Abstract
This review focuses on environmental implications and applications of engineered magnetite (Fe3O4) nanoparticles (MNPs) as a single phase or a component of a hybrid nanocomposite that exhibits superparamagnetism and high surface area. MNPs are synthesized via co-precipitation, thermal decomposition and combustion, hydrothermal process, emulsion, microbial process, and green approaches. Aggregation/sedimentation and transport of MNPs depend on surface charge of MNPs and geochemical parameters such as pH, ionic strength, and organic matter. MNPs generally have low toxicity to humans and ecosystem. MNPs are used for constructing chemical/biosensors and for catalyzing a variety of chemical reactions. MNPs are used for air cleanup and carbon sequestration. MNP nanocomposites are designed as antimicrobial agents for water disinfection and flocculants for water treatment. Conjugated MNPs are widely used for adsorptive/separative removal of organics, dyes, oil, arsenic, phosphate, molybdate, fluoride, selenium, Cr(VI), heavy metal cations, radionuclides, and rare earth elements. MNPs can degrade organic/inorganic contaminants via chemical reduction or catalyze chemical oxidation in water, sediment, and soil. Future studies should further explore mechanisms of MNP interactions with other nanomaterials and contaminants, economic and green approaches of MNP synthesis, and field scale demonstration of MNP utilization.
Keywords: Fe3O4, Hybrid nanocomposites, Superparamagnetic, Catalyst, Remediation
1. Introduction
Over the past few years, the scientific community has witnessed a dramatic increase in the number of papers published on implications and applications of magnetite (Fe3O4) nanoparticles (MNPs). Magnetite is a common iron oxide that exhibits outstanding physico-chemical properties due to the presence of both Fe(II) and Fe(III) in its structure. It behaves as superparamagnetic as the particle size is reduced to a few nanometers. MNPs have been used for biomedical applications such as biosensing, cell tracking, tissue engineering, magnetic resonance imaging (MRI)/optical multimodal imaging, targeted drug delivery, and hyperthermia therapeutic cancer treatment [1], [2], [3], [4], [5], [6], [7], [8]. Whereas review articles have addressed biomedical applications of MNPs [1], [2], [3], [4], [5], [6], [7], there has been limited efforts on the literature review of environmental aspects of MNPs [9], [10]. A thorough synthesis of recent literature is needed with regard to environmental implications and applications of MNPs in order to understand the current status of research and to explore future opportunities. This review tries to meet that need.
2. Synthesis of nanoscale magnetite and its hybrid nanocomposites
Several previous review articles have covered the synthesis, protection, functionalization, and application of MNPs, as well as the magnetic properties of nanostructured systems [11], [12], [13]. Substantial progress in the size and shape control of MNPs has been made by developing methods such as co-precipitation, thermal decomposition and/or reduction, micelle synthesis, and hydrothermal synthesis. Corrosion protection strategies have been developed using surfactant/polymer coating, silica coating and carbon coating of MNPs or embedding them in a matrix/support [12]. Unique shape MNPs such as those of high aspect ratio can be achieved in two main synthetic routes: (i) direct synthesis (in which the anisotropic growth is directed by tuning the reaction conditions or by using templates) and (ii) assembly methods (in which the high aspect ratio is achieved by assembly from individual building blocks) [14]. Table 1 lists examples of reported methods for synthesis of MNPs and their hybrid composites.
Table 1.
Common methods and examples for synthesis of MNPs and their hybrids.
| Method | Reagents and conditions | Stabilizer | Product | Ref. |
|---|---|---|---|---|
| Co-precipitation | FeSO4·7H2O, FeCl3, NH4OH | PVA | Fe3O4, 4–10 nm | [15] |
| FeCl2, FeCl3, NH4OH, 25–80 °C, | Fe3O4, 20 nm | [16] | ||
| FeSO4·7H2O, FeCl3·6H2O, NaOH | Citrate or not | Fe3O4, 8 nm with citrate, 14–28 nm without citrate | [18] | |
| FeSO4·7H2O, FeCl3·6H2O, NH4OH, 80 °C | Phosphate | Fe3O4, 9–40 nm | [20] | |
| Fe(acac)3, Co(acac)2, Oleic acid, benzyl ether, 290 °C | CoxFe3–xO4, 35–110 nm | [21] | ||
| FeCl3, AlCl3·6H2O, CoCl2·6H2O, 70 °C | CoFe2–xAlxO4, 20–63 nm | [22] | ||
| Thermodecomposition | FeCl3·6H2O, 2-pyrrolidone, 245 °C, reflux 1–24 h | Fe3O4, 4–60 nm | [30] | |
| Fe(acac)3, 2-pyrrolidone, 200–240 °C | α,ω-dicarboxyl-terminated poly(ethylene glycol) | Fe3O4, 6–15 nm with average 11 nm | [31] | |
| Fe(acac)3, oleylamine and benzyl ether, | Fe3O4, 8 ± 0.4 nm | [36] | ||
| Combustion waves | Fe2O3 NPs, nitrocellulose, 740 °C | Fe3O4, 5–20 nm | [37] | |
| Hydrothermal synthesis | metal linoleate (solid), an ethanol–linoleic acid liquid phase, and a water–ethanol solution | Fe3O4, 9 nm CoFe2O4, 12 nm | [38] | |
| FeCl3, ethylene glycol, NaOAc, 200 °C | Fe3O4–graphite, 100 nm | [42] | ||
| Composition controlled synthesis | Various surfactants | MFe2O4, M = Mg, Fe, Co, Ni, Cu, Zn, 3 nm | [44] | |
| Emulsion synthesis | Fe3O4, histidine | Fe3O4–histidine composite, 500 nm | [46] | |
| Microbial synthesis | β-FeOOH, ZnxFe1–xOOH, Clostridium sp. | Fe3O4, 5–10 nm using β-FeOOH, ZnxFe3–xO4, 3–8 nm using ZnxFe1–xOOH | [47] | |
| β-FeOOH, S. putrefaciens CN32, 9,10-anthraquinone-2,6-disulfonate | Fe3O4, framboidal, 20–50 nm | [48] | ||
| Green synthesis | Natural biorenewable resources | Plant extracts | Fe3O4, hybrids | [87] |
2.1. Co-precipitation
Co-precipitation is a simple way to synthesize MNPs by titrating aqueous Fe2+/Fe3+ salt solutions with a base under inert atmosphere at room temperature or at an elevated temperature. The size, shape, and composition of the magnetic NPs depend on the type of salts used (e.g., chlorides, sulfates, nitrates), the Fe2+/Fe3+ ratio, the reaction temperature, the pH value and ionic strength of the media [15], [16]. The use of organic additives as stabilization and/or reducing agents such as polyvinlyalcohol (PVA) facilitates preparation of monodisperse MNPs (4–10 nm) [15]. While elevated temperature is often used in the synthesis, room-temperature air-atmosphere co-precipitation method can also be used to tune the magnetic properties of iron oxide NPs with the compositional control between magnetite and maghemite (γ-Fe2O3) [17].
Organic ligands influence co-precipitation products. Citrate helps produce smaller MNPs (8 nm) compared to without citrate (14–28 nm) and better dispersion and strong magnetic performance [18]. Citrate also prevents oxidation of magnetite to produce hematite. In another study, the primary particle size of magnetite is tuned from 5 nm to 15 nm by varying the citrate/iron precursor ratio during the normal phase hydrolysis reaction, while the second iteration of citrate stabilizes the nanoclusters [19]. Inorganic ligands are also used to modify the products. Water dispersible MNPs with a phosphate monolayer capping has been synthesized by a single-step coprecipitation method using Fe2+/Fe3+ salt solutions, ammonia and H3PO4. The phosphate capped MNPs show excellent long term stability (>2 years of shelf life) [20].
Substituted MNPs can be synthesized via co-precipitation. For example, cubic cobalt-substituted magnetite CoxFe3−xO4 nanocubes (35–110 nm) with uniform composition distributions of Co, Fe and O, have been obtained via solution synthesis [21]. NPs of the ferrite system CoFe2−xAlxO4 (x = 0.0, 0.3, 0.7 and 1.0) have been synthesized through the co-precipitation technique to form a nano-size (20–63 nm) single spinel phase [22]. Magnetic properties such as coercivity and saturation magnetization can be controlled by changing the non-magnetic Al3+ ions content. In another study, mixed-ferrites produced from electroplating wastewater for metal recovery is used as the magnetic agents. With the addition of lanthanides and F− ions, a novel magnetite near infrared photocatalyst of Er3+/Tm3+/Yb3+-(CaF2/ZnFe2O4/ZnO) (ETY-FCZ) is further synthesized, which results in higher removal rates of methyl orange and salicylic acid compared to those of Ca-Zn magnetite precursor [23]. Hybrid Fe3O4-graphite composites [24] and hollow Fe3O4–Fe NPs with graphene sheets [25] have been prepared by in-situ chemical precipitation as high-performance electromagnetic wave absorbing materials. A recent study has explored low energy and chemically-mild process of co-nanoprecipitation using superparamagnetic iron oxide nanoparticles (SPIONs) and homopolymers or amphiphilic block copolymers, of varying architecture and hydrophilic/hydrophobic balance, which efficiently generates near monodisperse SPION-containing polymer NPs with complete retention of magnetism, and highly reversible aggregation and re-dispersion behavior [26]. One advantage of this method is that the quality of MNPs is reproducible. The drawback is that the size distribution of MNPs prepared by co-precipitation tend to be rather polydisperse.
2.2. Thermal decomposition and combustion
Thermal decomposition of organometallic compounds in high-boiling organic solvents containing stabilizing surfactants have been used to prepare monodisperse magnetic nanocrystals with smaller size [27], [28], [29]. Water soluble MNPs have been prepared using FeCl3·6H2O and 2-pyrrolidone as coordinating solvent under reflux at 245 °C [30]. The mean particle size is 4, 12, and 60 nm, respectively, when the reflux time is 1, 10, and 24 h. The shapes of the particles change from spherical at early stage to cubic morphologies with increasing reflux time. The same group has also developed a one-pot synthesis of water-soluble MNPs prepared under similar reaction conditions by the addition of α,ω-dicarboxyl-terminated poly(ethylene glycol) as a surface capping agent [31]. Most applications require a stable presentation of a defined surface chemistry; therefore, the native shell has to be completely exchanged for dispersants with irreversible affinity to the NP surface. A multiple exchange scheme has been developed to completely and irreversibly replace ligand on monodisperse MNPs synthesized with a strongly bound ligand shell of oleic acid [32]. Ligand exchange employing either citric acid or meso-2,3-dimercaptosuccinic acid (DMSA) ligand has been used to change monodisperse hydrophobic MNPs to hydrophilic MNPs produced by thermal decomposition of Fe(acac)3 in benzyl ether [33]. Novel-morphological Fe3O4 nanosheets with magnetochromatic property has been prepared by a modified solvothermal method. Such nanosheets could form one-dimension photonic crystal under an external magnetic field. The Fe3O4 nanosheets suspension could strongly diffract visible light and display varied colors with changing the intensity of the magnetic field [34]. Monodisperse superparamagnetic MNPs coated with oleic acid have been prepared by thermal decomposition of Fe(III) glucuronate. To make the MNPs dispersible in water, the particle surface is modified with α-carboxyl-ω-bis(ethane-2,1-diyl)phosphonic acid-terminated poly(3-O-methacryloyl-α-d-glucopyranose) (PMG–P). The PMG–P&Fe3O4 NPs have the potential to be used as contrast agents for MRI [35]. Monodisperse 8 nm MNPs have been synthesized by the thermal decomposition of iron(III) acetylacetonate in oleylamine and then are deposited onto n-type silicon wafer having the Al ohmic contact. The Au/Fe3O4 NPs/n-Si/Al heterojunction device shows unique electrical properties [36].
A facile one-pot synthesis method has been developed using combustion waves that simultaneously achieves fast reduction and direct formation of carbon coating layers on iron oxide nanostructures [37]. Hybrid composites of Fe2O3 NPs and nitrocellulose are fabricated by a wet impregnation process. Self-propagating combustion waves along interfacial boundaries between the surface of the metal oxide and the chemical fuels enable the release of oxygen from Fe2O3. This accelerated reaction directly transforms Fe2O3 into Fe3O4 nanostructures with a carbon coating layers of 5–20 nm thickness. The use of combustion waves may permit the precise manipulation of the chemical compositions of nanostructures, as well as the formation of organic/inorganic hybrid nanostructures.
2.3. Hydrothermal synthesis
A liquid–solid–solution reaction has been used under hydrothermal conditions to synthesize a broad range of nanostructured materials including magnetite. The system consists of metal linoleate (solid), an ethanol–linoleic acid liquid phase, and a water–ethanol solution at different reaction temperatures under hydrothermal conditions. Nanocrystals of Fe3O4 and CoFe2O4 can be prepared in very uniform sizes of about 9 and 12 nm, respectively [38]. In another study, Fe3O4-implanted ZnO and pristine ZnO nanosheets are synthesized hydrothermally. The composite nanosheets are photostable, reusable, and magnetically recoverable, revealing potential application in mineralization of organic pollutants [39]. A one-step hydrothermal process with silk fibroin (SF) nanofibers as the template and coating has been developed to synthesize core–shell magnetite/SF NPs with limited controllable sizes [40]. Monodispersed Fe3O4 vesicular nanospheres (160 nm) have been fabricated solvothermally in the mixed solution of ethylene glycol (EG) and ethylenediamine (EN) with the surfactant polyvinyl pyrrolidone (PVP) [41]. A Fe3O4–graphite composite has been synthesized using a one-step solvothermal method in which graphite powder in EG is mixed with FeCl3 dissolved in EG and NaOAc followed by heating at 200 °C for 10 h under autogenous pressure. The Fe3O4–graphite composite is used as a heterogeneous Fenton-like catalyst for the degradation of antibiotic levofloxacin (LEV) in an aqueous solution [42].
An easy mixed solvent solvothermal/hydrothermal method has been developed for the one-step synthesis of monodisperse Fe3O4 and α-Fe2O3 NPs. The morphologies can be varied from spherical, to octahedral, to rice like, and even to fusiform; the size can be continuously tuned to a range within 30–290 nm. The morphology-, dimension-, and phase-controlled growth of FexOy nanocrystals can be achieved by tuning kinetic factors, such as the H2O volume fraction, Fe3+ concentration, reaction temperature, and the ratio of alkali/Fe3+ [43]. In a separate study, a generalized hydrothermal method has been developed for the synthesis of single-crystalline magnetic spinel ferrite NPs (MFe2O4, M = Mg, Fe, Co, Ni, Cu, and Zn) to the smallest size (i.e., 3 nm) [44]. A facile hydrothermal method has been developed to control the thickness of the carbon coating formation on the surface of magnetic NPs from sucrose by using CH3COONH4 as a structure guiding agent and by adjusting the reaction time [45].
2.4. Emulsion synthesis
A combination strategy of the inverse emulsion crosslinking approach and the colloidal assembly technique has been tested to synthesize Fe3O4/histidine composite nanoclusters as new-type magnetic porous nanomaterials (NMs). The nanoclusters possess uniform morphology, high magnetic content and excellent protein adsorption capacity, exhibiting their great potential for bio-separation [46].
2.5. Microbial synthesis
Microbial synthesis of MNPs and Zn-substituted MNPs has been achieved by iron-reducing bacteria (Clostridium sp.) using akaganeite (β-FeOOH) or Zn-substituted akaganeite (β-ZnxFe1-xOOH) during glucose fermentation [47]. In another study, framboidal magnetite is formed as a major product in the presence of an electron shuttle [9,10-anthraquinone-2,6-disulfonate (AQDS)] via akaganeite (β-FeOOH) bioreduction by the iron(III)-reducing bacterium (IRB) Shewanella putrefaciens CN32 [48]. Microbial synthesis has been tested for preparing magnetically recoverable noble metal NP catalysts. Pd/Fe3O4, Au/Fe3O4, and PdAu/Fe3O4 nanocomposites are biosynthesized by Shewanella oneidensis MR-1. Microbial cells firstly transform akaganeite into magnetite, which then serves as support for the further synthesis of Pd, Au and PdAu NPs from respective precursor salts [49]. Compared with engineered MNPs synthesized by chemical approaches, bacterial MMPs have the properties of large production, monodispersity, high crystallinity, and close-to-bulk magnetization, which enable them to be the highly promising MNPs for use in nanobiotechnology [50]. A drawback of microbial synthesis is that the MNPs are likely coated with biomolecules produced by microbial activities that may not be desirable for certain applications. Removal or exchange of such coatings may be necessary.
2.6. Hybrid magnetite nanocomposites
Increasing efforts have been focused on the synthesis and applications of hybrid MNPs by taking advantages of individual NPs and their positive interactions. For example, a combination of superstructure-forming amphiphilic block copolymers and SPION produces new nano/microcomposites with unique size-dependent properties (Fig. 1) [51]. Graphene has been used to form nanocomposites with MNPs to make tunable ferromagnet [52]. A one-pot synthesis of reduced graphene oxide (RGO)/Fe3O4 composites has been reported. By the electrostatic interaction of exfoliated GO and Fe3+ ions, GO/Fe3+ ions are prepared in a diethylene glycol. In situ formation of Fe3O4 NPs on GO sheets and reduction of GO are then achieved simultaneously by the thermal decomposition reaction of Fe(acac)3 at high temperature [53]. The growth mechanism of magnetic NPs in the presence of graphite oxide (GO) has been investigated by varying the iron precursor dosage and reaction time. The synthesized magnetic NPs are anchored on the GO sheets due to the abundant oxygen-containing functionalities on the GO sheets such as carboxyl, hydroxyl and epoxy functional groups [54]. Superparamagnetic monodisperse Fe3O4/GO nanocomposite has been synthesized by a facile one-pot solvothermal synthesis using FeCl3·6H2O as the iron resource, EG as the reaction solvent, NaHCO3 as the precipitant and adding a GO suspension to EG solvent [55]. Nanocomposites with ultra-small magnetite (Fe3O4) NPs (~3 nm) uniformly anchored on RGO nanosheets have been successfully synthesized for anodes in sodium-ion batteries by a novel single-step high-temperature coprecipitation approach [56]. An in situ synthesis of very small MNPs (<12 nm) on the surface of graphene sheets has been achieved through the direct, liquid-phase exfoliation of crystalline graphite and the subsequent chemical functionalization of the graphene sheets via the well-established 1,3-dipolar cycloaddition reaction [57]. A magnetic composite of multi-walled carbon nanotubes (MWCNTs) and iron oxide NPs (10–30 nm) has been prepared by chemical precipitation. Transmission electron microscopy (TEM) observation indicates that the iron oxide NPs are highly dispersed on the outer walls of the MWCNTs [58].
Figure 1.
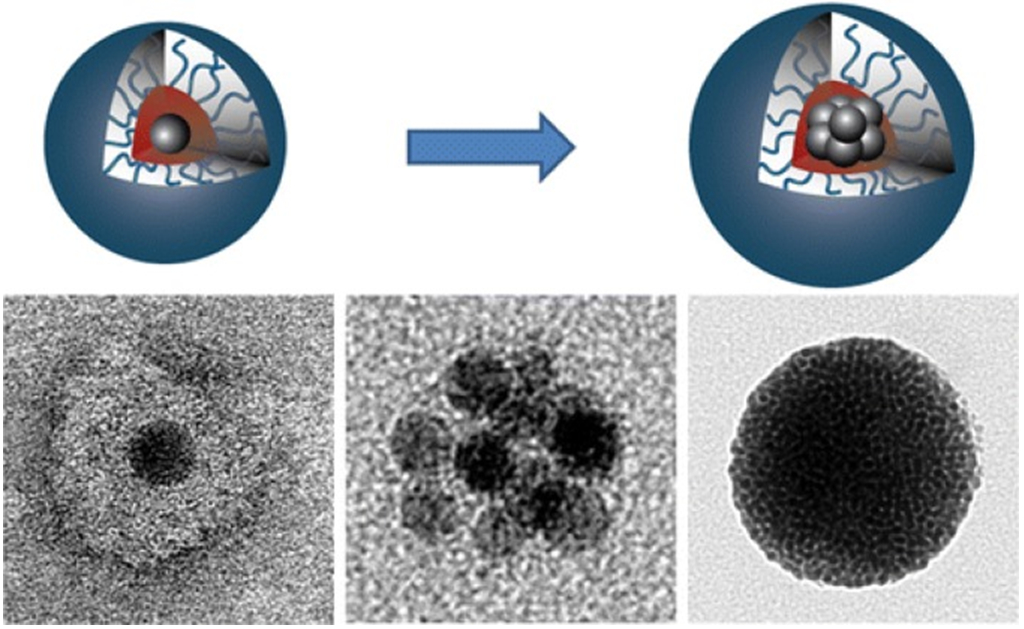
Polymer-assisted self-assembly of superparamagnetic iron oxide nanoparticles into well-defined clusters that controls the collective magnetic properties. Reprinted from Ref. [51]. Copyright 2014 American Chemical Society.
The combination of Fe3O4 with RGO generates a new hybrid substrate for the dispersion of noble metal NPs. For example, well-dispersed silver (Ag) NPs loaded on the surface of Fe3O4 modified RGO have been evaluated with the reduction of 4-nitrophenol (4-NP) into 4-aminophenol with excellent catalytic stability and much higher catalytic activity than the corresponding RGO/Ag catalyst [59]. A novel ternary ZnO/AgI/Fe3O4 nanocomposite show superior photocatalytic activity in degradation of rhodamine B (RhB), methylene blue (MB) and methyl orange than that of the ZnO/Fe3O4 nanocomposite due to more visible-light absorption ability and efficiently separation of the charge carriers [60].
A simple protocol for covalent immobilization of biotin onto the surface of MNPs for improving the biocompatibility of original MNPs has been realized. MNPs are first prepared by co-precipitation method which is subsequently anchored with functionalized biotin [61]. Nobel metals have been incorporated into MNPs to increase their reactivity. A simple single-step thermal decomposition of Fe(III) acetylacetonate complex, with the presence of silver seeds formed in the same reaction mixture, gives rise to novel compact heterostructures: brick-like Ag@Fe3O4 core–shell NPs (Fig. 2) [62]. Magnetic NPs have been synthesized and coated with tetraethyl orthosilicate and aminosilane to create functional amino groups before moxifloxacin is conjugated to the modified MNPs using glutaraldehyde as crosslinker [63].
Figure 2.
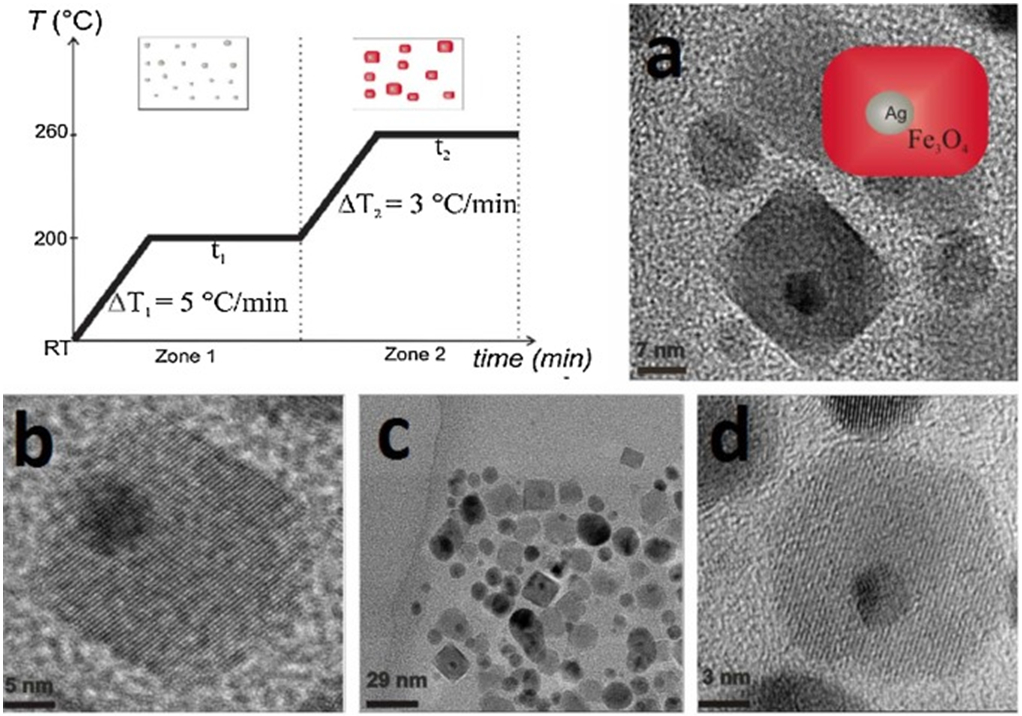
Temperature profile of the temperature-paused single-step thermal decomposition synthesis. Boxes sketch the expected predominant structures for each time zone. Typically, both waiting times are 120 min. Images: TEM images of brick-like nanoparticles (BLNs) obtained following the temperature-paused single-step protocol. Ag corresponds to the dark contrast, while lighter particles correspond to magnetite. Plain magnetite nanoparticles which are formed are also shown in (c). (a, b and d) are different amplifications of BLNs in order to understand the structure. Reprinted from Ref. [62]. Copyright 2015 American Scientific Publishers.
Starch-coated MNPs have been synthesized by the precipitation–oxidation of ferrous hydroxide method. The size of the as-prepared MNPs is tuned from 15 to 100 nm by changing the time of addition of a starch solution on the reaction system. Also, the starch-coating over MNPs assures good water-dispersibility, stability, and possible biocompatibility (Fig. 3) [64]. A spherical core–shell magnetic agarose nanocomposite has been prepared using a mixture of Fe(II) and Fe(III) salts solution, Span 85 as surfactant and cyclohexane as organic solvent [65]. Coatings have been also applied to microsized magnetite, for example, polypyrrole (PPy)–coated Fe3O4 hybrid particles have been synthesized under sonication [66].
Figure 3.
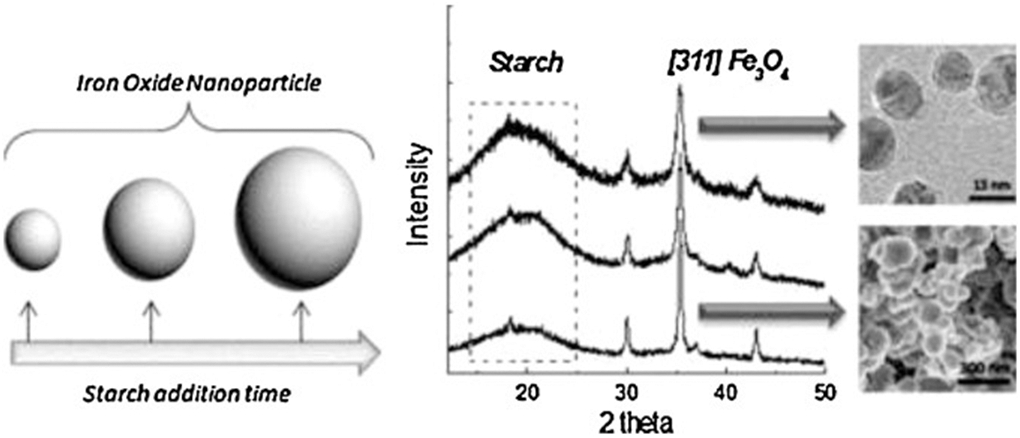
Starch coated Fe3O4 nanoparticles are synthesized by the precipitation oxidation method. Starch is employed as an effective control agent to tune the nanoparticle size and starch coated nanoparticles are water dispersible and forms a ferrofluid. Reprinted from Ref. [64]. Copyright 2015 Taylor & Francis.
Uniquely shaped MNP composites have been synthesized. A hairy core–shell structure is synthesized by combining distillation–precipitation polymerization with subsequent surface-initiated atom transfer radical polymerization [67]. A facile synthesis of rose-like Au/Pd–Fe3O4 nanocomposites has been reported via controlled thermal decomposition of Fe(CO)5 and reduction of Pd(OAc)2, followed by the immobilization of Au NPs onto the Pd–Fe3O4 supports [68]. Magnetic iron oxide@SiO2–Au@C particles with different shapes, such as pseduocube, ellipsoid, and peanut, have been synthesized using hematite as templates and precursors of magnetic iron oxide (Fig. 4) [69]. The as-obtained magnetic particles demonstrate uniform sizes, shapes, and well-designed core–shell nanostructures. Catalytic performance of the peanut-like particles keeps almost unchanged without a noticeable decrease in the reduction of 4-nitrophenol (4-NP) in 8 min even after 7 cycles, indicating excellent reusability of the particles [69].
Figure 4.
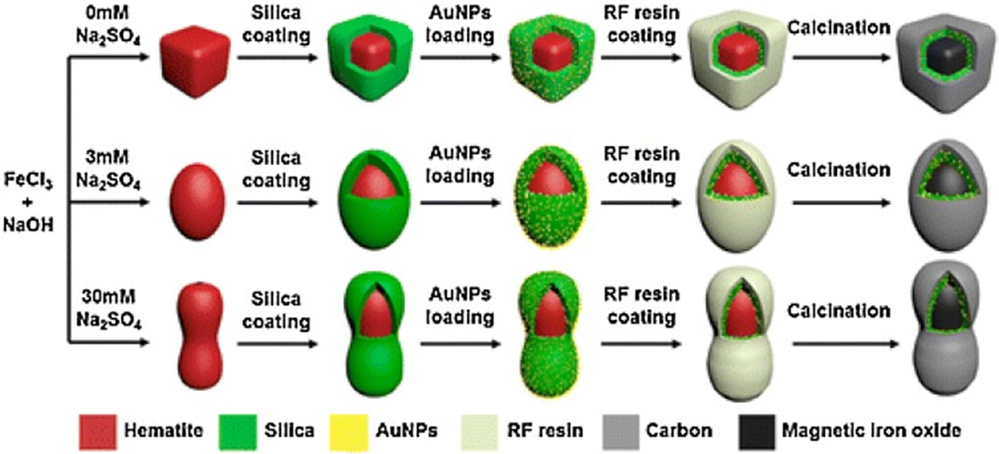
Magnetic iron oxide@SiO2–Au@C particles with shapes of pseduocube, ellipsoid, and peanut, are synthesized using hematite as templates and precursors of magnetic iron oxide. The Au NPs of ~6 nm are uniformly distributed between the silica and carbon layers, preventing the aggregation and reduced the loss of the metal nanocrystals during recycling. Reprinted from Ref. [69]. Copyright 2015 American Chemical Society.
Composite thermoplastic elastomers (CTPEs) of copolymer-grafted MNPs have been synthesized and characterized to generate magnetic CTPEs, which combines the magnetic property of MNPs and the thermoplastic elasticity of the grafted amorphous polymer matrix [70]. Calcination of hydrated iron salts in the pores of both spherical and rod-shaped mesoporous silica NPs changes the internal structure from an ordered 2D hexagonal structure into a smaller number of large voids in the particles with sizes ranging from large hollow cores down to ten nanometer voids. The phase of the iron oxide NPs is α-Fe2O3 when annealed at 500 °C, and Fe3O4 when annealed at lower temperatures [71]. Fe3O4/SiO2 porous nanorods with one-dimensional core/shell structure and high aspect ratio have been synthesized by a wet chemical method. Based on the nanorods, flexible Fe3O4/SiO2/PVDF nanocomposites have been first prepared by embedding the Fe3O4/SiO2 nanorods in a polyvinylidene fluoride (PVDF) matrix. The Fe3O4/SiO2/PVDF nanocomposite shows excellent microwave absorption performance [72]. In another study, the pre-shell-post-core route has been used to synthesize the Fe3O4@SiO2 rattle-type nanocomposite [73].
One-dimensional Ag–Fe3O4 core–shell heteronanowires have been synthesized by a facile and effective coprecipitation method, in which silver nanowires (AgNWs) are used as the nucleation site for growth of Fe3O4 in aqueous solution [74]. In a separate study, a layer of MNPs (50 nm) are synthesized and simultaneously coated on the surface of the polyethylene terephthalate (PET) fabric using FeSO4 as the precursor, NaOH as the precipitant, and sodium dodecyl benzene sulfonate as the dispersant at controlled pH during hydrothermal treatment. The magnetite-coated PET fabric is then modified with silane coupling agent A-151. The capabilities of magnetic response remain unchanged indicating durable washing [75]. Superparamagnetic poly(urea-urethane), PUU, nanocomposites have been obtained by interfacial miniemulsion polymerization with high encapsulation efficiency [76]. Magnetic PVA@Fe3O4/CA/AS nanocomposite films (PVA = poly(vinyl alcohol), CA = citric acid, AS = ascorbic acid) containing 4, 8, and 12 wt% of the modified NPs have been successfully manufactured under ultrasonic irradiation [77]. In a separate study, the detailed characterization of core/shell iron-oxide/silica NPs reveals how the superparamagnetic systems are actually composed of a Fe3O4 inner core, γ-Fe2O3 outer core, iron orthosilicate interphase layer, and exterior silica shell [78]. Chain-like core–shell structure Fe3O4@SiO2@chitosan composite NPs have been synthesized by a two-step coating and following crosslinking glutaraldehyde on chitosan shell. The composite particles (105 nm) are nearly monodisperse with a core diameter of 80 nm and chitosan shell thickness of 12 nm (Fig. 5). The composite nanoparticles show a decreasing adsorption in the order, Hg2+ > Pb2+ > Cu2+ in water [79].
Figure 5.
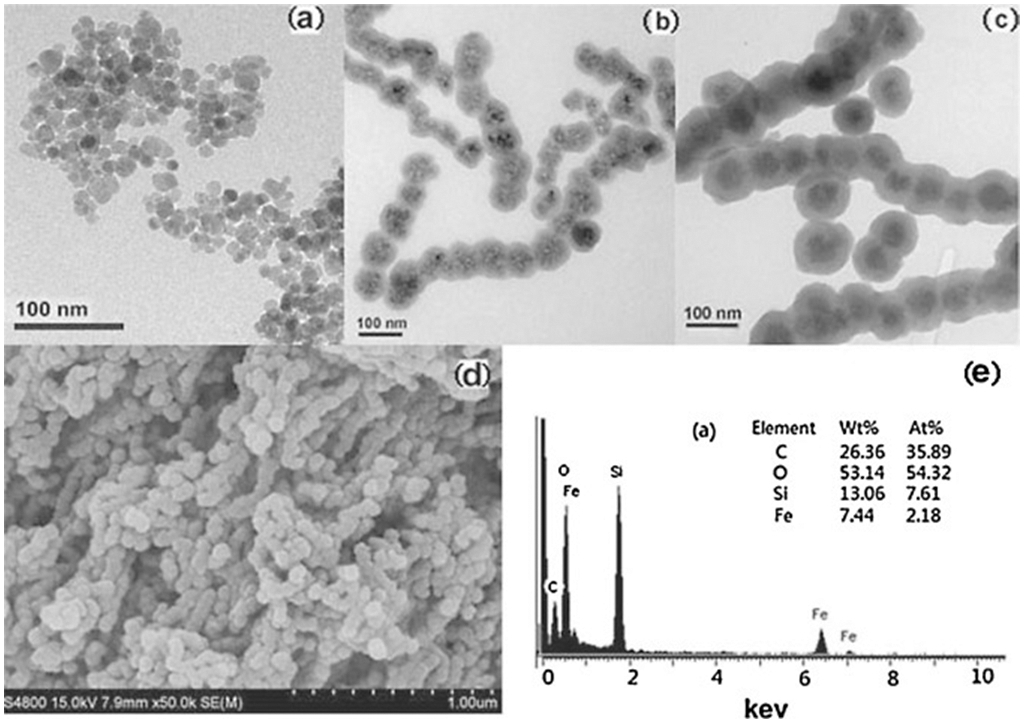
The TEM of (a), Fe3O4 (22 nm), (b), Fe3O4@SiO2 (core 60 nm, shell 10 nm) and (c), Fe3O4@SiO2@chitosan (total 105 nm, thickness of chitosan 12 nm) and (d), the SEM of Fe3O4@SiO2@chitosan, and (e), the EDS of sample-chitosan. The composite NPs having spherical core–shell and chain-like structure with a good dispersion are effective adsorbents for aquosus Hg2+, Pb2+, and Cu2+. Reprinted from Ref. [79]. Copyright 2016 American Scientific Publishers.
Flexible polydopamine (PDA)–Co0.3Ni0.7Fe2O4@SiO2 nanofibrous membranes (NFMs) with a hierarchical structure and strong magnetism have been prepared by gelation, calcination and dopamine self-polymerization on electrospun SiO2 NFMs [80]. Magnetic Fe3O4–PDA hybrid hollow microspheres, in which MNPs are firmly incorporated in the cross-linked PDA shell, have been prepared through the formation of core/shell polystyrene spheres/Fe3O4–PDA composites based on template-induced covalent assembly method, followed by core removal in a tetrahydrofuran solution. The Fe3O4–PDA hybrid hollow microspheres exhibit intrinsic peroxidase-like activity, as they could quickly catalyze the oxidation of typical substrates 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of H2O2 [81]. MNPs have been incorporated into Mg–Al–CO3 layered double hydroxide (LDH) to remove Cd2+ from water by magnetic separation of the immobilized Cd2+ via CdCO3 precipitation, surface adsorption and surface complexation [82].
New strategies for the controllable synthesis of complex hollow structures and their application in environmental remediation have been developed. Chinese researchers have reported a facile in situ growth process of SiO2@Fe3O4@MnO2, followed by an etching method to synthesize a hierarchical hollow structure, namely Fe3O4@MnO2 ball-in-ball hollow spheres (Fe3O4@MnO2 BBHs) for degradation of MB by catalytic generation of active radicals from peroxymonosulfate (PMS) [83]. A recent study reports fabrication of rationally-designed multicomponent nanocomposites comprising a magnetite core and an outer silver-decorated anatase shell and their application for visible-light hotodegradation of organic compounds [84]. As research continues, more high performance MNP hybrids are expected to be developed that might open up new application fields in biodetection, biocatalysis, and environmental monitoring.
2.7. Green synthesis
Green chemistry aims at minimizing environmental impact of production and utilization of chemicals including NMs. Eco-friendly green synthesis of NMs involves the use of alternate energy inputs (such as microwave irradiation) and benign reaction media (such as plant extracts and polyphenolic antioxidants) for sustainable applications and environmental remediation [85], [86]. MNPs can be used as a versatile support for functionalization of metals, organocatalysts, N-heterocyclic carbenes, and chiral catalysts. It is used as a support for important homogeneous catalytically active metals such as Pd, Pt, Cu, Ni, Co, Ir, etc. to obtain stable and magnetically recyclable heterogeneous catalysts [85]. Research led by Dr. Rajender S. Varma of the United States Environmental Protection Agency (U.S. EPA) has led to the utilization of natural biorenewable resources in nanoparticle synthesis, such as plant extracts and polyphenolic antioxidants from various sources, biodegradable polymers, such as cellulose, reducing sugars, and agricultural residues (beet juice), waste material (red grape pomace) from winery waste, and glycerol (biodiesel byproduct) [87]. His work avoids the use of toxic agents, such as borohydrides, hydrazines, or PVP, producing them in the matrix in which they are to be used thus reducing the risk of exposure or eliminating the use and generation of hazardous substances normally used. His group has developed NPs with a magnetic core, a property that renders them recoverable for reuse [88]. Other groups of researchers have also explored the benefits of green synthesis. For instance, a RGO/Fe3O4 based nanocomposite with Pd NPs has been synthesized via a green route by Withania coagulans leaf extract as a reducing and stabilizing agent and its catalytic activity has been tested for the reduction of 4-NP in water with good results. The hydroxyl groups of phenolics in W. coagulans leaf extract is directly responsible for the reduction of Pd2+, Fe3+ ions and GO [89]. A green synthesis of the Cu/Fe3O4 NPs using Silybum marianum L. seeds extract and their application as magnetically separable nanocatalyst for the reduction of nitroarenes have been reported [90]. In general, green synthesis is clean, nontoxic, and environment friendly. The synthesized nanocatalyst is recoverable by magnetic decantation and could be reused several times without significant loss in catalytic activity.
3. Fate, transport, and toxicity in the environment
3.1. Dispersion stability
Unlike ferrihydrite NPs that can be dispersed in water to form stable suspension due to their small size [91], suspensions of MNPs are usually unstable in the absence of a stabilizer, although one study claims a stable MNP hydrosol with no added dispersants [92]. The colloidal stability of MNPs is determined by electrostatic or steric repulsion, depending on the stabilizers. Dispersion with tertramethylammonium hydroxide (TMAH) results in a slower settling of the MNPs; however it is not recommended due to its toxicity [93]. Perfluorinated ligand-passivated MNPs have been prepared through a biphasic ligand exchange method. Reducing the O2 content of the fluorous phase by N2 bubbling results in a highly stable dispersion of MNPs with a single-particle size distribution maintained due to elimination of O2 competition for adsorption against carboxylate moieties of ligands during exchange [94].
MNPs are prone to oxidation by oxygen, or corrosion by acid or base. Protection strategies have been developed with a core–shell structure, that is, the naked MNPs as a core is coated by a shell, isolating the core against the environment. Coating materials can be organic compounds such as surfactant and polymers or inorganic substances such as silica, carbon, precious metals or oxides [11]. A technique to solubilize fine magnetic inorganic particles in general organic solvents has been proposed via surfaces modification by long-chain carboxylic acids [95]. A versatile mussel-inspired multidentate block copolymer with pendant multiple catechol groups is an excellent stabilizer of colloidal ultrasmall superparamagnetic MNPs as promising MRI contrast agents [96]. Other polymeric dispersants (PAA: polyacrylic acid, PMA: polymethacrylic acid, PAAMA: polymaleic acid-co-acrylic acid) on the dispersion stability of MNPs have been investigated by the settling test, the transmittance, zeta-potential, and particle size measurements. It is observed that the critical concentration for maximizing the dispersion stability of MNPs is in the range of concentration ratio (dispersant/MNPs) of 0.1–0.01 and the dispersion-stability of MNPs is not improved when the dispersant concentration is above this critical value [97].
3.2. Fate and transport of engineered magnetite nanoparticles
Upon release of engineered NPs into the aqueous environment, their fate and transport and hence their potential environmental and public health impacts will largely depend on how stable these NPs are as suspended particles. Factors such as humic acid (HA) affect surface charge status and aggregation potential of MNPs. At low loadings, the presence of HA can induce a shift in the point of zero charge (PZC) of MNPs from pH 7.1 to lower values due to partial neutralization of the positive charges on MNPs. At high loadings, however, HA is capable of completely cover magnetite particles giving rise to a suspension Zeta potential similar to its own [98]. Laboratory-scale SPION transport experiments have been conducted using quartz sand columns to study the influence of primary particle size (20 nm and 80 nm) and surface functionalization (plain, –COOH and –NH2 groups) on particle mobility. Particle surface properties (surface functionalization and resulting zeta potential) have a major influence while their primary particle size turns out to be less relevant. In particular, the mobility of SPION is significantly increased in the presence of natural organic matter (NOM) due to the sorption of NOM onto the particle surface resulting in a more negative zeta potential [99]. Compared to nonmagnetic NPs, stability and transport of magnetic NPs are influenced also by the magnetically induced aggregation and retention in that magnetically induced aggregation and subsequent straining results in greater retention [100].
The impact of aggregation state on transport and deposition of MNPs is not fully understood. Instruments such as small-angle X-ray scattering (SAXS) and cryogenic transmission electron microscopy (cryo-TEM) can be used to directly observe the aggregate structure of iron oxides such as ferrihydrite NPs and show how the aggregate structure responds to changing ionic strength [101]. The same approaches can be applied to MNPs. Column tests on ferrihydrite indicate that, for usual ionic strength in European aquifers (2–5 mM), under natural flow condition ferrihydrite NPs are likely to be transported for 5–30 m. For higher ionic strength, corresponding to contaminated aquifers, (e.g., 10 mM) the travel distance decreases to a few meters [91]. MNPs are expected to travel less than ferrihydrite NPs in the same aquifers although such tests are yet to be undertaken. For future applications to aquifer remediation using iron-based NPs, ionic strength and injection rate may be used as tuning parameters to control their mobility in the subsurface and therefore the radius of influence during field injections.
A comparison between laboratory- and field-based investigations into the size-dependent reactivity of MNP has been conducted. Synthetic MNPs (~6 nm, ~44 nm, and ~90 nm) are emplaced in the subsurface of a landfill. Laboratory analog dissolution experiments are conducted using synthetic groundwater. Field results indicate that an organic coating develops on the particle surfaces largely inhibiting reactivity. Limited dissolution of MNPs occur, with the amount of dissolution decrease as particle size decrease. Conversely, the laboratory analogs without organics reveal greater dissolution of the smaller particles. These results show that the presence of dissolved organics leads to a nearly complete reversal in the size-dependent reactivity trends displayed between the field and laboratory experiments indicating that size-dependent trends observed in laboratory investigations may not be relevant in organic-rich natural systems [102]. Sedimentation dynamics and aggregate formation of MNPs for different water flow velocities and starting particle concentrations have been studied recently. Dependence of the sedimentation time on the particle concentration does not follow the trend predicted by theory, the disagreement might be caused by the formation of fractal-like structures [103]. Reactions of goethite and magnetite with sulfide solutions under CH4 and/or CO2 atmospheres are monitored in situ with Raman spectroscopy. The iron (oxyhydr)oxide minerals are inert to sulfide solution under CH4 atmosphere, and that the addition of CO2 to this system triggers the sulfidization reactions and produces iron monosulfide, mackinawite and pyrrhotite [104]. More such detailed studies are needed to shed more light on the fate and transformation of engineered MNPs in the field and their roles in coupled contaminant degradation in the environment.
3.3. Fate and transport of naturally occurring magnetite nanoparticles
Redox-induced cycling of iron is primarily controlled by microorganisms in the environment. Bacteria grow using both Fe(II) and Fe(III) bound in solid-phase iron minerals, and changing environmental conditions enable the sharing of electrons in mixed-valent iron oxides between bacteria with different metabolisms. This is shown through magnetic and spectroscopic measurements that the phototrophic Fe(II)-oxidizing bacterium Rhodopseudomonas palustris TIE-1 oxidizes MNPs using light energy. This process is reversible in co-cultures by the anaerobic Fe(III)-reducing bacterium Geobacter sulfurreducens [105]. The activity of microorganisms is a key component of the biogeochemical cycle of iron in natural systems, where MNPs and green rusts are often observed as products of microbially driven redox processes [48]. Transport of naturally occurring MNPs has been rarely studied. More investigation is needed to understand fully how they move, relative to their synthetic counterparts, through geomedia in aquatic environments.
3.4. Toxicity assessment
A few studies have addressed the effects of MNPs on plants. Pumpkin plants (Cucurbita maxima), grown in an aqueous medium containing MNPs (20 nm), can absorb, translocate, and accumulate the particles in the plant tissues [106]. The uptake of NPs also depends on plant species as no uptake of MNPs is found to occur by treated lima bean plants (Phaseolus limensis). On the other hand, a separate study [107] did not notice any uptake of 25 nm MNPs by the pumpkin plants although MNPs induced more oxidative stress than Fe3O4 bulk particles in the ryegrass and pumpkin roots and shoots as indicated by significantly increased: (i) superoxide dismutase and catalase enzyme activities, and (ii) lipid peroxidation. It has been hypothesized that large size NPs cannot penetrate through the cell wall and transportation across the plasma membranes is also limited. The cell wall pore sizes vary from 2 to 20 nm, while the size of ions and water molecules are about 0.28 nm [108].
The genotoxicity of MNP with different particle sizes (10, 30 nm) and surface coatings (PEG or polyethylene glycol, PEI or polyethylenimine) have been assessed using three standard genotoxicity assays, the Salmonella typhimurium reverse mutation assay (Ames test), the in vitro mammalian chromosome aberration test, and the in vivo micronucleus assay. The mutagenicity of MNPs depend on their particle size and surface coating. MNPs with PEG coating exhibit mutagenic activity without chromosomal and clastogenic abnormalities, and smaller MNPs (10 nm) have stronger mutagenic potential than larger ones (30 nm); whereas, MNPs with PEI coating are not genotoxic in all three standard genotoxicity assays [109]. In another study, SPIONs are found not toxic to cells in cell viability assays [110]. No human epithelial cell deaths occur in the presence of SPIONs coated with positively (–NH3+) and negatively (–COO−) charged shells, but cell proliferation is influenced by surface chemistry. Proliferation reduction is dose dependent and highest for bare SPIONs. Negatively charged SPIONs are the most biocompatible [111].
Administration of Fe3O4-ZnO core–shell NPs does not cause significant changes to mice with respect to mortality, clinical observations, body weight, food intake, water consumption, urinalysis, haematology, serum biochemistry, and organ weights [112]. A 52-day continuous semi-static waterborne exposure (test media renewed daily) regimen was employed to investigate the accumulation and elimination profiles of two iron oxide NMs (nano-Fe2O3 and nano-Fe3O4) in zebrafish (Danio rerio). The experiment reveal that: (1) high accumulation of nano-Fe2O3 and nano-Fe3O4 are found in zebrafish; (2) accumulated NPs in zebrafish can be eliminated efficiently when fish are moved to NP-free water; and (3) iron oxide NPs may be adsorbed via the gastrointestinal tract, and store for more than 12 days [113]. The Fe3O4 hexagons coated with a dopamine-based ligand to increase dispersibility in aqueous buffers are only minimally toxic to RAW264.7 cells [114]. Toxicity of sulfhydryl-modified Fe3O4@SiO2 core/shell NPs in mouse fibroblast (L-929) cell lines is low, and the material lacks hemolytic activity, indicating good biocompatibility of this Fe3O4@SiO2@DMSA nanocomposite [115].
The safety of MNPs to human health is still an important topic of debate. A recent study has detected the toxicity and biological behavior of bare MNPs on human umbilical vascular endothelial cells (HUVECs), and demonstrated that bare MNPs disturb the process of autophagy in HUVECs, and eventually lead to endothelial dysfunction and inflammation [116]. A recent literature review [117] suggests that local myocardial delivery of SPION-mediated therapeutic agents might produce myocardial iron overload, resulting in deterioration of myocardial injury and exacerbating cardiac function via oxidative stress mediated iron toxicity, and undermining therapeutic effects. Protective measures should be taken into consideration before developing clinical applications. Given that SPION toxicity mainly stems from oxidative stress, surface modification with an antioxidant (such as N-Acetylcysteine or Trolox) may be a new method to suppress oxidative damage and injury. The thiolated SPIONs with mercaptosuccinic acid (MSA) and cysteine (Cys) have no toxic effects for stem cells [118]. Long-term in vivo studies in murine models have shown that dimercaptosuccinic acid (DMSA)-coated MNPs accumulate in spleen, liver and lung tissues during extended periods of time (at least up to 3 months) without any significant signs of toxicity detected [119]. On the other hand, polyethylene glycol (NP-PEG-(NH2)2)-coated MNPs show a delay in the arrival of the particles to the liver tissue. Superparamagnetic Fe3O4 supraparticles@MIL-100(Fe) (MIL: Materials of Institut Lavoisier) core–shell nanostructure microspheres show a good biocompatibility in the in vitro cytotoxicity test [120]. More research is needed to fully understand the degree of toxicity of MNPs to humans and organisms.
4. Environmental analytical chemistry and catalysis
4.1. Sensors and measurements
A previous review has summarized and discussed various sensing applications of iron oxide NPs in biomedical fields (Fig. 6) [121]. Nanocomposites such as Fe3O4@Au hybrids offer novel opportunities for designing new electrochemical aptasensors with unique versatility for binding diverse targets, including proteins and peptides. MNP based immunosensors are used for the detection of biological and chemical pathogens, contaminants, and other important analytes [121]. A sensitive H2O2 biosensor for evaluation of oxidative stress has been fabricated on the basis of the RGO nanocomposites decorated with Au, Fe3O4, and Pt NPs (RGO/AuFe3O4/Pt) modified glassy carbon electrode (GCE) and used to detect the released H2O2 from cancer cells and assess the oxidative stress elicited from H2O2 in living cells [122]. A biosensor for glucose detection has been developed using water-dispersible and biocompatible chitosan-functionalized graphene (CG) and MNPs with a detection limit of 16 μM, and a linear detection range up to 26 mM glucose. This may allow for applications in more clinical areas such as in vivo biosensors and MRI agents [123]. A microscale well-plate colorimetric assay for the multiplexed detection of glucose and cholesterol in clinical human blood samples has been developed utilizing one-pot nanocomposite entrapping MNPs as peroxidase mimetics and glucose oxidase (GOx)/cholesterol oxidase (ChOx) in mesoporous silica [124]. A magnetic core-plasmonic shell nanoparticle attached to hybrid GO based multifunctional nanoplatform has the capability for highly selective separation of Alzheimer’s disease biomarkers from whole blood sample, followed by label-free surface enhanced Raman spectroscopy (SERS) identification in femto gram level [125]. MNPs have been used as a component to fabricate a bimodal nanohybrid for the luminescent ON/OFF detection of glutathione [126]. An electrochemical immunosensor based on MNPs has been developed to determine microcystin-(leucine-arginine) in river water [127]. Vanillin molecularly imprinted stir bar has been constructed based on Fe3O4@Polyaniline NPs with magnetic field-induced self-assembly process. The molecular imprinting stir bar shows superior selectivity and fast binding kinetics for vanillin, and is used for the enrichment of vanilla-flavor enhancers (vanillin, ethyl maltol and methyl vanillin) in infant milk powders before measurement by high performance liquid chromatography (HPLC)-UV [128]. A sensing methodology that combines magnetic separation (MS) and magnetic relaxation switching (MS-MRS) for one-step detection of bacteria and viruses has been reported. Compared with a traditional MRS sensor, an MS-MRS sensor shows enhanced sensitivity, better reproducibility, and convenient operation, thus providing a promising platform for point-of-care testing [129]. A protease-activated ratiometric fluorescent probe based on fluorescence resonance energy transfer between a pH-sensitive fluorescent dye and biocompatible MNPs has been constructed to image the pH of subcutaneous tumor xenografts [130].
Figure 6.
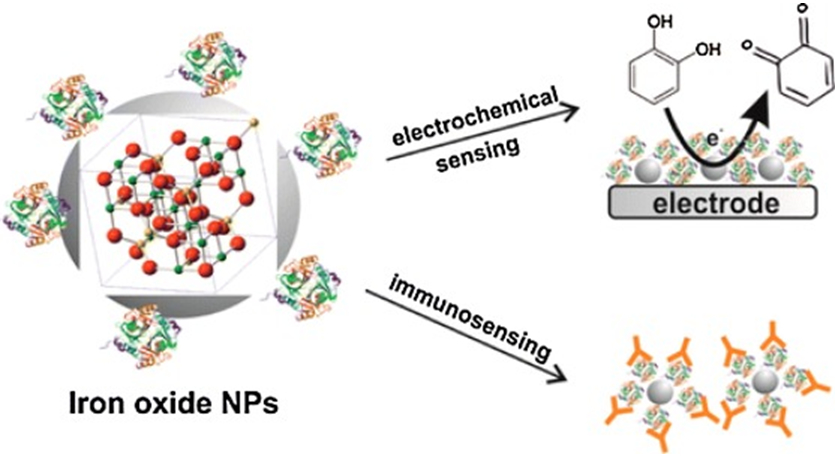
Iron oxide NPs such as nanomagnetite as a platform for electrochemical and chemical biosensors. Reprinted from Ref. [121]. Copyright 2014 American Chemical Society.
A multifunctional probe consists of a ternary nanocomposite of magnetite/ceria-codecorated titanoniobate nanosheet (MC-TiNbNS) is utilized for the enrichment and dephosphorylation of phosphopeptides [131]. A new type of magnetic halloysite nanotubes molecularly imprinted polymer (MHNTs@MIP) based on halloysite nanotubes (HNTs) with embedded MNPs has been prepared through surface imprinting technology, using 2,4-dichlorophenoxyacetic acid (2,4-D) as a template, 4-vinylpyridine as the monomer, divinylbenzene as cross-linking agents, and 2,2-azodiisobutyronitrile as initiator (Fig. 7) [132]. Magnetic cyclodextrin nanocomposites (β-CD-Fe3O4) could detect and remove β-naphthol from wastewater as a spectral probe [133].
Figure 7.
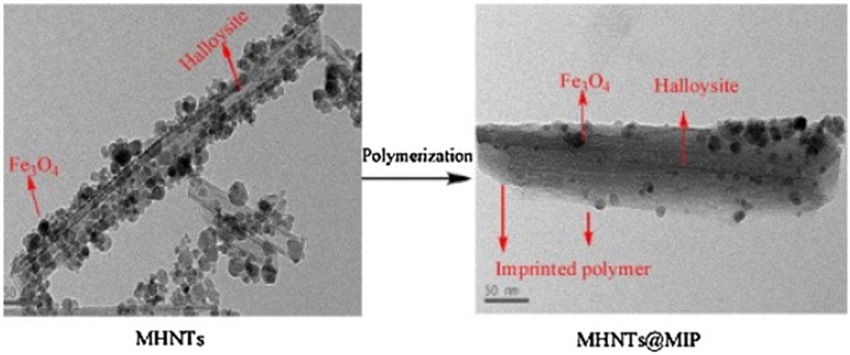
A new type of magnetic halloysite nanotubes molecularly imprinted polymer (MHNTs@MIP) based on halloysite nanotubes (HNTs) with embedded magnetic nanoparticles is prepared through surface imprinting technology, using 2,4-dichlorophenoxyacetic acid (2,4-D) as a template, 4-vinylpyridine as the monomer, divinylbenzene as cross-linking agents, and 2,2-azodiisobutyronitrile as initiator. MHNTs@MIP is applied as an adsorbent for sample pretreatment extraction followed by high-performance liquid chromatography detection of 2,4-D residue in water. Reprinted from Ref. [132]. Copyright 2014 Elsevier Ltd.
Selective and magnetic separation of various biological molecules can be accomplished by engineering the surface of MNPs. For example, MNPs with high magnetization and an amino-functionalized surface have been used to magnetically separate prevalent urinary crystals in the patient’s urine [134]. A MNP-based assay has been developed for the detection of bacteria (Escherichia coli, Proteus mirabilis and Pseudomonas aeruginosa) causing urinary tract infections in patient samples with a much improved efficiency [135]. MNPs are used to separate amyloid beta (Aβ) protein aggregates, which include fibrils and oligomers that are neurotoxic and are considered to cause Alzheimer’s disease from biological samples [136].
MNPs are also used in inorganic analytical chemistry. A new magnetic adsorbent, 3-mercaptopropionic acid coated 3-aminopropyl triethoxysilane modified MNPs, is used for the extraction and preconcentration of arsenic ions in aqueous solutions followed by determination using electrothermal atomic absorption spectrometry (AAS). Under the optimum conditions, wide linear range of 30–25,000 ng L−1 and low detection limit of 10 ng L−1 are obtained [137]. An coupled Au/Fe3O4/GO hybrid material improves the catalytic activity, stability, and separation capability of AuNPs and Hg2+ [138]. Multiwalled carbon nanotubes (MWCNTs) have been modified by MNPs with application for the preconcentration of cadmium [139], [140] and lead [139] prior to analysis by AAS. Hydrophobic ionic liquid as a green coating agent is deposited on the surface of synthesized MNPs to improve their extraction capability toward Pd(II) ions as diethyldithiocarbamate (DDTC) complex [141]. Magnetic core–shell silica NPs modified by 3-[2-(2-aminoethylamino) ethylamino]propyl-trimethoxysilane are used as a new adsorbent for simultaneous extraction and preconcentration of bismuth and lead ions through magnetic solid-phase extraction (MSPE) method. After adsorption, these ions are desorbed with nitric acid followed by determination with electrothermal AAS [142]. Novel dipyridile-modified MNPs have been used for the extraction and determination of low levels of Pb2+ ions in various samples of rice, baking powder, wheat, and other foodstuffs [143]. MNPs coated with either dopamine or glutathione have been developed as a new, simple and reliable method for the separation/pre-concentration of trace amounts of AgNPs followed by their quantification using inductively coupled plasma mass spectrometry (ICP-MS). The MNPs selectively captures AgNPs in a mixture containing both nano-particulate and ionic silver, which addresses the challenges of separation and quantification of AgNPs in addition to the total silver in environmental samples [144]. Chinese scientists have reported novel phosphatidylserine-functionalized Fe3O4@SiO2 NPs and enzyme-encapsulated liposomes for the visual detection of Cu2+ by employing phosphatidylserine for Cu2+ recognition and the enzymatic catalysis/oxidation of 3,3′,5,5′-tetramethylbenzidine sulfate (TMB) as a signal generator. It can be used to detect as little as 0.1–0.5 μM of Cu2+ in river water using naked-eye observation and 0.05 μM of Cu2+ in river water using UV–vis spectrophotometry. These detection limits are much lower than the maximum allowable level of Cu2+ (~20 μM) in drinking water defined by the USEPA [145].
MNPs have been used to construct a novel nitrite sensor by electropolymerization of alizarin red on the surface of glassy carbon electrode modified with Fe3O4-MWCNT composite nanofilm [146]. In another study, an electrochemical sensor has been developed based on the functionalized graphene anchored with MNPs and loaded on a glassy carbon electrode (GCE) for the detection of nitrite in tap, river and rain water samples [147]. Fluorescent chemosensors containing MNPs have been developed for detecting anions such as ClO− and SCN− [148]. A robust reusable fluoride sensor comprised of a receptor in charge of the chemical recognition and a fluorophore responsible for signal recognition has been designed. Highly fluorescent carbon quantum dot (CD) and magnetically separable nickel ethylenediaminetetraacetic acid (EDTA) complex bound-silica coated MNPs (Fe3O4@SiO2–EDTA–Ni) are used as fluorophore and fluoride ion receptor, respectively [149]. A highly efficient MRS system based on poly(vinyl alcohol) functionalized nanomagnetic iron oxide (PVA@NMIO) particles detects boric acid or borate ester (BA/BE) [150].
MNPs have been used as a component in the substrate for surface-enhanced Raman scattering (SERS) techniques. For example, Fe3O4@Au NPs are assembled into arrayed microstructures on a wafer scale with enhancement factors > 106 [151]. Ag-coated magnetic core–shell microspheres (Fe3O4@PEI@Ag) has been developed using polyethyleneimine (PEI) as an interlayer to construct a versatile SERS substrate, which is verified by the detection of adsorbed p-aminothiophenol molecules and human IgG with a detection limit as low as 10−11 M and 10−14 g mL−1, respectively (Fig. 8) [152]. Ag–Fe3O4 nanocomposites are assembled into an orderly arrayed SERS substrate holding clean and reproducible properties with an applied external magnetic field for detection of 4-mercaptobenzoic acid. The enhancement factors (EF) on the SERS substrate are up to 5.2 × 106 and the detection limit is down to ~10−10 M [153]. Rapid and sensitive SERS identification and quantification of arsenic species in multiple matrices have been realized using a Fe3O4@Ag magnetic substrate with a portable SERS sensor for onsite monitoring of arsenic speciation determination of As(III) and As(V). The entire process can be completed within 2 min. Moreover, the active SERS substrate can be used for As sensing in complex media such as juice, wine, and soils [154]. Quick and reliable measurements of contaminants in environmental media are in great need for monitoring and remediation decision-making. More fruitful discoveries can be made to take advantages of MNPs in sample pre-concentration and detection of a wider range of target compounds.
Figure 8.
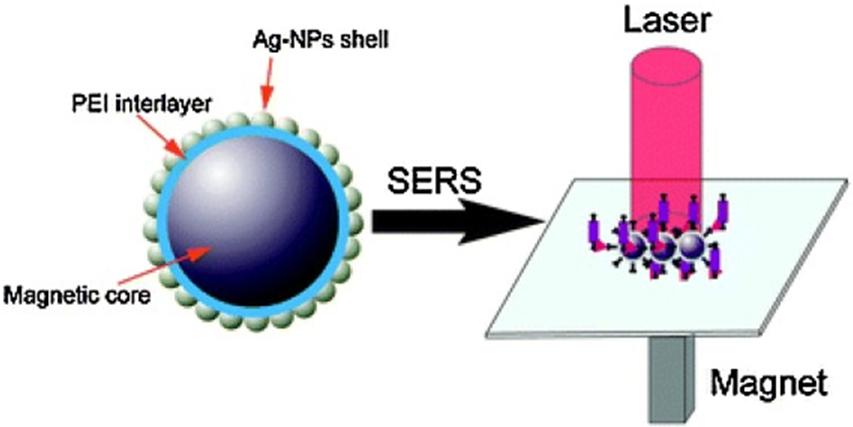
Novel Ag-coated magnetic core–shell microspheres (Fe3O4@PEI@Ag) with polyethyleneimine (PEI) as an interlayer is used to measure adsorbed PATP (p-aminothiophenol) molecules and human IgG with a detection limit as low as 10−11 M and 10−14 g mL−1, respectively. Reprinted from Ref. [152]. Copyright 2015 Royal Society of Chemistry.
4.2. Enzyme carrying and recycling
MNPs and their hybrids are known to adsorb proteins including enzymes. For example, uniform superparamagnetic Fe3O4/carboxymethyl chitosan composite nanospheres [155] and the core–shell structure Fe3O4/SiO2 magnetic icrospheres/nanospheres [156] show fast and efficient lysozyme adsorption. It is still a great challenge to deliver proteins and peptides with excellent bioactivity and controlled release. In a recent study, a pH-responsive delivery system has been obtained by anchoring 8-nm MNPs onto mesoporous support (SBA-15) (0.6–1 μm with a pore size of 6.2 nm). The pH-stimulative response is based on the interaction between the tris(aminomethyl)ethane (TAE) groups anchored onto the pore outlet of mesoporous silica scaffolds and the carboxybenzaldehyde (CBA) groups coated on the MNPs, which can lead to a rapid release under the acid condition (pH = 5) and a zero release with the increase of pH value (pH = 7.4) [157]. Enhancement of enzyme activity and enzyme recycling has been achieved by magnetic spherical polyelectrolyte brushes (MSPB) [158]. A magnetic enzyme nanosystem has been constructed with defined core–shell structure for rapid, efficient, and reusable tryptic digestion of proteins [159]. Magnetic chitosan/Fe3O4 NPs modified by 3-aminopropyltriethoxysilane have been used as carriers for the immobilization of pullulanase (a debranching enzyme) with high stability and activity. Immobilized pullulanase exhibits good resistance to metal ions and detergents [160]. Magnetic Fe3O4@SiO2 NPs have been prepared with molecular imprinting method using cellulase as the template. And the surface of the NPs is chemically modified with arginine. The prepared NPs are used as support for specific immobilization of cellulase. The half-life of the immobilized cellulase is 2-fold higher than that of the free enzyme at 50 °C [161]. These studies suggest that the prepared imprinted NPs have the potential industrial applications for the purification or immobilization of enzymes.
4.3. Recyclable magnetite catalysts and magnetite-supported catalysts
MNPs are popular materials used in the field of catalysis as they combine interesting reactivity with an easy, economical and environmentally benign mode of recovery. A recent review has summarized the recent progress made in the assembly of magnetic NPs and their direct application in catalysis with examples of such bare NPs including iron oxide (Fe2O3 and Fe3O4), metal ferrites (MFe2O4, M = Cu, Co and Ni), Fe0, Co0, Ni0, and multi-component NPs [162]. Another recent review has focused on the magnetic NP surface functionalization by different species such as inorganic, organic, silane reagents and biomolecules, biomolecules immobilization methods and their potential applications in catalysis [163]. Photocatalytic degradation of toxic organic pollutants in the magnetic iron oxide–semiconductor composite photocatalytic system can effectively break through the bottleneck of single-component semiconductor oxides with low activity under visible light and the challenging recycling of the photocatalyst from the final products [164]. There are crucial steps in precious- and nonprecious-metal-catalyzed CC and CX coupling reactions by using magnetically separable catalysts [165].
Halogenated organic compounds can be selectively removed from wastewater by using PdNPs on magnetite catalyst (Pd/Fe3O4), which is very active and magnetically extractable. This technique is sensitive to the presence of heavy metals and sulphides, which is a drawback. These components require removal before catalysis by Pd for dehalogenation [166]. A novel core–shell structural Fe3O4@MgAl-LDH@Au nanocatalyst exhibits excellent activity for the oxidation of 1-phenylethanol [167]. MNPs are effectively employed as heterogeneous catalyst for hydrogenation of ketone moiety to alcohol moiety by NaBH4 under the microwave radiation process [168]. An efficient and benign protocol has been reported for the synthesis of medicinally important pyrazole derivatives, 4-methoxyaniline, and Ullmann-type condensation reaction using MNPs (20–30 nm)-supported CuO NPs and the catalysts are recycled six times without loss in catalytic activity [169].
Water dispersible Fe3O4@carbon quantum dots (CQDs) hybrid nanoflowers show highly efficient photocatalytic activities because of their strong absorption in the visible light range and upconversion photoluminescence. The MNPs not only allow efficient magnetic separation and recycling of the photocatalyst, but also promote the photocatalytic activities of the CQDs [170]. MNPs coated with amino functional groups allow for amide bond formation with the carboxylic acid groups on zinc tetra- or octa-carboxyphthalocyanine (ZnTCPc or ZnOCPc respectively) [171]. Alumina-supported MNPs are used in hydrazine-mediated heterogeneously catalyzed reductions of nitroarenes to anilines [172]. Magnetically recoverable catalysts have been prepared by thermal decomposition of palladium acetylacetonate in the presence of MNPs. These NP mixtures are able to perform selective hydrogenation of 2-methyl-3-butyn-2-ol to 2-methyl-3-buten-2-ol, demonstrating clear differences in catalytic behavior depending on the catalyst structure (Fig. 9) [173]. Monodisperse magnetic sandwiched Fe3O4@Au//poly(ethyleneglycol methacrylate) (Fe3O4@Au/PEGDMA) core–shell microspheres show effective catalytic reduction of 4-NP to 4-aminophenol (4-AnP) [174].
Figure 9.
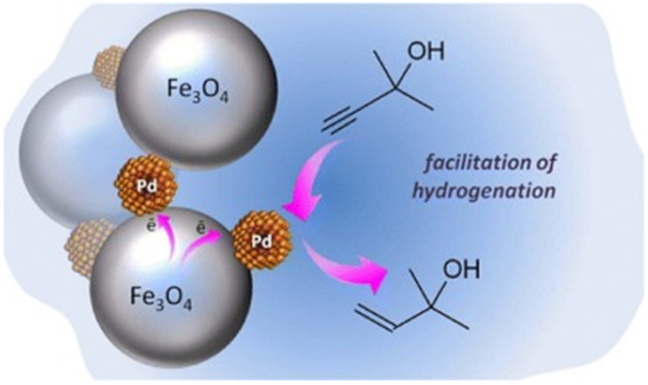
Fabrication of magnetically recoverable catalysts based on mixtures of Pd and magnetite nanoparticles for hydrogenation of alkyne alcohols. Reprinted from Ref. [173]. Copyright 2014 American Chemical Society.
Nanometer-sized magnetic stirring bars containing PdNPs (denoted as Fe3O4‐NC-PZS-Pd) for heterogeneous catalysis in microscopic system have been developed for the hydrogenation of styrene and Fe3O4‐NC-PZS-Pd show an activity similar to that of the commercial Pd/C catalyst, but much better stability. And the stirring bars display far better catalytic activity than the commercial Pd/C for the hydrogenation of MB [175]. The magnetically separable Pd nanocatalyst prepared by reacting palladium acetate with acetylacetonate-functionalized doubly silica-coated magnetic NPs is active for Suzuki cross-coupling reaction of acyl halides with boronic acids [176]. Surface modification of MNPs with triethoxyethylcyanide groups has been used for the immobilization of PdNPs to produce Fe3O4/Ethyl-CN/Pd for catalyzing the Suzuki cross-coupling reaction of various aryl halides (ArI, ArBr, ArCl) with phenylboronic acid in aqueous phase [177]. In another study, Pd-Fe3O4 composite NPs supported on graphene (Pd/Fe3O4/G) are used for Suzuki and Heck carbon–carbon coupling reactions [178]. A novel strategy to construct magnetic recyclable hollow capsules with Pd and MNPs embedded in polypyrrole (PPy) shell has been reported for the reduction of 4-NP by NaBH4. Compared with bare Pd and Fe3O4 NPs, the stability of both Pd and Fe3O4 NPs in hollow capsules is largely improved owing to the protection of PPy shell [179].
A novel nanomagnetic basic catalyst of Cs2CO3 supported on hydroxyapatite-coated Ni0.5Zn0.5Fe2O4 magnetic NPs (Ni0.5Zn0.5Fe2O4@HAP-Cs2CO3) has been used for the preparation of pyrazolo[1,2-b]phthalazine-5,10-diones [180]. A magnetic Fe3O4/BiOCl nanocomposite is prepared by a facile deposition–precipitation process that displays excellent catalytic activity for the photodegradation of RhB under simulated solar light irradiation [181]. Magnetically separable ternary g-C3N4/Fe3O4/BiOI nanocomposites show novel visible-light-driven photocatalysis in degradation of RhB, MB, and methyl orange under the visible-light irradiation. The excellent activity of the magnetic nanocomposite is attributed to more harvesting of the visible-light irradiation and efficiently separation of the electron–hole pairs [182]. Nanomagnetic Fe3O4‐Cu(II)-Schiff base is a highly active and selective catalyst for the oxidation of sulfides and thiols by H2O2 [183].
A facile Cu2O@Fe3O4‐catalyzed cyclization of ene-yne-ketone for the formation of CO and CC double bonds to prepare furans has been reported [184]. A series of magnetically separable catalysts based on Co–Rh bimetallic compounds supported on MNPs have been tested for dicyclopentadiene (DCPD) hydroformylation reactions, achieving excellent selectivity and conversion [185]. The hydrogenation of nitrobenzene is catalyzed over magnetically recoverable Pt deposited on MWCNTs with high yield of aniline [186]. A new family of Zn-containing magnetic oxides of different structures has been developed for syngas conversion to methanol [187]. A magnetic GO-loaded Ce-doped titania (MGO–Ce–TiO2) hybridized composite exhibits good adsorption capacity, high visible-light photoactive and magnetic separability as a novel photocatalyst in the degradation of antibiotic tetracyclines (TC) [188]. Maghemite/magnetite NPs functionalized with TiO2 show up to 89% of phenol photodegradation under UV irradiation [189]. So far most studies have been conducted in simple synthetic aqueous solutions. Information is lacking about the performance of the MNP based catalysts in real environmental media. Future investigations should focus on the improvement of the longevity of the catalysts in real world applications.
4.4. Activation of oxidants for organic contaminants degradation
MNPs have been studied for the activation of PMS to generate active radicals for degradation of pharmaceutical acetaminophen (APAP) in water. The MNPs effectively catalyze PMS for removal of APAP, and the reactions well follow a pseudo-first-order kinetics pattern. Higher MNP dose, lower initial APAP concentration, neutral pH, and higher reaction temperature favor the APAP degradation [190]. MNP-activated persulfate oxidation for trichloroethene (TCE) shows a great potential for the practical application of this technique in in situ TCE-contaminated groundwater remediation [191]. Dinitrotoluene is effectively degraded by H2O2 with MNPs at pH 1–9 with the optimal pH 3 [192]. Magnetic CuO-Fe3O4 composite is used as a heterogeneous catalyst for phenol degradation by persulfate [193]. A polyhydroquinone (PHQ)-coated Fe3O4/MWCNTs/PHQ catalyst has been used to degrade antibiotic flumequine (FLU) by persulfate oxidation from which two possible pathways are proposed involving hydroxylation, decarbonylation, and ring opening (Fig. 10) [194].
Figure 10.
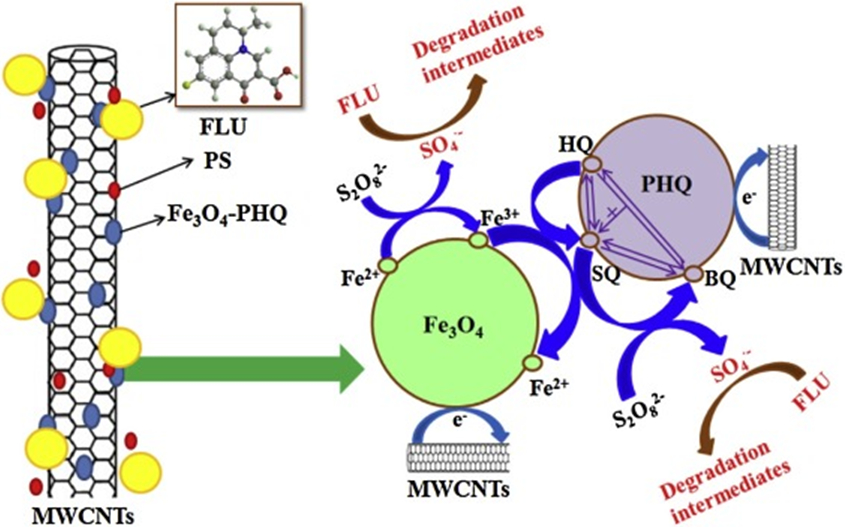
A novel heterogeneous catalyst, namely, polyhydroquinone-coated magnetite/multi-walled carbon nanotubes (Fe3O4/MWCNTs/PHQ) can be used as the efficient heterogeneous catalysts for persulfate oxidation of antibiotic flumequine (FLU). Reprinted from Ref. [194]. Copyright 2015 Elsevier Ltd.
Magnetic core/shell nanospheres (MCS, Fe3O4/carbon) have been synthesized by a hydrothermal method and their supported manganese oxide NPs (Mn/MCS) have been obtained by redox reactions between MCS and KMnO4. The Mn/MCS catalysts are able to effectively activate KHSO5 for phenol degradation in aqueous solutions. Sulfate radicals are suggested to be the primary reactive species generated from PMS for phenol catalytic oxidation (Fig. 11) [195]. 3D flower-like microspheres constructed by 2D Bi2WO6 nanosheets loaded with spherical MNPs have been used as a photocatalyst to degrade RhB under visible light irradiation assisted with H2O2 [196]. The heterogeneous UV/Fenton degradation of tetrabromobisphenol A (TBBPA) is catalyzed by nanocrystalline Fe3O4 and Fe2.04Cr0.96O4. The substitution of chromium greatly increases the BET specific surface area and surface hydroxyl amount, which improves the heterogeneous UV/Fenton catalytic activity of magnetite [197]. MNPs (~12 nm) show good oxidative capacity to promote heterogeneous Fenton-like reactions for the removal of nalidixic acid (NAL), a recalcitrant quinolone antibacterial agent. It is suggested that oxidative degradation of adsorbed NAL is by reactive oxygen species (ROS) produced via oxidation of both dissolved and structural Fe2+ ions [198]. Functionalized MNPs in alginate beads is used in the advanced Fenton oxidation of a malodorous compound (3 methyl-indole: 3-MI) without significant leaching of iron [199]. A magnetic photocatalytic CoFe2O4/g-C3N4 composite degrades MB in aqueous medium under visible light irradiation [200]. Novel CuFe2O4@C3N4 core–shell photocatalysts show photo Fenton-like discoloration of Orange II dye using H2O2 as an oxidant under visible-light irradiation [201]. MNPs prepared by steel pickling waste liquor to reduce the cost of preparation are comparable to those obtained by the common co-precipitation method for the heterogeneous Fenton-like catalytic degradation of bisphenol A (BPA) [202]. Oxidative removal of BPA involving PMS activated by CuFe2O4 magnetic NPs is through surface-bound, rather than free radicals generated by a surface catalyzed-redox cycle involving both Fe(III) and Cu(II) [203].
Figure 11.
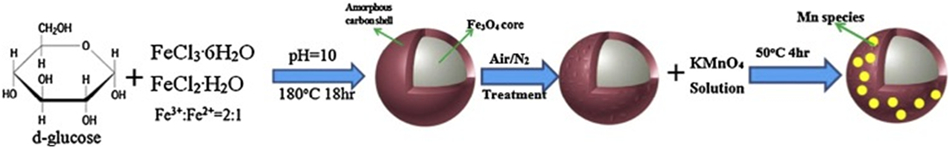
Synthesis procedure of magnetic Fe3O4@C supported manganese catalysts for oxidation of organics in water by peroxymonosulfate. Reprinted from Ref. [195]. Copyright 2015 Elsevier Ltd.
MNPs synthesized in diethylene glycol in the presence of carboxylic acids can be easily dispersed in aqueous media and other polar solvents due to being coated by a layer of hydrophilic polyol and carboxylic acid ligands in situ. They are an active, recyclable, and highly selective catalyst for the epoxidation of cyclic olefins with H2O2 [204]. Magnetic microspheres (Fe3O4/Cu) reduce and degrade RhB and MB in the presence of NaBH4 [205]. Fe3O4@SiO2@HPW (12-tungstophosphoric acid) NPs show excellent photocatalytic efficiency for the degradation of RhB under UV light irradiation [206]. Reusable TiO2/graphene nanocomposites responsive to external optical and magnetic fields are used to degrade MB in simulated solar light resulting in excellent photocatalytic performance [207]. Ferrite NPs similar to MNPs, such as CoFe2O4 catalyze the oxidation of benzyl alcohol (BzOH) to benzaldehyde (BzH) with H2O2 under solvent-free conditions [208]. In another study, with titanate nanotubes (TNTs) as catalyst support, a novel CoFe2O4/TNTs hybrid has been constructed for decomposition of RhB by PMS. Compared to the pure CoFe2O4, the as-prepared CoFe2O4/TNTs not only exhibits better performance, but also realizes higher total organic carbon removal and less cobalt leaching, which is attributed to the enhanced catalytic ability from smaller CoFe2O4 NPs and the unique ion-exchange ability from TNTs support [209]. The performance of MNP based catalysts for in situ oxidation of contaminants in soil and groundwater has not been fully evaluated. Laboratory tests give detailed information about possible mechanisms, yet field tests are required to validate the potential for practical applications.
4.5. Polymerization of organic compounds
The spontaneous surface induced polymerization of dissolved organic matter on environmental mineral particles has been demonstrated with carboxylated core–shell MNPs by using a ubiquitous polyphenolic precursor. Through the adsorption and in situ surface polymerization of gallic acid (GA), a polygallate (PGA) coating is formed on the MNPs (PGA@MNP) with possible antioxidant capacity. Observed also is the formation of ester and ether linkages between gallate monomers both in solution and in the adsorbed state. Higher polymers are formed in the course of several weeks both on the surface of MNPs and in the dispersion medium. The ratio of the absorbances of PGA supernatants at 400 and 600 nm (i.e., the E4/E6 ratio commonly used to characterize the degree of polymerization of humic materials) is determined to be 4.3, similar to that of HA [210]. The implications that the MNPs may be involved in the formation of humic substances in nature merits further investigation.
5. Air cleanup
Table 2 lists examples of MNP based NMs for environmental applications including air cleanup. MNPs have been used as an adsorption interface for the concurrent removal of gaseous benzene, toluene, ethylbenzene and m-xylene (BTEX), and SO2 [211]. Pt decorated octahedral Fe3O4 catalyst prepared by addition of a Pt4+ and Fe2+ mixture into a KOH solution leads to the simultaneous formation of an octahedral Fe3O4 and in situ reduction of Pt4+, which exhibits excellent catalytic activity for CO oxidation under moisture conditions [212]. In another study, Pd NP catalysts supported on Fe3O4, Co3O4, and Ni(OH)2 nanoplates are used in the low temperature oxidation of CO and the Pd/Co3O4 catalyst has particularly high activity for CO oxidation as a result of the strong interaction between the Pd NPs and the Co3O4 nanoplates [213]. PtNPs (1.2 ± 0.1 nm) synthesized in situ on MNPs (32 nm) previously deposited on alumina supports, is highly active towards the preferential oxidation of CO in a H2‐rich reformate stream and gives 100% CO conversion at 25 °C. Although the preferential oxidation of CO in H2‐rich stream is used in the production of clean H2 for H2‐based fuel cells, this catalyst can be used for CO removal from contaminated air [214]. Composites of magnetite and two-line ferrihydrite with GO have been tested as H2S adsorbents (Fig. 12) [215]. The addition of GO increases the surface area of the composites due to the formation of new micropores, leading to an increase in the H2S adsorption capacity; the opposite effect is recorded for the ferrihydrite composites [215]. Magnetic TiO2@SiO2@Fe3O4 and Cu/TiO2@SiO2@Fe3O4 photocatalysts show good catalytic activity in the CO2 reduction reaction to produce methanol and the addition of Cu improves the productivity [216].
Table 2.
Select examples of environmental applications of MNPs and their hybrids.
| Media and Treatment |
Contaminant s |
MNPs and hybrids | Mode of action |
Ref. |
|---|---|---|---|---|
| Air cleanup | BTEX | Fe3O4 | Adsorption | [211] |
| CO | Pt@Fe3O4 | Oxidation | [212], [216] | |
| CO | Pd@Fe3O4 | Oxidation | [213] | |
| H2S | Fe3O4@GO | Adsorption | [215] | |
| CO2 | TiO2@SiO2@Fe3O4 and Cu/TiO2@SiO2@Fe3O4 | Reduction to produce methanol | [216] | |
| Antimicrobial for water disinfection | E. coli, S. aureus | Fe3O4@GO–CNTs | Inactivation | [217] |
| E. coli | PQA@Fe3O4 | 100% biocidal | [218] | |
| E. coli, S. aureus | Fe3O4@PDA@Ag | Inactivation | [222] | |
| E. coli | Ag3PO4–TiO2–Fe3O4 | Photocatalytic inactivation | [223] | |
| M. aeruginosa | B. methylotrophicus, ZJU immobilized on Fe3O4 | Algicida | [229] | |
| Water treatment: Adsorptive organics removal | Dyes, | Fe3O4 | Separative Adsorption | [232] |
| Cationic dyes | AFMCNT | [233] | ||
| Congo Red, MB | Fe3O4@GO | [236] | ||
| BB | Fe3O4/chitosan | [239] | ||
| MB, MV | Fe3O4/MOF | [241] | ||
| Nitrofurans | Fe3O4/MWCNTs | [248] | ||
| PFOS | Fe3O4@SiO2@CTAB–SiO2) | [250] | ||
| Antibiotics (TC) | Fe3O4/polyacrylonitrial mat | [251] | ||
| Pharmaceuticals | MAA coated Fe3O4 | [256] | ||
| PCBs | Fe3O4@β-CD | [257] | ||
| BPA | DFMNPs | [260] | ||
| p-nitrophenol | Fe3O4@mSiO2/GO | [262] | ||
| 2,4,6–TCP | nFe3O4@NH2MIP | [264] | ||
| Herbicide (diquat) | Fe3O4@GO | [265] | ||
| Water treatment: Oxidative organics removal | APAP | Fe3O4, PMS | Catalytic oxidation | [190] |
| TCE | Fe3O4, persulfate | [191] | ||
| Dinitrotoluene | Fe3O4, H2O2 | [192] | ||
| Phenol | CuO–Fe3O4, persulfate | [193] | ||
| Antibiotic flumequine | Fe3O4/MWCNTs/PHQ, persulfate | [194] | ||
| Phenol | Fe3O4@C, PMS | [195] | ||
| RhB | Fe3O4–Bi2WO6, H2O2 | [196] | ||
| TBBPA | Fe2.04Cr0.96O4, UV/H2O2 | [197] | ||
| Antibacterial nalidixic acid | Fe3O4, H2O2 | [198] | ||
| 3 methyl-indole | Fe3O4–alginate, H2O2 | [199] | ||
| MB | CoFe2O4/g-C3N4, UV/VIS | [200] | ||
| Orange II | CuFe2O4/C3N4, H2O2 | [201] | ||
| BPA | Fe3O4, H2O2 | [202] | ||
| BPA | CuFe2O4, PMS | [203] | ||
| Cyclic olefins | Fe3O4–diethylene glycol, H2O2 | [204] | ||
| RhB, MB | Fe3O4–Cu, NaBH4 | [205] | ||
| RhB | Fe3O4@SiO2@HPW, UV | [206] | ||
| MB | Fe3O4–TiO2–GO, solar light | [207] | ||
| Benzyl alcohol | CoFe2O4, H2O2 | [208] | ||
| RhB | CoFe2O4/TNTs, PMS | [209] | ||
| Water treatment: Algae removal | S. dimorphus | PEI-coated Fe3O4 | Separative Adsorption | [267] |
| Algae | Steric acid–Fe3O4–ZnO | [268] | ||
| Water treatment: Oil spill cleanup | Crude oil, petroleum products | Fe3O4 conjugated with carbonaceous NMs | Separative Adsorption | [270], [271], [272], [273], [274], [275], [276] |
| Water treatment: Anionic inorganics removal | As(V), As(III) | Fe3O4, 12 nm | Separative Adsorption | [279] |
| As(V) | Fe3O4–coated sand | [289] | ||
| As(V) | Fe3O4, 98 nm | [291] | ||
| As(V) | Fe3O4–starch | [285] | ||
| As(V), As(III) | Fe3O4–TiO2 | [292] | ||
| As(III) | Fe3O4–chitosan | [287] | ||
| As(V), As(III) | Fe3O4–MnO2-GO | [298] | ||
| Phosphate | NH2–Al/SiO2/Fe3O4 | [304] | ||
| Phosphate | MFC@ZrO2 | [305] | ||
| Phosphate | Fe3O4@SiO2–lanthanum oxide | [306] | ||
| Phosphate | Fe3O4@LDH | [307] | ||
| Phosphate | MnFe2O4, 5 nm | [308] | ||
| Molybdate | Fe3O4 and ferric ions | [309] | ||
| Molybdate | ZnFe2O4 | [310] | ||
| Fluoride | CuCexFe2-xO4 (x = 0.0–0.5) | [312] | ||
| Selenate | Fe3O4, 10–20 nm | [313] | ||
| Cr(VI) | CD-E-MGO | [314] | ||
| Cr(VI) | Fe3O4@HNTs@C | Reduction | [322] | |
| Water treatment: Cationic heavy metal ions removal | Hg2+ | Fe3O4, 100 nm | Separative Adsorpton | [324] |
| Hg2+ | Fe3O4@SiO2 | [325] | ||
| Hg2+ | MCD–GO–R | [332] | ||
| Hg2+ | Tween20–AuNP–Fe3O4 | [334] | ||
| Hg2+, Pb2+, Cu2+ | CS-PAM-MCM | [335] | ||
| Hg2+, Pb2+, Cd2+ | Fe3O4@C | [339] | ||
| Hg2+, Pb2+, Cu2+ | Fe3O4@SiO2@chitosan | [340] | ||
| Pb2+ | MnFMC, CoFMC | [342] | ||
| Pb2+ | Fe3O4@Zr(OH)x | [343] | ||
| Cd2+ | Fe3O4@SiO2–poly(1-vinylimidazole) | [344] | ||
| Cd2+ | Fe3O4–sodium dodecyl sulfate | [345] | ||
| Zn2+ | Amino–Fe3O4@SiO2 | [346] | ||
| Cu2+ | Fe3O4–sodium alginate | [347] | ||
| Cu2+, Mn2+, Zn2+ | Fe3O4–PMMA | [348] | ||
| Cu2+, Ni2+, Co2+ | K2FeO4 | [349] | ||
| Cd2+, Pb2+, Co2+, Ni2+ | M–PAM–HA | [350] | ||
| Pb2+, Hg2+, Cu2+ | EDTA–MGO | [351] | ||
| Water treatment: Radionuclides removal | U(VI) | Fe3O4–TiO2 | Separative Adsorption | [354] |
| U(VI) | Amino–Fe3O4@GO | [355] | ||
| Sr(II), Th(II), U(VI) | Fungus–Fe3O4 | [356] | ||
| U(VI) | APTMS–MNP | [357] | ||
| Cs+ | Fe3O4–copper ferrocyanide | [359] | ||
| NpO2+ | Ti substituted Fe3O4 | Reduction | [360] | |
| Water treatment: Rare earth elements | Eu | Silane@Fe3O4 | Separative | [362] |
| La, Ce, Pr, Nd | Fe3O4–oleic acid | Adsorption | [363] | |
| Water treatment: Simultaneous organics and inorganics removal | 2,4-D, Pb2+ | Fe3O4–GO–LDH | Separative Adsorption | [364] |
| Phenanthrene, Cu2+, Zn2+, Pb2+ | Fe3O4–AC–biochar | [365] | ||
| 17β-estradiol, Pb2+ | Fe3O4–GO | [366] | ||
| Congo red, Cr(VI) | Co2.698Fe0.302O4 | [367] | ||
| MB, Hg2+ | Fe3O4–GO | [368] | ||
| Furazolidone, Cu2+ | Fe3O4–MWCNT | [369] | ||
| Pathogens, heavy metals, anions | Fe3O4–QAC | [370] | ||
| Congo red, methyl orange, Cu2+ | Fe3O4–PDA-LDH | [371] | ||
| Water treatment: Chemical reductive removal | PCBs | Fe3O4 under N2 | Reduction | [372] |
| PCB77 | Fe3O4 + NZVI | [373] | ||
| DDT | Pd–Fe3O4@nSiO2@mSiO2 | [247] | ||
| 4-NP | Ag–HNTS–Fe3O4 in NaBH4 | [375] | ||
| 2,4-dichlorophenol | MWCNTs–Fe3O4–Pd/Fe | [376] | ||
| CBZ, TC | Fe3O4–AC, Fe3O4–biochar, ball milling | [377] | ||
| Cr(VI) | Fe3O4, synthetic and natural | [378] | ||
| Cr(VI) | Fe3O4, synthetic and biogenic | [379] | ||
| Cr(VI) | Fe3O4@PmPDs | [380] | ||
| Nitrate | Fe3O4–ZVI | [381] | ||
| Sewage sludge stabilization | Cd2+, Co2+, Cu2+, Zn2+, Ni2+, Cr | Fe3O4–NZVI | Immobilization | [382] |
| Soil improvement | Fe3O4–biochar | Improvement of soil quality | [383] | |
| Soil remediation | PAHs | Fe3O4–AC, Fe3O4–biochar | Adsorption | [384] |
| As(V) | Fe3O4–starch | [385] | ||
| As | In situ biogenic Fe3O4 formation | [386] | ||
| Sediment remediation | Phosphate | Microsized ZVI with Fe3O4 as corrosion products | Immobilization | [387] |
| Cd2+ | Biogenic Fe3O4 | Adsorption | [388] | |
| Groundwater remediation | Chlorinated solvents | Synthetic and natural Fe3O4 | Reductive dechlorination | [389], [390], [391], [392], [393], [394], [395], [396], [397], [398] |
Figure 12.
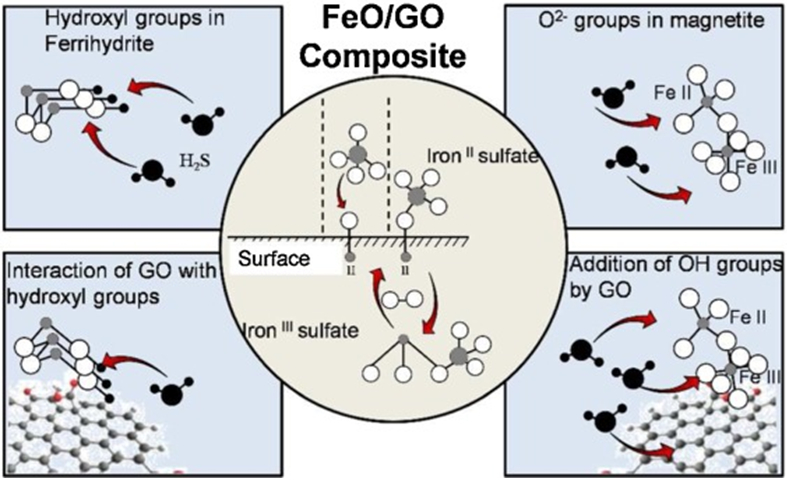
Composites of magnetite and two-line ferrihydrite with graphite oxide (GO) are synthesized and tested as hydrogen sulfide adsorbents. The addition of GO increased the surface area of the composites due to the formation of new micropores. Reprinted from Ref. [215]. Copyright 2015 American Chemical Society.
6. Wastewater and surface water treatment
6.1. Magnetic antimicrobial agents for water disinfection
Efficient disinfection and microbial control without harmful disinfection byproducts can be achieved with the use of MNPs based antibacterial material that can be recovered readily from matrix solution for reuse with an external magnetic field, avoiding waste of the disinfectant and possible contamination of the residual disinfectant to environment. Magnetic graphene–carbon nanotube iron nanocomposites inactivate Gram-positive (e.g., Staphylococcus aureus) and Gram-negative (e.g., E. coli) bacterial species [217]. Highly efficient recyclable antibacterial MNPs, consisting of a Fe3O4 core with an antibacterial poly quaternary ammonium (PQA) coating, show 100% biocidal against E. coli [218]. Magnetic GO (MGO) with MNPs well dispersed on GO nanosheets exert excellent antibacterial activity against E. coli. The inactivation mechanism of E. coli by M-GO includes both the membrane stress and oxidation stress during the incubation period [219]. PEG-coated MNPs functionalized with H-RWRWRW-NH2 ((RW)3), an antimicrobial peptide, is used as a novel magnetic-responsive support against E. coli and Bacillus subtilis 168 [220]. MNPs pre-coated with oleic acid and bearing novel antimicrobial N-heterocyclic choline analogues, namely O-, N- and O,N-bis-undecyl-substituted N-(2-hydroxyethyl)-1,2,3,4-tetrahydroisoquinolinium derivatives, are effective antimicrobial therapy for Gram-positive (S. aureus, Bacillus cereus) and Gram-negative (E. coli, P. aeruginosa, P. mirabili) bacterial strains and fungi (Candida albicans, Aspergillus niger) [221].
Conjugation with silver gives MNPs wider applications. MNPs are coated with a PDA layer through the autopolymerization of dopamine and subsequently, Ag+ ions are directly reduced into AgNPs by the PDA coating and the AgNPs (15 nm) are inlaid homogeneously and tightly onto the Fe3O4@PDA NPs to form Fe3O4@PDA@Ag magnetic composite. The obtained material has enhanced antibacterial ability due to the plenty of AgNPs immobilized on the PDA coating against E. coli and S. aureus [222]. The antibacterial film prepared from Ag3PO4/TiO2/Fe3O4 nanocomposite presents excellent bactericidal activity and recyclability toward E. coli cells under visible-light irradiation. In addition to the intrinsic cytotoxicity of silver ions, the elevated bactericidal efficiency of Ag3PO4/TiO2/Fe3O4 is largely attributed to its highly enhanced photocatalytic activity. The photogenerated hydroxyl radicals and superoxide ions on the formed Ag/Ag3PO4/TiO2 interfaces cause considerable morphological changes in the microorganism’s cells and lead to the death of the bacteria [223]. AgNPs deposited onto mesoporous composite particles of carbon inlaid with MNPs (Ag–Fe3O4@carbon) with a novel bowl structure show efficient antibacterial activities to E. coli and S. aureus, as well as high catalytic activity to the reduction of 4-NP by NaBH4 [224]. AgNP-decorated magnetic GO (MGO-Ag) exhibits excellent antibacterial activity against E. coli and S. aureus [225]. Ag-CoFe2O4-GO exhibits excellent antibacterial activity against E. coli and S. aureus compared with CoFe2O4, Ag-CoFe2O4, and CoFe2O4-GO composite. This superior disinfecting effect is possibly attributed to the combination of GO nanosheets and AgNPs [226]. Cu2O NPs functionalized with NiFe2O4 induce magnetomechanical stress and alteration of cellular membrane integrity and programmed cell death signaling in the yeast, Saccharomyces cerevisiae [227]. Chitosan is a naturally occurring biopolymer that is biocompatible, antifungal and antibacterial with wound healing ability, anticancerous property, anticholesteremic properties, and immunoenhancing effect. MNP doped chitosan beads are reusable agents against E. coli and S. aureus [228]. Algicidal bacteria offer a promising option for killing cyanobacteria. In a recent study, a newly isolated strain of Bacillus methylotrophicus, ZJU, has been used to control Microcystis aeruginosa. The strain ZJU as an algicidal agent is embedded in MNPs and wheat bran in the process of immobilization. The magnetization enables efficient re-collection of the immobilized bacteria by magnetic means [229].
6.2. Modification of nanocomposite membrane and flocculation of wastewater
MNP doped nanocomposite membrane has been made by synthesizing MNPs through co-precipitation, blending with polysulfone/N-methylpyrrolidone solution, and finally, dispersing in membrane structure after coagulation of casted polymeric solution. An increase in MNP concentration in membrane matrix causes permission flux to rise. On the other side, any increases in MNP content improves dyes dispersion and polyethylene glycol rejection as organic contaminant [230]. Economic and rapid removing of turbidity and reduction of sludge water content in sewage wastewater could be achieved with MNPs [231].
6.3. Adsorptive and separative removal of organic contaminants
Separation of ionic dye pollutants from aqueous solutions is often necessary in environmental cleanup. MNPs show MB dye adsorption [232]. Amine functionalized magnetic CNTs effectively remove cationic dyes from binary systems [233]. HA coated MNPs remove MB from aqueous solution [234]. Mesoporous-silica-capped Fe3O4 mesoporous clusters exhibit excellent adsorption of MB from aqueous solution and is easily regenerated by the photo-Fenton degradation reaction as a result of its high specific surface area, accessible pore channels, and good magnetic separation properties [235]. Fe3O4/GO nanocomposites effectively remove Congo red as an anionic dye and MB as a cationic dye in aqueous solutions [236]. A C18-functionalized core–shell magnetic mesoporous silica composite (Fe3O4/mSiO2-C18) has excellent adsorption ability toward MB dye due to the large surface area and the abundant hydrophobic C18 groups [237]. Reversible pH-responsive behavior and controlled adsorption/desorption of MB have been demonstrated on hybrid materials consisting of polyacid brushes using poly(itaconic acid) (PIA) and poly(acrylic acid) (PAA) at the amine functional groups of chitosan and MNPs [238].
Magnetic Fe3O4 chitosan NPs (m-Fe3O4-CNs) adsorb bromothymol blue (BB) from aqueous solutions [239]. Sunset yellow anionic dye is removed by aminopropyltriethoxysilane modified MNPs [240]. A Fe3O4/metal-organic frameworks (MOFs) composite adsorbs MB and methyl violet from an aqueous solution and the used Fe3O4/MOFs could be regenerated by acetonitrile [241]. Magnetic modified corncobs show good adsorption performances for Congo red [242]. MWCNTs decorated with MNPs are modified by polyaniline to remove methyl orange and Congo red [243]. Magnetically responsive RGO is used to prepare Pickering emulsions especially for water-in-oil emulsions that is used to remove Nile red dye from dodecane [244]. Fe3O4@3-aminophenol-formaldehyde (Fe3O4@APF) core–shell resin polymer magnetic nanocomposites are excellent adsorbent for the removal of methyl blue [245]. Fe3O4/chitosan/TiO2 nanocomposites show good adsorptive, photocatalytic, regenerated, and magnetic properties for degradation of MB in wastewater [246].
A novel multifunctional microsphere with an iron oxide-improved mesoporous silica shell and a Fe3O4@SiO2 core (Fe3O4@nSiO2@mSiO2-Fe core–shell) are utilized as a catalyst for the removal of 1,1,1-trichloro-2,2-bis(4-chlorophenyl) ethane (DDT) and its derivatives, i.e., 1,1-dichloro-2,2-bis(4-chlorophenyl) ethane (DDD) and 1,1-dichloro-2,2-bis(4-chlorophenyl) ethylene (DDE) [247]. Magnetic MWCNTs nanocomposite has been used for removal of four nitrofurans, namely, furazolidone (FZD), nitrofurazone (NFZ), nitrofurantoin (NFT), and furaltadone (FTD) from aqueous solution [248]. Magnetic core–shell Fe3O4@layered double oxide (Fe3O4@LDO) microspheres remove more 2,5-dihydroxybenzoic acid (2,5-DHBA) from aqueous samples compared with Fe3O4@LDH (Fig. 13) [249]. Novel mesoporous cetyltrimethylammonium bromide (CTAB)-functionalized magnetic microspheres with a core/shell structure (mesoporous Fe3O4@SiO2@CTAB-SiO2) effectively remove perfluorooctane sulfonates (PFOS) from water at acidic conditions (pH = 3) [250].
Figure 13.
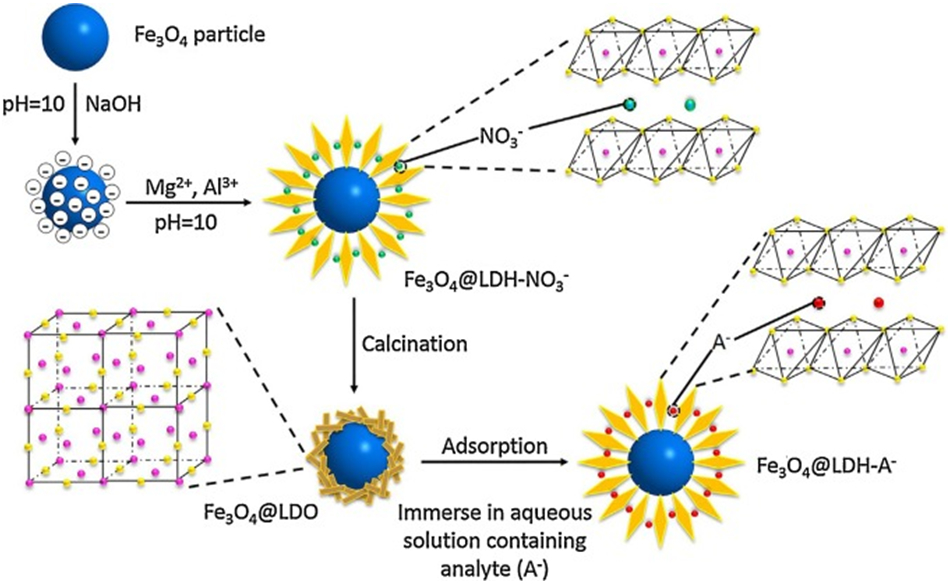
Schematic depicting the synthesis of Fe3O4@LDO microspheres, and regeneration of the LDH structure with intercalated analyte anion during the removal experiments. Reprinted from Ref. [249]. Copyright 2015 Elsevier Ltd.
Pharmaceuticals such as antibiotics in aquatic environments poses potential risks to the ecology and human health even at trace levels. A novel adsorbent, Fe3O4 incorporated polyacrylonitrile nanofiber mat (Fe-NFM) is able to remove tetracycline (TC), a typical class of antibiotics, from aqueous solution. Compared to conventional NP adsorbents which have difficulties in downstream separation, the novel nanofiber mat can be simply installed as a modular compartment and easily separated from the aqueous medium, promising its potential in drinking and wastewater treatment for micro-pollutant removal [251], [252]. Four types of organic acid-functionalized (oleic, undecenoic, caprylic or hexanoic acid) MNPs have been tested to remove TC from aqueous solution. The undecenoic acid-coated MNPs (UA-MNPs) exhibit the highest adsorption efficiency. Moreover, the UA-MNPs possess excellent ability to adsorb the other three major types of TC antibiotics, including chlortetracycline, oxytetracycline and doxycycline [253]. Removal of sulfonamide antibiotics is performed using oriented immobilized laccase from Echinodontium taxodii onconcanavalin A-activated Fe3O4 NPs. These NPs show higher enzyme loading and activity recovery compared with conventional covalent binding [254]. The removal of three widely detected and abundant pharmaceuticals, namely, ibuprofen, diclofenac, and sulfadiazine, by two Fe3O4-exchange resins show potential application for water purification [255]. Carbamazepine (CBZ) and diatrizoate (DTZ) are from the group of pharmaceutical and personal care products known to be persistent and non-biodegradable in wastewater treatment. Adsorptive removal of CBZ and DTZ using MNPs (10 nm), along with those coated with either methacrylic acid (MAA), Al(OH)3, or SiO2 have been evaluated. Results show that maximum DTZ adsorption capacities of MAA-, Al(OH)3-, and SiO2-coated MNPs are much higher than that of the uncoated MNPs [256].
Polychlorinated biphenyls (PCBs) present challenging issues due to human health concerns. MNPs are covered by a silica layer bonded β-cyclodextrin (β-CD) to construct core–shell Fe3O4@β-CD composites for removing PCB28 and PCB52 in aqueous solutions with promising results [257]. The removal of BPA is inhibited in the presence of NOM. The fabrication of powdered activated carbons impregnated with iron oxide NPs (IONPACs) allows for the simultaneous removal of BPA and NOM. The adsorption capacities of BPA and NOM are in the following sequence: bare PAC < hematite/PAC < magnetite/PAC < ferrihydrite/PAC [258]. Aqueous HA is removed with aminopropyl functionalized silica coated MNPs that show excellent desorption and regeneration properties. Zeta potential and XPS analysis reveal that electrostatic interaction and surface complexation between protonated amino groups and the disassociated HA molecules are the main adsorption mechanism [259]. Double functionalized magnetic NPs (DFMNPs) have been developed for extraction of BPA in an aqueous phase. In the preparation of DFMNPs, amide and pyridine groups are simultaneously introduced into the surface of magnetic NPs [260].
A magnetic adsorbent has been synthesized by encapsulation of magnetic functionalized NPs using alginate as a green biopolymer matrix to remove p-nitrophenol. A cationic surfactant, cetylpyridinium chloride (CPyCl), is used to confer a hydrophobic character to the magnetic beads and thus to promote their adsorption efficiency [261]. Magnetic porous silica–GO hybrid composites (Fe3O4@mSiO2/GO) show good performance in removing p-nitrophenol from aqueous solution [262]. Eendocrine-disrupting chemicals 4-n-nonylphenol (4-n-NP) and BPA are adsorbed by M-GO with excellent results [263]. Four novel amino functionalized core–shell molecularly imprinted magnetic polymers (nFe3O4@NH2MIP) have been synthesized using different amines, i.e., ethylenediamine (EDA), diethylenetriamine (DETA), triethylentetramine (TETA) and tetraethylenepentamine (TEPA), for adsorption of 2,4,6-TCP [264]. An herbicide diquat is removed by GO nanocomposite (GO-Fe3O4) [265]. Compared to conventional drinking water treatment (CT), a nano-treatment (NT) with quaternized magnetic microspheres reduces the concentrations of dissolved organic carbon, organic halogens (AOX), bromide and disinfection by-products more effectively. The NT completely eliminates the cytotoxicity, and greatly reduce the genotoxicity and oxidative stress of all raw water. In contrast, the CT increases the cytotoxicity of Taihu Lake and the Zhongshan River water, genotoxicity of Taihu Lake and the Mangshe River water, as well as the levels of superoxide dismutase and malondialdehyde of the Mangshe River water because CT increases disinfection by-products such as trihalomethane (THMs) and haloacetic acids as compared to raw river waters [266]. Adsorption does not destroy organic contaminants, rather it transfers contaminants from one media to the other. If complete destruction is required, alternative treatment such as chemical oxidation or reduction can be used.
6.4. Separative removal of algae from surface water
Magnetophoretic separation of algal biomass from water takes advantage of colloidal interactions between functionalized MNPs and algal cells. MNPs coated with PEI significantly reduce the energy barrier between MNPs and algae and thereby increased their heteroaggregation and algal harvesting efficiency in the separation of Scenedesmus dimorphus as well as in the recovery efficiency of MNPs from algal biomass through a chemical-free ultrasonic method [267]. A steric acid-coated Fe3O4–ZnO nanocomposite shifts hydrophobicity under UV365 irradiation to make magnetic nanocomposites detach from the concentrated algal biomass. The detachment is partially induced by the oxidation of steric acid coating layers due to the generation of radicals (e.g., OH) by ZnO under UV365 illumination. Consequently, the nanocomposite surface shifted from hydrophobic to hydrophilic, which significantly reduced the adhesion between magnetic particles and algae as predicted by the extended Derjaguin and Landau, Verwey, and Overbeek (EDLVO) theory. Such unique hydrophobicity shift may also find many other potential applications that require recovery, recycle, and reuse of valuable NMs to increase sustainability and economically viability (Fig. 14) [268].
Figure 14.
A steric acid-coated Fe3O4–ZnO nanocomposite shifts hydrophobicity under UV365 irradiation that leads to magnetic nanocomposites detachment from the concentrated algal biomass. Such unique hydrophobicity shift may also find many other potential applications that require recovery, recycle, and reuse of valuable nanomaterials to increase sustainability and economically viability. Reprinted from Ref. [268]. Copyright 2015 American Chemical Society.
6.5. Reusable sorbents for oil spill cleanup
MNPs conjugated with carbonaceous NMs (RGO film and graphene foam) offer a facile approach for the cleanup of pollution by crude oil, petroleum products, and toxic organic solvents (Fig. 15) [269]. The magnetic polymer-based graphene foam (MPG) for oil–water separation has been fabricated by the synergistic effects of the deposition of Fe3O4 on graphene sheets and the self-assembly of graphene on polyurethane (PU) sponge. The resulting MPG exhibits superhydrophobicity and superoleophilicity with the water contact angle (CA) of 158 ± 1° and the oil CA of 0°. A broad variety of oils and organic solvents can be removed from oil–water mixtures under manipulation by a magnet bar with high absorption capacity and selectivity. Significantly, the MPG can remain high absorption capacity for oil-water separation after several absorption/desorption cycles, demonstrating its good reusability [270]. MNP functionalized MWCNTS display excellent adsorption for toluene, ethylbenzene, and xylene [271]. Hybrid inorganic/organic composite materials synthesized from the coupling of amine-functionalized MNPs and pre-established shell cross-linked knedel-like polymer nanoconstructs show good capability to capture crude oil. These materials could be used in the cleaning of water contaminated during the process of drilling, extraction, and transport of crude oil [272]. MNPs prepared by micro-emulsion method and functionalized by organic species are effective for oil spill removal from the water surface. This is due to the fact that the small nano size, low density, hydrophobic character and high surface area of Fe3O4 facilitate the penetration process of NPs inside the oil. The contents of oil spill are simultaneously aggregated and easily removed by an external magnetic field [273]. PVP-coated MNPs separate a reference MC252 oil from oil–water mixture under environmentally relevant conditions with a high removal efficiency [274].
Figure 15.
Schematics showing the strategies to prepare graphene foams and their application for the removal of oils from water. Reprinted from Ref. [269]. Copyright 2014 Elsevier Ltd.
A recent study reports on fabrication of superhydrophobic and superoleophilic microparticles with magnetic property by combining the oxidation and self-polymerization of dopamine and formation of MNPs on the surface of the PDA particles, followed by modification with low surface energy material. The modified PDA/Fe3O4 particles show high water repellency with CA measured at 153.7 ± 1.6° and high oil affinity. Superhydrophobic sponge is prepared by modifying with the achieved microparticles. The sponge exhibits high absorption capability of oil, with weight gains ranging from 1348% to 7268% [275]. Researchers have explored utilization of low-cost clay minerals conjugated with MNPs for oil removal. For example, three organic–inorganic hybrid materials (vinyl benzene linear polymer modified SBA-15, attapulgite and halloysite nanotubes) in the shape of powder and the corresponding magnetic polysulfone microcapsules have been developed for removal of oil and dyes from environmental aqueous samples, respectively. The developed magnetic polysulfone microcapsules exhibit high adsorption capacity of 13.8–17.3 g g−1 for oil [276]. High performance and low-cost MNP based NMs for oil removal merits further investigation.
6.6. Separative removal of inorganics, radionuclides, and rare earth elements
6.6.1. Common inorganics
MNPs and their hybrids are very attractive candidates for low-cost adsorbents for the effective co-removal of heavy metals/metalloids from contaminated water due to their reusability, ease of magnetic separation, high removal efficiency, high surface area, and fast kinetics (Table 2). Examples of studies showing the adsorption capacities for select metalloids and metal ions are listed in Table 3. Notably, Chinese scientists have developed low-priced HA-coated MNP sorbent material for removing heavy metals from water with greatly enhanced stability of the material and increased removal efficiency with negligible leaching of NPs [277]. Compared to cationic pollutants, the removal of anionic pollutants by adsorption is more difficult because most adsorbents carry predominantly negative charges in neutral and alkaline environments.
Table 3.
Adsorption capacity of select MNPs and their hybrids for select metalloids (As) and metal ions (i: initial; e: equilibrium; RT: room temperature; rpm: revolutions per minute of shaking; qe: equilibrium adsorption; qm: maximum adsorption capacity calculated using Langmuir isotherm; qm(L): maximum adsorption capacity calculated using Freundlich isotherm).
| Adsorbents | Reaction conditions | Adsorption capacity (mg g−1) |
Ref. |
|---|---|---|---|
| Fe3O4, 11.72 nm, 20 nm, 300 nm, 0.011–2.5 g L−1 | pH 4.8–8.0, [As]i 0–34 mg L−1, RT, 4 rpm, 24 h | qm As(V): 0.75–172 qm As(III): 1.6–135 | [280] |
| Fe3O4–γ-Fe2O3, 0.4 g L−1 | pH 2, [As(V)]i and [As(III)]i 1.5 mg L−1, [Cr(VI)]i 1.0 mg L−1, RT, 28 rpm, 24 h | Without phosphate qe As(V): 3.7 qe As(III): 3.7 qe Cr(VI): 2.4 With Phosphate at 3–5 mg L−1, adsorption decreased by 40–50% | [281] |
| Fe3O4–CTAB, Fe3O4, 0.1 g L−1 | pH 6.0, [As(V)]i 0.1–7 mg L−1, 25 °C, 200 rpm, 2 h | qm: 23 for Fe3O4–CTAB, qm: 7.6 for Fe3O4 only | [282] |
| Fe3O4–graphene-LDH, LDH, 0.5 g L−1 | pH 6.0, [As(V)]i 0–160 mg L−1, RT, shaking, 24 h | qm: 73 for Fe3O4–graphene-LDH qm: 38 for LDH | [283] |
| Fe3O4–GO, Fe3O4–rGO 0.1 g L−1 | pH 7, [Asi] 0–600 mg L−1, 25 °C, shaking but no rpm reported, 24 h | Fe3O4–GO qm As(V): 18.8 qm As(III): 42.9 Fe3O4–rGO qm As(V): 8.42 qm As(III): 29.8 | [284] |
| Fe3O4–starch, 1.0 g L−1 as Fe | pH 5.0, pH 6.9, [As(V)]i 38–617 mg L−1, RT, 200 rpm, 48 h | qe: 248 at pH 5.0 qe: 198 at pH 6.9 | [285] |
| AC–MnFe2O4 | Optimum pH 4.0 for As(V), pH 7.0 for As(III), 30 °C, 70 min | qm As(V): 1314 qm As(III): 1253 | [293] |
| Mesoporous Fe3O4, 2.0 g L−1 | pH 4.0 for Cr(VI), pH 5.0 for Pb2+, [M]i 0–100 mg L−1, 25 °C, 35 °C, 45 °C, | qm Pb2+: 13.4, 14.1, 18.5 qm Cr(VI): 6.64, 7.31, 8.90 | [318] |
| Fe3O4/Mg–Al–CO3–LDH, Mg–Al–CO3–LDH, 4.0 g L−1 | No pH adjusting, [Cd2+]e 0–200 mg L−1, 30–50 °C, 1–5 h, | qm Cd2+: 45.6–54.7 for Fe3O4/Mg–Al–CO3–LDH, qm Cd2+: 61.4–70.2 for Mg–Al–CO3–LDH | [82] |
| Fe3O4–humic acid, 0.01 g L−1 | pH 6.0, [M]i 0.01–5 mg L−1, 20 °C, stirring, 0.5 h | qm Cu2+: 46.3 qm Cd2+: 50.4 qm Pb2+: 92.4 qm Hg2+: 97.7 | [277] |
| DSMA–Fe3O4, 0.002 g L−1 | pH 8.1, [Hg2+]e 0–2 mg L−1 | qm Hg2+: 227 | [288] |
| SH–Fe3O4–NMPs, 0.5 g L−1 | pH 3.0, [Hg2+]i 20–500 mg L−1 | qm(L) Hg2+: 523 | [326] |
| Fe3O4–polyethylenimine, 1.0 g L−1 | pH 6, [M]i 0–5 mmol L−1, 23–25 °C, 150 rpm, 12 h | qm Zn2+: 68 qm Pb2+: 168 | [327] |
| N–MSU–F–S/Fe3O4, NN–MSU–F–S/Fe3O4, NNN–MSU–F–S/Fe3O4 | pH 5.75, pH 5.7, [M]i 10–200 mg L−1, RT, 10 rpm, 24 h | qm Pb2+: 222, 219, 191 qm Cu2+: 57.9, 50.6, 53.3 | [328] |
| Fe3O4–Si–N = Gelatin | pH 4.0 for Cd2+, pH 7.0 for Pb2+, RT, shaking but no rpm reported, 0.5 h | qe Cd2+: 49.5 qe Pb2+: 82.9 | [329] |
| MHC/OMCNTs | pH 5.0, [Pb2+]i 10–200 mg L−1 2h | qm Pb2+: 116 | [336] |
| ZnO/ZnFe2O4/C | qm Pb2+: 345 | [338] | |
| MnFe2O4–MoS2–CD, CoFe2O4–MoS2–CD, | pH 1–6, 25 °C | qm Pb2+: 588 qm Pb2+: 661 | [342] |
| Fe3O4@SiO2@chitosan | pH 5.4–8.5, [M]i 10 mg L−1, 25 °C, 4 h | qe Hg2+: 8.5–8.9 qe Pb2+: 6.0–6.9 qe Cu2+: 3.4–7.0 | [79] |
6.6.1.1. Arsenic, phosphate, molybdate, fluoride, selenium
Arsenic is a known carcinogenic element and its removal from water supplies is needed to reduce its exposure through drinking water and food consumption. Magnetite is known to be an As adsorbent [278]. MNPs are more promising adsorbents for As removal because of their great adsorption capacity and easy separation. For example, using the high specific surface area of MNPs (12 nm), it is able to reduce the mass of waste associated with As(V) and As(III) removal from water by orders of magnitude [279]. Arsenic adsorption increases with decreasing MNP particle size [280] and phosphate competes against As for adsorption [281]. CTAB modified MNPs adsorb more As(V) than MNPs alone [282]. Fe3O4–graphene–LDH removes almost twice as much As(V) as LDH alone [283]. Fe3O4–GO adsorbs more As than Fe3O4–RGO because more functional groups exist on the Fe3O4–GO. As(III) is more favorably adsorbed than As(V) onto both adsorbents. This is advantageous, in terms of the process energy consumption, because energy saving could be achieved via omitting the reduction process to fabricate Fe3O4–RGO from Fe3O4–GO and the pre-oxidation process to convert As(III) to As(V) [284]. Functionalized MNPs show improved performance. For example, starch-bridged MNPs at an Fe-to-As molar ratio of 7.6 remove 100% As(V) from an initial 300 mg L−1 As(V) spent ion exchange brine (6% W/W NaCl), compared to only 20% removal when bare MNPs are used [285]. The starch acts as a stabilizer to prevent the NPs from agglomerating and as a bridging agent allowing the NPs to flocculate and precipitate while maintaining their high As sorption capacity. An earlier study has shown that starch-stabilized MNPs offer a much faster sorption rate and greater capacity than carboxymethyl cellulose (CMC)-stabilized magnetite [286]. MNPs (20 nm) show fast As(V) removal (reaching equilibrium within 2 h) that is not significantly influenced by pH (pH 7.7–8.3), ionic strength (0–0.1 M KNO3) and temperature (20–30 °C) [287]. Smaller MNPs (5.8 ± 0.9 nm) exhibits excellent distribution coefficient Kd (5800 mL g−1) for As and are also effective sorbents for toxic soft metals such as Hg, Ag, Pb, Cd, and Tl [288]. MNPs coated sand shows efficient As(V) removal from drinking water [289].
A recent study shows As adsorption by MNPs increases with increasing temperatures. As(V) predominantly forms bidentate binuclear corner-sharing complexes (2C), and As(III) forms tridentate hexanuclear corner-sharing (3C) complexes on MNP surfaces. More intriguingly, XANES and XPS results reveal complex redox transformation of the adsorbed As on MNPs exposed to air: Concomitant with the oxidation of MNPs, the oxidation of As(III) and MNPs is expected, but the observed As(V) reduction is surprising because of the role played by the reactive Fe(II) [290]. A pilot column test of a commercially available, food-grade magnetite (98 nm) has demonstrated removal of As(V) from Guanajuato, Mexico groundwater [291]. A magnetically separable nanomaterial Fe3O4–TiO2 eliminates the disadvantages of MNP agglomeration and iron leaching and takes advantage of the fact that TiO2 NPs are also effective As(V) and As(III) adsorbent [292]. A granular AC/MnFe2O4 composite with a mass ratio of 2:1 shows maximum Langmuir adsorption capacities of 1253 mg g−1 for As(III) and 1314 mg g−1 for As(V) at 30 °C and 70 min contact time. These adsorption capacities are among the highest (Table 3). The results show that As(III) and As(V) removal is strongly pH-dependent with an optimum pH value of 7.0 and 4.0, respectively [293]. Multiwalled boron nitride nanotubes (BNNTs) functionalized with MNPs are used for As(V) removal [294]. MNPs surface-coated with 3-mercaptopropanoic acid (3-MPA) show twice the As(V) adsorption capacity as compared to MNPs alone, which is attributed to the increase of active adsorption sites [295]. Other promising adsorbents include CNTs functionalized with MNPs for As(V) and As(III) [296] and chitosan-coated MNPs for As(III) [297].
GO modified with Fe3O4 and MnO2 NPs provides more adsorption sites for removing As(III) and As(V) by reducing the aggregation of Fe3O4 and MnO2 NPs. In addition, the MnO2 NPs promote the oxidation of As(III) to As(V) and simultaneously participate in the adsorption of arsenic with Fe3O4. Furthermore, arsenic adsorption remain stable in a wide pH range of 2–10, which is a limitation in most adsorbents [298]. Magnetic porous Fe–Mn binary oxide nanowires have been fabricated to remove As(III) with the adsorption capacity increasing with increasing manganese oxide in the composite, and with an initial Fe:Mn molar ratio of 1:3 exhibiting the highest adsorption capacity [299]. A novel composite adsorbent (HBC–Fe3O4–MnO2) has been synthesized by combining honeycomb briquette cinders (HBC) with Fe3O4 and MnO2 through a co-precipitation process for enhanced As(III) and As(V) removal. More As(III) is removed than As(V). Co-ions HCO3− and PO43− inhibit As removal; whereas, Cl−, NO3−, and SO42− have no influence [300]. The hybrids of single-layer GO with MnFe2O4 NPs show excellent adsorption properties for efficient removal of Pb(II), As(III), and As(V) from contaminated water [301]. Dihalogen crosslinked Fe3O4 core–shell NPs synthesized by crosslinking amine functionalized Fe3O4@SiO2 core–shell NPs with 1,2-bromochloroethane are loaded on to the RGO to obtain nanocomposites for removing As(V), As(III) and Hg(II) [302]. Magnetite occurs naturally as NPs. Four natural magnetite samples from an iron ore deposit and two synthetic commercial reference samples, show a widely variable As(V) adsorption behavior with no clear predictable pattern among samples [303].
Phosphate removal via adsorption is an effective means for controlling eutrophication in water bodies. A magnetic adsorbent, amine-functionalized silica magnetite (NH2–Al/SiO2/Fe3O4) behaves as a cationic adsorbent by adjusting the pH value of the aqueous solution to make amino groups protonated. The NH2–Al/SiO2/Fe3O4 adsorbs dissolved phosphate with the maximum adsorption at pH 3.0. Desorption of phosphate occurs in 0.05 M NaOH [304]. A magnetic core–shell composite with a Fe3O4 core and a carbon shell (denoted as MFC) is further functionalized by ZrO2 (denoted as MFC@ZrO2) for phosphate adsorption. Negligible phosphate adsorption is observed on MFC, while ZrO2 functionalization leads to markedly enhanced phosphate adsorption [305]. Fe3O4@SiO2 core/shell magnetic NPs coated with hydrous lanthanum oxide have been tested for separation of phosphate from water with a removal efficiency of higher than 95% attained for real effluent of a wastewater treatment plant. The adsorbed phosphate could be nearly completely desorbed with NaOH solution for further use [306]. Magnetic core–shell Fe3O4@LDHs composites show good phosphate adsorption efficiency with the maximum adsorption capacity following the order: Fe3O4@Zn–Al–LDH > Fe3O4@Mg–Al–LDH > Fe3O4@Ni–Al–LDH [307]. A MnFe2O4 (5.1 nm) exhibits good phosphate removal. Adsorbed phosphate is desorbed using 15 w/v% NaOH solution and recovered via hydroxyapatite crystallization in the desorption solution [308]. A novel combination of magnetic adsorptive and coagulative strategy has been used to remove molybdate from surface water. The ferromagnetic nanoclusters and ferromagnetic nanoclusters-ferric flocs composite coagulant show decreasing removal rate: ferromagnetic nanoclusters-FeCl3 > FeCl3 > AlCl3 > TiCl4 [309]. In another study, ZnFe2O4 NPs are used for removing and recovering molybdate from water [310].
A novel adsorbent of γ-AlOOH@CS (pseudoboehmite and chitosan shell) magnetic NPs shows good performance at pH 4–10 to remove fluoride from drinking water [311]. A series of doped and un-doped magnetic adsorbents CuCexFe2-xO4(x = 0.0–0.5) prepared with the micro-emulsion method have been compared for fluoride removal from water. Doped ferrites (x = 0.1–0.5) are superior to un-doped ferrites (x = 0) regarding the active sites, functional groups and fluoride adsorption [312]. Compared with either natural magnetite (<5 μm) or nanaosized zerovalent iron (NZVI) (~10 nm), MNP (10–20 nm) is a better adsorbent for selenite, while NZVI shows better adsorption performance for selenate [313]. Competition of anions for adsorption on MNPs and their hybrids needs to be more fully evaluated for practical applications. What works well in the laboratory setting may not work well in the field. More field-scale research is required.
6.6.1.2. Cr(VI) and Cr(III)
A novel β-cyclodextrin (β-CD) polymer adsorbent named β-cyclodextrin/ethylenediamine/M-GO (CD-E-MGO) removes Cr(VI) from water with good results [314]. Easily separable HA coated magnetite (HA-Fe3O4) NPs effectively adsorbs and reduces Cr(VI) to nontoxic Cr(III) (Fig. 16) [315]. No detectable transformation of the Fe3O4 core occurs during Cr(VI) adsorption and reduction, suggesting HA on the surface of HA-Fe3O4 is responsible for the reduction of Cr (VI) to Cr(III). The functional groups associated with HA act as ligands leading to the Cr(III) complex via a coupled reduction–complexation mechanism [315]. Magnetic carbon–iron nanoadsorbents fabricated by carbonizing cellulose and reducing MNPs or Fe(NO3)3 (the products are denoted as MC–O and MC–N, respectively) have demonstrated great Cr(VI) removal via Cr(VI) reduction and precipitation of Cr(III) [316]. A cross-linked chitosan derivative with quaternary ammonium and magnetic properties (QM-chitosan) removes both Cr (VI) and phosphate from aqueous environments [317]. Magnetite nanospheres with hollow interiors [318] and MNPs synthesized by chemical co-precipitation [319] both successfully remove Cr(VI) and Pb2+ from water [320]. Magnetic iron oxide/mesoporous silica nanocomposites consisting of iron oxide NPs embedded within mesoporous silica (MCM-41) and modified with aminopropyl functional groups exhibit a superior equilibrium Cr(III) adsorption than that for unmodified mesoporous silica [321]. Single ion kinetic studies have revealed Cr(VI) to be better adsorbed than Cd2+ onto citrate-coated MNPs. Otherwise Cd2+ adsorption ratio is improved with Cr(VI) in the binary mixture [322]. In a recent work, a novel “dumbbell-like” magnetic Fe3O4/halloysite nanohybrid (Fe3O4/HNTs@C) with oxygen-containing organic group grafting on the surface of natural halloysite nanotubes (HNTs) and homogeneous Fe3O4 nanospheres selectively aggregating at the tips of modified halloysite nanotubes has been successfully synthesized. The Fe3O4/halloysite nanohybrid Exhibits 100 times higher adsorption ability than that of unmodified halloysite nanotubes. More importantly, with the reduction of Fe3O4 and electron–donor effect of oxygen-containing organic groups, Cr(VI) ions are easily reduced into low toxicity Cr(III) and then adsorbed onto the surface of halloysite nanohybrid [323]. Compared to other reductants for Cr(VI) reduction to Cr(III) such as NZVI, MNPs have the advantage of remaining to be magnetic for easy separation; whereas, NZVI corrodes rapidly in oxic environments to form non-magnetic iron oxides.
Figure 16.
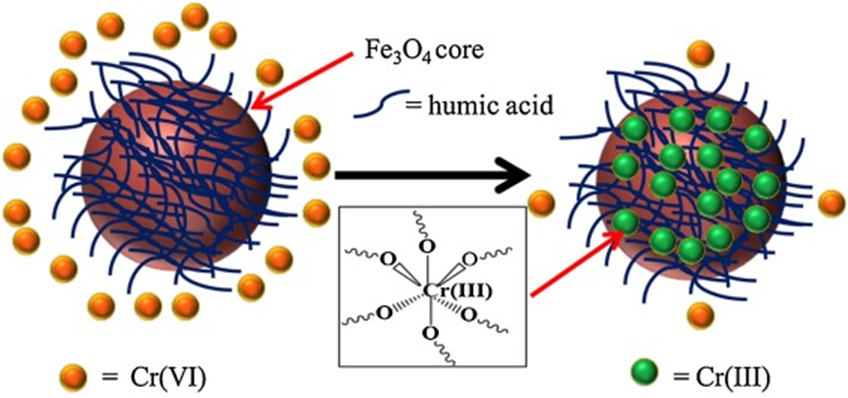
Schematics showing reduction of Cr(VI) by magnetite-coated humic acid. Reprinted from Ref. [315]. Copyright 2014 American Chemical Society.
6.6.1.3. Cationic heavy metal ions Hg2+, Pb2+, Cd2+, Cu2+, Zn2+, Co2+, Ni2+
Cationic heavy metal ions are persistent pollutants in wastewater and their removal is required to meet regulatory standards. MNPs and their hybrids are effective sorbents for their removal with high adsorption capacities (Table 3). For example, bare MNPs and γ-Fe2O3 NPs are able to remove Hg2+effiently [324]. Fe3O4@SiO2 magnetic NPs modified by grafting poly(1-vinylimidazole) oligomer (FSPV) show excellent removal of Hg2+ at pH 4–10 even at the presence of HA. Common ions including Na+, K+, Ca2+, Mg2+, Cl−, NO3−, and SO42− (up to 100 mM ionic strength) slightly increase the adsorption of Hg2+ by FSPV [325]. Mercapto modified MNPs show excellent Hg2+ removal [326]. Imine functionalized MNPs show selective removal of Pb2+ or Zn2+ by varying the concentrations of EDTA [327], and multi-amine grafted mesoporous silica embedded with nano-magnetite removes Pb2+ and Cu2+ [328]. A novel magnetic nano-sorbent has been synthesized by Schiff’s base formation via covalent bonding of gelatin to the surface of nano-magnetite-immobilized-3-aminopropyltrimethoxysilane (Nano-Fe3O4-Si-N = Gelatin) and it has the maximum capacity of Cd2+ and Pb2+ as 49.5 and 82.9 mg g−1, respectively [329]. Dithiocarbamate functionalized silica coated MNPs display great affinity for Hg2+, but they are not effective at removing As and Cd from seawater [330]. MNPs with a surface functionalization of dimercaptosuccinic acid (DMSA) are an effective sorbent material for Hg2+, Ag+, Pb2+, Cd2+, and Tl+, which effectively bind to the DMSA ligands and for As, which binds to the iron oxide lattices. DMSA-Fe3O4 has a capacity of 227 mg of Hg g−1, a 30-fold larger value than conventional resin based sorbents (GT-73) [288]. A novel adsorbent, 2-mercaptobenzamide modified itaconic acid-grafted-magnetite nanocellulose composite [P(MB-IA)-g-MNCC] selectively removes Hg2+ at the optimum pH of 8.0 [331]. Resin loaded magnetic β-cyclodextrin bead and GO sheet (MCD-GO-R) is an excellent adsorbent for Hg2+ removal at pH 4–10 [332]. A novel 3-aminopropyltriethoxysilane modified graphite nanosheets (GNS) decorated with Fe3O4 NPs (Fe3O4-GNS-NH2 hybrids) has been developed for adsorption of Hg2+. The main adsorption mechanism for Hg2+ is the formation of N-metal ions chemical complex, and the solution pH value has a major impact on Hg2+ adsorption with optimal removal at pH 4–6 [333]. Another method has been developed for removing Hg2+ from a high-salt matrix using Tween-20-stabilized gold NP (Tween 20-Au NPs) as Hg2+ adsorbents and composites of RGO and MNPs as NP collectors. Citrate ions adsorbed on the surface of the Tween 20-Au NPs reduce Hg2+ to Hg0, resulting in the deposition of Hg0 on the surface of the NPs. Compared with the reported NP-based methods for removing Hg2+, Tween 20-Au NPs offer the rapid (within 30 min), efficient (>99% elimination efficiency), durable (>10 cycles), and selective removal of Hg2+, CH3Hg+, and C2H5Hg+ in a high-salt matrix without the interference of other metal ions [334]. A novel magnetic composite microsphere (MCM) based on polyacrylamide (PAM)-grafted chitosan and silica-coated MNPs (CS-PAM-MCM) has been applied as an efficient adsorbent for the removal of Hg2+, Pb2+, and Cu2+ in respective single, binary, and ternary metal systems. Compared with chitosan magnetic composite microsphere (CS-MCM) without modification, CS-PAM-MCM show improved adsorption capacity for each metal ion and highly selective adsorption for Hg2+ from Pb2+ and Cu2+ [335]. Magnetic hydroxypropyl chitosan/oxidized MCNTs (MHC/OMCNTs) composites remove Pb2+, exhibiting an optimal pH 5.0 [336]. A ternary nanocomposite hydrogel consisting of magnetic attapulgite/fly ash/poly(acrylic acid) (ATP/FA/PAA) exhibits good adsorption selectivity toward Pb2+, and the adsorbed ion could be completely desorbed with HCl aqueous solution [337]. Nanoporous adsorbents of ZnO/ZnFe2O4/C show high Pb2+ adsorption performance. XRD and XPS data suggest that Zn2+ is substituted by a portion of Pb2+ on the surface of ZnO nanocrystals. This adsorbent may have limited applications if Zn2+ is also present in the wastewater [338].
Core–shell magnetic Fe3O4@C NPs functionalized with sulfonic and carboxylic acid groups remove Pb2+, Hg2+, and Cd2+ ions quickly to reach equilibrium within 5 min, with the maximum adsorption capacities Pb2+ > Hg2+ > Cd2+ [339]. Chain-like core–shell structure Fe3O4@SiO2@chitosan composite shows a decreasing adsorption in the order, Hg2+ > Pb2+ > Cu2+ [340]. Hybrid MNP sorbents synthesized through surface encapsulation of MNPs by o-phenylenediamine via cross-linking using formaldehyde and glutaraldhyde show superior selectivity for Pb2+ removal and good adsorption of Cd2+ [341]. A recent study has demonstrated a one-pot solvothermal synthetic method for fabricating MFe2O4 (M = Mn, Co)–MoS2–carbon dot (CD) nanohybrid composites (MnFMC, CoFMC) with excellent Pb2+ removal ability. The as-prepared composites exhibit high adsorption performance and show preferential Pb2+ sorption behavior with a high level concentration of competing cations (Ca2+/Mg2+) [342]. In another study, Fe3O4@Zr(OH)x yolk–shell nanospheres (YSNs) and another adsorbent without hollow cavities, i.e., Fe3O4@SiO2@Zr(OH)x core–shell nanospheres (CSNs), have been used for the removal of Pb2+. The Fe3O4@Zr(OH)x YSNs exhibits higher Pb2+ adsorption capacity as compared to that of Fe3O4@SiO2@Zr(OH)x CSNs due to the existence of cavities between Fe3O4 cores and Zr(OH)x shells [343]. Poly(1-vinylimidazole)-grafted Fe3O4@SiO2 magnetic NPs remove both free Cd2+ and nitrilotriacetic acid (NTA) complexed Cd2+ at pH 5–11, and the adsorbent could be regenerated with 10 mM HCl in 10 min, and the removal of both types of Cd2+ maintains above 95% in five consecutive adsorption/regeneration cycles [344]. Sodium dodecyl sulfate-modified MNPs also show good Cd2+ removal [345].
Amino-functionalized Fe3O4@SiO2 magnetic nano-adsorbent shows highly selective removal of Zn2+ from hot-dip galvanizing pickling waste that mainly contains ZnCl2 and FeCl2 in aqueous HCl media. The maximum adsorption occurs at pH 5 and Fe2+ does not interfere [346]. Sodium alginate-MNP nanocomposite beads adsorbs Cu2+ from aqueous solutions [347]. SPIONs coated with poly(methylmethacrylate) (PMMA) by emulsion polymerization process show some selectivity for Cu2+, Mn2+, and Zn2+ with the removal efficiency decreasing in the order Cu2+ > Mn2+ > Zn2+ > Cd2+ > Pb2+ > Co2+ > Ni2+ [348]. Potassium ferrate(VI) (K2FeO4, Fe(VI)) removes Co2+, Ni2+, and Cu2+ by the formation of MFe2O4 spinel phase and partially through their structural incorporation into octahedral positions of γ-Fe2O3 (maghemite) NP. In contrast, Cd2+ ions either do not form the spinel ferrite structure or are not incorporated into the lattic of iron(III) oxide phase due to the distinct electronic structure and ionic radius (Fig. 17) [349]. A novel magnetic hydroxamic acid modified polyacrylamide/Fe3O4 adsorbent (M-PAM-HA) have been prepared with acrylamide by microemulsion polymerization and then nucleophilic substitution of hydroxamic acid for the removal of Cd2+, Pb2+, Co2+ and Ni2+ [350]. EDTA functionalized MGO (EDTA-MGO) is a good adsorbent for Pb2+, Hg2+ and Cu2+ in that metal chelation and electrostatic attraction improve the adsorption capacity [351]. The presence of anions show positive effect on Cu2+ removal by aminated Fe3O4/GO (AMGO). In the single-ion systems, Cl−, ClO4−, and NO3− slightly increase the Cu2+ adsorption onto AMGO at low pH, while the Cu2+ adsorption is largely enhanced by the presence of SO42−, CO32−, and HPO42−. The enhancing effects is in the following sequence: HPO42− > CO32− > Cl− > SO42− > NO3− = ClO4− [352]. Adsorbents that can remove multiple heavy metal ions simultaneously are perhaps more useful for practical applications since the metals are usually co-contaminants in wastewater. Selective removal of a particular metal ion is sometimes required for recovery. Thus different types of adsorbents using MNP as a major component may be further developed for a specific purpose.
Figure 17.
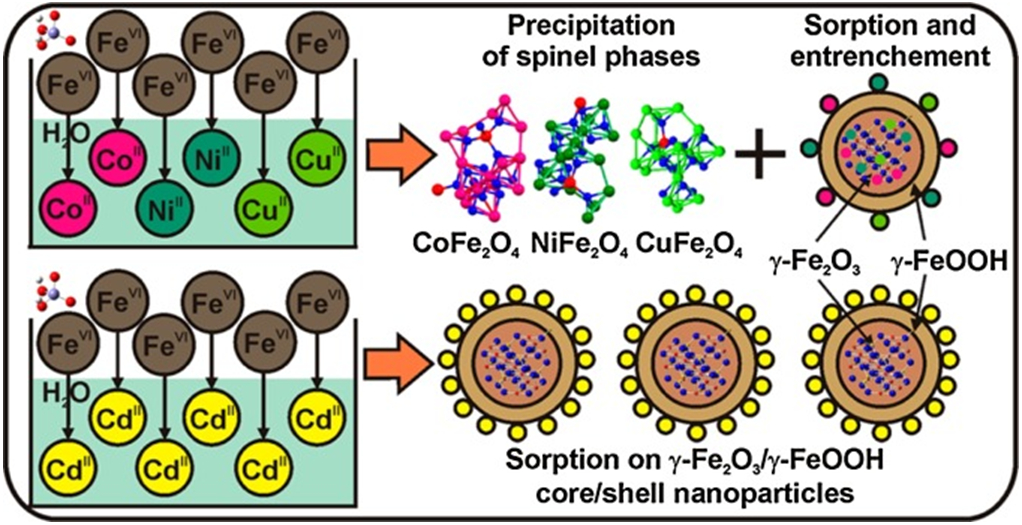
Ferrate(VI)-prompted removal of metals in aqueous media with enhanced efficiency via metal entrenchment in magnetic oxides. Reprinted from Ref. [349]. Copyright 2015 American Chemical Society.
6.6.2. Radionuclides
Uranium (VI) adsorption by oxine functionalized MNPs is strongly dependent on pH and independent of ionic strength, indicating that the sorption is mainly dominated by inner-sphere surface complexation [353]. Fe3O4@TiO2 nanocomposites remove U(VI) endothermically and spontaneously [354]. Amino functionalized magnetic GO composite displays a high efficiency for the removal of U(VI) [355]. The bio-nanocomposites of fungus-Fe3O4 adsorbs Sr(II), Th(IV), and U(VI), which is independent of ionic strength, indicating that inner-sphere surface complexation with oxygen-containing functional groups dominates their sorption. The maximum sorption capacities of fungus-Fe3O4 are for Sr(II) and U(VI) at pH 5.0, and Th(IV) at pH 3.0 [356]. Four new MNP containing different chelating groups as ester, amide, and azacrown moieties at the lower rim of calix[4]arenes have been prepared by the reaction of formylated calix[4]arenes and amino propyl modified MNPs (APTMS-MNP). MNPs containing picolinamide units exhibit superior and more efficient extraction percentage for uranium ions at lower pH values [357]. Uranium incorporation into magnetite and its behaviour during subsequent oxidation has been investigated at high pH to determine the uranium retention mechanism(s) on formation and oxidative perturbation of magnetite in systems relevant to radioactive waste disposal. Ferrihydrite is exposed to U(VI)aq containing cement leachates (pH 10.5–13.1) and crystallization of magnetite is induced via addition of Fe(II)aq. A combination of XRD, chemical extraction and XAS techniques provide direct evidence that U(VI) is reduced and incorporated into the magnetite structure, possibly as U(V), with a significant fraction recalcitrant to oxidative remobilization. Immobilization of U(VI) by reduction and incorporation into magnetite at high pH, and with significant stability upon reoxidation, has clear and important implications for limiting uranium migration in geological disposal of radioactive wastes [358]. No information about the size of magnetite was given in this study, but it may be relevant to MNPs as well. Copper ferrocyanide-functionalized MNPs show the effectiveness of the nano-adsorbents toward the removal of Cs [359]. Neptunium-237 is a radionuclide of great interest owing to its long half-life (2.14 × 106 years) and relative mobility as the neptunyl ion (NpO2+) under many surface and groundwater conditions. Reduction to Np(IV) is beneficial due to its low solubility. Natural magnetites often contain titanium impurities which have been shown to enhance radionuclide sorption via titanium’s influence on the Fe2+/Fe3+ ratio in the absence of oxidation. New evidence shows that Ti-substituted MNPs reduce NpO2+ to Np(IV) under reducing conditions. No evidence of NpO2 NP precipitation is obtained. Instead XAS confirms the nearly exclusive presence of Np(IV) on the titanomagnetite surface and provides supporting data indicating preferential binding of Np to terminal Ti–O sites as opposed to Fe–O sites [360]. Radioactive Tc(IV) has been shown experimentally and computationally to be able to favorably incorporate into magnetite but not trevorite (NiFe2O4) structure [361]. It remains to be seen if such substituted magnetite is stable over long periods of time for immobilization of radioactive elements at contaminated sites.
6.6.3. Rare earth elements
Nanocomposite of GO and silane modified magnetic NPs (silane@Fe3O4) have been synthesized in a form of dendritic structure that is used as a monomer for synthesis of europium ion imprinted polymer. The imprinted polymer modified silica fiber is first validated in the aqueous and blood samples for successful extraction and detection of europium. The imprinted polymer modified silica fiber is also used for preconcentration and separation of europium metal ion from various soil samples of coal mine areas. However, the same silica fiber is also used for wastewater treatment and shows 100% performance for europium removal. The findings suggest that dendritic nanocomposite could be potentially used as a highly effective material for the enrichment and preconcentration of europium or other trivalent lanthanides/actinides in nuclear waste management [362]. An adsorbent prepared based on the attachment of organophosphorus acid extractants to the surface of MNPs has been coated with oleic acid for adsorption of lanthanum, cerium, praseodymium, and neodymium [363].
6.7. Simultaneous separative removal of organic and inorganic contaminants
Mixed organic/inorganic contaminants often occur in aquatic environments. For example, potentially toxic metals and dyes commonly coexist in industrial wastewaters, posing a serious threat to public health and the environment and making the treatment more challenging. Magnetic composites consisting of Fe3O4, GO, and Mg3Al–OH LDH, denoted as MGL composites, are able to remove the heavy-metal Pb2+ and the hydrophobic organic pesticide 2,4-dichlorophenoxyacetic acid (2,4-D) from aqueous solutions [364]. Magnetic activated carbons and biochars produced by wet impregnation with iron oxides show strong sorption of the hydrophobic phenanthrene and copper, zinc, and lead [365]. Fe3O4/GO nanocomposites synthesized using a novel solvothermal method remove 17β-estradiol (E2) and Pb2+ synergistically [366]. Porous magnetic CoFe oxide nanosheets (Co2.698Fe0.302O4) show fast removal ability and good adsorption capacity for both organic waste Congo red and Cr(VI) [367]. Xanthate functionalized MGO is an ideal adsorbent for Hg2+ and MB removal with a higher adsorption capacity [368]. A magnetic MWCNTs nanocomposite shows high efficiency for simultaneous removal of furazolidone (FZD) and Cu2+ from aqueous solutions. The Cu2+ suppresses FZD binding in the simultaneous adsorption and FZD preloading experiment; whereas, the impact of FZD on Cu2+ desorption is almost negligible [369]. A new versatile MNP conjugated with quaternary ammonium compounds (QACs) (M-QAC) has been developed by the Chinese researchers that exhibits excellent disinfection and adsorption performance at the same time. The M-QAC is constructed by a Fe3O4 core surrounded by a polyethylenimine-derived corona. When dispersed in water, the M-QAC particles are able to interact simultaneously with multiple contaminants, including pathogens and heavy metallic cations and anions, in minutes. Subsequently, the M-QACs along with those contaminants can be easily removed and recollected by using a magnet [370]. Scientists from China have also developed a novel magnetic PDA–LDH (MPL) bifunctional material, which is fabricated by an easy and green approach for the simultaneous removal of Cu2+ and anionic dyes, methyl orange and Congo red [371]. Such a green and facile synthesis method, efficient removal performance and superior reusability suggest that the MPL assemblies have practical application potential for integrative and efficient treatment of coexisting toxic pollutants. More research should be conducted to develop more versatile adsorbents that can remove multiple organic and inorganic co-contaminants for greater technical merits and economic efficiency.
6.8. Chemical reductive removal of contaminants
The decomposition efficiency of polychlorinated biphenyls (PCBs) has been compared using Fe0 and three iron (hydr)oxides, i.e., α-Fe2O3, Fe3O4, and α-FeOOH, as catalysts under an inert, oxidizing or reducing atmosphere composed of N2, N2 + O2, or N2 + H2. N2 is the optimum atmosphere for PCB decomposition using iron or iron compounds. No matter which PCB congener is tested, Fe3O4 shows the highest activity. Reactive characteristics of Cl atoms on benzene rings followed para > meta > ortho [372]. Nano-sized hybrid Fe0 and Fe3O4 efficiently degrade 3,3′,4,4′-tetrachlorobiphenyl (PCB77). The degradation of PCB77 by Fe0/Fe3O4 NPs is a dechlorination process. Fe3O4 provides Fe2+ and Fe3+ for enhancing the PCB77 degradation by NZVI, suggesting a synergy between Fe0 and Fe3O4 [373]. A triple-functional nanocomposite has been prepared by integrating superparamagnetic Fe3O4 and Pd NPs onto mesoporous Fe3O4@nSiO2@mSiO2 nanospheres to degrade 1,1,1-trichloro-2,2-bis(4-chlorophenyl) ethane (DDT), a highly persistent organochlorine pollutant in environment. These magnetic mesoporous materials display excellent capabilities of capturing and catalytically degrading DDT in water at temperatures as low as 150 °C. The nanocomposites can be magnetically separated from the dispersion after adsorption, and then be easily regenerated which is accompanied by catalytic reaction. The whole treatment process is convenient, energy-saving, and just requires ambient pressure and mild reaction conditions [247]. The same Fe3O4@nSiO2@mSiO2 catalyst without Pd NPs doping requires a higher temperature (350 °C) to completely degrade DDT [374]. Ag/halloysite nanotubes/Fe3O4 (Ag/HNTs/Fe3O4) nanocatalyst has been synthesized in which Fe3O4 NPs (5–8 nm) are loaded in the internal hollow lumen of HNTs and Ag NP (20 nm) are randomly deposited on the external surface of HNTs/Fe3O4. The resultant Ag/HNTs/Fe3O4 exhibits excellent catalytic activity (with conversion of 100% in 38 min) to the reduction of 4-NP with NaBH4 [375]. MWCNTs-Fe3O4–Pd/Fe nanohybrids provide rapid adsorption, gradual dechlorination of 2,4-dichlorophenol, and final desorption of phenol. MWCNTs-Fe3O4–Pd/Fe nanohybrids outperform unsupported Pd/Fe NPs, which are difficult to retrieve, and are easily passivated and aggregated [376]. Ball milling has been used to prepare two ultrafine magnetic biochar/Fe3O4 and activated carbon (AC)/Fe3O4 hybrid materials targeted for use in pharmaceutical removal by adsorption and mechanochemical degradation of pharmaceutical compounds of CBZ and TC [377].
Redox sensitive contaminants such as Cr(VI) can be remedied using magnetite via chemical reduction to the very insoluble Cr(III) species. An recent study has investigated the Cr(VI) reduction performance of synthetic and natural magnetites of different particle size and found that only the finest magnetite show considerable Cr(VI) reduction yields. Mechanochemical mixing of the finer magnetites with 5% micron-sized Fe0 increases dramatically their reductive reactivity despite the fact that the same quantity of Fe0 added by itself reduces negligible amounts of Cr(VI) [378]. Biogenic MNPs synthesized by the Fe(III)-reducing bacterium Geobacter sulfurreducens, has been tested for the potential to remediate alkaline Cr(VI) contaminated waters associated with chromite ore processing residue (COPR). The performance of this biomaterial is compared to NZVI. Both NPs exhibit a considerable capacity for the remediation of COPR related Cr(VI) contamination, with the NZVI demonstrating greater reactivity than the biogenic MNPs. However, the biosynthesized MNP is also capable of significant Cr(VI) reduction and demonstrates a greater efficiency for the coupling of its electrons towards Cr(VI) reduction than the NZVI [379]. Magnetic Fe3O4@poly(m-phenylenediamine) particles (Fe3O4@PmPDs) with well-defined core–shell structure show high Cr(VI) removal that is attributed to the adsorption of Cr(VI) on protonated imino groups and the efficient reduction of Cr(VI) to Cr(III) by amine, followed by Cr(III) chelated on imino groups [380]. MNPs have been used as an additive material in a ZVI reaction to reduce nitrate in groundwater resulting in markedly increased nitrate reduction, with the rate proportionally increasing with MNP loading. MNPs act as a corrosion promoter for Fe0 corrosion as well as an electron mediator that facilitated electron transport from Fe0 to adsorbed nitrate [381]. The performance of MNPs for chemical reduction of these and other redox sensitive contaminants merits further investigation.
7. Sewage sludge stabilization, soil improvement, soil, sediment, and groundwater remediation
Anaerobic digestion (AD) is a widely used process to stabilize waste sewage sludge and produce biogas renewable energy. A recent study has tested NZVI and MNPs used in the mesophilic AD processes (37 ± 1 °C) to improve biogas production and heavy metal (Cd, Co, Cu, Zn, Ni and Cr) immobilization. Compared with AD without iron NPs, the application of iron NPs at dose of 0.5% shows positive impact not only on biogas production, but also on improvement of metals stabilization in the digestate. Metals are found concentrated in Fe–Mn bound and residual fractions and little is accumulated in the liquid digestate and most mobile fractions of solid digestate (water soluble, exchangeable, and carbonates bound). In addition, iron nanoparticles could promote the immobilization of phosphorus within the sludge during AD [382]. A biochar-mineral complexes (BMCs) containing Fe3O4 has been developed as a soil improvement material. It has a higher mineral content, surface functionality, exchangeable cations, high MNP, and higher water-extractable organic compounds. Two biochars produced under different pyrolysis conditions are activated with a phosphoric acid treatment. A mixture of clay, chicken litter, and minerals are added to the biochar, and then this composite is torrefied at either 180 or 220 °C. The BMCs improve mycorrhizal colonisation, wheat growth and nutrient uptake, and soil quality [383].
Degradation of polycyclic aromatic hydrocarbons (PAHs) in contaminated soils through Fenton-like oxidation catalyzed by magnetite has been reported [398]. PAHs degradation is improved in soils pre-treated with availability-enhancement agents such as ethanol or cyclodextrin. This study shows the promising efficiency of magnetite for PAHs oxidation at circumneutral pH over soluble Fe2+ in contaminated soils. Furthermore, MNPs are magnetic sorbents so that the adsorbed pollutants can be recovered. A coal-based magnetic activated carbon (MAC) is identified as the strongest of four AC and BC derived magnetic sorbents for PAHs remediation. An 8.1% MAC amendment (w/w, equal to 5% AC content) is found to be as effective as 5% (w/w) pristine AC in reducing aqueous PAHs within three months by 98% [384]. A recent study has investigated effectiveness of starch-stabilized MNPs for in situ enhanced sorption and immobilization of As(V) in a model sandy loam soil. Batch tests show that the NPs offer an As(V) distribution coefficient of 10,000 L g−1, which is >3 orders of magnitude greater than that of the soil. Batch and column experimental results reveal that the NP treatment greatly reduce water-leachable As(V) and the leachability of As(V) remaining in the soil per TCLP (Toxicity Characteristic Leaching Procedure) analysis. While the NPs are deliverable in the soil, the effective travel distance of the NPs can be manipulated by controlling the injection flow rate. Under natural groundwater flow conditions (velocity ≤2.4 × 10−4 cm s−1), the delivered MNPs are confined within a limited distance (<6.1 cm) [385]. Magnetite strongly retains As, and is relatively stable under Fe(III)-reducing conditions common in aquifers that release As. A laboratory microcosm experiment has investigated a potential As remediation method involving magnetite formation, using groundwater and sediments from the Vineland Superfund site. The microcosms are amended with various combinations of nitrate, FeSO4, and lactate, and are incubated for more than 5 weeks. In the microcosms enriched with 10 mM nitrate and 5 mM Fe(II)aq, black MNPs are produced, and As removal from solution is observed even under sustained Fe(III) reduction stimulated by the addition of 10 mM lactate. The enhanced As retention is mainly attributed to co-precipitation within magnetite and adsorption on a mixture of magnetite and ferrihydrite. Sequential chemical extraction, XAS and magnetic susceptibility measurements show that these minerals form at pH 6–7 following nitrate-Fe(II) addition, and As-bearing magnetite is stable under reducing conditions. SEM and XRD indicate that MNPs are produced as coatings on fine sediments, and no aging effect is detected on morphology over the course of incubation. These results suggest that a magnetite based strategy may be a long-term remedial option for As-contaminated aquifers [386].
Microsized magnetic particles (97.5% Fe, mostly ZVI) have been tested as P adsorbents in a microcosm experiment in a context of lake restoration. MPs are added to sediment cores from a hypertrophic lake, at Fe:PMobile molar ratio of 285:1 and 560:1 under both, oxic and anoxic conditions. It has been found that, under anoxic conditions (anoxic), magnetic particls are able to reduce P release rate from the sediment to the overlying water and to reduce sedimentary PMobile concentration (a 22–25% reduction within 0–4 cm depth compared to controls). Under oxic conditions, the addition of MPs do not affect P fluxes across the sediment and water interface since the lake sediment is naturally rich in iron oxides. However a measured reduction in sedimentary PMobile concentration (12–16% reduction in 0–10 cm depth) contributes to a potential reduction in long-term P efflux [387]. Because magnetite is a major corrosion product of ZVI, part of the P removing capacity could be derived from magnetite, either microscale or nanoscale. Polyferric sulphate has been widely used for emergent control on incidental release of heavy metals such as Cd2+ to surface water, causing precipitation of Cd-loaded polyferric flocs to the sediment. To date, little is known about whether the dissolution of the flocs in the presence of dissimilatory iron reducing bacteria (DIRB) can occur and how the dissolution influences the fate of Fe and Cd in the sediment. A recent study has demonstrated that Shewanella oneidensis MR-1, as representative DIRB, has the ability to reduce the flocs, resulting in the release of Fe2+ and Cd2+ to the solution and subsequent formation of iron minerals such as goethite and magnetite as a consequence of microbial Fe(III) reduction. The newly formed goethite and magnetite can re-immobilize Cd (Fig. 18) [388]. Studies like this provide solid evidence for natural attenuation of metals in the sediment.
Figure 18.
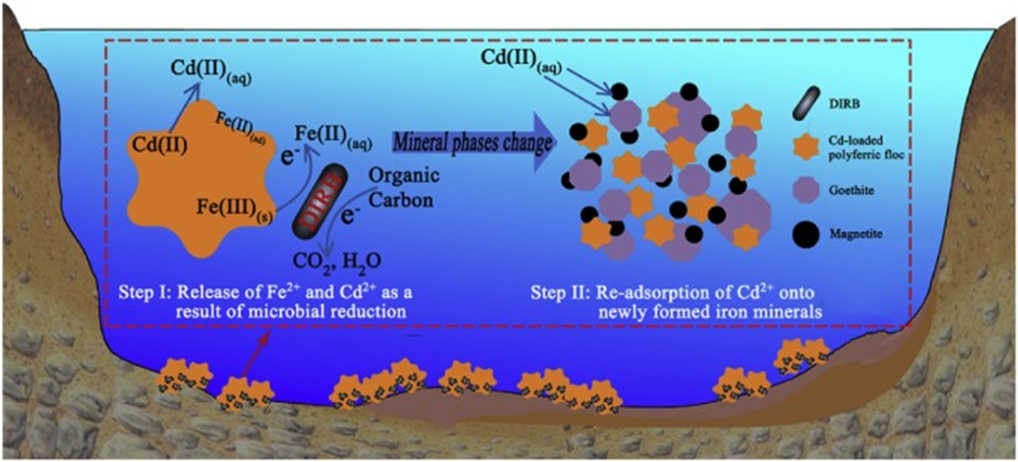
Proposed schematic illustrating the fate of Fe and Cd as a consequence of microbial reduction of Cd-loaded polyferric flocs by Shewanella oneidensis MR-1. Reprinted from Ref. [388]. Copyright 2016 Elsevier Ltd.
Chlorinated solvents are common groundwater contaminants due to their wide use in industry and commerce. Magnetite is known to be able to degrade certain chlorinated solvents under laboratory conditions [389], [390], [391], [392], [393], [394]. A study also shows that 0.3% magnetite in aquifer sediment was responsible for the natural attenuation of cis-dichloroethene (cis-DCE) in a groundwater plume [395]. A recent study has reported CCl4 degradation by different minerals with the normalized rate constant in the order of Fe0 > GR > Fe(II) sorbed goethite > magnetite [396]. Another study found the rate constants for cis-DCE degradation in the order of GR > Fe(OH)2 > mackinawite = magnetite [397]. More recently, a general trend of mineral reactivity toward chlorinated solvent degradation has been approximated as follows: disordered FeS > FeS > Fe0 > FeS2 > sorbed Fe2+ > GR = magnetite > biotite = vermiculite [398]. Compared to Fe0, iron sulfides, and GR, magnetite is less reactive in dechlorination; however, magnetite is more resistant to oxidation than the other minerals and its role in dechloration can be significant if magnetite is present in large quantities in aquifers.
8. Discussion
The potential number of hybrid nanocomposites that may conjugate MNPs with other NMs could be astronomical and it may be impractical to try out every possible combinations. Thus it is imperative that optimal experimental design is conceived to be scientifically feasible and economically cost-effective. Scale-up of synthesis methods may be challenging for making large quantities of MNP hybrid catalysts and reactants for environmental applications. The development of a low-cost, fast, and large-scale process for the synthesis and manipulation of MNPs is essential for incorporating materials with diverse practical applications. Green synthesis is an area in need of further investigation. More economical raw materials may be exploited for synthesizing MNPs. For example, a recent study has attempted to make MNPs using ferric iron recovered from waste iron ore tailing and reagent grade FeCl2 by coprecipitation, for the decolourisation of RhB under visible light (VL)/sunlight [399]. Euphorbia stracheyi Boiss plant root extract has been used as a natural source of reducing and stabilizing agent for supporting Pd NPs on MNPs. The Pd/Fe3O4 NPs can efficiently catalyze the reductive amination of aldehydes at room temperature [400]. Clay minerals are cheap and abundant. Eco-friendly pillared montmorillonites, in which the pillars consist of a magnetic iron oxide (γ-Fe2O3) have been synthesized using mild reaction conditions and benign products. The composite shows catalytic degradation of dichlorophenol (DCP) in the presence of PMS or H2O2 and peracetic acid [401]. A method has been reported to synthesize magnetic expanded perlite composite through a co-precipitation method using single iron source and yellow pea as a reducing agent. The suggested method is environment friendly, easy, and biogenic. The combination of MNPs with expanded perlite and its modification with ibuprofen results in a new, cost-effective, and eco-friendly magnetic adsorbent for dye (Direct Red-81) removal from contaminated streams [402].
Because magnetite is a major component of the passivation layer of granular ZVI, and because magnetite (both nanosized and microsized) is also a Fe0 corrosion product when NZVI is used for source zone treatment of chlorinated solvents [403], [404], magnetite is an important link with respect to contaminant degradation and removal. Researchers have explored means of depassivation of aged ZVI by divalent cations. For example, a recent study has evaluated dechlorination of trichloroethylene (TCE) by aged Fe0 in the presence of a series of divalent cations with the result that while no significant degradation of TCE is observed in Milli-Q water or in solutions of Ba2+, Sr2+, or Ca2+, very effective TCE removal is observed in solutions containing Mg2+, Mn2+, Co2+, Fe2+, Ni2+, Zn2+, Cu2+, or Pb2+. The depassivation process is proposed to involve (i) surface complexation of cations on surface coatings of aged Fe0, (ii) dissolution of the hydrated surface as a consequence of magnetite exposure, and (iii) transport of electrons from underlying Fe0 via magnetite to TCE, resulting in TCE dechlorination and, for some cations (Co2+, Ni2+, Cu2+, and Pb2+), reduction to their zero or +1 valence state (with potential for these reduced metals to enhance TCE degradation) [405]. Surfactants have been used to increase reactivity of NZVI-based materials. In a recent study, pentachlorophenol (PCP) is treated with Ni/Fe NPs. An increase in the Ni/Fe dosage enhances the removal of PCP. The most effective nickel percentage is 0.5%. Among the selected surfactants including carboxymethyl cellulose, Triton X-100, and CTAB, CTAB markedly enhances the removal of PCP by Ni/Fe [406]. Short-chain organic acids such as oxalic acid have been used as a complexation agent for ferrous iron to depassivate the iron oxide layer to generate fresh NZVI surfaces for PCP degradation by NZVI [407]. Oxalic acid is a greener treatment agent than doping metal on NZVI.
More research should be conducted on the field-scale impact of magnetite on groundwater contaminant degradation. NZVI corrodes away within a few months in groundwater but magnetite can be persistent [404]. More work is needed to characterize the formation and transformation of magnetite in groundwater systems where active remediation takes place. More laboratory and field tests could be performed to evaluate MNPs for soil treatment. Other NPs have been investigated, for example, a new class of stabilized Fe–Mn binary oxide nanoparticles is prepared with a water-soluble starch or CMC as a stabilizer [408]. The NPs are characterized and tested with respect to sorption of As(III) and As(V) from water and for immobilization of As(III) in soil. While arsenic sorption capacities are comparable for bare, or stabilized Fe–Mn nanoparticles, particle stabilization enables the NPs to be delivered into soil for in situ immobilization of As(III). High As(III) sorption capacity is observed at pH 5–9. Column breakthrough tests demonstrate soil mobility of CMC-stabilized NPs. Once delivered, the NPs remain virtually immobile in soil under typical groundwater conditions, serving as a fixed sink for arsenic [408].
More attention should be paid to evaluation of the role magnetite plays in the natural attenuation of contaminants in soil and groundwater. Microorganisms (e.g., G. metallireducens) can reduce hydrous ferric oxide to magnetite and/or to Fe(II) surface species bound to the magnetite surface under Fe(III)-reducing conditions. These biogenic magnetite and sorbed Fe(II) surface species could be regenerated by Fe(II) produced by microorganisms after their oxidation by chlorinated solvents. Thus, abiotic degradation by biogenic minerals may be long lasting if reducing conditions are maintained. This relationship can be used to design “in situ permeable reactive barriers” systems [398].
Magnetite had been considered as a stable end product of the bioreduction of Fe(III) minerals (e.g., ferrihydrite, lepidocrocite, hematite) or of the biological oxidation of Fe(II) compounds (e.g., siderite), with GR as a mixed Fe(II)–Fe(III) hydroxide intermediate. Biotic transformation of magnetite to GR had not been demonstrated until now. A recent study has investigated the capability of an iron-educing bacterium, Shewanella putrefaciens, to reduce magnetite at circumneutral pH in the presence of dihydrogen as sole inorganic electron donor. During incubation, GR and/or siderite (Fe(II)CO3) formation occurs as secondary iron minerals, resulting from the precipitation of Fe(II) species produced via the bacterial reduction of Fe(III) species present in magnetite. Taking into account the exact nature of the secondary iron minerals and the electron donor source is necessary to understand the exergonic character of the biotic transformation of magnetite to GR, which had been considered to date as thermodynamically unfavorable at circumneutral pH. This finding reinforces the hypothesis that GR would be the cornerstone of the microbial transformations of iron-bearing minerals in the anoxic biogeochemical cycle of iron and opens up new possibilities for the interpretation of the evolution of Earth’s history and for the understanding of biocorrosion processes in the field of applied science [409]. This may also have important implications when engineered MNPs are used for environmental remediation.
9. Summary
This review covers only a selective portion of a huge and ever increasing body of recent literature on environmental implications and applications of MNPs. MNPs are attractive and promising materials for helping solve environmental contamination problems due to their paramagnetism that leads to easy separation from the environmental media and recyclability of use, as well as the large surface area for contaminant adsorption and immobilization. MNPs possess chemical reducing power that can reduce toxic chemicals to less toxic or non-toxic forms. MNPs are widely used as reusable catalysts for oxidative degradation of recalcitrant contaminants. The following are main points of this review.
There are a variety of synthesis methods for making MNPs and their hybrids each with its own advantages and disadvantages. A suitable method has to be the one that produces the desired MNPs with specific size, shape, and shelf life.
Fate and transport of MNPs and their hybrids depend on the surface charge properties and properties of the porous media and solution chemistry.
MNPs have low to none toxicity to humans and ecosystems. Surface modification has to be made to make them biocompatible.
MNPs are important advanced anode materials for lithium-ion batteries.
MNPs are widely used in making biosensors, chemical sensors, and recyclable catalysts.
MNPs are widely used as adsorptive and separative removal of a wide range of contaminants from wastewater.
MNPs may be important in active remediation and natural attenuation of contaminants.
Further studies are needed to explore ways to cost effectively synthesize MNPs and to develop practical methods for soil and groundwater remediation.
Highlights.
Environmental impact of engineered MNPs.
MNPs and their hybrids explored for use in energy, analytical chemistry, and catalysis.
Surface modification to MNPs allow biocompatible applications.
Adsorptive and separative removal of a wide range of contaminants from aquatic environments.
Active remediation and natural attenuation of contaminants in soil and groundwater using MNPs.
Acknowledgements
The work upon which this paper is based was supported by the U.S. Environmental Protection Agency through its Office of Research and Development. This work has not been subjected to agency review and, therefore, doesn’t necessarily reflect the views of the agency and no official endorsement should be inferred. Any product or trade name mentioned here is for information purposes only and not to constitute endorsement.
Abbreviations
- AC
activated carbon
- AD
anaerobic digestion
- AAS
atomic absorption spectrometry
- APAP
Acetaminophen
- AQDS
9,10-anthraquinone-2,6-disulfonate
- BB
bromothymol blue
- BC
bacterial cellulose
- BMCs
biochar-mineral complexes
- BPA
bisphenol A
- BTEX
benzene, toluene, ethylbenzene and xylenes
- CA
contact angle
- CBZ
carbamazepine
- β-CD
β-cyclodextrin
- CG
chitosan-functionalized grapheme
- CMC
carboxymethyl cellulose
- CNF
carbon nanofiber
- CNTs
carbon nanotubes
- CQDs
carbon quantum dots
- CSN
core–shell nanospheres
- CTAB
cetyltrimethylammonium bromide
- CTPEs
composite thermoplastic elastomers
- 2,4-D
2,4-dichlorophenoxyacetic acid
- DCP
dichlorophenol
- DCPD
dicyclopentadiene
- DFMNPs
double functionalized magnetic nanoparticles
- DIRB
dissimilatory iron reducing bacteria
- DMSA
dimercaptosuccinic acid
- DSPE
dispersed solid-phase extraction
- EDTA-MGO
ethylenediaminetetraacetic acid-magnetic graphene oxide
- EG
ethylene glycol
- FLU
flumequine
- GA
gallic acid
- GO
graphene/graphite oxide
- GR
green rust
- HA
humic acid
- HNTs
halloysite nanotubes
- HPLC
high performance liquid chromatography
- HUVEC
human umbilical vein endothelial cells
- ICP-MS
inductively coupled plasma-mass spectrometry
- LDH
layered double hydroxide
- MAA
methacrylic acid
- MB
methylene blue
- MCM
magnetic composite microsphere
- MCS
magnetic core/shell
- MC-TiNbNS
magnetite/ceria-codecorated titanoniobate nanosheet
- m-Fe3O4-CN
magnetic Fe3O4 chitosan nanoparticles
- MGO
magnetic graphene oxide
- MNCs
magnetic nanocomposites
- MNPs
magnetite nanoparticles
- M-QAC
magnetite-quaternary ammonium compounds
- MWCNTs
multi-walled carbon nanotubes
- MS-MRS
magnetic separation and magnetic relaxation switching
- NMs
nanomaterials
- NFMs
nanofibrous membranes
- NOM
natural organic matter
- 4-NP
4-nitrophenol
- 4-n-NP
4-n-nonylphenol
- NPs
nanoparticles
- NZVI
nanosized zerovalent iron
- PAHs
polycyclic aromatic hydrocarbons
- PCP
pentachlorophenol
- PDA
polydopamine
- PET
polyethylene terephthalate
- PEG
polyethylene glycol
- PEI
polyethylenimine
- PFOS
perfluorooctane sulfonates
- PGA@MNP
polygallate coated magnetic nanoparticles
- PHQ
polyhydroquinone
- PMMA
poly(methylmethacrylate)
- PMS
peroxymonosulfate
- PPy
polypyrrole
- PQA
poly quaternary ammonium
- PUU
poly(urea-urethane)
- PVA
polyvinlyalcohol
- PVP
poly(vinylpyrrolidone)
- PVDF
polyvinylidene fluoride
- PZC
point of zero charge
- QAC
quaternary ammonium compounds
- RGO
reduced graphene oxide
- RhB
rhodamine B
- ROS
reactive oxygen species
- SEM
scanning electron microscope
- SERS
surface enhanced Raman spectroscopy
- SPE
solid-phase extraction
- SPION
superparamagnetic iron oxide nanoparticles
- TBBPA
tetrabromobisphenol A
- TC
tetracycline
- TCE
trichloroethene
- TCLP
toxicity characteristic leaching procedure
- TEM
transmission electron microscopy
- TEPA
tetraethylenepentamine
- TETA
triethylentetramine
- XAS
X-ray absorption spectroscopy
- XANES
X-ray adsorption near edge structure
- XPS
X-ray photoelectron spectroscopy
- XRD
X-ray diffraction
- YSN
Yolk–shell nanospheres
- ZVI
Zerovalent iron
References
- [1].Wu W, Wu Z, Yu T, Jiang C, Kim W-S Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications Sci. Technol. Adv. Mater, 16 (2015), pp. 23501–23543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sun S-N, Chao W, Zhu Z-Z, Hou Y-L, Venkatraman SS, Xu Z-C Magnetic iron oxide nanoparticles: synthesis and surface coating techniques for biomedical applications Chin. Phys. B, 23 (2014), p. 037503 [Google Scholar]
- [3].Shi D, Sadat ME, Dunn AW, Mast DB Photo-fluorescent and magnetic properties of iron oxide nanoparticles for biomedical applications Nanoscale, 7 (2015), pp. 8209–8232 [DOI] [PubMed] [Google Scholar]
- [4].Sharifi S, Seyednejad H, Laurent S, Atyabi F, Saei AA, Mahmoudi M Superparamagnetic iron oxide nanoparticles for in vivo molecular and cellular imaging Contrast Media Mol. Imaging, 10 (2015), pp. 329–355 [DOI] [PubMed] [Google Scholar]
- [5].Revia RA, Zhang M Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances Mater. Today, 19 (2016), pp. 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Talelli M, Aires A, Marciello M Protein-modified magnetic nanoparticles for biomedical applications Curr. Org. Chem, 20 (2016), pp. 1252–1261 [Google Scholar]
- [7].Sonmez M, Georgescu M, Alexandrescu L, Gurau D, Ficai A, Ficai D, Andronescu E Synthesis and applications of Fe3O4/SiO2 core–shell materials Curr. Pharm. Des, 21 (2015), pp. 5324–5335 [DOI] [PubMed] [Google Scholar]
- [8].Dutta S, Parida S, Maiti C, Banerjee R, Mandal M, Dhara D Polymer grafted magnetic nanoparticles for delivery of anticancer drug at lower pH and elevated temperature J. Colloid Interface Sci, 467 (2016), pp. 70–80 [DOI] [PubMed] [Google Scholar]
- [9].Horst MF, Lassalle V, Ferreira ML Nanosized magnetite in low cost materials for remediation of water polluted with toxic metals, azo- and antraquinonic dyes Front. Environ. Sci. Eng, 9 (2015), pp. 746–769 [Google Scholar]
- [10].Sharma VK, McDonald TJ, Kim H, Garg VK Magnetic graphene–carbon nanotube iron nanocomposites as adsorbents and antibacterial agents for water purification Adv. Colloid Interface Sci, 225 (2015), pp. 229–240 [DOI] [PubMed] [Google Scholar]
- [11].Lu AH, Salabas EL, Schuth F Magnetic nanoparticles: synthesis protection, functionalization, and application Angew. Chem. Int. Ed, 46 (2007), pp. 1222–1244 [DOI] [PubMed] [Google Scholar]
- [12].Wu W, He Q, Jiang C Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies Nanoscale Res. Lett, 3 (2008), pp. 397–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Unsoy G, Gunduz U, Oprea O, Ficai D, Sonmez M, Radulescu M, Alexie M, Ficai A Magnetite: from synthesis to applications Curr. Topics Med. Chem, 15 (2015), pp. 1622–1640 [DOI] [PubMed] [Google Scholar]
- [14].Fratila RM, Mitchell SG, del Pino P, Grazu V, de la Fuente JM Strategies for the biofunctionalization of gold and iron oxide nanoparticles Langmuir, 30 (2014), pp. 15057–15071 [DOI] [PubMed] [Google Scholar]
- [15].Lee J, Isobe T, Senna M Magnetic properties of ultrafine magnetite particles and their slurries prepared via in-situ precipitation Colloids Surf. A: Physicochem. Eng. Aspects, 109 (1996), pp. 121–127 [Google Scholar]
- [16].Kim JH, Kim SM, Kim YI Properties of magnetic nanoparticles prepared by co-precipitation J. Nanosci. Nanotechnol, 14 (2014), pp. 8739–8744 [DOI] [PubMed] [Google Scholar]
- [17].Vikram S, Dhakshnamoorthy M, Vasanthakumari R, Rajamani AR, Rangarajan M, Tsuzuki T Tuning the magnetic properties of iron oxide nanoparticles by a room-temperature air-atmosphere (RTAA) co-precipitation method J. Nanosci. Nanotechnol, 15 (2015), pp. 3870–3878 [DOI] [PubMed] [Google Scholar]
- [18].Chen J, Yuan TJ Effects of citrate on preparation and properties of nano-Fe3O4 J. Mater. Eng, 43 (2015), pp. 85–89 [Google Scholar]
- [19].Yoon KY, Xue Z, Fei Y, Lee JH, Cheng V, Bagaria HG, Huh C, Bryant SL, Kong SD, Ngo VW, Rahmani A-R, Ahmadian M, Ellison CJ, Johnston KP Control of magnetite primary particle size in aqueous dispersions of nanoclusters for high magnetic susceptibilities J. Colloid Interface Sci, 462 (2016), pp. 359–367 [DOI] [PubMed] [Google Scholar]
- [20].Muthukumaran T, Philip J A single pot approach for synthesis of phosphate coated iron oxide nanoparticles J. Nanosci. Nanotechnol, 15 (2015), pp. 2715–2725 [DOI] [PubMed] [Google Scholar]
- [21].Yang W, Yu Y, Wang L, Yang C, Li H Controlled synthesis and assembly into anisotropic arrays of magnetic cobalt-substituted magnetite nanocubes Nanoscale, 7 (2015), pp. 2877–2882 [DOI] [PubMed] [Google Scholar]
- [22].Zaki HM, Al-Heniti SH, Al-Hadeethi Y, Alsanoosi AM Magnetic nanoparticles: synthesis, characterization and magnetic properties of cobalt aluminum ferrite J. Nanosci. Nanotechnol, 16 (2016), pp. 4733–4741 [DOI] [PubMed] [Google Scholar]
- [23].Huang S, Wang H, Zhu N, Lou Z, Li L, Shan A, Yuan H Metal recovery based magnetite near-infrared photocatalyst with broadband spectrum utilization property Appl. Catal. B: Environ, 181 (2016), pp. 456–464 [Google Scholar]
- [24].Li XA, Wang CS, Han XJ Electromagnetic wave absorbing property of composite Fe3O4-graphite prepared by in-situ chemical precipitation J. Mater. Eng, 43 (2015), pp. 44–49 [Google Scholar]
- [25].Qu B, Zhu C, Li C, Zhang X, Chen Y Coupling hollow Fe3O4–Fe nanoparticles with graphene sheets for high-performance electromagnetic wave absorbing material ACS Appl. Mater. Interfaces, 8 (2016), pp. 3730–3735 [DOI] [PubMed] [Google Scholar]
- [26].Giardiello M, Hatton FL, Slater RA, Chambon P, North J, Peacock AK, He T, McDonald TO, Owen A, Rannard SP Stable, polymer-directed and SPION-nucleated magnetic amphiphilic block copolymer nanoprecipitates with readily reversible assembly in magnetic fields Nanoscale, 8 (2016), pp. 7224–7231 [DOI] [PubMed] [Google Scholar]
- [27].Park J, An K, Hwang Y, Park J-G, Noh H-J, Kim J-Y, Park J-H, Hwang N-M, Hyeon T Ultra-large-scale syntheses of monodisperse nanocrystals Nat. Mater, 3 (2004), pp. 891–895 [DOI] [PubMed] [Google Scholar]
- [28].Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles J. Am. Chem. Soc, 126 (2004), pp. 273–279 [DOI] [PubMed] [Google Scholar]
- [29].Redl FX, Black CT, Papaefthymiou GC, Sandstrom RL, Yin M, Zeng H, Murray CB, O’Brien SP Magnetic, electronic, and structural characterization of nonstoichiometric iron oxides at the nanoscale J. Am. Chem. Soc, 126 (2004), pp. 14583–14599 [DOI] [PubMed] [Google Scholar]
- [30].Li Z, Sun Q, Gao M Preparation of water-soluble magnetite nanocrystals from hydrated ferric salts in 2-pyrrolidone: mechanism leading to Fe3O4 Angew. Chem, 117 (2005), pp. 125–128 [DOI] [PubMed] [Google Scholar]
- [31].Hu FQ, Wei L, Zhou Z, Ran YL, Li Z, Gao MY Preparation of biocompatible magnetite nanocrystals for in vivo magnetic resonance detection of cancer Adv. Mater, 18 (2006), pp. 2553–2556 [Google Scholar]
- [32].Bixner O, Lassenberger A, Baurecht D, Reimhult E Complete exchange of the hydrophobic dispersant shell on monodisperse superparamagnetic iron oxide nanoparticles Langmuir, 31 (2015), pp. 9198–9204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Palma SICJ, Marciello M, Carvalho A, Veintemillas-Verdaguer S, del Puerto Morales M, Roque ACA Effects of phase transfer ligands on monodisperse iron oxide magnetic nanoparticles J. Colloid Interface Scie, 437 (2015), pp. 147–155 [DOI] [PubMed] [Google Scholar]
- [34].Zhuang L, Zhang W, Zhao Y, Shen H, Lin H, Liang J Preparation and characterization of Fe3O4 particles with novel nanosheets morphology and magnetochromatic property by a modified solvothermal method Sci. Rep, 5 (2015), p. 9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Patsula V, Kosinová L, Lovrić M, Hamzić LF, Rabyk M, Konefal R, Paruzel A, Šlouf M, Herynek V, Gajović S, Horák D Superparamagnetic Fe3O4 nanoparticles: synthesis by thermal decomposition of iron(III) glucuronate and application in magnetic resonance imaging ACS Appl. Mater. Interfaces, 8 (2016), pp. 7238–7247 [DOI] [PubMed] [Google Scholar]
- [36].Deniz AR, Çaldıran Z, Metin Ö, Meral K, Aydoğan Ş The investigation of the electrical properties of Fe3O4/n-Si heterojunctions in a wide temperature range J. Colloid Interface Sci, 473 (2016), pp. 172–181 [DOI] [PubMed] [Google Scholar]
- [37].Shin J, Lee KY, Yeo T, Choi W Facile one-pot transformation of iron oxides from Fe2O3 nanoparticles to nanostructured Fe3O4@C core–shell composites via combustion waves Sci. Rep, 6 (2016), p. 21792, 10.1038/srep21792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang X, Zhuang J, Peng Q, Li Y A general strategy for nanocrystal synthesis Nature, 437 (2005), pp. 121–124 [DOI] [PubMed] [Google Scholar]
- [39].Karunakaran C, Vinayagamoorthy P, Jayabharathi J Nonquenching of charge carriers by Fe3O4 core in Fe3O4/ZnO nanosheet photocatalyst Langmuir, 30 (2014), pp. 15031–15039 [DOI] [PubMed] [Google Scholar]
- [40].Sheng W, Liu J, Liu S, Lu Q, Kaplan DL, Zhu H One-step synthesis of biocompatible magnetite/silk fibroin core–shell nanoparticles J. Mater. Chem. B, 2 (2014), pp. 7394–7402 [DOI] [PubMed] [Google Scholar]
- [41].Song H, Liu M, Li S, Chen L, Lin C, Zhang L Polyvinyl pyrrolidone-assisted solvothermal synthesis of Fe3O4 vesicular nanospheres J. Nanosci. Nanotechnol, 15 (2015), pp. 3998–4002 [DOI] [PubMed] [Google Scholar]
- [42].Wang L, Zhao Q, Hou J, Yan J, Zhang F, Zhao J, Ding H, Li Y, Ding L One-step solvothermal synthesis of magnetic Fe3O4–graphite composite for Fenton-like degradation of levofloxacin J. Environ. Sci. Health Part A, 51 (2016), pp. 52–62 [DOI] [PubMed] [Google Scholar]
- [43].Tong G, Liu Y, Wu T, Tong C, Du F H2O-steered size/phase evolution and magnetic properties of large-scale, monodisperse FexOy nanomaterials J. Mater. Chem. C, 3 (2015), pp. 5506–5515 [Google Scholar]
- [44].Tiano AL, Papaefthymiou GC, Lewis CS, Han J, Zhang C, Li Q, Shi C, Abeykoon AMM, Billinge SJL, Stach E, Thomas J, Guerrero K, Munayco P, Munayco J, Scorzelli RB, Burnham P, Viescas AJ, Wong SS Correlating size and composition-dependent effects with magnetic Mössbauer, and pair distribution function measurements in a family of catalytically active ferrite nanoparticles Chem. Mater, 27 (2015), pp. 3572–3592 [Google Scholar]
- [45].Zhao H, Cui H-J, Fu M-L A general and facile method for improving carbon coat on magnetic nanoparticles with a thickness control J. Colloid Interface Sci, 461 (2016), pp. 20–24 [DOI] [PubMed] [Google Scholar]
- [46].Yang Q, Lan F, Yi Q, Wu Y, Gu Z A colloidal assembly approach to synthesize magnetic porous composite nanoclusters for efficient protein adsorption Nanoscale, 7 (2015), pp. 17617–17622 [DOI] [PubMed] [Google Scholar]
- [47].Kim Y, Yul Y Microbial synthesis and characterization of superparamagnetic Zn-substituted magnetite nanoparticles J. Nanosci. Nanotechnol, 15 (2015), pp. 6129–6132 [DOI] [PubMed] [Google Scholar]
- [48].O’Loughlin EJ, Gorski CA, Scherer MM Effects of phosphate on secondary mineral formation during the bioreduction of akaganeite (β-FeOOH): green rust versus framboidal magnetite Curr. Inorg. Chem, 5 (2015), pp. 214–224 [Google Scholar]
- [49].Tuo Y, Liu G, Dong B, Zhou J, Wang A, Wang J Microbial synthesis of Pd/Fe3O4 Au/Fe3O4 and PdAu/Fe3O4 nanocomposites for catalytic reduction of nitroaromatic compounds Sci. Rep, 5 (2015), p. 13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chen C, Wang P, Li L Applications of bacterial magnetic nanoparticles in nanobiotechnology J. Nanosci. Nanotechnol, 16 (2016), pp. 2164–2171 [DOI] [PubMed] [Google Scholar]
- [51].Schmidtke C, Eggers R, Zierold R, Feld A, Kloust H, Wolter C, Ostermann J, Merkl J-P, Schotten T, Nielsch K, Weller H Polymer-assisted self-assembly of superparamagnetic iron oxide nanoparticles into well-defined clusters: controlling the collective magnetic properties Langmuir, 30 (2014), pp. 11190–11196 [DOI] [PubMed] [Google Scholar]
- [52].Lin AL, Rodrigues JNB, Su C, Milletari M, Loh KP, Wu T, Chen W, Castro Neto AH, Adam S, Wee ATS Tunable room-temperature ferromagnet using an iron-oxide and graphene oxide nanocomposite Sci. Rep, 5 (2015), p. 11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lee J, Kwon SG, Park J-G, Hyeon T Size dependence of metal-insulator transition in stoichiometric Fe3O4 nanocrystals Nano Lett., 15 (2015), pp. 4337–4342 [DOI] [PubMed] [Google Scholar]
- [54].Wang Y, He Q, Qu H, Zhang X, Guo J, Zhu J, Zhao G, Colorado HA, Yu J, Sun L, Bhana S, Khan MA, Huang X, Young DP, Wang H, Wang X, Wei S, Guo Z Magnetic graphene oxide nanocomposites: nanoparticles growth mechanism and property analysis J. Mater. Chem. C, 2 (2014), pp. 9478–9488 [Google Scholar]
- [55].Fu M, Li J One-pot solvothermal synthesis and adsorption property of Pb(II) of superparamagnetic monodisperse Fe3O4/graphene oxide nanocomposite Nanosci. Nanotechnol. Lett, 6 (2014), pp. 1116–1122 [Google Scholar]
- [56].Zhang S, Li W, Tan B, Chou S, Li Z, Dou S One-pot synthesis of ultra-small magnetite nanoparticles on the surface of reduced graphene oxide nanosheets as anodes for sodium-ion batteries J. Mater. Chem. A, 3 (2015), pp. 4793–4798 [Google Scholar]
- [57].Tsoufis T, Syrgiannis Z, Akhtar N, Prato M, Katsaros F, Sideratou Z, Kouloumpis A, Gournis D, Rudolf P In situ growth of capping-free magnetic iron oxide nanoparticles on liquid-phase exfoliated grapheme Nanoscale, 7 (2015), pp. 8995–9003 [DOI] [PubMed] [Google Scholar]
- [58].Cao Y Preparation and magnetic properties of a multi-walled carbon nanotube-iron oxide nanoparticle composite Fullerenes Nanotubes Carbon Nanostruct., 23 (2015), pp. 623–626 [Google Scholar]
- [59].Ji Z, Shen X, Yue X, Zhou H, Yang J, Wang Y, Ma L, Chen K Facile synthesis of magnetically separable reduced graphene oxide/magnetite/silver nanocomposites with enhanced catalytic activity J. Colloid Interface Sci, 459 (2015), pp. 79–85 [DOI] [PubMed] [Google Scholar]
- [60].Shekofteh-Gohari M, Habibi-Yangjeh A Ultrasonic-assisted preparation of novel ternary ZnO/AgI/Fe3O4 nanocomposites as magnetically separable visible-light-driven photocatalysts with excellent activity J. Colloid Interface Sci, 461 (2016), pp. 144–153 [DOI] [PubMed] [Google Scholar]
- [61].Islam MR, Bach LG, Vo TS, Lim KT Covalent immobilization of biotin on magnetic nanoparticles: synthesis, characterization, and cytotoxicity studies J. Nanosci. Nanotechnol, 15 (2015), pp. 176–180 [DOI] [PubMed] [Google Scholar]
- [62].Brollo MEF, Lopez-Ruiz R, Muraca1 D, Figueroa SJA, Pirota KR, Knobel M Compact Ag@Fe3O4 core–shell nanoparticles by means of single-step thermal decomposition reaction Sci. Rep, 4 (2014), p. 6839, 10.1038/srep06839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Guldu OK, Unak P, Medine EI, Barlas FB, Muftuler FZB, Timur S Radioiodinated magnetic nanoparticles conjugated with moxifloxacin: synthesis and in vitro biological affinities Int. J. Polym. Mater. Polym. Biomater, 64 (2015), pp. 253–259 [Google Scholar]
- [64].Tancredi P, Botasini S, Moscoso-Londoño O, Méndez E, Socolovsky L Polymer-assisted size control of water-dispersible iron oxide nanoparticles in range between 15 and 100 nm Colloids Surf. A: Physicochem. Eng. Aspects, 464 (2015), pp. 46–51 [Google Scholar]
- [65].Serenjeh FN, Hashemi P, Rasoolzadeh F A simple method for the preparation of spherical core–shell nanomagnetic agarose particles Colloids Surf. A: Physicochem. Eng. Aspects, 465 (2015), pp. 47–53 [Google Scholar]
- [66].Park DE, Chae HS, Choi HJ, Maity A Magnetite–polypyrrole core–shell structured microspheres and their dual stimuli-response under electric and magnetic fields J. Mater. Chem. C, 3 (2015), pp. 3150–3158 [Google Scholar]
- [67].Yan X, Kong J, Yang C, Fu G Facile synthesis of hairy core–shell structured magnetic polymer submicrospheres and their adsorption of bovine serum albumin J. Colloid Interface Sci, 445 (2015), pp. 9–15 [DOI] [PubMed] [Google Scholar]
- [68].Woo H, Park JC, Park S, Park KH Rose-like Pd–Fe3O4 hybrid nanocomposite-supported Au nanocatalysts for tandem synthesis of 2-phenylindoles Nanoscale, 7 (2015), pp. 8356–8360 [DOI] [PubMed] [Google Scholar]
- [69].Li M, Li X, Qi X, Luo F, He G Shape-controlled synthesis of magnetic iron oxide@SiO2–Au@C particles with core–shell nanostructures Langmuir, 31 (2015), pp. 5190–5197 [DOI] [PubMed] [Google Scholar]
- [70].Jiang F, Zhang Y, Wang Z, Wang W, Xu Z, Wang Z Combination of magnetic and enhanced mechanical properties for copolymer-grafted magnetite composite thermoplastic elastomers ACS Appl. Mater. Interfaces, 7 (2015), pp. 10563–10575 [DOI] [PubMed] [Google Scholar]
- [71].Song HM, Zink JI, Khashab NM Engineering the internal structure of magnetic silica nanoparticles by thermal control Part. Part. Syst. Charact, 32 (2015), pp. 307–312 [Google Scholar]
- [72].Liu X, Chen Y, Cui X, Zeng M, Yu R, Wang G-S Flexible nanocomposites with enhanced microwave absorption properties based on Fe3O4/SiO2 nanorods and polyvinylidene fluoride J. Mater. Chem. A, 3 (2015), pp. 12197–12204 [Google Scholar]
- [73].Chen X, Tan L, Meng X Synthesis of magnetic rattle-type silica with controllable magnetite and tunable size by pre-shell-post-core method J. Nanosci. Nanotechnol, 16 (2016), pp. 3003–3008 [DOI] [PubMed] [Google Scholar]
- [74].Ma J, Wang K, Zhan M Growth mechanism and electrical and magnetic properties of Ag–Fe3O4 core–shell nanowires ACS Appl. Mater. Interfaces, 7 (2015), pp. 16027–16039 [DOI] [PubMed] [Google Scholar]
- [75].Zhang H, Liu Y, Zhou Y Preparation of magnetic PET fabric loaded with Fe3O4 nanoparticles by hydrothermal method J. Text. Inst, 106 (2015), pp. 1078–1088 [Google Scholar]
- [76].Chiaradia V, Valério A, Feuser PE, de Oliveira D, Araújo PHH, Sayer C Incorporation of superparamagnetic nanoparticles into poly(urea-urethane) nanoparticles by step growth interfacial polymerization in miniemulsion Colloids Surf. A: Physicochem. Eng. Aspects, 482 (2015), pp. 596–603 [Google Scholar]
- [77].Mallakpour S, Javadpour M An innovative strategy for the production of novel magnetite poly(vinyl alcohol) nanocomposite films with double-capped synthesized Fe3O4 nanoparticles with citric acid and vitamin C Compos. Interfaces, 22 (2015), pp. 867–884 [Google Scholar]
- [78].Rowe MP, Sullivan S, Desautels RD, Skoropata E, van J Lierop Rational selection of superparamagnetic iron oxide/silica nanoparticles to create nanocomposite inductors J. Mater. Chem. C, 3 (2015), pp. 9789–9793 [Google Scholar]
- [79].Shi H, Yang J, Zhu L, Yang Y, Yuan H, Yang Y, Liu X Removal of Pb2+ Hg2+, and Cu2+ by chain-like Fe3O4@SiO2@chitosan magnetic nanoparticles J. Nanosci. Nanotechnol, 16 (2016), pp. 1871–1882 [DOI] [PubMed] [Google Scholar]
- [80].Zhu Z, Li G, Zeng G, Chen X, Hu D, Zhang Y, Sun Y Fast capture of methyl-dyes over hierarchical amino-Co0.3Ni0.7Fe2O4@SiO2 nanofibrous membranes J. Mater. Chem. A, 3 (2015), pp. 22000–22004 [Google Scholar]
- [81].Liu S, Fu J, Wang M, Yan Y, Xin Q, Cai L, Xu Q Magnetically separable and recyclable Fe3O4–polydopamine hybrid hollow microsphere for highly efficient peroxidase mimetic catalysts J. Colloid Interface Sci, 469 (2016), pp. 69–77 [DOI] [PubMed] [Google Scholar]
- [82].Shan R-R, Yan L-G, Yang K, Hao Y-F, Du B Adsorption of Cd(II) by Mg–Al–CO3- and magnetic Fe3O4/Mg–Al–CO3-layered double hydroxides: kinetic, isothermal, thermodynamic and mechanistic J. Hazard. Mater, 299 (2015), pp. 42–49 [DOI] [PubMed] [Google Scholar]
- [83].Zhang S, Fan Q, Gao H, Huang Y, Liu X, Li J, Xu X, Wang X Formation of Fe3O4@MnO2 ball-in-ball hollow spheres as a high performance catalyst with enhanced catalytic performances J. Mater. Chem. A, 4 (2016), pp. 1414–1422 [Google Scholar]
- [84].Wang Y, Pan F, Dong W, Xu L, Wu K, Xu G, Chen W Recyclable silver-decorated magnetic titania nanocomposite with enhanced visible-light photocatalytic activity Appl. Catal. B: Environ, 189 (2016), pp. 192–198 [Google Scholar]
- [85].Gawande MB, Branco PS, Varma RS Nano-magnetite (Fe3O4) as support for recyclable nano-catalysts in the development of sustainable methodologies Chem. Soc. Rev, 42 (2013), pp. 3371–3393 [DOI] [PubMed] [Google Scholar]
- [86].Varma RS Journey on greener pathways: from the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation Green Chem., 16 (2014), pp. 2027–2041 [Google Scholar]
- [87].Sharma VK Festschrift in honor of Rajender S. Varma ACS Sustain. Chem. Eng, 4 (2016), pp. 640–642 [Google Scholar]
- [88].Nasir Baig RB, Varma RS Organic synthesis via magnetic attraction: benign and sustainable protocols using magnetic nanoferrites Green Chem., 15 (2013), pp. 398–417 [Google Scholar]
- [89].Atarod M, Nasrollahzadeh M, Sajadi SM Green synthesis of Pd/RGO/Fe3O4 nanocomposite using Withania coagulans leaf extract and its application as magnetically separable and reusable catalyst for the reduction of 4-nitrophenol J. Colloid Interface Sci, 465 (2016), pp. 249–258 [DOI] [PubMed] [Google Scholar]
- [90].Sajadi SM, Nasrollahzadeh M, Maham M Aqueous extract from seeds of Silybum marianum L. as a green material for preparation of the Cu/Fe3O4 nanoparticles: a magnetically recoverable and reusable catalyst for the reduction of nitroarenes J. Colloid Interface Sci, 469 (2016), pp. 93–98 [DOI] [PubMed] [Google Scholar]
- [91].Tosco T, Bosch J, Meckenstock RU, Sethi R Transport of ferrihydrite nanoparticles in saturated porous media: role of ionic strength and flow rate Environ. Sci. Technol, 46 (2012), pp. 4008–4015 [DOI] [PubMed] [Google Scholar]
- [92].Drozdov AS, Ivanovski V, Avnir D, Vinogradov VV A universal magnetic ferrofluid: nanomagnetite stable hydrosol with no added dispersants and at neutral pH J. Colloid Interface Sci, 467 (2016), pp. 307–312 [DOI] [PubMed] [Google Scholar]
- [93].Peeters K, Lespes G, Zuliani T, Ščančar J, Milačič R The fate of iron nanoparticles in environmental waters treated with nanoscale zero-valent iron, FeONPs and Fe3O4NPs Water Res., 94 (2016), pp. 315–327 [DOI] [PubMed] [Google Scholar]
- [94].Kim Y, Lee S, Kim S Preparation of fluorous solvent-dispersed Fe3O4 nanocrystals: role of oxygen in ligand exchange Langmuir, 32 (2016), pp. 3348–3353 [DOI] [PubMed] [Google Scholar]
- [95].Fujimori A, Ohmura K, Honda N, Kakizaki K Creation of high-density and low-defect single-layer film of magnetic nanoparticles by the method of interfacial molecular films Langmuir, 31 (2015), pp. 3254–3261 [DOI] [PubMed] [Google Scholar]
- [96].Li P, Chevallier P, Ramrup P, Biswas D, Vuckovich D, Fortin M-A, Oh JK Mussel-inspired multidentate block copolymer to stabilize ultrasmall superparamagnetic Fe3O4 for magnetic resonance imaging contrast enhancement and excellent colloidal stability Chem. Mater, 27 (2015), pp. 7100–7109 [Google Scholar]
- [97].Song G-D, Kim M-H, Maeng W-Y Optimization of polymeric dispersant concentration for the dispersion-stability of magnetite nanoparticles in water solution J. Nanosci. Nanotechnol, 14 (2014), pp. 9525–9533 [DOI] [PubMed] [Google Scholar]
- [98].Hu JD, Zevi Y, Kou X-M, Xiao J, Wang X-J, Jin Y Effect of dissolved organic matter on the stability of magnetite nanoparticles under different pH and ionic strength conditions Sci. Total Environ, 408 (2010), pp. 3477–3489 [DOI] [PubMed] [Google Scholar]
- [99].Cuny L, Herrling MP, Guthausen G, Horn H, Delay M Magnetic resonance imaging reveals detailed spatial and temporal distribution of iron-based nanoparticles transported through water-saturated porous media J. Contam. Hydrol, 182 (2015), pp. 51–62 [DOI] [PubMed] [Google Scholar]
- [100].Hong Y, Honda RJ, Myung NV, Walker SL Transport of iron-based nanoparticles: role of magnetic properties Environ. Sci. Technol, 43 (2009), pp. 8834–8839 [DOI] [PubMed] [Google Scholar]
- [101].Legg BA, Zhu M, Comolli LR, Gilbert B, Banfield JF Impacts of ionic strength on three-dimensional nanoparticle aggregate structure and consequences for environmental transport and deposition Environ. Sci. Technol, 48 (2014), pp. 13703–13710 [DOI] [PubMed] [Google Scholar]
- [102].Swindle AL, Madden ASE, Cozzarelli IM, Benamara M Size-dependent reactivity of magnetite nanoparticles: a field-laboratory comparison Environ. Sci. Technol, 48 (2014), pp. 11413–11420 [DOI] [PubMed] [Google Scholar]
- [103].Bakhteeva I, Medvedeva I, Byzov I, Zhakov S, Yermakov A, Uimin M, Shchegoleva N Magnetic field-enhanced sedimentation of nanopowder magnetite in water flow Environ. Technol, 36 (2015), pp. 1828–1836 [DOI] [PubMed] [Google Scholar]
- [104].Wang M, Chou I-M, Lu W, De B Vivo Effects of CH4 and CO2 on the sulfidization of goethite and magnetite: an in situ Raman spectroscopic study in high-pressure capillary optical cells at room temperature Eur. J. Mineral, 27 (2015), pp. 193–201 [Google Scholar]
- [105].Byrne JM, Klueglein N, Pearce C, Rosso KM, Appel E, Kappler A Redox cycling of Fe(II) and Fe(III) in magnetite by Fe-metabolizing bacteria Science, 347 (2015), pp. 1473–1476 [DOI] [PubMed] [Google Scholar]
- [106].Zhu H, Han J, Xiao JQ, Jin Y Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants J. Environ. Monit, 10 (2008), pp. 713–717 [DOI] [PubMed] [Google Scholar]
- [107].Wang H, Kou X, Pei Z, Xiao JQ, Shan X, Xing B Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants Nanotoxicology, 5 (2011), pp. 30–42 [DOI] [PubMed] [Google Scholar]
- [108].Srivastava N Iron nanoparticles induced toxicity in Sesbania cannabina: A morphological aspect Adv. Sci. Focus, 2 (2014), pp. 135–139 [Google Scholar]
- [109].Liu Y, Xia Q, Liu Y, Zhang S, Cheng F, Zhong Z, Wang L, Li H, Xiao K Genotoxicity assessment of magnetic iron oxide nanoparticles with different particle sizes and surface coatings Nanotechnology, 25 (2014), pp. 425101–425111 [DOI] [PubMed] [Google Scholar]
- [110].Mbeh DA, Javanbakht T, Tabet L, Merhi Y, Maghni K, Sacher E, L’Hocine Yahia Protein corona formation on magnetite nanoparticles: effects of culture medium composition, and its consequences on superparamagnetic nanoparticle cytotoxicity J. Biomed. Nanotechnol, 11 (2015), pp. 828–840 [DOI] [PubMed] [Google Scholar]
- [111].Mbeh DA, Mireles LK, Stanicki D, Tabet L, Maghni K, Laurent S, Sacher E, L’Hocine Yahia Human alveolar epithelial cell responses to core–shell superparamagnetic iron oxide nanoparticles (SPIONs) Langmuir, 13 (2015), pp. 3829–3839 [DOI] [PubMed] [Google Scholar]
- [112].Yun J-W, Yoon J-H, Kang B-C, Cho N-H, Seok SH, Min SK, Min JH, Che JH, Kim YK The toxicity and distribution of iron oxide–zinc oxide core–shell nanoparticles in C57BL/6 mice after repeated subcutaneous administration J. Appl. Toxicol, 35 (2015), pp. 593–602 [DOI] [PubMed] [Google Scholar]
- [113].Zhang Y, Zhu L, Zhou Y, Chen J Accumulation and elimination of iron oxide nanomaterials in zebrafish (Danio rerio) upon chronic aqueous exposure J. Environ. Sci, 30 (2015), pp. 223–230 [DOI] [PubMed] [Google Scholar]
- [114].Wang H, Shrestha TB, Basel MT, Pyle M, Toledo Y, Konecny A, Thapa P, Ikenberry M, Hohn KL, Chikan V, Troyer DL, Bossmann SH Hexagonal magnetite nanoprisms: preparation: characterization and cellular uptake J. Mater. Chem. B, 3 (2015), pp. 4647–4653 [DOI] [PubMed] [Google Scholar]
- [115].Guo X, Mao F, Wang W, Yang Y, Bai Z Sulfhydryl-modified Fe3O4@SiO2 core/shell nanocomposite: synthesis and toxicity assessment in vitro ACS Appl. Mater. Interfaces, 7 (2015), pp. 14983–14991 [DOI] [PubMed] [Google Scholar]
- [116].Zhang L, Wang X, Miao Y, Chen Z, Qiang P, Cui L, Jing H, Guo Y Magnetic ferroferric oxide nanoparticles induce vascular endothelial cell dysfunction and inflammation by disturbing autophagy J. Hazard. Mater, 304 (2016), pp. 186–195 [DOI] [PubMed] [Google Scholar]
- [117].Shen Y, Huang Z, Liu X, Qian J, Xu J, Yang X, Sun A, Ge J Iron-induced myocardial injury: an alarming side effect of superparamagnetic iron oxide nanoparticles J. Cell Mol. Med, 19 (2015), pp. 2032–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Haddad PS, Britos TN, Li LM, Li LDS Preparation, characterization and tests of incorporation in stem cells of superparamagnetic iron oxide J. Phys. Conf. Ser, 617 (2015), pp. 12002–12010 [Google Scholar]
- [119].Ruiz A, Gutiérrez L, Cáceres- Vélez PR, Santos D, Chaves SB, Fascineli ML, Garcia MP, Azevedo RB, Morales MP Biotransformation of magnetic nanoparticles as a function of coating in a rat model Nanoscale, 7 (2015), pp. 16321–16329 [DOI] [PubMed] [Google Scholar]
- [120].Yu S, Wan J, Chen K A facile synthesis of superparamagnetic Fe3O4 supraparticles@MIL-100(Fe) core–shell nanostructures: preparation, characterization and biocompatibility J. Colloid Interface Sci, 461 (2016), pp. 173–178 [DOI] [PubMed] [Google Scholar]
- [121].Urbanova V, Magro M, Gedanken A, Baratella D, Vianello F, Zboril R Nanocrystalline iron oxides composites, and related materials as a platform for electrochemical, magnetic, and chemical biosensors Chem. Mater, 26 (2014), pp. 6653–6673 [Google Scholar]
- [122].Wang L, Zhang Y, Cheng C, Liu X, Jiang H, Wang X Highly sensitive electrochemical biosensor for evaluation of oxidative stress based on the nanointerface of graphene nanocomposites blended with gold Fe3O4, and platinum nanoparticles ACS Appl. Mater. Interfaces, 33 (2015), pp. 18441–18449 [DOI] [PubMed] [Google Scholar]
- [123].Zhang W, Li X, Zou R, Wu H, Shi H, Yu S, Liu Y Multifunctional glucose biosensors from Fe3O4 nanoparticles modified chitosan/graphene nanocomposites Sci. Rep, 5 (2015), p. 11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kim M.l., Cho D, Park HG Colorimetric quantification of glucose and cholesterol in human blood using a nanocomposite entrapping magnetic nanoparticles and oxidases J. Nanosci. Nanotechnol, 15 (2015), pp. 7955–7961 [DOI] [PubMed] [Google Scholar]
- [125].Demeritte T, Nellore BPV, Kanchanapally R, Sinha SS, Pramanik A, Chavva SR, Ray PC Hybrid graphene oxide based plasmonic-magnetic multifunctional nanoplatform for selective separation and label-free identification of alzheimer’s disease biomarkers ACS App. Mater. Interfaces, 7 (2015), pp. 13693–13700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Nguyen P-D, Cong VT, Son SJ, Min J Fabrication of magnetic upconversion nanohybrid for luminescent resonance energy transfer-based detection of glutathione J. Nanosci. Nanotechnol, 15 (2015), pp. 7950–7954 [DOI] [PubMed] [Google Scholar]
- [127].Wang L, Kang T-F, Lu L-P, Zhang J-G, Xue R, Cheng S-Y Microcystin-(leucine-arginine) immunosensor based on iron(II, III) magnetic nanoparticles Anal. Lett, 47 (2014), pp. 2939–2949 [Google Scholar]
- [128].Wu J, Yang Z, Chen N, Zhu W, Hong J, Huang C, Zhou X Vanillin-molecularly targeted extraction of stir bar based on magnetic field induced self-assembly of multifunctional Fe3O4@Polyaniline nanoparticles for detection of vanilla-flavor enhancers in infant milk powders J. Colloid Interface Sci, 442 (2015), pp. 22–29 [DOI] [PubMed] [Google Scholar]
- [129].Chen Y, Xianyu Y, Wang Y, Zhang X, Cha R, Sun J, Jiang X One-step detection of pathogens and viruses: combining magnetic relaxation switching and magnetic separation ACS Nano, 9 (2015), pp. 3184–3191 [DOI] [PubMed] [Google Scholar]
- [130].Hou Y, Zhou J, Gao Z, Sun X, Liu C, Shangguan D, Yang W, Gao M Protease-activated ratiometric fluorescent probe for pH mapping of malignant tumors ACS Nano, 9 (2015), pp. 3199–3205 [DOI] [PubMed] [Google Scholar]
- [131].Min Q, Li S, Chen X, Abdel-Halim ES, Jiang L-P, Zhu J-J Magnetite/ceria-codecorated titanoniobate nanosheet: a 2D catalytic nanoprobe for efficient enrichment and programmed dephosphorylation of phosphopeptides ACS Appl. Mater. Interfaces, 7 (2015), pp. 9563–9572 [DOI] [PubMed] [Google Scholar]
- [132].Zhong S, Zhou C, Zhang X, Zhou H, Li H, Zhu X, Wang Y A novel molecularly imprinted material based on magnetic halloysite nanotubes for rapid enrichment of 2,4-dichlorophenoxyacetic acid in water J. Hazard. Mater, 276 (2014), pp. 58–65 [DOI] [PubMed] [Google Scholar]
- [133].Song X-J, Qin Z-Q, Wang X-B, Yang F, Fang Q-L, She C-G β-Cyclodextrin modified with magnetic nanoparticles noncovalently for β-naphthol removal from wastewater Synth. React. Inorg. Met.-Org. Nano-Met. Chem, 46 (2016), pp. 143–146 [Google Scholar]
- [134].Chiu Y-C, Chen P-A, Chang P-Y, Hsu C-Y, Tao C-W, Huang C-C, Chiang HK Enhanced Raman sensitivity and magnetic separation for urolithiasis detection using phosphonic acid-terminated Fe3O4 nanoclusters J. Mater. Chem. B, 3 (2015), pp. 4282–4290 [DOI] [PubMed] [Google Scholar]
- [135].Mezger A, Fock J, Antunes P, Østerberg FW, Boisen A, Nilsson M, Hansen MF, Ahlford A, Donolato M Scalable DNA-based magnetic nanoparticle agglutination assay for bacterial detection in patient samples ACS Nano, 9 (2015), pp. 7374–7382 [DOI] [PubMed] [Google Scholar]
- [136].Bu T, Zako T, Zeltner M, Sörgjerd KM, Schumacher CM, Hofer CJ, Stark WJ, Maeda M Adsorption and separation of amyloid beta aggregates using ferromagnetic nanoparticles coated with charged polymer brushes J. Mater. Chem. B, 3 (2015), pp. 3351–3357 [DOI] [PubMed] [Google Scholar]
- [137].Pourghazi K, Amoli-Diva M, Beiraghi A Speciation of ultra-trace amounts of inorganic arsenic in water and rice samples by electrothermal atomic absorption spectrometry after solid-phase extraction with modified Fe3O4 nanoparticles Int. J. Environ. Anal. Chem, 95 (2015), pp. 324–338 [Google Scholar]
- [138].Zhang S, Li H, Wang Z, Liu J, Zhang H, Wang B, Yang Z A strongly coupled Au/Fe3O4/GO hybrid material with enhanced nanozyme activity for highly sensitive colorimetric detection, and rapid and efficient removal of Hg2+ in aqueous solutions Nanoscale, 7 (2015), pp. 8495–8502 [DOI] [PubMed] [Google Scholar]
- [139].Ju S, Yu J, Ma Y, Yang Y, Liu M Rapid determination of cadmium and lead in maca (Lepidium meyenii) by magnetic solid-phase extraction and flame atomic absorption spectrometry Anal. Lett, 48 (2015), pp. 2566–2580 [Google Scholar]
- [140].Wang L, Hang X, Chen Y, Wang Y, Feng X Determination of cadmium by magnetic multiwalled carbon nanotube flow injection preconcentration and graphite furnace atomic absorption spectrometry Anal. Lett, 49 (2016), pp. 818–830 [Google Scholar]
- [141].Bayat M, Shemirani F, Hossein B, Mostafa D, Farahani M Ionic liquid-modified Fe3O4 nanoparticle combined with central composite design for rapid preconcentration and determination of palladium ions Desalin. Water Treat, 56 (2015), pp. 814–825 [Google Scholar]
- [142].Naghizadeh M, Taher MA, Behzadi M, Moghaddam FH Simultaneous preconcentration of bismuth and lead ions on modified magnetic core–shell nanoparticles and their determination by ETAAS Chem. Eng. J, 281 (2015), pp. 444–452 [Google Scholar]
- [143].Karimi M, Aboufazeli F, Lotfi Zadeh Zhad HR, Sadeghi O, Najafi E Use of dipyridile amine functionalized magnetic nanoparticles for preconcentration and determination of lead ions in food samples J. AOAC Int, 97 (2014), pp. 1446–1451 [DOI] [PubMed] [Google Scholar]
- [144].Mwilu SK, Siska E, Nasir Baig RB, Varma RS, Heithmar E, Rogers KR Separation and measurement of silver nanoparticles and silver ions using magnetic particles Sci. Total Environ, 472 (2014), pp. 316–323 [DOI] [PubMed] [Google Scholar]
- [145].He Y, Li M, Jiang W, Yang W, Lin L, Xu L, Fu F Phosphatidylserine-functionalized Fe3O4@SiO2 nanoparticles combined with enzyme-encapsulated liposomes for the visual detection of Cu2+ J. Mater. Chem. B, 4 (2016), pp. 752–759 [DOI] [PubMed] [Google Scholar]
- [146].Qu J, Dong Y, Wang Y, Lou T, Du X, Qu J Novel nanofilm sensor based on poly-(alizarin red)/Fe3O4 magnetic nanoparticles-multiwalled carbon nanotubes composite material for determination of nitrite J. Nanosci. Nanotechnol, 16 (2016), pp. 2731–2736 [DOI] [PubMed] [Google Scholar]
- [147].Bharath G, Madhu R, Chen S-M, Veeramani V, Mangalaraj D, Ponpandian N Solvent-free mechanochemical synthesis of graphene oxide and Fe3O4-reduced graphene oxide nanocomposites for sensitive detection of nitrite J. Mater. Chem. A, 3 (2015), pp. 15529–15539 [DOI] [PubMed] [Google Scholar]
- [148].Zhi L, Wang Z, Liu J, Liu W, Zhang H, Chen F, Wang B White emission magnetic nanoparticles as chemosensors for sensitive colorimetric and ratiometric detection, and degradation of ClO− and SCN− in aqueous solutions based on a logic gate approach Nanoscale, 7 (2015), pp. 11712–11719 [DOI] [PubMed] [Google Scholar]
- [149].Mohapatra S, Sahu S, Nayak S, Ghosh SK Design of Fe3O4@SiO2@carbon quantum dot based nanostructure for fluorescence sensing, magnetic separation, and live cell imaging of fluoride ion Langmuir, 31 (2015), pp. 8111–8120 [DOI] [PubMed] [Google Scholar]
- [150].Zhang G, Lu S, Qian J, Zhong K, Yao J, Cai D, Cheng Z, Wu Z Magnetic relaxation switch detecting boric acid or borate ester through one-pot synthesized poly(vinyl alcohol) functionalized nanomagnetic iron oxide ACS Appl. Mater. Interfaces, 7 (2015), pp. 16837–16841 [DOI] [PubMed] [Google Scholar]
- [151].Ye M, Wei Z, Hu F, Wang J, Ge G, Hu Z, Shao M, Lee S-T, Liu J Fast assembling microarrays of superparamagnetic Fe3O4@Au nanoparticle clusters as reproducible substrates for surface-enhanced Raman scattering Nanoscale, 7 (2015), pp. 13427–13437 [DOI] [PubMed] [Google Scholar]
- [152].Wang C, Xu J, Wang J, Rong Z, Li P, Xiao R, Wang S Polyethylenimine-interlayered silver-shell magnetic-core microspheres as multifunctional SERS substrates J. Mater. Chem. C, 3 (2015), pp. 8684–8693 [Google Scholar]
- [153].Zhu S, Fan C, Wang J, He J, Liang E, Chao M Realization of high sensitive SERS substrates with one-pot fabrication of Ag–Fe3O4 nanocomposites J. Colloid Interface Sci, 438 (2015), pp. 116–121 [DOI] [PubMed] [Google Scholar]
- [154].Du J, Cui J, Jing C Rapid in situ identification of arsenic species using a portable Fe3O4@Ag SERS sensor Chem. Commun, 50 (2014), pp. 347–349 [DOI] [PubMed] [Google Scholar]
- [155].Yang Q, Lan F, Liu Z, Ma S, Li W, Wu Y, Gu Z Uniform superparamagnetic Fe3O4/CMCS composite nanospheres for lysozyme adsorption J. Nanosci. Nanotechnol, 16 (2016), pp. 2233–2238 [DOI] [PubMed] [Google Scholar]
- [156].Ni Q, Chen B, Dong S, Tian L, Bai Q Preparation of core–shell structure Fe3O4@SiO2 superparamagnetic microspheres immoblized with iminodiacetic acid as immobilized metal ion affinity adsorbents for His-tag protein purification Biomed. Chromatogr, 30 (2016), pp. 566–573 [DOI] [PubMed] [Google Scholar]
- [157].Gan Q, Zhu J, Yuan Y, Liu C pH-responsive Fe3O4 nanopartilces-capped mesoporous silica supports for protein delivery J. Nanosci. Nanotechnol, 16 (2016), pp. 1533–4880 [DOI] [PubMed] [Google Scholar]
- [158].Xu Y, Wang S, Han H, Chen K, Qin L, Xu J, Wang J, Li L, Guo X Enhancement of enzymatic activity by magnetic spherical polyelectrolyte brushes: a potential recycling strategy for enzymes Langmuir, 30 (2014), pp. 11156–11164 [DOI] [PubMed] [Google Scholar]
- [159].Cheng G, Zheng S-Y Construction of a high-performance magnetic enzyme nanosystem for rapid tryptic digestion Sci. Rep, 4 (2014), p. 6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Long J, Li X, Wu Z, Xu E, Xu X, Jin Z, Jiao A Immobilization of pullulanase onto activated magnetic chitosan/Fe3O4 nanoparticles prepared by in situ mineralization and effect of surface functional groups on the stability Colloids Surf. A: Physicochem. Eng. Aspects, 472 (2015), pp. 69–77 [Google Scholar]
- [161].Tao Q-L, Li Y, Shi Y, Liu R-J, Zhang Y-W, Guo J Application of molecular imprinted magnetic Fe3O4@SiO2 nanoparticles for selective immobilization of cellulose J. Nanosci. Nanotechnol, 16 (2016), pp. 6055–6060 [DOI] [PubMed] [Google Scholar]
- [162].Hudson R, Feng Y, Varma RS, Moores A Bare magnetic nanoparticles: sustainable synthesis and applications in catalytic organic transformations Green Chem., 16 (2014), pp. 4493–4505 [Google Scholar]
- [163].Thangaraj B, Muniyandi B, Ranganathan S, Xin H Functionalized magnetic nanoparticles for catalytic application—a review Rev. Adv. Sci. Eng, 4 (2015), pp. 106–119 [Google Scholar]
- [164].Wu W, Jiang C, Vellaisamy ALR Recent progress in magnetic iron oxide–semiconductor composite nanomaterials as promising photocatalysts Nanoscale, 7 (2015), pp. 38–58 [DOI] [PubMed] [Google Scholar]
- [165].Karimi B, Mansouri F, Mirzaei HM Recent applications of magnetically recoverable nanocatalysts in CC and CX coupling reactions ChemCatChem, 7 (2015), pp. 1736–1789 [Google Scholar]
- [166].Hildebrand H, Mackenzie K, Kopinke FD Pd/Fe3O4 nano-catalysts for selective dehalogenation in wastewater treatment processes-influence of water constituents Appl. Catal. B: Environ, 91 (2009), pp. 389–396 [Google Scholar]
- [167].Mi F, Chen X, Ma Y, Yin S, Yuan F, Zhang H Facile synthesis of hierarchical core–shell Fe3O4@MgAl–LDH@Au as magnetically recyclable catalysts for catalytic oxidation of alcohols Chem. Commun, 47 (2011), pp. 12804–12806 [DOI] [PubMed] [Google Scholar]
- [168].Shah MT, Balouch A, Rajar K, Sirajuddin IAB, Umar AA Selective heterogeneous catalytic hydrogenation of ketone (C=O) to alcohol (OH) by magnetite nanoparticles following Langmuir–Hinshelwood kinetic approach ACS Appl. Mater. Interfaces, 7 (2015), pp. 6480–6489 [DOI] [PubMed] [Google Scholar]
- [169].Shelke SN, Bankar SR, Mhaske GR, Kadam SS, Murade DK, Bhorkade SB, Rathi AK, Bundaleski N, Teodoro OMND, Zboril R, Varma RS, Gawande MB Iron oxide-supported copper oxide nanoparticles (Nanocat-Fe-CuO): magnetically recyclable catalysts for the synthesis of pyrazole derivatives, 4-methoxyaniline, and Ullmann-type condensation reactions ACS Sustain. Chem. Eng, 2 (2014), pp. 1699–1706 [Google Scholar]
- [170].Wang H, Wei Z, Matsui H, Zhou S Fe3O4/carbon quantum dots hybrid nanoflowers for highly active and recyclable visible-light driven photocatalyst J. Mater. Chem. A, 2 (2014), pp. 15740–15745 [Google Scholar]
- [171].Modisha P, Antunes E, Nyokong T Photophysical properties of zinc tetracarboxy phthalocyanines conjugated to magnetic nanoparticles J. Nanosci. Nanotechnol, 15 (2015), pp. 3688–3696 [DOI] [PubMed] [Google Scholar]
- [172].Moghaddam MM, Pieber B, Glasnov T, Kappe CO Immobilized iron oxide nanoparticles as stable and reusable catalysts for hydrazine-mediated nitro reductions in continuous flow ChemSusChem, 7 (2014), pp. 3122–3131 [DOI] [PubMed] [Google Scholar]
- [173].Easterday R, Leonard C, Sanchez-Felix O, Losovyj Y, Pink M, Stein BD, Morgan DG, Lyubimova NA, Nikoshvili L.Zh., Sulman EM, Mahmoud WE, Al- Ghamdi AA, Bronstein LM Fabrication of magnetically recoverable catalysts based on mixtures of Pd and iron oxide nanoparticles for hydrogenation of alkyne alcohols ACS Appl. Mater. Interfaces, 6 (2014), pp. 21652–21660 [DOI] [PubMed] [Google Scholar]
- [174].Zhang W, Liu B, Zhang B, Bian G, Qi Y, Yang X, Li C Synthesis of monodisperse magnetic sandwiched gold nanoparticle as an easily recyclable catalyst with a protective polymer shell Colloids Surf. A: Physicochem. Eng. Aspects, 466 (2015), pp. 210–218 [Google Scholar]
- [175].Yang S, Cao C, Sun Y, Huang P, Wei F, Song W Nanoscale magnetic stirring bars for heterogeneous catalysis in microscopic systems Angew. Chem, 127 (2015), pp. 2699–2702 [DOI] [PubMed] [Google Scholar]
- [176].Hajipour A, Azizi G Fabrication of covalently functionalized mesoporous silica core–shell magnetite nanoparticles with palladium(II) acetylacetonate: application as a magnetically separable nanocatalyst for Suzuki cross-coupling reaction of acyl halides with boronic acids Appl. Organometal. Chem, 29 (2015), pp. 247–253 [Google Scholar]
- [177].Khakiani BA, Pourshamsian K, Veisi H A highly stable and efficient magnetically recoverable and reusable Pd nanocatalyst in aqueous media heterogeneously catalysed Suzuki C–C cross-coupling reactions Appl. Organometal. Chem, 29 (2015), pp. 259–265 [Google Scholar]
- [178].Elazab HA, Siamaki AR, Moussa S, Gupton BF, El- Shall MS Highly efficient and magnetically recyclable graphene-supported Pd/Fe3O4 nanoparticle catalysts for Suzuki and Heck cross-coupling reactions Appl. Catal. A: Gen, 491 (2015), pp. 58–69 [Google Scholar]
- [179].Yao T, Zuo Q, Wang H, Wu J, Xin B, Cui F, Cui T A simple way to prepare Pd/Fe3O4/polypyrrole hollow capsules and their applications in catalysis J. Colloid Interface Sci, 450 (2015), pp. 366–373 [DOI] [PubMed] [Google Scholar]
- [180].Maleki B, Chalaki SBN, Ashrafi SS, Seresht ER, Moeinpour F, Khojastehnezhad A, Tayebee R Caesium carbonate supported on hydroxyapatite-encapsulated Ni0.5Zn0.5Fe2O4 nanocrystallites as a novel magnetically basic catalyst for the one-pot synthesis of pyrazolo[1,2-b]phthalazine-5,10-diones Appl. Organometal. Chem, 29 (2015), pp. 290–295 [Google Scholar]
- [181].Bai J, Zhao R, Diao G Fabrication of a novel Fe3O4/BiOCl nanocomposite as magnetic visible light photocatalytic materials Curr. Nanosci, 11 (2015), pp. 186–190 [Google Scholar]
- [182].Mousavi M, Habibi-Yangjeh A Magnetically separable ternary g-C3N4/Fe3O4/BiOI nanocomposites: novel visible-light-driven photocatalysts based on graphitic carbon nitride J. Colloid Interface Sci, 465 (2016), pp. 83–92 [DOI] [PubMed] [Google Scholar]
- [183].Ghorbani-Choghamarani A, Darvishnejad Z, Norouzi M Cu(II)–Schiff base complex-functionalized magnetic Fe3O4 nanoparticles: a heterogeneous catalyst for various oxidation reactions Appl. Organometallic Chem, 29 (2015), pp. 170–175 [Google Scholar]
- [184].Liu Y, Liu Z, Cui Y An efficient nanoparticle‐supported and magnetically recoverable copper(I) catalyst for synthesis of furans from ene‐yne‐ketone Chin. J. Chem, 33 (2015), pp. 175–180 [Google Scholar]
- [185].Wang L, Ma Y, Qing S, Gao Z, Eli W, Wang T Magnetically separable Fe3O4 supported Co–Rh bimetallic catalysts for dicyclopentadiene hydroformylation to value-added fine chemicals Energy Environ. Focus, 4 (2015), pp. 334–339 [Google Scholar]
- [186].Fan G-Y, Huang W-J Solvent-free hydrogenation of nitrobenzene catalyzed by magnetically recoverable Pt deposited on multiwalled carbon nanotubes Synth. React. Inorg. Met.-Org. Nano-Met. Chem, 45 (2015), pp. 1819–1825 [Google Scholar]
- [187].Baird N, Losovyj Y, Yuzik-Klimova EY, Kuchkina NV, Shifrina ZB, Pink M, Stein BD, Morgan DG, Wang T, Rubin MA, Sidorov AI, Sulman EM, Bronstein LM Zinc-containing magnetic oxides stabilized by a polymer: one phase or two? ACS Appl. Mater. Interfaces, 8 (2016), pp. 891–899 [DOI] [PubMed] [Google Scholar]
- [188].Cao M, Wang P, Ao Y, Wang C, Hou J, Qian J Visible light activated photocatalytic degradation of tetracycline by a magnetically separable composite photocatalyst: graphene oxide/magnetite/cerium-doped titania J. Colloid Interface Sci, 467 (2016), pp. 129–139 [DOI] [PubMed] [Google Scholar]
- [189].Herrera A, Reyes A, Colina-Márquez J Evaluation of the photocatalytic activity of iron oxide nanoparticles functionalized with titanium dioxide J. Phys.: Conf. Ser, 687 (2016), pp. 12034–12037 [Google Scholar]
- [190].Tan C, Gao N, Deng Y, Deng J, Zhou S, Li J, Xin X Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate J. Hazard. Mater, 15 (2014), pp. 452–460 [DOI] [PubMed] [Google Scholar]
- [191].Ruan X, Gu X, Lu S, Qiu Z, Sui Q Trichloroethylene degradation by persulphate with magnetite as a heterogeneous activator in aqueous solution Environ. Technol, 36 (2015), pp. 1389–1397 [DOI] [PubMed] [Google Scholar]
- [192].Jiang S, Zhu J, Ding Y, Bai S, Guan Y, Wang J Degradation effect and mechanism of dinitrotoluene wastewater by magnetic nano-Fe3O4/H2O2 Fenton-like Ozone: Sci. Eng, 38 (2016), pp. 225–232 [Google Scholar]
- [193].Lei Y, Chen C-S, Tu Y-J, Huang Y-H, Zhang H Heterogeneous degradation of organic pollutants by persulfate activated by CuO-Fe3O4: mechanism, stability, and effects of pH and bicarbonate ions Environ. Sci. Technol, 49 (2015), pp. 6838–6845 [DOI] [PubMed] [Google Scholar]
- [194].Feng M, Qu R, Zhang X, Sun P, Sui Y, Wang L, Wang Z Degradation of flumequine in aqueous solution by persulfate activated with common methods and polyhydroquinone-coated magnetite/multi-walled carbon nanotubes catalysts Water Res., 85 (2015), pp. 1–10 [DOI] [PubMed] [Google Scholar]
- [195].Wang Y, Sun H, Ang HM, Tade MO, Wang S Synthesis of magnetic core/shell carbon nanosphere supported manganese catalysts for oxidation of organics in water by peroxymonosulfate J. Colloid. Interface Sci, 433 (2014), pp. 68–75 [DOI] [PubMed] [Google Scholar]
- [196].Shan G, Fu Y, Chu X, Chang C, Zhu L Highly active magnetic bismuth tungstate/magnetite composite under visible light irradiation in the presence of hydrogen peroxide J. Colloid Interface Sci, 444 (2015), pp. 123–131 [DOI] [PubMed] [Google Scholar]
- [197].Zhong Y, Liang X, He Z, Tan W, He H, Zhu R, Zhong Y, Zhu J, Yuan P, Jiang Z The UV/Fenton degradation of tetrabromobisphenol a catalyzed by nanocrystalline chromium substituted magnetite J. Nanosci. Nanotechnol, 14 (2014), pp. 7307–7314 [DOI] [PubMed] [Google Scholar]
- [198].Ardo SG, Nélieu S, Ona-Nguema G, Delarue G, Brest J, Pironin E, Morin G Oxidative degradation of nalidixic acid by nano-magnetite via Fe2+/O2-mediated reactions Environ. Sci. Technol, 49 (2015), pp. 4506–4514 [DOI] [PubMed] [Google Scholar]
- [199].Hammouda SB, Adhoum N, Monser L Synthesis of magnetic alginate beads based on Fe3O4 nanoparticles for the removal of 3-methylindole from aqueous solution using Fenton process J. Hazard. Mater, 294 (2015), pp. 128–136 [DOI] [PubMed] [Google Scholar]
- [200].Huang S, Xu Y, Xie M, Xu H, He M, Xia J, Huang L, Li H Synthesis of magnetic CoFe2O4/g-C3N4 composite and its enhancement of photocatalytic ability under visible-light Colloids Surf. A: Physicochem. Eng. Aspects, 478 (2015), pp. 71–80 [Google Scholar]
- [201].Yao Y, Lu F, Zhu Y, Wei F, Liu X, Lian C, Wang S Magnetic core–shell CuFe2O4@C3N4 hybrids for visible light photocatalysis of Orange II J. Hazard. Mater, 297 (2015), pp. 224–233 [DOI] [PubMed] [Google Scholar]
- [202].Huang R, Fang Z, Fang X, Tsang EP Ultrasonic Fenton-like catalytic degradation of bisphenol A by ferroferric oxide (Fe3O4) nanoparticles prepared from steel pickling waste liquor J. Colloid Interface Sci, 436 (2014), pp. 258–266 [DOI] [PubMed] [Google Scholar]
- [203].Xu Y, Ai J, Zhang H The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process J. Hazard. Mater, 309 (2016), pp. 87–96 [DOI] [PubMed] [Google Scholar]
- [204].Hosseini-Monfared H, Parchegani F, Alavi S Carboxylic acid effects on the size and catalytic activity of magnetite nanoparticles J. Colloid Interface Sci, 437 (2015), pp. 205–210 [DOI] [PubMed] [Google Scholar]
- [205].Wang Z-Z, Zhai S-R, Zhai B, An Q-D One‐step green synthesis of multifunctional Fe3O4/Cu nanocomposites toward efficient reduction of organic dyes Eur. J. Inorg. Chem, 10 (2015), pp. 1692–1699 [Google Scholar]
- [206].Hou Y, Ma J, Wang T, Fu Q Phosphotungstic acid supported on magnetic core–shell nanoparticles with high photocatalytic activity Mater. Sci. Semicond. Process, 39 (2015), pp. 229–234 [Google Scholar]
- [207].Zhang P, Mo Z-L, Zhang C, Han L-J, Zheng L Preparation and photocatalytic properties of magnetic responsive TiO2/graphene nanocomposites J. Mater. Eng, 43 (2015), pp. 72–77 [Google Scholar]
- [208].Nasrollahzadeh M, Bagherzadeh M, Karimi H Preparation, characterization and catalytic activity of CoFe2O4 nanoparticles as a magnetically recoverable catalyst for selective oxidation of benzyl alcohol to benzaldehyde and reduction of organic dyes J. Colloid Interface Sci, 465 (2016), pp. 271–278 [DOI] [PubMed] [Google Scholar]
- [209].Du Y, Ma W, Liu P, Zou B, Ma J Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants J. Hazard. Mater, 308 (2016), pp. 58–66 [DOI] [PubMed] [Google Scholar]
- [210].Tóth IY, Szekeres M, Turcu R, Sáringer S, Illés E, Nesztor D, Tombácz E Mechanism of in situ surface polymerization of gallic acid in an environmental-inspired preparation of carboxylated core–shell magnetite nanoparticles Langmuir, 30 (2014), pp. 15451–15461 [DOI] [PubMed] [Google Scholar]
- [211].Ye CZ, Ariya PA Co-adsorption of gaseous benzene, toluene, ethylbenzene, m-xylene (BTEX) and SO2 on recyclable Fe3O4 nanoparticles at 0–101% relative humidities J. Environ. Sci, 31 (2015), pp. 164–174 [DOI] [PubMed] [Google Scholar]
- [212].Li G, Li L, Wu B, Li J, Yuan Y, Shi J Controlled one-step synthesis of Pt decorated octahedral Fe3O4 and its excellent catalytic performance for CO oxidation Nanoscale, 7 (2015), pp. 17855–17860 [DOI] [PubMed] [Google Scholar]
- [213].Elazab HA, Moussa S, Gupton BF, El-Shall MS Microwave-assisted synthesis of Pd nanoparticles supported on Fe3O4 Co3O4, and Ni(OH)2 nanoplates and catalysis application for CO oxidation J. Nanopart. Res, 16 (2014), p. 2477 [Google Scholar]
- [214].Lopez A, Larrea A, Sebastian V, Calatayud MP, Irusta S, Santamaria J Ultrasmall platinum nanoparticles on Fe3O4: a low‐temperature catalyst for the preferential oxidation reaction ChemCatChem, 8 (2016), pp. 1479–1484 [Google Scholar]
- [215].Arcibar-Orozco JA, Wallace R, Mitchell JK, Bandosz TJ Role of surface chemistry and morphology in the reactive adsorption of H2S on iron (hydr)oxide/graphite oxide composites Langmuir, 31 (2015), pp. 2730–2742 [DOI] [PubMed] [Google Scholar]
- [216].Shi D, Li Y, Ji S Synthesis of magnetically recyclable TiO2@SiO2@Fe3O4 photocatalysts and their performance of photochemical reduction of CO2 Energy Environ. Focus, 4 (2015), pp. 87–94 [Google Scholar]
- [217].Sharma VK, McDonald TJ, Kim H, Garg VK Magnetic graphene-carbon nanotube iron nanocomposites as adsorbents and antibacterial agents for water purification Adv. Colloid Interface Sci, 225 (2015), pp. 229–240 [DOI] [PubMed] [Google Scholar]
- [218].Dong H, Huang J, Koepsel RR, Ye P, Russell AJ, Matyjaszewski K Recyclable antibacterial magnetic nanoparticles grafted with quaternized poly (2-dimethylamino) ethyl methacrylate brushes Biomacromolecules, 12 (2011), pp. 1305–1311 [DOI] [PubMed] [Google Scholar]
- [219].Deng C-H, Gong J-L, Zeng G-M, Niu C-G, Niu Q-Y, Zhang W, Liu H-Y Inactivation performance and mechanism of Escherichia coli in aqueous system exposed to iron oxide loaded graphene nanocomposites J. Hazard. Mater, 276 (2014), pp. 66–76 [DOI] [PubMed] [Google Scholar]
- [220].Pina AS, Batalha ÍL, Fernandes CSM, Aoki MA, Roque ACA Exploring the potential of magnetic antimicrobial agents for water disinfection Water Res., 66 (2014), pp. 160–168 [DOI] [PubMed] [Google Scholar]
- [221].Zablotskaya A, Segal I, Popelis Y, Mishnev A, Maiorov M, Zablotsky D, Blums E, Nikolajeva V, Eze D Iron oxide superparamagnetic nanocarriers bearing amphiphilic N-heterocyclic choline analogues as potential antimicrobial agents Appl. Organometal. Chem, 29 (2015), pp. 376–383 [Google Scholar]
- [222].Zhang X, Wang W, Zhang Y, Yan J, Jia C, Chang L facile and green synthesis of silver nanoparticles immobilized magnetite@polydopamine nanocomposite with enhanced antibacterial activity Energy Environ. Focus, 3 (2014), pp. 309–315 [Google Scholar]
- [223].Xu J-W, Gao Z-D, Han K, Liu Y, Song Y-Y Synthesis of magnetically separable Ag3PO4/TiO2/Fe3O4 heterostructure with enhanced photocatalytic performance under visible light for photoinactivation of bacteria ACS Appl. Mater. Interfaces, 6 (2014), pp. 15122–15131 [DOI] [PubMed] [Google Scholar]
- [224].Yu Q, Fu A, Li H, Liu H, Lv R, Liu J, Guo P, Zhao XS Synthesis and characterization of magnetically separable Ag nanoparticles decorated mesoporous Fe3O4@carbon with antibacterial and catalytic properties Colloids Surf. A: Physicochem. Eng. Aspects, 457 (2014), pp. 288–296 [Google Scholar]
- [225].Zhang HZ, Zhang C, Zeng GM, Gong JL, Ou XM, Huan SY Easily separated silver nanoparticle-decorated magnetic graphene oxide: synthesis and high antibacterial activity J. Colloid Interface Sci, 471 (2016), pp. 94–102 [DOI] [PubMed] [Google Scholar]
- [226].Ma S, Zhan S, Jia Y, Zhou Q Highly efficient antibacterial and Pb(II) removal effects of Ag-CoFe2O4-GO nanocomposite ACS Appl. Mater. Interfaces, 7 (2015), pp. 10576–10586 [DOI] [PubMed] [Google Scholar]
- [227].Giannousi K, Menelaou M, Arvanitidis J, Angelakeris M, Pantazaki A, Dendrinou-Samara C Hetero-nanocomposites of magnetic and antifungal nanoparticles as a platform for magnetomechanical stress induction in Saccharomyces cerevisiae J. Mater. Chem. B, 3 (2015), pp. 5341–5351 [DOI] [PubMed] [Google Scholar]
- [228].Giri MN, Agarwala V Synthesis and characterization of novel magnetic chitosan bead and their antibacterial applications J. Bionanosci, 9 (2015), pp. 276–280 [Google Scholar]
- [229].Sun P, Hui C, Wang S, Khan RA, Zhang Q, Zhao Y-H Enhancement of algicidal properties of immobilized Bacillus methylotrophicus ZJU by coating with magnetic Fe3O4 nanoparticles and wheat bran J. Hazard. Mater, 301 (2016), pp. 65–73 [DOI] [PubMed] [Google Scholar]
- [230].Homayoonfal M, Mehrnia MR, Shariaty-Niassar M, Akbari A, Sarrafzadeh MH, Ismail AF Fabrication of magnetic nanocomposite membrane for separation of organic contaminant from water Desalin. Water Treat, 54 (2015), pp. 3603–3609 [Google Scholar]
- [231].Hwang J-H, Han D-W Optimization and modeling of reduction of wastewater sludge water content and turbidity removal using magnetic iron oxide nanoparticles (MION) J. Environ. Sci. Health Part A, 50 (2015), pp. 1307–1315 [DOI] [PubMed] [Google Scholar]
- [232].Hernandez JST, Muriel AA, Tabares JA, Alcázar GAP, Bolaños A Preparation of Fe3O4 nanoparticles and removal of methylene blue through adsorption J. Phys.: Conf. Ser, 614 (2015), pp. 12007–12010 [Google Scholar]
- [233].Mahmoodi NM, Bagherpour F, Nariyan E Amine functionalized magnetic carbon nanotube: synthesis and binary system dye removal Desalin. Water Treat, 56 (2015), pp. 107–120 [Google Scholar]
- [234].Chen RP, Zhang YL, Wang XY, Zhu CY, Ma AJ, Jiang WM Removal of methylene blue from aqueous solution using humic-acid coated magnetic nanoparticles Desalin. Water Treat, 55 (2015), pp. 539–548 [Google Scholar]
- [235].Tan X, Lu L, Wang L, Zhang J Facile synthesis of bimodal mesoporous Fe3O4@SiO2 composite for efficient removal of methylene blue Eur. J. Inorg. Chem, 18 (2015), pp. 2928–2933 [Google Scholar]
- [236].Kim DW, Bach LG, Hong S-S, Park CL, Kwon T A facile route towards the synthesis of Fe3O4/graphene oxide nanocomposites for environmental applications Mol. Cryst. Liquid Cryst, 599 (2014), pp. 43–50 [Google Scholar]
- [237].Zhang X, Zeng T, Wang S, Niu H, Wang X, Cai Y One-pot synthesis of C18-functionalized core–shell magnetic mesoporous silica composite as efficient sorbent for organic dye J. Colloid Interface Sci, 448 (2015), pp. 189–196 [DOI] [PubMed] [Google Scholar]
- [238].Dolatkhah A, Wilson LD Magnetite/polymer brush nanocomposites with switchable uptake behavior toward methylene blue ACS Appl. Mater. Interfaces, 8 (2016), pp. 5595–5607 [DOI] [PubMed] [Google Scholar]
- [239].Akın D, Yakar A, Gündüz U Synthesis of magnetic Fe3O4-chitosan nanoparticles by ionic gelation and their dye removal ability Water Environ. Res, 87 (2015), pp. 425–436 [DOI] [PubMed] [Google Scholar]
- [240].Rajabi HR, Arjmand H, Hoseini SJ, Nasrabadi H Surface modified magnetic nanoparticles as efficient and green sorbents: synthesis, characterization, and application for the removal of anionic dye J. Mag. Mag. Mater, 394 (2015), pp. 7–13 [Google Scholar]
- [241].Li L, Yuan LJ, Hong W, Fan L, Mao LB, Liu L Hybrid Fe3O4/MOFs for the adsorption of methylene blue and methyl violet from aqueous solution Desalin. Water Treat, 55 (2015), pp. 1973–1980 [Google Scholar]
- [242].Dong L, Zhipeng Z, Yigang D A Simple method to prepare magnetic modified corncobs and its application for Congo red adsorption J. Dispers. Sci. Technol, 37 (2016), pp. 73–79 [Google Scholar]
- [243].Zhao Y, Chen H, Li J, Chen C Hierarchical MWCNTs/Fe3O4/PANI magnetic composite as adsorbent for methyl orange removal J. Colloid Interface Sci, 450 (2015), pp. 189–195 [DOI] [PubMed] [Google Scholar]
- [244].Lin KY, Yang H Magnetically controllable Pickering emulsion prepared by a reduced graphene oxide-iron oxide composite J. Colloid Interface Sci, 438 (2015), pp. 296–305 [DOI] [PubMed] [Google Scholar]
- [245].Zhao J, Luque R, Qi W, Lai J, Gao W, Gilani MRHS, Xu G Facile surfactant-free synthesis and characterization of Fe3O4@3-aminophenol–formaldehyde core–shell magnetic microspheres J. Mater. Chem. A, 3 (2015), pp. 519–524 [Google Scholar]
- [246].Xiang Y, Wang H, He Y, Song G Efficient degradation of methylene blue by magnetically separable Fe3O4/chitosan/TiO2 nanocomposites Desalin. Water Treat, 55 (2015), pp. 1018–1025 [Google Scholar]
- [247].Tian H, Chen J, He J, Liu F Pd-loaded magnetic mesoporous nanocomposites: a magnetically recoverable catalyst with effective enrichment and high activity for DDT and DDE removal under mild conditions J. Colloid Interface Sci, 457 (2015), pp. 195–202 [DOI] [PubMed] [Google Scholar]
- [248].Zhao Z-Y, Xiong Z-H Removal of four nitrofurans drugs from aqueous solution by magnetic multi-wall carbon nanotubes Fullerenes Nanotubes Carbon Nanostruct, 23 (201) (2016), pp. 640–648 [Google Scholar]
- [249].Tang S, Chia GH, Lee HK Magnetic core–shell iron(II,III) oxide@layered double oxide microspheres for removal of 2,5-dihydroxybenzoic acid from aqueous solutions J. Colloid. Interface Sci, 437 (2015), pp. 316–323 [DOI] [PubMed] [Google Scholar]
- [250].Li K, Zeng Z, Xiong J, Yan L, Guo H, Liu S, Dai Y, Chen T Fabrication of mesoporous Fe3O4@SiO2@CTAB–SiO2 magnetic, microspheres with a core/shell structure and their efficient adsorption performance for the removal of trace PFOS from water Colloids Surf. A: Physicochem. Eng. Aspects, 465 (2015), pp. 113–123 [Google Scholar]
- [251].Liu Q, Zheng Y, Zhong L, Cheng X Removal of tetracycline from aqueous solution by a Fe3O4 incorporated PAN electrospun nanofiber mat J. Environ. Sci, 28 (2015), pp. 29–36 [DOI] [PubMed] [Google Scholar]
- [252].Liu Q, Zhong LB, Zhao QB, Frear C, Zheng YM Synthesis of Fe3O4/polyacrylonitrile composite electrospun nanofiber mat for effective adsorption of tetracycline ACS Appl. Mater. Interfaces, 27 (2015), pp. 14573–14583 [DOI] [PubMed] [Google Scholar]
- [253].Guo L, Liang Y, Chen X, Xu W, Wu K, Wei H, Xiong Y Effective removal of tetracycline from aqueous solution by organic acid-coated magnetic nanoparticles J. Nanosci. Nanotechnol, 16 (2016), pp. 2218–2226 [DOI] [PubMed] [Google Scholar]
- [254].Shi L, Ma F, Han Y, Zhang X, Yu H Removal of sulfonamide antibiotics by oriented immobilized laccase on Fe3O4 nanoparticles with natural mediators J. Hazard. Mater, 30 (2014), pp. 203–2011 [DOI] [PubMed] [Google Scholar]
- [255].Jiang M, Yang W, Zhang Z, Yang Z, Wang Y Adsorption of three pharmaceuticals on two magnetic ion-exchange resins J. Environ. Sci, 31 (2015), pp. 226–234 [DOI] [PubMed] [Google Scholar]
- [256].Yoon SU, Mahanty B, Kim CG Preparation of superparamagnetic iron oxide nanoparticles and evaluation of their adsorption capacity toward carbamazepine and diatrizoate Desalin. Water Treat, 57 (2016), pp. 7789–7800 [Google Scholar]
- [257].Wang M, Liu P, Wang Y, Zhou D, Ma C, Zhang D, Zhan J Core–shell superparamagnetic Fe3O4@β-CD composites for host–guest adsorption of polychlorinated biphenyls (PCBs) J. Colloid Interface Sci, 47 (2015), pp. 1–7 [DOI] [PubMed] [Google Scholar]
- [258].Park H-S, Koduru JR, Choo K-H, Lee B Activated carbons impregnated with iron oxide nanoparticles for enhanced removal of bisphenol A and natural organic matter J. Hazard. Mater, 286 (2015), pp. 315–324 [DOI] [PubMed] [Google Scholar]
- [259].Wang J, Tian H, Ji Y Adsorption behavior and mechanism of humic acid on aminated magnetic nanoadsorbent Sep. Sci. Technol, 50 (2015), pp. 1285–1293 [Google Scholar]
- [260].Sheng Y, Guan H, Zhang Y, Zhang X, Zhou Q, Lin Z Double-functionalised magnetic nanoparticles for efficient extraction of bisphenol A from river water Environ. Chem, 13 (2015), pp. 43–49 [Google Scholar]
- [261].Obeid L, Kolli NE, Talbot D, Welschbillig M, Bée A Influence of a cationic surfactant on adsorption of p-nitrophenol by a magsorbent based on magnetic alginate beads J. Colloid Interface Sci, 457 (2015), pp. 218–224 [DOI] [PubMed] [Google Scholar]
- [262].Liu F, Wu Z, Wang D, Yu J, Jiang X, Chen X Magnetic porous silica–graphene oxide hybrid composite as a potential adsorbent for aqueous removal of p-nitrophenol Colloids Surf. A: Physicochem. Eng. Aspects, 490 (2016), pp. 207–214 [Google Scholar]
- [263].Jin Z, Wang X, Sun Y, Ai Y, Wang X Adsorption of 4-n-nonylphenol and bisphenol-A on magnetic reduced graphene oxides: a combined experimental and theoretical studies Environ. Sci. Technol, 49 (2015), pp. 9168–9175 [DOI] [PubMed] [Google Scholar]
- [264].Shen H-Y, Chen Z-X, Li Z-H, Hu M-Q, Dong X-Y Controlled synthesis of 2,4,6-trichlorophenol-imprinted amino-functionalized nano-Fe3O4-polymer magnetic composite for highly selective adsorption Colloids Surf. A: Physicochem. Eng. Aspects, 481 (2015), pp. 439–450 [Google Scholar]
- [265].Hao Y, Wang Z, Gou J, Wang Z Kinetics and thermodynamics of diquat removal from water using magnetic graphene oxide nanocomposite Can. J. Chem. Eng, 93 (2015), pp. 1713–1720 [Google Scholar]
- [266].Shi P, Ma R, Zhou Q, Li A, Wu B, Miao Y, Chen X, Zhang X Chemical and bioanalytical assessments on drinking water treatments by quaternized magnetic microspheres J. Hazard. Mater, 285 (2015), pp. 53–60 [DOI] [PubMed] [Google Scholar]
- [267].Ge S, Agbakpe M, Zhang W, Kuang L Heteroaggregation between PEI-coated magnetic nanoparticles and algae: effect of particle size on algal harvesting efficiency ACS Appl. Mater. Interfaces, 7 (2015), pp. 6102–6108 [DOI] [PubMed] [Google Scholar]
- [268].Ge S, Agbakpe M, Zhang W, Kuang L, Wu Z, Wang X Recovering magnetic Fe3O4-ZnO nanocomposites from algal biomass based on hydrophobicity shift under UV irradiation ACS Appl. Mater. Interfaces, 7 (2015), pp. 11677–11682 [DOI] [PubMed] [Google Scholar]
- [269].Yang S, Chen L, Mu L, Ma P-C Magnetic graphene foam for efficient adsorption of oil and organic solvents J. Colloid Interface Sci, 430 (2014), pp. 337–344 [DOI] [PubMed] [Google Scholar]
- [270].Liu C, Yang J, Tang Y, Yin L, Tang H, Li C Versatile fabrication of the magnetic polymer-based graphene foam and applications for oil–water separation Colloid Surf. A, 468 (2015), pp. 10–16 [Google Scholar]
- [271].Yu F, Ma J, Wang J, Zhang M, Zheng J Magnetic iron oxide nanoparticles functionalized multi-walled carbon nanotubes for toluene, ethylbenzene and xylene removal from aqueous solution Chemosphere, 146 (2016), pp. 162–172 [DOI] [PubMed] [Google Scholar]
- [272].Flores JA, Pavía-Sanders A, Chen Y, Pochan DJ, Wooley KL Recyclable hybrid inorganic/organic magnetically active networks for the sequestration of crude oil from aqueous environments Chem. Mater, 27 (2015), pp. 3775–3782 [Google Scholar]
- [273].Saber O, Mohamed NH, Al Jaafari AA Synthesis of magnetic nanoparticles and nanosheets for oil spill removal Nanosci. Nanotechnol.—Asia, 5 (2015), pp. 32–43 [Google Scholar]
- [274].Mirshahghassemi S, Lead JR Oil recovery from water under environmentally relevant conditions using magnetic nanoparticles Environ. Sci. Technol, 49 (2015), pp. 11729–11736 [DOI] [PubMed] [Google Scholar]
- [275].Zhang L, Li L, Dang Z-M Bio-inspired durable, superhydrophobic magnetic particles for oil/water separation J. Colloid Interface Sci, 463 (2016), pp. 266–271 [DOI] [PubMed] [Google Scholar]
- [276].Pan Y, Wang J, Sun C, Liu X, Zhang H Fabrication of highly hydrophobic organic–inorganic hybrid magnetic polysulfone microcapsules: a lab-scale feasibility study for removal of oil and organic dyes from environmental aqueous samples J. Hazard. Mater, 309 (2016), pp. 65–76 [DOI] [PubMed] [Google Scholar]
- [277].Liu J, Zhao Z, Jiang G Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water Environ. Sci. Technol, 42 (2008), pp. 6949–6954 [DOI] [PubMed] [Google Scholar]
- [278].Su C, Puls RW Arsenate and arsenite sorption on magnetite: relations to groundwater arsenic remediation using zerovalent iron and natural attenuation Water Air Soil Pollut., 193 (2008), pp. 65–78 [Google Scholar]
- [279].Yavuz CT, Mayo JT, Yu WW, Prakash A, Falkner JC, Yean S, Cong L, Shipley HJ, Kan A, Tomson M, Natelson D, Colvin VL Low-field magnetic separation of monodisperse Fe3O4 nanocrystals Science, 314 (2006), pp. 964–967 [DOI] [PubMed] [Google Scholar]
- [280].Yean S, Cong L, Yavuz CT, Mayo JT, Yu WW, Kan AT, Colvin VL, Tomson MB Effects of magnetite particle size on adsorption and desorption of arsenite and arsenate J. Mater. Res, 20 (2005), pp. 3255–3264 [Google Scholar]
- [281].Chowdhury SR, Yanful EK Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal J. Environ. Manage, 91 (2010), pp. 2238–2247 [DOI] [PubMed] [Google Scholar]
- [282].Jin Y, Liu F, Tong M, Hou Y Removal of arsenate by cetyltrimethylammonium bromide modified magnetic nanoparticles J. Hazard. Mater, 227–228 (2012), pp. 461–468 [DOI] [PubMed] [Google Scholar]
- [283].Wu X-L, Wang L, Chen C-L, Xu A-W, Wang X-K Water-dispersible magnetite-graphene-LDH composits for efficient arsenate removal J. Mater. Chem, 21 (2011), pp. 17353–17359 [Google Scholar]
- [284].Yoon Y, Park WK, Hwang T-M, Yoon DH, Yang WS, Kang J-W Comparative evaluation of magnetite–graphene oxide and magnetite-reduced graphene oxide composite for As(III) and As(V) removal J. Hazard. Mater, 304 (2016), pp. 96–204 [DOI] [PubMed] [Google Scholar]
- [285].An B, Liang QQ, Zhao DY Removal of arsenic(V) from spent ion exchange brine using a new class of starch-bridged magnetite nanoparticles Water Res., 45 (2011), pp. 1961–1972 [DOI] [PubMed] [Google Scholar]
- [286].Liang QQ, Zhao DY, Qian TW, Freeland K, Feng YC Effects of stabilizers and water chemistry on arsenate sorption by polysaccharide-stabilized magnetite nanoparticles Ind. Eng. Chem. Res, 51 (2011), pp. 2407–2418 [Google Scholar]
- [287].Shipley HJ, Yean S, Kan AT, Tomson MB Adsorption of arsenic to magnetite nanoparticles: effect of particle concentration pH, ionic strength, and temperature Environ. Toxicol. Chem, 28 (2009), pp. 509–515 [DOI] [PubMed] [Google Scholar]
- [288].Yantasee W, Warner CL, Sangvanich T, Addleman RS, Carter TG, Wiacek RJ, Fryxell GE, Timchalk C, Warner MG Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles Environ. Sci. Technol, 41 (2007), pp. 5114–5119 [DOI] [PubMed] [Google Scholar]
- [289].Kango S, Kumar R Magnetite nanoparticles coated sand for arsenic removal from drinking water Environ. Earth Sci, 75 (2016), pp. 381–392 [Google Scholar]
- [290].Liu CH, Chuang YH, Chen TY, Tian Y, Li H, Wang MK, Zhang W Mechanism of arsenic adsorption on magnetite nanoparticles from water: thermodynamic and spectroscopic studies Environ. Sci. Technol, 49 (2015), pp. 7726–7734 [DOI] [PubMed] [Google Scholar]
- [291].Farrell JW, Fortner J, Work S, Avendano C, Gonzalez-Pech NI, Araiza RZ, Li Q, Alvarez PJJ, Colvin V, Kan A, Tomson M Arsenic removal by nanoscale magnetite in Guanajuato Mexico Environ. Eng. Sci, 31 (2014), pp. 393–402 [Google Scholar]
- [292].Beduk F Superparamagnetic nanomaterial Fe3O4–TiO2 for the removal of As(V) and As(III) from aqueous solutions Environ. Technol, 37 (2016), pp. 1790–1801 [DOI] [PubMed] [Google Scholar]
- [293].Podder MS, Majumder CB Studies on the removal of As(III) and As(V) through their adsorption onto granular activated carbon/MnFe2O4 composite: isotherm studies and error analysis Compos. Interfaces, 23 (2016), pp. 327–372 [Google Scholar]
- [294].Chen R, Zhi C, Yang H, Bando Y, Zhang Z, Sugiur N, Golberg D Arsenic (V) adsorption on Fe3O4 nanoparticle-coated boron nitride nanotubes J. Colloid Interface Sci, 359 (2011), pp. 261–268 [DOI] [PubMed] [Google Scholar]
- [295].Morillo D, Uheida A, Pérez G, Muhammed M, Valiente M Arsenate removal with 3-mercaptopropanoic acid-coated superparamagnetic iron oxide nanoparticles J. Colloid Interface Sci, 438 (2015), pp. 227–234 [DOI] [PubMed] [Google Scholar]
- [296].Chen B, Zhu Z, Ma J, Yang M, Hong J, Hu X, Qiu Y, Chen J One-pot, solid-phase synthesis of magnetic multiwalled carbon nanotube/iron oxide composites and their application in arsenic removal J. Colloid Interface Sci, 434 (2014), pp. 9–17 [DOI] [PubMed] [Google Scholar]
- [297].Abdollahi M, Zeinali S, Nasirimoghaddam S, Sabbaghi S Effective removal of As(III) from drinking water samples by chitosan-coated magnetic nanoparticles Desalin. Water Treat, 56 (2015), pp. 2092–2104 [Google Scholar]
- [298].Luo X, Wang C, Luo S, Dong R, Tu X, Zeng G Adsorption of As(III) and As(V) from water using magnetite Fe3O4-reduced graphite oxide–MnO2 nanocomposites Chem. Eng. J, 187 (2012), pp. 45–52 [Google Scholar]
- [299].Cui H-J, Cai J-K, Zhao H, Yuan B, Ai C-L, Fu M-L Fabrication of magnetic porous Fe–Mn binary oxide nanowires with superior capability for removal of As(III) from water J. Hazard. Mater, 279 (2014), pp. 26–31 [DOI] [PubMed] [Google Scholar]
- [300].Zhu J, Baig SA, Sheng T, Lou Z, Wang Z, Xu X Fe3O4 and MnO2 assembled on honeycomb briquette cinders (HBC) for arsenic removal from aqueous solutions J. Hazard. Mater, 286 (2015), pp. 220–228 [DOI] [PubMed] [Google Scholar]
- [301].Kumar S, Nair RR, Pillai PB, Gupta SN, Iyengar MAR, Sood AK Ggraphene oxide–MnFe2O4 magnetic nanohybrids for efficient removal of lead and arsenic from water ACS Appl. Mater. Interfaces, 6 (2014), pp. 17426–17436 [DOI] [PubMed] [Google Scholar]
- [302].Babu CM, Palanisamy B, Sundaravel B, Shanthi K, Murugesan V Dihalogen crosslinked Fe3O4-reduced graphene oxide nanocomposites for arsenic and mercury adsorption Sci. Adv. Mater, 7 (2015), pp. 794–805 [Google Scholar]
- [303].Salazar-Camacho C, Villalobos M, de la Luz Rivas-Sánchez M, Arenas-Alatorre J, Alcaraz-Cienfuegos J, Gutiérrez-Ruiz ME Characterization and surface reactivity of natural and synthetic magnetites Chem. Geol, 347 (2013), pp. 233–245 [Google Scholar]
- [304].Chiou C-S, Lin Y-F, Chen H-W, Chang C-C, Chang S-H Adsorption of phosphate in aqueous solution by magnetite modified with diethylenetriamine J. Nanosci. Nanotechnol, 15 (2015), pp. 2850–2857 [DOI] [PubMed] [Google Scholar]
- [305].Wang W, Zhang H, Zhang L, Wan H, Zheng S, Xu Z Adsorptive removal of phosphate by magnetic Fe3O4@C@ZrO2 Colloids Surf. A: Physicochem. Eng. Aspects, 469 (2015), pp. 100–106 [Google Scholar]
- [306].Lai L, Xie Q, Chi L, Gu W, Wu D Adsorption of phosphate from water by easily separable Fe3O4@SiO2 core/shell magnetic nanoparticles functionalized with hydrous lanthanum oxide J. Colloid Interface Sci, 465 (2016), pp. 76–82 [DOI] [PubMed] [Google Scholar]
- [307].Yan LG, yang K, Shan RR, Yan T, Wei J, Yu SJ, Yu HQ, Du B Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core–shell Fe₃O₄@LDHs composites with easy magnetic separation assistance J. Colloid Interface Sci, 448 (2015), pp. 508–516 [DOI] [PubMed] [Google Scholar]
- [308].Xia S, Xu X, Xu C, Wang H, Zhang X, Liu G Preparation characterization, and phosphate removal and recovery of magnetic MnFe2O4 nano-particles as adsorbents Environ. Technol, 37 (2016), pp. 795–804 [DOI] [PubMed] [Google Scholar]
- [309].Ma W, Sha X, Gao L, Cheng Z, Meng F, Cai J, Tan D, Wang R Effect of iron oxide nanocluster on enhanced removal of molybdate from surface water and pilot scale test Colloids Surf. A: Physicochem. Eng. Aspects, 478 (2015), pp. 45–53 [Google Scholar]
- [310].Tu Y-J, Chan T-S, Tu H-W, Wang S.-Li., You C-F, Chang C-K Rapid and efficient removal/recovery of molybdenum onto ZnFe2O4 nanoparticles Chemosphere, 148 (2016), pp. 452–458 [DOI] [PubMed] [Google Scholar]
- [311].Wan Z, Chen W, Liu C, Liu Y, Dong C Preparation and characterization of γ-AlOOH@CS magnetic nanoparticle as a novel adsorbent for removing fluoride from drinking water J. Colloid Interface Sci, 443 (2015), pp. 115–124 [DOI] [PubMed] [Google Scholar]
- [312].Rehman MA, Yusoff I, Alias Y Fluoride adsorption by doped and un-doped magnetic ferrites CuCexFe2-xO4: preparation, characterization, optimization and modeling for effectual remediation technologies J. Hazard. Mater, 299 (2015), pp. 316–324 [DOI] [PubMed] [Google Scholar]
- [313].Wei X, Bhojappa S, Lin L-S, Viadero RC Jr. Performance of nano-magnetite for removal of selenium from aqueous solutions Environ. Eng. Sci, 29 (2012), pp. 526–532 [Google Scholar]
- [314].Wang H, Liu Y-G, Zeng G-M, Hu X-J, Hu X, Li T-T, Li H-Y, Wang Y-Q, Jiang L-H Grafting of β-cyclodextrin to magnetic graphene oxide viaethylenediamine and application for Cr(VI) removal Carbohydr. Polym, 113 (2014), pp. 166–173 [DOI] [PubMed] [Google Scholar]
- [315].Jiang W, Cai Q, Xu W, Yang M, Cai Y, Dionysiou DD, O’shea KE Cr(VI) adsorption and reduction by humic acid coated on magnetite Environ. Sci. Technol, 48 (2014), pp. 8078–8085 [DOI] [PubMed] [Google Scholar]
- [316].Qiu B, Gu H, Yan X, Guo J, Wang Y, Sun D, Wang Q, Khan M, Zhang X, Weeks BL, Young DP, Guo Z, Wei S Cellulose derived magnetic mesoporous carbon nanocomposites with enhanced hexavalent chromium removal J. Mater. Chem. A, 2 (2014), pp. 17454–17462 [Google Scholar]
- [317].Yao W, Rao P, Lo IMC, Zhang W, Zheng W Preparation of cross-linked magnetic chitosan with quaternary ammonium and its application for Cr(VI) and P(V) removal J. Environ. Sci, 26 (2014), pp. 2379–2386 [DOI] [PubMed] [Google Scholar]
- [318].Kumari M, Pittman CU, Mohan D Heavy metals [chromium(VI) and lead(II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres J. Colloid Interface Sci, 442 (2015), pp. 120–132 [DOI] [PubMed] [Google Scholar]
- [319].Rajput S, Pittman CU, Mohan D Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water J. Colloid Interface Sci, 468 (2016), pp. 334–346 [DOI] [PubMed] [Google Scholar]
- [320].Swindle AL, Cozzarelli IM, Madden ASE Using chromate to investigate the impact of natural organics on the surface reactivity of nanoparticulate magnetite Environ. Sci. Technol, 49 (2015), pp. 2156–2162 [DOI] [PubMed] [Google Scholar]
- [321].Egodawatte S, Datt A, Burns EA, Larsen SC Chemical insight into the adsorption of chromium(III) on iron oxide/mesoporous silica nanocomposites Langmuir, 31 (2015), pp. 7553–7562 [DOI] [PubMed] [Google Scholar]
- [322].Silva-Silva MJ, Mijangos-Ricardez OF, Vázquez-Hipólito V, Martinez-Vargas S, López-Luna J Single and mixed adsorption of Cd(II) and Cr(VI) onto citrate-coated magnetite nanoparticles Desalin. Water Treat, 57 (2016), pp. 4008–4017 [Google Scholar]
- [323].Tian X, Wang W, Tian N, Zhou C, Yang C, Komarneni S Cr(VI) reduction and immobilization by novel carbonaceous modified magnetic Fe3O4/halloysite nanohybrid J. Hazard. Mater, 309 (2016), pp. 151–156 [DOI] [PubMed] [Google Scholar]
- [324].Vélez E, Campillo GE, Morales G, Hincapié C, Osorio J, Arnache O, Uribe JI, Jaramillo F Mercury removal in wastewater by iron oxide nanoparticles J. Phys.: Conf. Ser, 687 (2016), pp. 12050–12053 [Google Scholar]
- [325].Shan C, Ma Z, Tong M, Ni J Removal of Hg(II) by poly(1-vinylimidazole)-grafted Fe3O4@SiO2 magnetic nanoparticles Water Res., 69 (2015), pp. 252–260 [DOI] [PubMed] [Google Scholar]
- [326].Pan S, Shen H, Xu Q, Luo J, Hu M Surface mercapto engineered magnetic Fe3O4 nanoadsorbent for the removal of mercury from aqueous solutions J. Colloid Interface Sci, 365 (2012), pp. 204–212 [DOI] [PubMed] [Google Scholar]
- [327].Zeng G, Pang Y, Zeng Z, Tang L, Zhang Y, Liu Y, Zhang J, Lei X, Li Z, Xiong Y, Xie G Removal and recovery of Zn2+ and Pb2+ by imine-functionalized magnetic nanoparticles with tunable selectivity Langmuir, 28 (2012), pp. 468–473 [DOI] [PubMed] [Google Scholar]
- [328].Chung J, Chun J, Woo J, Lee SH, Lee YJ, Hong SW Sorption of Pb(II) and Cu(II) onto multi-amine grafted mesoporous silica embedded with nano-magnetite: effects of steric factors J. Hazard. Mater, 239–240 (2012), pp. 183–191 [DOI] [PubMed] [Google Scholar]
- [329].Mahmoud ME, Rashad AR Enhanced separation and extraction of cadmium and lead by a novel magnetite-immobilized-gelatin nano-sorbent Sep. Sci. Technol, 51 (2016), pp. 767–777 [Google Scholar]
- [330].Mohmood I, Lopes CB, Lopes I, Tavares DS, Soares AMVM, Duarte AC, Trindade T, Ahmad I, Pereira E Remediation of mercury contaminated saltwater with functionalized silica coated magnetite nanoparticles Sci. Total Environ, 557–558 (2016), pp. 712–721 [DOI] [PubMed] [Google Scholar]
- [331].Anirudhan TS, Shainy F Effective removal of mercury(II) ions from chlor-alkali industrial wastewater using 2-mercaptobenzamide modified itaconic acid-grafted-magnetite nanocellulose J. Colloid Interface Sci, 456 (2015), pp. 22–31 [DOI] [PubMed] [Google Scholar]
- [332].Cui L, Wang Y, Gao L, Hu L, Wei Q, Du B Removal of Hg(II) from aqueous solution by resin loaded magnetic β-cyclodextrin bead and graphene oxide sheet: synthesis, adsorption mechanism and separation J. Colloid Interface Sci, 456 (2015), pp. 42–49 [DOI] [PubMed] [Google Scholar]
- [333].Ma Y, La P, Lei W, Lu C, Du X Adsorption of Hg(II) from aqueous solution using amino-functionalized graphite nanosheets decorated with Fe3O4 nanoparticles Desalin. Water Treat, 57 (2016), pp. 5004–5012 [Google Scholar]
- [334].Shih Y-C, Ke C-Y, Yu C-J, Lu C-Y, Tseng W-L Combined tween 20-stabilized gold nanoparticles and reduced graphite oxide–Fe3O4 nanoparticle composites for rapid and efficient removal of mercury species from a complex matrix ACS Appl. Mater. Interfaces, 6 (2014), pp. 17437–17445 [DOI] [PubMed] [Google Scholar]
- [335].Li K, Wang Y, Huang M, Yan H, Yang H, Xiao S, Li A Preparation of chitosan-graft-polyacrylamide magnetic composite microspheres for enhanced selective removal of mercury ions from water J. Colloid Interface Sci, 455 (2015), pp. 261–270 [DOI] [PubMed] [Google Scholar]
- [336].Wang Y, Shi L, Gao L, Wei Q, Cui L, Hu L, Yan L, Du B The removal of lead ions from aqueous solution by using magnetic hydroxypropyl chitosan/oxidized multiwalled carbon nanotubes composites J. Colloid Interface Sci, 451 (2015), pp. 7–14 [DOI] [PubMed] [Google Scholar]
- [337].Jiang L, Liu P Design of magnetic attapulgite/fly ash/poly(acrylic acid) ternary nanocomposite hydrogels and performance evaluation as selective adsorbent for Pb2+ ion ACS Sustain. Chem. Eng, 2 (2014), pp. 1785–1794 [Google Scholar]
- [338].Chen D, Shen W, Wu S, Chen C, Luo X, Guo L Ion exchange induced removal of Pb(II) by MOF-derived magnetic inorganic sorbents Nanoscale, 8 (2016), pp. 7172–7179 [DOI] [PubMed] [Google Scholar]
- [339].Chen Z, Geng Z, Zhang Z, Ren L, Tao T, Yang R, Guo Z Synthesis of magnetic Fe3O4@C nanoparticles modified with SO3H and COOH groups for fast removal of Pb2+ Hg2+, and Cd2+ ions Eur. J. Inorg. Chem, 2014 (2014), pp. 3172–3177 [Google Scholar]
- [340].Shi H, Yang J, Zhu L, Yang Y, Yuan H, Yang Y, Liu X Removal of Hg2+ Pb2+, and Cu2+ by chain-like Fe3O4@SiO2@chitosan magnetic nanoparticles J. Nanosci. Nanotechnol, 16 (2016), pp. 1871–1882 [DOI] [PubMed] [Google Scholar]
- [341].Mahmouda ME, Abdelwahaba MS, Abdoua AEH Enhanced removal of lead and cadmium from water by Fe3O4-cross linked-O-phenylenediamine nano-composite Sep. Sci. Technol, 51 (2016), pp. 237–247 [Google Scholar]
- [342].Wang J, Zhang W, Yue X, Yang Q, Liu F, Wang Y, Zhang D, Li Z, Wang J One-pot synthesis of multifunctional magnetic ferrite–MoS2–carbon dot nanohybrid adsorbent for efficient Pb(II) removal J. Mater. Chem. A, 4 (2016), pp. 3893–3900 [Google Scholar]
- [343].Pan S, Li J, Wan G, Liu C, Fan W, Wang L Nanosized yolk–shell Fe3O4@Zr (OH)x spheres for efficient removal of Pb(II) from aqueous solution J. Hazard. Mater, 309 (2016), pp. 1–9 [DOI] [PubMed] [Google Scholar]
- [344].Shan C, Ma Z, Tong M Efficient removal of free and nitrilotriacetic acid complexed Cd(II) from water by poly(1-vinylimidazole)-grafted Fe3O4@SiO2 magnetic nanoparticles J. Hazard. Mater, 299 (2015), pp. 479–485 [DOI] [PubMed] [Google Scholar]
- [345].Babaei AA, Bahrami M, Firouzi AF, Esfahani AR, Alidokht L Adsorption of cadmium onto modified nanosized magnetite: kinetic modeling, isotherm studies, and process optimization Desalin. Water Treat, 56 (2015), pp. 3380–3392 [Google Scholar]
- [346].Bao S, Tang L, Li K, Ning P, Peng J, Guo H, Zhu T, Liu Y Highly selective removal of Zn(II) ion from hot-dip galvanizing pickling waste with amino-functionalized Fe3O4@SiO2 magnetic nano-adsorbent J. Colloid Interface Sci, 462 (2016), pp. 235–242 [DOI] [PubMed] [Google Scholar]
- [347].Bakr AA, Moustafa YM, Khalil MMH, Yehia MM, Motawea EA Magnetic nanocomposite beads: synthesis and uptake of Cu(II) ions from aqueous solutions Can. J. Chem, 93 (2015), pp. 289–296 [Google Scholar]
- [348].Wanna Y, Chindaduang A, Tumcharern G, Phromyothin D, Porntheerapat S, Nukeaw J, Hofmann H, Pratontep S Efficiency of SPIONs functionalized with polyethylene glycol bis(amine) for heavy metal removal J. Magn. Magn. Mater, 414 (2016), pp. 32–37 [Google Scholar]
- [349].Prucek R, Tuček J, Kolařík J, Hušková I, Filip J, Varma RS, Sharma VK, Zbořil R Ferrate(VI)-prompted removal of metals in aqueous media: mechanistic delineation of enhanced efficiency via metal entrenchment in magnetic oxides Environ. Sci. Technol, 49 (2015), pp. 2319–2327 [DOI] [PubMed] [Google Scholar]
- [350].Zhao F, Tang WZ, Zhao D, Meng Y, Yin D, Sillanpaa M Adsorption kinetics, isotherms and mechanisms of Cd(II) Pb(II), Co(II) and Ni(II) by a modified magnetic polyacrylamide microcomposite adsorbent J. Water Process Eng, 4 (2014), pp. 47–57 [Google Scholar]
- [351].Cui L, Wang Y, Gao L, Hu L, Yan L, Wei Q, Du B EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: adsorption mechanism and separation property Chem. Eng. J, 281 (2015), pp. 1–10 [Google Scholar]
- [352].Hu X-J, Liu Y-G, Zeng G-M, Wang H, You S-H, Hu X, Tan X-F, Chen A-W, Guo F-Y Effects of inorganic electrolyte anions on enrichment of Cu(II) ions with aminated Fe3O4/graphene oxide: Cu(II) speciation prediction and surface charge measurement Chemosphere, 127 (2015), pp. 35–41 [DOI] [PubMed] [Google Scholar]
- [353].Tan L, Wang J, Liu Q, Sun Y, Zhang H, Wang Y, Jing X, Liu J, Song D Facile preparation of oxine functionalized magnetic Fe3O4 particles for enhanced uranium(VI) adsorption Colloids Surf. A: Physicochem. Eng. Aspects, 466 (2015), pp. 85–91 [Google Scholar]
- [354].Tan L, Zhang X, Liu Q, Jing X, Liu J, Song D, Hu S, Liu L, Wang J Synthesis of Fe3O4@TiO2 core–shell magnetic composites for highly efficient sorption of uranium(VI) Colloids Surf. A: Physicochem. Eng. Aspects, 469 (2015), pp. 279–286 [Google Scholar]
- [355].Chen L, Zhao D, Chen S, Wang X, Chen C One-step fabrication of amino functionalized magnetic graphene oxide composite for uranium(VI) removal J. Colloid Interface Sci, 472 (2016), pp. 99–107 [DOI] [PubMed] [Google Scholar]
- [356].Ding C, Cheng W, Sun Y, Wang X Novel fungus-Fe3O4 bio-nanocomposites as high performance adsorbents for the removal of radionuclides J. Hazard. Mater, 295 (2015), pp. 127–137 [DOI] [PubMed] [Google Scholar]
- [357].Ozcan F, Bayrakcı M, Ertul Synthesis S and preparation of novel magnetite nanoparticles containing calix[4]arenes with different chelating group towards uranium anions J. Macromol. Sci. Part A, 52 (2015), pp. 599–608 [Google Scholar]
- [358].Marshall TA, Morris K, Law GTW, Mosselmans JFW, Bots P, Roberts H, Shaw S Uranium fate during crystallization of magnetite from ferrihydrite in conditions relevant to the disposal of radioactive waste Mineral. Mag, 79 (2015), pp. 1265–1274 [Google Scholar]
- [359].Yang H-M, Hong SB, Choi YS, Lee K-W, Seo B-K, Moon J-K Copper ferrocyanide-functionalized magnetic adsorbents using polyethyleneimine coated Fe3O4 nanoparticles for the removal of radioactive cesium J. Nanosci. Nanotechnol, 16 (2016), pp. 3067–3070 [DOI] [PubMed] [Google Scholar]
- [360].Wylie EM, Olive DT, Powell BA Effects of titanium doping in titanomagnetite on neptunium sorption and speciation Environ. Sci. Technol, 50 (2016), pp. 1853–1858 [DOI] [PubMed] [Google Scholar]
- [361].Smith FN, Um W, Taylor CD, Kim D-S, Schweiger MJ, Kruger AA Computational investigation of technetium(IV) incorporation into inverse spinels: magnetite (Fe3O4) and Trevorite (NiFe2O4) Environ. Sci. Technol, 50 (2016), pp. 5216–5224 [DOI] [PubMed] [Google Scholar]
- [362].Patra S, Roy E, Madhuri R, Sharma PK Fast and selective preconcentration of europium from wastewater and coal soil by graphene oxide/silane@Fe3O4 dendritic nanostructure Environ. Sci. Technol, 49 (2015), pp. 6117–6126 [DOI] [PubMed] [Google Scholar]
- [363].Basualto C, Gaete J, Molina L, Valenzuela F, Yañez C, Marco JF Lanthanide sorbent based on magnetite nanoparticles functionalized with organophosphorus extractants Sci. Technol. Adv. Mater, 16 (2015), pp. 35010–35018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Zhang F, Song Y, Song S, Zhang R, Hou W Synthesis of magnetite-graphene oxide-layered double hydroxide composites and applications for the removal of Pb(II) and 2,4-dichlorophenoxyacetic acid from aqueous solutions ACS Appl. Mater. Interfaces, 7 (2015), pp. 7251–7263 [DOI] [PubMed] [Google Scholar]
- [365].Han Z, Sani B, Mrozik W, Obst M, Beckingham B, Karapanagioti HK, Werner D Magnetite impregnation effects on the sorbent properties of activated carbons and biochars Water Res., 70 (2015), pp. 394–403 [DOI] [PubMed] [Google Scholar]
- [366].Bai X, Feng R, Hua Z, Zhou L, Shi H Adsorption of 17β-estradiol (E2) and Pb(II) on Fe3O4/graphene oxide (Fe3O4/GO) nanocomposites Environ. Eng. Sci, 32 (2015), pp. 370–378 [Google Scholar]
- [367].Ge X, Gu CD, Wang XL, Tu JP Spinel type CoFe oxide porous nanosheets as magnetic adsorbents with fast removal ability and facile separation J. Colloid Interface Sci, 454 (2015), pp. 134–143 [DOI] [PubMed] [Google Scholar]
- [368].Cui L, Guo X, Wei Q, Wang Y, Gao L, Yan L, Yan T, Du B Removal of mercury and methylene blue from aqueous solution by xanthate functionalized magnetic graphene oxide: sorption kinetic and uptake mechanism J. Colloid Interface Sci, 439 (2015), pp. 112–120 [DOI] [PubMed] [Google Scholar]
- [369].Liu J, Wang C, Xiong Z Adsorption behavior of magnetic multiwalled carbon nanotubes for the simultaneous adsorption of furazolidone and Cu(II) from aqueous solutions Environ. Eng. Sci, 32 (2015), pp. 960–969 [Google Scholar]
- [370].Zhang X, Qian J, Pan B Fabrication of novel magnetic nanoparticles of multifunctionality for water decontamination Environ. Sci. Technol, 50 (2016), pp. 881–889 [DOI] [PubMed] [Google Scholar]
- [371].Li J, Fan Q, Wu Y, Wang X, Chen C, Tang Z, Wang X Magnetic polydopamine decorated with Mg–Al LDH nanoflakes as a novel bio-based adsorbent for simultaneous removal of potentially toxic metals and anionic dyes J. Mater. Chem. A, 4 (2016), pp. 1737–1746 [Google Scholar]
- [372].Sun Y, Liu X, Kainuma M, Wang W, Takaoka M, Takeda N Dechlorination of polychlorinated biphenyls by iron and its oxides Chemosphere, 137 (2015), pp. 78–86 [DOI] [PubMed] [Google Scholar]
- [373].Wang Y, Si X, Si Y Dechlorination of 3,3′,4,4′-tetrachlorobiphenyl in aqueous solution by hybrid Fe0/Fe3O4 nanoparticle system J. Exp. Nanosci, 10 (2015), pp. 1166–1179 [Google Scholar]
- [374].Tian H, Liu F, He J Multifunctional Fe3O4@nSiO2@mSiO2-Fe core–shell microspheres for highly efficient removal of 1,1,1-trichloro-2,2-bis(4-chlorophenyl) ethane (DDT) from aqueous media J. Colloid Interface Sci, 431 (2014), pp. 90–96 [DOI] [PubMed] [Google Scholar]
- [375].Gan M, Huang Y, Zhang Y, Pan J, Shi W, Yan Y Fabrication of Ag/halloysite nanotubes/Fe3O4 nanocatalyst and their catalytic performance in 4-nitrophenol reduction Desalin. Water Treat, 56 (2015), pp. 425–434 [Google Scholar]
- [376].Xu J, Liu X, Lowry GV, Cao Z, Zhao H, Zhou JL, Xu X Dechlorination mechanism of 2,4-dichlorophenol by magnetic MWCNTS supported Pd/Fe nanohybrids: rapid adsorption, gradual dechlorination, and desorption of phenol ACS Appl. Mater. Interfaces, 8 (2016), pp. 7333–7342 [DOI] [PubMed] [Google Scholar]
- [377].Shan D, Deng S, Zhao T, Wang B, Wang Y, Huang J, Yu G, Winglee J, Wiesner MR Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling J. Hazard. Mater, 305 (2016), pp. 156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Villacís-García M, Villalobos M, Gutiérrez- Ruiz M Ruiz Optimizing the use of natural and synthetic magnetites with very small amounts of coarse Fe(0) particles for reduction of aqueous Cr(VI) J. Hazard. Mater, 281 (2015), pp. 77–86 [DOI] [PubMed] [Google Scholar]
- [379].Watts MP, Coker VS, Parry SA, Pattrick RAD, Thomas RAP, Kalin R, Lloyd JR Biogenic nano-magnetite and nano-zero valent iron treatment of alkaline Cr(VI) leachate and chromite ore processing residue Appl. Geochem, 54 (2015), pp. 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [380].Wang T, Zhang L, Li C, Yang W, Song T, Tang C, Meng Y, Dai S, Wang H, Chai L, Luo J Synthesis of core–shell magnetic Fe3O4@poly(m-phenylenediamine) particles for chromium reduction and adsorption Environ Sci. Technol, 49 (2015), pp. 5654–5662 [DOI] [PubMed] [Google Scholar]
- [381].Cho D-W, Song H, Schwartz FW, Kim B, Jeon B-H The role of magnetite nanoparticles in the reduction of nitrate in groundwater by zero-valent iron Chemosphere, 125 (2015), pp. 41–49 [DOI] [PubMed] [Google Scholar]
- [382].Suanon F, Sun Q, Mama D, Li J, Dimon B, Yua C-P Effect of nanoscale zero-valent iron and magnetite (Fe3O4) on the fate of metals during anaerobic digestion of sludge Water Res, 88 (2016), pp. 897–903 [DOI] [PubMed] [Google Scholar]
- [383].Joseph S, Anawar HM, Storer P, Blackwell P, Chia C, Lin Y, Munroe P, Donne S, Horvat J, Wang J, Solaiman ZM Effects of enriched biochars containing magnetic iron nanoparticles on mycorrhizal colonisation plant growth, nutrient uptake and soil quality improvement Pedosphere, 25 (2015), pp. 749–760 [Google Scholar]
- [384].Usman M, Faure P, Ruby C, Hanna K Remediation of PAH-contaminated soils by magnetite catalyzed Fenton-like oxidation Appl. Catal. B: Environ, 117–118 (2012), pp. 10–17 [Google Scholar]
- [385].Liang QQ, Zhao DY Immobilization of arsenate in a sandy loam soil using starch-stabilized magnetite nanoparticles J. Hazard. Mater, 271 (2014), pp. 16–23 [DOI] [PubMed] [Google Scholar]
- [386].Sun J, Chillrud SN, Mailloux BJ, Stute M, Singh R, Dong H, Lepre CJ, Bostick BC Enhanced and stabilized arsenic retention in microcosms through the microbial oxidation of ferrous iron by nitrate Chemosphere, 144 (2016), pp. 1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [387].Funes A, de Vicente J, Cruz-Pizarro L, Álvarez-Manzaneda I, de Vicente I Magnetic microparticles as a new tool for lake restoration: a microcosm experiment for evaluating the impact on phosphorus fluxes and sedimentary phosphorus pools Water Res., 89 (2016), pp. 366–374 [DOI] [PubMed] [Google Scholar]
- [388].Li C, Yi X, Dang Z, Yu H, Zeng T, Wei C, Feng C Fate of Fe and Cd upon microbial reduction of Cd-loaded polyferric flocs by Shewanella oneidensis MR-1 Chemosphere, 144 (2016), pp. 2065–2072 [DOI] [PubMed] [Google Scholar]
- [389].Lee W, Batchelor B Abiotic reductive dechlorination of chlorinated ethylenes by iron-bearing soil minerals. 1. Pyrite and magnetite Environ. Sci. Technol, 36 (2002), pp. 5147–5154 [DOI] [PubMed] [Google Scholar]
- [390].McCormick ML, Adriaens P Carbon tetrachloride transformation on the surface of nanoscale biogenic magnetite particles Environ. Sci. Technol, 38 (2004), pp. 1045–1053 [DOI] [PubMed] [Google Scholar]
- [391].Hanoch RJ, Shao H, Butler EC Transformation of carbon tetrachloride by bisulfide treated goethite, hematite, magnetite, and kaolinite Chemosphere, 63 (2006), pp. 323–334 [DOI] [PubMed] [Google Scholar]
- [392].Danielsen K, Hayes KF pH dependence of carbon tetrachloride reductive dechlorination by magnetite Environ. Sci. Technol, 38 (2004), pp. 4745–4752 [DOI] [PubMed] [Google Scholar]
- [393].Zwank L, Elsner M, Aeberhard A, Schwarzenbach RP, Haderlein SB Carbon isotope fractionation in the reductive dehalogenation of carbon tetrachloride at iron (hydr)oxide and iron sulfide minerals Environ. Sci. Technol, 39 (2005), pp. 5634–5641 [DOI] [PubMed] [Google Scholar]
- [394].Vikesland PJ, Heathcock AM, Rebodos RL, Makus KE Particle size and aggregation effects on magnetite reactivity toward carbon tetrachloride Environ. Sci. Technol, 41 (2007), pp. 5277–5283 [DOI] [PubMed] [Google Scholar]
- [395].Ferrey ML, Wilkin RT, Ford RG, Wilson JT Non-biological removal of cis-dichloroethylene and 1,1-dichloroethylene in aquifer sediment containing magnetite Environ. Sci. Technol, 38 (2004), pp. 1746–1752 [DOI] [PubMed] [Google Scholar]
- [396].Ayala-Luis KB, Cooper NG, Koch CB, Hansen HC Efficient dechlorination of carbon tetrachloride by hydrophobic green rust intercalated with dodecanoate anions Environ. Sci. Technol, 46 (2012), pp. 3390–3397 [DOI] [PubMed] [Google Scholar]
- [397].Jeong HY, Anantharaman K, Hyun SP, Son M, Hayes KF pH impact on reductive dechlorination of cis-dichloroethylene by Fe precipitates: an X-ray absorption spectroscopy study Water Res., 47 (2013), pp. 6639–6649 [DOI] [PubMed] [Google Scholar]
- [398].He YT, Wilson JT, Su C, Wilkin RT Review of abiotic degradation of chlorinated solvents by reactive iron minerals in aquifers Groundwater Monit. Remediat, 35 (2015), pp. 57–75 [Google Scholar]
- [399].Giri SK, Das NN Visible light induced photocatalytic decolourisation of rhodamine B by magnetite nanoparticles synthesised using recovered iron from waste iron ore tailing Desalin. Water Treat, 57 (2016), pp. 900–907 [Google Scholar]
- [400].Nasrollahzadeh M, Sajadi SM Preparation of Pd/Fe3O4 nanoparticles by use of Euphorbia stracheyi Boiss root extract: a magnetically recoverable catalyst for one-pot reductive amination of aldehydes at room temperature J. Colloid Interface Sci, 464 (2016), pp. 147–152 [DOI] [PubMed] [Google Scholar]
- [401].Virkutyte J, Varma RS Eco-friendly magnetic iron oxide pillared montmorillonite for advanced catalytic degradation of dichlorophenol ACS Sustain. Chem. Eng, 2 (2014), pp. 1545–1550 [Google Scholar]
- [402].Shirkhodaie M, Beyki MH, Shemirani F Biogenic synthesis of magnetic perlite@iron oxide composite: application as a green support for dye removal Desalin. Water Treat, 25 (2016), pp. 11859–11871 [Google Scholar]
- [403].Su C, Puls RW, Krug TA, Watling MT, O’Hara SK, Quinn JW, Ruiz NE A two and half-year-performance evaluation of a field test on treatment of source zone tetrachloroethene and its chlorinated daughter products using emulsified zero valent iron nanoparticles Water Res., 46 (2012), pp. 5071–5084 [DOI] [PubMed] [Google Scholar]
- [404].Su C, Puls RW, Krug TA, Watling MT, O’Hara SK, Quinn JW, Ruiz NE Travel distance and transformation of injected emulsified zero valent iron nanoparticles in the subsurface during two and half years Water Res., 47 (2013), pp. 4095–4106 [DOI] [PubMed] [Google Scholar]
- [405].Liu T, Li X, Waite TD Depassivation of aged Fe0 by divalent cations: correlation between contaminant degradation and surface complexation constants Environ. Sci. Technol, 48 (2014), pp. 14564–14571 [DOI] [PubMed] [Google Scholar]
- [406].Lin C-H, Shih Y-H, MacFarlane J Amphiphilic compounds enhance the dechlorination of pentachlorophenol with Ni/Fe bimetallic nanoparticles Chem. Eng. J, 262 (2015), p. 59 [Google Scholar]
- [407].Ou Y-H, Wei C-Y, Shih Y-H Short-chain organic acids increase the reactivity of zerovalent iron nanoparticles to polychlorinated aromatic pollutants in water Chem. Eng. J, 284 (2016), pp. 372–379 [Google Scholar]
- [408].An B, Zhao DY Immobilization of As(III) in soil and groundwater using a new class of polysaccharide stabilized Fe-Mn oxide nanoparticles J. Hazard. Mater, 211 (2012), pp. 332–341 [DOI] [PubMed] [Google Scholar]
- [409].Etique M, Jorand FPA, Ruby C Magnetite as a precursor for green rust through the hydrogenotrophic activity of the ironreducing bacteria Shewanella putrefaciens Geobiology, 14 (2016), pp. 237–254 [DOI] [PubMed] [Google Scholar]


