Abstract
Group I metabotropic glutamate receptors (mGluRs) play important roles in various neuronal functions and have also been implicated in multiple neuropsychiatric disorders like fragile X syndrome, autism, and others. mGluR trafficking not only plays important roles in controlling the spatiotemporal localization of these receptors in the cell but also regulates the activity of these receptors. Despite this obvious significance, the cellular machineries that control the trafficking of group I metabotropic glutamate receptors in the central nervous system have not been studied in detail. The post-synaptic scaffolding protein tamalin has been shown to interact with group I mGluRs and also with many other proteins involved in protein trafficking in neurons. Using a molecular replacement approach in mouse hippocampal neurons, we show here that tamalin plays a critical role in the ligand-dependent internalization of mGluR1 and mGluR5, members of the group I mGluR family. Specifically, knockdown of endogenous tamalin inhibited the ligand-dependent internalization of these two receptors. Both N-terminal and C-terminal regions of tamalin played critical roles in mGluR1 endocytosis. Furthermore, we found that tamalin regulates mGluR1 internalization by interacting with S-SCAM, a protein that has been implicated in vesicular trafficking. Finally, we demonstrate that tamalin plays a critical role in mGluR-mediated internalization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, a process believed to be the cellular correlate for mGluR-dependent synaptic plasticity. Taken together, these findings reveal a mechanistic role of tamalin in the trafficking of group I mGluRs and suggest its physiological implications in the brain.
Keywords: endocytosis, trafficking, internalization, G-protein coupled receptor, metabotropic glutamate receptors, neurotransmitter receptors, tamalin, S-SCAM, synaptic plasticity, endocytosis
In the central nervous system, the major excitatory neurotransmitter glutamate acts through ionotropic glutamate receptors and metabotropic glutamate receptors (mGluRs) (1–3). Based on sequence similarity, second-messenger pathways, and pharmacology, mGluRs have been classified into three groups (3–5). Group I mGluRs (mGluR1 and mGluR5) are predominantly localized at the post-synaptic neurons, and they are positively coupled with the Gαq/11 heterotrimeric G proteins (4, 6). These receptors have been implicated in various forms of synaptic plasticity, including learning and memory, as well as in many neuropsychiatric disorders, like fragile X syndrome, autism, etc. (4, 7–13). Similar to many other G-protein-coupled receptors, these receptors also undergo “desensitization” following stimulation by the ligand, and desensitization represents an essential negative-feedback mechanism that protects the receptor from chronic overstimulation (2, 3, 14–16). Subsequent to that, group I mGluRs internalize and recycle back to the cell surface, a mechanism for the “resensitization” of these receptors (17–22). Therefore, trafficking of these receptors, which is a tightly regulated process, controls both the spatiotemporal localization and activity of these receptors. Despite the obvious significance of trafficking of mGluRs, the molecular mechanisms underlying these processes are not well understood.
Group I mGluRs are tightly regulated by a macromolecular protein complex at the post-synaptic membrane, a key component of which is the scaffolding protein tamalin. Tamalin is a 394-amino-acid protein comprising multiple protein-protein interaction domains. It is composed of a PDZ domain, a proline-rich region, a leucine zipper region, and a C-terminal PDZ-binding motif. The PDZ domain of tamalin interacts with the C terminus of group I mGluRs (23). Furthermore, tamalin also interacts with many other important scaffold proteins involved in post-synaptic organization and protein trafficking in neurons. For example, tamalin binds to PSD-95 and also interacts with proteins implicated in trafficking, including MINT2 and GRP-1 (24). The C terminus of tamalin interacts with S-SCAM, another scaffolding protein which directly interacts with motor proteins and thus regulates cargo delivery to and from the membrane (24). In light of all the above observations, we investigated the role of tamalin, if any, in the trafficking of group I mGluRs in primary hippocampal neurons.
We studied the role of tamalin in ligand-mediated trafficking of group I mGluRs using a “molecular replacement” approach that allows simultaneous shRNA-mediated acute knockdown of endogenous tamalin and expression of various forms of recombinant tamalin in primary hippocampal neurons (22, 25, 26). This approach has two important advantages. First, developmental compensatory adaptations that may occur during synaptogenesis and synapse maturation as a result of the loss of endogenous tamalin are minimized. Second, the function of heterologous constructs can be studied without the necessity of a dominant effect, which is required by a standard overexpression approach. We show here that tamalin plays a critical role in the trafficking of mGluR1 and mGluR5. We observed that knockdown of the endogenous tamalin led to the inhibition in the ligand-mediated endocytosis of these receptors. Both N-terminal and C-terminal regions of tamalin were critical for the internalization of mGluR1. Importantly, tamalin regulated the endocytosis of mGluR1 through interaction with S-SCAM. Knockdown of S-SCAM also resulted in the inhibition of the ligand-dependent endocytosis of group I mGluRs. Finally, we show that tamalin also controls mGluR-mediated AMPAR internalization, which is the cellular correlate for the mGluR-dependent synaptic plasticity, through interaction with S-SCAM.
Results
Knockdown of tamalin inhibits the endocytosis of group I mGluRs
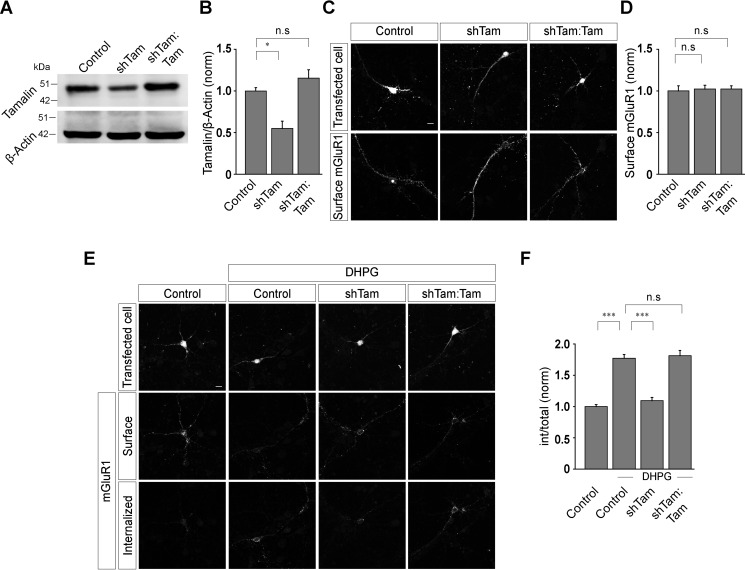
In order to investigate the role of tamalin in the trafficking of group I mGluRs, we employed the molecular replacement strategy. This strategy allows simultaneous shRNA-mediated acute knockdown of the endogenous tamalin and expression of mutant forms of recombinant tamalin in primary hippocampal neurons. To examine the role of tamalin in the ligand-induced endocytosis of mGluR1, we initially screened and identified a shRNA against tamalin (shTam) that could effectively knock down the endogenous tamalin. In the cultured neurons, the shTam construct was effective in knocking down endogenous tamalin, as evidenced by the significant knockdown of the endogenous tamalin measured through Western blotting 3 to 4 days after transfection (control, 1 ± 0.04; shTam, 0.55 ± 0.09; shTam:Tam, 1.15 ± 0.1) (Fig. 1, A and B). We then examined the effect of tamalin knockdown on the surface expression of Myc-mGluR1. shTam-mediated knockdown of tamalin did not have any effect on the surface expression of Myc-mGluR1 (Fig. 1, C and D). In addition, simultaneous expression of both shTam and HA-tagged WT tamalin (shTam resistant) also had no effect on the surface expression of Myc-mGluR1 (control, 1 ± 0.06; shTam, 1.02 ± 0.05; shTam:Tam, 1.02 ± 0.04) (Fig. 1, C and D). We subsequently investigated the effect of the knockdown of endogenous tamalin on the ligand-mediated trafficking of Myc-mGluR1. Primary hippocampal neurons were cotransfected with Myc-mGluR1 and shTam or shTam:Tam constructs. Live cells expressing Myc-mGluR1 were stained with anti-Myc primary antibody. Subsequent application of 100 μm R,S-DHPG, an agonist of mGluR1, led to the internalization of Myc-mGluR1 at 30 min in control cells. However, cells expressing shTam did not show significant internalized receptors on 100 μm R,S-DHPG application at 30 min, and most of the receptors were observed to be localized at the cell surface (control, 1 ± 0.03; control + DHPG, 1.77 ± 0.06; shTam + DHPG, 1.09 ± 0.05) (Fig. 1, E and F). Replacement of the endogenous tamalin with WT tamalin (shTam resistant) rescued the normal endocytosis of the receptor, as was evident from the presence of the endocytosed receptors in the intracellular compartments at 30 min after 100 μm R,S-DHPG application (shTam:Tam + DHPG, 1.81 ± 0.08), indicating that the observed phenotype was not due to nonspecific effects of shTam (Fig. 1, E and F). We chose 30 min as the time point because our previous studies suggested that R,S-DHPG-mediated internalization of Myc-mGluR1 reaches a maximum level at 30 min after ligand application (20, 22). On the other hand, the inhibition of the R,S-DHPG-mediated internalization of Myc-mGluR1 due to the knockdown of the endogenous tamalin could not be rescued by replacing the endogenous tamalin with another post-synaptic density protein that interacts with the group I mGluRs, Norbin, suggesting the specificity of the rescue experiment (control, 1 ± 0.05; control + DHPG, 1.58 ± 0.09; shTam + DHPG, 0.95 ± 0.03; shTam:Norbin + DHPG, 1.13 ± 0.06) (Fig. S1, A and B) (27).
Figure 1.
Knockdown of endogenous tamalin inhibits the ligand-mediated endocytosis of mGluR1. A and B, Western blotting (A) and quantitation of the Western blots (B), showing the efficient knock-down of endogenous tamalin by shTam and replacement of the endogenous tamalin with full-length tamalin. C and D, Representative images (C) and quantitation of surface Myc-mGluR1 (D) showing that knockdown of endogenous tamalin with shTam and expression of the WT tamalin replacement construct had no effect on the surface expression of Myc-mGluR1 (n values: control, 35; shTam, 39; shTam:Tam, 38). E, Representative examples of surface and internalized Myc-mGluR1, 30 min after application of 100 μm R,S-DHPG in control cells, shTam-expressing cells, and shTam- and WT-tamalin-expressing cells. F, Quantitation of the endocytosis index suggested that knockdown of endogenous tamalin inhibited the R,S-DHPG-mediated internalization of Myc-mGluR1 and expression of WT tamalin rescued the normal trafficking of the receptor (n values: control, 35; control + DHPG, 36; shTam + DHPG, 38; shTam:Tam + DHPG, 39). The results are presented as means ± S.E. from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; *, p < 0.05; n.s, p > 0.05.
We subsequently investigated the role of tamalin, if any, in the ligand-mediated endocytosis of the other member of the group I mGluR family, viz., mGluR5. Knockdown of the endogenous tamalin had no effect on the surface expression of Myc-mGluR5 (control, 1 ± 0.04; shTam, 0.98 ± 0.04; shTam:Tam, 0.98 ± 0.06) (Fig. S2, A and B). On the other hand, 100 μm R,S-DHPG-mediated internalization of Myc-mGluR5 was inhibited upon knockdown of the endogenous tamalin and replacement of the endogenous tamalin with WT tamalin rescued the normal internalization of the receptor (control, 1 ± 0.05; control + DHPG, 2 ± 0.06; shTam + DHPG, 1.03 ± 0.04; shTam:Tam + DHPG, 1.96 ± 0.05) (Fig. S2, C and D). These results suggest that tamalin plays a critical role in the ligand-mediated internalization of both members of group I mGluRs, viz., mGluR1 and mGluR5. Since trafficking of both mGluR1 and mGluR5 was regulated by tamalin, we concentrated on mGluR1 for rest of the study.
Role of tamalin domains in the trafficking of mGluR1
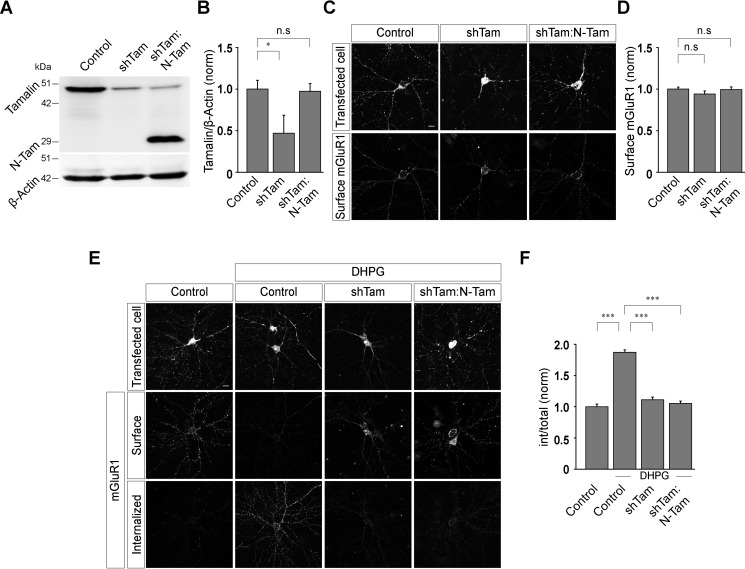
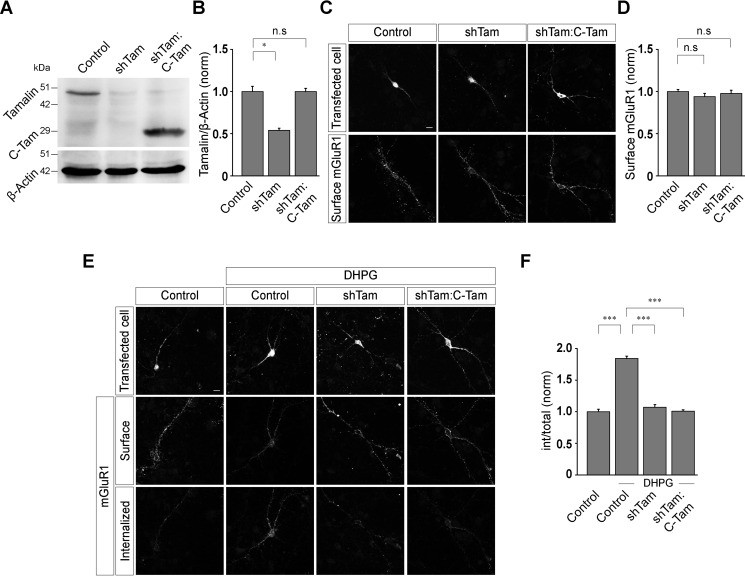
Tamalin is a scaffold protein that comprises multiple protein-interacting domains (23). For example, the PDZ domain of tamalin binds to the carboxyl-terminal tail of group I mGluRs, and the leucine zipper region of tamalin binds the coiled-coil region of guanine nucleotide exchange factor cytohesins (23). In order to determine the role of tamalin in group I mGluR trafficking, we first investigated the effect of various deletion mutants of tamalin in mGluR1 trafficking. To examine whether the N-terminal region of tamalin is sufficient for the normal trafficking of group I mGluRs, we first replaced the endogenous tamalin with a form of tamalin (N-Tam) having only the N-terminal region (amino acids 1 to 209 of tamalin) and lacking the C-terminal region of the protein. N-Tam was observed to have normal expression levels in primary neurons (control, 1 ± 0.11; shTam, 0.47 ± 0.21; shTam:N-Tam, 0.97 ± 0.09) (Fig. 2, A and B). Acute knockdown of the endogenous tamalin and expression of shTam:N-Tam did not have any effect on the surface expression of Myc-mGluR1 (control, 1 ± 0.02; shTam, 0.94 ± 0.04; shTam:N-Tam, 0.99 ± 0.03) (Fig. 2, C and D). However, unlike WT tamalin, N-Tam did not rescue the R,S-DHPG-mediated endocytosis of Myc-mGluR1, and the receptor did not internalize on application of 100 μm R,S-DHPG in shTam:N-Tam-expressing cells, similar to what was observed in cells where endogenous tamalin was knocked down (control, 1 ± 0.04; control + DHPG, 1.87 ± 0.04; shTam + DHPG, 1.11 ± 0.04; shTam:N-Tam + DHPG, 1.05 ± 0.04) (Fig. 2, E and F). Subsequently, we investigated whether the C-terminal region of tamalin alone was sufficient for the ligand-mediated internalization of mGluR1 by replacing the WT tamalin with a form of tamalin (C-Tam) containing the C-terminal region alone (amino acids 173 to 394 of tamalin) and lacking the N-terminal region of the protein. Similar to other constructs of tamalin, C-Tam also appeared to express properly, as observed by Western blotting (control, 1 ± 0.06; shTam, 0.54 ± 0.03; shTam:C-Tam, 1 ± 0.04) (Fig. 3, A and B). Expression of this replacement construct (shTam:C-Tam) had no effect on the surface expression of Myc-mGluR1 (control, 1.0 ± 0.02; shTam, 0.94 ± 0.04; shTam:C-Tam, 0.98 ± 0.04) (Fig. 3, C and D). Similar to N-Tam, C-Tam also did not rescue the R,S-DHPG-mediated internalization of Myc-mGluR1. No significant internalization of Myc-mGluR1 was observed 30 min after the application of 100 μm R,S-DHPG in shTam:C-Tam-expressing cells, similar to what was observed in tamalin knockdown cells (control, 1 ± 0.04; control + DHPG, 1.84 ± 0.04; shTam + DHPG, 1.07 ± 0.04; shTam:C-Tam + DHPG, 1 ± 0.02) (Fig. 3, E and F). These results suggest that both the N-terminal and C-terminal regions of tamalin play a critical role in the ligand-induced trafficking of mGluR1.
Figure 2.
N-terminal domain of tamalin alone is not sufficient for the ligand-mediated endocytosis of mGluR1. A and B, Western blotting (A) and quantitation of the Western blots (B), showing the effective knockdown of endogenous tamalin by shTam and expression of the N-Tam replacement construct. C and D, Representative images (C) and quantitation (D) showing that expression of shTam and shTam:N-Tam had no effect on the surface expression of Myc-mGluR1 (n values: control, 44; shTam, 43; shTam:N-Tam, 42). E and F, Representative cells (E) and quantitation (F) of 100 μm R,S-DHPG-induced internalization of Myc-mGluR1 suggested that knockdown of endogenous tamalin led to the inhibition of ligand-mediated endocytosis of Myc-mGluR1, and expression of the N-Tam replacement construct did not rescue the normal endocytosis of the receptor (n values: control, 42; control + DHPG, 43; shTam + DHPG, 42; shTam:N-Tam + DHPG, 44). The results are presented as means ± S.E. from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; *, p < 0.05; n.s, p > 0.05.
Figure 3.
The C-terminal domain of tamalin alone is not sufficient for the ligand-mediated endocytosis of mGluR1. A and B, Acute knockdown of endogenous tamalin and replacement of endogenous tamalin with C-Tam, as shown by Western blotting (A) and quantitation of the Western blots (B). C and D, Representative images (C) and quantitation (D) showing no effect on the surface expression of Myc-mGluR1 in shTam and shTam:C-Tam-expressing neurons (n values: control, 44; shTam, 43; shTam:C-Tam, 44). E and F, Representative images (E) and quantitation (F) suggested that knockdown of the endogenous tamalin inhibited the R,S-DHPG-mediated endocytosis of Myc-mGluR1, and replacement of the endogenous tamalin with C-Tam failed to rescue the normal internalization of the receptor (n values: control, 44; control + DHPG, 44; shTam + DHPG, 42; shTam:C-Tam + DHPG, 42). The results are presented as means ± S.E. from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; *, p < 0.05; n.s, p > 0.05.
The last 8 amino acids of tamalin play a critical role in the ligand-mediated endocytosis of mGluR1
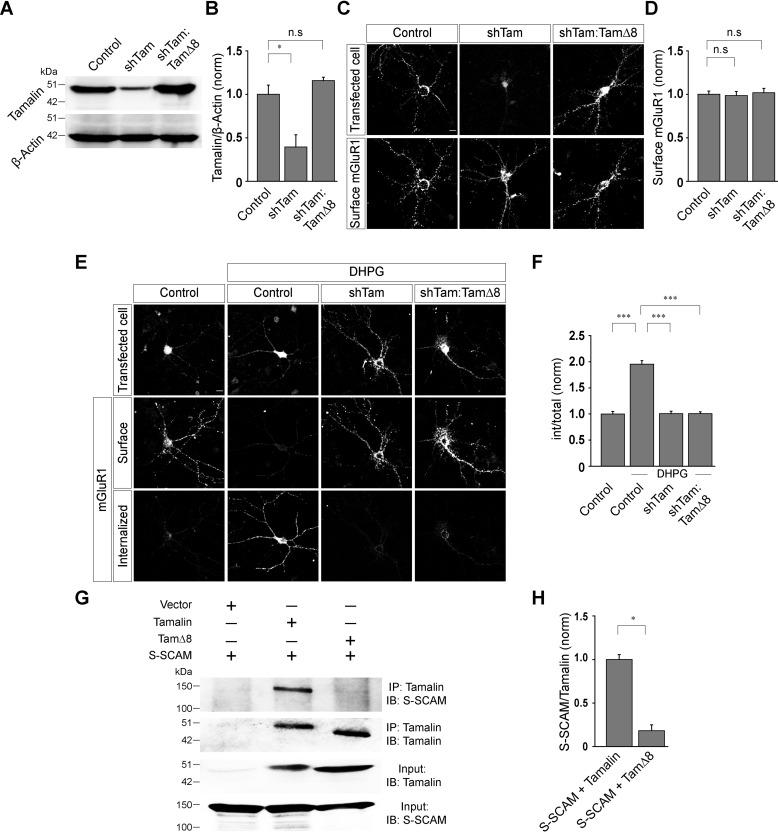
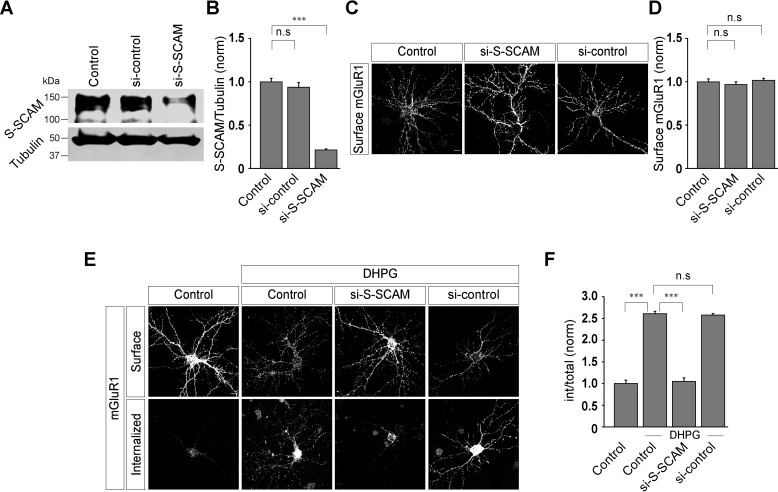
It has been reported that the C-terminal region of tamalin interacts with S-SCAM, another scaffolding protein which directly interacts with motor proteins and thus regulates the cargo delivery to and from the membrane (24, 28). In order to ascertain what role, if any, this domain has in the trafficking of group I mGluRs, the last 8 amino acids of the tamalin protein were deleted (TamΔ8) and a replacement construct was generated (shTam:TamΔ8). This replacement construct showed normal expression levels in Western blotting (control, 1 ± 0.11; shTam, 0.39 ± 0.14; shTam:TamΔ8: 1.16 ± 0.04) (Fig. 4, A and B). Initially, the role of this domain in the surface expression of Myc-mGluR1 was investigated. Deletion of the last 8 amino acids of tamalin had no effect on the surface expression of Myc-mGluR1 (control, 1.0 ± 0.04; shTam, 0.99 ± 0.05; shTam:TamΔ8, 1.02 ± 0.05) (Fig. 4, C and D). However, unlike WT tamalin, TamΔ8 did not rescue the ligand-mediated endocytosis of Myc-mGluR1. In shTam:TamΔ8-transfected cells, the receptors did not endocytose upon application of 100 μm R,S-DHPG, similar to what was observed in shTam-transfected cells (control, 1 ± 0.05; control + DHPG, 1.95 ± 0.07; shTam + DHPG, 1 ± 0.05; shTam:TamΔ8 + DHPG, 1 ± 0.03) (Fig. 4, E and F). We subsequently investigated whether binding of tamalin and S-SCAM is important for the ligand-mediated trafficking of mGluR1 (24, 28). Our data indicated that deletion of the last 8 amino acids of tamalin disrupted the binding of S-SCAM to tamalin, suggesting that the interaction of tamalin with S-SCAM is critical for the trafficking of mGluR1 (S-SCAM + tamalin, 1 ± 0.06; S-SCAM + TamΔ8, 0.18 ± 0.07) (Fig. 4, G and H). These results suggest that the last 8 amino acids in the C-terminal region of tamalin play a critical role in the ligand-mediated trafficking of mGluR1 through interaction with S-SCAM.
Figure 4.
The last 8 amino acids of tamalin play a critical role in the ligand-mediated internalization of mGluR1. A and B, Western blotting (A) and quantitation of the Western blots (B), showing the knockdown of the endogenous tamalin by shTam and expression of the TamΔ8 replacement construct. C and D, Representative images (C) and quantitation (D) of the surface-localized Myc-mGluR1 suggested that acute knockdown of the endogenous tamalin by shTam and replacement of the endogenous tamalin with TamΔ8 had no effect on the surface expression of Myc-mGluR1 (n values: control, 42; shTam, 42; shTam + TamΔ8; 43). E and F, Representative cells (E) and quantitation (F) suggested that knockdown of the endogenous tamalin resulted in the inhibition of the R,S-DHPG-mediated endocytosis of Myc-mGluR1, and the TamΔ8 replacement construct did not rescue the normal internalization of the receptor (n values: control, 42; control + DHPG, 45; shTam + DHPG, 42; shTam:TamΔ8 + DHPG, 48). G, Coimmunoprecipitation assays demonstrated that deletion of the last 8 amino acids of tamalin disrupted binding of S-SCAM to tamalin. H, Quantitation of the coimmunoprecipitation assays. All the results are presented as means ± S.E. from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; *, p < 0.05; n.s, p > 0.05.
Role of S-SCAM in the ligand-mediated trafficking of group I mGluRs
Thus far, our results suggested that tamalin controls the ligand-mediated trafficking of mGluR1 and that the last 8 amino acids at the C-terminal tail of tamalin play a critical role in this process. These last 8 amino acids of tamalin interact with the scaffolding protein S-SCAM, which directly regulates the cargo delivery to and from the membrane via interaction with the motor proteins (24, 28). To determine whether S-SCAM plays any role in the ligand-induced trafficking of mGluR1, we knocked down the endogenous S-SCAM in primary hippocampal neurons. Briefly, cells were cotransfected with Myc-mGluR1 cDNA and siRNA against the endogenous S-SCAM (si-S-SCAM) (ON-TARGETplus) or scrambled siRNA (si-control) at 8 to 9 days in vitro. Subsequently, experiments were conducted when the cells were at 12 to 14 days in vitro. The si-S-SCAM efficiently knocked down the endogenous S-SCAM in primary neurons (control, 1 ± 0.04; si-control, 0.94 ± 0.06; si-S-SCAM, 0.21 ± 0.01) (Fig. 5, A and B). Acute knockdown of the endogenous S-SCAM did not have any effect on the surface expression of Myc-mGluR1 (control, 1 ± 0.03; si-S-SCAM, 0.97 ± 0.03; si-control, 1.02 ± 0.02) (Fig. 5, C and D). Importantly, the receptor did not internalize within 30 min after 100 μm R,S-DHPG application in si-S-SCAM-transfected cells (Fig. 5, E and F). On the other hand, in both control cells and si-control-transfected cells, Myc-mGluR1 was observed to be in the internal compartment at 30 min after ligand application (control, 1 ± 0.08; control + DHPG, 2.61 ± 0.06; si-SCAM + DHPG, 1.05 ± 0.08; si-control + DHPG, 2.58 ± 0.04) (Fig. 5, E and F). We subsequently investigated the effect of S-SCAM knockdown on the surface expression and ligand-mediated endocytosis of Myc-mGluR5. Acute knockdown of the endogenous S-SCAM had no effect on the surface expression of Myc-mGluR5 (control, 1 ± 0.05; si-S-SCAM, 0.99 ± 0.05; si-control, 0.99 ± 0.04) (Fig. S3, A and B). On the other hand, 100 μm R,S-DHPG-mediated internalization of Myc-mGluR5 was inhibited in si-S-SCAM-transfected cells. Control cells and si-control-transfected cells showed normal internalization of Myc-mGluR5 upon application of 100 μm R,S-DHPG (control, 1 ± 0.09; control + DHPG, 2.7 ± 0.06; si-S-SCAM + DHPG, 1.04 ± 0.07; si-control + DHPG, 2.71 ± 0.06) (Fig. S3, C and D). These results suggest that S-SCAM plays a critical role in the ligand-mediated endocytosis of both mGluR1 and mGluR5, probably through the interaction with tamalin.
Figure 5.
Knockdown of S-SCAM inhibits the ligand-mediated internalization of mGluR1. A and B, Western blotting analyses (A) and quantitation of the Western blots (B), showing the efficient knockdown of endogenous S-SCAM by si-S-SCAM. C and D, Representative images (C) and quantitation (D) suggested that acute knockdown of endogenous S-SCAM had no effect on the surface expression of Myc-mGluR1 (n values: control, 36; si-S-SCAM, 35; si-control, 36). E and F, Representative cells (E) and quantitation of the endocytosis index (F) showing that knockdown of the endogenous S-SCAM led to the inhibition in the R,S-DHPG-mediated internalization of Myc-mGluR1, whereas in control cells and si-control-transfected cells, the receptor internalized normally (n values: control, 36; control + DHPG, 38; si-S-SCAM + DHPG, 37; si-control + DHPG, 37). The results are presented as means ± S.E. from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; n.s, p > 0.05.
Expression profile and synaptic localization of various mutants of tamalin
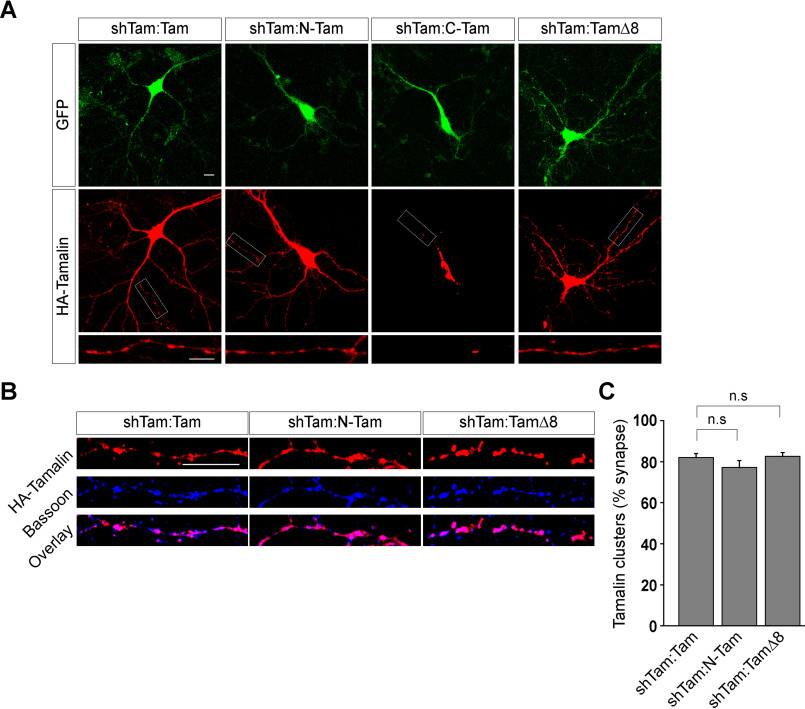
In order to check whether the various tamalin mutants used in this study were expressed and targeted properly in neurons, we first checked the expression profile of each of them using an immunostaining method. As stated before, each of these constructs was tagged with HA at the N terminus, and upon expression of these constructs, they produced recombinant proteins fused with HA at the N terminus of the protein. Each of these constructs was transfected into primary hippocampal neurons at 5 to 7 days in vitro using the calcium phosphate method. Cells were then stained with the anti-HA rat polyclonal antibody (1:500) at 12 to 15 days in vitro followed by the application of Alexa Fluor 568-conjugated goat anti-rat immunoglobulin secondary antibody (1:500). Our data suggested that the expression patterns of the N-Tam protein and TamΔ8 protein were similar to the expression pattern of the WT tamalin replacement protein (Fig. 6A). In contrast, the C-Tam protein did not target properly and was predominantly localized at the cell body of the neuron (Fig. 6A). In order to investigate whether the mutants of tamalin that were used in this study localized at the synapse, the proportion of synapses containing detectable amounts of these mutants of tamalin was quantified by staining for HA-containing clusters and counterstaining for Bassoon, a core component of the active zone that is commonly used to identify presynaptic terminals (29). Our data suggested that both N-Tam and TamΔ8 localized at the synapse, very similar to the WT tamalin protein (WT tamalin, 82.01 ± 1.97%; N-Tam, 77.19 ± 3.42%; TamΔ8, 82.65 ± 1.85%) (Fig. 6, B and C). Since the C-Tam construct did not target properly at the dendrites and was mostly localized in the cell body region, we did not study its colocalization with Bassoon. These results together suggested that deletion of the N-terminal domain of tamalin mislocalized the protein. Furthermore, both the N-terminal domain of tamalin and tamalin lacking the last 8 amino acids were targeted properly at the synapse.
Figure 6.
Synaptic localization of tamalin constructs. A, Representative images showing that WT tamalin is expressed throughout the hippocampal neuron and targeted to the dendrites. N-Tam and TamΔ8 expression was also observed throughout the hippocampal neuron, and they were also seen to be localized in the dendrites, similar to the WT tamalin. In contrast, C-Tam did not target properly to the dendrites of the neuron. B, Representative images showing colocalization of Bassoon, an active zone synaptic marker with various forms of tamalin (WT, N-Tam, and TamΔ8). All these constructs were found to colocalize with Bassoon, suggesting that they were targeted to the synapse. C, Quantitation also suggested that all the above forms of tamalin were localized at the synapse to a similar extent (n values: shTam:Tam, 34; shTam:N-Tam, 34; shTam:TamΔ8, 33). Scale bar, 10 μm. n.s, p > 0.05.
Tamalin plays a critical role in mGluR-mediated AMPAR endocytosis
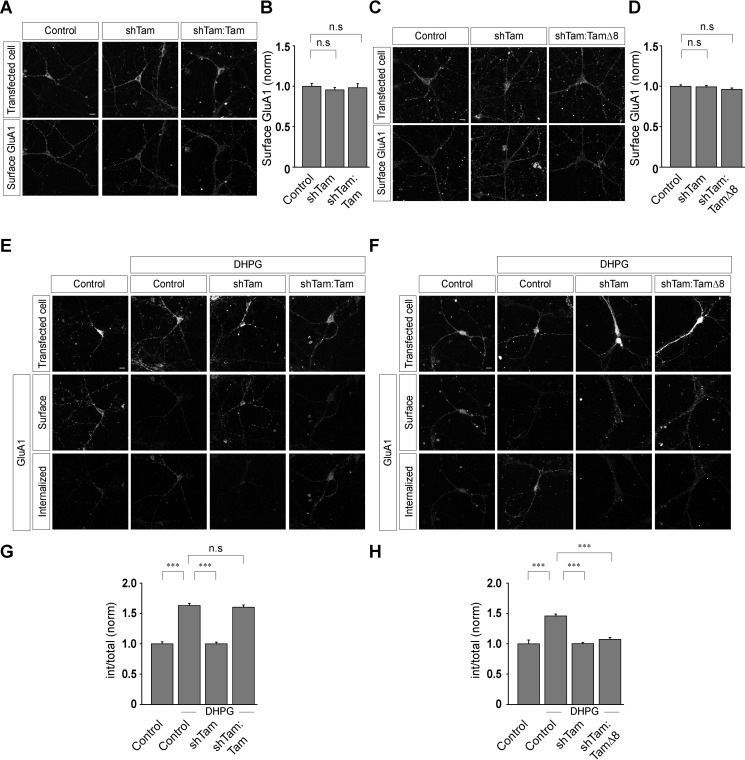
Rapid endocytosis of surface AMPARs can be triggered in cultured hippocampal neurons by application of various glutamate receptor agonists, including glutamate itself, N-methyl-d-aspartate, AMPA, and group I mGluR agonists (21, 22, 25, 26, 30, 31). mGluR-mediated AMPAR endocytosis is believed to be the cellular correlate for the mGluR-dependent synaptic plasticity (3, 4, 11, 12, 32). Because the goal of this study was to elucidate the role of tamalin in group I mGluR trafficking and, in turn, its effect on mGluR-dependent synaptic AMPAR endocytosis, we initially studied the effect of acute knockdown of tamalin on mGluR-dependent synaptic AMPAR endocytosis. We employed the protocol that results in the mGluR-mediated endocytosis of synaptic AMPARs (21, 22). To examine whether tamalin plays any role in mGluR-mediated AMPAR endocytosis, we acutely knocked down the endogenous tamalin in primary hippocampal neurons. Tamalin knockdown did not have any effect on the surface expression of GluA1-containing receptors (control, 1 ± 0.04; shTam, 0.96 ± 0.03; shTam:Tam, 0.98 ± 0.05) (Fig. 7, A and B). We subsequently studied the role of tamalin in mGluR-dependent AMPAR endocytosis. In control cells, application of R,S-DHPG (100 μm for 5 min) in the presence of 1 μm TTX, 20 μm DNQX, and 50 μm APV resulted in the endocytosis of AMPARs (control, 1 ± 0.03; control + DHPG, 1.63 ± 0.04) (Fig. 7, E and G). On the other hand, knockdown of the endogenous tamalin inhibited the AMPAR endocytosis triggered by the application of 100 μm R,S-DHPG (Fig. 7, E and G). The mGluR-mediated AMPAR endocytosis was rescued when endogenous tamalin was replaced by the WT tamalin (shTam + DHPG, 1 ± 0.03; shTam:Tam + DHPG, 1.6 ± 0.03) (Fig. 7, E and G).
Figure 7.
Tamalin regulates mGluR-mediated AMPAR endocytosis. A and B, Representative images (A) and quantitation (B) of surface AMPARs (GluA1-containing receptors) suggested that knockdown of the endogenous tamalin had no effect on the surface expression of these receptors (n values: control, 46; shTam, 45; shTam:Tam, 44). C and D, Representative images (C) and quantitation (D) of the surface AMPARs suggested that knockdown of the endogenous tamalin and replacement of the endogenous tamalin with TamΔ8 had no effect on the surface expression of the GluA1-containing receptors (n values: control, 28; shTam, 30; shTam:TamΔ8, 31). E and G, Representative images (E) and quantitation (G) of mGluR-mediated AMPAR endocytosis suggested that in control cells, the receptors internalized when they were stimulated with 100 μm R,S-DHPG for 5 min. In contrast, application of 100 μm R,S-DHPG did not cause endocytosis of GluA1-containing receptors in tamalin knockdown cells. Expression of WT tamalin rescued the R,S-DHPG-mediated endocytosis of the receptor (n values: control, 46; control + DHPG, 45; shTam + DHPG, 49; shTam:Tam + DHPG, 44). F and H, The inhibition of the mGluR-mediated AMPAR endocytosis due to the knockdown of the endogenous tamalin was not rescued by replacing the endogenous tamalin with TamΔ8, as observed from representative images (F) and quantitation (H) (n values: control, 28; control + DHPG, 24; shTam + DHPG, 30; shTam:TamΔ8 + DHPG, 27). The results are presented as means ± S.E. from three independent experiments. Scale bar, 10 μm. ***, p < 0.001; n.s, p > 0.05.
Our earlier data suggested that the C-terminal 8 amino acids of tamalin play a critical role in the interaction of tamalin with S-SCAM and that this interaction is essential for the ligand-mediated trafficking of group I mGluRs. In order to investigate whether interaction of tamalin with S-SCAM is also required for the mGluR-mediated AMPAR endocytosis, we studied the effect of TamΔ8 replacement in this process. Acute knockdown of the endogenous tamalin and expression of TamΔ8 had no effect on the surface expression of GluA1-containing receptors (control, 1 ± 0.02; shTam, 0.99 ± 0.02; shTam:TamΔ8, 0.96 ± 0.02) (Fig. 7, C and D). Importantly, knockdown of the endogenous tamalin inhibited the AMPAR endocytosis induced by 100 μm R,S-DHPG, and replacement of the endogenous tamalin with TamΔ8 did not rescue the mGluR-mediated AMPAR endocytosis (control, 1 ± 0.06; control + DHPG, 1.46 ± 0.03; shTam + DHPG, 1 ± 0.02; shTam:TamΔ8 + DHPG, 1.07 ± 0.03) (Fig. 7, F and H). These results suggest that tamalin also regulates the mGluR-mediated AMPAR endocytosis through the interaction with S-SCAM.
Discussion
Trafficking of group I mGluRs plays critical roles in the regulation of the activity of these receptors as well as their proper spatiotemporal localization in the neuron. Inappropriate trafficking of the receptor could result in abnormal signaling, which often has serious pathological consequences. For these reasons, in the last few years, understanding the molecular mechanisms of group I mGluR trafficking and their physiological significance has become a major area of research. In the present study, we defined a novel role for tamalin in the ligand-mediated trafficking of group I mGluRs. We showed earlier that subsequent to desensitization, group I mGluRs undergo internalization through a ubiquitin-dependent pathway (19–21). Following that, the receptors enter the recycling compartment, and they recycle back to the cell surface at 2.5 h after ligand application (19, 20, 22). Tamalin is a scaffold protein that contains multiple protein-interacting domains, including a PDZ domain, a proline-rich region, a leucine zipper region and a C-terminal PDZ-binding motif. The PDZ domain and the leucine zipper region of tamalin bind to the carboxyl-terminal tail of group I mGluRs (23). Moreover, tamalin interacts with many other proteins present in the post-synaptic density and involved in the post-synaptic organization. Some of those molecules have been implicated in the trafficking of proteins in neurons. For example, tamalin binds to PSD-95 and also interacts with MINT2 and GRP-1, proteins implicated in the trafficking process (24). The C terminus of tamalin interacts with S-SCAM, another scaffolding protein which directly interacts with motor proteins and thus regulates the cargo delivery to and from the membrane (24). In light of all the above observations, we investigated what role, if any, tamalin has in the ligand-mediated trafficking of group I mGluRs in primary hippocampal neurons. Our data suggest that tamalin plays a critical role in the ligand-mediated endocytosis of mGluR1 and mGluR5. Knockdown of the endogenous tamalin led to the inhibition of mGluR1/mGluR5 internalization upon application of R,S-DHPG. Subsequently, using a strategy in which mutant forms of tamalin replace the endogenous tamalin that has been knocked down by shRNA, we found that tamalin controls the ligand-mediated internalization of group I mGluRs through the interaction with S-SCAM.
Both the N-terminal region and the C-terminal region of tamalin seem to be critical for the normal endocytosis of mGluR1. As stated before, knockdown of the endogenous tamalin led to inhibition of the R,S-DHPG-mediated internalization of the receptor. Importantly, the ligand-mediated endocytosis of mGluR1 was still inhibited when the endogenous tamalin was replaced with the N-terminal region of tamalin (N-Tam) and the C-terminal region of tamalin (C-Tam). The N-terminal region of tamalin contains an alanine-rich region and a PDZ domain. On the other hand, the C-terminal region of tamalin contains a leucine zipper and a proline-rich region and a glycine-rich region (23). The PDZ domain of tamalin binds to the carboxyl-terminal tail of group I mGluRs, and the leucine zipper region binds the coiled-coil region of guanine nucleotide exchange factor cytohesins. Importantly, our data suggest that the C-Tam construct was not targeted properly at the dendritic region and thus was mislocalized. On the other hand, the N-Tam construct was targeted properly at the synapse. Since we were interested in investigating the mechanisms through which tamalin and its downstream binding partners modulate the trafficking of mGluR1, we studied how the C-terminal region of tamalin plays a critical role in the trafficking of mGluR1. It has been reported that the extreme C-terminal region of tamalin interacts with another scaffolding protein, S-SCAM (24). S-SCAM directly interacts with motor proteins and thus regulates the cargo delivery to and from the membrane. We therefore investigated whether the interaction of tamalin with S-SCAM is critical for the ligand-dependent internalization of mGluR1. Importantly, our data suggest that the last 8 amino acids of tamalin at the C-terminal region are necessary for the interaction of tamalin with S-SCAM. Furthermore, these last 8 amino acids at the C-terminal region of tamalin were also found to be critical for the ligand-mediated endocytosis of mGluR1. The internalization of the receptor was still inhibited when the endogenous tamalin was replaced with TamΔ8. TamΔ8 was targeted properly at the synapse and showed synaptic localization similar to that of WT tamalin. We therefore hypothesize at this point that the interaction of S-SCAM with tamalin is critical for the normal trafficking of mGluR1. Our hypothesis was strengthened by the observation that acute knockdown of endogenous S-SCAM also resulted in the inhibition of the R,S-DHPG-mediated internalization of mGluR1 and mGluR5. Importantly, we show here that knockdown of tamalin also inhibited mGluR-dependent AMPAR endocytosis, which is a prerequisite for mGluR-mediated synaptic plasticity. Our data also suggest that tamalin regulates mGluR-mediated AMPAR endocytosis through the interaction with S-SCAM. These results indicate that tamalin plays a critical role in both ligand-mediated internalization of group I mGluRs and mGluR-dependent AMPAR endocytosis.
In conclusion, we have shown here that ligand-dependent trafficking of group I mGluRs is mediated by tamalin through the interaction with S-SCAM in primary hippocampal neurons. Both the N-terminal region and the C-terminal region of tamalin seem to play critical roles in the trafficking of mGluR1. The last 8 amino acids of the C-terminal region of tamalin are critical for the normal trafficking of mGluR1, since S-SCAM interacts with tamalin through this region. Although we show here that the N-terminal region of tamalin also plays a critical role in the trafficking of mGluR1, the mechanism by which this region regulates the trafficking of group I mGluRs needs to be investigated. As stated before, inappropriate trafficking of group I mGluRs could lead to improper signaling, with pathological consequences. Inappropriate signaling of group I mGluRs has been suggested to be involved in the pathophysiology of multiple cognitive disorders, such as fragile X syndrome, autism, etc. Indeed, our data suggest that in the absence of tamalin, the receptor does not internalize upon application of the ligand. This might have a profound effect on the activity of the receptor. We showed earlier that the internalization and recycling of mGluR1 are required for the resensitization of the receptor (21, 22). Therefore, inhibition in the internalization of mGluR1 in tamalin knockdown cells might affect the resensitization of the receptor; this still needs to be tested. Taken together, our results reveal a critical role of tamalin in controlling the normal trafficking of group I mGluRs, dysregulation of which could contribute to these disorders by altering the activity as well as spatiotemporal localization of the receptor. For these reasons, further study in the near future of the regulation of group I mGluRs by tamalin and tamalin-interacting proteins is of paramount importance.
Materials and methods
Materials
The Myc-mGluR1 and Myc-mGluR5 constructs in which the Myc epitope was tagged at the N terminus of the full-length mGluR1/mGluR5 were a generous gift from Kathrine Roche (NIH, USA). Full-length mouse tamalin was obtained from Shigetada Nakanishi (Osaka Bioscience Institute, Japan), full-length human Norbin was a gift from Heidi Welch (Babraham Institute, UK), and rat Myc–S-SCAM was obtained from Addgene (USA). Reagents for cell culture were purchased from Invitrogen (USA). Polyethylenimine, floxuridine, PFA, poly-d-lysine, and Fluoromount aqueous mounting medium were obtained from Sigma (USA). Protein G beads were from GenScript (USA). R,S-DHPG, APV, and DNQX were purchased from Tocris (UK). TTX was purchased from Adooq Biosciences (USA). Fine chemicals were obtained from Life Technologies (USA) and Merck Ltd. (USA). The primary antibodies were purchased from following companies: anti-Myc antibody from Abcam (UK), anti-HA antibody from Roche (USA), anti-GFP antibody from Life Technologies (USA), anti-tamalin antibody from Rockland Immunochemicals (USA), anti-S-SCAM antibody from Millipore (USA), anti-β-actin antibody from Santa Cruz Biotechnology (USA), anti-tubulin antibody from Sigma (USA), and anti-GluA1 antibody from Calbiochem (USA). All secondary antibodies were purchased from Invitrogen (USA). An ECL Western blotting detection kit was obtained from GE Healthcare (USA). ON-TARGETplus SMARTpool siRNA against S-SCAM was obtained from Thermo Scientific Dharmacon (USA).
Construct preparation
We first identified a highly effective shRNA to tamalin (shTam) that robustly reduced the endogenous tamalin levels in dissociated neuron cultures when assessed by immunocytochemistry and Western blotting. The shRNA against tamalin (shTam) was cloned in a multipromoter vector under the control of the H1 promoter targeting the tamalin sequence GCATCTATGACACACTGGAGT. Enhanced GFP expression, which was under the control of the internal ribosome entry site, was used to identify transfected cells. We observed that shTam was very effective in knocking down the endogenous tamalin in primary neurons (Fig. S4). The replacement constructs were cloned under the ubiquitin promoter of the vector containing shTam. These replacement constructs include shTam:Tam (full-length tamalin), shTam:N-Tam (amino acids 1 to 209 of tamalin were present), shTam:C-Tam (amino acids 173 to 394 of tamalin were present), and shTam:TamΔ8 (the last 8 amino acids of tamalin were deleted). A schematic diagram of all the tamalin constructs used in this study is presented in Fig. S5. Silent mutations were introduced into the tamalin target region of shTam to generate the above-described replacement constructs. These silent mutations in the shTam-binding region in the replacement tamalin prevented the knockdown of the replacement constructs by shTam, but simultaneously the endogenous tamalin was down-regulated. All replacement constructs were tagged with the HA epitope at the N terminus of the protein.
Dissociated hippocampal neuron cultures
Studies done on mouse primary hippocampal neuron cultures were approved by the Institutional Animal Ethics Committee of the Indian Institute of Science Education and Research Mohali. Dissociated primary hippocampal neuron cultures were prepared from C57BL/6 P0 mouse pups of both sexes as described previously with minor changes (19, 21, 22). Briefly, P0 mouse pups were sacrificed, and hippocampi were obtained from them. Subsequently, papain treatment was done to dissociate the tissue. Approximately 150,000 cells were then plated on coverslips coated with poly-d-lysine in sodium borate (50 μg/ml poly-d-lysine plus 0.1 M sodium borate) in 12-mm wells of a 24-well plate. Cultures were maintained in neurobasal medium with 0.5 mm glutamine and B27 supplement. Glial growth was inhibited by adding floxuridine on the 4th day of culture.
HEK293 cell culture and transfection
HEK293 cells were cultured in DMEM supplemented with 10% FBS and an antibiotic-antimycotic mix at 37°C and 5% CO2. Transfection of the cells was performed on 60-mm dishes coated with 50 μg/ml poly-d-lysine using polyethyleneimine and DNA in a 3:1 ratio in plain DMEM. Experiments in HEK293 cells were carried out 24 h posttransfection.
Transfection in primary hippocampal neurons
Primary hippocampal neurons were cotransfected with Myc-mGluR1/Myc-mGluR5, shTam, and various tamalin replacement constructs at 8 to 9 days in vitro using calcium phosphate (33). Experiments were carried out when the cells were at 12 to 14 days in vitro. In order to study the effect of the knockdown of S-SCAM on the trafficking of group I mGluRs, ON-TARGETplus SMARTpool siRNA against S-SCAM and scrambled siRNA were cotransfected with Myc-mGluR1/Myc-mGluR5 cDNA into primary hippocampal neurons at 8 to 9 days in vitro using Lipofectamine 3000 following the manufacturer's instructions. Experiments were carried out in cells at 12 to 14 days in vitro.
mGluR endocytosis assay
Primary hippocampal neurons were cotransfected with the Myc-mGluR1/Myc-mGluR5 cDNA and with either shTam or shTam containing tamalin replacement constructs. Experiments were carried out 4 to 6 days posttransfection. Live cells were incubated with mouse anti-Myc primary antibody (1:250) for 20 min at 37°C. Following that, 100 μm R,S-DHPG, which is a specific agonist of group I mGluRs, was applied for 5 min. Subsequently, R,S-DHPG was removed, and cells were incubated at 37°C for various time periods in plain neurobasal medium in the absence of the ligand. Cells were then fixed without permeabilization using ice-cold 4% PFA for 15 min on ice. Receptors localized at the cell surface were labeled with the saturating concentration of Alexa Fluor 568-conjugated goat anti-mouse immunoglobulin secondary antibody (1:100) for 1 h at 37°C. After that, cells were permeabilized with 0.1% Triton X-100 for 30 min at room temperature. The endocytosed receptors were then labeled by the application of Alexa Fluor 647-conjugated goat anti-mouse immunoglobulin secondary antibody (1:800) for 1 h at 37°C. Subsequently, the cotransfected shRNA constructs containing the enhanced-GFP- and HA-tagged replacement constructs were stained with rabbit anti-GFP antibody (1:500) and rat anti-HA antibody (1:500), respectively, overnight at 4°C. Next, appropriate Alexa Fluor 488-conjugated secondary antibodies against the respective primary antibodies were applied. The coverslips were mounted on glass slides and scanned under the confocal microscope. In order to ensure that the secondary antibody that we used to label the internalized receptors, viz., the Alexa Fluor 647-conjugated secondary antibody, did not label any detectable surface receptors in our experiments, we performed control experiments to determine the saturating concentration of the first secondary antibody, similar to those described in our earlier studies (19–22, 34). The control experiments suggested that in our assays, Alexa Fluor 647-conjugated secondary antibody did not label any detectable amount of surface receptors, and thus, it stained the internalized receptors only (data not shown).
AMPAR endocytosis assay
For investigation of the role of tamalin in the mGluR-mediated AMPAR endocytosis, primary hippocampal neurons were transfected with shTam, shTam:Tam, or shTam:TamΔ8 as described above, and mGluR-mediated AMPAR endocytosis was assayed using the protocol we used before (21, 22). To study mGluR-mediated AMPAR endocytosis, cells were preincubated with appropriate mixtures of antagonists: 1 μm TTX (presynaptic release blocker), 20 μm DNQX (antagonist for AMPARs), and 50 μm APV (antagonist for N-methyl-d-aspartate receptors) for 30 min. Subsequently, surface GluA1-containing AMPARs were labeled in live neurons by 15 min incubation at 37°C with a rabbit polyclonal antibody directed against the N terminus of the GluA1 subunit (1:20). After removal of the primary antibody, 100 μm R,S-DHPG was applied for 5 min. The agonist was then washed out, and cells were further incubated in the presence of the antagonists for a total of 15 min at 37°C. Following the completion of the incubation period, cells were fixed in 4% PFA for 15 min on ice without permeabilization, and surface receptors were labeled by application of the saturating amount of goat-anti rabbit Alexa Fluor 568-conjugated secondary antibody (1:100). Subsequently, cells were permeabilized with 0.1% Triton X-100 for 30 min at room temperature, and the internalized receptors were stained with the goat anti-rabbit Alexa Fluor 647-conjugated secondary antibody (1:750). Again, in order to make sure that the Alexa Fluor 647-conjugated secondary antibody did not label any detectable surface receptors in our assays, we determined the saturating concentration of the first secondary antibody through a control experiment similar to that described in our earlier studies (21, 22, 25).
Colocalization assay
In order to investigate whether different mutants of tamalin are targeted at the synapse, the extent of colocalization of the mutants with the presynaptic protein Bassoon were measured. Bassoon is a core component of the active zone that is commonly used to identify presynaptic terminals (29). Briefly, primary hippocampal neurons were transfected with different HA-tagged tamalin mutants using calcium phosphate on day 6 or 7 in vitro. At 12 to 14 days in vitro, cells were fixed with ice-cold 4% PFA on ice for 15 min. Subsequently, cells were permeabilized by 0.1% Triton X-100 for 30 min at room temperature. Cells were then stained with rat anti-HA primary antibody (1:500) and rabbit anti-Bassoon antibody (1:500) by incubating overnight at 4°C. Afterward, cells were incubated with goat anti-rat Alexa Fluor 568- and goat anti-rabbit immunoglobulin Alexa Fluor 647-conjugated secondary antibodies for 1.5 h at 37°C to visualize the tamalin constructs and Bassoon, respectively. Coverslips were then mounted on glass slides and imaged under a confocal microscope.
Immunoprecipitation and Western blotting analysis
For the detection of the shRNA-mediated knockdown of endogenous tamalin and expression of replacement constructs, primary neurons were transfected with the respective constructs. At 3 to 4 days posttransfection, neurons were lysed in radioimmunoprecipitation assay lysis buffer containing a protease inhibitor mixture. The samples were then boiled in 5× Laemmli sample buffer for 10 min and subjected to SDS-PAGE by loading an equal amount of protein in each lane. Subsequently, they were transferred to a PVDF membrane and blocked with 5% skim milk in 0.05% PBS-Tween for 2 h at room temperature. The membrane was then incubated with either anti-tamalin rabbit polyclonal antibody (1:500) and anti-β-actin (1:1000) antibody at 4°C overnight. Membranes were washed and incubated in horseradish peroxidase-conjugated secondary antibodies for 45 min at room temperature. Blots were developed using an ECL Western detection kit, and images were acquired in ImageQuant LAS 4000. Knockdown of the endogenous S-SCAM was studied by transfecting primary neurons with either siRNA against S-SCAM (ON-TARGETplus SMARTpool) or scrambled siRNA (si-control) (Dharmacon, Thermo Scientific, Lafayette, CO, USA). At 72 h posttransfection, neurons were lysed in radioimmunoprecipitation assay lysis buffer containing a protease inhibitor mixture. Western blotting was carried out in a manner similar to that described above by using anti-S-SCAM rabbit polyclonal antibody (1:1000) and anti-tubulin mouse mAb (1:1000).
In order to check for the effect of the deletion of the last 8 amino acids at the C-terminal region of tamalin on the interaction of tamalin with S-SCAM, HEK293 cells were plated on a 60-mm culture dish. Cotransfection of HA-tagged full-length tamalin and TamΔ8 with S-SCAM was performed the next day. At 24 h posttransfection, cells were washed with ice-cold PBS and lysed using TAP lysis buffer (20 mm Tris (pH 8.0), 150 mm NaCl, 0.5% NP-40, 1 mm MgCl2, 1 mm Na3VO4, 1× protease inhibitor mixture). The lysate was centrifuged at 16,000 rpm for 15 min. Then 80 µl of supernatant was collected as input. Immunoprecipitation was done using HA antibody-bound protein G beads. The immunoprecipitates pulled by HA beads were eluted and boiled in 2× Laemmli sample buffer at 99°C for 10 min. The samples were then subjected to SDS-PAGE followed by Western blotting as described above. The immunoblotting of tamalin mutants and S-SCAM protein was carried out using anti-HA antibody (1:1000) and anti-S-SCAM antibody (1:500), respectively.
Image acquisition and analysis
Cells were imaged in Zeiss LSM 780 confocal laser scanning microscope using a 63× oil immersion objective (numerical aperture, 1.4). Each experiment was repeated at least three times. Images from all the conditions in a particular experiment were acquired using identical parameters. All the analysis procedures are described in our previous studies (19–22, 34). Briefly, raw images were used for all analyses, and quantitation was done using ImageJ software (NIH, USA) (35). Raw images were maximally projected and thresholded using identical values for a particular experiment. The thresholded areas occupied by the fluorescence of the labeled surface and internalized receptors were subsequently measured. The internalization index was then calculated by dividing the value contributed by the internal fluorescence by the value contributed by the total fluorescence (surface plus internal). These values were then normalized with respect to their controls. To measure the surface receptors in all our assays, surface fluorescence was divided by the cell area, which was determined by measuring background fluorescence using a low threshold level. These values were then normalized with respect to the control cells. All the data represent dendritic values for primary hippocampal neurons, which was defined by the area 10 μm away from the soma. Synaptic localization of various tamalin constructs was defined by visual colocalization with presynaptic Bassoon puncta along 30-μm portions of dendrites. The quantitative data from all the experiments are presented as a combination of results from all three repeats of that particular experiment. The raw images were processed in Adobe Photoshop software using identical values of brightness and contrast to obtain the representative images. All the Western blots were also quantified using ImageJ software.
Statistical analysis
As stated above, each experiment was repeated three times. Data are presented as means ± S.E. Experimental group results were compared with each other using Student's t test or one-way analysis of variance followed by Tukey's posttest. A p value of >0.05 was considered nonsignificant.
Data availability
All data are contained within the manuscript.
Supplementary Material
Acknowledgments
We thank Dr. Kathrine Roche (NIH, USA) for the generous gift of Myc-mGluR1 and Myc-mGluR5 constructs. We are also thankful to Dr. Shigetada Nakanishi (Osaka Bioscience Institute, Japan) for the mouse tamalin construct. We are grateful to Dr. Heidi Welch (Babraham Institute, UK) for the human Norbin construct. We acknowledge members of our laboratory for valuable input. We appreciate the help of Qingjun Tian for support in hippocampal neuronal cultures.
This article contains supporting information.
Author contributions—S. P., N. R., and S. B. conceptualization; S. P., N. R., R. S., R. G., and P. O. data curation; S. P., N. R., R. S., R. G., P. O., and S. B. formal analysis; S. P., N. R., and S. B. funding acquisition; S. P., N. R., R. S., R. G., P. O., and S. B. validation; S. P., N. R., R. S., R. G., and P. O. investigation; S. P., N. R., R. S., R. G., and P. O. visualization; S. P., N. R., R. S., R. G., P. O., and S. B. methodology; S. P., N. R., R. S., R. G., W. L., and S. B. writing—original draft; S. P., N. R., and S. B. project administration; S. P., N. R., R. S., R. G., P. O., W. L., and S. B. writing—review and editing; S. P., N. R., P. O., W. L., and S. B. resources; S. B. supervision.
Funding and additional information—This work was supported by Indian Institute of Science Education and Research Mohali and a grant from Department of Science and Technology, India (Grant EMR/2016/005249). S.P. acknowledges receipt of a CSIR fellowship. N.R, R.S., and R.G. acknowledge receipt of UGC fellowships. P.O. acknowledges receipt of a fellowship from the Indian Institute of Science Education and Research Mohali. W.L. is supported by National Institutes of Health, NINDS intramural research program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- mGluRs
- metabotropic glutamate receptors
- AMPAR
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- APV
- d-(−)-2-amino-5-phosphonopentanoic acid
- DNQX
- 6,7-dinitroquinoxaline-2,3-dione
- PFA
- paraformaldehyde
- R,S-DHPG
- (R,S)-3,5-dihydroxyphenylglycine
- S.E.
- standard error
- TTX
- tetrodotoxin.
References
- 1. Pin J. P., and Duvoisin R. (1995) The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34, 1–26 10.1016/0028-3908(94)00129-G [DOI] [PubMed] [Google Scholar]
- 2. Dhami G. K., and Ferguson S. S. (2006) Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol. Ther. 111, 260–271 10.1016/j.pharmthera.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharyya S. (2016) Inside story of group I metabotropic glutamate receptors (mGluRs). Int. J. Biochem. Cell Biol. 77, 205–212 10.1016/j.biocel.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 4. Mahato P. K., Ramsakha N., Ojha P., Gulia R., Sharma R., and Bhattacharyya S. (2018) Group I metabotropic glutamate receptors (mGluRs): ins and outs. Adv. Exp. Med. Biol. 1112, 163–175 10.1007/978-981-13-3065-0_12 [DOI] [PubMed] [Google Scholar]
- 5. Conn P. J., and Pin J. P. (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 37, 205–237 10.1146/annurev.pharmtox.37.1.205 [DOI] [PubMed] [Google Scholar]
- 6. Kim C. H., Lee J., Lee J. Y., and Roche K. W. (2008) Metabotropic glutamate receptors: phosphorylation and receptor signaling. J. Neurosci. Res. 86, 1–10 10.1002/jnr.21437 [DOI] [PubMed] [Google Scholar]
- 7. Huber K. M., Gallagher S. M., Warren S. T., and Bear M. F. (2002) Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. U.S.A. 99, 7746–7750 10.1073/pnas.122205699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bear M. F., Huber K. M., and Warren S. T. (2004) The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 10.1016/j.tins.2004.04.009 [DOI] [PubMed] [Google Scholar]
- 9. Anwyl R. (2006) Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog. Neurobiol. 78, 17–37 10.1016/j.pneurobio.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 10. Dolen G., Osterweil E., Rao B. S., Smith G. B., Auerbach B. D., Chattarji S., and Bear M. F. (2007) Correction of fragile X syndrome in mice. Neuron 56, 955–962 10.1016/j.neuron.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Citri A., and Malenka R. C. (2008) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33, 18–41 10.1038/sj.npp.1301559 [DOI] [PubMed] [Google Scholar]
- 12. Gladding C. M., Fitzjohn S. M., and Molnar E. (2009) Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol. Rev. 61, 395–412 10.1124/pr.109.001735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michalon A., Sidorov M., Ballard T. M., Ozmen L., Spooren W., Wettstein J. G., Jaeschke G., Bear M. F., and Lindemann L. (2012) Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 74, 49–56 10.1016/j.neuron.2012.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Francesconi A., and Duvoisin R. M. (2000) Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain. Proc. Natl. Acad. Sci. U.S.A. 97, 6185–6190 10.1073/pnas.97.11.6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sallese M., Salvatore L., D'Urbano E., Sala G., Storto M., Launey T., De Blasi A., Nicoletti F., and Knopfel T. (2000) The G-protein-coupled receptor kinase GRK4 mediates homologous desensitization of metabotropic glutamate receptor 1. FASEB J. 14, 2569–2580 10.1096/fj.00-0072com [DOI] [PubMed] [Google Scholar]
- 16. Dale L. B., Babwah A. V., and Ferguson S. S. (2002) Mechanisms of metabotropic glutamate receptor desensitization: role in the patterning of effector enzyme activation. Neurochem. Int. 41, 319–326 10.1016/S0197-0186(02)00073-6 [DOI] [PubMed] [Google Scholar]
- 17. Mundell S. J., Pula G., McIlhinney R. A., Roberts P. J., and Kelly E. (2004) Desensitization and internalization of metabotropic glutamate receptor 1a following activation of heterologous Gq/11-coupled receptors. Biochemistry 43, 7541–7551 10.1021/bi0359022 [DOI] [PubMed] [Google Scholar]
- 18. Choi K. Y., Chung S., and Roche K. W. (2011) Differential binding of calmodulin to group I metabotropic glutamate receptors regulates receptor trafficking and signaling. J. Neurosci. 31, 5921–5930 10.1523/JNEUROSCI.6253-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahato P. K., Pandey S., and Bhattacharyya S. (2015) Differential effects of protein phosphatases in the recycling of metabotropic glutamate receptor 5. Neuroscience 306, 138–150 10.1016/j.neuroscience.2015.08.031 [DOI] [PubMed] [Google Scholar]
- 20. Pandey S., Mahato P. K., and Bhattacharyya S. (2014) Metabotropic glutamate receptor 1 recycles to the cell surface in protein phosphatase 2A-dependent manner in non-neuronal and neuronal cell lines. J. Neurochem. 131, 602–614 10.1111/jnc.12930 [DOI] [PubMed] [Google Scholar]
- 21. Gulia R., Sharma R., and Bhattacharyya S. (2017) A critical role for ubiquitination in the endocytosis of glutamate receptors. J. Biol. Chem. 292, 1426–1437 10.1074/jbc.M116.752105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharma R., Gulia R., and Bhattacharyya S. (2018) A critical role for sorting nexin 1 in the trafficking of metabotropic glutamate receptors. J. Neurosci. 38, 8605–8620 10.1523/JNEUROSCI.0454-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitano J., Kimura K., Yamazaki Y., Soda T., Shigemoto R., Nakajima Y., and Nakanishi S. (2002) Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. J. Neurosci. 22, 1280–1289 10.1523/JNEUROSCI.22-04-01280.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kitano J., Yamazaki Y., Kimura K., Masukado T., Nakajima Y., and Nakanishi S. (2003) Tamalin is a scaffold protein that interacts with multiple neuronal proteins in distinct modes of protein-protein association. J. Biol. Chem. 278, 14762–14768 10.1074/jbc.M300184200 [DOI] [PubMed] [Google Scholar]
- 25. Bhattacharyya S., Biou V., Xu W., Schluter O., and Malenka R. C. (2009) A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat. Neurosci. 12, 172–181 10.1038/nn.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Citri A., Bhattacharyya S., Ma C., Morishita W., Fang S., Rizo J., and Malenka R. C. (2010) Calcium binding to PICK1 is essential for the intracellular retention of AMPA receptors underlying long-term depression. J. Neurosci. 30, 16437–16452 10.1523/JNEUROSCI.4478-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang H., Westin L., Nong Y., Birnbaum S., Bendor J., Brismar H., Nestler E., Aperia A., Flajolet M., and Greengard P. (2009) Norbin is an endogenous regulator of metabotropic glutamate receptor 5 signaling. Science 326, 1554–1557 10.1126/science.1178496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sugi T., Oyama T., Muto T., Nakanishi S., Morikawa K., and Jingami H. (2007) Crystal structures of autoinhibitory PDZ domain of tamalin: implications for metabotropic glutamate receptor trafficking regulation. EMBO J. 26, 2192–2205 10.1038/sj.emboj.7601651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dieck S., Sanmartí-Vila L., Langnaese K., Richter K., Kindler S., Soyke A., Wex H., Smalla K.-H., Kämpf U., Fränzer J.-T., Stumm M., Garner C. C., and Gundelfinger E. D. (1998) Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. J. Cell Biol. 142, 499–509 10.1083/jcb.142.2.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Biou V., Bhattacharyya S., and Malenka R. C. (2008) Endocytosis and recycling of AMPA receptors lacking GluR2/3. Proc. Natl. Acad. Sci. U.S.A. 105, 1038–1043 10.1073/pnas.0711412105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Citri A., Soler-Llavina G., Bhattacharyya S., and Malenka R. C. (2009) N-methyl-D-aspartate receptor- and metabotropic glutamate receptor-dependent long-term depression are differentially regulated by the ubiquitin-proteasome system. Eur. J. Neurosci. 30, 1443–1450 10.1111/j.1460-9568.2009.06950.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niswender C. M., and Conn P. J. (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322 10.1146/annurev.pharmtox.011008.145533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharma R., Gulia R., and Bhattacharyya S. (2019) Analysis of ubiquitination and ligand-dependent trafficking of group I mGluRs. Methods Cell Biol. 149, 107–130[ 10.1016/bs.mcb.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 34. Trivedi R. R., and Bhattacharyya S. (2012) Constitutive internalization and recycling of metabotropic glutamate receptor 5 (mGluR5). Biochem. Biophys. Res. Commun. 427, 185–190 10.1016/j.bbrc.2012.09.040 [DOI] [PubMed] [Google Scholar]
- 35. Schneider C. A., Rasband W. S., and Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript.