To The Editor,
Coronavirus disease‐2019 (COVID‐19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has spread to 163 countries/areas since December 2019. As of 17 March 2020, it has infected over 170 000 people and resulted in death toll 7904 over the world. 1 , 2 The symptoms of SARS‐CoV‐2 infection vary widely, mild cases were self‐limited, and even did not have clinical symptoms, but severe cases progressed rapidly, and would die from acute respiratory distress syndrome and multisystem organ failure.
Previous studies have reported that the SARS‐CoV‐2 could be detected in sputum, feces, tears, urine, and other specimens of infected patients. 3 , 4 In the case report, the novel coronavirus was found in the bile specimen from a patient with severe COVID‐19. On 28 January 2020, a 59‐year‐old man presented with fever and cough after contact with his wife who had returned from Wuhan, China, not long before. The patient then visited the hospital on 2 February 2020 and was soon diagnosed with COVID‐19 after comprehensive consideration of his epidemiological history, clinical manifestations, characteristic blood profile, lung computed tomography (CT) images, and etiological examination. He was immediately admitted to specified infection wards and received supplemental oxygen through a nasal tube, interferon alfa‐2b and darunavir plus arbidol as an antiviral treatment, and piperacillin to prevent secondary infection. Besides, methylprednisolone (40 mg twice daily) was administered to relieve symptoms and attenuate lung inflammation. On day 6 after admission, chest CT showed progressive infiltration in both lungs (Figure 1A,B), and serum bilirubin and serum creatinine levels elevated progressively (Figure 1C). Therefore, he was transferred to the intensive care unit, received invasive ventilator support, and artificial liver support system treatment. On day 14 after admission, his hypoxemia worsened, and he developed acute respiratory distress syndrome with kidney injury and hepatic failure; he was soon administered extracorporeal membrane oxygenation treatment.
Figure 1.
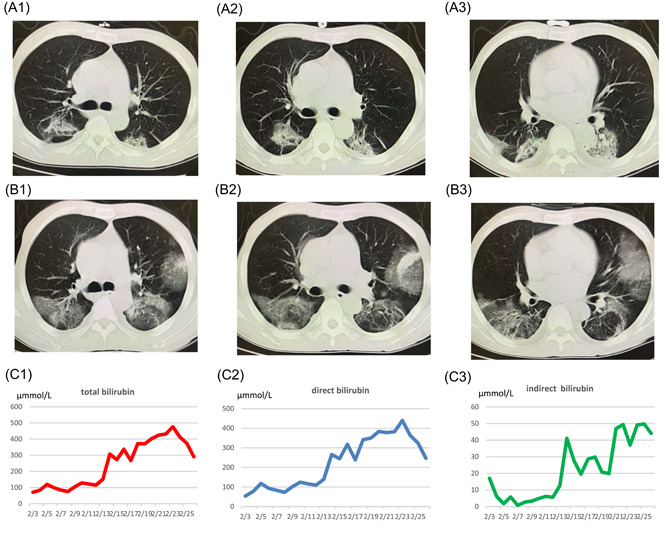
A and B, The lung CT imaging of the patient. A1, A2, and A3 are represent the lung CT preformed at day 1 of illness, panels B1, B2, and B3 are representing the lung CT preformed at day 6 of illness, it is obvious that chest CT showed progressive infiltrate in both lungs, so he was transferred to the ICU and received invasive ventilator support. Panels C1, C2, and C3 represent the total bilirubin, direct bilirubin, and indirect bilirubin of the patient during the stay of hospital. Despite the medication and artificial liver therapy, there was no significant decline in the levels of serum bilirubin while hospitalized, combining the patient's medical history, the attending doctor considered common bile duct of the patient obstruction was one of the reasons, and patient was given a endoscopic retrograde cholangiopancreatography (ERCP) treatment in February 24, then the levels of serum bilirubin dropped. CT, computed tomography; ICU, intensive care unit
Although he had been receiving artificial liver support system treatment during this hospitalization period, the serum level of bilirubin continued to rise significantly, and the maximum levels of total bilirubin and direct bilirubin were 476.7 and 437.9 μmmol/L, respectively. By asking the medical history, doctors learned that this patient had a history of undergoing liver transplantation 2 years prior and received an implantation of four plastic biliary stents. In view of the patient's medical history, the attending doctor considered bile duct obstruction as one of the reasons for liver function failure. So on day 23 after admission, the patient received endoscopic retrograde cholangiopancreatography treatment. During the operation, doctors found that the biliary sludge was completely obstructed at the duodenal papilla, so they removed the biliary stents with a medical snare and performed endoscopic naso‐biliary drainage treatment. On the following day, 60 mL of yellow‐green bile was irrigated into a closed drainage bag (to avoid contamination) from a nasobiliary duct. The bile specimen later tested positive on real‐time fluorescent reverse transcription polymerase chain reaction for SARS‐CoV‐2, and the cycle threshold value were 23 in the bile specimen and 32 in the sputum specimen which was collected on the same day, which means that much higher viral load in the bile juice than in the sputum.
The potential causes of bile duct obstruction were as follows:
-
1.
The primary disease and stent occlusion was the primary cause of bile duct obstruction.
-
2.
Angiotensin‐converting enzyme 2 receptor expression is enriched in cholangiocytes, so infection with SARS‐CoV‐2 can injure cholangiocytes, and cause cholestasis. 5
-
3.
During the course, the patient had been treated with antipyretic agents and antiviral drugs. Including acetaminophen, oseltamivir, abidol or lopinavir, which may injure bile duct and cause cholestasis. 6 And there may more potential causes of bile duct obstruction, such as clotting effects of SARS‐CoV‐2, and this needs further investigation.
To our knowledge, this is the first case in which bile juice SARS‐CoV‐2 was detected. It is known that bile juice is secreted by liver cells and biliary tract cells, which promote the digestion of fat and absorption of fat‐soluble vitamins. Therefore, it is suggested that the hepatocytes and/or biliary tract cells were infected by SARS‐CoV‐2 in the patient. And we speculate that SARS‐CoV‐2 detected in fecal specimens may, at least partly, originate from bile juice. Unfortunately, we did not know whether the SARS‐CoV‐2 in the bile juice survived or was contagious.
CONFLICT OF INTERESTS
All the authors declare that there are no conflict of interests.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382:727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. 10.1038/s41586-020-2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS‐CoV‐2 infection [published online ahead of print February 26, 2020]. J Med Virol. 2020. 10.1002/jmv.25725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID‐19 with fatal outcome: results from a prospective, single‐center, clinicopathologic case series [published online ahead of print May 14, 2020]. Ann Intern Med. 2020. 10.7326/M20-2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feng G, Zheng KI, Yan QQ, et al. COVID‐19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8(1):18‐24. 10.14218/JCTH.2020.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]


