Childhood varicella-zoster virus (VZV) immunization induces immune memory responses that protect against primary VZV infection, chicken pox. In the United States, routine childhood VZV vaccination was introduced only 2 decades ago. Hence, there is limited information on the longevity of B and CD4 T cell memory, which are both important for protection. Here, we showed in 15 healthy young adults that VZV-specific B and CD4 T cell responses are detectable in bone marrow (BM) and blood up to 20 years after vaccination. Specifically, we measured antibody-secreting plasma cells in the BM and VZV-specific CD4 T cells in BM and blood. These findings suggest that childhood VZV vaccination induces long-lived immunity.
KEYWORDS: CD4 T cells, adaptive immunity, bone marrow, immune memory, plasma cells, varicella vaccines, varicella-zoster virus
ABSTRACT
Childhood immunization with the live-attenuated varicella-zoster virus (VZV) vaccine induces protective immune responses. Routine VZV vaccination started only 2 decades ago, and thus, there are few studies examining the longevity of vaccine-induced immunity. Here, we analyzed the quantity of VZV-specific plasma cells (PCs) and CD4 T cells in the bone marrow (BM) of healthy young adults (n = 15) following childhood VZV immunization. Long-lived BM resident plasma cells constitutively secrete antibodies, and we detected VZV-specific PCs in the BM of all subjects. Anti-VZV plasma antibody titers correlated positively with the number of VZV-specific BM PCs. Furthermore, we quantified the number of interferon gamma (IFN-γ)-producing CD4 T cells specific for VZV glycoprotein E and all other structural and nonstructural VZV proteins in both BM and blood (peripheral blood mononuclear cells [PBMCs]). The frequency of VZV-specific IFN-γ-producing CD4 T cells was significantly higher in PBMCs than BM. Our study shows that VZV-specific PCs and VZV-specific CD4 memory T cells persist up to 20 years after vaccination. These findings indicate that childhood VZV vaccination can elicit long-lived immune memory responses in the bone marrow.
IMPORTANCE Childhood varicella-zoster virus (VZV) immunization induces immune memory responses that protect against primary VZV infection, chicken pox. In the United States, routine childhood VZV vaccination was introduced only 2 decades ago. Hence, there is limited information on the longevity of B and CD4 T cell memory, which are both important for protection. Here, we showed in 15 healthy young adults that VZV-specific B and CD4 T cell responses are detectable in bone marrow (BM) and blood up to 20 years after vaccination. Specifically, we measured antibody-secreting plasma cells in the BM and VZV-specific CD4 T cells in BM and blood. These findings suggest that childhood VZV vaccination induces long-lived immunity.
INTRODUCTION
Varicella-zoster virus (VZV) is an alphaherpesvirus that causes chicken pox upon primary infection and presents as shingles when it reactivates. Primary infection can lead to severe complications in young children and adults (1). Hence, children in the United States have been vaccinated against chicken pox with a live-attenuated VZV vaccine since 1995 (2). In 2007, recommendations changed in favor of a two-dose regimen (3), leading to a vaccine effectiveness of ∼93% against chicken pox and ∼100% against severe disease (4).
VZV vaccination elicits antibody and CD4 T cell immune memory responses that play a role in mediating protection (5–8). After immunization, naive B cells encounter vaccine antigens primarily in follicles and germinal centers of secondary lymphoid organs, where they proliferate and ultimately differentiate into two main types of memory cells: antibody-secreting plasma cells (PCs) and memory B cells (MBCs). PCs predominantly reside in the bone marrow (BM), are long-lived, and constitutively secrete antigen-specific antibodies, thus maintaining serum antibody titers for decades or life (9). MBCs can be detected in the blood, become activated upon antigen reencounter, and can differentiate into antibody-secreting cells (10).
CD4 T cells are a crucial component of the humoral immune system. They are involved in initiation and maintenance of germinal centers and thus contribute to antibody affinity maturation and the generation of long-lived humoral memory. CD4 T cells also play a role in cell-mediated immunity and are relevant in the prevention of VZV infection and reactivation (reviewed in reference 11). The rate of recurrent VZV infections in HIV-positive children depends on the number of CD4 T cells at the time of first infection (12), and the incidence of VZV reactivation is higher when CD4 numbers are low (13). In the elderly, a decline in antigen-specific CD4 T cells is associated with an increased risk of VZV reactivation, and vaccination with the live-attenuated shingles vaccine leads to only a temporary increase in VZV-specific CD4 T cell numbers, which coincides with transient protection (14, 15).
To date, most studies have assessed the longevity of immune responses after childhood VZV vaccination by measuring antibody titers in the peripheral blood, and it has been reported that anti-VZV antibodies decrease over time (16). However, there are still gaps in our knowledge about vaccine-induced cellular immune responses in the bone marrow compartment, especially regarding the persistence of PCs, which are crucial for long-term maintenance of antibody titers.
Here, we obtained bone marrow and peripheral blood from young adults and examined the longevity of VZV-specific B and CD4 T cell memory up to 2 decades after their last VZV vaccination.
RESULTS
Bone marrow (BM) and whole blood samples (peripheral blood mononuclear cells [PBMCs]) were obtained from healthy young adults (n = 15; age range, 19 to 25 years) with a history of VZV vaccination but no history of documented chicken pox disease. Median time since the last VZV vaccination was 8.6 years (interquartile range [IQR], 6.7 to 9.6 years; range, 0.1 to 19.6 years) (Table 1).
TABLE 1.
Study population characteristicsa
| Demographic | Value for study subjects (n = 15) |
|---|---|
| Age [median (range)] (yrs) | 20 (19–25) |
| Gender [no. (%)] | |
| Female | 11 (73.3) |
| Male | 4 (26.7) |
| Ethnicity [no. (%)] | |
| Hispanic | 5 (33.3) |
| Race [no. (%)] | |
| White | 9 (60) |
| Black | 4 (26.7) |
| More than one race | 2 (13.3) |
| History of vaccination | |
| Age at first dose [median (IQR)] (yrs) | 4.3 (2.0–7.6) |
| 2 vaccine doses [no. (%)] | 13 (86.7) |
| Time since last dose [median (range)] [yrs] | 8.6 (0.1–19.6) |
All donors had received their first dose of Varivax before the age of 10 years, except one subject, who was 23 years old. Thirteen donors had received 2 vaccine doses. Median time between the last vaccination and specimen sampling was 8.6 years (IQR, 6.7 to 9.6 years). One donor received the last dose 41 days prior to specimen sampling.
VZV-specific B cell responses and memory CD4 T cells are detectable in the peripheral blood.
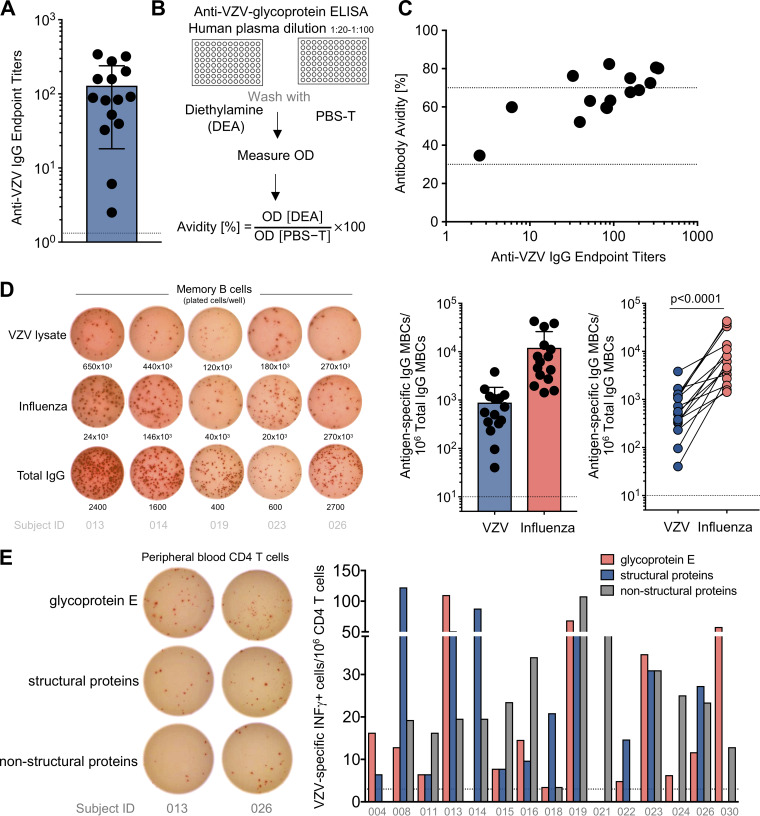
It has been documented that childhood VZV vaccination induces VZV-specific antibodies, which play a role in the protection against primary VZV infection (8, 17–19). We first evaluated in our cohort VZV-specific plasma IgG antibody responses against VZV lysate, which is derived from a VZV-infected human fibroblast cell line. All study subjects had measurable anti-VZV IgG antibodies; however, endpoint titers ranged largely from 1:2 to 1:340 (Fig. 1A). While the use of VZV lysate allows the detection of immune responses against the majority of VZV antigens, this mixture is not suitable for avidity testing by enzyme-linked immunosorbent assay (ELISA). Hence, we employed a preparation of multiple VZV glycoproteins to assess anti-VZV antibody avidity (Fig. 1B), which ranged from medium (30 to 70%) to high (>70%) (Fig. 1C). We next measured the number of VZV-specific memory B cells (MBCs) in the peripheral blood by enzyme-linked immunospot assay (ELISpot). MBCs become activated upon antigen reencounter and can differentiate into antibody-secreting cells. On average, 0.09% of IgG MBCs in the blood were VZV specific (∼900 VZV-specific IgG MBCs per million total IgG MBCs) (Fig. 1D, middle). As a positive control, we quantified influenza virus-specific MBC responses; influenza virus induces robust B cell memory due to repeated exposure to wild-type virus and seasonal vaccination. In all subjects, the number of influenza virus-specific IgG MBCs was consistently higher than that of VZV-specific IgG MBCs (Fig. 1D, right).
FIG 1.
VZV-specific humoral and CD4 T cell immune response in the peripheral blood. (A) The graph summarizes the endpoint titers of anti-VZV IgG antibodies of 15 previously VZV-vaccinated adults; each dot represents one subject. Means, standard deviations (SD), and the cutoff (dotted line) are shown. (B) Schematic showing the approach to test antibody avidity. (C) Anti-VZV antibody titers and avidity for each subject. The dashed lines delimit low (<30%), middle (30 to 70%), and high (>70%) antibody avidity. (D) Representative ELISpot wells of 5 subjects (left) and the numbers of VZV-specific and influenza virus-specific IgG MBCs per million IgG-producing MBCs (middle; means and SD are indicated), and numbers of VZV-specific (blue) and influenza virus-specific (red) IgG MBCs for each subject (P < 0.0001; paired Wilcoxon rank test) (right). (E) Representative ELISpot of 2 subjects showing IFN-γ-producing CD4 T cells and bar graph showing the number of antigen-specific CD4 T cells per million plated CD4 T cells for the 3 different peptide pools: glycoprotein E (red), structural proteins (excluding glycoprotein E; blue), and nonstructural proteins (gray). Subject IDs are below the bars.
In VZV infection, CD4 T cells are important for promoting cell-mediated immunity, and it has been recognized that the risk of VZV reactivation increases with reduced numbers of VZV-specific CD4 T cells (reviewed in reference 11). We thus measured VZV-specific CD4 T cells in the peripheral blood of our subjects. Purified CD4 T cells from blood were cocultured with autologous CD3-depleted PBMCs as antigen-presenting cells in an ELISpot assay and stimulated with three different peptide pools covering glycoprotein E, all other structural proteins, and nonstructural proteins. We could detect interferon gamma (IFN-γ)-producing VZV-specific CD4 T cells in all subjects, with variable but overall similar numbers of CD4 T cells specific for the different peptide pools (Fig. 1E). However, due to the limited amount of sample, it was not possible to deconvolute the peptide pools and identify individual epitopes.
In summary, we corroborated in our cohort the previously described persistence of VZV-specific B and CD4 T cell memory responses in the peripheral blood up to 20 years after live-attenuated childhood VZV vaccination.
VZV-specific plasma cells and CD4 T cells are detectable in the bone marrow.
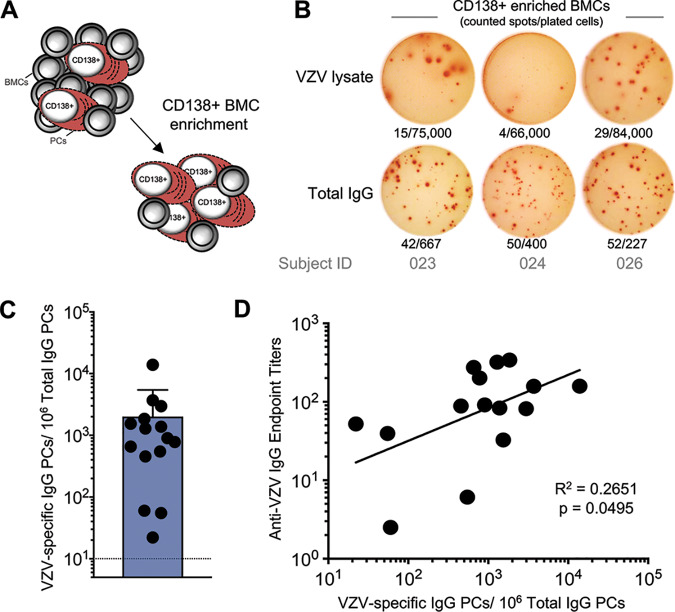
Antibodies are secreted by long-lived PCs that predominantly reside in the bone marrow (BM) (9) and are characterized by expression of the surface marker CD138. We next measured VZV-specific PCs in the BM of our VZV-vaccinated cohort. The frequency of BM PCs among total bone marrow cells (BMCs) is low (C. Davis and R. Ahmed, submitted for publication); hence, an enrichment of CD138+ PCs (Fig. 2A) prior to the assessment of antigen specificity by ELISpot was necessary. VZV-specific BM CD138+ PCs were observed in all subjects at variable frequencies, with a mean of 0.2% of IgG-producing PCs (Fig. 2B and C). After antigen encounter through infection or vaccination, antigen-specific antibody-secreting cells are initially measurable in the peripheral blood before they then die or migrate into the bone marrow to differentiate into PCs (20, 21). We could not detect any VZV-specific antibody-secreting cells in the peripheral blood of our cohort, implying no recent VZV antigen exposure. Consistent with the previously described role of BM PCs in the long-term maintenance of serum antibodies, the number of VZV-specific PCs in the BM correlated with VZV-specific antibody titers (Fig. 2D).
FIG 2.
VZV-specific plasma cell response decades after childhood vaccination. (A) CD138+ PCs from the bone marrow were enriched by positive selection. (B) Representative ELISpot wells of 3 subjects showing total IgG-producing and VZV-specific IgG-producing PCs. Number of counted spots/no. of plated cells are indicated below each well. (C) Numbers of VZV-specific IgG PCs per million IgG-producing PCs. Means and SD are presented. (D) Correlation between VZV-specific PCs and anti-VZV IgG endpoint titers. Linear regression of log-transformed values was performed, and a P value of <0.05 was considered statistically significant.
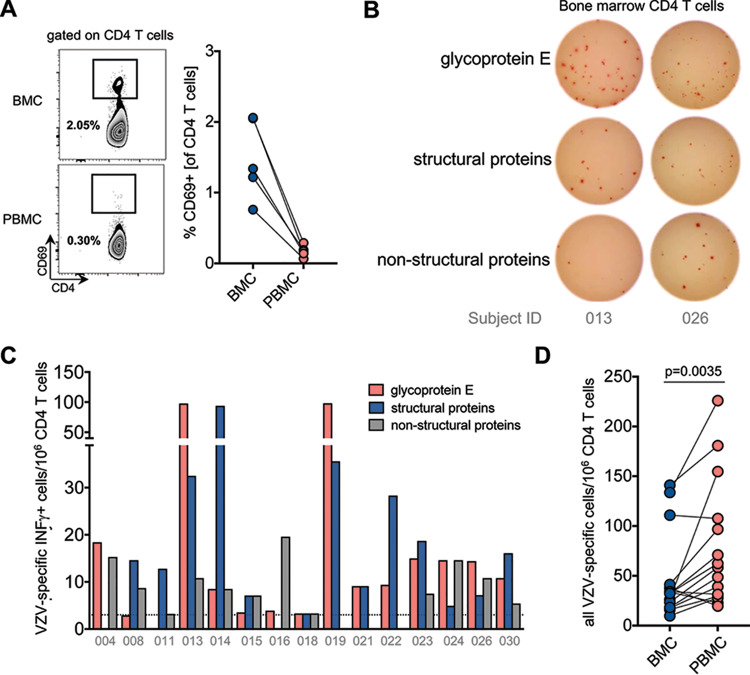
Memory CD4 T cells against various infectious agents have been shown to reside in the BM (22). We next asked whether VZV-specific CD4 memory T cells were also present in the BM following childhood VZV vaccination. First, and to identify potential peripheral blood contamination in our BM samples, we assessed previously described phenotypical differences between circulating CD4 T cells and those residing in the BM. We observed a slightly reduced frequency of CD4 T cells among total T cells in the BM, which translated into a lower CD4/CD8 ratio in the BM of all subjects compared to that in their peripheral blood (22). The activation marker CD69 has been reported as being expressed on BM-resident T cells (22, 23). We examined a subset of subjects and found that the frequency of CD69+ CD4 T cells is higher in the BM than in the blood (Fig. 3A). The majority of CD69+ CD4 T cells in the BM had an effector memory phenotype (CCR7neg CD45RAneg), whereas the few CD69+ CD4 T cells in the peripheral blood mostly exhibited a naive phenotype (CCR7pos CD45RApos). These findings of distinct phenotypes between BM and peripheral blood samples suggest that the examined BM CD4 T cells were predominantly from the BM compartment, with minimal contamination from peripheral blood. We next quantified VZV-specific BM CD4 T cells. As for the assessment of VZV-specific CD4 T cells in the peripheral blood, we stimulated purified BM CD4 T cells and autologous CD3-depleted PBMCs with three different peptide pools covering glycoprotein E, all other structural proteins, and nonstructural proteins. We measured comparable frequencies of IFN-γ-producing CD4 T cells against all 3 peptide pools (Fig. 3B and C). When antigen-specific CD4 T cell responses were pooled for each subject, we observed that the frequency of VZV-specific CD4 T cells was significantly higher in the peripheral blood than in the BM (paired Wilcoxon rank test; P = 0.0035) (Fig. 3D).
FIG 3.
VZV-specific CD4 T cells are detectable in bone marrow following childhood vaccination. (A) CD69 expression on CD4 T cells was determined by flow cytometry in a subset of 5 subjects, as illustrated in the flow plot (left). The summarizing graph (right) shows CD69 expression on CD4 T cells in the BM (blue) and blood (red). (B) Representative ELISpot of 2 subjects showing IFN-γ-producing CD4 T cells. (C) Numbers of antigen-specific IFN-γ-producing CD4 T cells per million plated CD4 T cells specific for glycoprotein E (red), other structural proteins (blue), and nonstructural proteins (gray). Subject IDs are below the bars. (D) Numbers of all VZV-specific CD4 T cells (pool of the 3 peptide pools) per one million plated CD4 T cells in BMCs (blue) and PBMCs (red) for individual subjects. Results for each subject are connected by a line (P = 0.0035; paired Wilcoxon rank test).
Two subjects were vaccinated only once, and most subjects received their last vaccine dose 6 to 10 years before sampling. We did not observe any striking association between time since last vaccination and the frequency of VZV-specific PCs, MBCs, antibody titers, or VZV-specific IFN-γ-producing CD4 T cells (Fig. 4). However, due to the limited heterogeneity of our cohort in terms of recent vaccinees (n = 3) and vaccine doses received, we cannot exclude their potential influence on immune memory. In summary, we show that B cell and CD4 T cell memory responses are detectable in BM and peripheral blood for up to 20 years after VZV vaccination.
FIG 4.
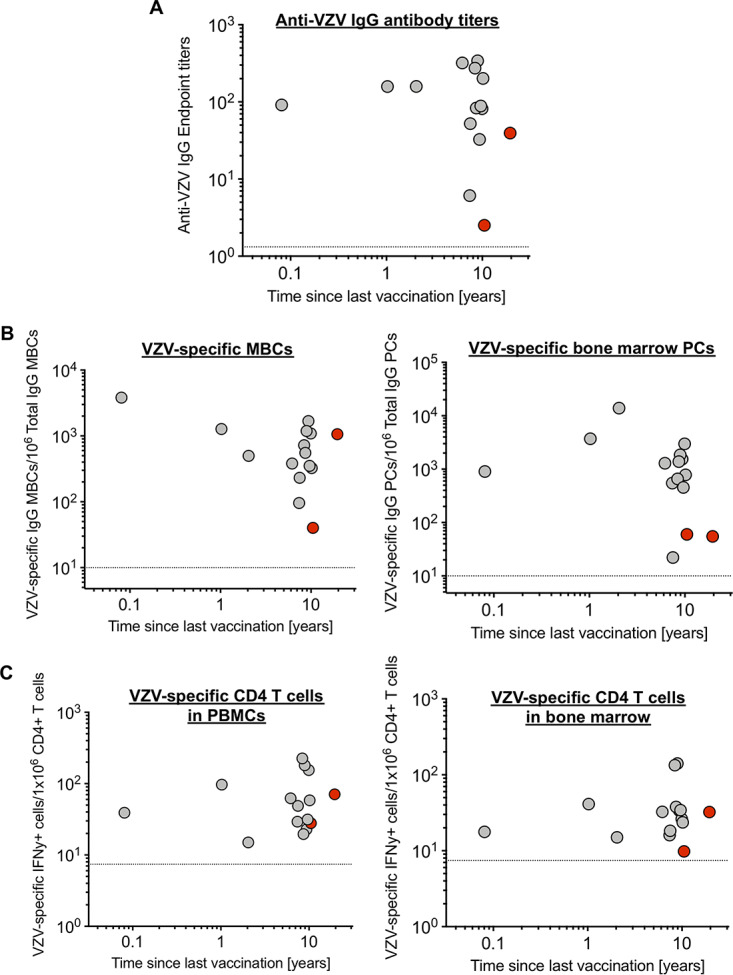
Time since last vaccination and magnitude of VZV-specific immune responses. Graphs show the time since last vaccination for each individual and report VZV-specific antibody titers (A), numbers of memory B cells and bone marrow plasma cells (B), and numbers of CD4 T cells in both peripheral blood and bone marrow (C). Red symbols indicate individuals who received only one vaccine dose. Dotted lines represent the limit of detection for each assay. Given the sample size and distribution, no statistical tests were performed.
DISCUSSION
Long-lived plasma cells reside in the bone marrow, constitutively secrete antibodies, and thus maintain serum antibody levels for decades or life. Here, we show that VZV-specific PCs are still detectable in the BM of young adults up to 2 decades after VZV vaccination and that they positively correlate with VZV-specific antibody titers. Given the limited number of study subjects, this correlation was strongly affected by three patients with antigen-specific PCs near the lower limit of detection.
Furthermore, we detected VZV-specific MBCs in all subjects, indicating the capacity to mount a recall response. The relatively low frequency of VZV-specific MBCs in comparison to influenza virus-specific MBCs can be explained by the different nature of viral antigen exposure and subsequent boosting: VZV vaccination results in a latent infection with limited endogenous antigen reexposure, and exposure to exogenous wild-type VZV has dramatically decreased in the United States since introduction of the childhood VZV vaccination (24). In contrast, influenza virus-specific MBCs are boosted throughout life by both recurrent exogenous exposure and seasonal vaccination. Our findings of persistent VZV-specific BM PCs and MBCs suggest that anti-VZV antibody titers might be durable and that in case of exposure, MBCs could elicit a recall response.
VZV-specific CD4 T cell memory plays a role in the protection from primary VZV infection (25) and reactivation (11). It is important that VZV vaccination elicits a durable CD4 T cell memory response, as there is still a risk for shingles after vaccination, although this risk is lower than after natural infection (26). We show that VZV-specific IFN-γ-producing CD4 T cells were present in BM and peripheral blood up to 2 decades after vaccination, indicating that cell-mediated immunity to VZV vaccination is also long-lived. VZV-specific CD4 T cells were more frequent in peripheral blood than in BM. However, the exact contribution of BM-resident versus circulating VZV-specific CD4 T cells to disease protection requires further investigation.
Childhood live-attenuated VZV vaccination leads to a primary infection, usually without any clinical symptoms, any detectable vaccine-strain viremia, or interindividual transmission (27, 28). However, similar to natural VZV infection, attenuated VZV can persist, and its capacity to reactivate has been demonstrated by cases of vaccine-strain shingles in children and adolescents (26). Following childhood VZV vaccination, we and others detected VZV-specific B cell and CD4 T cell memory (5–8), indicating that this vaccine induces potent immune responses. Memory responses in our cohort were heterogeneous. However, this cross-sectional study cannot address whether heterogeneous responses were induced at the time of vaccination or whether the observed differences evolved over time as a result of differential waning. Given the nature of latent infection, it is difficult to distinguish if VZV-specific memory cells were elicited only at the time of vaccination or if there was additional boosting through intermittent subclinical reactivation.
The attenuated VZV strain contained in the childhood vaccine (Varivax) is identical to the adult live-attenuated shingles vaccine (Zostavax). Although administered at around 14-fold-higher minimum potency (29), the shingles vaccine induces only poor immune responses, and antibody responses are lower in individuals with high anti-VZV antibody titers at the time of vaccination (30). While the childhood VZV vaccine is for VZV-naive individuals, the live-attenuated shingles vaccination is given to previously naturally infected adults with preexisting immune memory against VZV, which contributes to the reduced vaccine response in the elderly (31).
Overall, we showed in a cohort of 15 subjects that VZV-specific plasma cells, memory B cells, and IFN-γ-producing CD4 T cells are detectable in the bone marrow and peripheral blood up to 2 decades after vaccination. These findings indicate that childhood VZV vaccination can elicit long-lived memory responses.
MATERIALS AND METHODS
Patient cohort.
This was a single-center study at Emory University, Atlanta, GA, enrolling healthy adults over the age of 18 years who had received either one or two doses of Varivax without documented history of chicken pox. The study was approved by the institutional review board of Emory University (IRB number 00094420), and sample size was based on feasibility. Bone marrow (BM) and whole-blood samples were collected from 15 healthy adult donors from June 2017 to August 2018. Demographics are detailed in Table 1. Briefly, all donors with a median age of 20 years (range, 19 to 25 years) had received their first dose of Varivax before the age of 10 years, except one subject, who was 23 years old. Thirteen donors had received 2 vaccine doses. Median time between the last vaccination and specimen sampling was 8.6 years (IQR, 6.7 to 9.6 years; range, 0.1 to 19.6 years). Three donors had been vaccinated within the last 2 years, with one donor having been vaccinated 41 days prior to bone marrow sampling.
Plasma cell and memory B cell assessment using ELISpot.
Bone marrow mononuclear cells (BMCs) and peripheral blood mononuclear cells (PBMCs) were isolated according to standard protocols (30), and CD138+ plasma cells (PC) from the bone marrow were magnetically enriched by positive selection (Stemcell EasySep Human CD138 positive selection kit II, no. 17877).
ELISpot plates were coated overnight at 4°C with VZV lysate (Meridian Life Sciences no. 7740; 2.9 μg/well), quadrivalent seasonal inactivated influenza vaccine (Fluarix seasons 2016/17 and 2017/18; 0.15 μg/strain/well), or anti-human IgG (1 μg/well; Jackson no. 709-005-149). Plates were blocked with RPMI medium containing 10% fetal bovine serum (FBS) (R10, supplemented with 1% penicillin, streptomycin, and l-glutamine) for at least 2 h at 37°C and 5% CO2. Fresh enriched CD138+ BM plasma cells (1 × 102 to 3 × 105) were plated in a 3-fold dilution and incubated for 12 to 16 h at 37°C and 5% CO2. Biotinylated human IgG-specific antibodies (Jackson ImmunoResearch no. 709-066-098) followed by horseradish peroxidase (HRP)-conjugated avidin D (Vector Laboratories no. A-2004) were used to detect IgG-secreting cells. Plates were developed with 3-amino-9-ethylcarbazole substrate, data were acquired (ELISpot reader; CTL), and spots were manually counted. For memory B cell assays, cryopreserved PBMCs were thawed and stimulated with interleukin 2 (IL-2) (Peprotech; 10 ng/ml) and R848 (Sigma; 1 μg/ml) at 37°C and 5% CO2 for 5 days, and 3 × 102 to 10 × 105 cells were plated in a 3-fold serial dilution. ELISpot was performed as described above with a reduced incubation time of 6 h.
Antibody assessment by ELISA.
Nunc MaxiSorp flat-bottomed 96-well ELISA plates were coated with VZV antigen (Meridian Life Sciences no. 7740; 0.3 μg/well) and incubated overnight at 4°C. Plates were blocked with phosphate-buffered saline (PBS) containing 10% FBS and 0.05% Tween 20 at room temperature (RT). Plasma samples were serially 3-fold diluted, starting at 1:30. Plates were incubated at RT for 90 min, IgG antibodies were detected with secondary goat anti-human IgG-HRP (Jackson no. 109-036-098), and plates were developed with the SigmaFast OPD tablet set. The reaction was stopped after 8 min by adding 1 M HCl and read at 490 nm on a microplate reader (Bio-Rad). VZV lysate-specific IgG levels were reported as endpoint titers, determined by repetitive analysis of a seronegative sample (mean + 2 standard deviations [SD]). Data were analyzed with GraphPad Prism 7.0 software. Antibody avidity was tested as previously reported (32). Briefly, 96-well ELISA plates were coated in duplicate with lectin-purified VZV glycoproteins and normal tissue control antigen (Merck & Co, West Point, PA). Plasma was used at dilutions of 1:20 to 1:100, depending on the antibody titer. One plate was treated as for conventional ELISA (wash with PBS plus 0.05% Tween 20), and the second plate was washed with PBS plus 0.05% Tween 20 containing 35 mM diethylamine (DEA). Plates were developed as described above, and optical density (OD) readings were acquired. The avidity index was calculated as (mean OD with DEA ÷ mean OD without DEA) × 100.
VZV-peptide CD4 T cell megapools.
Three CD4 peptide megapools were designed, encompassing peptides from VZV glycoprotein E (gE), all other structural VZV proteins, and nonstructural VZV proteins. The gE megapool is composed of 123 15-mer peptides, overlapping by 10 amino acids and derived from the gE protein of the Dumas reference sequence (NC_001348.1), available in the viral ExPASy database (https://viralzone.expasy.org/). The VZV structural and nonstructural megapools are composed of 151 and 150 predicted HLA class II binding peptides, respectively, for an overall number of 301 peptides. Of those, 258 were predicted based on the Dumas reference sequence (NC_001348.1) by using TepiTool (33). Specifically, we applied the NetMHCIIpan 3.2 prediction algorithm on 7 HLA class II alleles that are expected to represent and cover 50% of the class II responses worldwide, as previously described (34). In this analysis, we applied a median cutoff of <5, ensuring that at least two peptides per protein were selected. Additionally, we included 44 already experimentally defined epitopes, retrieved by querying the IEDB (Immune Epitope Database) with the search parameters “positive assay only, No B cell assays, No MHC ligand assay, Host: Homo Sapiens and MHC restriction class II.” The peptide composition of the structural and the nonstructural megapools are detailed in Table S1 in the supplemental material. Peptides were synthetized as crude material (A&A, San Diego, CA), resuspended in dimethyl sulfoxide (DMSO), pooled, and sequentially lyophilized as previously described (35).
CD4 T cell ELISpot.
CD4 T cells were isolated from cryopreserved BMCs and PBMCs by negative selection (EasySep Human CD4+ T cell isolation kit, no. 17952; Stemcell) and plated with CD3 T cell-depleted (EasySep Human CD3 positive selection kit, no. 17851; Stemcell), autologous PBMCs at a ratio of 1:1 to 1:3 on ELISpot plates coated with anti-IFN-γ capture antibody (BD no. 51-2555KZ). Plated cells were stimulated overnight with one of the three above-described peptide pools (VZV-gE, structural VZV proteins, and nonstructural VZV proteins, each at 2 μg/ml). IFN-γ-secreting cells were detected with a biotinylated anti-IFN-γ detection antibody (51-1890KZ; BD), followed by avidin-HRP (Vector Laboratories no. A-2004). Plates were developed as described for the B-cell ELISpot.
Flow cytometry.
Cryopreserved PBMCs and BMCs were thawed, and cells were stained in PBS–2% FBS with a LIVE/DEAD fixable near-IR dead cell staining kit (Life Technologies) with anti-CD3 (clone SK7; BioLegend), anti-CD4 (clone OKT4; BioLegend), anti-CD8 (clone RPA-T8; BioLegend), anti-CCR7 (clone 150503; BD), anti-CD45RA (clone HI100; BioLegend), and anti-CD69 (clone FN50; BioLegend) antibodies and fixed with FACS (fluorescence-activated cell sorting) lysing solution (BD no. 349202). All data were acquired the same day on an LSR II cytometer (BD Biosciences) and analyzed using FlowJo software (V10; Tree Star).
Statistical tests.
Data were analyzed with GraphPad Prism 7.0 software, and means, medians, and standard deviations were determined. For correlations, equal distribution after log transformation was assessed, linear regression was calculated, and R2 was reported. The paired Wilcoxon test was used for comparison of paired data where specified. Statistical significance was set at a P value of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants U19AI090023 6556 and U19AI057266 7533 (to R.A. and to B.P.), Fondation Eugenio Litta (to C.S.E.), and Fond national suisse de la recherche scientifique (to C.S.E.).
We are grateful to the individuals who participated in the study. We thank Michele Paine McCullough, Mary Bower, and Eileen Osinski for screening and enrolling participants and for logistical support. We also thank Congrong Miao for technical assistance on the study and Hong Wu for technical support.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Papaloukas O, Giannouli G, Papaevangelou V. 2014. Successes and challenges in varicella vaccine. Ther Adv Vaccines 2:39–55. doi: 10.1177/2051013613515621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1996. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 45:1–36. [PubMed] [Google Scholar]
- 3.Marin M, Guris D, Chaves SS, Schmid S, Seward JF, Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. 2007. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 56:1–40. [PubMed] [Google Scholar]
- 4.WHO Strategic Advisory Group of Experts on Immunisation (SAGE). 2014. Systematic review of available evidence on effectiveness and duration of protection of varicella vaccines. http://www.who.int/immunization/sage/meetings/2014/april/4_Systematic_review_on_effectiveness_and_duration_of_protection_of_varicella_vaccines.pdf. Accessed 7 February 2019.
- 5.Nader S, Bergen R, Sharp M, Arvin AM. 1995. Age-related differences in cell-mediated immunity to varicella-zoster virus among children and adults immunized with live attenuated varicella vaccine. J Infect Dis 171:13–17. doi: 10.1093/infdis/171.1.13. [DOI] [PubMed] [Google Scholar]
- 6.Habermehl P, Lignitz A, Knuf M, Schmitt HJ, Slaoui M, Zepp F. 1999. Cellular immune response of a varicella vaccine following simultaneous DTaP and VZV vaccination. Vaccine 17:669–674. doi: 10.1016/s0264-410x(98)00249-7. [DOI] [PubMed] [Google Scholar]
- 7.Zerboni L, Nader S, Aoki K, Arvin AM. 1998. Analysis of the persistence of humoral and cellular immunity in children and adults immunized with varicella vaccine. J Infect Dis 177:1701–1704. doi: 10.1086/517426. [DOI] [PubMed] [Google Scholar]
- 8.White CJ, Kuter BJ, Ngai A, Hildebrand CS, Isganitis KL, Patterson CM, Capra A, Miller WJ, Krah DL, Provost PJ. 1992. Modified cases of chickenpox after varicella vaccination: correlation of protection with antibody response. Pediatr Infect Dis J 11:19–23. doi: 10.1097/00006454-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Slifka MK, Antia R, Whitmire JK, Ahmed R. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 10.Giesecke C, Frolich D, Reiter K, Mei HE, Wirries I, Kuhly R, Killig M, Glatzer T, Stolzel K, Perka C, Lipsky PE, Dorner T. 2014. Tissue distribution and dependence of responsiveness of human antigen-specific memory B cells. J Immunol 192:3091–3100. doi: 10.4049/jimmunol.1302783. [DOI] [PubMed] [Google Scholar]
- 11.Levin MJ. 2012. Immune senescence and vaccines to prevent herpes zoster in older persons. Curr Opin Immunol 24:494–500. doi: 10.1016/j.coi.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 12.von Seidlein L, Gillette SG, Bryson Y, Frederick T, Mascola L, Church J, Brunell P, Kovacs A, Deveikis A, Keller M. 1996. Frequent recurrence and persistence of varicella-zoster virus infections in children infected with human immunodeficiency virus type 1. J Pediatr 128:52–57. doi: 10.1016/s0022-3476(96)70427-4. [DOI] [PubMed] [Google Scholar]
- 13.Gershon AA, Mervish N, LaRussa P, Steinberg S, Lo SH, Hodes D, Fikrig S, Bonagura V, Bakshi S. 1997. Varicella-zoster virus infection in children with underlying human immunodeficiency virus infection. J Infect Dis 176:1496–1500. doi: 10.1086/514147. [DOI] [PubMed] [Google Scholar]
- 14.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan IS, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A, Veterans Affairs Cooperative Studies Program Shingles Prevention Study Investigators. 2008. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg A, Lazar AA, Zerbe GO, Hayward AR, Chan IS, Vessey R, Silber JL, MacGregor RR, Chan K, Gershon AA, Levin MJ. 2010. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis 201:1024–1030. doi: 10.1086/651199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan JR, Witkop CT, Webber BJ, Costello AA. 2017. Varicella seroepidemiology in United States air force recruits: a retrospective cohort study comparing immunogenicity of varicella vaccination and natural infection. Vaccine 35:2351–2357. doi: 10.1016/j.vaccine.2017.03.054. [DOI] [PubMed] [Google Scholar]
- 17.Lau YL, Vessey SJ, Chan IS, Lee TL, Huang LM, Lee CY, Lin TY, Lee BW, Kwan K, Kasim SM, Chan CY, Kaplan KM, Distefano DJ, Harmon AL, Golie A, Hartzel J, Xu J, Li S, Matthews H, Sadoff JC, Shaw A. 2002. A comparison of safety, tolerability and immunogenicity of Oka/Merck varicella vaccine and VARILRIX™ in healthy children. Vaccine 20:2942–2949. doi: 10.1016/s0264-410x(02)00245-1. [DOI] [PubMed] [Google Scholar]
- 18.Kuter BJ, Ngai A, Patterson CM, Staehle BO, Cho I, Matthews H, Provost PJ, White CJ. 1995. Safety, tolerability, and immunogenicity of two regimens of Oka/Merck varicella vaccine (Varivax) in healthy adolescents and adults. Oka/Merck Varicella Vaccine Study Group. Vaccine 13:967–972. doi: 10.1016/0264-410X(95)00046-4. [DOI] [PubMed] [Google Scholar]
- 19.White CJ, Kuter BJ, Hildebrand CS, Isganitis KL, Matthews H, Miller WJ, Provost PJ, Ellis RW, Gerety RJ, Calandra GB. 1991. Varicella vaccine (VARIVAX) in healthy children and adolescents: results from clinical trials, 1987 to 1989. Pediatrics 87:604–610. [PubMed] [Google Scholar]
- 20.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink K. 2012. Origin and runction of circulating plasmablasts during acute viral infections. Front Immunol 3:78. doi: 10.3389/fimmu.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okhrimenko A, Grün JR, Westendorf K, Fang Z, Reinke S, von Roth P, Wassilew G, Kühl AA, Kudernatsch R, Demski S, Scheibenbogen C, Tokoyoda K, McGrath MA, Raftery MJ, Schönrich G, Serra A, Chang H-D, Radbruch A, Dong J. 2014. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc Natl Acad Sci U S A 111:9229–9234. doi: 10.1073/pnas.1318731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herndler-Brandstetter D, Landgraf K, Jenewein B, Tzankov A, Brunauer R, Brunner S, Parson W, Kloss F, Gassner R, Lepperdinger G, Grubeck-Loebenstein B. 2011. Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL-15-producing cells. J Immunol 186:6965–6971. doi: 10.4049/jimmunol.1100243. [DOI] [PubMed] [Google Scholar]
- 24.Lopez AS, Zhang J, Marin M. 2016. Epidemiology of varicella during the 2-dose varicella vaccination program—United States, 2005–2014. MMWR Morb Mortal Wkly Rep 65:902–905. doi: 10.15585/mmwr.mm6534a4. [DOI] [PubMed] [Google Scholar]
- 25.Vossen MT, Gent MR, Weel JF, de Jong MD, van Lier RA, Kuijpers TW. 2004. Development of virus-specific CD4+ T cells on reexposure to varicella-zoster virus. J Infect Dis 190:72–82. doi: 10.1086/421277. [DOI] [PubMed] [Google Scholar]
- 26.Weinmann S, Chun C, Schmid DS, Roberts M, Vandermeer M, Riedlinger K, Bialek SR, Marin M. 2013. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J Infect Dis 208:1859–1868. doi: 10.1093/infdis/jit405. [DOI] [PubMed] [Google Scholar]
- 27.Asano Y, Itakura N, Hiroishi Y, Hirose S, Ozaki T, Kuno K, Nagai T, Yazaki T, Yamanishi K, Takahashi M. 1985. Viral replication and immunologic responses in children naturally infected with varicella-zoster virus and in varicella vaccine recipients. J Infect Dis 152:863–868. doi: 10.1093/infdis/152.5.863. [DOI] [PubMed] [Google Scholar]
- 28.Diaz PS, Au D, Smith S, Amylon M, Link M, Smith S, Arvin AM. 1991. Lack of transmission of the live attenuated varicella vaccine virus to immunocompromised children after immunization of their siblings. Pediatrics 87:166–170. [PubMed] [Google Scholar]
- 29.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Sullivan NL, Rouphael N, Yu T, Banton S, Maddur MS, McCausland M, Chiu C, Canniff J, Dubey S, Liu K, Tran V, Hagan T, Duraisingham S, Wieland A, Mehta AK, Whitaker JA, Subramaniam S, Jones DP, Sette A, Vora K, Weinberg A, Mulligan MJ, Nakaya HI, Levin M, Ahmed R, Pulendran B. 2017. Metabolic phenotypes of response to vaccination in humans. Cell 169:862–877 e17. doi: 10.1016/j.cell.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan NL, Eberhardt CS, Wieland A, Vora KA, Pulendran B, Ahmed R. 2019. Understanding the immunology of the Zostavax shingles vaccine. Curr Opin Immunol 59:25–30. doi: 10.1016/j.coi.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Weinmann S, Chun C, Mullooly JP, Riedlinger K, Houston H, Loparev VN, Schmid DS, Seward JF. 2008. Laboratory diagnosis and characteristics of breakthrough varicella in children. J Infect Dis 197(Suppl 2):S132–S138. doi: 10.1086/522148. [DOI] [PubMed] [Google Scholar]
- 33.Paul S, Sidney J, Sette A, Peters B. 2016. TepiTool: a pipeline for computational prediction of T cell epitope candidates. Curr Protoc Immunol 114:18.19.1–18.19.24. doi: 10.1002/cpim.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul S, Lindestam Arlehamn CS, Scriba TJ, Dillon MB, Oseroff C, Hinz D, McKinney DM, Carrasco Pro S, Sidney J, Peters B, Sette A. 2015. Development and validation of a broad scheme for prediction of HLA class II restricted T cell epitopes. J Immunol Methods 422:28–34. doi: 10.1016/j.jim.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrasco Pro S, Sidney J, Paul S, Lindestam Arlehamn C, Weiskopf D, Peters B, Sette A. 2015. Automatic generation of validated specific epitope sets. J Immunol Res 2015:763461. doi: 10.1155/2015/763461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.