Abstract
Traumatic brain injury (TBI) and severe blood loss (SBL) frequently co-occur in human trauma, resulting in high levels of mortality and morbidity. Importantly, each of the individual post-injury cascades is characterized by complex and potentially opposing pathophysiological responses, complicating optimal resuscitation and therapeutic approaches. Large animal models of poly-neurotrauma closely mimic human physiology, but a systematic literature review of published models has been lacking. The current review suggests a relative paucity of large animal poly-neurotrauma studies (N = 52), with meta-statistics revealing trends for animal species (exclusively swine), characteristics (use of single biological sex, use of juveniles) and TBI models. Although most studies have targeted blood loss volumes of 35–45%, the associated mortality rates are much lower relative to Class III/IV human trauma. This discrepancy may result from potentially mitigating experimental factors (e.g., mechanical ventilation prior to or during injury, pausing/resuming blood loss based on physiological parameters, administration of small volume fluid resuscitation) that are rarely associated with human trauma, highlighting the need for additional work in this area.
Keywords: Hypovolemia, Shock, Traumatic brain injury, Poly-neurotrauma, Large animal models, Common data elements
1. General introduction
Although trauma affects multiple organ systems, the two largest medical risks in polytrauma are severe blood loss (SBL), the associated hemorrhagic shock (HS), and concurrent traumatic brain injury (TBI; Eastridge et al., 2012; Jin et al., 2012a). Among civilians, 26% of more severely injured TBI patients present with concurrent HS (Manley et al., 2001), with much higher rates (e.g., 80%; Chambers et al., 2005) observed in military patients due to the higher incidence of extremity/penetrating injuries (Ling et al., 2009; Nelson et al., 2006). Up to 50% of deaths from either SBL/HS or central nervous system trauma occur within 60 min, indicating a short window for intervention (Pope et al., 1999). Although it has been known for decades that outcomes after combined TBI and HS (hereafter referred to as poly-neurotrauma) are worse than after TBI alone (Walsh et al., 1991), the optimal resuscitation approaches and potential consequent exacerbation of neuronal injury remain actively debated (Cannon, 2018; Fulop et al., 2013).
The choice of animal species is particularly important as a result of the complex, and sometimes opposing, physiological derangements that occur and are difficult to capture in small animal models (Cho et al., 2009; Hildebrand et al., 2013; Majde, 2003). Although the role of swine in polytrauma studies has recently been discussed (Hildebrand et al., 2013), a systematic review of large animal poly-neurotrauma models has not been published. The current review addresses this gap by providing an overview of TBI and SBL/HS pathophysiology, followed by a comprehensive review of the literature to date, along with associated meta-statistics. As the typical poly-neurotrauma scenario begins with a traumatic injury event followed by blood loss, we review TBI models first followed by SBL/HS (SBL leads to HS; terms used interchangeably throughout).
2. Review of traumatic brain injury models
Approximately 1.7 million people sustain a traumatic brain injury (TBI) each year in the United States alone, resulting in 235,000 hospitalizations, 50,000 deaths and 90,000 new patients with permanent disability (Faul et al., 2010; Gardner et al., 2018). The cumulative population of permanently disabled TBI survivors is approximately 5 million (Langlois et al., 2003), with the direct and indirect cost for TBI care estimated at over $77 billion. Currently, there are no efficacious treatments for moderate to severe TBI beyond acute care management (e.g., intracranial pressure management, craniotomy; Skolnick et al., (2014). The neurometabolic cascade of acute trauma is multifaceted and includes abrupt neuronal depolarization, a release of excitatory neurotransmitters, disruption of ionic balance, changes in glucose metabolism, vascular trauma, altered cerebral blood flow/neurovascular coupling, and impaired axonal function (Smith et al., 2015; Xiong et al., 2013). Secondary injury sequelae include ischemia, hypotension, cerebral hypoxia, cerebral edema and increased intracranial pressure. Thus, from the outset, it is critical to recognize that no one animal model will be able to cover the spectrum of injuries and pathologies that characterizes human TBI.
A variety of molecular and cellular changes also occur following TBI (Blennow et al., 2012; Shively et al., 2012; Smith et al., 2013). These include the aggregation of neurofibrillary tangles (NFTs), development of amyloid-β (Aβ) plaques and an increase in inflammatory markers such as glial fibrillary acidic protein, the interleukin family of cytokines and tumor necrosis factor (Kovesdi et al., 2010; McKee et al., 2009; Woodcock and Morganti-Kossmann, 2013). A recent study of brain samples from 39 survivors of a single moderate to severe TBI demonstrated a greater density and wider distribution of NFTs compared to age-matched controls (Johnson et al., 2012). Prior to NFT formation, elevated phosphorylated-Thr231 tau levels in cerebrospinal fluid may serve as a biomarker for TBI (Rubenstein et al., 2014). In a rat model of parasagittal fluid percussion TBI, Hawkins et al. (2013) demonstrated a rapid increase of oligomeric and phosphorylated tau aggregates as soon as 4 h and up to 2 weeks after TBI compared to sham control rats.
Aβ is a 40–42 amino acid long peptide generated by successive cleavage of amyloid pre-cursor protein (APP) by β-secretase followed by γ-secretase (Karran and De, 2016). Aβ can oligomerize and aggregate as fibrils to eventually form senile plaques, which are a common neuropathological hallmark of Alzheimer’s disease (Luhrs et al., 2005; Mattson, 2004; Selkoe and Schenk, 2003; Urbanc et al., 2010). Aβ plaques represent a common biomarker of diffuse axonal injury and are frequently cited as a potential pathophysiological link between TBI and the subsequent development of dementia. In a seminal study of patients who died 4 h to 2.5 years following severe head injury, Aβ plaques were found in 30% of brains examined (Roberts et al., 1994) and were more common in gray rather than white matter. Experiments using transgenic rodent models have demonstrated increases in intra-axonal soluble, insoluble and oligomeric Aβ following injury (Washington et al., 2014). As in Alzheimer’s disease, whether Aβ plaques are deleterious or play a neuroprotective role in sequestering harmful oligomeric amyloid species following TBI has yet to be established (Johnson et al., 2010).
TBI initiates a pro-inflammatory cascade associated with changes in vascular integrity (Franzblau et al., 2013). In a rat model of TBI (Glushakova et al., 2014), microbleeds were detected up to 3 months after injury along with blood-brain barrier/endothelial damage, activation of microglia and a macrophage-mediated inflammatory response. Similarly, evidence of neuroinflammation (reactive microglia) has also been observed in the corpus callosum and surrounding gray-matter regions in postmortem human brains of individuals that expired during the chronic (28% of surveyed cases) but not in the acute phase of TBI (Johnson et al., 2013). Further, TBI-induced vascular damage and the associated breakdown of the blood-brain barrier (Wojnarowicz et al., 2017) may stimulate cleavage of APP to release toxic species of Aβ. These injury mechanisms also interact, with evidence suggesting that neuroinflammation can accelerate hyperphosphorylation of microtubule-associated protein tau and cognitive impairment in a mouse model of tauopathy (Bhaskar et al., 2010). In a test of the hypothesis that Aβ activity occurs upstream to abnormal tau formation after TBI, treatment with an inhibitor of gamma-secretase, a proteolytic enzyme necessary for Aβ formation, effectively blocked Aβ production in these mice with no effect on tau, suggesting that TBI has independent effects on both Aβ and tau pathology (Tran et al., 2011).
There is some evidence suggesting that TBI may increase neurodegeneration and/or result in an earlier age of onset (Gilbert et al., 2014; Nemetz et al., 1999; Schofield et al., 1997), as well as a higher prevalence of pathological features characteristic of dementia upon autopsy (Jellinger et al., 2001). The risk for long-term sequelae of TBI likely follows a dose-response curve, with more severe injury and greater numbers of repetitive traumas leading to greater long-term risk (Smith et al., 2013). Investigations into the biological basis between TBI and the later development of dementia have implicated many of the previously discussed pathways including the pathogenic aggregates of proteins (Aβ, tau, TDP-43), vascular insult, white matter damage and neuroinflammatory responses (Blennow et al., 2012; Shively et al., 2012; Smith et al., 2013). However, another study reported minimal links between Alzheimer’s dementia and TBI, instead reporting associations between head injury and the development of Lewy bodies and Parkinson’s disease (Crane et al., 2016).
Injury models were initially characterized as either producing a focal injury or affecting the brain more diffusely, with both injuries typically associated with secondary injury cascades (Johnson et al., 2015; Smith et al., 2015; Xiong et al., 2013). However, this dichotomous classification scheme is no longer recognized as being valid given that most animal injury models induce multiple pathologies simultaneously (Smith et al., 2015; Xiong et al., 2013). Currently, there are four well-recognized methods for modeling TBI in animals: 1) contusional (e.g., weight drop, controlled cortical impact [CCI], fluid percussion injury [FPI]), 2) blast, 3) penetrating and 4) dynamic (impact and non-impact) head acceleration (Galgano et al., 2015; Smith et al., 2015; Xiong et al., 2013). Pharmaceutical, thermic, laser and freeze-induced lesion models of TBI have been used historically (Smith et al., 2015; Xiong et al., 2013), but are not discussed in the current review due to their limited human translation. A thorough discussion of the role and history of preclinical models for understanding human TBI has recently been provided (Wojnarowicz et al., 2017) and several excellent reviews of animal models of TBI exist (Johnson et al., 2015; Smith et al., 2015; Xiong et al., 2013). Thus, explicit details regarding animal models are only briefly recapitulated in the sections that follow.
Historically, CCI, weight drop and FPI represent the most widely used models across the phylogeny (Smith et al., 2015; Xiong et al., 2013) and are capable of producing both focal contusions in experimenter-selected cortical regions and more diffuse injury. FPI, for example, can result in increased shearing forces and multifocal injury depending on lateral versus midline positioning (Vink, 2018). The use of craniotomy in most FPI and CCI studies result in prominent tissue destruction (i.e., a contusion) in the absence of hematoma and skull fracture (Shultz et al., 2016), a constellation of findings that is infrequently observed in human injury. Contusional models traditionally fixed the position of the head (Xiong et al., 2013), which rarely occurs in human trauma settings. In addition, the longer animal preparation time (surgical) and exposure to long-acting anesthetic agents in these models can also interfere with rapid neurological assessment after injury. More recently, variations of the CCI and even projectile strike models have been developed without a craniotomy to more realistically model the full injury spectrum (i.e., concussions to severe TBI), as well as repetitive closed head injury (Galgano et al., 2015; Johnson et al., 2015; Smith et al., 2015; Xiong et al., 2013).
Blast models of TBI were generally understudied until Operations Enduring and Iraqi Freedom, during which TBI was recognized as the signature wound of these wars (Hoge et al., 2008). Blast involves primary (i.e., as a result of blast wave), secondary (i.e., as a result of projectiles), tertiary (i.e., as a result of rapid acceleration/deceleration forces) and quaternary (i.e., chemical exposure and burns) injuries, as well as reflective wave injury (Cernak, 2005). Advantages of the blast model include minimal preparation time and no craniotomy (Vink, 2018). However, effects of animal positioning (inside or outside the blast tube), tube construction (e.g., square versus circular opening), and the type of medium used to expose animals (Smith et al., 2015; Xiong et al., 2013) have all been shown to affect outcomes in blast-related trauma. For example, there has been debate as to whether a primary injury only (typically achieved by placing the animal inside the tube with head restraint) is sufficient for producing neuronal pathology and/or neurobehavioral sequelae (Cernak, 2014; Goldstein et al., 2012; Saljo et al., 2011). In contrast, an unrestrained mouse head moves at approximately 954krad/s2 during blast (tertiary injury), purportedly recapitulating forces similar to dynamic acceleration injuries in humans that cause tauopathy (Goldstein et al., 2012; Goldstein and McKee, 2012).
Penetrating injuries are relatively rare in civilian trauma but are associated with higher rates of mortality and a worse prognosis (Kazim et al., 2011). Although penetrating injuries occur more frequently in military settings (i.e., secondary damage from blast and ballistics), the annual incidence rate represents only a small fraction (typically less than 1%) of the total military TBI incidence, even during times of war (see yearly Defense and Veterans Brain Injury Center reports at https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi). Penetrating injuries are, by nature, more difficult to standardize and model due to challenges associated with variable ballistic impact and trajectory (Smith et al., 2015; Xiong et al., 2013), as well as the extensive tissue damage and cavitation that occurs as a result of energy dispersion (Finnie et al., 2002). Due to the higher military relevance, there has been a focus on developing and standardizing a non-lethal penetrating ballistic-like brain injury model as part of a pipeline to test various therapeutic agents in rodents by the United States Department of Defense (Kochanek et al., 2018).
Dynamic acceleration/deceleration (similar to tertiary injuries) of the head was historically considered to be the best model for generating diffuse injury and modeling subsequent loss of consciousness (Gennarelli et al., 1982). Dynamic acceleration/deceleration injuries can be produced with or without a mechanism of impact both in the laboratory and in human trauma (Vink, 2018). Initially, dynamic acceleration impact injuries were conducted with sheep as the large animal of choice, with outcomes focused on the relationship between vascular injury and intracranial pressure (Vink, 2018). More recently, there has been growing interest in models that combine blunt force trauma with a minimally restrained head to mimic impact induced dynamic acceleration/deceleration in rodents (Galgano et al., 2015). Protective mechanisms (e.g., helmet, steel plates, padding) are more frequently being utilized in these models to mimic sport-related injuries and reduce the likelihood of skull fracture (i.e., improve survivability).
The dynamic acceleration/deceleration non-impact model was originally developed for primates (Gennarelli et al., 1982), but has most recently been adapted for neonatal, juvenile and adult swine (Browne et al., 2011; Friess et al., 2009; Meaney et al., 1995; Smith et al., 2000). This model requires only 15–20 min to initialize post-anesthesia and does not require a craniotomy (Cullen et al., 2016). A critical parameter of all dynamic acceleration/deceleration models is whether the head is allowed to rotate in a single or multiplanar fashion, with the plane of rotation influencing clinical (i.e., loss of consciousness) and pathological (i.e., number of hematomas) outcomes in a species-dependent fashion. The primary critiques of dynamic acceleration/deceleration models (Vink, 2018; Xiong et al., 2013) are related to issues of reproducibility (especially for freely rotating heads), financial cost of the instrumentation (non-impact model only) and incidence of skull fracture (impact models).
Similar to models of hemorrhagic shock, the various TBI models (FPI, penetrating injury, dynamic acceleration/deceleration injury, etc.) were originally developed in larger animal species (e.g., primates, felines, and canines) and later adapted to small animal models (Vink, 2018). Small animals (rodents) have provided tremendous insight into the molecular basis of TBI (Smith et al., 2015), and are commonly used across a broad range of scientific research due to ethical reasons, cost-effectiveness, short breeding time, short lifespan, and the availability of transgenic animals for determining the genetic basis of disease. However, there are several limitations to small animal models that are critical to consider in the context of TBI research. Specifically, small animal models fail to replicate many of the microscopic and molecular pathologies of TBI in humans. For example, rodents consistently fail to show the formation of Ap plaques that are typically observed in both human (Johnson et al., 2010) and swine (Smith et al., 1999) TBI models up to 6 months following experimental trauma. Similarly, the extent of gliosis and the ratio of glial cells to neurons as well as cellular morphology varies across species (Ventura-Antunes et al., 2013), the former of which has significant ramifications for secondary neuroinflammatory responses.
More apparent are macroscopic differences in the brain across small and large animal species. The smooth (lissencephalic) brains of mice and rats compared with the convoluted (gyrencephalic) brains of large animals and humans result in differences in both the magnitude (greater deformation in gyrencephalic) and location (gyrencephalic = sulcal depths; lissencephalic = surface) for the accumulation of mechanical shear/stress forces following injury (Smith et al., 2015; Vink, 2018). A lissencephalic versus gyrencephalic cortex is directly attributable to differences in species scaling between grey/white ratios (Ventura-Antunes et al., 2013), which has been posited to be the primary driver for the subsequent mechanical deformation of tissue associated with the increased surface area of gyrencephalic brains (Van Essen, 1997; Ventura-Antunes et al., 2013). A second major macroscopic difference across animal species relates to brain mass, which was first discussed in the classic work of Holbourn (Holbourn, 1943). For example, it has been estimated that approximately 500–630% scaling factor has to be applied to the initial loading forces in large animal species to mimic the forces typically experienced by humans (Johnson et al., 2015). Thus, only animals with sufficient brain mass can realistically be used for modeling dynamic acceleration/deceleration injuries (Meaney et al., 1995), whereas contusional injury may scale better across small and large animal models (Smith et al., 2015).
Third, there are other gross morphological differences across species in terms of the distribution and shape of bony protuberances of the skull (e.g., sphenoid ridge), overall skull thickness, material properties of different anatomical substrates (e.g., rigidity of the tentorium cerebelli), and cerebrovascular distribution such as the cerebral arterial circle (Ashwini et al., 2008; Vink, 2018). All of these morphological factors likely interact in a non-linear fashion for determining injury biomechanics, the likelihood of skull fracture/associated hemorrhage, as well as influencing key secondary injuries such as intracranial pressure (reviewed in Vink, 2018). As a concrete example of species-related differences in neuroanatomy and biomechanics, the relationship between plane of rotation and loss of consciousness varies across primate (Gennarelli et al., 1982) and swine (Browne et al., 2011) in the same dynamic acceleration/deceleration non-impact model.
In summary, there are a multitude of factors that affect the severity of injury in animal models, including but not limited to the size/location of the craniotomy, the subsequent decision regarding cranioplasty, the angle of impact, the location of impact, the depth of penetration of the striking device, the duration of contact time between the device and the cortex, the material properties (e.g., rigid versus soft plastics, mass, and shape) and velocity/peak pressure of impacting device (Smith et al., 2015). Each TBI model has its unique advantages/disadvantages and relevance to particular types of brain injury, with no one model fully capturing the spectrum and heterogeneity of human injury (Cernak, 2005; Povlishock and Katz, 2005; Thompson et al., 2005). Perhaps the largest limitation for all animal models is the lack of standardization and the difficulty of directly translating findings to humans. Indeed, there are descriptions of both “mild” animal injuries that produce large and vacuous lesions as well as “severe” injuries in which the animal behaviorally recovers within hours of injury (Smith et al., 2015; Xiong et al., 2013), neither of which mirrors the pathoanatomical or behavioral sequelae that are observed in their human counterparts.
3. Review of severe blood loss models
It has been estimated that up to 50% of early deaths following trauma result from SBL and associated HS (Holcomb and Hoyt, 2015; Mathers et al., 2006). The lethal triad of hypothermia, acidosis and coagulopathy are characteristic complications of HS that variably contribute to shock pathogenesis and poor outcomes (Cannon, 2018). Even in patients who are successfully resuscitated, the early hypotension associated with SBL can lead to multiple organ failure and development of secondary infections (e.g., pneumonia or sepsis) that occur in sub-acute and more chronic disease stages (Durham et al., 2003; Franklin et al., 2000; Heckbert et al., 1998). Multiple organ failure may be irreversible, even following successful fluid resuscitation, and results in a chronic, systemic inflammatory response (Tsukamoto and Pape, 2009). Because of the heterogeneous nature of the disease process (metabolic imbalance versus organ failure versus ischemia versus coagulation), the systemic events that lead to and predict death in humans are still highly debated (Duchesne et al., 2010).
From a mechanistic perspective, hypovolemia is first detected by baroreceptors in the aortic arch and left atrium, leading to an immediate sympathetic response to increase cardiac contractility and peripheral vascular resistance, followed by the release of catecholamines, antidiuretic hormones and atrial natriuretic peptide (Cannon, 2018; Chaudry et al., 1993). The end result is an increase in heart rate and vasoconstriction that preferentially shunts oxygenated blood to vital organs such as the heart, lungs, and brain at the expense of peripheral organs, such as the skin, kidneys, liver and gastrointestinal tract, ultimately resulting in tissue acidosis for prolonged periods of HS (Link et al., 1981; Rossaint et al., 2006). Thus, the duration of HS represents another critical variable for determining physiologic outcome, with sustained hypotensive states (e.g., 60 min) resulting in significantly greater metabolic/hematologic derangements and increased tissue necrosis relative to shorter (e.g., 30 min) hypotensive states (Sheppard et al., 2018). While hyperventilation partially compensates for the metabolic acidosis, a continued state of oxygen debt results in progression to an uncompensated systemic acidemia state, the release of reactive oxygen species, pro-inflammatory immune responses, and eventually cell death (Hierholzer and Billiar, 2001).
The fundamental pathology for all states of HS is therefore a relative imbalance between systemic oxygen delivery and tissue oxygen consumption (oxygen debt), resulting from a simultaneous reduction in both circulating blood volume and red blood cell mass (American College of Surgeons Committee on Trauma, 2004; Cairns, 2001; Peitzman et al., 1995). However, the pathophysiology of HS is also influenced by both environmental and host-derived variables (e.g., age, sex, genetic background, and pre-existing health conditions; Angele et al., 2008). During uncontrolled hemorrhage (the most frequent scenario in human trauma), the amount and rate of blood loss depends on the nature, location and severity of the insult. In the absence of mechanical intervention, the rate of blood loss is dynamic, beginning very rapidly and decreasing exponentially in response to vasoconstriction, coagulation, and reduced mean arterial pressure (Hirshberg et al., 2006).
Concurrent with the hemodynamic and metabolic changes, trauma-related tissue damage triggers additional cellular responses to promote hemostasis and tissue repair, and to minimize infection (Chaudry, 1983; Hildebrand et al., 2013). The post-traumatic immune response is rapid and mainly regulated by the early release of cytokines and chemokines to recruit polymorphonuclear cells to the injured site. While this inflammatory cascade is necessary for tissue repair and immune defense against infection at the site of injury, overly exuberant inflammatory responses can contribute to a hyperinflammatory state, which further exacerbates other systemic imbalances and organ dysfunction distant from the site of injury (Hildebrand et al., 2006). Moreover, an acute hyperinflammatory state can lead to immune system exhaustion and subsequent immunosuppression, which paradoxically pre-disposes patients to secondary infections (Keel and Trentz, 2005).
Local vascular endothelial damage at the site of injury also triggers activation of the coagulation cascade, during which platelets and coagulation factors are recruited and activated to slow bleeding (Cannon, 2018). However, if hemorrhage is severe, progressive consumption of these limited factors leads to paradoxical hypocoagulation, which can be exacerbated by fluid resuscitation because of dilution effects. As a result, clotting time is increased, re-bleeding events are more likely to occur, and the patient may enter the lethal state of disseminated intravascular coagulopathy (Armand and Hess, 2003). Each systemic response that is triggered upon injury will progress variably based on the rate and volume of blood loss, the resulting mean arterial blood pressure, and the duration of the shock episode prior to therapeutic intervention (Holcomb et al., 2007).
Unlike TBI, there are several avenues of treatment that have been shown to be efficacious during HS in both humans and animal models. However, given the multisystemic involvement and evolving nature of HS, it is unlikely that a single therapy or therapeutic protocol will be universally effective, and there has been a shift over the past decade toward more individualized, and physiologic-based therapies (Bonanno, 2012). Basic resuscitation strategies for patients presenting with HS are focused on bleeding control, maintenance of tissue oxygenation, coagulation support, and maintenance of body temperature (Cannon, 2018). Current treatment protocols rely primarily on replacing blood volume with donor blood products (e.g., red blood cells, plasma, platelets) or whole blood (Cannon et al., 2017), although the ratios and quantities of these products, as well as the timing of resuscitation in patients without definitive hemostasis, are highly debated (Cannon, 2018). In general, aggressive therapy is successful in mitigating hypoxemia and hypovolemia when initiated quickly following moderate hemorrhage, but can be contraindicated in cases of noncompressible hemorrhage, when patients are at risk of re-bleeding following rapid restoration of blood volume (Morrison et al., 2011). The chronic effects of persistent inflammation, immunosuppression, and protein catabolism syndrome have resulted in an increased focus on identifying factors and therapeutic targets that can improve long-term outcomes (Mira et al., 2018). Concurrently, remote damage control resuscitation strategies are being investigated to advance various small volume resuscitation therapies, hemoglobin-based oxygen carriers, and lyophilized plasma and platelet products that can be delivered easily in the field (Naumann et al., 2018).
Similar to TBI, animal models of SBL and HS have greatly contributed to our current knowledge of this disease process and continue to serve an important role in preclinical testing of new treatments (Fulop et al., 2013; Hildebrand et al., 2013; Lomas-Niera et al., 2005; Moochhala et al., 2009). The vast majority of animal models are based on protocols in which blood loss is “controlled” by either 1) removing an estimated percentage of total blood volume (eTBV) or 2) maintaining physiological variables such as mean arterial pressure (MAP) or relative oxygen debt (Fulop et al., 2013; Moochhala et al., 2009). Blood is typically removed through a cannulated vessel in controlled models, which creates a ‘closed-circulation’ hypotension model and minimizes the confounding effects of tissue trauma and the subsequent inflammation on disease pathogenesis (Hildebrand et al., 2013). In contrast, “uncontrolled” blood loss models are typically used by studies that aim to re-create more realistic field scenarios of SBL. Several excellent reviews of SBL and associated HS already exist (Cannon, 2018; Fulop et al., 2013; Hildebrand et al., 2013; Lomas-Niera et al., 2005; Moochhala et al., 2009). Thus, the benefits and limitations of the most common animal models are only briefly reviewed in the sections that follow.
Even though the quantitative loss of blood is extremely difficult to characterize in most human trauma scenarios, the clinical definition of HS has been traditionally graded on a 4 point scale according to the Advanced Trauma Life Support (ATLS) recommendations for percentage (Class I = < 15%, Class II = 15–30%, Class III = 30–40%, and Class IV = > 40%) of circulating blood volume loss (American College of Surgeons Committee on Trauma, 2004). This clinical practice, in turn, led to the widespread adoption of fixed-volume hemorrhage models for animal studies (Fulop et al., 2013; Hildebrand et al., 2013). Most fixed-volume animal models of HS attempt to re-create Class III and IV hemorrhage, which consistently results in hypotension and organ dysfunction, and is associated with approximately 30% mortality in humans (American College of Surgeons Committee on Trauma, 2004). The greatest strengths of fixed-volume loss models are easy standardization and experimental control, resulting in increased reproducibility across multiple laboratories (Fulop et al., 2013; Moochhala et al., 2009).
However, several decades ago it was recognized that there can be variable hemodynamic responses (e.g., heart rate and MAP) to the same percentage of total blood loss, primarily driven by host-derived factors (Jacobsen et al., 1990; Pacagnella et al., 2013). Concordantly, the philosophy on clinical management of HS has shifted away from sole reliance on ATLS classifications to an emphasis on the modulation of animal specific physiologic response (Bonanno, 2012). In the most popular variant of this model, blood is removed until MAP reaches a pre-determined threshold, typically ranging between 20 and 55 mmHg (Fulop et al., 2013; Moochhala et al., 2009). Once the target pressure is achieved, the hypotensive state is maintained for a fixed period (ranging from 15 min to over 3 h) by titrating blood volume via either subsequent blood removal or small volume resuscitation (e.g., shed blood, crystalloid fluids, plasma). The fixed-pressure HS model has been instrumental in differentiating reversible from irreversible HS, and in defining the ‘golden hour’ for initiating treatment to prevent permanent tissue injury and death (Hildebrand et al., 2013).
As previously discussed, oxygen debt represents the final, and most specific, level of experimental control in animal models of HS, and can be standardized to a produce a pre-defined state of relative hypoxia at the tissue level (American College of Surgeons Committee on Trauma, 2004; Cairns, 2001; Peitzman et al., 1995). Relative oxygen debt forces cells into anaerobic metabolism, resulting in increased lactic acidemia and decreased base excess, both of which can be measured from blood (Cannon, 2018). Importantly, oxygen debt and the associated metabolic correlates are strongly predictive of tissue damage, organ failure and clinical outcome following HS (Crowell and Smith, 1964; Dunham et al., 1991; Rixen and Siegel, 2005). Both canine (Dunham et al., 1991) and swine (Rixen et al., 2001) models have demonstrated an exponential relationship between oxygen debt and the probability of death following 60 min of HS, suggesting there is a critical threshold of oxygen debt that is incompatible with life. Finally, therapeutic reversal of oxygen debt and metabolic normalization are much stronger predictors of successful resuscitation than either the volume of fluid replacement or resulting changes in blood pressure (Rixen et al., 2001; Siegel et al., 2003).
Other experimental variables, including the rate and location of the hemorrhage, as well as the duration of the subsequent shock episode, have been shown to have a significant impact on clinical outcomes in controlled injury models (Frankel et al., 2007; Hildebrand et al., 2013; Sondeen et al., 2007). For example, rapid blood loss, which is more representative of injuries to large vessels or organs, results in a different response than gradual blood loss, which is more representative of blunt trauma and injury to smaller vessels (Cannon, 2018). Removing a fixed volume (e.g., 30mL/kg) dynamically over 20 min results in more pronounced physiologic response than removal of the same percentage of blood at a constant rate over 20 min (Frankel et al., 2007). Thus, animal models of controlled HS should follow dynamic blood loss protocols that mimic the non-linear uncontrolled hemorrhage rates that most frequently occur in human trauma. A potential weakness of all controlled hemorrhage models is that trauma-induced inflammatory responses are dependent on the degree of blood loss (Wichmann et al., 1996; Zellweger et al., 1995), which can significantly influence disease pathogenesis and clinical outcome if animals are recovered (Faist, 1996). Thus, while controlled models provide tractable systems to evaluate candidate therapies, the clinical application is often restricted to very specific shock conditions, and caution should be exercised when translating results more broadly.
Animal models of uncontrolled hemorrhage represent the most realistic model of human trauma, but provide the least level of experimental control over clinical outcomes, with the same injury mechanism producing highly variable results across animals (Kiraly et al., 2006; Tsukamoto and Pape, 2009; Varicoda et al., 2003). During uncontrolled hemorrhage, the quantity of blood loss is typically estimated by weighing absorbent materials (Fulop et al., 2013), with the rate and severity of blood loss dictated by organ perfusion, the size of the vascular defect, and the pressure gradient across the vessel wall (Eddy et al., 1968). The most common injury sites for uncontrolled hemorrhage include the liver, spleen, and main arteries, including the aorta, as well as the femoral and carotid arteries (Hildebrand et al., 2013; Moochhala et al., 2009). Owing to their anatomic and physiologic similarities to humans, liver lacerations or vessel tears resulting in non-compressible torso hemorrhage are frequently modeled in swine (Davidson et al., 2017; Ross et al., 2014). Perforation of the inferior vena cava (Sava et al., 2003) or the abdominal aorta (Kheirabadi et al., 2005; Sondeen et al., 2003) in uncontrolled models has been instrumental in refining resuscitation protocols to maintain adequate tissue perfusion while preventing re-bleeding events.
Similar to TBI, both small and large animal models of HS have been characterized and each model has tremendous utility for modeling certain aspects of disease. Rodent models of HS have been widely adopted as important platforms for characterizing shock pathogenesis due to availability, ease of use, and relatively low cost (Chaudry et al., 1993; Fulop et al., 2013; Moochhala et al., 2009). Canines represent the oldest model of HS, but fail to reproduce many of the cardiovascular responses observed in humans (Fulop et al., 2013; Moochhala et al., 2009). More recently, swine have replaced dogs as the gold standard large animal species for HS across a variety of different models (Hildebrand et al., 2013). Finally, nonhuman primates are the closest genetic relatives to humans, and thus have the most similar physiology, but are less commonly used due to high cost and ethical considerations (Fulop et al., 2013).
There are several important species-specific differences to consider when selecting a HS animal model. Perhaps of greatest importance for modeling HS is the ability to estimate total blood volume, which can be more accurately predicted based on body weight in larger animal models and more closely mirrors the total composition found in humans due to similarities in overall body mass (Fulop et al., 2013; Hauser, 2005). Also related to their larger blood volume, surgical interventions can be more easily performed in larger animals and there is less restriction on the volume and frequency of blood sampling. Therefore, the type and complexity of HS studies that can be performed in rodent species is much more limited (Frink et al., 2011).
Other important advantages to large animal models of HS are their anatomic, physiologic, and genetic similarities to humans. The anatomy and physiology of the cardiovascular system is very species-dependent. Outside of non-human primates, swine have cardiovascular systems that most closely resemble humans in terms of homology in hemostatic mechanisms and distribution of coronary blood supply (Swindle et al., 2012; Tsukamoto and Pape, 2009). These factors are particularly critical in uncontrolled blood loss models, where physiological species-related variations in pressure gradients and blood supply can play a larger role (Fulop et al., 2013; Moochhala et al., 2009). Further, the development and maturation of these systems in large animal species is also more similar to humans and immature swine exhibit almost identical cardiovascular, cerebrovascular, hematologic, and electrolyte profiles to young humans (Tsukamoto and Pape, 2009). Finally, genetic relatedness between large animals and humans contributes to their value in modeling the immunologic responses to HS. Owing to their genetic similarity, NHPs offer the most realistic model for studying the delayed systemic inflammatory response syndrome that occurs in human trauma patients (Bograd et al., 2015). This is in contrast to mice (Seok et al., 2013), and even swine (Baker et al., 2012), which poorly predict the human inflammatory response to trauma (but see Brownstein et al., 2006).
Although all animal models serve a useful purpose, large animal models more accurately reproduce the hemodynamic, metabolic, and inflammatory imbalances that occur during HS in humans and, therefore, provide tractable systems for evaluating new treatments (Fulop et al., 2013; Hildebrand et al., 2013; Moochhala et al., 2009). Using these models, investigators can define basic HS pathophysiology, identify useful physiologic triggers, and test the efficacy of candidate therapeutics in a controlled setting. However, it is important to recognize that any study with comparative animal models has limitations, as the methods often used to control experimental variables (e.g., removal of blood through intravenous catheters) do not accurately mimic the clinical scenario of uncontrolled bleeding that characterizes human trauma (Fulop et al., 2013; Moochhala et al., 2009). It is, therefore, critical for investigators to recognize the limitations of animal models and choose the model that is most appropriate for the scientific goals and clinical application of the study (Fulop et al., 2013; Hildebrand et al., 2013).
4. Pathophysiology of concurrent TBI and SBL models
After one of the original reports on the complicating effects of HS on TBI (Miller and Becker, 1982), many other clinical and laboratory studies confirmed that systemic arterial hypotension worsens neurologic outcome, approximately doubling the mortality rate following TBI (Chesnut et al., 1993a, b; Coates et al., 2005; Cooper et al., 2004; Manley et al., 2001). Specifically, systemic arterial hypotension results in earlier and more severe reductions in cerebral blood flow, hypoxia and a doubling of the contusion volume when compared with normotensive patient controls (Bryan et al., 1995; DeWitt et al., 1992; Giri et al., 2000; Matsushita et al., 2001). During poly-neurotrauma, normally well-tolerated arterial pressure reductions can cause severe cerebral blood flow reductions that contribute to secondary hypoxia/ischemia, even after mild TBI (Navarro et al., 2012; Strebel et al., 1997).
Both systemic and cerebrovascular hemodynamic regulatory mechanisms are disrupted in poly-neurotrauma. Specifically, TBI exacerbates the cardiovascular decompensation that occurs during HS by decreasing blood pressure regulation (Yuan and Wade, 1992) and by reducing cardiovascular compensatory mechanisms (Goldstein et al., 1998; McMahon et al., 2008, 2011; Yuan et al., 1991; Yuan and Wade, 1991), including the modulation of systemic vascular tone (Law et al., 1996). Similarly, TBI induces inflammatory processes (Hazeldine et al., 2015; Hellewell et al., 2016; Witcher et al., 2015) that are exacerbated by systemic pathophysiological changes with HS (Gyoneva and Ransohoff, 2015; Liu et al., 2015), further contributing to systemic organ failure through microvascular shunting, coagulopathy, blood stasis, capillary occlusion and acidosis (Hinson et al., 2015; Lozano et al., 2015). Among these effects, the most detrimental are the mediators of inflammatory and oxidative stress (e.g., reactive oxygen species), which affect all organs including the brain (Namas et al., 2009).
Coincident TBI also interferes with compensatory mechanisms that reestablish normal physiologic processes and restore tissue homeostasis, which exacerbates disease and complicates treatment strategies. Moreover, correction of systemic imbalances that prevent or treat injuries associated with one condition may exacerbate injuries associated with the other. For example, while rapid fluid restoration to restore tissue perfusion may prevent HS-induced hypoxic/ischemic damage in the kidneys or gastrointestinal tract, it has the potential to worsen TBI-related brain pathology (Falk, 1995; Ramming et al., 1994). Knowledge of pathogenesis of combined TBI and SBL is essential for improving diagnostic and prognostic assays and for refining treatment protocols, but the interaction of these disease processes remains poorly understood.
5. Literature review criteria
A comprehensive audit of the literature was conducted through PubMed for all peer-reviewed original research articles (no case studies or reviews) featuring large animal models of combined TBI and SBL published or e-published in English prior to December 1st, 2017 (see Fig. 1 for Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram). The following search parameters returned a total of 154 publications: “(large animal OR swine OR horse OR sheep OR pig) AND (exsanguination OR blood loss OR hemorrhagic shock) AND traumatic brain injury.” The abstracts of all articles were subsequently screened, with full texts consulted as needed. A total of 106 articles were then excluded due to 1) lack of concurrent TBI injury; 2) no concurrent SBL; 3) neither TBI nor SBL; 4) non-representative TBI model; 5) review article; 6) not performed in large animal model; 7) duplicate entry; or 8) use of an experimental cohort drawn from multiple other existing publications. The non-representative TBI models utilized the direct application of liquid nitrogen to the brain to create a lesion, which does not reflect the types of injuries that are typically observed in human models (Vink, 2018). Therefore, a total of 48 published articles met the requirements for inclusion in the systematic literature review.
Fig. 1.
Review Process. Fig. 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram detailing the identification, screening, evaluation, and inclusion and exclusion of published studies at each stage of the current systematic review.
The primary focus of the current review is on methodology (Table 1) and outcomes (Supplemental Table 1) in large animal studies of poly-neurotrauma. Table 1 includes core recommended common data elements for preclinical TBI models including basic animal characteristics (e.g., species, age, sex) and methods for randomization (Smith et al., 2015). Extracted TBI data included model type, parameters relating to injury severity, number of injuries, and injury location (laterality and region). Extracted blood loss data included model type (e.g., uncontrolled hemorrhage versus blood loss based on volume, or physiological parameters), injury site(s), rate, eTBV conversion factor (e.g., 60–75 ml/kg), model lethality, and modifications made to the hemorrhage phase to limit mortality. Finally, the therapeutic agent or group assignment, time of agent administration, survival time following resuscitation, and outcome of the study are reported in Supplemental Table 1, while all extracted parameter information can be found in Supplemental Table 2.
Table 1.
Summary of TBI + SBL large animal poly-neurotrauma models.
| Author/Year | Animal model | TBI Model + SBL Site | Blood Loss Model/Rate | Other Physiological Mitigations | Model Lethality Rate |
|---|---|---|---|---|---|
| Bambakidis et al., 2016 | 10 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 30; saline to MAP > 35 | 0% |
| Bambakidis et al., 2017 | 10 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 27; saline to MAP > 30–35 | 0% |
| Brodhun et al., 2001 | 24 JuvS Farmǂ (sex NS) | (L/Pa)FPI + FA | 38.5%a (2.1%/min) | None | NS |
| Crookes et al., 2004 | 22 JuvS M/F Farm | (L/FrP)FPI + FA | 45% (1.50%/min) | None | 18.18% |
| 31 JuvS M/F Farm | (L/FrP)FPI + FA | 45% (1.50%/min) | None | 35.48% | |
| Dekker et al., 2014a | 10 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 30; saline to MAP > 35 | 0% |
| Dekker et al., 2014b | 10 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 30; saline to MAP > 35 | 0% |
| Dekker et al., 2014c | 15 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.00%/min) | None | 0% |
| Fabian and Proctor, 2002 | 28 JuvE M/F Farm | (L/FrP)FPI + FAǂ | 40% tri-phasic (1.30%/min) | 10 min pause between steps | EtOH: 9.09%; H2O: 23.53% |
| 20 JuvE M/F Farm | (L/FrP)FPI + FAǂ | 45% tri-phasic (1.50%/min) | 10 min pause between steps | All groups: 0% | |
| Feinstein et al., 2005 | 39 JuvE M/F Farm | (L/FrP)FPI + FA | MAP < 30 held 30 min (46.2%a; rate NS) | None | 5.13% |
| Fritz et al., 2005 | 13 JuvS F Farmǂ | (R/Pa)FPI + FAǂ | 30% (1.67%/min) | None | All groups: 0% |
| Georgoff et al., 2017 | 15 JuvE F Yrk | (R/Fr)CCI + FA + Poly | 40% bi-phasic (1.50%/min pre-Poly; 1.67%/min post) | Pause MAP < 30; saline to MAP > 35 | 20% |
| Gibson et al., 2002 | 26 JuvE M/F Farm | (L/FrP)FPI + CA | 30% tri-phasic (0.60%/min) | None | 33.30% |
| 11 JuvE M/F Farm | (L/FrP)FPI + CA | 35% tri-phasicǂ (1.17%/min) | None | 0% | |
| Glass et al., 1999 | 12 JuvS M/F swine (strain NS) | (Lǂ/FrP)FPI + FA | 1) 35% tri-phasic (1.67%/min); or 2) 40% tri-phasic (1.33%/min) | None | NS |
| Glass et al., 2001 | 10 JuvS M/F Farm | (L/FrP)FPI + FA | 40% (1.33%/min) | None | NS |
| Halaweish et al., 2015a | 10 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 27; saline to MAP > 30–35 | 0% |
| Halaweish et al., 2015b | 10 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | None | 0% |
| Halaweish et al., 2016 | 10 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 27; saline to MAP > 30–35 | 0% |
| Hariri et al., 1993 | 13 F Yuc (age NS) | (R/Fr)FPI + FA | MAP = 50–55 | None | NS |
| Hwabejire et al., 2013a | 10 JuvE F Yrk | (R/Fr)CCI + Poly then FA | Free, then to 40% (3.15%/min) | Pause MAP < 30; saline to MAP > 35 | 0%ǂ |
| Hwabejire et al., 2013b | 9 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 30; saline to MAP > 35 | 0%ǂ |
| Imam et al., 2013a | 15 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 30; saline to MAP > 35 | 10% |
| Imam et al., 2013b | 15 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.00%/min) | Pause MAP < 30; saline to MAP > 35 | 0% |
| Imam et al., 2015 | 12 JuvE F Yrk | (R/Fr)CCI + LL then FA | 250 mL free, then to 40% (3.15%/min) | Pause MAP < 30; saline to MAP > 35 | 0% |
| Jin et al., 2012a / Sillensen et al., 2017 | 15 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 30; saline to MAP > 35 | 10% |
| Jin et al., 2012b | 21 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 30; saline to MAP > 35 | 10% |
| Johnson et al., 2017 | 21 Juv/AdE M/F Cross | (R/Fr)CCI + JV | 25% (rate NS) | None | NS |
| King et al., 2004 | 28 Juv/AdE M/F Farm | (L/FrP)FPI + FA | MAP = 25 held 30 min (43.1%a; rate NS) | None | 14.29% |
| 15 Juv/AdE M/F Farm | (L/FrP)FPI + FA | BIS > 1 (43.1%a; 7.2%/minǂ) | None | 20% | |
| King et al., 2005 | 21 Juv/AdE M/F Farm | (L/FrP)FPI + FA | MAP = 30 held 30 min (48.8%a; 7.7%/min to target) | None | 28.57% |
| 8 Juv/AdE M/F Farm | (L/FrP)FPI + FA | MAP = 30 held 30 min (48.8%a; 7.7%/min to target) | None | 25% | |
| Malhotra et al., 2004 | 33 JuvE M/F Farm | (L/Pa)FPI + FA | 45% (1.50%/min) | None | 0% |
| Nikolian et al., 2017 | 10 JuvE F Yrk | (R/Fr)CCI + FA + Poly | 40% bi-phasic (1.50%/min pre-Poly; 1.67%/min post) | Pause MAP < 30; saline to MAP > 30 | 10% |
| Patel et al., 2006 | 26 JuvE M/F Farm | (L/FrP)FPI + FA | MAP = 20 held 30 min (47.3%a; rate NS) | None | 23.10% |
| Rosenthal et al., 2008 | 20 JuvE M Yrk | (R/Fr)CCI + FA | MAP = 40 held 40 min (1.65%/min to target) | None | 0% |
| Sanui et al., 2006 | 19 JuvE Farm (sex NS) | (L/FrP)FPI + FA | BIS > 1 (50.0%a; 8.3%/min) | None | 16% |
| 14 JuvE Farm (sex NS) | (L/FrP)FPI + FA | BIS > 1 (50.0%a; 8.3%/min) | None | 29% | |
| Sillesen et al., 2013a | 12 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 30; allowed time to recover | NS |
| Sillesen et al., 2013b / Sillesen et al., 2014b | 27 JuvE F Yrk | (R/Fr)CCI + FA | 40% (3.15%/min) | Pause MAP < 30; saline to MAP > 35 | NS |
| Sillesen et al., 2014a | 12 JuvE F Yrk | (R/Fr)CCI + Poly, then FA | 250 mL free, then to 40% (3.15%/min) | Pause MAP < 30; saline to MAP > 35 | 0% |
| Stern et al., 2000 | 24 JuvS swine (sex/strain NS) | (R/Pa)FPI + FA, then AT | MAP = 50 (1.8%/mina rolling), then AT to MAP = 30 | None | 4.17% |
| Stern et al., 2009 | 32 JuvS F Yrk | (R/FrP)FPI + LL | 15 min free (34.6%a; 2.3%/min); 75 min bleeding during resuscitation | None | 0% |
| Teranishi et al., 2012 | 26 JuvS F Yrk | (R/FrP)FPI + LL | 15 min free (34.6%a; 2.3%/min); 15 min bleeding during resuscitation | None | 0% |
| Vrettos et al., 2016 | 12 JuvS F swine (strain NS) | (M/Pa)FPI + AT | MAP = 30 (rate NS) | None | 0% |
| White et al., 2013 | 21 JuvS F Cross | (R/Pa)FPI + FA, then AT | MAP = 50 (21.5%a; rolling), then AT to MAP < 30 | None | NS |
| Zink et al., 1998a | 22 JuvS M/F swine (strain NS) | (R/Pa)FPI + FA | 38.5%a (rolling rate) | None | EtOH: 91%; Saline: 65% |
| Zink et al., 1998b | 18 JuvS Yrk (sex NS) | (R/Pa)FPI + FA | 46.2%a (rolling rate) | None | EtOH: 56%; Saline: 0% |
| Zink et al., 1999 | 26 JuvS Yrk (sex NS) | (R/Pa)FPI + FA | 46.2%a (rolling rate) | None | EtOH: 30.77%; Saline: 7.69% |
| Zink et al., 2001 | 40 JuvS swine (sex/strain NS) | (R/Pa)FPI + FA | 46.2%a (rolling rate) | None | EtOH: 15%; Saline: 0% |
| Zink et al., 2006 | 34 JuvS swine (sex/strain NS) | (R/Pa)FPI + FA then AT | MAP = 50 (46.2%a; rolling), then AT to MAP = 30 | None | EtOH: 7.14%; H2O: 0%; Control: NS |
Notes: This table contains results of a literature review of large animal models of combined traumatic brain injury and severe blood loss (TBI + SBL). As the focus is therapeutic effects within the combined model, article reports cover only methodology pertaining specifically to TBI + SBL groups. Where assumptions were made based on incomplete or non-explicit information, cells have been marked with a ǂ. If body weight conversion factor was not provided in the article, 0.65 was used to calculate approximate blood volume and subsequent loss total and/or rate given other provided information and marked with a a. For blood loss model, the primary target is indicated outside of the parentheses. Abbreviations include– for age: juvenile as specified in the article (JuvS), juvenile estimated based on reported weight range and strain (JuvE), or mixed juvenile/adult estimate by range (Juv/AdE); for sex: female (F), male (M), or mixed (M/F); for strain: cross-bred (Cross), farm variety (Farm), Yorkshire (Yrk), or miniature Yucatan (Yuc); for injury site: left (L), midline (M), or right (R) in frontal (Fr), fronto-parietal (FrP), or parietal (Pa) lobe brain areas; for TBI model: controlled cortical impact (CCI) or fluid percussion injury (FPI); for SBL site: aortic tear (AT), common carotid artery (CA), femoral artery (FA), liver laceration (LL), polytrauma (Poly), or jugular vein (JV); for blood loss target: bispectral index (BIS), removed estimated total blood volume (%) and rate (%/min), or mean arterial pressure (MAP; reported in mmHg); for model lethality: water (H2O) and alcohol (EtOH); across most columns: information not specified (NS).
Each article was independently reviewed by two raters (AD/BW/DS/PS), with a third rater providing resolution for any conflicts. Cells in Table 1 were marked with a ≠ whenever assumptions were made based on incomplete or non-explicit information for several fields. Finally, to avoid erroneous inflation of quantitative meta-statistics, we determined which studies utilized either completely unique (N = 44 articles) or overlapping samples (N = 4 articles, 2 samples for meta-statistics). Overlap was determined based on comparisons of previous publications from the same group or through direct contact with the corresponding author. Six of the articles included multiple experiments, each utilizing a unique sample, and were rated separately for inclusion in meta-statistics for a total of 52 unique experiments (Fig. 1).
6. Literature summary
A total of twenty experiments with unique samples have been published by a group based out of the University of Michigan Hospital (Bambakidis et al., 2016, 2017; Dekker et al., 2014a, b; Dekker et al., 2014c; Georgoff et al., 2017; Halaweish et al., 2015a, b; Halaweish et al., 2016; Hwabejire et al., 2013a, b; Imam et al., 2015, 2013a; Imam et al., 2013b; Jin et al., 2012a, b; Nikolian et al., 2017; Sillesen et al., 2013a, b; Sillesen et al., 2014a, b; Sillesen et al., 2017). The Michigan group evaluated the efficacy of various medical countermeasures in a swine model of combined CCI and SBL of approximately 40%. In this model, juvenile female Yorkshire swine were subjected to controlled blood loss from the femoral artery following a CCI. In several of these experiments (Georgoff et al., 2017; Hwabejire et al., 2013a; Imam et al., 2015; Nikolian et al., 2017; Sillesen et al., 2014a), TBI was combined with additional trauma, including liver or spleen laceration, rib fracture and rectus abdominis muscle crush. Blood loss from the femoral artery was typically controlled at a rate of 3.0–3.2% removed eTBV (reTBV)/minute. In the majority of experiments, blood loss was paused when MAP dropped below a critical threshold (either 27 or 30 mmHg), at which time IV fluid therapy was initiated until the MAP was partially restored (i.e., > 30–35 mmHg, study-dependent). Animals were mechanically ventilated throughout all study procedures.
When reported, this model was associated with either no (Bambakidis et al., 2016, 2017; Dekker et al., 2014a, b; Dekker et al., 2014c; Halaweish et al., 2015a, b; Halaweish et al., 2016; Hwabejire et al., 2013a, b; Imam et al., 2015, 2013b; Sillesen et al., 2014a) or low (i.e., 10–20%; Georgoff et al., 2017; Imam et al., 2013a; Jin et al., 2012a, b; Nikolian et al., 2017; Sillesen et al., 2017) mortality, presumably because of the physiological mitigations that prevented lethal hypovolemic shock (e.g., mechanical ventilation, pausing of blood loss based on physiological parameters, and small volume resuscitation). Large volume experimental therapies were typically initiated 1–2 h following the completion of blood loss. The Michigan group studies have been instrumental in characterizing the effects of various crystalloid and colloid replacement fluid therapies on coagulation and gene expression profiles in the brain. Further, these studies have provided preliminary evidence to support the use of valproic acid (VPA), a histone deacetylase inhibitor, as a pre-hospital small volume bolus to modulate gene expression and platelet function in the brain and increase survival following poly-neurotrauma.
Five studies from the Michigan group (Georgoff et al., 2017; Halaweish et al., 2015a, b; Halaweish et al., 2016; Nikolian et al., 2017) have also assessed cognitive ability following resuscitation from TBI + SBL using a food-motivated learning task (Alam et al., 2002). In general, these studies showed that most treatments beyond basic isotonic saline solution resulted in both lower impairment in the ability to learn or remember the task near the time of the injury and a quicker recovery towards pre-injury performance.
In addition to the Michigan laboratory, two other independent studies (Johnson et al., 2017; Rosenthal et al., 2008) have been published using the CCI poly-neurotrauma model. Johnson and colleagues (2017) used mixed-sex Yorkshire-cross swine to test the therapeutic efficacy of partial or complete aortic occlusion as compared with fluid resuscitation or no treatment. Following TBI by CCI, animals were subjected to blood loss of 25% reTBV from the jugular vein at an unspecified rate. Therapeutic intervention (i.e., occlusion) began 30 min following blood loss, with whole blood resuscitation starting at 90 min. Rosenthal et al. (2008) used juvenile male Yorkshire swine to test the therapeutic efficacy of a small-volume hemoglobin-based oxygen-carrying solution to improve cerebral perfusion and oxygen delivery to mitigate secondary brain injury. In this study, CCI preceded blood withdrawal from the femoral artery at a rate of 1.65% reTBV/min until MAP decreased to 40 mmHg. Additional blood was withdrawn in order to maintain this MAP for a 40-min hypotensive period, and therapy was initiated 15 min following the end of blood loss.
Several other groups have modeled combined FPI and SBL to test various medical countermeasures related to poly-neurotrauma. In studies performed at the University of Miami School of Medicine (Crookes et al., 2004; Fabian and Proctor, 2002; Feinstein et al., 2005; Gibson et al., 2002; Glass et al., 1999, 2001; King et al., 2004, 2005; Malhotra et al., 2004; Patel et al., 2006; Sanui et al., 2006), juvenile mixed-sex farm swine were subjected to lateral FPI of variable severity followed by blood loss from either the femoral or common carotid artery. Blood removal was determined by either fixed volume or by pre-determined physiological parameters, including MAP or neurologic activity (measured by bispectral index). For the experiments in which blood loss was fixed by volume (Crookes et al., 2004; Fabian and Proctor, 2002; Gibson et al., 2002; Glass et al., 1999, 2001; Malhotra et al., 2004), total blood loss ranged from 30 to 45% reTBV, and was often divided into three phases, with a decreased percentage of blood being withdrawn during each sequential phase. This stepwise protocol is designed to mimic the dynamic rate of blood loss that occurs during free hemorrhage (Frankel et al., 2007). In each of these experiments, the average rate of blood loss ranged from 0.6 to 1.67% reTBV/min.
Other experiments by the Miami group utilized MAP as a physiological marker for determining blood loss (Feinstein et al., 2005; King et al., 2004, 2005; Patel et al., 2006), withdrawing blood rapidly (7.7% reTBV/min) until the MAP dropped to a designated threshold (i.e., 20–30 mmHg experiment-dependent), after which additional blood was withdrawn to maintain MAP for a 30-min hypotensive period. Similarly, for experiments in which blood loss was determined based on neurologic activity (King et al., 2004; Sanui et al., 2006), blood was withdrawn until a designated bispectral index score was reached, indicating a suppressed level of consciousness. Mortality, when reported, ranged from 0 to 35% across these experiments. The Miami model has been useful for evaluating the therapeutic value of hemoglobin-based oxygen carriers (King et al., 2005; Malhotra et al., 2004; Patel et al., 2006), arginine vasopressin (Feinstein et al., 2005; Sanui et al., 2006), and phenylephrine (Feinstein et al., 2005; Patel et al., 2006) in maintaining cerebral perfusion and improving survival following combined poly-neurotrauma.
Unique to the Miami group studies is use of a modified version of the Veterinary Coma Scale (Hilton et al., 1993) to assess neurological impairment (motor and respiratory function, eyelid reflexes) during the recovery period (Gibson et al., 2002; Glass et al., 1999, 2001; King et al., 2004, 2005). Generally, between-treatment findings related to this scale were inconclusive due to low severity of injury (Gibson et al., 2002; Glass et al., 1999) or insufficient survivability from which to draw conclusions (King et al., 2005). When scale differences were present, it was most obvious in motor function rather than respiratory or eyelid response (Glass et al., 2001; King et al., 2004).
In studies performed by the University of Washington laboratory (Stern et al., 2009, 2000; Teranishi et al., 2012; White et al., 2013; Zink et al., 1998a, b; Zink et al., 1999, 2001; Zink et al., 2006), lateral FPI preceded controlled blood loss by either the femoral artery with or without an aortic tear, or by a grade 3 liver laceration. Animals used in these studies were juvenile Yorkshires (Stern et al., 2009; Teranishi et al., 2012; Zink et al., 1998b, 1999) or an unspecified swine breed (Stern et al., 2000; White et al., 2013; Zink et al., 1998a, 2001, 2006). Two studies utilized uncontrolled hemorrhage via liver laceration 15 min prior to administration of resuscitative fluids, with either an additional 15 (Teranishi et al., 2012) or 75 (Stern et al., 2009) min delay preceding surgical repair of the liver injury. Controlled hemorrhage lasted for 30 min in some studies (Zink et al., 1998a, b; Zink et al., 1999, 2001) or until a preliminary target MAP (i.e., 50 mmHg) was attained followed by uncontrolled hemorrhage from an aortic tear down to a secondary target (MAP =30 mmHg; Stern et al., 2000; White et al., 2013; Zink et al., 2006).
For the controlled blood loss studies conducted at the University of Washington (Stern et al., 2000; White et al., 2013; Zink et al., 1998a, b; Zink et al., 1999, 2001; Zink et al., 2006), the rate of blood removal was dynamic, starting high and decreasing exponentially throughout the procedure, with average rates of 1.3%–1.8% reTBV/min. The rate of uncontrolled blood loss through liver lacerations over 15 min was reported to be 2.3% reTBV/min (Stern et al., 2009; Teranishi et al., 2012). Because many of these studies were designed to measure survivability (Stern et al., 2009, 2000; Zink et al., 1998a, b), the mortality rate using this model was reported to be as high as 65% for vehicle-treated controls (Zink et al., 1998a). This model has been advantageous in characterizing the detrimental effects of ethanol on the outcome of combined poly-neurotrauma (Zink et al., 1998a, b; Zink et al., 1999, 2001; Zink et al., 2006).
In addition to the Miami and Washington laboratories, several other groups (Brodhun et al., 2001; Fritz et al., 2005; Hariri et al., 1993; Vrettos et al., 2016) have independently developed variations of the FPI poly-neurotrauma model. A German group (Brodhun et al., 2001; Fritz et al., 2005) and a group from New York (Hariri et al., 1993) have used the combined lateral FPI and SBL model to characterize the effects of fluid resuscitation on intracranial pressure and neuronal injury (Fritz et al., 2005; Hariri et al., 1993). The German group used juvenile cross-bred swine in a combined FPI and SBL model, in which blood (30–38.5% reTBV) was withdrawn from the femoral artery for evaluating the therapeutic effect of mild hypothermia on intracranial pressure and neuronal damage after 24 h (Brodhun et al., 2001). Hariri et al. (1993) instead used miniature Yucatan swine, and blood was removed incrementally until a target MAP of 50–55 mmHg was reached. Each of these models permitted prolonged monitoring up to 6 (Hariri et al., 1993) or 24 (Brodhun et al., 2001; Fritz et al., 2005) h post-injury. Finally, Vrettos et al. (2016) used juvenile female swine in a combined midline FPI and partial aortic tear model, with uncontrolled hemorrhage continuing to a target MAP of 30 mmHg. This study evaluated the effect of permissive hypotension versus fluid resuscitation on survival.
7. Metastatistics
Meta-statistics based on the total number of unique published articles only (N = 48) will be identified using the term “studies”, whereas meta-statistics based on all unique samples of animals (N = 52) will be identified using the term “experiments”. To illustrate the importance of common reporting standards (Smith et al., 2015), statistics that were not explicitly stated in experiments (e.g., a failure to report biological sex of animals or the rate of blood withdrawal) were included in the denominator to denote missing data and are represented in all figures. A review of the literature suggests that 88.5% of the 52 combined TBI and SBL experiments were performed by three scientific groups, with relatively consistent use of the same animal model among each respective laboratory. Interestingly, all reviewed studies describe the exclusive use of swine, confirming them as the preferred large-animal species for poly-neurotrauma research. Although canine (Balbino et al., 2010) and sheep (Lehmann et al., 1995; Wisner et al., 1989) poly-neurotrauma models have been described, these studies were excluded during the review process due to the use of atypical TBI methods (e.g., pharmaceutical injury). Among the 52 swine experiments, the majority specify the use of Yorkshire, farm or cross-bred strains. Only a single study utilized miniature swine (Yucatan) and another 11.5% (6/52) of experiments failed to specify strain. Only 47.1% (24/51) of experiments explicitly mention the assignment of animals to treatment groups prior to initiating TBI and SBL, potentially increasing the risk for experimental bias.
Somewhat surprising, approximately two-thirds of experiments (Fig. 2A) were either based on the utilization of a single sex (51.9%; 27/52) or did not specify the biological sex of the animals (15.4%). Sex and associated steroid hormones have known effects on the response to shock (Hildebrand et al., 2013; Mizushima et al., 2000) and neurotrauma (Xiong et al., 2013). Although less well-studied than the cardiovascular system, emerging evidence suggests a complex and sexually divergent neurodevelopmental profile in swine (Conrad et al., 2012) that has also been characterized in humans (Mayer et al., 2018). It is, therefore, recommended that future large animal poly-neurotrauma studies involve both biological sexes to improve clinical translation. Another unexpected finding from the review was that 88.5% (46/52) of all TBI and SBL experiments were conducted on sexually immature, juvenile swine (Fig. 2B).
Fig. 2.
Demographic Characteristics. Fig. 2 presents demographic information of swine utilized in the included experiments (N = 52). Panel A displays proportional data of biological sex of swine: female only (F; blue), male only (M; red), and both male and female (M/F; green). Studies that did not specify (NS; yellow) sex of swine are represented as well. Panel B presents age categories of swine: juvenile (either specified [JuvS; green] or estimated based on reported weight range [JuvE; blue]), mixed juvenile and adult estimated based on weight (Juv/AdE; red), or NS. NS studies neither specified age category nor provided a weight range from which to estimate age based on animal strain. Panel C presents the strain of swine used: Yorkshire (Yrk; cyan), Farm (red), Cross-bred (Cross; blue), miniature Yucatan (Yuc; green), and NS. Panel D presents weight information reported in alphabetical order by the included experiments (N = 52). These are delineated dependent on if the experiment supplied weight range (red line), mean (blue triangle) and two times the standard deviation (2SD; blue line), or an approximation (e.g., ~20) of weight (black dot). Those that did not report any information for weight are represented as blank spaces.
From a common data elements perspective (Smith et al., 2015), 69.23% (36/52) of experiments did not explicitly specify sexual maturity such that this critical metastatistic had to be estimated based on weight and strain. It was assumed that Yorkshire or farm strains of swine less than or equal to 50 kg at the time of the study were sexually immature juveniles (using entire range presented in article), based on published weight ranges for laboratory swine (Swindle et al., 2012; Tummaruk et al., 2008). Weight varies as a function of strain (Fig. 2C), age, and biological sex, as well as feeding schedule, but is critical variable for estimating total blood volume (Swindle and Smith, 2015). The majority (88.5%; 46/52) of experiments specifically reported utilizing animals between the weights of 20–50 kg across various strains (Fig. 2D).
While the use of juveniles swine facilitates animal housing and handling due to lower body weight, there are known differences for both heart rate (inversely related to age) and heart to body mass ratio (Swindle et al., 2012). Various strains of miniature swine (Yucatan, Gottingen, Hanford, etc.) provide the benefit of smaller size at sexual maturity, with Hanford swine exhibiting the closest match in terms of cardiovascular morphology to humans at sexual maturity (Swindle et al., 2012). Cardiovascular maturation (Swindle et al., 2012) and neural development (Finnie, 2012) occurs at approximately the same rate in swine as humans, rendering it an ideal animal model for comparative studies on development. To date, only a handful of studies have examined the effect of age on polytrauma in large animal models (reviewed in Hildebrand et al., 2013). However, based on these developmental timelines, the majority of published poly-neurotrauma studies in swine may more appropriately translate to human children and young adolescents rather than adults. Similarly, only 21.2% (11/52 experiments) performed any type of behavioral or neurological assay to quantify the effects of poly-neurotrauma on outcomes. Thus, future studies would benefit from an increased focus in this area to improve translation to human studies.
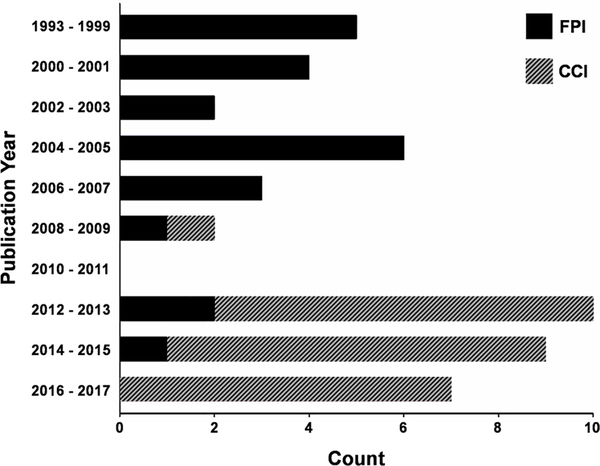
Fig. 3 indicates that 40.4% of the combined TBI and SBL large animal experiments were published in the 5 years prior to our literature review (December of 2017), suggesting the growing importance of the field. All studies utilized either FPI (57.7%; solid bars in Fig. 3) or CCI (hatched bars in Fig. 3) to initiate the TBI, with both models noted for their high levels of reproducibility (Xiong et al., 2013). Across both models, TBI exposures occurred in a lateral (98.1%; 51/52) rather than midline (1.9%; 1/52) plane, with exact injury focus occurring in frontal (44.2%; 23/52), parietal (21.2%; 11/52) or fronto-parietal (34.6%; 18/52) sites (Fig. 4). Although injury severity represents a challenging construct to quantify in both preclinical and clinical research (Smith et al., 2015; Vink, 2018; Xiong et al., 2013), a plurality of reviewed poly-neurotrauma studies classified (per author specification) their TBI as “moderate” or “severe”. Importantly, the FPI and CCI models all involved a craniotomy, and thus may not accurately mirror the types of closed head injuries that characterize the majority of human TBI.
Fig. 3.
Number of Articles per Year with TBI Model. Fig. 3 presents the frequency of publications for included studies (N = 48) regarding large animal models of traumatic brain injury (TBI) with combined severe blood loss. Except for the first bin (1993–1999), years are grouped into 2-year intervals. Bars are shaded depending on the proportion of studies utilizing a fluid percussion injury (FPI; solid) or a controlled cortical impact (CCI; hatched) TBI model.
Fig. 4.
TBI Impact Locations. Fig. 4 plots the number of experiments (N = 52) which produced traumatic brain injury (TBI) at various regions on the brain. Injury locations were categorized both in terms of hemisphere [left (L), right (R) or midline (M)] and general lobe [frontal (Fr), fronto-parietal (FP), or parietal (Pa)] of the brain. Locations are meant to be representative and may not reflect exact areas across all categorized experiments. Picture adapted from skull scanned by the University of Texas High-Resolution X-ray CT Facility (NSF IIS-0208675) located at http://digimorph.org/specimens/Sus_scrofa/skull/.
Most (90.9%; 20/22) of CCI experiments were performed by the Michigan group (Fig. 3). All of the Michigan experiments can be further sub-divided into two general patterns based on reported model parameters (see Supplemental Table 2 for detailed parameter information). Lower target parameters (4 m/s velocity; 100 ms dwell time; 8 mm penetration; 12.65 mm impact tip diameter) were used in 6 studies (27.3%). The more severe target (14 studies; 63.6%) maintained the same impact velocity and dwell time, but had deeper penetration (12 mm) and a larger impact tip (15 mm). The remaining two CCI experiments had similar impact velocity and penetration to the Michigan target, but with either shorter (200 μs; Johnson et al., 2017) or longer (400 ms; Rosenthal et al., 2008) dwell times. FPI parameter reporting was more inconsistent. The majority of FPI experiments (90%; 27/30) reported some targeted or measured injury pressure, but these encompassed a broad range (2.5–10 ATM) and were variably classified as “mild,” “moderate,” or “severe”. When specified, the bore tube diameter was typically less than or equal to 16 mm.
Fig. 5A indicates the percentage of experiments that utilized volume loss, MAP, bispectral index, uncontrolled hemorrhage, bispectral index plus MAP or a combination of models in their poly-neurotrauma experiments. Most of experiments achieved blood loss through withdrawal from the femoral (73.1%) or carotid (3.8%) artery only (Fig. 5B), with others adopting models simulating trauma to a single (5.8%; 3/52) or multiple (15.4%; 8/52) body organs. For volume-based or combination models, 83.3% (30/36) of studies simulated hemorrhage between 35–45% (i.e., Class III or IV trauma) of estimated total blood volume (Fig. 5C). Further, most experiments used either a fixed (51.9%; 27/52) or a gradually decreasing (13.5%; 7/52), controlled rate of blood withdrawal. Other experiments utilized uncontrolled blood loss (5.8%; 3/52), a mixture of both controlled and uncontrolled blood loss (11.5%; 6/52) or did not provide enough information to determine the type of model (17.3%; 9/52).
Fig. 5.
Blood Loss Models and Volume Loss. Fig. 5 presents experimental (N = 52) variables of severe blood loss models including removed estimated total blood volume (% reTBV). Panel A presents the proportion of experiments primarily utilizing various blood loss models including to a specific target volume (Vol; cyan), mean arterial pressure (MAP; yellow), or bispectral index score (BIS; blue), a combination of BIS + MAP targets (red), and free hemorrhage (green). Importantly, some models combined blood loss with a specific MAP target (e.g., titrating withdrawal if a certain MAP was reached). Panel B presents information on site of blood loss including femoral artery (FA; green), femoral artery plus additional trauma (FA + Poly; yellow), common carotid artery (CA; red), jugular vein (JV; cyan), via a liver laceration (LL; black) or via an aortic tear (AT; blue). Panel C demonstrates the range of % reTBV (bin widths of 5%) reported by the various blood loss models.
The average rate of blood loss was 2.18% reTBV/min among the fixed rate hypovolemic models, with 7/27 of the fixed rate experiments simulating blood loss in a biphasic/triphasic fashion. Of note, 76.9% (40/52) of experiments did not specify exactly how total blood volume was estimated. As demonstrated in Fig. 6, the utilization of scaling factors ranging between 60–75 ml/kg (Fulop et al., 2013; Swindle et al., 2012) can result in up to a 25% difference in the amount of blood that is withdrawn, representing an important and controllable parameter in the literature that should be reported in all experiments.
Fig. 6.
Weight to Blood Volume Ratio. Fig. 6 plots the relationship between weight and estimated total blood volume (eTBV) as a function of utilizing different conversion scalars [60 ml/kg (dashed-dotted line), 65 ml/kg (solid line), 70 ml/kg (dotted line), and 75 ml/kg (long-dashed line)] reported in the literature. The dispersion between eTBV increases in proportion to weight and can result in a difference of 25% more blood removal across the various scalars. The conversion scalar used for the calculation of eTBV was not reported in 40/52 experiments.
Finally, the overall pre-treatment lethality rate was relatively low (mean = 9.28% range = 0–65%), even though animals remained completely “untreated” for 1–2 h post blood loss in 46.2% (24/52) of experiments. This experimental rate is significantly lower than the approximate 30% mortality rate that has been estimated to occur in Class III and IV human trauma, even with shorter average treatment times (American College of Surgeons Committee on Trauma, 2004). The inflated survival rates in animal poly-neurotrauma models are likely owed to various experimental methodologies that were utilized, which are typically not a component of the “untreated” phase of human poly-neurotrauma (reviewed in Hildebrand et al., 2013).
For example, all studies utilized some form of anesthesia (e.g., isoflurane was used in some capacity for 61.5% of experiments), which is required for the ethical conduct of animal research but is not present prior to/during human injury. Anesthetic protocols are variable, and extremely difficult to standardize across animals. Animals are frequently also pretreated with agents like heparin to facilitate medical procedures and these procedures can potentially have protective (Rana et al., 1992) or deleterious downstream consequences. Moreover, 90.4% of experiments utilized mechanical ventilation (typically with a volume-cycled ventilator) either throughout the entire poly-neurotrauma protocol or on an “as needed” basis. An additional 7.7% of studies reported exclusively using mechanical ventilation either before or after the blood loss procedure, with the latter more closely mirroring what would potentially happen in a typical trauma scenario (i.e., ventilation during treatment phase rather than during injury).
These first two methodological confounds are not unrelated, as mechanical ventilation is frequently used to counter the known respiratory depressive properties of most anesthetic agents. However, respiratory depression is the most common cause of death in isolated, moderate-to-severe TBI models, which is frequently experimentally mitigated by the use of mechanical ventilation either prior to or immediately post-injury (Xiong et al., 2013). Mechanical ventilation also interferes with the typical pathophysiological cascade of TBI and SBL, by overriding the compensatory hyperventilation response that is critical to restoring systemic acid-base balance during HS (Gutierrez et al., 2004, 2013). Therefore, depending on the ventilation protocol and the animal’s physiological state, mechanical ventilation may be either beneficial or detrimental to the animal’s survival (Hildebrand et al., 2013).
A third set of mitigating experimental procedures included starting and stopping hemorrhage based on either physiological parameters (e.g., MAP, heart rate) or the passage of a set time window, as well as performing small volume fluid resuscitation based on critical physiological markers. Each of these study designs are atypical of most human poly-neurotrauma scenarios, and may account for the overall higher survival rates when compared with human clinical data. To more clearly delineate lethality rates in large animal poly-neurotrauma studies, experiments that did not report lethality rates (15.4%; 8/52) were first excluded. Remaining experiments were then reclassified into two separate categories (Fig. 7A) that either utilized physiological mitigations plus mechanical ventilation (18/52; red symbols in Fig. 7) or those that only utilized mechanical ventilation (26/52; blue symbols in Fig. 7). In experiments that administered drugs/therapies prior to poly-neurotrauma (7/52), only rates from control arms were utilized in subsequent lethality analyses.
Fig. 7.
Lethality Rate Information. Fig. 7 presents information of the distribution of the lethality rate between experiments utilizing mechanical ventilation (MV) and MV plus physiological mitigations (MV + Aid), and the relationship between lethality rate and removed estimated total blood volume (% reTBV). Panel A presents the scatterplot distribution of lethality rates in experiments that utilized MV only (blue circles) or those that used MV + Aid (red triangles). Panel B represents the association of % reTBV with lethality rate for those studies where it was specified or estimable based on provided information (N = 41). Larger symbols are used to denote data points where multiple studies overlapped. A linear fit between lethality rate and % reTBV is also plotted.
Although methodologies varied widely across studies, results show a trend toward decreased lethality in experiments that used additional physiological mitigations (U = 170.5, p = .098). Fig. 7B plots lethality both as a function of physiological mitigations and % reTBV loss for experiments that specified this value or where it could be estimated (41/44 studies reporting lethality). Minimal variance in mortality rate was explained by % reTBV (β = 0.53; t = 1.12; r = 0.18; p = 0.27), suggesting that there are a multitude of other factors that influence survivability in complicated large animal poly-neurotrauma models. Collectively, these results suggest that current large animal models may be under-estimating pre-treatment mortality rates associated with poly-neurotrauma events.
Increased survival rate is financially more appealing, reduces ethical concerns that are frequently present in large animal models and reduces potential bias associated with treatment of non-viable animals. However, these manipulations also may complicate the translation of findings from poly-neurotrauma models into the clinic. Importantly, in most of the reviewed studies, the various procedures (mechanical ventilation, temporary cessation of bleeding, small volume resuscitation) were carefully controlled across experimental groups. Thus, while they may have ultimately influenced survival time and lethality rate, they likely had minimal impact on the primary study aims related to therapeutic efficacy.
8. Updates to the literature
One year passed between the initial literature search and the review of this article. During this period, four additional large animal TBI and SBL studies were published from the Michigan group (Georgoff et al., 2018; Nikolian et al., 2018a, b; Williams et al., 2019) and one from the Washington group (Dickson et al., 2018). Importantly, none of these studies report any degree of pre-treatment lethality (i.e., all 0%) and all used juvenile female Yorkshire swine exclusively.
In keeping with their previous publications, all Michigan group publications used a CCI model of TBI and 40% eTBV removed, with three articles (Nikolian et al., 2018a, b; Williams et al., 2019) performing controlled blood loss and the final (Georgoff et al., 2018) using a mixture of uncontrolled (liver/spleen laceration) and controlled blood loss with additional trauma (rectus abdominus crush). In at least two of these publications, animals received supplemental aid during hypovolemic shock (Georgoff et al., 2018; Williams et al., 2019). Williams et al. (2019) also had a longer survival time (30 days) relative to the other articles (6 h) and assessed NSS following treatment with exosomes derived from mesenchymal stem cells, observing faster recovery in exosome-treated animals. Collectively, these newer publications by the Michigan group provide further evidence supporting the use of VPA as a pre-hospital small volume bolus to modulate gene expression and increase survival following poly-neurotrauma.
Similar to other studies from the group (Stern et al., 2000; White et al., 2013; Zink et al., 2006), the new Washington publication (Dickson et al., 2018) used an FPI and a rolling rate of controlled blood loss to a MAP target (50 mmHg) followed by uncontrolled blood loss through an aortic tear down to a secondary target (MAP 30 mmHg), also including a femur fracture. This study used mechanical ventilation on an as-needed basis during hypovolemia. Importantly, the study determined that adding vasopressin as a treatment to increase blood pressure does not also increase brain perfusion, likely due to the accompanying increase in internal hemorrhage volume.
9. Conclusions and summary
In summary, the current systematic review provides a comprehensive analysis of the variables that contribute to large animal poly-neurotrauma models. Most of studies describe total blood loss volumes of less than 45% eTBV. Overall, published models are associated with very low lethality rates, but describe other potentially mitigating experimenter factors (e.g., mechanical ventilation prior to or during injury, start/stopping of blood loss based on physiological parameters, administration of small volume fluid resuscitation) that are rarely a component of human trauma. An inherent dichotomy exists between the necessity for experimental control and the preservation of a realistic model of poly-neurotrauma. Important interactions exist between comparative neuroanatomy, biomechanics and injury severity across species (Xiong et al., 2013), and different physiological/behavioral responses have even been reported within the same species across different murine strains following TBI (Reid et al., 2010; Tan et al., 2009). Large animal models serve as important platforms for characterizing the complex and multifaceted pathophysiology of poly-neurotrauma and provide an essential bridge for advancing promising therapeutics into humans.
Although no animal model can completely emulate human field trauma scenarios, attaining the closest approximation of these conditions requires consideration of the experimental factors that deviate from reality. Animal poly-neurotrauma models should, therefore, require minimal preparation time (i.e., reducing undue exposure to anesthesia and associated complications) and the TBI and SBL should occur in close temporal proximity. In addition to previously published criteria (Cho et al., 2009; Hildebrand et al., 2013; Majde, 2003), the selected poly-neurotrauma model should also: 1) utilize TBI and SBL models that have been independently validated in large animals; 2) produce a reliable injury; 3) produce acceleration/deceleration forces that closely mirror human injury; and 4) be readily scalable to humans. Our systematic review reinforces the need for more standardized reporting of basic animal characteristics (e.g., sex, age/sexual maturity) and injury variables for all poly-neurotrauma studies (Smith et al., 2015). Increasing standardization will not only improve scientific rigor and reproducibility, but it will also facilitate the translation of both mechanistic and therapeutic findings across multiple animal species and into humans. The latter gap has yet to be bridged in the field of TBI treatment, and any effective therapies for isolated TBI will eventually require independent confirmation in poly-neurotrauma models due to the competing pathophysiological responses of SBL.
Supplementary Material
Acknowledgements
This research was supported by grants from the Departments of Defense (W81XWH-17-2-0052) and National Institutes of Health [https://www.nih.gov; grant numbers NIH 01 R01 NS098494-01A1 and -03S1A1 and 01 P20 GM109089-01] to Andrew R. Mayer. This work was also partially supported by Department of Defense Contract W911QY-15-C-0134 to Irshad H. Chaudry. Denis Bragin was partially supported by 17-15-01263. The Department of Defense and National Institutes of Health had no role in study review, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.neubiorev.2019.06.024.
References
- Alam HB, Bowyer MW, Koustova E, Gushchin V, Anderson D, Stanton K, Kreishman P, Cryer CM, Hancock T, Rhee P, 2002. Learning and memory is preserved after induced asanguineous hyperkalemic hypothermic arrest in a swine model of traumatic exsanguination. Surgery 132, 278–288. [DOI] [PubMed] [Google Scholar]
- American College of Surgeons Committee on Trauma, 2004. Advanced Trauma Life Support Program for Doctors. American College of Surgeons, Chicago, IL. [Google Scholar]
- Angele MK, Schneider CP, Chaudry IH, 2008. Bench-to-bedside review: latest results in hemorrhagic shock. Crit. Care 12, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand R, Hess JR, 2003. Treating coagulopathy in trauma patients. Transfus. Med. Rev. 17, 223–231. [DOI] [PubMed] [Google Scholar]
- Ashwini CA, Shubha R, Jayanthi KS, 2008. Comparative anatomy of the circle of Willis in man, cow, sheep, goat, and pig. Neuroanatomy 7, 54–65. [Google Scholar]
- Baker TA, Romero J, Bach HH, Strom JA, Gamelli RL, Majetschak M, 2012. Systemic release of cytokines and heat shock proteins in porcine models of polytrauma and hemorrhage. Crit. Care Med. 40, 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbino M, Capone NA, Prist R, Ferreira AT, Poli-de-Figueiredo LF, 2010. Fluid resuscitation with isotonic or hypertonic saline solution avoids intraneural calcium influx after traumatic brain injury associated with hemorrhagic shock. J. Trauma 68, 859–864. [DOI] [PubMed] [Google Scholar]
- Bambakidis T, Dekker SE, Halaweish I, Liu B, Nikolian VC, Georgoff PE, Piascik P, Li Y, Sillesen M, Alam HB, 2017. Valproic acid modulates platelet and coagulation function ex vivo. Blood Coagul. Fibrinolysis 28, 479–484. [DOI] [PubMed] [Google Scholar]
- Bambakidis T, Dekker SE, Sillesen M, Liu B, Johnson CN, Jin G, de Vries HE, Li Y, Alam HB, 2016. Resuscitation with valproic acid alters inflammatory genes in a porcine model of combined traumatic brain injury and hemorrhagic shock. J. Neurotrauma 33, 1514–1521. [DOI] [PubMed] [Google Scholar]
- Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb T, 2010. Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hardy J, Zetterberg H, 2012. The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899. [DOI] [PubMed] [Google Scholar]
- Bograd BA, Radowsky JS, Vicente DA, Lee EH, Davis TA, Elster EA, 2015. An evolving uncontrolled hemorrhage model using Cynomolgus macaques. Shock 44 (Suppl 1), 123–128. [DOI] [PubMed] [Google Scholar]
- Bonanno FG, 2012. Hemorrhagic shock: the “physiology approach. J. Emerg. Trauma Shock 5, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodhun M, Fritz H, Walter B, Antonow-Schlorke I, Reinhart K, Zwiener U, Bauer R, Patt S, 2001. Immunomorphological sequelae of severe brain injury induced by fluid-percussion in juvenile pigs–effects of mild hypothermia. Acta Neuropathol. 101, 424–434. [DOI] [PubMed] [Google Scholar]
- Browne KD, Chen XH, Meaney DF, Smith DH, 2011. Mild traumatic brain injury and diffuse axonal injury in swine. J. Neurotrauma 28, 1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein BH, Logvinenko T, Lederer JA, Cobb JP, Hubbard WJ, Chaudry IH, Remick DG, Baker HV, Xiao W, Mannick JA, 2006. Commonality and differences in leukocyte gene expression patterns among three models of inflammation and injury. Physiol. Genomics 24, 298–309. [DOI] [PubMed] [Google Scholar]
- Bryan RM Jr., Cherian L, Robertson C, 1995. Regional cerebral blood flow after controlled cortical impact injury in rats. Anesth. Analg. 80, 687–695. [DOI] [PubMed] [Google Scholar]
- Cairns CB, 2001. Rude unhinging of the machinery of life: metabolic approaches to hemorrhagic shock. Curr. Opin. Crit. Care 7, 437–443. [DOI] [PubMed] [Google Scholar]
- Cannon JW, 2018. Hemorrhagic shock. N. Engl. J. Med. 378, 370–379. [DOI] [PubMed] [Google Scholar]
- Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, Dubose JJ, Fox EE, Inaba K, Rodriguez CJ, Holcomb JB, Duchesne JC, 2017. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 82, 605–617. [DOI] [PubMed] [Google Scholar]
- Cernak I, 2005. Animal models of head trauma. NeuroRx. 2, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, 2014. Blast-induced neurotrauma models and their requirements. Front. Neurol. 5, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers LW, Rhee P, Baker BC, Perciballi J, Cubano M, Compeggie M, Nace M, Bohman HR, 2005. Initial experience of US Marine Corps forward resuscitative surgical system during Operation Iraqi freedom. Arch. Surg. 140, 26–32. [DOI] [PubMed] [Google Scholar]
- Chaudry IH, 1983. Cellular mechanisms in shock and ischemia and their correction. Am. J. Physiol. 245, R117–R134. [DOI] [PubMed] [Google Scholar]
- Chaudry IH, Wang P, Singh G, Hauptman JG, Ayala A, 1993. Rat and Mouse Models of Hypovolemic-traumatic Shock, in: Pathophysiology of Shock, Sepsis, and Organ Failure. Springer, pp. 371–383. [Google Scholar]
- Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA, 1993a. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma 34, 216–222. [DOI] [PubMed] [Google Scholar]
- Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF, 1993b. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir. Suppl (Wien.) 59, 121–125. [DOI] [PubMed] [Google Scholar]
- Cho SD, Holcomb JB, Tieu BH, Englehart MS, Morris MS, Karahan ZA, Underwood SA, Muller PJ, Prince MD, Medina L, Sondeen J, Shults C, Duggan M, Tabbara M, Alam HB, Schreiber MA, 2009. Reproducibility of an animal model simulating complex combat-related injury in a multiple-institution format. Shock 31, 87–96. [DOI] [PubMed] [Google Scholar]
- Coates BM, Vavilala MS, Mack CD, Muangman S, Suz P, Sharar SR, Bulger E, Lam AM, 2005. Influence of definition and location of hypotension on outcome following severe pediatric traumatic brain injury. Crit. Care Med. 33, 2645–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad MS, Dilger RN, Johnson RW, 2012. Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: a longitudinal MRI study. Dev. Neurosci. 34, 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DJ, Myles PS, McDermott FT, Murray LJ, Laidlaw J, Cooper G, Tremayne AB, Bernard SS, Ponsford J, 2004. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA 291, 1350–1357. [DOI] [PubMed] [Google Scholar]
- Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, Keene CD, Sonnen J, Montine TJ, Bennett DA, Leurgans S, Schneider JA, Larson EB, 2016. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 73, 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookes BA, Cohn SM, Bonet H, Burton EA, Nelson J, Majetschak M, Varon AJ, Linden JM, Proctor KG, 2004. Building a better fluid for emergency resuscitation of traumatic brain injury. J. Trauma 57, 547–554. [DOI] [PubMed] [Google Scholar]
- Crowell JW, Smith EE, 1964. Oxygen deficit and irreversable hemorrhagic shock. Am. J. Physiol. 206, 313–316. [DOI] [PubMed] [Google Scholar]
- Cullen DK, Harris JP, Browne KD, Wolf JA, Duda JE, Meaney DF, Margulies SS, Smith DH, 2016. A porcine model of traumatic brain injury via head rotational acceleration. Methods Mol. Biol. 1462, 289–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Russo RM, Ferencz SE, Cannon JW, Rasmussen TE, Neff LP, Johnson MA, Williams TK, 2017. Incremental balloon deflation following complete resuscitative endovascular balloon occlusion of the aorta results in steep inflection of flow and rapid reperfusion in a large animal model of hemorrhagic shock. J. Trauma Acute Care Surg. 83, 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker SE, Bambakidis T, Sillesen M, Liu B, Johnson CN, Jin G, Li Y, Alam HB, 2014a. Effect of pharmacologic resuscitation on the brain gene expression profiles in a swine model of traumatic brain injury and hemorrhage. J. Trauma Acute Care Surg. 77, 906–912. [DOI] [PubMed] [Google Scholar]
- Dekker SE, Sillesen M, Bambakidis T, Andjelkovic AV, Jin G, Liu B, Boer C, Johansson PI, Linzel D, Halaweish I, Alam HB, 2014b. Treatment with a histone deacetylase inhibitor, valproic acid, is associated with increased platelet activation in a large animal model of traumatic brain injury and hemorrhagic shock. J. Surg. Res. 190, 312–318. [DOI] [PubMed] [Google Scholar]
- Dekker SE, Sillesen M, Bambakidis T, Jin G, Liu B, Boer C, Johansson PI, Halaweish I, Maxwell J, Alam HB, 2014c. Normal saline influences coagulation and endothelial function after traumatic brain injury and hemorrhagic shock in pigs. Surgery 156, 556–563. [DOI] [PubMed] [Google Scholar]
- DeWitt DS, Prough DS, Taylor CL, Whitley JM, Deal DD, Vines SM, 1992. Regional cerebrovascular responses to progressive hypotension after traumatic brain injury in cats. Am. J. Physiol. 263, H1276–H1284. [DOI] [PubMed] [Google Scholar]
- Dickson JM, Wang X, St John AE, Lim EB, Stern SA, White NJ, 2018. Damage control resuscitation supplemented with vasopressin in a severe polytrauma model with traumatic brain injury and uncontrolled internal hemorrhage. Mil. Med. [DOI] [PubMed] [Google Scholar]
- Duchesne JC, McSwain NE Jr., Cotton BA, Hunt JP, Dellavolpe J, Lafaro K, Marr AB, Gonzalez EA, Phelan HA, Bilski T, Greiffenstein P, Barbeau JM, Rennie KV, Baker CC, Brohi K, Jenkins DH, Rotondo M, 2010. Damage control resuscitation: the new face of damage control. J. Trauma 69, 976–990. [DOI] [PubMed] [Google Scholar]
- Dunham CM, Fabian M, Siegel JH, Gettings L, 1991. Relationship of plasma amino acids to oxygen debt during hemorrhagic shock. Circ. Shock 35, 87–95. [PubMed] [Google Scholar]
- Durham RM, Moran JJ, Mazuski JE, Shapiro MJ, Baue AE, Flint LM, 2003. Multiple organ failure in trauma patients. J. Trauma 55, 608–616. [DOI] [PubMed] [Google Scholar]
- Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH, 2012. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J. Trauma Acute Care Surg. 73, S431–S437. [DOI] [PubMed] [Google Scholar]
- Eddy DM, Wangensteen SL, Ludewig RM, 1968. The kinetics of fluid loss from leaks in arteries tested by an experimental ex vivo preparation and external counter-pressure. Surgery 64, 451–458. [PubMed] [Google Scholar]
- Fabian MJ, Proctor KG, 2002. Hemodynamic actions of acute ethanol after resuscitation from traumatic brain injury. J. Trauma 53, 864–875. [DOI] [PubMed] [Google Scholar]
- Faist E, 1996. The mechanisms of host defense dysfunction following shock and trauma. Curr. Top. Microbiol. Immunol. 216, 259–274. [DOI] [PubMed] [Google Scholar]
- Falk JL, 1995. Fluid resuscitation in brain-injured patients. Crit. Care Med. 23, 4–6. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG, 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Atlanta (GA). [Google Scholar]
- Feinstein AJ, Patel MB, Sanui M, Cohn SM, Majetschak M, Proctor KG, 2005. Resuscitation with pressors after traumatic brain injury. J. Am. Coll. Surg. 201, 536–545. [DOI] [PubMed] [Google Scholar]
- Finnie JW, 2012. Comparative approach to understanding traumatic injury in the immature, postnatal brain of domestic animals. Aust. Vet. J. 90, 301–307. [DOI] [PubMed] [Google Scholar]
- Finnie JW, Manavis J, Blumbergs PC, Summersides GE, 2002. Brain damage in sheep from penetrating captive bolt stunning. Aust. Vet. J. 80, 67–69. [DOI] [PubMed] [Google Scholar]
- Frankel DA, Acosta JA, Anjaria DJ, Porcides RD, Wolf PL, Coimbra R, Hoyt DB, 2007. Physiologic response to hemorrhagic shock depends on rate and means of hemorrhage. J. Surg. Res. 143, 276–280. [DOI] [PubMed] [Google Scholar]
- Franklin GA, Boaz PW, Spain DA, Lukan JK, Carrillo EH, Richardson JD, 2000. Prehospital hypotension as a valid indicator of trauma team activation. J. Trauma 48, 1034–1037. [DOI] [PubMed] [Google Scholar]
- Franzblau M, Gonzales-Portillo C, Gonzales-Portillo GS, Diamandis T, Borlongan MC, Tajiri N, Borlongan CV, 2013. Vascular damage: a persisting pathology common to Alzheimer’s disease and traumatic brain injury. Med. Hypotheses 81, 842–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess SH, Ichord RN, Ralston J, Ryall K, Helfaer MA, Smith C, Margulies SS, 2009. Repeated traumatic brain injury affects composite cognitive function in piglets. J. Neurotrauma 26, 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frink M, Andruszkow H, Zeckey C, Krettek C, Hildebrand F, 2011. Experimental trauma models: an update. J. Biomed. Biotechnol. 2011, 797383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz HG, Walter B, Holzmayr M, Brodhun M, Patt S, Bauer R, 2005. A pig model with secondary increase of intracranial pressure after severe traumatic brain injury and temporary blood loss. J. Neurotrauma 22, 807–821. [DOI] [PubMed] [Google Scholar]
- Fulop A, Turoczi Z, Garbaisz D, Harsanyi L, Szijarto A, 2013. Experimental models of hemorrhagic shock: a review. Eur. Surg. Res. 50, 57–70. [DOI] [PubMed] [Google Scholar]
- Galgano M, Russell T, Mc Gillis S, Toshkezi G, Chin L, Zhao LR, 2015. A review of traumatic brain injury animal models: are we lacking adequate models replicating chronic traumatic encephalopathy. J. Neurol. Neurobiol. 2. [Google Scholar]
- Gardner RC, Dams-O’Connor K, Morrissey MR, Manley GT, 2018. Geriatric traumatic brain injury: epidemiology, outcomes, knowledge gaps, and future directions. J. Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP, 1982. Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 12, 564–574. [DOI] [PubMed] [Google Scholar]
- Georgoff PE, Nikolian VC, Halaweish I, Chtraklin K, Bruhn PJ, Eidy H, Rasmussen M, Li Y, Srinivasan A, Alam HB, 2017. Resuscitation with lyophilized plasma is safe and improves neurological recovery in a long-term survival model of swine subjected to traumatic brain injury, hemorrhagic shock, and polytrauma. J. Neurotrauma 34, 2167–2175. [DOI] [PubMed] [Google Scholar]
- Georgoff PE, Nikolian VC, Higgins G, Chtraklin K, Eidy H, Ghandour MH, Williams A, Athey B, Alam HB, 2018. Valproic acid induces prosurvival transcriptomic changes in swine subjected to traumatic injury and hemorrhagic shock. J. Trauma Acute Care Surg. 84, 642–649. [DOI] [PubMed] [Google Scholar]
- Gibson JB, Maxwell RA, Schweitzer JB, Fabian TC, Proctor KG, 2002. Resuscitation from severe hemorrhagic shock after traumatic brain injury using saline, shed blood, or a blood substitute. Shock 17, 234–244. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Snyder C, Corcoran C, Norton MC, Lyketsos CG, Tschanz JT, 2014. The association of traumatic brain injury with rate of progression of cognitive and functional impairment in a population-based cohort of Alzheimer’s disease: the Cache County Dementia Progression Study. Int. Psychogeriatr. 26, 1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri BK, Krishnappa IK, Bryan RM Jr., Robertson C, Watson J, 2000. Regional cerebral blood flow after cortical impact injury complicated by a secondary insult in rats. Stroke 31, 961–967. [DOI] [PubMed] [Google Scholar]
- Glass TF, Fabian MJ, Schweitzer JB, Weinberg JA, Proctor KG, 1999. Secondary neurologic injury resulting from nonhypotensive hemorrhage combined with mild traumatic brain injury. J. Neurotrauma 16, 771–782. [DOI] [PubMed] [Google Scholar]
- Glass TF, Fabian MJ, Schweitzer JB, Weinberg JA, Proctor KG, 2001. The impact of hypercarbia on the evolution of brain injury in a porcine model of traumatic brain injury and systemic hemorrhage. J. Neurotrauma 18, 57–71. [DOI] [PubMed] [Google Scholar]
- Glushakova OY, Johnson D, Hayes RL, 2014. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J. Neurotrauma 31, 1180–1193. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Toweill D, Lai S, Sonnenthal K, Kimberly B, 1998. Uncoupling of the autonomic and cardiovascular systems in acute brain injury. Am. J. Physiol. 275, R1287–R1292. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC, 2012. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 4, 134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, McKee AC, 2012. Response to comment on”chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model”. Sci. Transl. Med. 4, 157lr5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez G, Das A, Ballarino G, Beyzaei-Arani A, Turkan H, Wulf-Gutierrez M, Rider K, Kaya H, Amdur R, 2013. Decreased respiratory rate variability during mechanical ventilation is associated with increased mortality. Intensive Care Med. 39, 1359–1367. [DOI] [PubMed] [Google Scholar]
- Gutierrez G, Reines HD, Wulf-Gutierrez ME, 2004. Clinical review: hemorrhagic shock. Crit. Care 8, 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoneva S, Ransohoff RM, 2015. Inflammatory reaction after traumatic brain injury: therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol. Sci. 36, 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaweish I, Bambakidis T, Chang Z, Wei H, Liu B, Li Y, Bonthrone T, Srinivasan A, Bonham T, Chtraklin K, Alam HB, 2015a. Addition of low-dose valproic acid to saline resuscitation provides neuroprotection and improves long-term outcomes in a large animal model of combined traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 79, 911–919. [DOI] [PubMed] [Google Scholar]
- Halaweish I, Bambakidis T, He W, Linzel D, Chang Z, Srinivasan A, Dekker SE, Liu B, Li Y, Alam HB, 2015b. Early resuscitation with fresh frozen plasma for traumatic brain injury combined with hemorrhagic shock improves neurologic recovery. J. Am. Coll. Surg. 220, 809–819. [DOI] [PubMed] [Google Scholar]
- Halaweish I, Bambakidis T, Nikolian VC, Georgoff P, Bruhn P, Piascik P, Buckley L, Srinivasan A, Liu B, Li Y, Alam HB, 2016. Early resuscitation with lyophilized plasma provides equal neuroprotection compared with fresh frozen plasma in a large animal survival model of traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 81, 1080–1087. [DOI] [PubMed] [Google Scholar]
- Hariri RJ, Firlick AD, Shepard SR, Cohen DS, Barie PS, Emery III JM, Ghajar JB, 1993. Traumatic brain injury, hemorrhagic shock, and fluid resuscitation: effects on intracranial pressure and brain compliance. J. Neurosurg. 79, 421–427. [DOI] [PubMed] [Google Scholar]
- Hauser CJ, 2005. Preclinical models of traumatic, hemorrhagic shock. Shock 24 (Suppl 1), 24–32. [DOI] [PubMed] [Google Scholar]
- Hawkins BE, Krishnamurthy S, Castillo-Carranza DL, Sengupta U, Prough DS, Jackson GR, DeWitt DS, Kayed R, 2013. Rapid accumulation of endogenous tau oligomers in a rat model of traumatic brain injury: possible link between traumatic brain injury and sporadic tauopathies. J. Biol. Chem. 288, 17042–17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeldine J, Lord JM, Belli A, 2015. Traumatic brain injury and peripheral immune suppression: primer and prospectus. Front. Neurol. 6, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckbert SR, Vedder NB, Hoffman W, Winn RK, Hudson LD, Jurkovich GJ, Copass MK, Harlan JM, Rice CL, Maier RV, 1998. Outcome after hemorrhagic shock in trauma patients. J. Trauma 45, 545–549. [DOI] [PubMed] [Google Scholar]
- Hellewell S, Semple BD, Morganti-Kossmann MC, 2016. Therapies negating neuroinflammation after brain trauma. Brain Res. 1640, 36–56. [DOI] [PubMed] [Google Scholar]
- Hierholzer C, Billiar TR, 2001. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch. Surg. 386, 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand F, Andruszkow H, Huber-Lang M, Pape HC, van GM, 2013. Combined hemorrhage/trauma models in pigs-current state and future perspectives. Shock 40, 247–273. [DOI] [PubMed] [Google Scholar]
- Hildebrand F, Hubbard WJ, Choudhry MA, Frink M, Pape HC, Kunkel SL, Chaudry IH, 2006. Kupffer cells and their mediators: the culprits in producing distant organ damage after trauma-hemorrhage. Am. J. Pathol. 169, 784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton DL Jr., Einhaus SL, Meric AL III, White RP, Schweitzer JB, Park MR, Robertson JT, 1993. Early assessment of neurologic deficits in the fluid percussion model of brain injury. J. Neurotrauma 10, 121–133. [DOI] [PubMed] [Google Scholar]
- Hinson HE, Rowell S, Schreiber M, 2015. Clinical evidence of inflammation driving secondary brain injury: a systematic review. J. Trauma Acute Care Surg. 78, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshberg A, Hoyt DB, Mattox KL, 2006. Timing of fluid resuscitation shapes the hemodynamic response to uncontrolled hemorrhage: analysis using dynamic modeling. J. Trauma 60, 1221–1227. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA, 2008. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463. [DOI] [PubMed] [Google Scholar]
- Holbourn AHS, 1943. Mechanics of head injuries. Lancet 242, 438–441. [Google Scholar]
- Holcomb JB, Hoyt DB, 2015. Comprehensive injury research. JAMA 313, 1463–1464. [DOI] [PubMed] [Google Scholar]
- Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, Flaherty SF, Grathwohl KW, Spinella PC, Perkins JG, Beekley AC, McMullin NR, Park MS, Gonzalez EA, Wade CE, Dubick MA, Schwab CW, Moore FA, Champion HR, Hoyt DB, Hess JR, 2007. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J. Trauma 62, 307–310. [DOI] [PubMed] [Google Scholar]
- Hwabejire JO, Imam AM, Jin G, Liu B, Li Y, Sillesen M, Jepsen CH, Lu J, Demoya MA, Alam HB, 2013a. Differential effects of fresh frozen plasma and normal saline on secondary brain damage in a large animal model of polytrauma, hemorrhage and traumatic brain injury. J. Trauma Acute Care Surg. 75, 968–974. [DOI] [PubMed] [Google Scholar]
- Hwabejire JO, Jin G, Imam AM, Duggan M, Sillesen M, Deperalta D, Jepsen CH, Lu J, Li Y, Demoya MA, Alam HB, 2013b. Pharmacologic modulation of cerebral metabolic derangement and excitotoxicity in a porcine model of traumatic brain injury and hemorrhagic shock. Surgery 154, 234–243. [DOI] [PubMed] [Google Scholar]
- Imam A, Jin G, Sillesen M, Dekker SE, Bambakidis T, Hwabejire JO, Jepsen CH, Halaweish I, Alam HB, 2015. Fresh frozen plasma resuscitation provides neuroprotection compared to normal saline in a large animal model of traumatic brain injury and polytrauma. J. Neurotrauma 32, 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam AM, Jin G, Duggan M, Sillesen M, Hwabejire JO, Jepsen CH, Deperalta D, Liu B, Lu J, Demoya MA, Socrate S, Alam HB, 2013a. Synergistic effects of fresh frozen plasma and valproic acid treatment in a combined model of traumatic brain injury and hemorrhagic shock. Surgery 154, 388–396. [DOI] [PubMed] [Google Scholar]
- Imam AM, Jin G, Sillesen M, Duggan M, Jepsen CH, Hwabejire JO, Lu J, Liu B, Demoya MA, Velmahos GC, Alam HB, 2013b. Early treatment with lyophilized plasma protects the brain in a large animal model of combined traumatic brain injury and hemorrhagic shock. J. Trauma Acute Care Surg. 75, 976–983. [DOI] [PubMed] [Google Scholar]
- Jacobsen J, Sofelt S, Sheikh S, Warberg J, Secher NH, 1990. Cardiovascular and endocrine responses to haemorrhage in the pig. Acta Physiol. Scand. 138, 167–173. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Paulus W, Wrocklage C, Litvan I, 2001. Traumatic brain injury as a risk factor for Alzheimer disease. Comparison of two retrospective autopsy cohorts with evaluation of ApoE genotype. BMC Neurol. 75, 511–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Demoya MA, Duggan M, Knightly T, Mejaddam AY, Hwabejire J, Lu J, Smith WM, Kasotakis G, Velmahos GC, Socrate S, Alam HB, 2012a. Traumatic brain injury and hemorrhagic shock: evaluation of different resuscitation strategies in a large animal model of combined insults. Shock 38, 49–56. [DOI] [PubMed] [Google Scholar]
- Jin G, Duggan M, Imam A, Demoya MA, Sillesen M, Hwabejire J, Jepsen CH, Liu B, Mejaddam AY, Lu J, Smith WM, Velmahos GC, Socrate S, Alam HB, 2012b. Pharmacologic resuscitation for hemorrhagic shock combined with traumatic brain injury. J. Trauma Acute Care Surg. 73, 1461–1470. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Williams TK, Ferencz SE, Davidson AJ, Russo RM, O’Brien WT, Galante JM, Grayson JK, Neff LP, 2017. The effect of resuscitative endovascular balloon occlusion of the aorta, partial aortic occlusion and aggressive blood transfusion on traumatic brain injury in a swine multiple injuries model. J. Trauma Acute Care Surg. 83, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Meaney DF, Cullen DK, Smith DH, 2015. Animal models of traumatic brain injury. Handb. Clin. Neurol. 127, 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W, 2013. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH, 2010. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer’s disease? Nat. Rev. Neurosci. 11, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH, 2012. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 22, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E, De SB, 2016. The amyloid cascade hypothesis: are we poised for success or failure? J. Neurochem. 139 (Suppl 2), 237–252. [DOI] [PubMed] [Google Scholar]
- Kazim SF, Shamim MS, Tahir MZ, Enam SA, Waheed S, 2011. Management of penetrating brain injury. J. Emerg. Trauma Shock 4, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel M, Trentz O, 2005. Pathophysiology of polytrauma. Injury 36, 691–709. [DOI] [PubMed] [Google Scholar]
- Kheirabadi BS, Acheson EM, Deguzman R, Sondeen JL, Ryan KL, Delgado A, Dick EJ Jr., Holcomb JB, 2005. Hemostatic efficacy of two advanced dressings in an aortic hemorrhage model in Swine. J. Trauma 59, 25–34. [DOI] [PubMed] [Google Scholar]
- King DR, Cohn SM, Proctor KG, 2004. Changes in intracranial pressure, coagulation, and neurologic outcome after resuscitation from experimental traumatic brain injury with hetastarch. Surgery 136, 355–363. [DOI] [PubMed] [Google Scholar]
- King DR, Cohn SM, Proctor KG, 2005. Resuscitation with a hemoglobin-based oxygen carrier after traumatic brain injury. J. Trauma 59, 553–560. [PubMed] [Google Scholar]
- Kiraly LN, Differding JA, Enomoto TM, Sawai RS, Muller PJ, Diggs B, Tieu BH, Englehart MS, Underwood S, Wiesberg TT, Schreiber MA, 2006. Resuscitation with normal saline (NS) vs. Lactated ringers (LR) modulates hypercoagulability and leads to increased blood loss in an uncontrolled hemorrhagic shock swine model. J. Trauma 61, 57–64. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Bramlett HM, Dixon CE, Dietrich WD, Mondello S, Wang KKW, Hayes RL, Lafrenaye A, Povlishock JT, Tortella FC, Poloyac SM, Empey P, Shear DA, 2018. Operation brain trauma therapy: 2016 update. Mil. Med. 183, 303–312. [DOI] [PubMed] [Google Scholar]
- Kovesdi E, Luckl J, Bukovics P, Farkas O, Pal J, Czeiter E, Szellar D, Doczi T, Komoly S, Buki A, 2010. Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir. (Wien) 152, 1–17. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Kegler SR, Butler JA, Gotsch KE, Johnson RL, Reichard AA, Webb KW, Coronado VG, Selassie AW, Thurman DJ, 2003. Traumatic brain injury-related hospital discharges. Results from a 14-state surveillance system, 1997. MMWR Surveill. Summ. 52, 1–20. [PubMed] [Google Scholar]
- Law MM, Hovda DA, Cryer HG, 1996. Fluid-percussion brain injury adversely affects control of vascular tone during hemorrhagic shock. Shock 6, 213–217. [PubMed] [Google Scholar]
- Lehmann U, Reif W, Hobbensiefken G, Seekamp A, Regel G, Sturm JA, Dwenger A, Schweitzer G, Mann D, Ellerbeck M, 1995. [Effect of primary fracture management on craniocerebral trauma in polytrauma. An animal experiment study]. Unfallchirurg 98, 437–441. [PubMed] [Google Scholar]
- Ling G, Bandak F, Armonda R, Grant G, Ecklund J, 2009. Explosive blast neurotrauma. J. Neurotrauma 26, 815–825. [DOI] [PubMed] [Google Scholar]
- Link DP, Foerster JM, Lantz BM, Holcroft JW, 1981. Assessment of peripheral blood flow in man by video dilution technique: a preliminary report. Invest. Radiol. 16, 298–304. [DOI] [PubMed] [Google Scholar]
- Liu H, Xiao X, Sun C, Sun D, Li Y, Yang M, 2015. Systemic inflammation and multiple organ injury in traumatic hemorrhagic shock. Front Biosci. (Landmark. Ed) 20, 927–933. [DOI] [PubMed] [Google Scholar]
- Lomas-Niera JL, Perl M, Chung CS, Ayala A, 2005. Shock and hemorrhage: an overview of animal models. Shock 24 (Suppl 1), 33–39. [DOI] [PubMed] [Google Scholar]
- Lozano D, Gonzales-Portillo GS, Acosta S, de IP, Tajiri N, Kaneko Y, Borlongan CV, 2015. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 11, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhrs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Dobeli H, Schubert D, Riek R, 2005. 3D structure of Alzheimer’s amyloid-beta(1–42) fibrils. Proc. Natl. Acad. Sci. U. S. A. 102, 17342–17347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majde JA, 2003. Animal models for hemorrhage and resuscitation research. J. Trauma 54, S100–S105. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Schweitzer JB, Fox JL, Fabian TC, Proctor KG, 2004. Cerebral perfusion pressure elevation with oxygen-carrying pressor after traumatic brain injury and hypotension in Swine. J. Trauma 56, 1049–1057. [DOI] [PubMed] [Google Scholar]
- Manley G, Knudson MM, Morabito D, Damron S, Erickson V, Pitts L, 2001. Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Arch. Surg. 136, 1118–1123. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Lopez AD, Murray CJL, 2006. The Burden of Disease and Mortality by Condition: Data, Methods, and Results for 2001. [PubMed] [Google Scholar]
- Matsushita Y, Bramlett HM, Kuluz JW, Alonso O, Dietrich WD, 2001. Delayed hemorrhagic hypotension exacerbates the hemodynamic and histopathologic consequences of traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 21, 847–856. [DOI] [PubMed] [Google Scholar]
- Mattson MP, 2004. Pathways towards and away from Alzheimer’s disease. Nature 430, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Kaushal M, Dodd AB, Hanlon FM, Shaff NA, Mannix R, Master CL, Leddy JJ, Stephenson D, Wertz CJ, Suelzer EM, Arbogast KB, Meier TM, 2018. Advanced biomarkers of pediatric mild traumatic brain injury: progress and perils. Neurosci. Biobehav. Rev. 94, 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA, 2009. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CG, Kenny R, Bennett K, Kirkman E, 2008. Modification of acute cardiovascular homeostatic responses to hemorrhage following mild to moderate traumatic brain injury. Crit. Care Med. 36, 216–224. [DOI] [PubMed] [Google Scholar]
- McMahon CG, Kenny R, Bennett K, Little R, Kirkman E, 2011. Effect of acute traumatic brain injury on baroreflex function. Shock 35, 53–58. [DOI] [PubMed] [Google Scholar]
- Meaney DF, Smith DH, Shreiber DI, Bain AC, Miller RT, Ross DT, Gennarelli TA, 1995. Biomechanical analysis of experimental diffuse axonal injury. J. Neurotrauma 12, 689–694. [DOI] [PubMed] [Google Scholar]
- Miller JD, Becker DP, 1982. Secondary insults to the injured brain. J. R. Coll. Surg. Edinb. 27, 292–298. [PubMed] [Google Scholar]
- Mira JC, Nacionales DC, Loftus TJ, Ungaro R, Mathias B, Mohr AM, Moldawer LL, Efron PA, 2018. Mouse injury model of polytrauma and shock. Methods Mol. Biol. 1717, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima Y, Wang P, Jarrar D, Cioffi WG, Bland KI, Chaudry IH, 2000. Estradiol administration after trauma-hemorrhage improves cardiovascular and hepatocellular functions in male animals. Ann. Surg. 232, 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moochhala S, Wu J, Lu J, 2009. Hemorrhagic shock: an overview of animal models. Front Biosci. (Landmark. Ed) 14, 4631–4639. [DOI] [PubMed] [Google Scholar]
- Morrison CA, Carrick MM, Norman MA, Scott BG, Welsh FJ, Tsai P, Liscum KR, Wall MJ Jr., Mattox KL, 2011. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J. Trauma 70, 652–663. [DOI] [PubMed] [Google Scholar]
- Namas R, Ghuma A, Hermus L, Zamora R, Okonkwo DO, Billiar TR, Vodovotz Y, 2009. The acute inflammatory response in trauma/hemorrhage and traumatic brain injury: current state and emerging prospects. Libyan J. Med. 4, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann DN, Khan MA, Smith JE, Rickard R, Woolley T, 2018. Future strategies for remote damage control resuscitation after traumatic hemorrhage. J. Trauma Acute Care Surg. [DOI] [PubMed] [Google Scholar]
- Navarro JC, Pillai S, Cherian L, Garcia R, Grill RJ, Robertson CS, 2012. Histopathological and behavioral effects of immediate and delayed hemorrhagic shock after mild traumatic brain injury in rats. J. Neurotrauma 29, 322–334. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Wall DB, Stedje-Larsen ET, Clark RT, Chambers LW, Bohman HR, 2006. Predictors of mortality in close proximity blast injuries during Operation Iraqi Freedom. J. Am. Coll. Surg. 202, 418–422. [DOI] [PubMed] [Google Scholar]
- Nemetz PN, Leibson C, Naessens JM, Beard M, kokmen E, Annegers JF, Kurland LT, 1999. Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am. J. Epidemiol. 149, 32–40. [DOI] [PubMed] [Google Scholar]
- Nikolian VC, Dekker SE, Bambakidis T, Higgins GA, Dennahy IS, Georgoff PE, Williams AM, Andjelkovic AV, Alam HB, 2018a. Improvement of Blood-Brain Barrier Integrity in Traumatic Brain Injury and Hemorrhagic Shock Following Treatment With Valproic Acid and Fresh Frozen Plasma. Crit. Care Med. 46, e59–e66. [DOI] [PubMed] [Google Scholar]
- Nikolian VC, Dennahy IS, Higgins GA, Williams AM, Weykamp M, Georgoff PE, Eidy H, Ghandour MH, Chang P, Alam HB, 2018b. Transcriptomic changes following valproic acid treatment promote neurogenesis and minimize secondary brain injury. J. Trauma Acute Care Surg. 84, 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolian VC, Georgoff PE, Pai MP, Dennahy IS, Chtraklin K, Eidy H, Ghandour MH, Han Y, Srinivasan A, Li Y, Alam HB, 2017. Valproic acid decreases brain lesion size and improves neurologic recovery in swine subjected to traumatic brain injury, hemorrhagic shock, and polytrauma. J. Trauma Acute Care Surg. 83, 1066–1073. [DOI] [PubMed] [Google Scholar]
- Pacagnella RC, Souza JP, Durocher J, Perel P, Blum J, Winikoff B, Gulmezoglu AM, 2013. A systematic review of the relationship between blood loss and clinical signs. PLoS One 8, e57594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MB, Feinstein AJ, Saenz AD, Majetschak M, Proctor KG, 2006. Prehospital HBOC-201 after traumatic brain injury and hemorrhagic shock in swine. J. Trauma 61, 46–56. [DOI] [PubMed] [Google Scholar]
- Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, Simmons RL, 1995. Hemorrhagic shock. Curr. Probl. Surg. 32, 925–1002. [DOI] [PubMed] [Google Scholar]
- Pope A, French G, Longnecker DE, 1999. Protocols of Care at the Site of Injury, in: Fluid Resuscitation: State of the Science for Treating Combat Casualties and Civilian Injuries. Inst Medicine, Washington, DC. [PubMed] [Google Scholar]
- Povlishock JT, Katz DI, 2005. Update of neuropathology and neurological recovery after traumatic brain injury. J. Head Trauma Rehabil. 20, 76–94. [DOI] [PubMed] [Google Scholar]
- Ramming S, Shackford SR, Zhuang J, Schmoker JD, 1994. The relationship of fluid balance and sodium administration to cerebral edema formation and intracranial pressure in a porcine model of brain injury. J. Trauma 37, 705–713. [DOI] [PubMed] [Google Scholar]
- Rana MW, Singh G, Wang P, Ayala A, Zhou M, Chaudry IH, 1992. Protective effects of preheparinization on the microvasculature during and after hemorrhagic shock. J. Trauma 32, 420–426. [DOI] [PubMed] [Google Scholar]
- Reid WM, Rolfe A, Register D, Levasseur JE, Churn SB, Sun D, 2010. Strain-related differences after experimental traumatic brain injury in rats. J. Neurotrauma 27, 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixen D, Raum M, Holzgraefe B, Sauerland S, Nagelschmidt M, Neugebauer EA, 2001. A pig hemorrhagic shock model: oxygen debt and metabolic acidemia as indicators of severity. Shock 16, 239–244. [DOI] [PubMed] [Google Scholar]
- Rixen D, Siegel JH, 2005. Bench-to-bedside review: oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care 9, 441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M, Graham DI, 1994. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol. Neurosurg. Psychiatry 57, 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal G, Morabito D, Cohen M, Roeytenberg A, Derugin N, Panter SS, Knudson MM, Manley G, 2008. Use of hemoglobin-based oxygen-carrying solution-201 to improve resuscitation parameters and prevent secondary brain injury in a swine model of traumatic brain injury and hemorrhage: laboratory investigation. J. Neurosurg. 108, 575–587. [DOI] [PubMed] [Google Scholar]
- Ross JD, Burns CJ, Sagini EM, Zarzabal LA, Morrison JJ, 2014. A laparoscopic swine model of noncompressible torso hemorrhage. J. Trauma Acute Care Surg. 77, S77–S82. [DOI] [PubMed] [Google Scholar]
- Rossaint R, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, Stahel PF, Hunt BJ, Neugebauer E, Spahn DR, 2006. Key issues in advanced bleeding care in trauma. Shock 26, 322–331. [DOI] [PubMed] [Google Scholar]
- Rubenstein R, Chang B, Davies P, Wagner AK, Robertson CS, Wang KK, 2014. A novel, ultrasensitive assay for tau: potential for assessing traumatic brain injury in tissues and biofluids. J. Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saljo A, Mayorga M, Bolouri H, Svensson B, Hamberger A, 2011. Mechanisms and pathophysiology of the low-level blast brain injury in animal models. Neuroimage. 54 (Suppl 1), S83–S88. [DOI] [PubMed] [Google Scholar]
- Sanui M, King DR, Feinstein AJ, Varon AJ, Cohn SM, Proctor KG, 2006. Effects of arginine vasopressin during resuscitation from hemorrhagic hypotension after traumatic brain injury. Crit. Care Med. 34, 433–438. [DOI] [PubMed] [Google Scholar]
- Sava J, Velmahos GC, Karaiskakis M, Kirkman P, Toutouzas K, Sarkisyan G, Chan L, Demetriades D, 2003. Abdominal insufflation for prevention of exsanguination. J. Trauma 54, 590–594. [DOI] [PubMed] [Google Scholar]
- Schofield PW, Tang M, Marder K, Bell K, Dooneief G, Chun M, Sano M, Stern Y, Mayeux R, 1997. Alzheimer’s disease after remote head injury: an incidence study. J. Neurol. Neurosurg. Psychiatry 62, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Schenk D, 2003. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 43, 545–584. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U. S. A. 110, 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard FR, Macko AR, Glaser JJ, Vernon PJ, Burdette AJ, Paredes RM, Koeller CA, Pusateri AE, Tadaki DK, Cardin S, 2018. Nonhuman primate (Rhesus macaque) models of severe pressure-targeted hemorrhagic and polytraumatic hemorrhagic shock. Shock 49, 174–186. [DOI] [PubMed] [Google Scholar]
- Shively S, Scher AI, Perl DP, Diaz-Arrastia R, 2012. Dementia resulting from traumatic brain injury: what is the pathology? Arch. Neurol. 69, 1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, McDonald SJ, Vonder HC, Meconi A, Vink R, van DP, Taneja C, Iverson GL, Christie BR, 2016. The potential for animal models to provide insight into mild traumatic brain injury: translational challenges and strategies. Neurosci. Biobehav. Rev. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Fabian M, Smith JA, Kingston EP, Steele KA, Wells MR, Kaplan LJ, 2003. Oxygen debt criteria quantify the effectiveness of early partial resuscitation after hypovolemic hemorrhagic shock. J. Trauma 54, 862–880. [DOI] [PubMed] [Google Scholar]
- Sillesen M, Bambakidis T, Dekker SE, Li Y, Alam HB, 2017. Fresh frozen plasma modulates brain gene expression in a swine model of traumatic brain injury and shock: a network analysis. J. Am. Coll. Surg. 224, 49–58. [DOI] [PubMed] [Google Scholar]
- Sillesen M, Jin G, Oklu R, Albadawi H, Imam AM, Jepsen CH, Hwabejire JO, Ostrowski SR, Johansson PI, Rasmussen LS, Alam HB, 2013a. Fresh-frozen plasma resuscitation after traumatic brain injury and shock attenuates extracellular nucleosome levels and deoxyribonuclease 1 depletion. Surgery 154, 197–205. [DOI] [PubMed] [Google Scholar]
- Sillesen M, Johansson PI, Rasmussen LS, Jin G, Jepsen CH, Imam A, Hwabejire JO, Deperalta D, Duggan M, deMoya M, Velmahos GC, Alam HB, 2014a. Fresh frozen plasma resuscitation attenuates platelet dysfunction compared with normal saline in a large animal model of multisystem trauma. J. Trauma Acute Care Surg. 76, 998–1007. [DOI] [PubMed] [Google Scholar]
- Sillesen M, Johansson PI, Rasmussen LS, Jin G, Jepsen CH, Imam AM, Hwabejire J, Lu J, Duggan M, Velmahos G, deMoya M, Alam HB, 2013b. Platelet activation and dysfunction in a large-animal model of traumatic brain injury and hemorrhage. J. Trauma Acute Care Surg. 74, 1252–1259. [DOI] [PubMed] [Google Scholar]
- Sillesen M, Rasmussen LS, Jin G, Jepsen CH, Imam A, Hwabejire JO, Halaweish I, deMoya M, Velmahos G, Johansson PI, Alam HB, 2014b. Assessment of coagulopathy, endothelial injury, and inflammation after traumatic brain injury and hemorrhage in a porcine model. J. Trauma Acute Care Surg. 76, 12–19. [DOI] [PubMed] [Google Scholar]
- Sillesen M, Bambakidis T, Dekker SE, Li Y, Alam HB, 2017. Fresh frozen plasma modulates brain gene expression in a swine model of traumatic brain injury and shock: a network analysis. J. Am. Surg. 224, 49–58. [DOI] [PubMed] [Google Scholar]
- Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, Nelson NR, Stocchetti N, 2014. A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 371, 2467–2476. [DOI] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Nonaka M, Trojanowski JQ, Lee VM, Saatman KE, Leoni MJ, Xu BN, Wolf JA, Meaney DF, 1999. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J. Neuropathol. Exp. Neurol. 58, 982–992. [DOI] [PubMed] [Google Scholar]
- Smith DH, Hicks RR, Johnson VE, Bergstrom DA, Cummings DM, Noble LJ, Hovda D, Whalen M, Ahlers ST, LaPlaca M, Tortella FC, Duhaime AC, Dixon CE, 2015. Pre-clinical traumatic brain injury common data elements: toward a common language across laboratories. J. Neurotrauma 32, 1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Stewart W, 2013. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 9, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Nonaka M, Miller R, Leoni M, Chen XH, Alsop D, Meaney DF, 2000. Immediate coma following inertial brain injury dependent on axonal damage in the brainstem. J. Neurosurg. 93, 315–322. [DOI] [PubMed] [Google Scholar]
- Sondeen JL, Coppes VG, Holcomb JB, 2003. Blood pressure at which rebleeding occurs after resuscitation in swine with aortic injury. J. Trauma 54, S110–S117. [DOI] [PubMed] [Google Scholar]
- Sondeen JL, Dubick MA, Holcomb JB, Wade CE, 2007. Uncontrolled hemorrhage differs from volume- or pressure-matched controlled hemorrhage in swine. Shock 28, 426–433. [DOI] [PubMed] [Google Scholar]
- Stern S, Rice J, Philbin N, McGwin G, Arnaud F, Johnson T, Flournoy WS, Ahlers S, Pearce LB, McCarron R, Freilich D, 2009. Resuscitation with the hemoglobin-based oxygen carrier, HBOC-201, in a swine model of severe uncontrolled hemorrhage and traumatic brain injury. Shock 31, 64–79. [DOI] [PubMed] [Google Scholar]
- Stern SA, Zink BJ, Mertz M, Wang X, Dronen SC, 2000. Effect of initially limited resuscitation in a combined model of fluid-percussion brain injury and severe uncontrolled hemorrhagic shock. J. Neurosurg. 93, 305–314. [DOI] [PubMed] [Google Scholar]
- Strebel S, Lam AM, Matta BF, Newell DW, 1997. Impaired cerebral autoregulation after mild brain injury. Surg. Neurol. 47, 128–131. [DOI] [PubMed] [Google Scholar]
- Swindle MM, Makin A, Herron AJ, Clubb FJ Jr., Frazier KS, 2012. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 49, 344–356. [DOI] [PubMed] [Google Scholar]
- Swindle MM, Smith AC, 2015. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. CRC Press. [Google Scholar]
- Tan AA, Quigley A, Smith DC, Hoane MR, 2009. Strain differences in response to traumatic brain injury in Long-Evans compared to Sprague-Dawley rats. J. Neurotrauma 26, 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi K, Scultetus A, Haque A, Stern S, Philbin N, Rice J, Johnson T, Auker C, McCarron R, Freilich D, Arnaud F, 2012. Traumatic brain injury and severe uncontrolled haemorrhage with short delay pre-hospital resuscitation in a swine model. Injury 43, 585–593. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK, 2005. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma 22, 42–75. [DOI] [PubMed] [Google Scholar]
- Tran HT, LaFerla FM, Holtzman DM, Brody DL, 2011. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J. Neurosci. 31, 9513–9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Pape HC, 2009. Animal models for trauma research: what are the options? Shock 31, 3–10. [DOI] [PubMed] [Google Scholar]
- Tummaruk P, Tantasuparuk W, Kunavongkrit A, 2008. Age at Puberty in Landrace, Yorkshire, Duroc and Crossbred Landrace X Yorkshire Gilts Kept in Evaporative Cooling System in a Commercial Herd in Thailand. In Bangkok, Thailand. [Google Scholar]
- Urbanc B, Betnel M, Cruz L, Bitan G, Teplow DB, 2010. Elucidation of amyloid beta-protein oligomerization mechanisms: discrete molecular dynamics study. J. Am. Chem. Soc. 132, 4266–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, 1997. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313–318. [DOI] [PubMed] [Google Scholar]
- Varicoda EY, Poli de Figueiredo LF, Cruz RJ Jr., Silva LE, Silva Rochae, 2003. Blood loss after fluid resuscitation with isotonic or hypertonic saline for the initial treatment of uncontrolled hemorrhage induced by spleen rupture. J. Trauma 55, 112–117. [DOI] [PubMed] [Google Scholar]
- Ventura-Antunes L, Mota B, Herculano-Houzel S, 2013. Different scaling of white matter volume, cortical connectivity, and gyrification across rodent and primate brains. Front. Neuroanat. 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink R, 2018. Large animal models of traumatic brain injury. J. Neurosci. Res. 96, 527–535. [DOI] [PubMed] [Google Scholar]
- Vrettos T, Poimenidi E, Athanasopoulos P, Balasis S, Karagiorgos N, Siklis T, Gatzounis G, Fligkou F, 2016. The effect of permissive hypotension in combined traumatic brain injury and blunt abdominal trauma: an experimental study in swines. Eur. Rev. Med. Pharmacol. Sci. 20, 620–630. [PubMed] [Google Scholar]
- Walsh JC, Zhuang J, Shackford SR, 1991. A comparison of hypertonic to isotonic fluid in the resuscitation of brain injury and hemorrhagic shock. J. Surg. Res. 50, 284–292. [DOI] [PubMed] [Google Scholar]
- Washington PM, Morffy N, Parsadanian M, Zapple DN, Burns MP, 2014. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer’s disease mouse model. J. Neurotrauma 31, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ, Wang X, Bradbury N, Moon-Massat PF, Freilich D, Auker C, McCarron R, Scultetus A, Stern SA, 2013. Fluid resuscitation of uncontrolled hemorrhage using a hemoglobin-based oxygen carrier: effect of traumatic brain injury. Shock 39, 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Williams C, Chaudry IH, 1996. Immune function is more compromised after closed bone fracture and hemorrhagic shock than hemorrhage alone. Arch. Surg. 131, 995–1000. [DOI] [PubMed] [Google Scholar]
- Williams AM, Dennahy IS, Bhatti UF, Halaweish I, Xiong Y, Chang P, Nikolian VC, Chtraklin K, Brown J, Zhang Y, Zhang ZG, Chopp M, Buller B, Alam HB, 2019. Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J. Neurotrauma 36, 54–60. [DOI] [PubMed] [Google Scholar]
- Wisner D, Busche F, Sturm J, Gaab M, Meyer H, 1989. Traumatic shock and head injury: effects of fluid resuscitation on the brain. J. Surg. Res. 46, 49–59. [DOI] [PubMed] [Google Scholar]
- Witcher KG, Eiferman DS, Godbout JP, 2015. Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci. 38, 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnarowicz MW, Fisher AM, Minaeva O, Goldstein LE, 2017. Considerations for experimental animal models of concussion, traumatic brain injury, and chronic traumatic encephalopathy-these matters matter. Front. Neurol. 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock T, Morganti-Kossmann MC, 2013. The role of markers of inflammation in traumatic brain injury. Front. Neurol. 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M, 2013. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XQ, Wade CE, 1991. Influences of traumatic brain injury on the outcomes of delayed and repeated hemorrhages. Circ. Shock 35, 231–236. [PubMed] [Google Scholar]
- Yuan XQ, Wade CE, 1992. Traumatic brain injury attenuates the effectiveness of lactated Ringer’s solution resuscitation of hemorrhagic shock in rats. Surg. Gynecol. Obstet. 174, 305–312. [PubMed] [Google Scholar]
- Yuan XQ, Wade CE, Clifford CB, 1991. Suppression by traumatic brain injury of spontaneous hemodynamic recovery from hemorrhagic shock in rats. J. Neurosurg. 75, 408–414. [DOI] [PubMed] [Google Scholar]
- Zellweger R, Ayala A, DeMaso CM, Chaudry IH, 1995. Trauma-hemorrhage causes prolonged depression in cellular immunity. Shock 4, 149–153. [DOI] [PubMed] [Google Scholar]
- Zink BJ, Schultz CH, Stern SA, Mertz M, Wang X, Johnston P, Keep RF, 2001. Effects of ethanol and naltrexone in a model of traumatic brain injury with hemorrhagic shock. Alcohol. Clin. Exp. Res. 25, 916–923. [PubMed] [Google Scholar]
- Zink BJ, Schultz CH, Wang X, Mertz M, Stern SA, Betz AL, 1999. Effects of ethanol on brain lactate in experimental traumatic brain injury with hemorrhagic shock. Brain Res. 837, 1–7. [DOI] [PubMed] [Google Scholar]
- Zink BJ, Sheinberg MA, Wang X, Mertz M, Stern SA, Betz AL, 1998a. Acute ethanol intoxication in a model of traumatic brain injury with hemorrhagic shock: effects on early physiological response. J. Neurosurg. 89, 983–990. [DOI] [PubMed] [Google Scholar]
- Zink BJ, Stern SA, McBeth BD, Wang X, Mertz M, 2006. Effects of ethanol on limited resuscitation in a model of traumatic brain injury and hemorrhagic shock. J. Neurosurg. 105, 884–893. [DOI] [PubMed] [Google Scholar]
- Zink BJ, Stern SA, Wang X, Chudnofsky CC, 1998b. Effects of ethanol in an experimental model of combined traumatic brain injury and hemorrhagic shock. Acad. Emerg. Med. 5, 9–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.