Abstract
Increasing evidence indicates that decellularized extracellular matrices (dECMs) derived from cartilage tissues (T-dECMs) or chondrocytes/stem cells (C-dECMs) can support proliferation and chondrogenic differentiation of cartilage-forming cells. However, few review papers compare the differences between these dECMs when they serve as substrates for cartilage regeneration. In this review, after an introduction of cartilage immunogenicity and decellularization methods to prepare T-dECMs and C-dECMs, a comprehensive comparison focuses on the effects of T-dECMs and C-dECMs on proliferation and chondrogenic differentiation of chondrocytes/stem cells in vitro and in vivo. Key factors within dECMs, consisting of microarchitecture characteristics and micromechanical properties as well as retained insoluble and soluble matrix components, are discussed in-depth for potential mechanisms underlying the functionality of these dECMs in regulating chondrogenesis. With this information, we hope to benefit dECM based cartilage engineering and tissue regeneration for future clinical application.
Keywords: Cartilage regeneration, Chondrocyte, Chondrogenic differentiation, Decellularized matrix, Extracellular matrix, Proliferation, Stem cell
1. Introduction
Cartilage is an avascular load-bearing tissue consisting of chondrocytes distributed throughout a dense extracellular matrix (ECM), which is a structurally complex environment composed of many components including collagen, glycosaminoglycans (GAGs), proteoglycans and other elements such as fibronectin and laminin [1,2]. The low cellularity and avascular properties of cartilage result in the limited potential for self-repair following cartilage injury [3]. Current repair strategies, including microfracture, osteochondral autograft or mosaicplasty and autologous chondrocyte implantation, have achieved success in regenerating functional cartilage [4–7]. However, the limited availability of graft tissue, donor site morbidity, graft subsidence at the surface and fibrocartilage formation affect the quality of repair [4,8–10]. Recently, cartilage tissue engineering, combining cartilage-forming cells (chondrocytes and stem cells), growth factors and scaffolds, has provided promising approaches for cartilage regeneration [11,12].
As a basic element in cartilage tissue engineering, scaffolds play an important role in providing structural support and a micromechanical environment as well as biochemical cues for cell growth and chondrogenic differentiation. Numerous synthesized and natural materials, such as poly(l-lactic acid), poly(l-lactic-co-glycolic acid) (PLGA), collagen derivatives and fibrin glue [13], have been used as scaffolds for cartilage regeneration [12,14,15]. Increasing evidence has shown that ECM can provide not only physical support but also biological signals to cells that can facilitate cell attachment, proliferation and differentiation [16–19]. Decellularized ECMs (dECMs) from various tissues, such as heart, skin, bladder, nerves and tendons, have been used for tissue engineering applications with promising results [20,21]. Tissue-specific ECM derived from target tissues was reported to promote cell proliferation and lineage-specific differentiation through retaining biophysical and biochemical cues within native tissues [22,23]. For instance, dECMs derived from cartilage tissues (T-dECMs) have been extensively investigated as biological scaffolds for cartilage engineering due to their inherent components and unique structure and micromechanical properties, which provide a niche-like nanostructured microenvironment to aid in chondrogenesis [24–27].
Recent evidence showed that, different from T-dECMs that induce chondrogenic differentiation directly, dECMs derived from chondrocyte/stem cells (C-dECMs) benefited cartilage regeneration by promoting expanded cell proliferation and chondrogenic potential [25,27–32]. However, few review papers are available comparing the differences between these two dECMs when they serve as substrates for cartilage regeneration. In this review, cartilage immunogenicity and decellularization methods of T-dECMs and C-dECMs are introduced followed by a comprehensive comparison of the roles of T-dECMs and C-dECMs on proliferation and chondrogenic differentiation of cartilage-forming cells in vitro and in vivo. Also discussed are the potential influential factors within dECMs, including microarchitecture characteristics and micromechanical properties as well as retained insoluble and soluble matrix components such as collagen, GAGs and bioactive factors, which may contribute to differences between these two dECMs in regulating chondrogenesis (Figure 1).
Figure 1.
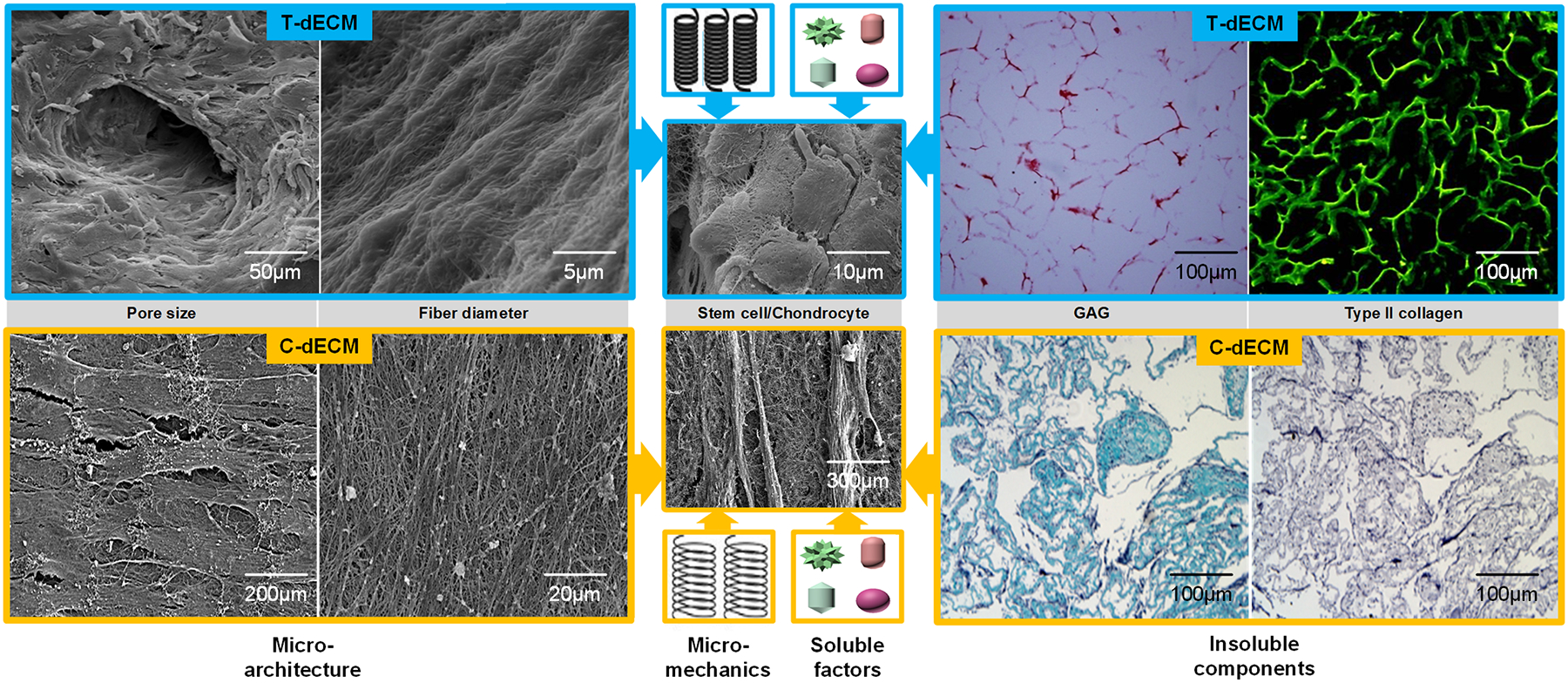
Diagram design. Decellularized extracellular matrix (dECM) from either tissue (T-dECM) or cell (C-dECM) supported chondrogenesis of stem cells and chondrocytes. Inherent properties and components of dECM scaffolds, such as microarchitecture characteristics including pore size and fiber diameter, micromechanical properties, insoluble components including glycosaminoglycan (GAG) and collagen, and soluble factors, were involved in the regulation of cell proliferation and chondrogenic differentiation. Cartilage T-dECMs with retained type II collagen and GAGs as well as larger pore sizes were more likely to facilitate seeded cells’ chondrogenic differentiation, which resulted in a round or elliptic morphology similar to chondrocyte-like cells [27]. C-dECMs with negligible levels of type II collagen and GAGs as well as smaller pore size had a superior tendency to support cell proliferation and chondrogenic potential, which led to a small and fibroblast-like shape [31]. Reprints with permission from “He, F.; Chen, X.; Pei, M. Tissue Eng. Part A 2009, 15, 3809. Copyright (2009) Mary Ann Liebert, Inc. Publications” and “Yang, Q., Peng, J., Guo, Q., Huang, J., Zhang, L., Yao, J., et al., Biomaterials 2008, 29, 2378. Copyright (2008) Elsevier Publications”.
2. Decellularization approach
Cartilage tissue properties, such as avascularity and high density, are unique which render its decellularization more complicated than other connective tissues.
2.1. Cartilage immunogenicity and necessity of decellularization
The avascular and dense nature of articular cartilage has led to the prediction that articular cartilage is immunoprivileged, whereby the cartilage’s immune system is limited because the cells are deeply encapsulated within the matrix and not easily reachable to immune cells [2,33–38]. Allogeneic cartilage transplantation from cadaveric origin is well tolerated and clinical results have validated a high success rate (60–95%) as construed by graft survival and good/excellent patient evaluations [38]. Animal studies also showed that chondrocytes of cartilage grafts maintained within their matrix are nearly nonimmunogenic [39]. Moreover, engineered cartilage using allogeneic chondrocytes with natural and synthetic scaffolds demonstrated successful repair of cartilage defects without significant signs of rejection and immune response [40–41].
However, the immunoprivileged nature of cartilage has been challenged by other findings showing that both chondrocytes and their embedded ECM contain antigens and elicit varying degrees of immune reactions [34,38,43–45]. Chondrocytes are liable to attack by natural killer cells [43,46] and also express major histocompatibility class (MHC) II antigens to trigger CD4 T lymphocytes and provoke cell or antibody-mediated immune responses [47–49]. Various degrees of immune response were reported after implantation of allogeneic chondrocytes grown on engineered scaffolds in osteochondral defects [50,51]. Interestingly, physically devitalized cartilage fragments supported chondrogenesis without significant inflammation in vivo [52]. Therefore, a threshold amount of cellular material remains in implanted scaffolds that can trigger a severe immune response. Eliminating donor cells through the decellularization processes is thought to be desirable to reduce the risk of immune response from recipients, particularly for xenogeneic or allogeneic donor tissues [53].
Furthermore, due to the intrinsic nature of cartilage tissue that consists of dense ECM with nanosized pores, chondrocytes/stem cells are unable to infiltrate and repopulate a cartilage scaffold in its native form. The matrix alone may not be adequate for tissue regeneration, while the low porosity limits cell infiltration which, in turn, limits new matrix deposition. Therefore, the decellularization process is necessary to remove cell components and immunogenic antigens as well as to improve reseeded cell infiltration for subsequent cartilage regeneration by using physical treatment, chemical agents and biological nucleases [25,26].
2.2. Decellularization protocols and challenges
Various methods used to prepare dECMs for cartilage regeneration have been reviewed [20,21,25,54]. Chemical agents, including but not limited to sodium dodecyl sulfate, Triton X-100, ethylenediaminetetraacetic acid and Tris-Hydrochloride, are used to remove cellular components and immunogenic material [2,25,55]. Biological nucleases such as DNases and RNases are also commonly used to degrade residual DNA or RNA [25,26]. Since cartilage is a dense and compact connective tissue with low porosity, to improve the efficiency of chemical decellularization, physically breaking down cartilage tissues into fragments has been applied to increase surface area and enhance permeation of chemical agents into cartilage [2,25]. Physical treatments were demonstrated to disrupt cellular membranes and nuclei, indicative of the ability to remove cellular components through decellularization protocols [2,13,56]. In addition, devitalization through tissue homogenization followed by retrieval of tissue particles, freezing and lyophilization has achieved porous and devitalized ECM-derived biomaterials [57,58]. The use of chemical agents to decellularize cartilage not only results in a significant reduction in the amount of whole cells, cell nuclei and DNA, but also impacts the biochemical composition of the dECM, including a reduction in GAG content, destruction of macrostructure and alteration of micromechanical properties [2,59,60].
Similar decellularization methods for cartilage tissue have also been applied for C-dECMs [32,54,61,62], such as mild chemical agents and nucleases that are used to effectively remove cellular components and degrade residual DNA or RNA. Three-dimensional (3D) C-dECM scaffolds were fabricated by depositing chondrocyte/stem cell secreted ECM onto a polymer surface followed by leaving or removing the polymer through the use of chemical decellularization [63,64]. Supplementation with ascorbic acid in the cell culture environment facilitated ECM deposition [65]. Because cell-derived ECM is less dense than native cartilage, it is usually unnecessary to use physical treatment paired with chemical agents [2,66]. Moreover, the decellularization process is generally shorter and more efficient for cell removal, which also prevents a reduction of aggregate modulus of dECM due to long decellularization protocols [2,67].
The decellularization process is essential for excluding cellular components and antigenicity from tissue explants concerning escaping from disease transmission, reducing inflammatory and immune responses toward the scaffold, particularly with xenogeneic or allogeneic donor tissues [21]. DNA and the cell surface oligosaccharide molecule α-Gal (also known as “Gal epitope”) are two typical antigens recognized to trigger an inflammatory response against biological scaffolds. Therefore, incomplete decellularization may result in residual DNA or the cell surface oligosaccharide molecule α-Gal being present, which leads to inflammatory or immune responses [25,68]. Unlike cellular material, ECM components prevalently conserved through species are well tolerated when employed as allografts or xenografts [25].
Currently there is no standard method of decellularization for cartilage. Reduction of sulfated GAGs [2,60], loss of inherent collagen content [59] as well as decreased biomechanical properties [60] of dECMs indicated that the decellularization process itself can affect the microarchitecture, micromechanical properties, and residual matrix components [31,55,69]. Therefore, optimal decellularization methods that can effectively remove cellular components with only minimal disruption to other components, such as collagen, GAGs and growth factors, can help maintain ECM ultrastructure and micromechanical properties.
3. Cartilage T-dECMs and chondrogenesis
An increasing number of studies demonstrate that cartilage T-dECMs, which retain most of the native structure and inherent components, direct cartilage-forming cells toward chondrogenesis by promoting cell proliferation (Table 1) and chondrogenic differentiation (Table 2).
Table 1.
The effect of cartilage T-dECMs on chondrocyte/stem cell proliferation.
| dECM origin | Seeded cell type | Treatment | Analysis | Results | Reference |
|---|---|---|---|---|---|
| bovine AC | human BMSC | grown in soluble dECMs in a 2D culture system | MTS assay/cell metabolism | enhanced cell proliferation | 22 |
| bovine AC | rabbit BMSC | grown in conditioned medium from dECM extraction | BrdU analysis | 10 mg/mL dECM promoted cell proliferation at day 6 and 9 | 55 |
| bovine meniscus | human BMSC | grown in conditioned medium with dECM extraction from menisci | MTS assay | enhanced cell proliferation | 72 |
| human AC | human SDSC | grown in conditioned medium from dECM extraction | WST-1 (activity) and LDH (death) analyses | supported cell proliferation | 70 |
| porcine AC | human ADSC | seeded onto the genipin-crosslinked physically prepared dECM in a culture medium without exogenous growth factors | DNA content | crosslinked scaffold with genipin supported cell proliferation | 220 |
| porcine AC | human and porcine chondrocyte | seeded onto physically prepared dECM scaffolds in a culture medium | DNA content | supported cell growth and proliferation | 221 |
| porcine AC | human BMSC | cultured on dECM scaffolds in a chondrogenic medium | DNA content | supported cell proliferation | 76 |
| porcine AC | human IPFSC | cultured on dECM scaffolds in a chondrogenic medium | DNA content | supported cell proliferation | 74 |
| porcine AC | human IPFSC | seeded onto physically prepared dECM scaffolds in a chondrogenic medium | DNA content | supported greater proliferation within the scaffolds fabricated using 250 mg/mL cartilage slurry concentrations | 57 |
| porcine AC (immature and mature) | human IPFSC | seeded onto dECMs and cultured in a chondrogenic medium | DNA content | supported cell proliferation | 222 |
| porcine AC | rat BMSC | treated with DCC in a pellet culture system | DNA content | supported cell proliferation (superior to DVC at day 7) | 223 |
| porcine meniscus | human chondrocyte | cultured on dECM scaffolds in a culture medium | DNA content | enhanced cell proliferation | 73 |
| porcine NP | human ADSC | cultured on dECM scaffolds | cell number | supported cell proliferation | 75 |
Abbreviations:2D: two-dimensional; AC: articular cartilage; ADSC: adipose derived stem cell; BMSC: bone marrow stromal cell; DCC: chemically decellularized cartilage particles; dECM: decellularized extracellular matrix; DVC: physically devitalized cartilage particles; ECM: extracellular matrix; IPFSC: infrapatellar fat pad derived stem cell; LDH: lactate dehydrogenase; NP: nucleus pulposus; SDSC: synovium derived stem cell.
Table 2.
Effect of cartilage T-dECMs on chondrogenic differentiation.
| dECM origin | Seeded cell type | Treatment | Results | Reference |
|---|---|---|---|---|
| bovine AC | bovine chondrocyte | cultured in rigid dishes coated with silicone rubber or monotonically expanded on high extension silicone rubber dishes functionalized with dECM extract | dECM supported enhanced preservation of chondrocyte phenotype and redifferentiation potential | 224 |
| bovine AC | human BMSC | treated with soluble dECM in 2D culture, pellet system or on aligned nanofibrous scaffolds; or seeded in dECM-encapsulated-GelMA hydrogels with or without exposure to TGF-β3 | both dECM alone or in combination with prochondrogenic factors promoted chondrogenic differentiation | 22 |
| bovine AC | human BMSC | suspended in dECM hydrogels with or without methacrylate and cultured in chondrogenic medium for up to 42 days | functionalization of pepsin-soluble dECM hydrogels compromised chondroinductivity, did not enhance BMSC chondrogenesis | 88 |
| bovine AC | rabbit BMSC | loaded onto dECMs to repair cartilage defects in a rabbit model | significantly improved the repair of cartilage defects after 12 weeks of implantation | 55 |
| bovine EC | human BMSC | seeded on dECMs and cultured in chondrogenic medium | supported chondrogenic differentiation | 71 |
| bovine meniscus | human BMSC | treated with urea-soluble extracts of dECMs from inner and outer meniscal regions in 2D culture; or culture in 3D dECM-GelMA hydrogels with chondrogenic induction | supported fibrochondrogenic differentiation in 2D culture; accelerated chondrogenic differentiation by the addition of soluble dECM fractions onto GelMA hydrogels | 72 |
| equine AC | equine chondrocyte and BMSC | seeded onto physically prepared dECM with chondrogenic induction | supported chondrogenic differentiation; BMSCs significantly outperformed chondrocytes in producing cartilaginous matrix | 225 |
| equine AC | equine BMSC | embedded in dECM particles and GelMA hydrogels with chondrogenic induction followed by subcutaneous implantation into a rat model | stimulated in vitro cartilage formation, but subsequently remodeled into endochondral bone formation in vivo | 226 |
| equine AC | human BMSC | seeded onto dECMs and cultured in chondrogenic medium followed by subcutaneous implantation into immunocompromised rats | supported in vitro chondrogenesis, but formed in vivo ectopic endochondral bone | 227 |
| human AC | canine chondrogenic BMSC | seeded onto dECMs and cultured in vitro for 3 days and then implanted subcutaneously in nude mice for 4 weeks | supported chondrogenic differentiation in vitro and formed cartilage-like tissues in vivo | 27 |
| human AC | human SDSC | grown on dECM-collagen constructs with or without treatment of growth factors (TGF-β3 and BMP-2) | promoted chondrogenesis and synergistically enhanced by growth factor induction | 70 |
| human AC | rabbit ADSC | seeded onto dECMs with chondrogenic induction followed by implantation to repair rabbit cartilage defects | supported in vitro cartilage formation and high-quality in vivo cartilage repair | 80 |
| human donor trachea | human epithelial cell and BMSC-derived-chondrocytes from recipient | colonized on dECMs to replace recipient’s left main bronchus after 5-year follow-up | produced engineered airway without risk of rejection | 82 |
| human donor trachea | human epithelial cell and BMSC derived chondrocyte from recipient | colonized on dECMs to replace recipient’s left main bronchus after 5-year follow-up | supported the re-population of the implanted airway matrix | 81 |
| porcine NSC | human nasal chondrocyte | seeded on dECMs and cultured in chondrocyte induction medium | supported chondrogenic differentiation | 60 |
| porcine NSC | human nasal septal chondrocyte | seeded on dECMs and cultured in chondrocyte induction medium | supported chondrocyte differentiation | 194 |
| porcine AC | human ADSC | seeded on physically prepared dECM without exogenous growth factors | promoted chondrogenic differentiation without exogenous growth factors | 56 |
| porcine AC | human ADSC | seeded on physically prepared dECM-PCL composite scaffolds in a culture medium | promoted ASC differentiation and chondrogenesis in vitro | 228 |
| porcine AC | human ADSC | seeded on to the genipin-crosslinked physically prepared dECM in culture medium without exogenous growth factors | crosslinked scaffold using the 0.05% genipin solution supported chondrogenic differentiation in vitro culture | 220 |
| porcine AC | human ADSC | Seeded on physically prepared dECM-PCL scaffolds with chondrogenic induction | enhanced chondrogenesis in vitro | 229 |
| porcine AC | human and porcine chondrocyte | seeded onto physically prepared dECM in a culture medium | supported chondrogenesis in the absence of exogenous growth factors | 221 |
| porcine AC | human ADSC and BMSC | seeded on physically prepared dECM in a chondrogenic medium consisting TGF-β3 and BMP-6 | supported chondrogenic differentiation | 230 |
| porcine AC | human BMSC | seeded on physically prepared dECM with various crosslinking treatments followed in chondrogenic induction containing human TGF-β3 | supported significant chondrogenic differentiation | 231 |
| porcine AC | human BMSC | seeded on dECM hemisphere scaffolds and cultured in chondrogenic medium | supported chondrogenic differentiation and prevented hypertrophy | 76 |
| porcine AC | human FPSC | encapsulated into physically prepared dECM functionalized fibrin hydrogels followed by culture in chondrogenic medium or seeded on physically prepared dECM functionalized fibrin hydrogels followed by implantation in nude mice | supported robust chondrogenesis in vitro and in vivo in the presence of TGF-β3 | 13 |
| porcine AC | human IPFSC | seeded on physically prepared dECM with chondrogenic induction | promoted robust chondrogenesis in the presence of TGF-β3 | 58 |
| porcine AC | human IPFSC | seeded onto physically prepared dECM with chondrogenic induction containing TGF-β3 | supported greater chondrogenesis within the scaffolds fabricated using 250 mg/mL cartilage slurry concentrations in vitro | 57 |
| porcine AC | porcine IPFSC | seeded onto a TGF-β3 eluting physically prepared dECM followed by implantation into nude mice | supported cartilage-like tissue formation in vivo | 57 |
| porcine AC | human IPFSC | seeded on dECMs and cultured chondrogenically under either static or rotational conditions for 10 days | supported chondrogenic differentiation | 74 |
| porcine AC (immature and mature) | human IPFSC | seeded on dECMs and cultured in chondrogenic medium | supported cartilage formation in vitro | 222 |
| porcine AC | rabbit BMSC | seeded onto dECM scaffolds and cultured in chondrogenic media for 7 weeks followed by implantation to treat rabbit tracheal defects | produced neocartilage and reconstructed partial tracheal defects | 232 |
| porcine AC | rat BMSC | seeded on DCC-encapsulated or coated PLGA microspheres and cultured for 6 weeks | DCC-encapsulated scaffolds induced chondrogenesis better than TGF-β encapsulated and DCC-coated scaffolds | 233 |
| porcine AC | rat BMSC | cultured on MeHA hydrogel incorporating with DVC and DCC microparticles followed with or without exposure to TGF-β3 over a 6-week culture period | DVC was superior to DCC in chondroinductivity and rheological performance of hydrogel precursors | 59 |
| porcine AC | rat BMSC | encapsulated in MeSDCC hydrogels and cultured without growth factors for 6 weeks | supported chondrogenic differentiation | 234 |
| porcine EC | porcine newborn chondrocyte | seeded on dECM sheets by stacking 20 layers and cultured for 4 weeks followed by either continuing 12-week culture or subcutaneous implantation into nude mice for 12 weeks | supported the formation of cartilage-like tissues both in vitro and in vivo | 77 |
| porcine EC | porcine newborn BMSC | seeded on dECM sheets by stacking 20 layers and cultured with or without chondrogenic factors followed by implantation into nude mice for another 4 weeks | promoted chondrogenic differentiation, further enhanced by chondrogenic factors | 78 |
| porcine hemi larynx | human BMSC | seeded onto dECMs and implanted into a sternomastoid muscle fascial pocket for 1 month, and then covered with a tissue-engineered oral mucosal sheet for relocating into a full-thickness defect in cricoid cartilage of immune-suppressed pig | remodeled in vivo cartilage by initiation of chondrogenesis | 83 |
| porcine meniscus | human chondrocyte/ BMSC | chondrocytes seeded on dECMs and BMSCs seeded on dECMs with chondrogenic induction | promoted chondrogenic differentiation | 73 |
| porcine NP | human ADSC | seeded on dECM hydrogels with or without chondrogenic induction | supported differentiation toward an NP-like cell phenotype, further enhanced by chondrogenic induction | 85 |
| porcine NSC | rat nasal septum chondrocyte | seeded on dECMs and implanted to repair nasal septum defects in a rat model | supported cartilage defect repair | 79 |
| porcine trachea | BM MNC and epithelial cell | seeded on dECMs and conditioned with growth and regenerative factors followed by implantation to replace recipients’ cervical trachea | supported in vivo cartilage regeneration of transplanted trachea | 235 |
| rabbit trachea | rabbit ADSC | seeded onto dECMs to replace rabbit recipient tracheas | autologous cell treatment generated tracheas to repair tracheal injuries | 236 |
| rabbit trachea | rabbit chondrocyte | seeded onto dECMs and cultured for 2 weeks followed by either maintaining for another 6 weeks or subcutaneously implanting into nude mice for 12 weeks | regenerated tubular cartilage | 84 |
Abbreviations:2D: two-dimensional; 3D: three-dimensional; AC: articular cartilage; ADSC: adipose derived stem cell; BM MNC: bone marrow (BM) mononuclear cell; BMP: bone morphogenetic protein; BMSC: bone marrow stromal cell; DCC: chemically decellularized cartilage particles; dECM: decellularized extracellular matrix; DVC: physically devitalized cartilage particles; EC: ear cartilage; ECM: extracellular matrix; GelMA: methacrylated gelatin; IPFSC: infrapatellar fat pad derived stem cell; MeHA: methacrylated hyaluronic acid; MeSDCC: methacrylated solubilized decellularized cartilage; NP: nucleus pulposus; NSC: nasal septal cartilage; PCL: poly(ε-caprolactone); SDSC: synovium derived stem cell; TGF: transforming growth factor.
3.1. Proliferation
Cartilage T-dECMs with a 3D interconnected porous environment facilitate cellular infiltration and support cell proliferation during chondrogenesis. It has been reported that cartilage T-dECMs were non-cytotoxic for chondrocytes and adult stem cells by contact and extract cytotoxicity analysis [27,55,60,70,71], suggesting that these scaffolds have good biocompatibility to support cell growth. Recent studies have shown that the extraction of T-dECMs from articular cartilage and meniscus exerted significant roles in promoting bone marrow stromal cell (BMSC) proliferation [55,72]. Enhanced cell proliferation was also observed when chondrocytes or adult stem cells were seeded on T-dECM scaffolds in either culture medium or chondrogenic medium [73–76]. These studies indicated that cartilage T-dECMs could provide a 3D environment to support cartilage-forming cell proliferation.
3.2. Chondrogenic differentiation
Recent findings have demonstrated that soluble T-dECMs from hyaline cartilage or meniscus promoted chondrogenic differentiation of stem cells as a medium supplementation in two-dimensional (2D) culture [22,72]. Supplementation with soluble T-dECMs also promoted chondrogenic differentiation when human BMSCs were cultured in a pellet culture system or on aligned nanofibers [22], indicating that cartilage T-dECMs provide an appropriate cue for chondrogenic inductivity.
An increasing number of studies demonstrated that hyaline cartilage T-dECMs alone [22,70,77] or in combination with prochondrogenic factors [27,60,76,78] facilitated in vitro chondrogenesis of reseeded chondrocytes/stem cells. Furthermore, implantation of cartilage T-dECMs seeded by chondrocytes/stem cells in an in vivo model also confirmed the formation of cartilage tissues and the repair of cartilage defects [27,55,77–80]. In addition, decellularized human trachea readily colonized by epithelial cell and BMSC derived chondrocytes supported remodeling and produced an engineered airway after a five-year follow-up of a left main bronchus replacement [81,82]. Decellularized trachea or larynx also promoted stem cells/chondrocytes to regenerate cartilage when these cell-dECMs were implanted in vivo [83,84], suggesting that airway remodeling may trigger initiation of chondrogenesis.
T-dECMs from meniscus and nucleus pulposus (NP) tissue also supported chondrogenic differentiation. Soluble ECM fractions from decellularized menisci incorporating methacrylated gelatin (GelMA) hydrogels accelerated chondrogenic differentiation of human BMSCs [72]. Human BMSCs/chondrocytes cultured in porcine decellularized meniscus promoted chondrocyte differentiation and secreted ECM in addition to supporting chondrogenesis of human BMSCs [73]. Moreover, porcine decellularized NP tissues supported human adipose derived stem cell (ADSC) differentiation toward an NP-like cell phenotype, even in the absence of differentiation media [85]. Interestingly, it was found that cartilage T-dECMs without supplementation of chondrocytes/stem cells also supported new hyaline cartilage formation when implanted in vivo [86,87]. These studies indicated that cartilage T-dECMs could support chondrogenic differentiation in vitro and in vivo.
However, contrary studies found that cartilage T-dECMs had a limited or failed capacity to induce chondrogenic differentiation [88,89]. For example, only a small portion of cartilaginous and fibrous tissue was formed when decellularized pig ears seeded with porcine BMSCs without chondrogenic induction were implanted into nude mice. These findings indicate that acellular cartilage is not strong enough to induce BMSCs to form homogenous cartilage in vivo, although it does possess chondrogenic activity [78]. A 72% failure rate within the first two years of implantation was observed at two institutions when patients were treated with a decellularized osteochondral allograft (Chondrofix; ZimmerBiomet) implant for knee cartilage injuries [90]. Many complicating factors, such as reseeded cells, species sensitivity, the decellularization process, initial biomechanical loading as well as the limited micromechanical properties of scaffolds, may be involved in the failure of T-ECM based chondrogenesis [89]. The functionalization of cartilage ECM hydrogels with methacrylate that compromised in vitro chondroinductivity also resulted in the failure of T-dECMs in enhancing chondrogenesis [88].
4. dECMs from chondrocytes/stem cells and chondrogenesis
Various types of cartilage-forming cells have been used as a source for C-dECMs, including chondrocytes and stem cells, to support cell proliferation (Table 3) and chondrogenic differentiation (Table 4) [91,92]. Interestingly, these C-dECMs alone or in combination with other factors exert varied capacities in promoting chondrogenesis in vitro and in vivo.
Table 3.
Effect of C-dECMs on chondrocyte/stem cell proliferation.
| dECM origin | Seeded cell type | Treatment | Analysis | Results | Reference |
|---|---|---|---|---|---|
| human BMSC | human BMSC | seeded onto dECMs in the expansion medium for 14 days | cell number | strongly promoted proliferation with a low level of ROS | 96 |
| human BMSC | human BMSC | seeded on dECMs | cell number | promoted cell proliferation | 95 |
| human BMSC | human BMSC | cultured on TCPS coated with dECMs | DNA content | dECMs extracted in urea dramatically enhanced cell proliferation compared to collagen and HPMECM coating | 97 |
| human BMSC (fetal and adult) | human adult BMSC | cultured on dECMs from fetal and adult BMSCs | cell number | superior proliferation on fetal dECMs compared to other conditions including TCPS and adult dECMs | 101 |
| human BMSC | human BMSC | cultured on dECMs supplemented with melatonin | DNA content | promoted cell proliferation and improved melatonin-mediated cell proliferation | 100 |
| human IFPSC | human IFPSC | expanded on dECMs | proliferation index | promoted stem cell proliferation | 65 |
| human SDSC | human SDSC | expanded on dECMs by exposing to varied concentrations of H2O2 | proliferation index and cell number | promoted proliferation and alleviated oxidative stress-mediated proliferation reduction by lowering apoptosis rate and elevating G1 transition | 237 |
| human SDSC (fetal and adult) | human adult SDSC | expanded on dECMs from fetal and adult SDSCs | cell number/ apoptotic percentage | promoted proliferation and decreased apoptosis; fetal dECM was higher proliferation than adult dECM | 69 |
| human SDSC | human SDSC | expanded on dECMs | cell number | promoted cell proliferation | 140 |
| human SDSC | human SDSC | expanded on dECMs and treated with sb203580 | cell number | enhanced proliferation and further enhanced by sb203580 preconditioning | 238 |
| human UC-MSC | human UC-MSC | cultured on dECMs | DNA content | promoted UC-MSC proliferation compared to TCPS | 239 |
| porcine chondrocyte | human BMSC | cultured on dECM–collagen microspheres | live/dead viability | supported cell survival after 7-day culture | 102 |
| porcine chondrocyte | rabbit chondrocyte | seeded on physically prepared dECM in a culture medium | DNA content | supported chondrocyte proliferation | 240 |
| porcine chondrocyte | rabbit chondrocyte | seeded on physically prepared dECM in a culture medium for 2 days, 2 weeks and 4 weeks | DNA content | supported chondrocyte proliferation | 241 |
| porcine SDSC | porcine chondrocyte | cultured on dECMs | cell number | enhanced chondrocyte expansion | 99 |
| porcine SDSC | porcine NPC | cultured on dECMs | cell number | enhanced NPC proliferation | 98 |
| porcine SDSC | porcine SDSC | cultured on dECM | cell number | promoted proliferation compared to TCPS | 31 |
| porcine SDSC | porcine SDSC | seeded on dECMs | cell number | greatly enhanced cell proliferation | 242 |
| porcine SDSC | porcine SDSC | expanded on dECMs | p-cyclin D1 expression | enhanced SDSC proliferation | 217 |
| porcine SDSC and chondrocyte | porcine SDSC and chondrocyte | expanded on dECMs from both SDSCs and chondrocytes | cell number | promoted cell proliferation during expansion on both dECMs, especially on SDSC-derived dECMs | 92 |
| porcine SDSC and IFPSC | porcine IFPSC | expanded on dECMs from SDSCs/IPFSCs | cell number | enhanced cell proliferation | 91 |
| porcine SDSC and NPC | porcine SDSC | cultured on dECMs from SDSCs, NPCs and SDSCs/NPCs that were fabricated under normoxia or hypoxia | cell number | enhanced in vitro proliferation, similar in normoxia- and hypoxia-made dECMs | 94 |
| rat chondrocyte | rat chondrocyte | seeded on dECMs | cell number | promoted chondrocyte proliferation | 93 |
Abbreviations:BMSC: bone marrow stromal cell; dECM: decellularized extracellular matrix; HPMECM: pepsin digested mesenchymal stem cell-derived dECM; IPFSC: infrapatellar fat pad derived stem cell; NPC: nucleus pulposus cell; ROS: reactive oxygen species; SDSC: synovium derived stem cell; TCPS: tissue culture polystyrene; UC-MSC: umbilical cord derived mesenchymal stem cell.
Table 4.
Effect of C-dECMs on chondrocyte/stem cell chondrogenic differentiation.
| dECM origin | Seeded cell type | Treatment | Results | Reference |
|---|---|---|---|---|
| calf chondrocyte | rabbit BMSC | seeded on dECM-PCL scaffolds and cultured in a medium with the addition of TGF-β1 | supported chondrogenic differentiation | 243 |
| bovine chondrocyte and rabbit MSC | rabbit BMSC | cultured on physically prepared dECM/PCL scaffolds in chondrogenic medium with or without TGF-β3 | cocultures of chondrocytes and BMSCs on PCL scaffold coated with cartilage-like ECM supported chondrogenic differentiation | 244 |
| human BMSC | human BMSC | seeded on physically prepared dECM with chondrogenic induction | ECM scaffold mimicking early stage chondrogenesis promoted more chondrogenic differentiation than that of the ECM scaffold mimicking late stage chondrogenesis | 29 |
| human BMSC | human BMSC | seeded on physically prepared dECM fabricated by human BMSC sheets cultured with TGF-β1 in the media or with TGF-β1-loaded microspheres | enhanced in vitro chondrogenesis | 30 |
| human BMSC | human IPFSC | seeded on physically prepared dECM from porcine ECM (Native), human engineered sheets (Eng) and from human engineered sheets with microspheres (Eng-MS) followed by chondrogenic induction | supported chondrogenesis of IPFSCs; higher chondrogenesis within native ECM scaffolds compared to engineered ECM scaffolds | 28 |
| human BMSC | human BMSC | expanded on dECMs followed by a pellet culture with chondrogenic induction | enhanced expanded stem cell chondrogenic potential | 95 |
| human BMSC (fetal and adult) | human adult BMSC | cultured on dECMs followed by 3D spheroid culture with chondrogenic induction | fetal dECMs enhanced chondrogenic potential of late-passage BMSCs | 101 |
| human BMSC | human chondrocyte (OA and healthy) | cultured on dECM-PCL scaffolds in chondrogenic medium; dECMs were deposited by BMSCs that were grown in chondrogenic medium (CM) or basic medium (BM) | BM-derived scaffolds had superior effect on chondrogenic differentiation of healthy chondrocytes compared to CM; no significant influence of dECMs on chondrogenic differentiation of OA chondrocytes | 64 |
| human BMSC and chondrocyte | human BMSC | grown on dECMs that were first coated on PLGA mesh discs followed by removal of PLGA and cultured in chondrogenic induction medium for 4 weeks | promoted chondrogenic differentiation; dECMs from BMSCs were better than from chondrocytes | 63 |
| human BMSC and USC | human BMSC | expanded on dECMs followed by a pellet culture with chondrogenic induction | dECMs from repeated passage BMSCs decreased chondrogenic rejuvenation ability; dECMs from USCs strengthened repeated passage BMSC chondrogenic potential | 106 |
| human chondrocyte | human PDMSC | mixed with dECMs followed by a pellet culture with chondrogenic induction (BMP-6, TGF-β3) | enhanced chondrogenic differentiation | 104 |
| human IPFSC | human IPFSC | expanded on dECMs that were fabricated using various doses and durations of AA followed by a pellet culture with chondrogenic induction | promoted chondrogenic potential | 65 |
| human SDSC | human SDSC | expanded on dECMs followed by a pellet culture with chondrogenic induction under hypoxia or normoxia | promoted chondrogenic potential and further enhanced by low oxygen (5%) during pellet culture | 245 |
| human SDSC | human SDSC | expanded on dECMs and followed by a pellet culture with chondrogenic induction | promoted chondrogenic potential and further enhanced after treatment with H2O2 in both proliferation and chondrogenic phases | 237 |
| human SDSC (fetal and adult) | human adult SDSC | expanded on dECMs deposited by SDSCs from fetal and adult donors followed by a pellet culture with chondrogenic induction | enhanced chondrogenic potential; fetal dECM was superior to adult dECMs | 69 |
| human SDSC | human SDSC | expanded on dECMs followed by a pellet culture with chondrogenic induction | promoted SDSC chondrogenic potential | 140 |
| human SDSC | human SDSC | expanded on dECMs followed by a pellet culture with chondrogenic induction; treatment of sb203580 either in proliferation or differentiation phases | dECMs enhanced chondrogenic potential which was further enhanced by sb203580 preconditioning; sb203580 preconditioning promoted dECM rejuvenated SDSCs’ ability against inflammation during chondrogenic induction | 238 |
| porcine chondrocyte | human BMSC | seeded on dECM-type I collagen microspheres and cultured in the medium without chondrogenic induction | supported chondrogenic differentiation | 102 |
| porcine chondrocyte | rabbit BMSC | seeded on dECM pellets with chondrogenic induction but no TGF-β addition followed by implantation in nude mice | supported in vitro chondrogenic differentiation; maintained longer chondrogenic phenotypes in vivo compared to PGA scaffolds | 1 |
| porcine chondrocyte | rabbit chondrocyte | seeded on physically prepared dECM in a culture medium | promoted cartilage formation in vitro | 240 |
| porcine chondrocyte | rabbit chondrocyte | seeded on physically prepared dECM and implanted in rabbit osteochondral defects | supported chondrogenesis in vitro, repaired the osteochondral defects in vivo | 241 |
| porcine chondrocyte | rabbit chondrocyte | seeded onto physically prepared dECM followed by implantation into the nude mouse | produced a hyaline-like cartilage tissue in vivo | 246 |
| porcine SDSC | porcine chondrocyte | expanded on dECMs followed by a pellet culture with chondrogenic induction | delayed dedifferentiation, retained redifferentiation capacity and enhanced redifferentiation | 99 |
| porcine SDSC | porcine NPC | grown on dECMs followed by a pellet culture with chondrogenic induction | enhanced and restored redifferentiation capacity | 98 |
| porcine SDSC | porcine SDSC | grown on dECM-coated TCPS followed by a pellet culture with chondrogenic induction | enhanced chondrogenic potential and prevented hypertrophic differentiation | 31 |
| porcine SDSC | porcine SDSC | expanded on dECMs in hypoxia or normoxia with or without FGF2, followed by a pellet culture with chondrogenic induction | promoted chondrogenic potential and enhanced when combined with hypoxia and FGF2; downregulated hypertrophic differentiation | 242 |
| porcine SDSC | porcine SDSC | expanded on dECMs followed by a pellet culture with chondrogenic induction in vitro, or injected into pig knees with cartilage defects | enhanced chondrogenic potential in vitro and enhanced SDSCs in vivo cartilage regeneration | 217 |
| porcine SDSC and chondrocyte | porcine SDSC and chondrocyte | expanded on dECMs followed by a pellet culture with chondrogenic induction | enhanced cell chondrogenic potential, particularly for cells expanded on dECM deposited by SDSCs | 92 |
| porcine SDSC and IPFSC | porcine IPFSC | expanded on dECMs followed by a pellet culture with chondrogenic induction (TGF-β3 alone or combined with BMP-6) | enhanced chondrogenic capacity and decreased hypertrophic differentiation; combined with BMP-6 further enhanced chondrogenic capacity | 91 |
| porcine SDSC and/or NPC | porcine SDSC/NPC | expanded on dECMs that were fabricated under normoxia or hypoxia followed by a pellet culture with chondrogenic induction | enhanced chondrogenic potential | 94 |
| rabbit BMSC | rabbit BMSC | seeded on physically prepared dECM with chondrogenic induction with or without TGF-β3 in vitro followed with implantation into nude mice | promoted chondrogenic differentiation of BMSCs without any exogenous growth factors in vitro and in vivo | 247 |
| rabbit chondrocyte | human BMSC | cultured on dECMs with chondrogenic induction containing BMP-2 | promoted chondrogenic differentiation | 103 |
| rabbit NPC | human BMSC, rabbit BMSC | human BMSCs were reseeded in dECM-collagen microspheres; rabbit BMSCs were seeded in dECM-collagen microspheres followed by injection in a pilot rabbit disc degeneration model | promoted differentiation toward a NPC-like lineage in vitro and in vivo | 105 |
| rat chondrocyte | rat chondrocyte | seeded on dECMs for 2D monolayer culture and 3D pellet culture in a chondrogenic medium without chondrogenic growth factors | supported chondrocyte re-differentiation in 2D culture and 3D pellet culture | 93 |
Abbreviations:2D: two-dimensional; 3D: three-dimensional; AA: L-ascorbic acid; BMSC: bone marrow stromal cell; BMP: bone morphogenetic protein; dECM: decellularized extracellular matrix; ECM: extracellular matrix; FGF2: basic fibroblast growth factor; IPFSC: infrapatellar fat pad derived stem cell; NPC: nucleus pulposus cells; OA: osteoarthritis; PCL: poly(ε-caprolactone); PDMSC: placenta derived mesenchymal stem cell; PGA: polyglycolic acid; PLGA: polymer lactic-glycolic acid; SDSC: synovium derived stem cell; TCPS: tissue culture polystyrene; TGF: transforming growth factor; USC: urine derived stem cell.
4.1. Proliferation
C-dECMs fabricated by chondrocytes and stem cells offer superior platforms to expand chondrocytes/stem cells for cartilage regeneration in contrast to traditional methods [92–94]. Many studies have demonstrated that culturing on C-dECMs substantially elevated the proliferation capacity of cartilage-forming cells relative to culturing on tissue culture polystyrene (TCPS). For example, our group has repeatedly demonstrated enhanced proliferation of BMSCs, synovium derived stem cells (SDSCs), infrapatellar fat pad derived stem cells (IPFSCs) and chondrocytes when cultured on C-dECMs [31,91,92,95]. Other researchers also reported similar findings that C-dECMs promoted cell proliferation by analyzing cell count and DNA content [93,96,97]. In addition to autologous cell derived C-dECMs, C-dECMs from allogeneic cells also supported the expansion of corresponding stem cells and chondrocytes [91,92,98,99]. These studies indicated that C-dECMs promoted the proliferation of stem cells and chondrocytes, possibly through the downregulation of intracellular reactive oxygen species (ROS) [91,95,96,100]
Interestingly, there exists the varying ability of C-dECMs in promoting proliferation of cartilage-forming cells. Li et al. [69] demonstrated that both fetal and adult C-dECMs from human SDSCs could promote adult human SDSC proliferation, but fetal C-dECMs had a higher proliferation capacity than adult C-dECMs. The findings were in agreement with another study where Ng et al. [101] found that fetal C-dECMs from human BMSCs were more effective in promoting adult human BMSC expansion than adult C-dECMs. Moreover, late-passage adult human BMSCs cultured on TCPS for six passages and subsequently cultured on fetal C-dECMs were significantly improved in terms of proliferation, suggesting that C-dECMs from fetal stem cells have the ability to enhance and rescue the proliferation of older cells.
4.2. Chondrogenic differentiation
Increasing evidence demonstrates that C-dECMs provide a 3D microenvironment for promoting chondrogenic potential. Our group repeatedly demonstrated that SDSCs, BMSCs, NP cells, IPFSCs and chondrocytes, expanded on autograft or allograft C-dECMs, exerted enhanced chondrogenic differentiation when these cells were subsequently subjected to chondrogenic induction in a pellet culture system, indicating that C-dECMs could promote chondrogenic potential [31,91,94,95,98,99]. Other studies showed that C-dECM expansion also promoted chondrogenic differentiation in a 3D culture system with or without supplementation of chondrogenic inductive factors [93,102–104]. C-dECMs could support chondrogenic differentiation of stem cells and NP cells after in vivo implantation in animal models [1,105]. These studies indicate that C-dECMs alone or in combination with chondroinductive factors could enhance chondrogenic capacity in vitro and in vivo.
Chondrogenic capacity varied according to the C-dECM properties, which may be influenced by different cell sources. For example, C-dECMs from porcine SDSCs had a comparable effect in enhancing chondrogenic potential of porcine IPFSCs compared to that of C-dECMs from porcine IPFSCs [91]. C-dECMs from human BMSCs showed a stronger stimulatory effect in promoting chondrogenic differentiation of human BMSCs than those from human chondrocytes, perhaps due to the easy dedifferentiation of chondrocytes during in vitro expansion culture [63]. Moreover, Pei et al. [106] showed that, human urine derived stem cells (USCs) did not differentiate into chondrocytes; however, C-dECM deposited by human USCs could enhance senescent human BMSCs toward a chondrogenic capacity, suggesting that trophic factors that were released by human USCs immobilized in C-dECMs contributed to the other stem cells’ chondrogenic capacity.
Interestingly, C-dECMs from repeated passage human BMSCs had a limited effect on chondrogenic differentiation of human BMSCs [106], while C-dECMs from fetal human BMSCs significantly improved differentiation potential in late-passage adult human BMSCs compared to TCPS [101], implying a superior role of C-dECMs from young cells to old cells. Li et al. [69] further demonstrated that expansion on C-dECMs deposited by fetal SDSCs (FECM) was superior to C-dECM deposited by adult SDSCs in promoting chondrogenic potential of adult human SDSCs in vitro. These results may be associated with unique protein components and the lower elasticity of FECM that was responsible for the enhancement of chondrogenic differentiation. Moreover, early stage chondrogenesis-mimicking dECM scaffold facilitated more chondrogenesis in human BMSCs than that of late stage chondrogenesis-mimicking dECM scaffold [29]. Devitalized tissue engineered cartilaginous sheets formed in the presence of growth factor releasing microspheres were considered to be more developmentally immature tissue and to support comparable levels of GAG synthesis to native cartilage ECM scaffolds [28]. The varying effects of ECM scaffolds on the chondrogenic differentiation of MSCs may be due to differences in their composition. The immature cartilage contained appropriate chondroinductive components that are not available in mature cartilage to provide a milieu of features more conducive to chondrogenesis [28,29].
5. Potential influential factors of dECMs on chondrogenesis
Although both T-dECMs and C-dECMs supported chondrogenesis, increasing studies indicated that cartilage T-dECMs were more likely to facilitate chondrogenic differentiation [27], while C-dECMs had a superior tendency to support cell proliferation and chondrogenic potential [31]. However, potential influential factors resulting in the differences in these two dECMs on chondrogenesis are unclear and there is a lack of direct evidence. Previous studies demonstrated that inherent properties and components of scaffolds, such as microarchitecture characteristics, micromechanical properties and biochemical components, were involved in the regulation of cell proliferation and chondrogenic differentiation [26,107–109], providing clues to elucidate the different effects of dECMs on chondrogenesis.
5.1. Microarchitecture
Much evidence has shown that the porous and nano-fibrous structure provided an initial microenvironment to support chondrogenesis [107,108]. Various parameters of the microarchitecture of scaffolds, such as pore size and fiber diameter, influence cellular behaviors such as proliferation and chondrogenic differentiation.
Pore size
Scaffolds with various pore sizes fabricated from synthetic and natural materials, such as PLGA [110], calcium polyphosphate [111], gelatin [112], collagen [113,114] and chitosan [115], provide 3D architectural support for stem cell chondrogenesis. There are still conflicting results as to which scaffold mean pore size is optimal for chondrogenesis. For example, higher cell proliferation was observed when chondrocytes/stem cells were cultured on collagen-hyaluronic acid (HA) scaffolds with pore sizes of 300 μm [108], poly-L-lactide-co-trimethylene carbonate scaffolds with pore sizes of 175 μm [116] and chitosan scaffolds with pore sizes of 70–120 μm [115], suggesting that larger pore sizes improved better proliferation compared with smaller pore sizes. These findings may be partially explained by larger pore sizes providing ample space, thus improving the distribution of cells and nutrients throughout the scaffolds and facilitating cell proliferation. In contrast, other studies demonstrated that type I collagen scaffolds with pore sizes of 20 μm, poly(ε-caprolactone) scaffold with pore sizes of 100 or 200 μm, as well as poly(ε-caprolactone) cylindrical scaffolds with pore sizes of 90–105 μm supported superior proliferation of chondrocytes and stem cells compared to those with larger pore sizes [113,117,118]. The abovementioned studies indicate that scaffolds having a higher surface area (smaller pore size) possess a larger cell adhesion area, thus enabling better cell attachment, migration and growth.
Recent findings demonstrated that scaffolds with smaller pore sizes (20–150 μm) facilitated greater levels of chondrogenic differentiation compared to scaffolds with larger pore sizes [113,116,119,120]. Stem cells seeded on scaffolds with smaller pore sizes outperformed larger pore sizes in promoting cartilaginous tissue formation in vitro and in vivo [119,121]. Scaffolds with smaller pore sizes also demonstrated a significantly greater ability to maintain chondrocyte morphology and exerted superior chondrogenic differentiation of chondrocytes compared to scaffolds with larger pore sizes [113,116]. These findings suggest that smaller pore sizes lead to closely packed cells and 3D cell aggregation as well as a lower oxygen environment, which may correlate positively with chondrogenic differentiation.
However, contrary studies exist. An increasing number of studies revealed that scaffolds with larger pore sizes (250–500 μm) had superior effects in promoting chondrogenic differentiation of chondrocytes/stem cells by producing cartilage ECM [108,117,118,122,123]. Interestingly, some studies demonstrated superior effects of smaller pore sizes in terms of cell proliferation, although they failed to outperform larger pore sizes in enhancing chondrogenic differentiation [117,118], indicating that smaller pore size initially allows greater cell attachment to enhance proliferation. Moreover, chondrocytes in the smaller pores often display a dedifferentiated form while the chondrocyte phenotype is maintained better in larger pores, suggesting that larger pore sizes are more likely to support chondrogenic differentiation [122].
Interestingly, cartilage T-dECMs with larger pore sizes promoted obvious chondrogenic differentiation of adult stem cells and formed cartilage-like tissue [27,55], while C-dECMs with smaller pore sizes effectively improved chondrocyte attachment and proliferation [93], further indicating that scaffold pore sizes may be responsible for the differences in chondrogenesis. Therefore, it is believed that cartilage T-dECMs with larger pore sizes more likely facilitate cell infiltration for chondrogenic differentiation, while C-dECMs with relatively smaller pore sizes may tend to support cell proliferation and chondrogenic potential.
Fiber diameter
The nanofibrous structure of scaffolds, morphologically similar in dimension to native collagen fibrils, has been demonstrated to increase chondrocyte expansion and maintain chondrocyte phenotype [124,125] as well as promote chondrogenic ECM deposition [126]. Nanofiber scaffolds could enhance in vitro chondrogenesis of BMSCs and repair cartilage defects after implantation into animal models [127–131], suggesting that nanofiber scaffolds support chondrogenesis of adult stem cells in vitro and in vivo. Recent studies also demonstrated that microfiber scaffolds supported chondrocyte proliferation and promoted cartilage ECM production [125,132,133]. Moreover, human BMSCs cultured on microfiber scaffolds exerted higher levels of cellular proliferation and chondrogenic differentiation in comparison to cells on nanofiber scaffolds [134,135], suggesting a superior role of microfiber diameter in supporting chondrogenesis compared to nanofiber diameter. The strategies for dispersing nanofibers into the microfiber scaffolds that have improved micromechanical properties also provide favorable conditions to enhance cell adhesion and proliferation [136,137]. These studies indicated that both nanofiber and microfiber scaffolds could support chondrogenesis.
It has been reported that the fiber diameter could influence cell behaviors such as proliferation, migration and differentiation [135,138,139]. For example, tube diameters of 15–20 nm strongly enhanced cellular activities, while cell proliferation, migration and differentiation were seriously damaged on nanotube layers with a tube diameter larger than 50 nm, particularly for 100 nm [139]. Moreover, small (around 30 nm diameter) nanotubes boosted adhesion without clear differentiation, whereas larger (70–100 nm diameter) nanotubes more likely triggered dramatic cytoskeletal stress and differentiation of human BMSCs [138]. Interestingly, some studies showed higher levels of chondrogenesis with nanofiber scaffolds compared with microfiber scaffolds [125,131], despite contrasting studies showing that chondrogenic gene expression was significantly enhanced on microfiber scaffolds compared with nanofiber scaffolds [134,135]. Recent studies have demonstrated that chondrocytes/stem cells cultured on cartilage T-dECMs and C-dECMs, which were made of nanoscale ECM fibers, exerted obvious cell proliferation and chondrogenic differentiation [31,140,141], also indicating that fiber diameter may be attributed to the differences in chondrogenesis.
Increasing evidence shows that fiber diameter could influence scaffold pore size which, in turn, affects chondrogenesis. Smaller diameter fibers generating smaller average pore sizes decreased cellular infiltration and reduced the advantages of 3D culture by limiting cell growth to the top of scaffolds. Larger diameter fibers resulting in larger pore size have been shown to improve cell infiltration and promote higher levels of stem cell differentiation [107,135]. Therefore, cells seeded onto fiber matrices with smaller pore sizes tend to spread, attach and, in some cases, extend along the length of the fibers, which facilitates cell proliferation. In contrast, fiber scaffolds with larger pore sizes achieve a 3D microenvironment for cellular infiltration, which more likely supports chondrogenic differentiation.
5.2. Micromechanical properties
Numerous studies have demonstrated that micromechanical properties of substrates, such as elasticity and stiffness, could influence cell adhesion, spreading and differentiation [142–144]. A well-known example is that human BMSCs efficaciously differentiated into bone, muscle or neuronal lineages, respectively, when cultured on stiff, medium or soft substrates [143], suggesting that inherent stiffness similar to that of corresponding native tissues more likely induced stem cells to differentiate into targeted tissues. Substrates with the lowest stiffness (0.5 kPa) directed rat BMSCs toward a chondrogenic lineage, but the stiffest scaffolds (1.5 kPa) resulted in BMSCs that differentiated toward an osteogenic lineage [145], suggesting that substrate elasticity can affect stem cell differentiation.
Studies on various stiffnesses of substrates, fabricated by hydrogels [146–153] and porous/fibrous materials [145,154,155], indicate that soft substrates more likely support chondrogenesis compared to stiffer substrates [156]. Interestingly, human SDSCs expanded on C-dECMs have greatly enhanced proliferation and chondrogenic potential compared to TCPS whose elasticity could be considered as infinite, suggesting that a lower stiffness of C-dECMs may be responsible for enhanced chondrogenic potential of expanded cells [31,140]. Moreover, lower elasticity of C-dECMs deposited by fetal SDSCs maintained better chondrogenic potential compared to those of adult C-dECMs or TCPS that had higher elasticity [69], suggesting that controlled elasticity of dECM provides great potential in regulating reseeded cell chondrogenesis.
Interestingly, higher stiffness of materials supported better cartilage regeneration compared to those with less stiffness [157–159]. For example, a storage modulus of about 45 Pa (weaker than naturally occurring cartilage) of HA:poly(ethylene glycol) hydrogel supported better chondrogenic differentiation of human BMSCs compared to lower stiffnesses of other scaffolds [157]. Chondroitin sulfate-containing hydrogels with a stiffness of ~7–33 kPa resulted in neocartilage formation after subcutaneous implantation into nude mice by co-encapsulating human ADSCs and calf chondrocytes; in contrast, soft stiffness (~1kPa) scaffolds failed to maintain structural integrity and broke into pieces in vivo [159], indicating that minimal initial stiffness is required to retain micromechanical integrity over time.
Despite the fact that micromechanical properties of natural cartilage varied due to different types of cartilage [160,161], compared to the native tissue, decreased linear modulus of meniscus/nasal septum T-dECM [60] and equilibrium and dynamic modulus of cartilage T-dECM [74] after decellularization supported chondrogenic differentiation. Furthermore, Abdelgaied and coworkers found a lower compressive elastic modulus in meniscus T-dECM compared to the nature structure, which could be explained by a 60% loss of GAG content [162]. The extraction of GAGs during decellularization resulted in the loss of water [163,164], contributing to an increase of stiffness [165,166]. An increasing number of studies showed that micromechanical properties of the scaffolds, which match native cartilage, supported chondrogenic differentiation by directing stem cells into the chondrogenic lineage and enhancing cartilage formation in vitro and in vivo [158,167,168]. Therefore, the micromechanical properties may be involved in regulating chondrogenesis of chondrocytes/stem cells by designing scaffolds with appropriate elasticity to control chondrogenic differentiation.
5.3. Chemical components
In addition to the abovementioned microstructural-micromechanical functionality, ECM is also composed of insoluble components such as collagen and GAGs as well as soluble factors such as growth factors, which play a significant role in cell-ECM interaction and cell proliferation and lineage-specific differentiation.
Insoluble factors
Collagens are primary insoluble components of cartilage ECM that form a tensile meshwork with a high compressive strength. Among various collagens, type II collagen is a dominant component of cartilage, while type I collagen and type X collagen are primarily located in the fibrocartilage and calcified regions, respectively [169,170]. Many studies have shown that collagens contribute to chondrogenic differentiation of chondrocytes/stem cells in vitro and in vivo [171,172]. For example, type II collagen alone promoted GAG level and the re-expression of cartilaginous marker mRNAs in human senescent chondrocytes (Passage 7) in 2D culture in a dose-dependent fashion [173]. Chondrocytes cultured in scaffolds, made by processing both type I and type II collagen, maintained chondrocyte phenotypes and accumulated cartilaginous ECMs during the culture period [171,173,174]. These collagen-based scaffolds also supported stem cell chondrogenesis as evidenced by promotion of chondrogenic differentiation and production of cartilaginous ECMs [175–177]. Furthermore, incorporation of collagens with natural polymers and synthetic polymers, such as agarose [178], poly(epsilon-caprolactone) [179] and chitosan [180], could enhance stem cell chondrogenesis in both micromechanical and biological properties. Though type I collagen is extensively used in cartilage engineering, interestingly, chondrogenic differentiation was more prominent in type II collagen when chondrocytes/stem cells were cultured in these collagen-based scaffolds [173,181,182]. These studies indicate that collagens can be biomaterials for providing chondroinductive cues to support chondrogenic differentiation for cartilage regeneration.
GAGs are polysaccharides that either link to protein cores to form proteoglycans or are free within cartilage ECM. Important types of GAGs include sulfated chondroitin sulfate, non-sulfated HA, keratan sulfate, sulfated heparin sulfate, heparin and dermatan sulfate [183,184]. Many studies have demonstrated that GAGs may regulate chondrogenic differentiation of chondrocytes/stem cells. For example, exogenous heparin sulfate stimulated chondrogenic differentiation of mesenchymal cells from embryonic chick limb buds and murine mesenchymal stem cells (MSCs) [185,186]. However, exogenous chondroitin sulfate did not show a significant effect on chondrocytes (Passage 7), while HA inhibited the expression of SRY (sex determining region Y)-box 9 and aggrecan mRNAs [173]. These findings suggest that various types of GAGs exert different capacities in regulating chondrogenic differentiation.
Recent studies showed that incorporation of GAG with hydrogel or PLGA could induce chondrogenic differentiation of stem cells in vitro and synthesize cartilage tissues in vivo [187–189]. Importantly, collagen-GAG scaffolds used to fabricate biocompatible scaffolds supported substantial chondrogenesis of chondrocytes/stem cells [108,109,145,190,191]. Interestingly, collagen-GAG scaffolds exerted various capacities in promoting chondrogenesis due to GAG types and contents [145,192]. For instance, incorporating HA into the scaffolds significantly enhanced chondrogenic differentiation compared to chondroitin sulfate, suggesting that HA may be more suitable for cartilage engineering [145,193]. Although HA supplementation into type I collagen hydrogels promoted chondrogenic differentiation of human ADSCs and chondrocytes, 1% HA showed the best overall effect compared to 5% HA [192]. These studies indicate that a combination of collagens and GAGs could promote chondrogenesis, perhaps depending on GAG types and contents within collagen-based scaffolds.
Increasing evidence indicates that collagens and GAGs in cartilage T-dECMs were preserved or partially reduced, maintaining an ECM-rich microenvironment to provide structural support and chondroinductive cues [22,27,59,73]. Reduced GAGs or collagens resulting in a 3D porous structure with decreased micromechanical properties after decellularization [27,55,60,194] may facilitate chondrogenic differentiation. However, C-dECMs were predominantly composed of type I collagen with negligible levels of type II collagen and GAGs [31], which might be responsible for promoting reseeded cell expansion and chondrogenic potential rather than chondrogenic differentiation [61].
Soluble factors
Numerous studies have demonstrated that native cartilage ECM scaffolds can act as reservoirs of bioactive factors, which influence cell behavior and regulate ECM production [57,58,78,195]. The soluble factors, such as transforming growth factor beta (TGF-β) [196–198], bone morphogenetic protein (BMP) [199,200], fibroblast growth factor (FGF) [201–204], insulin-like growth factor I (IGF-I) [205] and growth and differentiation factor 5 (GDF-5)[206,207], influenced cell proliferation and chondrogenic differentiation. Soluble factors individually and synergistically supported chondrocytes/stem cells toward chondrogenesis in both monolayer and pellet culture [91,99,200,208,209]. Supplementation with soluble factors, such as TGF-β, BMP and Mechano Growth Factor (MGF) IGF-I, into various scaffolds promoted efficient cartilage formation in human MSCs [210,211], indicating that these soluble factors could enhance chondrogenesis. Furthermore, it has been reported that TGF-β could bind to heparin sulfate [212] while GAGs stimulated the release of free and bioactive IGF-I in vitro [213], indicating that the interaction of soluble factors and GAGs may potentially influence chondrogenic differentiation.
Recent studies demonstrated that various soluble factors resulted in obvious differences in chondrogenesis. For example, TGF-β was always required for chondrogenic differentiation [198,209], while BMP-2, −4, −6, −7, acid FGF and IGF-I (10 ng/mL) alone lacked sufficient chondrogenic inductivity [214]. TGF-β3 supported more efficient chondrogenesis [215], while human BMSCs differentiated in a pellet culture system with TGF-β1 had significantly less mineralization than those cultured with TGF-β3 [216], despite the fact that both TGF-β isoforms performed similarly in directing porcine SDSC chondrogenesis [217]. Interestingly, some soluble factors, such as BMP-2, GDF-5 and TGF-β1, induced chondrogenic differentiation of chondrocytes/stem cells that was accompanied by hypertrophic expression, such as type X collagen and matrix metallopeptidase 13 (MMP13) [206,218,219]. These studies indicate that various soluble factors support chondrogenesis but exert different capacities.
Despite few reports to evaluate and compare the effect of soluble factors in dECM, Xue et al. found that soluble factors such as TGF-β1, basic FGF, IGF-I and BMP-2 remained in T-dECM after decellularization [78]. dECM from porcine NP cells partially retained TGF-β and its membrane bound receptor TGF-β receptor I after decellularization, which promoted differentiation toward a NP cell-like lineage in vitro and in vivo, suggesting that the presence of these soluble factors may contribute to the altered phenotype in reseeded MSCs [105]. Recently, a region-specific distribution was found with the existence of basic FGF only in outer meniscus dECM but higher TGF-β concentrations in inner meniscus dECM following decellularization [72], indicative of a site-dependent influence of these soluble factors on chondrogenesis.
6. Conclusion and perspective
As novel biomaterials used for cartilage regeneration, dECMs from cartilage tissues and cartilage-forming cells can be fabricated through physical, chemical and/or enzymatic methods. Both types of dECM demonstrate biocompatibility to support chondrogenesis by regulating cell proliferation and chondrogenic differentiation. Interestingly, cartilage T-dECMs are more likely to facilitate chondrogenic differentiation, while C-dECMs support chondrocyte/stem cell proliferation and promote chondrogenic potential. Various parameters, including microarchitecture such as mean pore size and fiber diameter, micromechanical properties, insoluble components (such as collagen and GAGs) and soluble factors, may be responsible for differences in these dECMs in supporting chondrogenesis. The above data suggest that both C-dECMs and T-dECMs may be ideal biomaterials to support the sequential chondrogenesis of reseeded cells, providing optimal scaffolds for the treatment of cartilage diseases.
However, some limitations exist to prevent further investigation into potential clinical applications. Currently there is no standard method of decellularization for cartilage. Despite an exploration of varying methods in the literature, decellularization of cartilage dECM may not only alter matrix architecture and micromechanical properties, but also remove some important components in native ECM, which may affect the capacity of dECMs to support chondrogenesis. dECMs with large pore size and soft micromechanical properties more efficiently support chondrogenesis, while increasing exposure time to decellularization agents results in a decrease in micromechanical integrity and structure as well as the loss of native components. Furthermore, both C-dECMs and T-dECMs during chondrogenesis exert unique capacities in supporting proliferation and chondrogenic differentiation. Cartilage T-dECMs with a larger pore size mostly retained type II collagen and GAGs [27], while negligible levels of type II collagen and GAGs as well as smaller pore sizes were observed in C-dECMs [31,91,93]. Other parameters such as fiber diameter, micromechanical properties and retention of soluble factors also influence chondrogenesis. Thus, the key components in dECMs should be further investigated to elucidate the variables that direct reseeded cells toward chondrogenesis.
Acknowledgements
We thank Suzanne Danley for editing the manuscript. This work was supported by Research Grants from the Musculoskeletal Transplant Foundation (MTF) and the National Institutes of Health (1R01AR067747-01A1) to M.P., Study Abroad Scholarship from Jiangsu Province and Jiangsu Province Youth Medical Talents (QNRC2016344) to Y.S., and Natural Science Foundation of China (81601889) to S.C..
Footnotes
Disclosure Statement
No competing financial interests exist.
Reference
- [1].Choi KH, Choi BH, Park SR, Kim BJ, Min BH, The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes, Biomaterials 31 (2010) 5355–5365. [DOI] [PubMed] [Google Scholar]
- [2].Sutherland AJ, Converse GL, Hopkins RA, Detamore MS, The bioactivity of cartilage extracellular matrix in articular cartilage regeneration, Adv. Healthc. Mater 4 (2015) 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Correa D, Lietman SA, Articular cartilage repair: Current needs, methods and research directions, Semin. Cell Dev. Biol 62 (2017) 67–77. [DOI] [PubMed] [Google Scholar]
- [4].Bedi A, Feeley BT, Williams RJ 3rd, Management of articular cartilage defects of the knee, J. Bone Joint Surg. Am 92 (2010) 994–1009. [DOI] [PubMed] [Google Scholar]
- [5].Karnes J, Zhang Y, Pei M, Cell Therapy for the Creation of Cartilage and Related Clinical Trials. N. S. Templeton, Gene and Cell Therapy: Therapeutic Mechanisms and Strategies, 4th Edition, Taylor & Francis/CRC Press, 2015, pp. 1123–1135. [Google Scholar]
- [6].Hunziker EB, Lippuner K, Keel MJ, Shintani N, An educational review of cartilage repair: precepts & practice--myths & misconceptions--progress & prospects, Osteoarthritis Cartilage 23 (2015) 334–350. [DOI] [PubMed] [Google Scholar]
- [7].Redman SN, Oldfield SF, Archer CW, Current strategies for articular cartilage repair, Eur. Cell Mater 9 (2005) 23–32; discussion 23–32. [DOI] [PubMed] [Google Scholar]
- [8].Bartlett W, Gooding CR, Carrington RW, Skinner JA, Briggs TW, Bentley G, Autologous chondrocyte implantation at the knee using a bilayer collagen membrane with bone graft, A preliminary report, J. Bone Joint Surg. Br 87 (2005) 330–332. [DOI] [PubMed] [Google Scholar]
- [9].Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle JA, A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee, J. Bone Joint Surg. Br 85 (2003) 223–230. [DOI] [PubMed] [Google Scholar]
- [10].Smith GD, Knutsen G, Richardson JB, A clinical review of cartilage repair techniques, J. Bone Joint Surg. Br 87 (2005) 445–449. [DOI] [PubMed] [Google Scholar]
- [11].Mamidi MK, Das AK, Zakaria Z, Bhonde R, Mesenchymal stromal cells for cartilage repair in osteoarthritis, Osteoarthritis Cartilage 24 (2016) 1307–1316. [DOI] [PubMed] [Google Scholar]
- [12].Pei M, Solchaga LA, Seidel J, Zeng L, Caplan AI, Vunjak-Novakovic G, Freed LE, Bioreactors mediate the effectiveness of tissue engineering scaffolds, FASEB. J 16 (2002) 1691–1694. [DOI] [PubMed] [Google Scholar]
- [13].Almeida HV, Eswaramoorthy R, Cunniffe GM, Buckley CT, O’Brien FJ, Kelly DJ, Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration, Acta Biomater. 36 (2016) 55–62. [DOI] [PubMed] [Google Scholar]
- [14].Liao J, Shi K, Ding Q, Qu Y, Luo F, Qian Z, Recent developments in scaffold-guided cartilage tissue regeneration, J. Biomed. Nanotechnol 10 (2014) 3085–3104. [DOI] [PubMed] [Google Scholar]
- [15].Nooeaid P, Salih V, Beier JP, Boccaccini AR, Osteochondral tissue engineering: scaffolds, stem cells and applications, J. Cell Mol. Med 16 (2012) 2247–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bradham DM, Passaniti A, Horton WE Jr., Mesenchymal cell chondrogenesis is stimulated by basement membrane matrix and inhibited by age-associated factors, Matrix Biol. 14 (1995) 561–571. [DOI] [PubMed] [Google Scholar]
- [17].Gattazzo F, Urciuolo A, Bonaldo P, Extracellular matrix: a dynamic microenvironment for stem cell niche, Biochim. Biophys. Acta 1840 (2014) 2506–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lynch K, Pei M, Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies, Organogenesis 10 (2014) 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sun Y, Wang TL, Toh WS, Pei M, The role of laminins in cartilaginous tissues: from development to regeneration, Eur, Cell Mater. 34 (2017) 40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Crapo PM, Gilbert TW, Badylak SF, An overview of tissue and whole organ decellularization processes, Biomaterials 32 (2011) 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gilbert TW, Sellaro TL, Badylak SF, Decellularization of tissues and organs, Biomaterials 27 (2006) 3675–3683. [DOI] [PubMed] [Google Scholar]
- [22].Rothrauff BB, Yang G, Tuan RS, Tissue-specific bioactivity of soluble tendon-derived and cartilage-derived extracellular matrices on adult mesenchymal stem cells, Stem Cell Res. Ther 8 (2017) 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Y, He Y, Bharadwaj S, Hammam N, Carnagey K, Myers R, Atala A, Van Dyke M, Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype, Biomaterials 30 (2009) 4021–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Benders KE, van Weeren PR, Badylak SF, Saris DB, Dhert WJ, Malda J, Extracellular matrix scaffolds for cartilage and bone regeneration, Trends Biotechnol. 31 (2013) 169–176. [DOI] [PubMed] [Google Scholar]
- [25].Cheng CW, Solorio LD, Alsberg E, Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering, Biotechnol. Adv 32 (2014) 462–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kiyotake EA, Beck EC, Detamore MS, Cartilage extracellular matrix as a biomaterial for cartilage regeneration, Ann. N. Y. Acad. Sci 1383 (2016) 139–159. [DOI] [PubMed] [Google Scholar]
- [27].Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, Yang F, Wang S, Xu W, Wang A, Lu S, A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells, Biomaterials 29 (2008) 2378–2387. [DOI] [PubMed] [Google Scholar]
- [28].Almeida HV, Dikina AD, Mulhall KJ, O’Brien FJ, Alsberg E, Kelly DJ, Porous Scaffolds Derived from Devitalized Tissue Engineered Cartilaginous Matrix Support Chondrogenesis of Adult Stem Cells, ACS Biomater. Sci. Eng 3 (2017) 1075–1082. [DOI] [PubMed] [Google Scholar]
- [29].Cai R, Nakamoto T, Kawazoe N, Chen G, Influence of stepwise chondrogenesis-mimicking 3D extracellular matrix on chondrogenic differentiation of mesenchymal stem cells, Biomaterials 52 (2015) 199–207. [DOI] [PubMed] [Google Scholar]
- [30].Dikina AD, Almeida HV, Cao M, Kelly DJ, Alsberg E, Scaffolds Derived from ECM Produced by Chondrogenically Induced Human MSC Condensates Support Human MSC Chondrogenesis, ACS Biomater. Sci. Eng 3 (2017) 1426–1436. [DOI] [PubMed] [Google Scholar]
- [31].He F, Chen X, Pei M, Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering, Tissue Eng. Part A 15 (2009) 3809–3821. [DOI] [PubMed] [Google Scholar]
- [32].Zhang W, Zhu Y, Li J, Guo Q, Peng J, Liu S, Yang J, Wang Y, Cell-Derived Extracellular Matrix: Basic Characteristics and Current Applications in Orthopedic Tissue Engineering, Tissue Eng. Part B Rev 22 (2016) 193–207. [DOI] [PubMed] [Google Scholar]
- [33].Arzi B, DuRaine GD, Lee CA, Huey DJ, Borjesson DL, Murphy BG, Hu JCY, Baumgarth N, Athanasiou KA, Cartilage immunoprivilege depends on donor source and lesion location, Acta Biomater. 23 (2015) 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bolano L, Kopta JA, The immunology of bone and cartilage transplantation, Orthopedics 14 (1991) 987–996. [DOI] [PubMed] [Google Scholar]
- [35].Fujihara Y, Takato T, Hoshi K, Immunological response to tissue-engineered cartilage derived from auricular chondrocytes and a PLLA scaffold in transgenic mice, Biomaterials 31 (2010) 1227–1234. [DOI] [PubMed] [Google Scholar]
- [36].Pei M, He F, Boyce BM, Kish VL, Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs, Osteoarthritis Cartilage 17 (2009) 714–722. [DOI] [PubMed] [Google Scholar]
- [37].Ramallal M, Maneiro E, López E, Fuentes-Boquete I, López-Armada MJ, Fernández-Sueiro JL, Galdo F, De Toro FJ, Blanco FJ, Xeno-implantation of pig chondrocytes into rabbit to treat localized articular cartilage defects: an animal model, Wound Repair Regen. 12 (2004) 337–345. [DOI] [PubMed] [Google Scholar]
- [38].Revell CM, Athanasiou KA, Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defects, Tissue Eng. Part B Rev 15 (2009) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Langer F, Gross AE, Immunogenicity of allograft articular cartilage, J. Bone Joint Surg. Am 56 (1974) 297–304. [PubMed] [Google Scholar]
- [40].Masuoka K, Asazuma T, Ishihara M, Sato M, Hattori H, Ishihara M, Yoshihara Y, Matsui T, Takase B, Kikuchi M, Nemoto K, Tissue engineering of articular cartilage using an allograft of cultured chondrocytes in a membrane-sealed atelocollagen honeycomb-shaped scaffold (ACHMS scaffold), J. Biomed. Mater. Res. B Appl. Biomater 75 (2005) 177–184. [DOI] [PubMed] [Google Scholar]
- [41].Shangkai C, Naohide T, Koji Y, Yasuji H, Masaaki N, Tomohiro T, Yasushi T, Transplantation of allogeneic chondrocytes cultured in fibroin sponge and stirring chamber to promote cartilage regeneration, Tissue Eng. 13 (2007) 483–492. [DOI] [PubMed] [Google Scholar]
- [42].Tanaka T, Komaki H, Chazono M, Fujii K, Use of a biphasic graft constructed with chondrocytes overlying a beta-tricalcium phosphate block in the treatment of rabbit osteochondral defects, Tissue Eng. 11 (2005) 331–339. [DOI] [PubMed] [Google Scholar]
- [43].Malejczyk J, Osiecka A, Hyc A, Moskalewski S, Effect of immunosuppression on rejection of cartilage formed by transplanted allogeneic rib chondrocytes in mice, Clin. Orthop. Relat. Res 269 (1991) 266–273. [PubMed] [Google Scholar]
- [44].Moskalewski S, Hyc A, Osiecka-Iwan A, Immune response by host after allogeneic chondrocyte transplant to the cartilage, Microsc. Res. Tech 58 (2002) 3–13. [DOI] [PubMed] [Google Scholar]
- [45].Stevenson S, Dannucci GA, Sharkey NA, Pool RR, The fate of articular cartilage after transplantation of fresh and cryopreserved tissue-antigen-matched and mismatched osteochondral allografts in dogs, J. Bone Joint Surg. Am 71 (1989) 1297–1307. [PubMed] [Google Scholar]
- [46].Yamaga KM, Kimura LH, Plymyer MR, Glant TT, Lance EM, Differentiation antigens of human articular chondrocytes and their tissue distribution as assessed by monoclonal antibodies, J. Autoimmun 7 (1994) 203–217. [DOI] [PubMed] [Google Scholar]
- [47].Huey DJ, Sanchez-Adams J, Willard VP, Athanasiou KA, Immunogenicity of bovine and leporine articular chondrocytes and meniscus cells, Tissue Eng. Part A 18 (2012) 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lance EM, Immunological reactivity towards chondrocytes in rat and man: relevance to autoimmune arthritis, Immunol. Lett 21 (1989) 63–73. [DOI] [PubMed] [Google Scholar]
- [49].Romaniuk A, Malejczyk J, Kubicka U, Hyc A, Olszewski WL, Moskalewski S, Rejection of cartilage formed by transplanted allogeneic chondrocytes: evaluation with monoclonal antibodies, Transpl. Immunol 3 (1995) 251–257. [DOI] [PubMed] [Google Scholar]
- [50].Kawabe N, Yoshinao M, The repair of full-thickness articular cartilage defects. Immune responses to reparative tissue formed by allogeneic growth plate chondrocyte implants, Clin. Orthop. Relat. Res 268 (1991) 279–293. [PubMed] [Google Scholar]
- [51].Schreiber RE, Ilten-Kirby BM, Dunkelman NS, Symons KT, Rekettye LM, Willoughby J, Ratcliffe A, Repair of osteochondral defects with allogeneic tissue engineered cartilage implants, Clin. Orthop. Relat. Res 367 Suppl (1999) S382–395. [DOI] [PubMed] [Google Scholar]
- [52].Wang Y, Huang YC, Gertzman AA, Xie L, Nizkorodov A, Hyzy SL, Truncale K, Guldberg RE, Schwartz Z, Boyan BD, Endogenous regeneration of critical-size chondral defects in immunocompromised rat xiphoid cartilage using decellularized human bone matrix scaffolds, Tissue Eng. Part A 18 (2012) 2332–2342. [DOI] [PubMed] [Google Scholar]
- [53].Badylak SF, Taylor D, Uygun K, Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds, Annu. Rev. Biomed. Eng 13 (2011) 27–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Li J, Pei M, A Protocol to Prepare Decellularized Stem Cell Matrix for Rejuvenation of Cell Expansion and Cartilage Regeneration, Methods Mol. Biol (2017) doi: 10.1007/7651_2017_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yang Z, Shi Y, Wei X, He J, Yang S, Dickson G, Tang J, Xiang J, Song C, Li G, Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold, Tissue Eng. Part C Methods 16 (2010) 865–876. [DOI] [PubMed] [Google Scholar]
- [56].Cheng NC, Estes BT, Awad HA, Guilak F, Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix, Tissue Eng. Part A 15 (2009) 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Almeida HV, Cunniffe GM, Vinardell T, Buckley CT, O’Brien FJ, Kelly DJ, Coupling Freshly Isolated CD44(+) Infrapatellar Fat Pad-Derived Stromal Cells with a TGF-ß3 Eluting Cartilage ECM-Derived Scaffold as a Single-Stage Strategy for Promoting Chondrogenesis, Adv. Healthc. Mater 4 (2015) 1043–1053. [DOI] [PubMed] [Google Scholar]
- [58].Almeida HV, Liu Y, Cunniffe GM, Mulhall KJ, Matsiko A, Buckley CT, O’Brien FJ, Kelly DJ, Controlled release of transforming growth factor-beta3 from cartilage-extra-cellular-matrix derived scaffolds to promote chondrogenesis of human-joint-tissue derived stem cells, Acta Biomater. 10 (2014) 4400–4409. [DOI] [PubMed] [Google Scholar]
- [59].Beck EC, Barragan M, Libeer TB, Kieweg SL, Converse GL, Hopkins RA, Berkland CJ, Detamore MS, Chondroinduction from Naturally Derived Cartilage Matrix: A Comparison Between Devitalized and Decellularized Cartilage Encapsulated in Hydrogel Pastes, Tissue Eng. Part A 22 (2016) 665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schwarz S, Koerber L, Elsaesser AF, Goldberg-Bockhorn E, Seitz AM, Dürselen L, Ignatius A, Walther P, Breiter R, Rotter N, Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications, Tissue Eng. Part A 18 (2012) 2195–2209. [DOI] [PubMed] [Google Scholar]
- [61].Pei M, Environmental preconditioning rejuvenates adult stem cells’ proliferation and chondrogenic potential, Biomaterials 117 (2017) 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Pei M, Li JT, Shoukry M, Zhang Y, A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering, Eur. Cell Mater 22 (2011) 333–343. [DOI] [PubMed] [Google Scholar]
- [63].Lu H, Hoshiba T, Kawazoe N, Koda I, Song M, Chen G, Cultured cell-derived extracellular matrix scaffolds for tissue engineering, Biomaterials 32 (2011) 9658–9666. [DOI] [PubMed] [Google Scholar]
- [64].Thakkar S, Ghebes CA, Ahmed M, Kelder C, van Blitterswijk CA, Saris D, Fernandes HA, Moroni L, Mesenchymal stromal cell-derived extracellular matrix influences gene expression of chondrocytes, Biofabrication 5 (2013) 025003. [DOI] [PubMed] [Google Scholar]
- [65].Pizzute T, Zhang Y, He F, Pei M, Ascorbate-dependent impact on cell-derived matrix in modulation of stiffness and rejuvenation of infrapatellar fat derived stem cells toward chondrogenesis, Biomed. Mater 11 (2016) 045009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Levorson EJ, Hu O, Mountziaris PM, Kasper FK, Mikos AG, Cell-derived polymer/extracellular matrix composite scaffolds for cartilage regeneration, Part 2: construct devitalization and determination of chondroinductive capacity, Tissue Eng. Part C Methods 20 (2014) 358–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Elder BD, Eleswarapu SV, Athanasiou KA, Extraction techniques for the decellularization of tissue engineered articular cartilage constructs, Biomaterials 30 (2009) 3749–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Badylak SF, Gilbert TW, Immune response to biologic scaffold materials, Semin. Immunol 20 (2008) 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Li J, Hansen KC, Zhang Y, Dong C, Dinu CZ, Dzieciatkowska M, Pei M, Rejuvenation of chondrogenic potential in a young stem cell microenvironment, Biomaterials 35 (2014) 642–653. [DOI] [PubMed] [Google Scholar]
- [70].Chang CH, Chen CC, Liao CH, Lin FH, Hsu YM, Fang HW, Human acellular cartilage matrix powders as a biological scaffold for cartilage tissue engineering with synovium-derived mesenchymal stem cells, J. Biomed. Mater. Res. A 102 (2014) 2248–2257. [DOI] [PubMed] [Google Scholar]
- [71].Utomo L, Pleumeekers MM, Nimeskern L, Nürnberger S, Stok KS, Hildner F, van Osch GJ, Preparation and characterization of a decellularized cartilage scaffold for ear cartilage reconstruction, Biomed. Mater 10 (2015) 015010. [DOI] [PubMed] [Google Scholar]
- [72].Rothrauff BB, Shimomura K, Gottardi R, Alexander PG, Tuan RS, Anatomical region-dependent enhancement of 3-dimensional chondrogenic differentiation of human mesenchymal stem cells by soluble meniscus extracellular matrix, Acta. Biomater 49 (2017) 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chen YC, Chen RN, Jhan HJ, Liu DZ, Ho HO, Mao Y, Kohn J, Sheu MT, Development and Characterization of Acellular Extracellular Matrix Scaffolds from Porcine Menisci for Use in Cartilage Tissue Engineering, Tissue Eng. Part C Methods 21 (2015) 971–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Luo L, Eswaramoorthy R, Mulhall KJ, Kelly DJ, Decellularization of porcine articular cartilage explants and their subsequent repopulation with human chondroprogenitor cells, J. Mech. Behav. Biomed. Mater 55 (2016) 21–31. [DOI] [PubMed] [Google Scholar]
- [75].Mercuri JJ, Gill SS, Simionescu DT, Novel tissue-derived biomimetic scaffold for regenerating the human nucleus pulposus, J. Biomed. Mater. Res. A 96 (2011) 422–435. [DOI] [PubMed] [Google Scholar]
- [76].Rowland CR, Colucci LA, Guilak F, Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds, Biomaterials 91 (2016) 57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gong YY, Xue JX, Zhang WJ, Zhou GD, Liu W, Cao Y, A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes, Biomaterials, 32 (2011) 2265–2273. [DOI] [PubMed] [Google Scholar]
- [78].Xue JX, Gong YY, Zhou GD, Liu W, Cao Y, Zhang WJ, Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets, Biomaterials 33 (2012) 5832–5840. [DOI] [PubMed] [Google Scholar]
- [79].Elsaesser AF, Bermueller C, Schwarz S, Koerber L, Breiter R, Rotter N, In vitro cytotoxicity and in vivo effects of a decellularized xenogeneic collagen scaffold in nasal cartilage repair, Tissue Eng. Part A 20 (2014) 1668–1678. [DOI] [PubMed] [Google Scholar]
- [80].Kang H, Peng J, Lu S, Liu S, Zhang L, Huang J, Sui X, Zhao B, Wang A, Xu W, Luo Z, Guo Q, In vivo cartilage repair using adipose-derived stem cell-loaded decellularized cartilage ECM scaffolds, J. Tissue Eng. Regen. Med 8 (2014) 442–453. [DOI] [PubMed] [Google Scholar]
- [81].Gonfiotti A, Jaus MO, Barale D, Baiguera S, Comin C, Lavorini F, Fontana G, Sibila O, Rombolà G, Jungebluth P, Macchiarini P, The first tissue-engineered airway transplantation: 5-year follow-up results, Lancet 383 (2014) 238–244. [DOI] [PubMed] [Google Scholar]
- [82].Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA, Clinical transplantation of a tissue-engineered airway, Lancet 372 (2008) 2023–2030. [DOI] [PubMed] [Google Scholar]
- [83].Ansari T, Lange P, Southgate A, Greco K, Carvalho C, Partington L, Bullock A, MacNeil S, Lowdell MW, Sibbons PD, Birchall MA, Stem Cell-Based Tissue-Engineered Laryngeal Replacement, Stem Cells Transl. Med 6 (2017) 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Xu Y, Li D, Yin Z, He A, Lin M, Jiang G, Song X, Hu X, Liu Y, Wang J, Wang X, Duan L, Zhou G, Tissue-engineered trachea regeneration using decellularized trachea matrix treated with laser micropore technique, Acta. Biomater 58 (2017) 113–121. [DOI] [PubMed] [Google Scholar]
- [85].Mercuri JJ, Patnaik S, Dion G, Gill SS, Liao J, Simionescu DT, Regenerative potential of decellularized porcine nucleus pulposus hydrogel scaffolds: stem cell differentiation, matrix remodeling, and biocompatibility studies, Tissue Eng. Part A 19 (2013) 952–966. [DOI] [PubMed] [Google Scholar]
- [86].Grevemeyer B, Bogdanovic L, Canton S, St Jean G, Cercone M, Ducharme NG, Brown BN, Regenerative medicine approach to reconstruction of the equine upper airway, Tissue Eng. Part A 20 (2014) 1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Novak T, Fites Gilliland K, Xu X, Worke L, Ciesielski A, Breur G, Neu CP, In Vivo Cellular Infiltration and Remodeling in a Decellularized Ovine Osteochondral Allograft, Tissue Eng. Part A 22 (2016) 1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rothrauff BB, Coluccino L, Gottardi R, Ceseracciu L, Scaglione S, Goldoni L, Tuan RS, Efficacy of thermoresponsive, photocrosslinkable hydrogels derived from decellularized tendon and cartilage extracellular matrix for cartilage tissue engineering, J. Tissue Eng. Regen. Med (2017) doi: 10.1002/term.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Vindas Bolaños RA, Cokelaere SM, Estrada McDermott JM, Benders KE, Gbureck U, Plomp SG, Weinans H, Groll J, van Weeren PR, Malda J, The use of a cartilage decellularized matrix scaffold for the repair of osteochondral defects: the importance of long-term studies in a large animal model, Osteoarthritis Cartilage 25 (2017) 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Farr J, Gracitelli GC, Shah N, Chang EY, Gomoll AH, High Failure Rate of a Decellularized Osteochondral Allograft for the Treatment of Cartilage Lesions, Am. J. Sports Med 44 (2016) 2015–2022. [DOI] [PubMed] [Google Scholar]
- [91].He F, Pei M, Extracellular matrix enhances differentiation of adipose stem cells from infrapatellar fat pad toward chondrogenesis, J. Tissue Eng. Regen. Med 7 (2013) 73–84. [DOI] [PubMed] [Google Scholar]
- [92].Pei M, He F, Wei L, Three-Dimensional Cell Expansion Substrate For Cartilage Tissue Engineering And Regeneration: A Comparison In Decellularized Matrix Deposited By Synovium-Derived Stem Cells And Chondrocytes, J. Tissue Sci. Eng 2 (2011) 104. [Google Scholar]
- [93].Cha MH, Do SH, Park GR, Du P, Han KC, Han DK, Park K, Induction of re-differentiation of passaged rat chondrocytes using a naturally obtained extracellular matrix microenvironment, Tissue Eng. Part A 19 (2013) 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Pei M, Shoukry M, Li J, Daffner SD, France JC, Emery SE, Modulation of in vitro microenvironment facilitates synovium-derived stem cell-based nucleus pulposus tissue regeneration, Spine (Phila Pa 1976) 37 (2012) 1538–1547. [DOI] [PubMed] [Google Scholar]
- [95].Pei M, He F, Kish VL, Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential, Tissue Eng. Part A 17 (2011) 3067–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lai Y, Sun Y, Skinner CM, Son EL, Lu Z, Tuan RS, Jilka RL, Ling J, Chen XD, Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells, Stem Cells Dev. 19 (2010) 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lin H, Yang G, Tan J, Tuan RS, Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential, Biomaterials 33 (2012) 4480–4489. [DOI] [PubMed] [Google Scholar]
- [98].He F, Pei M, Rejuvenation of nucleus pulposus cells using extracellular matrix deposited by synovium-derived stem cells, Spine (Phila Pa 1976) 37 (2012) 459–469. [DOI] [PubMed] [Google Scholar]
- [99].Pei M, He F, Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation, J. Cell. Physiol 227 (2012) 2163–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].He F, Liu X, Xiong K, Chen S, Zhou L, Cui W, Pan G, Luo ZP, Pei M, Gong Y, Extracellular matrix modulates the biological effects of melatonin in mesenchymal stem cells, J. Endocrinol 223 (2014) 167–180. [DOI] [PubMed] [Google Scholar]
- [101].Ng CP, Sharif AR, Heath DE, Chow JW, Zhang CB, Chan-Park MB, Hammond PT, Chan JK, Griffith LG, Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM, Biomaterials 35 (2014) 4046–4057. [DOI] [PubMed] [Google Scholar]
- [102].Cheng HW, Tsui YK, Cheung KM, Chan D, Chan BP, Decellularization of chondrocyte-encapsulated collagen microspheres: a three-dimensional model to study the effects of acellular matrix on stem cell fate, Tissue Eng. Part C Methods 15 (2009) 697–706. [DOI] [PubMed] [Google Scholar]
- [103].Kwon SH, Lee TJ, Park J, Hwang JE, Jin M, Jang HK, Hwang NS, Kim BS, Modulation of BMP-2-induced chondrogenic versus osteogenic differentiation of human mesenchymal stem cells by cell-specific extracellular matrices, Tissue Eng. Part A 19 (2013) 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Park YB, Seo S, Kim JA, Heo JC, Lim YC, Ha CW, Effect of chondrocyte-derived early extracellular matrix on chondrogenesis of placenta-derived mesenchymal stem cells, Biomed. Mater 10 (2015) 035014. [DOI] [PubMed] [Google Scholar]
- [105].Yuan M, Yeung CW, Li YY, Diao H, Cheung KM, Chan D, Cheah K, Chan PB, Effects of nucleus pulposus cell-derived acellular matrix on the differentiation of mesenchymal stem cells, Biomaterials 34 (2013) 3948–3961. [DOI] [PubMed] [Google Scholar]
- [106].Pei M, Li J, Zhang Y, Liu G, Wei L, Zhang Y, Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells, Cell Tissue Res. 356 (2014) 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Dahlin RL, Kasper FK, Mikos AG, Polymeric nanofibers in tissue engineering, Tissue Eng. Part B Rev 17 (2011) 349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Matsiko A, Gleeson JP, O’Brien FJ, Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition, Tissue Eng. Part A 21 (2015) 486–497. [DOI] [PubMed] [Google Scholar]
- [109].Matsiko A, Levingstone TJ, Gleeson JP, O’Brien FJ, Incorporation of TGF-beta 3 within collagen-hyaluronic acid scaffolds improves their chondrogenic potential, Adv. Healthc. Mater 4 (2015) 1175–1179. [DOI] [PubMed] [Google Scholar]
- [110].Uematsu K, Hattori K, Ishimoto Y, Yamauchi J, Habata T, Takakura Y, Ohgushi H, Fukuchi T, Sato M, Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold, Biomaterials 26 (2005) 4273–4279. [DOI] [PubMed] [Google Scholar]
- [111].Lien SM, Liu CK, Huang TJ, A novel surface modification on calcium polyphosphate scaffold for articular cartilage tissue engineering, Mater. Sci. Eng. C 27 (2007) 127–134. [Google Scholar]
- [112].Lien SM, Li WT, Huang TJ, Genipin-crosslinked gelatin scaffolds for articular cartilage tissue engineering with a novel crosslinking method, Mater. Sci. Eng. C 28 (2008) 36–43. [Google Scholar]
- [113].Nehrer S, Breinan HA, Ramappa A, Young G, Shortkroff S, Louie LK, Sledge CB, Yannas IV, Spector M, Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes, Biomaterials 18 (1997) 769–776. [DOI] [PubMed] [Google Scholar]
- [114].Zhang Q, Lu H, Kawazoe N, Chen G, Pore size effect of collagen scaffolds on cartilage regeneration, Acta. Biomater 10 (2014) 2005–2013. [DOI] [PubMed] [Google Scholar]
- [115].Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL, Chitosan scaffolds: interconnective pore size and cartilage engineering, Acta. Biomater 2 (2006) 313–320. [DOI] [PubMed] [Google Scholar]
- [116].Nava MM, Draghi L, Giordano C, Pietrabissa R, The effect of scaffold pore size in cartilage tissue engineering, J. Appl. Biomater. Funct. Mater 14 (2016) e223–e229. [DOI] [PubMed] [Google Scholar]
- [117].Im GI, Ko JY, Lee JH, Chondrogenesis of adipose stem cells in a porous polymer scaffold: influence of the pore size, Cell Transplant. 21 (2012) 2397–2405. [DOI] [PubMed] [Google Scholar]
- [118].Oh SH, Kim TH, Im GI, Lee JH, Investigation of pore size effect on chondrogenic differentiation of adipose stem cells using a pore size gradient scaffold, Biomacromolecules 11 (2010) 1948–1955. [DOI] [PubMed] [Google Scholar]
- [119].Matsiko A, Levingstone TJ, O’Brien FJ, Gleeson JP, Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering, J. Mech. Behav. Biomed. Mater 11 (2012) 41–52. [DOI] [PubMed] [Google Scholar]
- [120].Stenhamre H, Nannmark U, Lindahl A, Gatenholm P, Brittberg M, Influence of pore size on the redifferentiation potential of human articular chondrocytes in poly(urethane urea) scaffolds, J. Tissue Eng. Regen. Med 5 (2011) 578–588. [DOI] [PubMed] [Google Scholar]
- [121].Duan P, Pan Z, Cao L, He Y, Wang H, Qu Z, Dong J, Ding J, The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits, J. Biomed. Mater. Res. A 102 (2014) 180–192. [DOI] [PubMed] [Google Scholar]
- [122].Lien SM, Ko LY, Huang TJ, Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering, Acta. Biomater 5 (2009) 670–679. [DOI] [PubMed] [Google Scholar]
- [123].Yamane S, Iwasaki N, Kasahara Y, Harada K, Majima T, Monde K, Nishimura S, Minami A, Effect of pore size on in vitro cartilage formation using chitosan-based hyaluronic acid hybrid polymer fibers, J. Biomed. Mater. Res. A 81 (2007) 586–593. [DOI] [PubMed] [Google Scholar]
- [124].Li WJ, Danielson KG, Alexander PG, Tuan RS, Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds, J. Biomed. Mater. Res. A 67 (2003) 1105–1114. [DOI] [PubMed] [Google Scholar]
- [125].Li WJ, Jiang YJ, Tuan RS, Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size, Tissue Eng. 12 (2006) 1775–1785. [DOI] [PubMed] [Google Scholar]
- [126].da Silva MA, Crawford A, Mundy J, Martins A, Araújo JV, Hatton PV, Reis RL, Neves NM, Evaluation of extracellular matrix formation in polycaprolactone and starch-compounded polycaprolactone nanofiber meshes when seeded with bovine articular chondrocytes. Tissue Eng. Part A 15 (2009) 377–385. [DOI] [PubMed] [Google Scholar]
- [127].Coburn JM, Gibson M, Monagle S, Patterson Z, Elisseeff JH, Bioinspired nanofibers support chondrogenesis for articular cartilage repair, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 10012–10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS, Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold, Biomaterials 26 (2005) 5158–5166. [DOI] [PubMed] [Google Scholar]
- [129].Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS, A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells, Biomaterials 26 (2005) 599–609. [DOI] [PubMed] [Google Scholar]
- [130].Li WJ, Chiang H, Kuo TF, Lee HS, Jiang CC, Tuan RS, Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: a pilot study, J. Tissue Eng. Regen. Med 3 (2009) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wise JK, Yarin AL, Megaridis CM, Cho M, Chondrogenic differentiation of human mesenchymal stem cells on oriented nanofibrous scaffolds: engineering the superficial zone of articular cartilage, Tissue Eng. Part A 15 (2009) 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Visser J, Melchels FP, Jeon JE, van Bussel EM, Kimpton LS, Byrne HM, Dhert WJ, Dalton PD, Hutmacher DW, Malda J, Reinforcement of hydrogels using three-dimensionally printed microfibres, Nat. Commun 6 (2015) 6933. [DOI] [PubMed] [Google Scholar]
- [133].Yodmuang S, McNamara SL, Nover AB, Mandal BB, Agarwal M, Kelly TA, Chao PH, Hung C, Kaplan DL, Vunjak-Novakovic G, Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair, Acta. Biomater 11 (2015) 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Bean AC, Tuan RS, Fiber diameter and seeding density influence chondrogenic differentiation of mesenchymal stem cells seeded on electrospun poly(ε-caprolactone) scaffolds, Biomed. Mater 10 (2015) 015018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Shanmugasundaram S, Chaudhry H, Arinzeh TL, Microscale versus nanoscale scaffold architecture for mesenchymal stem cell chondrogenesis, Tissue Eng. Part A 17 (2011) 831–840. [DOI] [PubMed] [Google Scholar]
- [136].Kim SJ, Jang DH, Park WH, Min BM, Fabrication and characterization of 3-dimensional PLGA nanofiber/microfiber composite scaffolds, Polymer 51 (2010) 1320–1327. [Google Scholar]
- [137].Park SH, Kim TG, Kim HC, Yang DY, Park TG, Development of dual scale scaffolds via direct polymer melt deposition and electrospinning for applications in tissue regeneration, Acta. Biomater 4 (2008) 1198–1207. [DOI] [PubMed] [Google Scholar]
- [138].Oh S, Brammer KS, Li YS, Teng D, Engler AJ, Chien S, Jin S, Stem cell fate dictated solely by altered nanotube dimension, Proc. Natl. Acad. Sci. U. S. A 106 (2009) 2130–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Park J, Bauer S, von der Mark K, Schmuki P, Nanosize and vitality: TiO2 nanotube diameter directs cell fate, Nano. Lett 7 (2007) 1686–1691. [DOI] [PubMed] [Google Scholar]
- [140].Zhang Y, Li J, Davis ME, Pei M, Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix, Acta. Biomater 20 (2015) 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zheng X, Yang F, Wang S, Lu S, Zhang W, Liu S, Huang J, Wang A, Yin B, Ma N, Zhang L, Xu W, Guo Q, Fabrication and cell affinity of biomimetic structured PLGA/articular cartilage ECM composite scaffold, J. Mater. Sci. Mater. Med 22 (2011) 693–704. [DOI] [PubMed] [Google Scholar]
- [142].Discher DE, Janmey P, Wang YL, Tissue cells feel and respond to the stiffness of their substrate, Science 310 (2005) 1139–1143. [DOI] [PubMed] [Google Scholar]
- [143].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification, Cell 126 (2006) 677–689. [DOI] [PubMed] [Google Scholar]
- [144].Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, Chen X, Roberts CJ, Stevens MM, Substrate stiffness affects early differentiation events in embryonic stem cells, Eur. Cell Mater 18 (2009) 1–13; discussion 13–14. [DOI] [PubMed] [Google Scholar]
- [145].Murphy CM, Matsiko A, Haugh MG, Gleeson JP, O’Brien FJ, Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds, J. Mech. Behav. Biomed. Mater 11 (2012) 53–62. [DOI] [PubMed] [Google Scholar]
- [146].Allen JL, Cooke ME, Alliston T, ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation, Mol. Biol. Cell 23 (2012) 3731–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kwon HJ, Yasuda K, Chondrogenesis on sulfonate-coated hydrogels is regulated by their mechanical properties, J. Mech. Behav. Biomed. Mater 17 (2013) 337–346. [DOI] [PubMed] [Google Scholar]
- [148].Liu SQ, Tian Q, Hedrick JL, Po Hui JH, Ee PL, Yang YY, Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage, Biomaterials, 31 (2010) 7298–7307. [DOI] [PubMed] [Google Scholar]
- [149].Nicodemus GD, Skaalure SC, Bryant SJ, Gel structure has an impact on pericellular and extracellular matrix deposition, which subsequently alters metabolic activities in chondrocyte-laden PEG hydrogels, Acta. Biomater 7 (2011) 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S, The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β, Biomaterials 32 (2011) 3921–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Schuh E, Kramer J, Rohwedel J, Notbohm H, Müller R, Gutsmann T, Rotter N, Effect of matrix elasticity on the maintenance of the chondrogenic phenotype, Tissue Eng. Part A 16 (2010) 1281–1290. [DOI] [PubMed] [Google Scholar]
- [152].Wang T, Lai JH, Han LH, Tong X, Yang F, Chondrogenic differentiation of adipose-derived stromal cells in combinatorial hydrogels containing cartilage matrix proteins with decoupled mechanical stiffness, Tissue Eng. Part A 20 (2014) 2131–2139. [DOI] [PubMed] [Google Scholar]
- [153].Xue R, Li JY, Yeh Y, Yang L, Chien S, Effects of matrix elasticity and cell density on human mesenchymal stem cells differentiation, J. Orthop. Res 31 (2013) 1360–1365. [DOI] [PubMed] [Google Scholar]
- [154].Nam J, Johnson J, Lannutti JJ, Agarwal S, Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers, Acta. Biomater 7 (2011) 1516–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Zhu C, Li J, Liu C, Zhou P, Yang H, Li B, Modulation of the gene expression of annulus fibrosus-derived stem cells using poly(ether carbonate urethane)urea scaffolds of tunable elasticity, Acta. Biomater 29 (2016) 228–238. [DOI] [PubMed] [Google Scholar]
- [156].Zhang Y, Chen S, Pei M, Biomechanical signals guiding stem cell cartilage engineering: from molecular adaption to tissue functionality, Eur. Cell Mater 31 (2016) 59–78. [DOI] [PubMed] [Google Scholar]
- [157].Dorcemus DL, George EO, Dealy CN, Nukavarapu SP, Harnessing External Cues: Development and Evaluation of an In Vitro Culture System for Osteochondral Tissue Engineering, Tissue Eng. Part A 23 (2017) 719–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Hendriks JA, Moroni L, Riesle J, de Wijn JR, van Blitterswijk CA, The effect of scaffold-cell entrapment capacity and physico-chemical properties on cartilage regeneration, Biomaterials 34 (2013) 4259–4265. [DOI] [PubMed] [Google Scholar]
- [159].Wang T, Lai JH, Yang F, Effects of Hydrogel Stiffness and Extracellular Compositions on Modulating Cartilage Regeneration by Mixed Populations of Stem Cells and Chondrocytes In Vivo, Tissue Eng. Part A 22 (2016) 1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Little CJ, Bawolin NK, Chen X, Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng. Part B Rev 17 (2011) 213–227. [DOI] [PubMed] [Google Scholar]
- [161].Moutos FT, Freed LE, Guilak F, A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage, Nat. Mater 6 (2007) 162–167. [DOI] [PubMed] [Google Scholar]
- [162].Abdelgaied A, Stanley M, Galfe M, Berry H, Ingham E, Fisher J, Comparison of the biomechanical tensile and compressive properties of decellularised and natural porcine meniscus, J. Biomech 48 (2015) 1389–1396. [DOI] [PubMed] [Google Scholar]
- [163].Schmidt MB, Mow VC, Chun LE, Eyre DR, Effects of proteoglycan extraction on the tensile behavior of articular cartilage, J. Orthop. Res 8 (1990) 353–363. [DOI] [PubMed] [Google Scholar]
- [164].Zhu W, Mow VC, Koob TJ, Eyre DR, Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments, J. Orthop. Res 11 (1993) 771–781. [DOI] [PubMed] [Google Scholar]
- [165].Azhim A, Ono T, Fukui Y, Morimoto Y, Furukawa K, Ushida T, Preparation of decellularized meniscal scaffolds using sonication treatment for tissue engineering, Conf. Proc. IEEE. Eng. Med. Biol. Soc 2013 (2013) 6953–6956. [DOI] [PubMed] [Google Scholar]
- [166].Maier D, Braeun K, Steinhauser E, Ueblacker P, Oberst M, Kreuz PC, Roos N, Martinek V, Imhoff AB, In vitro analysis of an allogenic scaffold for tissue-engineered meniscus replacement, J. Orthop. Res 25 (2007) 1598–1608. [DOI] [PubMed] [Google Scholar]
- [167].Müller WE, Neufurth M, Wang S, Tolba E, Schröder HC, Wang X, Morphogenetically active scaffold for osteochondral repair (polyphosphate/alginate/N,O-carboxymethyl chitosan), Eur. Cell Mater 31 (2016) 174–190. [DOI] [PubMed] [Google Scholar]
- [168].Younesi M, Goldberg VM, Akkus O, A micro-architecturally biomimetic collagen template for mesenchymal condensation based cartilage regeneration, Acta. Biomater 30 (2016) 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Aigner T, Stöve J, Collagens--major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair, Adv. Drug Deliv. Rev 55 (2003) 1569–1593. [DOI] [PubMed] [Google Scholar]
- [170].Ashhurst DE, Collagens synthesized by healing fractures, Clin. Orthop. Relat. Res (255) (1990) 273–283. [PubMed] [Google Scholar]
- [171].Kimura T, Yasui N, Ohsawa S, Ono K, Chondrocytes embedded in collagen gels maintain cartilage phenotype during long-term cultures, Clin. Orthop. Relat. Res (186) (1984) 231–239. [PubMed] [Google Scholar]
- [172].Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M, Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees, Osteoarthritis Cartilage 10 (2002) 199–206. [DOI] [PubMed] [Google Scholar]
- [173].Chiu LH, Chen SC, Wu KC, Yang CB, Fang CL, Lai WF, Tsai YH, Differential effect of ECM molecules on re-expression of cartilaginous markers in near quiescent human chondrocytes, J. Cell Physiol 226 (2011) 1981–1988. [DOI] [PubMed] [Google Scholar]
- [174].Claus S, Mayer N, Aubert-Foucher E, Chajra H, Perrier-Groult E, Lafont J, Piperno M, Damour O, Mallein-Gerin F, Cartilage-characteristic matrix reconstruction by sequential addition of soluble factors during expansion of human articular chondrocytes and their cultivation in collagen sponges, Tissue Eng. Part C Methods 18 (2012) 104–112. [DOI] [PubMed] [Google Scholar]
- [175].Calabrese G, Forte S, Gulino R, Cefalì F, Figallo E, Salvatorelli L, Maniscalchi ET, Angelico G, Parenti R, Gulisano M, Memeo L, Giuffrida R, Combination of Collagen-Based Scaffold and Bioactive Factors Induces Adipose-Derived Mesenchymal Stem Cells Chondrogenic Differentiation In vitro, Front. Physiol 8 (2017) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Bornes TD, Jomha NM, Mulet-Sierra A, Adesida AB, Optimal Seeding Densities for In Vitro Chondrogenesis of Two- and Three-Dimensional-Isolated and -Expanded Bone Marrow-Derived Mesenchymal Stromal Stem Cells Within a Porous Collagen Scaffold, Tissue Eng. Part C Methods 22 (2016) 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Yang J, Chen X, Yuan T, Yang X, Fan Y, Zhang X, Regulation of the secretion of immunoregulatory factors of mesenchymal stem cells (MSCs) by collagen-based scaffolds during chondrogenesis, Mater. Sci. Eng. C Mater. Biol. Appl 70 (2017) 983–991. [DOI] [PubMed] [Google Scholar]
- [178].Tiruvannamalai Annamalai R, Mertz DR, Daley EL, Stegemann JP, Collagen Type II enhances chondrogenic differentiation in agarose-based modular microtissues, Cytotherapy 18 (2016) 263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Kim HJ, Lee JH, Im GI, Chondrogenesis using mesenchymal stem cells and PCL scaffolds, J. Biomed. Mater. Res. A 92 (2010) 659–666. [DOI] [PubMed] [Google Scholar]
- [180].Raftery RM, Woods B, Marques AL, Moreira-Silva J, Silva TH, Cryan SA, Reis RL, O’Brien FJ, Multifunctional biomaterials from the sea: Assessing the effects of chitosan incorporation into collagen scaffolds on mechanical and biological functionality, Acta. Biomater 43 (2016) 160–169. [DOI] [PubMed] [Google Scholar]
- [181].Nehrer S, Breinan HA, Ramappa A, Shortkroff S, Young G, Minas T, Sledge CB, Yannas IV, Spector M, Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro, J. Biomed. Mater. Res 38 (1997) 95–104. [DOI] [PubMed] [Google Scholar]
- [182].Veilleux NH, Yannas IV, Spector M, Effect of passage number and collagen type on the proliferative, biosynthetic, and contractile activity of adult canine articular chondrocytes in type I and II collagen-glycosaminoglycan matrices in vitro, Tissue Eng. 10 (2004) 119–127. [DOI] [PubMed] [Google Scholar]
- [183].Melrose J, Shu C, Whitelock JM, Lord MS, The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation, Matrix. Biol 52–54 (2016) 363–383. [DOI] [PubMed] [Google Scholar]
- [184].Freudenberg U, Liang Y, Kiick KL, Werner C, Glycosaminoglycan-Based Biohybrid Hydrogels: A Sweet and Smart Choice for Multifunctional Biomaterials, Adv. Mater 28 (2016) 8861–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Fisher MC, Li Y, Seghatoleslami MR, Dealy CN, Kosher RA, Heparan sulfate proteoglycans including syndecan-3 modulate BMP activity during limb cartilage differentiation, Matrix. Biol 25 (2006) 27–39. [DOI] [PubMed] [Google Scholar]
- [186].Jha AK, Yang W, Kirn-Safran CB, Farach-Carson MC, Jia X, Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release, Biomaterials 30 (2009) 6964–6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Park SH, Choi BH, Park SR, Min BH, Chondrogenesis of rabbit mesenchymal stem cells in fibrin/hyaluronan composite scaffold in vitro, Tissue Eng. Part A 17 (2011) 1277–1286. [DOI] [PubMed] [Google Scholar]
- [188].Solchaga LA, Gao J, Dennis JE, Awadallah A, Lundberg M, Caplan AI, Goldberg VM, Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle, Tissue Eng. 8 (2002) 333–347. [DOI] [PubMed] [Google Scholar]
- [189].Wu SC, Chang JK, Wang CK, Wang GJ, Ho ML, Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment, Biomaterials 31 (2010) 631–640. [DOI] [PubMed] [Google Scholar]
- [190].Farrell E, O’Brien FJ, Doyle P, Fischer J, Yannas I, Harley BA, O’Connell B, Prendergast PJ, Campbell VA, A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes, Tissue Eng. 12 (2006) 459–468. [DOI] [PubMed] [Google Scholar]
- [191].van Susante JLC, Pieper J, Buma P, van Kuppevelt TH, van Beuningen H, van Der Kraan PM, Veerkamp JH, van den Berg WB, Veth RPH. Linkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro, Biomaterials 22 (2001) 2359–2369. [DOI] [PubMed] [Google Scholar]
- [192].Amann E, Wolff P, Breel E, van Griensven M, Balmayor ER, Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures, Acta. Biomater 52 (2017) 130–144. [DOI] [PubMed] [Google Scholar]
- [193].Banu N, Tsuchiya T, Markedly different effects of hyaluronic acid and chondroitin sulfate-A on the differentiation of human articular chondrocytes in micromass and 3-D honeycomb rotation cultures, J. Biomed. Mater. Res. A 80 (2007) 257–267. [DOI] [PubMed] [Google Scholar]
- [194].Schwarz S, Elsaesser AF, Koerber L, Goldberg-Bockhorn E, Seitz AM, Bermueller C, Dürselen L, Ignatius A, Breiter R, Rotter N, Processed xenogenic cartilage as innovative biomatrix for cartilage tissue engineering: effects on chondrocyte differentiation and function, J. Tissue Eng. Regen. Med 9 (2015) E239–251. [DOI] [PubMed] [Google Scholar]
- [195].Blaney Davidson EN, Vitters EL, van der Kraan PM, van den Berg WB, Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation, Ann. Rheum. Dis 65 (2006) 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Murdoch AD, Grady LM, Ablett MP, Katopodi T, Meadows RS, Hardingham TE, Chondrogenic differentiation of human bone marrow stem cells in transwell cultures: generation of scaffold-free cartilage, Stem Cells 25 (2007) 2786–2796. [DOI] [PubMed] [Google Scholar]
- [197].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR, Multilineage potential of adult human mesenchymal stem cells, Science 284 (1999) 143–147. [DOI] [PubMed] [Google Scholar]
- [198].Sekiya I, Vuoristo JT, Larson BL, Prockop DJ, In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis, Proc. Natl. Acad. Sci. U. S. A 99 (2002) 4397–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Iwakura T, Sakata R, Reddi AH, Induction of chondrogenesis and expression of superficial zone protein in synovial explants with TGF-β1 and BMP-7, Tissue Eng. Part A 19 (2013) 2638–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Murphy MK, Huey DJ, Hu JC, Athanasiou KA, TGF-β1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells, Stem Cells 33 (2015) 762–773. [DOI] [PubMed] [Google Scholar]
- [201].Pizzute T, Li J, Zhang Y, Davis ME, Pei M, Fibroblast Growth Factor Ligand Dependent Proliferation and Chondrogenic Differentiation of Synovium-Derived Stem Cells and Concomitant Adaptation of Wnt/Mitogen-Activated Protein Kinase Signals, Tissue Eng. Part A 22 (2016) 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF, FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells, J. Cell Physiol 203 (2005) 398–409. [DOI] [PubMed] [Google Scholar]
- [203].Solchaga LA, Penick K, Goldberg VM, Caplan AI, Welter JF, Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells, Tissue Eng. Part A 16 (2010) 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Correa D, Somoza RA, Lin P, Greenberg S, Rom E, Duesler L, Welter JF, Yayon A, Caplan AI, Sequential exposure to fibroblast growth factors (FGF) 2, 9 and 18 enhances hMSC chondrogenic differentiation, Osteoarthritis Cartilage 23 (2015) 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Frisch J, Venkatesan JK, Rey-Rico A, Schmitt G, Madry H, Cucchiarini M, Influence of insulin-like growth factor I overexpression via recombinant adeno-associated vector gene transfer upon the biological activities and differentiation potential of human bone marrow-derived mesenchymal stem cells, Stem Cell Res. Ther 5 (2014) 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Coleman CM, Vaughan EE, Browe DC, Mooney E, Howard L, Barry F, Growth differentiation factor-5 enhances in vitro mesenchymal stromal cell chondrogenesis and hypertrophy, Stem Cells Dev. 22 (2013) 1968–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Zhang B, Yang S, Sun Z, Zhang Y, Xia T, Xu W, Ye S, Human mesenchymal stem cells induced by growth differentiation factor 5: an improved self-assembly tissue engineering method for cartilage repair, Tissue Eng. Part C Methods 17 (2011) 1189–1199. [DOI] [PubMed] [Google Scholar]
- [208].Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, Sekiya I, Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans, Arthritis Rheum. 54 (2006) 843–853. [DOI] [PubMed] [Google Scholar]
- [209].Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F, Multipotent stromal cells derived from the infrapatellar fat pad of the knee, Clin. Orthop. Relat. Res (412) (2003) 196–212. [DOI] [PubMed] [Google Scholar]
- [210].Crecente-Campo J, Borrajo E, Vidal A, Garcia-Fuentes M, New scaffolds encapsulating TGF-β3/BMP-7 combinations driving strong chondrogenic differentiation, Eur. J. Pharm. Biopharm 114 (2017) 69–78. [DOI] [PubMed] [Google Scholar]
- [211].Luo Z, Jiang L, Xu Y, Li H, Xu W, Wu S, Wang Y, Tang Z, Lv Y, Yang L, Mechano growth factor (MGF) and transforming growth factor (TGF)-β3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model, Biomaterials 52 (2015) 463–475. [DOI] [PubMed] [Google Scholar]
- [212].Lyon M, Rushton G, Gallagher JT, The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific, J. Biol. Chem 272 (1997) 18000–18006. [DOI] [PubMed] [Google Scholar]
- [213].Møller AV, Jørgensen SP, Chen JW, Larnkjaer A, Ledet T, Flyvbjerg A, Frystyk J, Glycosaminoglycans increase levels of free and bioactive IGF-I in vitro, Eur. J. Endocrinol 155 (2006) 297–305. [DOI] [PubMed] [Google Scholar]
- [214].Weiss S, Hennig T, Bock R, Steck E, Richter W, Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells, J. Cell Physiol 223 (2010) 84–93. [DOI] [PubMed] [Google Scholar]
- [215].Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF, Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow, Tissue Eng. 4 (1998) 415–428. [DOI] [PubMed] [Google Scholar]
- [216].Cals FL, Hellingman CA, Koevoet W, Baatenburg de Jong RJ, van Osch GJ, Effects of transforming growth factor-β subtypes on in vitro cartilage production and mineralization of human bone marrow stromal-derived mesenchymal stem cells, J. Tissue Eng. Regen. Med 6 (2012) 68–76. [DOI] [PubMed] [Google Scholar]
- [217].Pei M, He F, Li J, Tidwell JE, Jones AC, McDonough EB, Repair of large animal partial-thickness cartilage defects through intraarticular injection of matrix-rejuvenated synovium-derived stem cells, Tissue Eng. Part A 19 (2013) 1144–1154. [DOI] [PubMed] [Google Scholar]
- [218].Caron MM, Emans PJ, Cremers A, Surtel DA, Coolsen MM, van Rhijn LW, Welting TJ, Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7, Osteoarthritis Cartilage 21 (2013) 604–613. [DOI] [PubMed] [Google Scholar]
- [219].Narcisi R, Quarto R, Ulivi V, Muraglia A, Molfetta L, Giannoni P, TGF β−1 administration during ex vivo expansion of human articular chondrocytes in a serum-free medium redirects the cell phenotype toward hypertrophy, J. Cell Physiol 227 (2012) 3282–3290. [DOI] [PubMed] [Google Scholar]
- [220].Cheng NC, Estes BT, Young TH, Guilak F, Genipin-crosslinked cartilage-derived matrix as a scaffold for human adipose-derived stem cell chondrogenesis, Tissue Eng. Part A 19 (2013) 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Cheng NC, Estes BT, Young TH, Guilak F, Engineered cartilage using primary chondrocytes cultured in a porous cartilage-derived matrix, Regen. Med 6 (2011) 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Luo L, Chu JYJ, Eswaramoorthy R, Mulhall KJ, Kelly DJ, Engineering Tissues That Mimic the Zonal Nature of Articular Cartilage Using Decellularized Cartilage Explants Seeded with Adult Stem Cells, A.C.S. Biomater. Sci. Eng (2016). DOI: 10.1021/acsbiomaterials.6b00020. [DOI] [PubMed] [Google Scholar]
- [223].Sutherland AJ, Beck EC, Dennis SC, Converse GL, Hopkins RA, Berkland CJ, Detamore MS, Decellularized cartilage may be a chondroinductive material for osteochondral tissue engineering, PLoS One 10 (2015) e0121966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Rosenzweig DH, Solar-Cafaggi S, Quinn TM, Functionalization of dynamic culture surfaces with a cartilage extracellular matrix extract enhances chondrocyte phenotype against dedifferentiation, Acta. Biomater 8 (2012) 3333–3341. [DOI] [PubMed] [Google Scholar]
- [225].Benders KE, Boot W, Cokelaere SM, Van Weeren PR, Gawlitta D, Bergman HJ, Saris DB, Dhert WJ, Malda J, Multipotent Stromal Cells Outperform Chondrocytes on Cartilage-Derived Matrix Scaffolds, Cartilage 5 (2014) 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Visser J, Gawlitta D, Benders KE, Toma SM, Pouran B, van Weeren PR, Dhert WJ, Malda J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles, Biomaterials 37 (2015) 174–182. [DOI] [PubMed] [Google Scholar]
- [227].Gawlitta D, Benders KE, Visser J, van der Sar AS, Kempen DH, Theyse LF, Malda J, Dhert WJ, Decellularized cartilage-derived matrix as substrate for endochondral bone regeneration, Tissue Eng. Part A 21 (2015) 694–703. [DOI] [PubMed] [Google Scholar]
- [228].Moutos FT, Estes BT, Guilak F, Multifunctional hybrid three-dimensionally woven scaffolds for cartilage tissue engineering, Macromol. Biosci 10 (2010) 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Garrigues NW, Little D, Sanchez-Adams J, Ruch DS, Guilak F, Electrospun cartilage-derived matrix scaffolds for cartilage tissue engineering, J. Biomed. Mater. Res. A 102 (2014) 3998–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F, Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix, Tissue Eng. Part A 16 (2010) 523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Rowland CR, Lennon DP, Caplan AI, Guilak F, The effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs, Biomaterials 34 (2013) 5802–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Shin YS, Choi JW, Park JK, Kim YS, Yang SS, Min BH, Kim CH, Tissue-engineered tracheal reconstruction using mesenchymal stem cells seeded on a porcine cartilage powder scaffold, Ann. Biomed. Eng 43 (2015) 1003–1013. [DOI] [PubMed] [Google Scholar]
- [233].Sutherland AJ, Detamore MS, Bioactive Microsphere-Based Scaffolds Containing Decellularized Cartilage, Macromol. Biosci 15 (2015) 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Beck EC, Barragan M, Tadros MH, Gehrke SH, Detamore MS, Approaching the compressive modulus of articular cartilage with a decellularized cartilage-based hydrogel, Acta. Biomater 38 (2016) 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Jungebluth P, Bader A, Baiguera S, Möller S, Jaus M, Lim ML, Fried K, Kjartansdóttir KR, Go T, Nave H, Harringer W, Lundin V, Teixeira AI, Macchiarini P, The concept of in vivo airway tissue engineering, Biomaterials 33 (2012) 4319–4326. [DOI] [PubMed] [Google Scholar]
- [236].Batioglu-Karaaltin A, Karaaltin MV, Ovali E, Yigit O, Kongur M, Inan O, Bozkurt E, Cansiz H, In vivo tissue-engineered allogenic trachea transplantation in rabbits: a preliminary report, Stem Cell Rev. 11 (2015) 347–356. [DOI] [PubMed] [Google Scholar]
- [237].Pei M, Zhang Y, Li J, Chen D, Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis, Stem Cells Dev. 22 (2013) 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Zhang Y, Pizzute T, Li J, He F, Pei M, sb203580 preconditioning recharges matrix-expanded human adult stem cells for chondrogenesis in an inflammatory environment - A feasible approach for autologous stem cell based osteoarthritic cartilage repair, Biomaterials 64 (2015) 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Liu X, Zhou L, Chen X, Liu T, Pan G, Cui W, Li M, Luo ZP, Pei M, Yang H, Gong Y, He F, Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl 61 (2016) 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Jin CZ, Choi BH, Park SR, Min BH, Cartilage engineering using cell-derived extracellular matrix scaffold in vitro, J. Biomed. Mater. Res. A 92 (2010) 1567–1577. [DOI] [PubMed] [Google Scholar]
- [241].Jin CZ, Cho JH, Choi BH, Wang LM, Kim MS, Park SR, Yoon JH, Oh HJ, Min BH, The maturity of tissue-engineered cartilage in vitro affects the repairability for osteochondral defect, Tissue Eng. Part A 17 (2011) 3057–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Li J, Pei M, Optimization of an in vitro three-dimensional microenvironment to reprogram synovium-derived stem cells for cartilage tissue engineering, Tissue Eng. Part A 17 (2011) 703–712. [DOI] [PubMed] [Google Scholar]
- [243].Liao J, Guo X, Grande-Allen KJ, Kasper FK, Mikos AG, Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering, Biomaterials 31 (2010) 8911–8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Levorson EJ, Mountziaris PM, Hu O, Kasper FK, Mikos AG, Cell-derived polymer/extracellular matrix composite scaffolds for cartilage regeneration, Part 1: investigation of cocultures and seeding densities for improved extracellular matrix deposition, Tissue Eng. Part C Methods 20 (2014) 340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Li J, He F, Pei M, Creation of an in vitro microenvironment to enhance human fetal synovium-derived stem cell chondrogenesis, Cell Tissue Res. 345 (2011) 357–365. [DOI] [PubMed] [Google Scholar]
- [246].Jin CZ, Park SR, Choi BH, Park K, Min BH, In vivo cartilage tissue engineering using a cell-derived extracellular matrix scaffold, Artif. Organs 31 (2007) 183–192. [DOI] [PubMed] [Google Scholar]
- [247].Tang C, Jin C, Xu Y, Wei B, Wang L, Chondrogenic Differentiation Could Be Induced by Autologous Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Matrix Scaffolds Without Exogenous Growth Factor, Tissue Eng. Part A 22 (2016) 222–232. [DOI] [PubMed] [Google Scholar]


