Abstract
There is overwhelming evidence that sleep is crucial for memory consolidation. Patients with schizophrenia and their unaffected relatives have a specific deficit in sleep spindles, a defining oscillation of non-rapid eye movement (NREM) Stage 2 sleep that, in coordination with other NREM oscillations, mediate memory consolidation. In schizophrenia, the spindle deficit correlates with impaired sleep-dependent memory consolidation, positive symptoms, and abnormal thalamocortical connectivity. These relations point to dysfunction of the thalamic reticular nucleus (TRN), which generates spindles, gates the relay of sensory information to the cortex, and modulates thalamocortical communication. Genetic studies are beginning to provide clues to possible neurodevelopmental origins of TRN-mediated thalamocortical circuit dysfunction and to identify novel targets for treating the related memory deficits and symptoms. By forging empirical links in causal chains from risk genes to thalamocortical circuit dysfunction, spindle deficits, memory impairment, symptoms, and diagnosis, future research can advance our mechanistic understanding, treatment and prevention of schizophrenia.
Keywords: cognition, endophenotype, genetics, memory, schizophrenia sleep, spindles
1. Paradigm shift: Not all our learning happens while we are awake
Human beings spend about a third of their lives sleeping, yet the functions of sleep are still not clear. Over the last 20 years there has been a virtual explosion in research on the role of sleep in memory (Figure 1). This has produced a wealth of molecular, cellular, neural network, brain activation, and behavioral evidence from birds, rodents, cats, and humans of an evolutionarily conserved function for sleep in the consolidation of multiple forms of memory. This body of work demonstrates that to understand memory one must also understand sleep. It has also revealed the breadth of offline memory processing and its importance to cognition. These realizations have led to a paradigm shift: Far from being simply a passive restorative state, sleep is an active period of cognitive functioning that plays an essential role in memory.
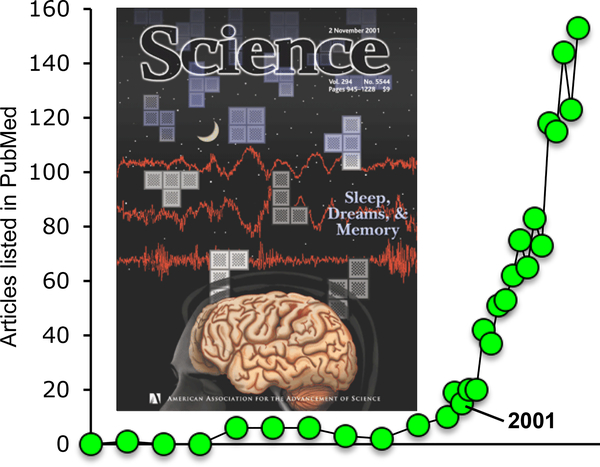
Figure 1.
Exponential growth of research on sleep and memory. Articles on sleep and learning listed in PubMed when searching for (memory[Title] OR learning[Title] AND sleep[Title]) have increased exponentially since the publication of a review on the topic in Science in 2001 (Stickgold et al. 2001), with a doubling time of just under 4 years. Cover reproduced with permission from Stickgold et al. 2001.
Following active encoding, memory consolidation proceeds offline, during both wake and sleep, without requiring conscious intent, effort, or awareness (Stickgold & Walker 2007). The term memory consolidation was originally introduced to explain how labile memories are thought to stabilize and become resistant to interference over time. Early studies of the effects of electroconvulsive therapy on recently formed memories fixed the time required for consolidation at one to four hours (Duncan 1949). We now know that there are also slower processes that take “weeks, months or even years” (p 55, Dudai 2004), and that the sleeping brain not only stabilizes recently learned materials but also contributes to their enhancement and integration into existing semantic networks, selectively maintains their emotional elements, extracts rules, and enhances the gist at the expense of details, all of which usually results in a more useful memory (Stickgold & Walker 2013). Recognition of these slower processes that work in concert over time and particularly during sleep has led to the proposal that the term “memory consolidation” be replaced with the more expansive “memory evolution,” which better captures the complexity and scope of the processes involved (Walker & Stickgold 2010). The goal of this review is to consider the relevance of both sleep physiology and these sleep-dependent processes to understanding the pathophysiology, symptoms, and cognitive deficits of schizophrenia.
2. Cognitive deficits are the most persistent, disabling, and treatment-refractory feature of schizophrenia
Schizophrenia is a neurodevelopmental disorder in which genetic and environmental risk factors interact and lead to the emergence of symptoms, usually in late adolescence or early adulthood. It is defined by positive symptoms such as hallucinations and delusions and negative symptoms such as amotivation, social withdrawal, and apathy. Schizophrenia is also characterized by a wide range of cognitive deficits. One criterion for identifying the core cognitive deficits—those that hold the most promise for illuminating pathophysiology—is that they are also present in healthy relatives. This would indicate that they are markers of genetic vulnerability to schizophrenia rather than epiphenomena of psychosis. Meta-analytic studies of first-degree relatives show the largest effect sizes for impairments on tasks with high executive function demands, such as task set-switching, inhibition of prepotent responses, and working memory (Snitz et al. 2006). In schizophrenia, these deficits often predate the onset of symptoms and persist throughout its course, even after the florid psychotic symptoms have been effectively controlled with antipsychotic drugs (APDs). This persistence in patients and presence in unaffected family members has led some investigators to call for a redefinition of schizophrenia as a cognitive disorder, with psychosis as a late and potentially preventable consequence (Cohen & Insel 2008).
Perhaps because of their persistence, cognitive deficits are better predictors of social function and outcome than the defining symptoms of schizophrenia (Green et al. 2000). As a result of impaired cognition, only ~20% of individuals with schizophrenia work (Insel 2009). Because schizophrenia strikes young people and affects ~1% of the population worldwide, this has staggering economic and psychosocial costs (Cloutier et al. 2016). Consequently, finding effective treatment for cognitive deficits is a priority of the schizophrenia research community and the focus of large-scale studies (e.g., Buchanan et al. 2007, Marder et al. 2004). An important limitation of these efforts is that cognition is measured in cross section at a single point in time. Whereas this approach provides a valid snapshot of function, it neglects the critical role of offline and particularly sleep-dependent processing in the evolution of memory. This omission is consequential because we lack effective treatments for cognitive deficits and studying the role of sleep has the potential to reveal pathophysiology and identify therapeutic targets. If we could identify sleep or cognitive markers of schizophrenia that precede and predict psychosis, we could transform our approach to treatment from ameliorative APDs to the development of preemptive interventions that could forestall or even prevent psychosis (Cohen & Insel 2008). In the early 2000s, the emerging revolution in our understanding of the role of sleep in cognition, together with the long association of schizophrenia with abnormal sleep, motivated investigations of sleep-dependent cognition in schizophrenia (Goder et al. 2004, Manoach et al. 2004). Today, a growing body of research points to impairments in sleep-dependent memory consolidation as an important and potentially treatable contributor to cognitive deficits in schizophrenia. Below, we review the current understanding of the role of sleep in memory evolution as a prelude to considering its relevance to schizophrenia.
3. Sleep plays diverse roles in memory evolution
3.1. Sleep is not a unitary state
Sleep is traditionally divided into rapid eye movement (REM) sleep and non-rapid eye movement (NREM) sleep. NREM sleep is subdivided into three stages: N1, N2, and N3 (Figure 2) (see Iber et al. 2007). Each stage contributes to different aspects of memory evolution (Stickgold & Walker 2013). Stabilization of hippocampus-dependent memories usually correlates with time spent in the deepest stage of NREM sleep, N3 (Plihal & Born 1997), although emotional memories tend to be associated with REM sleep (Wagner et al. 2001). REM sleep also facilitates complex cognitive processing, for example the extraction of rules (Barsky et al. 2015) or the formation of new associations for creative problem solving (Cai et al. 2009). Enhancement of performance on procedural motor memory tasks—memory for how to perform a task—often correlates with time spent in N2 sleep (e.g., Walker et al. 2002). Correlations between time in N1, which can be less than 10 min per night, and memory have not been identified, although task-related dream reports obtained from N1 predict postsleep improvement on a navigation task (Wamsley et al. 2010). Thus, each sleep stage has been associated with distinct forms of memory, leading to the strong hypothesis that sleep stages evolved to provide the optimal environments for specific components of memory evolution.
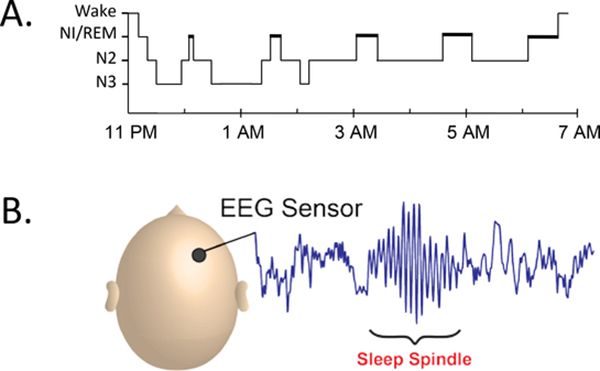
Figure 2.
A good night’s sleep. (a) Hypnogram showing the distribution of sleep stages across a typical night. A typical night consists of five 90-min cycles that include REM sleep. Most of the deep slow-wave sleep (N3) occurs early in the night, and most of the REM sleep occurs later in the night. N1 is a transitional state from wake to sleep, characterized by the disappearance of 8–12 Hz (alpha) waves from the EEG and the appearance of >0.5-s slow, rolling eye movements (Iber et al. 2007). N2 is defined by the presence of isolated K-complexes—sharp negative waves followed by a positive component lasting >0.5-s—and sleep spindles. N3 is defined by the presence of large (>75-μV peak to peak), slow (1–4 Hz) waves occupying at least 20% of each 30-s epoch. (b) A typical sleep spindle recorded from a scalp EEG sensor. Abbreviations: EEG, electroencephalogram; REM, rapid eye movement.
3.2. Neurophysiological characteristics of individual sleep stages set the stage for differential memory processing
Sleep stages differ in their spectral content (pattern, amplitude, and frequency of electrophysiological brain activity). During REM sleep 5–10-Hz theta activity is dominant in the hippocampus and is hypothesized to facilitate the transfer of information from hippocampus to neocortex (Poe et al. 2000). During NREM sleep, cortically generated slow oscillations (0.5–1 Hz) and slow waves (1–4 Hz), thalamic sleep spindles (11–16 Hz) (Figure 2b), and hippocampal sharp-wave ripples (100–200 Hz) act in concert to mediate memory consolidation (Latchoumane et al. 2017) (see Section 7). Slow oscillations and slow waves are also thought to mediate synaptic homeostasis, reversing the daytime strengthening of cortical synapses to facilitate new learning the following day (Tononi & Cirelli 2006). Synaptic down-selection, a refinement of the synaptic homeostasis hypothesis, suggests that homeostatic mechanisms may selectively weaken spurious synapses and thereby increase the signal-to-noise ratio of memory representations (Nere et al. 2013), although this interpretation has been questioned (Frank 2012). Although the molecular and synaptic mechanisms remain unknown, it is clear that sleep supports mechanisms of brain plasticity important for memory evolution.
3.3. Memory triage determines which memories endure
Not all memories endure, some rapidly fade. If a memory is to endure, it must survive from the time of encoding until the next sleep period and then undergo sleep-dependent consolidation. The brain is hypothesized to use a process of memory triage to identify those memories that are important enough to retain (Stickgold & Walker 2013). However, major questions about this process remain: First, when are memories tagged for retention—during wake, sleep, or both? Animal studies suggest that memories are selected for retention by synaptic tagging with plasticity-related proteins immediately after learning (Cassini et al. 2013). Second, what determines which memories are selected for retention? This may depend on a calculation of their future relevance. For example, postlearning instructions informing participants that the following day they will be rewarded for accurate recall of a subset of the materials they learned lead to selectively enhanced sleep-dependent consolidation of those materials (Rauchs et al. 2011). Third, once selected, how are memories retained throughout the day for subsequent processing during sleep? Recent studies suggest that synchronized brain activity within the networks that were involved in learning retains new memories across the day, outside of awareness. Functional magnetic resonance imaging (fMRI) studies show that learning a task changes this wakeful resting state activity in task-relevant networks (Albert et al. 2009), and that these changes predict better recognition of the learned material later that same day (Tambini et al. 2010) and even predict enhancement of memory after a night of sleep (Gregory et al. 2014). For example, learning a motor procedural memory task increased resting state connectivity (a measure of the synchronization of neural activity) in the motor network immediately after learning, and this increase predicted the amount of sleep-dependent performance improvement tested the following day (Gregory et al. 2014). These findings suggest that resting state activity retains memories of recent experiences over the course of the day for later processing during sleep. Finally, how is the form of sleep-dependent memory evolution determined? Sleep can either stabilize encoded memories in their original form, as it does with word pairs (Baran et al. 2018), or forego details and instead extract the gist, as it does for word lists (Payne et al. 2009). How the brain selects one form of processing over another is still completely unknown (Stickgold & Walker 2013).
4. Abnormal sleep is a key feature of schizophrenia
Neuropsychiatric disorders are primarily defined by waking phenomena, but sleep disturbances are a prominent feature. While usually viewed as secondary, sleep deprivation can precipitate psychosis and trigger or aggravate a range of disorders, and treating sleep can improve both symptoms and cognition (for a review, see Manoach & Stickgold 2009). This suggests that abnormal sleep is not merely epiphenomenal but can directly contribute to neuropsychiatric disorders. In schizophrenia, sleep disturbances have been described since Kraepelin (1919). They are common throughout the course of illness, including in individuals with prodromal symptoms (Lunsford-Avery et al. 2013). They are also reported in non-psychotic first degree relatives (Keshavan et al. 2004). Sleep disturbances often herald the initial onset of psychosis and predict relapse in remitted patients (Dencker et al. 1986). Reports of sleep disturbances in unmedicated and antipsychotic-naïve patients (Chouinard et al. 2004) indicate that disturbed sleep is not merely a medication side effect. In fact, APDs tend to normalize sleep (Krystal et al. 2008), and withdrawal is associated with a progressive deterioration of sleep quality (Nofzinger et al. 1993), which in turn is associated with increased positive symptoms (Chemerinski et al. 2002) and relapse (Dencker et al. 1986).
The most common subjective sleep disturbance in schizophrenia is insomnia (i.e., difficulty initiating and maintaining sleep) (see Chouinard et al. 2004). Polysomnographic (PSG) studies—that is, sleep electroencephalograms (EEG) taken together with recordings of eye movements and muscle tone—often show worse sleep quality in terms of reduced sleep efficiency (the fraction of time in bed spent asleep), increased sleep onset latencies, and increased wake time after sleep onset (for meta-analyses see, Benca et al. 1992, Chan et al. 2017, Chouinard et al. 2004). These studies also document diverse abnormalities of sleep architecture (i.e., the distribution of time spent in different sleep stages). Medicated and APD-naïve schizophrenia patients as well as first-degree relatives show N3 abnormalities, including reduced duration and power of the large delta waves (~1–4 Hz) that characterize N3 (for review, see Manoach & Stickgold 2009). Reduced duration and latency of REM sleep are also reported, but although one meta-analysis has found N3 and REM abnormalities (Chan et al. 2017), others have not found systematic differences in schizophrenia patients compared with healthy or psychiatric controls (Benca et al. 1992, Chouinard et al. 2004).
There are also reports of altered circadian rhythms and increased rates of sleep disorders, including obstructive sleep apnea, movement disorders, parasomnias, and hypersomnolence (for a review, see Benson 2015). In summary, despite the long association of various forms of disturbed sleep with schizophrenia, it has been difficult to pinpoint the exact nature of the problem and its relation to pathophysiology, cognitive deficits, and symptoms. Inconsistent findings may stem from sample differences (e.g., early-course versus chronic patients), inconsistent definitions and measurements of sleep parameters, and, perhaps most importantly, heterogeneity in the underlying pathophysiology. More recent studies have gone beyond measures of sleep quality and architecture to examine the spectral content of sleep. A relatively consistent literature has now emerged showing a specific reduction of sleep spindles in schizophrenia (for a review, see Manoach et al. 2016). Sleep spindles, a defining oscillation of N2 sleep, are seen in the EEG as brief (~1 s) powerful bursts of 11–16 Hz activity organized in a waxing/waning envelope (Figure 2b). Compelling evidence from both correlational and manipulation studies supports an important role for spindles in memory evolution. In schizophrenia, sleep spindle deficits have been associated with impaired sleep-dependent consolidation of both procedural and declarative memory.
5. Sleep spindles mediate memory evolution
5.1. Sleep spindles are a key facilitator of the synaptic plasticity involved in memory
In experimental models, spindle-like activity induces massive influxes of calcium ions into cortical pyramidal cells and triggers the intracellular mechanisms that are involved in long-term potentiation, a neural mechanism of memory (Sejnowski & Destexhe 2000). In humans, spindles correlate with the sleep-dependent consolidation of both procedural and declarative memory (for a review, see Fogel & Smith 2011). EEG, magnetoencephalography, intracortical electrocorticography, and simultaneous fMRI and EEG studies show that learning a task induces changes in sleep spindle activity specifically in the regions that were involved in learning, and that these changes correlate with sleep-dependent memory evolution (Bang et al. 2014, Clemens et al. 2006, Johnson et al. 2012, Nishida & Walker 2007, Tamaki et al. 2013). These findings suggest that spindle activity in brain networks involved in learning mediates the reactivation, transformation, and strengthening of memory traces acquired during the day.
5.2. Sleep spindles have a more general role in cognition
Based on their correlations with a variety of measures of learning ability and IQ (for a review, see Fogel & Smith 2011), spindles have been labeled “a biological marker of human intelligence” (Fang et al. 2017, p. 167). The direction of causality in these relationships is unclear. They may be mediated by memory: Individuals with more spindles and better sleep-dependent memory may score higher on IQ tests. Alternatively, higher IQ could lead to more learning, which could increase spindles, or, because sleep spindles protect sleep from disruption by external stimuli (Dang-Vu et al. 2010), they may enhance sleep quality, which could lead to better cognitive function (Nebes et al. 2009). Findings that spindles correlate with reasoning ability even when circadian and sleep quality effects are statistically controlled suggest that this is unlikely to be the whole story (Fang et al. 2017). Finally, the relation of spindles with intelligence may be due to a third factor such as the integrity of the thalamic reticular nucleus (TRN), which both initiates spindles and gates the relay of sensory information to the cortex to enhance information processing. Investigating the mechanisms underlying these relations could enhance our understanding of the neural bases of intelligence.
6. Sleep spindle deficits impair memory evolution in schizophrenia
6.1. A growing literature documents spindle deficits in schizophrenia
Chronic medicated patients show reduced spindle density (spindles/min) (see Ferrarelli et al. 2007, 2010; Manoach et al. 2010) that correlates with impaired sleep-dependent memory consolidation (Goder et al. 2015, Wamsley et al. 2012). Spindle deficits are also seen in medicated adolescents with early-onset schizophrenia spectrum disorder (Tesler et al. 2015). Importantly, except for two studies reporting increased sleep onset latency (Ferrarelli et al. 2007, 2010), research shows that the spindle deficit occurrs in the context of normal sleep quality and architecture, demonstrating that, at least in chronic medicated patients, it is not secondary to sleep disruption.
6.2. Spindle deficits in schizophrenia are not due to antipsychotic drugs or sleep disruption
The effects of APDs on sleep spindles have seldom been studied. Whereas a single dose of olanzapine in schizophrenia reduced spindle density (Goder et al. 2008), acute administration of haloperidol to healthy participants did not affect spindle density (Hirshkowitz et al. 1982). There are no studies of long-term treatment. Findings of normal spindle activity in other psychiatric groups taking APDs (Ferrarelli et al. 2010) and of spindle deficits in early course APD-naïve schizophrenia and in young nonpsychotic first-degree relatives of patients with schizophrenia (D’Agostino et al. 2018, Manoach et al. 2014) indicate that the spindle deficit is not a side effect of APDs. Unlike chronic patients, however, relatives and early-course patients exhibit spindle deficits in the context of disrupted sleep quality and architecture. Two findings demonstrate that reduced spindle activity is not secondary to sleep disruption or psychosis in these groups: (a) Early-course psychotic patients with diagnoses other than schizophrenia had disrupted sleep but no spindle deficits (Manoach et al. 2014), and (b) spindle deficits have been observed in first-degree relatives of schizophrenia patients in the context of normal sleep quality and architecture (Schilling et al. 2016). In summary, the spindle deficit in schizophrenia is not due to APDs, sleep disruption, psychosis, or chronicity and may instead be an endophenotype (i.e., a trait indicating genetic vulnerability) of schizophrenia that contributes to cognitive dysfunction (Figure 3) (see Gottesman & Gould 2003).
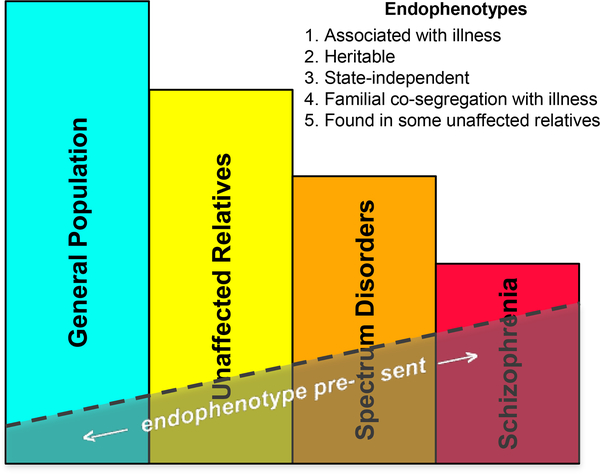
Figure 3.
A schematic illustration of the endophenotype concept in psychiatry. Endophenotypes are measurable traits, invisible to the unaided eye, along the pathway between disease and genotype (see Figure 7). They are simpler manifestations of the genetic underpinnings of a disorder than the syndrome itself. Shaded areas indicate the expected presence of an endophenotype (e.g., sleep spindle deficits) in individuals with schizophrenia, those with spectrum disorders, syndromally unaffected family members, and the general population. Existing evidence shows that sleep spindle deficits meet criteria 1, 2, and 5. Although spindles are trait-like across nights in studies of healthy individuals (e.g., Cox et al. 2017), stability of the deficit in longitudinal studies of individuals with schizophrenia across prodromal, psychotic, and remitted states would satisfy criterion 3. No study to date has addressed criterion 4. Definition and criteria adapted from Gottesman & Gould (2003).
6.3. Sleep spindle deficits are associated with impaired memory evolution in schizophrenia
Early studies of sleep-dependent memory evolution in schizophrenia used the finger-tapping motor sequence task (MST) (Figure 4a) (see Karni et al. 1998), a well-validated probe of sleep-dependent motor procedural memory. In healthy individuals, significant MST performance improvements occur after periods of sleep but not wake, and they correlate with both the duration of N2 sleep (Walker et al. 2002) and sleep spindle density (Albouy et al. 2013, Nishida & Walker 2007). In contrast, as a group, chronic medicated patients with schizophrenia fail to show significant overnight improvement despite a normal rate and amount of learning during training (Figure 4b) (see Manoach et al. 2004). Later studies replicated the failure of overnight MST improvement in schizophrenia (Genzel et al. 2015, Manoach et al. 2010) and found that it correlated with reduced sleep spindles (Figure 4c) (see Wamsley et al. 2012). Reduced sleep-dependent motor procedural memory in schizophrenia in the context of reduced spindle density has also been demonstrated using a mirror-tracing task (Seeck-Hirschner et al. 2010). Importantly, in these studies, participants with schizophrenia showed intact learning during training, implicating memory consolidation rather than initial learning in the reduced overnight improvement.
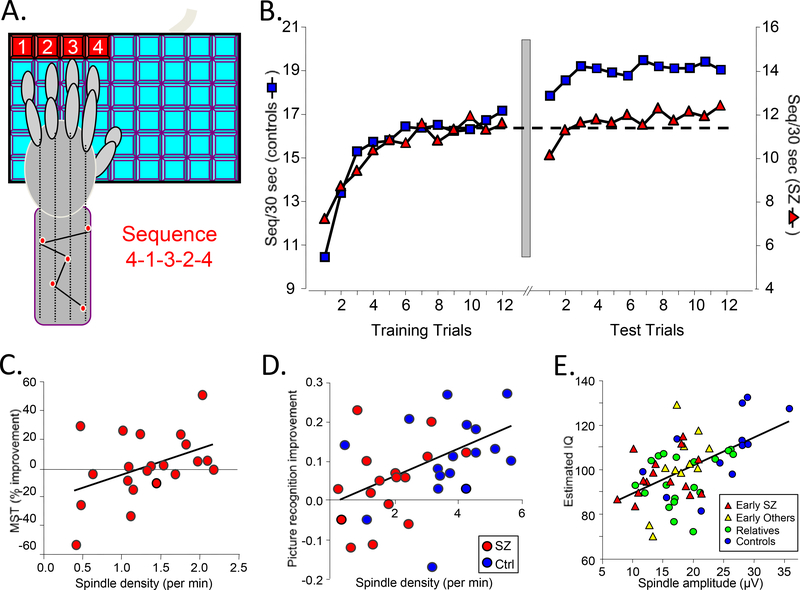
Figure 4.
Sleep in relation to cognition in schizophrenia. (a) The finger-tapping motor sequence task (MST) requires participants to repeatedly type a five-digit sequence (e.g., 4–1-3–2-4) on a keyboard with the left hand, as quickly and accurately as possible, for 12 30-s trials separated by 30-s rest periods. Participants train before sleep and test on an additional 12 trials after sleep. The primary outcome measure is overnight improvement, calculated as the percent increase in correctly typed sequences from the last three training trials to the first three test trials (Walker et al. 2002). (b) MST performance in schizophrenia (Manoach et al. 2004). The y axis is scaled separately for controls (left) and patients (right) to highlight the similarity of learning curves on day 1 and the failure of overnight improvement in the schizophrenia group only. The dashed line is positioned at the mean value of the last three training trials for both the control and patient groups. The break between the plots represents the passage of 24 hours, including a night of sleep. Patients and controls did not differ in the amount of learning during training, but only controls showed significant overnight improvement. (c) Sleep-dependent improvement in schizophrenia correlates with spindle density during N2 sleep in the posttraining night (r = 0.45, p = 0.04) (Wamsley et al. 2012). (d) Declarative memory evolution and sleep spindles. Compared to a day of wake, a night of sleep resulted in a 12% improvement in picture recognition accuracy (p < 0.001) in healthy participants. Schizophrenia patients showed no sleep-dependent benefit, and despite having comparable sleep architecture, they showed a 54% reduction in spindle density (p < 0.001). In both groups, the sleep-dependent change in recognition accuracy correlated with spindle density (Goder et al. 2015). (e) Regressions of estimated verbal IQ against spindle amplitude for early-course antipsychotic-naïve patients with schizophrenia, those with other psychotic disorders, young first-degree relatives of schizophrenia patients, and healthy controls (Manoach et al. 2014).
More recent work extends the sleep-dependent memory consolidation deficit in schizophrenia to declarative memory, tested with both picture recognition (Goder et al. 2015) and word-pair recall tasks (Baran et al. 2018). Compared to a day of wake, a night of sleep improved picture recognition accuracy in healthy participants but not schizophrenia patients, who, despite having comparable sleep architecture, showed reduced spindle density. In both groups, the sleep-dependent change in recognition accuracy correlated with spindle density (Figure 4d). Similarly, on a cued word-pair recall task, a night of sleep allowed controls, but not patients, to maintain their memory.
Spindle deficits also correlate with poorer executive function, worse memory, and lower IQ (Figure 4e) in APD-naïve, early course patients with both schizophrenia and other psychotic disorders, as well as in nonpsychotic first-degree relatives of schizophrenia patients (see Manoach et al. 2014, Schilling et al. 2017), supporting a more general role of spindles in cognitive deficits.
A limitation of these studies is that their small sample sizes may leave them underpowered to detect meaningful effects and may contribute to inconsistent findings. For example, in one MST study the correlation between spindle density and overnight improvement in schizophrenia was significant (r21 = 0.45, p = 0.04) (Wamsley et al. 2012), but in a smaller study with a similar effect size it was not (r14 = 0.46, p =0.10) (Manoach et al. 2010). The large basic literature showing robust correlations of spindle density with both procedural and declarative memory and a range of cognitive measures including IQ (Fogel & Smith 2011), together with the finding that manipulating spindles affects memory in animals and humans (see Section 9), support the hypothesis of a causal role for spindle deficits in impaired sleep-dependent memory consolidation in schizophrenia. In summary, there is growing evidence that spindle deficits contribute to cognitive dysfunction in APD-naïve, early-course, and chronic medicated patients with schizophrenia as well as their first-degree relatives.
7. Spindle deficits in schizophrenia implicate thalamic reticular nucleus and thalamocortical circuit dysfunction
7.1. Sleep spindles are initiated in the Thalamic Reticular Nucleus, which is abnormal in schizophrenia
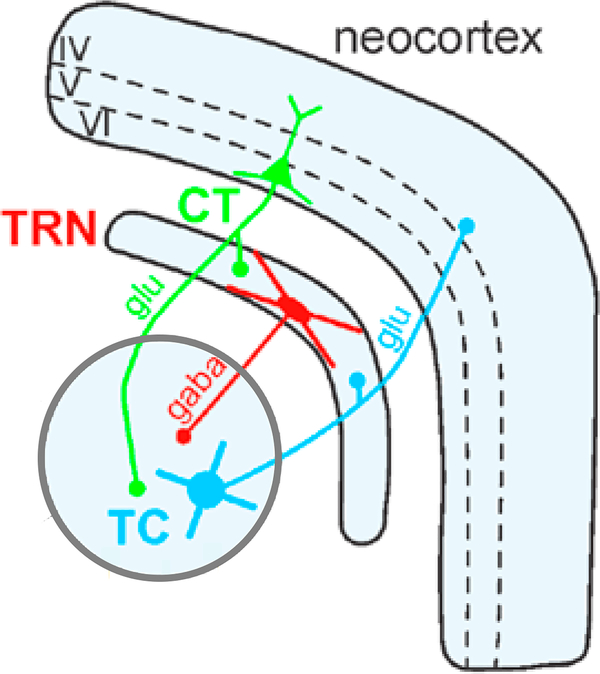
The thalamus is the gateway by which information from the senses reaches the cortex, and the TRN is considered the guardian of that gateway (Crick 1984). The TRN is a thin net-like structure that is strategically positioned between other thalamic nuclei and the cortex, which enables it to modulate thalamocortical communication (Figure 5). The TRN, which is comprised entirely of gamma-amino butyric acid (GABA) neurons, is the major inhibitory nucleus of the thalamus. It powerfully inhibits excitatory glutamatergic thalamic projection neurons to gate the relay of information to the cortex during wake and to initiate spindles during sleep (Pinault 2004). Sleep spindle initiation by the TRN depends on powerful and prolonged inhibition of thalamocortical neurons (Steriade 2003) followed by rebound spike bursts that entrain cortical neurons to oscillate at spindle frequency (Contreras & Steriade 1996). The TRN, in turn, receives modulatory input from the thalamus and cortex via collaterals from thalamocortical and corticothalamic neurons. Whereas the thalamus can generate sleep spindles in isolation, their propagation and synchronization across the cortex require reciprocal interactions within thalamocortical feedback loops (Contreras et al. 1996). Thus, the expression of sleep spindles depends on glutamatergic and GABAergic neurotransmission within thalamocortical circuitry, which are implicated in current pathophysiological models of schizophrenia. In schizophrenia, postmortem studies reveal TRN abnormalities, including decreased nicotinic receptor binding (Court et al. 1999), increased expression of excitatory amino acid transporters (Smith et al. 2001), and reductions in parvalbumin-expressing neurons and perineuronal nets—extracellular structures that support, modulate, and protect neurons (Steullet et al. 2017). A consequent impairment of TRN-mediated inhibition of thalamocortical neurons in schizophrenia could decrease the rebound bursting that generates spindles and impair the gating of information flow to the cortex.
Figure 5.
TRN circuitry for generating and synchronizing sleep spindles. The TRN, a net-like nucleus that sits between the rest of the thalamus and the neocortex, modulates thalamocortical communication. The TRN receives projections from thalamocortical and corticothalamic neurons. GABAergic TRN neurons inhibit thalamocortical relay neurons. Glutamatergic corticothalamic neurons send projections back to the TRN and other thalamic nuclei. Roman numerals indicate cortical layers. Abbreviations: GABA, gamma-amino butyric acid; Glu, glutamate; TRN, thalamic reticular nucleus. Figure adapted with permission from Pinault (2004).
7.2. TRN-mediated thalamocortical circuit abnormalities may contribute to the waking manifestations of schizophrenia
The TRN also plays an important role in waking cognition, acting as an “attentional searchlight” (Crick 1984, p. 4,586) that selectively intensifies or suppresses the flow of information to specific cortical regions. Rather than being a monolithic structure, the TRN is comprised of sectors that receive distinct inputs from the thalamus and cortex and have distinct projections to thalamic nuclei (Pinault 2004, Zikopoulos & Barbas 2012). It is the TRN neurons that project to sensory rather than limbic thalamic nuclei that participate in spindle generation, inhibit sensory processing during sleep, and augment sensory processing during attention-demanding tasks (Halassa et al. 2014). Impaired TRN-mediated inhibition of thalamocortical neurons in schizophrenia might result in increased and less filtered forwarding of sensory information during wake and a consequent fragmentation of attention.
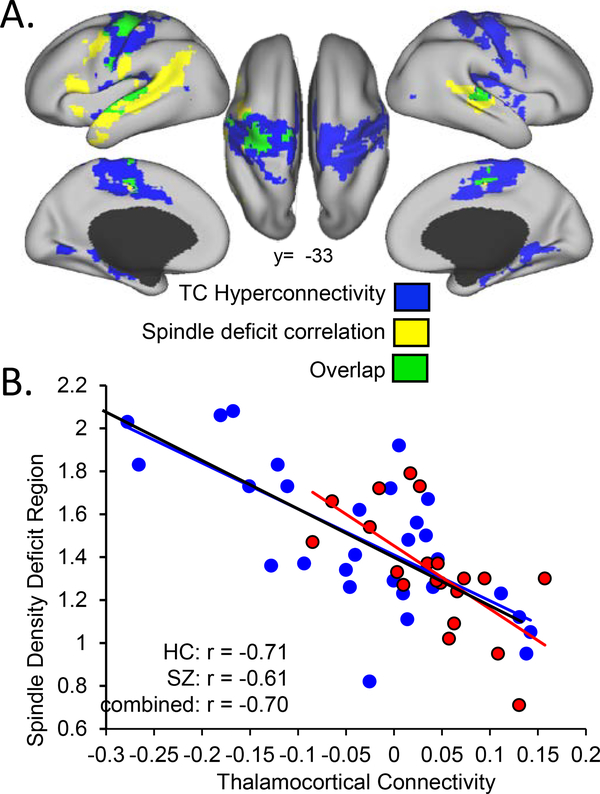
Resting-state functional connectivity MRI (rs-fcMRI) studies provide indirect evidence of increased thalamocortical information flow in schizophrenia. Such studies consistently report increased functional connectivity between the thalamus and motor and sensory cortex in schizophrenia (Anticevic et al. 2014, Ferri et al. 2018, Klingner et al. 2014, Skatun et al. 2017, Woodward et al. 2012) and in individuals at clinical high risk for schizophrenia, in whom it predicts conversion to illness (Anticevic et al. 2015). Recent work shows that this hyperconnectivity in schizophrenia correlates with reduced spindle density (Figure 6) (see Baran et al. 2016). Like prior studies, this study found thalamic hyperconnectivity with motor and somatosensory cortex, a selectivity that may reflect the anatomical organization of thalamocortical circuits, which can be divided into thalamic matrix versus core pathways (Jones 1998). Matrix neurons are widespread throughout the thalamus and project diffusely to multiple cortical regions. Core neurons, in contrast, have restricted, topographically organized cortical projections and are the primary input to sensory and motor regions. Core neurons are thought to initiate focal spindles in sensory and motor regions, which have been associated with memory consolidation (Clemens et al. 2006, Johnson et al. 2012), whereas matrix neurons are thought to play a greater role in initiating widely distributed spindles and in synchronizing spindles across the cortex (Bonjean et al. 2012). Thus, thalamocortical hyperconnectivity in motor and sensory regions and a correlated reduction in spindle density in schizophrenia are most consistent with abnormalities of the core pathway.
Figure 6.
Spindle deficits correlate with TC hyperconnectivity in schizophrenia. (a) Blue regions show significant TC hyperconnectivity in schizophrenia and include motor and somatosensory cortex. Yellow regions show a correlation of TC connectivity with average spindle density. Green regions show both significant hyperconnectivity in schizophrenia and a correlation of connectivity with average spindle density. (b) Average spindle density is plotted against TC connectivity in motor and somatosensory regions showing a significant inverse correlation. There were no regions of significantly positive correlation, and the slopes of the relations did not differ by group. Abbreviations: HC, healthy controls; SZ, schizophrenia; TC, thalamocortical.
Both thalamocortical hyperconnectivity (Avram et al. 2018, Ferri et al. 2018) and spindle deficits (Ferrarelli et al. 2010, Manoach et al. 2014, Wamsley et al. 2012) have also been shown to correlate with positive symptoms including hallucinations, which reflect difficulty distinguishing externally from internally generated auditory stimuli. Other signs of schizophrenia are consistent with defective TRN modulation of thalamocortical information flow (Pinault 2004, Vukadinovic 2011) including deficient sensory gating, which may result in sensory overload (Clementz et al. 1998), impaired attentional modulation, and abnormal corollary discharge (i.e., reduced suppression of sensations resulting from one’s own actions) (see Mathalon & Ford 2008). In healthy young people, reduced spindle density correlates with both elevated scores on scales of psychosis proneness and increased thalamic glutamine/glutamate levels, further supporting mechanistic links between spindles, heightened thalamic excitation, and psychosis risk (Lustenberger et al. 2015). Collectively, these findings are consistent with the hypothesis that TRN dysfunction in schizophrenia contributes to spindle deficits during sleep and to signs, symptoms, and cognitive dysfunction during wake. If this is true, interventions targeting TRN dysfunction could improve both cognitive deficits and symptoms. To realize this goal, it is necessary to understand the mechanisms of TRN dysfunction.
8. Thalamic Reticular Nucleus and spindle abnormalities in schizophrenia may have genetically mediated neurodevelopmental origins
8.1. Spindles are a heritable component of the sleep electroencephalogram
Genetic studies provide unprecedented opportunities to advance our understanding of spindle deficits and can guide the development of treatments targeted to specific mechanisms. EEG spectral power in the 11–16-Hz sigma band, which corresponds to sleep spindles, is highly heritable based on twin studies, and it shows both high interindividual variability and within-individual stability, leading to its description as an “electrophysiological fingerprint” (Ambrosius et al. 2008, De Gennaro et al. 2008). Based on 730 participants in a familial study of sleep (Dean et al. 2016), 33–48% of the variances in 12–15-Hz spindle density, frequency, and amplitude were genetically determined (Purcell et al. 2017). Despite this evidence of a strong genetic contribution to sleep spindles, relatively little is known about their genetic underpinnings.
8.2. Recent studies have identified risk genes for neurodevelopmental disorders that may contribute to spindle deficits and illuminate their mechanisms
CACNA1I, a risk gene for schizophrenia that is implicated in both common (Schizophr. Work. Group Psychiatr. Genom. Consort. 2014) and rare (Gulsuner et al. 2013) variation, encodes a calcium channel (Cav3.3) that is expressed only in the brain and preferentially in the TRN and hippocampus (see Allen Mouse Brain Atlas, at http://mouse.brain-map.org, and GTex, at http://www.gtexportal.org). Cav3.3 is the major spindle pacemaker in the thalamus, and mouse CACNA1I knockouts have a specific spindle deficit (Astori et al. 2011). One de novo CACNA1I mutation found in schizophrenia disrupts Cav3.3 channel activity in the TRN. In an experimental model, this disruption reduced the rebound burst firing necessary for spindle generation, suggesting Cav3.3 channel activity as a potential treatment target (Andrade et al. 2016).
Similarly, PTCHD1, which is mutated in ~1% of individuals with autism spectrum disorder and intellectual disability, is selectively expressed in the TRN early in postnatal development. Deletion of PTCHD1 in mouse TRN causes spindle deficits and learning impairment. Importantly, these deficits can be rescued by drugs that correct signaling in the affected channel, identifying another potential treatment target for ameliorating spindle deficits (Wells et al. 2016).
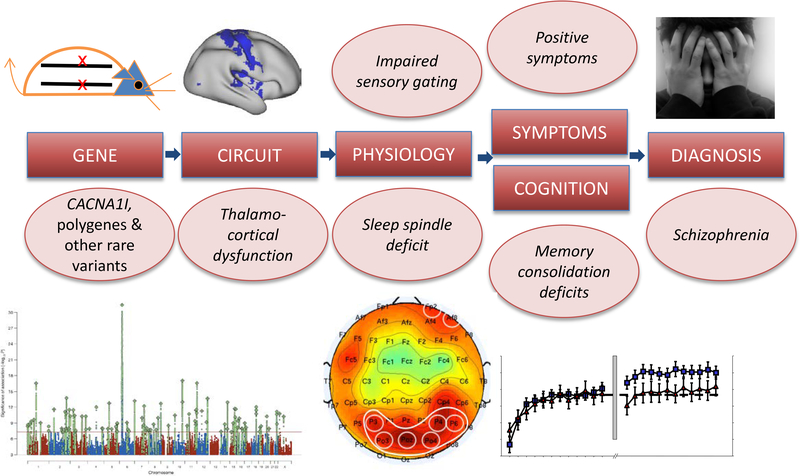
These studies show that genetic variation associated with neurodevelopmental disorders affects TRN function and results in physiological and behavioral phenotypes reminiscent of those seen in humans with these disorders and are potentially treatable. This evidence places reduced sleep spindle activity, a heritable component of the sleep EEG, in a hypothetical causal chain from risk genes to diagnosis (Figure 7).
Figure 7.
From genes to diagnosis: a hypothetical causal chain. Risk genes result in thalamic reticular nucleus–mediated thalamocortical dysfunction, which, in turn, gives rise to candidate endophenotypes of schizophrenia including sleep spindle and sensory gating deficits, which contribute to fundamental cognitive deficits, symptoms, and ultimately diagnosis. Illustrations from left to right represent a strand of DNA, a Manhattan plot identifying risk genes, thalamocortical hyperconnectivity, a topographic electroencephalogram map of reduced spindle density, and the sleep-dependent memory consolidation deficit.
8.3. The Thalamic Reticular Nucleus and spindle activity participate in the development and refinement of thalamocortical circuitry
Genetic variation affecting the TRN during neurodevelopment could predispose to schizophrenia. Rodent studies show that during gestation, axons that pass between the cortex and the thalamus all pass through the TRN, which helps guide them to their terminations (Mitrofanis & Guillery 1993). As early as the first postnatal week, spindle bursts—precursors of adult sleep spindles that are similar in shape, frequency, and origin (Lindemann et al. 2016)—appear and refine reciprocal thalamocortical glutamatergic connections to somatosensory and motor cortex (Cirelli & Tononi 2015). Although causal experiments are needed to examine the effects of perturbing migration and synaptic refinement on the establishment of thalamocortical circuitry, one might speculate that by disrupting the development of this circuitry, genetically mediated TRN abnormalities could set the stage for neurodevelopmental disorders.
9. Sleep oscillations are modifiable targets for improving cognition, but timing matters
9.1. Recent work supports a causal role for spindles in memory consolidation
Although most data linking sleep spindles to cognition are correlational, there is a burgeoning literature in humans suggesting a causal role for spindles. Increasing spindles or sigma power either pharmacologically via zolpidem (Kaestner et al. 2013, Mednick et al. 2013) or via transcranial stimulation (Del Felice et al. 2015, Marshall et al. 2006) improves sleep-dependent memory consolidation (for a meta-analysis see, Barham et al. 2016), including for the MST (Lustenberger et al. 2016), whereas stimulation that decreases sigma power impairs memory (Marshall et al. 2011). This work provides an impetus to develop novel therapies for spindle deficits to improve cognition.
A few studies have attempted to improve cognition in schizophrenia by manipulating spindles. In a small sample of patients, transcranial stimulation during N2 did not significantly alter sleep parameters but improved word-list recall (Goder et al. 2013). In another study that did not include PSG, the sleep drug eszopiclone (Lunesta) improved working memory in schizophrenia but not symptoms (Tek et al. 2014). Eszopiclone prolongs inhibitory postsynaptic currents through GABAA receptors on TRN neurons, which increases the burst firing necessary for spindle initiation (Jia et al. 2009). Our group has examined the effects of eszopiclone on spindles and sleep-dependent memory consolidation using the MST in schizophrenia. An initial study showed that eszopiclone significantly increased spindles in schizophrenia, but it may have been underpowered to detect an effect on memory (Wamsley et al. 2013). In a larger randomized clinical trial, eszopiclone again significantly increased spindles, and spindles correlated with memory; disappointingly, however, eszopiclone failed to improve memory in either patients or healthy controls (Baran et al. 2017). Recent findings may explain this failure.
9.2. Sleep-dependent memory consolidation relies not only on spindles but also on their precise temporal coordination with cortical slow oscillations and hippocampal sharp-wave ripples
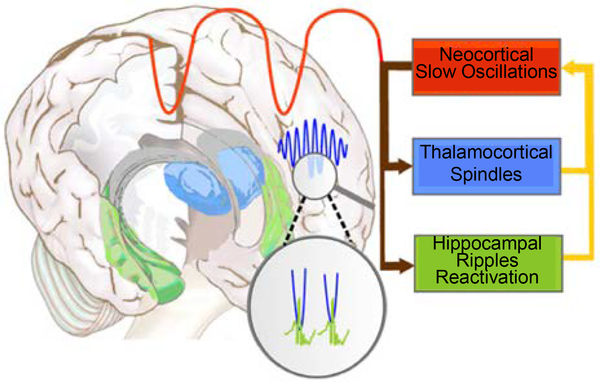
Sleep spindles do not act in isolation to mediate memory. During NREM sleep, cortical slow oscillations coordinate spindle generation in the TRN and the reactivation of memory representations during high frequency (100–200 Hz) hippocampal sharp-wave ripples (Ji & Wilson 2007, Wilson & McNaughton 1994). Hippocampal ripples nest in the troughs of spindles, which preferentially occur during the up states of slow oscillations (Figure 8). The orchestration of these three cardinal oscillations of NREM sleep is thought to support the transfer of new memories from temporary dependence on the hippocampus to longer-term representation in the cortex (Born & Wilhelm 2012, Siapas & Wilson 1998). The importance of this coordination for memory is supported by recent findings of both human and animal studies. In mice, optogenetic induction of spindles on the rising phase of slow oscillations leads to enhanced memory consolidation, whereas suppression of spindles at this phase impairs consolidation (Latchoumane et al. 2017). In older people, the coupling of slow oscillations with spindles degrades, and this reduction is associated with increased forgetting (Helfrich et al. 2018). Finally, in schizophrenia, although the coupling of spindle and slow oscillations appears to be intact, both the number of spindles and their temporal coordination with slow oscillations predict MST memory consolidation. Together, spindle density and timing in relation to slow oscillations predict memory consolidation significantly better than either variable alone, suggesting that both are important for memory consolidation (Demanuele et al. 2017). This body of work suggests that to improve memory in schizophrenia, an intervention needs to not only increase spindles but also to preserve or enhance slow oscillations and hippocampal ripples and their temporal coordination with spindles. It would be beneficial to characterize drug effects on these three oscillations and on memory prior to embarking on large, expensive, and lengthy clinical trials. Because hippocampal ripples are difficult to measure noninvasively in humans, drug screening will require complementary animal and human studies.
Figure 8.
The coordination of sleep spindles with hippocampal ripples and neocortical slow oscillations in the service of consolidating new memories during sleep. During non-rapid eye movement sleep, neocortical slow oscillations drive the reactivation of hippocampal memory representations during sharp-wave ripples in the hippocampus together with spindles in the thalamic reticular nucleus. Hippocampal ripples nest in the troughs of spindles, which occur during the up states of slow oscillations. This dialogue between slow oscillations, spindles, and hippocampal ripples is thought to mediate the transfer of selected new memories from temporary dependence on the hippocampus to longer-term representation in the neocortex (Siapas & Wilson 1998). Figure adapted with permission from Born & Wilhelm (2012).
10. Methodological and measurement challenges in sleep spindle research
10.1. Spindle definitions vary
Whereas the fundamental definition of sleep spindles as brief (~1 s) oscillations triggered by the TRN is unquestioned, there is debate about how they should be studied. This is in part due to historical reasons: Sleep spindles are defined in the American Academy of Sleep Medicine sleep scoring manual as distinct human scalp EEG “waves with frequency 11–16 HZ (most commonly 12–14 Hz with a duration ≥0.5 sec” (Iber et al. 2007, p. 23). Several features of this definition are noteworthy. Sleep spindles are defined as EEG events (i.e., without reference to their brain origin) based on visual recognition of the waveforms filtered at 0.3–35 HZ. Thus, spindle detection requires relatively high amplitude waveforms in the absence of excessive interference from higher-frequency activity. Whereas the minimum duration is 0.5 s, no upper limit is specified.
In current practice, EEG frequency ranges assigned to spindles vary substantially from 9 to 16 Hz. In addition, spindles are often divided into fast spindles, which dominate in central and parietal electrodes, and slow spindles, expressed most strongly in frontal electrodes. Again, definitions vary, and slow spindle ranges may not even overlap (e.g., 9–12 Hz in Ayoub et al. 2013 and 12–13.5 Hz in Wamsley et al. 2013. Some researchers have determined fast and slow spindle frequency ranges on a per-subject basis, given the considerable variability across individuals (Cox et al. 2017). Because spindle frequency ranges are based on scalp EEG, none has the face validity that could be obtained from concurrent thalamic recordings. Similar issues arise when looking at the relations of spindles with slow oscillations. The latter can be called either slow waves or slow oscillations, and they have been variably defined as <1 Hz (Molle et al. 2011), <1.25 Hz (Helfrich et al. 2018), or even 0.5–4 Hz (Demanuele et al. 2017). These different definitions complicate comparison across studies.
10.2. There is no agreed-upon method for quantifying spindle activity
Once a spindle frequency range has been chosen, the next issue is how to identify and count spindles. The classic method is hand counting, usually using EEG channels C3, C4, or both. The EEG may be displayed with standard filtering (e.g., 0.3–35 Hz) (see Iber et al. 2007) or after filtering specifically for spindle activity (e.g., 12–16 Hz). The latter method removes much extraneous activity and allows the detection of spindles with substantially lower amplitude. Hand counting requires training, is time consuming, is not feasible for high-density EEG arrays, and results in large interscorer variability, even among sleep experts (Warby et al. 2014). More recently, spindles have been counted using automated computer algorithms, which also produce substantially different outcomes (Warby et al. 2014). One solution to the problem of identifying and counting spindles is to instead quantify the spectral power within the spindle (sigma) frequency band. A more sophisticated approach involves the use of multitaper spectral analysis, which eliminates the need for sleep staging and permits the quantification of sigma activity in time-frequency plots of the EEG (Prerau et al. 2017).
10.3. Spindle activity changes dramatically across the lifespan
Another source of variation in spindle measurement is normal developmental changes, which are thought to reflect the maturation and later the development or disruption of thalamocortical regulatory mechanisms (Clawson et al. 2016). In a study of 11,630 individuals aged 4 to 97 years (Purcell et al. 2017), spindle density increased minimally (4%) between age 4 and its peak at age ~20, and then it declined almost by half (~45%) over the next 60 years. Spindle topography and morphology also change. For example, peak sigma frequency increased from early childhood into adolescence from a modal value of 12.0 Hz at 10 years to13.5 Hz at age 20, and then it stabilized at 14.0 Hz for ages 30–70 (Purcell et al. 2017). Across development, the correlations of spindle parameters with cognitive ability, learning, and memory also vary. For example, middle-aged and older adults show overnight MST improvement, but unlike what is seen in young adults, improvement does not correlate with spindle number or density (Tucker et al. 2011, Wamsley et al. 2012). Whether and how the developmental trajectory of spindle expression and function is altered is schizophrenia are open questions.
10.4. Adding magnetoencephalography to electroencephalography provides a more complete characterization of spindles
Based on their biophysical characteristics, MEG and EEG are differentially sensitive to spindles (Dehghani et al. 2010, 2011). Only about half the spindles detected by MEG are also seen in EEG, and MEG misses about 15% of spindles detected by EEG (see Dehghani et al. 2011). Spindles detected by MEG alone tend to have a focal onset and not to spread across the cortex. In contrast, spindles seen in both modalities are detected earlier in MEG and are only detected by EEG after spreading and becoming more coherent and powerful. Thus, MEG sees more spindles and is more sensitive to the emergence of focal spindles due to its more confined sensitivity patterns. In contrast, EEG, with its more diffuse sensitivity that is independent of the orientation of the source, is more likely to detect spindles from radial sources and those that rapidly cover extended areas of the cortex. For these reasons, MEG is more sensitive to oscillations generated via the core thalamocortical pathway, whose projections dominate in motor and sensory cortical areas and, as we hypothesized above, may be culpable in the spindle deficits of schizophrenia. EEG is more sensitive to oscillations generated by the matrix pathway, which projects broadly. Disadvantages of MEG include its cost and the requirement that subjects sleep with their head in the bore of the machine.
10.5. Different spindle types may have different functions
In addition to the difference between core and matrix spindles, the distinction between fast and slow spindles also has functional relevance. It is fast centroparietal spindles (≥12 Hz) that are most associated with memory consolidation (Molle et al. 2011) and are abnormal in schizophrenia (Ferrarelli et al. 2007, Wamsley et al. 2012). Fast and slow spindles have different hemodynamic correlates (e.g., greater hippocampal activation for fast spindles, which is consistent with memory processing); respond differently to drugs (Ayoub et al. 2013); are differentially synchronized with slow oscillations (Molle et al. 2011); and have different profiles with respect to age, sex, topography, and dynamics across the night. Moreover, fast and slow spindles are not genetically correlated. All these differences are consistent with fast and slow spindles having distinct origins and functions (Purcell et al. 2017). Another distinction relevant for memory is whether spindles are coupled with slow oscillations and hippocampal ripples. Unfortunately, ripples in the human hippocampus can only be observed with invasive techniques such as intracranial EEG, so it is not known whether it is specifically ripple-coupled spindles that are implicated in memory deficits in schizophrenia.
11. Future directions in research on sleep spindles in schizophrenia
11.1. Well-powered human genetic studies are necessary to decipher the genetic architecture of sleep spindles
To evaluate the spindle deficit as an endophenotype, it is important to determine its specificity to schizophrenia and to establish its heritability and genetic architecture in large genomic studies. Emerging findings herald a new era in neuropsychiatry in which we are beginning to forge empirical links in causal chains connecting risk genes, brain circuit dysfunction, physiology, and clinical syndromes (Figure 7). This work will enable us to develop mechanistically targeted treatments. Specifically, genetic studies can reveal mechanisms of TRN dysfunction and spindle deficits in schizophrenia and other disorders. Like most human traits, sleep spindles likely have a complex genetic architecture, with allelic variants in many genes combining to influence spindle expression. Genome-wide association studies (GWAS) with large sample sizes can capture genetic variation due to common alleles. Alleles identified with statistical confidence can then help to establish the broader gene networks that produce spindles and underlie their variation across populations. It is now also feasible to sequence the entire exome or genome in large numbers of individuals—an approach that can identify rare variants that may have larger effects on spindles because rare variants are likely to have arisen recently (and might even be de novo in the proband) and are less subject than common variants to natural selection (Veltman & Brunner 2012). A more fundamental challenge to genetic studies is that spindle activity cannot unambiguously be reduced to a single quantitative trait, because it likely represents a complex and possibly genetically heterogeneous set of processes (Purcell et al. 2017).
A major methodological challenge to deciphering the genetic architecture of sleep spindles is that it is prohibitively expensive and time consuming to conduct overnight PSG along with genetic characterization in large samples. A potential shortcut is the use of afternoon naps instead of overnight sleep (Mednick et al. 2003). Prior work has shown that memory improvement following a nap correlates with spindles (Schmidt et al. 2006) and that the architecture of postlunch naps resembles that of a night of sleep (Monk 2005), but studies demonstrating that spindles during nap and overnight recordings have the same trait-like features are lacking, and naps still require a considerable investment.
A potentially more fruitful goal for future work is to identify more scalable biomarkers of the thalamocortical pathology that gives rise to spindle deficits in schizophrenia. This is challenging because TRN function during wake in humans is poorly understood, partly because its size and location of the TRN make it largely inaccessible to neuroimaging. Consequently, animal models may be necessary to illuminate the contribution of the TRN to waking cognition (Halassa et al. 2014). A possible electrophysiological readout of TRN function is sensory gating (Krause et al. 2003), which attenuates redundant or irrelevant sensory stimuli, can be elicited using the P50 and prepulse inhibition event-related potential paradigms, and is deficient in schizophrenia patients and their first-degree relatives (Clementz et al. 1998, Swerdlow et al. 2008). The TRN is also thought to contribute to both evoked and spontaneous cortical gamma band oscillations (30–80 Hz, typically ~40 Hz (see Pinault 2004), which are strongly associated with working memory and performance of attention-demanding cognitive tasks and are abnormal in schizophrenia (Uhlhaas & Singer 2010).
11.2. It is important to define the scope and consequences of sleep-dependent memory deficits in schizophrenia
Dissociations such as reduced sleep-dependent consolidation of motor procedural memory in the context of intact spatial declarative memory consolidation (Seeck-Hirschner et al. 2010) and reduced consolidation of word-pair memory in the presence of intact sleep-dependent visuoperceptual procedural memory enhancement (Baran et al. 2018) suggest that only certain memory types are affected in schizophrenia, perhaps those that rely on spindles. Recognition that impaired sleep-dependent memory consolidation is a key contributor to cognitive disability in schizophrenia is growing, but there are still relatively few studies and those that exist have small sample sizes.
One of the challenges to studying sleep-dependent memory consolidation in schizophrenia is identifying tasks on which patients can reach normal levels of encoding during training. This is an important consideration in study design, because it allows for any deficit in sleep-dependent improvement to be attributed to sleep rather than worse encoding. Both the MST (Manoach et al. 2004) and mirror-tracing (Seeck-Hirschner et al. 2010) procedural learning tasks meet this criterion, as does the word-pair declarative memory task (Baran et al. 2018), although patients require more drilling to reach the same level of encoding as controls.
It is also important to determine how sleep-dependent memory deficits affect daily function. Sleep-dependent procedural memory deficits represent a breakdown of task automation (Manoach et al. 2004, Manoach & Stickgold 2009), which normally renders performance not only faster and less variable but also less dependent on voluntary attention. A failure of sleep-dependent automation leaves fewer attentional resources available for higher-order task demands and could thereby contribute to the generalized cognitive deficits that are a hallmark of schizophrenia (Chapman & Chapman 1978). Since treating deficits in sleep-dependent memory processing could improve overall cognitive functioning, measures of sleep-dependent memory consolidation should be included in clinical trials.
11.3. Spindle deficits may be relevant to the pathophysiology, manifestation, and treatment of other disorders
To evaluate the spindle deficit as diagnostic biomarker, it is important to determine its specificity to schizophrenia. In one study, spindle deficits were observed in APD-naïve patients with schizophrenia but not in those with other psychotic disorders (Manoach et al. 2014). Two other studies reported spindle deficits in schizophrenia but not in a mixed psychiatric control group taking APDs (Ferrarelli et al. 2010) or in individuals with a history of depression (Ferrarelli et al. 2007), consistent with another report of normal spindles in depression (Plante et al. 2013). There are scattered reports, however, of spindle abnormalities in other neurodevelopmental and neurodegenerative disorders characterized by cognitive impairment, including mental retardation (Shibagaki et al. 1982), Williams syndrome (Bodizs et al. 2012), autism (Farmer et al. 2018), Parkinson’s disease with dementia (Latreille et al. 2015), and Alzheimer’s disease (Gorgoni et al. 2016). However, the literature on these disorders is not as extensive or consistent as it is for schizophrenia, and whether the associated spindle deficits have unique characteristics and consequences remains to be determined. In conclusion, abnormal sleep is a prominent and understudied feature of neurodevelopmental, neuropsychiatric, and neurodegenerative disorders that may contribute to the defining symptoms and cognitive deficits and is a potential target for treatment. As has been demonstrated in major depression (Fava et al. 2006), treating sleep can be more effective than targeting symptoms alone. To identify treatment targets, future work must define sleep abnormalities in different disorders, understand how they contribute to disability, and illuminate their mechanisms.
Summary Points.
Rather than being simply a passive, restorative state, sleep is an active period of cognitive functioning that is essential to learning and memory.
Patients with schizophrenia and their nonpsychotic first-degree relatives have sleep spindle deficits that are not secondary to antipsychotic drugs or sleep disruption and instead may be endophenotypes.
Spindle deficits in schizophrenia may impair memory consolidation and are associated with lower IQ, executive dysfunction, and positive symptoms.
The associations of sleep spindle deficits in schizophrenia with thalamocortical hyperconnectivity and positive symptoms suggests that reduced inhibition of thalamocortical neurons by the thalamic reticular nucleus (TRN) is a common mechanism.
TRN dysfunction and abnormal sleep spindle activity may contribute to the neurodevelopment of schizophrenia via their roles in establishing and refining thalamocortical circuitry.
Thalamic sleep spindles need to be temporally coordinated with cortical slow oscillations and hippocampal sharp-wave ripples to mediate memory consolidation during sleep.
These three cardinal oscillations of non-rapid eye movement sleep can be manipulated pharmacologically and with noninvasive stimulation to enhance memory, and they constitute novel treatment targets.
Our growing understanding of the pathophysiology of abnormal sleep spindle activity in schizophrenia opens new avenues for research on treatment and prevention.
Future Directions.
Genetic studies that reveal mechanisms of sleep spindle deficits in schizophrenia can identify specific treatment targets.
To translate these advances, we need to develop efficient methods to select the most promising drugs for clinical trials based on their effects on the coordination of sleep oscillations and memory.
In recognition of their importance to understanding both the manifestations and the pathophysiology of schizophrenia, measures of spindle activity and sleep-dependent cognition should be included in clinical trials.
Cross-disciplinary research is necessary to forge empirical links in causal chains from schizophrenia risk genes to spindle deficits to diagnosis. These collaborative efforts provide unprecedented opportunities to advance our understanding of the genetics and pathophysiology of schizophrenia and to improve treatment, early identification of individuals at risk, and efforts at prevention.
References
- Albert NB, Robertson EM, Miall RC. 2009. The resting human brain and motor learning. Curr Biol 19: 1023–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, et al. 2013. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS One 8: e52805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosius U, Lietzenmaier S, Wehrle R, Wichniak A, Kalus S, et al. 2008. Heritability of sleep electroencephalogram. Biol Psychiatry 64: 344–8 [DOI] [PubMed] [Google Scholar]
- Andrade A, Hope J, Allen A, Yorgan V, Lipscombe D, Pan JQ. 2016. A rare schizophrenia risk variant of CACNA1I disrupts CaV3.3 channel activity. Scientific reports 6: 34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, et al. 2014. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex 24: 3116–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, et al. 2015. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA psychiatry 72: 882–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, et al. 2011. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A 108: 13823–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram M, Brandl F, Bauml J, Sorg C. 2018. Cortico-thalamic hypo- and hyperconnectivity extend consistently to basal ganglia in schizophrenia. Neuropsychopharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub A, Aumann D, Horschelmann A, Kouchekmanesch A, Paul P, et al. 2013. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity. Sleep 36: 905–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang JW, Khalilzadeh O, Hamalainen M, Watanabe T, Sasaki Y. 2014. Location specific sleep spindle activity in the early visual areas and perceptual learning. Vision Res 99: 162–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran B, Correll D, Vuper TC, Morgan A, Durrant SJ, et al. 2018. Spared and impaired sleep-dependent memory consolidation in schizophrenia. Schizophr Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran B, Demanuele C, Vuper TC, Seicol B, Fowler R, et al. APSS, Boston, 2017.
- Baran B, Karahanoglu FI, Demanuele C, Fowler RA, Vuper TC, et al. 2016. The role of thalamocortical connectivity in sleep spindles in schizophrenia. Presented at First International Conference on Sleep Spindling, Budapest, Hungary [Google Scholar]
- Barham MP, Enticott PG, Conduit R, Lum JA. 2016. Transcranial electrical stimulation during sleep enhances declarative (but not procedural) memory consolidation: Evidence from a meta-analysis. Neurosci Biobehav Rev 63: 65–77 [DOI] [PubMed] [Google Scholar]
- Barsky MM, Tucker MA, Stickgold R. 2015. REM sleep enhancement of probabilistic classification learning is sensitive to subsequent interference. Neurobiol Learn Mem 122: 63–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. 1992. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry 49: 651–68; discussion 69–70 [DOI] [PubMed] [Google Scholar]
- Benson KL. 2015. Sleep in Schizophrenia: Pathology and Treatment. Sleep medicine clinics 10: 49–55 [DOI] [PubMed] [Google Scholar]
- Bodizs R, Gombos F, Kovacs I. 2012. Sleep EEG fingerprints reveal accelerated thalamocortical oscillatory dynamics in Williams syndrome. Research in developmental disabilities 33: 153–64 [DOI] [PubMed] [Google Scholar]
- Bonjean M, Baker T, Bazhenov M, Cash S, Halgren E, Sejnowski T. 2012. Interactions between core and matrix thalamocortical projections in human sleep spindle synchronization. J Neurosci 32: 5250–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Wilhelm I. 2012. System consolidation of memory during sleep. Psychological research 76: 192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Freedman R, Javitt DC, Abi-Dargham A, Lieberman JA. 2007. Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophr Bull 33: 1120–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. 2009. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A 106: 10130–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassini LF, Sierra RO, Haubrich J, Crestani AP, Santana F, et al. 2013. Memory reconsolidation allows the consolidation of a concomitant weak learning through a synaptic tagging and capture mechanism. Hippocampus 23: 931–41 [DOI] [PubMed] [Google Scholar]
- Chan MS, Chung KF, Yung KP, Yeung WF. 2017. Sleep in schizophrenia: A systematic review and meta-analysis of polysomnographic findings in case-control studies. Sleep Med Rev 32: 69–84 [DOI] [PubMed] [Google Scholar]
- Chapman LJ, & Chapman JP 1978. The measurement of differential deficit. J Psychiatr Res 14: 303–11 [DOI] [PubMed] [Google Scholar]
- Chemerinski E, Ho BC, Flaum M, Arndt S, Fleming F, Andreasen NC. 2002. Insomnia as a predictor for symptom worsening following antipsychotic withdrawal in schizophrenia. Compr Psychiatry 43: 393–6 [DOI] [PubMed] [Google Scholar]
- Chouinard S, Poulin J, Stip E, Godbout R. 2004. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull 30: 957–67 [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. 2015. Cortical Development, Electroencephalogram Rhythms, and the Sleep/Wake Cycle. Biol Psychiatry 77: 1071–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson BC, Durkin J, Aton SJ. 2016. Form and Function of Sleep Spindles across the Lifespan. Neural Plast 2016: 6936381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens Z, Fabo D, Halasz P. 2006. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett 403: 52–6 [DOI] [PubMed] [Google Scholar]
- Clementz BA, Geyer MA, Braff DL. 1998. Poor P50 suppression among schizophrenia patients and their first-degree biological relatives. Am J Psychiatry 155: 1691–4 [DOI] [PubMed] [Google Scholar]
- Cloutier M, Aigbogun MS, Guerin A, Nitulescu R, Ramanakumar AV, et al. 2016. The Economic Burden of Schizophrenia in the United States in 2013. J Clin Psychiatry 77: 764–71 [DOI] [PubMed] [Google Scholar]
- Cohen JD, Insel TR. 2008. Cognitive neuroscience and schizophrenia: translational research in need of a translator. Biol Psychiatry 64: 2–3 [DOI] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. 1996. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274: 771–4 [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. 1996. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol 490 ( Pt 1): 159–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court J, Spurden D, Lloyd S, McKeith I, Ballard C, et al. 1999. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem 73: 1590–7 [DOI] [PubMed] [Google Scholar]
- Cox R, Schapiro AC, Manoach DS, Stickgold R. 2017. Individual Differences in Frequency and Topography of Slow and Fast Sleep Spindles. Front Hum Neurosci 11: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F 1984. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A 81: 4586–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino A, Castelnovo A, Cavallotti S, Casetta C, Marcatili M, et al. 2018. Sleep endophenotypes of schizophrenia: slow waves and sleep spindles in unaffected first-degree relatives. NPJ Schizophr 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM. 2010. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol 20: R626–7 [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Marzano C, Fratello F, Moroni F, Pellicciari MC, et al. 2008. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol 64: 455–60 [DOI] [PubMed] [Google Scholar]
- Dean DA 2nd, Goldberger AL, Mueller R, Kim M, Rueschman M, et al. 2016. Scaling Up Scientific Discovery in Sleep Medicine: The National Sleep Research Resource. Sleep 39: 1151–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani N, Cash SS, Chen CC, Hagler DJ Jr., Huang M, et al. 2010. Divergent cortical generators of MEG and EEG during human sleep spindles suggested by distributed source modeling. PLoS One 5: e11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani N, Cash SS, Halgren E. 2011. Emergence of synchronous EEG spindles from asynchronous MEG spindles. Hum Brain Mapp 32: 2217–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Felice A, Magalini A, Masiero S. 2015. Slow-oscillatory Transcranial Direct Current Stimulation Modulates Memory in Temporal Lobe Epilepsy by Altering Sleep Spindle Generators: A Possible Rehabilitation Tool. Brain stimulation 8: 567–73 [DOI] [PubMed] [Google Scholar]
- Demanuele C, Bartsch U, Baran B, Khan S, Vangel MG, et al. 2017. Coordination of Slow Waves with Sleep Spindles Predicts Sleep-Dependent Memory Consolidation in Schizophrenia. Sleep 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker SJ, Malm U, Lepp M. 1986. Schizophrenic relapse after drug withdrawal is predictable. Acta Psychiatr Scand 73: 181–5 [DOI] [PubMed] [Google Scholar]
- Dudai Y 2004. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55: 51–86 [DOI] [PubMed] [Google Scholar]
- Duncan CP. 1949. The retroactive effect of electroshock on learning. J Comp Physiol Psychol 42: 32–44 [DOI] [PubMed] [Google Scholar]
- Fang Z, Sergeeva V, Ray LB, Viczko J, Owen AM, Fogel SM. 2016. Sleep Spindles and Intellectual Ability: Epiphenomenon or Directly Related? J Cogn Neurosci: 1–16 [DOI] [PubMed] [Google Scholar]
- Farmer CA, Chilakamarri P, Thurm AE, Swedo SE, Holmes GL, Buckley AW. 2018. Spindle activity in young children with autism, developmental delay, or typical development. Neurology 91: e112–e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, McCall WV, Krystal A, Wessel T, Rubens R, et al. 2006. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry 59: 1052–60 [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, et al. 2007. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry 164: 483–92 [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, et al. 2010. Thalamic Dysfunction in Schizophrenia Suggested by Whole-Night Deficits in Slow and Fast Spindles. Am J Psychiatry 167: 1339–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri J, Ford JM, Roach BJ, Turner JA, van Erp TG, et al. 2018. Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol Med: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. 2011. The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev 35: 1154–65 [DOI] [PubMed] [Google Scholar]
- Frank MG. 2012. Erasing synapses in sleep: is it time to be SHY? Neural Plast 2012: 264378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzel L, Dresler M, Cornu M, Jager E, Konrad B, et al. 2015. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol Psychiatry 77: 177–86 [DOI] [PubMed] [Google Scholar]
- Goder R, Baier PC, Beith B, Baecker C, Seeck-Hirschner M, et al. 2013. Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophr Res 144: 153–4 [DOI] [PubMed] [Google Scholar]
- Goder R, Boigs M, Braun S, Friege L, Fritzer G, et al. 2004. Impairment of visuospatial memory is associated with decreased slow wave sleep in schizophrenia. J Psychiatr Res 38: 591–9 [DOI] [PubMed] [Google Scholar]
- Goder R, Fritzer G, Gottwald B, Lippmann B, Seeck-Hirschner M, et al. 2008. Effects of olanzapine on slow wave sleep, sleep spindles and sleep-related memory consolidation in schizophrenia. Pharmacopsychiatry 41: 92–9 [DOI] [PubMed] [Google Scholar]
- Goder R, Graf A, Ballhausen F, Weinhold S, Baier PC, et al. 2015. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med 16: 564–9 [DOI] [PubMed] [Google Scholar]
- Gorgoni M, Lauri G, Truglia I, Cordone S, Sarasso S, et al. 2016. Parietal Fast Sleep Spindle Density Decrease in Alzheimer’s Disease and Amnesic Mild Cognitive Impairment. Neural Plast 2016: 8376108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. 2003. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160: 636–45 [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. 2000. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 26: 119–36 [DOI] [PubMed] [Google Scholar]
- Gregory M, Agam Y, Selvadurai C, Nagy A, Vangel M, et al. 2014. Resting state connectivity immediately following learning correlates with subsequent sleep-dependent enhancement of motor task performance. NeuroImage 102P2: 666–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, et al. 2013. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell 154: 518–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, et al. 2014. State-dependent architecture of thalamic reticular subnetworks. Cell 158: 808–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich RF, Mander BA, Jagust WJ, Knight RT, Walker MP. 2018. Old Brains Come Uncoupled in Sleep: Slow Wave-Spindle Synchrony, Brain Atrophy, and Forgetting. Neuron 97: 221–30 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshkowitz M, Thornby JI, Karacan I. 1982. Sleep spindles: pharmacological effects in humans. Sleep 5: 85–94 [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Quan SF. 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, Ill: American Academy of Sleep Medicine. [Google Scholar]
- Insel TR. 2009. Translating scientific opportunity into public health impact: a strategic plan for research on mental illness. Arch Gen Psychiatry 66: 128–33 [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. 2007. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10: 100–7 [DOI] [PubMed] [Google Scholar]
- Jia F, Goldstein PA, Harrison NL. 2009. The modulation of synaptic GABA(A) receptors in the thalamus by eszopiclone and zolpidem. J Pharmacol Exp Ther 328: 1000–6 [DOI] [PubMed] [Google Scholar]
- Johnson LA, Blakely T, Hermes D, Hakimian S, Ramsey NF, Ojemann JG. 2012. Sleep spindles are locally modulated by training on a brain-computer interface. Proc Natl Acad Sci U S A 109: 18583–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. 1998. Viewpoint: the core and matrix of thalamic organization. Neuroscience 85: 331–45 [DOI] [PubMed] [Google Scholar]
- Kaestner EJ, Wixted JT, Mednick SC. 2013. Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories. J Cogn Neurosci 25: 1597–610 [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, et al. 1998. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A 95: 861–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Stanley JA, Pettegrew JW. 2004. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychiatry 3: 163–8 [PMC free article] [PubMed] [Google Scholar]
- Klingner CM, Langbein K, Dietzek M, Smesny S, Witte OW, et al. 2014. Thalamocortical connectivity during resting state in schizophrenia. European archives of psychiatry and clinical neuroscience 264: 111–9 [DOI] [PubMed] [Google Scholar]
- Kraepelin E 1919. Dementia praecox and paraphrenia. Edinburgh, Scotland: E.S. Livingston. [Google Scholar]
- Krause M, Hoffmann WE, Hajos M. 2003. Auditory sensory gating in hippocampus and reticular thalamic neurons in anesthetized rats. Biol Psychiatry 53: 244–53 [DOI] [PubMed] [Google Scholar]
- Krystal AD, Goforth HW, Roth T. 2008. Effects of antipsychotic medications on sleep in schizophrenia. Int Clin Psychopharmacol 23: 150–60 [DOI] [PubMed] [Google Scholar]
- Latchoumane CV, Ngo HV, Born J, Shin HS. 2017. Thalamic Spindles Promote Memory Formation during Sleep through Triple Phase-Locking of Cortical, Thalamic, and Hippocampal Rhythms. Neuron 95: 424–35 e6 [DOI] [PubMed] [Google Scholar]
- Latreille V, Carrier J, Lafortune M, Postuma RB, Bertrand JA, et al. 2015. Sleep spindles in Parkinson’s disease may predict the development of dementia. Neurobiol Aging 36: 1083–90 [DOI] [PubMed] [Google Scholar]
- Lindemann C, Ahlbeck J, Bitzenhofer SH, Hanganu-Opatz IL. 2016. Spindle Activity Orchestrates Plasticity during Development and Sleep. Neural Plast 2016: 5787423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsford-Avery JR, Orr JM, Gupta T, Pelletier-Baldelli A, Dean DJ, et al. 2013. Sleep dysfunction and thalamic abnormalities in adolescents at ultra high-risk for psychosis. Schizophr Res 151: 148–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustenberger C, Boyle M, Alagapan S, Mellin J, Vaughn B, Frolich F. 2016. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Current Biology August [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustenberger C, O’Gorman RL, Pugin F, Tushaus L, Wehrle F, et al. 2015. Sleep spindles are related to schizotypal personality traits and thalamic glutamine/glutamate in healthy subjects. Schizophr Bull 41: 522–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. 2004. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry 56: 951–6 [DOI] [PubMed] [Google Scholar]
- Manoach DS, Demanuele C, Wamsley EJ, Vangel M, Montrose DM, et al. 2014. Sleep spindle deficits in antipsychotic-naïve early course schizophrenia and in non-psychotic first-degree relatives. Frontiers in Human Neuroscience 8: 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Pan JQ, Purcell SM, Stickgold R. 2016. Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition? Biol Psychiatry 80: 599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Stickgold R. 2009. Does abnormal sleep impair memory consolidation in schizophrenia? Frontiers in Human Neuroscience 3: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, et al. 2010. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res 44: 112–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Fenton W, Youens K. 2004. Schizophrenia, IX: Cognition in schizophrenia--the MATRICS initiative. Am J Psychiatry 161: 25. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. 2006. Boosting slow oscillations during sleep potentiates memory. Nature 444: 610–3 [DOI] [PubMed] [Google Scholar]
- Marshall L, Kirov R, Brade J, Molle M, Born J. 2011. Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans. PLoS One 6: e16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM. 2008. Corollary discharge dysfunction in schizophrenia: evidence for an elemental deficit. Clin EEG Neurosci 39: 82–6 [DOI] [PubMed] [Google Scholar]
- Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, et al. 2013. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci 33: 4494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrofanis J, Guillery RW. 1993. New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci 16: 240–5 [DOI] [PubMed] [Google Scholar]
- Molle M, Bergmann TO, Marshall L, Born J. 2011. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep 34: 1411–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH. 2005. The post-lunch dip in performance. Clin Sports Med 24: e15–23, xi-xii [DOI] [PubMed] [Google Scholar]
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. 2009. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci 64: 180–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nere A, Hashmi A, Cirelli C, Tononi G. 2013. Sleep-dependent synaptic down-selection (I): modeling the benefits of sleep on memory consolidation and integration. Front Neurol 4: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Walker MP. 2007. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE 2: e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA, van Kammen DP, Gilbertson MW, Gurklis JA, Peters JL. 1993. Electroencephalographic sleep in clinically stable schizophrenic patients: two-weeks versus six-weeks neuroleptic-free. Biol Psychiatry 33: 829–35 [DOI] [PubMed] [Google Scholar]
- Payne JD, Schacter DL, Propper RE, Huang LW, Wamsley EJ, et al. 2009. The role of sleep in false memory formation. Neurobiol Learn Mem 92: 327–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D 2004. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev 46: 1–31 [DOI] [PubMed] [Google Scholar]
- Plante DT, Goldstein MR, Landsness EC, Peterson MJ, Riedner BA, et al. 2013. Topographic and sex-related differences in sleep spindles in major depressive disorder: a high-density EEG investigation. J Affect Disord 146: 120–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plihal W, Born J. 1997. Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience 9: 534–47 [DOI] [PubMed] [Google Scholar]
- Poe GR, Nitz DA, McNaughton BL, Barnes CA. 2000. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res 855: 176–80 [DOI] [PubMed] [Google Scholar]
- Prerau MJ, Brown RE, Bianchi MT, Ellenbogen JM, Purdon PL. 2017. Sleep Neurophysiological Dynamics Through the Lens of Multitaper Spectral Analysis. Physiology (Bethesda) 32: 60–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Manoach DS, Demanuele C, Cade BE, Mariani S, et al. 2017. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat Commun 8: 15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchs G, Feyers D, Landeau B, Bastin C, Luxen A, et al. 2011. Sleep contributes to the strengthening of some memories over others, depending on hippocampal activity at learning. J Neurosci 31: 2563–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling C, Schlipf M, Spietzack S, Rausch F, Eisenacher S, et al. 2016. Fast sleep spindle reduction in schizophrenia and healthy first-degree relatives: association with impaired cognitive function and potential intermediate phenotype. European archives of psychiatry and clinical neuroscience [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics C. 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511: 421–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, et al. 2006. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci 26: 8976–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeck-Hirschner M, Baier PC, Sever S, Buschbacher A, Aldenhoff JB, Goder R. 2010. Effects of daytime naps on procedural and declarative memory in patients with schizophrenia. J Psychiatr Res 44: 42–7 [DOI] [PubMed] [Google Scholar]
- Sejnowski TJ, Destexhe A. 2000. Why do we sleep? Brain Res 886: 208–23 [DOI] [PubMed] [Google Scholar]
- Shibagaki M, Kiyono S, Watanabe K. 1982. Spindle evolution in normal and mentally retarded children: a review. Sleep 5: 47–57 [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. 1998. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21: 1123–8 [DOI] [PubMed] [Google Scholar]
- Skatun KC, Kaufmann T, Brandt CL, Doan NT, Alnaes D, et al. 2017. Thalamo-cortical functional connectivity in schizophrenia and bipolar disorder. Brain Imaging Behav [DOI] [PubMed] [Google Scholar]
- Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. 2001. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry 158: 1393–9 [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW 3rd, Carter CS. 2006. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull 32: 179–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M 2003. The corticothalamic system in sleep. Front Biosci 8: d878–99 [DOI] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Bukhari SA, Ardelt MI, Pantazopoulos H, et al. 2017. The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. 2007. Sleep-dependent memory consolidation and reconsolidation. Sleep Med 8: 331–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. 2013. Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci 16: 139–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. 2008. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 199: 331–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki M, Huang TR, Yotsumoto Y, Hamalainen M, Lin FH, et al. 2013. Enhanced spontaneous oscillations in the supplementary motor area are associated with sleep-dependent offline learning of finger-tapping motor-sequence task. J Neurosci 33: 13894–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. 2010. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 65: 280–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tek C, Palmese LB, Krystal AD, Srihari VH, DeGeorge PC, et al. 2014. The impact of eszopiclone on sleep and cognition in patients with schizophrenia and insomnia: a double-blind, randomized, placebo-controlled trial. Schizophr Res 160: 180–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesler N, Gerstenberg M, Franscini M, Jenni OG, Walitza S, Huber R. 2015. Reduced sleep spindle density in early onset schizophrenia: a preliminary finding. Schizophr Res 166: 355–7 [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. 2006. Sleep function and synaptic homeostasis. Sleep Med Rev 10: 49–62 [DOI] [PubMed] [Google Scholar]
- Tucker M, McKinley S, Stickgold R. 2011. Sleep optimizes motor skill in older adults. J Am Geriatr Soc 59: 603–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. 2010. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11: 100–13 [DOI] [PubMed] [Google Scholar]
- Veltman JA, Brunner HG. 2012. De novo mutations in human genetic disease. Nature reviews. Genetics 13: 565–75 [DOI] [PubMed] [Google Scholar]
- Vukadinovic Z 2011. Sleep abnormalities in schizophrenia may suggest impaired trans-thalamic cortico-cortical communication: towards a dynamic model of the illness. Eur J Neurosci 34: 1031–9 [DOI] [PubMed] [Google Scholar]
- Wagner U, Gais S, Born J. 2001. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem 8: 112–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. 2002. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35: 205–11. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. 2010. Overnight alchemy: sleep-dependent memory evolution. Nat Rev Neurosci 11: 218; author reply 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley E, Tucker MA, Shinn AK, Ono KE, McKinley S, et al. 2012. Reduced sleep spindles and spindle coherence in schizophrenia: Mechanisms of impaired memory consolidation? Biol Psychiatry 71: 154–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Shinn AK, Tucker MA, Ono KE, McKinley SK, et al. 2013. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep 36: 1369–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker M, Payne JD, Benavides JA, Stickgold R. 2010. Dreaming of a learning task is associated with enhanced sleep-dependent memory consolidation. Curr Biol 20: 850–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warby SC, Wendt SL, Welinder P, Munk EG, Carrillo O, et al. 2014. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nature methods 11: 385–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MF, Wimmer RD, Schmitt LI, Feng G, Halassa MM. 2016. Thalamic reticular impairment underlies attention deficit in Ptchd1(Y/-) mice. Nature 532: 58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265: 676–9 [DOI] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. 2012. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry 169: 1092–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. 2012. Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. J Neurosci 32: 5338–50 [DOI] [PMC free article] [PubMed] [Google Scholar]