Abstract
Aims
Since December 2019, the novel coronavirus SARS‐CoV‐2 has spread rapidly throughout China and keeps the world in suspense. Cardiovascular complications with myocarditis and embolism due to COVID‐19 have been reported. SARS‐CoV‐2 genome detection in the heart muscle has not been demonstrated so far, and the underlying pathophysiological mechanisms remain to be investigated.
Methods and results
Endomyocardial biopsies (EMBs) of 104 patients (mean age: 57.90 ± 16.37 years; left ventricular ejection fraction: 33.7 ± 14.6%, sex: n = 79 male/25 female) with suspected myocarditis or unexplained heart failure were analysed. EMB analysis included histology, immunohistochemistry, and detection of SARS‐CoV‐2 genomes by real‐time reverse transcription polymerase chain reaction in the IKDT Berlin, Germany. Among 104 EMBs investigated, five were confirmed with SARS‐CoV‐2 infected by reverse real‐time transcriptase polymerase chain reaction. We describe patients of different history of symptoms and time duration. Additionally, we investigated histopathological changes in myocardial tissue showing that the inflammatory process in EMBs seemed to permeate vascular wall leading to small arterial obliteration and damage.
Conclusions
This is the first report that established the evidence of SARS‐CoV‐2 genomes detection in EMBs. In these patients, myocardial injury ischaemia may play a role, which could explain the ubiquitous troponin increases. EMB‐based identification of the cause of myocardial injury may contribute to explain the different evolution of complicated SARS‐CoV‐2‐infection and to design future specific and personalized treatment strategies.
Keywords: SARS‐CoV‐2‐infection, COVID‐19, Endomyocardial biopsy, Myocarditis, Heart failure
Introduction
In December 2019, a novel coronavirus with potential zoonotic origin, named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was identified as the causative agent of a cluster of suspicious pneumonia cases in Wuhan, Hubei, China. The incredible fast worldwide spread of the coronavirus disease 2019 (COVID‐19) prompt the World Health Organization (WHO) to declare COVID‐19 as a pandemic on 11 March 2020. 1 More than 1 776 867 confirmed cases of COVID‐19 and more than 111 828 fatalities in 185 countries have been attributed to SARS‐CoV‐2 as of 14 April 2020 (https://who.sprinklr.com/).
Molecular tests (real‐time reverse transcriptase polymerase chain reaction, RT‐qPCR) were generally used to confirm the clinical diagnosis of COVID‐19. A recent report showed that SARS‐CoV‐2 could be detected in different types of clinical specimens such as Broncho alveolar lavage, sputum, nasal swabs, feces, blood, and urine. 2 The ubiquitous distribution of the main viral entry receptor angiotensin converting enzyme 2 (ACE2) for SARS‐CoV‐2 entry into the target cells led to the hypothesis of the involvement of other potential target organs for SARS‐CoV‐2 besides the respiratory tract, for example, the heart, the liver, the brain, the pancreas, or the kidneys. 3
Infection with the SARS‐CoV‐2 is associated with systemic illness by hyper‐inflammation. 4 Cardiovascular complications with embolism due to COVID‐19 have been reported recently. 5 , 6 , 7 , 8 Acute myocardial injury associated with COVID‐19 manifested as an increase of high‐sensitivity cardiac troponin levels. 1 However, direct SARS‐CoV‐2 RNA in the heart muscle has not been demonstrated so far. The damage caused by SARS‐CoV‐2 to the cardiovascular system and the underlying mechanisms remain to be investigated. Accordingly, we prospectively analysed endomyocardial biopsies (EMBs) from a cohort of 104 samples of patients with suspected myocarditis or unexplained heart disease for the presence of SARS‐CoV‐2 RNA by RT‐qPCR and hints for histopathological injury.
Methods
Up to 8 EMBs each of 104 patients [mean age: 57.90 ± 16.37 years; left ventricular ejection fraction (LVEF): 33.7 ± 14.6%, sex: n = 79 male/25 female] with suspected myocarditis or unexplained heart failure were analysed between 3 February and 26 March 2020 in German clinical centres in accordance with SARS‐CoV2 spread in Germany. EMBs were routinely taken from left ventricle. In 60.4% a hypertrophy was seen according possible due to a cardiac oedema. Coronary artery disease was excluded angiographically in all patients prior to EMB. The suspected diagnosis had been made by clinicians. EMBs were send for further diagnosis to the laboratory IKDT (Institute for Cardiac Diagnostic and Therapy Berlin, Germany). Analysis included histology, immunohistochemistry, and molecular virology. Following EMB extraction, samples were transferred to formalin for histological analyses and to RNAlater™ solution (Thermo Fisher Scientific, Waltham, MA, USA) for immunohistological and molecular analyses. DNA was extracted by Puregene Core Kit A (Qiagen, Hilden, Germany) according to manufacturer's instructions. Total RNA from one EMB was isolated using TRIzol Reagent™ (Thermo Fisher Scientific, Waltham, MA, USA), solubilized in DEPC‐H2O, and treated with DNAse (PeqLab, Erlangen, Germany) to remove any traces of DNA followed by reverse transcription with High‐Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA).
Random hexamer primers (5 μM) were used in addition to specific primers targeting the E‐gene of SARS‐CoV2 (0.2 μM each). DNA and cDNA concentrations were measured by PCR‐based Quantifiler™ Human DNA Quantification Kit (Thermo Fisher Scientific, Waltham, MA, USA) or by expression of housekeeping gene HPRT, respectively. Detection of other cardiotropic viruses (Enteroviruses, Adenoviruses, Human Herpesvirus 6, Epstein‐Barr virus, parvovirus B19) using (RT‐) qPCR or nested PCR was applied as described elsewhere. 9 , 10 Commercially available RT‐qPCR kits targeting the E‐gene and RdRp‐gene (TIB MOLBIOL, Roche Diagnostics, Germany) and the assay N2 (N‐gene) published by the CDC were chosen to initially screen samples for presence of SARS‐CoV2 genomes. As aforementioned assays were proven to be the most robust, RdRp‐gene assay was used for confirmation of results. 11
All RT‐qPCR assays were performed using TaqMan Universal PCR MasterMix (Thermo Fisher Scientific, Waltham, MA, USA) in a 20 μL reaction mix consisting of 2× PCR buffer including enzyme mix, primers, and probes concentrations as recommended by manufacturers and 21.5 μL and 5 μL of cDNA. Thermal cycling was carried out as recommended by manufacturers on either ABI QuantStudio 12k flex or BIORAD CFX96 thermal cyclers. In brief, real‐time RT‐PCR was performed with 45 cycles using 1.5 μL of cDNA. However, for validation of our PCR results additional PCR runs were performed with 5 μL of cDNA not altering the results obtained by the 1.5 μL approach. Synthetic in vitro RNA of E‐gene and RdRP‐gene assays were diluted 1:10 prior to cDNA synthesis and plasmid positive control of N‐gene assay was diluted 1:104 prior to RT‐qPCR to account for an expected low yield in total RNA extracted from EMB samples.
Histology was developed from formalin‐fixed tissue by haematoxylin & eosin (HE); Azan, and Periodic acid–Schiff (PAS) staining in light microscopy. For immunohistological evaluation, specimens were RNAlater fixed, embedded in Tissue Tec (SLEE, Mainz, Germany) and immediately snap‐frozen in methyl butane which had been cooled in liquid nitrogen and then stored at −80°C until processing. Embedded specimens were cut into cryosections placed on 10% poly‐l‐lysine‐precoated slides. Myocardial inflammation was diagnosed by CD3+ t‐lymphocytes/mm2 (Dako, Glostrup, Denmark), CD11a+/LFA‐1+ lymphocytes/mm2 (Immuno Tools, Friesoythe, Germany), CD11b+/Mac‐1+ macrophages/mm2 (ImmunoTools, Friesoythe, Germany), CD45R0+ t memory cells (Dako, Glostrup, Denmark), perforin+ cytotoxic cells/mm2 (BD Bioscience, San Jose, California). In addition, we stained intercellular adhesion molecules and MHC class II cell surface receptor (CD54/ICAM‐1 and HLADR, Immunotools, Friesoythe, Germany). Staining were quantified by digital image analysis. 12
Ethical approval
Approval was not required.
Results
Endomyocardial biopsy results of total patient cohort are summarized in Table 1 . Out of the 104 EMB samples, five patients were positive for SARS‐CoV‐2 E‐gene specific sequences indicating the first description of SARS‐CoV‐2 presents in a case series.
TABLE 1.
Clinical characteristics and biopsy findings
| Group | All patients |
|---|---|
| Number of patients, n (%) | 104 (100) |
| Men, n (%) | 79 (76) |
| Age at diagnosis, mean ± SD (years) | 57.9 ± 16.4 |
| LVEF at diagnosis, mean ± SD (%) | 33.7 ± 14.6 |
| Diagnosis, n (%) | |
| • Active myocarditis | 14 (13.4) |
| • Inflammatory cardiomyopathy | 34 (32.6) |
| • Borderline myocarditis | 3 (2.9) |
| • Dilated cardiomyopathy | 43 (41.3) |
| • Amyloidosis | 10 (9.6) |
| EMB results | |
| CD3+ count in EMB | 24.1 ± 54.0 |
| Mean ± SD (cells/mm2) | |
| CD45R0+ count in EMB | 87.9 ± 96.4 |
| Mean ± SD (cells/mm2) | |
| LFA‐1+ count in EMB | 29.9 ± 48.3 |
| Mean ± SD (cells/mm2) | |
| Mac‐1+ count in EMB | 70.3 ± 106.7 |
| Mean ± SD (cells/mm2) | |
| Perforin+ count in EMB | 1.3 ± 3.8 |
| Mean ± SD (cells/mm2) | |
| CD54+ count in EMB | 2.7 ± 1.5 |
| Mean ± SD (%Area fraction) | |
| HLADR+ count in EMB | 4.6 ± 2.0 |
| Mean ± SD ((%Area fraction) | |
| SARS‐CoV‐2, n (%) | 5 (4.8) |
| B19V, n (%) | 70 (67.3) |
| HHV6, n (%) | 8 (7.7) |
| ADV, n (%) | 0 (0.0) |
| EBV, n (%) | 4 (3.8) |
| COX, n (%) | 1 (1.0) |
EMB, endomyocardial biopsy; LVEF, left ventricular ejection fraction. Immunohistological marker: CD3, T‐lymphocytes; LFA‐1, leukocyte function antigen‐1; Mac‐1, macrophage‐1 antigen; CD45R0 (UCHL1), leucocyte common antigen; perforin, cytotoxic cells; CD54/ICAM‐1, intercellular adhesion molecule‐1; HLADR, MHC class II cell surface receptor; B19V, Parvovirus B19; HHV6, Human Herpesvirus 6; ADV, Adenovirus; EBV, Epstein–Barr‐Virus; COX, Coxsackivirus. The data are presented as mean ± standard deviation.
Besides latent infection with parvovirus B19, no other viral pathogens were detectable in SARS‐CoV‐2 positive samples.
Based on the clinical history, the clinicians expressed a suspicion of a previous COVID‐19 infection, but they were not tested with throat swab sample during admission to the hospital. The clinical courses of the five patients were different and showed highly acute to mild forms.
Patient 1
Patient 1 was a 48‐year‐old male with newly diagnosed heart failure and significantly reduced systolic function (EF 22%). Suspected diagnosis was acute myocarditis. He described sudden onset of high‐grade fever and dyspnoea within a few days. In addition, he suffered from thrombi and embolia. He reported a prior vacation in Tyrol, Austria. This patient showed a highly acute status was admitted to the intensive care unit (ICU) and due to severe infection. The diagnosis of a small‐vessel vasculitis was established, and cyclophosphamide and additional steroids were initiated. The patient recovered adequately. After receiving EMB results, immunosuppressive treatment was stopped immediately.
Patient 2
Patient 2 was a 62‐year‐old male with mildly reduced EF (40%) and moderate LV‐hypertrophy, and without respiratory infect. This patient had a new cardiac impairment of LV function since January 2020. The cause was unknown, so a possible myocarditis was assumed. With the exception of cardiac symptoms, this patient had a mild course and did not need to be monitored by ICU.
Patient 3
Patient 3 was a 60‐year‐old female with heart failure symptoms but preserved EF (60%) with pronounced LV‐hypertrophy. Initially, she was admitted to the ICU with severe acute respiratory syndrome. Blood tests revealed elevated levels of markers of myocyte injury (see Table 2 ), which remained positive during the first days of her hospitalization. After respiratory improvement the EMB was carried out 4 weeks after onset of syndromes. In this interesting case, the cardiac symptoms occurred with a pronounced relapse after the initial event.
TABLE 2.
Characteristics of patients
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age at diagnosis (years) | 48 | 62 | 60 | 36 | 39 |
| Clinical suspected diagnosis | Acute myocarditis | Unexplained heart failure | Unexplained heart failure | Inflammatory cardiomyopathy | Acute myocarditis |
| Diagnosis | Active myocarditis | Inflammatory cardiomyopathy | Inflammatory cardiomyopathy | Inflammatory cardiomyopathy | Borderline‐myocarditis |
| Sex | M | M | F | M | M |
| LVEF at diagnosis (%) | 22 | 40 | 60 | 25 | 55 |
| Laboratory parameters: | |||||
| High sensitive Troponin (pg/mL) | 3264 | ‐ | 83 | 56 | 379 |
| BNP (pg/mL) | 12 232 | ‐ | 113 | 258 | 109 |
| EMB analysis: | |||||
| Myocyte diameter (μm) | 18 | 18 | 32 | 22 | 19 |
| CD3+ count in EMB (cells/mm2) | 106.98 | 7.0 | 20.54 | 4.97 | 18.74 |
| CD45R0+ count in EMB (cells/mm2) | 156.23 | 14.0 | 96.15 | 61.47 | 162.38 |
| LFA‐1+ count in EMB (cells/mm2) | 83.15 | ‐ | 24.36 | 16.95 | 102.6 |
| Mac‐1+ count in EMB (cells/mm2) | 155.34 | 39,5 | 91.56 | 49.09 | 154.35 |
| Perforin+ count in EMB (cells/mm2) | 1.79 | ‐ | 1.74 | 0.00 | 4.01 |
| CD54+ count in EMB (%Area Fraction) | 6.42 | ‐ | 1.91 | 1.90 | 4.05 |
| HLADR+ count in biopsy (%Area Fraction) | 7.25 | 0,5 | 3.78 | 7.14 |
EMB, endomyocardial biopsy; LVEF, left ventricular ejection fraction. Immunohistological marker: CD3, T‐lymphocytes; LFA‐1, leukocyte function antigen‐1; Mac‐1, macrophage‐1 antigen; CD45R0 (UCHL1), leucocyte common antigen; perforin, cytotoxic cells; CD54/ICAM‐1, intercellular adhesion molecule‐1; HLADR, MHC class II cell surface receptor.
Patient 4
Patient 4 was a 36‐year‐old male with a significantly reduced systolic function (EF 25%) with a history of mild respiratory infect 3 weeks ago. The clinical course developed without complications and ICU surveillance. During hospitalization, the levels of troponin decreased laboratory values on day 15 were in reference range, and he recovered during this time.
Patient 5
Patient 5 was a 39‐year‐old male with heart failure symptoms but preserved EF with suspected diagnosis of acute myocarditis. The patient had a history of upper airway infection with headache and fever up to 4 weeks before admission. He suffered from shortness of breath, T‐wave inversions in the anterolateral leads in ECG, elevated cardiac troponin I, and cardiac magnetic resonance imaging compatible with myocarditis. The course of this patient was acute and required ICU treatment.
In patients 2–5, treatment strategies were not modified after receiving the result of SARS‐CoV‐2 RT‐qPCR in EMB. They were treated symptomatically, in part with initiation of guideline‐directed medication for heart failure.
Patient characteristics and EMB results are summarized in Table 2 .
Analysis of endomyocardial biopsies
SARS‐CoV‐2 loads determined in the EMBs were low (Ct values: 36.66 ± 1.99) corresponding to less than 100 to 500 viral copies/reaction. Viral loads were determined from the internal SARS‐CoV‐2 positive control with a Ct value of 32.73 ± 1.12 corresponding to approximately 10E + 4 copies/reaction while the Ct values of SARS‐CoV‐2 negative samples were below 40 cycles and thus below detection limit. Results from RT‐qPCR are shown in Table 3 .
TABLE 3.
RT‐qPCR results of SARS‐CoV‐2 detection in EMBs
| Patient | Ct (E‐Gen) |
|---|---|
| 1 | 36.79 ± 2.48 |
| 2 | 38.65 ± 8,9 |
| 3 | 33.36 ± 1.9 |
| 4 | 37.6 |
| 5 | 36.91 ± 3.7 |
| positive control | 32.73 ± 1.12 |
Ct, threshold cycle; RT‐qPCR, quantitative polymerase chain reaction.
Histological assessment of EMBs revealed an active myocarditis according to the Dallas criteria in patient 1 13 , 14 (Figure 1 A ). Histological analysis could also show necrosis of myocytes and interstitial tissue and granulation tissue in the periphery of necrosis of the type observed after an infarction (Figure 1 A ).
Figure 1.
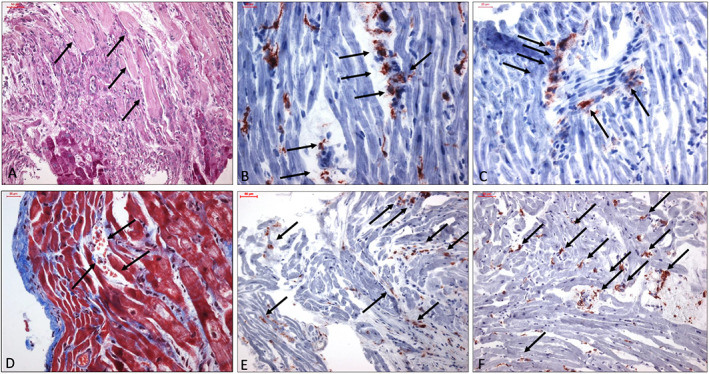
(A) Myocardium with recent necrosis (arrows) in the upper part, below granulation tissue with moderate infiltrates of inflammatory cells. At the bottom persisting myocytes. PAS. Bar 50 μm. (B) Immunohistochemical staining of CD11b + macrophages. Vessels are involved in the inflammation process. Bar 20 μm. (C) Perforin positive cells in the neighbourhood of an oblique cut blood vessel (arrow) within the myocardium. Staining by antibody against perforin, hemalum. Bar 20 μm. (D) Five to six myocyte layers below the endocardium ruptured capillary and bleeding (arrow). Azan staining. Bar 20 μm. (E) Increase of T‐lymphocytes stained by CD3 antibody. Bar 50 μm. (F) Increase of CD45R0 positive T memory cells. Bar 50 μm.
Immunohistochemical EMB analysis confirmed pronounced intramyocardial inflammation. Analysis of immune cell infiltrates of SARS‐CoV‐2 genome positive EMBs showed elevated number of T‐cells, macrophages, lymphocytes, and T‐memory cells (CD45R0) in four of the five patients (Figure 1 A–C, E, F ). Moreover, all SARS‐CoV‐2 patients exhibited an elevated number of cell adhesion molecules (CD54/ICAM‐1). Patient 2 showed inflammatory response on limit values.
We could show that the inflammatory process in cardiac tissue seemed to permeate vascular wall. The inflammatory process was leading to arterial obliteration and damage (Figure 1B–D). The final mechanism of tissue damage in consequence of vascular obliteration appears to be similar to systemic forms of vasculitis leading to ischaemia. The neighbouring myocardium displayed vacuoles in myocytes as a sign of restricted metabolism. Perivascular fibrosis with variation of fibre densities could be seen in cases 2 to 5 (not shown in figures). This phenomenon indicated relicts of previous damage.
Discussion
In this study, we established for the first time the evidence of SARS‐CoV‐2 genome detection in 5 of 104 EMBs of patients with suspected myocarditis or unexplained heart failure.
After the first cases describing pneumonia of unknown origin in Wuhan, China, SARS‐CoV‐2 rapidly spread worldwide with critical challenges for the public health and medical communities. Cardiovascular involvement in COVID‐19 seems to be a notable complication. First single case reports could show viral particles in interstitial cytopathic macrophages and their surroundings in EMB of a severe COVID‐19 shock patient by electron microscopic analyses. Whether direct myocardial injury due to viral involvement or the effect of systemic inflammation appear to be the most common mechanisms responsible for cardiogenic shock situation needs to be further investigated. 19
In the analytic stage, real‐time RT‐PCR assays remain the molecular test of choice for the aetiologic diagnosis of SARS‐CoV‐2 infection. Specificity of E‐gene and RdRP‐gene assays tested with clinical respiratory samples and SARS‐CoV and MERS‐CoV did not result in cross‐reactivity and false positive results. High sensitivity of both assays as indicated by low PCR limit of detection for purified RNA could also be confirmed for RNA spiked into and extracted from swab samples. 15 However, direct SARS‐CoV‐2 RNA detection in the myocardium has not been demonstrated so far. Herein, we demonstrated by RT‐qPCR that SARS‐CoV‐2 genomes is present in different cases.
In this study, we described series of different histories of cardiovascular patients admitted to the hospital. One main clinical finding is that cardiac involvement with positive SARS‐CoV‐2 genomes in EMBs can either occur acutely or with latency after onset of symptoms of infection.
Based on the results of currently published research, it seems important to discuss the manifestations and characteristics of myocardial damage induced by COVID‐19. 16 Herewith, we validated the direct cardiac involvement associated with intramyocardial inflammation in patients with SARS‐CoV‐2 genome positivity in EMBs. In patient 1, we could show an active myocarditis and in patient 5 a borderline‐myocarditis according the Dallas criteria. In the remaining patients, an inflammatory cardiomyopathy was determined.
Recent literature data have shown that cardiac Troponin I concentration is increased in all patients with SARS‐CoV‐2 infection, and values exceeding the 99th percentile in the upper reference limit can be observed in 8–12% of positive cases. 17 Moreover, patients with COVID‐19 are known to be at higher risk of acute pulmonary embolism, and elevated d‐dimer levels on admission are predictive of adverse outcomes for patients with COVID‐19. 5 The first vascular sign has been referred to as ‘vascular thickening’ or ‘vascular congestion’ in the lung. Bai et al. 18 reported vascular thickening to be significantly associated with COVID‐19 compared with non–COVID‐19 pneumonia (59% vs. 22%, P < 0.001). The physiopathologic mechanisms behind these changes remain unclear, but their role in diagnosis and possible future treatment strategies is substantial.
In this regard, a very recent report showed that pericytes demonstrating high ACE‐2 expression might act as target cells for SARS‐CoV‐2, while pericyte injury can result in endothelial cell dysfunction. 22 Recent reports showed that besides pericyctes ACE‐2 is expressed to different levels also in cardiomyocytes, endothelial cells, fibroblasts, and leucocytes. 23 However, ACE‐2 expression does not argue for permissive infection of a respective target cell by SARS‐CoV‐2. On the other hand, recent reports have demonstrated that SARS‐CoV‐2 genomes could be detected besides airway epithelium cells also in the intestinal enterocytes, spleen, liver, kidney, and heart. 2 , 24 In addition, recent histologically post‐mortem analyses in COVID‐10 positive patients revealed lymphocytic endotheliitis in different organs with evidence of direct viral infection, indicating endothelial dysfunction as a possible principle determination of microvascular dysfunction by shifting the vascular equilibrium towards more vasoconstriction with subsequent organ ischaemia and inflammation. 21 Although nearly all organs seemed to be affected by COVID‐19, we currently do not know in‐depth details about the organ‐specific infection by SARS‐CoV‐2. In this regard, Tavazzi and coworkers have shown recently in their case description using electron microscopy on EMBs of a patient with COVID‐19 in cardiogenic shock that SARS‐CoV‐2 particles could be localized to interstitial macrophages and their surroundings but not in cardiomyocytes. 19 As to whether this observation is due to a transient viraemia or infected macrophage migration from the lung has to be evaluated. Our finding of SARS‐CoV‐2 genome detection in EMBs of patients suffering from myocarditis/inflammatory cardiomyopathy cannot rule out or confirm the infection of cardiac cells but revealed incremental insights into organ‐specific infection of SARS‐CoV‐2 using possibly macrophage migration as a shuttle from the lung to the heart.
In this study, we investigated histopathological changes in myocardial tissue in the series of SARS‐CoV‐2 positive EMBs. In line with the recently published study, we could show that the inflammatory process in EMBs seemed to permeate vascular wall leading to small arterial obliteration. The final mechanism of tissue damage in consequence of vascular obliteration appears to be similar to systemic forms of vasculitis. We therefore hypothesize that in these patients, myocardial injury ischaemia may play a role, which could explain the ubiquitous troponin increases. As a result, this ischaemia could trigger possible cardiac arrhythmias.
Limitation of study
A limitation of this study is that we did not had enough material for SARS‐CoV‐2 genome in depth analysis to certainly exclude cross reaction with other corona virus strains due to the limited material available by the EMBs. However, the high sensitivity and specificity of the used PCR systems to detect solely SARS‐CoV‐2 genomes have been demonstrated recently. 15
Another limitation is that we cannot completely exclude that the detection of SARS‐CoV‐2 genomes in the heart might result from contamination of circulating blood. Unfortunately, we have no blood samples to the corresponding EMBs on hand to analyse this aspect. However, SARS‐CoV‐2 load in blood seemed to be low in comparison with other clinical types of specimens. 2
Nevertheless, SARS CoV‐2 can potentially bind to its cellular ACE2 receptor in heart tissue cells and can therefore be detected in the heart muscle. In this regard, a recent report showed that pericytes in the heart demonstrating high ACE‐2 expression might act as target cells for SARS‐CoV‐2 while pericyte injury can result in endothelial cell dysfunction. 20 , 22 If SARS CoV‐2 can replicate in these target cells of the heart, this has to be investigated in subsequent analysis. The low detection rate and low viral loads of SARS‐CoV‐2 genomes may be due to the limited number, size, and quantity of EMBs. Heart tissue cells (e.g. pericytes) are not the main target cells of SARS‐CoV‐2 while specimens of the main target the lung of infected patients are easier and in larger quantity to obtain than EMBs and may contribute to the low detection rate in EMBs. However, we showed that SARS‐CoV‐2 is detectable in the heart muscle but can only speculate about the clinical relevance of SARS‐CoV‐2 infection of the heart. As to whether SARS‐CoV‐2 infection may induce myocarditis is questionable, however, may trigger an ongoing progress to myocarditis of other reason.
In conclusion, in this study, we could show for the first‐time evidence of SARS‐CoV‐2 genome detection in 5 of 104 patients with suspected myocarditis or unexplained heart failure with different history of symptoms and time duration. In addition to inflammation and consequential damage, one possible histopathological mechanism may be vascular involvement with arterial obliteration which can lead to ischaemia. A possible SARS‐CoV‐2 infection should therefore be considered in patients with acute unexplained heart failure or new cardiac arrhythmias. We believe that recognition by the scientific community of myocarditis as a possible complication associated with COVID‐19 may be helpful for strict monitoring of affected patients. EMB‐based identification of the cause of myocardial injury may contribute to explain the different evolution of complicated SARS‐CoV‐2‐infection and to design future specific treatment strategies. An antiviral therapy is not yet available. Based on our histopathological results, possible anticoagulant/antiaggregation therapy should be investigated.
Conflict of interest
None declared.
Acknowledgements
All nurses, clinicians, and infectious diseases specialists working hard in this difficult period are greatly acknowledged for their efforts and daily care for patients suffering from SARS‐CoV‐2 infection. This work was done within a ProFIT grant of the Investitionsbank Berlin (ProFIT No. 10169028, Berlin, Germany). For their excellent technical assistance, we thank K. Winter, C. Seifert, S. Ochmann, C. Liebig, and K. Errami (IKDT Berlin, Germany).
Escher, F. , Pietsch, H. , Aleshcheva, G. , Bock, T. , Baumeier, C. , Elsaesser, A. , Wenzel, P. , Hamm, C. , Westenfeld, R. , Schultheiss, M. , Gross, U. , Morawietz, L. , and Schultheiss, H.‐P. (2020) Detection of viral SARS‐CoV‐2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Failure, 7: 2440–2447. 10.1002/ehf2.12805.
References
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA 2020; 323: 1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, Lou Y, Gao D, Yang L, He D, Wang MH. Preliminary estimation of the basic reproduction number of novel coronavirus (2019‐nCoV) in China, from 2019 to 2020: a data‐driven analysis in the early phase of the outbreak. Int J Infect Dis The Author(s). Published by Elsevier Ltd on behalf of International Society for Infectious Diseases 2020; 92: 214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK . COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020; 395: 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng Y‐Y, Ma Y‐T, Zhang J‐Y, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol 2020; 17: 259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sotiriou E, Heiner S, Jansen T, Brandt M, Schmidt KH, Kreitner K‐F, Emrich T, Schultheiss H‐P, Schulz E, Münzel T, Wenzel P. Therapeutic implications of a combined diagnostic workup including endomyocardial biopsy in an all‐comer population of patients with heart failure: a retrospective analysis. ESC Hear Fail 2018; 5: 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuhl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, Schultheiss H‐P. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 2005; 112: 1965–1970. [DOI] [PubMed] [Google Scholar]
- 11. Casto AM, Roychoudhury P, Xie H, Selke S, Perchetti GA, Wofford H, Huang M‐L, Verjans GMGM, Gottlieb GS, Wald A, Jerome KR, Koelle DM, Johnston C, Greninger AL. Large, stable, contemporary interspecies recombination events in circulating human herpes simplex viruses. J Infect Dis 2019; 221: 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schultheiss H‐P, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, Liu PP, Matsumori A, Mazzanti A, McMurray J, Priori SG. Dilated cardiomyopathy. Nat Rev Dis Primers 2019; 5: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol 1987; 18: 619–624. [DOI] [PubMed] [Google Scholar]
- 14. Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss H‐P, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM, European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648 2648a‐2648d. [DOI] [PubMed] [Google Scholar]
- 15. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DGJC, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette J‐L, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MPG, Drosten C. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill European Centre for Disease Prevention and Control (ECDC) 2020; 25: 2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020. 10.1001/jamacardio.2020.1286 [DOI] [PubMed] [Google Scholar]
- 17. Lippi G, Lavie CJ, Sanchis‐Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID‐19): evidence from a meta‐analysis. Prog Cardiovasc Dis 2020. 10.1016/j.pcad.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, Pan I, Shi L‐B, Wang D‐C, Mei J, Jiang X‐L, Zeng Q‐H, Egglin TK, Hu P‐F, Agarwal S, Xie F, Li S, Healey T, Atalay MK, Liao W‐H. Performance of radiologists in differentiating COVID‐19 from viral pneumonia on chest CT. Radiology Radiological Society of North America 2020: 200823 10.1148/radiol.2020200823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail 2020. 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS‐CoV‐2. Cardiovasc Res 2020; 116: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID‐19. The Lancet 2020; 395: 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Attwell D, Mishra A, Hal CN, O'Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab 2016; 36: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M, Holubec T, Walther T, Zeiher AM, Dimmelere S. Cell type‐specific expression of the putative SARS‐CoV‐2 receptor ACE2 in human hearts. Eur Heart J 2020: ehaa311; 41: 1804–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli R, van Schayck JP, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schippers D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS‐CoV‐2 productively infects human gut enterocytes. Science 2020: eabc1669 Published online 2020 May 1. 10.1126/science.abc1669 [DOI] [PMC free article] [PubMed] [Google Scholar]


