Abstract
Osteoarthritis (OA) is a common clinical degenerative disease characterized by the destruction of articular cartilage, which has an increasing impact on people's lives and social economy. The pathogenesis of OA is complex and unclear, and there is no effective way to block its progress. The study of the pathogenesis of OA is the prerequisite for the early diagnosis and effective treatment of OA. To define the pathogenesis of OA, this review considers the pathological mechanism of OA that involves microRNA, lncRNA, and exosomes. More and more evidence shows that microRNA, lncRNA, and exosomes are closely related to OA. MicroRNA inhibits the target gene by binding to the 3′‐ untranslated region of the targets. LncRNA usually competes with microRNA to regulate the expression level of downstream genes, while exosomes, as a carrier of intercellular information transfer, transmit the biological information of mother cells to target cells, and the effect of exosomes secreted by different cells on OA are different. In this review, we emphasized that different microRNA, lncRNA, and exosomes have different regulatory effects on chondrocyte proliferation and apoptosis, extracellular matrix degradation and inflammation. Besides, we classified and analyzed these molecules according to their effects on the progress of OA. Based on the analysis of the reported literature, this review reveals some pathogenesis of OA, and emphasizes that microRNA, lncRNA, and exosomes have great potential to assist early diagnosis and effective treatment of OA.
Keywords: Exosomes, LncRNA, MicroRNA, Osteoarthritis
Introduction
Osteoarthritis (OA) is a common degenerative disease related to age, obesity, gender, weight, and trauma1. It is characterized by synovial hyperplasia, osteophyte formation, subchondral osteosclerosis, progressive articular cartilage destruction, and cartilage loss caused by the imbalance of extracellular matrix synthesis and catabolism2. According to statistics, OA has an impact on the lives of 250 mn people around the world, bringing an annual economic burden of more than US$89.1bn3. At present, the treatment strategy for early and middle stage OA is to relieve joint pain and intra‐articular injection, and the treatment strategy for late stage OA is joint replacement surgery. However, although these treatments can alleviate the symptoms of OA and improve the quality of life of patients to a certain extent, they have little effect on blocking the progressive development of OA. The specific pathogenesis of OA is still not clear. The existing evidence shows that the pathogenesis of OA is related to inflammatory factors, abnormal apoptosis of chondrocytes, and degradation of extracellular matrix. During the development of OA, TNF‐a, IL‐1, IL‐6, and other inflammatory factors were abnormally expressed, which led to the increase of chondrocyte apoptosis and the degradation of extracellular matrix1, 4. At present, there is still a lack of effective means for the early diagnosis and treatment of OA.
MicroRNA (miRNA) are a kind of multifunctional non‐coding RNA molecule with 22–25 bases encoded by endogenous genes. MiRNA regulate the stability and translation of mRNA, inhibit splicing and translation, inhibit target gene expression, and regulate downstream pathway by fully complementary binding with the 3′‐ untranslated region (3′‐ UTR) of target mRNA5, 6, 7. Long non‐coding RNA (LncRNA) is a kind of non‐coding RNA with a length of more than 200 bases. It does not encode proteins. It is a regulatory molecule. According to their positions relative to the protein coding genes, it can be divided into five categories: (i) antisense lncRNA; (ii) enhancer lncRNA; (iii) large International non‐coding RNA; (iv) bidirectional lncRNA; and (v) intronic script lncRNA (intron lncRNA)8, 9. LncRNA binds and isolates microRNA away from the sites that act on mRNA, thereby reducing the effect of microRNA on mRNA expression10. Figure 1 illustrates the interaction mechanism of LncRNA, microRNA, and mRNA. Exosomes (Exo) is a kind of vesicle with double plasmalemma structure, which is secreted by cells. It can express CD63, CD9, and other marker proteins on its surface. The membrane contains protein molecules, mRNA, miRNA, lncRNA, and other signal substances, carries the specific cytokines of mother cells, and targets the proximal cells through autocrine and paracrine, or the distal cells through the circulatory system tissue, information exchange between cells11, 12.
Figure 1.
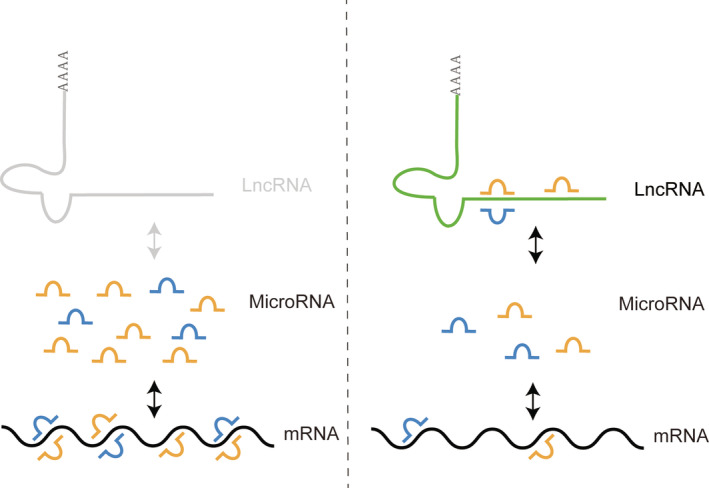
Interaction mechanism of LncRNA, microRNA and mRNA: LncRNA binds and isolates microRNA away from the sites that act on mRNA, thereby reducing the effect of microRNA on mRNA expression.
In recent years, with the development of molecular biology technology, the important role of miRNA, lncRNA, and Exo in disease progression has been gradually discovered by researchers. MicroRNA, lncRNA, and Exo are also expected to be an important way to explain the pathogenesis, early diagnosis, and treatment of OA. The purpose of this review is to summarize the key role of microRNA, lncRNA, and Exo in the development of OA. All the information is extracted from the high‐quality literature retrieved in the PubMed database.
Methods
With the help of the library platform of Hunan University of Traditional Chinese Medicine, PubMed database was searched. The literature from 2010 to 2019 were searched with the keywords “osteoarthritis,” “microRNA,” “lncRNA,” “exosomes,” and the language was English. Figure 2 illustrates the flow chart of searched results.
Figure 2.
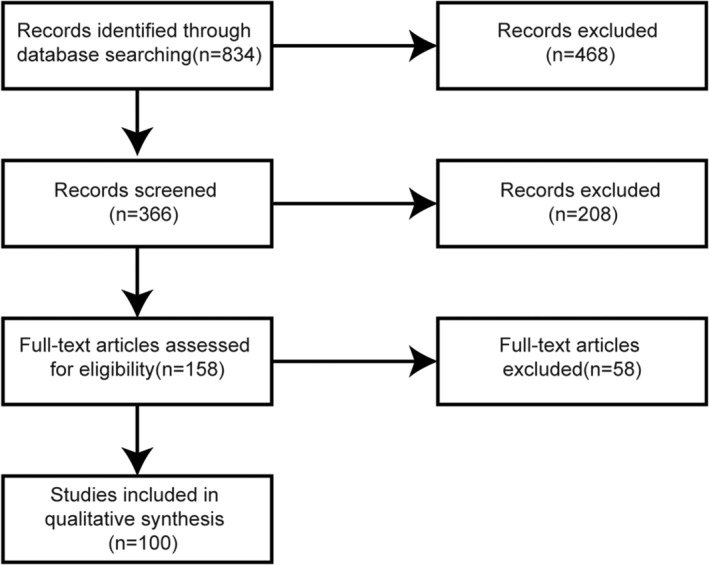
The flow chart of literature search and screening.
Eight hundred and thirty‐four related citations were obtained by literature search. Three hundred and sixty‐six related citations were obtained by taking experimental studies related to the pathological process of OA as the selection criteria and deleting the duplicate part. The two authors independently screened the titles and abstracts of each article, excluding the comprehensive research, clinical research, meeting report, and case report. The screening process was completed by the Rayyan QCRI software, and the dispute part was solved by the third author independently. A total of 158 documents have been full‐text reviewed. Through the full‐text review, there are 100 documents in line with our selection criteria, including 35 documents related to microRNA and OA, 54 documents related to lncRNA and OA, and 11 documents related to exosomes and OA. A descriptive analysis was performed on included studies.
Results
MicroRNA
In the pathogenesis of OA, miRNA has the biological functions of regulating chondrocyte apoptosis and proliferation, extracellular matrix metabolism, inflammatory response, and so on13, 14, 15, 16. The keywords of miRNA and osteoarthritis were searched in the PubMed database, 39 of which were research literature on molecular mechanism. It was found that there were 16 kinds of miRNA inhibiting OA process and 14 kinds of miRNA promoting OA process (Table 1).
Table 1.
classification of miRNA in OA process
| Inhibiting OA process (16 kinds) | Promoting OA process(14 kinds) |
|---|---|
| miR‐13217, 18, miR‐10719, miR‐149‐5p20, miR‐93‐5p21, miR‐335‐5p22, miR‐478423, miR‐106a‐5p24, miR‐14525, miR‐14026, 27, miR‐22128, miR‐38129, miR‐10530, miR‐21031, miR‐29a27, miR‐48832, miR‐125b33, miR‐10134 | miR‐146b35, 36, miR‐34a37, 38, miR‐181a37, 39, 40, miR‐582‐5p41, miR‐324‐5p42, miR‐21‐5p43, miR‐483‐5p44, 45, miR‐384‐5p46, miR‐15539, miR‐9847, miR‐127‐5p48, miR‐16‐5p49, miR‐10150, miR‐146a51 |
Apoptosis is a form of programmed cell death, which involves a series of gene activation, expression, and regulation52. Out of control apoptosis can cause cancer, autoimmune diseases, and degenerative diseases. Over apoptosis of chondrocytes is the main pathological manifestation of OA53. MiRNA promote or inhibit chondrocyte apoptosis by regulating the expression of key molecules involved in apoptosis signaling pathways. MiR‐34a and miR‐108a have a synergistic relationship in chondrocytes, which can jointly promote the activity of p50 NF‐ κB, reduce the expression of Bcl‐2, and promote chondrocyte apoptosis37; in addition, miR‐98 can also inhibit the transcription of Bcl‐2 and the apoptosis of chondrocytes47. According to the research of Zhao et al.40, miR‐181a can inhibit the expression of Glycerol‐3‐Phosphate Dehydrogenase 1‐Like Protein (GPD1L) and accelerate the apoptosis of chondrocytes by binding with the 3′‐ UTR end of GPD1L. In addition, miR‐181a may have a cooperative relationship with miR‐15539. There are other targets in miR‐34a, and Yang et al.38 have proved that miR‐34a targets cysteine‐rich angiogenic inductor 61 (CYR61), and inhibiting the expression of miR‐34a can promote the proliferation of chondrocytes. MiR‐146b can inhibit the expression of alpha‐2‐macroglobulin (A2M), improve the activity of proteolytic enzyme, promote cell apoptosis, and accelerate the development of OA35. MiR‐384‐5p inhibit cell proliferation and induce apoptosis by targeting Sox946. MiR‐127‐5p targeting combined with OPN inhibit chondrocyte proliferation48. Li et al.31 showed that miR‐210 inhibit the expression of HIF‐3 α and promoted the proliferation of chondrocytes. MiR‐146a target Smad4 to promote chondrocyte apoptosis51. MiR‐29a can upregulate the expression of type II collagen, reduce the expression of MMP13, resist the apoptosis of chondrocytes, and delay the development of OA27.
The extracellular matrix (ECM) of articular cartilage is mainly composed of proteoglycan and type II collagen, including a small number of chondrocytes. ECM contains a large number of signal molecules, which actively participate in the control of cell growth, polarity, shape, migration, and metabolism. The disruption of ECM catabolic balance will lead to the occurrence of OA54. Wang et al.45 found that miR‐483‐5p directly target Matn3 and TIMP2 to promote the degradation of ECM, chondrocyte hypertrophy, and cartilage angiogenesis, and accelerate the process of OA. Zheng et al.28 confirmed that miR‐221 was down regulate in OA, and miR‐221 could inhibit the degradation of cartilage extracellular matrix through SDF1 / CXCR4 signaling pathway. MiR‐145 inhibit the phosphorylation of MMK4 and alleviate the degradation of cartilage extracellular matrix caused by TNF ‐ α stimulation25. Liu et al.23 found that the expression of miR‐4784 is low in the early stage of OA, overexpression of miR‐4784 can increase the expression of type II collagen in cartilage extracellular matrix, decrease the expression of MMP3, and inhibit the degradation of extracellular matrix. Li et al.49 found that the expression of miR‐16‐5p in OA tissue is higher than that in normal tissue and further experiments confirm that miR‐16‐5p can target Smad3 to promote the degradation of cartilage extracellular matrix and promote the development of OA. In the experiments of Dai et al.50, they found that silencing miR‐101 can increase the expression of Sox9 and type II collagen and proteoglycan, thus inhibiting the degradation of cartilage extracellular matrix. However, Gao et al.34 found that the expression of miR‐101 increases while that of Sox9 and Runx2 decreases.
Long Non‐Coding RNA
The pathogenesis of OA is still unclear, but a large amount of evidence shows that the interaction between lncRNA and miRNA plays an important role in the development of OA. LncRNA can competitively bind miRNA, act as competitive endogenous RNA (CeRNA), reduce the combination of miRNA and downstream genes, and increase the transcription and expression of downstream genes55, 56. LncRNA has become an early diagnosis and effective treatment target of OA. In the PubMed database, 45 relevant pieces of literature were searched with the keywords of lncRNA and osteoarthritis, and 34 kinds of lncRNA were involved in these studies, including 17 kinds of lncRNA molecules inhibiting the development of OA and 15 kinds of lncRNA molecules promoting the development of OA (Table 2).
Table 2.
classification of lncRNA in OA process
| LncRNA | Targets | Cell process | |
|---|---|---|---|
| Inhibiting OA process |
ANCR59 DILC60 MIR4435‐2HG61 SNHG162 SNHG563 HULC64 PACER65
LINC0034168 ATB69 PMS2L270 MALAT171 ROR72 ZFAS173 GACAT374 UFC175 |
MiR‐27a/TLR457 MiR‐206/CCND158
MiR‐16‐5P MiR‐26a/Sox2 MiR‐101
MiR‐93/TGFBR266 MiR‐16/SAMD767 MiR‐141/YAF2 MiR‐223 MiR‐203 MiR‐150‐5p/AKT3
MiR‐34a |
Chondrocyte proliferation ( + ) Chondrocyte proliferation ( + ) Chondrocyte proliferation ( + ) Inflammation(‐) Chondrocyte proliferation ( + ) Inflammation (‐) Chondrocyte proliferation ( + ) Inflammation (‐) Chondrocyte apoptosis (‐) Degradation of extracellular matrix (‐) Chondrocyte proliferation ( + ) Chondrocyte apoptosis (‐) Inflammation (‐) Inflammation (‐) Chondrocyte proliferation ( + ) Chondrocyte apoptosis (‐) Chondrocyte proliferation ( + ) Chondrocyte proliferation ( + ) Chondrocyte apoptosis (‐) |
|
Promoting OA process |
MIAI17
TM1P378 CTD‐2574D22.479 TNFSF1080 LOC10192813481 CASA282 CHRF83 Nespas84 H1985 THRIL86 TUG87 P2188
XIST94 MBNL1‐AS195 HOTAIR96
FAS‐AS197 MSR98 PCGEM199 |
MiR‐132 MiR‐216a/JAK276 MiR‐577/Sphk277 MiR‐22/MMP13
MiR‐376/FGFR1
TL‐17 MiR‐146a/JAK1/STAT3
MiR‐130a MiR‐125b MiR‐195/MMP‐13 MiR‐130b/PTEN/AKT MiR‐130a/Bim89
MiR‐27b91 MiR‐14992 MiR‐48893 MiR‐211/CXR4/MAPK
MiR‐12496 MiR‐17‐5p/FUT2/β‐catenin96
MiR‐152 MiR‐770 |
Chondrocyte proliferation (‐) OA Chondrocyte proliferation and inflammation ( + ) OA Chondrocyte proliferation ( + ) Degradation of extracellular matrix ( + ) Chondrocyte apoptosis and inflammation ( + ) OA Chondrocyte proliferation and inflammation ( + ) Chondrocyte apoptosis ( + ) Chondrocyte apoptosis ( + ) Inflammation ( + ) Chondrocyte apoptosis ( + ) Chondrocyte apoptosis ( + ) Inflammation ( + ) Degradation of extracellular matrix ( + ) Chondrocyte apoptosis ( + ) Chondrocyte apoptosis ( + ) Chondrocyte autophagy ( + )90 Degradation of extracellular matrix ( + ) Degradation of extracellular matrix ( + ) Chondrocyte apoptosis ( + ) Chondrocyte apoptosis ( + ) Chondrocyte apoptosis ( + ) Inflammation ( + ) Degradation of extracellular matrix ( + ) Degradation of extracellular matrix ( + ) Degradation of extracellular matrix ( + ) Chondrocyte apoptosis ( + ) |
The combination of lncRNA and miRNA interferes with the inhibition of miRNAs on the expression of downstream target genes. That is to say, the expression of lncRNA is negatively correlated with the corresponding miRNAs and positively correlated with the expression of downstream target genes. Competitive binding of mRNA to miRNA is the main way for lncRNA to regulate biological functions. In the known research, it has been confirmed that some lncRNA have multi‐target and multi‐level regulatory effect. The study of Wang et al.57 confirmed that lncRNA FOXD2‐AS1 was low expression in OA patients, further experiments show that lncRNA FOXD2‐AS1 promote chondrocyte proliferation and inhibit the development of OA through miR‐27a / TLR4 axis. Cao et al.58 confirm that lncRNA FOXD2‐AS1 inhibit the expression of miR‐206 and upregulate the expression of CCND1, through miR‐206 / CCND1 axis, and it promote the survival and development of chondrocytes and hinder the process of OA, besides, lncRNA DANCR promote the proliferation of OA chondrocytes through miR‐216a / JAK2 axis 76 and miR‐577 / Sphk273 axis. LncRNA CIR can promote apoptosis through miR‐130a / Bim axis89, and inhibit the expression of miR‐27b91 to promote the degradation of extracellular matrix. LncRNA PVT1 target regulation of miR‐14992 and miR‐48893 expression promotes apoptosis, extracellular matrix degradation, and inflammatory response. LncRNA HOTAIR inhibit miR‐12496 expression or promote inflammatory response and apoptosis through miR‐17‐5p / FUT2 /β‐ catenin axis96.
Jiang et al.65 found that the expression of lncRNA PACER is low in OA and inhibited cell apoptosis. The negative correlation between the expression of PACER and lncRNA HOTAIR suggested that there is also a mechanism of mutual regulation between lncRNA. There are also some lncRNA that have been proved to be related to OA, but the specific regulatory mechanism is not clear. Li et al.59 found that lncRNA ANCR can promote chondrocyte proliferation in OA patients, and its expression was negatively correlated with TGF ‐β1. Huang et al.60 alleviate the inflammatory response in OA by inhibiting the expression of IL‐6. Yang et al.81 found that when the expression of lncRNA LOC101928134 is downregulated, the expression of IFN1 increase and activate the downstream JAK / STAT signal pathway, inhibiting the proliferation of synovium and cartilage destruction, but the specific regulatory mechanism of LOC101928134 is not clear. In addition, lncRNA ZFAS1 can inhibit the activation of Wnt3a pathway and promote the proliferation and migration of chondrocytes73. The expression of GACAT3 is negatively correlated with IL‐6, inhibits the activity of IL‐6 / STAT pathway, and promotes chondrocyte proliferation74. The study of Park et al.84 found that the overexpression of lncRNA Nespas can inhibit the expression of miR‐291a‐3p, miR‐196a‐5p, miR‐23a‐3p, miR‐24‐3p, and Let‐7a‐5p. After analysis, these miRNAs target ACSL6, but the transcriptional binding of these miRNAs with lncRNA Nespas and ACSL6 needs further experimental verification.
Interestingly, Zhang et al.76 found that the expression of lncRNA DANCR increases in OA patients, DANCR promotes the proliferation and inflammatory response of OA chondrocytes, inhibits apoptosis, and promotes the progression of OA. Fan et al.77 also found that the expression of DANCR increases in OA patients and promotes the proliferation of OA chondrocytes through miR‐577 / Sphk2 axis. The study of Huang et al.80 confirmed that lncRNA TNFSF10 promotes OA chondrocyte proliferation, inhibits apoptosis, and promotes inflammatory response through miR‐376 / FGFR1 axis. These experiments prove that OA chondrocytes are relate to inflammatory response, and promoting the proliferation of OA chondrocytes can accelerate the development of OA. In other words, the proliferation and inflammatory response of degenerated chondrocytes can accelerate the degeneration of cartilage100. OA chondrocytes have different biological functions from normal chondrocytes.
Exosomes
The biological characteristics of Exo, which is not secreted by cells, are different, and its effect on OA is also different. Domenis et al.101 extracted and identified synovial‐fluid‐derived exosomes of patients with osteoarthritis, and found that SF‐Exo can promote inflammatory response. The Exo secreted by IL‐1β stimulate synovial fibroblasts can increase the expression of MMP‐13 and ADAMTS5, decrease the expression of COL2A1 and ACAN, promote the degradation of cartilage extracellular matrix, and accelerate the development of OA102. Because Exo can carry the biological information of mother cells, more research regards Exo as a strategy of treatment. The study of Qi et al.103 confirms that Exo secrete by mesenchymal stem cells can promote Akt phosphorylation by inhibiting p38 and ERK phosphorylation, and inhibit chondrocyte apoptosis cause by mitochondrial dysfunction. Bone‐marrow‐mesenchymal‐stem‐cells Exo104, embryonic‐mesenchymal‐stem‐cells Exo105, and adipose‐mesenchymal‐stem‐cells Exo106 can promote the expression of type II collagen and proteoglycan, inhibit the expression of MMP‐13, ADAMTS5 and proinflammatory factors, and maintain the balance of cartilage extracellular matrix. Exosomes can also regulate the biological functions of target cells by carrying miRNA and lncRNA. Wu et al.107 found that miR‐100‐5p was abundant in the Exo of subpatellar fat pad mesenchymal stem cells, and the activity of mTOP autophagy pathway in chondrocytes is inhibited by miR‐100‐5p to adjust the gait of OA in a rat model. Sun et al.108 confirmed that Exo can promote chondrocyte proliferation and inhibit MMP‐13 expression through miR‐302c. The study of Mao et al.109 confirmed that the expression of miR‐92a in Exo of OA chondrocytes was lower than that of normal chondrocytes and further experiments show that human mesenchymal stem cells inhibit the expression of Wnt5a through miR‐92a and alleviate the degeneration of articular cartilage. The Exo secrete by miR‐140‐5p overexpress synovial stem cells can promote the proliferation and migration of chondrocytes110. Liu et al.111 confirmed that human mesenchymal stem cells could inhibit the apoptosis of chondrocytes induced by IL‐1β and promote cartilage repair through lncRNA KLF3‐AS1.
Conclusions
MicroRNA, lncRNA, and Exo have strong regulatory effects on the pathological process of OA, so they are expected to be the targets of early diagnosis and treatment of OA. Figure 3 illustrates the mechanism of the study. The interaction mechanism of these three factors may be as follows: Exo secreted by other cells contains different lncRNA, microRNA, or mRNA; when Exo contacts the cell membrane, these substances are transferred to the target cell; in the cell, microRNA can bind mRNA to inhibit its expression. However, lncRNA can competitively bind microRNA to alleviate the inhibition of microRNA on the expression of downstream genes. All in all, their regulatory role in cells is ultimately achieved by influencing mRNA expression downstream. From the current literature reports, the pathological research of OA involves microRNA, lncRNA, and Exo, which are basically in vitro experiments or animal model experiments. With the progress of molecular biotechnology in the later research, it is hoped that more clinical experimental reports will be published. In addition, due to the diversity of biological functions of microRNA and lncRNA and the characteristics of Exo information transmission media, research into the fusion of these three factors may effectively promote the research process of the pathological mechanism of OA.
Figure 3.
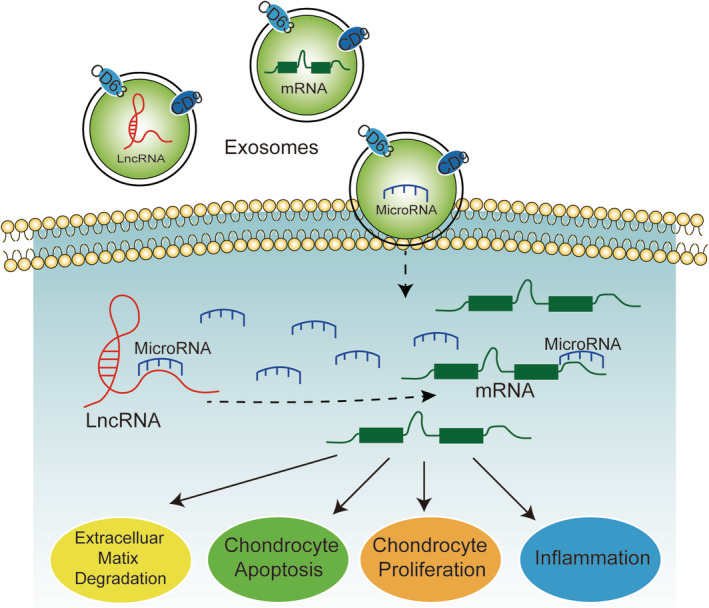
MicroRNA, IncRNA and Exo intervence the pathological process of OA through chondrocyte proliferation, chondrocyte apoptosis, extracellulsr matrix degradation and inflammation.
Authorship Declaration
All authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors, and all authors are in agreement with the manuscript.
Acknowledgments
The research was funded by the National Natural Science Foundation of China (Grant Number: 81874476), Hunan Administration of Traditional Chinese Medicine (Grant Number: 201902), and Changde Science and Technology Bureau (Grant Number: 2012SK06).
Disclosure: The authors have declared that no competing interest exists.
Contributor Information
Xian‐fang Shao, Email: 13973657615@139.com.
Guan‐bao Wu, Email: yhywgb@126.com.
References
- 1. Wang Y, Fan X, Xing L, Tian F. Wnt signaling: a promising target for osteoarthritis therapy. Cell Commun Signal, 2019, 17: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen D, Shen J, Zhao W, et al Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res, 2017, 5: 16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2016. Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet, 390: 1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodell‐May JE, Sommerfeld SD. Role of inflammation and the immune system in the progression of osteoarthritis. J Orthop Res, 2020, 38: 253–257. [DOI] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell, 2009, 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mo YY. MicroRNA regulatory networks and human disease. Cell Mol Life Sci, 2012, 69: 3529–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gebert L, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol, 2019, 20: 21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quinn JJ, Chang HY. Unique features of long non‐coding RNA biogenesis and function. Nat Rev Genet, 2016, 17: 47–62. [DOI] [PubMed] [Google Scholar]
- 9. Huynh NP, Anderson BA, Guilak F, McAlinden A. Emerging roles for long noncoding RNAs in skeletal biology and disease. Connect Tissue Res, 2017, 58: 116–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ballantyne MD, McDonald RA, Baker AH. lncRNA/MicroRNA interactions in the vasculature. Clin Pharmacol Ther, 2016, 99: 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng X, Zhang G, Zhang L, et al Mesenchymal stem cells deliver exogenous miR‐21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med, 2018, 22: 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol, 2014, 30: 255–289. [DOI] [PubMed] [Google Scholar]
- 13. Malemud CJ. MicroRNAs and osteoarthritis. Cell, 2018, 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al‐Modawi RN, Brinchmann JE, Karlsen TA. Multi‐pathway protective effects of MicroRNAs on human chondrocytes in an in vitro model of osteoarthritis. Mol Ther Nucleic Acids, 2019, 17: 776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le LT, Swingler TE, Clark IM. Review: the role of microRNAs in osteoarthritis and chondrogenesis. Arthritis Rheum, 2013, 65: 1963–1974. [DOI] [PubMed] [Google Scholar]
- 16. Swingler TE, Niu L, Smith P, et al The function of microRNAs in cartilage and osteoarthritis. Clin Exp Rheumatol, 2019, 37: 40–47. [PubMed] [Google Scholar]
- 17. Li C, Pan S, Song Y, Li Y, Qu J. Silence of lncRNA MIAT protects ATDC5 cells against lipopolysaccharides challenge via up‐regulating miR‐132. Artif Cells Nanomed Biotechnol, 2019, 47: 2521–2527. [DOI] [PubMed] [Google Scholar]
- 18. Zhou X, Luo D, Sun H, et al MiR‐132‐3p regulates ADAMTS‐5 expression and promotes chondrogenic differentiation of rat mesenchymal stem cells. J Cell Biochem, 2018, 119: 2579–2587. [DOI] [PubMed] [Google Scholar]
- 19. Lin SS, Yuan LJ, Niu CC, Tu YK, Yang CY, Ueng S. Hyperbaric oxygen inhibits the HMGB1/RAGE signaling pathway by upregulating Mir‐107 expression in human osteoarthritic chondrocytes. Osteoarthritis Cartilage, 2019, 27: 1372–1381. [DOI] [PubMed] [Google Scholar]
- 20. Çelik E, Bayram C, Denkbaş EB. Chondrogenesis of human mesenchymal stem cells by microRNA loaded triple polysaccharide nanoparticle system. Korean J Couns Psychother, 2019, 102: 756–763. [DOI] [PubMed] [Google Scholar]
- 21. Xue H, Tu Y, Ma T, et al miR‐93‐5p attenuates IL‐1β‐induced chondrocyte apoptosis and cartilage degradation in osteoarthritis partially by targeting TCF4. Bone, 2019, 123: 129–136. [DOI] [PubMed] [Google Scholar]
- 22. Zhong G, Long H, Ma S, Shunhan Y, Li J, Yao J. miRNA‐335‐5p relieves chondrocyte inflammation by activating autophagy in osteoarthritis. Life Sci, 2019, 226: 164–172. [DOI] [PubMed] [Google Scholar]
- 23. Liu J, Yu Q, Ye Y, Yan Y, Chen X. Abnormal expression of miR‐4784 in chondrocytes of osteoarthritis and associations with chondrocyte hyperplasia. Exp Ther Med, 2018, 16: 4690–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ji Q, Qi D, Xu X, et al Cryptotanshinone protects cartilage against developing osteoarthritis through the miR‐106a‐5p/GLIS3 Axis. Mol Ther Nucleic Acids, 2018, 11: 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu G, Zhao X, Wang C, et al MicroRNA‐145 attenuates TNF‐α‐driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis, 2017, 8: e3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Si HB, Zeng Y, Liu SY, et al Intra‐articular injection of microRNA‐140 (miRNA‐140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthritis Cartilage, 2017, 25: 1698–1707. [DOI] [PubMed] [Google Scholar]
- 27. Li X, Zhen Z, Tang G, Zheng C, Yang G. MiR‐29a and MiR‐140 protect chondrocytes against the anti‐proliferation and cell matrix signaling changes by IL‐1β. Mol Cells, 2016, 39: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng X, Zhao FC, Pang Y, et al Downregulation of miR‐221‐3p contributes to IL‐1β‐induced cartilage degradation by directly targeting the SDF1/CXCR4 signaling pathway. J Mol Med (Berl), 2017, 95: 615–627. [DOI] [PubMed] [Google Scholar]
- 29. Chen W, Sheng P, Huang Z, et al MicroRNA‐381 regulates chondrocyte hypertrophy by inhibiting histone Deacetylase 4 expression. Int J Mol Sci, 2016, 17: 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ji Q, Xu X, Xu Y, et al miR‐105/Runx2 axis mediates FGF2‐induced ADAMTS expression in osteoarthritis cartilage. J Mol Med (Berl), 2016, 94: 681–694. [DOI] [PubMed] [Google Scholar]
- 31. Li Z, Meng D, Li G, Xu J, Tian K, Li Y. Overexpression of microRNA‐210 promotes chondrocyte proliferation and extracellular matrix deposition by targeting HIF‐3α in osteoarthritis. Mol Med Rep, 2016, 13: 2769–2776. [DOI] [PubMed] [Google Scholar]
- 32. Song J, Kim D, Lee CH, Lee MS, Chun CH, Jin EJ. MicroRNA‐488 regulates zinc transporter SLC39A8/ZIP8 during pathogenesis of osteoarthritis. J Biomed Sci, 2013, 20: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsukawa T, Sakai T, Yonezawa T, et al MicroRNA‐125b regulates the expression of aggrecanase‐1 (ADAMTS‐4) in human osteoarthritic chondrocytes. Arthritis Res Ther, 2013, 15: R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao F, Peng C, Zheng C, Zhang S, Wu M. miRNA‐101 promotes chondrogenic differentiation in rat bone marrow mesenchymal stem cells. Exp Ther Med, 2019, 17: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu X, Liu L, Zhang H, et al MiR‐146b accelerates osteoarthritis progression by targeting alpha‐2‐macroglobulin. Aging, 2019, 11: 6014–6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Budd E, de Andrés MC, Sanchez‐Elsner T, Oreffo R. MiR‐146b is down‐regulated during the chondrogenic differentiation of human bone marrow derived skeletal stem cells and up‐regulated in osteoarthritis. Sci Rep, 2017, 7: 46704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheleschi S, Tenti S, Mondanelli N, et al MicroRNA‐34a and MicroRNA‐181a mediate Visfatin‐induced apoptosis and oxidative stress via NF‐κB pathway in human osteoarthritic chondrocytes. Cell, 2019, 8: 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang B, Ni J, Long H, Huang J, Yang C, Huang X. IL‐1β‐induced miR‐34a up‐regulation inhibits Cyr61 to modulate osteoarthritis chondrocyte proliferation through ADAMTS‐4. J Cell Biochem, 2018, 119: 7959–7970. [DOI] [PubMed] [Google Scholar]
- 39. De Palma A, Cheleschi S, Pascarelli NA, Giannotti S, Galeazzi M, Fioravanti A. Hydrostatic pressure as epigenetic modulator in chondrocyte cultures: a study on miRNA‐155, miRNA‐181a and miRNA‐223 expression levels. J Biomech, 2018, 66: 165–169. [DOI] [PubMed] [Google Scholar]
- 40. Zhai X, Meng R, Li H, et al miR‐181a modulates chondrocyte apoptosis by targeting Glycerol‐3‐phosphate dehydrogenase 1‐like protein (GPD1L) in osteoarthritis. Med Sci Monit, 2017, 23: 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang P, Dong R, Wang B, et al Genome‐wide microRNA screening reveals miR‐582‐5p as a mesenchymal stem cell‐specific microRNA in subchondral bone of the human knee joint. J Cell Physiol, 2019, 234: 21877–21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woods S, Barter MJ, Elliott HR, et al miR‐324‐5p is up regulated in end‐stage osteoarthritis and regulates Indian hedgehog signalling by differing mechanisms in human and mouse. Matrix Biol, 2019, 77: 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang XB, Zhao FC, Yi LH, et al MicroRNA‐21‐5p as a novel therapeutic target for osteoarthritis. Rheumatology (Oxford), 2019, 58: 1485–1497. [DOI] [PubMed] [Google Scholar]
- 44. Wang H, Zhang H, Sun Q, et al Chondrocyte mTORC1 activation stimulates miR‐483‐5p via HDAC4 in osteoarthritis progression. J Cell Physiol, 2019, 234: 2730–2740. [DOI] [PubMed] [Google Scholar]
- 45. Wang H, Zhang H, Sun Q, et al Intra‐articular delivery of Antago‐miR‐483‐5p inhibits osteoarthritis by modulating Matrilin 3 and tissue inhibitor of metalloproteinase 2. Mol Ther, 2017, 25: 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Zhang W, Cheng P, Hu W, et al Inhibition of microRNA‐384‐5p alleviates osteoarthritis through its effects on inhibiting apoptosis of cartilage cells via the NF‐κB signaling pathway by targeting SOX9. Cancer Gene Ther, 2018, 25: 326–338. [DOI] [PubMed] [Google Scholar]
- 47. Wang J, Chen L, Jin S, et al Altered expression of microRNA‐98 in IL‐1β‐induced cartilage degradation and its role in chondrocyte apoptosis. Mol Med Rep, 2017, 16: 3208–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tu M, Li Y, Zeng C, et al MicroRNA‐127‐5p regulates osteopontin expression and osteopontin‐mediated proliferation of human chondrocytes. Sci Rep, 2016, 6: 25032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li L, Jia J, Liu X, et al MicroRNA‐16‐5p controls development of osteoarthritis by targeting SMAD3 in chondrocytes. Curr Pharm des, 2015, 21: 5160–5167. [DOI] [PubMed] [Google Scholar]
- 50. Dai L, Zhang X, Hu X, Zhou C, Ao Y. Silencing of microRNA‐101 prevents IL‐1β‐induced extracellular matrix degradation in chondrocytes. Arthritis Res Ther, 2012, 14: R268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li J, Huang J, Dai L, et al miR‐146a, an IL‐1β responsive miRNA, induces vascular endothelial growth factor and chondrocyte apoptosis by targeting Smad4. Arthritis Res Ther, 2012, 14: R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li D, Ni S, Miao KS, Zhuang C. PI3K/Akt and caspase pathways mediate oxidative stress‐induced chondrocyte apoptosis. Cell Stress Chaperones, 2019, 24: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Musumeci G, Castrogiovanni P, Trovato FM, et al Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci, 2015, 16: 20560–20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guilak F, Meyer BC, Ratcliffe A, Mow VC. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage, 1994, 2: 91–101. [DOI] [PubMed] [Google Scholar]
- 55. Sun H, Peng G, Ning X, Wang J, Yang H, Deng J. Emerging roles of long noncoding RNA in chondrogenesis, osteogenesis, and osteoarthritis. Am J Transl Res, 2019, 11: 16–30. [PMC free article] [PubMed] [Google Scholar]
- 56. Chen WK, Yu XH, Yang W, et al lncRNAs: novel players in intervertebral disc degeneration and osteoarthritis. Cell Prolif, 2017, 1: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang Y, Cao L, Wang Q, Huang J, Xu S. LncRNA FOXD2‐AS1 induces chondrocyte proliferation through sponging miR‐27a‐3p in osteoarthritis. Artif Cells Nanomed Biotechnol, 2019, 47: 1241–1247. [DOI] [PubMed] [Google Scholar]
- 58. Cao L, Wang Y, Wang Q, Huang J. LncRNA FOXD2‐AS1 regulates chondrocyte proliferation in osteoarthritis by acting AS a sponge of miR‐206 to modulate CCND1 expression. Biomed Pharmacother, 2018, 106: 1220–1226. [DOI] [PubMed] [Google Scholar]
- 59. Li Q, Zhang Z, Guo S, Tang G, Lu W, Qi X. LncRNA ANCR is positively correlated with transforming growth factor‐β1 in patients with osteoarthritis. J Cell Biochem, 2019, 120: 14226–14232. [DOI] [PubMed] [Google Scholar]
- 60. Huang J, Liu L, Yang J, Ding J, Xu X. lncRNA DILC is downregulated in osteoarthritis and regulates IL‐6 expression in chondrocytes. J Cell Biochem, 2019, 120: 16019–16024. [DOI] [PubMed] [Google Scholar]
- 61. Xiao Y, Bao Y, Tang L, Wang L. LncRNA MIR4435‐2HG is downregulated in osteoarthritis and regulates chondrocyte cell proliferation and apoptosis. J Orthop Surg Res, 2019, 14: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lei J, Fu Y, Zhuang Y, Zhang K, Lu D. LncRNA SNHG1 alleviates IL‐1β‐induced osteoarthritis by inhibiting miR‐16‐5p‐mediated p38 MAPK and NF‐κB signaling pathways. Biosci Rep, 2019, 39: BSR20191523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shen H, Wang Y, Shi W, Sun G, Hong L, Zhang Y. LncRNA SNHG5/miR‐26a/SOX2 signal axis enhances proliferation of chondrocyte in osteoarthritis. Acta Biochim Biophys Sin, 2018, 50: 191–198. [DOI] [PubMed] [Google Scholar]
- 64. Chu P, Wang Q, Wang Z, Gao C. Long non‐coding RNA highly up‐regulated in liver cancer protects tumor necrosis factor‐alpha‐induced inflammatory injury by down‐regulation of microRNA‐101 in ATDC5 cells. Int Immunopharmacol, 2019, 72: 148–158. [DOI] [PubMed] [Google Scholar]
- 65. Jiang M, Liu J, Luo T, Chen Q, Lu M, Meng D. LncRNA PACER is down‐regulated in osteoarthritis and regulates chondrocyte apoptosis and lncRNA HOTAIR expression. Biosci Rep, 2019, 39: BSR20190404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen K, Zhu H, Zheng MQ, Dong QR. LncRNA MEG3 inhibits the degradation of the extracellular matrix of chondrocytes in osteoarthritis via targeting miR‐93/TGFBR2 Axis. Cartilage, 2019: 1947603519855759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu J, Xu Y. The lncRNA MEG3 downregulation leads to osteoarthritis progression via miR‐16/SMAD7 axis. Cell Biosci, 2017, 7: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang Q, Li X, Zhou Y, Fu W, Wang J, Wei Q. A LINC00341‐mediated regulatory pathway supports chondrocyte survival and may prevent osteoarthritis progression. J Cell Biochem, 2019, 120: 10812–10820. [DOI] [PubMed] [Google Scholar]
- 69. Ying H, Wang Y, Gao Z, Zhang Q. Long non‐coding RNA activated by transforming growth factor beta alleviates lipopolysaccharide‐induced inflammatory injury via regulating microRNA‐223 in ATDC5 cells. Int Immunopharmacol, 2019, 69: 313–320. [DOI] [PubMed] [Google Scholar]
- 70. Li X, Yu M, Chen L, et al LncRNA PMS2L2 protects ATDC5 chondrocytes against lipopolysaccharide‐induced inflammatory injury by sponging miR‐203. Life Sci, 2019, 217: 283–292. [DOI] [PubMed] [Google Scholar]
- 71. Zhang Y, Wang F, Chen G, He R, Yang L. LncRNA MALAT1 promotes osteoarthritis by modulating miR‐150‐5p/AKT3 axis. Cell Biosci, 2019, 9: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yang Z, Tang Y, Lu H, et al Long non‐coding RNA reprogramming (lncRNA‐ROR) regulates cell apoptosis and autophagy in chondrocytes. J Cell Biochem, 2018, 119: 8432–8440. [DOI] [PubMed] [Google Scholar]
- 73. Ye D, Jian W, Feng J, Liao X. Role of long noncoding RNA ZFAS1 in proliferation, apoptosis and migration of chondrocytes in osteoarthritis. Biomed Pharmacother, 2018, 104: 825–831. [DOI] [PubMed] [Google Scholar]
- 74. Li X, Ren W, Xiao ZY, Wu LF, Wang H, Guo PY. GACAT3 promoted proliferation of osteoarthritis synoviocytes by IL‐6/STAT3 signaling pathway. Eur Rev Med Pharmacol Sci, 2018, 22: 5114–5120. [DOI] [PubMed] [Google Scholar]
- 75. Zhang G, Wu Y, Xu D, Yan X. Long Noncoding RNA UFC1 promotes proliferation of chondrocyte in osteoarthritis by acting as a sponge for miR‐34a. DNA Cell Biol, 2016, 35: 691–695. [DOI] [PubMed] [Google Scholar]
- 76. Zhang L, Zhang P, Sun X, Zhou L, Zhao J. Long non‐coding RNA DANCR regulates proliferation and apoptosis of chondrocytes in osteoarthritis via miR‐216a‐5p‐JAK2‐STAT3 axis. Biosci Rep, 2018, 38: BSR20181228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fan X, Yuan J, Xie J, et al Long non‐protein coding RNA DANCR functions as a competing endogenous RNA to regulate osteoarthritis progression via miR‐577/SphK2 axis. Biochem Biophys Res Commun, 2018, 500: 658–664. [DOI] [PubMed] [Google Scholar]
- 78. Li Y, Li Z, Li C, Zeng Y, Liu Y. Long noncoding RNA TM1P3 is involved in osteoarthritis by mediating chondrocyte extracellular matrix degradation. J Cell Biochem, 2019, 120: 12702–12712. [DOI] [PubMed] [Google Scholar]
- 79. Li L, Zhang L, Zhang Y, et al Inhibition of Long non‐coding RNA CTD‐2574D22.4 alleviates LPS‐induced apoptosis and inflammatory injury of chondrocytes. Curr Pharm des, 2019, 25: 2969–2974. [DOI] [PubMed] [Google Scholar]
- 80. Huang B, Yu H, Li Y, Zhang W, Liu X. Upregulation of long noncoding TNFSF10 contributes to osteoarthritis progression through the miR‐376‐3p/FGFR1 axis. J Cell Biochem, 2019, 120: 19610–19620. [DOI] [PubMed] [Google Scholar]
- 81. Yang DW, Zhang X, Qian GB, Jiang MJ, Wang P, Wang KZ. Downregulation of long noncoding RNA LOC101928134 inhibits the synovial hyperplasia and cartilage destruction of osteoarthritis rats through the activation of the Janus kinase/signal transducers and activators of transcription signaling pathway by upregulating IFNA1. J Cell Physiol, 2019, 234: 10523–10534. [DOI] [PubMed] [Google Scholar]
- 82. Huang T, Wang J, Zhou Y, Zhao Y, Hang D, Cao Y. LncRNA CASC2 is up‐regulated in osteoarthritis and participates in the regulation of IL‐17 expression and chondrocyte proliferation and apoptosis. Biosci Rep, 2019, 39: BSR20182454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yu C, Shi D, Li Z, Wan G, Shi X. Long noncoding RNA CHRF exacerbates IL‐6‐induced inflammatory damages by downregulating microRNA‐146a in ATDC5 cells. J Cell Physiol, 2019, 234: 21851–21859. [DOI] [PubMed] [Google Scholar]
- 84. Park S, Lee M, Chun CH, Jin EJ. The lncRNA, Nespas, is associated with osteoarthritis progression and serves as a potential new prognostic biomarker. Cartilage, 2019, 10: 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hu Y, Li S, Zou Y. Knockdown of LncRNA H19 relieves LPS‐induced damage by modulating miR‐130a in osteoarthritis. Yonsei Med J, 2019, 60: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu G, Wang Y, Zhang M, Zhang Q. Long non‐coding RNA THRIL promotes LPS‐induced inflammatory injury by down‐regulating microRNA‐125b in ATDC5 cells. Int Immunopharmacol, 2019, 66: 354–361. [DOI] [PubMed] [Google Scholar]
- 87. Tang LP, Ding JB, Liu ZH, Zhou GJ. LncRNA TUG1 promotes osteoarthritis‐induced degradation of chondrocyte extracellular matrix via miR‐195/MMP‐13 axis. Eur Rev Med Pharmacol Sci, 2018, 22: 8574–8581. [DOI] [PubMed] [Google Scholar]
- 88. Han W, Liu J. LncRNA‐p21 inhibited the proliferation of osteosarcoma cells via the miR‐130b/PTEN/AKT signaling pathway. Biomed Pharmacother, 2018, 97: 911–918. [DOI] [PubMed] [Google Scholar]
- 89. Lu Z, Luo M, Huang Y. lncRNA‐CIR regulates cell apoptosis of chondrocytes in osteoarthritis. J Cell Biochem, 2018, 120: 5. [DOI] [PubMed] [Google Scholar]
- 90. Wang CL, Peng JP, Chen XD. LncRNA‐CIR promotes articular cartilage degeneration in osteoarthritis by regulating autophagy. Biochem Biophys Res Commun, 2018, 505: 692–698. [DOI] [PubMed] [Google Scholar]
- 91. Li YF, Li SH, Liu Y, Luo YT. Long Noncoding RNA CIR promotes chondrocyte extracellular matrix degradation in osteoarthritis by acting as a sponge for Mir‐27b. Cell Physiol Biochem, 2017, 43: 602–610. [DOI] [PubMed] [Google Scholar]
- 92. Zhao Y, Zhao J, Guo X, She J, Liu Y. Long non‐coding RNA PVT1, a molecular sponge for miR‐149, contributes aberrant metabolic dysfunction and inflammation in IL‐1β‐simulated osteoarthritic chondrocytes. Biosci Rep, 2018, 38: BSR20180576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li Y, Li S, Luo Y, Liu Y, Yu N. LncRNA PVT1 regulates chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR‐488‐3p. DNA Cell Biol, 2017, 36: 571–580. [DOI] [PubMed] [Google Scholar]
- 94. Li L, Lv G, Wang B, Kuang L. The role of lncRNA XIST/miR‐211 axis in modulating the proliferation and apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK signaling. Biochem Biophys Res Commun, 2018, 503: 2555–2562. [DOI] [PubMed] [Google Scholar]
- 95. Li XF, Wang ZQ, Li LY, Zhao GQ, Yu SN. Downregulation of the long noncoding RNA MBNL1‐AS1 protects sevoflurane‐pretreated mice against ischemia‐reperfusion injury by targeting KCNMA1. Exp Mol Med, 2018, 50: 115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96. Hu J, Wang Z, Shan Y, Pan Y, Ma J, Jia L. Long non‐coding RNA HOTAIR promotes osteoarthritis progression via miR‐17‐5p/FUT2/β‐catenin axis. Cell Death Dis, 2018, 9: 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhu JK, He TD, Wei ZX, Wang YM. LncRNA FAS‐AS1 promotes the degradation of extracellular matrix of cartilage in osteoarthritis. Eur Rev Med Pharmacol Sci, 2018, 22: 2966–2972. [DOI] [PubMed] [Google Scholar]
- 98. Liu Q, Hu X, Zhang X, et al The TMSB4 Pseudogene LncRNA functions as a competing endogenous RNA to promote cartilage degradation in human osteoarthritis. Mol Ther, 2016, 24: 1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kang Y, Song J, Kim D, et al PCGEM1 stimulates proliferation of osteoarthritic synoviocytes by acting as a sponge for miR‐770. J Orthop Res, 2016, 34: 412–418. [DOI] [PubMed] [Google Scholar]
- 100. Goldring MB. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskeletal Dis, 2012, 4: 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Domenis R, Zanutel R, Caponnetto F, et al Characterization of the Proinflammatory profile of synovial fluid‐derived Exosomes of patients with osteoarthritis. Mediators Inflamm, 2017, 2017: 4814987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kato T, Miyaki S, Ishitobi H, et al Exosomes from IL‐1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther, 2014, 16: R163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Qi H, Liu DP, Xiao DW, Tian DC, Su YW, Jin SF. Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction‐induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. In Vitro Cell Dev Biol Anim, 2019, 55: 203–210. [DOI] [PubMed] [Google Scholar]
- 104. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep, 2017, 7: 16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang Y, Yu D, Liu Z, et al Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther, 2017, 8: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tofiño‐Vian M, Guillén MI, Pérez Del Caz MD, Castejón MA, Alcaraz MJ. Extracellular vesicles from adipose‐derived Mesenchymal stem cells Downregulate senescence features in osteoarthritic osteoblasts. Oxid Med Cell Longev, 2017, 2017: 7197598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wu J, Kuang L, Chen C, et al miR‐100‐5p‐abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials, 2019, 206: 87–100. [DOI] [PubMed] [Google Scholar]
- 108. Sun H, Hu S, Zhang Z, Lun J, Liao W, Zhang Z. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J Cell Biochem, 2019, 120: 171–181. [DOI] [PubMed] [Google Scholar]
- 109. Mao G, Zhang Z, Hu S, et al Exosomes derived from miR‐92a‐3p‐overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther, 2018, 9: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR‐140‐5p‐overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics, 2017, 7: 180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F. Exosomal KLF3‐AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J, 2018, 475: 3629–3638. [DOI] [PubMed] [Google Scholar]


