Abstract
Objective
To investigate the efficiency of anterior decompression on the proximal‐type cervical spondylotic amyotrophy patients.
Methods
This was a retrospective analysis. From January 2014 to November 2017, 21 patients with proximal‐type cervical spondylotic amyotrophy (CSA) underwent anterior decompression. There were 15 males and 6 females, aged 35–73 years with an average of 51.62 years. All the patients underwent surgery of anterior decompression (ACDF or ACCF). Among them, 12 patients underwent C4/5 single level ACDF, eight patients underwent C4/5 and C5/6 double level ACDF, and one patient underwent C5 anterior cervical corpectomy decompression and fusion surgery. Preoperative and postoperative clinical and radiologic parameters were assessed. The clinical examinations were reviewed, including muscle strength, neck disability index (NDI) score, cervical Japanese Orthopaedic Association (JOA) score, and improvement rate of manual muscle test (MMT) at the last follow‐up. Preoperative spinal cord or nerve impingement was assessed by magnetic resonance imaging (MRI) or computed tomography (CT) myelography. Postoperative lateral X‐ray radiographs were performed every 3 months after the surgery.
Results
Severe preoperative muscle atrophy of the deltoid or biceps muscles occurred in 21 patients included in the study. All of them involve impingements of the ventral nerve root and/or the anterior horn according to MRI and CT myelography. The preoperative duration of symptoms averaged 8.4 months. The average follow‐up for all patients was 13.2 months. At the final follow‐up, all patients showed statistically significant improvements in muscle strength and NDI scores (P < 0.05, P < 0.05). For the deltoid muscles force and C‐JOA scores, the average improvement rates were 66.49% ± 10.04% and 62.23% ± 9.23%, respectively. With respect to MMT, 12 proximal‐type patients were graded excellent, six were good, and three were fair, and the overall improvement rate was 85.7%.
Conclusions
For proximal‐type CSA patients with cervical radiculopathy, earlier anterior decompression surgery can achieve satisfactory results by significantly improving a patient's muscle strength and relieving compression symptoms.
Keywords: Cervical spondylotic amyotrophy, Anterior decompression, Surgical outcomes
Introduction
Cervical spondylotic amyotrophy (CSA) is an uncommon clinical syndrome which is characterized by muscle atrophy in the upper extremities with no or insignificant sensation deficits. Although such a clinical presentation has been described by Brain et al. in 1952 as muscle atrophy of the upper limbs without sensory disturbance1, the first case of cervical spondylotic amyotrophy as a clinical syndrome of “dissociated motor loss in the upper extremities with cervical spondylosis” was reported by Keegan in 19652, 3. Cervical spondylotic amyotrophy is classified into two subtypes according to the most predominantly affected muscle groups: proximal amyotrophy (deltoid and biceps) and distal amyotrophy (triceps, forearm, and hand muscles). Muscular atrophy of the proximal‐type patients is mainly localized in the C5 and C6 myotomes2, 4, 5, while for patients of the distal‐type, the responsible lesion involves the anterior horn at C7‐T1 myotomes. Clinically, most cervical spondylotic amyotrophy patients are involved in unilateral disorder whereas few others are bilateral symmetric disorder6.
Cervical spondylotic amyotrophy often follows a self‐limited course, which means that the manifestations usually keep steady for years after an initial progressive course. Typically, sensory loss or pyramidal signs are absent or insignificant in cervical spondylotic amyotrophy patients. Thus, diagnosis of cervical spondylotic amyotrophy mainly depends on a combination of radiographic examination, clinical manifestation, disease course, and electrophysiological findings. In the treatment of CSA, conservative intervention was once suggested at the onset of neurological symptom and seems to hamper disease progression for some proximal‐type cervical spondylotic amyotrophy. Whereas in some other studies, surgical intervention is recommended if conservative treatment has not been successful7, 8. In exploring the prognostic factors for cervical spondylotic amyotrophy, several recent studies have found positive correlations between the long duration of symptoms and the poor surgical outcome regardless of the proximal‐type or the distal‐type9, 10, 11. Thus early surgical intervention is recommended once diagnosed in a more recent study12.
Indeed, the underlying pathogenesis of cervical spondylotic amyotrophy is still not fully understood. The focus lies in whether cervical spondylotic amyotrophy is the result of selective damage to the ventral root or to anterior horn (Fig. 1), two essentially different pathogenesis for CSA13. Also, clinical efficiency of improvement in muscle strength of upper extremity varies across patients who underwent surgical treatments. Although predictive factors involving the prognosis of anterior decompression was investigated by some studies14, 15, yet few of these studies distinguish damage to the ventral nerve root or the anterior horn. Indeed, the two types of damage belong to different pathophysiologies16. In the clinical practice, patients diagnosed with cervical spondylotic amyotrophy often manifest either the myelopathy or radiculopathy2, 17, but it is difficult to distinguish underlying pathophysiologies from magnetic resonance imaging (MRI) for a large proportion of CSA patients. Thus, awareness is still lacking about clinical features of either type of cervical spondylotic amyotrophy, and associations between surgical procedures and outcomes have not been fully understood.
Figure 1.
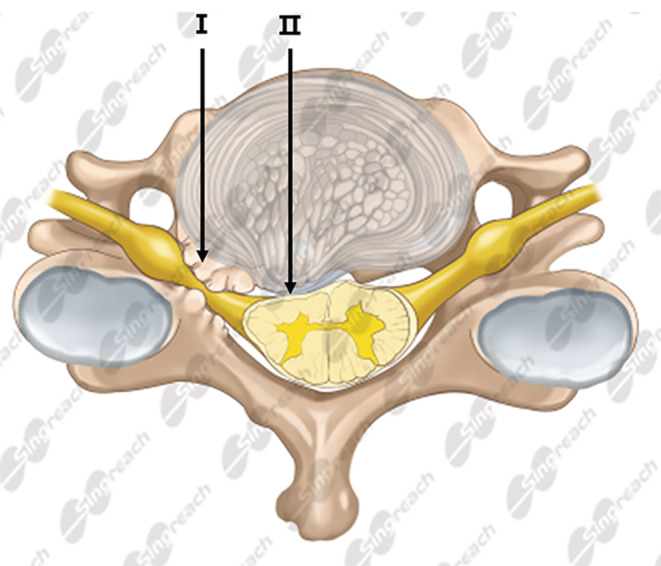
Possible pathogenesis of cervical spondylotic amyotrophy. I: impingement against ventral root; II: impingement against anterior horn.
In this study, we made an effort to review proximal‐type cervical spondylotic amyotrophy patients admitted to our hospital for treatment and identified the cases especially caused by radiculopathy on the basis of MRI findings. The purposes of this study were, firstly, to summarize current understanding and the clinical features of CSA, and secondly, to clarify the efficiency of anterior decompression approach for the proximal‐type cervical spondylotic amyotrophy patients with radiculopathy.
Material and Methods
Patients
Written informed consent was obtained from each patient and the study was approved by the Ethics Committee of Xijing Hospital, Xi'an, China. The inclusion criteria followed the PICOS principle: (i) patients diagnosed with CSA treated in our center; (ii) the CSA was treated by surgery of anterior decompression (ACDF or ACCF); (iii) preoperative and the postoperative comparisons were made with items including imaging data, muscle strength, neck disability index (NDI) score, cervical Japanese Orthopedic Association (JOA) score, and improvement rate of manual muscle test (MMT); (iv) improvements in the results of long‐term outcome between pre‐operation and post‐operation should be expected; and (v) the study design was a retrospective study. The exclusion criteria for this study are as follows: (i) brachial plexus and peripheral nerve injury (carpal tunnel syndrome, cubital tunnel syndrome, biceps tendonitis, thoracic outlet Syndrome, etc.); (ii) cervical flexion myelopathy multifocal motor neuropathy; and (iii) amyotrophic lateral sclerosis. Patients diagnosed as CSA must meet the following criteria: (i) the presence of unilateral muscle atrophy or impairment of the shoulder girdle muscles; (ii) mild or no sensory disturbance in the upper extremity; (iii) MRI or computed tomography (CT) indicating nerve root compression and/or spinal cord compression; and (iv) no gait disturbance.
From January 2014 to November 2017, 21 cases subjects in our department who had proximal‐type CSA were enrolled in our study. All patients complained of radicular pain or numbness in unilateral scapular area, shoulder or upper arm which lasted for 8.8 weeks (2–17 weeks) on average.
We collected data on patients' sex, age, surgical approaches, preoperative manual muscle test (MMT) results, C‐JOA scores, NDI score, duration of symptoms, levels of spinal canal stenosis, etiological diagnosis, and presence of high‐intensity zones on T2‐weighted MRI.
Surgical Procedure
All patients underwent general anesthesia in supine position using tracheal intubation. Surgeries began with incision of the right anterior cervical skin, then subcutis and muscular layer were bluntly dissected. The front of the vertebral body was exposed along the intermuscular space. Localized by fluoroscopy examination, the target intervertebral space was distracted and ACDF or ACCF surgery was performed according to the surgical plan. During the surgery, a complete decompression was performed to the Luschka joint especially in the affected side. Additional osteophytectomy was then performed to enlarge the intervertebral foramen, and the spinal canal or nerve root canal was carefully explored to see whether there was broken nucleus pulposus tissue. Finally, cages or titanium mash filled with autologous bone grains were placed into the intervertebral space and anterior titanium plate fixation was performed to obtain firm interfusion.
Among the 21 cases included in our analysis, 13 patients underwent C4/5 single‐segment ACDF surgery, seven patients underwent C4/5 and C5/6 dual‐segment ACDF surgery, and one underwent C5 vertebral ACCF surgery.
Assessment of Surgical Outcome
For these patients, we collected the basic information including operative procedure, operation time, blood loss during the cervical operation, and postoperative hospital stay. All imaging studies include plain radiographs (neutral, flexion, and extension views), CT and MRI examinations were performed to identify the presence of impingement of the ventral nerve root and corresponding intervertebral levels in each patient. To evaluate the effect of surgical treatment, we measured items including manual muscle testing (MMT), neck disability index (NDI), and cervical Japanese Orthopedic Association (C‐JOA) score.
Manual Muscle Testing (MMT)
Manual muscle testing (MMT) was adopted to quantify the muscle strength. MMT improvement rate = (last follow‐up muscle strength – preoperative muscle strength) / (5 – preoperative muscle strength). Improvements in muscle power of the most severely atrophic muscle were classified into four grades: excellent (more than two grades of recovery on manual muscle testing), good (one grade of improvement on manual muscle testing), fair (no improvement on manual muscle testing), and poor (worsening on manual muscle testing).
Neck Disability Index (NDI)
The Neck Disability Index (NDI) is a 10‐item questionnaire that assesses disability associated with neck pain and whiplash (NDI‐Vernon and Mior 1991). A score of less than 0–4 indicates no disability, 5–14 mild disability, 15–24 moderate disability, 25–34 severe disability, and scores greater than 35 complete disability.
Cervical Japanese Orthopaedic Association Score of Cervical Spine (C‐JOA)
C‐JOA is one of the most frequently used clinical outcome measures to quantify functional status in patients with cervical myelopathy. A score of 16–17 indicates normal function (best possible outcome), a score of 12–15 is grade 1, a score of 8–11 is grade 2, and a score of 0–7 is the most severe deficits. The recovery rate for the C‐JOA score = [(postoperative score – preoperative score)/(17‐preoperative score)]×100%.
Statistical Analysis
The statistical analysis of the clinical data was performed with software SPSS version 24.0 (SPSS, Inc., Chicago, IL). Measured data were expressed as mean ± standard deviation (x ± s). Variations of postoperative follow‐up were analyzed by Wilcoxon signed‐rank test, of which P < 0.05 was considered a significant difference.
Results
All the proximal‐type CSA patients in our study underwent anterior cervical discectomy and fusion (ACDF) or anterior cervical corpectomy and fusion (ACCF). Surgeries were performed by the same surgeon to ensure consistency in variables. After an average follow‐up of 13.2 months (10–32 months), four individuals were lost to follow‐up. Eventually, 21 subjects (15 males and six females) aged 35–73 years were included in this study, with an average age of 52.9 years.
The General Surgical Outcomes
The patients' characteristics and operation approaches are shown in Table 1. Of the 21 patients diagnosed as CSA, the average operation time was 136.7 min, the average intraoperative blood loss was 111.9 mL, and the postoperative hospital stay was 4.63 days on average. For 13 patients who underwent C4/5 ACDF, prolapse intervertebral disc can be found in the spinal canal, resulting in foramen stenosis or oppression of ipsilateral nerve root (Fig. 2). For seven patients who underwent C4/ 5, C5/6 two‐stage ACDF patients, different degrees of ipsilateral intervertebral disc protrusion in intervertebral space and nerve root compression can be found (Fig. 3). It should also be noted that in one case, aside from a huge deviation of the contralateral disc herniation, spinal canal stenosis was found during the surgical procedure (Fig. 4), thus C5 ACCF was performed to achieve a thorough decompression.
Table 1.
Clinical and imaging data
| Number | gender/age (years) | Lesion site | etiological diagnosis | Site of compression in MRI | duration of symptoms(months) | surgical approaches | operating time (min) | hemorrhage volume (mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | Male/41 | C4/5 C5/6 | CDH | NCR | 8 | C4‐6 ACDF | 140 | 150 |
| 2 | Male/48 | C4/5 | CDH | NCR | 2 | C4/5 ACDF | 135 | 70 |
| 3 | Female/59 | C4/5 C5/6 | CDH | SCC | 6 | C4‐6 ACDF | 156 | 100 |
| 4 | Male/54 | C4/5 | CDH | NCR | 10 | C4/5 ACDF | 121 | 180 |
| 5 | Male/60 | C4/5C5/6 | CDH | NCR | 16 | C4‐ 6 ACDF | 152 | 130 |
| 6 | Male/35 | C4/5 | CDH | NCR | 4 | C4/5 ACDF | 126 | 70 |
| 7 | Female/62 | C4/5 C5/6 | CDH | NCR and SCC | 12 | C4‐ 6 ACDF | 138 | 120 |
| 8 | Male/49 | C4/5 | CDH | NCR | 15 | C4/5 ACDF | 128 | 100 |
| 9 | Male/56 | C4/5 | CDH | SCC | 10 | C4/5 ACDF | 145 | 90 |
| 10 | Male/73 | C4/5 | CDH | NCR | 9 | C4/5 ACDF | 150 | 170 |
| 11 | Female/48 | C4/5 | CDH | NCR | 7 | C4/5 ACDF | 130 | 80 |
| 12 | Male/45 | C4/5 | CDH | NCR | 9 | C4/5 ACDF | 123 | 100 |
| 13 | Male/48 | C4/5 C5/6 | CDH | NCR and SCC | 17 | C5 ACCF | 171 | 210 |
| 14 | Male/59 | C4/5 | CDH | NCR | 3 | C4/5 ACDF | 108 | 60 |
| 15 | Female/39 | C4/5 | CDH | NCR | 4 | C4/5 ACDF | 119 | 100 |
| 16 | Male/71 | C4/5 C5/6 | CDH | ambiguous | 8 | C4‐ 6 ACDF | 143 | 120 |
| 17 | Male/49 | C4/5 | CDH | NCR | 6 | C4/5 ACDF | 124 | 70 |
| 18 | Female/53 | C4/5 | CDH | NCR | 7 | C4/5 ACDF | 108 | 70 |
| 19 | Male/50 | C4/5 C5/6 | CDH | SCC | 5 | C4‐ 6 ACDF | 169 | 130 |
| 20 | Female/42 | C4/5 C5/6 | CDH | ambiguous | 7 | C4‐ 6 ACDF | 170 | 150 |
| 21 | Male/43 | C4/5 | CDH | NCR | 4 | C4/5 ACDF | 115 | 80 |
NRC, nerve root compression; SCC, spinal cord compression.
Figure 2.

A 35‐year‐old male patient with proximal‐type CSA at the C4/5 level. (A) The pre‐operative X‐rays of the cervical spine showing narrowed intervertebral space of the C4/5. (B) Sagittal T2‐weighted magnetic resonance image showing cord compression at the C4‐C5 space; (C) Axial T2‐weighted magnetic resonance image at the C4‐C5 space showing impingement against the right ventral nerve root and anterior horn, with no abnormal signal intensity in the spinal cord. (D) CT of the cervical spine show that the center of the C4/5 disc protrudes to the right, the outlet of the right nerve root is narrowed. (E) postoperative lateral X‐ray of the cervical spine showing that the internal fixation is firm. (F) Follow‐up at 3 months, X‐ray of the cervical spine showing that the internal fixation is firm and the bony fusion is formed.
Figure 3.

A 41‐year‐old male patient with proximal‐type CSA at the C4/5 and C5/6 levels. (A) The pre‐operative X‐rays of the cervical spine showing narrowed intervertebral space of the C4/5 and C5/6 levels. (B) Sagittal T2‐weighted magnetic resonance image showing cord compression at the C4‐C6 space. (C) CT image at the C4‐C5 space showing impingement against C4/5 intervertebral disc on the left side. (An intraoperative exploration confirmed that multiple nucleus pulposus broke into the spinal canal and the nerve root outlet). (D) CT image at the C5‐C6 space showing that the central C5/6 disc protruded to the left and the outlet of the left nerve root narrowed. (E) postoperative lateral X‐ray of the cervical spine showing that the internal fixation is in good position. (F) Follow‐up at 6 months, X‐ray of the cervical spine showing that the internal fixation is firm and solid arthrodeses is formed.
Figure 4.

A 48‐year‐old male patient with proximal‐type CSA at the C4/5 and C5/6 levels. (A) The pre‐operative X‐rays of the cervical spine showing cervical hyperplasia bone hyperplasia, narrowed C4/5 and C5/6 intervertebral space. (B) Sagittal T2‐weighted magnetic resonance image showing cord compression at the C4‐C6 space. (C) Axial T2‐weighted magnetic resonance image at the C4‐C5 space showing both left ventral nerve root nerve root and spinal cord were compressed at the C4‐5 intervertebral level. (D) Axial T2‐ weighted magnetic resonance image at the C5‐C6 space showing narrowed segmental spinal canal and impingement against the left anterior horn nerve roots (white arrows). (E) Postoperative lateral X‐ray of the cervical spine showing that the internal fixation and the titanium cage were in appropriate position. (F) Follow‐up at 3 months, X‐ray of the cervical spine showing that the internal fixation is firm and maintained in appropriate position.
Radiographic Outcomes
For all patients, compression at the ventral nerve root or the spinal cord was found in 13 and four patients, respectively. Two patients were found to have compression at both sites, and the compression sites is difficult to be distinguished in two patients. During the postoperative follow‐up, no significant complications occurred. Reviewing the cervical X‐ray and lateral three‐dimensional reconstruction of CT showed that all surgical segments achieved bony fusion.
Functional Improvement Evaluated by Clinical Indexes
Muscle Strength and MMT Score
At the final follow‐up, the deltoid muscle strength of all participants was 4.09 ± 0.55 on average, which was significantly higher than the preoperative level of 2.29 ± 0.78 (P < 0.01). The recovery rate was 66.49% ± 10.04%. The average brachii muscle strength (4.21 ± 0.62) was significantly improved at the final follow‐up compared with the preoperative 3.16 ± 0.94. The recovery rate was 57.12% ± 12.37% (Table 2). Improvement of the most atrophic muscle by manual muscle testing (MMT) revealed that 12 proximal‐type patients were graded excellent, six were good, and three were fair. The improvement rate was 85.7%.
Table 2.
Statistical analysis of degree of improvement between before operation and last follow‐up
| Assessment items | Preoperative | Last follow‐up | P | Improvement rate (%) | |
|---|---|---|---|---|---|
| Muscle strength rating | Deltoid muscle | 2.29 ± 0.78 | 4.09 ± 0.55 | <0.01 | 66.49 ± 10.04 |
| Biceps brachii | 3.16 ± 0.94 | 4.21 ± 0.62 | <0.05 | 57.12 ± 12.37 | |
| NDI score | 38.25 ± 6.10 | 12.50 ± 2.42 | <0.05 | ||
| JOA score | 6.02 ± 2.11 | 12.91 ± 3.82 | <0.01 | 62.23 ± 9.23 | |
NDI
NDI scored an average of 12.50 ± 2.42 at the final follow‐up, which were significantly improved compared with preoperative score of 38.25 ± 6.10 C (Table 2).
C‐JOA
The mean C‐JOA score improved from 6.02 ± 2.11 preoperatively to 12.91 ± 3.82 postoperatively (P < 0.05). The average recovery rate for C‐JOA score at the final follow‐up was 62.23% ± 9.23% (Table 2).
Subgroup Analysis
Results of NDI, C‐JOA, and deltoid muscle strength showed no significant difference in analysis at 3 months vs 9 months postoperatively. The score of the biceps brachii strength improved from 3.71 ± 0.42 at 3 months to 4.09 ± 0.55 at 9 months (P < 0.05) (Table 3).
Table 3.
Subgroup analysis on the results of functional parameters at different follow‐up time points (NS, no statistically significant difference)
| Follow‐up time points | deltoid muscle strength | biceps brachii strength | NDI score | JOA score |
|---|---|---|---|---|
| 3 months | 3.94 ± 0.51 | 3.77 ± 0.42 | 11.40 ± 2.30 | 12.71 ± 3.92 |
| 9 months | 4.09 ± 0.55 | 4.21 ± 0.62 | 12.50 ± 2.42 | 12.91 ± 3.82 |
| P | NS | <0.05 | NS | NS |
Discussion
Although CSA has been documented for 60 years, the underlying pathogenesis to proximal‐type CSA is still unclear. Some researchers proposed that the impingements were mainly concentrated in ventral nerve root2; alternatively, others attribute it to impingement against the anterior horn6, 18. Studies by Fujiwara et al. and Shinomiya et al. proposed that the impingement against both the ventral nerve root and the anterior horn might cause disease10, 19. For the CSA patients in our study, although impingement against both sites were found though MRI examination, the distinction between them was ambiguous especially for those that involve multilevel spinal stenosis.
There is still much controversy around surgical options for patients with CSA. Previously, there were claims that posterior laminoplasty with or without foraminotomy have comparable results with anterior decompression and fusion. A recent study by Chen et al. recommended anterior decompression as first choice regardless of the number of spinal canal stenosis considering pathogenic lesion leading to CSA comes from the ventral side of the nerve root or anterior horn20. All the patients in our study adopted an anterior approach in the decompression procedure, which achieved satisfactory results in the proximal‐type CSA patients. Nevertheless, potentials of functional improvements in CSA patients after decompression are limited, and most clinical parameters showed no significant improvements at 9 months postoperatively compared with that at 3 months.
It is worth noting that in the decompression procedure of one case, disc tissue was absent in the C4/5 intervertebral space and a rupture was found in the posterior longitudinal ligament. After further intraoperative exploration, multiple broken nucleus pulposus were found at the outlet of C5 nerve root. Then a complete decompression was performed to the affected nerve roots. Thus, enlarging the intervertebral foramen is needed in such cases to achieve a complete decompression of the affected nerve roots.
It is generally accepted that upper limb muscles are innervated by several nerve roots21. C5 nerve roots mainly innervate the deltoid muscle, as well as part of the biceps muscle. Damage to C5 nerve root compression often results in restricted motion of shoulder and/or elbow, which has been reported to occur in C4/5 segment disc herniation. However, some scholars proposed that deltoid muscle spasm may also occur in patients with C3/4 or C5/6 single‐segment CDH22, 23. Han et al. reported a total of 14 patients with deltoid muscle paralysis caused by single‐segment cervical disc herniation and cervical spondylosis of the nerve root ranging from C3 to C6 levels24. Nevertheless, in some cases, the pathogenesis of decreased deltoid or biceps brachii muscle strength in the C3/4 or C5/6 segment of the CDH cannot be interpreted solely by clinical studies. Such phenomenon was interpreted as dissociation between the levels of spinal stenosis and atrophic muscles. In our study, eight of 21 patients with C5 nerve root paralysis were found to be involved with C4/5 and C5/6 double‐segmental lesions. After a two‐stage decompression, all patients achieved satisfactory results. For these patients, it is advisable to make a comprehensive surgical plan according to involved levels so as to achieve a complete decompression.
Several limitations of our study must be acknowledged. Firstly, the number of cases was small and therefore insufficient for statistically evaluating the surgical outcomes. This was mainly because CSA patients of this type were rare and there was loss of contact with patients in the follow‐up. Secondly, duration of follow‐up was short. The shortest follow‐up of the study was 10 months, with an average of 13.2 months, which is less persuasive compared with the long‐term clinical follow‐up studies reported in previous literature.
Conclusions
In this study, we demonstrated clinical features of proximal‐type CSA with cervical radiculopathy and evaluated the surgical outcomes of anterior approaches. On the basis of our analysis, we concluded that anterior decompression is effective and can achieve satisfying clinical improvement in both clinical syndrome and muscle strength of atrophic muscles in proximal type CSA patients with cervical radiculopathy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81672132 to Dr. Wei Lei) and the booster program of Xijing Hospital (XJZT18ML34 to Dr. Yang Zhang).
Disclosure: The authors declare that there is no conflict of interests regarding the publication of this paper.
Contributor Information
Yang Zhang, Email: zhangyang@fmmu.edu.cn.
Wei Lei, Email: leiwei@fmmu.edu.cn.
Reference
- 1. BRAIN WR, NORTHFIELD D, WILKINSON M. The neurological manifestations of cervical SPONDYLOSIS. Brain, 1952, 75: 187–225. [DOI] [PubMed] [Google Scholar]
- 2. Keegan JJ. The cause of dissociated motor loss in the upper extremity with cervical spondylosis. J Neurosurg, 1965, 23: 528–536. [DOI] [PubMed] [Google Scholar]
- 3. Akiyama N, Kitamura H, Yoshimura Y, Tsuchiya T, Shiokawa A. Dissociated motor loss in the upper extremities with cervical spondylosis, a report of autopsy case (author's transl). Nihon Seikeigeka Gakkai zasshi, 1980, 54: 303–310. [PubMed] [Google Scholar]
- 4. Itoh T, Tsuji H, Tamaki T, Miyasaka H, Toyoda A. The clinical consideration of the dissociated motor loss syndrome (Keegan) in diseases of the cervical spine (author's transl). Nihon Seikeigeka Gakkai zasshi, 1980, 54: 135–151. [PubMed] [Google Scholar]
- 5. Matsunaga S, Sakou T, Imamura T, Morimoto N. Dissociated motor loss in the upper extremities. Clinical features and pathophysiology. Spine, 1993, 18: 1964–1967. [DOI] [PubMed] [Google Scholar]
- 6. Gebere‐Michael SG, Johnston JC, Metaferia GZ, Wuhib MZ, Fernandez HH. Bilaterally symmetric cervical spondylotic amyotrophy: a novel presentation and review of the literature. J Neurol Sci, 2010, 290: 142–145. [DOI] [PubMed] [Google Scholar]
- 7. Inui Y, Miyamoto H, Sumi M, Uno K. Clinical outcomes and predictive factors relating to prognosis of conservative and surgical treatments for cervical Spondylotic Amyotrophy. Spine, 2011, 36: 794–799. [DOI] [PubMed] [Google Scholar]
- 8. Tauchi R, Imagama S, Inoh H, et al Appropriate timing of surgical intervention for the proximal type of cervical spondylotic amyotrophy. Eur J Orthop Surg Traumatol, 2015, 25: 107–113. [DOI] [PubMed] [Google Scholar]
- 9. Kaneko K, Taguchi T, Toyoda K, Kato Y, Azuma Y, Kawai S. Distal‐type cervical spondylotic amyotrophy: assessment of pathophysiology from radiological findings on magnetic resonance imaging and epidurally recorded spinal cord responses. Spine, 2004, 29: E185–E188. [DOI] [PubMed] [Google Scholar]
- 10. Fujiwara Y, Tanaka N, Fujimoto Y, Nakanishi K, Kamei N, Ochi M. Surgical outcome of posterior decompression for cervical Spondylosis with unilateral upper extremity Amyotrophy. Spine, 2006, 31: E728–E732. [DOI] [PubMed] [Google Scholar]
- 11. Kameyama T, Ando T, Yanagi T, Yasui K, Sobue G. Cervical spondylotic amyotrophy. Magnetic resonance imaging demonstration of intrinsic cord pathology. Spine, 1998, 23: 448–452. [DOI] [PubMed] [Google Scholar]
- 12. Wang HL, Li HC, Jiang JY, Lū FZ, Chen WJ, Ma XS. Evaluation of characteristics and surgical outcomes in cervical spondylotic amyotrophy. Indian J Orthop, 2014, 48: 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang SD, Jiang LS, Dai LY. Cervical spondylotic amyotrophy. Eur Spine J, 2011, 20: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imajo Y, Kato Y, Kanchiku T, et al Prediction of surgical outcome for proximal‐type cervical Spondylotic Amyotrophy novel mode of assessment using compound action potentials of deltoid and biceps Brachii and central motor conduction time. Spine, 2012, 37: E1444–E1449. [DOI] [PubMed] [Google Scholar]
- 15. Tauchi R, Imagama S, Inoh H, et al Risk factors for a poor outcome following surgical treatment of cervical spondylotic amyotrophy: a multicenter study. Eur Spine J, 2013, 22: 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kong LD, Wang LF, Zhang JT, Zhang YZ, Ding WY, Shen Y. Predictive factors relating to prognosis of anterior decompressive surgery for proximal‐type cervical spondylotic amyotrophy. J Back Musculoskelet Rehabil, 2015, 28: 261–266. [DOI] [PubMed] [Google Scholar]
- 17. Yanagi T. Clinical characteristics of cervical spondylotic amyotrophy. Clin Neurol, 1976, 16: 520. [PubMed] [Google Scholar]
- 18. Kenzo U, Hideaki N, Takafumi Y, et al Anterior and posterior decompressive surgery for progressive amyotrophy associated with cervical spondylosis: a retrospective study of 51 patients. J Neurosurg Spine, 2009, 11: 330–337. [DOI] [PubMed] [Google Scholar]
- 19. Shinomiya K, Komori H, Matsuoka T, Mutoh N, Furuya K. Neuroradiologic and electrophysiologic assessment of cervical spondylotic amyotrophy. Spine, 1994, 19: 21–25. [DOI] [PubMed] [Google Scholar]
- 20. Li T, Shi G, Shi L, Miao J, Chen D, Chen Y. Clinical features and long‐term surgical outcomes of patients with cervical Spondylotic Amyotrophy. World Neurosurg, 2019, 121: e172–e180. [DOI] [PubMed] [Google Scholar]
- 21. Gu Y, Shen L, Shen Z. Functional motor innervation of brachial plexus roots: an intraoperative electrophysiological study. Zhonghua Wai Ke Za Zhi, 1996, 34: 40–43. [PubMed] [Google Scholar]
- 22. Marzo JM, Simmons EH, Kallen F. Intradural connections between adjacent cervical spinal roots. Spine, 1987, 12: 964–968. [DOI] [PubMed] [Google Scholar]
- 23. Moriishi J, Otani K, Tanaka K, Inoue S‐I. The intersegmental anastomoses between spinal nerve roots. Anat Rec, 1989, 224: 110–116. [DOI] [PubMed] [Google Scholar]
- 24. Chang H, Park JB, Hwang JY, Song KJ. Clinical analysis of cervical radiculopathy causing deltoid paralysis. Eur Spine J, 2003, 12: 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]


