme that included age and sex, and the lead study coordinator was the only person with access to the randomization scheme. Due to staffing limitations, this study coordinator—who was unblinded to treatment allocation—was also responsible for administering the cognitive tests. All other study investigators were blinded to treatment allocation, and participants were reminded at study visits to not reveal their group allocation to these investigators.
Study procedures
All study procedures were approved by the University of Wisconsin – Madison Institutional Review Board (#2014-1434). The baseline visit took place over two days, within a 30-day window. Written informed consent was obtained at the beginning of the first day of the visit. The post-intervention visit was the same in structure and components as the baseline visit, with the addition of an exit questionnaire that was sent home with the participant and then mailed back to the study coordinator. In addition to being collected at baseline and post- intervention, accelerometry (see Accelerometry) was also recorded at the mid-point of the intervention (week 13). Participants in the Usual PA group also received monthly phone calls from the study coordinator, while participants in the Enhanced PA group had monthly in-person check-ins with the study coordinator at exercise sessions. The following procedures were completed at pre- and post- intervention study visits:
18F fluorodeoxyglucose (FDG) positron emission tomography (PET) scan
Cerebral glucose metabolism was a secondary outcome of this study. Participants underwent the scan after a 4 h fast from food, nicotine, caffeine, medications, and alcohol (but not water). If female and of childbearing potential, a pregnancy test was given prior to these scans. If a participant was to have blood drawn (see Clinical Assessment) on the day of PET scan, this was done prior to tracer injection. A blood glucose test was administered to measure blood glucose levels and had to be ≤180 mg/dL for FDG to be injected. Images were acquired using a PET scanner (EXACT HR+, Siemens, Erlangen, Germany) in 3-dimensional mode (septa retracted) using the Alzheimer’s Disease Neuroimaging Initiative protocol [31]. Prior to the PET scan, the participant’s blood pressure and pulse were taken. Participant were positioned head first, supine with the cantho-meatal line parallel to the in-plane field of view. An intravenous catheter was placed in one arm for injection of 5.0±0.5 mCi of 18F FDG and the participant was instructed to remain awake but relaxed in a quiet room. Imaging began 30 min after injection, and the scan was acquired as six, 5 min frames with the participant positioned head first, supine with the cantho-meatal line parallel to the in-plane field of view. A 5 min transmission scan was then acquired following the emission scan.
Magnetic resonance imaging (MRI) scan
Participants underwent the scan after a 4 h fast from food, nicotine, caffeine, alcohol, and medications with vasomodulatory properties (a requirement for the perfusion scans). The scan was preceded by an MRI safety screening interview to ensure participant safety. If a participant reported a possible history of metal in their eyes, or a history of working with metal (grinding, welding, etc.) they received an orbital x-ray to ensure eligibility for MRI. If female and of childbearing potential, a pregnancy test was given prior to the scan. The MRI scan was conducted using a 3.0T X750 high-field MRI scanner (General Electric, Waukesha, WI). The MRI protocol featured a T1 structural scan for quantifying brain volume [24] a pseudo-continuous ASL (pcASL) for assessing cerebral perfusion [25], a diffusion tensor imaging scan for examining white matter microstructure [32], a T2 fluid-attenuated inversion recovery (FLAIR) scan for quantifying white matter lesions [33], and isotropic-voxel radial projection imaging (pcVIPR) [34]. This protocol was completed in about 60 min. All MR images received radiological review from a neuroradiologist to screen for clinical abnormalities that may not have been previously detected; any clinically significant findings were communicated to the study team for clinical follow-up with the participant.
Cognitive and mood assessment
A trained technician administered a cognitive test battery and mood assessment with well-established psychometric properties at the WADRC. These tests targeted cognitive domains of episodic memory and executive function, which are known to be both impacted by AD [35] and preferentially remediable by engaging in PA [36]. The test battery included the Mini-Mental State Examination (MMSE) [37], California Verbal Learning Test-II (CVLT) [38], selected tests from the Delis-Kaplan Executive Function System (D-KEFS) (trail-making, sorting, verbal fluency, design fluency, tower, and color-word interference) [39], and Profile of Mood States (POMS) [40]. The POMS was also administered to participants in the Enhanced PA group every two weeks at the exercise facility throughout the intervention. If a participant had been fasting for another study procedure (e.g., MRI scan), a meal was provided prior to cognitive testing.
Physical activity and self-report questionnaires
A trained technician administered questionnaires assessing PA, sleep and other aspects of well-being. These questionnaires characterize quantity, frequency, intensity, and type of PA typically performed by the participant, perceived barriers to and benefits of exercise, and sleep quality and quantity. The assessments included the Community Healthy Activities Model Program for Seniors (CHAMPS) [41], International Physical Activity Questionnaire [42], Exercise Benefits/Barriers Scale [43], Barriers Self-Efficacy Scale (BARSE) [44], Epworth Sleepiness Scale [45], Insomnia Severity Index [46], Medical Outcomes Study Sleep Questionnaire [47], and Pittsburgh Sleep Quality Index [48]. The BARSE was also administered to participants in the Enhanced PA group every two weeks throughout the intervention to support Aim 1, assessment of study feasibility.
Cardiorespiratory fitness
A continuous graded maximal treadmill protocol was administered by a certified exercise physiologist under the supervision of a physician using standard American College of Sports Medicine guidelines to minimize risks and maximize safety. Before the exercise test the participant was asked a series of medical history questions related to cardiac and respiratory conditions and symptoms to ensure the test could be performed safely. Participants reported for the test in the morning after a 12 h fast from food, nicotine, caffeine, alcohol, and medications (but not water). Participants were advised to refrain from exercise on the day of the test, and to wear comfortable walking shoes and loose comfortable clothing to the test. Throughout the test, electrocardiography was used to monitor each participant and collect heart rate (HR). A metabolic cart and 2-way non-rebreathing valve (TrueOne 2400, ParvoMedics, Sandy, UT) were used to obtain continuous measurements of oxygen uptake (VO2), carbon dioxide production, and minute ventilation. A modified Balke protocol with self-selected walking speed was used, although the majority of participants walked at 3.5 miles per hour. The initial incline was 0%, and the treadmill incline increased by 2.5% every 2 min until the participant reached volitional exhaustion. Criteria for peak aerobic capacity were met if at least two of the following were achieved: 1) respiratory exchange ratio ≥1.1, 2) achievement of 90% of age-predicted maximum HR, 3) rating of perceived exertion (RPE) ≥17, and 4) a change in VO2 < 200 ml with an increase in incline. These methods were implemented to avoid potential age-driven relationships when volitional effort was not achieved [49]. Following volitional exhaustion, the speed and grade of the treadmill were reduced to 2 mph and 0% grade for a 3 min active recovery period.
Ultrasound imaging
The ultrasound imaging session was performed by registered diagnostic medical sonographers and included the following examinations; transcranial Doppler, carotid ultrasound and brachial artery reactivity testing. Ultrasound imaging was performed at baseline and at 26 weeks.
Transcranial doppler. The Acuson Siemens S2000 ultrasound system with a 4-1Vc transducer (Siemens Ultrasound, Malvern, PA, USA) was used to image the right and left middle cerebral artery at a depth of 45–60 mm from the temporal window. The peak systolic velocity, end diastolic velocity, mean velocity, resistive index, pulsatility index, and systolic/diastolic (S/D) ratio were recorded from the pulsed-wave Doppler waveform.
Carotid ultrasound imaging. The Acuson Siemens S2000 ultrasound system with a 9L4 transducer (Siemens Ultrasound, Malvern, PA, USA) was used to acquire all carotid images. The right and left common carotid artery (proximal, mid and distal segments), carotid bulb, internal carotid artery (proximal, mid and distal segments), external carotid artery and vertebral artery were imaged with B-mode (grayscale), color Doppler and pulsed wave spectral Doppler. B-mode imaging was performed to image the near and far wall of arterial segments and to examine for the presence of plaque [50]. Grayscale analyses of B-mode images of plaque (if present) were examined for plaque area, grayscale median value, presence of a black area near the border and discrete white areas [50, 51]. Grayscale analysis of the right and left distal one centimeter of the common carotid arterial walls was also performed from B-mode images to measure echogenicity (gray scale median value) and evaluate the texture features [52–54]. Grayscale analysis was performed with specialized software (LifeQ Medical, Nicosia, Cyprus). The peak systolic velocity, end-diastolic velocity, resistive index, pulsatility index and systolic to diastolic ratio were recorded from the pulsed wave Doppler waveform for each arterial segment. The distal one centimeter of the right common carotid artery was imaged to perform carotid distensibility and intima-media thickness (IMT) measurements [55–57]. Carotid IMT was measured on the far wall of the right common carotid artery from an anterior, lateral and posterior angle using the Meijer Arc [58, 59].
Brachial artery reactivity testing (BART). Ultrasound imaging of the right brachial artery was performed with the CX50 ultrasound system and the L12-3 MHz transducer (Philips Ultrasound, Andover, MA, USA). A blood pressure cuff was placed around the forearm and inflated to 250 mmHg for 5 min to induce ischemia [60, 61]. The arterial diameter was measured at end-diastole using AccessPoint Web software (Alpharetta, GA, USA) pre-cuff inflation (baseline image) and 60- and 90 s post cuff deflation. The percentage difference between the diameter pre-cuff inflation and post cuff deflation is the reactivity measure [62]. A baseline Doppler image and reactive hyperemia (RH) Doppler signal were also recorded, the baseline Doppler image was acquired prior to cuff inflation and the RH Doppler image was recorded at the time of cuff deflation.
Clinical assessment
Participants reported to the Clinical Research Unit in the morning after a 12 h fast from food, nicotine, caffeine, medications, and alcohol (but not water). Three blood pressure measurements were obtained after the participant was seated for 10 min and cuff size was chosen appropriate to the participant’s arm circumference. The average of the second and third readings were used for both systolic and diastolic blood pressure. Weight was measured to the nearest tenth of a kilogram using a medical grade scale and height was measured using a stadiometer. Waist and hip circumferences were measured to the nearest centimeter with an anthropometric tape while the participant was standing. The waist measurement was taken at the level of the umbilicus, and the hip measurement was taken at the level of the maximum gluteal muscle protrusion. Both waist and hip measurements were taken two times, and if the measurements differed by more than 1 centimeter, the measurement was repeated a third time. The smallest waist and largest hip measurements were used.
Fasting blood samples were collected following vital sign and anthropometric measurement. Participants were first asked about symptoms suggesting an acute inflammatory process that could interfere with measures of inflammation. If any such symptoms were present, the blood sample was taken, but this information was noted. Blood was collected into a 10 mL tube containing EDTA for plasma sampling, a 9 mL red-top tube containing a clot activator for serum sampling, and a 4 mL tube for a clinical lab panel. EDTA tubes were gently inverted 10–12 times and then centrifuged within 1 h of collection for 15 min at 3000 rpm at room temperature. Red-top tubes were allowed to clot for no more than 30 min and then centrifuged for 10 min at 3000 rpm at 4°C. Cell-free plasma and serum was then aliquoted into cryovials and stored at – 80°C until analysis. These samples were stored for analysis of peripheral neurotrophins and other factors implicated in the association between exercise and AD. Whole blood samples were sent to the UW clinical laboratories for analysis of lipids, insulin, glucose, and cortisol. All participants already had APOE genetic testing on record in a coded and secure database as part of participation in the WRAP or WADRC studies.
Accelerometry
Participants were fitted with a triaxial accelerometer (GT3X+, Actigraph, Pensacola, FL, USA) attached to an elastic belt that was worn on the hip for seven consecutive days to record free-living PA and sedentary time. They were also given a logbook to record when they put on or took off the device, when they exercised, completed REACH exercise sessions, and any other details about their PA that could help the study team analyze the data. Participants were instructed to wear the device during all waking hours, with the exception of bathing, swimming. In addition to the baseline and post-intervention timepoints, the accelerometer was also provided to each participant at the mid-point of the intervention (13 weeks). The accelerometer was mailed to each participant with instructions to wear the device for seven consecutive days for assessment of mid-intervention PA and sedentary time. Accelerometry was used to objectively measure PA because subjective PA assessment can be impacted by participant recall, social desirability bias, and even education level, sex, and age [63, 64].
Monthly check-in
A study coordinator called each Usual PA participant once per month to query recent adverse events, changes to medications, and travel plans, and to provide encouragement and support. These same questions were asked of Enhanced PA participants monthly in-person at an exercise session. In the event that a participant had an issue that they wanted to be addressed, the study coordinator was available by phone throughout the intervention.
Exit questionnaire (post-intervention visit only)
Participants completed an anonymous exit questionnaire that was sent home at the post-intervention visit and mailed back to the study coordinator. The goal of this assessment was to capture participants’ level of satisfaction with their participation, the study staff, and the training facility. The assessment was also designed to reveal the participants’ favorite aspects of the study, motivations for continuing, challenges to participation, and how the study could be improved. These responses would help refine the intervention protocol for a future, larger study.
Intervention implementation
After completing all baseline procedures, participants attended an orientation session at the facility where the Enhanced PA intervention was to take place. The unblinded study coordinator provided participants an envelope according to the randomization scheme that contained a letter indicating which group they were randomized to. All participants were reminded of the purpose of the study. Participants randomized to Enhanced PA were given details about what the intervention would entail. Specifically, they were told how to coordinate with the exercise specialist to establish their training schedule, the importance of attending all three weekly exercise sessions, what to wear during exercise, how to track exercise session completion, and how to report absences from exercise sessions. The Enhanced PA group was also given a “Tips and Tricks for Sticking to the REACH Exercise Program” handout containing strategies for overcoming setbacks or barriers to exercise. Participants randomized to the Usual PA group were told that they would be followed over the 26 weeks via monthly phone calls that would inquire into their PA habits. They also received educational materials developed by the National Institute on Aging on how to safely start an exercise program including “Exercise & Physical Activity: Your Everyday Guide from the National Institute on Aging” and “Go4Life Everyday Exercises.” No additional guidance or instruction about an exercise program was given to this group during the study.
Exercise training sessions in the Enhanced PA group occurred in a university gymnasium setting, and sessions were conducted in a semi-private studio to minimize potential interference and distractions. Some sessions occurred at the same time as other university exercise classes, but a partition was set up to separate the participant’s exercise studio. Participants were fully supervised by a trained exercise specialist during all sessions. Most sessions were conducted one-on-one between the participant and the supervising exercise specialist, although some sessions were held during which up to two participants were supervised by one trainer. Up to four participants exercised at once. The exercise specialist greeted the participants, accompanied them to the training studio, and conducted the exercise session. At the beginning of each exercise session, participants were provided a watch and heart rate strap to be used to monitor and maintain the prescribed heart rate.
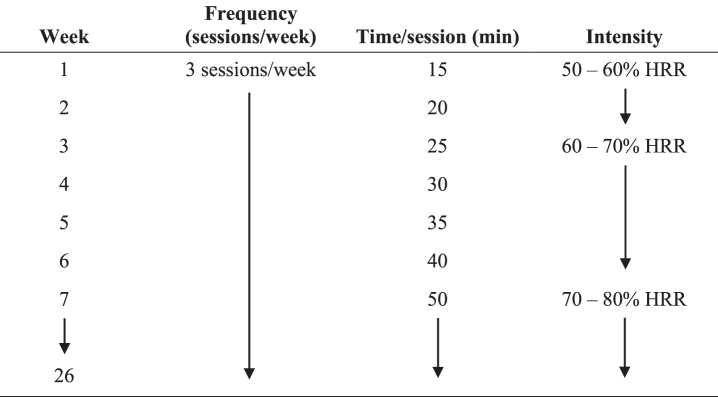
The exercise sessions were all completed on a treadmill. The initial training speed was the same as the self-selected walking pace used during the baseline continuous graded maximal treadmill protocol. Heart rate reserve (HRR) was calculated by the Karvonen method [65] as Target HR = [(HRmax – HRresting)×% Intensity] + HRresting, using the resting HR obtained at the baseline clinical assessment. Intensity was monitored by the exercise specialist using the watch and chest strap provided at the beginning of each session. Exercise intensity for weeks one and two was moderate at 50–60% of HRR, 60–70% HRR for weeks three through six, and 70–80% HRR for weeks seven through 26. This gradual increase in intensity was chosen so that by week seven, participants would be training at a vigorous intensity to ensure that they met or exceeded the minimum activity levels for health benefits according to public health recommendations [66]. Each session began with a warm-up featuring a gradual increase of treadmill speed and incline in order to reach the participant’s target percentage of HRR. The warm-up lasted approximately 5 min but was shortened or extended depending on when the participant’s HR reached the target % HRR. Once the participant’s HR entered the target zone, the exercise specialist started a timer to monitor the duration spent within the target heart rate zone. Each exercise session ended with a 5 min cool down period at a 0% incline and a speed lower than during the training session.
Participants trained three days per week throughout the 26-week intervention. During week one, participants exercised within the target HR zone for 15 min per session. Each subsequent week, session duration was increased by 5 min through week six, when participants exercised within the target HR zone for 40 min per session. Beginning in week seven, participants exercised within this zone for 50 min per session, thereby meeting or exceeding the current public health recommendation for a minimum of 150 min per week of moderate or 75 min per week of vigorous intensity aerobic exercise [66]. Figure 3 provides an outline of the progression of the Enhanced PA intervention.
Fig. 3.
Enhanced PA intervention progression. Participants in the Enhanced PA intervention completed a gradual buildup of exercise duration and intensity over the course of the first seven weeks; they maintained duration and relative intensity from weeks seven through 26. HRR, heart rate reserve.
Retention and adherence
The WRAP and WADRC IMPACT cohorts from which we recruited are exceptionally committed to research participation with >95% retention rate over 12 years of follow-up and 84% over 10 years of follow-up, respectively. Therefore, we expected low attrition (<10%) in this study. Still, the intervention presented a novel routine for these participants who had previously engaged in less than 150 min/week of PA, so we developed a comprehensive plan to promote retention and adherence.
Study staff received ongoing monitoring and training regarding treatment of participants with the utmost respect. Monthly phone calls and in-person check-ins were made to query adverse events, medication changes, and travel plans, and to remind participants of their upcoming study visits. If a participant missed an exercise session, study staff contacted the participant by phone to provide support for continuing in the study. Exercise specialists offered flexible hours for exercise sessions, allowing participants to incorporate workouts into their schedules. Parking was provided at the study locations, and transportation costs were reimbursed if the participant lived more than 10 miles from the exercise facility. In the event that a participant was unable to exercise at the study facility for an extended period of time due to travel plans, family obligations, or other circumstances, the participant was issued a heart rate monitor and exercise log materials and were encouraged to continue their exercise program while away and to record details (i.e., duration and average HR) of these sessions. These accommodations for exercise outside of the exercise facility did not occur for more than two weeks at a time and no more than three weeks total during the study.
Analysis
Effects reported in prior aerobic exercise studies [18, 27] indicate that our target sample size of 15 per group would provide sufficient power (80%, α= 0.05) for detecting treatment effects that are≥Cohen’s d of 0.84 for secondary outcomes.
As the primary aim of this pilot study was to obtain data on the feasibility and acceptability of the intervention in our cohort, our focus was on collecting metrics regarding: suitability of eligibility criteria; recruitment, retention, and adherence rates; barriers and motivators to adherence; safety and tolerability; dosing of exercise program; and treatment effect across secondary outcomes and variability of response. Acquisition of these data was critical to inform the design of a future, larger study. Bowen et al. [67] outlined eight factors to be addressed by feasibility studies, and we found five of them to be particularly relevant to our intervention. Specifically, the participants’ reaction or affinity for the intervention (acceptability), the likelihood of its use by the participants (demand), the extent to which it could be delivered as planned (implementation), the ability to deliver it given resource constraints (practicality), and the likelihood of intervention effect (limited efficacy). Our primary metrics were enrollment and completion rates for feasibility, and adherence for acceptability. Acceptability, demand, implementation, and practicality would also be evaluated by analyzing study completion rates and intervention adherence rates using frequency counts, crosstabs, and descriptive statistics, and by employing qualitative data analytic approaches from the post-intervention exit questionnaire.
For the secondary outcomes of brain glucose metabolism, cerebral blood flow, hippocampal volume, cognitive, mood, and cerebrovascular outcomes, we would use change score approaches or mixed effects regression to assess differential rate of change in secondary outcome measures between Enhanced PA and Usual PA. The models would include relevant covariates and use random effects to account for repeated measures and individual differences in baseline and change rate. To investigate the biological and behavioral mechanisms underpinning the secondary outcomes listed above, we would apply paired t-tests to baseline and post-intervention data to assess whether the intervention groups exhibit differential changes in circulating neurotrophins and cardiorespiratory fitness. Correlations and linear regression would be used to evaluate the strength of association between changes in protective factors (e.g., CRF, PA) with changes in outcomes (e.g., brain glucose metabolism, cognition). We would test interaction or stratified effects to assess whether the beneficial effects of aerobic exercise vary by PA levels outside the intervention. For these secondary outcomes, we would explore both intent-to-treat (primary) and per-protocol (secondary) approaches. The analyses of these secondary outcomes also allows for evaluation of the fifth measure of feasibility that we explore: limited efficacy [67].
Biospecimen storage and analysis
Blood samples were stored in a cryogenic, – 80°C secure storage facility equipped with backup power and a telephone alarm system. Tissue specimen banking was done to ensure samples were kept until all analyses for this project were complete or until the samples were exhausted.
Incidental findings and adverse events
If any incidental findings were identified during the exercise test or imaging procedures, the results were brought to the attention of the principal investigator and the participant was notified if the result was deemed clinically significant. Additionally, a copy of the incidental finding was provided to the participant’s primary physician if the participant consented to this disclosure.
During monthly check-ins with the participants (via phone for Usual PA or in-person for Enhanced PA), the study coordinator queried illnesses or injuries and assessed the seriousness of any events. Changes to medication were also assessed. Adverse events were reported to the appropriate institutional agency (i.e., IRB) and evaluated for proper follow-up.
Data quality checks
The MRI and PET imaging systems were used full time for research and were staffed with scientists who carefully monitor image quality. Standard procedures were used to ensure geometric stability and uniform image intensity of the MRI. A phantom was used to assess scanner distortion. All participant images were inspected for motion and other artifacts that may have affected the data. At acquisition, foam cushions around the head were used to minimize motion while maintaining comfort. For anatomic MRI, the images were inspected while the participant was in the scanner, and rescanning of particular sequences was done if necessary.
The graded maximal treadmill protocol was conducted under controlled environmental conditions at an altitude of 259 meters, 22–25°C, and 40–60% relative humidity. The metabolic cart was warmed up for at least 30 min prior to each test. It was then calibrated volumetrically using a three-liter piston syringe. The O2 and CO2 analyzers were calibrated using room air and known concentrations of standard gas (16.00% O2, 4.00% CO2), accounting for barometric pressure, temperature, and humidity (Vantage Vue, Davis Instruments, Hayward, CA, USA).
Ultrasound imaging was performed with the same ultrasound systems, transducers and presets throughout the study. Sonographers identified an anatomical landmark to match between scans for brachial artery and carotid imaging [58, 60]. In addition, the Meijer arc was also used to ensure that the carotid artery was imaged at the same angles at baseline and post-intervention visits [58]. After each ultrasound examination, the sonographer reviewed the ultrasound images for quality.
Precautions were taken to ensure the integrity of the data maintained in the study databases. Manual data entry into our databases was done by one member of the study team and subsequently reviewed by a second member of the study team to ensure accuracy. Physical records were stored in locked cabinets, and electronic records were stored on secure password-protected computers in password-protected documents.
DISCUSSION
This report details a feasibility study of aerobic exercise training in late-middle-aged individuals at risk for AD. To our knowledge, this was the first exercise training trial related to AD that consisted of only cognitively normal, late-middle-aged individuals with documented positive family history of AD. Because of their increased risk for AD, this population is a prime target for evaluating the effects of preventative therapies and, specifically, for determining whether aerobic exercise should be recommended before symptom onset in order to delay or prevent the underlying contributors to AD.
The Enhanced PA intervention was developed on the basis of current public health recommendations for exercise in adults: a minimum of 150 min per week of moderate intensity or 75 min of vigorous intensity exercise on at least three days of the week [66]. The intent of prescribing vigorous intensity exercise was three-fold. Vigorous intensity walking is a widely available form of exercise, achieved in many adults by brisk walking on an inclined treadmill or inclined terrain outdoors. Preliminary evidence suggests that moderate and vigorous, rather than light intensity exercise may be needed in order to realize benefit in brain glucose metabolism [68]. Finally, we wished to promote robust CRF adaption, which would be more likely by exceeding the minimum public health recommendations. A 26-week intervention allowed for six weeks of gradual progression of session intensity and duration, then followed by 20 weeks of full-dose exercise. The gradual progression of exercise was also meant to allow participants to adjust to a combination of increasing intensity and duration with the goal of promoting adherence and minimizing missed sessions or drop-outs that might occur when exercise is prescribed more aggressively. Prior research shows that 26 weeks of exercise training is sufficient in order to detect changes in structural MRI imaging in adults with mild cognitive impairment [69] and that shorter interventions may produce no detectable change [70]. The intervention comprised of aerobic exercise because of its established effect on cardiorespiratory fitness, while other activities such as stretching do not confer the same benefit for cardiorespiratory fitness [18].
The present study builds on the strengths of several other RCTs designed to test the effect of aerobic exercise on brain health. While prior studies have also utilized objective measures of CRF [18, 19, 27], they have not accounted for PA levels and sedentary behavior outside the intervention. This is an important limitation for two reasons. First, the effect of free-living PA on response to aerobic exercise training remains unknown. Second, aerobic exercise training has been shown to influence sedentary behavior outside the intervention [71], and sedentary behavior may influence cognitive health and glycemic control [72]. Thus, we measured PA levels and sedentary behavior outside of the intervention using accelerometry, the gold standard for this outcome. Prior studies recruited participants already experiencing cognitive decline or did not characterize disease risk in the cognitively normal individuals studied. Our participants’ documented family history makes our sample highly relevant to AD prevention. Furthermore, we implemented our intervention at an age when participants commonly cite lack of time as a barrier to exercise [73].
This study had several notable strengths. The single-site design of this study allowed us to tightly control intervention implementation and standardize outcome assessments. This also allowed us to develop close partnerships with the participants, enhancing understanding of factors important to feasibility (e.g., motivation, scheduling logistics). The intervention was overseen by a core staff of exercise specialists who received standardized training and ongoing supervision from study coordinators. Each secondary outcome measure was assessed by trained study personnel specific to the technique (e.g., MRI technician). Furthermore, the secondary outcomes were selected to evaluate changes in key AD biomarkers including cerebral glucose metabolism, memory function, and hippocampal volume. Cerebral glucose hypometabolism was a valid outcome because it is a core feature of AD [74] and may be improved with aerobic exercise [10, 68].
Despite its strengths, this study had limitations that bear consideration. A number of individuals declined to participate after receiving an invitation, contributing to possible self-selection bias. Nonetheless, self-selection does not preclude success in terms of feasibility, the primary aim of the study. Finally, due to staffing limitations, a member of the study staff remained unblinded to treatment allocation despite administering cognitive, mood, and PA assessments throughout the trial.
Several modifications would be made to future trials to address these limitations and to further improve on the study design. Increased funding would allow recruitment of more participants and increased staffing to ensure proper investigator blinding, both of which would increase confidence in the results. Future studies should characterize participants during baseline testing using the amyloid-β, neurofibrillary tau, and neurodegeneration [AT(N)] criteria proposed recently [75]. The present study tested the effect of current public health recommendations for older adults (i.e., a minimum of 150 min per week of moderate intensity or 75 min per week of vigorous intensity), but future trials should test whether there are minimum or maximum amounts of exercise needed to benefit brain glucose metabolism and other outcomes related to AD as is being tested for cognition [76]. The current study was not designed to test the effects of different exercise paradigms (e.g., sprint-interval versus vigorous intensity versus moderate intensity). Future studies should determine whether training intensity and paradigm impacts response. Finally, investigation into the interactions among exercise intensity and risk factors such as sex and APOE4 status will be needed.
REACH was a pilot trial of an aerobic exercise intervention in middle-aged persons at risk for AD. It tested the feasibility and acceptability of current public health exercise guidelines, and sought to preliminarily determine whether adherence to such an exercise regimen lessens biomarker accumulation associated with AD. This study was responsive to a national call to establish interventions that prevent or reduce the risk of AD [77]. Findings from this trial, including feasibility metrics, will serve as the basis for a larger study of the effect of aerobic exercise training on biomarkers and cognition in late-middle-aged individuals at risk for AD.
TRIAL STATUS
At the time of submission for publication, the REACH study was closed to enrollment.
TRIAL REGISTRATION
REACH was registered on ClinicalTrials.gov with the identifier NCT02384993.
CONFLICT OF INTEREST
C. Mitchell has an authorship contract with Davies Publishing, Inc, authorship with future royalties for textbook chapters with Elsevier and Wolters-Kluwer and contracted research grants from WL Gore to UW Madison.
ACKNOWLEDGMENTS
This work was supported by grants from the Alzheimer’s Association (NIRGD-305257), the Extendicare Foundation, and the National Institute on Aging (K23 AG045957, R01 AG027161, P50 AG033514, and UL1RR025011).
REFERENCES
- [1]. Brookmeyer R, Abdalla N, Kawas CH, Corrada MM (2018) Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States, Alzheimers Dement 14, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Zissimopoulos J, Crimmins E, St. Clair P (2014) The value of delaying Alzheimer’s disease onset, Forum Health Econ Policy 18, 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Alzheimer’s Association (2018) 2018 Alzheimer’s disease facts and figures, Alzheimers Dement 14, 367–429. [Google Scholar]
- [4]. Cummings JL, Morstorf T, Zhong K (2014) Alzheimer’s disease drug-development pipeline: few candidates, frequent failures, Alzheimers Res Ther 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Berk C, Sabbagh MN (2013) Successes and failures for drugs in late-stage development for Alzheimer’s disease, Drugs Aging 30, 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Sperling RA, Jack CR, Aisen PS (2011) Testing the right target and right drug at the right stage, Sci Transl Med 3, 111cm33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer’s disease prevalence, Lancet Neurol 10, 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Caspersen CJ, Powell KE, Christenson GM (1985) Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research, Public Health Rep 100, 126–131. [PMC free article] [PubMed] [Google Scholar]
- [9]. Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C (2007) Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association, Med Sci Sports Exerc 39, 1435–1445. [DOI] [PubMed] [Google Scholar]
- [10]. Okonkwo OC, Schultz SA, Oh JM, Larson J, Cook D, Koscik R, Gallagher CL, Dowling NM, Carlsson CM, Bendlin BB, LaRue A, Rowley HA, Christian BT, Asthana S, Hermann BP, Johnson SC, Sager MA (2014) Physical activity attenuates age-related biomarker alterations in preclinical AD, Neurology 83, 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Zhu N, Jacobs DR, Schreiner PJ, Yaffe K, Bryan N, Launer LJ, Whitmer RA, Sidney S, Demerath E, Thomas W, Bouchard C, He K, Reis J, Sternfeld B (2014) Cardiorespiratory fitness and cognitive function in middle age: the CARDIA study, Neurology 82, 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Boots EA, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, LaRue A, Asthana S, Hermann BP, Sager MA, Johnson SC, Okonkwo OC (2015) Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease, Brain Imaging Behav 9, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Dougherty RJ, Schultz SA, Boots EA, Ellingson LD, Meyer JD, Van Riper S, Stegner AJ, Edwards DF, Oh JM, Einerson J, Korcarz CE, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, Asthana S, Hermann BP, Sager MA, Stein JH, Johnson SC, Okonkwo OC, Cook DB (2017) Relationships between cardiorespiratory fitness, hippocampal volume, and episodic memory in a population at risk for Alzheimer’s disease, Brain Behav 7, e00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Spartano NL, Himali JJ, Beiser AS, Lewis GD, DeCarli C, Vasan RS, Seshadri S (2016) Midlife exercise blood pressure, heart rate, and fitness relate to brain volume 2 decades later, Neurology 86, 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Schultz SA, Boots EA, Almeida RP, Oh JM, Einerson J, Korcarz CE, Edwards DF, Koscik RL, Dowling MN, Gallagher CL, Bendlin BB, Christian BT, Zetterberg H, Blennow K, Carlsson CM, Asthana S, Hermann BP, Sager MA, Johnson SC, Stein JH, Okonkwo OC (2015) Cardiorespiratory fitness attenuates the influence of amyloid on cognition, J Int Neuropsychol Soc 21, 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Schultz SA, Boots EA, Darst BF, Zetterberg H, Blennow K, Edwards DF, Koscik RL, Carlsson CM, Gallagher CL, Bendlin BB, Asthana S, Sager MA, Hogan KJ, Hermann BP, Cook DB, Johnson SC, Engelman CD, Okonkwo OC (2017) Cardiorespiratory fitness alters the influence of a polygenic risk score on biomarkers of AD, Neurology 88, 1650–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory, Proc Natl Acad Sci U S A 108, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S (2010) Effects of aerobic exercise on mild cognitive impairment: a controlled trial, Arch Neurol 67, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, Wilkins HM, Brooks WM, Billinger SA, Swerdlow RH, Burns JM (2017) Aerobic exercise for Alzheimer’s disease: a randomized controlled pilot trial, PLoS One 12, e0170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Frederiksen KS, Larsen CT, Hasselbalch SG, Christensen AN, Høgh P, Wermuth L, Andersen BB, Siebner HR, Garde E (2018) A 16-week aerobic exercise intervention does not affect hippocampal volume and cortical thickness in mild to moderate Alzheimer’s disease, Front Aging Neurosci 10, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Lamb SE, Sheehan B, Atherton N, Nichols V, Collins H, Mistry D, Dosanjh S, Slowther AM, Khan I, Petrou S, Lall R (2018) Dementia and physical activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial, BMJ 361, k1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. van der Kleij LA, Petersen ET, Siebner HR, Hendrikse J, Frederiksen KS, Sobol NA, Hasselbalch SG, Garde E (2018) The effect of physical exercise on cerebral blood flow in Alzheimer’s disease, Neuroimage Clin 20, 650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Reiter K, Nielson KA, Smith TJ, Weiss LR, Alfini AJ, Smith JC (2015) Improved cardiorespiratory fitness is associated with increased cortical thickness in mild cognitive impairment, J Int Neuropsychol Soc 21, 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Okonkwo OC, Xu G, Dowling NM, Bendlin BB, LaRue A, Hermann BP, Koscik R, Jonaitis E, Rowley HA, Carlsson CM, Asthana S, Sager MA, Johnson SC (2012) Family history of Alzheimer disease predicts hippocampal atrophy in healthy middle-aged adults, Neurology 78, 1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Okonkwo OC, Xu G, Oh JM, Dowling NM, Carlsson CM, Gallagher CL, Birdsill AC, Palotti M, Wharton W, Hermann BP, LaRue A, Bendlin BB, Rowley HA, Asthana S, Sager MA, Johnson SC (2014) Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer’s disease, Cereb Cortex 24, 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Jarvik L, LaRue A, Blacker D, Gatz M, Kawas C, McArdle JJ, Morris JC, Mortimer JA, Ringman JM, Ercoli L, Freimer N, Gokhman I, Manly JJ, Plassman BL, Rasgon N, Roberts JS, Sunderland T, Swan GE, Wolf PA, Zonderman AB (2008) Children of persons with Alzheimer disease, Alzheimer Dis Assoc Disord 22, 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory, Proc Natl Acad Sci U S A 108, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Johnson SC, Koscik RL, Jonaitis EM, Clark LR, Mueller KD, Berman SE, Bendlin BB, Engelman CD, Okonkwo OC, Hogan KJ, Asthana S, Carlsson CM, Hermann BP, Sager MA (2018) The Wisconsin Registry for Alzheimer’s Prevention: a review of findings and current directions, Alzheimers Dement 10, 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Ellis RJ, Jan K, Kawas C, Koller WC, Lyons KE, Jeste DV, Hansen LA, Thal LJ (1998) Diagnostic validity of the Dementia Questionnaire for Alzheimer disease, Arch Neurol 55, 360–365. [DOI] [PubMed] [Google Scholar]
- [30]. Mayer CJ, Steinman L, Williams B, LoGerfo J, Topolski TD (2007) Developing a Telephone Assessment of Physical Activity (TAPA) questionnaire for older adults, Prev Chronic Dis 5, 1–7. [PMC free article] [PubMed] [Google Scholar]
- [31]. Jagust WJ, Bandy D, Chen K, Foster NL, Landau SM, Mathis CA, Price JC, Reiman EM, Skovronsky D, Koeppe RA (2010) The Alzheimer’s Disease Neuroimaging Initiative Positron Emission Tomography Core, Alzheimers Dement 6, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Bendlin BB, Ries ML, Canu E, Sodhi A, Lazar M, Alexander AL, Carlsson CM, Sager MA, Asthana S, Johnson SC (2010) White matter is altered with parental family history of Alzheimer’s disease, Alzheimers Dement 6, 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Debette S, Markus HS (2010) The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis, BMJ 341, c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Gu T, Korosec FR, Block WF, Fain SB, Turk Q, Lum D, Zhou Y, Grist TM, Haughton V, Mistretta CA (2005) PC VIPR: A high-speed 3D phase-contrast method for flow quantification and high- resolution angiography, AJNR Am J Neuroradiol 26, 743–749. [PMC free article] [PubMed] [Google Scholar]
- [35]. Baudic S, Barba G, Thibaudet M, Smagghe A, Remy P, Traykov L (2006) Executive function deficits in early Alzheimer’s disease and their relations with episodic memory, Arch Clin Neuropsychol 21, 15–21. [DOI] [PubMed] [Google Scholar]
- [36]. Hayes SM, Alosco ML, Hayes JP, Cadden M, Peterson KM, Allsup K, Forman DE, Sperling RA, Verfaellie M (2015) Physical activity is positively associated with episodic memory in aging, J Int Neuropsychol Soc 21, 780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician, J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [38]. Delis DC, Kramer JH, Kaplan E, Ober BA (2000) California Verbal Learning Test. [DOI] [PubMed]
- [39]. Delis DC, Kaplan E, Kramer JH (2001) Delis-Kaplan Executive Function System International Psychological Corporation, San Antonio, TX. [Google Scholar]
- [40]. McNair DM, Lorr M, Droppleman LF (1992) Edits Manual for the Profile of Mood States Educational and Industrial Testing Service, San Diego, CA. [Google Scholar]
- [41]. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL (2001) CHAMPS physical activity questionnaire for older adults: outcomes for interventions, Med Sci Sports Exerc 33, 1126–1141. [DOI] [PubMed] [Google Scholar]
- [42]. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P (2003) International Physical Activity Questionnaire: 12-country reliability and validity, Med Sci Sports Exerc 35, 1381–1395. [DOI] [PubMed] [Google Scholar]
- [43]. Sechrist KR, Walker SN, Pender NJ (1987) Development and psychometric evaluation of the exercise benefits/barriers scale, Res Nurs Health 10, 357–365. [DOI] [PubMed] [Google Scholar]
- [44]. McAuley E (1992) The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults, J Behav Med 15, 65–88. [DOI] [PubMed] [Google Scholar]
- [45]. Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale, Sleep 14, 540–545. [DOI] [PubMed] [Google Scholar]
- [46]. Bastien CH, Vallières A, Morin CM (2001) Validation of the Insomnia Severity Index as an outcome measure for insomnia research, Sleep Med 2, 297–307. [DOI] [PubMed] [Google Scholar]
- [47]. Stewart AL, Ware JE, Brook RH, Davies AR (1978) Conceptualization and measurement of health for adults in the Health Insurance Study. Vol II. Physical health in terms of functioning The RAND Corporation, Santa Monica, CA. [Google Scholar]
- [48]. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research, Psychiatry Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- [49]. Dougherty RJ, Lindheimer JB, Stegner AJ, Van Riper S, Okonkwo OC, Cook DB (2018) An objective method to accurately measure cardiorespiratory fitness in older adults who cannot satisfy widely used oxygen consumption criteria, J Alzheimers Dis 61, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Mitchell CC, Stein JH, Cook TD, Salamat S, Wang X, Varghese T, Jackson DC, Garcia CS, Wilbrand SM, Dempsey RJ (2017) Histopathological validation of grayscale carotid plaque characteristics related to plaque vulnerability, Ultrasound Med Biol 43, 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Mitchell C, Korcarz CE, Gepner AD, Kaufman JD, Post W, Tracy R, Gassett AJ, Ma N, McClelland RL, Stein JH (2018) Ultrasound carotid plaque features, cardiovascular disease risk factors and events: the multi-ethnic study of atherosclerosis, Atherosclerosis 276, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Mitchell C, Piper ME, Korcarz CE, Hansen K, Weber J, Fiore MC, Baker TB, Stein JH (2018) Echogenicity of the carotid arterial wall in active smokers, J Diagn Med Sonogr 34, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Mitchell CC, Korcarz CE, Gepner AD, Nye R, Young RL, Matsuzaki M, Post WS, Kaufman JD, McClelland RL, Stein JH (2019) Carotid artery echolucency, texture features, and incident cardiovascular disease events: the MESA study, J Am Heart Assoc 8, e010875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Mitchell CC, Korcarz CE, Tattersall MC, Gepner AD, Young RL, Post WS, Kaufman JD, McClelland RL, Stein JH (2018) Carotid artery ultrasound texture, cardiovascular risk factors, and subclinical arterial disease: the multi-ethnic study of atherosclerosis (MESA), BJR 91, 20170637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Gepner AD, Tedla Y, Colangelo LA, Tattersall MC, Korcarz CE, Kaufman JD, Liu K, Burke GL, Shea S, Greenland P, Stein JH (2017) Progression of carotid arterial stiffness with treatment of hypertension over 10 years: the multi-ethnic study of atherosclerosis, Hypertension 69, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Gepner AD, Korcarz CE, Colangelo LA, Hom EK, Tattersall MC, Astor BC, Kaufman JD, Liu K, Stein JH (2014) Longitudinal effects of a decade of aging on carotid artery stiffness: the multiethnic study of atherosclerosis, Stroke 45, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Stern R, Tattersall MC, Gepner AD, Korcarz CE, Kaufman J, Colangelo LA, Liu K, Stein JH (2015) Sex differences in predictors of longitudinal changes in carotid artery stiffness: the multi-ethnic study of atherosclerosis, Arterioscler Thromb Vasc Biol 35, 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Mitchell CKC, Aeschlimann SE, Korcarz CE (2004) Carotid intima-media thickness testing: technical considerations, J Am Soc Echocardiogr 17, 690–692. [DOI] [PubMed] [Google Scholar]
- [59]. Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS (2008) Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force endorsed by the Society for Vascular Medicine, J Am Soc Echocardiogr 21, 93–111. [DOI] [PubMed] [Google Scholar]
- [60]. Aeschlimann SE, Mitchell CKC, Korcarz CE (2004) Ultrasound brachial artery reactivity testing: technical considerations, J Am Soc Echocardiogr 17, 697–699. [DOI] [PubMed] [Google Scholar]
- [61]. Korcarz CE, Benca R, Barnet JH, Stein JH (2016) Treatment of obstructive sleep apnea in young and middle-aged adults: Effects of positive airway pressure and compliance on arterial stiffness, endothelial function, and cardiac hemodynamics, J Am Heart Assoc 5, e002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP (2002) Multi-ethnic study of atherosclerosis: objectives and design, Am J Epidemiol 156, 871–881. [DOI] [PubMed] [Google Scholar]
- [63]. Hagstromer M, Ainsworth BE, Oja P, Sjostrom M (2010) Comparison of a subjective and an objective measure of physical activity in a population sample, J Phys Act Health 7, 541–550. [DOI] [PubMed] [Google Scholar]
- [64]. Dyrstad SM, Hansen BH, Holme IM, Anderssen SA (2014) Comparison of self-reported versus accelerometer-measured physical activity, Med Sci Sports Exerc 46, 99–106. [DOI] [PubMed] [Google Scholar]
- [65]. Karvonen M, Kentala E, Mustala O (1957) The effects of training on heart rate; a longitudinal study, Ann Med Exp Biol Fenn 35, 307–315. [PubMed] [Google Scholar]
- [66]. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD (2018) The physical activity guidelines for Americans, JAMA 320, 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, Bakken S, Kaplan CP, Squiers L, Fabrizio C, Fernandez M (2009) How we design feasibility studies, Am J Prev Med 36, 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Dougherty RJ, Schultz SA, Kirby TK, Boots EA, Oh JM, Edwards D, Gallagher CL, Carlsson CM, Bendlin BB, Asthana S, Sager MA, Hermann BP, Christian BT, Johnson SC, Cook DB, Okonkwo OC (2017) Moderate physical activity is associated with cerebral glucose metabolism in adults at risk for Alzheimer’s disease, J Alzheimers Dis 58, 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. ten Brinke LF, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran-Khan K, Liu-Ambrose T (2015) Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial, Br J Sports Med 49, 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Matura S, Fleckenstein J, Deichmann R, Engeroff T, Füzéki E, Hattingen E, Hellweg R, Lienerth B, Pilatus U, Schwarz S, Tesky VA, Vogt L, Banzer W, Pantel J (2017) Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: results of the randomised controlled SMART trial, Transl Psychiatry 7, e1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Kozey-Keadle S, Staudenmayer J, Libertine A, Mavilia M, Lyden K, Braun B, Freedson P (2014) Changes in sedentary time and physical activity in response to an exercise training and/or lifestyle intervention, J Phys Act Health 11, 1324–1333. [DOI] [PubMed] [Google Scholar]
- [72]. Wheeler MJ, Dempsey PC, Grace MS, Ellis KA, Gardiner PA, Green DJ, Dunstan DW (2017) Sedentary behavior as a risk factor for cognitive decline? a focus on the influence of glycemic control in brain health, Alzheimers Dement 3, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Booth ML, Bauman A, Owen N, Gore CJ (1997) Physical activity preferences, preferred sources of assistance, and perceived barriers to increased activity among physically inactive Australians, Prev Med 26, 131–137. [DOI] [PubMed] [Google Scholar]
- [74]. Protas HD, Chen K, Langbaum JBS, Fleisher AS, Alexander GE, Lee W, Bandy D, de Leon MJ, Mosconi L, Buckley S, Truran-Sacrey D, Schuff N, Weiner MW, Caselli RJ, Reiman EM (2013) Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease, JAMA Neurol 70, 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease, Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Vidoni ED, Johnson DK, Morris JK, Van Sciver A, Greer CS, Billinger SA, Donnelly JE, Burns JM (2015) Dose-response of aerobic exercise on cognition: a community-based, pilot randomized controlled trial, PLoS One 10, e0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].U.S. Department of Health and Human Services (2018) National Plan to Address Alzheimer’s Disease: 2018 Update. U.S. Department of Health and Human Services, Washington, DC.