Mucosal immunity represented by pSIgA plays important roles in protection from IAV infection. Furthermore, IAV HA-specific pSIgA antibodies are thought to contribute to cross-protective immunity against multiple IAV subtypes. However, the mechanisms by which pSIgA exerts such versatile antiviral activity are not fully understood. In this study, we generated broadly cross-reactive recombinant IgG and pSIgA having the same antigen-recognition site and compared their antiviral activities in vitro. These recombinant antibodies did not show “classical” neutralizing activity, whereas pSIgA, but not IgG, significantly inhibited the production of progeny virus particles from infected cells. Plaque formation was also significantly reduced by pSIgA, but not IgG. These effects were seen in infection with IAVs of several different HA subtypes. Based on our findings, we propose an antibody-mediated host defense mechanism by which mucosal immunity may contribute to broad cross-protection from IAVs of multiple HA subtypes, including viruses with pandemic potential.
KEYWORDS: IgA, antibody, broadly cross-reactive, budding, cross-protective immunity, hemagglutinin, influenza A virus, nonneutralizing
ABSTRACT
IgA antibodies on mucosal surfaces are known to play an important role in protection from influenza A virus (IAV) infection and are believed to be more potent than IgG for cross-protective immunity against IAVs of multiple hemagglutinin (HA) subtypes. However, in general, neutralizing antibodies specific to HA are principally HA subtype specific. Here, we focus on nonneutralizing but broadly cross-reactive HA-specific IgA antibodies. Recombinant IgG, monomeric IgA (mIgA), and polymeric secretory IgA (pSIgA) antibodies were generated based on the sequence of a mouse anti-HA monoclonal antibody (MAb) 5A5 that had no neutralizing activity but showed broad binding capacity to multiple HA subtypes. While confirming that there was no neutralizing activity of the recombinant MAbs against IAV strains A/Puerto Rico/8/1934 (H1N1), A/Adachi/2/1957 (H2N2), A/Hong Kong/483/1997 (H5N1), A/shearwater/South Australia/1/1972 (H6N5), A/duck/England/1/1956 (H11N6), and A/duck/Alberta/60/1976 (H12N5), we found that pSIgA, but not mIgA and IgG, significantly reduced budding and release of most of the viruses from infected cells. Electron microscopy demonstrated that pSIgA deposited newly produced virus particles on the surfaces of infected cells, most likely due to tethering of virus particles. Furthermore, we found that pSIgA showed significantly higher activity to reduce plaque sizes of the viruses than IgG and mIgA. These results suggest that nonneutralizing pSIgA reactive to multiple HA subtypes may play a role in intersubtype cross-protective immunity against IAVs.
IMPORTANCE Mucosal immunity represented by pSIgA plays important roles in protection from IAV infection. Furthermore, IAV HA-specific pSIgA antibodies are thought to contribute to cross-protective immunity against multiple IAV subtypes. However, the mechanisms by which pSIgA exerts such versatile antiviral activity are not fully understood. In this study, we generated broadly cross-reactive recombinant IgG and pSIgA having the same antigen-recognition site and compared their antiviral activities in vitro. These recombinant antibodies did not show “classical” neutralizing activity, whereas pSIgA, but not IgG, significantly inhibited the production of progeny virus particles from infected cells. Plaque formation was also significantly reduced by pSIgA, but not IgG. These effects were seen in infection with IAVs of several different HA subtypes. Based on our findings, we propose an antibody-mediated host defense mechanism by which mucosal immunity may contribute to broad cross-protection from IAVs of multiple HA subtypes, including viruses with pandemic potential.
INTRODUCTION
Influenza A viruses (IAVs) belonging to the family Orthomyxoviridae have eight-segmented, negative-sense, single-stranded RNA genomes. IAVs have two envelope glycoproteins, hemagglutinin (HA) and neuraminidase (NA), on the viral surface. HA precursor HA0 is posttranslationally cleaved into HA1 and HA2 by host proteases (1, 2). HA1 mediates virus binding to sialic acids to initiate viral entry through endocytosis. The acidic pH in endosomes induces an irreversible conformational change in HA2 that mediates the fusion of the viral envelope and endosomal membranes (3). NA promotes the release of progeny virus particles from infected cells, as well as the penetration through host mucus by desialylation of viral and cellular surface glycans (4, 5). NA also plays a role in the initial stage of virus infection by facilitating virus motility on the cell surface (6).
HA and NA of IAVs are classified into 16 and 9 subtypes, respectively, based on their antigenicities (7). In addition, two novel influenza viruses were detected in bats captured in South and Central America and tentatively designated as new subtypes (H17N10 and H18N11) (8, 9). The HA molecule is the major target of neutralizing antibodies, which predominantly bind to highly variable antigenic regions surrounding the receptor binding site of HA and inhibit viral entry into the cells (10–12). Since the antigenicity of HA is principally determined by the structure of the antigenic sites, the majority of neutralizing antibodies naturally produced upon infection and/or vaccination are HA subtype specific, and only a small population of neutralizing antibodies is known to recognize multiple HA subtypes (13–17). HA-specific neutralizing antibodies that do not inhibit receptor binding, as well as nonneutralizing antibodies, are also induced to other antigenic regions (e.g., stem regions) conserved among multiple HA subtypes (14, 15, 18, 19).
IgA antibodies exist as monomeric and polymeric forms comprising two or more monomeric IgA (mIgA) units covalently linked by a joining (J) chain (20, 21). Polymeric IgA antibodies are intracellularly transferred to the apical membrane via transcytosis mediated by the polymeric immunoglobulin receptor (pIgR) expressed on the basolateral membrane, subsequently released from cells with the extracellular portion of pIgR, the so-called secretory component (SC), and function as polymeric secretory IgA (pSIgA) on mucosal surfaces (22). Although several different forms of IgA molecules (i.e., monomeric, dimeric, trimeric, and tetrameric) are known, the majority of pSIgA molecules are dimeric (23). pSIgA antibodies contribute to mucosal immunity against IAVs by blocking the initial virus infection of epithelial cells in the respiratory tract (23–25), as well as inhibiting the viral egress from infected cells, most likely due to tethering of the progeny virus particles (26). Interestingly, it has been shown that intranasal vaccination of mice with inactivated IAV particles provides cross-protective immunity against IAVs of multiple HA subtypes, whereas subcutaneous vaccination is only effective against the IAV homologous to the vaccine strain (27, 28). Since subcutaneous immunization predominantly induces a serum IgG response, whereas intranasal immunization induces both IgG and IgA responses, pSIgA antibodies are suggested to play an important role in this heterosubtypic immunity (27–29).
In the present study, we focused on HA-specific nonneutralizing pSIgA antibodies since nonneutralizing antibodies that bind to multiple HA subtypes are known to be generally present (18, 30, 31), whereas HA-specific cross-reactive antibodies with “classical” neutralizing capacity (i.e., inhibition of viral entry into cells) are limitedly induced. We generated recombinant IgG, mIgA, and pSIgA antibodies based on the sequence of a mouse anti-HA monoclonal antibody (MAb) 5A5 that had no neutralizing activity but showed broad binding capacity to multiple HA subtypes. We compared in vitro antiviral activities other than neutralization among the three forms of antibodies using IAVs of various HA subtypes.
RESULTS
Production of monoclonal IgG and IgA antibodies.
Mouse MAb 5A5, which is highly cross-reactive to multiple HA subtypes but has no neutralizing activity, was selected from the repository of our laboratory. To compare the antiviral activities of IgG and IgA forms of this antibody, mouse-human chimeric IgG and IgA antibodies were generated based on the sequence of the mouse MAb 5A5 variable region. Briefly, the genes encoding variable regions of this antibody were cloned into heavy and light chain expression plasmids and the constructed plasmids were subsequently transfected into Expi 293F cells. Note that SC and J chain expression plasmids were cotransfected to generate pSIgA antibodies. Recombinant IgG and IgA antibodies were purified from the supernatant by affinity chromatography. Then, gel filtration chromatography (GFC) enabled us to separate different forms of IgA antibodies based on their molecular weights (Fig. 1A). Fractions 1 to 9 and 12 to 14 were pooled for MAb 5A5 pSIgA and mIgA, respectively. Negative-control IgG and IgA antibodies were produced in the same manner based on the sequence of MAb B12, which was obtained from a healthy adult volunteer (32) (Fig. 1B). Fractions 1 to 8 and 10 to 14 were pooled for MAb B12 pSIgA and mIgA, respectively (Fig. 1B). The purified MAbs were validated for their purity and molecular weight (Fig. 1C and D) and used for further analyses.
FIG 1.
Purification of chimeric MAb 5A5 IgG and IgA. Recombinant IgG and IgA antibodies were purified from the supernatant by affinity chromatography. MAb 5A5 (A) and B12 (B) IgA antibodies were further fractionated by GFC with a Superose 6 10/300 GL column. A chromatogram demonstrating absorbance at 280 nm (shown in milli-absorbance units) revealed two major peaks. Fractions covering the two peaks were subjected to BN-PAGE. Equal amounts (5 μg) of purified IgG, mIgA, and pSIgA from MAb 5A5 (C) and B12 (D) were used for BN-PAGE.
Broad cross-binding capacity of MAb 5A5 IgG and IgA to multiple HA subtypes.
We investigated the binding capacities and specificities of recombinant MAb 5A5 IgG and IgA using HA antigens of H1 to H17 subtypes in enzyme-linked immunosorbent assay (ELISA) (Fig. 2). MAb 5A5 IgG, mIgA, and pSIgA showed similar binding patterns to HA subtypes (H1, H2, H4, H5, H6, H7, H8, H9, H10, H11, H12, H14, and H15), confirming that these recombinant IgG and IgA MAbs shared the antigen-binding site and recognized the same epitope. MAb 5A5 did not show reactivity to H3, H13, H16, H17, and B/Lee strains. To obtain information on the epitope of MAb 5A5, we generated two chimeric HAs (cHAs) between A/Puerto Rico/8/1934 (H1N1) (PR8) and A/little yellow-shouldered bat/Guatemala/060/2010 (H17N10) (Fig. 2): cH1/17 comprised of a part of the HA1 region (amino acid positions 52 to 277), the so-called the globular head region (33, 34), from PR8 and the other region from the H17N10 virus, and vice versa (Fig. 3A). We found that MAb 5A5 IgG, mIgA, and pSIgA recognized the globular head region of H1 HA in ELISA (Fig. 3B). Based on the reactivities of the antibodies, three IAV strains each were selected as representative human and avian isolates for further analyses: PR8, A/Adachi/2/1957 (H2N2) (Ad2), and A/Hong Kong/483/1997 (H5N1) (HK483) (human) and A/shearwater/South Australia/1/1972 (H6N5) (SA1), A/duck/England/1/1956 (H11N6) (Eng1), and A/duck/Alberta/60/1976 (H12N5) (Alb60) (avian), respectively.
FIG 2.
Reactivities of MAb 5A5 IgG, mIgA, and pSIgA to HA antigens from various subtypes. Reactivities of MAb 5A5 IgG, mIgA, and pSIgA to recombinant HAs were measured in ELISA. MAb 5A5 was diluted at 0.1 μg/ml before use. HAs of the following IAV strains were used: A/swine/Hokkaido/2/1981 (H1N1), A/Puerto Rico/8/1934 (H1N1), A/Kadoma/4/2006 (H1N1), A/Narita/1/2009 (H1N1), A/Adachi/2/1957 (H2N2), A/Aichi/2/1968 (H3N2), A/duck/Hokkaido/5/1977 (H3N2), A/duck/Czechoslovakia/1956 (H4N6), A/Hong Kong/483/1997 (H5N1), A/duck/Hong Kong/820/1980 (H5N3), A/shearwater/South Australia/1/1972 (H6N5), A/duck/Hokkaido/301/1978 (H7N2), A/seal/Massachusetts/1/1980 (H7N7), A/turkey/Ontario/6118/1968 (H8N4), A/Hong Kong/1073/1999 (H9N2), A/chicken/Germany/N/1949 (H10N7), A/duck/England/1/1956 (H11N6), A/duck/Alberta/60/1976 (H12N5), A/gull/Maryland/704/1977 (H13N6), A/mallard/Astrakhan/263/1982 (H14N5), A/duck/Australia/341/1983 (H15N8), A/black-headed gull/Sweden/5/1999 (H16N3), and A/little yellow-shouldered bat/Guatemala/060/2010 (H17N10). An influenza B virus strain, B/Lee/1940, was used as a negative control. Columns and error bars indicate the means and standard deviations of triplicate wells, respectively.
FIG 3.
(A) Reactivities of MAb 5A5 to cHAs between H1 and H17. H1, H17, and cHAs were assessed as described in Materials and Methods. H1 and H17 HAs were derived from A/Puerto Rico/8/1934 (H1N1) and A/little yellow-shouldered bat/Guatemala/060/2010 (H17N10), respectively. Numbers indicate the positions of amino acids according to H3 numbering. (B) Reactivities of MAb 5A5 IgG, mIgA, and pSIgA to the cHAs were measured using ELISA. Columns and error bars indicate the means and standard deviations of triplicate wells, respectively.
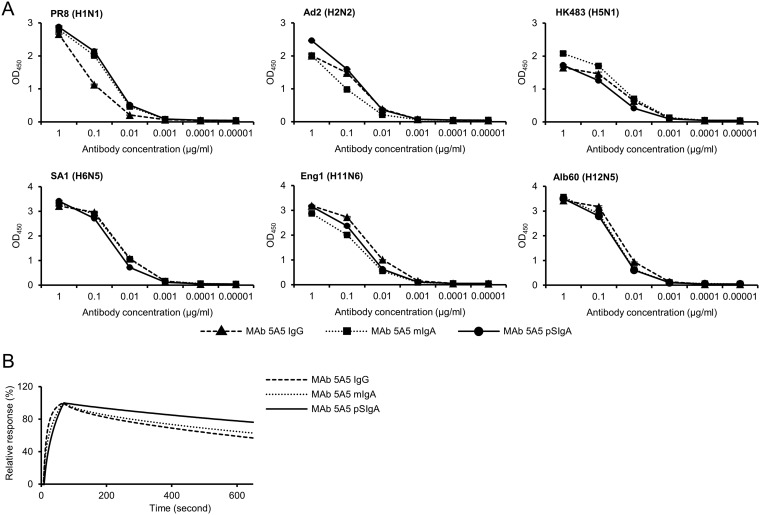
We then compared the binding activity among MAb 5A5 IgG, mIgA, and pSIgA against HAs derived from the selected 6 IAV strains. Although MAb 5A5 IgG showed slightly lower reactivity to PR8 HA than mIgA and pSIgA, there was no remarkable difference in the binding capacity to other HAs in ELISA (Fig. 4A). To quantify avidity of each MAb to PR8 HA, the binding dynamics of MAb 5A5 IgG, mIgA, and pSIgA was investigated by surface plasmon resonance (SPR) analysis. We found that MAb 5A5 pSIgA showed only a slightly lower dissociation rate than IgG and mIgA (Fig. 4B). These results indicated that the isotype difference between IgG1 and IgA1 backbones gave only limited effects on the binding activity of MAb 5A5 while polymerization might slightly enhanced the avidity of MAb 5A5.
FIG 4.
Comparison of avidity to HA antigens among MAb 5A5 antibodies. (A) Reactivities of IgG, mIgA, and pSIgA (0.00001 to 1 μg/ml) to recombinant HAs of PR8, Ad2, HK483, SA1, Eng1, and Alb60 were measured using ELISA. Binding dynamics of MAb 5A5 IgG, mIgA, and pSIgA against the recombinant trimeric PR8 HA (B). Sensorgrams were adjusted (x = 0, y = 0: baseline, y = 100: binding) to allow comparisons between different antibody forms in terms of the dissociation rate.
Reduction of viral particles released from IAV-infected cells in the presence of MAb 5A5 IgA.
We confirmed that MAb 5A5 IgG, mIgA, and pSIgA did not show neutralizing activity against any of the six IAVs tested (Fig. 5). Since nonneutralizing antibodies may have the potential to interfere with the virus budding/release process (18, 19), we then investigated inhibitory effects of these MAbs on virus release from Madin-Darby canine kidney (MDCK) cells infected with IAVs (Fig. 6). We detected significantly smaller amounts of the M1 protein in the supernatants of PR8-, HK483-, SA1-, and Eng1-infected cells incubated with MAb 5A5 mIgA and pSIgA than in those of MAb-untreated cells, and pSIgA decreased the M1 amount more significantly than IgG or mIgA (Fig. 6A). MAb 5A5 IgG showed inhibitory effects only against HK483 and Eng1. MAb B12 IgG, mIgA, and pSIgA showed no remarkable inhibitory effects. There was no significant difference in the intracellular expression levels of the matrix 1 (M1) protein of these six IAV strains among the antibody treatments, indicating that viral protein synthesis was not affected by the treatment with MAb 5A5 or the negative-control antibody MAb B12 (Fig. 6B).
FIG 5.
Neutralization tests of MAb 5A5 antibodies. Serial dilutions of MAb 5A5 IgG, mIgA, pSIgA, and positive-control neutralizing MAbs (0.01 to 100 μg/ml) were mixed with the respective IAV strains, followed by plaque assays as described in Materials and Methods. Means and standard deviations of plaque numbers were calculated from three individual experiments. Relative plaque numbers to each control sample (i.e., cells incubated without MAbs) are shown.
FIG 6.
Detection of the viral protein in supernatants and lysates of IAV-infected cells. MDCK cells were infected with IAVs at an MOI 2.0 and incubated with or without 5A5 and B12 MAbs (10 μg/ml). The M1 protein in supernatants (A) and cell lysates (B) was detected in Western blotting and beta-actin was also stained for cell lysate samples. The band intensities relative to each control sample (i.e., cells incubated without MAbs) are shown. Each experiment was performed three times, and averages and standard deviations are shown. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001) determined using one-way ANOVA, followed by Tukey’s multiple-comparison tests.
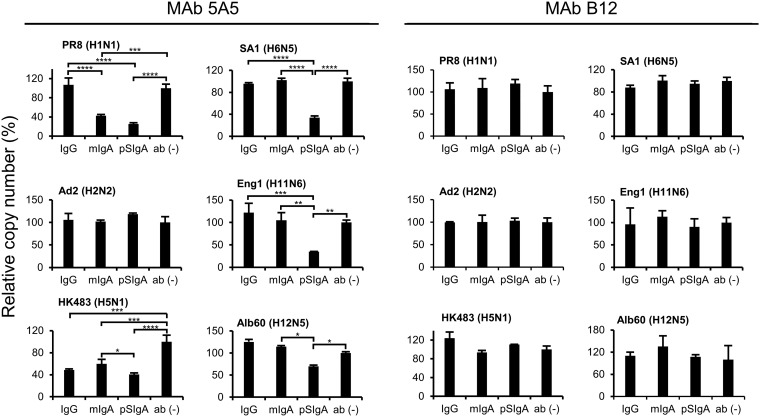
To further analyze the amounts of virus particles released into cell culture supernatants, viral RNAs (nucleoprotein [NP] gene) were quantified by real-time reverse transcription-PCR (RT-PCR) assays. We confirmed that the RNA copy numbers of the NP gene of PR8, HK483, SA1, Eng1, and Alb60 were significantly lower in the supernatants of the cells incubated with MAb 5A5 pSIgA than in those of the cells incubated with IgG or mIgA (Fig. 7). Taken together, these results indicated that MAb 5A5 pSIgA had greater potential than IgG and mIgA to reduce the number of IAV particles released from infected cells.
FIG 7.
Detection of the viral RNA genome in supernatants of IAV-infected cells. MDCK cells were infected with IAVs at an MOI 2.0 and incubated with or without 5A5 or B12 MAbs (10 μg/ml). The viral RNA genome was detected by real-time RT-PCR. Average copy numbers of the viral genome in the supernatant of IAV-infected cells incubated without any MAb were set to 100%. Each experiment was performed three times, and averages and standard deviations are shown. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001) determined using one-way ANOVA, followed by Tukey’s multiple-comparison tests.
Accumulation of virus particles on IAV-infected cells incubated in the presence of MAb 5A5 IgA.
To investigate the mechanism of the antiviral activities of the antibodies, IAV-infected MDCK cells incubated with MAb 5A5 IgG, mIgA, and pSIgA were observed by transmission electron microscopy (TEM). Unusual aggregation and accumulation of virus particles were found on the virus-infected cells cultured in the presence of MAb 5A5 mIgA and pSIgA, and this phenomenon was particularly prominent in the pSIgA-treated cells (Fig. 8). In contrast, lower numbers of virus particles in less proximity were found on the infected cells incubated with MAb 5A5 IgG and MAb-untreated cells, suggesting that efficient virus release from infected cells occurred. These data indicated that MAb 5A5 mIgA and pSIgA deposited newly produced virus particles on the cell surface more efficiently than IgG, resulting in reduced virus release from infected cells.
FIG 8.
Electron microscopy of virus particles on IAV-infected cells. DCK cells were infected with IAVs at an MOI 2.0 and incubated for 8 h with or without MAb 5A5 IgG, mIgA, or pSIgA. Randomly selected fields (10 to 20) were observed, and representative images are shown. Scale bars, 500 nm.
Since anti-HA antibodies that sterically hinder NA access to sialic acids were shown to possess NA inhibition (NI) activity (35), we investigated NI activity of MAb 5A5 IgA using an enzyme-linked lectin assay (ELLA) (36). We confirmed that both mIgA and pSIgA showed only minimum NI activities against the viruses even at the highest concentration (100 μg/ml) (Fig. 9). These results indicated that reduced virus release which was most likely caused by aggregation and accumulation of virus particles on the infected cells was not due to NI activity of MAb 5A5 IgA.
FIG 9.
NI activity of MAb 5A5 antibodies. Twofold serial dilutions of MAb 5A5 IgG, mIgA, and pSIgA (6.25 to 100 μg/ml) (A) and 10-fold serial dilutions of positive-control antisera (102 to 106-fold dilution) (B) were mixed with the respective IAV strains, followed by ELLA as described in Materials and Methods. Polyclonal chicken antisera against A/duck/Hokkaido/Vac-1/04 (H5N1), A/Singapore/1/1957 (H2N2), A/mallard/Astrakhan/263/1982 (H14N5), and Eng1 (H11N6) were used as positive controls for N1, N2, N5, and N6. Means and standard deviations of NA activity were calculated from triplicate wells. The NA activity values relative to each control sample (i.e., viruses incubated without MAbs) are shown.
Reduction in plaque size by MAb 5A5.
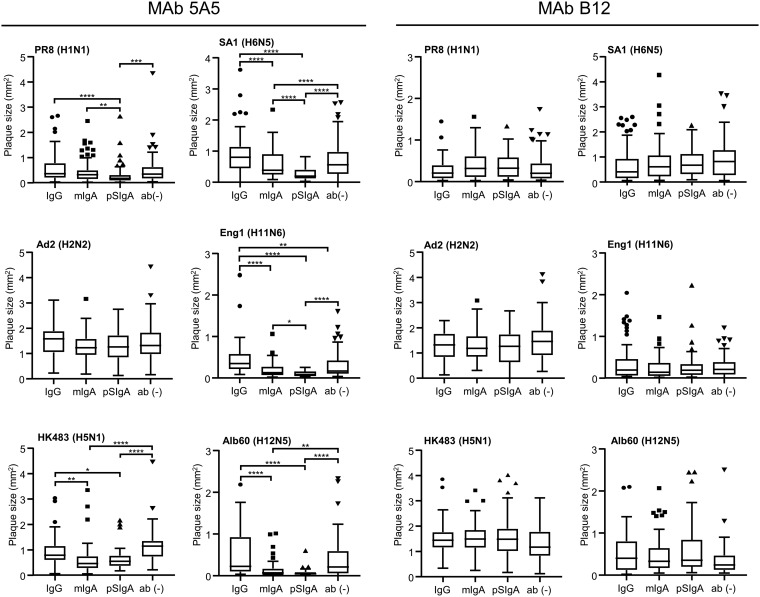
Since some antibodies having budding inhibition activity are known to reduce plaque sizes of IAVs (37, 38), we compared the ability to inhibit plaque formation among MAbs 5A5 IgG, mIgA, and pSIgA. MDCK cells infected with the IAVs were incubated with three different forms of 5A5 and B12 MAbs, and plaque sizes were measured (Fig. 10). We found that the plaque sizes of PR8, HK483, SA1, Eng1, and Alb60 were significantly reduced in the presence of pSIgA and that mIgA also significantly reduced the plaque sizes of HK483, SA1, and Alb60, whereas MAb 5A5 IgG showed no significant reduction (Fig. 10). Furthermore, as expected, MAb 5A5 pSIgA showed higher ability to reduce plaque size than mIgA against some of the IAV strains (PR8, SA1, and Eng1). Plaque size reduction was not observed with the control antibody, MAb B12. These results were consistent with the ability to reduce viral particle release from infected cells estimated by Western blotting and real-time RT-PCR analyses (Fig. 6 and 7).
FIG 10.
Reduced plaque size in the presence of 5A5 MAbs. MDCK cells were infected with IAVs and incubated with or without 5A5 or B12 MAbs (10 μg/ml). Plaques were stained as described in Materials and Methods (A), and plaque sizes were measured for each well (B). Each box with a horizontal black line represents the interquartile range (IQR) and the median. The marks represent outlying plots located over 1.5 × IQR from the upper quartile. Whiskers extend from the highest and lowest values within a fence. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001) determined using one-way ANOVA, followed by Tukey’s multiple-comparison tests.
DISCUSSION
It was reported that intranasal, but not subcutaneous, immunization of mice induced heterosubtypic immunity against multiple IAV HA subtypes, most likely owing to HA-specific nonneutralizing pSIgA antibodies (28, 29). It is also known that nonneutralizing antibodies are generally produced upon immunization/vaccination and that neutralizing activity is not the only indicator for protective antibodies against IAV infection (18, 39–42). Thus, the present study aimed at providing direct evidence for the potential role of such nonneutralizing antibodies in cross-protective immunity against IAVs, focusing on the IgA function during the viral egress from infected cells (26). Using anti-HA nonneutralizing MAbs (5A5 IgG, mIgA, and pSIgA) recognizing a single epitope involved in intersubtypic cross-reactivity, we compared their inhibitory effects on virus particle release and plaque formation in vitro.
We found that MAb 5A5 IgA inhibited virus particle release of multiple IAV strains of different HA subtypes more efficiently than IgG. The plaque sizes of the tested IAVs were accordingly reduced more significantly by MAb 5A5 IgA than by IgG (Fig. 10). Consistent with previous studies showing that pSIgA antibodies have more potential to neutralize IAVs than IgG (26) and that trimeric/tetrameric secretory IgA has stronger antiviral activity against IAVs than IgG and mIgA (23, 32, 43), our results indicated that pSIgA had enhanced ability to inhibit the release of IAV particles compared to IgG and mIgA (Fig. 6 to 8). Although MAb 5A5 IgG, mIgA, and pSIgA showed binding to Ad2 HA, they had limited effects on inhibiting virus particle release and plaque formation, suggesting that MAb 5A5 bound to Ad2 HA with a slightly different affinity from the other five IAV strains tested in this study.
Since HAs are abundantly expressed on IAV-infected cells (38, 44), MAb 5A5 is thought to bind to cell surface HAs and to tether the progeny virus particles newly produced by infected cells at the cell surface. Importantly, increased numbers of virus particles deposited on the cell surface were observed in the presence of MAb 5A5 pSIgA compared to the presence of IgG and mIgA (Fig. 7), which is consistent with our previous study showing that neutralizing IgA antibodies accumulated virus particles more efficiently than IgG (26). However, interestingly, we found no remarkable difference among MAb 5A5 IgG, mIgA, and pSIgA in the binding activity (i.e., avidity) to the viruses (Fig. 4). Because of the multiplicity of antigen binding sites in a single pSIgA molecule and the flexibility of the constant heavy chains of IgA antibodies resulting in higher affinity to the epitope (45), it is assumed that pSIgA has an advantage in the ability to tether the virus particles at the cell surface. Since NA inhibitors (e.g., oseltamivir, zanamivir, and peramivir) have well-known protective efficacy against IAVs by inhibiting the virus budding process (46–48), broadly reactive anti-HA IgA antibodies that inhibit viral release from infected cells may contribute to cross-protective immunity against IAVs of multiple HA subtypes even if they do not have “classical” neutralizing activity. It is known that intranasal immunization of live attenuated influenza vaccine (LAIV) provides broad cross-protective immunity in animal models, most likely due to the induction of nasal IgA antibodies (49, 50). However, detailed mechanisms how IgA antibodies contribute to the cross-protection remain unknown (51). Our findings suggest that cross-reactive nonneutralizing pSIgA induced by the intranasal vaccination may contribute to the protection. However, further studies are required to provide direct evidence of the importance of such IgA antibodies for cross-protective immunity in vivo.
In this study, MAb 5A5 was found to bind to the HA globular head region (Fig. 3). Some antibodies that inhibit virus release are known to bind to the HA globular head or stem region (17, 26, 42). Since MAb 5A5 has broad reactivities against multiple HA subtypes, we assume that its epitope is located in a highly conserved region among HA subtypes. Although it is difficult to determine the epitopes of nonneutralizing antibodies such as MAb 5A5 by obtaining amino acid sequences of escape mutants, identification of such epitopes on IAV HAs may provide profound understanding of the regions that are important for inhibition of virus release from infected cells and some other antibody functions (e.g., antibody-dependent cellular cytotoxicity).
Polymeric IgA antibodies are transferred intracellularly to the apical membrane by transcytosis and some IgA antibodies are known to inhibit viral protein functions intracellularly (52–54). Interestingly, IgA antibodies that do not have “classical” neutralizing activity effectively inhibit rotavirus and measles virus replication via this mechanism, which is called intracellular neutralization (52, 54). It has also been shown that anti-HA IgA, but not IgG, interacted with newly produced intracellular HA proteins in IAV-infected cells, thereby reducing viral titers (55). Nonneutralizing HA-specific IgA antibodies, therefore, may also have the ability to disturb the function or maturation of HA in infected cells. A recent study has demonstrated that intracellular neutralization against IAVs actually occurs in cells collected from patients’ nasopharyngeal aspirates (56). Further studies are needed to confirm whether nonneutralizing cross-reactive anti-HA IgA antibodies contribute to the intracellular neutralization.
In conclusion, our findings highlight a potential role of nonneutralizing pSIgA antibodies in intersubtypic cross-protective immunity against IAVs. Our study supports the idea that intranasal vaccination may provide heterosubtypic immunity to IAVs by inducing nonneutralizing but cross-reactive pSIgA antibodies that inhibit the release of viruses from infected cells. Further analyses on the in vivo function of pSIgA will provide new insights into mucosal vaccination to induce a broad spectrum of protective immunity against multiple IAV HA subtypes, including viruses with pandemic potential.
MATERIALS AND METHODS
Viruses.
A/Puerto Rico/8/1934 (H1N1) (PR8) and A/Adachi/2/1957 (H2N2) (Ad2) were propagated in MDCK cells. A/Hong Kong/483/1997 (H5N1) (HK483), A/shearwater/South Australia/1/1972 (H6N5) (SA1), A/duck/England/1/1956 (H11N6) (Eng1), and A/duck/Alberta/60/1976 (H12N5) (Alb60) were propagated in embryonated chicken eggs. All viruses were stored at −80°C until use. Infectious titers were determined by a plaque-forming assay with MDCK cells.
Cells.
MDCK cells were maintained in Eagle minimum essential medium (MEM; Wako) supplemented with 10% bovine serum (Gibco), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Gibco). After IAV inoculation, MDCK cells were cultured in MEM containing 0.3% bovine serum albumin (0.3% BSA/MEM) with 5 μg/ml trypsin (Gibco). Expi 293F cells were maintained in Expi293 expression medium (Thermo Fisher Scientific) as described in the manufacturer’s instructions. MDCK and Expi 293F cells were maintained at 37°C in 5% CO2 and at 37°C in 8% CO2 while being shaken with a 125 rpm orbital shaker, respectively. Human embryonic kidney (57) 293T cells were grown in Dulbecco modified Eagle medium (Sigma) supplemented with 10% fetal calf serum (Cell Culture Bioscience), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Gibco).
Production of mouse MAb 5A5.
Six-week-old female BALB/c mice were subcutaneously immunized twice at 2-week intervals with 100 μg of formalin (0.2%)-inactivated purified Eng1, together with complete Freund adjuvant (Difco Laboratories). At 3 weeks after the second immunization, the mice were intraperitoneally boosted with inactivated virus alone. Three days later, spleen cells from the mice and mouse myeloma P3U1 cells were fused and maintained according to a standard procedure (58). Hybridomas were screened for secretion of influenza virus-specific MAbs by ELISA, and then HA-specific MAbs were identified by Western blotting and immunostaining of 293T cells transfected with plasmids expressing Eng1 HA. MAbs reactive to Eng1 were further screened for their cross-reactivity to other HA subtypes by ELISA, and we obtained cross-reactive MAb 5A5 (IgG1). The hybridoma producing MAb 5A5 (IgG1) was cloned three times by limiting dilution of the cells.
5′-RACE–PCR and sequencing.
Total RNA was extracted from the hybridoma producing mouse MAb 5A5 using an RNeasy kit (Qiagen) and reverse transcribed with a SMARTer RACE 5′/3′ kit (Clontech) using 5′ RACE (5′-rapid amplification of cDNA ends) CDS primer A (Clontech). Subsequently, the variable gene segments for the heavy chain (VH) and light chain (VL) were amplified by PCR with the primer sets specific to VH and VL, respectively, using SeqAmp DNA polymerase according to the manufacturer’s instructions (TaKaRa). The PCR products for the VH and VL genes were cloned into a vector, pCR-Blunt II-TOPO (Invitrogen), and subjected to nucleotide sequencing. The nucleotide sequences were determined by using a BigDye Terminator sequencing kit, version 3.1 (Applied Biosystems), and an Applied Biosystems 3130xl genetic analyzer (Applied Biosystems). The primer sequences are available upon request.
Expression and purification of MAbs.
VH and VL genes of MAbs 5A5 and B12 were amplified with restriction enzyme cutting sites and ligated into α1H, γ1HC, and κLC vectors (32, 59) by using ligation mix (TaKaRa) as described in the manufacturer’s instructions. To express a human-mouse chimeric IgG antibody, Expi 293F cells were cotransfected with γ1HC- and κLC-expressing plasmids in a 1:1 ratio using an Expifectamine 293 transfection kit (Gibco). To express human-mouse chimeric IgA antibodies, Expi 293F cells were cotransfected with α1H-, κLC-, J chain-, and SC-expressing plasmids in a 2:2:1:1 ratio, respectively (32). Both IgG and IgA antibodies expressed by the plasmid vectors were isotype 1. After culture for 5 days, supernatants were collected and subjected to purification of the antibodies. IgG and IgA antibodies were purified from Expi 293F cell supernatants by using UNOsphere SUPrA (Bio-Rad) and CaptureSelect IgA (Invitrogen), respectively, according to the manufacturer’s instructions. Each antibody was concentrated using Amicon Ultra 30K (Merck). For IgG antibodies, elution buffer was replaced with phosphate-buffered saline (PBS) during the concentration process. Concentrated IgA antibodies were further subjected to GFC using a Superose 6 10/300 GL column (GE Healthcare) with an AKTA Avant 25 chromatography system (GE Healthcare). Fractions (200 μl each) were collected in PBS at a flow rate of 0.5 ml/min. Fractionated samples were analyzed by blue native-polyacrylamide gel electrophoresis (BN-PAGE) using NativePAGE 4 to 16% Bis-Tris protein gels (Invitrogen). NativeMark (Invitrogen) was used as a molecular weight standard. Considering the results of BN-PAGE, fractions were separated into two subsets: mIgA and pSIgA antibodies. Fractions for each IgA antibody subset were pooled and concentrated using Amicon Ultra 30K (Merck). All antibodies were stored at −80°C until use.
Expression of recombinant HA.
Recombinant HAs for ELISA antigens were prepared as previously reported (18). Briefly, HEK 293T cells transfected with the protein expression vector pCAGGS (60) encoding recombinant HAs using TransIT-LT1 (Mirus) were subjected to membrane protein extraction using a eukaryotic membrane protein extraction reagent kit (Thermo Fisher Scientific). HA protein antigens of the following IAVs were prepared: A/swine/Hokkaido/2/1981 (H1N1), PR8, A/Kadoma/4/2006 (H1N1), A/Narita/1/2009 (H1N1), Ad2, A/Aichi/2/1968 (H3N2), A/duck/Hokkaido/5/1977 (H3N2), A/duck/Czechoslovakia/1956 (H4N6), HK483, A/duck/Hong Kong/820/1980 (H5N3), SA1, A/duck/Hokkaido/301/1978 (H7N2), A/seal/Massachusetts/1/1980 (H7N7), A/turkey/Ontario/6118/1968 (H8N4), A/Hong Kong/1073/1999 (H9N2), A/chicken/Germany/N/1949 (H10N7), Eng1, Alb60, A/gull/Maryland/704/1977 (H13N6), A/mallard/Astrakhan/263/1982 (H14N5), A/duck/Australia/341/1983 (H15N8), A/black-headed gull/Sweden/5/1999 (H16N3), and A/little yellow-shouldered bat/Guatemala/060/2010 (H17N10). To generate cHAs (cHA H1/17 and cHA H17/1) between PR8 and A/little yellow-shouldered bat/Guatemala/060/2010 (H17N10), cDNAs corresponding to the HA globular head and the other regions were amplified and cloned into the pCAGGS vector (60) using an In-Fusion HD cloning kit (Clontech). The extracted membrane proteins were appropriately diluted (1 : 1000–8000) with PBS to give the highest optical density values at 450 nm for hyperimmune chicken antisera (repository of our laboratory) (18) specific to the respective HA subtypes and used as antigens for ELISA. The PCR conditions, including primer sequences, are available upon request.
Enzyme-linked immunosorbent assay.
ELISA plates (Nunc Maxisorp) were coated with the prepared HA antigens and blocked with PBS containing 3% skim milk (Becton Dickinson). MAb 5A5 diluted in 1% skim milk in PBS containing 0.05% Tween 20 (PBST) were plated in triplicate, and bound antibodies were detected using horseradish peroxidase (HRP)-conjugated goat anti-human IgG(H+L) (109-001-003; Jackson Immuno Research, 1:10,000) or HRP-conjugated goat anti-human IgA(H+L) (ab97215; Abcam, 1:10,000). The reaction was visualized by adding 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma-Aldrich), and the absorbance at 450 nm was measured.
Surface plasmon resonance assay.
The SPR assay was performed by using Biacore 3000 (GE Healthcare) as described in a previous study (32). Briefly, recombinant trimeric PR8 HA with a C-terminal His tag was immobilized on the surface of Sensor Chip NTA (GE Healthcare) by using the NTA reagent kit (GE Healthcare). After trimeric HA immobilization (10 μg/ml for 180 s), the molecular interaction of HA with MAb 5A5 IgG, mIgA, or pSIgA (50 μg/ml) was analyzed with a contact time of 60 s and a dissociation time of 650 s.
Neutralization assay.
IAVs (50 to 100 PFU) were incubated with serial dilutions of antibodies (0.01 to 100 μg/ml) prior to inoculation into MDCK cells. The following antibodies were used as positive-control neutralizing antibodies: anti-H1 MAb APH 269-5, anti-H2 MAb S139/1 (17, 26), anti-H5 MAb 36-1, anti-H6 MAb 85-2-2, anti-H11 MAb 9F5-2-4, and anti-H12 MAb 15-3-1. After incubation with the viruses, the cells were washed with PBS twice and overlaid with 0.3% BSA/MEM containing 1.2% Avicel RC 591 (FMC BioPolymer) (61), a processed mixture of microcrystalline cellulose and sodium carboxymethyl cellulose, and 5 μg/ml trypsin. After 20 h of incubation, the cells were fixed with methanol and blocked with PBS containing 1% BSA. Plaques were stained with a mouse anti-HA MAb, HRP-conjugated goat anti-mouse IgG(H+L) (115-035-062; Jackson Immuno Research), and a 3,3′-diaminobenzidine (62) substrate (Wako).
Sample collection of cell lysates and supernatants of infected cells.
MDCK cells seeded on 12-well plates (Corning) were incubated with IAVs at a multiplicity of infection (MOI) of 2.0 for an hour for adsorption and then washed with PBS three times. Subsequently, the infected cells were incubated with culture media containing 10 μg/ml of IgG and IgA forms of MAb 5A5 or the control antibody (MAb B12). Eight hours later, the supernatant was centrifuged (15,000 × g, 4°C, 10 min) and collected into new tubes. Laemmli sample buffer (Bio-Rad) containing 2-mercaptoethanol was added to the cells and collected into tubes.
Western Blotting.
Cells and supernatants that were mixed with Laemmli sample buffer (Bio-Rad) containing 2-mercaptoethanol were incubated at 95°C for 10 and 5 min, respectively. After SDS-PAGE using 12% sodium dodecyl sulfate (SDS), separated proteins were transferred onto polyvinylidene fluoride (polyvinylidene difluoride [PVDF]) membranes (Merck). The PVDF membranes were blocked with PBS containing 3% skim milk (Becton Dickinson) and washed with PBST. Each membrane was incubated with a mouse anti-M1 MAb (APH 6–23-1-6) (63) (1:2500) and a mouse anti-beta-actin antibody (ab6276; Abcam, 1:5,000) as primary antibodies and HRP-conjugated goat anti-mouse IgG(H+L) (115-035-062; Jackson Immuno Research, 1:10,000) as a secondary antibody. Antibodies were diluted with PBST containing 1.5% skim milk. After being washed with PBST, the bound antibodies were visualized with Immobilon Western (Millipore). The amount of the viral M1 protein was semiquantified from the intensity of the stained band using an Amersham Imager 600 (GE Healthcare).
Real-time RT-PCR.
Viral RNA was extracted from the cell supernatant using a QIAmp viral RNA minikit (Qiagen) and subjected to real-time RT-PCR-based IAV gene detection using a One-Step SYBR prime script RT-PCR kit II (TaKaRa). Primer sets specific for conserved regions of IAV NP genes (NP 972F [CAAGAGTCAGCTGGTGTGGA] and NP 1160R [GCCCAGTACCTGCTTCTCAG]) were used. Quantitation of the NP gene was performed using a standard curve generated by threshold cycle values obtained from 10-fold serial dilutions (covering 102 to 106 copies) of the PR8 NP gene inserted into the pHH21 vector (64). All samples were tested in triplicate. Average copy numbers of the viral genome in the supernatant of IAV-infected cells incubated without any MAb was set to 100%. Real-time RT-PCR conditions are available upon request.
Transmission electron microscopy.
TEM was performed as described previously (26, 65). Briefly, MDCK cells infected with IAVs at an MOI of 1.0 to 2.0 were cultured with or without MAb 5A5 IgG, mIgA, or pSIgA (10 μg/ml) for 8 h and fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4). The fixed cells were postfixed with 2% osmium tetroxide, dehydrated with series of ethanol gradients followed by propylene oxide, embedded in Epon 812 resin mixture (TAAB Laboratories Equipment, Ltd.), and polymerized. Ultrathin sections (50 nm) were stained with uranyl acetate and lead citrate and examined with a JEM-1210 (JEOL) electron microscope at 80 kV.
NA inhibition assay.
NA inhibition (NI) assays based on the ELLA method were performed as described previously (36, 66–68). Briefly, ELISA plates (Nunc Maxisorp) were coated with 100 μl of fetuin (Wako) at a concentration of 500 μg/ml and blocked with PBS containing 5% BSA. MAbs 5A5 IgG and IgA were 2-fold serially diluted from 200 μg/ml in PBS and reacted with the viruses diluted to 2 × 50% effective concentration (based on NA assay) in PBS containing 1% BSA. Chicken hyperimmune antisera against A/duck/Hokkaido/Vac-1/04 (H5N1), A/Singapore/1/1957 (H2N2), A/mallard/Astrakhan/263/1982 (H14N5), and Eng1 (H11N6) were used as positive-control antibodies. Each antibody-virus mixture was transferred to fetuin-coated plates. A secondary solution of peanut agglutinin conjugated with peroxidase (Sigma) was added at a concentration of 1 μg/ml in PBS. The reaction was visualized by adding the TMB (Sigma-Aldrich) solution, and the absorbance at 450 nm was measured.
Plaque size reduction assay.
Confluent monolayers of MDCK cells on 12-well plates (Corning) were infected with the viruses to give 50 to 100 PFU and overlaid with 0.3% BSA/MEM containing 5 μg/ml trypsin, 10 μg/ml of the IgG and IgA forms of MAb 5A5 and the control antibody (MAb B12), and 0.8% agarose S (Wako). After incubation with or without MAbs for 2 days at 35°C, plaques were visualized by immunostaining using the same methods as described above for the neutralizing assay. Plaque images on the wells were scanned, and the sizes of at least 30 to 50 plaques were measured using ImageJ analysis software.
Statistical analyses.
Data (band intensity, RNA copy number, and plaque size) were analyzed using Prism version 8 for Windows (GraphPad Software, San Diego, CA). One-way analysis of variance (ANOVA), followed by Tukey’s multiple-comparison test, was used to analyze each data set as indicated in the figure legends. The following statistical values and symbols are used throughout the manuscript (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
ACKNOWLEDGMENTS
This study was supported by Grants-in-Aid for JSPS Fellows from the Japan Society for the Promotion of Science (JSPS; JSPS KAKENHI grant number 18J20917), KAKENHI (grant numbers 24390110 and 16H02627), and the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from the Japan Agency for Medical Research and Development (AMED; JP18fm0108008).
REFERENCES
- 1.Klenk HD, Rott R, Orlich M, Blodorn J. 1975. Activation of influenza A viruses by trypsin treatment. Virology 68:426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- 2.Steinhauer DA. 1999. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 258:1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- 3.Skehel JJ, Wiley DC. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem 69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 4.Cohen M, Zhang XQ, Senaati HP, Chen HW, Varki NM, Schooley RT, Gagneux P. 2013. Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase. Virol J 10:321. doi: 10.1186/1743-422X-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palese P, Tobita K, Ueda M, Compans RW. 1974. Characterization of temperature-sensitive influenza virus mutants defective in neuraminidase. Virology 61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 6.Ohuchi M, Asaoka N, Sakai T, Ohuchi R. 2006. Roles of neuraminidase in the initial stage of influenza virus infection. Microbes Infect 8:1287–1293. doi: 10.1016/j.micinf.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Medina RA, Garcia-Sastre A. 2011. Influenza A viruses: new research developments. Nat Rev Microbiol 9:590–603. doi: 10.1038/nrmicro2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO. 2012. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A 109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Wu Y, Tefsen B, Shi Y, Gao GF. 2014. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol 22:183–191. doi: 10.1016/j.tim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. 1982. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 31:417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 11.Okada J, Ohshima N, Kubota-Koketsu R, Iba Y, Ota S, Takase W, Yoshikawa T, Ishikawa T, Asano Y, Okuno Y, Kurosawa Y. 2011. Localization of epitopes recognized by monoclonal antibodies that neutralized the H3N2 influenza viruses in man. J Gen Virol 92:326–335. doi: 10.1099/vir.0.026419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchiya E, Sugawara K, Hongo S, Matsuzaki Y, Muraki Y, Li ZN, Nakamura K. 2001. Antigenic structure of the haemagglutinin of human influenza A/H2N2 virus. J Gen Virol 82:2475–2484. doi: 10.1099/0022-1317-82-10-2475. [DOI] [PubMed] [Google Scholar]
- 13.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. 2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 14.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleury D, Barrere B, Bizebard T, Daniels RS, Skehel JJ, Knossow M. 1999. A complex of influenza hemagglutinin with a neutralizing antibody that binds outside the virus receptor binding site. Nat Struct Biol 6:530–534. doi: 10.1038/9299. [DOI] [PubMed] [Google Scholar]
- 16.Okuno Y, Matsumoto K, Isegawa Y, Ueda S. 1994. Protection against the mouse-adapted A/FM/1/47 strain of influenza A virus in mice by a monoclonal antibody with cross-neutralizing activity among H1 and H2 strains. J Virol 68:517–520. doi: 10.1128/JVI.68.1.517-520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida R, Igarashi M, Ozaki H, Kishida N, Tomabechi D, Kida H, Ito K, Takada A. 2009. Cross-protective potential of a novel monoclonal antibody directed against antigenic site B of the hemagglutinin of influenza A viruses. PLoS Pathog 5:e1000350. doi: 10.1371/journal.ppat.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muramatsu M, Yoshida R, Miyamoto H, Tomabechi D, Kajihara M, Maruyama J, Kimura T, Manzoor R, Ito K, Takada A. 2013. Heterosubtypic antiviral activity of hemagglutinin-specific antibodies induced by intranasal immunization with inactivated influenza viruses in mice. PLoS One 8:e71534. doi: 10.1371/journal.pone.0071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneemann A, Speir JA, Tan GS, Khayat R, Ekiert DC, Matsuoka Y, Wilson IA. 2012. A virus-like particle that elicits cross-reactive antibodies to the conserved stem of influenza virus hemagglutinin. J Virol 86:11686–11697. doi: 10.1128/JVI.01694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandtzaeg P. 1974. Presence of J chain in human immunocytes containing various immunoglobulin classes. Nature 252:418–420. doi: 10.1038/252418a0. [DOI] [PubMed] [Google Scholar]
- 21.Johansen FE, Braathen R, Brandtzaeg P. 2000. Role of J chain in secretory immunoglobulin formation. Scand J Immunol 52:240–248. doi: 10.1046/j.1365-3083.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- 22.Norderhaug IN, Johansen FE, Schjerven H, Brandtzaeg P. 1999. Regulation of the formation and external transport of secretory immunoglobulins. Crit Rev Immunol 19:481–508. [PubMed] [Google Scholar]
- 23.Suzuki T, Kawaguchi A, Ainai A, Tamura S, Ito R, Multihartina P, Setiawaty V, Pangesti KN, Odagiri T, Tashiro M, Hasegawa H. 2015. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc Natl Acad Sci U S A 112:7809–7814. doi: 10.1073/pnas.1503885112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould VMW, Francis JN, Anderson KJ, Georges B, Cope AV, Tregoning JS. 2017. Nasal IgA provides protection against human influenza challenge in volunteers with low serum influenza antibody titre. Front Microbiol 8:900. doi: 10.3389/fmicb.2017.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy BR, Clements ML. 1989. The systemic and mucosal immune response of humans to influenza A virus. Curr Top Microbiol Immunol 146:107–116. doi: 10.1007/978-3-642-74529-4_12. [DOI] [PubMed] [Google Scholar]
- 26.Muramatsu M, Yoshida R, Yokoyama A, Miyamoto H, Kajihara M, Maruyama J, Nao N, Manzoor R, Takada A. 2014. Comparison of antiviral activity between IgA and IgG specific to influenza virus hemagglutinin: increased potential of IgA for heterosubtypic immunity. PLoS One 9:e85582. doi: 10.1371/journal.pone.0085582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takada A, Kuboki N, Okazaki K, Ninomiya A, Tanaka H, Ozaki H, Itamura S, Nishimura H, Enami M, Tashiro M, Shortridge KF, Kida H. 1999. Avirulent Avian influenza virus as a vaccine strain against a potential human pandemic. J Virol 73:8303–8307. doi: 10.1128/JVI.73.10.8303-8307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. 2003. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine 21:3212–3218. doi: 10.1016/S0264-410X(03)00234-2. [DOI] [PubMed] [Google Scholar]
- 29.Tumpey TM, Renshaw M, Clements JD, Katz JM. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol 75:5141–5150. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo L, Wang D, Zhou H, Wu C, Gao X, Xiao Y, Ren L, Paranhos-Baccala G, Shu Y, Jin Q, Wang J. 2016. Cross-reactivity between avian influenza A (H7N9) virus and divergent H7 subtypic and heterosubtypic influenza A viruses. Sci Rep 6:22045. doi: 10.1038/srep22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Z, Gera L, Mant CT, Hirsch B, Yan Z, Shortt JA, Pollock DD, Qian Z, Holmes KV, Hodges RS. 2016. Platform technology to generate broadly cross-reactive antibodies to alpha-helical epitopes in hemagglutinin proteins from influenza A viruses. Biopolymers 106:144–159. doi: 10.1002/bip.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito S, Sano K, Suzuki T, Ainai A, Taga Y, Ueno T, Tabata K, Saito K, Wada Y, Ohara Y, Takeyama H, Odagiri T, Kageyama T, Ogawa-Goto K, Multihartina P, Setiawaty V, Pangesti KNA, Hasegawa H. 2019. IgA tetramerization improves target breadth but not peak potency of functionality of anti-influenza virus broadly neutralizing antibody. PLoS Pathog 15:e1007427. doi: 10.1371/journal.ppat.1007427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P. 2012. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol 86:5774–5781. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, Garcia-Sastre A, Palese P. 2010. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 1:e00018-10. doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YQ, Lan LY, Huang M, Henry C, Wilson PC. 2018. Hemagglutinin stalk-reactive antibodies interfere with influenza virus neuraminidase activity by steric hindrance. J Virol 93. doi: 10.1128/JVI.01526-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westgeest KB, Bestebroer TM, Spronken MI, Gao J, Couzens L, Osterhaus AD, Eichelberger M, Fouchier RA, de Graaf M. 2015. Optimization of an enzyme-linked lectin assay suitable for rapid antigenic characterization of the neuraminidase of human influenza A (H3N2) viruses. J Virol Methods 217:55–63. doi: 10.1016/j.jviromet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Job ER, Schotsaert M, Ibanez LI, Smet A, Ysenbaert T, Roose K, Dai M, de Haan CAM, Kleanthous H, Vogel TU, Saelens X. 2018. Antibodies directed toward neuraminidase N1 control disease in a mouse model of influenza. J Virol 92:e01584-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zebedee SL, Lamb RA. 1988. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol 62:2762–2772. doi: 10.1128/JVI.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, Winnall WR, Stratov I, Brooks AG, Reading PC, Kent SJ. 2013. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 190:1837–1848. doi: 10.4049/jimmunol.1201574. [DOI] [PubMed] [Google Scholar]
- 40.Krammer F, Palese P. 2013. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terajima M, Cruz J, Co MD, Lee JH, Kaur K, Wrammert J, Wilson PC, Ennis FA. 2011. Complement-dependent lysis of influenza a virus-infected cells by broadly cross-reactive human monoclonal antibodies. J Virol 85:13463–13467. doi: 10.1128/JVI.05193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamayoshi S, Uraki R, Ito M, Kiso M, Nakatsu S, Yasuhara A, Oishi K, Sasaki T, Ikuta K, Kawaoka Y. 2017. A broadly reactive human anti-hemagglutinin stem monoclonal antibody that inhibits influenza A virus particle release. EBioMedicine 17:182–191. doi: 10.1016/j.ebiom.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki T, Ainai A, Hasegawa H. 2017. Functional and structural characteristics of secretory IgA antibodies elicited by mucosal vaccines against influenza virus. Vaccine 35:5297–5302. doi: 10.1016/j.vaccine.2017.07.093. [DOI] [PubMed] [Google Scholar]
- 44.Nayak DP, Hui EK, Barman S. 2004. Assembly and budding of influenza virus. Virus Res 106:147–165. doi: 10.1016/j.virusres.2004.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tudor D, Yu H, Maupetit J, Drillet AS, Bouceba T, Schwartz-Cornil I, Lopalco L, Tuffery P, Bomsel M. 2012. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc Natl Acad Sci U S A 109:12680–12685. doi: 10.1073/pnas.1200024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim CU, Lew W, Williams MA, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen MS, Mendel DB, Tai CY, Laver WG, Stevens RC. 1997. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J Am Chem Soc 119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 47.Varghese JN, McKimm-Breschkin JL, Caldwell JB, Kortt AA, Colman PM. 1992. The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor. Proteins 14:327–332. doi: 10.1002/prot.340140302. [DOI] [PubMed] [Google Scholar]
- 48.von Itzstein M, Wu WY, Kok GB, Pegg MS, Dyason JC, Jin B, Van Phan T, Smythe ML, White HF, Oliver SW. 1993. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 49.Isakova-Sivak I, Matyushenko V, Kotomina T, Kiseleva I, Krutikova E, Donina S, Rekstin A, Larionova N, Mezhenskaya D, Sivak K, Muzhikyan A, Katelnikova A, Rudenko L. 2019. Sequential immunization with universal live attenuated influenza vaccine candidates protects ferrets against a high-dose heterologous virus challenge. Vaccines 7:61. doi: 10.3390/vaccines7030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ito R, Ozaki YA, Yoshikawa T, Hasegawa H, Sato Y, Suzuki Y, Inoue R, Morishima T, Kondo N, Sata T, Kurata T, Tamura S. 2003. Roles of anti-hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine 21:2362–2371. doi: 10.1016/S0264-410X(03)00078-1. [DOI] [PubMed] [Google Scholar]
- 51.Turner PJ, Abdulla AF, Cole ME, Javan RR, Gould V, O’Driscoll ME, Southern J, Zambon M, Miller E, Andrews NJ, Höschler K, Tregoning JS. 2020. Differences in nasal immunoglobulin A responses to influenza vaccine strains after live attenuated influenza vaccine (LAIV) immunization in children. Clin Exp Immunol 199:109–118. doi: 10.1111/cei.13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corthesy B, Benureau Y, Perrier C, Fourgeux C, Parez N, Greenberg H, Schwartz-Cornil I. 2006. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J Virol 80:10692–10699. doi: 10.1128/JVI.00927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, Nedrud JG. 1992. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci U S A 89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou D, Zhang Y, Li Q, Chen Y, He B, Yang J, Tu H, Lei L, Yan H. 2011. Matrix protein-specific IgA antibody inhibits measles virus replication by intracellular neutralization. J Virol 85:11090–11097. doi: 10.1128/JVI.00768-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazanec MB, Coudret CL, Fletcher DR. 1995. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol 69:1339–1343. doi: 10.1128/JVI.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kok TW, Costabile M, Tannock GA, Li P. 2018. Colocalization of intracellular specific IgA (icIgA) with influenza virus in patients’ nasopharyngeal aspirate cells. J Virol Methods 252:8–14. doi: 10.1016/j.jviromet.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 57.Poehling KA, Caspard H, Peters TR, Belongia EA, Congeni B, Gaglani M, Griffin MR, Irving SA, Kavathekar PK, McLean HQ, Naleway AL, Ryan K, Talbot HK, Ambrose CS. 2018. 2015–2016 vaccine effectiveness of live attenuated and inactivated influenza vaccines in children in the United States. Clin Infect Dis 66:665–672. doi: 10.1093/cid/cix869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kida H, Brown LE, Webster RG. 1982. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology 122:38–47. doi: 10.1016/0042-6822(82)90375-0. [DOI] [PubMed] [Google Scholar]
- 59.Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. 2008. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods 329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 61.Matrosovich M, Matrosovich T, Garten W, Klenk HD. 2006. New low-viscosity overlay medium for viral plaque assays. Virol J 3:63. doi: 10.1186/1743-422X-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dabaghian M, Latifi AM, Tebianian M, NajmiNejad H, Ebrahimi SM. 2018. Nasal vaccination with r4M2e.HSP70c antigen encapsulated into N-trimethyl chitosan (TMC) nanoparticulate systems: preparation and immunogenicity in a mouse model. Vaccine 36:2886–2895. doi: 10.1016/j.vaccine.2018.02.072. [DOI] [PubMed] [Google Scholar]
- 63.Nao N, Kajihara M, Manzoor R, Maruyama J, Yoshida R, Muramatsu M, Miyamoto H, Igarashi M, Eguchi N, Sato M, Kondoh T, Okamatsu M, Sakoda Y, Kida H, Takada A. 2015. A single amino acid in the M1 protein responsible for the different pathogenic potentials of H5N1 highly pathogenic avian influenza virus strains. PLoS One 10:e0137989. doi: 10.1371/journal.pone.0137989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez DR, Donis R, Hoffmann E, Hobom G, Kawaoka Y. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A 96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noda T, Sagara H, Suzuki E, Takada A, Kida H, Kawaoka Y. 2002. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J Virol 76:4855–4865. doi: 10.1128/JVI.76.10.4855-4865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cate TR, Rayford Y, Nino D, Winokur P, Brady R, Belshe R, Chen W, Atmar RL, Couch RB. 2010. A high dosage influenza vaccine induced significantly more neuraminidase antibody than standard vaccine among elderly subjects. Vaccine 28:2076–2079. doi: 10.1016/j.vaccine.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lambre CR, Terzidis H, Greffard A, Webster RG. 1991. An enzyme-linked lectin assay for sialidase. Clin Chim Acta 198:183–193. doi: 10.1016/0009-8981(91)90352-D. [DOI] [PubMed] [Google Scholar]
- 68.Lebarbenchon C, Albespy F, Brochet AL, Grandhomme V, Renaud F, Fritz H, Green AJ, Thomas F, van der Werf S, Aubry P, Guillemain M, Gauthier-Clerc M. 2009. Spread of avian influenza viruses by common teal (Anas crecca) in Europe. PLoS One 4:e7289. doi: 10.1371/journal.pone.0007289. [DOI] [PMC free article] [PubMed] [Google Scholar]