Abstract
This case series describes the cases of three adolescent patients with established inflammatory bowel disease (IBD) who experienced significant hypophosphataemia following intravenous infusion of ferric carboxymaltose as treatment for iron deficiency anaemia. Hypophosphataemia may cause a diverse range of symptoms and may be difficult to diagnose clinically due to their non-specific nature. Checking a baseline phosphate (PO4) prior to intravenous iron infusion may identify patients at higher risk for significant hypophosphataemia and perhaps allow the selection of an alternative iron preparation. The routine monitoring of PO4 levels postinfusion presents a greater challenge; with cases of asymptomatic hypophosphataemia likely to be uncovered, as in case 3. Clinicians, patients and families should be aware of the symptoms of hypophosphataemia, and symptomatic patients should have bloods checked to allow prompt identification and correction of abnormalities where required. Review of guidelines surrounding intravenous iron infusion and management of hypophosphataemia in paediatric patients is now required.
Keywords: paediatric gastroenterology, inflammatory bowel disease
Case series
Case 1
A 17-year-old female patient with established Crohn’s disease on thiopurine therapy received intravenous ferric carboxymaltose (1000 mg; 18 mg/kg) due to iron deficiency anaemia (IDA) (haemoglobin 109 g/L), following poor oral iron tolerance and ongoing dizziness and fatigue. She attended the emergency department 3 days following this infusion with abdominal pain, headaches and worsening dizziness. Her phosphate (PO4) was 0.67 mmol/L with no recent measurement for comparison. Reduced oral intake secondary to symptoms and weight loss (1.3 kg) were noted on admission (body mass index (BMI) Z-score −0.2); however, no concerns regarding refeeding syndrome were apparent. She was admitted and remained as an inpatient for 11 days with persistent hypophosphataemia despite nine high-dose PO4 infusions (figure 1). Calcium, renal function, vitamin D and parathyroid hormone (PTH) were within normal limits. The patient was switched to oral therapy for discharge; this was discontinued 20 days postpresentation after PO4 stabilised. Haemoglobin remained within the low-normal range from 1 month following ferric carboxymaltose infusion (111–127 g/L).
Figure 1.
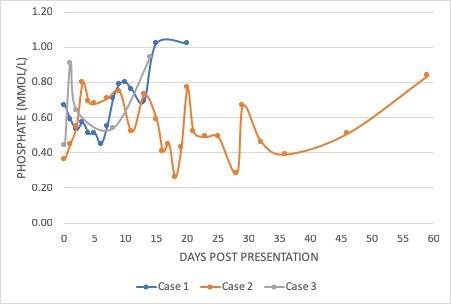
Graph demonstrating persistent hypophosphataemia in three patients with paediatric inflammatory bowel disease (cases 1, 2 and 3) following intravenous iron infusion.
Case 2
A 16-year-old female patient with Crohn’s disease and previous right hemicolectomy presented 3 days following ferric carboxymaltose infusion (1000 mg; 18 mg/kg; administered due to IDA (haemoglobin 115 g/L; ferritin 3.9 ug/L)) with severe back pain, muscle weakness and spasms beginning 1 day following infusion. PO4 was 0.36 mmol/L (Mg and K+ within normal limits and normal renal function), with normal bone biochemistry 2 months previously (PO4 1.21 mmol/L). No concerns regarding oral intake were reported (BMI Z-score +0.4). The patient received an intravenous PO4 infusion, increasing PO4 to 0.45 mmol/L and resolving symptoms. She was discharged but experienced diarrhoea secondary to oral PO4 leading to cessation; PO4 remained persistently low (figure 1) despite this and three further intravenous infusions. PTH was normal; calcium remained low to normal throughout this episode (2.09–2.29 mmol/L); vitamin D was found to be low (24 nmol/L) and was replaced as per regional guidelines. Paired urinary and serum electrolytes demonstrated a significantly diminished renal threshold for phosphate. Hypophosphataemia resolved at 8 weeks after initial infusion, with haemoglobin remaining within the low to normal range (121–132 g/L).
Case 3
A 17-year-old female inpatient with an acute flare of ulcerative colitis on intravenous steroid therapy was found to have a PO4 of 0.44 mmol/L (K+ 3.3 mmol/L; Mg 0.74 mmol/L; calcium within normal limits; vitamin D and PTH unavailable; no previous PO4 for comparison). The patient was asymptomatic and managing oral intake with stable weight (BMI Z-score 1.27). Two ferric carboxymaltose infusions had been administered 5 and 4 weeks previously (1 g and 390 mg; 20 mg/kg total dose) due to poor energy levels, IDA (serum iron 8 umol/L, transferrin saturations 13%) and poor oral iron tolerance. After an infusion of 0.7 mmol/kg sodium phosphate, repeat PO4 was 0.91 mmol/L and the patient was discharged. Oral PO4 replacement was commenced after identifying further low PO4 (0.64 mmol/L) at first outpatient review. This was discontinued after 3 days by the patient due to an increase in gastrointestinal symptoms; however, further review showed stable PO4 (0.94 mmol/L) despite discontinuation (figure 1). Repeat iron studies 6 weeks following second iron infusion showed low serum iron (5 umol/L) and transferrin saturations (11%).
A fourth patient presenting with symptomatic hypophosphataemia 5 weeks post-ferric carboxymaltose infusion was excluded from this series due to potentially confounding comorbidities.
Discussion
This report describes three adolescent patients within our population who developed hypophosphataemia with intravenous ferric carboxymaltose being the most likely precipitating cause between December 2017 and December 2018. Intravenous iron is increasingly utilised within paediatric inflammatory bowel disease (PIBD) and other diseases for the treatment of IDA, in particular where anaemia is severe or oral iron is poorly tolerated due to gastrointestinal side effects. The Royal Hospital for Children, Glasgow, is one of the largest PIBD units in the UK and is considered a large site as per international criteria, caring for more than 300 patients with PIBD. A recent audit within our department would estimate that this complication occurs in approximately 3%–5% of patients treated with intravenous ferric carboxymaltose. Previous studies have shown intravenous iron to have few reported side effects in paediatrics (table 1). While hypophosphataemia is a recognised side effect of intravenous iron in adults,1 2 we believe we report the first potential cases of this within a paediatric population. Patients with mild hypophosphataemia may remain asymptomatic; however, hypophosphataemia may also cause symptoms on a spectrum of severity such as fatigue; muscle weakness, cramps and spasm; myalgia; paraesthesia; seizures and arrhythmia.3 Chronic hypophosphataemia has long-term implications, including effects on bone health; and is therefore of critical importance in the paediatric population.3 The non-specific nature of the aforementioned symptoms means that hypophosphataemia secondary to iron infusion may be difficult to diagnose unless specifically suspected and symptoms may be attributed to other conditions, such as active IBD or IDA itself.
Table 1.
Summary of studies assessing the safety of ferric carboxymaltose in paediatric practice
| Study | Patients (n) | Infusions (n) | Dose (single infusion) | Adverse effects |
| Papadopoulos et al (2018) | 35 | 58 | 500–1000 mg | 2/35 (5.7% patients; 3.4% infusions) mild rash |
| Tan et al (2017) | 51 | Not noted | 50–1000 mg | No adverse effects reported |
| Mantadakis et al (2017) | 15 | 27 | 500–1000 mg | 1/15 (6.7% patients; 3.7% infusions) skin staining secondary to extravasation |
| Powers et al (2017) | 72 | 116 | 15 mg/kg; maximum 750 mg | 4/72 (5.6% patients; 3.4% infusions) urticaria/pruritus 1/72 (1.4% patients; 0.9% infusions) skin staining secondary to extravasation 1/72 (1.4% patients; 0.9% infusions) tingling localised to cannula site 1/72 (1.4% patients; 0.9% infusions) dyspnoea |
| Valério de Azevedo et al (2017) | 10 | 10 | 650–100mg | 1/10 (10.0% patients and infusions) minor headaches 1/10 (10.0% patients and infusions) fever |
| Laass et al (2014) | 72 | 147 | 50–1000 mg | 2/72 (2.8% patients; 1.4% infusions) mild urticaria 1/72 (1.4% patients; 0.7% infusions) mild oedema of hands and fingers |
Although the mechanism of hypophosphataemia secondary to intravenous iron is not fully clear, the involvement of fibroblast growth factor 23 (FGF-23), a phosphatonin secreted from osteocytes and osteoblasts, has been suggested.1 Parenteral iron has been shown to increase levels of active FGF-23 resulting in inhibition of renal PO4 reabsorption and consequent PO4 wasting,1 with the low point of serum PO4 occurring approximately 2 weeks following iron infusion and hypophosphataemia persisting for a median time of 84 days.4 Calculation of PO4 excretion using paired urinary and serum electrolytes, as undertaken in case 2, may highlight a diminished renal PO4 threshold following intravenous iron infusion.4 Patients receiving ferric carboxymaltose are at higher risk of developing hypophosphataemia and more severe hypophosphataemia when compared with other intravenous iron preparations,1 2 but with a lower risk of hypersensitivity reactions.2 We speculate that a pharmacogenomic relationship is present in patients with mutations in FGF genes or altered gene expression in those who develop this complication. This hypothesis would need to be explored in a larger number of patients.
While all of these cases were female, there is no compelling biological reason why females are more likely to develop hypophosphataemia following intravenous iron; it is worth noting, however, that severe iron deficiency is more common in females with IBD.5 Previous reports suggest that low preinfusion PO4 is a risk factor for the development of severe hypophosphataemia following intravenous iron.1 All three patients described above required intravenous PO4 replacement; with one requiring a prolonged inpatient stay (11 days) for repeated intravenous infusions and another requiring multiple readmissions due to refractory hypophosphataemia. Checking a baseline PO4 prior to intravenous iron infusion may identify at-risk patients and perhaps allow dose reduction or use of an alternative intravenous iron preparation, potentially reducing the incidence of significant postinfusion hypophosphataemia. The routine monitoring of PO4 levels following intravenous iron infusion presents a greater challenge; this is likely to uncover cases of asymptomatic hypophosphataemia, as within case 3, and raises the complex question of how and when to treat this. Spurious hypophosphataemia should be considered, especially in asymptomatic patients, and it is important to investigate other aetiologies of hypophosphataemia such as vitamin D deficiency or refeeding syndrome; in particular for malnourished patients who are also proportionally more likely to require intravenous iron supplementation.5
Conclusion/learning points
Clinicians, patients and their families should be made aware of hypophosphataemia as a side effect of intravenous iron therapy to increase symptom vigilance.
Hypophosphataemia should be considered in patients presenting with symptoms such as fatigue, generalised muscle weakness and myalgia following intravenous iron infusion; symptoms should trigger blood monitoring with prompt correction of electrolyte abnormalities undertaken if required.
Further investigation into the prevalence of hypophosphataemia following intravenous ferric carboxymaltose in paediatric patients is required.
Acknowledgments
REH’s Clinical Research Fellow role at the Royal Hospital for Children, Glasgow, is supported by the Catherine McEwan Foundation. RH and RKR are supported by NHS Research Scotland Senior fellowship awards.
Footnotes
Contributors: REH prepared the manuscript with comments and review from all authors. LA, LC, VG, LG, RT, RH and RKR provided critical review of the manuscript. All authors have approved the uploaded draft.
Funding: The IBD team at the Royal Hospital for Children, Glasgow are supported by the Catherine McEwan Foundation.
Competing interests: LC has received conference, accommodation and travel fees from Ferring and conference fees from Tillots. VG has received speaker’s fees from Ferring and conference fees from Abbvie. LG has participated in a research consultancy session with Lilly. RH is supported by an NHS Research Scotland Senior Research Fellowship, and has received speaker’s fees, travel support and/or participated in medical board meetings with MSD Immunology, Dr Falk, Nutricia & 4D Pharma. RKR is supported by an NHS Research Scotland Senior Research Fellowship and has received speaker’s fees, travel support and/or participated in medical board meetings with Nestle, MSD Immunology, AbbVie, Dr Falk, Takeda, Napp, Mead Johnson, Nutricia & 4D Pharma.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Imel EA, Econs MJ. Approach to the hypophosphatemic patient. J Clin Endocrinol Metab 2012;97:696–706. 10.1210/jc.2011-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schaefer B, Würtinger P, Finkenstedt A, et al. Choice of high-dose intravenous iron preparation determines hypophosphatemia risk. PLoS One 2016;11:e0167146 10.1371/journal.pone.0167146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bager P, Hvas CL, Dahlerup JF. Drug-specific hypophosphatemia and hypersensitivity reactions following different intravenous iron infusions. Br J Clin Pharmacol 2017;83:1118–25. 10.1111/bcp.13189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anand G, Schmid C. Severe hypophosphataemia after intravenous iron administration. BMJ Case Reports [Internet], 2017. Available: https://casereports.bmj.com/content/2017/bcr-2016-219160 [Accessed 7 March 2019]. [DOI] [PMC free article] [PubMed]
- 5. Gerasimidis K, Barclay A, Papangelou A, et al. The epidemiology of anemia in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2411–22. 10.1097/MIB.0b013e31829ed855 [DOI] [PubMed] [Google Scholar]


