In this article, we provide a new role for a commonly found bacterial pigment in controlling herpes simplex virus infection, for which diverse and multimodal antiviral agents are needed to prevent drug resistance. Serratia marcescens is a red pigment (prodigiosin)-producing Gram-negative bacillus that is naturally found in soil and water. It is associated with many kinds of human infections, including wound and eye infections, and meningitis. Taking cues from previous studies on prodigiosin, including possible proapoptotic anticancer properties, we investigated how it might affect HSV infection. Interestingly, we found that it is a potent virucidal compound that disrupts host signaling pathways needed for HSV growth and survival. The mode of antiviral action suggests potentially broad activity against enveloped viruses. Our results also indicate that interactions with commensal bacteria may inhibit HSV infection, underscoring the importance of studying these microbial metabolites and their implications for viral pathogenesis and treatment.
KEYWORDS: antiviral, herpesvirus, prodigiosin, cell signaling
ABSTRACT
Herpes simplex virus (HSV) is among the most prevalent viral infections worldwide and remains incurable. While nucleoside analogs are used to relieve symptoms of infection, they suffer from having serious adverse effects and are unable to abolish the virus from the host. Here, we demonstrate a unique antiviral effect of prodigiosin (PG), a natural secondary metabolite produced by Serratia marcescens, on HSV infection. We show that PG naturally exerts antiviral activity against HSV-1 and HSV-2 infections. PG treatment resulted in robust inhibition of viral replication in vitro and ex vivo in cultured porcine corneas. Additionally, PG protected against HSV-1 infection and disease progression in a murine model of ocular infection. In our quest to determine the molecular mechanisms of its antiviral activity, we show that PG specifically inhibits NF-κB and Akt signaling pathways and promotes accelerated cell death in HSV-infected cells. Our findings reveal novel antiviral properties of PG, suggesting its high potential as an alternative treatment for herpetic diseases. They also provide new information on antiviral effects of HSV-bacterial metabolite interactions.
IMPORTANCE In this article, we provide a new role for a commonly found bacterial pigment in controlling herpes simplex virus infection, for which diverse and multimodal antiviral agents are needed to prevent drug resistance. Serratia marcescens is a red pigment (prodigiosin)-producing Gram-negative bacillus that is naturally found in soil and water. It is associated with many kinds of human infections, including wound and eye infections, and meningitis. Taking cues from previous studies on prodigiosin, including possible proapoptotic anticancer properties, we investigated how it might affect HSV infection. Interestingly, we found that it is a potent virucidal compound that disrupts host signaling pathways needed for HSV growth and survival. The mode of antiviral action suggests potentially broad activity against enveloped viruses. Our results also indicate that interactions with commensal bacteria may inhibit HSV infection, underscoring the importance of studying these microbial metabolites and their implications for viral pathogenesis and treatment.
INTRODUCTION
Herpes simplex virus (HSV), a member of the Herpesviridae family, is a double-stranded DNA virus with an estimated worldwide prevalence of roughly 90%. Herpes simplex virus 2 (HSV-2) is commonly recognized as genital herpes, and herpes simplex virus 1 (HSV-1) often involves the oral mucosa or herpes labialis (cold sores). Most commonly presenting itself in the form of herpes labialis, HSV can cause genital herpes, epithelial and/or stromal keratitis and even encephalitis in rare cases (1). While nucleoside analogs such as acyclovir (ACV) are effective antiviral therapies, they only cause symptoms to subside without entirely clearing the virus from the host due to the ability of the virus to establish latency in the host sensory neurons (2). Trifluorothymidine (TFT) is most commonly administered topically to treat ocular HSV-1 infection. However, prolonged use of this drug can cause ocular disorders (3, 4). Additionally, with the emergence of HSV strains resistant to nucleoside analogs, treatment options become limited in those cases (5). Ultimately, the search for a better therapeutic and a cure is still under way and is much sought after.
Prodigiosin (PG) is a natural red pigment produced as a secondary metabolite by numerous bacterial species, including Serratia marcescens (6). Bacterial prodigiosins and their synthetic derivatives have displayed proapoptotic anticancer properties and exhibit cellular targets affecting the outcome of multiple pathways, including NF-κB, phosphatidylinositol 3-kinase (PI3K/Akt), and Wnt/β-catenin pathways (7–11). The PG analog obatoclax has been extensively investigated as an anticancer drug and underwent several clinical trials (12–14). Considering its evident role in common signaling cascades known to be involved in multiple diseases, including viral infections, we sought to investigate the unknown effect of PG on herpesvirus infection. In this study, we report PG as an antiviral agent against HSV-1, as well as HSV-2, infection. Our in vitro, ex vivo, and in vivo experiments demonstrate that PG is a potent antiviral against HSV-1 and -2 that antagonizes the NF-κB and Akt pathways to establish a proapoptotic state in HSV-infected cells.
RESULTS
Prodigiosin suppresses HSV-1 infection in human corneal epithelial cells in vitro and in ex vivo porcine corneas, as well as HSV-2 infection in vitro.
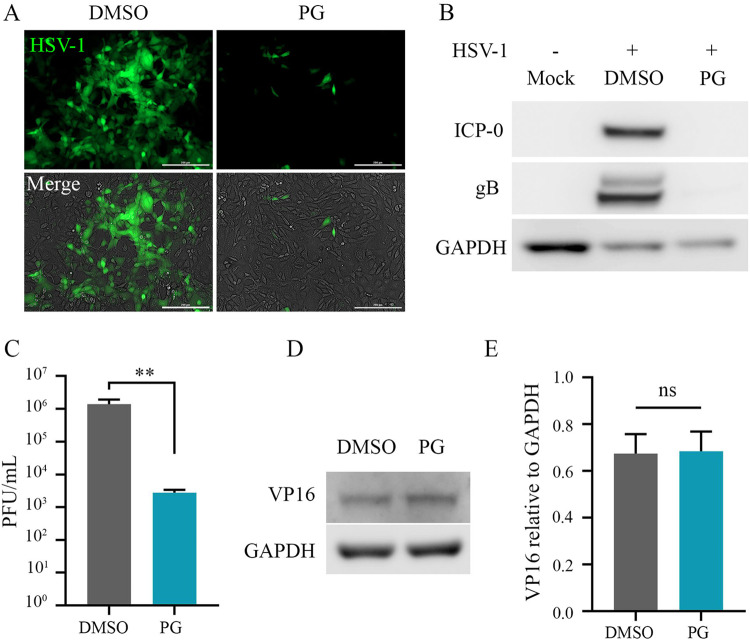
We initially aimed to understand the therapeutic potential of the natural pigment PG on HSV-1 infection by using human corneal epithelial (HCE) cells as a physiologically relevant in vitro model. HCE cells were infected with the HSV-1 KOS green fluorescent protein (GFP) mutant strain at a multiplicity of infection (MOI) of 0.1 and therapeutically treated with PG at 2 h postinfection (hpi) at various concentrations. Bright-field and fluorescence images were taken at 24 hpi show a robust suppression of viral replication following PG treatment in a dose-dependent manner, displaying an optimal therapeutic concentration of 2.5 μM (Fig. 1A). We also observed significant inhibition of transcription and protein production of both immediate early (infected-cell protein 0/4) and late (glycoprotein B) viral proteins (Fig. 1B to D). Furthermore, the treatment with PG inhibited the egress of infectious virus particles at all concentrations, including at an 8-fold dilution of the determined therapeutic concentration (Fig. 1E). Using the ex vivo porcine cornea culture model for ocular HSV-1 infection, similar antiviral effects of PG were observed as seen by the notably decreased levels of viral replication in the fluorescence images (Fig. 1F). We then sought to determine whether the antiviral effect of prodigiosin also extended to HSV-2. With the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, we also confirmed that PG does not exert any cytotoxic effects (Fig. 1G). Further, using the GFP-expressing HSV-2 333 strain, we infected HeLa cells at an MOI of 0.1 and therapeutically treated them with PG. Our findings were consistent in that PG inhibited HSV-2 replication, transcription, and protein production (see Fig. S1 in the supplemental material). Collectively, our findings clearly show PG as an effective inhibitor of HSV-1 and -2 replication, protein production, and virion formation.
FIG 1.
Prodigiosin inhibits HSV-1 infection. (A to E) HCE cells were infected with HSV-1 strain KOS tagged with GFP (MOI of 0.1), and at 2 hpi the cells were treated with different concentrations of PG and DMSO, which served as a vehicle control. The extent of HSV-1 infection was analyzed at 24 hpi. (A) Representative monographs of HCE cells, indicating the presence of HSV-1 infection (green). Images were captured using a BioTek Lionheart FX automated microscope under a 10× objective. (Scale bar, 200 μm.) (B) HSV-1 ICP-4 and gB transcripts were determined using qRT-PCR (PG, 2.5 μM). At 24 hpi, cells were collected to isolate RNA using the TRIzol method. HSV-1 transcripts were analyzed using qRT-PCR and are represented as a copy number relative to GAPDH. (C) Representative immunoblots for HSV-1 protein ICP0, gB, and GAPDH extracted from HCE cells at 24 hpi. (D) Graph showing quantitative protein expression relative to GAPDH. (E) Graph showing the generation of mature virus particles in PG-treated and DMSO-treated HCE cells at 24 hpi using plaque assay. (F) Representative micrograph of HSV-1-infected porcine corneas, showing the efficacy of prodigiosin ex vivo. The porcine corneas were infected with 106 PFU/ml of HSV-1 strain 17 (tagged with GFP) and incubated for 24 h. At 24 hpi, the corneas were treated with either DMSO or PG (2.5 μM). HSV-1 infection was analyzed by fluorescence imaging. (G) Graph showing the percent cell viability of HCE cells at different concentrations of PG.
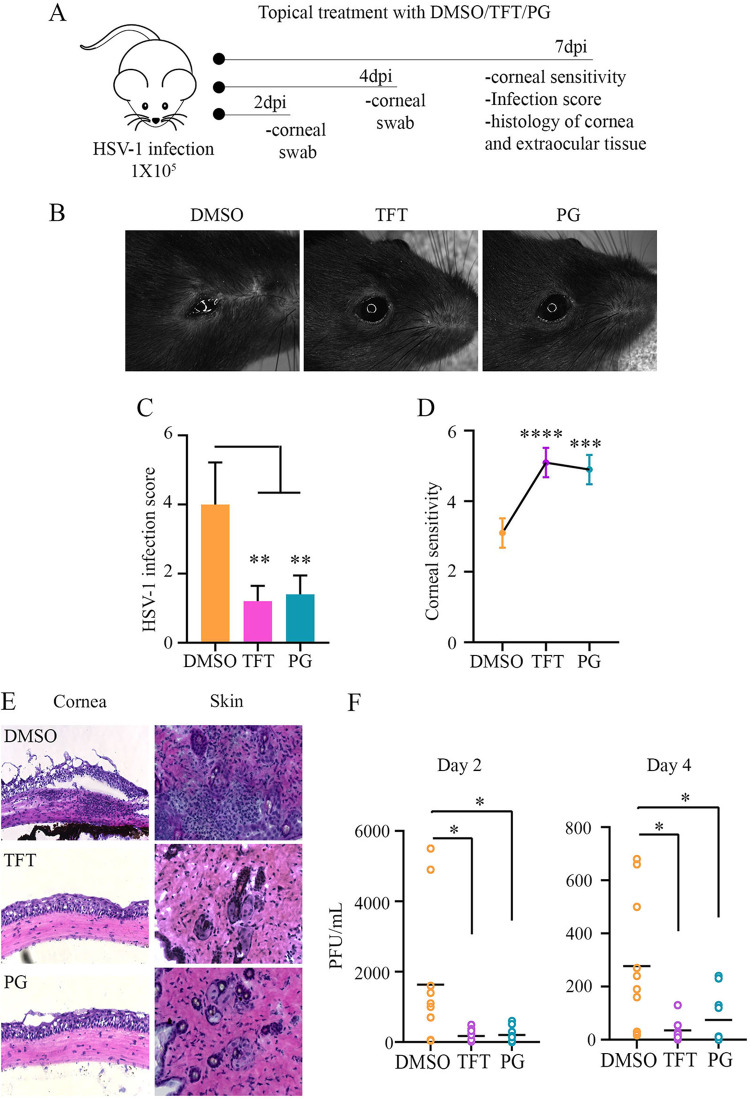
Topical treatment with PG suppresses HSV-1 infection in a mouse model and protects against epithelial inflammation.
We then sought to determine the therapeutic potential of topical treatment with PG using the murine model of ocular HSV-1 infection. Using dimethyl sulfoxide (DMSO) and TFT (50 μM), a commonly prescribed topical drug for ocular herpes infection, as controls, we investigated the antiviral potency of PG (50 μM). For 7 days, we monitored the infection and disease progression in all treatment groups (Fig. 2A). Levels of infectivity were visually assessed, and we found that at 3 days postinfection (dpi), the DMSO-treated mice began to develop signs of ocular infection as well as inflammation of surrounding extraocular tissue. A representative bright-field image taken at 7 dpi shows increased pathogenesis in the DMSO-treated mouse, whereas both PG- and TFT treated mice appear to be healthy (Fig. 2B). Furthermore, each mouse was given an ocular disease score based on clinical signs of acute HSV infection, and this showed that PG treatment significantly protected mice against disease development (Fig. 2C). Loss of corneal sensitivity is a result of damaged corneal sensory fibers as a result of developing keratitis and drug toxicity. DMSO-treated HSV-1-infected mice showed a severe loss of corneal sensitivity, whereas TFT and PG treatment protected against this (Fig. 2D). At 2 and 4 dpi, corneal swabs were collected from the infected eye and subjected to plaque assay to quantify the titer of viral secretion. The topical treatment of PG, as well as TFT, resulted in a significantly smaller amount of viral shedding at both 2 and 4 dpi compared to that in the DMSO-treated group (Fig. 2F). Furthermore, the pathology of the cornea and extent of damage resulting from the infection were assessed by histological staining (Fig. 2E). DMSO-treated mice displayed signs of severe corneal damage, including edema, thickening of the corneal epithelium, and focal inflammatory infiltrate consistent with herpes keratitis. Both TFT- and PG-treated mice displayed normal corneal pathology with no signs of necrotizing keratitis or excessive inflammation. Apart from the apparent corneal lesions and inflammation observed in the DMSO-treated mice, focal acute inflammatory lesions were also observed in the eyelid tissue with particularly significant levels of neutrophilic infiltrates. Eyelid tissue examined from TFT- and PG-treated mice showed some occurrences of slight acute inflammation, but these were not significant or indicative of severe disease. Overall, PG therapy displayed high antiviral efficacy and protection against inflammatory disease in mice.
FIG 2.
Topical treatment with prodigiosin suppresses corneal HSV-1 infection in mice. (A) Schematic representation of the in vivo experiment performed to analyze the antiviral efficacy of PG in BALB/c mice infected with HSV-1 strain McKrae at 105 PFU. The infected mice were topically treated with 5 μl of 2.5% DMSO (complexed with 10 mg/ml β-cyclodextrin), TFT (50 μM), and PG (50 μM, complexed with 10 mg/ml β-cyclodextrin). Treatment was performed three times a day until 7 dpi. (B) Representative pictures of infected eyes from different treatment groups. (C) Graph showing infection score based on visual observation. (D) Corneal sensitivity was measured using an esthesiometer, and the scores were recorded and plotted. (E) Representative micrographs of corneal histology sections, indicating corneal damage and infiltration of immune cells in corneas and extraocular tissue. (F) Graphs showing the secreted virus titers from eyewash samples collected from the infected eyes of different treatment groups on 2 and 4 dpi. Plaque assay was performed on Vero cells. Significance between the treatment and control groups was determined using the student unpaired t test.
PG exhibits prophylactic antiviral activity and does not interfere with viral entry.
We next performed a series of experiments to further understand at what stage of viral infection PG intervenes and exerts its antiviral activity. Initially, either PG or DMSO was incubated with GFP-tagged HSV-1 KOS strain (MOI, 0.1) for 1 h and then placed on a monolayer of HCE cells. Fluorescence images were taken at 24 hpi and are indicative of the robust inhibition of viral replication following prophylactic treatment with PG (Fig. 3A). Cell lysates were then processed for Western blot analysis, which showed that prophylactic treatment with PG inhibits viral protein production of both immediate early and late viral proteins (Fig. 3B). A significant decrease of PFU was also observed by plaque assay, suggesting that PG attenuates susceptibility to infection by targeting host factors that would otherwise assist in increased viral replication (Fig. 3C). By performing an entry assay, it was evident that this treatment did not interfere with the viral entry (Fig. 3D and E). Our results are conclusive that PG exerts its antiviral effect by targeting certain host pathways following viral entry.
FIG 3.
Prophylactic treatment with prodigiosin inhibits HSV-1. (A to C) HSV-1 (MOI, 0.1) was incubated with PG (2.5 μM), and after 1 h of incubation, the suspension was added over HCE cells and was replaced by DMEM (with 10% FBS and 1% penicillin-streptomycin). DMSO (0.1%) served as a vehicle control. The assay was terminated at 24 hpi, and the cells were collected to analyze HSV-1 infection. (A) Representative micrographs of HCE cells infected with HSV-1 KOS (GFP tagged) at 24 hpi (scale bar, 200 μm). (B) Representative immunoblots for HSV-1 protein ICP0, gB, and GAPDH extracted from HCE cells at 24 hpi. (C) Graph showing mature virus particles produced in the control and treatment groups. (D) Immunoblot of HSV-1 VP16 tegument protein after infecting HCE cells with HSV-1 strain KOS at an MOI of 10. (E) Quantification of HSV-1 VP16 protein, depicting virus entry in HCE cells.
Prodigiosin dampens NF-κB signal transduction and inhibits transcription of TNF-α during HSV-1 infection.
Modulation of NF-κB activity during HSV-1 infection to promote cell survival has been shown in multiple studies (15–17). To investigate potential host targets of PG that may be contributing to the establishment of an antiviral state and to better characterize its mechanism of action, we initially examined whether PG had an effect on NF-κB during infection. For these experiments, a suboptimal dose of PG (0.6 μM) was used to study its mechanism in a host environment where some amount of viral replication is permissible. Using an NF-κB-inducible luciferase reporter plasmid, we found that HSV-1 infection of HCE cells caused a robust increase in NF-κB activity, consistent with previous results. Interestingly, PG-treated cells showed a significant reduction of NF-κB activity (Fig. 4A). To further probe for factors contributing to the activation of NF-κB, we examined mRNA levels of tumor necrosis factor alpha (TNF-α), since TNF-α-induced NF-κB activity is a highly characterized pathway (18). In line with our findings, TNF-α mRNA levels were also shown to be downregulated as a result of PG treatment following infection (Fig. 4B). This indicates that PG may be providing antiviral relief through inhibition of TNF-α-induced antiapoptotic activity mediated through NF-κB regulation of prosurvival genes.
FIG 4.
The antiviral activity of prodigiosin is independent of interferon response, but it suppresses NF-κB activity. (A) Graph showing suppression of NF-κB activity by PG treatment. HCE cells transfected with an NF-κB reporter plasmid (which is able to show luminescence while NF-κB is active) were infected with HSV-1 at an MOI of 5 and treated with PG (2.5 μM) at 1 hpi; DMSO served as a vehicle control. The assay was terminated at 6 hpi, and NF-κB activity was analyzed by measuring relative luminescence units. (B) Graph showing fold change of TNF-α transcript in HCE cells uninfected or infected with HSV-1 in the PG treatment and control groups. (C) Representative immunoblot showing HSV-1-gB, IRF-3, IRF-7, and GAPDH. (D) Quantitation of protein expression (C) using ImageJ. (E and F) Graph showing fold change relative to GAPDH for IFN-α (E) and IFN-β (F) transcripts.
Moreover, Western blot analysis indicated that the antiviral activity of PG is independent of interferon response, as the expression of interferon regulatory factor 7 (IRF-7) was comparatively less in PG-treated and HSV-1-infected cells (Fig. 4C and D). Similarly, transcript levels of cytokines alpha interferon (IFN-α) and IFN-β were significantly less in PG-treated cells with or without infection than in DMSO-treated and infected cells (Fig. 4E and F). These findings indicate that PG interferes with the TNF-α/NF-κB pathway, and this may be contributing to the observed hindrance of the interferon response.
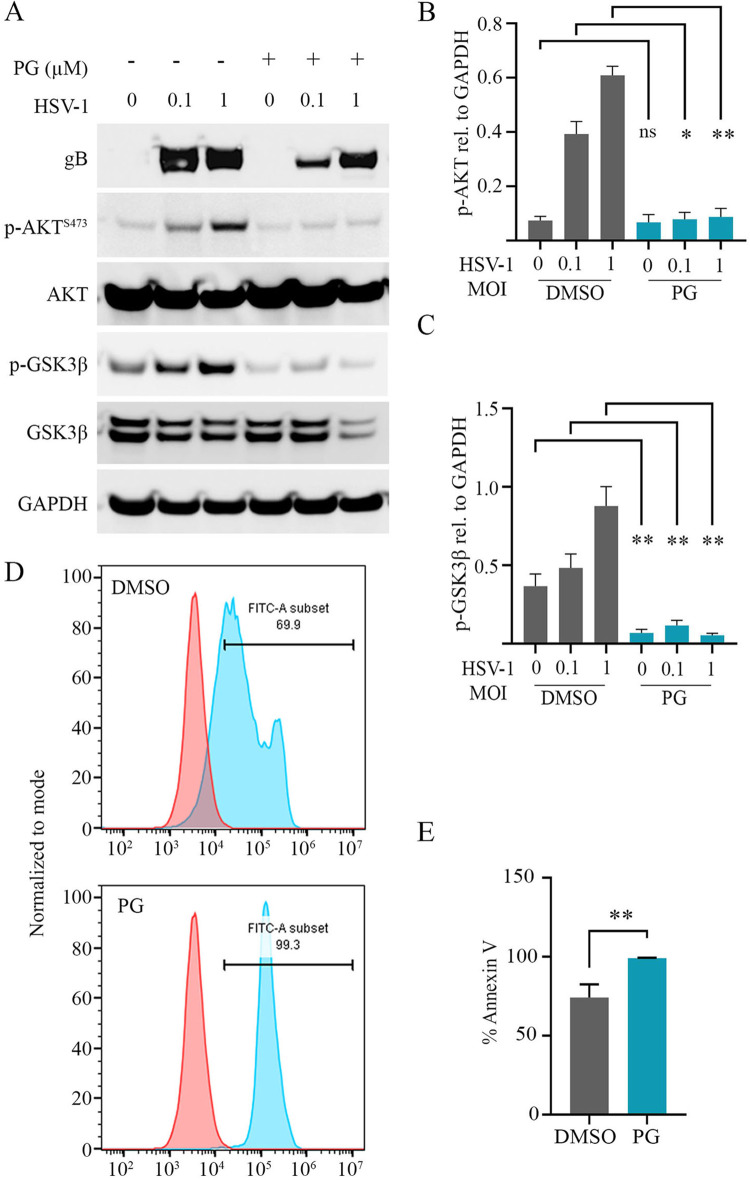
PG inhibits the Akt pathway during HSV-1 infection.
HSV modulates the PI3K/Akt pathway to block apoptosis, facilitate protein synthesis, and generate a host environment permissive for viral replication (19–21). During infection, a key target of Akt is the inhibition of glycogen synthase kinase 3 beta (GSK-3β), which contributes to the establishment of a prosurvival cellular state (22, 23). One of the proposed mechanisms for the anticancer properties of PG includes the inactivation of Akt and subsequent activation of GSK-3β (6, 10, 11). To assess whether this pathway was also being manipulated by PG in our system and under conditions of HSV-1 infection, we performed Western blot analysis to assess changes in phosphorylation of Akt and GSK-3β. Our data show that PG treatment following infection at MOIs of 0.1 and 1 inhibits Akt phosphorylation, leading to its inactivation and inability to phosphorylate and inactivate GSK-3β, which leads to subsequent activation of GSK-3β (Fig. 5A). Quantification of protein levels of phosphorylated Akt and GSK-3β reveals a significant reduction of expression following PG treatment (Fig. 5B and C). Moreover, using an annexin V-fluorescein isothiocyanate (FITC) apoptosis assay, we found that the PG treatment induces higher levels of FITC-positive cells, which is indicative of an increase in the proapoptotic cell population (Fig. 5D and E). In Fig. 5D, the dual peak appears to be due to differences in annexin V-FITC staining (and intensity) in the cells which are in different phases of apoptosis (early phase and late phase).
FIG 5.
Prodigiosin inhibits the Akt pathway during HSV-1 infection. (A) Immunoblot showing expression of HSV-1 gB, AKT, GSK-3β, GAPDH, and phosphorylated forms of AKT at S473 and GSK-3β. HCE cells were infected at MOIs of 0.1 and 1 of HSV-1 strain KOS and treated with PG (0.6 μM) at 2 hpi. (B) Quantification of the phosphorylated form of AKT using ImageJ software. (C) Quantification of the phosphorylated form of GSK-3β using ImageJ software. (D) Representative graphs of the percent proapoptotic population by using flow cytometry of annexin V-FITC-stained HCE cells infected with HSV-1 KOS at an MOI of 1 and treated either with PG or DMSO. (E) Quantification of the percent annexin V-FITC-stained population from panel D. Statistical significance between the treatment and control groups was determined using the unpaired Student t test.
DISCUSSION
This study demonstrates the antiviral potential of the natural secondary metabolite prodigiosin, produced by Serratia marcescens, on HSV-1 and HSV-2 infection. Using in vitro, ex vivo, and in vivo models of HSV infection, our findings conclude that PG shows high efficacy in inhibiting HSV viral replication. More importantly, our data highlight the nontoxic effect of PG at its therapeutic concentration and show protection against ocular HSV-1 infection and pathogenesis in mice. We show that, by targeting cell signaling pathways such as NF-κB and Akt, PG establishes an antiviral state through inhibition of prosurvival pathways.
PG has garnered much attention due to its high potential as a clinical drug with a breadth of application, including anticancer, antimicrobial, antimalarial, and immunosuppressive properties. Extensive studies on PG have revealed its anticancer properties through preferential induction of apoptosis in malignant cells (8–11). While the exact mechanism and direct targets of PG are unclear, several potential pathways have been implicated as targets of the metabolite, such as the PI3K/Akt/mTOR, NF-κB, and Wnt/β-catenin signaling pathways (7, 10, 11). Many of these pathways have also been implicated in driving herpesvirus infection. During infection, multiple cellular signaling pathways are known to be dysregulated or modulated by herpesviruses to evade host defense systems, including the inhibition of apoptosis and activation of cell survival pathways (15–21, 23). For this reason, we sought to determine whether PG exhibits any antiviral properties against HSV infection.
Initially, potent inhibition of HSV-1 and HSV-2 replication, viral protein production, and viral transcription was observed in HCE and HeLa cells, respectively, after therapeutic treatment with PG. In addition, we observed robust inhibition of viral replication upon prophylactic treatment with PG. Since prophylactic treatment did not affect viral entry, it is possible that PG may be targeting host proteins and establishing an antiviral cellular state prior to infection. It is also possible that when cells are infected with HSV-1, proviral host proteins that are typically used early in viral life cycle are targeted by PG. We then sought to determine whether PG elicited its apparent antiviral properties in vivo using a mouse model for ocular HSV-1 infection. To enhance drug delivery of the hydrophobic compound PG, it was used in combination with β-cyclodextrin, a cyclic molecule with a central hydrophobic cavity favorable for PG binding and an hydrophilic outer side that helps to deliver the cargo inside the cell. Mice treated with PG displayed significantly less viral shedding at 2 and 4 dpi, comparable to that of the DMSO-treated mice. PG, as well as TFT, protected the mice against the development of an ocular disease, inflammation of surrounding epithelium and damage of the cornea. We next examined the mechanism of PG’s antiviral activity and found that PG acts on host machinery to attenuate susceptibility to viral replication by blocking the dysregulation of multiple signaling pathways during infection. In particular, PG induces apoptosis in HSV-infected cells through the inhibition of Akt and NF-κB. These observations are in line with previous reports indicating the proapoptotic role of prodigiosin. The initiation of programmed cell death acts as an intrinsic antiviral defense mechanism at the cellular level and reduces the amount of virus replication by choking off the supply of viral progeny. Therefore, to generate a permissive environment, pathogens often rely on host survival pathways such as PI3K/AKT/mTOR. Activation of Akt signaling allows the virus to prolong apoptosis. Even though HSV-1 is reported to translate proteins necessary for its survival, the inhibition of Akt results in a reduction of viral load, highlighting the importance of the pathway in a productive infection (21, 24). Given that a variety of RNA and DNA viruses have been demonstrated to induce Akt activation, including adenoviruses, poxviruses, HIV, papillomaviruses, herpesviruses, and influenza A virus, PG may also show antiviral efficacy against these viruses due to its role in inhibiting Akt phosphorylation and activation (21, 25–27).
This study highlights a novel, antiviral property of PG against herpesvirus infection. This natural secondary metabolite is particularly promising due to its multimodal actions, which limit the chances of drug resistance development in the virus. Additionally, PG has undergone extensive research, including in preclinical trials for the treatment of pancreatic cancer. Analogs of PG have reached clinical trials for the treatment of chronic lymphocytic leukemia, and reports indicate that it was well tolerated in human subjects and that it does not exhibit genotoxicity. While more studies are required to fully understand the antiviral mechanism of PG and its efficacy in the clinic, the current data presented show the high potential of PG as an effective therapeutic against HSV infection. Our results also underscore the need to study viral interactions with commensal bacteria. They bring out the importance of studying microbial metabolites in persistent viral infections. They may also explain one possible reason for the self-containing nature of herpetic diseases at mucosal surfaces.
MATERIALS AND METHODS
Cell lines and viruses.
A human corneal epithelial (HCE) cell line (RCB1834 HCE-T) was used for in vitro HSV-1 infection, and it was procured from Kozaburo Hayashi (National Eye Institute, Bethesda, MD). Similarly, the HeLa cell line was used for HSV-2 experiments. The HCE cells were cultured in minimum essential medium (MEM) (Life Technologies, Carlsbad, CA), while HeLa cells were cultured in Dulbecco modified Eagle medium (DMEM) (Gibco). Both culture media were supplemented with 10% fetal bovine serum (FBS) (Life Technologies) and 1% penicillin-streptomycin (Life Technologies). The HSV-1 KOS and McKrae strains were procured from Patricia G. Spear (Northwestern University, Chicago, IL), whereas HSV-1 KOS dual-color ICP0p-GFP/gCp-RFP was a gift from Paul Kinchington (University of Pittsburgh). HSV-1 strain 17 was obtained from Richard Thompson (University of Cincinnati, Cincinnati, OH). The virus stocks were made and titrated in Vero cells and stored at –80°C.
Chemicals.
Prodigiosin from Serratia marcescens (Calbiochem no. R 529685-200UG) was purchased from Millipore Sigma (Germany).
Antibodies.
HSV-1 gB (ab6506) antibody was purchased from Abcam (Cambridge, United Kingdom). The following antibodies were used for Western blotting or immunofluorescence: AKT (9272S), p-AKTS473 (9271S), GSK-3β (9315), p-GSK-3β (9323), IRF-3 (D83B9), and IRF-7 (4920) purchased from Cell Signaling Technology (Danvers, MA); HSV-1 ICP0 (sc-53070) and HSV-1 VP16 (sc-7546) purchased from Santa Cruz Biotechnology (Dallas, TX); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (10494-1-AP) purchased from Proteintech Group, Inc. (Rosemont, IL).
Quantitative real-time PCR (qRT-PCR).
The TRIzol (Life Technologies) method was used to isolate RNA from reaction wells. The high-capacity cDNA reverse transcription kit (Applied Biosystems Foster City, CA) was used to reverse transcribe the isolated RNA. The resultant cDNA was further used for real-time quantitative PCR (qPCR) performed on a QuantStudio 7 Flex system (Invitrogen Life Technologies) using the Fast SYBR green master mix (Life Technologies).
The primers used in this study were as follows: GAPDH qPCR F, 5′-TCC ACT GGC GTC TTC ACC-3′; GAPDH qPCR R, 5′-GGC AGA GAT GAT GAC CCT TTT-3′; TNF-α F, 5′-AGC CCA TGT TGT AGC AAA CCC-3′; TNF-α R, 5′-GCA CCT GGG AGT AGA TGA GGT-3′; IFN-α F, 5′-GAT GGC AAC CAG TTC CAG AAG-3′; IFN-α R, 5′-AAA GAG GTT GAA GAT CTG CTG GAT-3′; IFN-β F, 5′-CTC CAC TAC AGC TCT TTC CAT-3′; IFN-β R, 5′-GTC AAA GTTC ATC CTG TCC TT-3′; HSV-1 ICP-4 F, 5′-ACG TTG TGG ACT GGG AAG G-3′; HSV-1 ICP-4 R, 5′-CGT CTT CGG GTT TCA CAA GC-3′; HSV-1 gB F, 5′-GCC TTT TGT GTG TGG G-3′; HSV-1 gB R, 5’AGG AAA GAG GAA ACA GGC C-3′; HSV-2 ICP27 F, 5′-TGT CGG AGA TCG ACT ACA CG-3′; HSV-2 ICP27 R, 5′-GGT GCG TGT CCA GTA TTT CA-3′; HSV-2 gB F, 5′-GTC TGC ACC ATG ACC AAG TG-3′; HSV-2 gB R, 5′-GTG GTG AAG GTG GTC GAG AT-3′.
Immunoblotting.
The cells in culture wells were dissociated and collected using Hanks cell dissociation buffer, and protein isolation was performed using radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich, St. Louis, MO). The appropriate volume of the protein samples was mixed with lithium dodecyl sulfate (LDS) sample loading buffer and β-mercaptoethanol (5%) (Bio-Rad, Hercules, CA). The proteins in the resultant mixture were denatured at 95°C for 9, min followed by an electrophoretic run on an Invitrogen Mini Gel tank (Fisher Scientific) through precast gels (4 to 12%). The proteins were then transferred onto a nitrocellulose membrane (Fisher Scientific) through iBlot (Invitrogen). Nonspecific sites on the membrane were blocked in a mixture of 5% milk and Tris-buffered saline–Tween 20 (TBS-T) for 1 h, followed by overnight incubation with the respective primary antibody at 4°C. The unbound antibodies were removed by washing with TBS-T and incubated with the respective horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. Secondary anti-rabbit antibodies (1:5,000) were used for the detection of all phosphoproteins. The membranes were washed again before exposing them to SuperSignal West Pico Plus chemiluminescent substrate (34577) and or SuperSignal West Femto maximum-sensitivity substrate. The protein bands were further visualized with an Image-Quant LAS 4000 biomolecular imager (GE Healthcare Life Sciences, Pittsburgh, PA).
Virus titer (plaque formation) assay.
HSV-1- or HSV-2-infected HCE or HeLa cells were dissociated with Hanks buffer and centrifuged at 3,000 rpm for 3 min. The pellets were then suspended in Opti-MEM (Thermo Fisher Scientific) and sonicated to release intracellular infectious virus particles. The monolayer of Vero cells was infected with the respective dilution of cell lysate in Opti-MEM. After 2 h postinfection, Opti-MEM was replaced by a complete medium (DMEM with 10% FBS and 1% penicillin-streptomycin) containing 0.5% (wt/vol) methylcellulose (Fisher Scientific). The plaque assay culture plate(s) was incubated at 37°C with 5% CO2 for 72 h. After incubation, the cells were fixed by adding 100% methanol directly into the medium. After fixation, all the medium was removed, and cells were stained with crystal violet solution. Numbers of plaques were counted visually as PFU/ml.
Cell viability (MTT) and apoptosis assays.
HCE cells were used to measure the percent viability of cells at different concentrations of PG. A 96-well plate of HCE cells was seeded and incubated overnight before treatment with PG at various concentrations starting from 20 μM; DMSO (at vehicle concentration) served as a control. After 24 h of treatment, the cell viability was tested using the MTT assay. In brief, 5 μl of MTT reagent (5 mg/ml) was added to each reaction well and incubated at 37°C in the dark. After 3 h, formazan crystals were dissolved in acidified isopropanol by shaking the plate at 25 rpm for 15 min in the dark. The supernatant was transferred to a new 96-well plate. The optical density of the resultant mixture was measured at 550 nm while the reference wavelength was 630 nm. The percent viability was calculated.
HCE cells were infected with HSV-1 at an MOI of 1 and harvested at 12 hpi. FITC-annexin V (Invitrogen A13199) was used to detect cells undergoing apoptosis. As per the manufacturer’s protocol, the sample pellets were washed with cold phosphate-buffered saline (PBS). The cell pellets were suspended in a 1× annexin binding buffer. The suspension was mixed with 5 μl FITC-annexin V and incubated for 15 min. The mixture was further diluted with 1× annexin binding buffer and analyzed by flow cytometry (BD Accuri C6 Plus flow cytometer). Flow data were analyzed using FlowJo software (Tree Star Inc.).
Entry assay.
HCE cells were cultured and infected with HSV-1 at an MOI of 10. The cells were simultaneously treated with either DMSO or PG (2.5 μM). To synchronize the virus entry, the culture plate was immediately moved onto ice, left for 30 min, and thereafter incubated at 37°C and 5% CO2 for 15 min. After incubation, the cells were washed with citrate buffer to remove the virus on the cell surface. The cells were collected in Hanks buffer, and proteins were isolated by RIPA. HSV-1 tegument protein VP16 was quantified in both samples by Western blotting.
Murine corneal HSV-1 infection.
To analyze the antiviral efficacy of PG in vivo, C57BL/6 mice were used. All animal care procedures were performed following the institutional and NIH guidelines, with prior approval of the Animal Care Committee at the University of Illinois at Chicago (ACC protocol 17-077). A total of 15 C57BL/6J mice were divided into three groups. The mice were anesthetized, and their corneas were sacrificed in a 3-by-3 grid using a 30-gauge needle. The corneas were infected with HSV-1 strain McKrae (1 × 105). Each group of animals was topically treated in three treatment groups: (i) DMSO (2.5% [vol/vol] in PBS containing 10 mg/ml β-cyclodextrin), (ii) PG (50 μM in PBS and complexed with 10 mg/ml β-cyclodextrin), and (iii) TFT (50 μM). The topical treatment (5 μl) was performed three times in a day and continued until day 7 postinfection. Eyewash samples were collected on day 2 and day 4 postinfection and were analyzed for the presence of mature virus particles through plaque assay.
Porcine corneal model of HSV-1 infection.
Whole pig eyes were provided by Park Packing, Inc., Chicago, IL. The cornea was carefully excised from the whole eye and rinsed in PBS containing 5% antibiotic-antimycotic (Gibco). The cornea was further dipped in serum-free MEM supplemented with 5% antibiotic-antimycotic and 1% insulin-transferrin-sodium selenite (Sigma-Aldrich) for 1 h at 37°C and 5% CO2. The cornea was further infected with 1 × 106 PFU of HSV-1 strain 17 (tagged with GFP) and incubated for 24 h. After incubation, the inoculum was replaced with a medium containing DMSO and PG (2.5 μM). Medium change was performed on each day. The extent of infection was analyzed by fluorescence imaging (SteREO Discovery.V20 [Zeiss, Germany]).
Histology.
After euthanizing the mice, whole eyes and extraocular tissues were dissected, embedded in Tissue-Plus O.C.T. (Fisher Healthcare), frozen on dry ice, and kept at –80°C until processing. Hematoxylin-and-eosin staining was performed as previously reported (28).
Statistical analysis.
Error bars in all figures represent the standard error of the mean (SEM) from three independent experiments (n = 3) unless otherwise specified. The experimental data for two groups were compared using the two-tailed unpaired Student t test. The P values are indicated in the figures (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). Differences between values were considered significant when the P value was 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health RO1 grants EY029426, AI139768, and EY024710 (to D.S.), an NEI core grant (EY001792), and the Illinois Society for the Prevention of Blindness.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Koujah L, Suryawanshi R, Shukla D. 2018. Pathological processes activated by herpes simplex virus-1 (HSV-1) infection in the cornea. Cell Mol Life Sci 76:405–419. doi: 10.1007/s00018-018-2938-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsatsos M, MacGregor C, Athanasiadis I, Moschos MM, Hossain P, Anderson D. 2016. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin Exp Ophthalmol 44:824–837. doi: 10.1111/ceo.12785. [DOI] [PubMed] [Google Scholar]
- 3.Jayamanne DG, Vize C, Ellerton CR, Morgan SJ, Gillie RF. 1997. Severe reversible ocular anterior segment ischaemia following topical trifluorothymidine (F3T) treatment for herpes simplex keratouveitis. Eye (Lond) 11:757–759. doi: 10.1038/eye.1997.193. [DOI] [PubMed] [Google Scholar]
- 4.Maudgal PC, Van Damme B, Missotten L. 1983. Corneal epithelial dysplasia after trifluridine use. Graefes Arch Clin Exp Ophthalmol 220:6–12. doi: 10.1007/bf02307009. [DOI] [PubMed] [Google Scholar]
- 5.Piret J, Boivin G. 2011. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother 55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darshan N, Manonmani HK. 2015. Prodigiosin and its potential applications. J Food Sci Technol 52:5393–5407. doi: 10.1007/s13197-015-1740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Li B, Zhou L, Yu S, Su Z, Song J, Sun Q, Sha O, Wang X, Jiang W, Willert K, Wei L, Carson DA, Lu D. 2016. Prodigiosin inhibits Wnt/beta-catenin signaling and exerts anticancer activity in breast cancer cells. Proc Natl Acad Sci U S A 113:13150–13155. doi: 10.1073/pnas.1616336113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llagostera E, Soto-Cerrato V, Montaner B, Pérez-Tomás R. 2003. Prodigiosin induces apoptosis by acting on mitochondria in human lung cancer cells. Ann N Y Acad Sci 1010:178–181. doi: 10.1196/annals.1299.030. [DOI] [PubMed] [Google Scholar]
- 9.Huh J-E, Yim J-H, Lee H-K, Moon E-Y, Rhee D-K, Pyo S. 2007. Prodigiosin isolated from Hahella chejuensis suppresses lipopolysaccharide-induced NO production by inhibiting p38 MAPK, JNK and NF-kappaB activation in murine peritoneal macrophages. Int Immunopharmacol 7:1825–1833. doi: 10.1016/j.intimp.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Soto-Cerrato V, Viñals F, Lambert JR, Kelly JA, Pérez-Tomás R. 2007. Prodigiosin induces the proapoptotic gene NAG-1 via glycogen synthase kinase-3beta activity in human breast cancer cells. Mol Cancer Ther 6:362–369. doi: 10.1158/1535-7163.MCT-06-0266. [DOI] [PubMed] [Google Scholar]
- 11.Espona-Fiedler M, Soto-Cerrato V, Hosseini A, Lizcano JM, Guallar V, Quesada R, Gao T, Pérez-Tomás R. 2012. Identification of dual mTORC1 and mTORC2 inhibitors in melanoma cells: prodigiosin vs. obatoclax. Biochem Pharmacol 83:489–496. doi: 10.1016/j.bcp.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Schimmer AD, Raza A, Carter TH, Claxton D, Erba H, DeAngel DJ, Tallman MS, Goard C, Borthakur G. 2014. A multicenter phase I/II study of obatoclax mesylate administered as a 3- or 24-hour infusion in older patients with previously untreated acute myeloid leukemia. PLoS One 9:e108694. doi: 10.1371/journal.pone.0108694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh SA, Kantarjian H, Schimmer A, Walsh W, Asatiani E, El-Shami K, Winton E, Verstovsek S. 2010. Phase II study of obatoclax mesylate (GX15-070), a small-molecule BCL-2 family antagonist, for patients with myelofibrosis. Clin Lymphoma Myeloma Leuk 10:285–289. doi: 10.3816/CLML.2010.n.059. [DOI] [PubMed] [Google Scholar]
- 14.Hwang JJ, Kuruvilla J, Mendelson D, Pishvaian MJ, Deeken JK, Siu LL, Berger MS, Viallet J, Marshall JL. 2010. Phase I dose finding studies of Obatoclax (GX15-070), a small molecule pan-BCL-2 family antagonist, in patients with advanced solid tumors or lymphoma. Clin Cancer Res 16:4038–4045. doi: 10.1158/1078-0432.CCR-10-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodkin ML, Ting JL, Blaho JA. 2003. NF-κB is required for apoptosis prevention during herpes simplex virus type 1 Infection. J Virol 77:7261–7280. doi: 10.1128/jvi.77.13.7261-7280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amici C, Rossi A, Costanzo A, Ciafrè S, Marinari B, Balsamo M, Levrero M, Santoro MG. 2006. Herpes simplex virus disrupts NF-κB regulation by blocking its recruitment on the IκBα promoter and directing the factor on viral genes. J Biol Chem 281:7110–7117. doi: 10.1074/jbc.M512366200. [DOI] [PubMed] [Google Scholar]
- 17.Patel A, Hanson J, McLean TI, Olgiate J, Hilton M, Miller WE, Bachenheimer SL. 1998. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology 247:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Zhou BP. 2010. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer 102:639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Cohen JI. 2015. The role of PI3K/Akt in human herpesvirus infection: from the bench to the bedside. Virology 479–480:568–577. doi: 10.1016/j.virol.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benetti L, Roizman B. 2006. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of ΔUS3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J Virol 80:3341–3348. doi: 10.1128/JVI.80.7.3341-3348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norman KL, Sarnow P. 2010. Herpes simplex virus is Akt-ing in translational control. Genes Dev 24:2583–2586. doi: 10.1101/gad.2004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermida MA, Dinesh Kumar J, Leslie NR. 2017. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv Biol Regul 65:5–15. doi: 10.1016/j.jbior.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Chuluunbaatar U, Roller R, Feldman ME, Brown S, Shokat KM, Mohr I. 2010. Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev 24:2627–2639. doi: 10.1101/gad.1978310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaishankar D, Yakoub AM, Yadavalli T, Agelidis A, Thakkar N, Hadigal D, Ames J, Shukla D. 2018. An off-target effect of BX795 blocks herpes simplex virus type 1 infection of the eye. Sci Transl Med 10:10:eaan5861. doi: 10.1126/scitranslmed.aan5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC. 2008. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol 6:266–275. doi: 10.1038/nrmicro1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werden SJ, McFadden G. 2010. Pharmacological manipulation of the Akt signaling pathway regulates myxoma virus replication and tropism in human cancer cells. J Virol 84:3287–3302. doi: 10.1128/JVI.02020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasquereau S, Kumar A, Abbas W, Herbein G. 2018. Counteracting Akt activation by HIV protease inhibitors in monocytes/macrophages. Viruses 10:190. doi: 10.3390/v10040190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agelidis A, Koujah L, Suryawanshi R, Yadavalli T, Mishra YK, Adelung R, Shukla D. 2019. An intra-vaginal zinc oxide tetrapod nanoparticles (ZOTEN) and genital herpesvirus cocktail can provide a novel platform for live virus vaccine. Front Immunol 10:500. doi: 10.3389/fimmu.2019.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.