Varicella-zoster virus (VZV) causes herpes zoster, a major health issue in the aging and immunocompromised populations. Small noncoding RNAs (sncRNA) are recognized as important actors in modulating gene expression. This study extends our previous work and shows that four VZVsncRNA clustering in and near ORF61 and antisense to the latency-associated transcript of VZV can positively influence productive VZV infection. The ability of multiple exogenous small oligonucleotides targeting VZVsncRNA to inhibit VZV replication strengthens the possibility that they may inform development of novel treatments for painful herpes zoster.
KEYWORDS: herpes zoster, noncoding RNA, varicella-zoster virus
ABSTRACT
Small noncoding RNAs (sncRNA), including microRNA (miR), are expressed by many viruses to provide an additional layer of gene expression regulation. Our work has shown that varicella-zoster virus (VZV; also called human herpesvirus 3 [HHV3]), the human alphaherpesvirus causing varicella and herpes zoster, expresses 24 virally encoded sncRNA (VZVsncRNA) in infected cells. Here, we demonstrate that several VZVsncRNA can modulate VZV growth, including four VZVsncRNA (VZVsncRNA10, -11, -12, and -13) that are antisense to VLT, a transcript made in lytic infections and associated with VZV latency. The influence on productive VZV growth and spread was assessed in epithelial cells transfected with locked nucleotide analog antagonists (LNAA). LNAA to the four VZVsncRNA antisense to VLT significantly reduced viral spread and progeny titers of infectious virus, suggesting that these sncRNA promoted lytic infection. The LNAA to VZVsncRNA12, encoded in the leader to ORF61, also significantly increased the levels of VLT transcripts. Conversely, overexpression of VZVsncRNA13 using adeno-associated virus consistently increased VZV spread and progeny titers. These results suggest that sncRNA antisense to VZV may regulate VZV growth, possibly by affecting VLT expression. Transfection of LNAA to VZVsncRNA14 and VZVsncRNA9 decreased and increased VZV growth, respectively, while LNAA to three other VZVsncRNA had no significant effects on replication. These data strongly support the conclusion that VZV replication is modulated by multiple virally encoded sncRNA, revealing an additional layer of complexity of VZV regulation of lytic infections. This may inform the development of novel anti-sncRNA-based therapies for treatment of VZV diseases.
IMPORTANCE Varicella-zoster virus (VZV) causes herpes zoster, a major health issue in the aging and immunocompromised populations. Small noncoding RNAs (sncRNA) are recognized as important actors in modulating gene expression. This study extends our previous work and shows that four VZVsncRNA clustering in and near ORF61 and antisense to the latency-associated transcript of VZV can positively influence productive VZV infection. The ability of multiple exogenous small oligonucleotides targeting VZVsncRNA to inhibit VZV replication strengthens the possibility that they may inform development of novel treatments for painful herpes zoster.
INTRODUCTION
Varicella-zoster virus (VZV) is a human-specific neurotropic alphaherpesvirus that causes varicella (or chicken pox) upon primary infection of nonvaccinated individuals, during which the virus establishes a lifelong state of latency in neurons of peripheral somatic and autonomic ganglia. Reactivation from latency is most often manifested as herpes zoster, a painful skin eruption with dermatomal distribution that occurs most often in the elderly and immunocompromised. Zoster is frequently complicated by chronic pain states (postherpetic neuralgia) and, more rarely, cranial nerve palsies, meningoencephalitis, myelopathy, ischemic strokes, vasculopathies, and ocular disease leading to vision loss.
Several types of noncoding RNAs are made by host cells and pathogens that provide an additional level of regulation of gene expression. These include long noncoding RNA (lncRNA), small noncoding RNAs (sncRNA), and microRNAs (miR). These RNA molecules can act by downregulating target protein expression through translation interference, degradation of mRNA, and other mechanisms. miR are known to recognize mRNA targets via a short seed recognition sequence, allowing the regulation of expression of many genes simultaneously. They are encoded by the genomes of most organisms, ranging from humans to small DNA and RNA viruses. Some sncRNA without definitive characteristics of miR have been shown to regulate gene expression (1). Of the human herpesviruses, seven are known to express RNAs with classic miR structure (reviewed, for example, in reference 2). Up to 29 miR have been reported to be encoded by herpes simplex virus 1 (HSV-1) and 44 miR by Epstein-Barr virus (2). Several HSV-1 miR are encoded by the HSV-1 latency-associated transcript (LAT), the only region of the genome highly expressed during latency, and these have been suggested to have roles in neuronal infection and latency. For example, it was suggested that HSV-1 miR-H2 was involved in establishment and maintenance of latency via its interaction with the RNA encoding the major transactivator ICP0 (3). A subsequent study (4) demonstrated that the effect of HSV-1 miR-H2 on ICP0 was minor and weaker than that of host miR-138 on ICP0 expression. Using mutational analysis, the authors of that study found that this miR had no effect on lytic replication or reactivation from latency and concluded that it may have only a subtle role in controlling gene expression rather than being a central factor in lytic replication and/or latency of HSV-1. Other HSV-1 miR have been shown to be expressed during productive infection and act by limiting viral growth and rates of spread (5).
miR located in the regions of latency-associated transcripts have been described and studied in other neurotropic alphaherpesviruses, including two nonhuman varicelloviruses more closely related to VZV than HSV: pseudorabies virus (PRV) and bovine herpesvirus 1 (BoHV1). In BoHV1 (and the related BoHV5), the latency-associated transcript was reported to express 12 mature miR, with interesting characteristics, such as bidirectional transcription and the location of some miR within the origin of replication (6). Overexpression of two of the miR located within the origin of replication partially repressed replication of the virus. In PRV, a cluster of 11 miR are encoded within the large latency transcript (7) that may be derived from RNAs made during latency, as described for HSV. Deletion of the entire cluster of PRV latency-associated-transcript-derived miR resulted in reduced virulence, suggesting that they are involved in PRV replication and latency (8).
No miR have yet been described for VZV. An early bioinformatic study attempting to predict miR in herpesvirus genomes did not yield any candidate VZV miR (9). Studies searching for small RNAs in next-generation sequencing (NGS) analyses of autopsy samples of latently infected human peripheral ganglia failed to detect any small RNA species, even though HSV miR (10) were detected. However, there are usually much lower levels of VZV genomes in latently infected ganglia than HSV genomes, which may contribute to making the VZV sncRNA or miRNA more difficult to detect (11, 12). Importantly, a recent unbiased NGS study of human ganglia coupled with enrichment techniques for viral RNA resulted in the detection of VZV latency-associated transcripts (VLT) that are genomically positioned in a manner similar to that of the HSV LAT RNA. Multiple splice variants of VLT have been found in cells productively infected with VZV, and some of these may encode protein.
Advances in NGS technology as well as in bioinformatics tools led us to reexamine the possibility that VZV encodes small RNAs. We analyzed NGS data obtained from sequencing small (<200-nucleotide [nt]) RNA from VZV-infected fibroblasts as well as productively and latently infected neurons in vitro and found 24 sequences of 20 to 24 nt with the potential to be VZV-encoded sncRNA (10). The presence of 7 of the VZVsncRNA in both VZV-infected fibroblasts and neurons was confirmed using stem-loop quantitative reverse transcription-PCR (qRT-PCR) (SL-PCR). One of these, VZVsncRNA23, was predicted to fold like a miR, and transfection of a locked nucleotide analog antagonist (LNAA) to it revealed that VZVsncRNA23 can decrease viral spread and replication during productive infection (14). We subsequently detected 23 of the 24 sequences in VZV-infected epithelial cells (ARPE-19 cells) and 19 in neurons (13) using SL-PCR. We also tested two additional LNA antagonists to VZVsncRNA and found that one increased VZV spread and replication, while a second had no significant effect on VZV growth.
The current study tested potential physiological roles of nine additional VZVsncRNA using specific LNAA, including four VZVsncRNA encoded antisense to the VLT RNA associated with VZV latency. Two VZVsncRNA lie within the coding region of open reading frame 61 (ORF61; the homolog of the gene for the HSV-1 regulatory protein ICP0), and two are predicted to be derived from the 5′ untranslated leader sequence of the mRNA, upstream of the ORF61 coding sequence. LNAA to these four VZVsncRNA encoded opposite VLT consistently reduced VZV plaque growth and infectious virus, suggesting that these VZVsncRNA have a positive effect on VZV growth. We also show that inhibiting VZVsncRNA14, presumably encoded by the ORF62 (HSV-1 ICP4) mRNA, also reduced VZV growth, while an inhibitor of VZVsncRNA9 presumably encoded within ORF59 had the opposite effect and increased viral replication. LNAA inhibition of three additional VZVsncRNA had no significant effect on VZV spread. These results indicate that VZV uses sncRNA as an added layer of control of viral growth in lytic infection.
RESULTS
The four VZVsncRNA antisense to VLT upregulate VZV spread and replication.
We previously reported that transfection of LNAA to VZVsncRNA23 and VZVsncRNA20 increased viral spread in productively infected ARPE-19 cells, while transfection of an LNAA to VZVsncRNA2 had no significant effect (13, 14). We chose now to focus on the VZVsncRNA mapping to the VZV ORF61 gene, because it was recently shown that there is a family of spliced transcripts, termed VLT, that are encoded antisense to ORF61 (10). That study also reported that VLT RNAs are also transcribed in productive VZV infections, and one has the potential to be translated into a small protein. Four VZVsncRNA (VZVsncRNA10 to -13) map antisense to VLT (10) (Fig. 1) and would be expected to be produced by the processing of the mRNA of the ORF61 gene, with two coming from the translated coding region of ORF61 (the VZV homolog of HSV-1 ICP0) and two from the untranslated 5′ RNA leader sequence of ORF61 mRNA.
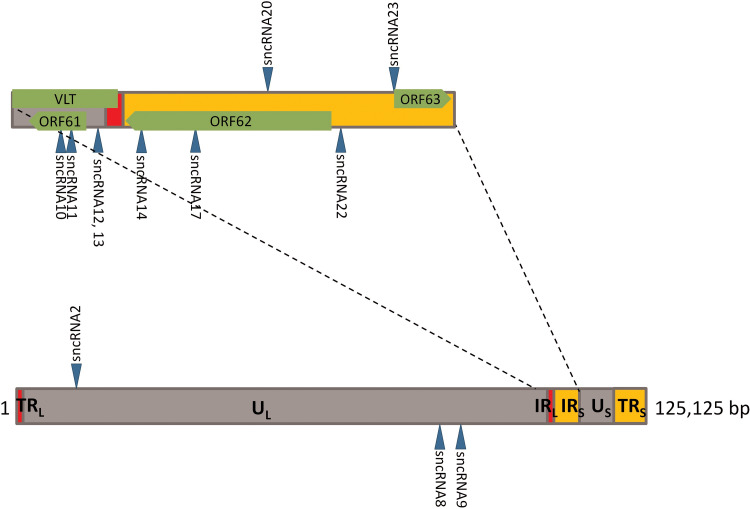
FIG 1.
Location in the viral genome of VZVsncRNA studied in this report. The lower diagram represents the entire VZV genome, while the top diagram is an expansion of the region containing most of the VZVsncRNA whose function was assayed for in the present study. Arrowheads facing upward indicate the VZVsncRNA coded by the bottom strand and deriving from right-to-left primary transcripts, while the arrowheads facing downward indicate the VZVsncRNA coded by the top strand of the viral genome in its standard configuration and deriving from RNAs transcribed left to right. The positions of relevant VZV ORFs and the VLT transcript coding region are indicated in green in the upper diagram.
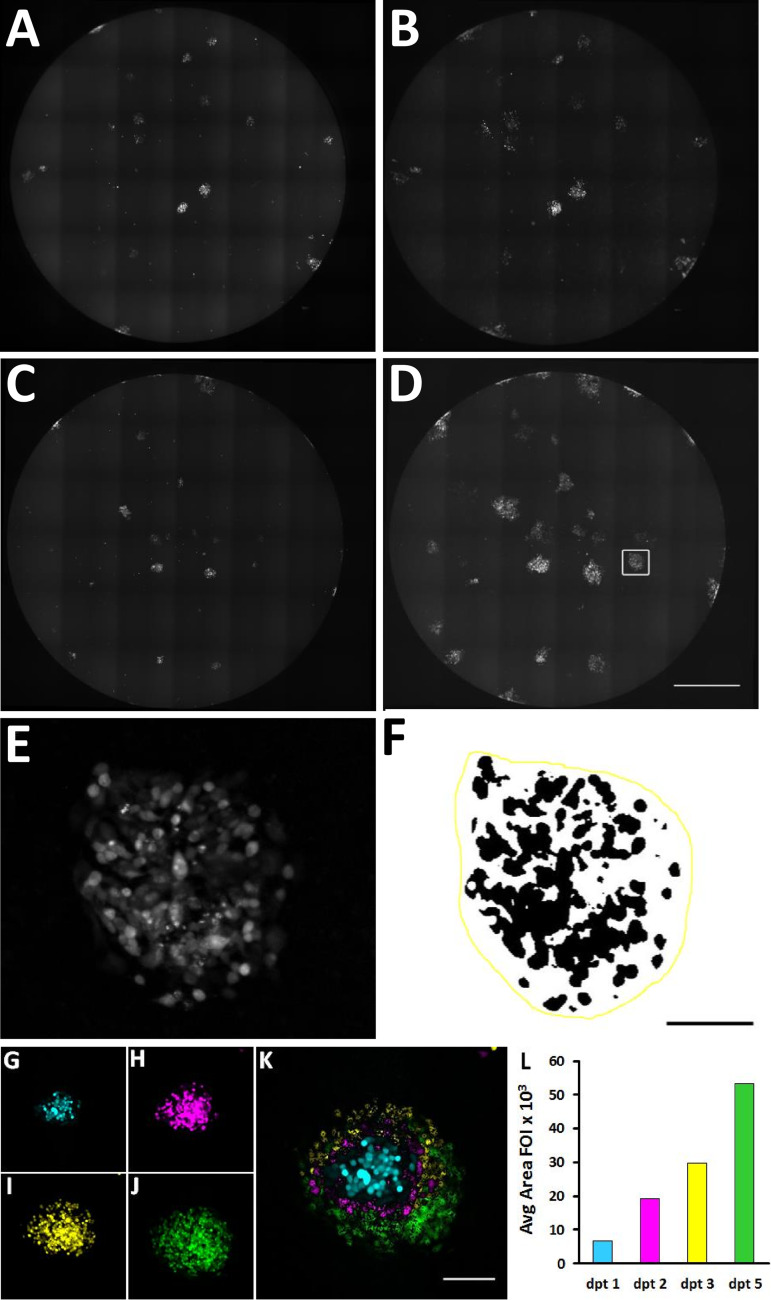
In order to investigate whether the VZVsncRNA antisense to VLT modulate VZV replication, we obtained custom LNAA to each of VZVsncRNA10, -11, -12, and -13. Naive ARPE-19 cells were infected with small numbers (10 to 30) of ARPE-19 cells previously infected with a recombinant VZV expressing red fluorescent protein (RFP) fused to ORF66 (VZVRFP66) (10, 13) in wells of 96-well plates. One day after small numbers of VZV-infected cells were seeded onto the naive cells, wells were transfected with LNAA or scrambled LNA RNA control. Cultures were photographed 1, 2, 3, and 5 days posttransfection (dpt) using an automated microscope that recorded more than 95% of the well area (Fig. 2A to D). The images were then processed as described in Materials and Methods and as detailed previously (10) to quantify the fluorescent area of individual foci of infection (FOI) (Fig. 2E and F) in order to monitor the spread of infection. An example of a FOI and its quantification from 1 to 5 dpt is shown in Fig. 2G to L.
FIG 2.
Method used for measuring growth of VZV infectious foci (FOI) in ARPE-19 cells. Ten to thirty ARPE-19 cells infected with VZV66RFP were added to 90% confluent wells of 96-well plates. At 24 h postinfection, cells were transfected either with an LNA antagonist to a scrambled RNA (A and B) or with VZVsncRNA9 (C and D). The RFP fluorescence of entire wells was photographed with an automated microscope, and the micrographs were assembled into a single image. (E) A single fluorescent VZV FOI after image processing. (F) FOI in panel E after application of a threshold for fluorescence (and inversion of the pixel luminance values): the black pixels represent the fluorescence indicating the presence of the virus. The yellow line demarcates the area within which black pixels were counted by the computer in order to generate a value for infection in an FOI. See Materials and Methods for more details. (G to L) Growth of a single focus of infection and its quantification. (G to J) Single FOI at 1 (blue), 2 (magenta), 3 (yellow), and 5 (green) days after transfection. (K) Images were pseudocolored and overlaid in order to visualize the FOI growth. (L) Graph of the quantification of this FOI on these days. Bars, 1 mm (A to D), 250 μm (E and F), and 100 μm (G to K).
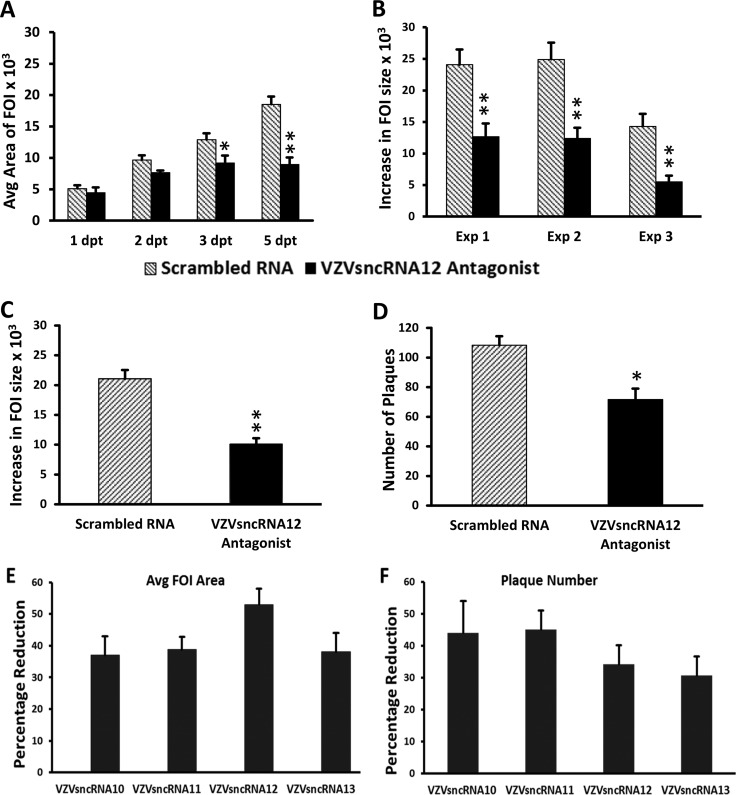
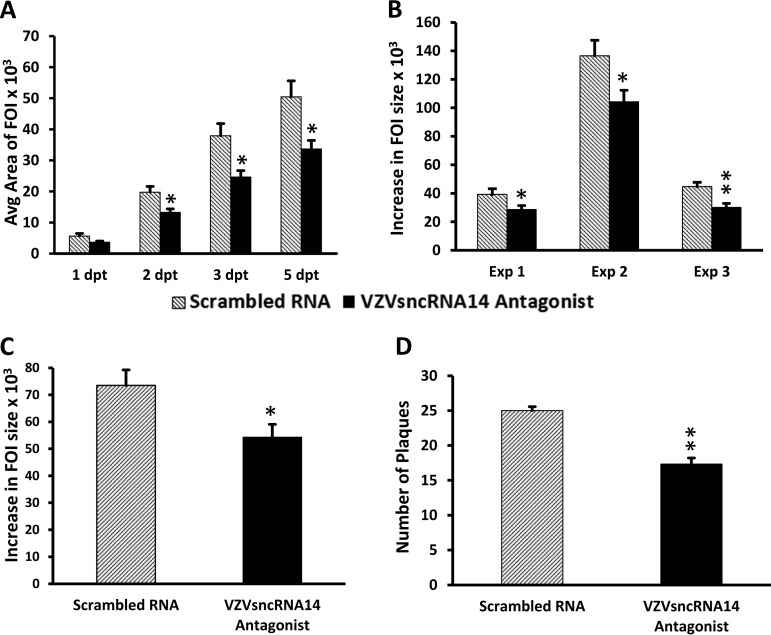
We first tested VZVsncRNA12, since it was detected in every preparation of small RNA extracted from ARPE-19 cells and human neurons by stem-loop reverse transcriptase PCR (SL-PCR) (13). Transfection of the LNAA resulted in a significant decrease in FOI growth in each of three experiments (including a total of 82 control and 98 antagonist-treated wells). The difference in size of the FOI was apparent from the first day posttransfection and was statistically significant at 3 dpt (P < 0.005) and thereafter (Fig. 3A to C). We also quantified the amounts of infectious cell-associated virus produced at 7 days after transfection of LNAA or scrambled RNA by trypsinizing cultures and using 10-fold dilutions of the cell suspension to reseed naive ARPE-19 cell monolayers. Plaques detected by crystal violet staining were counted 7 days after the seeding. The LNAA-treated cells yielded a significantly lower number of plaques (n = 3; P < 0.05) (Fig. 3D).
FIG 3.
Transfection of LNA antagonists antisense to VLT reduces growth of VZV infectious foci and infectious virus and increase VLT expression. An LNA antagonist to VZVsncRNA12 was transfected into wells of ARPE-19 cells 1 day after seeding of 10 to 30 VZV66RFP-infected ARPE-19 cells onto a 90% confluent culture of naive ARPE-19 cells (black bars). An LNA RNA oligonucleotide with a scrambled sequence was transfected as a control (gray bars). (A) Graph of the average size of FOI 1 to 5 dpt in a single experiment. Significant differences are indicated with asterisks (*, P < 0.05; **, P < 0.005). Errors bars represent the standard errors of the means (SEM) of the measurements from the three wells assayed within the same experiment. (B) Average increase in size of individual FOI between 1 to 5 dpt in arbitrary (pixel) units in three independent experiments. **, P < 0.005. (C) The transfection of an LNA antagonist to VZVsncRNA12 decreased the growth of the FOI by an average of 52% for the three experiments. **, P < 0.005. (D) Results from plaque assays of cells harvested from the LNA antagonist and scrambled RNA-transfected wells at 7 dpt. The antagonist reduced the plaque count by 34% compared to scrambled RNA (average from 3 independent experiments). The asterisk indicates statistical significance between control and LNAA-transfected wells (*, P < 0.05). (E and F) Effects of LNAA on all 4 VZVsncRNA antisense to the putative VZV latency-encoded transcript VLT on viral growth. LNA antagonists to VZVsncRNA10, -11, -12, and -13 and scrambled LNA RNA were transfected into VZV-infected ARPE-19 cells and analyzed as described in Materials and Methods and the legend to Fig. 2. All four antagonists reduced the growth of FOI between 1 and 5 dpt, by averages of 37%, 39%, 52%, and 38%, respectively (n = 3 for each antagonist). (F) Plaque-forming assays of cells harvested from the antagonist and scrambled-RNA wells. The average number of plaques obtained was reduced by 44%, 45%, 34%, and 30% for the LNA antagonists to VZVsncRNA10, -11, -12, and -13, respectively. All of the reductions in FOI growth and plaques were significant (P < 0.05).
This analysis was then extended to the other three VZVsncRNA antisense to VLT: VZVsncRNA10 and -11, mapping to distinct regions of the ORF61, and VZVsncRNA13, overlapping that of VZVsncRNA12 but detected as a distinct entity in NGS and SL-PCR. LNAA specific for VZVsncRNA10, -11, and -13 all reduced the plaque size of VZV and the number of progeny infectious cells at day 7 (n = 3 for each LNAA) (Fig. 3E and F). All of the reductions in FOI growth and plaque numbers were significant (P < 0.05). Taken together, these studies suggest that all four VZVsncRNA antisense to VLT have the effect of promoting VZV replication in epithelial cells. This effect on VZV growth is opposite of the effects of VZVsncRNA23 (14) and VZVsncRNA20 (13), which are predicted, from similar LNAA transfection experiments, to modestly but significantly downregulate productive VZV replication.
Transfection of an LNA antagonist to VZVsncRNA12 encoded antisense to the VLT leader sequence increases expression of VLT mRNA in lytically infected cells.
We postulated that VZVsncRNA12 expressed from the leader sequence region of ORF61 might function to regulate the levels of VLT. qRT-PCR for the levels of VLT mRNA in antagonist and scrambled RNA transfected cells were performed using primers that were detailed previously (10). Intriguingly, VLT transcript levels were found to be significantly increased by the introduction of the LNA antagonist into VZV-infected ARPE-19 cells at 5 dpt (Table 1) (n = 3; P < 0.03). This strongly suggests that while the VZVsncRNA are not predicted to fold like conventional miR (14), they may act by affecting the levels of specific viral transcripts.
TABLE 1.
An LNA antagonist to VZVsncRNA12 enhances expression of VLT in lytic infectionsa
| Expt | Level of expression of VLT transcripts (Cq) at 5 dpt |
|
|---|---|---|
| Scrambled RNA | VZVsncRNA12 LNAA | |
| 1 | 26.0 | 19.5 |
| 2 | 22.6 | 19.3 |
| 3 | 29.6 | 26.1 |
| Avgb | 26.1 ± 2.0 | 21.6 ± 2.3 |
RNA was extracted from VZV66RFP-infected ARPE-19 cells 5 days after transfection of an LNA antagonist to VZVsncRNA12, or a control scrambled LNA RNA (6 days postinfection, 5 dpt). Transfection of an LNAA to VZVsncRNA12 resulted in increased levels of VLT compared to control RNA infection in each individual experiment at 5 dpt, with an average difference of 4.5 cycles, which was statistically significant (P < 0.03).
Values are means ± SEM.
Some LNAA targeting VZVsncRNA not encoded antisense to VLT modulate viral replication, while others do not.
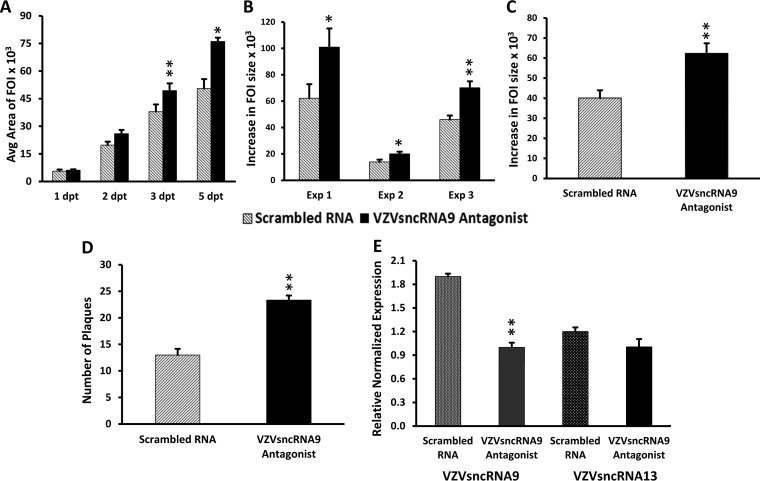
VZVsncRNA9 was present in the highest number of NGS reads in fibroblasts in our initial study (14) and was detected in all samples of RNA from VZV-infected ARPE-19 cells (13). Its sequence is within that encoding ORF59 (Fig. 1). We examined the effect of inhibiting VZVsncRNA9 on VZV replication and spread (Fig. 4A to D) using a specific LNAA and the image analysis and infectious-virus assays described above. Transfection of the LNAA to VZVsncRNA9 significantly increased FOI growth at times from 3 dpt on; results of a typical experiment are shown in Fig. 4A. The transfection of the LNAA resulted in a statistically significant increase in growth of FOI between 1 and 5 dpt in each of three independent experiments (Fig. 4B). Pooled and averaged results from three independent experiments revealed a 55% increase (81 control and 116 antagonist-treated foci; P < 0.005) (Fig. 4C). Counting infectious virus plaques that developed after reseeding of cells from wells receiving LNAA or scrambled LNA RNA revealed a significant increase (P < 0.005) in infectious virus generated in wells that received the LNAA (n = 3) (Fig. 4D). These results strongly suggest that VZVsncRNA9 downregulates viral growth in lytic infection.
FIG 4.
Administration of an LNAA to VZVsncRNA9 increases VZV FOI growth and number of infectious plaques. (A) The growth of FOI 1 to 5 days after transfection of an LNAA to VZVsncRNA9 (black bars) compared to control scrambled LNA RNA (gray bars) in a typical experiment is shown in arbitrary (pixel) units. A difference was observed at 2 dpt and became significant by 3 dpt. (B) Average increase in size of individual FOI between 1 and 5 dpt for each of the three experiments performed. (C) The transfection of an antagonist to VZVsncRNA9 increased the growth of FOI by an average of 55% for the three experiments. (D) Progeny virus yields quantified by plaque assays of cells harvested from the LNA antagonist and scrambled LNA RNA-transfected wells 7 dpt. The antagonist increased the plaque count compared to scrambled RNA by an average of 79% (n = 3). Asterisks indicate statistical significance between control and LNAA-transfected wells (*, P < 0.05; **, P < 0.005). (E) Transfection of an LNA antagonist to one VZVsncRNA reduced the levels of its target but not those of another VZVsncRNA. VZV-infected ARPE-19 cells were transfected with an LNA antagonist to VZVsncRNA9 or a scrambled LNA oligonucleotide control. At 7 dpt, cells were harvested, RNA was extracted, and stem-loop qRT-PCR was performed on small (<200-nt) RNAs for VZVsncRNA9 (targeted). VZVsncRNA13 was a nontargeted control. The antagonist reduced the level of the targeted VZVsncRNA9 by about 50% in two independent experiments (P < 0.05; 3 wells in each experiment). In contrast, the level of nontargeted VZVsncRNA13 from the same RNA preparation was not significantly different from that of the scrambled control.
LNAA are designed to bind to specific single-stranded sncRNA and to either remove it from the functional pool or cause it to be degraded. Both of these mechanisms would reduce the effective levels of the available sncRNA in the cell. In order to determine that transfected LNAA acted specifically on the VZVsncRNA they were designed to target, cells were infected and then transfected with the LNAA to VZVsncRNA or scrambled oligonucleotides as described above, and the small-RNA fraction was extracted at 7 dpt. Live-imaging analysis showed the expected increase in spread, similar to that presented in Fig. 4. We then performed stem-loop qRT-PCR to examine expression of available VZVsncRNA9 (the target) and VZVsncRNA13, whose level would not be expected to be affected by the LNAA to sncRNA9. The LNAA reduced the levels of the target VZVsncRNA9 by 47% compared to the scrambled-RNA-transfected cells, while the level of VZVsncRNA13 was not affected (Fig. 4E). Two additional independent experiments were performed with similar results. It can therefore be concluded that this LNAA depleted the available levels of the specific VZVsncRNA that it was designed to target.
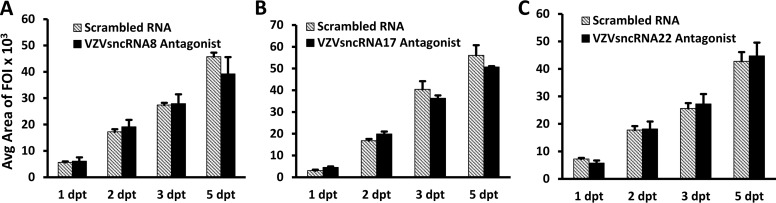
We subsequently evaluated an additional four LNAA for their effects on VZV growth and spread (Fig. 5 and 6). The antagonist to VZVsncRNA14 decreased the average growth of FOI by 19% and reduced the average plaque number by 30% (n = 3) (Fig. 5A to C). The levels of progeny virus obtained in plaque assays was also significantly reduced (Fig. 5D). In contrast, transfection of specific antagonists to VZVsncRNA8, VZVsncRNA17, and VZVsncRNA22 all gave results in the live-imaging assay that were not consistent in direction and not significantly different from those of controls (Fig. 6A to C). These results indicate that some but not all VZVsncRNA have effects on replication of VZV in ARPE-19 cells. However, it is possible that some have significant effects on viral growth in other cell types or in other assays.
FIG 5.
Inhibition of VZVsncRNA14 reduced growth of VZV infectious foci and infectious virus production. An LNA antagonist to VZVsncRNA14 or control LNA RNA was transfected 1 day after seeding of 10 to 30 VZV66RFP-infected ARPE-19 cells onto monolayers of naive ARPE-19 and analyzed as described in Materials and Methods and the legend to Fig. 3. (A) Increase in FOI size in a single experiment, in which a significant difference, determined by comparing averages of FOI growth from three wells with each oligonucleotide, appeared by 2 dpt. (B) Average change in size of individual FOI in 3 independent experiments. (C) A modest but significant 19% average decrease in FOI growth was measured in the three experiments whose results are shown in panel B. (D) Progeny virus measured 7 days after transfection, in which cells were harvested from the antagonist- and scrambled-RNA-transfected wells and seeded onto naive ARPE-19 cells. Analysis 7 days later revealed that transfection of the antagonist resulted in a 30% decrease in infectious plaque count (n = 3) compared to the control. Asterisks indicate statistical significance between control and LNAA-transfected wells (*, P < 0.05; **, P < 0.005).
FIG 6.
LNA antagonists to three VZVsncRNA did not have a consistent effect on FOI growth. LNA antagonists to VZVsncRNA8 (A), VZVsncRNA17 (B), and VZVsncRNA22 (C) were transfected 1 day after seeding of small numbers of VZV66RFP-infected ARPE-19 cells onto monolayers of ARPE-19. Controls and analysis were performed as described in Materials and Methods and the legend to Fig. 2. Average changes in FOI size from three independent experiments with each LNA VZVsncRNA antagonist are shown.
Overexpression of a VZVsncRNA antisense to VLT has an effect on VZV growth opposite that of the corresponding LNAA.
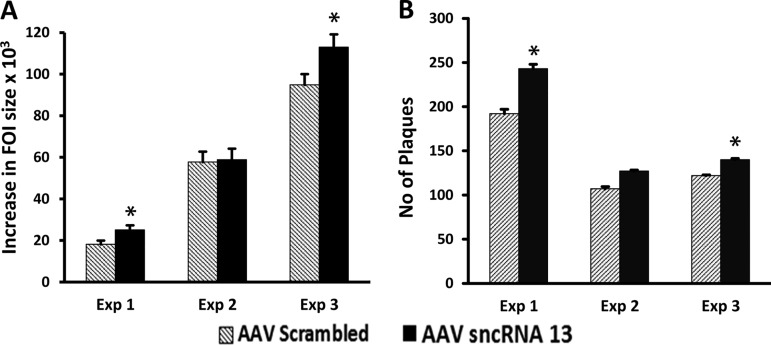
A prediction based on our experiments showing that the LNAA to the VZVsncRNA opposite VLT reduce viral replication is that expression of these VZVsncRNA should enhance the replication of VZV. In order to test this prediction, we constructed an adeno-associated virus (AAV) constitutively expressing VZVsncRNA13 or a scrambled sequence of the same length. ARPE-19 cells were transduced with each AAV and subsequently superinfected with small numbers of VZV-infected cells (Fig. 7). In two of three experiments, a modest but significant (P < 0.05) increase in both FOI growth (Fig. 7A) and infectious virus production (Fig. 7B) was observed in the cells overexpressing VZVsncRNA13 compared to controls. This result supports the hypothesis that this VZVsncRNA expressed antisense to VLT can enhance viral growth.
FIG 7.
Exogenous expression of VZVsncRNA13 by AAV promotes viral growth. ARPE-19 cells were transduced with AAV2 expressing VZVsncRNA13 or expressing a control RNA of the same length under the control of the U6 promoter and 1 day later infected with 10 to 30 VZV-infected cells. Analysis of FOI growth was performed as described in the legend to Fig. 2. Modest increases in FOI size (A) and plaque number (B) were obtained in the cells overexpressing VZVsncRNA13 relative to those expressing the scrambled RNA in two of three independent experiments. In experiment 2, no significant difference in FOI growth or increase in plaque number were observed, although a trend was apparent. The results from overexpression of this VZVsncRNA are the opposite of those obtained by transfecting its LNA antagonist (Fig. 3).
DISCUSSION
A great deal of experimental evidence has accumulated indicating that most human herpesviruses express long and short noncoding RNAs that exert an additional level of control of gene expression to regulate viral growth. We found 24 RNA sequences of 20 to 22 nt in an NGS analysis of small (<200-nt) in fibroblasts and neurons infected with VZV in vitro that we termed VZVsncRNA (14). Only one of these, VZVsncRNA23, was structurally predicted to fold as a miR, and transfection of an LNAA directed to it increased VZV replication in lytic infections (14). Thus, the VZVsncRNA found in our studies are able to affect VZV growth rates. This was supported by a second study, where we reported that LNAA to VZVsncRNA20 also increased viral replication (13), while an LNAA to sncRNA2 did not have a significant effect. In the present study, we examined the effects of LNAA to nine additional VZVsncRNA and found that most modulate VZV replication and spread (Table 2). These results strongly suggest that VZV encodes sncRNA that have both positive and negative influences on productive replication.
TABLE 2.
Summary of the effects of transfected LNA antagonists to VZVsncRNA on VZV productive infection measured using live-imaging and plaque assaysa
Orange highlighting indicates data from reference 14; green highlighting indicates data from reference 13. All antagonists were tested with the live-imaging FOI growth assay. “Not consistent” means that a consistent trend in the direction of effect on VZV growth in LNAA transfected cells was not found in 3 independent experiments. NT, not tested.
Importantly, four VZVsncRNA are presumably derived from the mRNA of ORF61 and antisense to the recently described spliced VZV latency-associated transcript VLT (10). In contrast to our previous studies, where transfection of LNAA to VZVsncRNA resulted in increased replication (and thus implied that the corresponding VZVsncRNA reduced spread and replication), all four appear to promote viral replication, because introduction of LNAA directed to them reduced FOI growth and infectious progeny. In other alphaherpesviruses, the roles of the miR within latency-associated transcripts have not been extensively analyzed in detail using LNAA, but several have been examined by mutation of the sequences or deletion of the miR regions. For example, deletion of the porcine varicellovirus PRV miR cluster reduced virulence (8), suggesting that PRV miR in this part of the genome enhance lytic replication. Several of the HSV mRNAs have been also analyzed by deletion analyses and shown to have effects on growth and models of pathogenesis ranging from none to significant (4, 15, 16; reviewed in reference 17). An unusual aspect of the VZVsncRNA10 to -13 is that they are encoded antisense to VLT. While the PRV miR cluster and many (but not all) of the HSV miR are encoded in the corresponding latency-associated transcript, some of the HSV miR and BoHV miR appear to be encoded from the opposite strand. While the function of VLT is not yet clear, it was postulated to suppress VZV infection, because cells overexpressing VLT showed increased resistance to support VZV replication (10). Several spliced forms of the VLT gene are also expressed in cells productively infected with VZV, and some may encode a small protein whose functions are yet to be determined. VLT RNA is spliced in both lytic and latent infections, but during a lytic infection, the 5′ ends of the VLT RNAs vary and splicing occurs in a more complex manner than that seen in latent infections in human ganglia (10). We speculate that the four VZVsncRNA antisense to the VLT transcript may regulate VLT stability, its regulation of infection, or its translation to the VLT protein, and work is now in progress to distinguish these possibilities.
Depledge et al. speculated that VLT downregulates ORF61 based on cotransfection and coexpression studies of VLT and ORF61 mRNA (10), although this was not examined in the context of VZV infection. Consistent with this hypothesis, we found that an LNAA to at least one of the four VZVsncRNA increases VLT expression in a productive infection using qRT-PCR (Table 1). A possible mechanism for the effects we observe upon inhibition of these four VZVsncRNA is that ORF61 mRNA may not only generate ORF61 protein but also may give rise to the 4 VZVsncRNA that autoregulate its own expression by counteracting the effects of the VLT. These VZVsncRNA may thus influence how VLT RNA acts to influence the levels of ORF61. This complex scenario of cross- and self-regulation may also influence putative VLT protein expression in productively infected cells. qRT-PCR experiments examining the levels of ORF61 transcripts in VZVsncRNA12 LNAA-transfected ARPE-19 cells showed a small (1 to 2 quantification cycles [Cq]) but consistent reduction in ORF61 expression (data not shown) (n = 3). However, these experiments looking for changes in ORF61 levels were performed on ARPE-19 cells, which support a robust VZV replication. It is possible that a stronger regulation of ORF61 may occur in other cell types, such as neurons, where decisions between lytic and latent outcomes of infection need to be carefully controlled.
The implied regulation of ORF61 is not surprising, since ORF61 encodes the VZV homolog of HSV ICP0, and ICP0 is a critical host cell modulator of the innate immune responses to viral infection that is also an important factor in lytic/latent decisions in neurons (18, 19). One study suggested that HSV-1 miR derived from LAT could bind to the ICP0 mRNA and regulate levels of ICP0 (3), while a later study suggested the effect upon HSV growth and pathogenesis (4). Our results regarding ORF61 are thus consistent with the small effect on ICP0 expression after mutation of HSV-1 miR-H2 obtained in this more recent study. The roles of the VZVsncRNA require study in in vitro models of VZV neuronal latency and reactivation. However, the study of the roles of VZVsncRNA in latency and reactivation is complicated by the known difficulty of transfecting neurons.
One approach to modulate VZVsncRNA expression without transfection would be to use viral vector transduction. In order to establish proof of principle, we developed an AAV expressing VZVsncRNA13. Overexpression experiments with this AAV supported the finding that VZVsncRNA13 modulates VZV infection. Overexpression is predicted to have the opposite effect of transfected LNAA, and indeed, this was the case: AAV VZVsncRNA13 consistently increased both plaque size and spread as well as the recovery of infectious virus-infected cells at day 7. The strength of the effect of exogenous expression was more modest than that of the LNAA, and this might be a result of several factors, such as the efficiency of AAV-mediated transduction of ARPE-19 cells and the possibility that expression levels of the natural VZVsncRNA from VZV are near their saturating levels in productive infection, among other possibilities. Regardless, given that the AAV-mediated expression of this sncRNA had the opposite effect of the LNA antagonist over controls, it supports the specificity of the effects of LNAA and exogenous expression on VZV infection and may provide a tool to examine the role of VZVsncRNA in latent and lytic infection in neurons.
In addition to the four VZVsncRNA in the VLT region, we also extended our study to investigate the effects of antagonists to several other VZVsncRNA. Transfection of an antagonist to VZVsncRNA9, which had the highest number of NGS counts in VZV-infected fibroblasts by VZV (14), increased VZV replication in ARPE-19 cells, similar to the effects of antagonists to VZVsncRNA23 and VZVsncRNA20 previously reported (13, 14). Thus, at least three VZVsncRNA may act to repress viral replication. Similar observations have been made for two HSV miR, miR-H28 and miR-H29, which apparently act to reduce productive infection (5). In contrast, the antagonist to VZVsncRNA14, which maps to the ORF62 region and is in the same direction as the four VLT RNA in the ORF61 gene, also significantly and consistently decreased VZV replication, suggesting that VZVsncRNA14 may also enhance VZV growth. We speculate that there are alternative forms of VLT in latently infected human ganglia that may also include some species that extend their primary transcription through ORF62, so this effect might also be through a VLT-related pathway. It is possible that these five molecules may act synergistically or are redundant and able to compensate for one another. Resolving these possibilities will require additional study.
We also found that the LNAA to VZVsncRNA8 and VZVsncRNA17 did not have a consistent effect on VZV growth: in some experiments, FOI increased, and in other experiments, it was reduced. This lack of consistency was in spite of both VZVsncRNA being detected in every sample of small RNA extracted from VZV-infected ARPE-19 and neurons by SL-PCR (13). VZVsncRNA17 is encoded within the transcriptional activator ORF62 (VZV analog of HSV ICP4) and presumably comes from its mRNA, while VZVsncRNA22 is encoded in the ORF62 untranslated 5′ leader sequence. The lack of activity contrasts with the strong effect of LNAA directed to VZVsncRNA14, also located in the ORF62 region. This may be due to a more subtle effect of these noncoding RNAs on infection, the actual levels of the sncRNA made, or cell type specificity of their actions. However, it is not surprising that LNAA to several VZVsncRNA do not show an effect on viral growth, since miR in other herpesviruses have weak or undetectable effects, as discussed above. It may be that their effects are compensated for by expression of other VZV RNAs or proteins, or their effect may be more substantial in specific cell types. In total, 12 of the 24 NGS VZVsncRNA have now been assayed for potential function by transfection with specific LNAA, with 9 of the 12 significantly modulating viral growth (Table 2).
With the exception of VZVsncRNA23, none are predicted to fold as miR, and we have not been able to precipitate them using Argonaute (AGO) immunoprecipitation. Since their mechanism of action may not be through the formation of RNA-induced silencing complexes (RISC) like conventional miR, an intriguing possibility is that they may represent a novel type of functional sncRNA generated through a yet-to-be-defined pathway. Our studies therefore may provide insights into novel mechanisms of small RNA metabolism and action in herpesvirus lytic infection. Indeed, the recent demonstration that both viral and host miR biosynthesis are blocked in HSV-1 lytic infection (20) suggests that the entire small-RNA environment is changed upon herpesvirus infection. We are therefore pursuing other experimental and bioinformatics techniques that are independent of AGO precipitation or seed sequence binding to look for targets of this family of VZVsncRNA.
All of the experiments in this and our previous studies on VZVsncRNA have examined only their effects in productive infections and in nonneuronal cells. VZV with deletions and/or mutations of these sequences and transgenic human embryonic stem cell (hESC) lines (which can be used to generate neurons) with potentially inducible expression of the VZVsncRNA are now being developed in order to evaluate the potential roles of these molecules in latent infection of neurons by VZV. It may be that VZVsncRNA that negatively regulate lytic infection initially act as a backup mechanism to ensure that a latent rather than lytic infection is promoted. In contrast, sncRNA made from ORF61 mRNA may act to promote reactivation, because ORF61 is a vital transactivator involved in lytic replication.
Finally, it is worth noting that miR antagonists, mimics, and small interfering RNAs (siRNAs) are new and exciting therapeutic modalities being explored as treatments for cancer as well as viral infections (21, 22; reviewed in reference 23). The ability of VZVsncRNA to modulate viral replication opens the possibility of developing drugs to treat VZV infections, especially painful zoster, using small, diffusible, and easily produced oligonucleotides.
MATERIALS AND METHODS
Cells, viruses, and infection.
ARPE-19 cells (human retinal pigmented epithelium; ATCC CRL-2302) were grown and maintained as described previously (13). Recombinant VZV (VZV66RFP) expressing monomeric red fluorescent protein (mRFP) as an N-terminal fusion in frame to ORF66 was generated by recombineering of the parent of the Oka bacterial artificial chromosome (pOka BAC) as described previously (24, 25). All infections with VZV were performed in a cell-associated manner.
Transfection of LNA antagonists to VZVsncRNA.
Monolayers of ARPE-19 cells in 96-well black-wall plates were infected with multiple dilutions of trituration-dispersed VZV66RFP-infected ARPE-19 cells in to obtain a minimum of 3 wells containing 10 to 30 foci of infection (FOI) in each well. Wells with this number permitted us to follow the growth of individual plaques without overlap. Cells were transfected 24 h after infection with a synthetic LNA antagonist RNA (Exiqon [now Qiagen]) to a VZVsncRNA or with a scrambled RNA control. Transfection was performed using Dharmafect2 transfection reagent (Dharmacon) according to the manufacturer’s instructions.
Live-imaging assay for spread of infection.
Details of the live-imaging method were described previously (14). In brief, an automated microscope (Olympus IX81) with a motorized stage (Marzhauser) was used to photograph entire wells (36 images/well) under fluorescence and bright-field illumination with a computer-controlled LED illumination system (CoolLED, Andover, United Kingdom), a cooled charge-coupled device (CCD) monochrome camera (QImaging, Surrey, Canada) and Micromanager 1.4 (26). Three wells receiving the LNAA and three wells receiving the control RNA were photographed 1, 2, 3, and 5 days posttransfection (dpt). The micrographs were assembled using Fiji (27) to make a montage covering more than 95% of each well in a 96-well culture plate. The montages were thresholded to generate binary images of the FOI, which were measured using Fiji. Statistical significance was tested using Student’s paired t test assuming equal variances in Microsoft Excel.
Measurement of viral yield by plaque assays.
Infected cells transfected with LNAA to VZVsncRNA or scrambled RNA were harvested by trypsinization 7 dpt. Tenfold serial dilutions of the cells were prepared and seeded onto naive ARPE-19 cells in 24-well plates in triplicate. The inocula were allowed to infect the naive ARPE-19 cells for 1 day, after which the medium was changed. Seven days after seeding, cultures were fixed and stained with 0.1% crystal violet in 10% formaldehyde, and plaques were counted.
qRT-PCR for mRNA and sncRNA.
VZV-infected ARPE-19 cells in 96-well plates were transfected with an LNA antagonist to VZVsncRNA12 or a scrambled control RNA, and cultures were maintained until 5 dpt. Cells were harvested and total RNA extraction performed using the GeneAll kit (number 325-150; GeneAll, Seoul, South Korea). cDNA was prepared, and primers crossing the splice site between exons 3 and 4 (10) (Table 3) were used for detection of the VLT transcript. Statistical significance was tested using Student’s paired t test. For quantification of VZVsncRNA levels, infection of the monolayer of ARPE-19 cells in 96-well black plates was performed as described above, in which six wells were transfected with the LNA antagonist to VZVsncRNA9 and six with scrambled oligonucleotides. The effect of the antagonist on FOI growth was confirmed using the imaging system as described above. At 7 dpt, the cells were harvested, and RNA was extracted using the Hybrid-R kit for large and small RNA (GeneAll). RNA was treated with DNase (AMPD1; Sigma-Aldrich). Primers (Table 3) were generated by Sigma-Aldrich (Rehovot, Israel), and the probe was LGC Biosearch Technologies (Petaluma, CA) number DLO-RFB-5. The cDNA for VZVsncRNA9, VZVsncRNA13, and hsa-mir26 was prepared from small RNA in separate reactions and converted to cDNA using Moloney murine leukemia virus (MMLV) reverse transcriptase (number M1701; Promega) at increasing temperatures: 25°C for 15 min, 37°C for 15 min, 42°C for 40 min, and finally 2 min at 95°C for enzyme inactivation. All qPCRs were performed in triplicate and averaged to compensate for pipetting errors. Host hsa-mir26 is expressed at high levels and shows little variation between cells infected by multiple viruses and uninfected cells (28), and it was used to normalize expression of small RNA (14). Two independent experiments were performed with similar results.
TABLE 3.
Primers and probes used in this studya
| Primer or probe | Sequence |
|---|---|
| VZVsncRNA9 SL Fwd | 5′-ATGAGTACCCGAGATCGATTGG-3′ |
| VZVsncRNA9 SL Rev | 5′-GGTCGTATGCAAGAGCAGGGTCCGAGGTATCCATCGCACGCATCGCACTGCATACGACCATAACACT-3′ |
| VZVsncRNA13 SL Fwd | 5′-ATTTGGATGCCCGGACAT-3′ |
| VZVsncRNA13 SL Rev | 5′-GGTCGTATGCAAGAGCAGGGTCCGAGGTATCCATCGCACGCATCGCACTGCATACGACCGTATTCTA-3′ |
| miR26a SL Fwd | 5′-GCATGGCGTTCAAGTAATCCAG-3′ |
| miR26a SL Rev | 5′-GGTCGTATGCAAGAGCAGGGTCCGAGGTATCCATCGCACGCATCGCACTGCATACGACCAGCCTATC-3′ |
| Universal SL Rev | 5′-GAGCAGGGTCCGAGG-3′ |
| Universal probe | FAM-5′-TCCATCGCACGCATCGCACT-3′-BHQ-1 |
| VLT exon 3Fwd | 5′-AATCGAGCCATACACCACCG-3′ |
| VLT exon 4Rev | 5′-TGTATTCGGGCATGGACCT-3′ |
| hGAPDH Fwd | 5′-CACATGGCCTCCAAGGAGTAA-3′ |
| hGAPDH Rev | 5′-TGAGGGTCTCTCTCTTCCTCTTGT-3′ |
AAV-based overexpression of VZVsncRNA.
AAV serotype 2 expressing either the sequence of VZVsncRNA13 or a scrambled control under the control of the human U6 small nuclear promoter were generated commercially (mammalian shRNA knockdown vectors; Vectorbuilder, Guangzhou Science City, China). The viruses also contained a cassette containing enhanced green fluorescent protein (eGFP) under the control of the cytomegalovirus (CMV) reporter for visually following infection. Analysis by SL-PCR of small RNA extracted from ARPE-19 cells 2 days after infection with these AAVs (n = 2) revealed that both VZVsncRNA13 (Cq, 27.5 and 24.3) and host miR miR26a (Cq, 24.1 and 23.52) were expressed in cells receiving the vector containing VZVsncRNA13, while mock-infected cells expressed only the host miR (Cq, 23 and 23.52), and the VZVsncRNA13 sequence was not detected (data not shown).
Monolayers of ARPE-19 cells in 96-well black plates were infected by AAV expressing VZVsncRNA13 or AAV expressing scrambled sncRNA using equal titers (108) of genomic copies per well. One day afterward, the medium was changed, and wells of ARPE-19 cells infected with AAV were incubated with 10 to 30 VZVORF66RFP-infected ARPE-19 cells as in the antagonist experiments. The effect of VZVsncRNA13 overexpression on VZV FOI growth and generation of infectious virus was quantified as described above.
ACKNOWLEDGMENTS
This research was supported by NIH grant R01 AI122640 and US-Israel Binational Science Foundation 2017259 to R.G. and P.R.K. R.G. was also supported by Israel Science Foundation grant 254/16. P.R.K. was also supported by P30 EY08098, Research to Prevent Blindness Inc. (New York, NY), and The Eye & Ear Foundation of Pittsburgh.
REFERENCES
- 1.Weinberg MS, Morris KV. 2016. Transcriptional gene silencing in humans. Nucleic Acids Res 44:6505–6517. doi: 10.1093/nar/gkw139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piedade D, Azevedo-Pereira JM. 2016. The role of microRNAs in the pathogenesis of herpesvirus infection. Viruses 8:156. doi: 10.3390/v8060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores O, Nakayama S, Whisnant AW, Javanbakht H, Cullen BR, Bloom DC. 2013. Mutational inactivation of herpes simplex virus 1 microRNAs identifies viral mRNA targets and reveals phenotypic effects in culture. J Virol 87:6589–6603. doi: 10.1128/JVI.00504-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan D, Pesola JM, Li G, McCarron S, Coen DM. 2017. Mutations inactivating herpes simplex virus 1 microRNA miR-H2 do not detectably increase ICP0 gene expression in infected cultured cells or mouse trigeminal ganglia. J Virol 91:e02001-16. doi: 10.1128/JVI.02001-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Z, Liu X, Chen X, Zhou X, Du T, Roizman B, Zhou G. 2016. miR-H28 and miR-H29 expressed late in productive infection are exported and restrict HSV-1 replication and spread in recipient cells. Proc Natl Acad Sci U S A 113:E894–E901. doi: 10.1073/pnas.1525674113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glazov EA, Horwood PF, Assavalapsakul W, Kongsuwan K, Mitchell RW, Mitter N, Mahony TJ. 2010. Characterization of microRNAs encoded by the bovine herpesvirus 1 genome. J Gen Virol 91:32–41. doi: 10.1099/vir.0.014290-0. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y-Q, Chen D-J, He H-B, Chen D-S, Chen L-L, Chen H-C, Liu Z-F. 2012. Pseudorabies virus infected porcine epithelial cell line generates a diverse set of host microRNAs and a special cluster of viral microRNAs. PLoS One 7:e30988. doi: 10.1371/journal.pone.0030988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Zhang M-M, Yan K, Tang Q, Wu Y-Q, He W-B, Chen H-C, Liu Z-F. 2018. The full-length microRNA cluster in the intron of large latency transcript is associated with the virulence of pseudorabies virus. Virology 520:59–66. doi: 10.1016/j.virol.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Umbach JL, Nagel MA, Cohrs RJ, Gilden DH, Cullen BR. 2009. Analysis of human alphaherpesvirus microRNA expression in latently infected human trigeminal ganglia. J Virol 83:10677–10683. doi: 10.1128/JVI.01185-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Depledge DP, Ouwendijk WJD, Sadaoka T, Braspenning SE, Mori Y, Cohrs RJ, Verjans G, Breuer J. 2018. A spliced latency-associated VZV transcript maps antisense to the viral transactivator gene 61. Nat Commun 9:1167. doi: 10.1038/s41467-018-03569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pevenstein SR, Williams RK, McChesney D, Mont EK, Smialek JE, Straus SE. 1999. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol 73:10514–10518. doi: 10.1128/JVI.73.12.10514-10518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K, Lau TY, Morales M, Mont EK, Straus SE. 2005. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single-cell level. J Virol 79:14079–14087. doi: 10.1128/JVI.79.22.14079-14087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golani-Zaidie L, Borodianskiy-Shteinberg T, Bisht P, Das B, Kinchington PR, Goldstein RS. 2019. Bioinformatically-predicted varicella zoster virus small non-coding RNAs are expressed in lytically-infected epithelial cells and neurons. Virus Res 274:197773. doi: 10.1016/j.virusres.2019.197773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markus A, Golani L, Ojha NK, Borodiansky-Shteinberg T, Kinchington PR, Goldstein RS. 2017. Varicella-zoster virus expresses multiple small noncoding RNAs. J Virol 91:e01710-17. doi: 10.1128/JVI.01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X, Brown D, Osorio N, Hsiang C, Li L, Chan L, BenMohamed L, Wechsler SL. 2015. A herpes simplex virus type 1 mutant disrupted for microRNA H2 with increased neurovirulence and rate of reactivation. J Neurovirol 21:199–209. doi: 10.1007/s13365-015-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamura Y, Bosch-Marce M, Tang S, Patel A, Krause PR. 2018. Herpes simplex virus 2 latency-associated transcript (LAT) region mutations do not identify a role for LAT-associated microRNAs in viral reactivation in guinea pig genital models. J Virol 92:e00642-18. doi: 10.1128/JVI.00642-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhela S, Rouse BT. 2018. Are miRNAs critical determinants in herpes simplex virus pathogenesis? Microbes Infect 20:461–465. doi: 10.1016/j.micinf.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alandijany T. 2019. Host intrinsic and innate intracellular immunity during herpes simplex virus type 1 (HSV-1) infection. Front Microbiol 10:2611. doi: 10.3389/fmicb.2019.02611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson AC, Mohr I. 2012. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol 20:604–611. doi: 10.1016/j.tim.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan D, Li G, Morris-Love J, Qi S, Feng L, Mertens ME, Jurak I, Knipe DM, Coen DM. 2019. Herpes simplex virus 1 lytic infection blocks microRNA (miRNA) biogenesis at the stage of nuclear export of pre-miRNAs. mBio 10:e02856-18. doi: 10.1128/mBio.02856-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Chen X, Zhou X, Roizman B, Zhou GG. 2018. miRNAs targeting ICP4 and delivered to susceptible cells in exosomes block HSV-1 replication in a dose-dependent manner. Mol Ther 26:1032–1039. doi: 10.1016/j.ymthe.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ors-Kumoglu G, Gulce-Iz S, Biray-Avci C. 2019. Therapeutic microRNAs in human cancer. Cytotechnology 71:411–425. doi: 10.1007/s10616-018-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee S-S. 2017. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids 8:132–143. doi: 10.1016/j.omtn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karstentischer B, von Einem J, Kaufer B, Osterrieder N. 2006. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques 40:191–197. doi: 10.2144/000112096. [DOI] [PubMed] [Google Scholar]
- 25.Markus A, Lebenthal-Loinger I, Yang IH, Kinchington PR, Goldstein RS. 2015. An in vitro model of latency and reactivation of varicella zoster virus in human stem cell-derived neurons. PLoS Pathog 11:e1004885. doi: 10.1371/journal.ppat.1004885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. 2010. Computer control of microscopes using μManager. Curr Protoc Mol Biol 92:14.20.1–14.20.17. doi: 10.1002/0471142727.mb1420s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson S, Mercier S, Bye C, Wilkinson J, Cunningham AL, Harman AN. 2007. Determination of suitable housekeeping genes for normalisation of quantitative real time PCR analysis of cells infected with human immunodeficiency virus and herpes viruses. Virol J 4:130. doi: 10.1186/1743-422X-4-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohrs RJ, Gilden DH. 2007. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J Virol 81:2950–2956. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]