Influenza viruses are responsible for up to 650,000 deaths per year through seasonal epidemics, and pandemics have caused tens of millions of deaths in the past. Most current therapeutics suffer from widespread resistance, creating a need for new drug targets against influenza virus. The virus encodes an RNA-dependent RNA polymerase, which replicates and transcribes the vRNA genome. The polymerase interacts with vRNA and the complementary replicative intermediate cRNA using several specific binding sites; however, the functions associated with these binding sites remain unknown. Here, we functionally characterize a binding site for the 3′ vRNA and cRNA promoters. Our data offer insight into the mechanism of viral genome transcription by the influenza virus polymerase and may be applicable to other related viruses.
KEYWORDS: RNA polymerases, influenza, promoters, transcription
ABSTRACT
Influenza viruses encode a viral RNA-dependent RNA polymerase (FluPol), which is responsible for transcribing and replicating the negative-sense viral RNA (vRNA) genome. FluPol transcribes vRNA using a host-capped mRNA primer and replicates it by synthesizing a positive-sense cRNA intermediate, which is copied back into vRNA. To carry out these functions, FluPol interacts with vRNA and cRNA using conserved promoter elements at the 5′ and 3′ termini. Recent structural studies have identified a new surface binding site for the 3′ vRNA and cRNA promoters on FluPol, referred to as the mode B site. However, the role of this binding site in FluPol function is unknown. In this study, we used a combination of cell-based and biochemical assays to show that the mode B site is important for both viral genome transcription and replication in influenza A virus. Furthermore, we show that the mode B site is not needed for initiating transcription in vitro but is required to synthesize a full-length product. This is consistent with a model in which the 3′ terminus of the vRNA template binds in the mode B site during elongation. Our data provide the first functional insights into the role of the mode B site on FluPol, which advances our understanding of FluPol function and influenza virus replication.
IMPORTANCE Influenza viruses are responsible for up to 650,000 deaths per year through seasonal epidemics, and pandemics have caused tens of millions of deaths in the past. Most current therapeutics suffer from widespread resistance, creating a need for new drug targets against influenza virus. The virus encodes an RNA-dependent RNA polymerase, which replicates and transcribes the vRNA genome. The polymerase interacts with vRNA and the complementary replicative intermediate cRNA using several specific binding sites; however, the functions associated with these binding sites remain unknown. Here, we functionally characterize a binding site for the 3′ vRNA and cRNA promoters. Our data offer insight into the mechanism of viral genome transcription by the influenza virus polymerase and may be applicable to other related viruses.
INTRODUCTION
Negative-strand RNA viruses cause several important human diseases, such as Ebola, influenza, and rabies. These viruses encode multifunctional RNA polymerases, which are responsible for both transcribing and replicating their viral RNA (vRNA) genomes. In order to carry out these processes, viral polymerases must bind to the vRNA genome (1). Recently, structures of La Crosse and influenza virus RNA polymerases bound to vRNA have become available; however, we still know relatively little about how these polymerases interact with vRNA during their transcription and replication processes (2–6).
The best studied negative-strand RNA virus polymerases are those from influenza viruses. Influenza viruses have negative-sense RNA genomes divided into 7 to 8 nonidentical segments, which each consist of an open reading frame for one or more proteins. These are flanked by noncoding regions, which include conserved 5′ and 3′ promoter elements of 13 and 12 nucleotides in length, respectively. Three of the viral genome segments encode polymerase basic 1 (PB1), polymerase basic 2 (PB2) and polymerase acidic (PA), which are subunits of the viral RNA-dependent RNA polymerase (FluPol). FluPol associates with negative-sense vRNA by binding to the 5′ and 3′ vRNA promoters. The circularized vRNA is also bound by nucleoprotein (NP), forming a viral ribonucleoprotein (vRNP) complex. Influenza vRNPs are released into the host cell upon infection and traffic to the nucleus (7). In the context of a vRNP, FluPol transcribes vRNA to produce viral mRNA early in infection. FluPol initiates viral genome transcription using fragments of host-capped mRNAs as primers, through a process known as cap snatching. This produces a capped viral mRNA transcript, which is then polyadenylated at the 3′ end by polymerase stuttering (8). In addition to transcription, FluPol carries out viral genome replication to produce vRNA. Viral genome replication is a two-step process and predominates over transcription later in the course of an infection. In the first step of replication, vRNA is used as a template to synthesize positive-sense cRNA. Similar to vRNA, cRNA is bound by FluPol and NP to form a complementary ribonucleoprotein (cRNP). FluPol then copies cRNA back into vRNA with the 5′ and 3′ terminal cRNA sequences acting as the cRNA promoter. Both steps of viral genome replication are initiated by FluPol in a primer-independent manner, though the initiation mechanism for vRNA synthesis differs from cRNA synthesis and requires FluPol dimerization (6, 7).
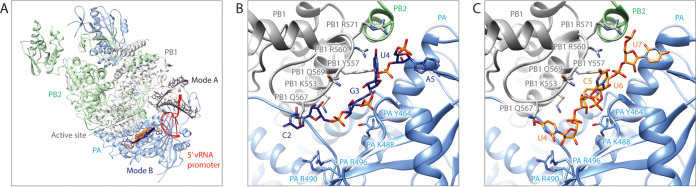
FluPol has specific binding sites for the 5′ and 3′ vRNA and cRNA promoters, which have been identified by crystallography and cryo-electron microscopy (cryo-EM) studies. The 5′ vRNA and cRNA promoters both form similar hook structures, which bind with high affinity in a single pocket formed by the PB1 and PA subunits (2, 6, 9). Conversely, the 3′ vRNA promoter has three distinct binding sites. The 3′ vRNA promoter can bind in the polymerase active site, where it acts as a template for RNA synthesis, or in one of two sites on the surface of FluPol (4, 6, 10) (Fig. 1A). The first of these surface binding sites to be observed was the “mode A site” for the influenza B virus FluPol (FluPolB), in which the 3′ vRNA promoter is located close to the entrance to the active site (4). More recently, another 3′ vRNA binding site termed the “mode B site” has been structurally characterized for FluPol (5, 6) (Fig. 1B). The mode B site is located in a groove further away from the polymerase active site entrance, with all three FluPol subunits contributing to it, and is similar to 3′ vRNA promoter binding sites present in the related La Crosse orthobunyavirus and Machupo mammarenavirus polymerases (3, 11). Structural studies have observed that the 3′ cRNA promoter can also bind in the mode B site (5, 6) (Fig. 1C).
FIG 1.
RNA binding sites of FluPolA. (A) Locations of RNA binding sites modeled on FluPolA (PDB accession number 6QNW). The 5′ vRNA promoter binds in a single site (red) (PDB accession number 4WRT), while the 3′ vRNA promoter can occupy the mode A site (gray) (PDB accession number 4WRT) or the mode B site (navy) (PDB accession number 6KUR). The 3′ cRNA promoter can also occupy the mode B site (gold) (PDB accession number 6QX3). (B) Close-up view of the 3′ vRNA promoter bound in the mode B site of FluPolA, with key interacting residues on the PB1 and PA subunits visible. The 3′ vRNA promoter residues C2 to A5 (navy) (PDB accession number 6KUR) are overlaid on FluPolA (PDB accession number 6QX3). (C) Close-up view of 3′ cRNA promoter residues U4 to U7 (gold) bound in the mode B site of FluPolA, with key interacting residues on the PB1 and PA subunits visible (PDB accession number 6QX3).
Despite detailed structural data describing 3′ vRNA and cRNA promoter binding in the mode B site, no functional analysis has been able to identify a role for this site. Here, we aimed to functionally characterize the mode B site for the influenza A virus FluPol (FluPolA) using a combination of cell-based and biochemical assays. We show that promoter binding in the mode B site is important for both replication and transcription of the viral genome and identify that mutating the mode B site inhibits transcription elongation. These data support a model in which the 3′ vRNA promoter binds in the mode B site during transcription elongation. Our results provide the first functional insights into the role of the FluPolA mode B promoter binding site and may be widely applicable to other negative-sense RNA viruses with similar 3′ RNA promoter binding sites.
RESULTS
Mutagenesis of the mode B 3′ RNA promoter binding site.
In order to study the function of 3′ RNA promoter binding in the mode B site, we mutated key residues identified in a structure of FluPolA in complex with 3′ cRNA promoter (6) (Fig. 1B and C). We targeted clusters of residues on the PB1 and PA subunits, as these interact most extensively with the 3′ cRNA promoter in the mode B site, and tested these mutants in FluPolA minigenome assays (Fig. 2). We did not observe a strong inhibition of FluPolA activity with any of the single alanine substitutions. We hypothesized that this could be because the mode B site is an extended groove involving many contacts between the 3′ promoter and FluPolA, some of which may be redundant. In order to more effectively disrupt the mode B site, we created three FluPolA constructs with multiple point mutations, each targeting different regions of the mode B site. Of these mutations, PB1K553A/Y557A/R560A and PAY464A/K488A/R490A/R496A significantly inhibited mRNA, cRNA, and vRNA accumulation by FluPolA in minigenome assays. Interestingly, the PB1Q567A/Q569A/R571A mutant had reduced mRNA synthesis but not vRNA or cRNA accumulation, indicating that these mutations specifically affect viral genome transcription.
FIG 2.

Mutagenesis of the mode B site in FluPolA. The effect of single and multiple mode B site mutations on FluPolA activity was determined using minigenome assays. Primer extension gels are shown (top) with quantitation from n = 3 independent transfections (middle). Western blotting was carried out to confirm the expression of mutant FluPolA PB1 and PA subunits (bottom). Data are mean ± standard error of the mean (SEM), analyzed by one-way analysis of variance (ANOVA). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Mode B site mutations affect both viral genome transcription and replication.
Next, we wanted to determine if these mutations inhibit the ability of FluPolA to carry out viral genome replication, transcription, or both. For this purpose, we took advantage of a previously described complementation assay which uses transcription-deficient FluPolA mutants (12). FluPolA synthesizes mRNA using vRNA as a template, so if vRNA accumulation is inhibited, then mRNA synthesis is always decreased, independently of whether FluPolA transcription is also inhibited. To overcome this limitation, we expressed transcription-deficient mutant FluPolA, which is able to support vRNA accumulation and then coexpressed each mode B site FluPolA mutant (Fig. 3). We used PB1K669A/R670A as the transcription-deficient FluPolA mutant for complementing PB1 mutants and PAD108A for complementing the PA mutant (13, 14). PB1K669A/R670A and PAD108A FluPolA mutants are unable to efficiently synthesize mRNA, so the mRNA signal observed when we coexpress mode B site FluPolA mutants is the result of mRNA synthesis by those mutants. When we coexpressed the PB1K553A/Y557A/R560A or the PB1Q567A/Q569A/R571A mutant with PB1K669A/R670A, we observed only weak mRNA signals. This indicates that the PB1K553A/Y557A/R560A and PB1Q567A/Q569A/R571A mutations inhibit viral genome transcription. However, when we coexpressed PAY464A/K488A/R490A/R496A with PAD108A, we observed a robust mRNA signal, indicating that the PAY464A/K488A/R490A/R496A mutations affect viral genome replication but not transcription. Collectively, these data show that mutations in the mode B site affect both viral genome transcription and replication.
FIG 3.

The mode B site is important for both viral genome transcription and replication. Transcription activity of mode B site FluPolA mutants was determined by complementation with transcription-deficient FluPolA PB1 and PA subunits in minigenome assays. Primer extension gels (top) and quantitation from n = 3 independent transfections (bottom) are shown. Data are mean ± SEM, analyzed by one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001. mRNA signals were compared by two-tailed unpaired t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Mode B site mutations disrupt 3′ vRNA and cRNA promoter binding.
To confirm that the mode B site mutations affect 3′ RNA promoter binding, we overexpressed affinity-tagged FluPolA with PB1K553A/Y557A/R560A, PB1Q567A/Q569A/R571A, or PAY464A/K488A/R490A/R496A mutations in HEK 293T cells and purified the recombinant FluPolA (Fig. 4A). We then incubated the purified FluPolA with radiolabeled 3′ vRNA or cRNA promoter in the presence of the corresponding 5′ RNA promoter, cross-linked the promoter RNAs to FluPolA, and determined the quantity of radiolabeled 3′ RNA promoter bound by SDS-PAGE and phosphorimaging (Fig. 4B). All of the mode B site mutations significantly reduced FluPolA binding to radiolabeled 3′ vRNA and cRNA promoters, with PB1Q567A/Q569A/R571A and PAY464A/K488A/R490A/R496A having the strongest effect. These results confirm that the mutations in the mode B site reduce the ability of FluPolA to bind to both the 3′ vRNA and 3′ cRNA promoters.
FIG 4.

Mode B site mutations reduce 3′ vRNA and cRNA promoter binding by FluPolA. (A) Mode B site FluPolA mutants were purified from HEK 293T cells and run on SDS-PAGE; size markers and FluPolA subunit bands are labeled. An unknown band, possibly Hsp90, is labeled with an asterisk (18). Note that the PB1Q567A/Q569A/R571A mutant FluPolA subunit migrated slightly faster than wild type, possibly due to the mutations introduced. (B) 3′ vRNA and cRNA promoter binding was determined by cross-linking radiolabeled 3′ RNA promoter to FluPolA and running SDS-PAGE followed by phosphorimaging (top). 3′ viral RNA promoters cross-link to multiple FluPolA subunits, so cross-linked products migrate as multiple bands. Quantitation is from n = 3 independent replicates (bottom), each with different recombinant FluPolA preparations. Data are mean ± SEM analyzed by one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Mode B site mutations in vRNPs inhibit transcription elongation.
To gain further insight into the role of the mode B site, we took advantage of the transcription-specific phenotype of the PB1Q567A/Q569A/R571A FluPolA mutant. These mutations do not affect viral genome replication, so PB1Q567A/Q569A/R571A mutant RNPs accumulate efficiently in cells and can be purified. We overexpressed affinity-tagged FluPolA with NP and a vRNA template in HEK 293T cells and then purified wild-type and PB1Q567A/Q569A/R571A mutant RNPs (Fig. 5A). We then tested the in vitro transcription activity of purified vRNPs by incubating them with a 5′ capped RNA primer in the presence or absence of nucleoside triphosphates (NTPs) and analyzed the reaction products by primer extension. Using a radiolabeled primer specific for the 5′ end of the mRNA product (NA 160*), we detected equal levels of mRNA product synthesized by wild-type and PB1Q567A/Q569A/R571A mutant vRNPs in vitro (Fig. 5B and C). We also performed reverse transcription and quantitative PCR (qPCR) of the reaction products using the same primer without a radiolabel (NA 160), which produced similar results to the primer extension assay (Fig. 5B and D).
FIG 5.

Mode B site mutations inhibit transcription elongation by vRNPs in vitro. (A) Mode B site mutant RNPs were purified from HEK 293T cells and run on SDS-PAGE; size markers and protein bands are labeled. (B) Schematic of the viral mRNA product with annealing positions of the NA 160 (top and middle) and NA 1394 primers (bottom), not to scale. An asterisk denotes the 32P labeling used in the primer extension assay (top), and positions of the reverse qPCR primers are shown (middle and bottom). (C) Recombinant RNP preparations were used for capped RNA-primed in vitro transcription assays, in presence or absence of NTPs. mRNA products were visualized by primer extension with the NA 160* primer. (D) In vitro transcription products were analyzed by reverse transcription with the NA 160 or NA 1394 primers followed by qPCR. RNP preparation concentrations were adjusted to normalize input vRNA levels. Quantitation of mRNA signal is from n = 3 to 4 independent reactions, using 2 different recombinant RNP preparations. Data are mean ± SEM, analyzed by one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Individual mRNA signals were compared by two-tailed unpaired t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To examine the ability of the PB1Q567A/Q569A/R571A mutant vRNPs to synthesize full-length mRNA, we then reverse-transcribed reaction products and performed qPCR using a primer directed against the 3′ end of the mRNA product (NA 1394). In contrast to results using the NA 160 primer, we found that PB1Q567A/Q569A/R571A mutant vRNPs produced significantly reduced full-length mRNA signal compared to that of the wild type (Fig. 5B and D). Collectively, these data indicate that the mode B site is not required for vRNPs to initiate mRNA synthesis; however, it is required for complete elongation of the mRNA product.
DISCUSSION
FluPol has multiple surface binding sites for the 3′ vRNA promoter; however, the functions associated with these sites are unknown. Here, we perform the first functional characterization of the recently identified mode B site. We find that the mode B site is necessary for both viral genome replication and transcription and show that while it is dispensable for initiating viral genome transcription, the mode B site is required to produce a full-length transcript.
These data support a model in which vRNPs initiate transcription independently of the mode B site (Fig. 6A), and then the 3′ vRNA promoter binds in the mode B site after it has passed through the active site as has been suggested previously (6) (Fig. 6B and C). Keeping the 3′ end of the vRNA template bound to FluPol throughout transcription would help to maintain the structural integrity of the vRNP, which may be required for FluPol to move along the vRNA template. This is consistent with previous studies, which have shown that FluPol is dependent on NP as an elongation factor to transcribe full-length vRNA templates, such as those present in vRNPs (15). In addition, recent cryo-EM data suggest that the double-helical vRNP arrangement is maintained during transcription elongation (16). Maintaining the structure of the vRNP during transcription would presumably be necessary for a single vRNP to be capable of subsequent rounds of viral genome transcription or for viral genome replication later in the course of an infection.
FIG 6.

Model for the function of the mode B site during viral genome transcription. (A) vRNPs initiate transcription of the viral genome using a capped mRNA primer, derived from host mRNAs by the cap-snatching function of FluPol. (B) FluPol extends the nascent capped mRNA using vRNA as a template. After it is copied, the 3′ end of the vRNA template exits the FluPol active site. (C) The 3′ end of the vRNA template binds in the mode B site, where it remains during transcription elongation.
It is likely that the mode B site has a similar role in viral genome replication as it does in transcription; however, our data do not rule out the possibility of additional functions. For example, previous studies have speculated that the 3′ cRNA promoter may bind in the mode B site during the initiation of vRNA synthesis (5, 6). Furthermore, the mode B site is located at a FluPolA dimer interface, raising the possibility that dimerization may have some interplay with 3′ cRNA promoter binding during viral genome replication (6). Interestingly, by mutating different clusters of residues in the mode B site, we observed different phenotypes; specifically, the PB1Q567A/Q569A/R571A mutations only affect viral genome transcription while the PB1K553A/Y557A/R560A mutations affect viral genome transcription and replication. However, the activity of mode B site FluPolA mutants did not correlate with 3′ vRNA or cRNA promoter binding, which could be due to other sites contributing to the total 3′ vRNA or cRNA promoter binding observed. Although the mode B site appears to be important for both transcription and replication by FluPolA, these data would also be consistent with the two processes having differences that result in dependence on different amino acid residues in the mode B site or require different affinities for the 3′ vRNA or cRNA promoter.
Our results provide the first insights into the function of a FluPolA 3′ RNA promoter binding site by showing that the mode B site is required for both viral genome transcription and replication. More detailed functional analyses indicate that the mode B site is important for transcription elongation by vRNPs, which improves mechanistic understanding of FluPolA and influenza A virus replication. Our findings may be further applicable to other influenza viruses and negative-strand RNA viruses with similar RNA polymerases.
MATERIALS AND METHODS
Cells and plasmids.
Human embryonic kidney (HEK) 293T cells were grown in Dulbecco’s modified Eagle medium (DMEM) plus 10% fetal calf serum (FCS). Plasmids pcDNA-PB1, pcDNA-PB1K669A/R670A, pcDNA-PB2, pcDNA-PB2-TAP (tandem affinity purification), pcDNA-PA, pcDNA-PAD108A, pcDNA-NP, and pPOLI-NA were derived from influenza A/WSN/33 virus and have been described previously (13, 14, 17–19). All mutations used in this study were generated by site-directed PCR mutagenesis from pcDNA plasmids and validated by Sanger sequencing.
FluPolA minigenome assay.
Approximately 0.2 × 106 HEK 293T cells in DMEM plus 10% FCS were transfected with 200 ng pcDNA-PB1, pcDNA-PB2, pcDNA-PA, pcDNA-NP, and pPOLI-NA plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Where indicated, the wild-type pcDNA plasmid was substituted for a mutant pcDNA plasmid. For complementation with transcription-deficient mutants, equal amounts of the mutant plasmid and pcDNA-PB1K669A/R670A or pcDNA-PAD108A were transfected. Twenty hours posttransfection, total cellular RNA was extracted using TRI Reagent (Sigma), and primer extension analysis was carried out.
Primer extension.
Primer extension analysis was carried out as described previously (20). Briefly, RNA was reverse transcribed with SuperScript III reverse transcriptase (Invitrogen) using 32P-labeled primers against positive-sense mRNA and cRNA and negative-sense vRNA, with a primer against cellular 5S rRNA included as a loading control. Products were separated by 6% denaturing PAGE with 7 M urea and visualized by phosphorimaging on an FLA-5000 scanner (Fuji). RNA signals were quantified using ImageJ and normalized to the 5S rRNA loading control. Input vRNA signal, estimated from the “-PB2” control, was subtracted from all subsequent lanes. Data were analyzed using Prism 8 (GraphPad).
Western blotting.
Western blotting was carried out using specific polyclonal antibodies produced in rabbits. Commercially available antibodies were used to blot PB1 (GeneTex) and actin (Sigma), and a custom-made antibody was used to blot PA (21). Goat anti-rabbit antibody conjugated to horseradish peroxidase (HRP) was used in all cases as a secondary antibody, and detection was carried out using Amersham ECL Western blotting detection reagents (GE).
Recombinant FluPolA purification.
Approximately 4 × 106 HEK 293T cells in DMEM plus 10% FCS were transfected with 3 μg pcDNA-PB1, pcDNA-PB2-TAP, and pcDNA-PA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Where indicated, the wild-type pcDNA plasmid was substituted for a mutant pcDNA plasmid. For vRNP preparations, pcDNA-NP and pPOLI-NA were also transfected. Forty-eight hours posttransfection, cells were lysed in 500 μl of lysis buffer (50 mM Tris-HCl, pH 8.0, 200 mM NaCl, 25% glycerol, 0.5% IGEPAL CA-630, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 1× protease inhibitor cocktail tablet [Sigma]), and the lysate was cleared by centrifugation at 17,000 × g for 5 min at 4°C. The supernatant was diluted in 2 ml 150 mM NaCl and incubated with washed IgG Sepharose beads (GE), which bind to the two protein A domains in PB2-TAP. Beads were washed 2× in wash buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 10% glycerol, 0.1% IGEPAL CA-630, 1 mM PMSF) and 1× in cleavage buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 40% glycerol, 0.1% IGEPAL CA-630, 1 mM DTT, 1 mM PMSF) before overnight cleavage at 4°C with AcTEV protease in 200 μl cleavage buffer. Cleavage of PB2-TAP removes the protein A domains, leaving a calmodulin-binding domain (CBD). Beads were cleared by centrifugation at 17,000 × g for 1 min and elutants were analyzed by SDS-PAGE and silver staining using SilverXpress (Invitrogen).
RNA cross-linking assay.
Purified FluPolA preparations in cleavage buffer (see above) were incubated for 10 min at 30°C with 1 mM DTT, 2 U/μl RNasin (Promega), 0.5 μM vRNA (5′-AGUAGAAACAAGGCC-3′) or cRNA (5′-AGCAAAAGCAGGCC-3′) 5′ RNA promoter, and 5 × 104 cpm 32P-labeled vRNA (5′-GGCCUGCUUUUGCU-3′) or cRNA (5′-GGCCUUGUUUCUACU-3′) 3′ RNA promoter in a total volume of 10 μl. The mixtures were then irradiated with 254 nm UV light in a UV Stratalinker (Stratagene) for 10 min in a 96-well plate and separated by 8% SDS-PAGE. Cross-linked complexes were visualized by phosphorimaging on an FLA-5000 scanner (Fuji), and images were analyzed using ImageJ and Prism 8 (GraphPad).
vRNP activity assay.
Purified vRNP preparations in cleavage buffer (see above) were incubated with 1 mM ATP, 0.5 mM UTP, 0.5 mM CTP, 0.5 mM GTP, 5 mM MgCl2, 1 mM DTT, 2 U/μl RNasin (Promega), and 25 nM synthetic capped RNA (m7GpppAAUCUAUAAUAG-3′) for 4 h at 30°C in a total reaction volume of 10 μl. RNA was extracted using TRI Reagent (Sigma), and primer extension or qPCR analysis of the reaction products was carried out.
Strand-specific quantitative PCR.
The qPCR analysis was carried out as described previously (22). Briefly, RNA was reverse transcribed with SuperScript III reverse transcriptase (Invitrogen) using strand-specific primers against the 5′ end (NA 160; 5′-TCCAGTATGGTTTTGATTTCCG-3′) or 3′ end (NA 1394; 5′-CCAGATCGTTCGAGTCCGTTTTTTTTTTTTTTTTTGAACAAACTAC-3′) of NA segment mRNA. Quantitative PCR was carried out on a QuantStudio 5 reverse transcriptase PCR (RT-PCR) machine (Applied Biosystems) with 2 μl cDNA and 1× reverse transcription-quantitative PCR (qRT-PCR) Brilliant III SYBR master mix (Agilent) in a 20 μl reaction mixture. Forward and reverse primers for NA 160 (5′-AGCAAAAGCAGGAGTTTAAATGAATCCAAACC-3′; 5′-CCGGTTTGAATTGAATGGCTAATCCATAT-3′) or NA 1394 (5′-TGAATAGTGATACTGTAGATTGGTCT-3′; 5′-CCAGATCGTTCGAGTCGT-3′) were present at 0.75 μM. Data presented are 2(−ΔCT), normalized to the wild-type sample.
ACKNOWLEDGMENTS
We thank George Brownlee for plasmids and helpful discussions.
This work was supported by Medical Research Council program grant MR/R009945/1 to E.F. and MRC Studentship to A.P.W.
A.P.W. and E.F. designed and conceived the experiments. A.P.W. and J.S. performed the experiments, and A.P.W. analyzed the data. A.P.W. and E.F. wrote the paper.
We have no conflicts of interest to declare.
REFERENCES
- 1.Ortín J, Martín-Benito J. 2015. The RNA synthesis machinery of negative-stranded RNA viruses. Virology 479–480:532–544. doi: 10.1016/j.virol.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Pflug A, Guilligay D, Reich S, Cusack S. 2014. Structure of influenza A polymerase bound to the viral RNA promoter. Nature 516:355–360. doi: 10.1038/nature14008. [DOI] [PubMed] [Google Scholar]
- 3.Gerlach P, Malet H, Cusack S, Reguera J. 2015. Structural insights into bunyavirus replication and its regulation by the vRNA promoter. Cell 161:1267–1279. doi: 10.1016/j.cell.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reich S, Guilligay D, Pflug A, Malet H, Berger I, Crépin T, Hart D, Lunardi T, Nanao M, Ruigrok RWH, Cusack S. 2014. Structural insight into cap-snatching and RNA synthesis by influenza polymerase. Nature 516:361–366. doi: 10.1038/nature14009. [DOI] [PubMed] [Google Scholar]
- 5.Peng Q, Liu Y, Peng R, Wang M, Yang W, Song H, Chen Y, Liu S, Han M, Zhang X, Wang P, Yan J, Zhang B, Qi J, Deng T, Gao GF, Shi Y. 2019. Structural insight into RNA synthesis by influenza D polymerase. Nat Microbiol 4:1750–1759. doi: 10.1038/s41564-019-0487-5. [DOI] [PubMed] [Google Scholar]
- 6.Fan H, Walker AP, Carrique L, Keown JR, Serna Martin I, Karia D, Sharps J, Hengrung N, Pardon E, Steyaert J, Grimes JM, Fodor E. 2019. Structures of influenza A virus RNA polymerase offer insight into viral genome replication. Nature 573:287–290. doi: 10.1038/s41586-019-1530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Te Velthuis AJW, Fodor E. 2016. Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis. Nat Rev Microbiol 14:479–493. doi: 10.1038/nrmicro.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker AP, Fodor E. 2019. Interplay between influenza virus and the host RNA polymerase II transcriptional machinery. Trends Microbiol 27:398–407. doi: 10.1016/j.tim.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thierry E, Guilligay D, Kosinski J, Bock T, Gaudon S, Round A, Pflug A, Hengrung N, El Omari K, Baudin F, Hart DJ, Beck M, Cusack S. 2016. Influenza polymerase can adopt an alternative configuration involving a radical repacking of PB2 domains. Mol Cell 61:125–137. doi: 10.1016/j.molcel.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reich S, Guilligay D, Cusack S. 2017. An in vitro fluorescence based study of initiation of RNA synthesis by influenza B polymerase. Nucleic Acids Res 45:3353–3368. doi: 10.1093/nar/gkx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng R, Xu X, Jing J, Wang M, Peng Q, Liu S, Wu Y, Bao X, Wang P, Qi J, Gao GF, Shi Y. 2020. Structural insight into arenavirus replication machinery. Nature 579:615–619. doi: 10.1038/s41586-020-2114-2. [DOI] [PubMed] [Google Scholar]
- 12.Mänz B, Schwemmle M, Brunotte L. 2013. Adaptation of avian influenza A virus polymerase in mammals to overcome the host species barrier. J Virol 87:7200–7209. doi: 10.1128/JVI.00980-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerry PS, Willsher N, Fodor E. 2008. A cluster of conserved basic amino acids near the C-terminus of the PB1 subunit of the influenza virus RNA polymerase is involved in the regulation of viral transcription. Virology 373:202–210. doi: 10.1016/j.virol.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Hara K, Schmidt FI, Crow M, Brownlee GG. 2006. Amino acid residues in the N-terminal region of the PA subunit of influenza A virus RNA polymerase play a critical role in protein stability, endonuclease activity, cap binding, and virion RNA promoter binding. J Virol 80:7789–7798. doi: 10.1128/JVI.00600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turrell L, Lyall JW, Tiley LS, Fodor E, Vreede FT. 2013. The role and assembly mechanism of nucleoprotein in influenza A virus ribonucleoprotein complexes. Nat Commun 4:1591. doi: 10.1038/ncomms2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coloma R, Arranz R, de la Rosa-Trevín JM, Sorzano COS, Munier S, Carlero D, Naffakh N, Ortín J, Martín-Benito J. 9 March 2020. Structural insights into influenza A virus ribonucleoproteins reveal a processive helical track as transcription mechanism. Nat Microbiol doi: 10.1038/s41564-020-0675-3. [DOI] [PubMed] [Google Scholar]
- 17.Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A. 1999. Rescue of influenza A virus from recombinant DNA. J Virol 73:9679–9682. doi: 10.1128/JVI.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng T, Sharps J, Fodor E, Brownlee GG. 2005. In vitro assembly of PB2 with a PB1-PA dimer supports a new model of assembly of influenza A virus polymerase subunits into a functional trimeric complex. J Virol 79:8669–8674. doi: 10.1128/JVI.79.13.8669-8674.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fodor E, Crow M, Mingay LJ, Deng T, Sharps J, Fechter P, Brownlee GG. 2002. A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase inhibits endonucleolytic cleavage of capped RNAs. J Virol 76:8989–9001. doi: 10.1128/jvi.76.18.8989-9001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robb NC, Smith M, Vreede FT, Fodor E. 2009. NS2/NEP protein regulates transcription and replication of the influenza virus RNA genome. J Gen Virol 90:1398–1407. doi: 10.1099/vir.0.009639-0. [DOI] [PubMed] [Google Scholar]
- 21.Engelhardt O, Smith M, Fodor E. 2005. Association of the influenza A virus RNA-dependent RNA polymerase with cellular RNA polymerase II. J Virol 79:5812–5818. doi: 10.1128/JVI.79.9.5812-5818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakami E, Watanabe T, Fujii K, Goto H, Watanabe S, Noda T, Kawaoka Y. 2011. Strand-specific real-time RT-PCR for distinguishing influenza vRNA, cRNA, and mRNA. J Virol Methods 173:1–6. doi: 10.1016/j.jviromet.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]