Herpes simplex virus 1 is the leading cause of infectious corneal blindness in the United States and Europe. Developing vaccines to prevent ocular disease is challenging because the eye is a relatively immune-privileged site. In this study, we compared a single-cycle viral vaccine candidate, which is unique in that it elicits predominantly nonneutralizing antibodies that activate Fc receptors and bind complement, and a glycoprotein D subunit vaccine that elicits neutralizing but not Fc receptor-activating or complement-binding responses. Only the single-cycle vaccine provided both active and passive protection against a lethal ocular challenge. These findings greatly expand our understanding of the types of immune responses needed to protect the eye and will inform future prophylactic and therapeutic strategies.
KEYWORDS: antibody-dependent cell-mediated cytotoxicity, herpes simplex virus, ocular herpes, vaccines
ABSTRACT
Herpes simplex virus 1 (HSV-1) is a leading cause of infectious blindness, highlighting the need for effective vaccines. A single-cycle HSV-2 strain with the deletion of glycoprotein D, ΔgD-2, completely protected mice from HSV-1 and HSV-2 skin or vaginal disease and prevented latency following active or passive immunization in preclinical studies. The antibodies functioned primarily by activating Fc receptors to mediate antibody-dependent cellular cytotoxicity (ADCC). The ability of ADCC to protect the immune-privileged eye, however, may differ from skin or vaginal infections. Thus, the current studies were designed to compare active and passive immunization with ΔgD-2 versus an adjuvanted gD subunit vaccine (rgD-2) in a primary lethal ocular murine model. ΔgD-2 provided significantly greater protection than rgD-2 following a two-dose vaccine regimen, although both vaccines were protective compared to an uninfected cell lysate. However, only immune serum from ΔgD-2-vaccinated, but not rgD-2-vaccinated, mice provided significant protection against lethality in passive transfer studies. The significantly greater passive protection afforded by ΔgD-2 persisted after controlling for the total amount of HSV-specific IgG in the transferred serum. The antibodies elicited by rgD-2 had significantly higher neutralizing titers, whereas those elicited by ΔgD-2 had significantly more C1q binding and Fc gamma receptor activation, a surrogate for ADCC function. Together, the findings suggest ADCC is protective in the eye and that nonneutralizing antibodies elicited by ΔgD-2 provide greater protection than neutralizing antibodies elicited by rgD-2 against primary ocular HSV disease. The findings support advancement of vaccines, including ΔgD-2, that elicit polyfunctional antibody responses.
IMPORTANCE Herpes simplex virus 1 is the leading cause of infectious corneal blindness in the United States and Europe. Developing vaccines to prevent ocular disease is challenging because the eye is a relatively immune-privileged site. In this study, we compared a single-cycle viral vaccine candidate, which is unique in that it elicits predominantly nonneutralizing antibodies that activate Fc receptors and bind complement, and a glycoprotein D subunit vaccine that elicits neutralizing but not Fc receptor-activating or complement-binding responses. Only the single-cycle vaccine provided both active and passive protection against a lethal ocular challenge. These findings greatly expand our understanding of the types of immune responses needed to protect the eye and will inform future prophylactic and therapeutic strategies.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) is a major global health problem with an estimated 3.72 billion people infected worldwide (1). HSV-1 causes oral and genital mucocutaneous disease and sporadic encephalitis and is the leading cause of infectious corneal blindness in the United States and Europe (2). HSV-1 results in 300,000 diagnoses of ocular disease in the United States annually and 40,000 new cases of severe visual impairment globally (3). Most of these epidemiological studies have been limited to developed nations, however, and thus may not accurately reflect the true global incidence. Ocular disease may occur in response to direct inoculation following primary exposure or, more commonly, following reactivation of virus that established latency in the trigeminal ganglia (TG) after oral infection. Primary or reactivating ocular infection may result in blepharitis, conjunctivitis, keratitis, retinitis, or, less commonly, scleritis. Keratitis is the most prevalent and may progress to involve the deeper stromal structures, resulting in herpes stromal keratitis (HSK), which can lead to blindness. HSK reflects both the cytolytic effects of viral replication as well as the immune and inflammatory response and is characterized by scarring, edema, neovascularization, and leukocyte infiltration (2).
The primary approach to HSV prevention has focused on genital disease and has centered on subunit protein vaccines comprised of HSV-2 envelope glycoprotein D (gD-2) combined with different adjuvants. The subunit protein vaccines primarily elicit antibodies that neutralize both HSV-1 and HSV-2 in cell culture assays. These vaccines exhibited variable protection in preclinical models of vaginal, skin, and ocular disease with either serotype (4–6) but failed to provide significant protection against genital HSV-2 infection or disease, which was the primary study outcome, in clinical trials (7, 8). Partial protection against genital HSV-1 disease was observed in the most recent trial and correlated with neutralizing titers in an analysis of a small subset of participants (9). No clinical trials have been conducted to assess vaccine efficacy against HSV-1 ocular disease.
We adopted a completely different approach and deleted the gene encoding gD-2, which is required for viral entry and cell-to-cell spread, to generate a single-cycle candidate HSV-2 vaccine strain designated ΔgD-2 (10). The virus is grown on complementing cells that express HSV-1 gD (VD60 cells) (11) to yield genotypically gD-null viruses that have incorporated gD-1 on their envelope, allowing for an initial cycle of infection. Vaccination with the complemented virus caused no disease in wild-type or immunodeficient mice because the viral progeny do not express gD on their envelope and, thus, are unable to infect new cells (12). The vaccine was highly immunogenic, eliciting high-titer, polyantigenic IgG responses that provided complete protection in mice following lethal vaginal (female) or skin (female and male) inoculation with clinical isolates of HSV-1 or HSV-2; notably, the vaccine also prevented the establishment of latency (5, 12, 13). Passive transfer studies demonstrated that the antibodies (Abs) alone were sufficient to protect wild-type mice but not Fc gamma receptor (FcγR) or neonatal Fc receptor (FcRn) knockout mice (12). The Abs had little complement-independent neutralizing activity but activated murine FcγRIII and FcγRIV to mediate antibody-dependent cell killing by cytolytic and phagocytic pathways (5, 12–14).
However, the ability of vaccines to prevent HSV ocular disease may differ because the eye is a relatively immune-privileged site. The notion of immune privilege was first identified in studies demonstrating a lack of an immune response to allografts (15). Mechanisms that may contribute to immune privilege include lack of direct lymphatic drainage, a blood-ocular barrier, as well as immunosuppressive and immunoregulatory responses (16). Additionally, the FcRn may preferentially transport immunoglobulin G (IgG) out of the eye into the systemic circulation (17), which could limit Ab-mediated protection. Building on this foundation, the current studies were designed to compare active and passive protection afforded by ΔgD-2 or an adjuvanted recombinant gD subunit protein vaccine against primary lethal corneal disease in a murine model.
(This research was conducted by N. L. M. Ramsey in partial fulfillment of the requirements for a Ph.D. from Albert Einstein College of Medicine, Bronx, NY, 2020.)
RESULTS
ΔgD-2 provides greater protection than rgD-2/Alum-MPL against lethal ocular HSV-1 infection.
Prior murine studies to assess vaccine efficacy in the ocular model were typically conducted in female BALB/c mice (6, 18–20) and relied on sublethal doses of laboratory-adapted HSV-1 strains, such as KOS or McKrae (6, 18, 20). To generate a more stringent and lethal model, we infected mice with different doses of the clinical isolate, HSV-1 (B3x1.1), which caused lethal disease following skin and vaginal challenge in adult mice and following intranasal infection of pups (5, 13, 14). B3x1.1 infection of the cornea resulted in consistent lethality at doses of 105 PFU or greater, and we used 105 or 106 PFU for subsequent vaccine studies.
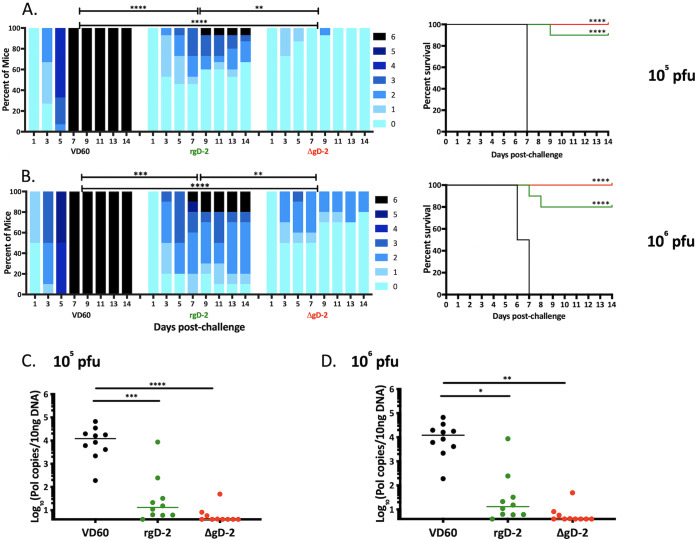
Female mice received two doses of ΔgD-2, rgD-2 combined with alum monophosphoryl lipid A (MPL) (rgD-2/Alum-MPL) which is similar to the vaccine used in the HerpeVac trial (7), or an uninfected VD60 cell lysate as a control prior to corneal challenge with 105 (Fig. 1A) or 106 (Fig. 1B) PFU of B3x1.1. Mice vaccinated with either ΔgD-2 or rgD-2/Alum-MPL exhibited significantly lower disease scores than the VD60-vaccinated controls (P < 0.001). The controls developed progressive ocular and neurologic disease and succumbed by day 7 postinfection (p.i.). However, there were significant differences between the two vaccines with disease scores significantly lower in ΔgD-2-vaccinated mice than rgD-2/Alum-MPL-vaccinated mice (P < 0.01), and this difference was magnified when the mice were challenged with 106 PFU of B3x1.1. All of the mice infected with this higher dose of virus exhibited signs of disease as early as day 3, but the ΔgD-2 vaccinated mice recovered quickly with no mortality. In contrast, 2/10 mice vaccinated with rgD-2/Alum-MPL succumbed, and most exhibited persistent signs of disease (scores > 2) throughout the 2-week monitoring period. Both vaccines provided significant protection against latency after challenge with 105 or 106 PFU, as evidenced by the quantity of HSV DNA detected in excised ipsilateral TG (Fig. 1C and D, respectively).
FIG 1.
ΔgD-2 vaccine protects against disease and latency in an ocular lethal HSV-1 challenge model. Female BALB/c mice received two doses of 5 × 106 PFU ΔgD-2 (red), 5 μg rgD-2 combined with alum and MPL (green), or an uninfected VD60 cell lysate as control (black) and were then challenged with 105 PFU (A) (15 mice per group, 3 independent experiments) or 106 PFU (B) (10 mice per group, 2 independent experiments) of HSV-1 (B3x1.1). Mice were scored for signs of disease, euthanized at a score of 5, and assigned a score of 6 the following day. Survival curves are shown to the right of disease scores. Viral spread to trigeminal ganglia was determined by quantitative PCR at time of demise because of disease or at day 28 postchallenge in survivors following infection with 105 PFU (C) or 106 PFU (D). Data is displayed as median copies per 10 ng of DNA. The disease scores were compared by two-way ANOVA with Sidak’s multiple comparison, survival by Gehan-Breslow-Wilcoxon test relative to controls, and viral DNA by ANOVA with Tukey’s multiple comparison (*, P < 0.05; **, P < 0.01; ****, P < 0.0001).
To assess how quickly virus was cleared from the site of inoculation, the eyes were swabbed and infectious virus quantified by plaque assay (n = 3 mice per group). Significantly less virus was detected in ΔgD-2-vaccinated mice than in control mice 2 days postinoculation (P = 0.02), and the amount of virus declined more rapidly over the next several days although the differences in clearance did not reach statistical significance (Fig. 2A). To assess the ability of the vaccines to prevent viral spread, replicating or latent virus was quantified in the contralateral TG by coculturing minced TG tissue with Vero cells (n = 5 mice per group). No infectious virus was detected in TG harvested from ΔgD-2-vaccinated mice even after 21 days of coculture, whereas virus was detected in all of the TG harvested from rgD-2/Alum-MPL-vaccinated mice by day 9 and within 48 to 72 h in all of the TG from control-vaccinated mice (Fig. 2B; P < 0.0001 for ΔgD-2 versus rgD-2 or VD60 control). Virus detected within 2 to 3 days presumably reflects lytic rather than latent virus.
FIG 2.
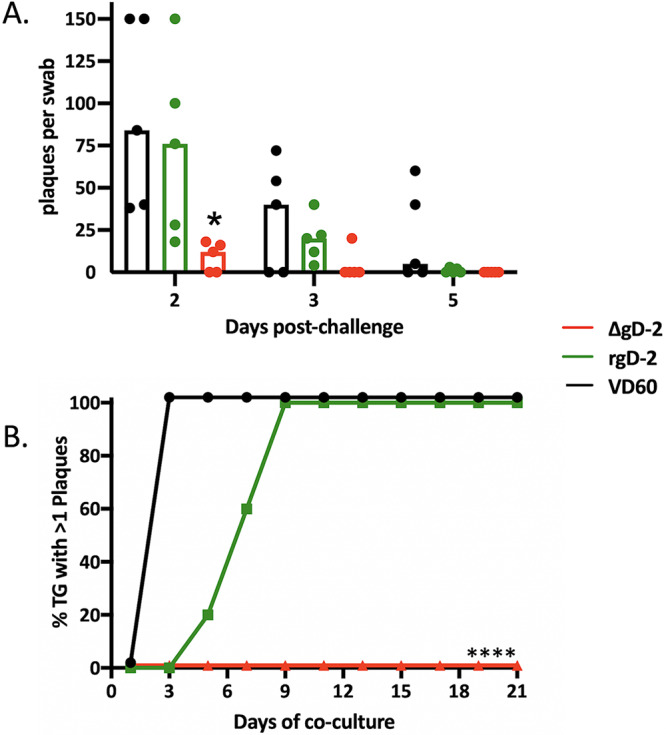
ΔgD-2 vaccination results in rapid clearance of virus and prevents spread to contralateral trigeminal ganglia. Female BALB/c mice received two doses of 5 × 106 PFU ΔgD-2 (red), 5 μg rgD-2 combined with alum and MPL (green), or an uninfected VD60 cell lysate as control (black) and were then challenged with 105 PFU HSV-1 (B3x1.1). (A) Eyes were swabbed on days 2, 3, and 5 and infectious virus quantified by performing a plaque assay on Vero cells. Results are presented as scatterplots with bar at median and compared at each time by ANOVA (*, P < 0.05). The upper limit of detection for plaques per well is 150. (B) Contralateral trigeminal ganglia were excised at time of death and cocultured with Vero cells to detect infectious or reactivating virus (n = 5 mice per group). Results are presented as percentage of positive cultures at each time point and compared using Gehan-Breslow-Wilcoxon test; ****, P < 0.0001 for ΔgD-2 compared to both VD60 and adjuvanted rgD-2.
Transfer of immune serum from ΔgD-2- but not rgD-2/Alum-MPL-vaccinated mice protects against subsequent ocular challenge.
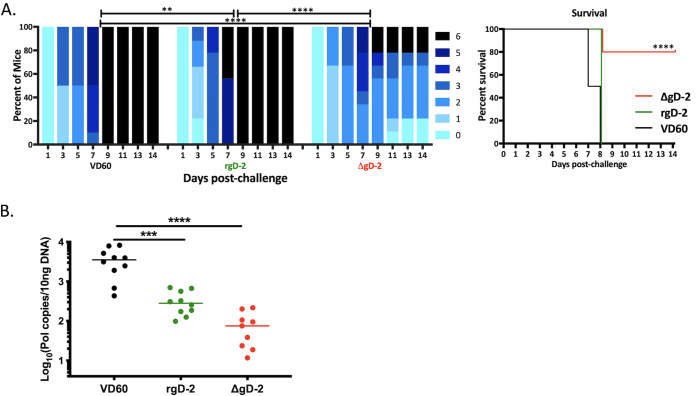
Previous studies demonstrated that immune serum from ΔgD-2-vaccinated mice passively protected naive animals from vaginal or skin challenge (12). To assess whether immune serum could also protect against ocular disease, serum from ΔgD-2-, rgD-2/Alum-MPL-, or control-immunized mice was pooled, and an equivalent amount of total IgG (750 μg) was administered intraperitoneally 1 day prior and 4 days following corneal challenge with 105 PFU of B3x1.1. Although all mice developed signs of disease, the majority (8/10) that received ΔgD-2 immune serum survived compared to none of the mice that received rgD-2/Alum-MPL or VD60 immune serum (Fig. 3A; P < 0.0001). Disease scores were higher and progressed more rapidly in the latter groups. Passive transfer of ΔgD-2 immune serum reduced the quantity of HSV-1 DNA detected in TG compared to VD60 (P < 0.0001). Pooled rgD-2/Alum-MPL immune serum also reduced the amount of latent TG virus compared to that of VD60 immune serum (P < 0.001) despite not providing any survival benefit (Fig. 3B).
FIG 3.
Passive transfer of immune serum from mice vaccinated with ΔgD-2 protects against lethal ocular disease. Female BALB/c mice received two doses of 5 × 106 PFU ΔgD-2 (red), 5 μg rgD-2 combined with alum and MPL (green), or an uninfected VD60 cell lysate (black) administered subcutaneously at 3-week intervals. Mice were bled 2 weeks and 4 weeks after the second vaccine dose, and the serum was pooled and total IgG quantified by ELISA. Naive mice were administered serum containing 750 μg IgG intraperitoneally 1 day prior and 4 days following corneal inoculation with 105 PFU HSV-1 (B3x1.1). (A) The disease scores (left) were compared by two-way ANOVA with Sidak’s multiple comparison and survival (right) by Gehan-Breslow-Wilcoxon test (n = 10 mice per group; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). (B) Viral spread to trigeminal ganglia was determined by quantitative PCR at time of demise because of disease or at day 21 postchallenge in survivors and is presented as HSV DNA copies per 10 ng of DNA (n = 9 to 10 mice/group in 2 independent experiments; ***, P < 0.001; ****, P < 0.0001).
Nonneutralizing antibodies protect against ocular disease.
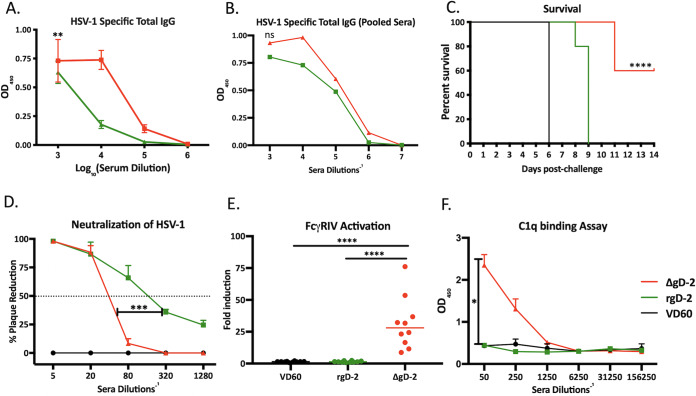
The striking loss in protection following passive compared to active immunization with rgD-2/Alum-MPL (0/10 versus 8/10 survivors, respectively) but not with ΔgD-2 (8/10 and 10/10 survivors) could be attributed to quantitative and/or qualitative differences in the antibody response and/or relative contribution of T cells to immune protection with the two vaccines. ΔgD-2 elicited higher total HSV-1-specific IgG responses compared to those of rgD-2/Alum-MPL (Fig. 4A). To assess whether this quantitative difference accounted for the lack of passive protection afforded by rgD-2/Alum-MPL, new pools were prepared by combining immune serum from individual mice with similar concentrations of HSV-1 antibody titer (measured by enzyme-linked immunosorbent assay [ELISA]), and the HSV-specific IgG in the new pools were compared (Fig. 4B). The passive transfer studies were repeated with these new pools and again showed significantly greater protection with ΔgD-2 than rgD-2 immune serum, indicating that the concentration of HSV-specific IgG does not fully explain the differences in protection (Fig. 4C). We then compared the functionality of the Ab responses. Consistent with prior studies (5), rgD-2/Alum-MPL elicited a higher neutralizing Ab titer (mean ± standard deviation [SD], 156 ± 39.7 compared to 33 ± 5.2 for ΔgD-2 and rgD-2, respectively; representative results from 3 mice are shown in Fig. 4D). Conversely, the Abs elicited by ΔgD-2 exhibited greater activation of the FcγRIV receptor, a biomarker of antibody-dependent cellular cytotoxicity (ADCC) (Fig. 4E). Moreover, the ΔgD-2-elicited Abs, but not the rgD-2/Alum-MPL Abs, also bound C1q (Fig. 4F).
FIG 4.
Differences in passive protection reflect distinct antibody function. (A) Immune serum was assayed for total HSV-specific IgG by ELISA. Results are presented as optical densitometry (OD) units at indicated serum dilution with mean ± standard error of the mean (SEM) (n = 5 mice per group); **, P < 0.01, linear regression. (B) Immune serum from mice with similar HSV-specific IgG titers were pooled, and the new pools were retested in the HSV ELISA (ns, no significant difference in curves by linear regression). (C) Passive transfer studies were repeated with new pools of immune serum containing similar total HSV-specific IgG (n = 5 mice per group). Survival curves were compared by Gehan-Breslow-Wilcoxon test; ****, P < 0.0001 compared to VD60 or adjuvanted rgD-2. (D) Neutralization of viral infection was determined by plaque assay with indicated serum dilution and is shown as percent reduction in PFU relative to control serum. The horizontal line at 50% indicates the dilution of serum that inhibits 50% of viral plaques (neutralization titer). The figure is the average obtained from a representative experiment with 3 individual mice per group (***, P < 0.001 unpaired t test with Welch’s correction area under curve). (E) ADCC was assayed using the murine FcγRIV activation assay with 1:5 dilution of immune serum; results are fold induction relative to controls and data shown as scatterplots (n = 10 mice per group). ****, P < 0.0001 ANOVA with Tukey’s multiple comparison. (F) C1q binding of immune serum was assayed by ELISA at the indicated dilutions, and results are shown as OD mean ± SEM (n = 4 mice per group). *, P < 0.0001 area under curve was compared by unpaired t test with Welch’s correction.
T cell depletion following active vaccination with either the subunit or single-cycle vaccine leads to a reduction in protection.
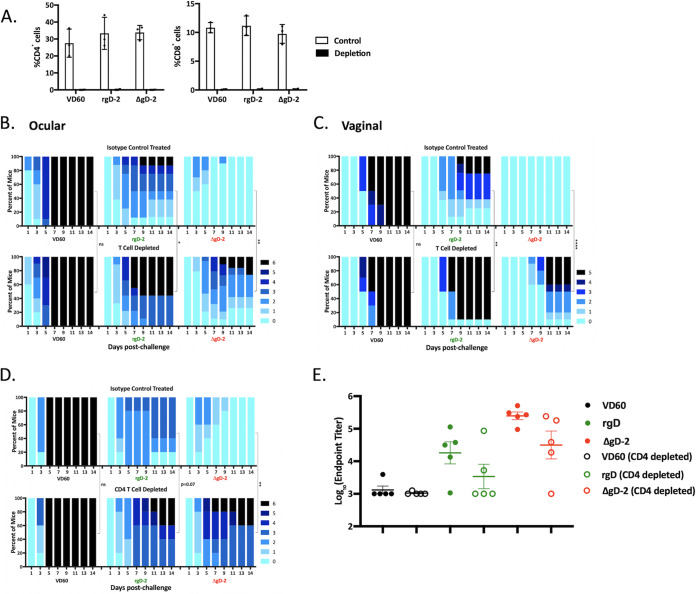
The greater discordance between active and passive protection provided by rgD-2/Alum-MPL compared to ΔgD-2 could also reflect a greater role for T cells in mediating subunit vaccine protection following active immunization. To address this, mice were actively immunized with 2 doses of each vaccine, and then T cells were depleted by treating the mice with anti-CD4 and anti-CD8 monoclonal antibodies (MAbs) 4 days and 2 days prior to ocular or, for comparison, vaginal challenge. Controls received an unrelated isotype-matched Ab. The depleting Abs resulted in a greater than 95% reduction in CD4 and CD8 T cell populations by flow cytometry (Fig. 5A). As expected, ΔgD-2 provided significantly greater protection against disease than rgD-2/Alum-MPL following ocular (Fig. 5B) or vaginal (Fig. 5C) challenge in the isotype control-treated mice. Depletion of T cells just prior to challenge, however, led to a significant increase in disease scores for both vaccines. Survival (score of <5) decreased from 100% to 80% and from 100% to 60% after ocular and vaginal challenge, respectively, for ΔgD-2 and from 90% to 50% and from 80% to 10% after ocular and vaginal challenge, respectively, for rgD-2/Alum-MPL. A similar increase in disease scores after ocular challenge was also observed when only CD4+ T cells were depleted, and this trended toward significance for the subunit vaccine (P = 0.07) and was significant for ΔgD-2 (P < 0.01) (Fig. 5D). Survival decreased from 100% to 60% for both vaccines. Depletion of CD4+ T cells was associated with a modest, but not statistically significant, decrease in HSV-specific Ab titers in the blood, consistent with a role for CD4 T cells in maintaining Ab levels in mice (Fig. 5E) (21, 22).
FIG 5.
Depletion of T cells in vaccinated mice prior to challenge results in reduction in protection for both vaccines. Mice received two doses (3 weeks apart) of 5 × 106 PFU ΔgD-2 (red), 5 μg rgD-2 combined with alum and MPL (green), or an uninfected VD60 cell lysate as control (black). Three weeks later, mice were treated with anti-CD4 and anti-CD8 monoclonal antibodies or an isotype control, which was administered intraperitoneally 4 and 2 days prior to challenge with 105 PFU HSV-1 (B3x1.1). (A) The percentage of CD4 and CD8 T cells in blood obtained 1 day prior to challenge was quantified by flow cytometry; results are presented as mean ± SEM (n = 3 per group). Mice were challenged on the cornea (B) or intravaginally (C) and disease scores monitored (n = 8 to 10 mice/group). (D) Mice were treated with anti-CD4 MAb or isotype control and challenged intraocularly (n = 5 mice per group). Disease scores were compared between mice treated with depleting antibodies or isotype control antibody by two-way ANOVA with Sidak’s multiple-comparison test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). (E) Immune serum was assayed for total HSV-specific IgG by ELISA using serum obtained 5 days postinfection; differences between mice treated with depleting anti-CD4 or isotype control were compared by t test.
DISCUSSION
Following active or passive immunization, ΔgD-2, a novel single-cycle candidate vaccine strain, provided significantly greater protection than an adjuvanted gD-2 subunit protein vaccine in a primary ocular murine challenge model using a clinical isolate of HSV-1 at lethal doses. The differences were more striking in the passive transfer studies and persisted after delivering a comparable amount of total HSV-specific IgG, thus providing insights into the relative contribution of functionally distinct Abs in mediating protection. The Abs elicited in response to ΔgD-2 bind and activate the murine FcγRIV, the primary mediator of ADCC (23, 24), and, as shown here for the first time, also bind C1q. The C1q and FcγR-binding sites on the Fc domain partially overlap (25), and both contribute to immune protection. Engagement of FcγRIV promotes ADCC and antibody-dependent phagocytosis, and binding to C1q activates the complement cascade, leading to complement-dependent cytotoxicity. In addition to directly activating complement-dependent cytolysis, the ability of the Fc portion of IgG to bind C1q may further promote engagement of the FcγR via bridging and thereby enhance Fc effector functions (26). The gD subunit vaccine, in contrast, induced an almost exclusively neutralizing Ab response with little or no FcγRIV activation or C1q binding. The inability of the gD-specific neutralizing Abs to provide as much protection as the nonneutralizing Abs may reflect the ability of HSV to spread from infected to uninfected cells across intercellular bridges, thereby escaping neutralization (27). The Abs also differ with respect to antigenic targets, with the subunit vaccine generating Abs to gD (28, 29), whereas ΔgD-2 elicits responses targeting multiple viral antigens (12). In addition, the Abs may differ in their ability to bind the Fc neonatal receptor and be retained within the cornea.
The more striking difference in protection against lethality following active compared to passive protection for rgD-2/Alum-MPL versus ΔgD-2 could not be explained by a differential role for T cells. Depletion of CD4+ and CD8+ T cells or CD4+ cells alone from actively immunized mice prior to challenge led to a reduction in protection for both vaccines. The loss of protection was observed following both ocular and vaginal challenges and was somewhat greater in the ΔgD-2-immunized mice. This could reflect a role for CD4 help in facilitating Ab localization to immune-privileged sites, including the eye and the peripheral nervous system. The cornea, peripheral nervous system, and brain are protected by a blood-ocular, blood-nerve, and blood-brain barrier. A recent study showed that CD4+ T cells contribute to the ability of Abs to access these sites by releasing interferon γ to promote an increase in vascular permeability (30). We speculate that this contributed to the decrease in protection observed when T cells were depleted just prior to challenge. Moreover, other immune cell populations, most notably CD4+ dendritic cells (DCs) may be depleted by anti-CD4 treatment (31). This could have further interfered with ΔgD-2 vaccine efficacy since DCs, which express FcγRIV, contribute to antibody-mediated cell killing in mice (23, 32). We found that depletion of CD11c+ cells led to a loss of protection in passive transfer studies with ΔgD-2 immune serum (C. B. Aschner and B. C. Herold, unpublished data).
The ocular model used in these studies differs from the more traditional model in which mice are challenged with sublethal doses of laboratory-adapted strains of HSV-1 that typically cause ∼50% or less mortality (6, 18, 20, 33). Potential advantages of the high-dose lethal challenge model include its stringency, but a disadvantage is that it does not reflect clinical ocular disease, which is not lethal and more often the result of repeated episodes of viral reactivation. However, the observation that vaccination with ΔgD-2 was associated with rapid clearance of virus, protection against dissemination of virus to the contralateral trigeminal ganglia, and a significant decrease in the latent reservoir, as measured by quantitative PCR (qPCR) in the ipsilateral trigeminal ganglia, provide strong indirect evidence that this different vaccine strategy would prevent or reduce the risk of reactivation. Although we did not attempt to induce reactivation with UV light or other stimulation in the ΔgD-2-vaccinated mice, no signs of ocular disease were clinically observed in any of the vaccinated mice who were monitored for up to 4-weeks postchallenge. Future studies with models of recurrent disease, such as rabbit models, may provide further insights (34–37).
Passive transfer of immune serum from ΔgD-2-vaccinated mice was less effective against ocular disease than against vaginal or skin disease. A single dose of pooled ΔgD-2 immune serum (containing 750 μg of total IgG) administered 1 day prior to challenge provided 100% protection against a lethal HSV-2 vaginal or skin challenge (12). Complete protection against lethality was also observed in male mice treated with a single dose of ΔgD-2 immune serum; notably, in that study, immune serum pooled from rgD-2/Alum-MPL-immunized mice provided no protection against lethal skin challenge (5). The reduction in efficacy with ΔgD-2 immune serum in the prevention of ocular compared to vaginal or skin disease, even when two doses were administered to address the relatively short half-life (4 to 8 days) of murine IgG (38, 39), likely reflects accumulation of less Ab in the eye, either because of differences in vascular permeability and/or ability of the FcRn to pump Ab out of the eye (17).
A recent study compared a different adjuvanted rgD-2 subunit protein vaccine to a live attenuated strain, HSV-1 (0ΔNLS), which has a deletion of the sequence for the nuclear localization signal on the viral ICP0 gene. The subunit vaccine used in that study contained only 2.5 μg of gD protein (compared to 5 μg here) and provided no significant increase in HSV neutralization titers or protection compared to that of control mice. Notably, the challenge dose was 104 PFU of HSV-1 (McKrae), which caused ∼50% mortality in the control mice. However, the authors did observe significant protection with the live attenuated vaccine strain, which correlated with neutralizing titers. Thus, neutralizing antibodies, whether elicited in response to a higher dose of adjuvanted rgD-2 as observed here or in response to a live attenuated vaccine strain, do confer some protection against ocular disease. The novelty of the current studies is that nonneutralizing Abs accumulate sufficiently in the immune-privileged eye following active or passive immunization to provide even greater protection against lethal ocular disease. These findings may have implications not only for development of strategies to prevent or treat ocular HSV but also may be relevant for other pathogens.
MATERIALS AND METHODS
Mice and ethics statement.
Female BALB/c mice were purchased from the Jackson Laboratory (JAX, Bar Harbor, ME). The use of mice in this study was approved by the Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine, protocol 2016-0908.
Cells, virus, and vaccines.
Vero (African green monkey kidney, ATCC CCL-81) and VD60 (11) cells were grown in Dulbecco modified Eagle medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT) and 1% penicillin-streptomycin (Invitrogen). ΔgD-2 was propagated in VD60 cells and viral titers (PFU/ml) were quantified on complementing VD60 and noncomplementing Vero cells (10). HSV-1 (B3x1.1), a clinical isolate of HSV-1, was originally obtained from the Montefiore Clinical Virology Lab and propagated on Vero cells (13). Recombinant HSV-2 gD protein (rgD-2) was synthesized by the Einstein Protein Core Facility, as previously described (5).
Immunization and challenge protocol.
Four- to six-week-old female mice were immunized subcutaneously (s.c.) with 5 × 106 PFU of ΔgD-2 (based on the titer on VD60 cells; note no plaques were detected on Vero cells), 5 μg of rgD-2 combined with 150 μg alum (Imject Alum; Pierce Biotechnology, Rockland, IL) and 12.5 μg monophosphoryl lipid A (MPL) (InvivoGen, San Diego, CA) (rgD-2/Alum-MPL), or an uninfected VD60 cell lysate as a control. Two doses of vaccine were administered at 3-week intervals, and 3 weeks after the second dose, mice were challenged on the right eye with 105 or 106 PFU of HSV-1 (Bx31.1) diluted in 5 μl of phosphate-buffered saline (PBS) after scarifying the cornea with a 24-guage needle. Mice were monitored daily for signs of ocular disease and scored as follows: 0, no disease; 1, minimal eyelid swelling; 2, moderate eyelid swelling, minimal ocular discharge, or minimal hair loss; 3, severe eyelid swelling, moderate ocular discharge, or severe hair loss; 4, eyes crusted shut; 5, signs of neurologic disease (poor grooming, hunched back, disequilibrium, paralysis, weight loss); 6, death. Mice were euthanized at a score of 5 and assigned a score of 6 the following day. In select experiments, vaginal challenges were conducted for comparison using previously described methods (12, 40). Vaccinated mice were treated with 2.5 mg of medroxyprogesterone administered s.c. 5 days prior to intravaginal challenge with 5 × 106 PFU of HSV1 (Bx31.1) diluted in 20 μl of PBS. Mice were monitored for 2 weeks and scored as follows: 0, no disease; 1, mild erythema or edema; 2, small lesion, moderate erythema, edema, or hair loss at inoculum site; 3, large lesion, multiple lesions, or hair loss and/or mild paresis, urinary retention, or fecal retention; 4, hind limb paralysis or severe urinary or fecal retention; 5, death. Mice with a score of 4 were euthanized and assigned a score of 5 the following day.
Passive transfer studies.
Serum was collected ∼2 weeks post-second vaccine dose, randomly pooled, and then assayed for total IgG content by ELISA (catalog number 88-50400-88; Invitrogen, Carlsbad, CA). In select experiments, serum was pooled based on HSV-specific IgG titers, which were quantified using ELISA against HSV-infected cell lysates as described below. Immune serum containing 750 μg of total IgG (final volume, 250 to 300 μl) was administered intraperitoneally to naive (not previously immunized) mice 24 h before and 4 days after corneal challenge with 105 PFU of Bx31.1.
Quantitation of HSV DNA in neuronal tissue.
At the time of euthanasia or when mice succumbed to disease, trigeminal or sacral dorsal root ganglia were harvested and DNA isolated using the Qiagen blood and tissue DNA isolation kit (Qiagen). Previously described primers and probes targeting the HSV polymerase gene were used to quantify viral DNA by quantitative PCR (qPCR) using 10 ng DNA per sample (5). Mouse β actin primers and probes were included as a loading control (Applied Biosystems, Foster City, CA), and qPCR was run in an Applied Biosystems QuantStudio 7 Flex. Samples with fewer than 4 copies detected were considered negative.
Detection of infectious virus by plaque assay.
On days 2, 3, and 5 postchallenge, infected eyes were swabbed with sterile cotton tipped applicators premoistened with DMEM. The swabs were placed into 1 ml of DMEM in Eppendorf tubes and vortexed for 15 s to elute virus before discarding the swab. Samples were frozen at –80°C until plaque assays on Vero cells were performed. Plaque assays were conducted by inoculating Vero cells grown on 24-well plates in duplicate with 250 μl of sample. Plaques were counted after 48 h.
To assess whether infectious virus had spread from inoculated eye to the contralateral side, contralateral TG were extracted at time of death or at day 28 postchallenge, minced, and plated onto Vero cell monolayers grown on 6-well plates. Cells were monitored microscopically daily for plaque formation as evidence of viral reactivation.
Antibody assays.
Total HSV-1-binding IgG titers were quantified by ELISA using HSV-1-infected Vero cell lysates as the antigen. Vero cells were infected with HSV-1 (B3x1.1) at a multiplicity of infection (MOI) of 0.1 PFU/cell, and after 24 h of incubation, the cells were harvested by scraping and sonicated for 30 s. Uninfected Vero cell lysates were prepared in parallel as controls. The protein concentration was determined by Pierce BCA protein assay (Thermo Fisher Scientific, Waltham, MA), and 10 μg of infected or uninfected lysate were added to individual wells of a 96-well MaxiSorp ELISA plate (Nunc, NY). Plates were incubated overnight at 4°C. The cells were then further permeabilized with 0.1% Triton X-100 in PBS and fixed with 1% formaldehyde. Serially diluted individual serum samples (10-fold dilutions from 1:1,000 to 1:1,000,000) were added in duplicate to wells and allowed to bind overnight at 4°C. Wells were washed 5 times with 0.05% Tween 20 buffer, incubated with goat anti-mouse IgG biotin-labeled secondary antibody (catalog number 553999; BD Pharmingen, San Jose, CA) for an additional 2 h at room temperature (RT), washed again, incubated with horseradish peroxidase (HRP)-conjugated streptavidin (BD Pharmingen, San Jose, CA) for 30 min at RT, and then developed with tetramethylbenzidine (TMB) substrate (BD OptEIA) for 5 min. The reaction was stopped with 2 N H2SO4 and absorbance at 450 nm read on a SpectraMax (M5 series) ELISA plate reader. The final absorbance was determined by subtracting values obtained for uninfected cell lysates from values obtained with infected cell lysates.
Neutralization assays were conducted as previously described (12). Serial 4-fold dilutions of heat-inactivated serum were incubated with HSV-1 (B3x1.1) (100 PFU/well) for 1 h at 37°C, and then the mixture was added to a Vero cell monolayer for 1 h at 37°C. Cells were fixed with methanol and stained with Giemsa after a 48-h incubation. Plaques were counted, and the neutralization titer was defined as the highest dilution to result in a 50% reduction in plaque numbers.
ADCC was determined using the mFcγRIV ADCC reporter bioassay (Promega, Madison, WI). Vero cells were infected with HSV-1 (B3x1.1) at an MOI of 0.1 PFU/cell for 12 h as targets for the assay, transferred to white, flat-bottomed 96-well plates, and incubated with heat-inactivated serum from immunized mice (1:5 dilution in DMEM) for 15 min at room temperature. FcγRIV-expressing reporter cells were added for 6 h at 37°C and 5% CO2, and FcγRIV activation was detected by the addition of luciferin substrate. Plates were read in a SpectraMax M5e (Molecular Devices). Fold induction was calculated relative to luciferase activity in the absence of serum.
C1q binding was assessed by ELISA. The 96-well ELISA plates were coated with B3x1.1-infected or uninfected (control) Vero cell lysates as targets for the ADCC assays. Wells were blocked for 1 h at RT with 5% dry skim milk in PBS with 0.1% IGEPAL CA-630 (Sigma-Aldrich, Germany) and then washed four times. Serial 4-fold dilutions of immune serum from individual mice were added to each well for an additional 2 h before washing. Murine C1q (Complement Technology, Tyler, TX) was added at 2 μg/ml and incubated for 2 h at RT before washing. Bound C1q was quantified by adding rat anti-mouse C1q-biotin (0.5 μg/ml) (Cedarlane, Canada) for 1 h at RT followed by incubation with HRP-conjugated streptavidin (BD Pharmingen, San Jose, CA).
T cell depletion post-active immunization.
Vaccinated mice were treated with 0.15 mg of anti-CD4 (clone GK1.5) alone or in combination with 0.15 mg of anti-CD8 (clone 2.43) monoclonal antibodies (MAbs) or an equivalent quantity of anti-rat keyhole limpet hemocyanin MAb as an isotype control administered intraperitoneally 4 days and 2 days prior to vaginal or ocular challenge. Depletion was assessed by flow cytometry. Tail blood was collected into Alsever’s solution and centrifuged at 1,500 rpm at 4°C for 5 min. Ammonium-chloride-potassium lysing buffer (Thermo Fisher Scientific, Waltham, MA) was added to the pellet to lyse red blood cells (RBCs), and samples were centrifuged at 1,500 rpm at 4°C for 5 min. Cell viability was assessed using the eFluor 450 fixable viability dye. Samples were incubated with anti-CD16/CD32 for 20 min at 4°C for FcR receptor blocking and stained with a cocktail of MAbs comprised of anti-CD45 (fluorescein isothiocyanate [FITC]), anti-CD3 (PE-Cy7), anti-CD4 (PerCP-Cy5.5), and anti-CD8 (APC-H7) in fluorescence-activated cell sorter (FACS) buffer (PBS supplemented with 2% [vol/vol] FBS) at 4°C for 30 min. Samples were washed 3 times and then fixed with 4% (vol/vol) paraformaldehyde (Electron Microscopy Science) for 20 min at RT. Samples were analyzed with LSRII flow cytometer (BD) and FlowJo software. All antibodies were purchased from eBioscience (Thermo Fisher Scientific, Waltham, MA).
Statistical analysis.
Analyses were performed using GraphPad Prism version 8.0 software (GraphPad Software Inc., San Diego, CA). A P value of 0.05 was considered statistically significant. Survival curves were compared using the Gehan-Breslow-Wilcoxon test, and other results were compared using analysis of variance (ANOVA) with multiple testing as indicated.
ACKNOWLEDGMENTS
We thank John Kim for technical assistance with mice and Mei Cong, Vanessa Ott, and Aileen Paguio at Promega for the mFcγRIV ADCC reporter bioassay. The authors also thank the Einstein Protein Core Facility for producing recombinant glycoprotein D.
N.L.M.R. was supported by NIH T32 AI007501, and C.B.A. was supported by a Howard Hughes Medical Institute International Student Research Fellowship. This work was also supported by NIAID R01 AI17321 (B.C.H. and W.R.J.) and the Center for AIDS Research (P30AI124414). W.R.J. and B.C.H. receive research support for development of the ΔgD-2 vaccine from X-Vax Inc., which holds the license for its development. The authors also serve on the Scientific Advisory Board for X-Vax.
REFERENCES
- 1.Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, Newman LM. 2015. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One 10:e0140765. doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobo AM, Agelidis AM, Shukla D. 2019. Pathogenesis of herpes simplex keratitis: the host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf 17:40–49. doi: 10.1016/j.jtos.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farooq AV, Shukla D. 2012. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol 57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, Stanberry LR. 2003. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis 187:542–549. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 5.Burn C, Ramsey N, Garforth SJ, Almo S, Jacobs WR, Herold BC. 2017. A herpes simplex virus (HSV)-2 single-cycle candidate vaccine deleted in glycoprotein D protects male mice from lethal skin challenge with clinical isolates of HSV-1 and HSV-2. J Infect Dis 217:754–758. doi: 10.1093/infdis/jix628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keadle TL, Laycock KA, Miller JK, Hook KK, Fenoglio ED, Francotte M, Slaoui M, Stuart PM, Pepose JS. 1997. Efficacy of a recombinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J Infect Dis 176:331–338. doi: 10.1086/514049. [DOI] [PubMed] [Google Scholar]
- 7.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Herpevac Trial for Women. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanberry LR, GlaxoSmithKline Herpes Vaccine Efficacy Study Group, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 9.Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, Deal CD. 2014. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis 209:828–836. doi: 10.1093/infdis/jit651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheshenko N, Trepanier JB, Stefanidou M, Buckley N, Gonzalez P, Jacobs W, Herold BC. 2013. HSV activates Akt to trigger calcium release and promote viral entry: novel candidate target for treatment and suppression. FASEB J 27:2584–2599. doi: 10.1096/fj.12-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson DC, Wisner TW, Wright CC. 2011. Herpes simplex virus glycoproteins gB and gD function in a redundant fashion to promote secondary envelopment. J Virol 85:4910–4926. doi: 10.1128/JVI.00011-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petro C, Gonzalez PA, Cheshenko N, Jandl T, Khajoueinejad N, Benard A, Sengupta M, Herold BC, Jacobs WR. 2015. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. Elife 4:e06054. doi: 10.7554/eLife.06054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petro CD, Weinrick B, Khajoueinejad N, Burn C, Sellers R, Jacobs WR, Herold BC. 2016. HSV-2 ΔgD elicits FcγR-effector antibodies that protect against clinical isolates. JCI Insight 1:e88529. doi: 10.1172/jci.insight.88529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao CM, Goymer J, Loh LN, Mahant A, Burn Aschner C, Herold BC. 2020. Murine model of maternal immunization demonstrates protective role for antibodies that mediate antibody-dependent cellular cytotoxicity in protecting neonates from herpes simplex virus type 1 and type 2. J Infect Dis 221:729−738. doi: 10.1093/infdis/jiz521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medawar PB. 1948. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol 29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor AW. 2009. Ocular immune privilege. Eye (Lond) 23:1885–1889. doi: 10.1038/eye.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H, Robinson SB, Csaky KG. 2009. FcRn receptor-mediated pharmacokinetics of therapeutic IgG in the eye. Mol Vis 15:2803–2812. [PMC free article] [PubMed] [Google Scholar]
- 18.Royer DJ, Carr MM, Gurung HR, Halford WP, Carr D. 2017. The neonatal Fc receptor and complement fixation facilitate prophylactic vaccine-mediated humoral protection against viral infection in the ocular mucosa. J Immunol 199:1898–1911. doi: 10.4049/jimmunol.1700316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royer DJ, Gurung HR, Jinkins JK, Geltz JJ, Wu JL, Halford WP, Carr D. 2016. A highly efficacious herpes simplex virus 1 vaccine blocks viral pathogenesis and prevents corneal immunopathology via humoral immunity. J Virol 90:5514–5529. doi: 10.1128/JVI.00517-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Lint AL, Torres-Lopez E, Knipe DM. 2007. Immunization with a replication-defective herpes simplex virus 2 mutant reduces herpes simplex virus 1 infection and prevents ocular disease. Virology 368:227–231. doi: 10.1016/j.virol.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker DC. 1993. T cell-dependent B cell activation. Annu Rev Immunol 11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Hamada T, Inoue N, Nagasawa H, Fujisaki K, Suzuki N, Mikami T. 2000. The role of CD4+ or CD8+ T cells in the protective immune response of BALB/c mice to Neospora caninum infection. Vet Parasitol 90:183–191. doi: 10.1016/s0304-4017(00)00238-7. [DOI] [PubMed] [Google Scholar]
- 23.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. 2005. FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity 23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Nimmerjahn F, Lux A, Albert H, Woigk M, Lehmann C, Dudziak D, Smith P, Ravetch JV. 2010. FcγRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci U S A 107:19396–19401. doi: 10.1073/pnas.1014515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, Scallon B, Teplyakov A, Malia TJ, Strohl WR. 2014. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods 65:114–126. doi: 10.1016/j.ymeth.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 26.Lee CH, Romain G, Yan W, Watanabe M, Charab W, Todorova B, Lee J, Triplett K, Donkor M, Lungu OI, Lux A, Marshall N, Lindorfer MA, Goff OR, Balbino B, Kang TH, Tanno H, Delidakis G, Alford C, Taylor RP, Nimmerjahn F, Varadarajan N, Bruhns P, Zhang YJ, Georgiou G. 2017. IgG Fc domains that bind C1q but not effector Fcγ receptors delineate the importance of complement-mediated effector functions. Nat Immunol 18:889–898. doi: 10.1038/ni.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sattentau Q. 2008. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol 6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 28.Halford WP. 2014. Antigenic breadth: a missing ingredient in HSV-2 subunit vaccines? Expert Rev Vaccines 13:691–710. doi: 10.1586/14760584.2014.910121. [DOI] [PubMed] [Google Scholar]
- 29.Whitbeck JC, Huang Z-Y, Cairns TM, Gallagher JR, Lou H, Ponce-De-Leon M, Belshe RB, Eisenberg RJ, Cohen GH. 2014. Repertoire of epitopes recognized by serum IgG from humans vaccinated with herpes simplex virus 2 glycoprotein D. J Virol 88:7786–7795. doi: 10.1128/JVI.00544-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iijima N, Iwasaki A. 2016. Access of protective antiviral antibody to neuronal tissues requires CD4 T-cell help. Nature 533:552–556. doi: 10.1038/nature17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung SR, Suprunenko T, Ashhurst TM, King NJC, Hofer MJ. 2018. Collateral damage: what effect does anti-CD4 and anti-CD8alpha antibody-mediated depletion have on leukocyte populations? J Immunol 201:2176–2186. doi: 10.4049/jimmunol.1800339. [DOI] [PubMed] [Google Scholar]
- 32.Haynes NM, Hawkins ED, Li M, McLaughlin NM, Hammerling GJ, Schwendener R, Winoto A, Wensky A, Yagita H, Takeda K, Kershaw MH, Darcy PK, Smyth MJ. 2010. CD11c+ dendritic cells and B cells contribute to the tumoricidal activity of anti-DR5 antibody therapy in established tumors. J Immunol 185:532–541. doi: 10.4049/jimmunol.0903624. [DOI] [PubMed] [Google Scholar]
- 33.Davido DJ, Tu EM, Wang H, Korom M, Gazquez Casals A, Reddy PJ, Mostafa HH, Combs B, Haenchen SD, Morrison LA. 2018. Attenuated herpes simplex virus 1 (HSV-1) expressing a mutant form of ICP6 stimulates a strong immune response that protects mice against HSV-1-induced corneal disease. J Virol 92:e01036-18. doi: 10.1128/JVI.01036-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esteves PJ, Abrantes J, Baldauf H-M, BenMohamed L, Chen Y, Christensen N, González-Gallego J, Giacani L, Hu J, Kaplan G, Keppler OT, Knight KL, Kong X-P, Lanning DK, Le Pendu J, de Matos AL, Liu J, Liu S, Lopes AM, Lu S, Lukehart S, Manabe YC, Neves F, McFadden G, Pan R, Peng X, de Sousa-Pereira P, Pinheiro A, Rahman M, Ruvoën-Clouet N, Subbian S, Tuñón MJ, van der Loo W, Vaine M, Via LE, Wang S, Mage R. 2018. The wide utility of rabbits as models of human diseases. Exp Mol Med 50:1–10. doi: 10.1038/s12276-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava R, Khan AA, Huang J, Nesburn AB, Wechsler SL, BenMohamed L. 2015. A herpes simplex virus type 1 human asymptomatic CD8+ T-cell epitopes-based vaccine protects against ocular herpes in a “humanized” HLA transgenic rabbit model. Invest Ophthalmol Vis Sci 56:4013–4028. doi: 10.1167/iovs.15-17074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dasgupta G, BenMohamed L. 2011. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8+-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine 29:5824–5836. doi: 10.1016/j.vaccine.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, Jester JV, Nesburn AB, Wechsler SL, BenMohamed L. 2010. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol 184:2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mankarious S, Lee M, Fischer S, Pyun KH, Ochs HD, Oxelius VA, Wedgwood RJ. 1988. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med 112:634–640. [PubMed] [Google Scholar]
- 39.Vieira P, Rajewsky K. 1988. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol 18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 40.Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest 70:369–380. [PubMed] [Google Scholar]