Abstract
Background
The pandemic of coronavirus disease 2019 (COVID‐19) has emerged as a relevant threat for humans worldwide. Abnormality in liver function tests (LFTs) has been commonly observed in patients with COVID‐19, but there is controversy on its clinical significance. The aim of this study was to assess the prevalence, the characteristics and the clinical impact of abnormal LFTs in hospitalized, non‐critically ill patients with COVID‐19.
Methods
In this multicentre, retrospective study, we collected data about 565 inpatients with COVID‐19. Data on LFTs were collected at admission and every 7 ± 2 days during the hospitalization. The primary outcome was a composite endpoint of death or transfer to intensive care unit (ICU).
Results
Upon admission 329 patients (58%) had LFTs abnormality. Patients with abnormal LFTs had more severe inflammation and higher degree of organ dysfunction than those without. During hospitalization, patients with abnormal LFTs had a higher rate of transfer to ICU (20% vs 8%; P < .001), acute kidney injury (22% vs 13%, P = .009), need for mechanical ventilation (14% vs 6%; P = .005) and mortality (21% vs 11%; P = .004) than those without. In multivariate analysis, patients with abnormal LFTs had a higher risk of the composite endpoint of death or transfer to ICU (OR = 3.53; P < .001). During the hospitalization, 86 patients developed de novo LFTs abnormality, which was associated with the use of tocilizumab, lopinavir/ritonavir and acetaminophen and not clearly associated with the composite endpoint.
Conclusions
LFTs abnormality is common at admission in patients with COVID‐19, is associated with systemic inflammation, organ dysfunction and is an independent predictor of transfer to ICU or death.
Keywords: liver injury, nCOV‐19, SARS‐CoV‐2, sepsis
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BT
body temperature
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- FiO2
fraction of inspired oxygen
- GGT
gamma‐glutamyltransferase
- HR
heart rate
- LFTs
liver function tests, ICU, intensive care unit
- NEWS‐2
national early warning scale 2
- NSAIDs
nonsteroidal anti‐inflammatory drugs
- OR
odds ratio
- qSOFA
quick SOFA
- RR
respiratory rate
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SatO2
pulse oxygen saturation
- SIRS
systemic inflammatory response syndrome
- SOFA
sequential organ failure assessment
- ULN
upper limit of normal
Key points.
Abnormalities of liver function tests are frequent in patients with COVID‐19, but there is uncertainty whether they are markers of severity of the disease or not. Herein we showed that: (a) more than one‐half of non‐critically ill patients with COVID‐19 has abnormal liver function tests at hospital admission; (b) abnormal liver function tests strongly predict a worse clinical course of the disease (need for intensive care unit and mortality) and (c) de novo abnormalities of liver function tests are frequently related to drug induced liver injury.
1. INTRODUCTION
The pandemic of coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has rapidly emerged as a relevant threat for humans worldwide. 1 The main clinical feature of COVID‐19 is pneumonia, which is characterized by a high mortality rate, however, increasing data suggest that COVID‐19 is a systemic disease affecting also other organs/systems including liver, heart, kidney and coagulation. 2 , 3 , 4 , 5 An increase in liver function tests (LFTs) has been found in patients with COVID‐19, ranging 14%‐75%. 2 , 3 , 6 , 7 , 8 , 9 , 10 , 11 Some studies found higher levels of transaminases in patients with severe COVID‐19 pneumonia and in patients dying for COVID‐19. 2 , 11 , 12 , 13 The clinical relevance of LFTs abnormalities has been controversial, with some studies suggesting its association with the severity of COVID‐19 pneumonia, whereas others not. 9 , 14 Some limitations affected those studies involving the lack of information about concomitant or previous use of hepatotoxic drugs among the others. Overall, there is a paucity of studies assessing the prevalence, the pattern (hepatocellular, cholestatic, mixed) and the clinical impact of LFTs, in particular in Caucasian patients. On clinical ground, it is still uncertain whether LFTs abnormalities should be considered a marker of severity of COVID‐19 or not. Thus, the aim of this study was to assess the prevalence, the clinical features and the clinical impact of abnormal LFTs in hospitalized, non‐critically ill patients with COVID‐19.
2. METHODS
2.1. Patients
Patients with COVID‐19 hospitalized in five internal medicine COVID‐Unit in two regions of Northern Italy from February 22nd to April 8th were retrospectively identified. Diagnosis of COVID‐19 was performed according to World Health Organization interim guidance. 15 Inclusion criteria were as follows: (a) patients hospitalized with a SARS‐CoV‐2 infection confirmed by real‐time reverse transcription polymerase chain reaction method; (b) age >18 years old. Exclusion criterion was patients admitted in the intensive care unit (ICU) within 12 hours from admission to emergency room. We excluded patients admitted to ICU within 12 hours, because they were likely to be already critically ill at admission and we wanted to target patients managed in regular ward. Permission for retrospective data analyses was obtained from local Ethics Committee. Verbal or written informed consent was obtained from patients surviving hospitalization. The study was performed according to the ethical guidelines of the Declaration of Helsinki (seventh revision).
2.2. Study design
Paper and electronic charts were reviewed and demographic, clinical, radiological and laboratory data were collected at admission. Information on medical history, including comorbidities, symptoms of infection and drugs taken in the previous 14 days before admission were collected. Vital signs (respiratory rate [RR], body temperature [BT], arterial blood pressure, heart rate [HR], pulse oxygen saturation [SatO2] and fraction of inspired oxygen [FiO2]) were collected. Laboratory tests including alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma‐glutamyltransferase (GGT), alkaline phosphatase (ALP) and bilirubin were collected at admission and every 7 ± 2 days during the admission. Scores of severity of the disease such as sequential organ failure assessment (SOFA), quick SOFA (qSOFA), systemic inflammatory response syndrome (SIRS) and national early warning scale 2 (NEWS‐2) were calculated at admission. 16 , 17 In patients without arterial blood gas test, the respiratory component of SOFA score was calculated using SatO2/FiO2 ratio as previously suggested. 18 Charlson comorbidity index was calculated as well. 19 Type of drugs administered during the hospitalization were collected, date of initiations and discontinuation were collected as well. The following events occurred during the hospitalization were collected: admission to the ICU, mechanical ventilation, non‐invasive ventilation, acute kidney injury, renal replacement therapy, treatment with vasopressors, occurrence of multiorgan failure, acute liver failure (jaundice, INR >1.5 and encephalopathy) and death. Data were collected until April 23rd, 2020 in an electronic case report form using the Research Electronic Data Capture Software REDCap 20 hosted at the Department of Medicine of the University of Padova (Padova, Italy).
2.3. Definitions
LFTs were considered abnormal when at least one among AST, ALT, GGT, ALP and bilirubin were above the upper limit of normal (ULN) of local reference laboratories. 21 The pattern of abnormal LFTs was defined hepatocellular when patients showed predominantly raised ALT and AST; cholestatic when patients showed predominantly raised ALP and GGT, and mixed when the extent of AST/ALT and ALP/GGT was similar. 21 De novo abnormality of LFTs was defined as the occurrence of abnormal LFTs in patients with normal LFTs at admission. Fever was defined as a BT >38°C. Acute kidney injury was defined according to the kidney disease improving global outcomes criteria. 22 The primary outcome of the study was a composite endpoint of death or transfer to the ICU during the hospitalization.
2.4. Statistical analysis
Continuous variables were presented as mean ± standard deviation or median with interquartile range (IQR) and compared with Student's t test and Mann‐Whitney U test according to normal or skewed distribution respectively. Categorical variables were presented as frequencies and percentages and compared with chi‐square test (with Yates’ correction) or Fisher's exact test when appropriate. Two multivariate logistic regression models were used to identify whether abnormal LFTs were independently associated with the composite outcome (transfer to ICU or death). Variables to be included in multivariate analysis were selected among variables deemed to be meaningful (age, sex, comorbidity, vital signs, radiological findings, biomarkers of inflammation and scores of organ dysfunction or acute illness). Results were expressed as odds ratio (OR) and 95% confidence interval (CI). Non‐normally distributed continuous variables were log‐transformed before to be included in logistic regression models. When SOFA score and NEWS‐2 scores were included in the model, their components were excluded to avoid multicollinearity. Survival curves were build using the Kaplan Meier methods and were compared with log rank test. Patients were censored at the time of discharge. Pearson's correlation coefficient was used to assess the correlation between AST and either ALT or creatine phosphokinase. All tests were two‐tailed and P‐values < .05 were considered significant. Statistical analysis was performed using SPSS statistical package (version 25).
3. RESULTS
3.1. Study population
During the study period we identified 615 inpatients with confirmed COVID‐19. Thirty‐one were excluded because admitted to ICU within 12 hours from admission to the emergency room. Among 584 patients admitted to general ward, 19 had no data on LFTs and were excluded, thus 565 patients were included in the analysis (Figure 1). The mean age was 66 ± 15 years old and two thirds of them were male (Table 1). The majority of patients were Caucasian (97%) and had at least one comorbidity (69%). The most common comorbidity was hypertension (52%), followed by diabetes (16%) and atrial fibrillation (11%). Thirty‐one patients (6%) had chronic liver disease, among which 17 had non‐alcoholic fatty liver disease, four had alcoholic liver disease, three HCV infection (in sustained virological response after treatment with direct acting antivirals); four patients (0.7%) had cirrhosis. In Table 1 drugs taken in the 14 days before admission were listed. Many patients took potential hepatotoxic drugs such as acetaminophen (15%, none more than 3 grams per day), antibiotics (24%), statins (15%) and nonsteroidal anti‐inflammatory drugs (NSAIDs, 3%). Most common symptoms of COVID‐19 were fever (87%), cough (60%), dyspnoea (49%) and fatigue (24%) and time from symptoms onset to admission was 8 (IQR = 5‐11) days. On admission, most of patients had lymphocytopenia and elevated levels of C‐reactive protein. More than one half had SIRS and two thirds had a SOFA score equal or higher than 2. The most common findings at chest x‐ray were interstitial pneumonia (39%) and bilateral (39%) or unilateral consolidation (18%). Proportion of missing data for each variable has been reported in Table S1.
Figure 1.
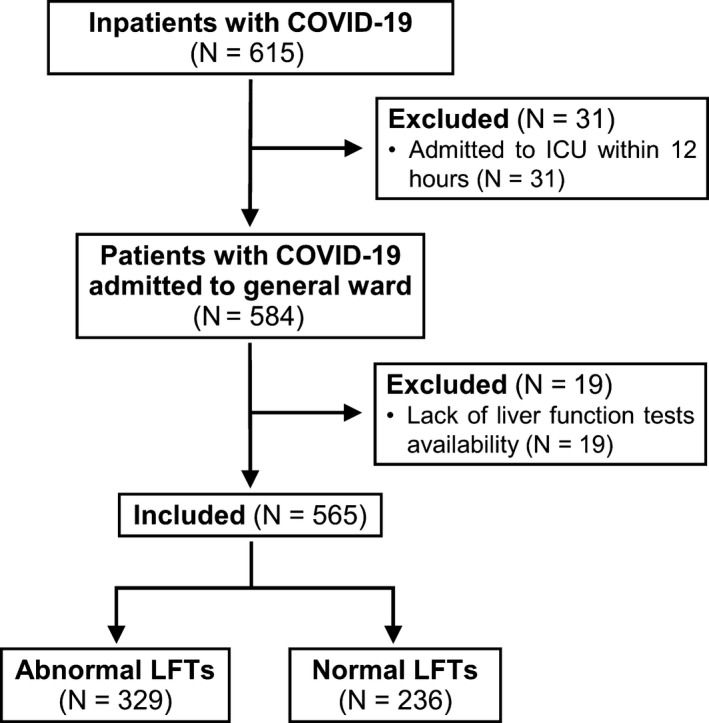
Flow chart of the study Legend: COVID‐19, coronavirus disease 2019; LFTs, liver function tests
Table 1.
Demographic, clinical, laboratory and radiological characteristics of patients with COVID‐19
| Variables | N = 565 |
|---|---|
| Age (years) – m (SD) | 66 (15) |
| Gender (male) – n (%) | 357 (63) |
| Ethnicity (Caucasian) – n (%) | 546 (97) |
| Comorbidity – n (%) | 388 (69) |
| Hypertension | 291 (52) |
| Diabetes | 90 (16) |
| Ischaemic heart disease | 45 (8) |
| Atrial fibrillation | 60 (11) |
| COPD | 45 (8) |
| Chronic kidney disease | 37 (7) |
| Chronic liver disease a | 31 (6) |
| Drug use in the previous 14 days – n (%) | 454 (80) |
| Acetaminophen | 87 (15) |
| Antibiotics | 135 (24) |
| ACE inhibitors | 103 (18) |
| ARBs | 93 (17) |
| Beta‐blockers | 118 (21) |
| Dihydropyridine CCBs | 64 (11) |
| Furosemide | 73 (13) |
| Statins | 86 (15) |
| NSAIDs | 18 (3) |
| Antiplatelet agents | 78 (14) |
| Oral anticoagulants | 50 (9) |
| Other | 221 (39) |
| Symptoms – n (%) | |
| Fever (>38°C) | 491 (87) |
| Dyspnoea | 278 (49) |
| Cough | 337 (60) |
| Fatigue | 138 (24) |
| Myalgia | 41 (7) |
| Nausea/vomiting | 32 (6) |
| Diarrhoea | 71 (13) |
| Headache | 19 (3) |
| Dysgeusia | 33 (6) |
| Hyposmia | 20 (4) |
| Confusion | 45 (8) |
| Time from symptoms to hospitalization (days) – M (IQR) | 8 (5‐11) |
| Alteration of consciousness – n (%) | 48 (9) |
| Body temperature (°C) – m (SD) | 37.7 (0.9) |
| SatO2/FiO2 ratio – M (IQR) | 426 (257‐457) |
| PaO2/FiO2 ratio – M (IQR)§ | 290 (202‐336) |
| Respiratory rate (breath/min) – M (IQR) | 20 (18‐24) |
| Mean arterial pressure (mmHg) – m (SD) | 93 (12) |
| Heart rate (beat/min) – m (SD) | 85 (14) |
| Hb (g/L) – M (IQR) | 13.4 (12.4‐14.5) |
| WBC (x109/L) – M (IQR) | 5.8 (4.0‐8.4) |
| Neutrophils (x109/L) – M (IQR) | 4.1 (2.5‐6.5) |
| Lymphocytes (x109/L) – M (IQR) | 0.9 (0.7‐1.2) |
| Platelets (x109/L) – M (IQR) | 182 (145‐233) |
| C‐reactive protein (mg/L) – M (IQR) | 64 (27‐120) |
| Procalcitonin (µg/L) – M (IQR) | 0.11 (0.05‐0.30) |
| INR – M (IQR) | 1.10 (1.05‐1.22) |
| D‐Dimer (µg/L) – M (IQR) | 217 (150‐584) |
| Fasting glucose (mg/dl) – M (IQR) | 110 (94‐135) |
| Serum creatinine (mg/dl) – M (IQR) | 0.92 (0.77‐1.12) |
| AST (U/L) – M (IQR) | 38 (28‐58) |
| ALT (U/L) – M (IQR) | 30 (20‐49) |
| GGT (U/L) – M (IQR) | 36 (21‐65) |
| ALP (U/L) – M (IQR) | 61 (48‐80) |
| Bilirubin (mg/dl) – M (IQR) | 0.54 (0.40‐0.77) |
| Albumin (g/dl) – m (SD) | 3.2 (0.5) |
| LDH (U/L) – M (IQR) | 301 (239‐394) |
| CPK (U/L) – M (IQR) | 106 (59‐206) |
| Troponin (ng/L) – M (IQR) | 12 (4‐33) |
| Ferritin (µg/L) – M (IQR) | 668 (302‐1167) |
| Lactates (mmol/L) – M (IQR) | 1.2 (1.0‐1.6) |
| SOFA score – m (SD) | 2 (1‐4) |
| SOFA ≥ 2 – n (%) | 375 (66) |
| qSOFA score – m (SD) | 1 (0‐1) |
| SIRS – n (%) | 294 (52) |
| NEWS‐2 score – m (SD) | 4 (3) |
| Chest X‐ray findings – n (%) | |
| Normal | 70 (12) |
| Interstitial pneumonia | 220 (39) |
| Ground glass opacity | 28 (5) |
| Local consolidation | 104 (18) |
| Bilateral consolidation | 218 (39) |
| Other | 54 (10) |
Abbreviations: ACE, angiotensin converting enzyme; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ARB, angiotensin receptor blockers; AST, aspartate aminotransferase; COPD, chronic obstructive pulmonary disease; CPK, creatine kinase; FiO2, fraction of inspired oxygen; GGT, gamma‐glutamyltransferase; Hb, haemoglobin; INR, international normalized ratio; IQR, interquartile range; LDH, lactate dehydrogenase; m, mean; M, median; n, number; NEWS‐2, national early warning scale 2; NSAIDs, non‐steroidal anti‐inflammatory agents; PaO2, partial pressure of arterial oxygen; qSOFA, quick sequential organ failure assessment; SatO2, pulse oxygen saturation; SD, standard deviation; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment; WBC, white blood cells.
17 NAFLD, 3 HCV infection; 4 HBV infection; 4 alcoholic liver disease; 3 autoimmune hepatitis; 1 primary biliary cholangitis; 4 patients had cirrhosis of the liver.
3.2. Characteristics of patients with abnormal liver function tests
On admission 329 patients (58%) had abnormal LFTs. AST, ALT, GGT, ALP and bilirubin above ULN in 44%, 32%, 34%, 5% and 8% of patients respectively. The pattern of LFTs abnormality was hepatocellular in 56%, cholestatic in 24% and mixed in 19%. Just four patients had an isolated abnormality of bilirubin (1%). Alteration of LFTs was below two times ULN in 65%, two to three times ULN in 22% and above three times ULN in 13% of patients with abnormal LFTs. Characteristics of patients with or without LFTs abnormalities has been reported in Table 2. Patients with abnormal LFTs had a trend toward higher rate of comorbidities, although no significant difference was found in the rate of known chronic liver disease (7% vs 3%; P = .095). Interestingly, no association was found between abnormal LFTs and the use of acetaminophen, antibiotics or NSAIDs during the 14 days before admission. Patients with abnormal LFTs had more frequently dyspnoea (55% vs 42%; P = .003), had a higher RR (22 vs 20 bpm; P < .001) and a lower SatO2/FiO2 and PaO2/FiO2 ratios. Time from symptoms onset to hospitalization was slightly higher in patients with abnormal LFTs. Patients with abnormal LFTs had also a more severe inflammation than those with normal tests, as shown by the higher levels of white blood cells, neutrophils, C‐reactive protein and procalcitonin. Interestingly, D‐dimer, lactate dehydrogenase, creatine‐kinase and troponin were higher in patients with abnormal LFTs than those without. Scores of severity of infection, such as SOFA score and NEWS‐2 score were significantly higher in patients with abnormal LFTs. Finally, patients with abnormal LFTs had more frequently bilateral consolidation at chest X‐ray than those without (44% vs 32%; P = .006). AST had a strong correlation with ALT (r = .751; P < .001) and a moderate correlation with CPK (r = .319; P < .001). Even in the subgroup of patients with abnormal AST (N = 249), AST was strongly correlated with ALT (r = .711; P < .001) and mildly correlated with creatine phosphokinase (r = .214; P = .005). As for GGT, it was strongly correlated with ALP (r = .540; P < .001), moderately correlated with ALT (r = .446; P < .001) and AST (r = .325; P < .001).
Table 2.
Characteristics of patients with or without abnormal liver function tests at admission
| Variables |
Normal liver function tests (N = 236) |
Abnormal liver function tests (N = 329) |
P |
|---|---|---|---|
| Age (years) – m (SD) | 65 (15) | 66 (15) | .432 |
| Gender (male) – n (%) | 149 (63) | 208 (63) | 1.000 |
| Ethnicity (caucasian) – n (%) | 226 (97) | 320 (98) | .443 |
| Comorbidity – n (%) | 152 (64) | 236 (72) | .069 |
| Hypertension | 119 (50) | 172 (52) | .726 |
| Diabetes | 39 (17) | 51 (16) | .833 |
| Ischaemic heart disease | 15 (6) | 30 (9) | .299 |
| Atrial fibrillation | 26 (11) | 34 (10) | .903 |
| COPD | 16 (7) | 29 (9) | .469 |
| Chronic kidney disease | 10 (4) | 27 (8) | .088 |
| Chronic liver disease | 8 (3) | 23 (7) | .096 |
| Charlsons comorbidity index – m (SD) | 1.1 (1.9) | 1.1 (1.7) | .894 |
| Drug use in the previous 14 days – n (%) | 184 (78) | 270 (82) | .270 |
| Acetaminophen | 34 (14) | 53 (16) | .664 |
| Antibiotics | 54 (23) | 81 (25) | .705 |
| ACE inhibitors | 34 (14) | 69 (21) | .060 |
| ARBs | 41 (17) | 52 (16) | .704 |
| Beta‐blockers | 47 (20) | 71 (22) | .707 |
| Dihydropyridine CCBs | 23 (10) | 41 (13) | .384 |
| Furosemide | 27 (11) | 46 (14) | .447 |
| Statins | 32 (14) | 54 (16) | .416 |
| NSAIDs | 4 (2) | 14 (4) | .143 |
| Antiplatelet agents | 32 (14) | 46 (14) | .984 |
| Oral anticoagulants | 23 (10) | 27 (8) | .624 |
| Others | 93 (39) | 128 (39) | .974 |
| Symptoms – n (%) | |||
| Fever (>38°C) | 205 (87) | 286 (87) | 1.000 |
| Dyspnoea | 98 (42) | 180 (55) | .003 |
| Cough | 139 (59) | 198 (60) | .826 |
| Fatigue | 59 (25) | 79 (24) | .865 |
| Myalgia | 19 (8) | 22 (7) | .651 |
| Nausea/vomiting | 18 (8) | 14 (4) | .127 |
| Diarrhoea | 31 (12) | 40 (13) | .828 |
| Headache | 8 (3) | 11 (3) | 1.000 |
| Dysgeusia | 13 (6) | 20 (6) | .918 |
| Hyposmia | 7 (3) | 13 (4) | .693 |
| Confusion | 15 (6) | 30 (9) | .299 |
| Time from symptoms to hospitalization (days) – M (IQR) | 7 (4‐10) | 8 (5‐11) | .027 |
| Alteration of consciousness – n (%) | 21 (9) | 27 (8) | .890 |
| Body temperature (°C) – m (SD) | 37.7 (0.9) | 37.7 (1.0) | .993 |
| SatO2/FiO2 ratio – M (IQR) | 448 (283‐462) | 343 (218‐452) | <.001 |
| PaO2/FiO2 ratio – M (IQR)§ | 314 (255‐352) | 269 (175‐324) | <.001 |
| Respiratory rate (bpm) – M (IQR) | 20 (16‐22) | 22 (18‐26) | <.001 |
| MAP (mmHg) – m (SD) | 93 (12) | 94 (12) | .397 |
| Heart rate (beats/min)– m (SD) | 83 (14) | 86 (15) | .057 |
| Hb (g/L) – M (IQR) | 13.5 (12.5‐14.4) | 13.4 (12.2‐14.6) | .997 |
| WBC (x109/L) – M (IQR) | 5.0 (3.7‐7.9) | 6.3 (4.5‐8.7) | .001 |
| Neutrophils (x109/L) – M (IQR) | 3.3 (2.3‐6.0) | 4.6 (2.8‐7.0) | <.001 |
| Lymphocytes (x109/L) – M (IQR) | 0.9 (0.6‐1.2) | 0.9 (0.7‐1.2) | .751 |
| Platelets (x109/L) – M (IQR) | 172 (142‐222) | 186 (148‐242) | .027 |
| C‐reactive protein (mg/L) – M (IQR) | 35 (16‐86) | 88 (45‐131) | <.001 |
| Procalcitonin (µg/L) – M (IQR) | 0.07 (0.04‐0.16) | 0.14 (0.07‐0.38) | <.001 |
| INR – M (IQR) | 1.10 (1.05‐1.20) | 1.12 (1.06‐1.24) | .108 |
| D‐Dimer (µg/L) – M (IQR) | 181 (150‐450) | 245 (150‐725) | .028 |
| Fasting glucose (mg/dl) – M (IQR) | 108 (92‐134) | 110 (95‐138) | .170 |
| Serum creatinine (mg/dl) – M (IQR) | 0.90 (0.76‐1.09) | 0.92 (0.78‐1.17) | .400 |
| AST (U/L) – M (IQR) | 28 (22‐34) | 52 (40‐73) | <.001 |
| ALT (U/L) – M (IQR) | 21 (16‐27) | 44 (28‐64) | <.001 |
| GGT (U/L) – M (IQR) | 21 (16‐32) | 56 (34‐94) | <.001 |
| ALP (U/L) – M (IQR) | 53 (42‐70) | 67 (52‐89) | <.001 |
| Bilirubin (mg/dl) – M (IQR) | 0.51 (0.39‐0.70) | 0.58 (0.40‐0.80) | .014 |
| Albumin (g/dl) – m (SD) | 3.4 (0.5) | 3.1 (0.5) | <.001 |
| LDH (U/L) – M (IQR) | 255 (200‐307) | 339 (274‐467) | <.001 |
| CPK (U/L) – M (IQR) | 90 (52‐163) | 129 (67‐285) | <.001 |
| Troponin (ng/L) – M (IQR) | 8 (4‐21) | 15 (5‐42) | .016 |
| Ferritin (µg/L) – M (IQR) | 398 (166‐731) | 876 (474‐1601) | <.001 |
| Lactates (mmol/L) – M (IQR) | 1.1 (0.9‐1.5) | 1.3 (1.0‐1.8) | <.001 |
| SOFA score – m (SD) | 2 (1‐3) | 3 (2‐4) | <.001 |
| SOFA ≥ 2 – n (%) | 138 (64) | 237 (77) | .001 |
| qSOFA score ≥ 2 – n (%) | 14 (7) | 26 (9) | .454 |
| SIRS – n (%) | 118 (52) | 176 (57) | .245 |
| NEWS‐2 score – m (SD) | 3 (3) | 5 (3) | <.001 |
| Chest X‐ray findings – n (%) | |||
| Normal | 37 (16) | 33 (10) | .060 |
| Interstitial pneumonia | 96 (41) | 124 (38) | .528 |
| Ground glass opacity | 12 (5) | 16 (5) | 1.000 |
| Local consolidation | 45 (19) | 59 (18) | .816 |
| Bilateral consolidation | 75 (32) | 143 (44) | .006 |
| Other | 24 (10) | 30 (9) | .784 |
Abbreviations: ACE, angiotensin converting enzyme; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ARB, angiotensin receptor blockers; AST, aspartate aminotransferase; bpm, breaths/min; COPD, chronic obstructive pulmonary disease; CPK, creatine kinase; FiO2, fraction of inspired oxygen; GGT, gamma‐glutamyltransferase; Hb, haemoglobin; INR, international normalized ratio; IQR, interquartile range; LDH, lactate dehydrogenase; m, mean; M, median; MAP, mean arterial pressure; n, number; NEWS‐2, national early warning scale 2; NSAIDs, non‐steroidal anti‐inflammatory agents; PaO2, partial pressure of arterial oxygen; qSOFA, quick sequential organ failure assessment; SatO2, pulse oxygen saturation; SD, standard deviation; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment; WBC, white blood cells.
3.3. COVID‐19 management and clinical course
During clinical course most of patients received antimalarial drugs, either hydroxychloroquine (73%) or chloroquine (16%). Among antiviral drugs 256 patients received lopinavir/ritonavir (45%) and 22 remdesivir (4%). Among antinflammatory drugs, 238 patients received corticosteroids (42%), whereas 69 received tolicizumab (12%). Antibiotics were administered in 87% of patients and the most used were azithromycin (56%), ceftriaxone (50%) and piperacillin tazobactam (24%). Acetaminophen was administered in 52% of patients, NSAIDs in 4%. At the end of observation period 452 patients (80%) were discharged, 19 (3%) were still hospitalized and 94 (17%) died. During hospital stay 83 patients (15%) were transferred to the ICU, 62 (11%) required mechanical ventilation, 48 (9%) required non‐invasive ventilation, 47 (8%) continuous positive airways pressure and 80 (14%) high flow oxygen supplementation. The median time from admission to the transfer to the ICU was 4 (IQR = 2‐6) days. The median length of hospital stay was 10 (IQR = 6‐16) days.
3.4. Outcomes of patients with or without abnormal liver function tests upon admission
Patients with abnormal LFTs had a higher rate of admission to the ICU (20% vs 8%; P < .001) and need for mechanical ventilation (14% vs 6%; P = .005), non‐invasive ventilation (11% vs 5%; P = .021) and vasopressors (14% vs 4%; P < .001) than those without (Table 3). The occurrence of acute kidney injury and the need for renal replacement therapy was significantly higher in patients with abnormal LFTs than those without (22% vs 13%, P = .009; 3% vs 0%, P = .012 respectively). Mortality rate was significantly higher in patients with abnormal LFTs than in those without (21% vs 11%; P = .004). Length of hospital stay was significantly longer in patients with abnormal LFTs. The composite outcome of transfer to ICU or death was significantly higher in patients with abnormal LFTs than those without (36% vs 17%; P < .001). Among patients with abnormal LFTs, those with mixed pattern of abnormalities had more commonly the composite outcome. Abnormalities of AST, ALT, GGT and bilirubin were significantly more frequent in patients with the composite outcome. Characteristics of patients with or without the composite outcome of death or transfer to the ICU are shown in Table 4. Patients with the composite outcome were older, had higher rate of comorbidities, dyspnoea and altered mentation. As expected, SatO2/FiO2 ratios and PaO2/FiO2 were lower in patients with the composite endpoint than in those without. The former had higher grade of inflammation as shown by the higher levels of white blood cell counts, C‐reactive protein and pro‐calcitonin and lower albumin concentration than the latter. SOFA and NEWS‐2 scores were significantly higher in patients admitted to the ICU or death. Finally, bilateral consolidation at chest X‐ray were more common in patients with the composite endpoint than those without.
Table 3.
Outcomes of patients with abnormal liver function tests
| Variable |
Normal liver function tests (N = 236) |
Abnormal liver function tests (N = 329) |
P |
|---|---|---|---|
| Transfer to ICU – n (%) a | 18 (8) | 65 (20) | <.001 |
| Mechanical ventilation – n (%) | 15 (6) | 47 (14) | .005 |
| Non‐invasive ventilation – n (%) | 12 (5) | 36 (11) | .021 |
| High flow nasal cannula ventilation – n (%) | 27 (11) | 53 (16) | .148 |
| Continuous positive airway pressure ventilation – n (%) | 18 (8) | 29 (9) | .613 |
| Acute kidney injury – n (%) | 30 (13) | 71 (22) | .009 |
| Renal replacement therapy – n (%) | 0 (0) | 9 (3) | .012 |
| Vasopressors use – n (%) | 10 (4) | 45 (14) | <.001 |
| Multi organ failure – n (%) | 5 (2) | 18 (5) | .053 |
| Mortality – n (%) b | 26 (11) | 68 (21) | .004 |
| Transfer to ICU or death – n (%) a | 40 (17) | 117 (36) | <.001 |
| Length of stay (days) – M (IQR) b | 9 (6‐15) | 11 (7‐18) | .003 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; M, median; n, number.
7 patients still hospitalized and not admitted to ICU at the end of the study.
19 patients still hospitalized at the end of the study.
Table 4.
Characteristics of patients with a composite outcome of death or transfer to ICU during the hospitalization
| Variables |
No composite outcome (N = 408) |
Composite outcome (N = 157) |
P |
|---|---|---|---|
| Age (years) – m (SD) | 63 (15) | 74 (13) | <.001 |
| Gender (male) – n (%) | 252 (62) | 105 (67) | .302 |
| Ethnicity (caucasian) – n (%) | 391 (96) | 155 (99) | .147 |
| Comorbidity – n (%) | |||
| Hypertension | 254 (62) | 134 (85) | <.001 |
| Diabetes | 190 (47) | 101 (64) | <.001 |
| Ischaemic heart disease | 57 (14) | 33 (21) | .055 |
| Atrial fibrillation | 23 (6) | 22 (14) | .002 |
| COPD | 32 (8) | 28 (18) | .001 |
| Chronic kidney disease | 22 (5) | 23 (15) | .001 |
| Chronic liver disease | 14 (3) | 23 (15) | <.001 |
| Charlson comorbidity index – m (SD) | 0.8 (1.5) | 2.0 (2.2) | <.001 |
| Symptoms – n (%) | |||
| Fever (>38°C) | 360 (88) | 131 (83) | .130 |
| Dyspnea | 179 (44) | 99 (63) | <.001 |
| Cough | 255 (63) | 82 (52) | .033 |
| Fatigue | 106 (26) | 32 (20) | .201 |
| Myalgia | 31 (8) | 10 (6) | .747 |
| Nausea/vomiting | 27 (5) | 7 (3) | .168 |
| Diarrhoea | 61 (15) | 10 (6) | .009 |
| Headache | 17 (4) | 2 (1) | .148 |
| Dysgeusia | 30 (7) | 3 (2) | .023 |
| Hyposmia | 20 (5) | 0 (0) | .010 |
| Confusion | 21 (5) | 24 (15) | <.001 |
| Time from symptoms to hospitalization (days) – M (IQR) | 8 (5‐11) | 6 (4‐10) | .003 |
| Alteration of consciousness – n (%) | 25 (6) | 23 (15) | .002 |
| Body temperature (°C) – m (SD) | 37.7 (0.9) | 37.5 (1.0) | .064 |
| SatO2/FiO2 ratio – M (IQR) | 448 (291‐462) | 263 (125‐399) | <.001 |
| PaO2/FiO2 ratio – M (IQR) | 314 (261‐351) | 192 (119‐282) | <.001 |
| Respiratory rate (bpm) – M (IQR) | 20 (16‐24) | 24 (20‐30) | <.001 |
| MAP (mmHg) – m (SD) | 94 (12) | 93 (12) | .435 |
| Heart rate (beats/min) – m (SD) | 84 (14) | 88 (15) | .013 |
| Hb (g/L) – M (IQR) | 13.6 (12.6‐14.6) | 12.8 (11.7‐14.3) | .001 |
| WBC (x109/L) – M (IQR) | 5.4 (3.9‐7.7) | 7.1 (4.7‐10.8) | <.001 |
| Neutrophils (x109/L) – M (IQR) | 3.6 (2.4‐5.7) | 5.8 (3.2‐9.4) | <.001 |
| Lymphocytes (x109/L) – M (IQR) | 1.0 (0.8‐1.3) | 0.7 (0.5‐1.0) | <.001 |
| Neutrophils/lymphocytes ratio (x109/L) – M (IQR) | 3.9 (2.2‐6.6) | 7.3 (4.2‐14.2) | <.001 |
| Platelets (x109/L) – M (IQR) | 183 (148‐237) | 174 (142‐222) | .221 |
| C‐reactive protein (mg/L)M (IQR) | 52 (21‐106) | 104 (57‐159) | <.001 |
| Procalcitonin (µg/L) —M (IQR) | 0.08 (0.04‐0.16) | 0.31 (0.12‐1.05) | <.001 |
| INR—M (IQR) | 1.10 (1.05‐1.20) | 1.15 (1.07‐1.30) | <.001 |
| D‐Dimer (µg/L)— M (IQR) | 179 (150‐391) | 385 (159‐1295) | <.001 |
| Fasting glucose (mg/dl)—M (IQR) | 106 (92‐126) | 120 (103‐167) | <.001 |
| Serum creatinine (mg/dl)— M (IQR) | 0.89 (0.75‐1.04) | 1.06 (0.86‐1.41) | <.001 |
| Abnormal LFTs—n (%) | 212 (52) | 117 (75) | <.001 |
| Abnormal AST—n (%) | 149 (37) | 100 (64) | <.001 |
| Abnormal ALT—n (%) | 119 (29) | 60 (38) | .038 |
| Abnormal GGT—n (%) | 124 (30) | 68 (43) | .004 |
| Abnormal ALP—n (%) | 17 (4) | 12 (8) | .093 |
| Abnormal bilirubin—n (%) | 23 (6) | 22 (14) | .001 |
| Pattern of alteration of LFTs—n (%) a | |||
| Normal | 196 (48) | 40 (26) | <.001 |
| Hepatocellular | 124 (31) | 60 (39) | |
| Cholestatic | 55 (14) | 24 (16) | |
| Mixed | 32 (8) | 33 (20) | |
| Extent of LFTs abnormality—n (%) | |||
| Normal | 205 (50) | 44 (28) | <.001 |
| 1‐1.99 ULN | 135 (33) | 65 (41) | |
| 2‐2.99 ULN | 41 (10) | 32 (20) | |
| ≥3 ULN | 27 (7) | 16 (10) | |
| Albumin (g/dl)— m (SD) | 3.4 (0.5) | 2.9 (0.5) | <.001 |
| LAD (U/L)— M (IQR) | 280 (224‐343) | 378 (300‐502) | <.001 |
| CPK (U/L)—M (IQR) | 98 (57‐186) | 140 (69‐378) | .007 |
| Troponin (ng/L)— M (IQR) | 9 (3‐19) | 35 (12‐104) | <.001 |
| Ferritin (µg/L)— M (IQR) | 594 (263‐1047) | 906 (483‐1877) | <.001 |
| Lactates (mmol/L)— M (IQR) | 1.1 (0.9‐1.5) | 1.4 (1.2‐2.1) | <.001 |
| SOFA score—m (SD) | 2 (1‐3) | 4 (3‐5) | <.001 |
| SOFA ≥ 2—n (%) | 244 (65) | 131 (89) | <.001 |
| qSOFA score ≥ 2—n (%) | 17 (5) | 23 (16) | <.001 |
| SIRS—n (%) | 212 (55) | 82 (55) | .988 |
| NEWS‐2 score—m (SD) | 4 (3) | 6 (3) | <.001 |
| Chest X‐ray findings—n (%) | |||
| Normal | 61 (15) | 9 (6) | .005 |
| Interstitial pneumonia | 154 (38) | 66 (42) | .400 |
| Ground glass opacity | 20 (5) | 8 (5) | 1.000 |
| Local consolidation | 73 (18) | 31 (20) | .698 |
| Bilateral consolidation | 142 (35) | 76 (48) | .004 |
| Other | 37 (9) | 17 (11) | .633 |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; bpm, breaths/min; COPD, chronic obstructive pulmonary disease; CPK, creatine kinase; FiO2, fraction of inspired oxygen; Hb, haemoglobin; INR, international normalized ratio; IQR, interquartile range; LDH, lactate dehydrogenase; LFTs, liver function tests; m, mean; M, median; MAP, mean arterial pressure; n, number; NEWS‐2, national early warning scale 2NSAIDs, non‐steroidal anti‐inflammatory agents; PaO2, partial pressure of arterial oxygen; qSOFA, quick sequential organ failure assessment; SatO2, pulse oxygen saturation; SD, standard deviation; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment; WBC, white blood cells.
patients with isolated abnormality of bilirubin excluded (n = 4).
We performed a multivariate logistic regression analysis to show whether abnormal LFTs were independently associated to the composite outcome. We explored two models to avoid multicollinearity among variables and scores of clinical severity (Table 5). In the first model (adjusted for age, gender, Charlson comorbidity index, NEWS‐2 score, neutrophils/lymphocytes ratio, C‐reactive protein, serum albumin and bilateral consolidation at X‐ray) abnormal LFTs were independently associated with the risk of the composite outcome of death or transfer to the ICU (OR = 3.53; 95% CI = 1.97‐6.35; P < .001).). In the second model (adjusted for age, gender, Charlson comorbidity index, SOFA score, RR, HR, neutrophils/lymphocytes ratio, C‐reactive protein, serum albumin and bilateral consolidation at X‐ray) abnormal LFTs were independently associated with the risk of the composite outcome (OR = 4.00; 95% CI = 2.15‐7.44; P < .001). Even in patients with 1 or 0 point in respiratory component of SOFA score (ie PaO2/FiO2 ratio ≥ 300 and/or a SatO2/FiO2 ratio ≥357; n = 300), those with abnormal LFTs were more likely to have the composite outcome (22% vs 8%; P = .002; Figure 2). Figure 3 shows the probability of developing the composite outcome of death or transfer to ICU in patients with or without abnormal LFTs. Among patients with abnormal LFTs, those with mixed pattern of abnormalities had the highest probability to be transferred to ICU (75%), whereas those with a cholestatic or hepatocellular pattern had an intermediate probability (63% and 64% respectively) and those with normal LFTs the lowest (40%; Figure S1).
Table 5.
Adjusted analysis of predictors of a composite outcome of death or transfer to ICU during the hospitalization
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Model 1 | |||
| Age (years) | 1.03 | 1.01‐1.05 | .003 |
| Gender (male) | 1.45 | 0.83‐2.53 | .195 |
| Charlson comorbidity index | 1.23 | 1.05‐1.43 | .009 |
| NEWS‐2 score | 1.15 | 1.04‐1.27 | .005 |
| Neutrophils/lymphocytes ratio (×109/L) a | 1.45 | 1.02‐2.06 | .037 |
| C‐reactive protein (mg/L)† | 1.04 | 0.74‐1.45 | .834 |
| Serum albumin (g/L) | 0.48 | 0.26‐0.87 | .016 |
| Bilateral consolidation at X‐ray | 0.83 | 0.48‐1.42 | .828 |
| Abnormal liver function tests | 3.53 | 1.97‐6.35 | <.001 |
| Model 2 | |||
| Age (years) | 1.03 | 1.01‐1.05 | .012 |
| Gender (male) | 1.42 | 0.80‐2.52 | .236 |
| Charlson comorbidity index | 1.21 | 1.03‐1.42 | .021 |
| SOFA score | 1.30 | 1.10‐1.54 | .002 |
| Respiratory rate (breath/min) a | 4.35 | 1.43‐13.17 | .009 |
| Heart rate (beat/min) | 1.01 | 0.99‐1.03 | .196 |
| Neutrophils/lymphocytes ratio (×109/L) a | 1.38 | 0.97‐1.98 | .075 |
| C‐reactive protein (mg/L) a | 1.00 | 0.71‐1.40 | .976 |
| Serum albumin (g/L) | 0.58 | 0.31‐1.08 | .083 |
| Bilateral consolidation at X‐ray | 0.73 | 0.42‐1.30 | .288 |
| Abnormal liver function tests | 4.00 | 2.15‐7.44 | <.001 |
Abbreviations: CI, confidence interval; NEWS‐2, national early warning scale 2; OR, odds ratio; SOFA, sequential organ failure assessment.
variables log transformed to be included in the analysis.
Figure 2.
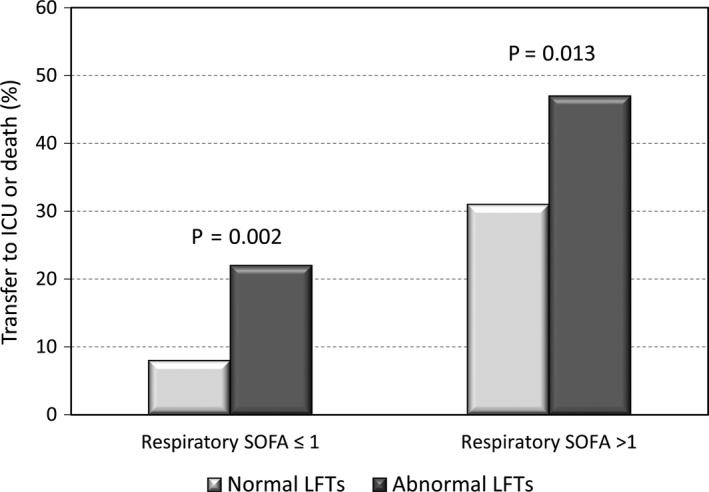
Composite outcome of transfer to intensive care unit or death according to abnormality of liver function tests and the baseline respiratory component of SOFA score. Legend: ICU, intensive care unit; LFTs, liver function tests; SOFA, sequential organ failure assessment
Figure 3.
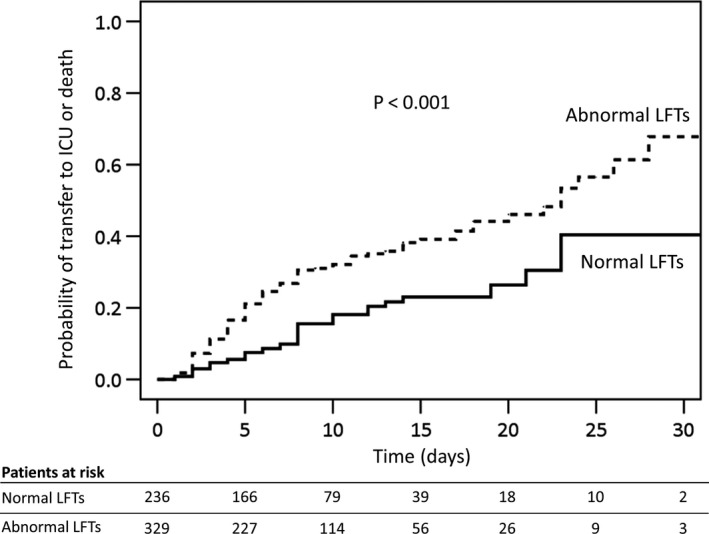
Probability of the composite outcome of transfer to intensive care unit or death according to abnormality of liver function tests. Legend: ICU, intensive care unit; LFTs, liver function tests
3.5. Characteristics and significance of abnormal liver function tests during the hospitalization
Follow‐up data on LFTs were available for 389 patients on day 7 ± 2, 14 ± 2 or 21 ± 2. Eighty‐six out of 153 patients with normal LFTs upon admission, developed de novo abnormalities of LFTs, thus, 415 patients (74%) had abnormal LFTs during the hospitalization. De novo abnormalities of LFTs were more commonly observed among patients receiving tocilizumab (82% vs 52%; P = .009), acetaminophen (63% vs 47%; P = .048), piperacillin/tazobactam (72% vs 50%; P = .013) and lopinavir/ritonavir (64% vs 48%; P = .045), whereas antimalarial drugs (57% vs 53%; p = NS) and azithromycin (55% vs 58%; p = NS) were not associated with de novo abnormalities of LFTs. Tocilizumab, lopinavir/ritonavir and acetaminophen were associated with hepatocellular (71%, 67% and 68% respectively) or mixed pattern (29%, 17% and 18% respectively) of de novo abnormalities. Among patients not yet admitted to ICU before reassessment of LFTs, those with de novo abnormalities of LFTs had a non‐significant trend to a higher rate of the composite endpoint (14% vs 5%; P = .069). Overall, the alteration of LFTs was below 2 times ULN in 33%, between 2‐3 times ULN in 18% and above 3 times ULN in 23% of patients. Fifty‐six patients (10%) had an alteration of LFTs >5 times ULN, 12 patients (2%) >10 times ULN. One patient (0.2%) developed a fulminant liver failure with encephalopathy and coagulopathy and died within 7 days. He had taken amoxicillin/clavulanic acid and acetaminophen the 14 days before the admission.
4. DISCUSSION
Abnormal LFTs have been commonly observed in patients with COVID‐19 although the clinical significance and the mechanism of liver injury is still to be clearly determined. Data from China showed higher levels of AST and ALT in patients with severe COVID‐19. 2 , 3 , 5 , 8 , 9 , 12 Most of these studies were not specifically designed to assess LFTs abnormalities in patients with COVID‐19 and did not take into account potential confounders, such as previous liver disease and/or concomitant treatments. Others considered abnormal LFTs as a whole during the hospitalization, a strategy that cannot answer to a relevant question: do patients with abnormal LFTs at admission have different clinical course than those with normal LFTs?
Herein, we showed that abnormalities of LFTs are observed in 58% of patients with COVID‐19 at admission to the hospital and they are independently associated to a composite endpoint of transfer to the ICU or death, in particular when the pattern of alteration is mixed. Thus, on clinical ground abnormal LFTs at admission should be considered as a marker of disease severity and should lead physicians to closely follow‐up these patients and to be prepared for potential rapid worsening of clinical conditions. This could anticipate the potentially need for ICU beds, which is relevant considering the shortage observed in some regions during COVID‐19 pandemic. 23
The abnormality of LFTs in patients with COVID‐19 can be caused by several mechanisms. SARS‐CoV‐2 binds to target cells through angiotensin‐converting enzyme 2, which occurs abundantly on liver and biliary epithelial cells. 24 Thus, the liver is a potential target for infection and abnormal LFTs may reflect a direct virus‐induced cytopathic effect. Although a postmortem histopathological study demonstrated the presence of viral genome in the liver, others did not confirm these results. 25 , 26 However, immune damage from the provoked inflammatory response is a potential concomitant or alternative mechanism by which the disease can cause liver injury. 7 Although the direct cytopathic effect is still to be determined, herein we showed that patients with abnormal LFTs had a more severe systemic inflammation as shown by the higher levels of leucocytes, neutrophils, C‐reactive protein and ferritin. Abnormal LFTs could also represent a sign of systemic involvement of the disease, indeed we found higher levels of SOFA score and markers of muscle and heart injury in patients with abnormal LFTs. Thus, the worse clinical course in patients with abnormal LFTs can be either because of the systemic spread of the virus to targeted organs other than the lungs, such as the liver or because of the severe systemic inflammatory response. Noteworthy, AST, which was the most commonly abnormal LFT, was strongly correlated with ALT and to a less extent to CPK, reasonably suggesting that AST abnormalities reflected a hepatic injury. This findings are in keeping with Bloom et al 27 One may correctly argue that respiratory failure is the main marker of severity and driver of transfer to ICU or death in patients with COVID‐19, however, we found that even in patients with adequate PaO2/FiO2 or SatO2/FiO2 ratio, abnormal LFTs are associated with the composite outcome, suggesting that they can precede the worsening of respiratory function.
During the hospitalization, de novo occurrence of LFTs abnormality was common, and associated with the use of potentially hepatotoxic drugs, such as lopinavir/ritonavir, tocilizumab, acetaminophen and antibiotics. Therefore, the ability of abnormal LFTs to predict transfer to ICU or death was dampened during the hospitalization, because of these relevant confounders. Our findings also highlight the need for well‐designed studies to prove the efficacy of antiviral and anti‐inflammatory drugs, which have been broadly used for treating COVID‐19, without clear evidence of their efficacy and with potential side effects. 28 , 29 One of the patients with COVID‐19 and abnormal LFTs had fulminant liver failure, however, we could not rule out the role of drug‐induced liver injury related to the previous administration of amoxicillin/clavulanic acid and/or acetaminophen.
Our study has strengths and limitation. It is one of largest studies specifically designed to assess prevalence and clinical impact of LFTs abnormality and the first one performed in Europe. We considered several potential confounders, including comorbidities and concomitant drugs. Among limitations, this is a retrospective study and other potential confounders could have been missed. We had no data on preadmission LFTs, therefore we cannot exclude that some patients without a diagnosis of chronic liver disease actually had LFTs abnormalities before COVID‐19. Furthermore, we did not collect data on dyslipidaemia as comorbidity. We decided to exclude patients admitted to the ICU within 12 hours from admission to the emergency room, because they were likely to be already critically ill at admission and, on clinical ground, abnormality of LFTs were expected to be useless in these patients. Liver biopsy specimen were not collected, thus the clear cytopathic effects of the virus on the liver could not be directly demonstrated. Finally, long‐term outcomes on liver health of patients with COVID‐19 and abnormal LFTs were not available.
In conclusion, we showed that LFTs abnormality is commonly observed on admission in patients with COVID‐19 and it is associated with systemic inflammation, organ dysfunction and is an independent predictor of transfer to the ICU or death during the hospitalization. Patients with LFTs abnormality at admission should be closely followed up for a potential worse outcome.
5. ETHICS STATEMENT
Permission for retrospective data analyses was obtained from local Ethics Committee. Verbal or written informed consent was obtained from patients surviving hospitalization. The study was performed according to the ethical guidelines of the Declaration of Helsinki (seventh revision).
CONFLICT OF INTERESTS
The authors states that they have no conflicts of interest regarding the content of this manuscript.
AUTHORS’ CONTRIBUTION
SP contributed to study concept and design, development of electronic case report form, data collection, analysis and interpretation of data, drafting of the manuscript and study supervision. AD, EV, DB, MM, AndM, RS, CGG, VF, LC, AnnM and MML involved in collection of data. AC, AD, EV, DB, MM, AndM, PA, RV and RS provided critical revision for important intellectual content. PA and RV also involved in study supervision and drafting of the manuscript.
Supporting information
Supplementary Material
Appendix 1.
COVID‐LIVER STUDY GROUP COLLABORATORS
Gaetano Arcidiacono, Department of Medicine – DIMED, University of Padova, Padova, Italy; Silvia Bettini, Department of Medicine – DIMED, University of Padova, Padova, Italy; Benedetta M. Bonora, Department of Medicine – DIMED, University of Padova, Padova, Italy; Giovanni Bucca, Department of Medicine – DIMED, University of Padova, Padova, Italy; Armando Gabrielli, Dipartimento di Scienze Cliniche e Molecolari, Università Politecnica delle Marche, Ancona, Italy; Francesca Ghirardini, Department of Medicine – DIMED, University of Padova, Padova, Italy; Umberto Gnudi, UOC Pronto Soccorso e Medicina d'Urgenza, Azienda Ospedaliera Ospedali Riuniti Marche Nord, Pesaro, Italy; Sara Mareso, Internal Medicine Unit, Dell'Angelo Hospital, ULSS3 Serenissima, Mestre, Italy; Marco Marini, Dipartimento di Scienze Cardiovascolari, AOU Ospedali Riuniti "Umberto I‐GM Lancisi‐G.Salesi", Ancona, Italy; Luciano Mucci, UOC Medicina Interna, Azienda Ospedaliera Ospedali Riuniti Marche Nord, Pesaro, Italy; Gian P. Perna, Dipartimento di Scienze Cardiovascolari, AOU Ospedali Riuniti "Umberto I‐GM Lancisi‐G.Salesi", Ancona; Fabio Presotto, Internal Medicine Unit, Dell'Angelo Hospital, ULSS3 Serenissima, Mestre, Italy; Caterina Sensi, Department of Medicine – DIMED, University of Padova, Padova, Italy; Francesco Soliani, Department of Medicine, University and Azienda Ospedaliera Universitaria Integrata of Verona, Verona, Italy; Roberta Stupia, Department of Medicine, University and Azienda Ospedaliera Universitaria Integrata of Verona, Verona, Italy; Mirko Zoncapè, Department of Medicine, University and Azienda Ospedaliera Universitaria Integrata of Verona, Verona, Italy.
Piano S, Dalbeni A, Vettore E, et al; the COVID-LIVER study group . Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19. Liver Int. 2020;40:2394–2406. 10.1111/liv.14565
Roberto Vettor and Paolo Angeli share senior authorship
Handling Editor: Luca Valenti
Funding information
No specific grant supported this study. The Department of Medicine of the University of Padova provided the website facilities for the electronic data capture.
REFERENCES
- 1. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan W, Ni Z, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020; published online Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu BO, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus‐Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020; published online March 26. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang C, Shi L, Wang F‐S. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020; published online March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020; published online March 14. 10.1111/liv.14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai Q, Huang D, Yu H, et al. COVID‐19: Abnormal liver function tests. J Hepatol. 2020; published online May 2. 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta‐analysis. Liver Int. 2020; published online April 4. 10.1111/liv.14465 [DOI] [PubMed] [Google Scholar]
- 11. Lei F, Liu Y‐M, Zhou F, et al. Longitudinal association between markers of liver injury and mortality in COVID‐19 in China. Hepatology. 2020; published online May 2. 10.1002/hep.31301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu C, Chen X, Cai Y, et al. Risk factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020; published online March 13. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou F, Yu T, Du R, et al. Articles Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China : a retrospective cohort study. Lancet. 2020;6736:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID‐19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020; published online April 2. 10.1111/liv.14455 [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected. 2020; 1‐21.
- 16. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA. 2016;315:801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Royal College of Physicians . National Early Warning Score (NEWS) 2: Standardizing the assessment of acute‐illness severity in the NHS. London RCP: Updated report of a working party; 2017. [Google Scholar]
- 18. Pandharipande PP, Shintani AK, Hagerman HE, et al. Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit Care Med. 2009;37:1317‐1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Newsome PN, Cramb R, Davison SM, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67:6‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2:1‐138. [Google Scholar]
- 23. Carenzo L, Costantini E, Greco M, et al. Hospital surge capacity in a tertiary emergency referral centre during the COVID‐19 outbreak in Italy. Anaesthesia. 2020; published online April 4. 10.1111/anae.15072 [DOI] [PubMed] [Google Scholar]
- 24. Chai X, Hu L, Zhang Y, et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019‐nCoV Infection. bioRxiv 2020. 2020;02(03):931766. [Google Scholar]
- 25. Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID‐19) through postmortem core biopsies. Mod Pathol. 2020. 10.1038/s41379-020-0536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bloom PP, Meyerowitz EA, Reinus Z, et al. Liver Biochemistries in Hospitalized Patients With COVID‐19. Hepatology. 2020; published online May. 10.1002/hep.31326 [DOI] [PubMed] [Google Scholar]
- 28. Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir‐Ritonavir in Adults Hospitalized with Severe Covid‐19. N Engl J Med. 2020; published online March 18. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020; published online May 2. 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


