A 78‐year‐old man with haemorrhagic signs (epistaxis and purpura) presented with severe thrombocytopenia, lymphopenia and circulating neutrophil precursors. A bone marrow aspirate showed increased plasma cells and an increase in pleomorphic megakaryocytes, consistent with peripheral thrombocytopenia. A few macrophages showing haemophagocytosis were also revealed (Fig 1A). Considering the outbreak of COVID‐19, 1 , 2 the combination of thrombocytopenia, lymphopenia and neutrophil precursors led to consideration and detection of SARS‐CoV‐2, although the patient did not have fever, cough, dyspnoea, diarrhoea, myalgia or headache. The diagnosis was confirmed by reverse transcriptase‐polymerase chain reaction (RT‐PCR) assay and chest X‐ray (CXR). Apart from thrombocytopenia and haemophagocytosis, this patient did not have other features of secondary haemophagocytic lymphohistiocytosis (sHLH). Clinical and laboratory features of the H‐score 3 were not met (Table I). Numerous large megakaryocytes in the bone marrow aspirate and the presence of platelet antibodies led to a diagnosis of autoimmune thrombocytopenic purpura (ITP), potentially related to COVID‐19. 4 The platelet count increased after treatment with intravenous immunoglobulin (from 6 to 87 × 109/l in 5 days). Corticosteroids were avoided in the context of COVID‐19. 5
Fig 1.
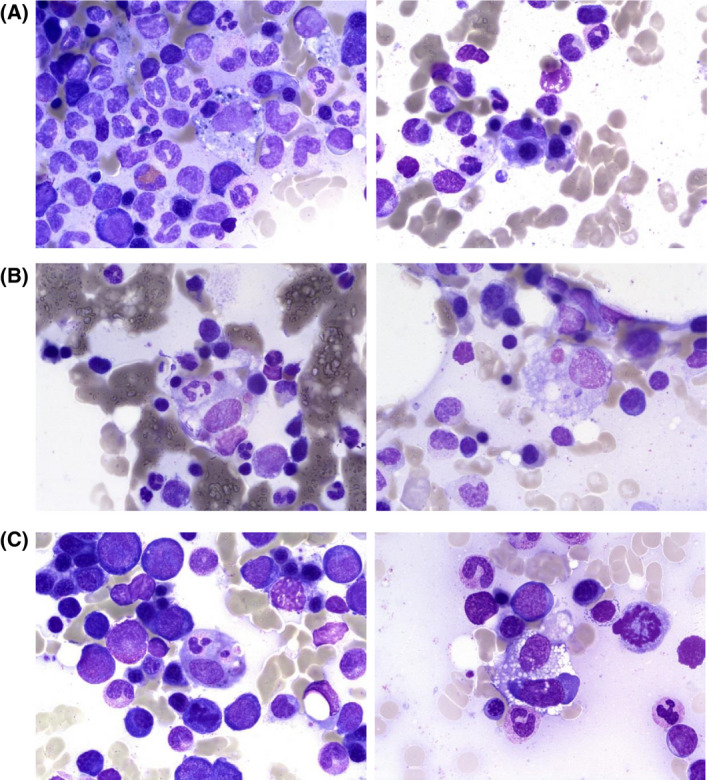
Haemophagocytosis in bone marrow aspirate. May–Grünwald–Giemsa staining of bone marrow aspirate shows histiocytes with engulfed nucleated cells or platelet.
Table I.
Demographic, clinical characteristics and laboratory findings.
| Characteristic | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age (year) | 78 | 67 | 63 |
| Sex | Male | Female | Male |
| Body mass index kg/m2 | 27 | 49 | 36 |
| Disease features at onset | Epistaxis, asthenia, anorexia | Worsening of the general state, cough, dyspnoea, fever | Worsening of the general state, cough, dyspnoea, hypoxaemia, fever |
| Imaging features | Diffuse bilateral pulmonary infiltrates | Diffuse bilateral pulmonary infiltrates | Diffuse bilateral pulmonary infiltrates |
| H‐score parameters | |||
| Fever | No | No | Yes |
| Hepatomegaly | No | No | No |
| Splenomegaly | No | No | No |
| Haemoglobin (g/l) | 124 | 104 | 85 |
| Leucocyte count (×109/l) | 5·84 | 12·65 | 23·65 |
| Platelet count (×109/l) | <5 | 26 | 89 |
| Serum ferritin (µg/l) | 624 | 620 | 4899 |
| Triglycerides (mmol/l) | 1·35 | 1·15 | 5·62 |
| Fibrinogen (g/l) | 6·6 | 1·6 | 2·1 |
| Aspartate aminotransferase (units/l) | 25 | 87 | 127 |
| Known underlying immunosuppression | No | No | No |
| Haemophagocytosis in bone marrow | Yes | Yes | Yes |
| H‐Score | 35 | 84 | 207 |
| Probability of sHLH (%) | <1 | <1 | 92 |
| Other laboratory findings | |||
| Neutrophil count (×109/l) | 7·09 | 9·34 | 20·81 |
| Lymphocyte count (×109/l) | 0·8 | 1·77 | 0·95 |
| Monocyte count (×109/l) | 0·57 | 0·97 | 0·24 |
| Neutrophil precursors count (×109/l) | 0·14 | 0·38 | 1·65 |
| C‐reactive protein (mg/l) | 12 | 204 | 357 |
| LDH (Units/l) | 219 | 584 | 588 |
| PT (%) | 82 | 61 | 99 |
| aPTT (sec) | 32·7 | 35·9 | 28·4 |
| D‐Dimer (mg/l) | 2177 | >25 000 | 17 994 |
sHLH, secondary haemophagocytic lymphohistiocytosis; C‐reactive protein, CRP; LDH, lactate dehydrogenase; PT, prothrombin time; aPTT, activated partial thromboplastin time.
Two other patients with severe COVID‐19 confirmed by RT‐PCR also had haemophagocytosis demonstrated in a bone marrow aspirate performed for cytopenia (Fig 1B,C). One of them (patient 2) was a 67‐year‐old obese woman with worsening of her general state, cough and fever, with a known SARS‐CoV‐2 contact. On admission, she had dyspnoea and tachycardia. CXR showed diffuse bilateral pulmonary infiltrates, and SARS‐CoV‐2 infection was confirmed by RT‐PCR. A bone marrow aspirate also revealed increased pleomorphic megakaryocytes and increased plasma cells, but with more prominent haemophagocytosis in this case (Fig 1B). Interestingly, haemophagocytosis often involved platelets. The H‐score (Table I) showed a low probability of sHLH (<1%). The patient died of refractory acute respiratory distress syndrome (ARDS). Patient 3 was a 63‐year‐old obese man with the same symptoms as patient 2 at disease onset. He was hospitalised in the intensive care unit for respiratory and renal failure. A bone marrow aspirate performed for cytopenia showed, once again, increased pleomorphic megakaryocytes, increased plasma cells and numerous haemophagocytic macrophages (Fig 1C). The high H‐score probability of 92% as well as the multiorgan failure leading to death confirmed a sHLH diagnosis in this setting.
Discussion
COVID‐19 may show varying presentation. 6 Our report highlights the presence of haemophagocytosis in these three cases of COVID‐19 presenting with different clinical features and severity: one ITP, one ARDS and one sHLH. Haemophagocytosis is neither necessary nor mandatory for the diagnosis of sHLH. 7 The H‐score, 3 including underlying immunoinsufficiency, body temperature, organomegaly, cytopenias, serum ferritin, triglycerides, fibrinogen and aspartate aminotransferase should be taken into account. Cytopenia, hyperferritinaemia and coagulopathy are described in many severe COVID‐19 pneumonia cases, suggesting that a subgroup of cases may have a macrophage activation syndrome. 8 In COVID‐19, the lungs are mainly involved, and the classical organomegaly pattern of sHLH is uncommonly reported. 9 , 10 Paradoxically, we found haemophagocytosis in the bone marrow aspirates of the two patients without features of sHLH. One of these patients did not have evidence of inflammation (low C‐reactive protein; see Table I). The macrophage activation in the bone marrow could partially explain the cytopenia in patient 2, with severe thrombocytopenia and activated macrophages engulfing mainly platelets. Furthermore, an autoimmune process may be involved, as in patient 1 with ITP.
Apart from these pathophysiological considerations, in laboratory practice haemophagocytosis in the bone marrow is usually observed in infection, autoimmune disease, myeloproliferative neoplasms, bone marrow failure and haemolysis. 11 Henceforth, whatever the clinical presentation, SARS‐CoV‐2 infection should be considered among the causes when haemophagocytosis is observed, probably even outside the context of a pandemic.
Author contributions
A.D. and I.H. performed data analysis. A.D. wrote the first draft. I.H., B.D. and J.Y.M. reviewed the manuscript. J.M., P.A., G.L., A.M., M.L. and K.K. provided patient care and data. All the authors reviewed the manuscript and provided final approval.
References
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Engl J Med. 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . World Health Organization. 2020. Available at: https://www.who.int/ [Accessed April 16, 2020].
- 3. Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, et al. Development and validation of the hscore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–20. [DOI] [PubMed] [Google Scholar]
- 4. Zulfiqar A‐A, Lorenzo‐Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient with Covid‐19. New Engl J Med. 2020;382(18):e43. 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta‐analysis. J Infect. 2020;S0163‐4453(20)30191‐2. 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan W‐J, Ni Z‐Y, Hu Y, Liang W‐H, Ou C‐Q, He J‐X, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382(18):1708–20. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lehmberg K, Ehl S. Diagnostic evaluation of patients with suspected haemophagocytic lymphohistiocytosis. Br J Haematol. 2013;160:275–87. [DOI] [PubMed] [Google Scholar]
- 8. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmun Rev. 2020;19(6):102537. 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID‐19) through postmortem core biopsies. Mod Pathol. 2020. 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Resp Med. 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Usmani GN, Woda BA, Newburger PE. Advances in understanding the pathogenesis of HLH. Br J Haematol. 2013;161:609–22. [DOI] [PubMed] [Google Scholar]


