Although new clinical importance has been revealed for human herpesviruses 6A (HHV-6A) and 6B, much is still unknown about the life cycles of these viruses in target cells. We identified a novel cellular factor, gp96, that is critical for both HHV-6A and -6B entry into host cells. As gp96 can function as an adjuvant in vaccine development for both infectious agents and cancers, it can be a potential therapeutic target for infection by these two viruses.
KEYWORDS: HHV-6, entry, gp96
ABSTRACT
Human herpesviruses 6A and 6B (HHV-6A and HHV-6B, respectively) are two virus species in the betaherpesvirus subfamily that exhibit T cell tropism. CD46 and CD134 are the cellular receptors for HHV-6A and HHV-6B, respectively. Interestingly, the efficiency of HHV-6A/6B entry is different among different types of target cells despite similar receptor expression levels on these cells. Here, we found that the cellular factor gp96 (also known as glucose-regulated protein 94 [GRP94]) is expressed on the cell surface and interacts with viral glycoprotein Q1 (gQ1) during virus entry. gp96 cell surface expression levels are associated with the efficiency of HHV-6A and HHV-6B entry into target cells. Both loss-of-function and gain-of-function experiments indicated that gp96 plays an important role in HHV-6 infection. Our findings provide new insight into the HHV-6 entry process and might suggest novel therapeutic targets for HHV-6 infection.
IMPORTANCE Although new clinical importance has been revealed for human herpesviruses 6A (HHV-6A) and 6B, much is still unknown about the life cycles of these viruses in target cells. We identified a novel cellular factor, gp96, that is critical for both HHV-6A and -6B entry into host cells. As gp96 can function as an adjuvant in vaccine development for both infectious agents and cancers, it can be a potential therapeutic target for infection by these two viruses.
INTRODUCTION
Human herpesvirus 6 (HHV-6), which consists of two virus species (HHV-6A and HHV-6B), now belongs to the betaherpesvirus subfamily. Initially, HHV-6A and HHV-6B were classified as two variants based on their different genetic, antigenic, and growth characteristics (1, 2). Later, they were officially classified as two different virus species by the International Committee on Taxonomy of Viruses (ICTV) (3). HHV-6 primary infection causes exanthem subitum (4, 5). HHV-6 mostly infects infants between 6 and 12 months of age and establishes lifelong latency (6). Reactivation of HHV-6 in immunosuppressed or immunocompromised patients can cause severe disease (7, 8).
Herpesvirus entry into host cells (generally referring to the steps of receptor binding, fusion of the virion and plasma membranes, and delivery of the tegument and capsid to the cytoplasm) requires the coordinated action of multiple viral and cellular factors. Numerous factors have been identified, and the general viral entry process can be summarized based on the prototype entry processes of herpes simplex viruses 1 and 2 (HSV-1 and HSV-2, respectively). After attachment of the virus to the cell surface, viral glycoprotein X (gX), where X is the glycoprotein of a given virus, or its complex, gH/gL/gX, binds to its cellular receptor, which plays a key role in virus entry. Subsequently, gH/gL and gB contribute to fusion of the viral envelope with the cellular membrane, which can occur either on the cell surface or intracellularly after endocytosis of viral particles. In addition to the cellular receptors for gX or the gH/gL/gX complex, other cellular factors that interact with viral glycoproteins (e.g., paired immunoglobulin-like type 2 receptor α [PILRα] and myelin-associated glycoprotein [MAG] for HSV-1 gB [9, 10]) have also been identified. Furthermore, multiple cellular receptors for a single viral ligand, e.g., gX or the gH/gL/gX complex, have also been reported (e.g., nectin-1, nectin-2, 3-O-sulfated heparan sulfate [3-OS HS], and herpesvirus entry mediator [HVEM] for HSV gD [11–14] and CD21 and CD35 for Epstein-Barr virus [EBV] gp350 [15, 16]). These factors bind independently to the viral ligands during virus entry. Novel cellular factors for herpesvirus glycoprotein binding have continued to be identified, not only enhancing our understanding of detailed entry processes of these viruses, especially with regard to viral tropism, but also aiding in the development of antiviral therapies (17–19).
Both HHV-6A and HHV-6B infect CD4 T cells efficiently, although the target cell ranges of these two viruses differ and include a variety of cell types (20–23). Initially, CD46 was identified as the cellular receptor for both HHV-6A and HHV-6B (24). Later, Mori et al. found that CD46 is the cellular receptor for HHV-6A but not for HHV-6B (25) (at least not for all HHV-6B strains [26]). Furthermore, the group found that CD134 is the authentic receptor for HHV-6B infection (27). Both viral ligands for these two cellular receptors are gH/gL/gQ1/gQ2 complexes, and they share high amino acid sequence identity (approximately 94% for gH and gL and approximately 70% for glycoprotein Q1 [gQ1] and gQ2) (28–30). Other than CD46 and CD134, few cellular factors have been identified.
In the present study, we sought to identify cellular binding partners of HHV-6 envelope glycoproteins by coimmunoprecipitation followed by mass spectrometry analysis. We found that the gp96 protein specifically binds to the HHV-6 gQ1 protein. gp96, also known as glucose-regulated protein 94 (GRP94), is a multifunctional glycoprotein. Although it belongs to the HSP90 family and localizes mainly within the lumen of the endoplasmic reticulum (ER), it is also expressed on the cell surface (31–33) and functions in both innate and adaptive immunity (34, 35). In addition, a variety of pathogens or their products (toxins) bind to gp96, which initiates the entry of these pathogens into target cells (36–39). Increased cell surface expression of gp96 has been observed to correlate with increased tumor immunogenicity; therefore, gp96 is an attractive therapeutic target for cancer (40).
In a previous study, Prusty et al. (41) reported that gp96 interacts with HHV-6A virions and contributes to HHV-6A attachment to target cells during infection of the HSB-2 cell line; they did not identify viral protein binding to gp96. The authors also found that HHV-6A infection promotes gp96 cell surface expression. In the present study, we performed detailed analyses on a set of HHV-6-permissive cell lines and primary cells and expanded the previous findings. We found that both HHV-6A and HHV-6B gQ1 interact with gp96, that cell surface expression of gp96 is relatively consistent with HHV-6 infection efficacy in target cells, and that gp96 facilitates the entry of both HHV-6A and HHV-6B into their target cells.
RESULTS
Identification of the interaction of gp96 with HHV-6 gQ1.
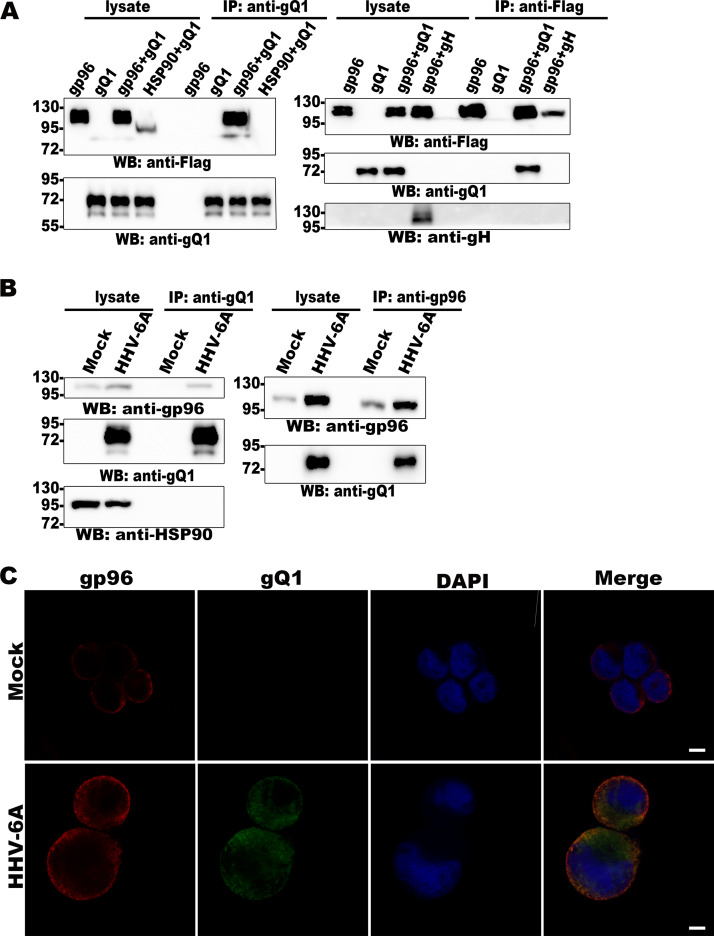
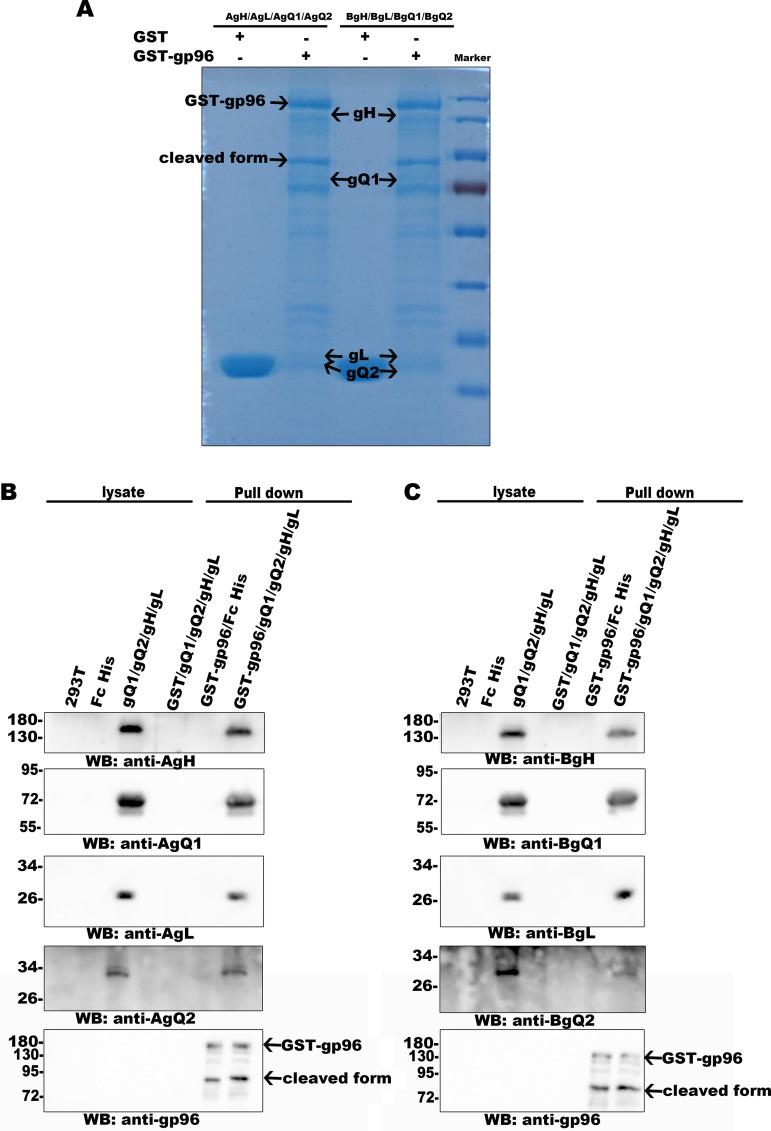
It is well known that some viral glycoproteins on enveloped virions play important roles in virus entry. To fulfil this role, most glycoproteins need to bind to cellular factors, and a single viral glycoprotein can sometimes bind to more than one cellular partner. Identification and characterization of these cellular factors will greatly contribute not only to our understanding of the virus entry process but also to the development of treatment methods for virus infection. In the present study, we performed a screen to identify cellular factors binding to HHV-6 glycoproteins and found that the factor gp96 bound to HHV-6 gQ1 by coimmunoprecipitation followed by mass spectrometry (Fig. 1A) and immunoblot analysis (Fig. 1B). HHV-6 gQ1 is a relatively large glycoprotein (approximately 80 kDa in HHV-6 virions [42]) that forms a complex with gH, gL, and gQ2 (the gH/gL/gQ1/gQ2 complex); this complex functions as a viral ligand that binds to CD46 or CD134 during HHV-6A or HHV-6B infection, respectively (24, 27, 42, 43). Other cellular binding partners of gQ1 have not yet been identified. We confirmed this finding with coimmunoprecipitation and Western blot analyses. gp96, but not its homologous family member HSP90, coprecipitated with gQ1, as determined with an anti-gQ1 antibody (Fig. 2A, left; Fig. 3A). In addition, gQ1 coprecipitated with gp96, as determined with an anti-Flag antibody for Flag-tagged gp96; however, another HHV-6 glycoprotein, gH, did not coprecipitate with gp96 (Fig. 2A, right; Fig. 3A). It has been reported that the secreted form of the gH/gL/gQ1/gQ2 complex produced by the individual complex component-expressing cells can bind to CD46 (HHV-6A) or CD134 (HHV-6B) (25, 27). Thus, we next tested whether gp96 could bind to this form of the gH/gL/gQ1/gQ2 complex by pulldown assay, and we found that GST-gp96, but not GST (glutathione S-transferase), could pull down both the HHV-6A and HHV-6B gH/gL/gQ1/gQ2 complexes (Fig. 4).
FIG 1.
Identification of gQ1 binding by immunoprecipitation and mass spectrometry analysis. (A) 293T cells were transfected with a plasmid for expression of gQ1. The cells were lysed and immunoprecipitated with an anti-gQ1 antibody or an isotype control antibody. The immunoprecipitates were separated in a denaturing gel and silver stained. (B) gQ1-expressing or its control cells were used for the immunoprecipitation assay using anti-gQ1 and an isotype control antibody followed by immunoblot analysis.
FIG 2.
HHV-6A gQ1 interacts with gp96. (A) 293T cells were transfected with gQ1, gH, gp96Flag, and HSP90Flag expression plasmids (indicated at the top of each lane). The cell lysates were subjected to immunoprecipitation using an anti-gQ1 (left) or anti-Flag (right) antibody, and immunoblot detection was performed with anti-gQ1, anti-Flag, and anti-gH antibodies. (B) Mock- or HHV-6A-infected HSB-2 cells were lysed and subjected to immunoprecipitation using anti-gQ1 (left) and anti-gp96 antibodies (right), and immunoblot detection of gQ1, gp96, and HSP90 was performed. (C) Mock- or HHV-6A-infected CBMCs were fixed and subjected to indirect IFA using anti-gQ1 (green) and anti-gp96 (red) antibodies and DAPI (blue). Scale bar = 5 μm.
FIG 3.
HHV-6B gQ1 interacts with gp96. (A) HHV-6B gQ1 coprecipitated with gp96. 293T cells were transfected with plasmids for expression of gQ1 and Flag-tagged gp96, as indicated at the top of each lane. The cells were lysed for immunoprecipitation using anti-gQ1 and anti-Flag antibodies, and immunoblot analysis was subsequently performed. (B) Mock- or HHV-6B-infected CBMCs were lysed and prepared for immunoprecipitation assays using anti-gQ1 and anti-gp96 antibodies. Immunoblot detection of gQ1 and gp96 was then performed. (C) Colocalization of gp96 with gQ1 in HHV-6B-infected MT4 cells. HHV-B-infected MT4 cells were fixed for indirect immunofluorescence assays using anti-gQ1 (green) and anti-gp96 (red) antibodies and DAPI (4′,6-diamidino-2-phenylindole) (blue). Scale bar = 5 μm.
FIG 4.
The secreted form of the gH/gL/gQ1/gQ2 complex binds to gp96. GST and gp96-GST were incubated with glutathione beads at 4°C for 8 h. Then, the beads were incubated with purified gH/gL/gQ1/gQ2 (secreted form) or Fc protein at 4°C for 8 h. Proteins that bound to the glutathione beads were eluted, separated by gel electrophoresis, and then stained with CBB (A). Immunoblot analysis with appropriate antibodies (HHV-6A gH/gL/gQ1/gQ2 complex [B]; HHV-6B gH/gL/gQ1/gQ2 complex [C]) was then performed.
The gQ1 and gp96 interaction in HHV-6A- and HHV-6B-infected cells was also confirmed in a coimmunoprecipitation assay using anti-gQ1 (Fig. 2B, left; Fig. 3B) and anti-gp96 (Fig. 2B, right; Fig. 3B) antibodies. Finally, the colocalization of these two proteins in infected cells was confirmed by immunofluorescence assay (IFA) (Fig. 2C, Fig. 3C).
gp96 expression is consistent with the efficiency of HHV-6 infection.
In fluorescence microscopy tests, the intracellular distribution of gp96 was dramatically altered in HHV-6-infected cells compared with uninfected cells. gp96 was diffusely expressed in uninfected cells. In HHV-6-infected cells, gp96 was expressed near/at the plasma membrane and colocalized with gQ1 (Fig. 2C, Fig. 3C). To analyze the mechanism in detail, we first measured the cell surface expression of gp96 in a set of HHV-6A- or HHV-6B-permissive cells by fluorescence-activated cell sorter (FACS) analysis and found that the gp96 expression levels dramatically differed among these cells. HSB-2 cells, MT4 cells, and umbilical cord blood mononuclear cells (CBMCs) showed relatively higher cell surface expression of gp96 than JJhan and Molt3 cells (Fig. 5A). We also tested whether cell surface expression of gp96 contributed to HHV-6 infection by detecting viral immediate early 1 (IE1) expression after virus entry. In addition to CBMCs, HHV-6A (GS strain) infected HSB-2 cells more efficiently than it infected JJhan cells, consistent with the gp96 cell surface expression results (Fig. 5B, left). HHV-6B (Z29 strain) infected MT4 cells more efficiently than it infected Molt3 cells (Fig. 5B, right). Another HHV-6A strain, U1102 (a JJhan-adapted virus strain) more efficiently infected MT4, JJhan, and HSB-2 cells than Molt3 cells (Fig. 5B, middle). These results indicate that gp96 is associated with HHV-6 infection efficiency; however, other factor(s) may also be involved.
FIG 5.
The expression of gp96 is consistent with the efficiency of HHV-6 infection. (A) Detection of gp96 cell surface expression in a panel of T cell lines and CBMCs. The cells were stained with anti-gp96 and secondary antibodies for FACS analysis. (B) The cells in panel A were infected with the HHV-6A GS strain (left) or the U1102 strain (middle) or with HHV-6B (right) for 24 h and then subjected to IE1 and actin immunoblot detection.
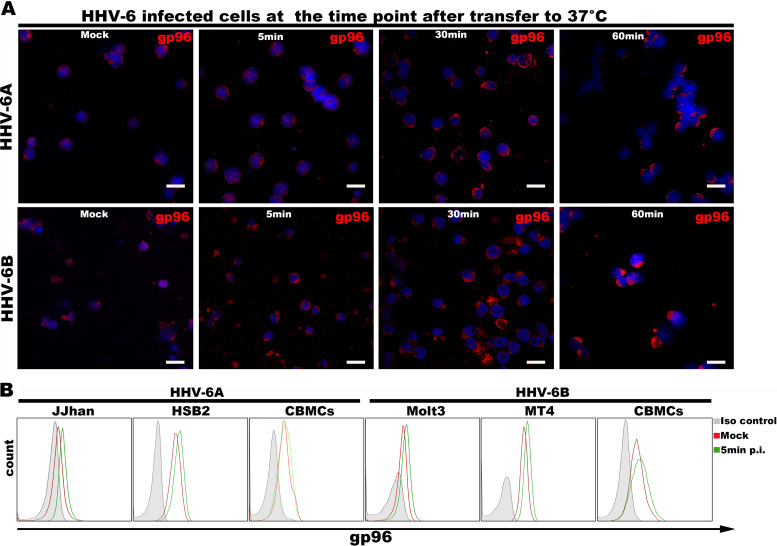
Next, we tested whether redistribution of gp96 in HHV-6-infected cells would enhance cell surface expression of gp96 by observing the overall cellular distribution of gp96 by fluorescence microscopy and analyzing the cell surface expression of gp96 by FACS analysis during the HHV-6 entry process. We found that gp96 was redistributed at a very early stage of virus entry, and this redistribution became more obvious after 30 min postinfection (Fig. 6A). Cell surface expression of gp96 was induced even at 5 min postinfection (Fig. 6B) (43).
FIG 6.
HHV-6 infection affects gp96 intracellular localization. (A) HSB-2 (top) or MT4 (bottom) cells were incubated with HHV-6 at 4°C for 1 h and then shifted to 37°C. The cells were harvested at 5 min, 30 min, and 60 min after transfer to 37°C and were then fixed and stained with anti-gp96 and secondary antibodies for fluorescence microscopy. Scale bar = 20 μm. (B) Detection of gp96 cell surface expression in HHV-6-infected cells. The cells were incubated with HHV-6 at 4°C for 1 h, transferred to 37°C for 5 min, and then prepared for FACS analysis.
Knockdown of gp96 inhibits HHV-6 infection.
As some HHV-6-permissive cells strongly expressed gp96 and could be induced to express more protein on the cell surface, we suspected that gp96 contributed to HHV-6 entry. Thus, we performed several experiments to investigate this hypothesis. First, we knocked down gp96 expression in HSB-2 cells, MT4 cells and CBMCs using a short hairpin (shRNA)-expressing lentivirus. Compared with the expression in control cells, the total expression (Fig. 7A to C) and cell surface expression (data not shown) of gp96 were significantly decreased in cells transduced with the gp96 shRNA-expressing lentivirus but not in cells transduced with a control shRNA-expressing lentivirus. We also confirmed that knockdown of gp96 in these cells had little effect on the cell surface expression of CD134 and CD46 (by FACS analysis; Fig. 7D) or on the endocytic pathway of the transferrin receptor (Fig. 7E and F) (44). We further infected the transduced cells with HHV-6A or HHV-6B and analyzed virus entry at 24 h postinfection by measuring IE1 protein and viral genome expression. Both viral genome and IE1 expression were dramatically decreased in gp96 knockdown cells compared with control cells, indicating that gp96 plays a role during HHV-6 infection (Fig. 7A to C and G). Then, we expressed exogenous gp96 in the knockdown cells and found that gp96 expression could only partially rescue the infection efficiency of HHV-6 in these cells (Fig. 7H and I; indicated as IE1 expression). We also tried to express gp96 in JJhan and Molt3 cells; however, we could not detect upregulated cell surface expression of gp96 (data not shown), suggesting that gp96 cell surface expression needed the assistance of other factors.
FIG 7.
Knockdown of gp96 inhibits HHV-6 infection, and exogenous expression of gp96 partially rescues it. (A to C) HSB-2 cells (A), MT4 cells (B), and CBMCs (C) were transduced with control shRNA- or gp96shRNA-expressing lentiviruses for 2 days and then infected with HHV-6A or HHV-6B. At 24 h postinfection, IE1 expression in these cells was detected using immunoblot analysis. (D) CD46 (left) or CD134 (right) expression in gp96 knockdown cells. Control shRNA- or gp96shRNA-expressing HSB-2 and MT4 cells were stained with an anti-CD46 or anti-CD134 antibody followed by a secondary antibody. Then, the cells were analyzed using FACS. (E to F) gp96 knockdown did not affect the endocytic pathway. gp96 shRNA-expressing cells or control cells were treated with 50 μg/ml Alexa 488-labeled transferrin for 1 h at 4°C and then incubated at 37°C for 15 min. After being washed with acid stripping buffer, the cells were analyzed with a fluorescence microscope (E) and FACS (F). Scale bar = 10 μm. (G) gp96 knockdown or control cells were infected with HHV-6 for 2 h at 37°C and were then washed and processed for reverse transcriptase quantitative PCR (RT-qPCR) analysis for viral DNA quantification. Mean ± standard error of the mean (SEM); n = 3. The data represented one of three independent experiments. (H) MT4 cells were transduced with gp96shRNA- or control shRNA-expressing lentiviruses. The gp96 knockdown cells were transduced with control- or gp96-expressing lentiviruses for rescue. The cells (indicated at the top of each lane) were lysed for immunoblot analysis with anti-gp96 and anti-actin antibodies. (I) The cells generated in panel H were infected with HHV-6B, and IE1 expression at 24 h postinfection was detected by immunoblot analysis.
Soluble gp96 protein blocks HHV-6 infection.
We constructed a soluble gp96 protein by deleting the ER retention signal of the full-length protein, fusing the truncated protein with the Fc fragment of human IgG1 and tagging the fusion protein with 6 histidine (His) at its C terminus for purification. We then incubated HHV-6 with different concentrations of recombinant gp96 or His-tagged Fc control protein before using these viruses to infect HSB-2 (Fig. 8A, top) or MT4 cells (Fig. 8A, bottom). Virus entry was determined at 24 h postinfection by detection of IE1 expression. We found that the soluble gp96 protein, but not the Fc protein, inhibited HHV-6 infection in a dose-dependent manner.
FIG 8.
gp96 is required for HHV-6 infection. (A) HHV-6A (top) and HHV-6B (bottom) were incubated with a series of concentrations of soluble gp96 protein, and then HSB-2 or MT4 cells were infected with these viruses for 24 h. The cell lysates were immunoblotted for HHV-6 IE1 and actin protein detection. (B) An anti-gp96 antibody inhibited HHV-6 infection. HSB-2 (top) and MT4 (bottom) cells were incubated with an anti-gp96 antibody and then infected with HHV-6A and HHV-6B, respectively. IE1 protein expression was detected at 24 h postinfection.
A gp96 antibody blocks HHV-6 infection.
We also generated an anti-gp96 antibody by immunizing mice with the soluble gp96 protein and analyzed whether the antibody could inhibit HHV-6 infection. HSB-2 and MT4 cells were incubated with anti-gp96 or control antibodies and then infected with HHV-6A and HHV-6B, respectively. At 24 h postinfection, IE1 expression was reduced in the anti-gp96 antibody-treated cells, which indicated that the antibody could inhibit HHV-6 infection in these target cells (Fig. 8B).
DISCUSSION
The virus entry process requires the cooperation of both cellular and viral factors. An individual virus must manipulate a unique set of host factors to establish infection. In the present study, we found that a novel cellular factor, gp96, is a binding partner of HHV-6 gQ1 and that the interaction between these molecules contributes to HHV-6 infection. Although gp96 was previously identified to interact with the HHV-6 virion, a viral partner was not found at that time (43).
Both knockdown of gp96 expression and blockade of gp96 with soluble gp96 or an antibody inhibited HHV-6 infection. These results reveal that the interaction between gp96 and gQ1 plays an important role in HHV-6 infection. As CD46 and CD134 have been reported to be HHV-6A and HHV-6B receptors, respectively, we speculated how gp96 might cooperate with these two receptors (24, 27). At least two mechanisms are possible if two or more cellular proteins bind to a viral ligand for virus entry. First, the mechanism could be similar to that by which HSV-1 gD binds to different cellular receptors (e.g., nectin-1 or HVEM) for virus entry into different types of cells (11–14). Second, the mechanism could be similar to that by which HIV-1 gp120/gp41 sequentially binds to CD4 and CCR5/CXCR4 molecules for HIV-1 infection into the same cells (45–47). Although evidence is still limited, binding of gQ1 to gp96 is more likely to follow the latter mechanism based on the following findings: (i) blockade of either CD46 or CD134 or gp96 can inhibit HHV-6 infection in the same cells (27); (ii) the binding sites of CD46/CD134 and gp96 on gQ1 (or the gH/gL/gQ1/gQ2 complex) do not completely overlap (an anti-CD46/CD134 antibody precipitated the gH/gL/gQ1/gQ2 complex and gp96 from the lysate of gH-, gL-, gQ1-, gQ2-, and gp96-expressing cells; data not shown); and (iii) gp96 alone cannot support HHV-6 infection. As shown in Fig. 5, although HSB-2 cells showed high gp96 cell surface expression, they were resistant to HHV-6B infection (27). Notably, it remains unclear how these cellular factors function together; this uncertainty will be a focus of our future studies.
Although intracellular gp96 is mainly restricted to the ER, gp96 is also expressed on the cell surface, at least in some cell types (31–33). In our present study, we found that gp96 was expressed on the cell surface in several cell lines and on primary CBMCs. Furthermore, additional cell surface gp96 expression could be induced during HHV-6 infection. However, it remained unclear how gp96 was trafficked to the cell surface. There should be other cellular factor(s) facilitating gp96 cell-surface transport, which needs further investigation.
A conformational structure formed by four components is required for the binding of the HHV-6 gH/gL/gQ1/gQ2 complex ligand to CD46; in contrast, a conformational structure formed by HHV-6B gQ1 and gQ2 is required and sufficient for binding to CD134 (24, 27). Here, we found that for both HHV-6A and HHV-6B, gQ1 alone could bind to gp96, despite the fact that gp96 naturally binds to the gH/gL/gQ1/gQ2 complex (Fig. 4); this finding is different from that regarding HHV-6 ligand binding to either CD46 or CD134. As HHV-6A and HHV-6B gQ1 share approximately 70% identity, it is highly likely that gp96 binds to the conserved region of these two gQ1 proteins; however, a more detailed analysis is required.The factor gp96 functions in both innate and acquired immune responses. The use of gp96 as an adjuvant has been a recent focus of studies on vaccine development for both infectious agents and cancer (35, 48–50). As there is still no available vaccine for HHV-6 infection, it would be useful to evaluate an HHV-6 vaccine based on the gQ1 (or complex) antigen with gp96 as an adjuvant.
MATERIALS AND METHODS
Cells and viruses.
JJhan, HSB-2, Molt3, and MT4 cells were maintained in RPMI 1640 (Gibco) supplemented with 8% fetal bovine serum (FBS; Gibco) at 37°C in 5% CO2. 293T cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% FBS. CBMCs were isolated by using Ficoll (Hao Yang Biological Manufacture Co.) and cultured in RPMI medium containing 10% FBS, phytohemagglutinin (5 μg/ml; Sigma), and interleukin-2 (IL-2) (2 ng/ml; Sigma). The HHV-6A strains U1102 and GS and the HHV-6B strain Z29 were propagated in CBMCs as described previously and infected target cells with a multiplicity of infection (MOI) of approximately 0.01 (51).
Plasmid construction.
The human gp96 gene (GenBank gene ID 7184) was amplified with PCR from human U87 cDNA using the primers 5′-ACACTCGAGAATTCGCCACCATGAGGGCCCTGTGG-3′ and 5′-ACACTCGAGTTACTTGTCGTCATCGTCTTTGTAGTCCAATTCATCTTTTTC-3′ (containing a Flag tag sequence) and cloned into the pCAGGS vector to generate pCAGGSgp96Flag. To construct the secreted proteins, an Fc fragment of human IgG1 was mutated at residues 266 and 267 (the two leucines were mutated to alanine and glutamine, respectively) to reduce the binding affinity to cellular Fc receptors, and this fragment was tagged with the IL-2 signal sequence. To generate the secreted form of gp96 (base pairs 64 to 2412), the sequence for ectodomain expression was amplified from the pCAGGSgp96Flag vector using the primers 5′-ACAAGATCTGACGATGAAGTTGATG-3′ and 5′-ACAAGATCTCAATTCATCTTTTTCAG-3′, ligated with the Fc fragment containing a 6 histidine sequence at its 3′ end, and cloned into the pCAGGS plasmid. The plasmid pCAGGSHSP90Flag was used for expression of HSP90Flag and was constructed by amplifying the sequence from U87 cDNA with the primers 5′-ACAAGATCTGCCACCATGCCTGAGGAAGTGC-3′ and 5′-ACAGATCTTACTTGTCGTCATCGTCTTTGTAGTCATCGACTTCTTCCATG-3′ (containing a Flag tag sequence). The gene sequences for gQ1, gQ2, gH, and gL expression have been described previously; these sequences were cloned into the pCAGGS plasmid (gQ2 was tagged with a streptavidin-binding peptide and gL with HA tags at their C termini) (25, 43). For the knockdown experiment, we modified the original pLKO.1 plasmid by amplifying the plasmid with the primers 5′-ACAGAATTCACCGGTGTTTCGTCCTTTC-3′ and 5′-ACAGAATTCTCGACCTCGAG-3′, digesting the PCR product with EcoRI (TaKaRa), and ligating the digested DNA fragment, which resulted in new plasmids containing EcoRI and AgeI (TaKaRa) sites for oligonucleotide insertion. The oligonucleotides 5′-GATCCCAAGTTGATGTGGATGGTACAGTTCAAGAGACTGTACCATCCACATCAACTTTTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAAAAGTTGATGTGGATGGTACAGTCTCTTGAACTGTACCATCCACATCAACTTGG-3′ for gp96 were annealed and cloned into the AgeI and EcoRI sites of the pLKO.1 vector. The control oligonucleotides 5′-CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTT-3′ and 5′-AATTAAAAACAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTG-3′ were also annealed and cloned into the pLKO.1 vector. HHV-6 IE1 plasmids for antigen protein expression were constructed as previously described (52, 53). The plasmids for CD46 and CD134 expression were constructed as previously described (27).
Fc fusion protein.
The plasmids for Fc or Fc fusion protein expression were transfected into 293T cells using polyethyleneimine (PEI; Polysciences) according the manufacturer’s instructions. Two days after transfection, the soluble proteins in the culture medium were purified using Ni-NTA (30210; Qiagen) affinity chromatography.
Antibodies.
To generate anti-gp96 antibodies, BALB/c mice were subcutaneously immunized with gp96Fc fusion proteins via the pads of their hind feet. Inguinal lymph nodes were subsequently retrieved and fused with SP2 myeloma cells. Hybridomas secreting gp96Fc-specific antibodies were selected. UV-inactivated virions (GS strain) were used as antigens for immunization of mice to raise anti-gH and anti-gQ1 antibodies. To generate antibodies for HHV-6 IE1, recombinant HHV-6A and HHV-6B IE1 proteins were purified from Escherichia coli cells (BL21 strain) and used to immunize mice. Mouse IgG isotype control (A7028; Beyotime) and mouse monoclonal antibodies against β-actin (60008-1; Proteintech), CD46 (J4.48; Abnova Corporation), CD134 (ACT35; CST), and Flag (F1804; Sigma) were purchased.
Coimmunoprecipitation.
Antibodies were bound to protein G-Sepharose (GE Healthcare) by incubation at 4°C for 8 h and then cross-linked with protein G-Sepharose with dimethyl pimelimidate (DMP; Thermo Scientific) according to the manufacturer’s instructions. The cells were lysed in TNE buffer (10 mM Tris · HCl [pH 7.8], 0.15 M NaCl, 1 mM EDTA, and 1% Nonidet P-40 [Nacalai Tesque] containing protease inhibitor cocktail [Sigma-Aldrich]) for 30 min at 4°C. After centrifugation at 13,000 × g for 1 h at 4°C, the supernatant was incubated with the appropriate protein G-Sepharose-bound antibody at 4°C for 8 h. The bound proteins were eluted with 0.1 M glycine (pH 2.8) at 4°C, collected, and neutralized with 1 M Tris-HCl (pH 9.0) to pH 7.0 to 7.4. The samples were suspended in 5× SDS sample buffer, boiled for 5 min at 100°C, and then analyzed by Western blotting with the indicated antibodies.
Mass spectrometry.
The above immunoprecipitates were electrophoretically separated in a denaturing gel and visualized with silver staining. The remaining immunoprecipitates were digested with trypsin and analyzed using mass spectrometry analysis on an AB Sciex TripleTOF 5600+ system (Novogene, China) (54).
Western blot analysis.
Samples were separated by SDS-PAGE, electrotransferred onto polyvinylidene difluoride (PVDF) membranes, and reacted with primary antibodies. The reactive bands were detected using horseradish peroxidase-conjugated secondary antibodies (Santa Cruz) and visualized using enhanced chemiluminescence (ECL) reagents.
Establishment of cell lines stably expressing shRNA against human gp96.
A gp96 shRNA-expressing lentivirus and its control were generated by transfecting 293T cells with packaging plasmids (pCAG-HIV-gag and pCMV-VSV-G-RSV-Rev) and the pLKO.1gp96 plasmid or a pLKO.1 control plasmid. The culture media containing the viruses were harvested at 3 days after transfection and centrifuged at 12,000 × g for 1 h. Cells were transduced with the lentiviruses for 1 day and selected with puromycin (0.2 to 1 μg/ml) in maintenance medium.
Transferrin uptake assay.
Control and gp96 knockdown cells were treated with 50 μg/ml Alexa 488-labeled transferrin (T13342; Thermo Scientific) for 1 h at 4°C and then incubated at 37°C for 15 min. After being washed with citric acid buffer (0.1 M, pH 3.0), the cells were analyzed with FACS analysis.
Quantitative real-time PCR analysis for viral genome quantification.
Factor gp96 knockdown or corresponding control cells (5 × 105 cells for each sample) were infected with HHV-6 for 2 h at 37°C and then washed with 0.25% trypsin-EDTA. Total DNA was extracted from the infected cells with a nucleic acid extraction kit (DP304; Tiangen). Viral DNA was quantified using U22 primers (5′-CGCTCGGAAAGGAAACATTA-3′ and 5′-AAGTGGAACTGCTTGGTGGC-3′) on a 7500 fast PCR system (Applied Biosystems) using TB Green Premix Ex Taq (TaKaRa). The data were normalized to the expression of actin (forward primer: 5′-TGGCACCCAGCACAATGAA-3′, reverse primer: 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′).
Pulldown assay.
The secreted form of the gH/gL/gQ1/gQ2 complex (The ectodomain of gH was tagged with Fc and 6× His.) and the Fc protein were purified from the culture medium of their expression cells as described previously (27, 55). GST and GST-gp96 were incubated with glutathione beads (17-5130; GE Healthcare) at 4°C for 8 h. The beads were washed 5 times with phosphate-buffered saline (PBS) and then incubated with purified gH/gL/gQ1/gQ2 complex and/or Fc protein at 4°C for 8 h. The beads were washed with PBS three times, and the proteins bound to the beads were eluted with elution buffer (C600325; Sangon Biotech) for Coomassie brilliant blue (CBB) staining and immunoblot analysis with appropriate antibodies.
Infection inhibition assay.
To analyze the inhibitory effect of an anti-gp96 antibody on HHV-6 infection, MT4 and HSB-2 cells were pretreated with the anti-gp96 polyclonal antibody or control serum at 37°C for 30 min and then inoculated with cell-free HHV-6A and HHV-6B, respectively. After viral adsorption for 60 min, the inoculum was removed, and the cells were refed with the appropriate medium for 24 h. Then, these samples were lysed with radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris · HCl [pH 7.4], 0.15 M NaCl, 1 mM EDTA, 0.1% SDS, 0.1% sodium deoxycholate, and 1% Nonidet P-40 [Nacalai Tesque] containing protease inhibitor cocktail [Sigma]) and used for immunoblot analysis.
To analyze the inhibitory effect of soluble gp96 on HHV-6 infection, cell-free HHV-6A and HHV-6B were incubated with gp96Fc (diluted 4-fold from 20 μg) for 30 min, and then these viruses were used to infect MT4 and HSB-2 cells (5 × 105), respectively, at 37°C for 1 h. The Fc protein at the same concentration was used as a control. The cells were cultured in 1 ml of medium for 24 h and then lysed with RIPA buffer for immunoblot analysis.
Immunofluorescence confocal microscopy.
Mock- or HHV-6A/6B-infected cells were fixed and stained with the indicated primary antibodies. After being washed three times, the cells were incubated with secondary antibodies, washed three more times, and then fixed. Confocal images were acquired using a Zeiss LSM 710 confocal laser scanning microscope.
Cell surface expression assay.
Cells were incubated with an isotype control or with an anti-gp96, anti-CD46, or anti-CD134 antibody at 4°C for 1 h, washed one time with cold PBS, and incubated with a secondary antibody at 4°C for another 1 h. After being washed twice, the cells were analyzed on a flow cytometer (CytoFLEX; Beckman). Cell surface-bound fluorescence was detected with a solid-state laser (488 nm) and analyzed with FlowJo 10 software.
ACKNOWLEDGMENTS
We thank Yasuko Mori (Kobe University), Junichi Miyazaki (Osaka University, Japan), and Hiroyuki Miyoshi (RIKEN BRC, Japan) for providing reagents.
This work was supported by the National Natural Science Foundation of China (grants 81571979 and 81273235), the Plan of the Jiangsu Innovative and Entrepreneurial Team (303073227), and the Natural Science Foundation of Jiangsu Province of China (grant BK20171489).
REFERENCES
- 1.Aubin JT, Collandre H, Candotti D, Ingrand D, Rouzioux C, Burgard M, Richard S, Huraux JM, Agut H. 1991. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J Clin Microbiol 29:367–372. doi: 10.1128/JCM.29.2.367-372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campadelli-Fiume G, Guerrini S, Xiaoming L, Foa-Tomasi L. 1993. Monoclonal antibodies to glycoprotein B differentiate human herpesvirus 6 into two clusters, variants A and B. J General Virology 74:2257–2262. doi: 10.1099/0022-1317-74-10-2257. [DOI] [PubMed] [Google Scholar]
- 3.Ablashi D, Agut H, Alvarez-Lafuente R, Clark DA, Dewhurst S, Diluca D, Flamand L, Frenkel N, Gallo R, Gompels UA, Hollsberg P, Jacobson S, Luppi M, Lusso P, Malnati M, Medveczky P, Mori Y, Pellett PE, Pritchett JC, Yamanishi K, Yoshikawa T. 2014. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol 159:863–870. doi: 10.1007/s00705-013-1902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates M, Monze M, Bima H, Kapambwe M, Clark D, Kasolo FC, Gompels UA. 2009. Predominant human herpesvirus 6 variant A infant infections in an HIV-1 endemic region of sub-Saharan Africa. J Med Virol 81:779–789. doi: 10.1002/jmv.21455. [DOI] [PubMed] [Google Scholar]
- 5.Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet 1:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 6.Okuno T, Takahashi K, Balachandra K, Shiraki K, Yamanishi K, Takahashi M, Baba K. 1989. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J Clin Microbiol 27:651–653. doi: 10.1128/JCM.27.4.651-653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark DA. 2002. Human herpesvirus 6 and human herpesvirus 7: emerging pathogens in transplant patients. Int J Hematol 76(Suppl 2):246–252. doi: 10.1007/bf03165124. [DOI] [PubMed] [Google Scholar]
- 8.Zerr DM, Corey L, Kim HW, Huang ML, Nguy L, Boeckh M. 2005. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis 40:932–940. doi: 10.1086/428060. [DOI] [PubMed] [Google Scholar]
- 9.Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, Arase H. 2010. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc Natl Acad Sci U S A 107:866–871. doi: 10.1073/pnas.0913351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh T, Arii J, Suenaga T, Wang J, Kogure A, Uehori J, Arase N, Shiratori I, Tanaka S, Kawaguchi Y, Spear PG, Lanier LL, Arase H. 2008. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell 132:935–944. doi: 10.1016/j.cell.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 12.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 13.Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery RI, Warner MS, Lum BJ, Spear PG. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 15.Tanner J, Weis J, Fearon D, Whang Y, Kieff E. 1987. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell 50:203–213. doi: 10.1016/0092-8674(87)90216-9. [DOI] [PubMed] [Google Scholar]
- 16.Ogembo JG, Kannan L, Ghiran I, Nicholson-Weller A, Finberg RW, Tsokos GC, Fingeroth JD. 2013. Human complement receptor type 1/CD35 is an Epstein-Barr Virus receptor. Cell Rep 3:371–385. doi: 10.1016/j.celrep.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu K, Oberstein A, Wang W, Shenk T. 2018. Role of PDGF receptor-alpha during human cytomegalovirus entry into fibroblasts. Proc Natl Acad Sci U S A 115:E9889–E9898. doi: 10.1073/pnas.1806305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Martin N, Marcandalli J, Huang CS, Arthur CP, Perotti M, Foglierini M, Ho HD, Dosey AM, Shriver S, Payandeh J, Leitner A, Lanzavecchia A, Perez L, Ciferri C. 2018. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell 174:1158–1171.e19. doi: 10.1016/j.cell.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Sathiyamoorthy K, Zhang X, Schaller S, Perez White BE, Jardetzky TS, Longnecker R. 2018. Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus. Nat Microbiol 3:172–180. doi: 10.1038/s41564-017-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, Sonoda S, Higashi K, Kondo T, Takahashi H, Takahashi M, Yamanishi K. 1989. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J Virol 63:3161–3163. doi: 10.1128/JVI.63.7.3161-3163.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lusso P, Malnati MS, Garzino-Demo A, Crowley RW, Long EO, Gallo RC. 1993. Infection of natural killer cells by human herpesvirus 6. Nature 362:458–462. doi: 10.1038/362458a0. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Popescu N, Woodworth C, Berneman Z, Corbellino M, Lusso P, Ablashi DV, DiPaolo JA. 1994. Human herpesvirus 6 infects cervical epithelial cells and transactivates human papillomavirus gene expression. J Virol 68:1173–1178. doi: 10.1128/JVI.68.2.1173-1178.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cermelli C, Concari M, Carubbi F, Fabio G, Sabbatini AM, Pecorari M, Pietrosemoli P, Meacci M, Guicciardi E, Carulli N, Portolani M. 1996. Growth of human herpesvirus 6 in HEPG2 cells. Virus Res 45:75–85. doi: 10.1016/s0168-1702(96)01364-0. [DOI] [PubMed] [Google Scholar]
- 24.Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- 25.Tang H, Hayashi M, Maeki T, Yamanishi K, Mori Y. 2011. HHV-6 glycoprotein complex formation is required for folding and trafficking of the gH/gL/gQ1/gQ2 complex and its cellular receptor binding. J Virol 85:11121–11130. doi: 10.1128/JVI.05251-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen AS, Bundgaard BB, Biltoft M, Rossen LS, Hollsberg P. 2017. Divergent tropism of HHV-6AGS and HHV-6BPL1 in T cells expressing different CD46 isoform patterns. Virology 502:160–170. doi: 10.1016/j.virol.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 27.Tang H, Serada S, Kawabata A, Ota M, Hayashi E, Naka T, Yamanishi K, Mori Y. 2013. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proc Natl Acad Sci U S A 110:9096–9099. doi: 10.1073/pnas.1305187110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isegawa Y, Mukai T, Nakano K, Kagawa M, Chen J, Mori Y, Sunagawa T, Kawanishi K, Sashihara J, Hata A, Zou P, Kosuge H, Yamanishi K. 1999. Comparison of the complete DNA sequences of human herpesvirus 6 variants A and B. J Virol 73:8053–8063. doi: 10.1128/JVI.73.10.8053-8063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominguez G, Dambaugh TR, Stamey FR, Dewhurst S, Inoue N, Pellett PE. 1999. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J Virol 73:8040–8052. doi: 10.1128/JVI.73.10.8040-8052.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gompels UA, Nicholas J, Lawrence G, Jones M, Thomson BJ, Martin ME, Efstathiou S, Craxton M, Macaulay HA. 1995. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- 31.Hou J, Li X, Li C, Sun L, Zhao Y, Zhao J, Meng S. 2015. Plasma membrane gp96 enhances invasion and metastatic potential of liver cancer via regulation of uPAR. Mol Oncol 9:1312–1323. doi: 10.1016/j.molonc.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Sun L, Hou J, Gui M, Ying J, Zhao H, Lv N, Meng S. 2015. Cell membrane gp96 facilitates HER2 dimerization and serves as a novel target in breast cancer. Int J Cancer 137:512–524. doi: 10.1002/ijc.29405. [DOI] [PubMed] [Google Scholar]
- 33.Robert J, Ménoret A, Srivastava P, Cohen N. 1997. Cell surface expression of the endoplasmic reticular heat shock protein gp96 is phylogenetically conserved. J Immunology 21:99–4139. doi: 10.1016/S0145-305X(97)88534-5. [DOI] [PubMed] [Google Scholar]
- 34.Gulic T, Laskarin G, Redzovic A, Eminovic S, Haller H, Rukavina D. 2013. The significance of heat-shock protein gp96 and its receptors’ CD91 and Toll-like receptor 4 expression at the maternal foetal interface. Am J Reprod Immunol 70:10–23. doi: 10.1111/aji.12096. [DOI] [PubMed] [Google Scholar]
- 35.Ju Y, Fan H, Liu J, Hu J, Li X, Li C, Chen L, Gao Q, Gao GF, Meng S. 2014. Heat shock protein gp96 adjuvant induces T cell responses and cross-protection to a split influenza vaccine. Vaccine 32:2703–2711. doi: 10.1016/j.vaccine.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 36.Trusch F, Loebach L, Wawra S, Durward E, Wuensch A, Iberahim NA, de Bruijn I, MacKenzie K, Willems A, Toloczko A, Dieguez-Uribeondo J, Rasmussen T, Schrader T, Bayer P, Secombes CJ, van West P. 2018. Cell entry of a host-targeting protein of oomycetes requires gp96. Nat Commun 9:2347. doi: 10.1038/s41467-018-04796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittal R, Prasadarao NV. 2011. gp96 expression in neutrophils is critical for the onset of Escherichia coli K1 (RS218) meningitis. Nat Commun 2:552. doi: 10.1038/ncomms1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabanes D, Sousa S, Cebria A, Lecuit M, Garcia-del Portillo F, Cossart P. 2005. Gp96 is a receptor for a novel Listeria monocytogenes virulence factor, Vip, a surface protein. EMBO J 24:2827–2838. doi: 10.1038/sj.emboj.7600750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bloor S, Maelfait J, Krumbach R, Beyaert R, Randow F. 2010. Endoplasmic reticulum chaperone gp96 is essential for infection with vesicular stomatitis virus. Proc Natl Acad Sci U S A 107:6970–6975. doi: 10.1073/pnas.0908536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ansa-Addo EA, Thaxton J, Hong F, Wu BX, Zhang Y, Fugle CW, Metelli A, Riesenberg B, Williams K, Gewirth DT, Chiosis G, Liu B, Li Z. 2016. Clients and oncogenic roles of molecular chaperone gp96/grp94. Curr Top Med Chem 16:2765–2778. doi: 10.2174/1568026616666160413141613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prusty BK, Siegl C, Gulve N, Mori Y, Rudel T. 2014. GP96 interacts with HHV-6 during viral entry and directs it for cellular degradation. PLoS One 9:e113962. doi: 10.1371/journal.pone.0113962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akkapaiboon P, Mori Y, Sadaoka T, Yonemoto S, Yamanishi K. 2004. Intracellular processing of human herpesvirus 6 glycoproteins Q1 and Q2 into tetrameric complexes expressed on the viral envelope. J Virol 78:7969–7983. doi: 10.1128/JVI.78.15.7969-7983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawabata A, Oyaizu H, Maeki T, Tang H, Yamanishi K, Mori Y. 2011. Analysis of a neutralizing antibody for human herpesvirus 6B reveals a role for glycoprotein Q1 in viral entry. J Virol 85:12962–12971. doi: 10.1128/JVI.05622-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hackett BA, Yasunaga A, Panda D, Tartell MA, Hopkins KC, Hensley SE, Cherry S. 2015. RNASEK is required for internalization of diverse acid-dependent viruses. Proc Natl Acad Sci U S A 112:7797–7802. doi: 10.1073/pnas.1424098112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amess PN, Baudin J, Townsend J, Meek J, Roth SC, Neville BG, Wyatt JS, Stewart A. 2008. Epilepsy in very preterm infants: neonatal cranial ultrasound reveals a high-risk subcategory. Dev Med Child Neurol 40:724–730. doi: 10.1111/j.1469-8749.1998.tb12339.x. [DOI] [PubMed] [Google Scholar]
- 46.Wyatt R, Sodroski J. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science ( 280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 47.Freed EO, Myers DJ, Risser R. 1990. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A 87:4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang K, Peng Z, Huang X, Qiao Z, Wang X, Wang N, Xi H, Cui J, Gao Y, Huang X, Gao H, Wei B, Chen L. 2017. Phase II trial of adjuvant immunotherapy with autologous tumor-derived Gp96 vaccination in patients with gastric cancer. J Cancer 8:1826–1832. doi: 10.7150/jca.18946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pishraft-Sabet L, Kosinska AD, Rafati S, Bolhassani A, Taheri T, Memarnejadian A, Alavian SM, Roggendorf M, Samimi-Rad K. 2015. Enhancement of HCV polytope DNA vaccine efficacy by fusion to an N-terminal fragment of heat shock protein gp96. Arch Virol 160:141–152. doi: 10.1007/s00705-014-2243-8. [DOI] [PubMed] [Google Scholar]
- 50.Ding Y, Zheng H, Feng C, Wang B, Liu C, Mi K, Cao H, Meng S. 2016. Heat-shock protein gp96 enhances T cell responses and protective potential to bacillus Calmette-Guérin vaccine. Scand J Immunol 84:222–228. doi: 10.1111/sji.12463. [DOI] [PubMed] [Google Scholar]
- 51.Dhepakson P, Mori Y, Jiang YB, Huang HL, Akkapaiboon P, Okuno T, Yamanishi K. 2002. Human herpesvirus-6 rep/U94 gene product has single-stranded DNA-binding activity. J Gen Virol 83:847–854. doi: 10.1099/0022-1317-83-4-847. [DOI] [PubMed] [Google Scholar]
- 52.Huang H, Li Y, Sadaoka T, Tang H, Yamamoto T, Yamanishi K, Mori Y. 2006. Human herpesvirus 6 envelope cholesterol is required for virus entry. J Gen Virol 87:277–285. doi: 10.1099/vir.0.81551-0. [DOI] [PubMed] [Google Scholar]
- 53.Mori Y, Seya T, Huang HL, Akkapaiboon P, Dhepakson P, Yamanishi K. 2002. Human herpesvirus 6 variant A but not variant B induces fusion from without in a variety of human cells through a human herpesvirus 6 entry receptor, CD46. J Virol 76:6750–6761. doi: 10.1128/jvi.76.13.6750-6761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D, Cheng L, Xia W, Liu X, Guo Y, Yang X, Guo X, Xu EY. 2020. LYPD4, mouse homolog of a human acrosome protein, is essential for sperm fertilizing ability and male fertility. Biology of Reprod doi: 10.1093/biolre/ioaa018. [DOI] [PubMed] [Google Scholar]
- 55.Jasirwan C, Furusawa Y, Tang H, Maeki T, Mori Y. 2014. Human herpesvirus-6A gQ1 and gQ2 are critical for human CD46 usage. Microbiol Immunol 58:22–30. doi: 10.1111/1348-0421.12110. [DOI] [PubMed] [Google Scholar]