Porcine circovirus type 2 (PCV2) is a small DNA virus that depends heavily on host cells for its infection. This study reports the close relationship between subcellular localization of host high-mobility-group box 1 (HMGB1) protein and viral replication during PCV2 infection. Restriction of PCV2 replication by nuclear HMGB1 is the early step of host defense at the host-pathogen interface. PCV2 then upregulates host reactive oxygen species (ROS) to prevent sequestration of its genome by expelling nuclear HMGB1 into the cytosol. It will be interesting to study if a similar evasion strategy is employed by other circoviruses such as beak and feather disease virus, recently discovered PCV3, and geminiviruses in plants. This study also provides insight into the justification and pharmacological basis of antioxidants as an adjunct therapy in PCV2 infection or possibly other diseases caused by the viruses that deploy the ROS-HMGB1 interaction favoring their replication.
KEYWORDS: high-mobility-group box 1 protein, porcine circovirus type 2, reactive oxygen species, viral replication
ABSTRACT
Porcine circovirus type 2 (PCV2) is an important swine pathogen that causes significant economic losses to the pig industry. PCV2 interacts with host cellular factors to regulate its replication. High-mobility-group box 1 (HMGB1) protein, a major nonhistone protein in the nucleus, was recently discovered to participate in viral infections. Here, we demonstrate that nuclear HMGB1 negatively regulated PCV2 replication as shown by overexpression of HMGB1 or blockage of its nucleocytoplasmic translocation with ethyl pyruvate. The B box domain was essential in restricting PCV2 replication. Nuclear HMGB1 restricted PCV2 replication by sequestering the viral genome via binding to the Ori region. However, PCV2 infection induced translocation of HMGB1 from cell nuclei to the cytoplasmic compartment. Elevation of reactive oxygen species (ROS) induced by PCV2 infection was closely associated with cytosolic translocation of nuclear HMGB1. Treatment of PCV2-infected cells with ethyl pyruvate or N-acetylcysteine downregulated PCV2-induced ROS production, suppressed nucleocytoplasmic HMGB1 translocation, and decreased PCV2 replication. Collectively, these findings offer new insight into the mechanism of the PCV2 evasion strategy: PCV2 manages to escape restriction of its replication by nuclear HMGB1 by inducing ROS to trigger the nuclear-to-cytoplasmic translocation of HMGB1.
IMPORTANCE Porcine circovirus type 2 (PCV2) is a small DNA virus that depends heavily on host cells for its infection. This study reports the close relationship between subcellular localization of host high-mobility-group box 1 (HMGB1) protein and viral replication during PCV2 infection. Restriction of PCV2 replication by nuclear HMGB1 is the early step of host defense at the host-pathogen interface. PCV2 then upregulates host reactive oxygen species (ROS) to prevent sequestration of its genome by expelling nuclear HMGB1 into the cytosol. It will be interesting to study if a similar evasion strategy is employed by other circoviruses such as beak and feather disease virus, recently discovered PCV3, and geminiviruses in plants. This study also provides insight into the justification and pharmacological basis of antioxidants as an adjunct therapy in PCV2 infection or possibly other diseases caused by the viruses that deploy the ROS-HMGB1 interaction favoring their replication.
INTRODUCTION
Porcine circovirus 2 (PCV2), member of the genus Circovirus in the family Circoviridae, is a small, icosahedral, and nonenveloped virus containing a covalently closed, circular single-stranded DNA (1, 2). PCV2 is the primary causative agent of porcine circovirus-associated diseases (PCVAD), characterized by conditions such as postweaning multisystemic wasting syndrome (PMWS), congenital tremors, respiratory disorders, enteritis, and reproductive failures, causing severe economic losses to the pig industry worldwide (3, 4).
PCV2 has an ambisense genome organization containing two major open reading frames (ORFs): ORF1 encodes the replicase protein (Rep) that participates in the rolling-circle replication of viral genome (5, 6), and ORF2 encodes the capsid protein (Cap) of high immunogenicity (7, 8). The Rep and Cap genes are oriented in opposite directions, with an intergenic region between their 5′ ends as the origin of virus replication (Ori) that is characterized by a stem-loop structure necessary for initiation of viral DNA replication (2, 6). Recent studies revealed that three other viral proteins (ORF3, ORF4, and ORF5) are involved in host-virus interaction and multiple cellular signaling pathways to regulate and utilize host responses beneficial for PCV2 replication and infection process (9–15).
The PCV2 genome replicates via the rolling-circle replication (RCR) mechanism (6, 16), and its replication largely depends upon host cell factors because of its small genome size and limited coding capacity (6). Previous studies showed that high-mobility-group box type 1 (HMGB1), a nonhistone chromosomal protein, promotes viral rolling-circle replication (17, 18) or inhibits viral integration into the genome by binding viral DNA (19–21). HMGB1 is a highly conserved protein belonging to the high-mobility-group box (HMGB) protein family (22). HMGB1 consists of two tandem HMG boxes (A and B boxes) and a C-terminal acidic tail that contains a continuous array of aspartic acid and glutamic acid residues (23). HMG boxes are DNA-binding domains characterized by three α-helices arranged in an L-shaped conformation (24–26). The A and B boxes of HMGB1 can bind DNA with preference for the noncanonical DNA structures such as single-stranded DNA and DNA-containing cruciform or bent structures and enhance DNA flexibility by looping (27–30).
HMGB1 is a multifunctional protein with various roles in different cellular compartments. It is normally located in the nucleus to act as a chromosome guardian and DNA chaperone involved in DNA replication, gene transcription, DNA repair, nucleosome stability, and telomere homeostasis (30). Reactive oxygen species (ROS) are known to promote HMGB1 translocation to the cytosol (31–33). In the cytoplasm, HMGB1 can competitively interact with beclin1 to dissociate the Bcl-2-beclin1 complex, thus promoting autophagy and limiting apoptosis (34). Extracellular HMGB1 is considered the damage-associated molecular pattern (DAMP) molecule because it can interact with multiple receptors (e.g., Toll-like receptor 4 [TLR4]) and take part in a wide range of physiological and pathological processes such as inflammation, immune responses, cell migration and proliferation, tissue regeneration, and angiogenesis (35).
Accumulating evidence has revealed that HMGB1 translocation occurs in response to virus infection and plays an important role in the progression of viral infection. HMGB1 translocation triggered by porcine reproductive and respiratory syndrome virus (PRRSV) and Newcastle disease virus (NDV) infection potentiates virus-induced inflammatory responses (36, 37). HMGB1 released by hepatitis C virus (HCV) infection can inhibit virus propagation to uninfected cells (38), while intracellular HMGB1 might interact with HCV RNA to promote viral genome replication (39). Intranuclear replication of the influenza virus can be enhanced by the interaction of nuclear HMGB1 with the viral nucleoprotein (40).
However, it remains unknown whether and how HMGB1 is involved in PCV2 infection. Here, we demonstrate that nuclear HMGB1 inhibited PCV2 replication by direct binding to the Ori region of the PCV2 genome. We further show that PCV2 was able to drive HMGB1 out of the nucleus to benefit its replication by inducing an elevation of cellular ROS. Treatment of PCV2-infected cells with ROS scavengers inhibited nucleocytoplasmic translocation of HMGB1 and repressed PCV2 replication.
RESULTS
PCV2 infection induced nucleocytoplasmic translocation of HMGB1 in PK-15 and porcine alveolar macrophage cells.
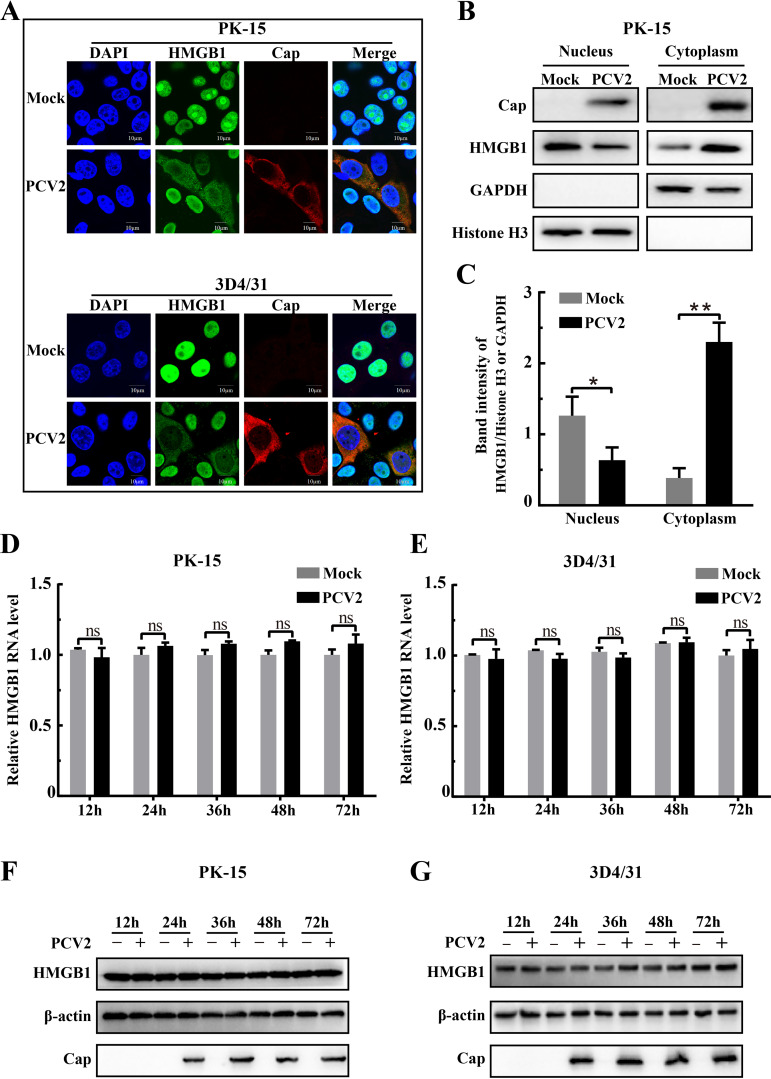
Previous studies reported that viruses, such as respiratory syncytial virus (RSV), HCV, PRRSV, and HIV-1, trigger nuclear HMGB1 translocation into the cytoplasmic compartment (36, 38, 41, 42). We first analyzed expression and distribution of HMGB1 in PK-15 cells and 3D4/31 cells (a porcine alveolar macrophage cell line) infected with PCV2 by confocal imaging and Western blotting. Confocal microscopy revealed that HMGB1 was present in both the nuclear and cytoplasmic compartments in PCV2-infected cells while it was mainly confined in the nuclear compartment in uninfected control cells (Fig. 1A). Lower abundance of HMGB1 in the nuclear fraction was accompanied by its higher abundance in the cytoplasmic fraction of PCV2-infected cells, while an opposite distribution of HMGB1 in these two fractions was seen in mock-infected cells (P < 0.05 or 0.01 between the two treatments in either fraction) (Fig. 1B and C). PCV2 infection did not affect transcription and translation of HMGB1 during viral infection up to 72 h in both cell lines (Fig. 1D to G). These data indicate that PCV2 infection promoted HMGB1 translocation from nuclei to cytoplasm.
FIG 1.
PCV2 infection led to translocation of HMGB1 from nuclei to cytoplasmic compartments. PK-15 cells and porcine monocytic cells (3D4/31) were infected for 36 h with PCV2 (MOI = 1) or mock infected as a control. (A) Confocal imaging of HMGB1 distribution in PCV2-infected cells immunostained with anti-HMGB1 (green) and anti-Cap (red) antibodies. Nuclei were labeled with DAPI (blue). Representative micrographic images are shown. (B) Immunoblotting of PCV2 Cap and HMGB1 in nuclear and cytoplasmic extracts from PCV2- or mock-infected PK-15 cells. Histone H3 and GAPDH were used as internal controls for nuclear and cytoplasmic fractions, respectively. (C) The intensity of protein bands was quantified densitometrically using Gel-Pro Analyzer. Ratios of nuclear or cytoplasmic HMGB1 to Histone H3 or GAPDH were quantified, respectively. (D and E) Quantification of hmgb1 mRNA by qPCR in PK-15 and 3D4/31 cells infected with PCV2 for different times using total RNA extracts from the cells. (F and G) Immunoblotting of HMGB1 and PCV2 Cap in the lysates of PK-15 and 3D4/31 cells infected with PCV2 for different times. β-Actin was used as a loading control. The data in panels A, B, F, and G are representative of three independent experiments. Bar charts in panels C, D, and E show means ± SDs from three independent experiments. ns, not significant; *, P < 0.05; **, P < 0.01.
HMGB1 exerted negative regulation of PCV2 replication.
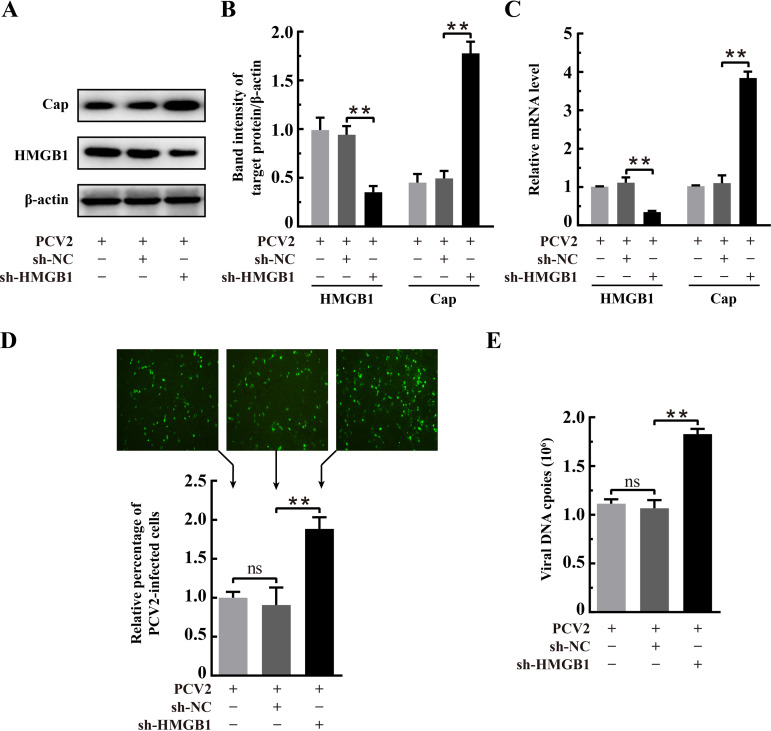
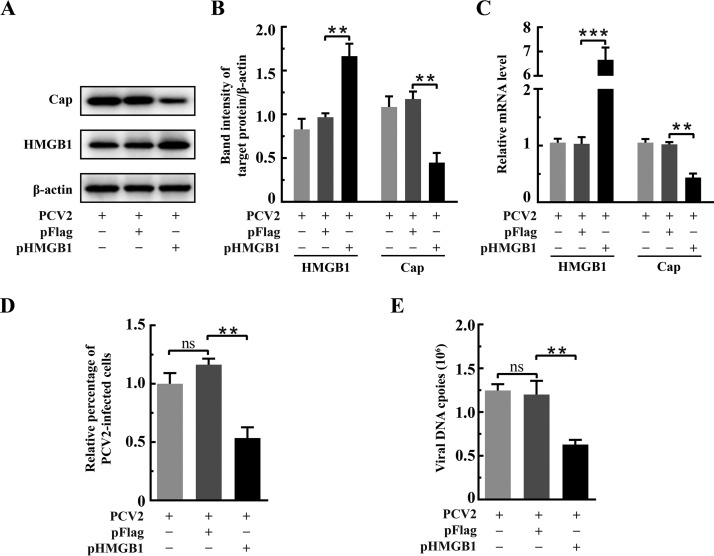
Some early studies have shown that HMGB1 has an impact on the replication of several viruses, binding to Rep and promoting Rep-mediated cleavage of DNA in adeno-associated virus (17) and initiating rolling-circle-type DNA replication at the hairpin origin in parvovirus (18). During productive PCV2 infection, the viral genome is delivered to the nucleus and begins to replicate at approximately 15 h postinfection (hpi), viral transcripts can be detected at 18 hpi, and progeny viruses begin to appear at about 30 hpi (43, 44). Thus, we chose 36 hpi for subsequent experiments to investigate the effects of HMGB1 on PCV2 replication in PK-15 cells by overexpression or specific short hairpin RNA (shRNA). Gene silencing (sh-HMGB1) was effective in downregulating HMGB1 expression, as shown either by quantitative PCR (qPCR) or by Western blotting (Fig. 2A to C) (P < 0.01 compared with that for the scrambled RNA control). In PCV2-infected cells with the sh-HMGB1 plasmid, there was significantly higher transcription and expression of the viral orf2 gene (Cap) (Fig. 2B and C) as well as increased numbers of viral genomic DNA copies and PCV2-infected cells (Fig. 2D and E) (P < 0.01 in all cases in comparison with control RNA [sh-NC] transfection). Figure 3A to C shows that HMGB1 was overexpressed in PK-15 cells (P < 0.01 compared with expression with the control vector pFlag). Contrary to findings with shRNA silencing, HMGB1 overexpression resulted in marked reduction of PCV2-infected cells, viral genome copies, and Cap expression (Fig. 3B to E) (P < 0.01 in all cases compared with those for the vector control). These results clearly show that HMGB1 exerts a negative impact on PCV2 replication.
FIG 2.
Downregulation of HMGB1 promoted PCV2 replication. PK-15 cells were transfected with hmgb1-specific RNA interference (RNAi) plasmid (sh-HMGB1) or control RNAi plasmid (sh-NC) for 24 h and then infected with PCV2 (MOI= 1) for 36 h. (A) Effect of hmgb1 knockdown on PCV2 Cap expression (β-actin used as a loading control) as shown by immunoblotting using protein samples from the whole-cell lysates. The gel shown is representative of three independent experiments. (B) The ratios of band intensity of HMGB1 or PCV2 Cap to β-actin (as shown in panel A). (C) Effect of hmgb1 knockdown on PCV2 orf2 (encoding Cap) transcription examined by qPCR using total RNA extracted from the whole-cell lysates. (D) PCV2 replication in hmgb1-silenced cells as assessed by indirect immunofluorescence. Representative fluorescence images are shown (top). Percentage of PCV2-infected cells was calculated by dividing the number of PCV2-infected cells by the total cell number in each group (n = 2 images for each experiment per group) that were counted using ImageJ software. Relative percentages of PCV2-infected cells in the hmgb1-silenced cells are shown with nontransfected but PCV2-infected cells set at 100% (bottom). (E) Effect of hmgb1 silencing on PCV2 genomic DNA copies measured by qPCR using total DNA extracts from whole-cell lysates. Bar charts in panels B, C, D, and E show means ± SDs from three independent experiments. ns, not significant; **, P < 0.01.
FIG 3.
Overexpression of HMGB1 inhibited PCV2 replication. PK-15 cells were transfected with recombinant plasmid expressing HMGB1 (pHMGB1) or control plasmid (pFlag) for 24 h and then infected with PCV2 (MOI = 1) for 36 h. (A) Effect of HMGB1 overexpression on PCV2 Cap expression as shown by immunoblotting using protein samples from the whole-cell lysates. β-Actin was used as a loading control. The gel shown is representative of three independent experiments. (B) The ratios of band intensity of HMGB1 or PCV2 Cap to β-actin (as shown in panel A). (C) Effect of HMGB1 overexpression on PCV2 orf2 (encoding Cap) transcription measured by qPCR using total RNA extracted from the whole-cell lysates. (D) PCV2 replication in cells overexpressing HMGB1 as assessed by indirect immunofluorescence. Percentages of PCV2-infected cells were calculated as described in the legend for Fig. 2. Relative percentages of PCV2-infected cells in the HMGB1 overexpressing cells are shown with nontransfected but PCV2-infected cells set at 100%. (E) PCV2 genomic DNA copies in cells overexpressing HMGB1 quantified by qPCR using total DNA extracts from whole-cell lysates. Bar charts in panels B, C, D, and E show means ± SDs from three independent experiments. ns, not significant; **, P < 0.01; ***, P < 0.001.
Nuclear HMGB1 influenced PCV2 replication.
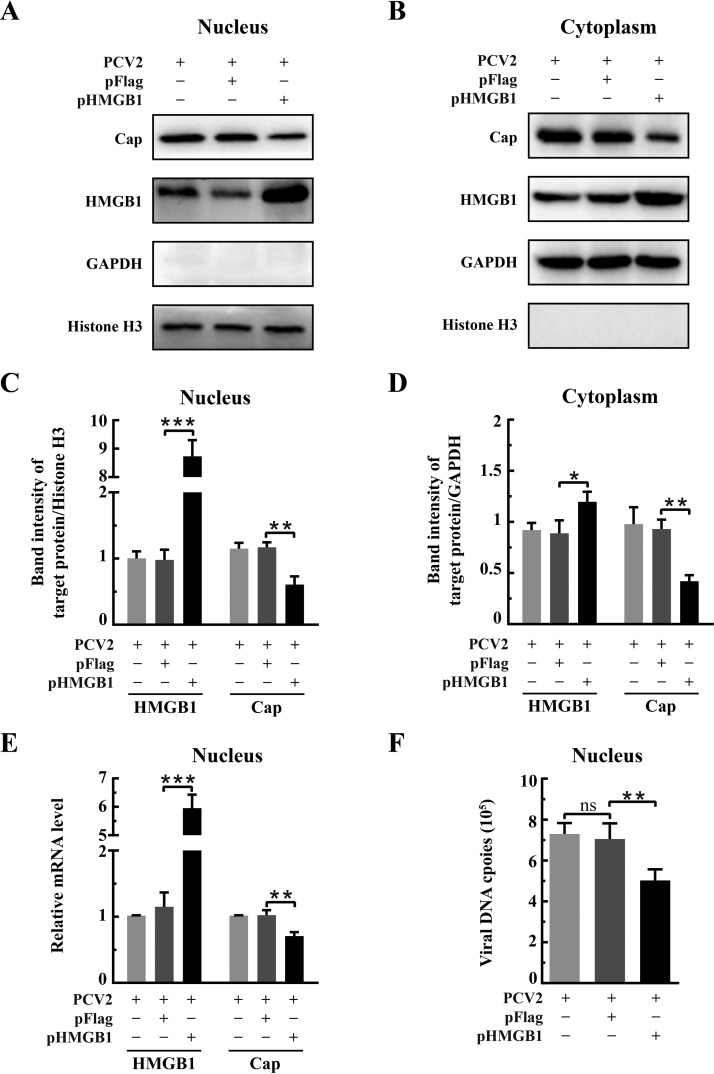
Because HMGB1 had a negative effect on PCV2 replication, we wondered if it was the nuclear HMGB1 that impacted PCV2 replication. In the life cycle of PCV2, viral genome replication, mRNA transcription, and virus assembly take place in the nuclear compartment (16). Thus, nuclear and cytoplasmic fractions were prepared from the PK-15 cells transfected with either control vector (pFlag) or pHMGB1 plasmid followed by PCV2 infection for examination of HMGB1 and viral protein levels by Western blotting and quantitative PCR. We found that HMGB1 was far more abundant in the nuclear fraction of PCV2-infected cells overexpressing HMGB1 (transfected with pHMGB1) than in those receiving the control vector (Fig. 4A and C) (P < 0.001). HMGB1 in the cytoplasm was also high as a result of overexpression, but to a lesser degree in comparison with vector control (Fig. 4B and D) (P < 0.05). Overexpression of HMGB1 resulted in decreased levels of the viral Cap protein in both fractions (Fig. 4C and D) (P < 0.01). There were also significant reductions of Cap mRNA and viral genome DNA copies in the nuclear fraction of HMGB1-overexpressed cells (Fig. 4E and F) (P < 0.01). Therefore, we postulate that nuclear HMGB1 contributes to repression of viral genome replication, hence the reduction of viral protein expression and virus particle formation.
FIG 4.
Nuclear HMGB1 repressed PCV2 replication. PK-15 cells were transfected with recombinant plasmid expressing HMGB1 (pHMGB1) or control plasmid (pFlag) for 24 h and then infected with PCV2 (MOI = 1) for 36 h. Nuclear and cytoplasmic extracts were prepared for immunoblotting as described in the legend for Fig. 1 Immunoblotting of HMGB1 and PCV2 Cap in the nuclear (A) and cytoplasmic (B) fractions. Histone H3 and GAPDH were used as internal controls for nuclear and cytoplasmic extracts, respectively. Representative images from three independent experiments are shown. The ratios of band intensities of HMGB1 or PCV2 Cap to those of histone H3 (as shown in panel A) in the nuclear fraction (C) or to GAPDH (as shown in panel B) in the cytoplasmic fraction (D). (E) Effect of HMGB1 overexpression on PCV2 orf2 transcription in the nuclei examined by qPCR using total RNA extracted from the nuclear fractions. Results were normalized to histone H3 mRNA in the same samples. (F) PCV2 genomic DNA replication in the nuclei of HMGB1-overexpressing cells quantified by qPCR using total DNA extracted from nuclear fractions. Bar charts in panels C to F show means ± SDs from three independent experiments. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
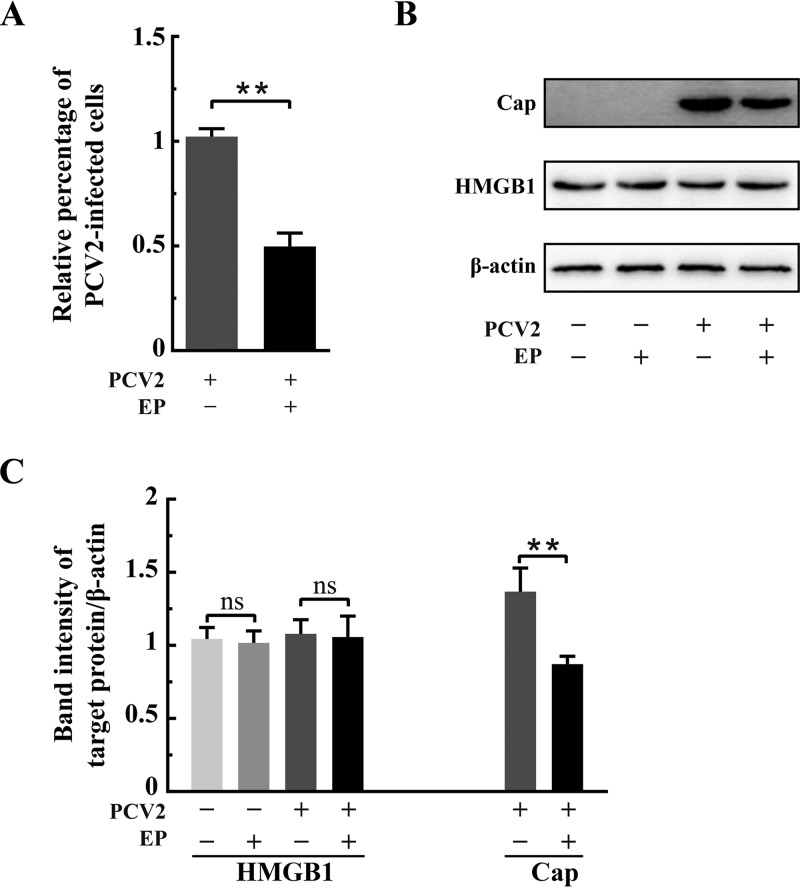
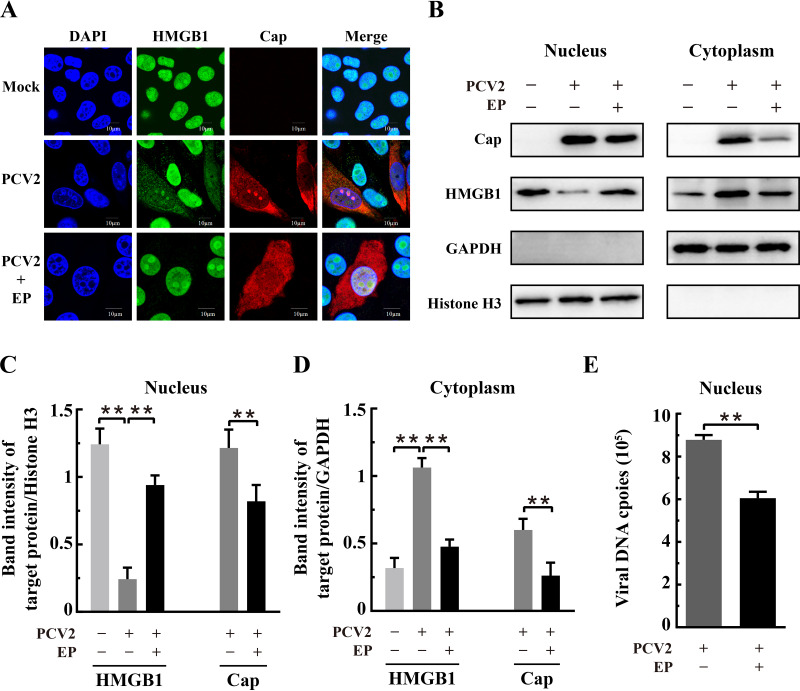
Ethyl pyruvate inhibited PCV2 replication by blocking HMGB1 translocation.
Ethyl pyruvate (EP), a simple derivative of pyruvic acid, has been found as a potent anti-inflammatory agent in a variety of in vitro and in vivo model systems and under various pathological conditions (45, 46). Recent studies have shown that EP inhibits nuclear-to-cytoplasmic translocation of HMGB1 (34, 45, 47). We treated the PCV2-infected cells with EP to investigate if retention of HMGB1 in the nuclei would impact PCV2 replication. Figure 5 shows that EP treatment, at a concentration that does not affect cell viability (96%), resulted in a significant decrease of PCV2-infected cells and reduced Cap expression in comparison with the untreated control (P < 0.01) without apparent change of total HMGB1. EP was effective in inhibiting nucleocytoplasmic translocation of HMGB1 in PCV2-infected cells (Fig. 6A). Western blotting revealed that EP treatment led to marked retention of HMGB1 within the nuclei of PCV2-infected cells, with a corresponding decrease of its distribution in the cytoplasm, compared with that in PCV2-infected control cells without EP (Fig. 6B to D) (P < 0.01). There was a significant reduction of PCV2 Cap expression in the nuclear and cytoplasmic fractions and marked decrease of viral genome DNA in the nuclei as a result of EP treatment (Fig. 6B to E) (P < 0.01 compared with that in untreated cells). These results further support the notion that nuclear HMGB1 contributes to repression of PCV2 replication, because EP effectively blocked HMGB1 translocation out of the cell nuclei.
FIG 5.
Ethyl pyruvate was inhibitory to PCV2 infection. PK-15 cells were mock infected or infected with PCV2 (MOI = 1) with or without ethyl pyruvate (EP; 7.5 mM) treatment. The cell samples were harvested at 36 hpi. (A) Effect of EP on PCV2 replication in PK-15 cells by immunofluorescence. Percentages of PCV2-infected cells were calculated as described in the legend for Fig. 2. Relative percentages of PCV2-infected cells in the EP-treated cells are shown with untreated but PCV2-infected cells set at 100%. (B) Immunoblotting of HMGB1 and PCV2 Cap in whole-cell lysates with β-actin used as a loading control. (C) The ratios of band intensities of HMGB1 or PCV2 Cap to β-actin (as shown in panel B). Bar charts in panels A and C show means ± SDs from three independent experiments. ns, not significant; **, P < 0.01.
FIG 6.
Ethyl pyruvate inhibited nucleocytoplasmic translocation of HMGB1 in PCV2-infected cells. PK-15 cells were mock infected or infected with PCV2 (MOI = 1) with or without ethyl pyruvate (EP; 7.5 mM) treatment. The cell samples were harvested at 36 hpi. (A) Confocal microscopic images show inhibition of nuclear HMGB1 migration into the cytosol by EP. Cells were immunostained for HMGB1 (green) and PCV2 Cap (red), with nuclei stained with DAPI (blue). Bars, 10 μm. (B) Immunoblotting of HMGB1 and PCV2 Cap in the nuclear and cytoplasmic fractions of PCV2-infected and EP-treated cells. Histone H3 and GAPDH were used as internal controls for nuclear and cytoplasmic extracts, respectively. Representative images from three independent experiments are shown. The ratios of band intensities of HMGB1 or PCV2 Cap to those of histone H3 (as shown in panel B, left) in the nuclear fraction (C) or to GAPDH (as shown in panel B, right) in the cytoplasmic fraction (D). (E) Effect of EP on PCV2 genomic DNA replication by qPCR using DNA extracted from nuclei of PCV2-infected cells treated with 7.5 mM EP. Bar charts in panels C, D, and E show means ± SDs from three independent experiments. ns, not significant; **, P < 0.01.
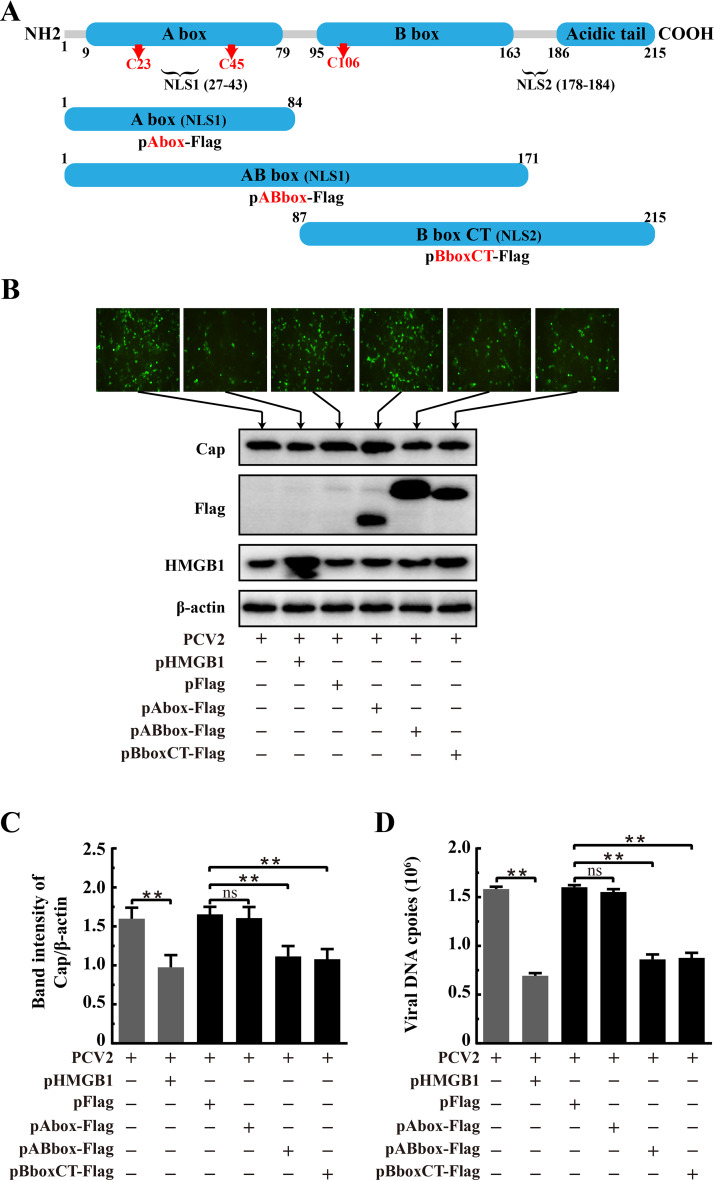
The DNA-binding domain B box of HMGB1 was responsible for inhibition of PCV2 replication.
HMGB1 of Sus scrofa (Pig) has high identity (98.6%) to its human homolog that is composed of two defined DNA-binding domains (A box and B box) and an acidic tail that contains a string of glutamic and aspartic acids (22). To characterize the critical domain of HMGB1 responsible for the restriction of PCV2 replication, truncated versions of HMGB1 were overexpressed in PCV2-infected cells to determine which part of the molecule would impair PCV2 replication. The full-length protein was split into three regions: the A box, AB box, and B box-C terminus (B boxCT), each containing a nuclear localization signal (NLS) (either NSL1 or NSL2) of its own (Fig. 7A) such that the truncated peptides would be localized in the nuclei. PK-15 cells were transfected with recombinant plasmids containing full-length and truncated versions of HMGB1 prior to PCV2 infection to examine their impact on viral replication.
FIG 7.
The B box domain of HMGB1 was involved in inhibition of PCV2 replication. (A) Schematic illustration of the full-length and truncated forms of porcine HMGB1 according to its human homolog. All truncated versions, A box, AB box, and B box plus C terminus (B boxCT), were flag tagged. The numbers indicate positions of amino acids. Arrows with C followed by numbers represent key cysteine residues. NLS, nuclear localization signal. PK-15 cells were transfected with recombinant plasmids expressing flag-tagged or full-length HMGB1 for 24 h and then infected with PCV2 (MOI = 1) for 36 h. (B) Numbers of PCV2-infected cells examined by immunofluorescence using anti-Cap monoclonal antibody as the probe (top). Expression of PCV2 Cap and different forms of HMGB1 as assessed by immunoblotting using the whole-cell lysates harvested at 36 hpi and antibodies against Flag, Cap, and HMGB1 (bottom). β-Actin was used as a loading control. The panel B images are representative of three individual experiments. (C) The ratios of band intensities of PCV2 Cap to those of β-actin (as shown at the bottom panel of B). (D) Effect of different HMGB1 truncations on PCV2 DNA replication estimated by qPCR using total DNA extracted from the whole-cell lysate. Bar charts in panels C and D show means ± SDs from three independent experiments. ns, not significant; **, P < 0.01.
Different HMGB1 regions in virus-infected cells were successfully expressed, as shown by Western blotting using the anti-Flag antibody (Fig. 7B). We found that the fluorescent punctate signals representing PCV2-infected cells were significantly decreased in cells expressing the AB box or B boxCT peptides or full-length HMGB1, but not in cells expressing the A box (Fig. 7B). Overexpression of the AB box and B boxCT led to marked inhibition of PCV2 capsid protein expression and viral genome copies in levels comparable to that of full-length HMGB1 (Fig. 7C and D) (P < 0.01 in all cases compared with those in the control vector). However, the A box alone did not have such an inhibitory effect on viral replication (similar to the control vector, P > 0.05). Therefore, we suppose that the HMGB1 B box domain is involved in repression of PCV2 replication.
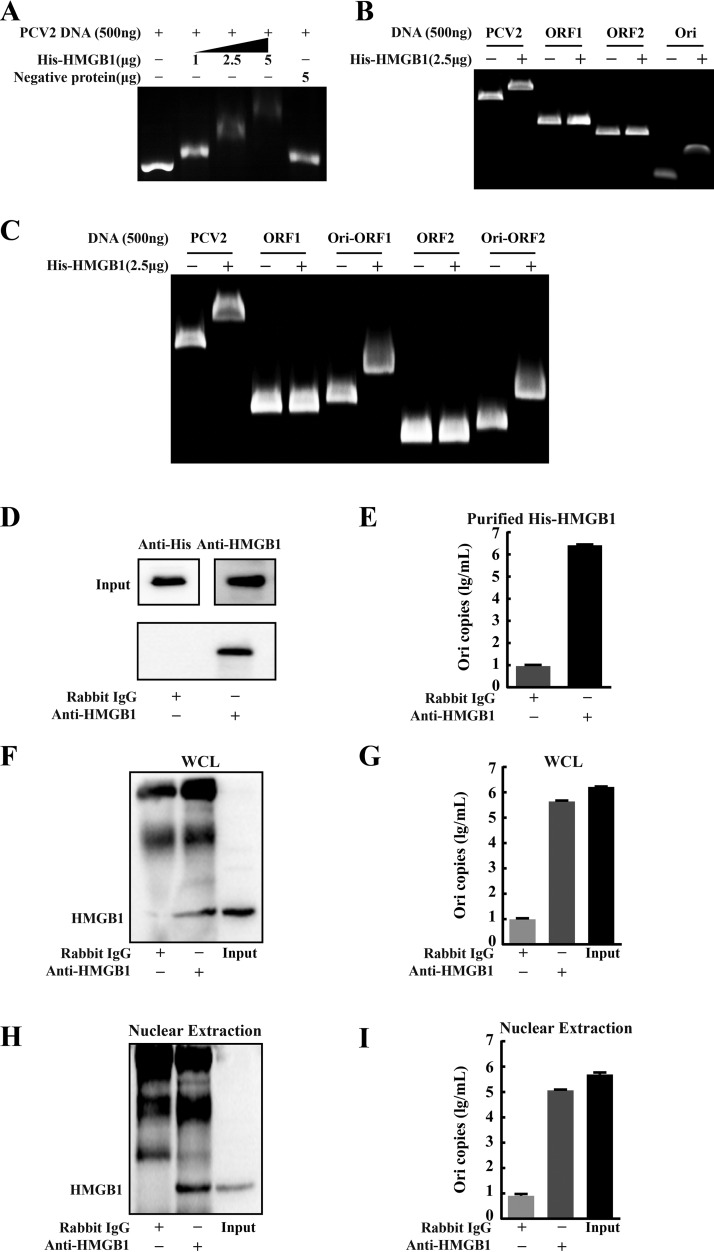
HMGB1 interacted with the Ori region of PCV2 genome.
Previous studies have reported that HMGB1 can inhibit viral integration into the genome by binding viral DNA (22). We hypothesized that nuclear HMGB1 might interact with PCV2 DNA by forming complexes to impair viral genome replication. The gel shift assay and immunoprecipitation coupled with quantitative PCR (qPCR) approaches were used to examine the possible interaction of HMGB1 with PCV2 genome DNA using His-tagged recombinant porcine HMGB1 (His-HMGB1) expressed in a baculovirus expression system. Incubation of different concentrations of His-HMGB1 with purified PCV2 genome DNA led to a retarded mobility pattern in a dose-dependent manner (Fig. 8A). Next, the full-length PCV2 genome was divided into three sections: orf1 (945 bp), orf2 (705 bp), and Ori (139 bp [including the 83-bp stem-loop structure]) to identify the region of the PCV2 genome that might interact with HMGB1. We found that HMGB1 hindered DNA motility when incubated with the Ori region, while no motility changes were seen when incubated with orf1 and orf2 (Fig. 8B). The circular PCV2 genome contains a stem-loop-structured Ori region. The 5′ ends of two main ORFs, ORF1 and ORF2, are separated by the Ori intergenic region (IR) (6). To further prove that the Ori region was responsible for binding with HMGB1, two additional DNA fragments, Ori-orf1 and Ori-orf2, were prepared for the gel shift assay. Figure 8C reveals that DNA motility was retarded when either Ori-orf1 or Ori-orf2, but not orf1 or orf2 alone, was incubated with recombinant HMGB1.
FIG 8.
HMGB1 bound to the Ori region of the PCV2 genome. (A) Binding of porcine HMGB1 to PCV2 DNA using the gel shift assay. PCV2 DNA (500 ng) and various concentrations (0 to 5 μg) of purified His-tagged recombinant HMGB1 were mixed in binding buffer. The DNA-protein mixtures were subjected to 0.8% agarose gel electrophoresis to visualize changes of the DNA motility. (B) Binding of HMGB1 to a specific region of PCV2 DNA: full-length and different fragments of PCV2 genome (orf1, orf2, and Ori) were incubated with recombinant HMGB1 protein to identify the region of PCV2 genome involved in HMGB1 binding. (C) To confirm the Ori region is required for HMGB1 binding, the Ori fragment was combined with orf1 or orf2 (Ori-orf1 or Ori-orf2) that were then compared with orf1 or orf2 alone by the gel shift assay. (D) Immunoprecipitation of purified HMGB1 protein (500 μg) and PCV2 DNA (500 ng) mixture by anti-HMGB1 antibody (rabbit IgG as control) and protein A/G agarose. The precipitates were probed with anti-His and anti-HMGB1 antibodies by immunoblotting. (E) Quantification of PCV2 genomic Ori copies by qPCR in DNA extracts from PCV2 DNA-HMGB1 precipitates (shown in panel D) after DNase pretreatment. (F) Blotting of HMGB1 in immunoprecipitates of whole-cell lysates (WCL) of the PK-15 cells infected with PCV2 (36 h) by anti-HMGB1 (rabbit IgG as control) and protein A/G agarose. (G) Quantification of PCV2 genomic Ori copies by qPCR in DNA extracts from immunoprecipitates of whole-cell lysates (shown in panel F) after DNase pretreatment. (H) Blotting of HMGB1 in immunoprecipitates of nuclear extracts of the PK-15 cells infected with PCV2. (I) Quantification of PCV2 genomic Ori copies in the precipitates shown in panel H. Bar charts in panels E, G, and I show means ± SDs from three independent experiments.
To confirm the observed binding of HMGB1 with PCV2 genomic Ori, an immunoprecipitation assay was used to capture the viral DNA bound by recombinant HMGB1. The precipitates were subjected to DNase digestion followed by qPCR using the primer pair targeting the Ori region (see Table S1 in the supplemental material). Immunoprecipitation was successful (Fig. 8D). A large number of viral Ori copies was detected in the HMGB1-bound precipitate (Fig. 8E). To further verify such in vitro interaction between HMGB1 and PCV2 Ori, the same immunoprecipitation assay was employed for the whole-cell lysates (WCL) and the nuclear extracts of PCV2-infected cells. HMGB1 was well precipitated (Fig. 8F and H), and a large number of PCV2 genomic Ori copies was present in the nuclear fraction and whole-cell lysate (Fig. 8G and I). Together, these data demonstrate that nuclear HMGB1 binds directly to the Ori region of the PCV2 genome in infected cells.
PCV2 infection increased ROS generation that facilitated nucleocytoplasmic translocation of HMGB1 and viral replication.
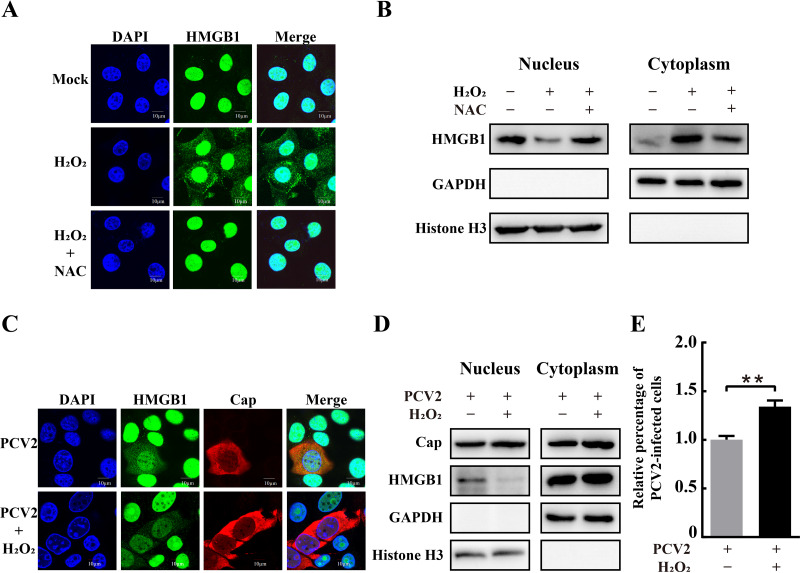
Several studies have shown that subcellular localization of HMGB1 is associated with increased ROS generation (32, 33). PCV2 infection was found to cause oxidative stress, thus favoring viral replication (48). Therefore, we sought to investigate the role of PCV2-induced ROS in the translocation of nuclear HMGB1 to the cytoplasmic compartment during infection and the possible mechanism by which ROS influences PCV2 replication. Confocal microscopy revealed that HMGB1 was diffusely present in the cytosolic compartments of H2O2-treated cells in addition to the nuclei, while it was predominantly located in the nuclear compartments in the untreated control cells (Fig. 9A). Western blotting showed increased HMGB1 in the cytoplasmic fraction and decreased HMGB1 in the nuclear fraction in H2O2-treated cells (Fig. 9B). Similar findings were seen for PCV2-infected cells (Fig. 1). Treatment of PCV2-infected cells with H2O2 further exacerbated nucleocytoplasmic translocation of HMGB1 and even facilitated viral replication (Fig. 9C to E).
FIG 9.
Effect of hydrogen peroxide treatment on subcellular localization of HMGB1 and PCV2 replication. (A) H2O2 treatment promoted nucleocytoplasmic translocation of HMGB1. PK-15 cells were treated with or without N-acetylcysteine (NAC; 10 mM) before adding 50 μM H2O2. Cells were fixed and immunostained with anti-HMGB1 (green) for confocal microscopy. Nuclei were labeled with DAPI (blue). (B) Immunoblotting of HMGB1 in the nuclear and cytoplasmic fractions of PK-15 cells treated with H2O2 and NAC. (C) Confocal imaging of PK-15 cells infected by PCV2 with or without 50 μM H2O2 treatment after immunostaining with anti-HMGB1 (green) and anti-Cap (red) antibodies. (D) Blotting of HMGB1 and PCV2 Cap in the nuclear and cytoplasmic extracts of PCV2-infected cells with or without H2O2 treatment. Histone H3 and GAPDH were used as internal controls for the nuclear and cytoplasmic fractions, respectively. (E) Percentages of PCV2-infected cells were calculated from immunofluorescence images as described in the legend for Fig. 2. Relative percentages of PCV2-infected cells in the H2O2-treated cells are shown with nontreated but PCV2-infected cells set at 100%. Bar chart in panel E shows means ± SDs from three independent experiments. **, P < 0.01.
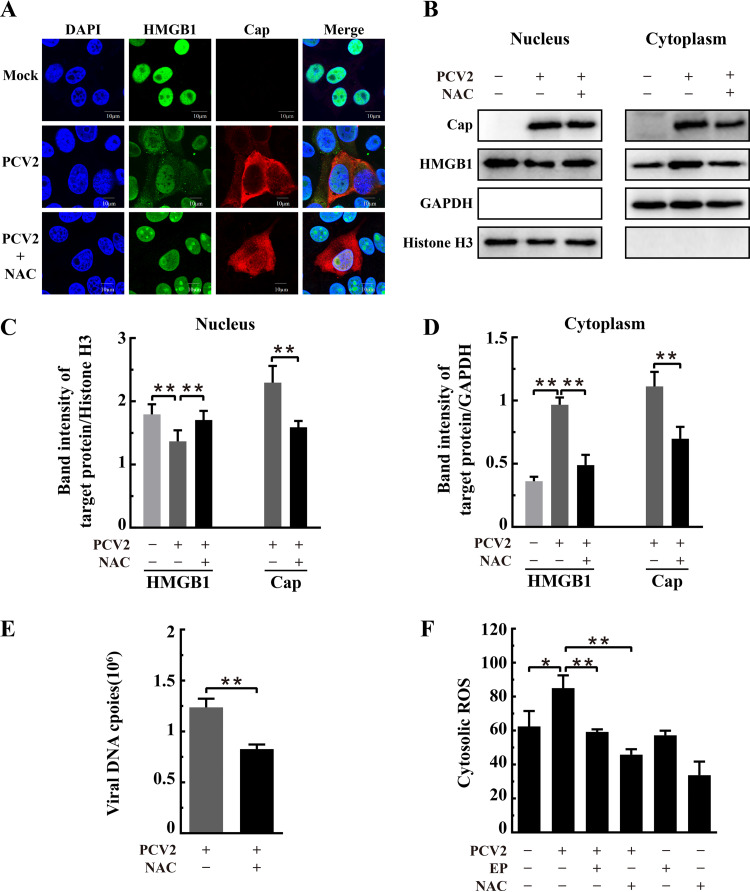
N-Acetylcysteine (NAC) treatment effectively blocked the translocation of nuclear HMGB1 to the cytoplasm compartment in H2O2-treated cells (Fig. 9A and B). The same effect was also seen in PCV2-infected cells: the migration of nuclear HMGB1 to the cytoplasm was significantly blocked by NAC treatment (Fig. 10A to C). Moreover, NAC treatment led to marked reduction (by about 50%) of viral capsid protein expression in both nuclear and cytoplasmic fractions (Fig. 10C and D) as well as fewer viral DNA copies in the nuclei (Fig. 10E). By flow cytometric analysis, we found that NAC or EP treatment resulted in marked reduction of cellular ROS arising from PCV2 infection (Fig. 10F). It is therefore clear that PCV2 infection induces elevation of cellular ROS and that increased ROS reduces the nuclear HMGB1 level by facilitating the migration of nuclear HMGB1 into the cytoplasmic compartment, thus derepressing PCV2 replication from suppressive nuclear HMGB1. Mitigation of ROS by antioxidants NAC or EP effectively increased nuclear HMGB1 retention, thereby inhibiting PCV2 replication.
FIG 10.
N-Acetylcysteine inhibited PCV2-induced HMGB1 translocation from nuclei to cytosol and repressed PCV2 replication. PK-15 cells were mock infected or infected with PCV2 (MOI = 1) for 12 h and then treated with 10 mM N-acetylcysteine (NAC). The cell samples were harvested at 36 hpi. (A) Confocal imaging of HMGB1 distribution in PCV2-infected and NAC-treated cells after the cells were fixed and immunostained for HMGB1 (green) and Cap (red). Nuclei were stained with DAPI (blue). Bars, 10 μm. (B) Blotting of HMGB1 and PCV2 Cap in the nuclear and cytoplasmic extracts of PCV2-infected cells with or without NAC treatment. Histone H3 and GAPDH were used as internal controls for the nuclear and cytoplasmic fractions, respectively. The figure is representative of three independent experiments. The ratios of band intensities of HMGB1 or PCV2 Cap to histone H3 (as shown in panel B, left) in the nuclear fraction (C) or to GAPDH (as shown in panel B, right) in the cytoplasmic fraction (D). (E) Effect of NAC on PCV2 genomic DNA replication by qPCR using DNA extracted from lysates of PCV2-infected cells treated with NAC. (F) Cytosolic ROS levels in PCV2-infected cells with or without treatment by NAC or ethyl pyruvate (EP) as measured by flow cytometry after probing with DCFH-DA. Bar charts in panels C, D, E, and F show means ± SDs from three independent experiments. *, P < 0.05; **, P < 0.01.
DISCUSSION
PCV2 is small but powerful not only in causing severe PCVAD but also in manipulating host responses in favor of its replication (2). Several studies have found that oxidative stress, unfolded protein response/endoplasmic reticulum (ER) stress, and autophagy induced by PCV2 promote viral replication (48–50). However, the underlying mechanisms for how PCV2 manipulates the host responses to enhance its replication remain unknown. Here, we provide clear evidence that HMGB1 in the nucleus inhibits PCV2 DNA replication by binding to the Ori region in the viral genome, thus repressing PCV2 replication, while PCV2 enhances its replication by inducing ROS to drive the suppressive HMGB1 out of the nucleus.
Protein movement between different subcellular compartments is an essential aspect of biological processes, including transcriptional regulation and immune or stress responses. Recent studies have revealed the importance of infection-induced protein translocations between organelles in the outcomes of infectious diseases (51). HMGB1 is one of such proteins that translocate from nuclei to the cytosol or are even released to the extracellular space via an active or passive release mechanism during infection, exerting various roles in different cellular compartments (22, 30). Two RNA viruses, HCV and influenza virus, interact with HMGB1 in different ways. HCV infection triggers HMGB1 translocation from nuclei to the cytoplasm, where it interacts with the stem-loop 4 in the viral 5′ untranslated region via its A box domain to promote HCV replication (39). For influenza virus, HMGB1 associates with the viral nucleoprotein in the nuclei of infected cells, enhances the activity of viral polymerase, and promotes virus growth (40). There are other viruses, such as RSV and hepatitis B virus (HBV), that induce nucleocytoplasmic translocation of HMGB1 via elevation of cytosol ROS or Ca2+, leading to more severe inflammatory responses or promoting hepatocellular carcinoma metastasis (41, 52). However, it remains unknown if and how HMGB1 impacts replication of DNA viruses, such as PCV2, in the infected cells.
Our initial experiments indicated that PCV2 replication was negatively regulated by HMGB1 (Fig. 2 and 3). Further study revealed that HMGB1 inhibited PCV2 replication through its B box domain (Fig. 7) and that nuclear HMGB1 inhibited PCV2 replication (Fig. 4 to 6) in PK-15 cells (and also in porcine alveolar macrophage cell line 3D4/31 cells) (see Fig. S1 in the supplemental material). This inhibition was realized by binding of HMGB1 in the nuclei to the stem-loop structured Ori region of the viral DNA but not to the major coding regions orf1 and orf2 (Fig. 8). This Ori region is critical for initiating replication of PCV2 as the binding site by the viral Rep and Rep′ complex (6). In other words, HMGB1 might function as an antiviral restriction factor against PCV2, probably by competing with the Rep complex for the Ori region. This seems to be different from early studies on other DNA viruses such as adeno-associated virus (AAV) and minute virus of mice (MMV) in the parvovirus family. In AAV, HMGB1 promotes the formation of Rep-DNA complexes and stimulates the activity of Rep (initiator endonuclease) in site- and strand-specific cleavage of DNA and the hydrolysis of ATP, functions required for viral gene regulation (17). Cotmore et al. showed that HMGB1 allows the NS1 (viral nickase) molecules of MMV positioned at each end of the origin of viral DNA to interact, thereby activating its endonuclease function (18).
It is well known that HMGB1 translocates from the nucleus to the cytoplasm to exert different functions during inflammation and apoptosis (53). The trafficking of HMGB1 between the nucleus and cytoplasm is modulated by acetylation (54, 55), phosphorylation (56), and redox regulation (32, 57) on the nuclear localization signals (NLS), where lysine, serine, and cysteine residues reside for such posttranslation modifications that were able to be examined by site-directed mutagenesis. Kwak et al. reported that H2O2-induced oxidation of HMGB1 catalyzed by peroxiredoxins I and II results in the formation of an intramolecular disulfide bond between Cys23 and Cys45 that is necessary and sufficient for its nucleocytoplasmic translocation (57). Because increased ROS generation in PCV2-infected cells was readily observed (Fig. 10) and treatment with N-acetylcysteine or ethyl pyruvate suppressed nucleocytoplasmic translocation of HMGB1 induced either by H2O2 or by PCV2 in PK-15 cells (Fig. 6, 9 and 10) or in 3D4/31 macrophage cells (see Fig. S2), we suppose that PCV2 managed to promote its replication in the cells by inducing ROS that are known to prompt the translocation of nuclear HMGB1 to the cytosol by oxidizing HMGB1 on its key cysteine residues (32, 57, 58).
HMGB1 can also be released into the extracellular space via a number of pathways, including pyroptosis, necroptosis, apoptosis, and secretory lysosome-mediated release (58). Extracellular HMGB1 functions as a damage-associated molecular pattern and interacts with TLR4 or receptor for advanced glycation end products (RAGE) to initiate antiviral innate immune responses (22) or even enhanced inflammatory responses as seen in infections by PRRSV (36), NDV (37), West Nile virus, and severe acute respiratory syndrome coronavirus (59). HMGB1-mediated antiviral immune responses, if any in PCV2-infected cells, might impact viral replication and have confounding effects on the elucidation of the mechanisms of HMGB1-mediated suppression of PCV2 replication. Because PCV2 induces mild forms of cellular responses, such as ER stress, autophagy, and apoptosis but not pyroptosis or necroptosis (9, 10, 48, 49), extracellular release of HMGB1 during PCV2 infection might involve apoptosis or lysosome-mediated secretion, although apoptosis does not seem to have a significant contribution (58). In order to examine if HMGB1, released by whatever means into the extracellular space, might have an impact on PCV2 replication, glycyrrhizin was used to inhibit extracellular HMGB1 activity (60). We did find effective inhibition of HMGB1 release into the culture supernatants but did not see any significant effect on PCV2 replication in PK-15 and 3D4/31 macrophage cells (see Fig. S3).
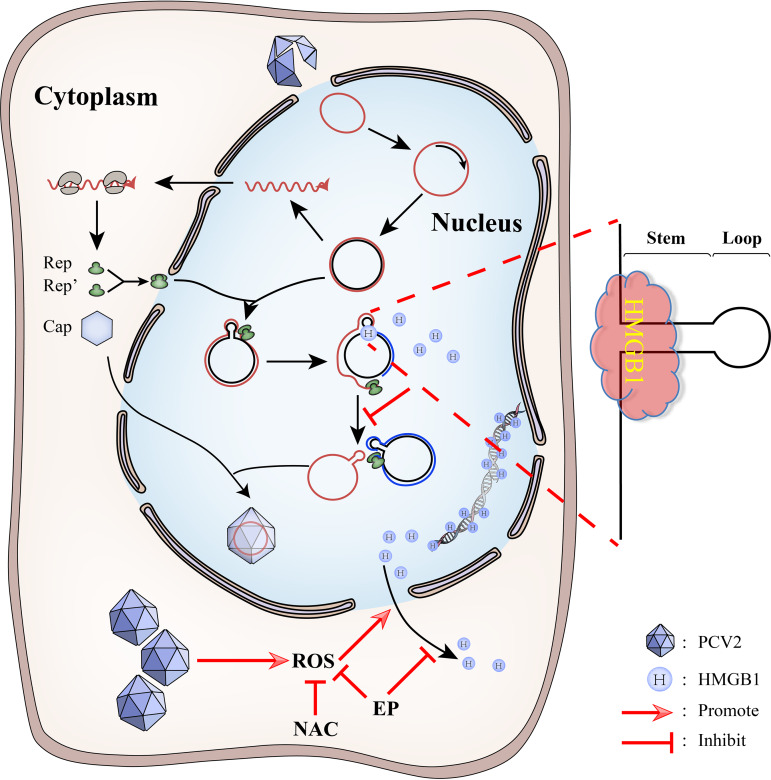
In conclusion, this study elucidates the molecular mechanism of activation of PCV2 replication by ROS: HMGB1 in the nucleus is an antiviral restriction factor against PCV2 replication by binding of its B box domain to the Ori region of the PCV2 genome. PCV2 induces and hijacks the host cell ROS to benefit its replication by promoting nucleocytoplasmic translocation of HMGB1 into the cytosol, thus preventing the viral genome from sequestration by the nuclear HMGB1. The antioxidant N-acetylcysteine can repress PCV2 replication indirectly by antagonizing ROS-induced nucleocytoplasmic translocation of HMGB1 (Fig. 11). Further research could examine if HMGB1 is also involved in enhanced viral replication during ER stress or autophagy in PCV2-infected cells, as observed in our recent work (49, 50), and if ROS is engaged in mobilizing HMGB1 translocation in these cellular responses, considering its well-known interplay with ER stress (61) or autophagy (62). It will be interesting to study if a similar evasion strategy is employed by other circoviruses such as beak and feather disease virus, recently discovered PCV3, and geminiviruses in plants. This study also provides insights into the pharmacological basis and justification of application of antioxidants as an adjunct therapy in PCV2 infection or possibly other diseases caused by the viruses that deploy the ROS-HMGB1 interaction favoring their replication.
FIG 11.
Schematic illustration of the interaction between PCV2 and HMGB1 in infected cells. HMGB1 in the nucleus restricts PCV2 replication by binding to the Ori region of the PCV2 genome. PCV2 infection causes increased generation of cellular ROS. Increased ROS promotes nucleocytoplasmic translocation of HMGB1 and lessens sequestration of the viral DNA by HMGB1 in the nucleus, thus enhancing PCV2 replication. N-Acetylcysteine (and probably ethyl pyruvate as well) scavenges PCV2-induced ROS and thus increases retention of HMGB1 in the nucleus, leading to sequestration of viral DNA and reduced PCV2 replication.
MATERIALS AND METHODS
Cell lines and virus.
Porcine kidney epithelial cell line PK-15 free of PCV1 and the porcine alveolar macrophage cell line 3D4/31 (PAM) were maintained in our laboratory. PK-15 cells were cultured at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, MA, USA) supplemented with 5% fetal bovine serum (FBS) (HyClone, UT, USA). 3D4/31 cells were grown in RPMI 1640 (Thermo) supplemented with 10% FBS at 5% CO2 and 37°C. PCV2 strain JH (PCV2d, GenBank accession no. MG245867), originally isolated from a pig with naturally occurring PCVAD in Zhejiang, China, was stored at −80°C and propagated in PK-15 cells prior to use. The viral stock had a titer of 1 × 105.75 50% tissue culture infective dose (TCID50)/ml. The cells were infected with PCV2 at a multiplicity of infection (MOI) of 1.0 for all experiments.
Construction of recombinant plasmids.
The pCMV-flag vector (Clontech Laboratories, CA, USA) and p3×FLAG-CMV-14 vector (Sigma-Aldrich Corporation, MO, USA) were used to construct recombinant mammalian cell expression vectors. The cDNA of Sus scrofa hmgb1 (GenBank accession number NM_001004034.1) was amplified by reverse transcription-PCR (RT-PCR) from total cellular RNA isolated from PK-15 cells and subsequently cloned into the pCMV-flag vector to generate pHMGB1. Truncated HMGB1 fragments, including the A box (1 to 84 amino acids [aa]), AB box (1 to 171 aa), and B box-C terminus (B boxCT) (87 to 215 aa), were amplified using the full-length hmgb1 cDNA as the template and cloned into the p3×FLAG-CMV-14 vector. All recombinant plasmids were verified by DNA sequencing. The primers used for plasmid construction are listed in Table S1 in the supplemental material.
RNA interference.
A short hairpin RNA (shRNA) plasmid used in the RNA interference assay was synthesized by GenePharma (Shanghai, China) for the target sequence of Sus scrofa hmgb1 (5′-GGACAAGGCCCGTTATGAAAG-3′) and cloned into pGPU6 (pGPU6-shHMGB1). Vector pGPU6-shNC containing a scrambled sequence, 5′-TTCTCCGAACGTGTCACGT-3′, was used as a negative control.
Reagents and antibodies.
The chemicals ethyl pyruvate (EP) (Sigma-Aldrich), N-acetylcysteine (NAC) (Beyotime, Shanghai, China), and hydrogen peroxide (H2O2) (Sangon Biotech, Shanghai, China) were used at the designated concentrations in appropriate experiments. The primary antibodies used included rabbit anti-HMGB1 monoclonal antibody (ab79823) (Abcam, MA, USA), rabbit anti-HMGB1 polyclonal antibody (chromatin immunoprecipitation [ChIP] grade, ab18256) (Abcam), mouse anti-Cap monoclonal antibody (D11-1, our laboratory storage), rabbit anti-histone H3 polyclonal antibody (ab1791) (Abcam), mouse anti-β-actin monoclonal antibody (Cell Signaling Technology [CST], MA, USA), and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (CST). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG were obtained from Sangon Biotech for immunoblotting. Secondary antibodies for immunofluorescence included fluorescein-labeled goat anti-mouse IgG(H+L) (KPL, MD, USA), and those for confocal imaging were Alexa Fluor 555-labeled donkey anti-mouse IgG(H+L) or Alexa Fluor 488-labeled donkey anti-rabbit IgG(H+L) (Thermo).
Virus infection, plasmid transfection, chemical treatments, and subcellular fractioning.
The PK-15 or PAM cells were grown to approximately 60% to 70% confluence and infected with PCV2. Infection was allowed to proceed at 37°C and 5% CO2 for 2 h, and the supernatants were then removed. The cell monolayers were rinsed with Hanks’ balanced salt solution to remove unattached virus particles and then incubated in the presence of fresh medium containing 2% or 5% FBS at 37°C and 5% CO2 for the designated time points to investigate the effects of PCV2 infection on HMGB1 expression and subcellular partitioning.
To examine the effects of HMGB1 overexpression or downregulation on PCV2 replication, PK-15 cells were transfected with recombinant expression vectors or shRNA plasmids using Lipofectamine 2000 (Thermo) according to the manufacturer’s instruction. After incubation at 37°C and 5% CO2 for 24 h, the cells were infected with PCV2 for 36 h. The same approach was used to examine the effects of different HMGB1 fragments on PCV2 replication using the recombinant vectors described above. To investigate the effects of chemical treatments on HMGB1 compartmentation and/or virus replication, PK-15 or PAM cells were infected with PCV2 for 36 h as described above and cultured at 37°C and 5% CO2 in fresh medium containing ethyl pyruvate (EP; 7.5 mM), N-acetylcysteine (NAC; 10 mM), hydrogen peroxide (H2O2; 50 μM), or glycyrrhizic acid (GA; 0.1 or 0.2 mM) for 24 h. Control cells without chemicals were included. For verification of the effect of elevated ROS on subcellular localization of HMGB1, PK-15 or PAM cells were stimulated by 50 μM H2O2 for 2 h with or without N-acetylcysteine (NAC; 10 mM) pretreatment. For confocal microscopic analysis, cells were grown on 10-mm coverslips in a 29-mm glass-bottom dish (Cellvis, Mountain View, CA, USA) for PCV2 infection and chemical treatments.
The infected and/or treated cells in the above-described experiments were collected at specified time points postinfection for immunoblotting, immunofluorescence, confocal imaging, and quantitative reverse transcription-PCR (qRT-PCR) as described below. Fractions of cytoplasmic and nuclear extracts, where necessary, were prepared using the NE-PER nuclear and cytoplasmic extraction reagents (Thermo) according to the manufacturer’s instructions.
Quantitative reverse transcription-PCR and quantitative PCR.
Total RNA of the samples was prepared with TRIzol reagent (Life Technologies Corporation, CA, USA), and cDNA was synthesized using the ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo Biotech, Osaka, Japan) according to the manufacturer’s instructions. qRT-PCR was conducted using the ChamQ Universal SYBR qPCR master mix (Vazyme Biotech, Nanjing, China) and Mx3005P qPCR system (Agilent Technologies, CA, USA). The primers used are listed in Table S1. The transcript levels were normalized using porcine β-actin or histone H3 as an internal control. Relative expression of mRNA was calculated and analyzed based on the comparative cycle threshold (2−ΔΔCT) method (63).
Quantification of viral DNA was performed as described previously (64). Briefly, PCV2 genomic DNA was extracted using an Ezup column virus DNA purification kit (Sangon Biotech) for quantitative PCR with the PCV2-specific primer pair (Table S1). A reference curve was established by using the recombinant plasmid containing PCV2 orf2 for quantification of viral DNA copies.
Immunoblotting.
Immunoblotting was carried out using the standard protocol as described previously (10). Briefly, cells in culture plates were washed with precooled phosphate-buffered saline (PBS; pH 7.2). Whole-cell lysate was extracted by incubating cells with ice-cold cell lysis buffer (Beyotime) supplemented with protease inhibitor cocktail (Roche, Mannheim, Germany) at 4°C for 30 min and scraping the culture plate. The lysates were centrifuged at 4°C and 14,000 × g to remove the detergent-insoluble cell debris, and the supernatant samples were collected. Total protein concentrations of the lysates were determined using the Bradford protein assay kit (Beyotime). Equivalent amounts of protein samples were loaded and separated on 12% or 15% SDS-PAGE gels. The target proteins in the gels were transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, MA, USA), followed by blocking for 1 h at 37°C with 5% (wt/vol) nonfat milk in TBST buffer (20 mM Tris, 150 mM NaCl, and 0.05% Tween 20, pH 7.6). Membranes were then incubated with different primary antibodies overnight at 4°C. After sufficient washing with PBS containing Tween 20 (PBST), the membranes were incubated with an appropriate secondary antibody conjugated to horseradish peroxidase (HRP) for 1 h at 37°C and then subjected to further washing. Immunoreactive bands on the membranes were visualized by using SuperSignal West Pico chemiluminescent substrate (Thermo) under the conditions recommended by the manufacturer. Images were captured in a Gel 3100 chemiluminescent imaging system (Sagecreation, Beijing, China). The blots were scanned and densitometric analysis was performed by using Gel-Pro Analyzer software (Media Cybernetics, MD, USA). Ratios of the band intensity between target proteins and reference proteins were used for relative quantitative purposes.
Indirect immunofluorescence and confocal microscopy.
PCV2 replication in the cells was quantified by indirect immunofluorescence assay (IFA). The PK-15 or PAM cells infected with PCV2 with or without vector transfection or chemical treatments as described above were washed twice with PBS and fixed with precooled 80% acetone at −20°C for 30 min. Fixed cells were blocked with PBS containing 5% nonfat milk at 37°C for 1 h and then incubated with a mouse anti-Cap monoclonal antibody (1:1,000 dilution) at 37°C for 1 h, followed by incubation with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG at 37°C for 1 h in the dark. After washing three times with PBS, cell images were captured under the IX81 fluorescence microscope (Olympus, Tokyo, Japan). The number of cells positive for PCV2 viral antigens was counted by using ImageJ software (National Institutes of Health, MD, USA). The percentage of PCV2-infected cells was calculated by dividing the numbers of PCV2-infected cells by the total cell numbers in each group (n = 2 images for each experiment per group). The relative percentages of PCV2-infected cells in treated groups are shown, with the group untreated but PCV2 infected set at 100% (N = 6 images/group).
To visualize subcellular localization of HMGB1 by confocal microscopy, cells were first fixed with 4% paraformaldehyde for 20 min at room temperature (25°C) and then permeabilized with 0.2% Triton X-100 in PBS for 15 min. Fixed cells were washed with PBS and blocked with 5% goat serum in PBS for 1 h at 37°C to prevent nonspecific binding. The cells were incubated with corresponding primary antibodies at 37°C for 1 h and stained with Alexa Fluor-conjugated secondary antibodies in the dark at 37°C for 1 h. After extensive washing, cellular nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Beyotime) for 5 min. The cells were washed three times and observed under a confocal laser scanning microscope IX81-FV1000 (Olympus). Cell images were captured using FluoView FV1000 software version 3.1.2.2 (Olympus).
HMGB1-PCV2 DNA binding and gel shift assay.
Porcine full-length HMGB1 protein as aforementioned was produced by using the Bac-to-Bac baculovirus expression system (Thermo) and purified by Ni-nitrilotriacetic acid (NTA) agarose column chromatography (Sangon Biotech). To examine the interaction between HMGB1 and PCV2 genomic DNA in vitro, 0.5 μg of purified PCV2 genomic DNA and various concentrations (0, 1, 2.5, or 5 μg) of the recombinant His-HMGB1 protein were incubated in binding buffer (50 mM Tris-HCl [pH 8.0], 250 mM NaCl, 5.0 mM MgCl2, 2.5 mM DTT [1,4-dithiothreitol], 2.5 mM EDTA, and 20% glycerol) for 20 min at 37°C and 10 min at 4°C. The samples were then mixed with 6× SuperStain loading buffer (CWBIO, Beijing, China) and subjected to electrophoresis on a 0.8% agarose gel. The images were taken under the JS-680B automatic gel imaging analysis system (P&Q S&T, Shanghai, China). The full-length genome of PCV2 was divided into five fragments (Ori, orf1, orf2, Ori-orf1, and Ori-orf2) that were prepared by PCR using primers listed in Table S1 to identify the region of the PCV2 genome that interacts with HMGB1.
Identification of HMGB1-PCV2 DNA binding by immunoprecipitation coupled with quantitative PCR.
PCV2-infected cells were washed with precooled PBS. The whole-cell lysates were extracted using the Western and IP lysis buffer (Beyotime) supplemented with protease inhibitor cocktail (Roche). Nuclear protein extraction was prepared by using NE-PER nuclear and cytoplasmic extraction reagents (Thermo). Protein concentrations in the samples were determined by the Bradford method. Approximately 1 mg of total cellular proteins or nuclear proteins was transferred to a 1.5-ml microcentrifuge tube and incubated with 4 μg of rabbit anti-HMGB1 polyclonal antibody (ChIP grade, ab18256) (Abcam) or normal rabbit IgG (Beyotime) for 2 h at 4°C. Forty microliters of precleaned protein A/G PLUS agarose (Santa Cruz Biotechnology, TX, USA) was then added and subjected to further incubation at 4°C on a rotating device overnight. The immunoprecipitates were collected by centrifugation at 1,000 × g for 5 min at 4°C and washed 5 times with 1 ml PBS. The protein-DNA complexes bound to the beads were eluted in PBS at 70°C for 30 min. The eluted complexes were treated with DNase (Beyotime) at 37°C for 30 min. After digestion, the remaining protein-bound DNA fragments were extracted with the Ezup column virus DNA purification kit (Sangon Biotech) and used as the templates for qPCR using the primers (Table S1) targeting the PCV2 genomic Ori region.
Measurement of cytosolic ROS.
Measurement of cytosolic ROS was performed as described previously (10). In brief, intracellular ROS levels in infected or treated cells were probed using 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) (Sigma-Aldrich). Flow cytometric analysis was performed with a BD FACSCalibur flow cytometer (BD Biosciences, NJ, USA) for measurement of intracellular ROS. Data were analyzed using FlowJo software version X (Tree Star Inc., OR, USA).
Statistical analyses.
Statistical analyses were performed on GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA). All results are presented as means ± standard deviations from three independent experiments. The Student's t test was used for comparisons between two groups. Differences were considered significant with P values of <0.05 and markedly significant with P values of <0.01.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the National Natural Science Foundation of China (31272534) and the Natural Science Foundation of Zhejiang Province (LQ19C180003).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Tischer I, Gelderblom H, Vettermann W, Koch MA. 1982. A very small porcine virus with circular single-stranded DNA. Nature 295:64–66. doi: 10.1038/295064a0. [DOI] [PubMed] [Google Scholar]
- 2.Meng XJ. 2013. Porcine circovirus type 2 (PCV2): pathogenesis and interaction with the immune system. Annu Rev Anim Biosci 1:43–64. doi: 10.1146/annurev-animal-031412-103720. [DOI] [PubMed] [Google Scholar]
- 3.Opriessnig T, Meng XJ, Halbur PG. 2007. Porcine circovirus type 2-associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest 19:591–615. doi: 10.1177/104063870701900601. [DOI] [PubMed] [Google Scholar]
- 4.Gillespie J, Opriessnig T, Meng XJ, Pelzer K, Buechner-Maxwell V. 2009. Porcine circovirus type 2 and porcine circovirus-associated disease. J Vet Intern Med 23:1151–1163. doi: 10.1111/j.1939-1676.2009.0389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mankertz A, Mankertz J, Wolf K, Buhk HJ. 1998. Identification of a protein essential for replication of porcine circovirus. J Gen Virol 79:381–384. doi: 10.1099/0022-1317-79-2-381. [DOI] [PubMed] [Google Scholar]
- 6.Cheung AK. 2012. Porcine circovirus: transcription and DNA replication. Virus Res 164:46–53. doi: 10.1016/j.virusres.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Nawagitgul P, Morozov I, Bolin SR, Harms FA, Sorden SD, Paul PS. 2000. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J Gen Virol 81:2281–2287. doi: 10.1099/0022-1317-81-9-2281. [DOI] [PubMed] [Google Scholar]
- 8.Trible BR, Suddith AW, Kerrigan MA, Cino-Ozuna AG, Hesse RA, Rowland RR. 2012. Recognition of the different structural forms of the capsid protein determines the outcome following infection with porcine circovirus type 2. J Virol 86:13508–13514. doi: 10.1128/JVI.01763-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Chen I, Kwang J. 2005. Characterization of a previously unidentified viral protein in porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J Virol 79:8262–8274. doi: 10.1128/JVI.79.13.8262-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YK, Sun RJ, Geng SC, Shan Y, Li XL, Fang WH. 2019. Porcine circovirus type 2 induces ORF3-independent mitochondrial apoptosis via PERK activation and elevation of cytosolic calcium. J Virol 93:e01784-18. doi: 10.1128/JVI.01784-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He JL, Cao JJ, Zhou N, Jin YL, Wu JS, Zhou JY. 2013. Identification and functional analysis of the novel ORF4 protein encoded by porcine circovirus type 2. J Virol 87:1420–1429. doi: 10.1128/JVI.01443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv QZ, Guo KK, Xu H, Wang T, Zhang YM. 2015. Identification of putative ORF5 protein of porcine circovirus type 2 and functional analysis of GFP-fused ORF5 protein. PLoS One 10:e0127859. doi: 10.1371/journal.pone.0127859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo KK, Xu L, Wu MM, Hou YF, Jiang YF, Lv JM, Xu PP, Fan ZX, Zhang RQ, Xing FS, Zhang YM. 2018. A host factor GPNMB restricts porcine circovirus type 2 (PCV2) replication and interacts with PCV2 ORF5 protein. Front Microbiol 9:3295. doi: 10.3389/fmicb.2018.03295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi CY, Choi YC, Park IB, Lee CH, Kang SJ, Chun T. 2018. The ORF5 protein of porcine circovirus type 2 enhances viral replication by dampening type I interferon expression in porcine epithelial cells. Vet Microbiol 226:50–58. doi: 10.1016/j.vetmic.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Zhang XL, Ma C, You JW, Dong M, Yun SF, Jiang P. 2016. Heat shock protein 90 is essential for replication of porcine circovirus type 2 in PK-15 cells. Virus Res 224:29–37. doi: 10.1016/j.virusres.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Finsterbusch T, Mankertz A. 2009. Porcine circoviruses–small but powerful. Virus Res 143:177–183. doi: 10.1016/j.virusres.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Costello E, Saudan P, Winocour E, Pizer L, Beard P. 1997. High mobility group chromosomal protein 1 binds to the adeno-associated virus replication protein (Rep) and promotes Rep-mediated site-specific cleavage of DNA, ATPase activity and transcriptional repression. EMBO J 16:5943–5954. doi: 10.1093/emboj/16.19.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotmore SF, Tattersall P. 1998. High-mobility group 1/2 proteins are essential for initiating rolling-circle-type DNA replication at a parvovirus hairpin origin. J Virol 72:8477–8484. doi: 10.1128/JVI.72.11.8477-8484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hindmarsh P, Ridky T, Reeves R, Andrake M, Skalka AM, Leis J. 1999. HMG protein family members stimulate human immunodeficiency virus type 1 and avian sarcoma virus concerted DNA integration in vitro. J Virol 73:2994–3003. doi: 10.1128/JVI.73.4.2994-3003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajino K, Yamamoto T, Hayashi J, Umeda T, Takahara T, Hino O. 2001. Recombination hot spot of hepatitis B virus genome binds to members of the HMG domain protein family and the Y box binding protein family; implication of these proteins in genomic instability. Intervirology 44:311–316. doi: 10.1159/000050063. [DOI] [PubMed] [Google Scholar]
- 21.Naghavi MH, Nowak P, Andersson J, Sonnerborg A, Yang H, Tracey KJ, Vahlne A. 2003. Intracellular high mobility group B1 protein (HMGB1) represses HIV-1 LTR-directed transcription in a promoter- and cell-specific manner. Virology 314:179–189. doi: 10.1016/S0042-6822(03)00453-7. [DOI] [PubMed] [Google Scholar]
- 22.Lotze MT, Tracey KJ. 2005. High-mobility group box 1 protein (HMGB): nuclear weapon in the immune arsenal. Nat Rev Immunol 5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi ME, Falciola L, Ferrari S, Lilley D. 1992. The DNA-binding site of Hmg1 protein Is composed of 2 similar segments (Hmg boxes), both of which have counterparts in other eukaryotic regulatory proteins. EMBO J 11:1055–1063. doi: 10.1002/j.1460-2075.1992.tb05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardman CH, Broadhurst RW, Raine ARC, Grasser KD, Thomas JO, Laue ED. 1995. Structure of the A-domain of HMG1 and its interaction with DNA as studied by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry 34:16596–16607. doi: 10.1021/bi00051a007. [DOI] [PubMed] [Google Scholar]
- 25.Stott K, Tang GSF, Lee KB, Thomas JO. 2006. Structure of a complex of tandem HMG boxes and DNA. J Mol Biol 360:90–104. doi: 10.1016/j.jmb.2006.04.059. [DOI] [PubMed] [Google Scholar]
- 26.Weir HM, Kraulis PJ, Hill CS, Raine ARC, Laue ED, Thomas JO. 1993. Structure of the Hmg box motif in the B-domain of Hmg1. EMBO J 12:1311–1319. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange SS, Vasquez KM. 2009. HMGB1: the jack-of-all-trades protein is a master DNA repair mechanic. Mol Carcinog 48:571–580. doi: 10.1002/mc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianchi ME, Beltrame M, Paonessa G. 1989. Specific recognition of cruciform DNA by nuclear-protein Hmg1. Science 243:1056–1059. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- 29.Webb M, Payet D, Lee KB, Travers AA, Thomas JO. 2001. Structural requirements for cooperative binding of HMG1 to DNA minicircles. J Mol Biol 309:79–88. doi: 10.1006/jmbi.2001.4667. [DOI] [PubMed] [Google Scholar]
- 30.Kang R, Chen RC, Zhang QH, Hou W, Wu S, Cao LZ, Huang J, Yu Y, Fan XG, Yan ZW, Sun XF, Wang HC, Wang QD, Tsung A, Billiar TR, Zeh HJ, Lotze MT, Tang DL. 2014. HMGB1 in health and disease. Mol Aspects Med 40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang DL, Shi YZ, Kang R, Li T, Xiao WM, Wang HC, Xiao XZ. 2007. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol 81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoppe G, Talcott KE, Bhattacharya SK, Crabb JW, Sears JE. 2006. Molecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1. Exp Cell Res 312:3526–3538. doi: 10.1016/j.yexcr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Tang DL, Kang R, Zeh HJ, Lotze MT. 2011. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal 14:1315–1335. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang DL, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, Lotze MT. 2010. Endogenous HMGB1 regulates autophagy. J Cell Biol 190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ugrinova I, Pasheva E. 2017. HMGB1 protein: a therapeutic target inside and outside the cell. Adv Protein Chem Struct Biol 107:37–76. doi: 10.1016/bs.apcsb.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Duan EZ, Wang D, Luo R, Luo JY, Gao L, Chen HC, Fang LR, Xiao SB. 2014. Porcine reproductive and respiratory syndrome virus infection triggers HMGB1 release to promote inflammatory cytokine production. Virology 468–470:1–9. doi: 10.1016/j.virol.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 37.Qu YR, Zhan Y, Yang S, Ren SH, Qiu XS, Rehamn ZU, Tan L, Sun YJ, Meng CC, Song CP, Yu SQ, Ding C. 2018. Newcastle disease virus infection triggers HMGB1 release to promote the inflammatory response. Virology 525:19–31. doi: 10.1016/j.virol.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Jung JH, Park JH, Jee MH, Keum SJ, Cho MS, Yoon SK, Jang SK. 2011. Hepatitis C virus infection is blocked by HMGB1 released from virus-infected cells. J Virol 85:9359–9368. doi: 10.1128/JVI.00682-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu R, Yang DR, Lei SH, Wang XH, Meng XH, Xue BB, Zhu HZ. 2015. HMGB1 promotes hepatitis C virus replication by interaction with stem-loop 4 in the viral 5′ untranslated region. J Virol 90:2332–2344. doi: 10.1128/JVI.02795-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moisy D, Avilov SV, Jacob Y, Laoide BM, Ge XY, Baudin F, Naffakh N, Jestin JL. 2012. HMGB1 protein binds to influenza virus nucleoprotein and promotes viral replication. J Virol 86:9122–9133. doi: 10.1128/JVI.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosakote YM, Brasier AR, Casola A, Garofalo RP, Kurosky A. 2016. Respiratory syncytial virus infection triggers epithelial HMGB1 release as a damage-associated molecular pattern promoting a monocytic inflammatory response. J Virol 90:9618–9631. doi: 10.1128/JVI.01279-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barqasho B, Nowak P, Abdurahman S, Walther-Jallow L, Sönnerborg A. 2010. Implications of the release of high-mobility group box 1 protein from dying cells during human immunodeficiency virus type 1 infection in vitro. J Gen Virol 91:1800–1809. doi: 10.1099/vir.0.016915-0. [DOI] [PubMed] [Google Scholar]
- 43.Cao JJ, Lin C, Wang HJ, Wang L, Zhou N, Jin YL, Liao M, Zhou JY. 2015. Circovirus transport proceeds via direct interaction of the cytoplasmic dynein IC1 subunit with the viral capsid protein. J Virol 89:2777–2791. doi: 10.1128/JVI.03117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung AK, Bolin SR. 2002. Kinetics of porcine circovirus type 2 replication. Arch Virol 147:43–58. doi: 10.1007/s705-002-8302-4. [DOI] [PubMed] [Google Scholar]
- 45.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. 2002. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A 99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fink MP. 2007. Ethyl pyruvate: a novel treatment for sepsis. Curr Drug Targets 8:515–518. doi: 10.2174/138945007780362791. [DOI] [PubMed] [Google Scholar]
- 47.Dave SH, Tilstra JS, Matsuoka K, Li FL, DeMarco RA, Beer-Stolz D, Sepulveda AR, Fink MP, Lotze MT, Plevy SE. 2009. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol 86:633–643. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen XX, Ren F, Hesketh J, Shi XL, Li JX, Gan FH, Hu ZH, Huang KH. 2013. Interaction of porcine circovirus type 2 replication with intracellular redox status in vitro. Redox Rep 18:186–192. doi: 10.1179/1351000213Y.0000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu BL, Zhou YS, Xu F, Shuai JB, Li XL, Fang WH. 2012. Porcine circovirus type 2 induces autophagy via the AMPK/ERK/TSC2/mTOR signaling pathway in PK-15 Cells. J Virol 86:12003–12012. doi: 10.1128/JVI.01434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou YS, Qi BZ, Gu YX, Xu F, Du HH, Li XL, Fang WH. 2016. Porcine circovirus 2 deploys PERK pathway and GRP78 for its enhanced replication in PK-15 cells. Viruses 8:56. doi: 10.3390/v8020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook KC, Cristea IM. 2019. Location is everything: protein translocations as a viral infection strategy. Curr Opin Chem Biol 48:34–43. doi: 10.1016/j.cbpa.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen S, Dong Z, Yang P, Wang X, Jin G, Yu H, Chen L, Li L, Tang L, Bai S, Yan H, Shen F, Cong W, Wen W, Wang H. 2017. Hepatitis B virus X protein stimulates high mobility group box 1 secretion and enhances hepatocellular carcinoma metastasis. Cancer Lett 394:22–32. doi: 10.1016/j.canlet.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Yang H, Antoine DJ, Andersson U, Tracey KJ. 2013. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol 93:865–873. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. 2003. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu B, Antoine DJ, Kwan K, Lundback P, Wahamaa H, Schierbeck H, Robinson M, Van Zoelen MA, Yang H, Li J, Erlandsson-Harris H, Chavan SS, Wang H, Andersson U, Tracey KJ. 2014. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci U S A 111:3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Youn JH, Shin JS. 2006. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol 177:7889–7897. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- 57.Kwak MS, Kim HS, Lkhamsuren K, Kim YH, Han MG, Shin JM, Park IH, Rhee WJ, Lee SK, Rhee SG, Shin JS. 2019. Peroxiredoxin-mediated disulfide bond formation is required for nucleocytoplasmic translocation and secretion of HMGB1 in response to inflammatory stimuli. Redox Biol 24:101203. doi: 10.1016/j.redox.2019.101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang YT, Zhao X, Antoine D, Xiao XZ, Wang HC, Andersson U, Billiar TR, Tracey KJ, Lu B. 2016. Regulation of posttranslational modifications of HMGB1 during immune responses. Antioxid Redox Signal 24:620–634. doi: 10.1089/ars.2015.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang HC, Ward MF, Fan XG, Sama AE, Li W. 2006. Potential role of high mobility group box 1 in viral infectious diseases. Viral Immunol 19:3–9. doi: 10.1089/vim.2006.19.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Musumeci D, Roviello GN, Montesarchio D. 2014. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther 141:347–357. doi: 10.1016/j.pharmthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Zeeshan HMA, Lee GH, Kim HR, Chae HJ. 2016. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci 17:327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wible DJ, Bratton SB. 2018. Reciprocity in ROS and autophagic signaling. Curr Opin Toxicol 7:28–36. doi: 10.1016/j.cotox.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 64.Zhu BL, Xu F, Li J, Shuai JB, Li XL, Fang WH. 2012. Porcine circovirus type 2 explores the autophagic machinery for replication in PK-15 cells. Virus Res 163:476–485. doi: 10.1016/j.virusres.2011.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.