Abstract
Background
A pandemic outbreak of COVID‐19 has been sweeping the world since December. It begins as a respiratory infection that, mainly in men with diabetes or renal impairment, evolves into a systemic disease, with SARDS, progressive endothelial cell damage, abnormal clotting and impaired cardiovascular and liver function. Some clinical trials are testing biological drugs to limit the immune system dysregulation, “cytokines storm,” that causes the systemic complications of COVID‐19. The contraindications of these drugs and their cost raise concerns over the implications of their widespread availability.
Objectives
Numerous clinical and experimental studies have revealed a role for the nitric oxide (NO)‐cyclic GMP‐phosphodiesterase type 5 (PDE5) pathway in modulating low‐grade inflammation in patients with metabolic diseases, offering cardiovascular protection. PDE5 inhibition favors an anti‐inflammatory response by modulating activated T cells, reducing cytokine release, lowering fibrosis, increasing oxygen diffusion, stimulating vascular repair. PDE5 is highly expressed in the lungs, where its inhibition improves pulmonary fibrosis, a complication of severe COVID‐19 disease.
Materials and methods
We performed a systematic review of all evidence documenting any involvement of the NO‐cGMP‐PDE5 axis in the pathophysiology of COVID‐19, presenting the ongoing clinical trials aimed at modulating this axis, including our own “silDEnafil administration in DiAbetic and dysmetaboLic patients with COVID‐19 (DEDALO trial).”
Results
The reviewed evidence suggests that PDE5 inhibitors could offer a new strategy in managing COVID‐19 by (i) counteracting the Ang‐II‐mediated downregulation of AT‐1 receptor; (ii) acting on monocyte switching, thus reducing pro‐inflammatory cytokines, interstitial infiltration and the vessel damage responsible for alveolar hemorrhage‐necrosis; (iii) inhibiting the transition of endothelial and smooth muscle cells to mesenchymal cells in the pulmonary artery, preventing clotting and thrombotic complications.
Discussion and Conclusion
If the ongoing trials presented herein should provide positive findings, the low cost, wide availability and temperature stability of PDE5 inhibitors could make them a major resource to combat COVID‐19 in developing countries.
Keywords: PDE5 inhibitors, cytokine storm, IL‐6, interstitial pneumonia, type 2 diabetes mellitus, pulmonary fibrosis
1. INTRODUCTION
COVID‐19 is now a worldwide pandemic, with exceeded 2 millions of confirmed cases worldwide and a high mortality rate among the minority of people with COVID‐19 who get severe disease (60.5% mortality for critical cases). 1 , 2 , 3
Up to 20% of infected patients develop severe pneumonia evolving into severe acute respiratory syndrome (SARS), ending with multiple organ failure. 1 , 3 , 4 The two most frequent comorbidities in deceased patients were diabetes mellitus and hypertension. 5 This is extremely important given that over 463 million people worldwide have diabetes. 6
Data from the Italian cohort highlight a significantly higher number of infections (approximately 4:1) and a higher fatality in men than in women: 8% of men died, compared with 5% of women (ISS ‐ Higher Health Institute of Rome analysis of 25 058 cases)(Integrated surveillance of COVID‐19 in Italy, https://www.epicentro.iss.it/coronavirus/).
The clinical worsening is apparently related to a hyperinflammation with excessive release of proinflammatory cytokines (TNF‐alfa, IL‐6), the cytokine storm, culminating in loss of control, immunosuppression, reduction in memory CD4+ T helper cells 7 , 8 , 9 , 10 and severe lung damage. 11 This may subsequently evolve to fibrosis, a potential sequela even after viral propagation has attenuated.
Comorbidities play a role in hampering the initial protective immune response, helping the virus spread, and facilitating the more severe phase through their previous dysregulation of the immune system.
The current designed protocols, including the use of chloroquine and tocilizumab for proinflammatory cytokine modulation, have significant side effects. 12 , 13
In developing a new therapeutic strategy, it is reasonable to attempt to boost immune response during the first immune‐mediated phase while suppressing the final cytokine‐mediated phase, 8 , 14 , 15 preventing progression from mild to severe form and mitigating fibrosis in patients overcoming the acute stage. 16 , 17
Androgen sensitivity could be a determinant of COVID‐19 disease severity. The androgen sensitivity model might explain why males are more likely to develop severe symptoms whereas children seem to be resistant to infection. Androgen sensitivity is determined by genetic variants of the androgen receptor which regulates transcription of the transmembrane protease, serine 2 (TMPRSS2). This is required for SARS‐CoV‐2 infectivity: Cell entry of SARS‐CoV‐2 depends on binding of the viral spike proteins to ACE2. ACE2 is a functional receptor for SARS‐CoV‐2. 18 TMPRSS2 primes the Spike protein of the virus, which has two consequences: diminishing viral recognition by neutralizing antibodies and activating SARS‐CoV‐2 for virus‐cell fusion. 19 , 20
This has led to the intuition that TMPRSS2 inhibition may work to block or decrease the severity of SARS‐CoV‐2 infections and a recent study on almost 5 thousands COVID‐19 patients demonstrated that those receiving androgen‐deprivation therapy (ADT) had a significantly lower risk of SARS‐CoV‐2 infection compared with patients who did not receive ADT (OR 4.05). 20
Given the presence of ACE2 receptor in testes also, COVID‐19 infectious‐related orchitis was examined and reported. 21 Although the presence of SARS‐CoV‐2 RNA in semen samples was reported in some, 22 but not all the studies, 23 data provide potential clues for further medical pathogenesis of COVID‐19‐related male infertility in infected patients.
The use of nitric oxide (NO) to treat COVID‐19‐related interstitial lung disease was approved by the FDA a few weeks ago (as reported by Bellerophon CEO Fabian Tenenbaum to BioWorld), 24 , 25 as already described in literature. 26 , 27
Phosphodiesterase type‐5 inhibitors (PDE5i) such as sildenafil were approved 20 years ago for the treatment of male erectile dysfunction, but they were originally tested as alternatives to nitrates for the relief of angina, as they enhance the nitric oxide/cyclic guanosine monophosphate (NO/cGMP) signaling pathway 28 , 29 with pleiotropic systemic effects.
Two different meta‐analyses on chronic oral use of different formulation of PDE5i have shown reduced mortality 30 and a good safety profile 31 in patients with type 2 diabetes mellitus (T2DM) and high cardiovascular risk, endorsing the beneficial cardioprotective properties and inflammatory cytokine modulation previously demonstrated in the CECSID trial after chronic (3 months) oral sildenafil (100 mg/daily) on T2DM patients with diabetic cardiomiopathy. 32 , 33 To note, as hypogonadism frequently complicates T2DM resulting in severe endothelial dysfunction, in these context PDE5i efficacy on sexual function could be reduced, given the testosterone regulation of PDE5 expression as one of the major mechanisms controlling vasodilator mechanisms in penile tissue. 34 , 35 , 36 , 37 , 38
Aside from pre‐clinical studies demonstrating respiratory and renal protective effects of PDE5i, 39 , 40 both acute 41 and chronic administration of oral sildenafil at different dose in human have confirmed the benefit on pulmonary compliance as well as anti‐remodeling effects, 31 , 39 , 40 , 42 , 43 , 44 especially in T2DM, where they improve the endothelial dysfunction that underpins various metabolic, cardiovascular and inflammatory diseases.
Chronic (6 months) PDE5 inhibition through vardenafil 10 mg/die has been shown to preserve endothelial function in T2DM patients, 45 probably due to an increase in angiogenic mediators, including Ang1. 46 These effects seem particularly important given that neutrophils isolated from patients with T2DM release larger amounts of proinflammatory cytokines, above all TNF‐α and IL‐6, 47 main players of the cytokine storm seen in COVID‐19 infection.
In mouse models of diabetes, sildenafil restores normal levels of circulating TIE2‐expressing monocytes (TEMs), limiting inflammation, and promoting tissue repair. 46 , 47 , 48 These findings were then confirm in T2DM patients where 3 months of oral sildenafil (100 mg/day) reduced the endothelial function marker P‐selectin. 49 In pre‐clinical, as well as in clinical studies using chronic PDE5i with sildenafil 100 mg daily, we and others have shown that the NO‐cGMP‐PDE5 pathway and related targets are involved in the pathogenesis of several complications of diabetes affecting the immune system, 46 , 47 , 48 adipose tissue, 50 , 51 and renal function. 39 A dose of 50mg of Sildenafil, given twice a week, was shown to improve surrogate markers of endothelial function (C‐reactive protein, endothelin‐1 and ICAM‐1, ANG‐1/TIE2 axis), modulating the number of circulating proangiogenic cells and exerting an anti‐inflammatory response in T2Dm patients. 52
PDE5 is predominantly expressed in the lungs, the organ most affected by COVID‐19.
Its expression in men 53 is even higher, making them more responsive to PDE5i, as revealed in pulmonary hypertension (PH) and fibrosis. 54 , 55 , 56 Sildenafil is approved for the treatment of PH and is currently indicated in patients with WHO functional class II and III PH. The approved posology is 20 mg three times per day. The use of PDE5i to improve endothelial function by the NO‐cGMP pathway could help repair alveolar‐vascular interface damage, 57 thus improving O2 diffusion, as demonstrated by 12 weeks treatment with sildenafil (20 mg/three times per day) in patients with idiopathic pulmonary fibrosis (IPF). 57 , 58 These studies documented the absence of a direct relaxant vascular effect, while an anti‐contractile and anti‐remodeling effect of sildenafil explaining its beneficial effects. The same mechanisms are proposed as the rationale for using inhaled NO for interstitial lung disease caused by COVID‐19. 57
Abnormal clotting and thrombosis in COVID‐19 patients significantly affect the incidence of complications and are one of the most important variables associated with mortality so much so that the prevention of venous thromboembolism in SARS‐CoV‐2 subjects has been recommended by the World Health Organization. Again, this evidence supports the use of sildenafil in COVID‐19 infected patients, as its role in inhibiting neointimal formation and platelet aggregation via the NO/cGMP/PKG pathway is well recognized. 59 Pro‐thrombotic clotting abnormalities are more evident in diabetics, and literature data suggest that the positive modulation by sildenafil of cytokines, inflammatory, and endothelial markers may have a beneficial effect on clotting in such patients. 60
We performed a systematic review to identify ongoing trials of COVID‐19 patients that target the NO‐cGMP‐PDE5 axis, finding 6 out of 1717 registered studies (https://clinicaltrials.gov).
We performed a web search on https://clinicaltrials.gov/ with COVID as fixed "condition or disease" search term and "sildenafil," "tadalafil," "vardenafil," "avanafil," "PDE5 inhibitor," "phosphodiesterase inhibitor" alternatively used as other terms.
Five of these focus on different NO inhalation therapy regimens, respectively, exploring: (a) inhaled NO (140‐300 ppm for 20‐30 minutes) versus placebo in 260 subjects (recruitment ended) to prevent the deterioration of mild COVID‐19 infection (NCT04338828); (b) inhaled NO (140 ‐ 180 ppm for 20‐30 minutes for 14 days) versus control in 240 subjects (recruiting) to prevent deterioration from mild to severe COVID‐19 infection (NCT04305457); (c) inhaled NO (160 ppm for 15 minutes) versus control in 470 healthcare professionals dedicated to care for patients with proven SARS‐CoV‐2 infection (not yet recruiting) to estimate the percentage of subjects with a positive test in the two groups (NCT04312243); (d) inhaled NO (concentration 80 ppm for 48 hours, followed by 40 ppm, followed by weaning before stop) versus control in 200 subjects (recruiting) to measure changes in arterial oxygenation at 48 hours from enrollment (NCT04306393); (e) inhaled NO (160 ppm for 26 days) in 20 subjects (recruiting) to measure the safety of enrolled patients (NCT03331445): Interestingly, this trial, which explores a new target therapy for Corona‐like virus lung infections, was first posted on November 6, 2017. The final trial (NCT04304313) is a pilot study exploring the effects of sildenafil 0.1 g/day for 14 days in 10 subjects, with the rate of disease remission as the primary outcome. Table S1 summarizes these studies.
Given these evidences, we believe that the administration of sildenafil, as an adjunct to the current standard protocols, to hospitalized men with metabolic syndrome and/or T2DM who have confirmed SARS‐CoV‐2 infection and mild‐to‐severe symptoms of COVID‐19 may offer a therapeutic benefit by targeting inflammation and fibrosis, and hence reducing the progression of lung disease and associated systemic complications. Our hypothesis is that sildenafil could act by: (a) counteracting the Ang‐II‐mediated downregulation of AT‐1 receptor (initial first phase); (b) acting on monocyte switching, thus reducing pro‐inflammatory cytokines, interstitial infiltration and the vessel damage responsible for alveolar hemorrhage‐necrosis; (c) inhibiting the transition of endothelial and smooth muscle cells to mesenchymal cells in the pulmonary artery by inhibition of extracellular kinase 1 and 2 (ERK1/2) and SMAD3 phosphorylation; (d) inhibiting intrapulmonary vasoconstriction caused by AT1 receptor downregulation due to SARS‐CoV‐2‐ACE2 binding alveolar cells, bronchial epithelium, and vascular endothelium, 9 , 61 see Figure 1. This hypothesis has been formalized in the Italian DEDALO project, whose authorization by the Italian Ministry of Health is pending.
Figure 1.
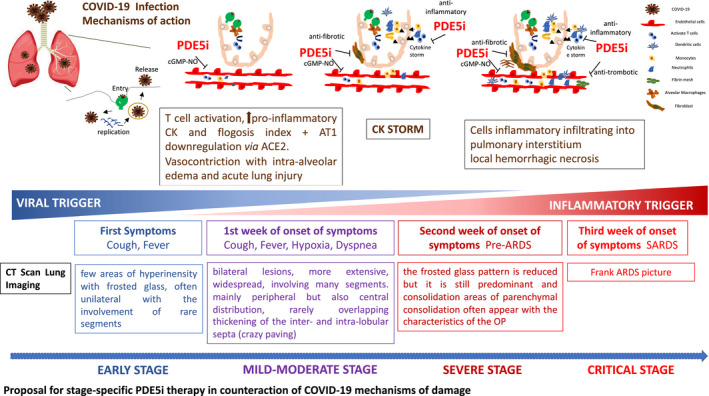
Proposal for stage‐specific PDE5i therapy in counteraction of COVID‐19 mechanisms of damage
The safety profile of long‐term PDE5 inhibitor use has been extensively investigated, 31 , 62 , 63 including in relation to antiretroviral drugs in HIV patients. 64 , 65 , 66 , 67 , 68 , 69
It is this context, in which we apply in late April at call COVID‐19 from the Italian Ministry of Health with our propose: “SilDEnafil administration in DiAbetic and dysmetabolic maLe patients with COVID‐19. The DEDALO trial’’ submitted as a phase 3 multicenter randomized, interventional, controlled trial. This trial aims to evaluate the effect of oral sildenafil on the rate of disease remission (defined as improvement in symptoms or lung imaging and/or SPO2 > 93% or PaO2/FiO2 > 300 mmHg without oxygen inhalation) in mild‐moderate patients, while in severe patients, rate of entering mechanical ventilation. Time for hospitalization, stabilization or increase of the P/F (over 15%), reduction in PEEP absolute value (over 20%), rate of symptoms, inflammatory/endothelial function markers and CKs or lung imaging recovery, rate of undetectable viral RNA (continuous twice), adverse events, infectious susceptibility, and the long‐term effect on lung damage will also detected.
The overall enrolment will include 100 Italian hospitalized diabetic and dysmetabolic men (at high risk) diagnosed with mild to moderate and severe COVID‐19 infection. They will be randomized to sildenafil citrate 60 mg (20 mg oral tablets, 1 pill TID) versus control for 8 weeks.
If the findings of the various ongoing clinical trials are positive, the low cost, wide availability, and temperature stability of PDE5 inhibitors could make them a major resource to combat COVID‐19, especially in developing countries.
2. CONCLUSIONS
There is strong evidence that PDE5 inhibitors could modulate the harmful effects resulting from over‐stimulation of the immune system, opening up a new scenario for their use in COVID‐19 patients. Given the devastating economic consequences of COVID‐19 on national health systems worldwide and the costs of the intensive care units needed to manage critical patients, the use of PDE5 inhibitors may offer a cheap, readily available and non‐experimental treatment strategy to stop the disease from progressing to its most severe final stages, in which current treatments are unfortunately not always effective.
CONFLICT OF INTEREST
Nothing.
AUTHORS’ CONTRIBUTIONS
AMI, EG, and RP conceived and designed the opinion. MAV, FC, and DG collected the data. AMI, EG, and RP co‐wrote the manuscript. CMM, Al, and Gd’E contributed to the revision of the manuscript. All authors have read and approved the final manuscript.
Supporting information
Table S1
ACKNOWLEDGEMENTS
We would like to thank Marie‐Hélène Hayles MITI for revision of the English text.
Isidori AM, Giannetta E, Pofi R, et al. Targeting the NO‐cGMP‐PDE5 pathway in COVID‐19 infection. The DEDALO project. Andrology.2021;9:33–38. 10.1111/andr.12837
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID‐19. Lancet. 2020;395(10229):1014‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bordi L, Nicastri E, Scorzolini L, et al. Differential diagnosis of illness in patients under investigation for the novel coronavirus (SARS‐CoV‐2), Italy, February 2020. Euro Surveill. 2020;25(8):2000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The ADA’s Standards of Medical Care . Introduction: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43(Suppl 1):S1‐S2. [DOI] [PubMed] [Google Scholar]
- 7. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi Y, Wang Y, Shao C, et al. COVID‐19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li JZ, Liu JW, Benito G, Siddiqui FA, Lian EC. Electron microscopic study of platelet agglutination induced by thrombotic thrombocytopenic purpura plasma containing 37‐KDa platelet agglutinating protein. Thromb Res. 1989;55(6):757‐766. [DOI] [PubMed] [Google Scholar]
- 10. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Reeth K, Van Gucht S, Pensaert M. In vivo studies on cytokine involvement during acute viral respiratory disease of swine: troublesome but rewarding. Vet Immunol Immunopathol. 2002;87(3–4):161‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3(4):e208857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lane JCE, Weaver J, Kostka K, et al.Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide‐spread use for COVID‐19: a multinational, network cohort and self‐controlled case series study. medRxiv. 2020:2020.2004.2008.20054551.
- 14. Isidori AM, Pofi R, Hasenmajer V, Lenzi A, Pivonello R. Use of glucocorticoids in patients with adrenal insufficiency and COVID‐19 infection. Lancet Diabetes Endocrinol. 2020;8(6):472‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Isidori AM, Arnaldi G, Boscaro M, et al. COVID‐19 infection and glucocorticoids: update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J Endocrinol Invest. 2020;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID‐ 19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo WY, Gou J, Li X, Sun Y, Li J, Liu L. Clinical Pathology of Critical Patient with Novel Coronavirus Pneumonia (COVID‐19). Preprints 2020, 2020020407. 2020.
- 18. Fu J, Zhou B, Zhang L, et al. Expressions and significances of the angiotensin‐converting enzyme 2 gene, the receptor of SARS‐CoV‐2 for COVID‐19. Mol Biol Rep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wambier CG, Goren A, Vano‐Galvan S, et al. Androgen sensitivity gateway to COVID‐19 disease severity. Drug Dev Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montopoli M, Zumerle S, Vettor R, et al. Androgen‐deprivation therapies for prostate cancer and risk of infection by SARS‐CoV‐2: a population‐based study (n=4532). Ann Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cardona Maya WD, Du Plessis SS, Velilla PA. SARS‐CoV‐2 and the testis: similarity with other viruses and routes of infection. Reprod Biomed Online. 2020;40(6):763‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA Netw Open. 2020;3(5):e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paoli D, Pallotti F, Colangelo S, et al. Study of SARS‐CoV‐2 in semen and urine samples of a volunteer with positive naso‐pharyngeal swab. J Endocrinol Invest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu R, Wang L, Kuo H‐C, et al. Update on current therapeutic drugs treating COVID‐19. Curr Pharmacol Rep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martel J, Ko YF, Young JD, Ojcius DM. Could nasal nitric oxide help to mitigate the severity of COVID‐19? Microbes Infect. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akerstrom S, Gunalan V, Keng CT, Tan YJ, Mirazimi A. Dual effect of nitric oxide on SARS‐CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keyaerts E, Vijgen L, Chen L, Maes P, Hedenstierna G, Van Ranst M. Inhibition of SARS‐coronavirus infection in vitro by S‐nitroso‐N‐acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis. 2004;8(4):223‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldstein I, Lue TF, Padma‐Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998;338(20):1397‐1404. [DOI] [PubMed] [Google Scholar]
- 29. Defeudis G, Gianfrilli D, Di Emidio C, et al. Erectile dysfunction and its management in patients with diabetes mellitus. Rev Endocr Metab Disord. 2015;16(3):213‐231. [DOI] [PubMed] [Google Scholar]
- 30. Anderson SG, Hutchings DC, Woodward M, et al. Phosphodiesterase type‐5 inhibitor use in type 2 diabetes is associated with a reduction in all‐cause mortality. Heart. 2016;102(21):1750‐1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giannetta E, Feola T, Gianfrilli D, et al. Is chronic inhibition of phosphodiesterase type 5 cardioprotective and safe? A meta‐analysis of randomized controlled trials. BMC Med. 2014;12:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hutchings DC, Anderson SG, Caldwell JL, Trafford AW. Phosphodiesterase‐5 inhibitors and the heart: compound cardioprotection? Heart. 2018;104(15):1244‐1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giannetta E, Isidori AM, Galea N, et al. Chronic Inhibition of cGMP phosphodiesterase 5A improves diabetic cardiomyopathy: a randomized, controlled clinical trial using magnetic resonance imaging with myocardial tagging. Circulation. 2012;125(19):2323‐2333. [DOI] [PubMed] [Google Scholar]
- 34. Aversa A, Francomano D, Lenzi A. Does testosterone supplementation increase PDE5‐inhibitor responses in difficult‐to‐treat erectile dysfunction patients? Expert Opin Pharmacother. 2015;16(5):625‐628. [DOI] [PubMed] [Google Scholar]
- 35. Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med. 2011;8(1):284‐293. [DOI] [PubMed] [Google Scholar]
- 36. Spitzer M, Basaria S, Travison TG, et al. Effect of testosterone replacement on response to sildenafil citrate in men with erectile dysfunction: a parallel, randomized trial. Ann Intern Med. 2012;157(10):681‐691. [DOI] [PubMed] [Google Scholar]
- 37. Aversa A, Jannini EA, Maggi M, Lenzi A. Effects of testosterone replacement on response to sildenafil citrate. Ann Intern Med. 2013;158(7):569‐570. [DOI] [PubMed] [Google Scholar]
- 38. Corona G, Isidori AM, Buvat J, et al. Testosterone supplementation and sexual function: a meta‐analysis study. J Sex Med. 2014;11(6):1577‐1592. [DOI] [PubMed] [Google Scholar]
- 39. Pofi R, Fiore D, De Gaetano R, et al. Phosphodiesterase‐5 inhibition preserves renal hemodynamics and function in mice with diabetic kidney disease by modulating miR‐22 and BMP7. Sci Rep. 2017;7:44584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Borgdorff MA, Bartelds B, Dickinson MG, et al. Sildenafil treatment in established right ventricular dysfunction improves diastolic function and attenuates interstitial fibrosis independent from afterload. Am J Physiol Heart Circ Physiol. 2014;307(3):H361‐369. [DOI] [PubMed] [Google Scholar]
- 41. Lewis GD, Lachmann J, Camuso J, et al. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115(1):59‐66. [DOI] [PubMed] [Google Scholar]
- 42. Guazzi M. Is sildenafil neutral on cardiopulmonary performance in group 2 pulmonary hypertension? More details for interpretation. Eur J Heart Fail. 2017;19(5):691. [DOI] [PubMed] [Google Scholar]
- 43. Barnes H, Brown Z, Burns A, Williams T. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst Rev. 2019;1:CD012621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Behr J, Nathan SD, Harari S, et al. Sildenafil added to pirfenidone in patients with advanced idiopathic pulmonary fibrosis and risk of pulmonary hypertension: A Phase IIb, randomised, double‐blind, placebo‐controlled study ‐ Rationale and study design. Respir Med. 2018;138:13‐20. [DOI] [PubMed] [Google Scholar]
- 45. Santi D, Granata ARM, Guidi A, et al. Six months of daily treatment with vardenafil improves parameters of endothelial inflammation and of hypogonadism in male patients with type 2 diabetes and erectile dysfunction: a randomized, double‐blind, prospective trial. Eur J Endocrinol. 2016;174(4):513‐522. [DOI] [PubMed] [Google Scholar]
- 46. Venneri MA, Barbagallo F, Fiore D, et al. PDE5 inhibition stimulates tie2‐expressing monocytes and angiopoietin‐1 restoring angiogenic homeostasis in diabetes. J Clin Endocrinol Metab. 2019;104(7):2623‐2636. [DOI] [PubMed] [Google Scholar]
- 47. Hughes MJ, McGettrick HM, Sapey E. Shared mechanisms of multimorbidity in COPD, atherosclerosis and type‐2 diabetes: the neutrophil as a potential inflammatory target. Eur Respir Rev. 2020;29(155):190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Venneri MA, Giannetta E, Panio G, et al. Chronic inhibition of PDE5 limits pro‐inflammatory monocyte‐macrophage polarization in streptozotocin‐induced diabetic mice. PLoS One. 2015;10(5):e0126580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mandosi E, Giannetta E, Filardi T, et al. Endothelial dysfunction markers as a therapeutic target for Sildenafil treatment and effects on metabolic control in type 2 diabetes. Expert Opin Ther Targets. 2015;19(12):1617‐1622. [DOI] [PubMed] [Google Scholar]
- 50. Fiore D, Gianfrilli D, Cardarelli S, et al. Chronic phosphodiesterase type 5 inhibition has beneficial effects on subcutaneous adipose tissue plasticity in type 2 diabetic mice. J Cell Physiol. 2018;233(11):8411‐8417. [DOI] [PubMed] [Google Scholar]
- 51. Fiore D, Gianfrilli D, Giannetta E, et al. PDE5 inhibition ameliorates visceral adiposity targeting the miR‐22/SIRT1 pathway: evidence from the CECSID Trial. J Clin Endocrinol Metab. 2016;101(4):1525‐1534. [DOI] [PubMed] [Google Scholar]
- 52. Morano S, Mandosi E, Fallarino M, et al. Antioxidant treatment associated with sildenafil reduces monocyte activation and markers of endothelial damage in patients with diabetic erectile dysfunction: a double‐blind, placebo‐controlled study. Eur Urol. 2007;52(6):1768‐1774. [DOI] [PubMed] [Google Scholar]
- 53. Corbin JD, Beasley A, Blount MA, Francis SH. High lung PDE5: a strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun. 2005;334(3):930‐938. [DOI] [PubMed] [Google Scholar]
- 54. Hassoun PM, Nathan SD. Sildenafil for pulmonary hypertension complicating idiopathic pulmonary fibrosis: a rationale grounded in basic science. Eur Respir J. 2016;47(6):1615‐1617. [DOI] [PubMed] [Google Scholar]
- 55. Hayton C, Craig C, Chaudhuri N. Treatment of severe idiopathic pulmonary fibrosis‐is sildenafil the next (in)stage? J Thorac Dis. 2019;11(2):339‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marashi SM, Nasri‐Nasrabadi Z. Is there a role for sildenafil in the management of paraquat‐induced lung fibrosis? Arh Hig Rada Toksikol. 2016;67(2):167‐168. [DOI] [PubMed] [Google Scholar]
- 57. Milara J, Escriva J, Ortiz JL, et al. Vascular effects of sildenafil in patients with pulmonary fibrosis and pulmonary hypertension: an ex vivo/in vitro study. Eur Respir J. 2016;47(6):1737‐1749. [DOI] [PubMed] [Google Scholar]
- 58. Idiopathic Pulmonary Fibrosis Clinical Research N , Zisman DA, Schwarz M, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363(7):620‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang H‐M, Jin S, Jang H, et al. Sildenafil reduces neointimal hyperplasia after angioplasty and inhibits platelet aggregation via activation of cGMP‐dependent protein kinase. Sci Rep. 2019;9(1):7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Santi D, Giannetta E, Isidori AM, Vitale C, Aversa A, Simoni M. Therapy of endocrine disease. Effects of chronic use of phosphodiesterase inhibitors on endothelial markers in type 2 diabetes mellitus: a meta‐analysis. Eur J Endocrinol. 2015;172(3):R103‐114. [DOI] [PubMed] [Google Scholar]
- 61. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Olschewski H, Ardeschir ghofrani H, Walmrath D, et al. Inhaled prostacyclin and iloprost in severe pulmonary hypertension secondary to lung fibrosis. Am J Respir Crit Care Med. 1999;160(2):600‐607. [DOI] [PubMed] [Google Scholar]
- 63. Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328(6):399‐405. [DOI] [PubMed] [Google Scholar]
- 64. Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493‐2537. [DOI] [PubMed] [Google Scholar]
- 65. Muirhead GJ, Wulff MB, Fielding A, Kleinermans D, Buss N. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br J Clin Pharmacol. 2000;50(2):99‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sekar V, Lefebvre E, De Marez T, et al. Effect of repeated doses of darunavir plus low‐dose ritonavir on the pharmacokinetics of sildenafil in healthy male subjects: phase I randomized, open‐label, two‐way crossover study. Clin Drug Investig. 2008;28(8):479‐485. [DOI] [PubMed] [Google Scholar]
- 67. Chinello P, Cicalini S, Cortese A, Cicini MP, Petrosillo N. Bosentan and sildenafil in the treatment of HIV‐associated pulmonary hypertension. Infect Dis Rep. 2011;3(2):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chinello P, Cicalini S, Pichini S, Pacifici R, Tempestilli M, Petrosillo N. Sildenafil plasma concentrations in two HIV patients with pulmonary hypertension treated with ritonavir‐boosted protease inhibitors. Curr HIV Res. 2012;10(2):162‐164. [DOI] [PubMed] [Google Scholar]
- 69. Chinello P, Cicalini S, Pichini S, et al. Sildenafil and bosentan plasma concentrations in a human immunodeficiency virus‐ infected patient with pulmonary arterial hypertension treated with ritonavir‐boosted protease inhibitor. Infect Dis Rep. 2015;7(1):5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


