Abstract
The members of the family Bromoviridae have spherical or bacilliform virions with tri-segmented, single-stranded genomic RNAs, packaged in separate particles. Six genera including Alfamovirus, Anulavirus, Bromovirus, Cucumovirus, Ilarvirus, and Oleavirus are part of the family. RNA1 and RNA2 code for the replicase whereas RNA3 codes for movement and coat proteins. Genomic RNAs are infectious, but some species also require CP for infectivity. Members can encapsidate/accumulate sub-genomic RNAs, and/or defective or satellite RNAs. RNA replication occurs inside membranous spherules, and the role of host factors in RNA replication have been documented. Frequent RNA-RNA recombination and segment reassortment processes were observed among bromovirids. Transmission occurs mechanically, via pollen, seeds or insects. Host range varies from narrow to wide, infecting herbaceous plants, shrubs and trees, with some members causing major epidemics.
Keywords: Brome mosaic virus, Bromoviridae, Host factor, Host range, Infectious cDNA clones, Positive-strand RNA virus, RNA recombination, Tripartite RNA genome, Viral gene expression, Viral genome replication, Virion structure, Virus-host interactions
Glossary
- Cross Protection
It describes a phenomenon in that an initial infection of a host plant with a mild strain of a virus induces resistance in that plant to the infection of another, closely related virus potentially protecting the plant from disease caused by a more virulent isolate.
- Genome Activation
Ilarviral genomic RNAs alone are unable to establish infection in plants, unless the coat protein is present. This function of the coat protein is termed genome activation and is specific for ilarviruses and closely related alfamoviruses. The event is triggered by binding of the coat protein RNA binding domain to the 3′ terminus of genomic RNA.
- RNA Silencing
A fundamental, evolutionarily conserved and sequence-specific mechanism that is triggered by double-stranded RNA and regulates gene expression in eukaryotes. It is a primary antiviral defense mechanism in plants and other living organisms.
- RNA Silencing Suppressor
A countermeasure to RNA silencing, often a protein encoded by a virus which interrupts a single, or multiple steps in the RNA silencing pathway such as binding to small interfering RNA and thereby preventing their incorporation into the RNA induced silencing complex.
- Sub-genomic RNA
A segment of RNA generated from a genomic RNA via an internal promoter that has the same 3′ end as the genomic RNA, but has a deletion at the 5′ end. The sub-genomic RNA makes it possible to efficiently translate the downstream open reading frame of the genomic RNA.
- Tripartite Genome
A viral genome consisting of three genomic fragments, which are encapsidated into three separate virions.
Introduction
The family Bromoviridae contains important genera of plant viruses, with host ranges varying from narrow to very wide, and infecting herbaceous plants, shrubs and even trees. Several of them are responsible for major epidemics in fodder crops such as tomato, cucurbits, bananas, or alfalfa. The members of the family Bromoviridae have spherical or bacilliform particles with a tri-segmented, positive-sense, single-stranded RNA (ssRNA) genome, packaged in separate virions. Bromovirids can be transmitted mechanically, via the pollen, seeds or by insect vectors.
As shown in Tables 1 and 2, the Bromoviridae family includes six genera: Alfamovirus (one member, type species: Alfalfa mosaic virus, AMV), Anulavirus (two members, type species: Pelargonium zonate spot virus, PZSV), Bromovirus (six members, type species: Brome mosaic virus, BMV), Cucumovirus (four members, type species: Cucumber mosaic virus, CMV), Ilarvirus (22 members, type species: Tobacco streak virus, TSV), and Oleavirus (one member, type species: Olive latent virus 2, OLV-2). Genus demarcation criteria are based on natural host range, method of transmission, detailed morphology and properties of particles, organization of RNA genome, replication schemes and producing defective RNAs and satellite RNAs.
Table 1.
Main characteristics of the RNA genome in six genera of the family Bromoviridaea
| Genus | Acronym | RNA1 | RNA2 | RNA3 | 3′UTR | sgRNA/diRNA |
|---|---|---|---|---|---|---|
| Alfamovirus | ||||||
| Alfalfa mosaic virus | (AMV) | 3644 | 2593 | 2037 | Complex | –/– |
| Anulavirus | ||||||
| Pelargonium zonate spot virus | (PZSV) | 3383 | 2435 | 2639 | Complex | –/– |
| Bromovirus | ||||||
| Brome mosaic virus | (BMV) | 3234 | 2865 | 2117 | tRNA-like | +/– |
| Cucumovirus | ||||||
| Cucumber mosaic virus | (CMV) | 3357 | 3050 | 2216 | tRNA-like | +/+ |
| llarvirus | ||||||
| Tobacco streak virus | (TSV) | 3491 | 2926 | 2205 | Complex | –/– |
| Oleavirus | ||||||
| Olive latent virus 2 | (OLV-2) | 3126 | 2734 | 2438 | Complex | +/– |
Partial sequence.
Table 2.
List of genera and species in the family Bromoviridae. Type species are written in bold
| Genus | Species | Acronym | GenBank accession no. |
||
|---|---|---|---|---|---|
| RNA 1 (P1) | RNA 2 (P2) | RNA 3 (MP and CP) | |||
| Alfamovirus | Alfalfa mosaic virus | (AMV) | NC_001495 | NC_002024 | NC_002024 |
| Anulavirus | Amazon lily mild mottle virus | (ALMMoV) | NC_018402 | NC_018403 | NC_018404 |
| Pelargonium zonate spot virus | (PZSV) | NC_003649 | NC_003650 | NC_003651 | |
| Tentative species | Cassava Ivorian bacilliform virus | (CsIBV) | NC_025482 | NC_025483 | NC_025484 |
| Bromovirus | Broad bean mottle virus | (BBMV) | NC_004006 | NC_004007 | NC_004008 |
| Brome mosaic virus | (BMV) | NC_002026 | NC_002027 | NC_002028 | |
| Cassia yellow blotch virus | (CsYBV) | NC_006999 | NC_007000 | NC_007001 | |
| Cowpea chlorotic mottle virus | (CCMV) | NC_003543 | NC_003541 | NC_003542 | |
| Melandrium yellow fleck virus | (MeYFV) | NC_013266 | NC_013267 | NC_013268 | |
| Spring beauty latent virus | (SBLV) | NC_004120 | NC_004121 | NC_004122 | |
| Cucumovirus | Cucumber mosaic virus | (CMV) | NC_002034 | NC_002035 | NC_001440 |
| Gayfeather mild mottle virus | (GMMoV) | NC_012134 | NC_012135 | NC_012136 | |
| Peanut stunt virus | (PSV) | NC_002038 | NC_002039 | NC_002040 | |
| Tomato aspermy virus | (TAV) | NC_003837 | NC_003838 | NC_003836 | |
| Ilarvirus | |||||
| Subgroup 1 | |||||
| Ageratum latent virus | (ALV) | NC_022127 | NC_022128 | NC_022129 | |
| Blackberry chlorotic ringspot virus | (BChRSV) | NC_011553 | NC_011554 | NC_011555 | |
| Parietaria mottle virus | (PaMoV) | NC_005848 | NC_005849 | NC_005854 | |
| Privet ringspot virus | (PrRSV) | NC_027928 | NC_027929 | NC_027930 | |
| Strawberry necrotic shock virus | (StNSV) | NC_008706 | NC_008707 | NC_008708 | |
| Tobacco streak virus | (TSV) | NC_003844 | NC_003842 | NC_003845 | |
| Subgroup 2 | |||||
| Asparagus virus 2 | (AsV2) | NC_011808 | NC_011809 | NC_011807 | |
| Citrus leaf rugose virus | (CiLRV) | NC_003548 | NC_003547 | NC_003546 | |
| Citrus variegation virus | (CVV) | NC_009537 | NC_009538 | NC_009536 | |
| Elm mottle virus | (EMoV) | NC_003569 | NC_003568 | NC_003570 | |
| Lilac ring mottle virus | (LRMoV) | EU919668a | NC_038777 | NC_038776 | |
| Spinach latent virus | (SpLV) | NC_003808 | NC_003809 | NC_003810 | |
| Tomato necrotic streak virus | (ToNSV) | NC_039075 | NC_039074 | NC_039076 | |
| Tulare apple mosaic virus | (TAMV) | NC_003833 | NC_003834 | NC_003835 | |
| Subgroup 3 | |||||
| Apple mosaic virus | (ApMV) | NC_003464 | NC_003465 | NC_003480 | |
| Blueberry shock virus | (BlSV) | NC_022250 | NC_022251 | NC_022252 | |
| Lilac leaf chlorosis virus | (LiLChV) | NC_025477 | NC_025478 | NC_025481 | |
| Prunus necrotic ringspot virus | (PNRSV) | NC_004362 | NC_004363 | NC_004364 | |
| Subgroup 4 | |||||
| Fragaria chiloensis latent virus | (FCLV) | NC_006566 | NC_006567 | NC_006568 | |
| Prune dwarf virus | (PDV) | NC_008039 | NC_008037 | NC_008038 | |
| Unassigned species | |||||
| American plum line pattern virus | (APLPV) | NC_003451 | NC_003452 | NC_003453 | |
| Apple necrotic mosaic virus | (ANMV) | NC_040469 | NC_040471 | NC_040470 | |
| Cape gooseberry virus 1 | (CGV1) | NC_040393 | NC_040392 | NC_040394 | |
| Humulus japonicus latent virus | (HuJLV) | NC_006064 | NC_006065 | NC_006066 | |
| Tea plant line pattern virus | (TPLPV) | NC_040435 | NC_040436 | NC_040437 | |
| Oleavirus | Olive latent virus 2 | (OLV) | NC_003673 | NC_003674 | NC_003671 |
Partial sequence.
The two prototype genera, Bromovirus and Cucumovirus, are the genera mostly related, with the latter being agriculturally important. Both bromo- and cucumoviruses share such properties like the molecular and genetic features of their tripartite RNA genome, the number of encoded proteins and similar virion structure. The computer-assisted comparisons of aa sequences reveal significant similarity among their RNA replication proteins, much beyond the presence of characteristic GDD motif for 2a or for helicase/transferase domains in 1a. More broadly, the replication proteins share aa sequence similarity within the alphavirus-like super-family of positive-strand RNA viruses, which includes numerous plant and important animal/human viruses. The type members of different genera, such as CMV, BMV and AMV, have and continue to constitute excellent models for molecular research on viral gene expression, RNA replication, virion assembly, RNA recombination, epidemiology or the role of cellular genes in basic virology.
Phylogeny and Biodiversity of the Family Bromoviridae
Although RNA1, 2 and 3 overall keep a great similitude in their sequences, clearly rearrangements of RNAs has been done for members of the Bromoviridae. Both RNA recombination but also segment reassortment played a major role as the sources of variation in shaping the bromovirids member groups, being important contributors to the evolutionary history of the family, especially for the genera Bromovirus, Cucumovirus and Ilarvirus (Fig. 1, Fig. 2 ). However, doubts have been shed on the biological significance of the official taxonomy of the family Bromoviridae. To better understand the taxonomy, attempts have been made to reconcile the incongruences observed in the viruses’ evolutionary radiation caused by recombination and reassortment. These two processes could create new genetic variability and then these primary variants would undergo further selection for functional genomes of individual viruses. Consequently, the variants generated by reassortment and recombination events must have been initially viable which represents the first selective filter while further directional selection fine tunes the newly created RNAs. RNA segment reassortment was probably common at the origin of the bromoviruses and cucumoviruses as well as at the origin of Alfalfa mosaic virus, American plum line pattern virus and Citrus leaf rugose virus. Furthermore, recombination analyzes done for each of the three genomic RNAs revealed very common crossovers within the members of the genera Bromovirus, Cucumovirus and Ilarvirus, but also mixed recombination involving species from different genera. It seems that bromoviruses and cucumoviruses did split from a common ancestor forming distinct clades due to crossover events in RNA3, whereas protein 2b promoted the selection of a CMV-TAV RNA1/2-RNA3 recombinant. In the 5′ untranslated regions (UTR) of CMV RNA3 the sequence rearrangements have likely been the precursors of the radiation of three cucumovirus subgroups. The results illustrated in Fig. 2 confirm a clear separation between the genera Bromovirus and Cucumovirus, while the ilarviruses constitute their own cluster; two other genera (Anulavirus and Oleavirus) are more unique within the family. Although these results suggest that AMV should be included in the ilarviruses, its unequivocal assignment has yet to be resolved, especially because AMV differs from other ilarviruses with the mode of transmission, by aphids versus by pollen and thrips. Bromovirids are members of a larger alpha-like supergroup based upon both 1a and 2a proteins whereas 3a proteins cluster the bromovirids together with other viral groups into a separate pool of movement-associated proteins. In general, the constructed phylogenetic network not only reflects the initial genetic exchanges but also confirms the taxonomic status of the different genera within the family Bromoviridae, notwithstanding the phylogenetic disturbances caused by genetic exchange.
Fig. 1.

Phylogenetic trees obtained for the three genomic segments RNA1, RNA2 and RNA3, by concatenating coding and non-coding regions and fitting a heterogeneous nucleotide-substitution model using CODEML. See Table 2 for virus names and abbreviations. The three trees have been aligned with a color-coded system for each genus to show similitudes and differences.
The trees are reproduced from Codoner, F.M., Elena, S.F., 2008. The promiscuous evolutionary history of the family Bromoviridae. Journal of General Virology 89, 1739–1747 with permission (modified by C. Fauquet).
Fig. 2.
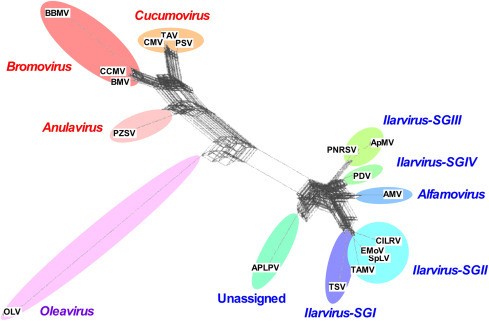
Evolution of the family Bromoviridae. Unrooted phylogenetic network illustrating the evolutionary history of the family Bromoviridae. The different viral species in the family are linked to each other via multiple paths of interconnecting network rather than as single tree, which suggests the effects of recombination and reassortment events.
Figure reproduced from Codoner, F.M., Elena, S.F., 2008. The promiscuous evolutionary history of the family Bromoviridae. Journal of General Virology 89, 1739–1747 with permission (modified by C. Fauquet).
Virion Properties and Structure
Virions of bromovirids are non-enveloped, being either spherical or pseudo-spherical, with T = 3 icosahedral symmetries, and a diameter of 26–35 nm (genera Anulavirus, Bromovirus, Cucumovirus and Ilarvirus), whereas genera Alfamovirus and some ilarviruses have bacilliform virions, of diameters 18–26 nm and lengths of 30–85 nm. In Oleavirus virions have different shape.
In genus Bromovirus three types of icosahedral particles are composed of 180 molecules of a 20 kDa CP, and encapsidate different RNA components: RNA1 (Mr c. 1.1 × 106), RNA2 (Mr c. 1.0 × 106) and RNA3 plus sgRNA4 (Mr c. 0.75 × 106 and 0.3 × 106). In addition to viral RNAs, BMV virions have been recently reported to package small amounts of host RNAs, with their functions yet to be determined. The members of the genus Cucumovirus, in addition to three genomic RNAs, encapsidate two sub-genomic RNAs (sgRNAs) and a satellite RNA.
The crystal structures of both BMV and CCMV have been resolved showing very similar organization (Fig. 3 ), with the CP subunits folded into a beta-barrel core organized within the protruding both pentameric and hexameric capsomers. The interactions among hydrophobic aa residues stabilize the capsomers, and the hexameric subunits are further stabilized via interactions between N-terminal portions, where six short beta-strands form a tubule called beta-hexamer. Mutational analysis demonstrated that beta-hexamer was not required for virion formation but rather modulated virus spread in planta. In addition, the capsomers are hold together by interactions through C-terminal portions that extend radially from the capsid. The C-termini are anchored between the beta-barrel core and the N-proximal loop, and this interaction might be responsible for initiation of assembly of CCMV capsids. The structure of BMV is organized similarly to that of CCMV such that both capsids undergo well-studied reversible structural transitions where shifting pH from 5.0 to 7.0 causes capsid expansion. However, some CP mutations can further stabilize the capsids. Capsids are also stabilized by metals at multiple biding sites that coordinate the amino acids from adjacent CP subunits.
Fig. 3.

Montage of transmission electron microscopic photographs and computer rendering of molecular particle structures of bromovirids. A & B. Surface capsid structure of the Brome mosaic virus (BMV) (A) and Cowpea chlorotic mottle virus (CCMV) (B). The hexameric and the pentameric structural elements are visible. Both pictures are from the virion picture collection at the web-site of the Institute for Molecular Virology at the University of Wisconsin-Madison. C–F. Negative-contrast electron micrograph of particles of (C) Brome mosaic virus (Bromovirus), (D) Prunus necrotic ringspot virus (Ilarvirus), (E) Alfalfa mosaic virus (Alfamovirus), and (F) Olive latent virus 2 (Oleavirus).
Photographs A-C IMV-Michigan State University, 1994 Thorben Lundsgaard, C.J. Woolston and Ed Rybicki, and Photograph D courtesy of A. Paredes, NCTR/ORA, Arkansas USA. Photographs E and F, courtesy of A. De Stradis, IPSP-CNR, Bari, Italy. Bars = 50 nm.
The packaged RNAs interact with the basic N-terminal aa of the CP in the torus-shaped sub-shell inside the BMV capsid so to neutralize the phosphate groups. Other sites of RNA interaction localize to the internally-proximal basic aa of the CP subunits. The RNA encapsidation signals have been mapped on BMV RNAs, especially to both the 3′ UTR and a central sequence in RNA3. The co-packaging of sgRNA4 is contingent upon both RNA replication and translation of CP.
The detailed knowledge of CCMV capsids provided opportunities in nanotechnology, e.g., for the reversible pH-dependent gating, useful during the regulation of size-constrained biomimetic mineralization. The interior surface of CCMV capsids can be engineered of as differentially functionalized CP subunits, to act e.g., as a ferritin surrogate that spatially constrains the formation of iron oxide nanoparticles. Also, the electrostatically driven adsorption on Si and amine-functionalized Si as well as the fabrication of multilayer CCMV films have been reported. The electrostatically patchy protein cages of CCMV can be used to direct the assembly of super lattices for RNA encapsulation.
Genome Organization and Expression
The total length of the Bromoviridae RNA genome is approximately 8 kb, with three RNA segments capped at the 5′ terminus. Whereas the highly conserved within members, the not polyadenylated 3′ termini fold into strong secondary structures. These structures are either aminoacylable tRNA-like (genera Bromovirus and Cucumovirus) or forming other not aminoacylated arrangements (genera Alfamovirus, Anulavirus, Ilarvirus and Oleavirus) (Table 1). RNAs 1 and 2 are monocistronic and they code, respectively, for viral replicase proteins 1a and 2a (Fig. 4 ). Protein 1a has two distinct domains of guanylyl transferase and helicase, and it is involved in anchoring the replicase complex to the endoplasmic reticulum membrane, and induces the formation of membranous vesicular mini-organelles called spherules where RNA replication occurs. In AMV the replicase proteins interact with the tonoplast. Protein 2a is the actual RNA-dependent RNA polymerase enzyme that interacts with protein 1a and synthesizes the vRNAs. Mutations and deletions in 1a/2a ORFs helped to identify the regions active in BMV RNA replication as well as regions responsible for interaction with the cellular membrane or for interactions between 1a/2a polypeptides. An active BMV replicase preparation has been extracted. The anulavirus encodes the smallest RdRp (2a) protein within the family. For cucumoviruses and in some ilarviruses RNA2 is dicistronic encoding a protein 2b, that is part of the C-terminal region of the protein 2a. Protein 2b was found to act as suppressor of RNA interference, being involved in the inhibition of viral gene silencing but also in systemic movement and affecting the symptoms.
Fig. 4.

Genome organizations representatives of the six genera of the family Bromoviridae: (A) genus Alfamovirus, Bromovirus, Ilarvirus subgroups 3 and 4 and Oleavirus. (B) genus Anulavirus. (C) genus Cucumovirus and Ilarvirus subgroups 1 and 2. The 3′ termini form either tRNA-like (B) or complex structures (A, C) shown as black square boxes.
Figure from Bujarski, J., Gallitelli, D., García-Arenal, F., et al., 2019. ICTV Virus Taxonomy Profile: Bromoviridae. Journal of General Virology 100, 1206–1207.
RNA3 encodes the movement (MP) and coat (CP) proteins, the latter being translated from the subgenomic (sg)RNA4. BMV CP is a multi-functional protein. In addition to its structural/encapsidation role, it also coordinates the viral infection processes including (1) participation in the formation of replication factories, (2) repression of RNA replication but also translation, and (3) stimulation of BMV RNA accumulation at lower CP levels. Moreover, the BMV CP participates in RNA recombination events; an analogous function to that of BMV CP was assigned to nucleocapsid proteins in retroviruses and coronaviruses. The multiple functions of CP are exercised by effective binding to several distinct sites in RNA3, including the 3′ non-coding hairpin, two central regions, and possibly at the 5′ end. The contact BMV CP amino acids have been mapped at distinct parts of the CP monomers. Since BMV replicase complex also binds to most of these sites it has been postulated that the CP is involved in the regulation of BMV RNA replication such that the repression of RNA accumulation and translation occurs at higher levels of BMV CP, while stimulation of BMV RNA accumulation at the lower CP levels. While CP is usually translated from the encapsidated 3′ sgRNA4, Olive latent virus 2 encapsidates a sgRNA with no apparent messenger activity whereas CP is translated from another non-encapsidated sgRNA4. The purified genomic RNAs are directly infectious but for some bromovirids the presence of CP is necessary (e.g., for AMV or ilarviruses).
The 32 kDa MP of bromovirids has a conserved RNA binding domain that binds to vRNA and assists with viral transport. There are two groups among the members of the family Bromoviridae regarding the cell-to-cell transport; those that do not require participation of virions and those that transport whole virions. In the first group only the MP-RNA entity is transported (non-virion transport) in a form of either the complex of MP and vRNA, or a triple complex of vRNA, MP and CP. For the first group, the prototype example is TMV whereas members of the genus Bromovirus have a similar mechanism, which has been demonstrated for CCMV. The MP of these viruses belongs to the 30K superfamily of MPs that appear to interact with cellular microtubules. In the second subtype, where the transported complex is vRNA-MP-CP, the typical example is CMV, a comovirus. In this case, the CMV MP exhibits the binding affinity to the actine microfilaments. The second group transports whole virions intercellularly through plasmodesmata inside the microtubules (virion transport). BMV and AMV are the members of two genera that are transported this way. Also for ilarviruses such as PDV or PNRSV, their MPs likely assist during translocation of the entire viral particles alongside the tubular structures. It appears that MPs of these viruses interact with virion CP subunits via a 44C-terminal key aa domain.
For long distance transport, most viruses require the CP which suggests that they are transported in the form of viral particles (virions). This has been demonstrated by showing that bromovirids require unmodified CP and the wild type C-terminus of MP for long distance spread, such as AMV, BMV and CMV. Very likely the CP-MP interactions enhance the systemic transport, independently of the mechanism of short distance (cell-to-cell) transport.
Genomic RNA Replication and Recombination
Replication of bromovirus RNAs are the best studied among the members of the family Bromoviridae. Only viral proteins 1a (helicase and methyl-transferase domains) and 2a (RdRp), but not the proteins 3a or CP, are required for RNA synthesis (Fig. 4), first demonstrated for BMV, both in plant and in yeast cells. The cytoplasmic RNA replicase complex localizes to the endoplasmic reticulum membranes called spherules. The extracted bromoviral RdRp preparations have allowed for mapping in vitro on three genomic BMV RNAs the promoter of (–) strand synthesis within the 3′ UTR. The promoters of (+) strand synthesis have also been mapped to the 5′ proximal non-coding region in BMV RNAs. Likewise, the sub-genomic promoter (sgp) has been localized as a 100 nt subset of the 250 nt intercistronic region in (–) strand in BMV RNA3, being responsible for synthesis (transcription) of sgRNA4. In addition, in the plus strand the 250 nt intercistronic sequence supports other functions including the efficient RNA3 replication, the maintenance of a proper ratio of (+) to (–) strands of RNA3, stabilization of RNA3 via interaction with protein 1a, synthesis (transcription) of sgRNA4, and the assembly of the active RNA replication complex. It also serves as an efficient RNA recombination hot spot. Some bromoviruses as well as togaviruses carry the internal poly(A) tract, as part of the intercistronic region of the RNA3 segment. Besides viral RNA sequences and viral proteins, a variety of essential host genes affecting BMV RNA replication have been identified, by using yeast knockout libraries, as apparently the yeast cells can support a complete replication cycle of this virus Fig. 5 .
Fig. 5.

Severe outbreaks of Cucumber mosaic virus (CMV; Cucumovirus) containing the necrogenic satRNA (A) and Pelargonium zonate spot virus (PZSV; Anulavirus) (B) in crops of canning tomato in southern Italy. Insets show disease symptoms on fruits.
Photographs courtesy of ICTV.
Both homologous and non-homologous RNA-RNA crossovers has been demonstrated between bromoviral RNAs during infection. Homologous recombination has also been shown during co-infection between two strains of BMV, with some distinct hot spots localized within both the coding and non-coding regions. The role in recombination of proteins 1a and 2a as well as of the CP have been demonstrated in BMV, suggesting the template switching as a likely mechanism for RNA recombination. For the cucumoviruses, the control of recombination frequency resides mainly in the 2a gene.
Members of the genera Bromovirus and Cucumovirus are capable of producing the defective (d)RNAs and defective-interfering (di)RNAs during infection (Table 1). In particular, strains of Broad bean mottle virus (BBMV) do accumulate RNA2-derived deletion variants after serial passages through broad bean. In BMV, both replicating and non-replicating truncated RNA2-derived artificial diRNAs have been shown to interfere with BMV RNAs in protoplasts. For CMV, several types of RNA3-derived diRNAs have been described.
Biology
The family Bromoviridae is one of the most important families of plant RNA viruses, with some members widely distributed in the world. In its entirety, the family has a wide host range (more than 10,000 species) and some members are causing agronomically important diseases. However, the host range of members of individual genera ranges from significantly narrow (genera Bromovirus, Oleavirus) to extremely broad (genus Cucumovirus). CMV can infect one of the largest number of plant species among plant viruses. Some of these viruses cause major disease epidemics in vegetables, fodder and fruit crops, e.g., in tomato, cucurbits, bananas, or alfalfa, and in fruit trees (ilarviruses). Different virus species are transmitted mechanically, via pollen/thrips, through seeds or by insect vectors like aphids or beetles. It has been speculated that lack of inefficient vectors evolved some bromovirids toward producing larger concentration of viral particles. For CMV, although the virus is prone to recombination, the recombinants are rare during infection, suggesting the presence of strong selection bottlenecks. No direct correlation between virus yield and symptom severity have been observed, but rather the symptoms seem to be associated with changes in specific regions in the RNA genome, as it has been mapped by using the natural strains of BMV, BBMV or CCMV.
Nomenclature
- aa
Amino acid(s)
- CP
Coat protein or capsid protein
- ELISA
Enzyme-linked immuno-sorbent assay
- ER
Endoplasmic reticulum
- kb
Kilobases; the size of a ssDNA or ssRNA molecule
- kDa
Kilodaltons; the size of a protein
- MP
Movement protein
- Mr
Relative molecular mass
- NGS
Next generation sequencing
- nm
Nanometer(s)
- ORF
Open reading frame(s)
- PCR
Polymerase chain reaction
- RBD
RNA binding domain
- RdRp
RNA-dependent RNA polymerase
- RT-PCR
Reverse transcription polymerase chain reaction
- sgp
Sub-genomic promoter
- sgRNA
Sub-genomic RNA
- ssRNA
Single-stranded ribonucleic acid
- TLS
Transfer RNA-like structures
- UTR
Untranslated region
- VIGS
Virus-induced gene silencing
- VRC
Virus replication complexes
Further Reading
- Bujarski J., Gallitelli D., García-Arenal F., et al. ICTV Virus Taxonomy Profile: Bromoviridae. Journal of General Virology. 2019;100:1206–1207. doi: 10.1099/jgv.0.001282. [DOI] [PubMed] [Google Scholar]
- Chaturvedi S., Rao A. Molecular and biological factors regulating the genome packaging in single-strand positive-sense tripartite RNA plant viruses. Current Opinion in Virology. 2018;33:113–119. doi: 10.1016/j.coviro.2018.07.019. [DOI] [PubMed] [Google Scholar]
- Codoner F.M., Elena S.F. The promiscuous evolutionary history of the family Bromoviridae. Journal of General Virology. 2008;89:1739–1747. doi: 10.1099/vir.0.2008/000166-0. [DOI] [PubMed] [Google Scholar]
- Diaz A., Wang X. Bromovirus-induced remodeling of host membranes during viral RNA replication. Current Opinion in Virology. 2014;9:104–110. doi: 10.1016/j.coviro.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Gancarz B.L., Hao L., He Q., Newton M.A., Ahlquist P. Systematic identification of novel, essential host genes affecting bromovirus RNA replication. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostiainen M.A., Hiekkataipale P., Laiho A., et al. Electrostatic assembly of binary nanoparticle superlattices using protein cages. Nature Nanotechnology. 2013;8:52–56. doi: 10.1038/nnano.2012.220. [DOI] [PubMed] [Google Scholar]
- Pallas V., Aparicio F., Herranz M.C., Sanchez-Navarro J.A., Scott S.W. The molecular biology of ilarviruses. Advances in Virus Research. 2013;87:139–181. doi: 10.1016/B978-0-12-407698-3.00005-3. [DOI] [PubMed] [Google Scholar]
- Schoelz J.E., Harries P.A., Nelson R.S. Intracellular transport of plant Viruses: Finding the door out of the cell. Molecular Plant. 2011;4(5):813–831. doi: 10.1093/mp/ssr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztuba-Solińska J., Urbanowicz A., Figlerowicz M., Bujarski J.J. RNA-RNA recombination in plant virus replication and evolution. The Annual Review of Phytopathology. 2011;49:415–443. doi: 10.1146/annurev-phyto-072910-095351. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Matsuo Y., Suzuki M., et al. Impact of a defective RNA 3 from cucumber mosaic virus on helper virus infection dynamics. Virology. 2009;389:59–65. doi: 10.1016/j.virol.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Weber P.H., Bujarski J.J. Multiple functions of capsid proteins in (+) stranded RNA viruses during plant-virus interactions. Virus Research. 2015;196:140–149. doi: 10.1016/j.virusres.2014.11.014. [DOI] [PubMed] [Google Scholar]


