Abstract
Introduction
Endoscopic therapy for the management of patients with Barrett’s oesophagus (BE) neoplasia has significantly developed in the past decade; however, significant variation in clinical practice exists. The aim of this project was to develop expert physician-lead quality indicators (QIs) for Barrett’s endoscopic therapy.
Methods
The RAND/UCLA Appropriateness Method was used to combine the best available scientific evidence with the collective judgement of experts to develop quality indicators for Barrett’s endotherapy in four subgroups: pre-endoscopy, intraprocedure (resection and ablation) and postendoscopy. International experts, including gastroenterologists, surgeons, BE pathologist, clinical nurse specialist and patient representative, participated in a three-round process to develop 15 QIs that fulfilled the RAND/UCLA definition of appropriateness.
Results
17 experts participated in round 1 and 20 in round 2. Of the 24 proposed QIs in round 1, 20 were ranked as appropriate (put through to round 2) and 4 as uncertain (discarded). At the end of round 2, a final list of 15 QIs were scored as appropriate.
Conclusions
This UK national consensus project has successfully developed QIs for patients undergoing Barrett’s endotherapy. These QIs can be used by service providers to ensure that all patients with BE neoplasia receive uniform and high-quality care.
Keywords: Barrett’s Oesophagus, endoscopy, Endoscopic Mucosal Resection (EMR), Endoscopic Submucosal Dissection (ESD), Radio Frequency Ablation (RFA)
Significance of this study.
What is already known on this topic
Endoscopic eradication therapy for Barrett’s neoplasia has revolutionised the management of patients with Barrett’s neoplasia; however, despite various societal guidelines, there still exists a great variation in clinical practice that results in variable patient outcomes.
What this study adds and how might it impact on clinical practice in the foreseeable future
Quality indicators for Barrett’s endotherapy have been published recently in the USA.
Adherence to these quality indicators has shown improvement in dysplasia detection rate; however, quality indicators for Barrett’s endotherapy in the UK and Europe are lacking.
These quality indicators identify important steps for providing a unified high-quality care based on the best available evidence and expert opinion.
These quality indicators may also be used for the training of the new generation of advanced endoscopists, and adherence to these measures would ultimately result in improving patient outcomes.
Introduction
The past decade has seen significant advancement in minimally invasive endoscopic treatment modalities for Barrett’s oesophagus (BE) neoplasia. Short-term and long-term data report high eradication rates, acceptable disease durability and good safety profile that are comparable with the outcomes of surgical treatment.1 There has been great emphasis on targeting patients at earlier disease stages amenable to endoscopic eradication therapy (EET). EET for early neoplastic BE has been recommended by various major international guidelines.2
EET for BE neoplasia has revolutionised the management of patients with BE neoplasia and is increasingly used at high-volume tertiary referral centres and smaller district general hospitals. Adherence to quality indicators (QIs) introduced by the American Gastroenterological Association for the endoscopic management of patients with BE has been shown to improve dysplasia detection rate. Despite various societal guidelines,2 there still exists a great variation in clinical practice that results in variable patient outcomes.
It is important to note that the management of patients with BE neoplasia is confined not just to the endoscopic procedure. It requires case discussion in a dedicated multidisciplinary team (MDT) meeting with careful explanations to patients of their disease status and available therapies prior to and after endotherapy.
The current endoscopic management of BE neoplasia consists of endoscopic resection (ER) of visible lesions for accurate staging and risk stratification of patients, followed by field ablation of the remaining areas of flat BE to prevent the development of metachronous neoplasia. It is therefore important that cases are carefully selected for endoscopic therapy following discussion in MDTs with appropriate choice of therapy (after discussion with the patient), with strict follow-up of these cases to ensure high-quality service provision and better patient outcomes.
It is essential that medical resources are used appropriately and that health provision is shaped and maintained at the highest standard in order to ensure the best possible patient outcomes. Healthcare systems and providers will therefore need to be aligned to ensure a streamlined, efficient and high-quality service provision to all patients. QIs for Barrett’s endotherapy (BET) in the UK and Europe are lacking and have led to variable outcomes in the past.3
The aim of this project was to develop physician-led quality indicators for Barrett’s endotherapy (QBET) to define standardised clinical practice and achieve optimal clinical outcomes for all patients with BE neoplasia.
The aim from this project is not to replace existing guidelines but to create an adjunct so that clinicians can measure performance in a systematic way.
Methods
This project was not a clinical trial and there was no search conducted on humans.
The RAND/UCLA Appropriateness Method
The RAND/University of California, Los Angeles Appropriateness Methodology (RAND/UCLA Appropriateness Method (RAM)) was developed in the 1980s as part of the RAND Corporation/UCLA Health Services Utilisation Study. It is a tool used to measure the overuse and underuse of resources. In RAM an appropriate measure refers to one in which the expected health benefit exceeds the expected negative consequences by a wide margin, such that the procedure is worth performing without considering the cost.4 This methodology is used in situations where there is no adequate high-quality research (eg, randomised controlled trials) to guide clinical practice, and therefore the best available evidence is combined with expert opinion, in order to develop QIs. RAM is a modified Delphi method that gives experts the opportunity to have a face-to-face discussion. RAM has been used in various clinical specialties including gastroenterology.5 This methodology was successfully used in establishing similar quality measures in EET in the USA endorsed by the American Society for Gastrointestinal Endoscopy (ASGE) and the American College of Gastroenterology (ACG).5
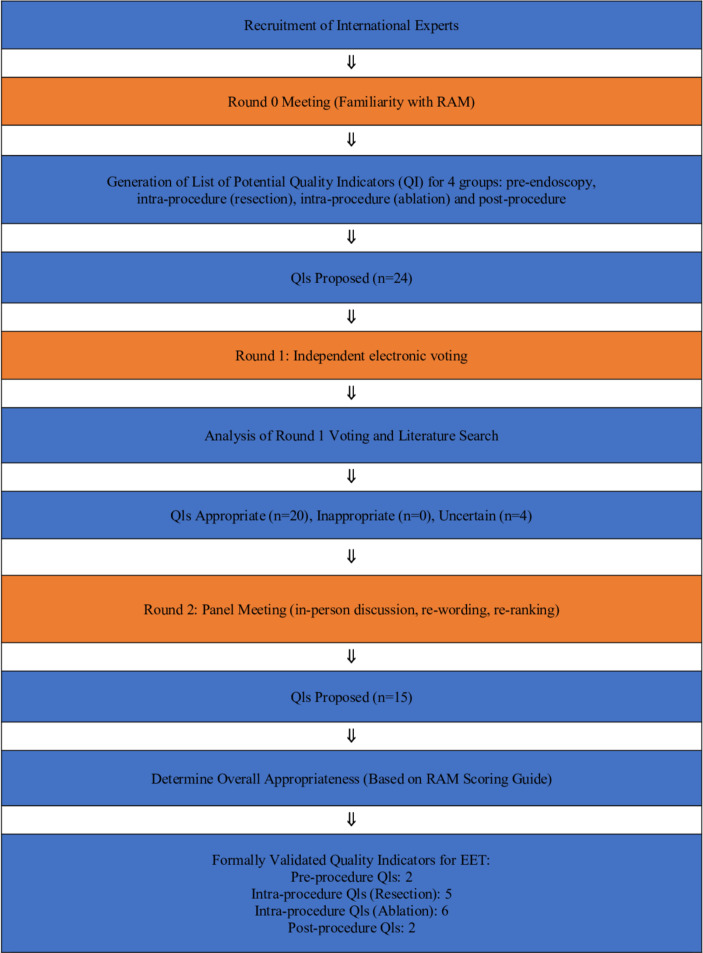
We used RAM to combine the best available scientific evidence with the collective judgement of experts to develop QBET in four subgroups that are integral to patient selection, treatment and follow-up in BET (figure 1). The expert panel was selected based on membership in the UK radiofrequency ablation registry and publication history in the field of BE and BET. In addition, geographical variation was considered to ensure expert representation from all regions in the UK, which could be representative of the European variation in practice. The experts consisted of gastroenterologists and therapeutic endoscopists (n=20), including two surgeons performing surgery for advanced oesophageal adenocarcinoma (OAC) and providing BET, and one BE expert pathologist. We also had participation from a BE clinical nurse specialist, a medical statistician and a patient representative. We developed QIs in four subgroups, as follows:
Figure 1.
RAND/UCLA Appropriateness Method (RAM): summary. EET, endoscopic eradication therapy.
Pre-endoscopy.
Intraprocedure (resection).
Intraprocedure (ablation).
Postendoscopy.
Round 0
RAND/UCLA uses three rounds as shown in figure 1. In round 0, experts were introduced to the project methodology and objectives (via teleconference on 18 September 2017 by RH, DA and KR) and familiarised with the RAM process. In addition, one expert was allocated as lead for each subgroup to facilitate the discussions during the face-to-face meeting (round 2). After round 0, the core group leading the project (RH, DA, KR, PS, OP) met to collate a list of potential QIs. These were then reviewed with the project leads, and the project leads (consisting of national and international experts) then proposed potential QIs for each of the four subgroups, which were put forward for ranking at round 1 (24 QIs in total).
Round 1
In round 1, 17 experts had the opportunity to rank each of the 24 QIs electronically in an independent fashion. This was done without interaction with other colleagues. The proposed QIs were sent to all the participating experts via a REDCap (Research Electronic Data Capture) database.
Study data were collected and managed using REDCap electronic data capture tools hosted at University College London Hospital.6 7 REDCap is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources.
Instructions were also sent to the panel indicating that each QI should be scored by each expert based on their current expertise and knowledge on the topic. The experts were advised to score each QI as it would be applied to an average patient presenting to an average medical facility and to an average physician without the consideration for cost or feasibility of applying the QI in clinical practice. Each QI was ranked from 1 to 9 as per the RAM protocol.
Score of 1, 2, 3: inappropriate QI.
Score of 4, 5, 6: uncertain QI.
Score of 7, 8, 9: appropriate QI.
Following round 1 voting, all the scores were collected and analysed using four statistical methods by an expert statistician with knowledge of the RAM process.
In addition, an extensive literature search on PubMed on the topic of BE and BET was performed around the proposed QIs. The literature search was limited to publications from 1 January 1990 to 23 January 2018.
Prior to the round 2 face-to-face interaction and voting, the following were sent to all the investigators:
A summary copy of the literature search for each QI.
A document showing the distribution of all the responses from round 1, including the investigator’s personal response.
Round 2
Only QIs that were deemed appropriate at round 1 (based on round 1 voting and statistical analysis) were put forward for discussion at round 2. The round 2 meeting (face-to-face meeting) took place on 14 March 2018 in London. At this meeting 20 investigators were provided with individual iPads containing all the overall results of the round 1 voting, a summary of all the literature searches around the QIs, and full text copies of all manuscripts and references for reference and discussion. The lead for each subgroup led the discussion for each QI in that subgroup during this meeting. Each QI was discussed in detail taking into account the opinion from all those present and the available scientific literature. QIs were reworded, deleted and new QIs were developed (where necessary) for each of the four subgroups.
At the end of round 2 meeting, a set of 15 QIs were finalised and scored by each investigator (pre-endoscopy 2 QIs; intraprocedure (resection) 5 QIs; intraprocedure (ablation) 6 QIs; and postprocedure 2 QIs). The experts also agreed on setting performance thresholds for each QI (if indicated) in order to set aspirational targets for all service providers. The median score (and range) of suggested performance thresholds is included with each QI. There were no set aspirational targets for QIs with predefined performance target in the text (eg, intraprocedural (ablation) QI number 4). The expert panel recognised that some performance targets had to be set cautiously in order to avoid undermining established efficient practices, and therefore aspirational targets were set to encourage centres to work towards enhancing their practice and performance.
There were no attempts to force the expert panel to reach a consensus, and each expert had the opportunity to score the finalised QIs independently.
Statistical method
First, summaries of the number of responses in three categories were produced. Each response was categorised into one of the following categories:
Inappropriate: score 1–3.
Uncertain: score 4–6.
Appropriate: score 7–9.
In addition to the categorisation, the median score for each QI was calculated and summarised.
The deviation in the responses between the panel members was assessed using a number of different methods. First, deviation was assessed by the MAD-M statistics. This is the mean absolute deviation from the median (MAD-M). Higher values of MAD-M indicate more spread in responses between the panel. A second measure was based on the BIOMED Concerted Action on Appropriateness definition. This method calculates the number of raters outside of the response category (ie, inappropriate, uncertain, appropriate) containing the median response. Disagreement was assumed if the number of raters outside this category meets a predefined threshold. In the RAND/UCLA handbook guidance is given for panel sizes up to 16 raters, but none is provided for 20 raters, as per this panel. Although there were no set guidelines for this number of raters, the decision was based on the same criteria as for a 16-rater panel (agreement if ≤4 raters outside the category). The third measure used the RAND method that tests hypotheses about the distribution of ratings in a hypothetical population of repeated ratings. It is hypothesised that 90% of the hypothetical population of repeated ratings are within one of two extra wide regions (1–6 or 4–9). The binomial test was used to calculate the probability (p value) that that ‘true’ value is below 90%. If the calculated probability is below the predetermined level of 0.10, the conclusion will be reached that there is disagreement among raters. The final measure of deviation uses the interpercentile range adjusted for symmetry (IPRAS) method. This method is based on the interpercentile range (IPR) between the 30th and 70th percentiles. The IPRAS is a statistics based on the IPR which is adjusted for symmetry. Disagreement was assumed if the IPRAS was larger than the IPR.
An additional set of analyses examined the threshold values for questions where these were appropriate. Median values and ranges were calculated for the thresholds.
The measures of spread included the following:
The count of responses in each three-point region (1, 2, 3 – 4, 5, 6 – 7, 8, 9).
MAD-M.
Appropriateness was measured using the following:
Median rating.
BIOMED Concerted Action on Appropriateness definition.
P value.
IPRAS.
A QI was deemed appropriate if it met the definition of appropriateness, using all defined statistical methods.
Results
The summary of responses from round 2 for each individual QI is shown in table 1. At round 2, 20 investigators ranked 15 QIs that were all deemed appropriate and are shown in tables 2–5 with corresponding aspirational performance target (if indicated) and evidence summary. During round 1, 17 investigators ranked 24 QIs, of which 20 were deemed appropriate and 4 uncertain (table 6).
Table 1.
Summary of responses from round 2 to individual QI
| Group | QI | Inappropriate n (%) |
Uncertain n (%) |
Appropriate n (%) |
Median | Median interpretation |
| Pre-endoscopy | 1 | 0 (0) | 0 (0) | 20 (100) | 9 | Appropriate |
| 2 | 0 (0) | 0 (0) | 20 (100) | 9 | Appropriate | |
| Intraprocedure (EMR) |
1 | 0 (0) | 0 (0) | 20 (100) | 9 | Appropriate |
| 2 | 0 (0) | 0 (0) | 20 (100) | 9 | Appropriate | |
| 3 | 0 (0) | 0 (0) | 20 (100) | 9 | Appropriate | |
| 4 | 0 (0) | 0 (0) | 20 (100) | 8.5 | Appropriate | |
| 5 | 0 (0) | 0 (0) | 20 (100) | 8.5 | Appropriate | |
| Intraprocedure (RFA) |
1 | 0 (0) | 0 (0) | 20 (100) | 9 | Appropriate |
| 2 | 0 (0) | 0 (0) | 20 (100) | 9 | Appropriate | |
| 3 | 0 (0) | 0 (0) | 20 (100) | 8 | Appropriate | |
| 4 | 0 (0) | 0 (0) | 20 (100) | 8 | Appropriate | |
| 5 | 0 (0) | 0 (0) | 20 (100) | 9 | Appropriate | |
| 6 | 0 (0) | 0 (0) | 20 (100) | 8 | Appropriate | |
| Postendoscopy | 1 | 0 (0) | 0 (0) | 20 (100) | 9 | Appropriate |
| 2 | 0 (0) | 0 (0) | 20 (100) | 8 | Appropriate |
EMR, endoscopic mucosal resection; QI, quality indicator; RFA, radiofrequency ablation.
Table 2.
Appropriate pre-endoscopy QIs after round 2 voting with the median score, MAD-M, BIOMED analysis, p value, IPRAS analysis and the performance threshold
| Pre-endoscopy QIs | Median score | MAD-M | BIOMED analysis | P value | IPRAS analysis | Performance threshold, median % (range) |
| BET should be performed in high-volume centres within a local cancer network to meet efficacy and safety standards. | 9 | 0.2 | No disagreement | 1 | No disagreement | 100 (90–100) |
|
Aspirational performance target: 100% (range: 90–100).
Evidence summary: Endoscopic training should start with knowledge acquisition, followed by resection and ablation in animal models, before training in human subjects. Endoscopist proficiency increases with the number of treatment sessions performed.8 Adherence to BE surveillance biopsy protocol in non-tertiary centres is poor, resulting in reduced dysplasia detection rate. Adherence to this protocol is further reduced with an increasing length of BE segment. Advanced imaging with HD-WLE and NBI has been shown to improve the detection rate of early neoplasia in patients with BE. The majority of gastroenterologists from academic centres use high definition white light endoscopy (HD-WLE) to classify BE as per guidelines and perform significantly more EET procedures per month, in comparison with those in district general hospitals. These factors favour the referral of patients with BE neoplasia to dedicated high-volume centres. In addition, data from the UK RFA registry have shown that increasing experience in performing EET is associated with significantly improved CR-D and CR-IM rates, less number of rescue EMRs and faster protocol completion. At the start of the registry and at a time when only less than 20 patients were enrolled, the documented CR-D and CR-IM after completing EET were 79.8% and 71.3%, respectively; however, with increasing experience (ie, once >40 patients enrolled), the study was able to show significantly better CR-D (91%) and CR-IM (83.9%) (p<005).9 These data support improvement in experience and outcomes with increase in the number of procedures performed. The expert panel has therefore suggested that endoscopic therapy should be performed in high-volume referral centres to optimise outcomes. Hospitals performing >40 EET cases per year may therefore be suitable centres for performing BE endoscopic eradication therapy. | ||||||
| Patients considered for BET should be discussed in an oesophagogastric MDT. | 9 | 0.3 | No disagreement | 1 | No disagreement | 93 (85–100) |
|
Aspirational performance target: 93% (range: 85–100).
Evidence summary: The National Institute for Health and Care Excellence (August 2010) guidelines on ablative therapy for the treatment of BE recommends to discuss the MDT’s views on the range of appropriate treatments with the patient. It also recommends giving patients verbal and written information about their diagnosis, available treatments, patient support groups and the uncertainty of the long-term outcomes of ablative therapies.10 In addition the BSG recommends that the treatment of patients with BE neoplasia should be discussed in a dedicated GI specialist MDT taking into account patient comorbidities, nutritional status, patient preferences and staging.2 Patients should be provided with information on all treatment options and offered verbal and written information on support groups available to them,2 including clinical nurse specialists. Despite little evidence, the expert panel advocates an MDT approach (consisting of an expert BE pathologist) for these patients in order to safeguard against incorrect use of BET in patients with more advanced disease and to ensure that the case management provided is directed to best patient interest. | ||||||
BE, Barrett’s oesophagus; BET, Barrett’s endotherapy; BSG, British Society of Gastroenterology; CR-D, complete remission of dysplasia; CR-IM, complete remission of intestinal metaplasia; EET, endoscopic eradication therapy; EMR, endoscopic mucosal resection; GI, gastrointestinal; IPRAS, interpercentile range adjusted for symmetry; MAD-M, mean absolute deviation from the median; MDT, multidisciplinary team; NBI, narrow band imaging; QI, quality indicator; RFA, radiofrequency ablation.
Table 3.
Appropriate intraprocedure (resection) QIs after round 2 voting with the median score, MAD-M, BIOMED analysis, p value, IPRAS analysis and the performance threshold
| Intraprocedure QIs (resection) | Median score | MAD-M | BIOMED analysis | P value | IPRAS analysis | Performance threshold, median % (range) |
| Adherence to the Prague and Paris classifications is mandatory. | 9 | 0.1 | No disagreement | 1 | No disagreement | 95 (80–100) |
|
Aspirational performance target: 95% (range: 80–100).
Evidence summary: Several studies have investigated the validity of the Prague circumferential and maximum length classification showing high overall validity for the endoscopic assessment of visualised BE lengths among expert endoscopists, community hospital endoscopists and trainees. The BSG guidelines recommend endoscopic reporting be performed using the Prague criteria.2 Description of lesion morphology using the Paris classification is based on the Japanese system used to classify early gastric cancer. This provides information on the likelihood of invasion of cancer and helps communication between endoscopists. Description of lesion morphology using the Paris classification improves lesion recognition at the time of endoscopic therapy. It gives an indication of the likelihood of invasive cancer and aids communication between clinicians. The BSG recommends the use of Paris classification for all visible lesions; therefore, adherence to the Prague and Paris classifications is recommended. | ||||||
| All patients undergoing BET and follow-up should have assessment with high-definition white light endoscopy with (virtual) chromoendoscopy. | 9 | 0.4 | No disagreement | 1 | No disagreement | 93 (80–100) |
|
Aspirational performance target: 93% (range: 80–100).
Evidence summary: Endoscopy in patients with BE should be performed with careful inspection of the columnar-lined oesophagus using HD-WLE, with biopsy of any suspicious areas followed by four-quadrant biopsies of the BE metaplasia. The use of the HD-WLE is associated with improved detection of dysplasia during routine BE surveillance. In addition, chromoendoscopy allows for detailed imaging of the mucosal and vascular surface patterns in BE. Recent studies have shown that imaging techniques such as chromoendoscopy or virtual chromoendoscopy increase the diagnostic yield for identification of dysplasia or cancer in patients with BE; however, the evidence for advanced endoscopy boosting dysplasia detection rate on a per-patient basis is slim.11 The application of a dilute AA solution to the BE mucosa results in mucosal colour change and highlights mucosal patterns more clearly, facilitating sensitive and specific identification of potentially neoplastic areas. Furthermore, the premature loss of aceto-whitening in areas of the mucosa and the speed at which it disappears are also associated with the presence of early neoplasia. The efficacy of AA chromoendoscopy has been demonstrated in few studies showing a sensitivity and specificity of up to 98% and 96%, respectively. Three main virtual chromoendoscopy modalities are currently available: NBI (Olympus), the i-Scan imaging system (Pentax) and BLI (Fujifilm). Recent studies have indicated the potential of NBI as a replacement for AA chromoendoscopy with an accuracy of 92%, and sensitivity and specificity of 91% and 93%, respectively, in the identification of early dysplastic lesions on still images.12 Other studies have also shown that i-Scan can improve neoplasia detection in patients with BE with an impressive accuracy and sensitivity of up to 94% and 83%, respectively. The use of i-Scan in combination with zoom magnified endoscopy and the addition of AA can also provide further improvement in dysplasia detection rate. A recent study by Subramaniam et al validated a classification system for BLI which identifies dysplastic BE tissue with sensitivity and specificity of 96%, based on both increased pit pattern irregularity and the presence of disordered and dilated microvessels.13 Currently, only AA and NBI have reached the ASGE PIVI requirement. The current data on advanced imaging modalities in improving dysplasia yield are encouraging, but the data do not provide evidence on how these modalities can impact EET. Most studies to date have either been performed using still images or have been limited to high-volume BE referral centres. The expert panel has therefore suggested that all patients undergoing BET and follow-up should have assessment with HD-WLE with chromoendoscopy or virtual chromoendoscopy. | ||||||
| All visible lesions should be entirely resected with EMR or ESD. | 9 | 0.3 | No disagreement | 1 | No disagreement | 93 (80–100) |
|
Aspirational performance target: 93% (range: 80–100).
Evidence summary: ER is the cornerstone of endoscopic therapy of early oesophageal neoplasia which aims to provide accurate histological staging with therapeutic intent. ER of early BE neoplasia with multiband mucosectomy is effective and safe. A large number of studies have shown long-term complete remission rate of 85%–96%, with bleeding rates ranging from 0.7% to 7.9% and perforation rates ranging from 0.2% to 2.3%.14 EMR of all visible lesions has been shown to upgrade the pathological diagnosis in 39% of all patients. Most of the change was associated with upgrading of grade of dysplasia and neoplasia. EMR for all visible lesions has been recommended by the ASGE. In addition the provision of EMR specimens to the pathology department results in an improvement in interobserver agreement among pathologists compared with biopsy specimens only. ESD for early-stage BE neoplasia is also a feasible treatment option as it allows en-bloc resection and accurate histopathological analysis of lateral resection margins in BE neoplasia. Multiple studies have shown high en-bloc resection rates ranging from 89% to 98.6% and R0 resection rates ranging from 72.4% to 87%, with acceptable perforation (0%–8.3%), bleeding (1.4%–1.7%) and stricture rates (2.1%–11.6%). When curative resections are achieved, good oncological outcomes are likely in the management of early-stage BE neoplasia by ESD. The ESGE recommendations (2015) state that EMR is acceptable for resecting lesions confined to the mucosa, regardless of the size, but ESD may be considered for lesions larger than 15 mm, poorly lifting tumours and lesions at risk for SM invasion.15 These data show that EMR and ESD are effective treatment modalities in the staging and treatment of early BE neoplasia with acceptable side effect profiles. It is however important to mention that operator skill and experience will have significant effect on patient outcome, and therefore good training is paramount. | ||||||
| The use of EUS is not routinely recommended for patients undergoing BET. | 8.5 | 0.6 | No disagreement | 1 | No disagreement | 90 (70–100) |
| Aspirational performance target: 90% (range: 70–100).
Evidence summary: A systematic review and meta-analysis by Qumseya et al 16 showed that EUS was able to detect only 14% of patients presenting with advanced disease and 4% of patients with advanced disease in the absence of nodules. A prospective study by May et al compared staging of early oesophageal neoplasia using HR endoscopy with staging using HR endosonography. The accuracy of the endoscopic and endosonographic staging was 83.4% and 79.6%, respectively. Sensitivity for mucosal tumours was more than 90% (EUS 91.2%, endoscopy 94.1%), while sensitivity for submucosal tumours was 48% for EUS and 56% for endoscopic staging. A combination of the two techniques increased the sensitivity for submucosal tumours to 60%. The overall diagnostic accuracy of both HR endoscopy and HR endosonography in early oesophageal cancer is approximately 80% with no significant differences between the two techniques.17 EUS can provide staging in patients with BE neoplasia; however, there is a significant degree of overstaging and understaging when compared with ER. The expert panel agreed that EUS is not recommended for the work-up of patients with early oesophageal neoplasia, but only to exclude T2 disease or nodal involvement. | ||||||
| Lesions with SM invasion are only to be considered for curative BET if deemed to present a low risk of metastasis. | 8.5 | 0.6 | No disagreement | 1 | No disagreement | 90 (80–100) |
|
Aspirational performance target: 90% (range: 80–100).
Evidence summary: Neoplastic lesions confined to the mucosa have a better prognosis when compared with those invading the submucosa. LN metastasis and recurrence of the tumour correlate with the depth of invasion of the lesion into deeper tissue layers. Depth of tumour invasion, grade of differentiation and lymphatic involvement are important decision-making factors. Lesions confined to the mucosa and SM1 have a very low risk of lymphovascular invasion; however, invasion beyond SM1 (>500 μm measured from the deepest fibre of the muscularis mucosae) is at increased risk of developing recurrent disease within 5 years.18 EET is used to treat superficial neoplasms in BE, but cannot cure cancers that have metastasised to LN. The risk of occult LN metastases for patients with mucosal neoplasms in BE is in the range of 1%–2%.19 Oesophagectomy has a mortality rate that often exceeds 2% with substantial morbidity. Therefore, the risk of LN metastases alone does not warrant the choice of oesophagectomy over ET for HGD and IMC in BE.19 A study by Manner et al concluded that the rate of LN metastasis in pT1b SM1 early adenocarcinoma with histologically low-risk pattern was 2%, which was lower than the mortality rate of oesophagectomy (3%); high-risk lesions, however, had an LN metastasis risk of 9%, suggesting that ET may be used as an alternative to surgery in low-risk lesions only.20 The expert panel has therefore recommended that only low-risk lesions with SM invasion should be considered for curative BET, and all patients with high-risk SM lesions should be considered for surgery (unless not suitable due to comorbidities) following discussion at MDT and with the patient. | ||||||
AA, acetic acid; ASGE, Americal society for gastrointestinal endoscopy; BE, Barrett’s oesophagus; BET, Barrett’s endotherapy; BLI, blue laser imaging; BSG, British Society of Gastroenterology; EET, endoscopic eradication therapy; EMR, endoscopic mucosal resection; ER, endoscopic resection; ESD, endoscopic submucosal dissection; ESGE, European Society of Gastrointestinal Endoscopy; ET, endoscopic therapy; EUS, rndoscopic ultrasound; HD-WLE, high definition white light endoscopy; HGD, high grade dysplasia; HR, high resolution; IMC, intramucosal carcinoma; IPRAS, interpercentile range adjusted for symmetry; LN, lymph node; MAD-M, mean absolute deviation from the median; MDT, multidisciplinary team; NBI, narrow band imaging; PIVI, Preservation and incorporation of valuable endoscopic innovations; QI, quality indicator; SM, submucosal.
Table 4.
Appropriate intraprocedure (ablation) QIs after round 2 voting with the median score, MAD-M, BIOMED analysis, p value, IPRAS analysis and the performance threshold
| Intraprocedure QIs (ablation) | Median score | MAD-M | BIOMED analysis | P value | IPRAS analysis | Performance threshold, median % (range) |
| Low-grade and high-grade dysplasia without visible lesions should undergo endoscopic ablation. | 9 | 0.4 | No disagreement | 1 | No disagreement | 95 (80–100) |
|
Aspirational performance target: 95% (range: 80–100).
Evidence summary: The multicentre EURO II study showed that RFA can achieve CR-D and CR-IM rates of 92% and 87%, respectively,21 in patients with early BE neoplasia. A systematic review by Desai et al 22 also showed that ET of BE neoplasia with resection of visible lesions followed by ablation of the remaining segment of BE can achieve CR-D rate of 93.4% and CR-IM rate of 73.1%. ET for early BE neoplasia should therefore be offered after appropriate discussion with the patient as ET is associated with high rates of CR-D and CR-IM and reduction in disease progression and development of cancer. The efficacy and safety profile of RFA suggests that it is the best ablative modality currently available for patients with LGD and HGD without visible lesions. The diagnosis of dysplasia should be reproduced and confirmed by expert BE pathologists prior to consideration for EET. Recent meta-analysis by Qumseya et al studied the progression rates in patients with LGD based on review by an expert GI pathologist. The group was able to show that the rate of progression from LGD to HGD/OAC was significantly higher among studies where expert GI pathologist confirmed the diagnosis of LGD compared with studies that did not use a GI pathologist.23 | ||||||
| Following endoscopic resection, patients undergo ablative therapy every 2–4 months in order to achieve CR-IM. | 9 | 0.3 | No disagreement | 1 | No disagreement | 90 (80–100) |
|
Aspirational performance target: 90% (range: 80–100).
Evidence summary: The initial UK RFA registry of 335 patients with BE and neoplasia that received ER for visible lesion followed by RFA every 3 months until all areas of BE were ablated or cancer developed showed that by 12 months after initial RFA treatment CR-D was achieved in 81% and CR-IM in 62% of patients.24 The registry’s later report in 2015 (consisting of 508 patients) showed CR-D and CR-IM rates of 92% and 83%, respectively.3 There is increasing evidence to support the use of RFA25 post-ER of any visible lesion in order to achieve CR-IM in the first 12–18 months post initial endoscopic ablation. Data are lacking on how often and at which interval RFA should be provided to these patients; however, our panel of experts suggests that an interval of 2–4 months would be acceptable practice. | ||||||
| For patients undergoing RFA with a focal device, the dosimetry and treatment regimen is 12 J/cm2×3, without interval cleaning, and for patients undergoing RFA with a circumferential device the dosimetry and treatment regimen is 10 J/cm2–clean–10 J/cm2. | 8 | 0.4 | No disagreement | 1 | No disagreement | N/A |
|
Aspirational performance target: N/A.
Evidence summary: Focal application of RFA without cleaning in between each ablation has been shown to be effective with 94% CR-D and 87% CR-IM, with a stenosis rate of 11%.26 A multicentre randomised trial by van Vilsteren et al 27 showed that a simplified ablative regimen (3×15 J/cm2–no clean) is highly effective and can achieve higher complete remission of residual BE islands (73% vs 67%) than the standard method (2×15 J/cm2–clean–2×15 J/cm2) at 2 months. The same group was also able to show that the simplified regimen without cleaning was able to achieve higher BE surface regression (88% vs 83%) in comparison with the standard regimen in circumferential balloon-based RFA with significantly shorter ablation time with the simplified technique (p<0.01).28 Furthermore, a multicentre RCT on focal RFA for dysplastic BE showed that the simplified RFA regimen (3×12 J/cm2, without cleaning) is non-inferior to the standard regimen (2×15 J/cm2, followed by cleaning, followed by 2×15 J/cm2), and therefore is the preferred RFA regimen for the management of patients with BE dysplasia.29 The volume of evidence supporting the use of the circumferential RFA device in published literature is increasing. Recent data have shown a regression of 78% of BE segment at 3 months postablation with the circumferential device using a dose of 12 J and 85% regression with 10 J.30 Furthermore a randomised trial in the Netherlands assessed treatment regimens for the 360 Express RFA balloon catheter (360 Express) using standard (1×10 J/cm2–clean–1×10 J/cm2), simple-double (2×10 J/cm2–no clean) and simple-single ablation regimen (1×10 J/cm2–no clean). The simple-double arm of the study was terminated early as a result of significant severe stenosis; however, the study was able to show higher median BE regression in the standard arm compared with the simple-single group: 85% (IQR 75–94), 95% CI 78% to 92% vs 73% (IQR 48–90), 95% CI 59% to 85% (p=0.009).30 It would therefore be appropriate to consider standard regimen (1×10 J/cm2–clean–1×10 J/cm2) for the use of the circumferential RFA device. | ||||||
| Centres undertaking BET should achieve CR-D ≥90% and CR-IM ≥80% within 18 months after the first treatment. | 8 | 0.4 | No disagreement | 1 | No disagreement | N/A |
|
Aspirational performance target: N/A.
Evidence summary: The Ablation of Intestinal Metaplasia Containing Dysplasia trial included a 5-year follow-up analysis of patients with BE and dysplasia managed by RFA in a randomised controlled trial. Data showed BE recurrence after CR-IM by RFA in almost one-third of patients with baseline dysplastic BE. Most recurrences occurred during the first year after CR-IM. However, patients who achieved CR-IM and remained BE-free at 1 year after RFA had a low risk of BE recurrence.31 In addition, data from the UK RFA registry, the multicentre community practice registry and the multicentre interventional EURO II study have all shown that ET is capable of achieving CR-D in 81%–92% and CR-IM in 72%–87% of patients with BE neoplasia at 12 months.3 Recent systematic reviews and a meta-analysis have also shown that EMR followed by RFA in patients with early BE neoplasia can achieve CR-D of 91%–93% and CR-IM of 73%–78% with 5%–10% stricture rate, 1% bleeding rate and 0.2% perforation rate.22 Based on recent studies, the expert panel suggests that centres undertaking BET should aim for CR-D >90% and CR-IM >80% at 18 months after the first treatment, and end of treatment should be confirmed by two successive negative endoscopies, after which patients should receive follow-up endoscopies at appropriate intervals stratified according to risk of recurrence. The expert panel agreed that an 18-month time point is appropriate as standard clinical practice cannot always ensure timely visits and a 12-month time point would be too restrictive. | ||||||
| Patients with residual dysplasia after 18 months are to be rediscussed at an oesophagogastric MDT. | 9 | 0.7 | No disagreement | 1 | No disagreement | 90 (80–100) |
|
Aspirational performance target: 90% (range: 80–100).
Evidence summary: The recurrence of neoplasia after ER can be significantly reduced if the residual BE is completely ablated. A prospective study by Pech et al showed a significant (96.6%) response to ET in patients with BE neoplasia. However, metachronous lesions in the BE segment developed in 21.5% of patients within 2 years. The risk factors most frequently associated with recurrence were piecemeal resection, long-segment BE, no ablative therapy of BE after complete response, time until complete response achieved >10 months and multifocal neoplasia.32 It is therefore recommended that all patients with residual BE neoplasia after 18 months of endotherapy are discussed in a dedicated OG neoplasia MDT and considered for further investigation and treatment. | ||||||
| Post-BET symptomatic stricture rate should not exceed 10%–15%. | 8 | 0.5 | No disagreement | 1 | No disagreement | N/A |
|
Aspirational performance target: N/A.
Evidence summary: The documented SSR from several major studies range from 2.1% to 14%,33 requiring a median of 2–4 dilatations post-therapy. These also include data from EURO II study (SSR=6%),21 UK RFA registry (SSR=6.2%),3 and the meta-analyses by Yang et al (SSR=11.6%)33 and Qumseya et al (SSR=5.6%).34 EMR and ESD are increasingly used in the management of BE neoplasia, and stricture rates are expected to rise accordingly. It is therefore reasonable to suggest that all centres undertaking BET should not have SSR exceeding 10%–15% post-BET. | ||||||
BE, Barrett’s oesophagus; BET, Barrett’s endotherapy; CR-D, complete remission of dysplasia; CR-IM, complete remission of intestinal metaplasia; EET, endoscopic eradication therapy; EMR, endoscopic mucosal resection; ER, endoscopic resection; ESD, endoscopic submucosal dissection; ET, endoscopic therapy; GI, gastrointestinal; HGD, high grade dysplasia; IPRAS, interpercentile range adjusted for symmetry; LGD, low grade dysplasia; MAD-M, mean absolute deviation from the median; MDT, multidisciplinary team; N/A, not applicable; OAC, oesophageal adenocarcinoma; OG, Oesophago-gastric; QI, quality indicator; RCT, randomised controlled trial; RFA, radiofrequency ablation; SSR, symptomatic stricture rate.
Table 5.
Appropriate postprocedure QIs after round 2 voting with the median score, MAD-M, BIOMED analysis, p value, IPRAS analysis and the performance threshold
| Postprocedure QIs | Median score | MAD-M | BIOMED analysis | P value | IPRAS analysis | Performance threshold, median % (range) |
| Following successful BET, patients undergo follow-up endoscopies at appropriate intervals stratified according to risk of recurrence. | 9 | 0.6 | No disagreement | 1 | No disagreement | 90 (80–100) |
|
Aspirational performance target: 90% (range: 80–100).
Evidence summary: ET does not eliminate the need for continued endoscopic surveillance or completely eliminate the risk of synchronous or metachronous disease. Particular concern remains over IM, which is buried under the neosquamous epithelium after ET. This is a rare but recognised finding. The identification of these cases indicates the need for continued surveillance following RFA therapy, even after CR-IM. Increasing age and length of BE segment are associated with a longer time to achieve CR-IM. It is therefore essential to continue surveillance after RFA. By dividing patients into simple categories, clinicians may stratify risk to choose the appropriate surveillance regimen. A large prospective study by Shaheen et al 25 has shown impressive CR-D and CR-IM rates at 2 years (CR-D 95% and CR-IM 93%) and 3 years (CR-D 98% and CR-IM 91%) post initial BET with an annual rate of neoplastic progression of 1.37% per patient-years.25 Phoa et al 35 also showed a 90% remission at 5 years post-BET.35 The UK RFA registry has demonstrated a risk of neoplasia recurrence of 19% at 5 years with a predicted risk of IM recurrence of 13% at 26 months and a 32% risk of IM recurrence at 5 years.3 The literature supports an IM/neoplasia recurrence rate between 10% and 32% at 5 years. Therefore, follow-up postendoscopic therapy of BE neoplasia is needed to exclude recurrence and to deliver further therapy as needed.2 A recent study by Cotton et al 36 provided evidence-based surveillance intervals after completion of ET in patients with BE neoplasia. For patients with LGD the group proposed surveillance endoscopy at 1 and 3 years after achieving CR-IM with ET. For patients with HGD or IMC, the proposed surveillance endoscopy was at 3 months, 6 months and 1 year and then annually (for 5 years) after achieving CR-IM with ET.36 Based on recent evidence, our expert panel felt that it would be reasonable to consider endoscopic follow-up proposed by Cotton et al.36 | ||||||
| At follow-up endoscopy, biopsies should be taken from the squamocolumnar junction and within the extent of the original BE length, for the first 2 years; thereafter, biopsies should be taken from the squamocolumnar junction and any visible lesion. | 8 | 0.6 | No disagreement | 1 | No disagreement | 90 (80–100) |
|
Aspirational performance target: 90% (range: 80–100).
Evidence summary: Adherence to biopsy protocol will significantly increase the detection rate of dysplasia in patients with BE. IM can reoccur at the gastro-oesophageal junction in the absence of visible BE following the successful eradication of BE neoplasia. Recent studies have suggested evidence of buried glands post-BET in 5.5%–7% of patients, but the majority of these were not detectable at subsequent endoscopies.37 Our expert panel suggests that endoscopic follow-up should include biopsies at the GOJ and within the previous extent of the BE epithelium.2 This should include a high-resolution gastroscope to assess the treated and remaining area of BE.5 In order to exclude synchronous neoplastic lesions, four-quadrant biopsies should be performed at 1–2 cm intervals throughout the entire BE segment.5 | ||||||
BE, Barrett’s oesophagus; BET, Barrett’s endotherapy; CR-D, complete remission of dysplasia; CR-IM, complete remission of intestinal metaplasia; ET, endoscopic therapy; GOJ, gastro oesophageal junction; HGD, high grade dysplasia; IM, intestinal metaplasia; IMC, intramucosal carcinoma; IPRAS, interpercentile range adjusted for symmetry; LGD, low grade dysplasia; MAD-M, mean absolute deviation from the median; QI, quality indicator; RFA, radiofrequency ablation.
Table 6.
Quality indicators ranked as uncertain after round 1 voting with the median score, MAD-M, BIOMED analysis, p value and IPRAS analysis
| Median score | MAD-M | BIOMED analysis | P value | IPRAS analysis | ||
| Pre-endoscopy | Before undertaking EET, endoscopists need to have attended BET academia platforms. | 7 | 1.2 | Disagreement | 0.83 | No disagreement |
| It is recommended that prior to starting BET, a minimum of 30 supervised cases of endoscopic resection and 30 cases of endoscopic ablation should be performed to acquire competence in technical skills, management pathways and complications. | 7 | 1.2 | Disagreement | 0.83 | No disagreement | |
| Intraprocedure (ablation) | For patients undergoing RFA with a circumferential device, the recommended dose is 10 J/cm2–clean–10 J/cm2 (Express). | 7 | 1.5 | Disagreement | 0.83 | No disagreement |
| Postprocedure | Following successful eradication after BET, patients should undergo follow-up surveillance endoscopies at 3, 6, 9 and 21 months and then annually (if fit for endoscopy). | 8 | 1.7 | Disagreement | 0.51 | No disagreement |
BET, Barrett’s endotherapy; EET, endoscopic eradication therapy; IPRAS, interpercentile range adjusted for symmetry; MAD-M, mean absolute deviation from the median; RFA, radiofrequency ablation.
Discussion
Endoscopic treatment for dysplastic BE and early OAC has been recommended by various major societal guidelines; however, QIs for the management of patients with BE neoplasia have been lacking. This piece of work delivers a UK-based collection of QIs that will allow streamlined and accountable delivery of best clinical practice to patients undergoing BET.
This nationwide project combined the best available evidence with the collective judgement of national and international experts in order to develop a set of formally validated QIs for the management of patients with BE neoplasia using a rigorous and validated methodology (RAM). RAM, unlike the original Delphi, provides the expert panel with the opportunity to have a face-to-face discussion in round 2. Unlike guidelines which use a consensus methodology, RAM reduces the possibility of results being influenced by the opinion of the most senior or most vocal member of the panel.
These UK-based QIs reflect those recently published QIs in BET in the USA5; we were able to develop QIs for the intraprocedure component of patient care and for the management of patients at the pre-endoscopy and postendoscopy stages. In addition this UK-based project covered various aspects of patient care, including the importance of formal training of endoscopists prior to service provision, the use of high-quality endoscopic imaging modalities for lesion recognition in BE surveillance and the need for individual patient discussion at dedicated MDTs.
Adherence to Prague classification is known to result in improved dysplasia detection in patients with BE. This may be influenced by data from tertiary centres where diagnosis was obtained by expert BE endoscopists that are more likely to adhere to Prague classification with access to better endoscopic equipment, including high-definition endoscopy and virtual chromoendoscopy.
Our expert panel acknowledged the importance of ER modalities (endoscopic mucosal resection and endoscopic submucosal dissection (ESD)) for the management of visible lesions in BE neoplasia. ESD is a feasible treatment option that allows en-bloc resection for histological staging and treatment of patients with early BE neoplasia. ESD is likely to expand in the near future, and these QIs may need to evolve in order to cater for that in due course.
It is important that the clinical community recognises the balance between performing BET and the rate of success and stenosis. Therefore the expert group emphasised the importance of minimising stricture rates (not exceeding 10%–15%) post-BET and the need for discussion of patients’ care in MDTs prior to BET and when BET fails to achieve successful outcomes.
The current published evidence in BET3 provides data that are confined to a limited time period (less than 10 years); however, BET is expanding rapidly and therefore we need to continue long-term follow-up in these patients and monitor outcomes, which will provide us with essential information that will shape our future practice.
In this project we were also able to set aspirational performance thresholds to ensure that patient care is of highest standard. Regulatory and accrediting agencies as well as hospitals and clinicians may use these QIs to measure performance and highlight areas for improvement. The regular audit of outcomes and adverse events will ensure the efficacy and safety of endoscopic therapy for patients with early BE neoplasia. Auditing results may be used to implement changes in routine practice nationally, allowing comparison of local practices with national standards. These QIs may also be used for teaching, service development and standardisation of care at all hospitals performing BET. Future studies will need to investigate the positive and the negative impact of these QIs on patient outcomes.
There were some limitations to this study. First, high-quality evidence such as randomised controlled trials in the literature was not available for some QIs; however, this situation is common in many aspects of healthcare, and it was the very reason that the expert panel methodology such as RAM was developed.4 Second, some healthcare centres in the country may not be equipped with high-quality endoscopic modalities, and therefore these QIs may have a negative impact on their practice. Third, there was lack of validation of these QIs by an external committee and our expert panel voted on QIs that they developed themselves; hence, all the QIs in round 2 voting performed very well. Finally, the expert panel failed to determine the number of procedures needed to be performed by a centre to qualify as high-volume centre and also failed to determine the adequate number of procedures needed by an endoscopist prior to performing independent BET.
In conclusion, this is the first UK national consensus project that has used a validated methodology to successfully develop process-based QIs for patients undergoing endoscopic treatment for early BE neoplasia. These indicators identify meaningful and important steps for providing a unified high-quality care based on the best available evidence and expert opinion. These QIs may also be used for the training of the new generation of advanced endoscopists, and adherence to these measures would ultimately result in improving patient outcomes.
Footnotes
Presented at: This study was previously presented as an abstract at UEGW 2018.
Contributors: DA is the first author, and has written and submitted the manuscript, and was directly involved with the planning, analysis, data search and critique of this paper. KR, SW, IDP, NJT, MJ, MB, PrB, AJM, RW, PhB, HLS, NR, JD, CG, JM, IM, MdP, AMV, ST, CM, ME, SS, DG, SA, OP, PS and LBL were directly involved with the planning and critique of this paper and provided intellectual input to this paper. PaB significantly contributed to the statistical analysis and critique of this paper. RH was directly involved in the planning, analysis, data search and critique of this paper, and is the senior author. All authors contributed to the refinement of the paper and approved the final manuscript.
Funding: This project received funding from the BSG to cover travel expenses endured by the investigators in order to attend the face-to-face meeting in London for round 2 voting.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Wani S, Drahos J, Cook MB, et al. Comparison of endoscopic therapies and surgical resection in patients with early esophageal cancer: a population-based study. Gastrointest Endosc 2014;79:224–32. 10.1016/j.gie.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7–42. 10.1136/gutjnl-2013-305372 [DOI] [PubMed] [Google Scholar]
- 3. Haidry RJ, Butt MA, Dunn JM, et al. Improvement over time in outcomes for patients undergoing endoscopic therapy for Barrett's oesophagus-related neoplasia: 6-year experience from the first 500 patients treated in the UK patient registry. Gut 2015;64:1192–9. 10.1136/gutjnl-2014-308501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fitch K, Bernstein SJJ, Aguilar MDD, et al. The RAND / UCLA Appropriateness Method User’s Manual, 2001: 109 p. [Google Scholar]
- 5. Wani S, Muthusamy VR, Shaheen N, et al. 212 development of quality indicators for endoscopic eradication therapies (EET) in Barrett's esophagus: the TREAT-BE (treatment with resection and endoscopic ablation techniques for Barrett's esophagus) Consortium. Gastrointest Endosc 2016;83:AB129–AB130. 10.1016/j.gie.2016.03.054 [DOI] [PubMed] [Google Scholar]
- 6. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pasricha S, Cotton C, Hathorn KE, et al. Effects of the Learning Curve on Efficacy of Radiofrequency Ablation for Barrett’s Esophagus. Gastroenterology 2015;149:890–6. 10.1053/j.gastro.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lipman G, Gupta A, Banks M, et al. Specialist centre patient volume does not impact on endoscopic outcomes for treatment of barrett’s dysplasia. Results from the UK RFA registry. BSG annual conference, 2016. [Google Scholar]
- 10. NICE guideline Barrett’s Oesophagus: Ablative Therapy for the treatment of Barrett’s Oesophagus. National Institute for Health and Clinical Excellence, 2010. [PubMed] [Google Scholar]
- 11. Qumseya BJ, Wang H, Badie N, et al. Dysplasia and Neoplasia in Patients with Barrett’s Esophagus: Meta-Analysis and Systematic Review. Clinical Gastroenterology and Hepatology 2013;6:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma P, Bergman JJGHM, Goda K, et al. Development and Validation of a Classification System to Identify High-Grade Dysplasia and Esophageal Adenocarcinoma in Barrett’s Esophagus Using Narrow-Band Imaging. Gastroenterology 2016;150:591–8. 10.1053/j.gastro.2015.11.037 [DOI] [PubMed] [Google Scholar]
- 13. Subramaniam S, Kandiah K, Chedgy F, et al. Blue light imaging for barrett’s neoplasia classification (blinc): the development and validation of a new endoscopic classification system to identify barrett’s neoplasia, 2017. [Google Scholar]
- 14. Tomizawa Y, Konda VJA, Coronel E, et al. Efficacy, durability, and safety of complete endoscopic mucosal resection of Barrett esophagus: a systematic review and meta-analysis. J Clin Gastroenterol 2017;00:1–7. [DOI] [PubMed] [Google Scholar]
- 15. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection : European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829–54. [DOI] [PubMed] [Google Scholar]
- 16. Qumseya BJ, Brown J, Abraham M, et al. Diagnostic performance of EUS in predicting advanced cancer among patients with Barrett’s esophagus and high-grade dysplasia/early adenocarcinoma: systematic review and meta-analysis. Gastrointest Endosc 2015;81:865–74. 10.1016/j.gie.2014.08.025 [DOI] [PubMed] [Google Scholar]
- 17. May A, Günter E, Roth F, et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut 2004;53:634–40. 10.1136/gut.2003.029421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Westerterp M, Koppert LB, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Archiv 2005;446:497–504. 10.1007/s00428-005-1243-1 [DOI] [PubMed] [Google Scholar]
- 19. Kerry B, Dunbar SJS. The Risk of Lymph Node Metastases in Patients with High Grade Dysplasia or Intramucosal Carcinoma in Barrett’s Esophagus: A Systematic Review. Am J Gastroenterol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schölvinck D, Künzli H, Meijer S, et al. Management of patients with T1b esophageal adenocarcinoma: a retrospective cohort study on patient management and risk of metastatic disease. Surg Endosc 2016;30:4102–13. 10.1007/s00464-016-5071-y [DOI] [PubMed] [Google Scholar]
- 21. Phoa KN, Pouw RE, Bisschops R, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of an European multicentre study (EURO-II). Gut 2016;65:555–62. 10.1136/gutjnl-2015-309298 [DOI] [PubMed] [Google Scholar]
- 22. Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017;85:482–95. 10.1016/j.gie.2016.09.022 [DOI] [PubMed] [Google Scholar]
- 23. Wani S, Qumseya B, Sultan S, et al. Endoscopic eradication therapy for patients with Barrett’s esophagus–associated dysplasia and intramucosal cancer. Gastrointest Endosc 2018;87:907–31. 10.1016/j.gie.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 24. Haidry RJ, Dunn JM, Butt MA, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic Barrett's esophagus and early esophageal adenocarcinoma: outcomes of the UK national halo RFA registry. Gastroenterology 2013;145:87–95. 10.1053/j.gastro.2013.03.045 [DOI] [PubMed] [Google Scholar]
- 25. Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology 2011;141:460–8. 10.1053/j.gastro.2011.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Künzli H, Schölvinck D, Meijer S, et al. Efficacy of the CryoBalloon Focal Ablation System for the eradication of dysplastic Barrett’s esophagus islands. Endoscopy 2016. [DOI] [PubMed] [Google Scholar]
- 27. van Vilsteren FGI, Phoa KN, Alvarez Herrero L, et al. A simplified regimen for focal radiofrequency ablation of Barrett's mucosa: a randomized multicenter trial comparing two ablation regimens. Gastrointest Endosc 2013;78:30–8. 10.1016/j.gie.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 28. van Vilsteren FGI, Phoa KN, Alvarez Herrero L, et al. Circumferential balloon-based radiofrequency ablation of Barrett's esophagus with dysplasia can be simplified, yet efficacy maintained, by omitting the cleaning phase. Clin Gastroenterol Hepatol 2013;11:491–8. 10.1016/j.cgh.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 29. Pouw RE, Künzli HT, Bisschops R, et al. Simplified versus standard regimen for focal radiofrequency ablation of dysplastic Barrett's oesophagus: a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol 2018;3:566–74. 10.1016/S2468-1253(18)30157-2 [DOI] [PubMed] [Google Scholar]
- 30. Belghazi K, Pouw RE, Koch AD, et al. 477 self-sizing radiofrequency ablation balloon for eradication of barrett’s esophagus: results of an international multicenter randomized trial comparing three different treatment regimens. Gastrointest Endosc 2018;87:AB81 10.1016/j.gie.2018.04.086 [DOI] [PubMed] [Google Scholar]
- 31. Cotton CC, Wolf WA, Overholt BF, et al. Late recurrence of barrett’s esophagus after complete eradication of intestinal metaplasia is rare: final report from ablation in intestinal metaplasia containing dysplasia trial. Gastroenterology 2017;153:681–8. 10.1053/j.gastro.2017.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pech O, Behrens A, May A, et al. Long-Term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200–6. 10.1136/gut.2007.142539 [DOI] [PubMed] [Google Scholar]
- 33. Yang D, Zou F, Xiong S, et al. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc 2018;87:1383–93. 10.1016/j.gie.2017.09.038 [DOI] [PubMed] [Google Scholar]
- 34. Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett's esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:1086–95. 10.1016/j.cgh.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 35. Phoa KN, Pouw RE, van Vilsteren FGI, et al. Remission of Barrett's esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology 2013;145:96–104. 10.1053/j.gastro.2013.03.046 [DOI] [PubMed] [Google Scholar]
- 36. Cotton CC, Haidry R, Thrift AP, et al. Development of Evidence-Based Surveillance Intervals After Radiofrequency Ablation of Barrett’s Esophagus. Gastroenterology 2018;155:316–26. 10.1053/j.gastro.2018.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781–9. 10.1055/s-0030-1255779 [DOI] [PubMed] [Google Scholar]