Abstract
Severe coronavirus disease (COVID‐19) is characterized by an excessive proinflammatory cytokine storm, resulting in acute lung injury and development of acute respiratory distress syndrome (ARDS). The role of corticosteroids is controversial in severe COVID‐19 pneumonia and associated hyper‐inflammatory syndrome. We reported a case series of six consecutive COVID‐19 patients with severe pneumonia, ARDS and laboratory indices of hyper‐inflammatory syndrome. All patients were treated early with a short course of corticosteroids, and clinical outcomes were compared before and after corticosteroids administration. All patients evaded intubation and intensive care admission, ARDS resolved within 11.8 days (median), viral clearance was achieved in four patients within 17.2 days (median), and all patients were discharged from the hospital in 16.8 days (median). Early administration of short course corticosteroids improves clinical outcome of patients with severe COVID‐19 pneumonia and evidence of immune hyperreactivity.
Keywords: ARDS, corticosteroids, COVID‐19, cytokine storm, hyper‐inflammatory syndrome
Research Highlights
Severe COVID‐19 is characterized by an excessive pro‐inflammatory cytokine storm resulting in acute lung injury and development of ARDS.
Early, short‐course of corticosteroids in severe COVID‐19 pneumonia and associated hyper‐inflammatory syndrome may be beneficial.
Routine use of corticosteroids in COVID‐19 is not recommended.
1. INTRODUCTION
In addition to respiratory failure, early studies on patients with severe coronavirus disease (COVID‐19) have reported significantly increased plasma levels of several cytokines and chemokines, and inflammatory markers, such as C‐reactive protein (CRP), interleukin 6, procalcitonin, and ferritin, appear to correlate with respiratory failure, ARDS, altered immune status, and fatal outcome. 1 , 2 Based on these findings, a dysregulated host immune response to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) lung infection leading to exuberant cytokine release (“cytokine storm”) and immune‐mediated lung injury has been postulated as a critical pathogenetic factor in the progression to ARDS. 3
We present a small series of six consecutive hospitalized COVID‐19 patients with rapidly deteriorating hypoxemia and laboratory indices of COVID‐19 associated hyper‐inflammatory syndrome (CAHS), all of which evaded intubation/intensive care unit (ICU) admission and recovered completely following a short course of high‐dose corticosteroid (CS).
2. METHODS
From 19th March to 24th April 2020, 38 patients were admitted to the 7th Department of Respiratory Medicine, “Sotiria” Chest Hospital, Athens, Greece, which was transformed to a COVID‐19 only care unit. All of them had confirmed COVID‐19, defined by a positive reverse‐transcriptase–polymerase chain reaction (RT‐PCR) assay for SARS‐CoV‐2 in a nasopharyngeal swab. Baseline characteristics and clinical, laboratory, radiological, and treatment data were prospectively collected as part of an observational cohort study approved by the research ethics committee of our hospital (10685/2020). Written informed consent was obtained from all patients included in this registry.
3. RESULTS
Eleven patients developed severe hypoxemia, fulfilling the diagnostic criteria of ARDS (PaO2/FiO2 < 300 mm Hg) either at admission or shortly after.
Six out of the eleven patients who developed severe hypoxemia, were middle‐aged (median, 58; range, 45‐64 years), mostly male (5/6), never or ex‐smokers with otherwise insignificant medical history. All presented with fever, cough, and mild dyspnea on exertion as well as pulmonary infiltrates several days after symptom onset (median, 7.8; range, 6‐12 days) and either had hypoxemia (median, PaO2/FiO2 190 mm Hg; range, 113‐252 mm Hg) on admission or developed it shortly after (median time from admission to ARDS 1.5; range, 0‐3 days), thus fulfilling the diagnostic criteria of ARDS (Table 1). Antigen tests for influenza (nasopharyngeal swabs), Streptococcus pneumoniae and Legionella pneumophila (urine samples) and sputum cultures were performed in all patients. On admission, lymphopenia was almost universally present (median, 635; range, 490‐1,930 lymphocytes/μL) as well as a low lymphocyte‐to‐CRP ratio, while increased levels of d‐dimers (median, 1.19; range, 0,45‐3,81 μg/mL) and transaminitis (median alanine aminotransferase, 60.5; range, 17‐343 IU/L) were noticed in four patients. CRP and ferritin in contrast with procalcitonin, were already significantly elevated at presentation and increased further in parallel with the development of hypoxemia (Figure 1).
Table 1.
Baseline characteristics, treatment, and outcome of six patients with severe COVID‐19 pneumonia treated with corticosteroids
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Baseline characteristics | ||||||
| Age, y | 45 | 62 | 64 | 60 | 45 | 56 |
| Sex | Male | Female | Male | Male | Male | Male |
| Smoking status, smoker | Ex | Never | Never | Ex | Ex | Never |
| Comorbidities | None | Hyper‐lipidemia | None | AH radical prostatectomy nephrolithiasis | Allergic rhinitis | None |
| Disease time course | ||||||
| Time from illness onset to admission, d | 7 | 8 | 12 | 7 | 7 | 6 |
| Time from admission to ARDS, d | 2 | 0 | 1 | 1 | 2 | 3 |
| Treatment | ||||||
| Hydroxychloroquine | Noa | Noa | Noa | Yes | Noa | Yes |
| Azithromycin | No | Yes | Yes | Yes | Yes | Yes |
| Time from admission to CS treatment onset, d | 3 | 4 | 1 | 2 | 4 | 3 |
| CS treatment duration, d | 3 | 3 | 3 | 3 | 5 | 5 |
| Outcome | ||||||
| Time from CS onset to PaO2/FiO2 > 300 mm Hg, d | 9 | 14 | 6 | 11 | 18 | 13 |
| CS side effects | None | None | None | None | Hyper‐glycemia | None |
| Length of hospital stay, d | 15 | 21 | 10 | 13 | 23 | 19 |
| Viral clearance, d | NA | 20 | NA | 13 | 19 | 17 |
Abbreviations: ARDS, acute respiratory distress syndrome; AH, arterial hypertension; CS, corticosteroids; NA, not available.
Glucose‐6‐phosphate dehydrogenase (G6PD) deficiency.
Figure 1.
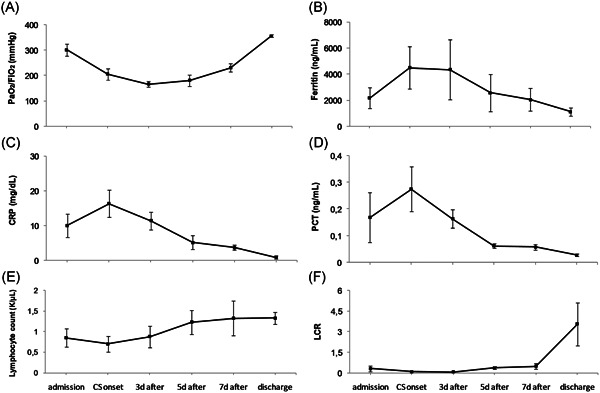
Comparative trend of inflammatory markers and oxygenation from hospital admission to CS treatment onset and on days 3, 5, and 7 post CS initiation and on discharge. Data are presented as mean ± standard error of the mean. C‐reactive protein (CRP): normal range <0.70 mg/dL. Lymphocyte absolute number: normal range 1.2 to 4.0 K/μL. Procalcitonin (PCT): normal range <0.25 ng/mL. Ferritin: normal range 21.81 to 274.6 ng/mL. CS, corticosteroids; LCR, lymphocyte‐to‐CRP ratio
Patients received treatment as per local protocol (combination of azithromycin, hydroxychloroquine, beta‐lactam, prophylaxis with low‐molecular‐weight heparin and oxygen therapy). One patient had also tested positive for influenza and received oseltamivir, while four patients did not receive hydroxychloroquine due to laboratory confirmed glucose‐6‐phosphate dehydrogenase deficiency. Treatment with CS (i.v. methylprednisolone 125 mg once daily) was started shortly after hypoxemia and evidence of cytokine storm had ensued.
In all six patients, fever subsided and dyspnea alleviated within 48 hours after CS initiation and by day 3 post treatment onset, four patients had attained significant reductions in the levels of most or all of the inflammatory markers measured and CS were discontinued. A more prolonged treatment course (5 days) was followed in the other two patients, in whom inflammatory markers began to decline on day 5 of CS therapy. Oxygenation gradually improved obviating the need for intubation in all six patients. The inflammatory profile amelioration tended to precede PaO2/FiO2 which was slow, with the time needed to reach a PaO2/FiO2 > 300 mm Hg ranging from 6 to 18 days (median, 11.8 days), indicating a slower rate of the inflammatory resolution in the pulmonary compared with systemic compartment (Figure 1). Overall, CS were well tolerated, with only one patient developing transient mild hyperglycemia, and RT‐PCR to confirm viral clearance on discharge was repeated in four patients and all had two negative tests at a 24‐hour interval (median, 17.2 days; range, 14‐23 days) (Table 1). Of the remaining five patients out of eleven (median, 74; range, 62‐91 years) who developed severe hypoxemia and did not receive CS, the first three were intubated and transferred to ICU, one was treated as cardiac failure and recovered, and another had terminal cancer and died of septic shock‐the only fatal outcome among our patients (Table S2).
4. DISCUSSION
In this uncontrolled case series of six middle‐aged patients with COVID‐19 associated hyper‐inflammatory syndrome and without significant comorbidities, administration of CS was followed by clinical improvement, and in four patients checked on discharge, viral clearance was documented, raising question whether or not CS may prolong viral shedding.
Real‐life data suggest that therapeutic trials of CS have already been applied quite extensively in COVID‐19, particularly in ICU patients with severe or critical disease, without clear evidence of benefit, although a recent study showed that early administration of CS reduced the need for intubation and mortality. 1 , 4 Moreover, inconclusive findings from previous studies on the efficacy of CS treatment in influenza, SARS and Middle East respiratory syndrome along with concerns of possible harm principally with respect to secondary infections and prolonged viral replication, render their use for COVID‐19 controversial at this point. 5 , 6
The rationale for CS use in this specific clinical phenotype of COVID‐19 patients with associated hyper‐inflammatory syndrome, was that the benefits of an early intervention to decrease the hyper‐inflammatory response and acute lung injury, that does not initially resembles that of classic ARDS, may outweigh the adverse effects of CS. 7
Theoretically, this CAHS could bear some resemblance to secondary haemophagocytic lymphohistiocytosis (sHLH), a reactive hyper‐ferritinemic hyper‐cytokinemic immune syndrome, most commonly triggered by viral infections, which may result in life‐threatening organ failure, including ARDS in approximately 50% of patients, 8 through an unpredictable (though potentially fulminant) course. Early administration of high‐dose CS with or without IV immunoglobulin is the mainstay of first‐line treatment for sHLH and a graded approach regarding CS dosage and duration tailored to the disease severity and course is recommended. 9
Therefore, understanding the pathophysiology of COVID‐19 in the different clinical stages of the disease is important, as distinct treatments may be needed in the different expressions of the disease and in what looks like a COVID‐19 triggered immune hyper‐reaction. 9 To corroborate this hypothesis, preliminary data from postmortem biopsies of severe COVID‐19 infection at different temporal stages of the disease, indicate that a lymphocytic alveolar/interstitial pattern is observed in the early period in contrast with acute fibrinous organizing pneumonia seen in the later period, culminating in diffuse alveolar damage. 10 , 11 This dynamic pathologic process may correlate with the degree of viral alveolar units infected, the rate of the hyper‐inflammatory syndrome and of thrombotic microangiopathy. 12 Ideally, lung biopsies (eg, transbronchial cryobiopsies) could provide useful information regarding pathologic features and viral presence in the different stages, particularly the acute/early one) of severe COVID‐19 infection that may guide therapeutic approach.
Although the study design and the limited sample size do not permit definitive conclusions, they do raise the awareness on the potential effectiveness of CS in COVID‐19 associated hyper‐inflammatory syndrome in younger patients, with lack of comorbidities. Only well‐designed randomized clinical trials may clarify the effect of CS, taking into account the timing, dosage, and duration of CS and any other treatment, nevertheless, while still awaiting results from large, randomized studies, and indisputable evidence, it may be worth considering CS use in selected patients with COVID‐19 associated hyper‐inflammatory syndrome.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
LK and KL conceived the study, performed the analysis, draft the manuscript, and designed the figures, SG, LV, AL, and EZ draft the manuscript. MG draft the manuscript and supervised the study.
Supporting information
Supporting information
Kolilekas L, Loverdos K, Giannakaki S, et al. Can steroids reverse the severe COVID‐19 induced “cytokine storm”? J Med Virol. 2020;92:2866–2869. 10.1002/jmv.26165
Lykourgos Kolilekas and Konstantinos Loverdos contributed equally to this work.
References
- 1. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038]. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pedersen SF, Ho YC. SARS‐CoV‐2: a storm is raging [published online ahead of print, 2020 Apr 13]. J Clin Invest. 2020;130:2202‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID‐19. Clin Infect Dis. 2020. 10.1093/cid/ciaa601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395:473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019‐nCoV pneumonia. Lancet. 2020;395:683‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rello J, Storti E, Belliato M, Serrano R. Clinical phenotypes of SARS‐CoV‐2: Implications for clinicians and researchers. Eur Respir J. 2020;55. 10.1183/13993003.01028-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133:2465‐2477. [DOI] [PubMed] [Google Scholar]
- 10. Copin MC, Parmentier E, Duburcq T, Poissy J, Mathieu D, Lille COVID‐19 ICU and Anatomopathology Group . Time to consider histologic pattern of lung injury to treat critically ill patients with COVID‐19 infection. Intensive Care Med. 2020. 10.1007/s00134-020-06057-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID‐19) through postmortem core biopsies. Mod Pathol. 2020;33:1007‐1014. 10.1038/s41379-020-0536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020. 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


