β-HPVs contribute to cSCC development in immunocompromised populations. However, it is unclear if these common cutaneous viruses are tumorigenic in the general population. Thus, a more thorough investigation of β-HPV biology is warranted. If β-HPV infections do promote cSCCs, they are hypothesized to destabilize the cellular genome. In vitro data support this idea by demonstrating the ability of the β-HPV E6 protein to disrupt DNA repair signaling events following UV exposure. We show that β-HPV E6 more broadly impairs cellular signaling, indicating that the viral protein dysregulates the HP. The HP protects genome fidelity by regulating cell growth and apoptosis in response to a myriad of deleterious stimuli, including failed cytokinesis. After failed cytokinesis, β-HPV 8E6 attenuates phosphorylation of the HP kinase (LATS). This decreases some, but not all, HP signaling events. Notably, β-HPV 8E6 does not limit senescence associated with failed cytokinesis.
KEYWORDS: cancer, cytokinesis, Hippo signaling pathway, human papillomavirus, skin cancer, apoptosis, senescence
ABSTRACT
Beta genus human papillomaviruses (β-HPVs) cause cutaneous squamous cell carcinomas (cSCCs) in a subset of immunocompromised patients. However, β-HPVs are not necessary for tumor maintenance in the general population. Instead, they may destabilize the genome in the early stages of cancer development. Supporting this idea, β-HPV’s 8E6 protein attenuates p53 accumulation after failed cytokinesis. This paper offers mechanistic insight into how β-HPV E6 causes this change in cell signaling. An in silico screen and characterization of HCT 116 cells lacking p300 suggested that the histone acetyltransferase is a negative regulator of Hippo pathway (HP) gene expression. HP activation restricts growth in response to stimuli, including failed cytokinesis. Loss of p300 resulted in increased HP gene expression, including proproliferative genes associated with HP inactivation. β-HPV 8E6 expression recapitulates some of these phenotypes. We used a chemical inhibitor of cytokinesis (dihydrocytochalasin B [H2CB]) to induce failed cytokinesis. This system allowed us to show that β-HPV 8E6 reduced activation of large tumor suppressor kinase (LATS), an HP kinase. LATS is required for p53 accumulation following failed cytokinesis. These phenotypes were dependent on β-HPV 8E6 destabilizing p300 and did not completely attenuate the HP. It did not alter H2CB-induced nuclear exclusion of the transcription factor YAP. β-HPV 8E6 also did not decrease HP activation in cells grown to a high density. Although our group and others have previously described inhibition of DNA repair, to the best of our knowledge, this marks the first time that a β-HPV E6 protein has been shown to hinder HP signaling.
IMPORTANCE β-HPVs contribute to cSCC development in immunocompromised populations. However, it is unclear if these common cutaneous viruses are tumorigenic in the general population. Thus, a more thorough investigation of β-HPV biology is warranted. If β-HPV infections do promote cSCCs, they are hypothesized to destabilize the cellular genome. In vitro data support this idea by demonstrating the ability of the β-HPV E6 protein to disrupt DNA repair signaling events following UV exposure. We show that β-HPV E6 more broadly impairs cellular signaling, indicating that the viral protein dysregulates the HP. The HP protects genome fidelity by regulating cell growth and apoptosis in response to a myriad of deleterious stimuli, including failed cytokinesis. After failed cytokinesis, β-HPV 8E6 attenuates phosphorylation of the HP kinase (LATS). This decreases some, but not all, HP signaling events. Notably, β-HPV 8E6 does not limit senescence associated with failed cytokinesis.
INTRODUCTION
The human papillomavirus (HPV) family includes over 200 double-stranded DNA viruses that are divided into five genera, all of which infect human epithelia (1). Upon infecting mucosal or cutaneous tissue, members of each genus can cause a broad array of pathologies. Of these, the most prominent diseases are the anogenital and oropharyngeal carcinomas caused by alpha genus HPVs (2, 3). Cutaneous beta genus HPVs (β-HPVs) have also been linked to tumorigenesis via high viral DNA loads in cutaneous squamous cell carcinomas (cSCCs) of immunocompromised patients, primarily in sun-exposed skin (4–6).
While β-HPV infections are common in immunocompetent individuals, their contribution to cSCCs is less clear. The main etiological factor in skin cancer pathogenesis is UV. Further, the characterizations of cSCCs in the general population do not include continued β-HPV expression (7–9). Viral loads decrease as lesions progress from precancerous actinic keratosis (AK) to cSCC (10–12). These data have led to the hypothesized “hit-and-run” mechanism of oncogenesis, where β-HPVs cooperate with UV to enhance genomic instability in the early stages of carcinogenesis (10, 13, 14). This elevated mutational load then increases the chances of tumor progression independent of continued viral gene expression.
While it is hard to prove the role of a transient viral infection in persistent cancer, β-HPVs are also a common resident of our skin and are frequently found in AKs. Despite the billions of dollars spent on sun care products annually, 58 million Americans still have one or more AKs. Moreover, over $1 billion is spent during 5.2 million outpatient visits each year for AK treatment (15, 16). The cost of these AKs for the patient, both financial and emotional, increases if these lesions develop into malignancies. Within 1 year of diagnosis, an estimated 0.6% of AKs progress to cSCCs. This progression expands to 2.6% of AKs 5 years after diagnosis (17). Because β-HPV infections are quite common, even a mild increase in cancer risk would be notable. Thus, it is important to understand their potential contribution to the genome instability that drives cSCC progression.
A great deal is known about the tumorigenic potential of β-HPV proteins, particularly the E6 protein. The presence of the putative oncogene E6 from β-HPV 8 (β-HPV 8E6) is enough to cause cancers in mice without UV exposure (18, 19). β-HPV 8E6 inhibits differentiation and promotes proliferation by targeting the NOTCH and TGF-β signaling pathways (20). Another central theme of β-HPV E6 proteins is their ability to bind the cellular histone acetyltransferase p300 (21–24). β-HPV 8E6 and the E6 from β-HPV 5 bind p300 strongly, leading to its destabilization and decreasing DNA damage repair (DDR) gene expression (22, 25, 26). β-HPV type 38’s E6 protein has a lower p300-binding affinity and cannot destabilize the cellular protein (27). Nevertheless, binding p300 is essential for HPV38-induced immortalization of human foreskin keratinocytes (HFKs) (28). This suggests that p300 binding may be a shared factor in β-HPV-promoted oncogenesis. Because p300 is a master regulator of gene expression (29, 30), other signaling pathways are likely to be altered by β-HPV 8E6’s destabilization of the histone acetyltransferase.
Approximately 10% of skin cells do not divide after entering mitosis (25, 31). β-HPV 8E6 allows these cells to divide by preventing p53 stabilization in a p300-dependent manner (25). p53 accumulation requires the activation of large tumor suppressor kinase (LATS), a kinase in the Hippo signaling pathway (HP) (32). This suggests that β-HPV 8E6 may attenuate LATS activity. The HP also prevents growth by inhibiting the proproliferative activity of YAP/TAZ (32–34). Our analysis of transcriptomic data from cell lines segregated by their relative p300 expression was consistent with p300 acting as a negative regulator of HP and HP-responsive gene expression. We confirm that p300 modulates HP gene expression using HCT 116 cells with and without the p300 gene locus. Expressing β-HPV 8E6 in HFKs recapitulated some, but not all, of these effects. p300 is also important for responding to dihydrocytochalasin B (H2CB)-induced failed cytokinesis. HCT 116 cells without p300 had reduced LATS activation and p53 accumulation. β-HPV 8E6’s destabilization of p300 similarly hindered LATS phosphorylation and p53 accumulation. Despite p53’s role in apoptosis, elevated p53 levels did not correlate with increased apoptosis until the drug was washed off and the cells were allowed to recover. During this recovery period, β-HPV 8E6 displayed some ability to reduce markers of apoptosis. β-HPV 8E6 did not completely abrogate the HP’s response to failed cytokinesis, as YAP was still excluded from the nucleus. β-HPV 8E6 also did not impede the HP induction in cells grown to a high density.
RESULTS
Loss of p300 alters Hippo pathway gene expression.
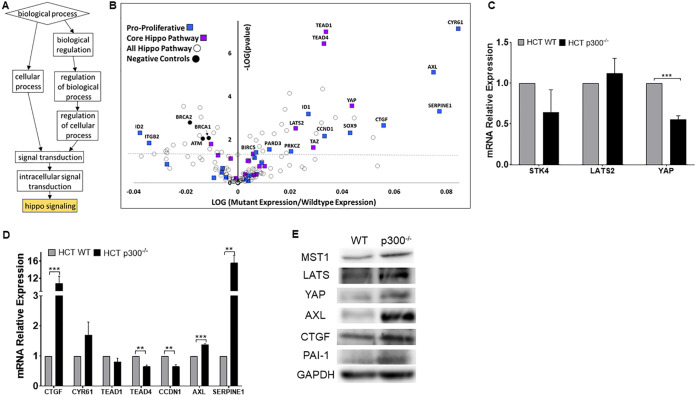
Animal models show that certain β-HPV E6 genes can contribute to UV-associated carcinogenesis (18, 19, 23). In vitro studies from our group and others have added molecular details by describing β-HPV E6’s ability to impair the DDR by destabilizing p300 (18, 22, 27, 28, 35, 36). Despite this focus on repair, there are DDR-independent pathways that protect genome fidelity (37–39). To identify p300-regulated pathways that could contribute to β-HPV E6-associated genome destabilization, we performed an in silico screen comparing RNA sequencing data among 1,020 cancer cell lines grouped by their relative p300 expression levels (Data Set S1 in the supplemental material) (40–42). The rationale for this approach is based on our prior observations that reducing p300 expression via RNA interference (RNAi) phenocopies β-HPV 8E6’s p300-dependent reduction of gene expression (22, 26). We compared expression in cell lines with and without low p300 expression (Z scores of less than −1.64 and greater than −1.64, respectively). Of the cell lines screened, 71 had low p300 expression. The remaining 949 cell lines were considered as not having low p300 expression. This identified 4,211 genes that had altered expression in cells with lower p300 expression. Next, gene ontology (GO) analysis was performed using Gene Ontology enRIchment anaLysis and visuaLizAtion tool (GOrilla) to identify pathways that were significantly altered when p300 expression was reduced (43, 44). Notably, HP was the only pathway identified by GOrilla as significantly changed (Fig. 1A). We then performed a more detailed analysis of HP, using the Kyoto Encyclopedia of Genes and Genomes (KEGG) to provide an unbiased definition of the pathway’s members. When p300 expression was reduced, many canonical HP genes were upregulated. However, the most striking changes occurred in proproliferative TEAD-responsive genes (e.g., CYR61, CTGF, AXL, and SERPINE1) (Fig. 1B). As expected, there was a significant reduction in the expression of genes (ATM, BRCA1, and BRCA2) that are dependent on p300 for robust transcription (26, 45, 46).
FIG 1.
Loss of p300 leads to changes in Hippo pathway gene expression. (A) Gene ontology of 1,020 cancer cell lines via GOrilla. Boxes show GO biological process terms. Boxes descend from general to specific functions. Gold color indicates P ≤ 0.001. (B) Volcano plot of 154 HP genes in 1,020 cancer cell lines with decreased EP300 expression. The colors blue, purple, and black represent proproliferative TEAD targets, core HP genes, and p300-negative controls, respectively. The horizontal line denotes P = 0.05. (C and D) Canonical HP genes (C) and TEAD-regulated mRNA expression (D) in HCT 116 WT and -p300−/− measured by RT-qPCR and normalized to β-actin mRNA. (E) Representative immunoblots of HP and TEAD-regulated proteins in HCT 116 WT and -p300−/−. Figures depict the mean ± the standard error of the mean; n ≥ 3. *, significant difference between indicated samples; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (Student's t test).
We used isogenic HCT 116 cells with (WT) or without the p300 gene (p300−/−) deleted to confirm our in silico analysis (47). The p300 status of these cells was verified by immunoblot (data not shown) before the expression of canonical HP genes (LATS2, STK4, and YAP1) was measured by quantitative real-time PCR (RT-qPCR). Note, STK4 is the gene that encodes the HP kinase, MST1. Of the three HP genes analyzed, the expression of YAP was significantly decreased by p300 loss (Fig. 1C). Next, we defined the abundance of TEAD and TEAD-responsive gene transcripts by RT-qPCR. Seven genes (CTGF, CYR61, TEAD1, TEAD4, CCND1, AXL, and SERPINE1) were chosen based on indications that they were negatively regulated by p300 in our computational screen. Some of these transcripts were more abundant in HCT 116 cells that lacked p300, with increased expression of CTGF, AXL, and SERPINE1 reaching statistical significance (Fig. 1D). Next, we turned to immunoblots to determine if p300 loss leads to changes at the protein level. These data show that increased canonical HP proteins are increased in the absence of p300 (Fig. 1E). The elevated levels extended to AXL, CTGF, and PAI-1 (the protein encoded by the SERPINE1 gene).
β-HPV E6 expression alters Hippo pathway gene expression.
β-HPV 8E6 destabilizes p300, but this does not result in complete loss of the histone acetyltransferase. We questioned if this decrease in p300 was enough to dysregulate the HP. To determine the extent that the reduction of p300 by β-HPV 8E6 increased HP gene expression, we defined the expression of canonical HP genes using RT-qPCR. β-HPV 8E6 did not increase expression of the canonical HP genes in human foreskin keratinocytes or HFKs (Fig. 2A). Immunoblots of these cells were consistent with these results except for LATS, which was more abundant when β-HPV 8E6 was expressed (Fig. 2B). We continued this analysis by defining the amount of TEAD and TEAD-responsive genes in HFKs expressing β-HPV 8E6. RT-qPCR comparing expression between vector control (LXSN) and β-HPV E6-expressing HFKs found β-HPV E6 increased expression of some TEAD-responsive genes (CTGF, CYR61, CCND1, AXL, and SERPINE1) (Fig. 2C). This was similar to our results in HCT 116 cells except for CCND1. Immunoblots were used to compare protein levels for TEAD-responsive genes, with elevated expression in HFKs expression in β-HPV 8E6. This demonstrated that β-HPV 8E6 increases CTGF, PAI-1, and AXL protein (Fig. 2D). A luciferase reporter assay showed a small but reproducible increase in luciferase expression driven from a TEAD-responsive promoter (data not shown). The increased expression of proproliferative TEAD-responsive genes correlated with increased proliferation (Fig. 2E). Consistent with a p300-dependent mechanism, β-HPV 8E6-driven changes in the HP were abrogated by the deletion of the p300-binding domain in a previously characterized mutant, β-HPV Δ8E6 (Fig. 2).
FIG 2.
β-HPV 8E6 alters Hippo pathway gene expression. Canonical HP genes (A) and TEAD-regulated mRNA expression (C) in HFKs LXSN, β-HPV 8E6, and β-HPV Δ8E6 measured by RT-qPCR and normalized to β-actin mRNA. Representative immunoblots of HP (B) and TEAD-regulated proteins (D) in HFKs LXSN, β-HPV 8E6, and β-HPV Δ8E6. (E) Relative growth recorded over a 5-day period. Figures depict mean ± standard error of the mean; n ≥ 3. *, significant difference between indicated samples; #, significant difference from LXSN; one symbol (* or #), P ≤ 0.05; two symbols (** or ##), P ≤ 0.01; three symbols (*** or ###), P ≤ 0.001 (Student's t test). In β-HPV Δ8E6, residues 132 to 136 were deleted.
p300 is necessary for a robust Hippo pathway response to failed cytokinesis.
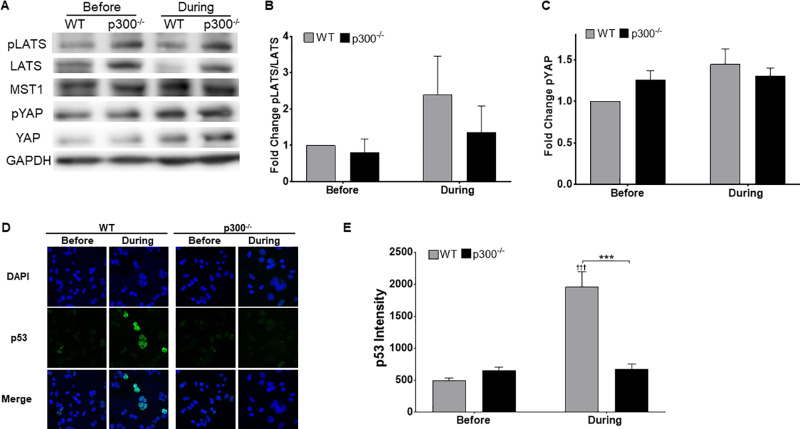
The HP typically restricts growth in response to adverse conditions. This includes failed cytokinesis, induced by dihydrocytochalasin B (H2CB), an inhibitor of actin polymerization (48). Because the loss of p300 promoted proliferation gene expression and dysregulated the HP, we hypothesized that p300 was required for the cellular response to H2CB exposure. Confirming previous data, LATS phosphorylation increased in HCT 116 cells with exposure to 4 μM H2CB (Fig. 3A and B). YAP phosphorylation was similarly elevated by H2CB treatment (Fig. 3A and C). Loss of p300 in HCT 116 cells reduced LATS in response to H2CB (Fig. 3A and B). When cells are treated with H2CB, LATS activation leads to p53 accumulation (32). To determine if p300 was necessary for this response, we used immunofluorescence microscopy to detect p53 in wild-type (WT) and p300 knockout (p300−/−) HCT 116 cells grown in H2CB-containing media. We were able to confirm previous reports of p53 buildup in response to the drug in WT HCT 116 cells (Fig. 3D and E). However, p53 did not accumulate in HCT 116 cells lacking p300.
FIG 3.
Loss of p300 impedes the Hippo pathway’s response to failed cytokinesis. (A) Representative immunoblot of HP proteins before and during H2CB treatment. (B and C) Densitometry of immunoblots described in panel A. GAPDH was used as a loading control. (D) Representative images of p53 (green)- and DAPI (blue)-stained HCT 116 cells before and during H2CB exposure. (E) Relative p53 intensity in HCT 116 cells. At least 150 cells/line were image across three independent experiments. Figures depict mean ± standard error of the mean; n ≥ 3. *, significant difference between indicated samples; †, significant difference relative to before H2CB; one symbol (* or †), P ≤ 0.05; two symbols (** or ††), P ≤ 0.01; three symbols (*** or †††), P ≤ 0.001 (Student's t test). In β-HPV Δ8E6, residues 132 to 136 were deleted.
β-HPV 8E6 attenuates LATS2 phosphorylation but does not impede nuclear exclusion of YAP.
These data suggest that β-HPV 8E6 alters H2CB induction of the HP. Before evaluating this possibility, we needed to confirm that β-HPV 8E6 did not impede H2CB-induced failed cytokinesis. The visualization of cells with more than one nucleus provides a straightforward measure of failed cytokinesis. We used bright-field and immunofluorescence microscopy to detect the presence of two or more nuclei in cells grown in media containing H2CB (Fig. 4A to D). The percentage of cells with supernumerary nuclei increased as a function of time in H2CB. This was true in both HFK and U2OS cells. The frequency of these abnormal cells was also not notably altered by β-HPV 8E6 or β-HPV Δ8E6. H2CB increased STK4 and YAP1 gene expression. Neither β-HPV 8E6 nor β-HPV Δ8E6 changed this (Fig. 4E). Consistent with our observations in HCT 116 cells, β-HPV 8E6 reduced LATS phosphorylation in cells exposed to H2CB (Fig. 4F). β-HPV Δ8E6 did not attenuate LATS phosphorylation. β-HPV 8E6’s restriction of HP signaling may be limited to reducing LATS phosphorylation. β-HPV 8E6 did not change YAP phosphorylation or the abundance of other HP proteins (data not shown). Moreover, immunofluorescence microscopy of YAP shows that β-HPV 8E6 did not hinder the nuclear exclusion of YAP associated with the protein’s phosphorylation (Fig. 4G) (49). As expected from these results, H2CB reduced TEAD-responsive promoter activity. β-HPV 8E6 did not prevent this decrease (data not shown).
FIG 4.
β-HPV 8E6 diminishes LATS phosphorylation during failed cytokinesis. Representative images of U2OS (A) and HFK (B) cells before and during H2CB exposure. Green and blue represent α-tubulin and DAPI, respectively. Quantification of U2OS (C) and HFK (D) cells with 2 or more nuclei as a function of time in H2CB. (E) STK4, LATS, and YAP1 expression before and after H2CB exposure measured by RT-qPCR; n = 2. (F) Representative immunoblot of pLATS and totals LATS protein levels in HFK cells before and during H2CB exposure. (G) Representative images of YAP (green)- and DAPI (blue)-stained HFK cells before and during H2CB treatment. At least 200 cells/line were imaged from three independent experiments. Figures depict mean ± standard error of the mean; n ≥ 3. †, significant difference relative to before H2CB; †, P ≤ 0.05; ††, P ≤ 0.01; †††, P ≤ 0.001 (Student's t test). In β-HPV Δ8E6, residues 132 to 136 were deleted.
β-HPV 8E6 attenuates p53 accumulation after failed cytokinesis.
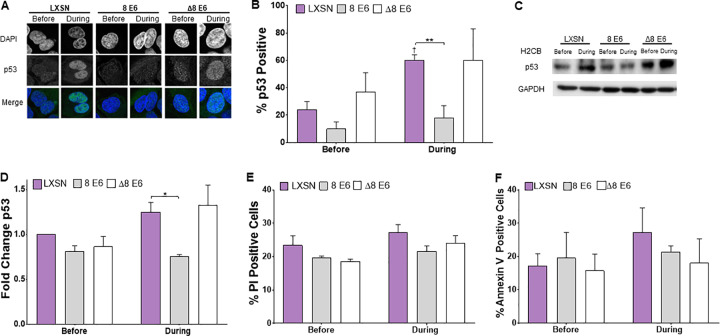
Seeing β-HPV 8E6 reduce LATS phosphorylation led us to hypothesize that β-HPV 8E6 would also reduce p53 accumulation in response to H2CB. To test this, we used immunofluorescence microscopy to detect p53 in U2OS grown in media containing H2CB. Consistent with our previous observations, H2CB increased the frequency of cells with more than one nucleus. H2CB increased p53 levels in vector control cells but not in cells expressing β-HPV E6 (Fig. 5A and B). This abrogation of p53 accumulation is likely dependent on p300 degradation, as β-HPV Δ8E6 expressing U2OS and vector control had a similar frequency of p53-stained cells. To validate these results, we used immunoblotting to detect p53 levels in cells grown in H2CB. These experiments also demonstrated that β-HPV 8E6 can repress p53 buildup in response to H2CB. Again, vector control U2OS and β-HPV Δ8E6-expressing U2OS behaved similarly in this assay (Fig. 5C and D). We speculated that the additional p53 found in cells grown with H2CB would result in apoptosis. Fluorescence-based detection of two apoptosis markers (propidium iodide and annexin V) were used to test this idea (50, 51). Surprisingly, we did not see exposure to H2CB associated with an increase in either of these apoptosis markers (Fig. 5E and F). There were also no differences in staining among vector control HFKs and HFKs expressing β-HPV 8E6 or β-HPV Δ8E6.
FIG 5.
β-HPV 8E6 attenuates p53 accumulation upon H2CB-induced failed cytokinesis. (A) Representative images of p53 and DAPI staining in cells before and during H2CB treatment. (B) Percent of p53-positive U2OS cells. (C) Representative immunoblot of p53 before and during H2CB exposure. (D) Densitometry of immunoblots described in panel C. GAPDH was used as a loading control. Data were normalized to p53 levels in untreated LXSN cells (set to 1). (E) Percent of propidium iodide-stained HFK cells before and during H2CB exposure. (F) Percent of annexin V-stained HFK cells before and during H2CB treatment. At least 200 cells/line were imaged from three independent experiments. Figures depict mean ± standard error of the mean; n ≥ 3. *, significant difference between indicated samples; †, significant difference relative to before H2CB; one symbol (* or †), P ≤ 0.05; two symbols (** or ††), P ≤ 0.01; three symbols (*** or †††), P ≤ 0.001 (Student's t test). In β-HPV Δ8E6, residues 132 to 136 were deleted.
H2CB stalls cytokinesis. It was important to understand if/how those cells recover once the drug is removed and cytokinesis is again possible. To this end, we compared HFKs grown in three conditions: without H2CB (before), grown with 4 days of continual H2CB (during), and grown in H2CB for 4 days followed by 3 additional days without H2CB (after). Figure 6A depicts our experimental setup. We used microscopy to determine the frequency of HFKs with supernumerary nuclei (two or more) in each of these conditions. β-HPV 8E6 did not make supernumerary nuclei less prevalent before or during H2CB exposure (Fig. 6B). However, β-HPV 8E6 decrease supernumerary nuclei after H2CB. This appears to be dependent on p300 destabilization, as supernumerary nuclei were similarly prevalent in HFKs with β-HPV Δ8E6 or vector control. We next used immunoblots to determine if β-HPV 8E6 maintained its ability to attenuate LATS phosphorylation after H2CB. While LATS phosphorylation was elevated in vector control HFKs after H2CB, they remained low in HFKs expressing β-HPV 8E6. This phenotype was not seen in HFKs expressing β-HPV Δ8E6. We used immunofluorescence microscopy as an additional way of detecting p53. These experiments complement the results from immunoblotting, as p53-positive cells were more frequent after H2CB in the vector control but not β-HPV 8E6-expressing HFKs (Fig. 6D and E). We repeated the detection of propidium iodide (PI) and annexin described in Fig. 5 after H2CB. β-HPV 8E6 reduced the percentage of PI-positive HFKs after H2CB compared to vector control and β-HPV Δ8E6-expressing HFKs (Fig. 6F). Annexin V staining was also reduced, but this change did not reach statistical significance (Fig. 6G).
FIG 6.
β-HPV 8E6 hinders LATS phosphorylation and p53 accumulation after failed cytokinesis. (A) Timeline for administration and removal of H2CB. (B) Percent of HFK cells with ≥ 2 nuclei per cell before, during, and after H2CB treatment. (C) Representative immunoblots of pLATS and totals LATS in HFKs before and after H2CB exposure. (D) Representative images of p53 and DAPI staining in cells before and after H2CB exposure. (E) Percent of p53-stained HFK cells before and after H2CB exposure. At least 200 cells/line were imaged across three independent experiments. (F) Percent of propidium iodide-stained HFK cells before and after H2CB exposure. (G) Percent of annexin V-stained HFK cells before and after H2CB treatment. Figures depict mean ± standard error of the mean; n ≥ 3. *, significant difference between indicated samples; †, significant difference relative to before H2CB; one symbol (* or †), P ≤ 0.05; two symbols (** or ††), P ≤ 0.01; three symbols (*** or †††), P ≤ 0.001 (Student's t test). In β-HPV Δ8E6, residues 132 to 136 were deleted.
β-HPV 8E6 does not completely abrogate the Hippo pathway.
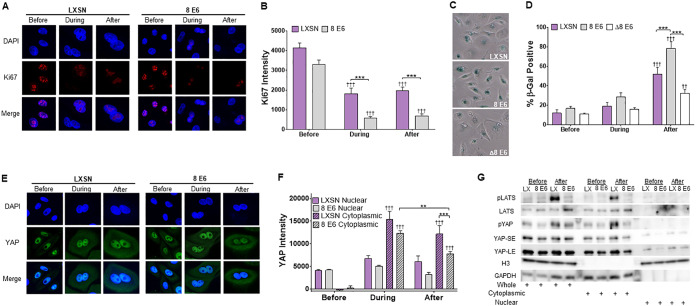
Having seen diminished LATS activation, we queried whether β-HPV 8E6 prevented the HP from restricting growth after H2CB was removed. We used immunofluorescence microscopy to detect Ki67, an established marker of proliferation. While we readily detected Ki67 in both vector control and β-HPV 8E6-expressing HFKs before H2CB, Ki67 was notably less abundant during and after H2CB (Fig. 7A). Ki67 staining intensity was also lower in HFKs expressing β-HPV 8E6 (Fig. 7B). Consistent with these results, HFKs were not capable of long-term proliferation with or without β-HPV 8E6 (data not shown). To understand what was happening to HFKs after H2CB, we stained for senescence-associated beta-galactosidase (SA β-Gal) activity as an indicator of cellular senescence. These data were consistent with our Ki67 staining experiments (Fig. 7C and D). HFKs were more likely to have SA β-Gal activity after H2CB, and β-HPV 8E6 expression amplified this phenotype.
FIG 7.
β-HPV 8E6 increases SA β-Gal staining and reduces YAP abundance after failed cytokinesis. (A) Representative images of Ki67 (red) and DAPI (blue) staining in HFK cells before, during, and after H2CB treatment. (B) Relative KI67 intensity in HFK cells before, during, and after H2CB treatment. At least 150 cells/line were imaged across three independent experiments. (C) Representative images of HFK cells stained for SA β-Gal activity (blue). (D) Percent of SA β-Gal-positive HFK cells before, during, and after H2CB exposure. (E) Representative images of HFK cells stained for YAP (green) and DAPI (blue). (F) Cytoplasmic and nuclear YAP intensity in HFK cells before, during, and after H2CB treatment. At least 205 images were imaged across three independent experiments. (G) Subcellular fractionation of HFKs harvested before and after H2CB treatment. Hippo pathway proteins were probed via immunoblotting. GAPDH and histone H3 serve as cytoplasmic and nuclear loading controls, respectively. YAP-SE and YAP-LE indicate short- and long-term exposure of YAP, respectively. Figures depict mean ± standard error of the mean; n ≥ 3. *, significant difference between indicated samples; †, significant difference relative to before H2CB; one symbol (* or †), P ≤ 0.05, two symbols (** or ††), P ≤ 0.01; three symbols (*** or †††), P ≤ 0.001 (Student's t test). In β-HPV Δ8E6, residues 132 to 136 were deleted.
The HP restricts growth by relocating YAP from the nucleus to the cytoplasm. We used immunofluorescence microscopy to define the subcellular localization of YAP in HFKs before, during, and after H2CB (Fig. 7E and F). Cytoplasmic YAP increased in HFKs during and after H2CB as expected. β-HPV 8E6 attenuated this only after H2CB exposure (Fig. 7F). Additionally, we saw a nominal decrease in nuclear-located YAP in HFKs expressing β-HPV 8E6 alone after H2CB treatment. To more precisely define YAP localization, we performed subcellular fractionation on HFKs before and after H2CB (Fig. 7G). Immunoblotting demonstrated that phosphorylated YAP was more abundant in the cytoplasm, particularly after H2CB. This was also true for phosphorylated LATS. After H2CB, β-HPV 8E6 decreased the amount of total YAP in the nuclear fraction, despite reducing total and cytoplasmic phospho-LATS1/2 (pLATS).
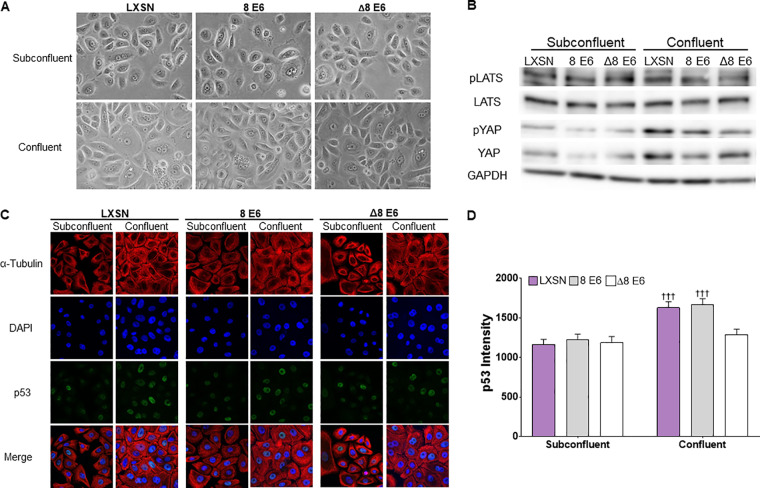
We wanted to determine the extent that β-HPV 8E6 could attenuate LATS phosphorylation in response to other stimuli. Because cell density is a commonly used activator of the HP, we compared LATS and YAP phosphorylation in confluent and subconfluent HFKs (Fig. 8A and B). Immunoblots of these cells demonstrate increased YAP phosphorylation in confluent HFKs compared to subconfluent HFKs. The phosphorylation of LATS did not increase under these conditions. Next, we used immunofluorescence microscopy to determine if HFKs increased p53 levels when grown to high confluence (Fig. 8C and D). As expected, p53 staining was more intense in confluent compared to subconfluent HFKs. In general, neither β-HPV 8E6 nor β-HPV Δ8E6 changed these responses. However, β-HPV Δ8E6 decreased p53 staining. We have no explanation of this observation, but it does demonstrate that β-HPV Δ8E6 is not universally inactive.
FIG 8.
β-HPV 8E6 does not inhibit the Hippo pathway’s response to cellular density. (A) Representative images of subconfluent and confluent HFKs. (B) Representative immunoblot of HP proteins in confluent and subconfluent HFKs. (C) Representative images of α-tubulin (red)-, p53 (green)-, and DAPI (blue)-stained subconfluent and confluent HFK cells. (D) Mean p53 intensity in HFK cells before and after confluence. At least 200 cells/line were imaged across 3 independent experiments; n = 2 for β-HPV Δ8E6. Figures depict mean ± standard error of the mean; n ≥ 3. †, significant difference relative to before H2CB; †, P ≤ 0.05; ††, P ≤ 0.01; †††, P ≤ 0.001 (Student's t test). In β-HPV Δ8E6, residues 132 to 136 were deleted.
DISCUSSION
Tumorigenesis is among the grave consequences associated with changes in ploidy. HP activation is one of the cellular mechanisms that prevents polyploidy by halting the proliferation of cells that do not divide after replication (32, 52). Despite the high stakes, cytokinesis fails in approximately 10% of skin cells that enter mitosis (25, 31). As a result, the HP may play an important role in preventing cSCCs. The growth arrest associated with HP activation is likely refractory to β-HPV replication, as HPV replicates in actively proliferating cells (53). Our work suggests that β-HPV 8E6 helps binucleated cells survive by mitigating HP activation. Presumably limiting HP signaling is beneficial to papillomaviruses in general, as genus α HPV oncogenes also dysregulate the Hippo pathway (54, 55).
The “evolutionary motivation” behind this could stem from the modest growth advantage that we report. However, this weak phenotype seems unlikely to drive convergent evolution toward HP dysregulation. Given the HP’s role in immunity, it is more enticing to speculate that targeting the HP helps HPVs avoid an immune response (56, 57). Indeed, an MST1 deficiency increased β-HPV infections (58). Since the HP was only discovered 14 years ago (59), there could also be other currently unknown advantages to be gained by disrupting the pathway.
Less speculatively, we extend the understanding of β-HPV 8E6 biology. β-HPV E6 disrupts multiple cell signaling pathways necessary for DNA repair and regulating differentiation. Much of β-HPV E6’s ability to disrupt DNA repair is linked to p300 destabilization. We demonstrate that changes in signaling associated with reduced p300 extend to the HP, as LATS phosphorylation is attenuated following failed cytokinesis. β-HPV 8E6 also displays some antiapoptotic properties in response to H2CB-induced failed cytokinesis. Together, these data demonstrate that β-HPV 8E6 is a versatile protein capable of a striking reprogramming of cellular signaling. We also extend the long history of using viral oncogenes to learn about cell biology by linking p300 to the HP- and TEAD-responsive gene expression.
The fact that most people get infected with β-HPV but a significantly lower number of those infections become cSCCs causes many to doubt that the virus is tumorigenic. β-HPV 8E6 may be more mutagenic in certain genetic backgrounds. For instance, β-HPV 8E6 reduces LATS phosphorylation after failed cytokinesis, but the cells still senesce. This suggests that mutations that help cells avoid senescence would augment β-HPVs tumorigenic potential. One could imagine any number of additional mutations that might synergize with β-HPV 8E6 to promote mutagenesis. Genetic landscapes where the opposite is true seem equally likely. Moreover, β-HPV E6 could also be more or less harmful when coexpressed with other β-HPV genes. Future studies are needed to evaluate these complexities.
MATERIALS AND METHODS
Cell cultures.
U2OS and HCT 116 cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin. Primary HFKs were derived from neonatal human foreskins. HFKs were grown in EpiLife medium supplemented with calcium chloride (60 μM), human keratinocyte growth supplement (Thermo Fisher Scientific), and penicillin-streptomycin. HPV genes were cloned, transfected, and confirmed as previously described (25). We carefully monitored cell density in all experiments. To avoid confounding our experiments by activating the Hippo pathway via contact inhibition, experiments were aborted if unintended differences in seeding resulted in cell densities that were more than 10% different among cell lines at the beginning of an experiment.
Proliferation assays and H2CB cell viability assays.
Cells were counted, and 4.0 × 104 cells were plated into 6 wells per cell line of 6-well tissue culture dishes. One well was trypsinized, resuspended, and counted 3 times via hemocytometer with trypan blue. For dihydrocytochalasin B (H2CB) cell viability assays, cells were grown for 24 h and then treated with 2/4 μM H2CB, and fresh H2CB was readministered every 2 days while cells were trypsinized and counted 3 times via hemocytometer with trypan blue.
RT-qPCR.
Cell were lysed using TRIzol (Invitrogen) and RNA isolated with the RNeasy kit (Qiagen).
Two micrograms of RNA were reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR (RT-qPCR) was performed in triplicate with the TaqMan FAM-MGB gene expression assay (Applied Biosystems) and C1000 touch thermal cycler (Bio-Rad). The following probes (Thermo Scientific) were used: ACTB (Hs01060665_g1), STK4 (Hs00178979_m1), LATS2 (referred to as LATS in the text) (Hs01059009_m1), YAP1 (Hs00902712_g1), CTGF (Hs00170014_m1), CYR61 (Hs00155479_m1), TEAD4 (Hs01125032_m1), TEAD1 (Hs00173359_m1), CCND1 (Hs00765553_m1), AXL (Hs01064444_m1), and SERPINE1 (Hs00167155_m1).
Immunoblotting.
After being washed with ice-cold phosphate-buffered saline (PBS), cells were lysed with radioimmunoprecipitation assay (RIPA) lysis buffer (VWR Life Science) supplemented with Phosphatase inhibitor cocktail 2 (Sigma) and protease inhibitor cocktail (Bimake). The Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Scientific) was used to determine protein concentration. Equal protein lysates were run on Novex 4-12% Tris-Glycine WedgeWell mini gels (Invitrogen) and transferred to Immobilon-P membranes (Millipore). Membranes were then probed with the following primary antibodies: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnologies; catalog no. sc-47724), LATS2 (Cell Signaling Technologies; clone D83D6), phospho-LATS1/2 (Ser909) (referred to as pLATS in the text) (Cell Signaling Technologies; product no. 9157), YAP (Cell Signaling Technologies; product no. 4912S), phospho-YAP (Ser127) (Referred to pYAP in the text) (Cell Signaling Technologies; product no. 4911S), MST1 (Cell Signaling Technologies; product no. 3682S), AXL (Cell Signaling Technologies; product no. 8661S), CTGF (Abcam; catalog no. ab6692), PAI-1 (Cell Signaling Technologies; product no. 11907S), p53 (Calbiochem; catalog no. OP43; 100 μg), p300 (Santa Cruz Biotechnologies; catalog no. sc-584), and histone H3 (Abcam; catalog no. ab1791). After exposure to the matching horseradish peroxidase (HRP)-conjugated secondary antibody, cells were visualized using SuperSignal West Femto maximum sensitivity substrate (Thermo Scientific).
cBioPortal and gene ontology analysis.
Software from www.cbioportal.org was used to recognize, analyze, and categorize mutations and transcriptomic data from over 1,000 cancer cell lines (40–42) and cutaneous squamous cell carcinomas (60, 61). Gene Ontology enRIchment anaLysis and visuaLizAtion tool (GOrilla) identified and visualized enriched GO terms from these data (43, 44). The Kyoto Encyclopedia of Genes and Genomes (KEGG) was used to identify genes specific to the Hippo signaling pathway (hsa04390).
Senescence-associated β-galactosidase staining.
Cells were seeded onto three 6-well plates and were grown for 24 h. Then, they were treated with 4 μM H2CB for stated times, after which cells were fixed and stained for senescence-associated β-galactosidase (β-Gal) expression according to the manufacturer’s protocol (Cell Signaling Technologies).
Immunofluorescence microscopy.
Cells were seeded onto either 96-well glass-bottom plates (Cellvis) or coverslips and grown overnight. Cells treated with H2CB for a specified time and concentration were fixed with 4% formaldehyde. Then, 0.1% Triton-X solution in PBS was used to permeabilize the cells, followed by blocking with 3% bovine serum albumin in PBS for 30 min. Cells were then incubated with the following: p53 (Cell Signaling Technologies; clone 1C12), YAP (Cell Signaling Technologies; product no. 4912S), Ki67 (Abcam; catalog no. ab15580), alpha-tubulin (Abcam; catalog no. ab18251), and α-tubulin (Cell Signaling Technologies; product no. 3873S). The cells were washed and stained with the appropriate secondary antibodies: Alexa Fluor 594 goat anti-rabbit (Thermo Scientific; catalog no. A11012) and Alexa Fluor 488 goat anti-mouse (Thermo Scientific A11001). After washing, the cells were stained with 28 μM 4′,6-diamidino-2-phenylindole (DAPI) in PBS and visualized with the Zeiss LSM 770 microscope. Images were analyzed using ImageJ techniques previously described in reference 62.
Apoptosis assay.
After H2CB treatment, HFKs were harvested via trypsinization and then counted while incubating at 37°C for 30 min. After incubation, cells were resuspended to 1 × 106 cells/ml. Next, cells were stained with 100 μg/ml of propidium iodide (PI) and 1× annexin-binding buffer following the protocol from Dead Cell apoptosis kit (Invitrogen; catalog no. V13242). Stained cells were imaged with the Countess II FL automated cell counter (Invitrogen). Images were processed using ImageJ software.
Subcellular fractionation.
Cells were seeded at 5.0 × 105 cells/10 cm2 plate and grown for 24 h. Cells were then treated with 4 μM H2CB for 3 days, washed with PBS, and recovered in fresh EpiLife for 3 days (after H2CB treatment). Before and after H2CB exposure, cells were washed with ice-cold PBS and divided into cytosolic and nuclear fractions via Abcam’s subcellular fractionation protocol. Afterward, lysates were treated the same as in the Immunoblotting section.
Statistical analysis.
Unless otherwise noted, statistical significance was determined by an unpaired Student's t test and was confirmed when appropriate by two-way analysis of variance (ANOVA) with Turkey’s correction. Only P values of less than 0.05 were reported as significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank and acknowledge the Kansas State University College of Veterinary Medicine (KSU-CVM) Confocal Core, especially Joel Sanneman, for assisting with our immunofluorescence imaging, Michael Underbrink for providing the telomerase reverse transcriptase (TERT)-immortalized HFKs, Stefano Piccolo for gifting the 8×GTIIC plasmid, along with Emily Burghardt and Jazmine Snow for their constructive criticism of the manuscript. Also, we thank members of the Zhilong Yang lab for their support.
This work was supported by Department of Defense grant CMDRP PRCRP CA160224 (to N.A.W.) and was made possible by generous support from the Les Clow family and the Johnson Cancer Research Center at Kansas State University.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Van Doorslaer K, Li Z, Xirasagar S, Maes P, Kaminsky D, Liou D, Sun Q, Kaur R, Huyen Y, McBride AA. 2017. The Papillomavirus Episteme: a major update to the papillomavirus sequence database. Nucleic Acids Res 45:D499–D506. doi: 10.1093/nar/gkw879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zur Hausen H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 3.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, WHO International Agency for Research on Cancer. 2005. Carcinogenicity of human papillomaviruses. Lancet Oncol 6:204. doi: 10.1016/S1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 4.Orth G, Jablonska S, Favre M, Croissant O, Jarzabek-Chorzelska M, Rzesa G. 1978. Characterization of two types of human papillomaviruses in lesions of Epidermodysplasia verruciformis. Proc Natl Acad Sci U S A 75:1537–1541. doi: 10.1073/pnas.75.3.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purdie KJ, Surentheran T, Sterling JC, Bell L, McGregor JM, Proby CM, Harwood CA, Breuer J. 2005. Human papillomavirus gene expression in cutaneous squamous cell carcinomas from immunosuppressed and immunocompetent individuals. J Invest Dermatol 125:98–107. doi: 10.1111/j.0022-202X.2005.23635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harwood CA, McGregor JM, Proby CM, Breuer J. 1999. Human papillomavirus and the development of non-melanoma skin cancer. J Clin Pathol 52:249–253. doi: 10.1136/jcp.52.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chahoud J, Semaan A, Chen Y, Cao M, Rieber AG, Rady P, Tyring SK. 2016. Association between β-genus human papillomavirus and cutaneous squamous cell carcinoma in immunocompetent individuals—a meta-analysis. JAMA Dermatol 152:1354–1364. doi: 10.1001/jamadermatol.2015.4530. [DOI] [PubMed] [Google Scholar]
- 8.Masini C, Fuchs PG, Gabrielli F, Stark S, Sera F, Ploner M, Melchi CF, Primavera G, Pirchio G, Picconi O, Petasecca P, Cattaruzza MS, Pfister HJ, Abeni D. 2003. Evidence for the association of human papillomavirus infection and cutaneous squamous cell carcinoma in immunocompetent individuals. Arch Dermatol 139:890–894. doi: 10.1001/archderm.139.7.890. [DOI] [PubMed] [Google Scholar]
- 9.Chitsazzadeh V, Coarfa C, Drummond JA, Nguyen T, Joseph A, Chilukuri S, Charpiot E, Adelmann CH, Ching G, Nguyen TN, Nicholas C, Thomas VD, Migden M, MacFarlane D, Thompson E, Shen J, Takata Y, McNiece K, Polansky MA, Abbas HA, Rajapakshe K, Gower A, Spira A, Covington KR, Xiao W, Gunaratne P, Pickering C, Frederick M, Myers JN, Shen L, Yao H, Su X, Rapini RP, Wheeler DA, Hawk ET, Flores ER, Tsai KY. 2016. Cross-species identification of genomic drivers of squamous cell carcinoma development across preneoplastic intermediates. Nat Commun 7:12601. doi: 10.1038/ncomms12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissenborn SJ, Nindl I, Purdie K, Harwood C, Proby C, Breuer J, Majewski S, Pfister H, Wieland U. 2005. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Invest Dermatol 125:93–97. doi: 10.1111/j.0022-202X.2005.23733.x. [DOI] [PubMed] [Google Scholar]
- 11.Howley PM, Pfister HJ. 2015. Beta genus papillomaviruses and skin cancer. Virology 479–480:290–296. doi: 10.1016/j.virol.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissenborn SJ, De Koning MNC, Wieland U, Quint WGV, Pfister HJ. 2009. Intrafamilial transmission and family-specific spectra of cutaneous betapapillomaviruses. J Virol 83:811–816. doi: 10.1128/JVI.01338-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akgül B, Cooke JC, Storey A. 2006. HPV-associated skin disease. J Pathol 208:165–175. doi: 10.1002/path.1893. [DOI] [PubMed] [Google Scholar]
- 14.Aldabagh B, Angeles JGC, Cardones AR, Arron ST. 2013. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol Surg 39:1–23. doi: 10.1111/j.1524-4725.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen T, Lebwohl MG. 2013. Prevalence and awareness of actinic keratosis: barriers and opportunities. J Am Acad Dermatol 68:S2–9. doi: 10.1016/j.jaad.2012.09.052. [DOI] [PubMed] [Google Scholar]
- 16.Warino L, Tusa M, Camacho F, Teuschler H, Fleischer AB, Feldman SR. 2006. Frequency and cost of actinic keratosis treatment. Dermatol Surg 32:1045–1049. doi: 10.1111/j.1524-4725.2006.32228.x. [DOI] [PubMed] [Google Scholar]
- 17.Criscione VD, Weinstock MA, Naylor MF, Luque C, Eide MJ, Bingham SF, Department of Veteran Affairs Topical Tretinoin Chemoprevention Trial Group. 2009. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 115:2523–2530. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- 18.Marcuzzi GP, Hufbauer M, Kasper HU, Weißenborn SJ, Smola S, Pfister H. 2009. Spontaneous tumour development in human papillomavirus type 8 E6 transgenic mice and rapid induction by UV-light exposure and wounding. J Gen Virol 90:2855–2864. doi: 10.1099/vir.0.012872-0. [DOI] [PubMed] [Google Scholar]
- 19.Schaper ID, Marcuzzi GP, Weissenborn SJ, Kasper HU, Dries V, Smyth N, Fuchs P, Pfister H. 2005. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res 65:1394–1400. doi: 10.1158/0008-5472.CAN-04-3263. [DOI] [PubMed] [Google Scholar]
- 20.Meyers JM, Uberoi A, Grace M, Lambert PF, Munger K. 2017. Cutaneous HPV8 and MmuPV1 E6 proteins target the NOTCH and TGF-β tumor suppressors to inhibit differentiation and sustain keratinocyte proliferation. PLoS Pathog 13:e1006171. doi: 10.1371/journal.ppat.1006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Underbrink MP, Howie HL, Bedard KM, Koop JI, Galloway DA. 2008. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J Virol 82:10408–10417. doi: 10.1128/JVI.00902-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace NA, Robinson K, Howie HL, Galloway DA. 2012. HPV 5 and 8 E6 abrogate ATR activity resulting in increased persistence of UVB induced DNA damage. PLoS Pathog 8:e1002807. doi: 10.1371/journal.ppat.1002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viarisio D, Mueller-Decker K, Kloz U, Aengeneyndt B, Kopp-Schneider A, Gröne H-J, Gheit T, Flechtenmacher C, Gissmann L, Tommasino M. 2011. E6 and E7 from beta HPV38 cooperate with ultraviolet light in the development of actinic keratosis-like lesions and squamous cell carcinoma in mice. PLoS Pathog 7:e1002125. doi: 10.1371/journal.ppat.1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshmukh J, Pofahl R, Pfister H, Haase I. 2016. Deletion of epidermal Rac1 inhibits HPV-8 induced skin papilloma formation and facilitates HPV-8- and UV-light induced skin carcinogenesis. Oncotarget 7:57841–57850. doi: 10.18632/oncotarget.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace NA, Robinson K, Galloway DA. 2014. Beta human papillomavirus E6 expression inhibits stabilization of p53 and increases tolerance of genomic instability. J Virol 88:6112–6127. doi: 10.1128/JVI.03808-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace NA, Gasior SL, Faber ZJ, Howie HL, Deininger PL, Galloway DA. 2013. HPV 5 and 8 E6 expression reduces ATM protein levels and attenuates LINE-1 retrotransposition. Virology 443:69–79. doi: 10.1016/j.virol.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howie HL, Koop JI, Weese J, Robinson K, Wipf G, Kim L, Galloway DA. 2011. Beta-HPV 5 and 8 E6 promote p300 degradation by blocking AKT/p300 association. PLoS Pathog 7:e1002211. doi: 10.1371/journal.ppat.1002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muench P, Probst S, Schuetz J, Leiprecht N, Busch M, Wesselborg S, Stubenrauch F, Iftner T. 2010. Cutaneous papillomavirus E6 proteins must interact with p300 and block p53-mediated apoptosis for cellular immortalization and tumorigenesis. Cancer Res 70:6913–6924. doi: 10.1158/0008-5472.CAN-10-1307. [DOI] [PubMed] [Google Scholar]
- 29.Dancy BM, Cole PA. 2015. Protein lysine acetylation by p300/CBP. Chem Rev 115:2419–2452. doi: 10.1021/cr500452k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan HM, Thangue N. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci 114:2363–2373. [DOI] [PubMed] [Google Scholar]
- 31.Soto M, García-Santisteban I, Krenning L, Medema RH, Raaijmakers JA. 2018. Chromosomes trapped in micronuclei are liable to segregation errors. J Cell Sci 131:jcs214742. doi: 10.1242/jcs.214742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganem NJ, Cornils H, Chiu S-Y, O’Rourke KP, Arnaud J, Yimlamai D, Théry M, Camargo FD, Pellman D. 2014. Cytokinesis failure triggers Hippo tumor suppressor pathway activation. Cell 158:833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stukenberg PT. 2004. Triggering p53 after cytokinesis failure. J Cell Biol 165:607–608. doi: 10.1083/jcb.200405089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinmura K, Bennett RA, Tarapore P, Fukasawa K. 2007. Direct evidence for the role of centrosomally localized p53 in the regulation of centrosome duplication. Oncogene 26:2939–2944. doi: 10.1038/sj.onc.1210085. [DOI] [PubMed] [Google Scholar]
- 35.Wallace NA, Robinson K, Howie HL, Galloway DA. 2015. β-HPV 5 and 8 E6 disrupt homology dependent double strand break repair by attenuating BRCA1 and BRCA2 expression and foci formation. PLoS Pathog 11:e1004687. doi: 10.1371/journal.ppat.1004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snow JA, Murthy V, Dacus D, Hu C, Wallace NA. 2019. β-HPV 8E6 attenuates ATM and ATR signaling in response to UV damage. Pathogens 8:267. doi: 10.3390/pathogens8040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganem NJ, Storchova Z, Pellman D. 2007. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev 17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Sen S. 2000. Aneuploidy and cancer. Curr Opin Oncol 12:82–88. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Meyers JM, Spangle JM, Munger K. 2013. The human papillomavirus type 8 E6 protein interferes with NOTCH activation during keratinocyte differentiation. J Virol 87:4762–4767. doi: 10.1128/JVI.02527-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV, Sonkin D, Reddy A, Liu M, Murray L, Berger MF, Monahan JE, Morais P, Meltzer J, Korejwa A, Jané-Valbuena J, Mapa FA, Thibault J, Bric-Furlong E, Raman P, Shipway A, Engels IH, Cheng J, Yu GK, Yu J, Aspesi P, de Silva M, Jagtap K, Jones MD, Wang L, Hatton C, Palescandolo E, Gupta S, Mahan S, Sougnez C, Onofrio RC, Liefeld T, MacConaill L, Winckler W, Reich M, Li N, Mesirov JP, Gabriel SB, Getz G, Ardlie K, Chan V, Myer VE, et al. . 2012. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. 2013. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. 2012. The cBio Cancer Genomics Portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. 2009. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eden E, Lipson D, Yogev S, Yakhini Z. 2007. Discovering motifs in ranked lists of DNA sequences. PLoS Comput Biol 3:e39. doi: 10.1371/journal.pcbi.0030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stauffer D, Chang B, Huang J, Dunn A, Thayer M. 2007. p300/CREB-binding protein interacts with ATR and is required for the DNA replication checkpoint. J Biol Chem 282:9678–9687. doi: 10.1074/jbc.M609261200. [DOI] [PubMed] [Google Scholar]
- 46.Ogiwara H, Kohno T. 2012. CBP and p300 histone acetyltransferases contribute to homologous recombination by transcriptionally activating the BRCA1 and RAD51 genes. PLoS One 7:e52810. doi: 10.1371/journal.pone.0052810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iyer NG, Chin S-F, Ozdag H, Daigo Y, Hu D-E, Cariati M, Brindle K, Aparicio S, Caldas C. 2004. p300 regulates p53-dependent apoptosis after DNA damage in colorectal cancer cells by modulation of PUMA/p21 levels. Proc Natl Acad Sci U S A 101:7386–7391. doi: 10.1073/pnas.0401002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maness PF, Walsh RC. Jr., 1982. Dihydrocytochalasin B disorganizes actin cytoarchitecture and inhibits initiation of DNA synthesis in 3T3 cells. Cell 30:253–262. doi: 10.1016/0092-8674(82)90031-9. [DOI] [PubMed] [Google Scholar]
- 49.Zhao B, Li L, Tumaneng K, Wang C-Y, Guan K-L. 2010. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCFβ-TRCP. Genes Dev 24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cornelissen M, Philippé J, De Sitter S, De Ridder L. 2002. Annexin V expression in apoptotic peripheral blood lymphocytes: an electron microscopic evaluation. Apoptosis 7:41–47. doi: 10.1023/a:1013560828090. [DOI] [PubMed] [Google Scholar]
- 51.Riccardi C, Nicoletti I. 2006. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 52.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O, Niso-Santano M, Shen S, Marino G, Criollo A, Boileve A, Job B, Ladoire S, Ghiringhelli F, Sistigu A, Yamazaki T, Rello-Varona S, Locher C, Poirier-Colame V, Talbot M, Valent A, Berardinelli F, Antoccia A, Ciccosanti F, Fimia GM, Piacentini M, Fueyo A, Messina NL, Li M, Chan CJ, Sigl V, Pourcher G, Ruckenstuhl C, Carmona-Gutierrez D, Lazar V, Penninger JM, Madeo F, Lopez-Otin C, Smyth MJ, Zitvogel L, Castedo M, Kroemer G. 2012. An immunosurveillance mechanism controls cancer cell ploidy. Science 337:1678–1684. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 53.McBride AA. 2008. Replication and Partitioning of Papillomavirus Genomes. Adv Virus Res 72:155–205. doi: 10.1016/S0065-3527(08)00404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He C, Mao D, Hua G, Lv X, Chen X, Angeletti PC, Dong J, Remmenga SW, Rodabaugh KJ, Zhou J, Lambert PF, Yang P, Davis JS, Wang C. 2015. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med 7:1426–1449. doi: 10.15252/emmm.201404976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morgan E, Patterson M, Lee SY, Wasson C, Macdonald A. 2018. High-risk human papillomaviruses down-regulate expression of the Ste20 family kinase MST1 to inhibit the Hippo pathway and promote transformation. bioRxiv doi: 10.1101/369447. [DOI]
- 56.Cheng J, Jing Y, Kang D, Yang L, Li J, Yu Z, Peng Z, Li X, Wei Y, Gong Q, Miron RJ, Zhang Y, Liu C. 2018. The role of MstI in lymphocyte homeostasis and function. Front Immunol 9:149. doi: 10.3389/fimmu.2018.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Zhang H, Zhao B. 2018. Hippo signaling in the immune system. Trends Biochem Sci 43:77–80. doi: 10.1016/j.tibs.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Crequer A, Picard C, Patin E, D’Amico A, Abhyankar A, Munzer M, Debré M, Zhang S-Y, Saint-Basile G. d, Fischer A, Abel L, Orth G, Casanova J-L, Jouanguy E. 2012. Inherited MST1 deficiency underlies susceptibility to EV-HPV infections. PLoS One 7:e44010. doi: 10.1371/journal.pone.0044010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan D. 2010. The Hippo signaling pathway in development and cancer. Dev Cell 19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li YY, Hanna GJ, Laga AC, Haddad RI, Lorch JH, Hammerman PS. 2015. Genomic analysis of metastatic cutaneous squamous cell carcinoma. Clin Cancer Res off J Am Assoc Cancer Res 21:1447–1456. doi: 10.1158/1078-0432.CCR-14-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pickering CR, Zhou JH, Lee JJ, Drummond JA, Peng SA, Saade RE, Tsai KY, Curry JL, Tetzlaff MT, Lai SY, Yu J, Muzny DM, Doddapaneni H, Shinbrot E, Covington KR, Zhang J, Seth S, Caulin C, Clayman GL, El-Naggar AK, Gibbs RA, Weber RS, Myers JN, Wheeler DA, Frederick MJ. 2014. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res off J Am Assoc Cancer Res 20:6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murthy V, Dacus D, Gamez M, Hu C, Wendel SO, Snow J, Kahn A, Walterhouse SH, Wallace NA. 2018. Characterizing DNA repair processes at transient and long-lasting double-strand DNA breaks by immunofluorescence microscopy. JoVE e57653. doi: 10.3791/57653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.