ABSTRACT
Background
Low serum magnesium (Mg) concentrations have been associated with higher coronary artery disease (CAD) risk. A previous Atherosclerosis Risk in Communities (ARIC) Study article that evaluated the Mg–CAD association, based on 319 events occurring over 4–7 y, identified a sex-interaction whereby the inverse Mg–CAD association was much stronger among women than men. More than 1700 additional ARIC CAD events have since accrued.
Objective
We aimed to test our hypothesis that serum Mg is inversely and independently associated with long-term CAD risk in ARIC and in a meta-analysis with other prospective studies.
Methods
A total of 14,446 ARIC study participants (baseline mean ± SD age: 54 ± 6 y, 57% women, 27% African American) were followed for incident CAD through 2017. CAD events were defined by myocardial infarction or CAD mortality. Serum Mg was modeled as quintiles based on mean visit 1 (1987–1989) and visit 2 (1990–1992) concentrations. Cox regression models were used. We also conducted a random-effects meta-analysis incorporating these contemporary ARIC findings.
Results
Over a median follow-up of 27 y, 2131 incident CAD cases accrued. Overall, low serum Mg was associated with higher CAD risk after adjustment for demographics, lifestyle factors, and other CAD risk factors than was higher serum Mg (HR Q1 compared with Q5: 1.28; 95% CI: 1.11, 1.47; P-linear trend <0.001). The association was stronger among women (HR Q1 compared with Q5: 1.53; 95% CI: 1.22, 1.92) than men (HR: 1.11; 95% CI: 0.92, 1.34) (P-interaction = 0.05). In the meta-analysis including 5 studies, the pooled RR (95% CI) for CAD in the lowest compared with the highest circulating Mg category was 1.18 (1.06, 1.31) (I2 = 22%, P-heterogeneity = 0.27).
Conclusions
In this large community-based cohort and updated meta-analysis, low circulating Mg was associated with higher CAD risk than was higher Mg. Whether increasing Mg concentrations within healthy limits is a useful strategy for CAD prevention remains to be seen.
Keywords: circulating magnesium, coronary artery disease, cohort study, observational prospective studies, meta-analysis
Introduction
Magnesium (Mg) is an abundant cation and micronutrient that plays many crucial roles in the body by activating enzymes, contributing to energy production, and regulating concentrations of calcium and related biomarkers (1). Serum total Mg has traditionally been used to assess Mg status in both research and clinical settings (2, 3). Low serum Mg has been associated with increased risk of many outcomes, including increased incidence of cardiovascular disease (CVD) (4, 5), hypertension, and diabetes (6) in observational studies. In meta-analyses of randomized controlled trials, Mg supplementation has inconsistently been associated with reductions in blood pressure (7, 8), and reductions in fasting glucose concentrations among individuals with diabetes (9). The strongest evidence that serum Mg may be causally related to CVD risk comes from a recent Mendelian randomization study, in which genetically predicted higher serum Mg was associated with lower risk of coronary artery disease (CAD) (10). The association between low Mg and CVD may arise through numerous physiological pathways, such as elevated blood pressure, chronic inflammation, hyperglycemia, or impaired vasomotor tone and peripheral blood flow (2, 11).
A previous publication from the Atherosclerosis Risk in Communities (ARIC) Study cohort evaluated serum Mg in relation to incident CAD. That analysis, based on 319 events occurring over 4–7 y, identified a sex-interaction whereby the inverse Mg–CAD association was much stronger among women than men (i.e., quartiles 4 compared with 1 HR in women: 0.55; 95% CI: 0.27, 1.14; men: 0.84; 95% CI: 0.53, 1.31) (12). Since then, associations between low circulating Mg and CAD risk have been inconsistently found in the NHANES Follow-up I (13), Prevention of Renal and Vascular End-Stage Disease study (14), Framingham Offspring Study (15), and the Nurses’ Health Study (16). Three meta-analyses on the association between serum Mg and CVD have been conducted; all incorporated the sex-stratified results from this early ARIC analysis and showed low serum Mg to be associated with greater risk of CAD (4–6). However, ∼1700 more incident CAD events over 22 additional years of follow-up have been ascertained since the original ARIC publication.
Given growing interest in the association between Mg and CAD, we sought to update the ARIC analysis on Mg and CAD risk in order to achieve greater precision and to conduct an updated meta-analysis. We hypothesized that serum Mg would be inversely associated with incidence of CAD.
Methods
The ARIC study
The ARIC study (17) is a prospective community-based cohort study which began in 1987–1989, and has >30 y of follow-up. Participants were recruited from 4 locations (suburbs of Minneapolis, MN; Forsyth County, NC; Jackson, MS; and Washington County, MD). ARIC has conducted continuous surveillance for hospitalizations by annual or semiannual follow-up telephone calls, and performed several postbaseline clinic visits (visit 2: 1990–1992; visit 3: 1993–1995; visit 4: 1996–1998; visit 5: 2011–2013; and visit 6: 2016–2017). Institutional review board approval was obtained at each study site for each visit. Participants provided written informed consent at each clinic visit.
Measurements
Participants were asked to fast for >8 h before each ARIC clinic visit. At each visit, participants were interviewed and underwent anthropomorphic measurements, sitting blood pressure measurements (after 5 min rest), and a blood draw. Participants were also asked to bring bottles of current medications to the visit; medication names and dosages were transcribed and coded. Systolic blood pressure was quantified based on the mean of the second and third blood pressure measurements. BMI was calculated as kg/m2. Physical activity (sports index) was quantified using the validated Baecke questionnaire (18).
Blood samples were frozen until analysis (soon after the clinic visit) at the University of Minnesota Central Chemistry Laboratory. Blind duplicate samples from the original samples taken at visit 1 were sent ∼1 wk apart in order to calculate the laboratory CV. Serum Mg was measured at visit 1 (1987–1989) using metallochromic dye calmagite [1-(1-hydroxy-4-methyl-2-phenylazo)-2-naphthol-4-sulfonic acid] based on the Gindler and Heth procedure (CV: 3.6%) (19). Serum Mg was measured at visit 2 (1990–1992) using similar procedures (CV: 3.6%). At both visits 1 and 2, serum Mg was reported to 1 decimal place.
Serum creatinine was measured using a modified kinetic Jaffe method (CV: 3.7%). Diabetes was defined as having a fasting glucose concentration ≥126 mg/dL, nonfasting glucose concentration ≥200 mg/dL, self-reported use of diabetes medication, or self-reported physician diagnosis. Estimated glomerular filtration rate (eGFR) was calculated using serum creatinine (20) and eGFR was categorized using established clinical cutoffs (≥90, 60 to <90, and 15 to <60 mL · min−1 · 1.73 m−2). Total cholesterol and HDL cholesterol were measured in plasma using enzymatic methods (21).
CAD ascertainment
Prevalent CAD at baseline was defined by self-reported previous physician diagnosis of myocardial infarction (MI), or prevalent MI by 12-lead electrocardiogram. Potential incident CAD events were identified by 1) recent hospitalizations identified during follow-up phone calls to participants, 2) ongoing surveillance of community hospital discharge lists and death certificates, and 3) linkage to state and national death indexes. International Classification of Diseases, 9th revision codes were recorded from all hospitalizations and possible hospitalized CAD events were abstracted onto standardized forms. Possible coronary deaths occurring out-of-hospital were investigated through physician questionnaires and next-of-kin interviews. All CAD events were adjudicated by physician review. CAD events were defined as either definite or probable MI or definite fatal CAD based on ARIC criteria (22). ARIC criteria for fatal CAD involved history of CAD or chest pain, underlying cause of death, or presence or absence of a noncardiac cause of death.
Statistical analysis
Prospective analysis of the ARIC study
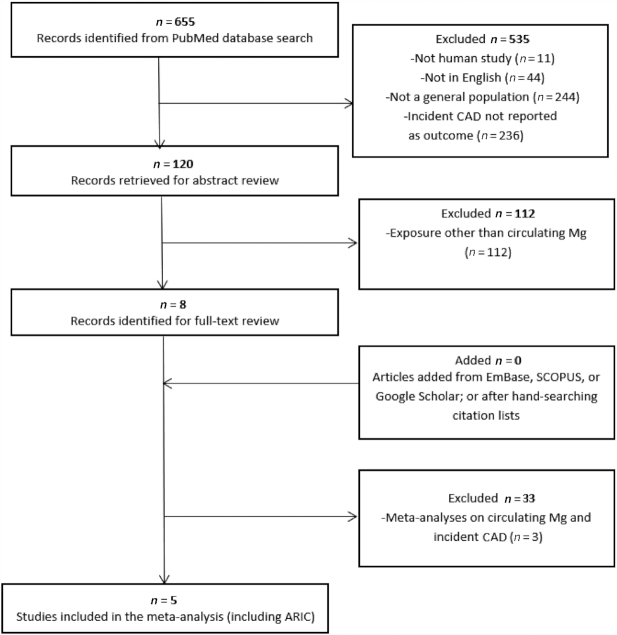
As depicted in Supplemental Figure 1, we excluded participants with prevalent CAD (n = 766) or missing information (n = 344) on prevalent CAD at visit 1, those missing a visit 1 serum Mg measurement (n = 122), and those with stage 5 chronic kidney disease (eGFR <15 mL · min−1 · 1.73 m−2) (n = 19). In addition, owing to small numbers, participants who reported a race other than white or African American (n = 44) as well as African Americans at the Minnesota and Washington County sites (n = 51) were excluded. A total of 14,446 ARIC study participants were included in this analysis.
Multivariable Cox proportional hazards regression models were used to estimate the association between serum Mg and CAD. Serum Mg was categorized into quintiles based on the mean of the visit 1 and 2 concentrations (to improve precision), with ties going to the lowest group. For individuals who did not attend visit 2 or were censored before visit 2, their visit 1 serum Mg measurement was used for ranking. For those individuals who attended both visits and were censored after visit 2, the mean value of their 2 serum Mg measurements was used for ranking. There were 1250 individuals who did not attend visit 2 and 70 nonfatal CAD events occurred between visits 1 and 2. Person-time was calculated from visit 1 to the CAD event, date of death, date of last contact, or through 31 December, 2017, whichever came first.
Restricted cubic splines were used to examine the presence of a dose–response association. We also tested for linear trend by including Mg quintiles in the models as an ordinal variable and per 1-SD (i.e., 0.14 mEq/L) decrement. We tested for violations of the proportional hazards assumption by assessing whether there was a multiplicative interaction between Mg and ln(time), and by visual inspection of ln(−ln) survival curves.
We employed multiple models to sequentially adjust for potential confounders of the association between Mg and CAD. In Model 1, we adjusted for demographic variables: age, sex, and race-center. In Model 2, we further adjusted for adiposity and behavioral confounders: BMI, education, smoking status (current/former/never), pack-years of smoking, physical activity sports index, drinking status (current/former/never), and ethanol intake (in grams per week). In Model 3, we further adjusted for traditional CAD risk factors: diabetes, HDL cholesterol, total cholesterol, use of lipid-lowering medications, systolic blood pressure, use of antihypertensive medications, and eGFR (categorized as ≥90, 60 to <90, or 15 to <60 mL · min−1 · 1.73 m−2), and, in women, the use of hormone therapy. We examined multiplicative interactions of Mg quintiles with age, race, sex, and eGFR (modeled continuously and categorically) by including cross-product terms in the models. Based on the early findings in ARIC suggesting a sex-interaction, we decided a priori to provide sex-stratified results.
To test the robustness of our findings, we conducted several sensitivity analyses. First, we excluded individuals on diuretics, which tend to decrease serum Mg concentrations. Second, we examined the association using the visit 1 serum Mg measurement only to see if the results were similar to the results using both visit 1 and visit 2 serum Mg measurements. Lastly, we looked at the associations of serum Mg with nonfatal CAD and fatal CAD as separate outcomes.
Two-tailed P values of 0.05 were used for tests of statistical significance. For tests of interactions, if this threshold was met, stratum-specific estimates were examined. SAS version 9.4 (SAS Institute) was primarily used for the ARIC analysis, whereas STATA version 14.1 (StataCorp LLC) was used to generate the restricted cubic splines.
Meta-analysis
In the meta-analysis, we include our ARIC findings and all prospective observational (cohort or nested case-control) studies of circulating Mg and incident CAD that were published before July 2019, were written in English, and included an effect estimate and a measure of statistical uncertainty. Published articles were identified via PubMed searches from inception until September 2018. The following Medical Subject Headings search terms were used: (“micronutrients” OR “magnesium” OR “magnesium deficiency”) AND (“cardiovascular disease” OR “myocardial infarction” OR “ischemic heart disease” OR “coronary heart disease”) AND (“cohort studies” OR “follow-up studies” OR “longitudinal studies” OR “prospective studies” OR “nested case-control studies”). We then used a similar approach with EmBase, SCOPUS, or Google Scholar, or after hand-searching citation lists. For eligible studies, we then extracted information related to inclusion criteria, an effect estimate, and a measure of variability from the fully adjusted model. Many of the prior studies published estimates comparing the highest category with the lowest category (referent). However, because low Mg may be problematic we felt it important to have high Mg as the reference group in the present ARIC analysis and meta-analysis. In order for high Mg to be the common reference group across all articles, for articles that were published with the lowest category as the reference group, we used a standard (23) approach to recalculate the RR estimate and CIs using the highest category as the reference. Study estimates for Mg categories were pooled using random-effects meta-analysis and in a sensitivity analysis using fixed effects. We used the Newcastle-Ottawa quality assessment scale to estimate the quality of the eligible studies (24). Heterogeneity was assessed using the I2 statistic. We examined results for publication bias, visually using funnel plots and statistically using Egger's (25) and Begg's (26) tests. STATA version 14.1 (StataCorp LLC) was used for the meta-analysis.
Results
Prospective analysis of the ARIC study
Participants’ mean ± SD age at baseline was 54 ± 6 y, 57% were women, and 27% were African American. Mean ± SD serum Mg at visit 1 and visit 2 was 1.62 ± 0.14 mEq/L overall, 1.62 ± 0.14 mEq/L among women, and 1.63 ± 0.14 mEq/L among men. Serum Mg at visit 1 and at visit 2 were moderately correlated (Pearson's r = 0.46, P value < 0.001). Those with lower serum Mg tended to be female, African American, have diabetes, and use antihypertensive medications (Table 1).
TABLE 1.
Unadjusted baseline characteristics by serum magnesium quintiles: the ARIC study, 1987–19891
| Quintiles of serum magnesium | |||||
|---|---|---|---|---|---|
| Characteristic | 1 | 2 | 3 | 4 | 5 |
| n | 3278 | 2669 | 3094 | 2726 | 2679 |
| Magnesium, mEq/L | 1.45 [0.60–1.50] | 1.55 [1.55–<1.60] | 1.65 [1.60–<1.70] | 1.70 [1.70–<1.75] | 1.80 [1.75–2.35] |
| Age, y | 54 ± 6 | 54 ± 6 | 54 ± 6 | 54 ± 6 | 54 ± 6 |
| Female | 1986 (60.6) | 1562 (58.5) | 1706 (55.1) | 1467 (53.8) | 1452 (54.2) |
| African American | 1439 (43.9) | 671 (25.1) | 745 (24.1) | 529 (19.4) | 442 (16.5) |
| Education | |||||
| <High school | 1029 (31.5) | 564 (21.2) | 625 (20.2) | 536 (19.7) | 541 (20.2) |
| High school graduate | 1243 (38.0) | 1125 (42.2) | 1329 (43.0) | 1144 (42.0) | 1080 (40.4) |
| >High school | 1000 (30.6) | 977 (36.7) | 1135 (36.7) | 1042 (38.3) | 1053 (39.4) |
| Sport index | 2.3 ± 0.8 | 2.4 ± 0.8 | 2.4 ± 0.8 | 2.5 ± 0.8 | 2.5 ± 0.8 |
| Smoking status | |||||
| Current | 927 (28.3) | 666 (25.0) | 813 (26.3) | 667 (24.5) | 680 (25.4) |
| Former | 974 (29.8) | 819 (30.7) | 977 (31.6) | 886 (32.5) | 885 (33.1) |
| Never | 1373 (41.9) | 1181 (44.3) | 1303 (42.1) | 1170 (43.0) | 1113 (41.6) |
| Pack-years smoking | 315 ± 446 | 296 ± 416 | 310 ± 419 | 299 ± 416 | 303 ± 406 |
| Drinking status | |||||
| Current | 1558 (47.8) | 1511 (56.8) | 1766 (57.2) | 1672 (61.7) | 1621 (60.7) |
| Former | 702 (21.5) | 459 (17.2) | 564 (18.3) | 432 (15.9) | 464 (17.4) |
| Never | 1002 (30.7) | 692 (26.0) | 755 (24.5) | 606 (22.4) | 586 (21.9) |
| Ethanol intake, g/wk | 40 ± 106 | 38 ± 87 | 44 ± 95 | 44 ± 93 | 45 ± 92 |
| Diabetes | 760 (23.3) | 260 (9.8) | 288 (9.3) | 168 (6.2) | 122 (4.6) |
| BMI, kg/m2 | 29 ± 6 | 28 ± 5 | 27 ± 5 | 27 ± 5 | 27 ± 5 |
| HDL-C, mg/dL | 53 ± 19 | 54 ± 18 | 53 ± 17 | 54 ± 17 | 54 ± 17 |
| LDL-C, mg/dL | 130 ± 42 | 133 ± 39 | 136 ± 41 | 136 ± 38 | 138 ± 39 |
| Total cholesterol, mg/dL | 213 ± 44 | 214 ± 41 | 215 ± 42 | 215 ± 40 | 217 ± 42 |
| Lipid-lowering medication use | 82 (2.5) | 57 (2.2) | 97 (3.2) | 59 (2.2) | 73 (2.7) |
| Systolic BP, mm Hg | 126 ± 20 | 121 ± 18 | 120 ± 18 | 119 ± 18 | 119 ± 17 |
| Antihypertension medication use | 1373 (41.9) | 726 (27.2) | 813 (26.3) | 609 (22.4) | 574 (21.4) |
| eGFR, mL · min−1 · 1.73 m−2 | 106 ± 18 | 104 ± 14 | 103 ± 14 | 101 ± 14 | 100 ± 15 |
| eGFR category | |||||
| ≥90 mL · min−1 · 1.73 m−2 | 2813 (85.8) | 2300 (86.2) | 2638 (85.3) | 2246 (82.4) | 2190 (81.8) |
| 60 to <90 mL · min−1 · 1.73 m−2 | 410 (12.5) | 348 (13.0) | 436 (14.1) | 451 (16.5) | 457 (17.1) |
| 15 to <60 mL · min−1 · 1.73 m−2 | 55 (1.7) | 21 (0.8) | 20 (0.7) | 29 (1.1) | 32 (1.2) |
| Hormone therapy use (in women only) | 407 (20.5) | 317 (20.3) | 295 (17.3) | 271 (18.5) | 232 (16.0) |
n = 14,446. Values are mean ± SD or median [range] for continuous variables and n (%) for categorical variables. ARIC, Atherosclerosis Risk in Communities; BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
Over a median follow-up of 27 y, 2131 incident CAD cases were identified. Overall, after adjustment for demographics, serum Mg was inversely associated with CAD risk at concentrations of ≤1.8 mEq/L; for Mg concentrations above this cutoff, there was no apparent association (Figure 1). The inverse Mg–CAD association was stronger in women than in men (Supplemental Figure 2). In quintile analyses, serum Mg was inversely and monotonically associated with CAD risk after demographic adjustment (Model 1 HR Q1 compared with Q5: 1.69; 95% CI: 1.47, 1.93; P value for linear trend <0.001; Table 2). A similar association between low serum Mg and higher CAD risk was observed after further adjustment for lifestyle factors (Model 2 HR Q1 compared with Q5: 1.55; 95% CI: 1.35, 1.79; P-trend < 0.001) and other CAD risk factors (Model 3 HR Q1 compared with Q5: 1.28; 95% CI: 1.11, 1.47; P-trend < 0.001) (Table 2). The attenuation of the effect estimate from Model 2 to Model 3 was primarily driven by adjustment for diabetes (i.e., in a model which included Model 2 covariates plus diabetes the HR was 1.33; 95% CI: 1.16, 1.54). The association was stronger among women (Model 3 HR Q1 compared with Q5: 1.53; 95% CI: 1.22, 1.92) than men (HR: 1.11; 95% CI: 0.92, 1.34) (Model 1 P-interaction = 0.03, Model 2 P = 0.04, Model 3 P = 0.05). No other meaningful multiplicative interactions were found between Mg quintiles and age, race, or eGFR in relation to CAD risk.
FIGURE 1.
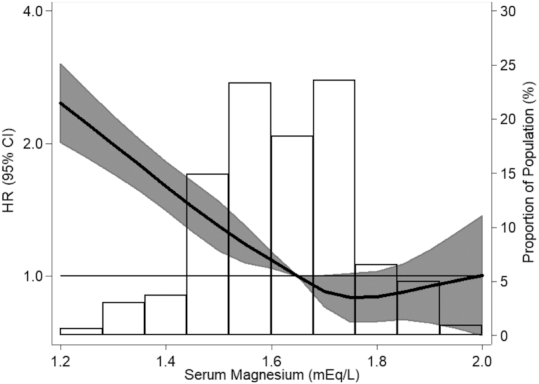
Association of serum magnesium with incidence of coronary artery disease, in the overall cohort: the Atherosclerosis Risk in Communities study, 1987–2017 (n = 14,446). Serum magnesium modeled as restricted cubic splines with knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles with adjustment for age, race-center, and sex. For ease of display, those <1 and >99th percentile have been removed.
TABLE 2.
HRs (95% CIs) for serum magnesium and risk of coronary artery disease, overall and stratified by sex: the ARIC study, 1987–20171
| Quintiles of serum magnesium | HR per 1-SD decrement3 | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P-trend2 | ||
| Median (range), mEq/L | 1.45 (0.60–1.50) | 1.55 (1.55 to <1.60) | 1.65 (1.60 to <1.70) | 1.70 (1.70 to <1.75) | 1.80 (1.75–2.35) | ||
| Overall (n = 14,446) | |||||||
| No. events/total | 599/3278 | 390/2669 | 430/3094 | 369/2726 | 343/2679 | ||
| Incidence rate4 | 8.76 | 6.29 | 6.13 | 5.87 | 5.47 | ||
| Model 1 | 1.69 (1.47, 1.93) | 1.20 (1.04, 1.39) | 1.13 (0.98, 1.31) | 1.08 (0.93, 1.25) | 1 (ref) | <0.001 | 1.23 (1.18, 1.29) |
| Model 2 | 1.55 (1.35, 1.79) | 1.17 (1.01, 1.36) | 1.12 (0.97, 1.29) | 1.09 (0.94, 1.27) | 1 (ref) | <0.001 | 1.19 (1.14, 1.24) |
| Model 3 | 1.28 (1.11, 1.47) | 1.12 (0.96, 1.30) | 1.08 (0.93, 1.25) | 1.08 (0.93, 1.26) | 1 (ref) | <0.001 | 1.10 (1.05, 1.15) |
| Women (n = 8173) | |||||||
| No. events/total | 318/1986 | 178/1562 | 177/1706 | 147/1467 | 124/1452 | ||
| Incidence rate4 | 7.25 | 4.69 | 4.37 | 4.21 | 3.47 | ||
| Model 1 | 2.17 (1.75, 2.68) | 1.46 (1.16, 1.84) | 1.33 (1.06, 1.67) | 1.26 (0.99, 1.60) | 1 (ref) | <0.001 | 1.36 (1.27, 1.44) |
| Model 2 | 1.98 (1.59, 2.46) | 1.45 (1.14, 1.83) | 1.29 (1.02, 1.63) | 1.26 (0.99, 1.62) | 1 (ref) | <0.001 | 1.31 (1.22, 1.39) |
| Model 3 | 1.53 (1.22, 1.92) | 1.36 (1.07, 1.72) | 1.18 (0.93, 1.49) | 1.23 (0.96, 1.57) | 1 (ref) | <0.001 | 1.17 (1.10, 1.25) |
| Men (n = 6273) | |||||||
| No. events/total | 281/1292 | 212/1107 | 253/1388 | 222/1259 | 219/1227 | ||
| Incidence rate4 | 11.48 | 8.80 | 8.54 | 7.94 | 8.10 | ||
| Model 1 | 1.40 (1.17, 1.68) | 1.06 (0.88, 1.28) | 1.03 (0.86, 1.24) | 0.98 (0.81, 1.18) | 1 (ref) | <0.001 | 1.13 (1.07, 1.20) |
| Model 2 | 1.31 (1.09, 1.58) | 1.03 (0.85, 1.24) | 1.01 (0.84, 1.22) | 0.99 (0.82, 1.19) | 1 (ref) | 0.005 | 1.11 (1.04, 1.18) |
| Model 3 | 1.11 (0.92, 1.34) | 0.98 (0.81, 1.19) | 1.02 (0.85, 1.23) | 0.99 (0.82, 1.20) | 1 (ref) | 0.36 | 1.04 (0.98, 1.11) |
n = 14,446. Model 1 was adjusted for age, sex, and race-center; not adjusted for sex in stratified analyses. Model 2 = Model 1 also adjusted for baseline BMI, education, physical activity, drinking status, ethanol intake (grams per week), smoking status, and pack-years of smoking. Model 3 = Model 2 also adjusted for diabetes, HDL cholesterol, total cholesterol, lipid-lowering medication use, systolic blood pressure, antihypertensive medication use, estimated glomerular filtration rate categories, and hormone therapy use (in women only). ARIC, Atherosclerosis Risk in Communities.
P values are based on linear trends across quintiles of serum magnesium.
One SD of serum magnesium = 0.14 mEq/L.
Unadjusted incidence rate per 1000 person-years.
In sensitivity analyses, associations were similar when we excluded diuretic users (n = 2454 excluded; Supplemental Table 1), used only visit 1 Mg as the exposure (Supplemental Table 2), and examined fatal and nonfatal CAD as separate outcomes (Supplemental Table 3). The proportional hazards assumption was violated statistically [Model 1 P value for Mg quintiles*ln(time) <0.001]. In ln(−ln) curves, serum Mg quintiles crossed over follow-up. HRs and 95% CIs for Mg quintiles and CAD risk stratified by follow-up time are presented in Supplemental Table 4. Briefly, the associations were similar in the extreme quintiles in the first 10 y (Model 3 HR Q1 compared with Q5: 1.42; 95% CI: 1.11, 1.83) and during the subsequent ∼20-y follow-up period (HR Q1 compared with Q5: 1.43; 95% CI: 1.21, 1.69). In addition, as shown in Supplemental Table 5, effect estimates were of a similar magnitude to the main results when stratified by baseline diabetes status, and by BMI categories (obese ≥30, overweight 25 to <30, healthy weight 18.5 to <25).
Meta-analysis
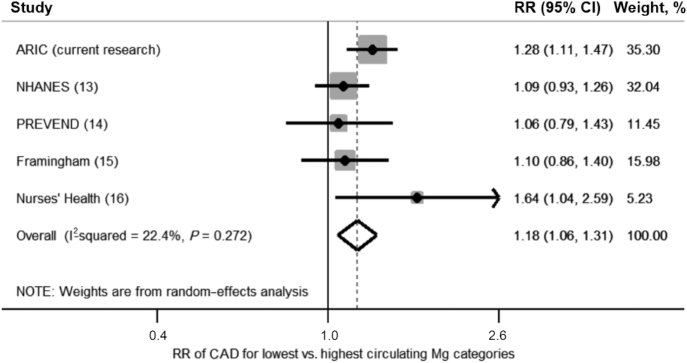
After evaluating 655 studies for inclusion (Figure 2), 5 studies (including these ARIC findings) were included in this meta-analysis on circulating Mg and CAD risk (Supplemental Table 6). A listing of the effect estimates and 95% CIs, as included in the meta-analysis, is provided in Table 3. There were 37,981 total participants with 5784 CAD events for longitudinal studies; in the nested case-control study, there were 458 cases and 458 controls. The lowest Mg category was associated with an 18% higher CAD risk than was the highest category (RR: 1.18; 95% CI: 1.06, 1.31; Figure 3). A similar pooled effect estimate was calculated using fixed-effects models (RR: 1.18; 95% CI: 1.08, 1.29; Supplemental Figure 3). There was moderate between-study heterogeneity (I2 = 22.4%; P-heterogeneity = 0.27). There was no evidence of publication bias in Egger's test (P = 0.80), Begg's test (P = 0.99), nor by visual inspection of a funnel plot (Supplemental Figure 4). Because some readers may be interested in a summary estimate comparing the highest with the lowest (referent) category of circulating Mg, we also present results of this comparison, using a random-effects meta-analysis, in Supplemental Figure 5.
FIGURE 2.
Screening and selection of articles on circulating magnesium and risk of coronary artery disease. ARIC, Atherosclerosis Risk in Communities; CAD, coronary artery disease.
TABLE 3.
Summary of articles and effect estimates as included in meta-analysis on circulating magnesium and risk of CAD1
| Study | Design | Follow-up, y | n total | n events or cases | Exposure assessment method | Exposure categorization2 | Outcome assessment | HR or RR3 (95% CI), low vs. high category | Covariate adjustments |
|---|---|---|---|---|---|---|---|---|---|
| ARIC study (current research) | Cohort | 30 | 14,446 | 2131 | Serum; metallo-chromic dye calmagite | Quintiles (mEq/L): Q1 0.60–1.50 (ref); Q2 1.55–<1.60; Q3 1.60–<1.70; Q4 1.70–<1.75; Q5 1.75–2.35 | Incident CAD = medical records; death certificates with next-of-kin interviews and physician questionnaire | HR Q1 vs. Q5: 1.28 (1.11, 1.47) | Age, sex, race-center, baseline BMI, education, physical activity, drinking status, ethanol intake (grams per week), smoking status, pack-years of smoking, diabetes, HDL cholesterol, total cholesterol, lipid-lowering medication use, SBP, antihypertensive medication use, eGFR, hormone therapy use (in women only) |
| NHANES Follow-up (13) | Cohort | 19 | 12,340 | 2637 | Serum; atomic absorption spectrophotometry | Quartiles (mEq/L): Quartile 1 <1.59 (ref); Quartile 2 1.59–<1.68; Quartile 3 1.68–<1.77; Quartile 4 ≥1.77 | Incident IHD = hospital records (ICD-9-CM codes 410–414) | HR* Q1 vs. Q4: 1.09 (0.93, 1.26) | Age, gender, race, education, smoking, serum cholesterol, SBP, antihypertensive medication, self-reported diabetes, BMI, leisure- and nonleisure time physical activity, alcohol consumption |
| PREVEND study (14) | Cohort | 11 | 6595 | 435 | Plasma; xylidyl blue | Quintiles (mmol/L): Q1 <0.77; Q2 0.77–0.79; Q3 0.80–0.82 (ref); Q4 0.83–0.85; Q5 >0.85 | Incident IHD = acute myocardial infarction (ICD-10 code I21), hospitalization for other acute IHD (I24), coronary artery bypass grafting, or percutaneous transluminal coronary angioplasty | HR Q1 vs. Q3: 1.06 (0.79, 1.43) | Age; smoking status; sex; BMI; ratio of total to HDL cholesterol; parental history of IHD; alcohol consumption; plasma calcium, sodium, and potassium concentrations |
| Framingham Offspring study (15) | Cohort | 20 | 3531 | 554 | Serum; colorimetric assay | Quartiles (mmol/L): Quartile 1 0.41–<0.80; Quartile 2 0.80–<0.84; Quartile 3 0.84–<0.89; Quartile 4 0.89–1.45 | Incident CVD = angina pectoris, coronary insufficiency, MI, stroke or TIA, HF, intermittent claudication, or death secondary to CVD | HR* Q1 vs. Q4: 1.10 (0.86, 1.40) | Age, sex, BMI, diabetes, SBP, total:HDL cholesterol, smoking, hemoglobin, albumin, eGFR |
| Nurses’ Health Study (16) | Nested case-control | n/a | 916 | 458 | Plasma; colorimetric assay | Quartiles (mg/dL): Quartile 1 ≤2.0; Quartile 2 2.1–2.1; Quartile 3 2.2–2.3; Quartile 4 ≥2.4 | Incident CAD = nonfatal MI (WHO criteria) or fatal CAD (autopsy or hospital records or primary cause of death) | RR* Q1 vs. Q4: 1.64 (1.04, 2.59) | Matching factors (age, smoking status, month of blood draw, fasting status), BMI, exercise, alcohol intake, family history of MI, eGFR, menopausal therapy, multivitamin use, intake of omega‐3 fats, polyunsaturated:saturated fat ratio, trans fat, dietary cholesterol, cereal fiber, calcium, potassium, vitamin D |
Study quality assessed using the Newcastle-Ottawa Scale (24): NHANES Follow-up = 9; PREVEND study = 9; Framingham Offspring study = 9; Nurses’ Health Study = 9. ARIC, Atherosclerosis Risk in Communities; CAD, coronary artery disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, International Classification of Diseases; IHD, ischemic heart disease; MI, myocardial infarction; n/a, not applicable; PREVEND, Prevention of Renal and Vascular End-Stage Disease; SBP, systolic blood pressure; TIA, transient ischemic attack.
To convert magnesium (milliequivalents per liter) to millimoles per liter or milligrams per deciliter, multiply by 0.5 and by 1.22, respectively.
HR* or RR* indicates that we inverted (23) the published HR or RR and corresponding 95% CI such that the highest category became the referent group to be consistent with the ARIC analyses.
FIGURE 3.
Forest plot of RR of nonfatal and fatal CAD in relation to circulating magnesium (lowest compared with highest categories) pooled using random-effects meta-analysis. The PREVEND study used Q3 as its referent group, so the RR presented is based on Q1 compared with Q3. ARIC, Atherosclerosis Risk in Communities; CAD, coronary artery disease; PREVEND, Prevention of Renal and Vascular End-Stage Disease; Q, quintile.
Discussion
In this large community-based cohort, serum Mg was inversely associated with CAD risk, with most of the excess risk at lower concentrations. This association was independent of traditional CAD risk factors, and was slightly stronger among women than men. In the updated meta-analysis, we found low circulating Mg was associated with a modestly higher CAD risk than were high circulating Mg concentrations. Of note, in our study, the favorable associations of higher circulating concentrations are for those within healthy limits, and who do not have hypermagnesemia (>2.6 mEq/L).
The strongest evidence that serum Mg may have a causal relation with heart disease comes from a recent Mendelian randomization study of Mg-related single nucleotide polymorphisms and CAD risk. Each 0.1-mmol/L (multiply millimoles per liter by 2 to convert to milliequivalents per liter; ∼1-SD) decrement of genetically predicted serum Mg was associated with 14% higher odds of CAD (OR: 1.14; 95% CI: 1.01, 1.28) [results in the original publication were presented as per 0.1-mmol/L increment higher circulating Mg (OR: 0.88; 95% CI: 0.78, 0.99)] (10). To be consistent with the direction of our analysis in ARIC and the meta-analysis (high Mg referent), the effect estimate was recalculated to represent per 0.1-mmol/L decrement. Sex-specific associations were not reported in the Mendelian randomization study. In the ARIC data, each 1-SD decrement was associated with a 19% higher risk of incident CAD (model 2 HR: 1.19; 95% CI: 1.14, 1.24). In our meta-analysis, the RR for lower compared with higher Mg was 1.18 (95% CI: 1.06, 1.31). Three prior meta-analyses yielded similar summaries of the prospective association of circulating Mg and CAD risk (Supplemental Table 7) (4–6). All incorporated the sex-stratified results from the early ARIC study (12), and they generally reported trends toward inverse associations between circulating Mg and CAD risk.
Low circulating Mg likely could increase CAD risk through traditional pathways, such as hypertension, elevated blood glucose, or chronic inflammation (2, 11). A meta-analysis of randomized controlled trials found that Mg supplementation (mean dosage: 300 mg/d) compared with placebo increased serum Mg by 0.10 mEq/L (95% CI: 0.06, 0.14 mEq/L) and decreased systolic and diastolic blood pressure by 2.0 mm Hg (95% CI: 3.6, 0.4 mm Hg) and 1.8 mm Hg (95% CI: 2.8, 0.7 mm Hg), respectively (27). Other meta-analyses have reported that oral Mg supplementation compared with placebo has beneficial effects on certain cardiometabolic biomarkers among individuals with type 2 diabetes (9, 28), for example, HDL cholesterol increased by 0.08 mmol/L (95% CI: 0.03, 0.14 mmol/L) and fasting glucose decreased by 0.56 mmol/L (95% CI: 1.10, 0.01 mmol/L) (9). Low serum Mg has also been associated cross-sectionally with markers of subclinical CVD, such as higher coronary artery calcification scores among Koreans with low CVD risk (29) and among Mexican individuals with diabetes (30), as well as with greater carotid intima media thickness and mitral valve calcification in diabetic patients with mild-to-moderate chronic kidney disease (31). Cross-sectionally in the ARIC study, low serum Mg was associated with greater carotid wall thickness among women (but not men) (32). Mechanisms accounting for the slightly stronger Mg–CAD association among women than men remain to be characterized.
There are also other proposed mechanisms connecting Mg and CAD. Mg plays roles in maintaining normal sinus rhythm (33), platelet formation (33, 34), vascular smooth muscle tone, endothelial function (33, 35), and in mitigating oxidative stress (33). In experimental studies, hypomagnesemia induced oxidative DNA damage in cardiac tissue (36). Among mice with high fat diet–induced type 2 diabetes, Mg supplementation in drinking water resulted in reduced mitochondrial reactive oxygen species and improved diastolic function (37). Mg also acts as a natural calcium antagonist in the setting of intracellular calcium overload during ischemia and helps regulate ion channel transport in cardiac cells (38). Tissue obtained during autopsy of male fatal CAD events had lower Mg within myocardial muscle compared with those who died from noncardiac causes (39). Significant drops in serum Mg have been reported immediately after an MI, with normal concentrations returning within 12 d after the infarction (40). Although high-dose intravenous Mg therapy is thought to have no survival benefit as a secondary prevention strategy among MI patients (41, 42), whether Mg administration or supplementation prevents incidence of CAD is unknown. In observational studies dietary Mg intake has generally not been linked to CAD incidence (43); however, given the complexity of Mg homeostasis, circulating Mg and estimates of dietary Mg intake tend to correlate poorly (e.g., in ARIC the correlation was only 0.03) (12, 16, 44).
There are limitations to these analyses. First, despite adjustment for numerous potential confounding characteristics, residual confounding of the association between Mg and CAD may remain. However, we conducted several sensitivity analyses and our findings were robust. Second, follow-up was long, and serum Mg concentrations at baseline may not be reflective of serum Mg over the life course or immediately preceding CAD events. However, misclassification of Mg concentrations would tend to obscure associations with CAD rather than exaggerate them. We also did not account for changes in confounding factors over time, which might have biased the observed associations. Third, circulating Mg may not necessarily reflect intracellular stores (45); in the body, <1% of Mg is circulating (2, 33). Last, specific to the meta-analysis, circulating Mg categories were not defined identically, which may bias the pooled effect estimate. Nevertheless, there are several notable strengths of these findings, including the large sample size and power to measure associations between serum Mg and CAD in a community-based population which is diverse in terms of both sex and race.
In conclusion, a growing number of epidemiologic studies—including the present updated analysis of ARIC—have documented inverse associations between circulating Mg and risk of cardiovascular outcomes. Although Mg homeostasis is complex, Mg concentrations can be intervened upon among those with hypomagnesemia through supplementation and diet modification (46), thereby suggesting that Mg supplementation could be a potential candidate for CAD prevention. An adequately powered randomized controlled trial is needed to test whether increasing Mg concentrations within healthy limits is a useful target for the primary prevention of CAD.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—MRR and PLL: designed the research; MRR: conducted the literature search, performed the statistical analysis, and had primary responsibility for the final content; and all authors: wrote the paper and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by National Heart, Lung, and Blood Institute (NHLBI) of the NIH award T32HL007779 (to MRR). The Atherosclerosis Risk in Communities Study has been funded in whole or in part with federal funds from the NHLBI, NIH, Department of Health and Human Services, under contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. CMR was supported by National Institute of Diabetes and Digestive and Kidney Diseases mentored research scientist development award K01 DK107782 and NHLBI grant R21 HL143089.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplemental Figures 1–5 and Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ARIC, Atherosclerosis Risk in Communities; CAD, coronary artery disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction.
References
- 1. Bryrd-Bredbenner C, Moe G, Berning J, Kelley D. Wardlaw's perspectives in nutrition updated with 2015–2020 Dietary Guidelines for Americans. 10th ed. New York, NY:McGraw-Hill Education; 2016. [Google Scholar]
- 2. Costello RB, Elin RJ, Rosanoff A, Wallace TC, Guerrero-Romero F, Hruby A, Lutsey PL, Nielsen FH, Rodriguez-Moran M, Song Y et al.. Perspective: the case for an evidence-based reference interval for serum magnesium: the time has come. Adv Nutr. 2016;7(6):977–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costello RB, Nielsen F. Interpreting magnesium status to enhance clinical care: key indicators. Curr Opin Clin Nutr Metab Care. 2017;20(6):504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Del Gobbo LC, Imamura F, Wu JH, de Oliveira Otto MC, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2013;98(1):160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qu X, Jin F, Hao Y, Li H, Tang T, Wang H, Yan W, Dai K. Magnesium and the risk of cardiovascular events: a meta-analysis of prospective cohort studies. PLoS One. 2013;8(3):e57720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu J, Xun P, Tang Q, Cai W, He K. Circulating magnesium levels and incidence of coronary heart diseases, hypertension, and type 2 diabetes mellitus: a meta-analysis of prospective cohort studies. Nutr J. 2017;16(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jee SH, Miller ER, Guallar E, Singh VK, Appel LJ, Klag MJ. The effect of magnesium supplementation on blood pressure: a meta-analysis of randomized clinical trials. Am J Hypertens. 2002;15(8):691–6. [DOI] [PubMed] [Google Scholar]
- 8. Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr. 2012;66(4):411–18. [DOI] [PubMed] [Google Scholar]
- 9. Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006;23(10):1050–6. [DOI] [PubMed] [Google Scholar]
- 10. Larsson SC, Burgess S, Michaëlsson K. Serum magnesium levels and risk of coronary artery disease: Mendelian randomisation study. BMC Med. 2018;16(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rude RK. Magnesium. in: Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD. Encyclopedia of dietary supplements. 2nd ed. New York, NY: Informa Healthcare; 2010. p. 527–37. [Google Scholar]
- 12. Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 1998;136(3):480–90. [DOI] [PubMed] [Google Scholar]
- 13. Ford ES. Serum magnesium and ischaemic heart disease: findings from a national sample of US adults. Int J Epidemiol. 1999;28(4):645–51. [DOI] [PubMed] [Google Scholar]
- 14. Joosten MM, Gansevoort RT, Mukamal KJ, van der Harst P, Geleijnse JM, Feskens EJ, Navis G, Bakker SJ; The PREVEND Study Group. Urinary and plasma magnesium and risk of ischemic heart disease. Am J Clin Nutr. 2013;97(6):1299–306. [DOI] [PubMed] [Google Scholar]
- 15. Khan AM, Sullivan L, McCabe E, Levy D, Vasan RS, Wang TJ. Lack of association between serum magnesium and the risks of hypertension and cardiovascular disease. Am Heart J. 2010;160(4):715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiuve SE, Sun Q, Curhan GC, Taylor EN, Spiegelman D, Willett WC, Manson JE, Rexrode KM, Albert CM. Dietary and plasma magnesium and risk of coronary heart disease among women. J Am Heart Assoc. 2013;2(2):e000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 18. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–42. [DOI] [PubMed] [Google Scholar]
- 19. The ARIC Investigators. Manual 10. Clinical chemistry determinations. [Internet] 1987. The National Heart, Blood, and Lung Institute of the National Institutes of Health; Available from: http://www.cscc.unc.edu/aric/pubuse/manual/Clinical_Chemistry_Determinations.1_10.pdf. [Google Scholar]
- 20. Levey AS. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The ARIC Investigators. Manual 8. Lipid and lipoprotein determinations. [Internet] 1987. The National Heart, Blood, and Lung Institute of the National Institutes of Health; Available from: http://www.cscc.unc.edu/aric/visit/Lipid_and_Lipoprotein_Determinations.1_8.pdf. [Google Scholar]
- 22. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–33. [DOI] [PubMed] [Google Scholar]
- 23. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Internet] 2019. Ottawa, Ontario: Ottawa Hospital Research Institute; Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 27. Zhang X, Li Y, Del Gobbo LC, Rosanoff A, Wang J, Zhang W, Song Y. Effects of magnesium supplementation on blood pressure: a meta-analysis of randomized double-blind placebo-controlled trials. Hypertension. 2016;68(2):324–33. [DOI] [PubMed] [Google Scholar]
- 28. Veronese N, Watutantrige-Fernando S, Luchini C, Solmi M, Sartore G, Sergi G, Manzato E, Barbagallo M, Maggi S, Stubbs B. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: a systematic review and meta-analysis of double-blind randomized controlled trials. Eur J Clin Nutr. 2016;70(12):1354–9. [DOI] [PubMed] [Google Scholar]
- 29. Lee SY, Hyun YY, Lee KB, Kim H. Low serum magnesium is associated with coronary artery calcification in a Korean population at low risk for cardiovascular disease. Nutr Metab Cardiovasc Dis. 2015;25(11):1056–61. [DOI] [PubMed] [Google Scholar]
- 30. Posadas-Sánchez R, Posadas-Romero C, Cardoso-Saldaña G, Vargas-Alarcón G, Villarreal-Molina MT, Pérez-Hernández N, Rodríguez-Pérez JM, Medina-Urrutia A, Jorge-Galarza E, Juárez-Rojas JG et al.. Serum magnesium is inversely associated with coronary artery calcification in the Genetics of Atherosclerotic Disease (GEA) study. Nutr J. 2016;15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Silva AP, Gundlach K, Buchel J, Jeronimo T, Fragoso A, Silva C, Guilherme P, Santos N, Faisca M, Neves P. Low magnesium levels and FGF-23 dysregulation predict mitral valve calcification as well as intima media thickness in predialysis diabetic patients. Int J Endocrinol. 2015:308190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma J, Folsom AR, Melnick SL, Eckfeldt JH, Sharrett AR, Nabulsi AA, Hutchinson RG, Metcalf PA. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. J Clin Epidemiol. 1995;48(7):927–40. [DOI] [PubMed] [Google Scholar]
- 33. de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1–46. [DOI] [PubMed] [Google Scholar]
- 34. Ravn HB, Kristensen SD, Vissinger H, Husted SE. Magnesium inhibits human platelets. Blood Coagul Fibrinolysis. 1996;7(2):241–4. [DOI] [PubMed] [Google Scholar]
- 35. Shechter M. Magnesium and cardiovascular system. Magnes Res. 2010;23(2):60–72. [DOI] [PubMed] [Google Scholar]
- 36. Shah NC, Shah GJ, Li Z, Jiang XC, Altura BT, Altura BM. Short-term magnesium deficiency downregulates telomerase, upregulates neutral sphingomyelinase and induces oxidative DNA damage in cardiovascular tissues: relevance to atherogenesis, cardiovascular diseases and aging. Int J Clin Exp Med. 2014;7(3):497–514. [PMC free article] [PubMed] [Google Scholar]
- 37. Liu M, Jeong E-M, Liu H, Xie A, So EY, Shi G, Jeong GE, Zhou A, Dudley SC Jr. Magnesium supplementation improves diabetic mitochondrial and cardiac diastolic function. JCI Insight. 2019;4(1):123182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mubagwa K, Gwanyanya A, Zakharov S, Macianskiene R. Regulation of cation channels in cardiac and smooth muscle cells by intracellular magnesium. Arch Biochem Biophys. 2007;458(1):73–89. [DOI] [PubMed] [Google Scholar]
- 39. Johnson CJ, Peterson DR, Smith EK. Myocardial tissue concentrations of magnesium and potassium in men dying suddenly from ischemic heart disease. Am J Clin Nutr. 1979;32(5):967–70. [DOI] [PubMed] [Google Scholar]
- 40. Abraham A, Eylath U, Weinstein M, Czaczkes E. Serum magnesium levels in patients with acute myocardial infarction. N Engl J Med. 1977;296(15):862–3. [DOI] [PubMed] [Google Scholar]
- 41. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58 050 patients with suspected acute myocardial infarction. Lancet. 1995;345(8951):669–82. [PubMed] [Google Scholar]
- 42. Antman E. Early administration of intravenous magnesium to high-risk patients with acute myocardial infarction in the Magnesium in Coronaries (MAGIC) trial: a randomised controlled trial. Lancet. 2002;360(9341):1189–96. [DOI] [PubMed] [Google Scholar]
- 43. Song Y, Manson JE, Cook NR, Albert CM, Buring JE, Liu S. Dietary magnesium intake and risk of cardiovascular disease among women. Am J Cardiol. 2005;96:1135–41. [DOI] [PubMed] [Google Scholar]
- 44. Misialek JR, Lopez FL, Lutsey PL, Huxley RR, Peacock JM, Chen LY, Soliman EZ, Agarwal SK, Alonso A. Serum and dietary magnesium and incidence of atrial fibrillation in whites and in African Americans—Atherosclerosis Risk in Communities (ARIC) study. Circ J. 2013;77(2):323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Witkowski M, Hubert J, Mazur A. Methods of assessment of magnesium status in humans: a systematic review. Magnes Res. 2011;24(4):163–80. [DOI] [PubMed] [Google Scholar]
- 46. Zhang X, Del Gobbo LC, Hruby A, Rosanoff A, He K, Dai Q, Costello RB, Zhang W, Song Y. The circulating concentration and 24-h urine excretion of magnesium dose- and time-dependently respond to oral magnesium supplementation in a meta-analysis of randomized controlled trials. J Nutr. 2016;146(3):595–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.