Abstract
There has been a spurt in structural neuroimaging studies of the effect of hearing loss on the brain. Specifically, magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) technologies provide an opportunity to quantify changes in gray and white matter structures at the macroscopic scale. To date, there have been 32 MRI and 23 DTI studies that have analyzed structural differences accruing from pre- or peri-lingual pediatric hearing loss with congenital or early onset etiology and postlingual hearing loss in pre-to-late adolescence. Additionally, there have been 15 prospective clinical structural neuroimaging studies of children and adolescents being evaluated for cochlear implants. The results of the 70 studies are summarized in two figures and three tables. Plastic changes in the brain are seen to be multifocal rather than diffuse, that is, differences are consistent across regions implicated in the hearing, speech and language networks regardless of modes of communication and amplification. Structures in that play an important role in cognition are affected to a lesser extent. A limitation of these studies is the emphasis on volumetric measures and on homogeneous groups of subjects with hearing loss. It is suggested that additional measures of morphometry and connectivity could contribute to a greater understanding of the effect of hearing loss on the brain. Then an interpretation of the observed macroscopic structural differences is given. This is followed by discussion of how structural imaging can be combined with functional imaging to provide biomarkers for longitudinal tracking of amplification.
This article is categorized under:
Developmental Biology > Developmental Processes in Health and Disease Translational, Genomic, and Systems Medicine > Translational Medicine Laboratory Methods and Technologies > Imaging
Keywords: brain mapping, computational anatomy, deafened brain, morphometry
1 ∣. INTRODUCTION
Sensorineural hearing loss is the most common type of deafness resulting in degraded transmission of acoustic information from the dysfunctional cochleae in the inner ears to the primary auditory cortex and secondary or association cortices in the brain (see Section 2). Such sensory deprivation results in a brain that is structurally different from one with normal hearing. This difference is likely to be related to the degree of hearing loss as well as plasticity induced by the brain adapting to auditory stimuli provided by a hearing aid or cochlear implant and/or visual stimuli provided by lipreading (or speechreading). Further differences can accrue from developing cognitive strategies to compensate for the hearing loss. Thus, the deafened brain has been both an attractive and challenging target of neuroimaging studies since the turn of the new millennium. These studies have been fueled by advances in technologies such as positron emission tomography (PET), magnetic resonance imaging (MRI), functional MRI (fMRI), diffusion tensor imaging (DTI), functional near-infrared spectroscopy (fNIRS), and cortical auditory evoked potential (CAEP) to name but a few.
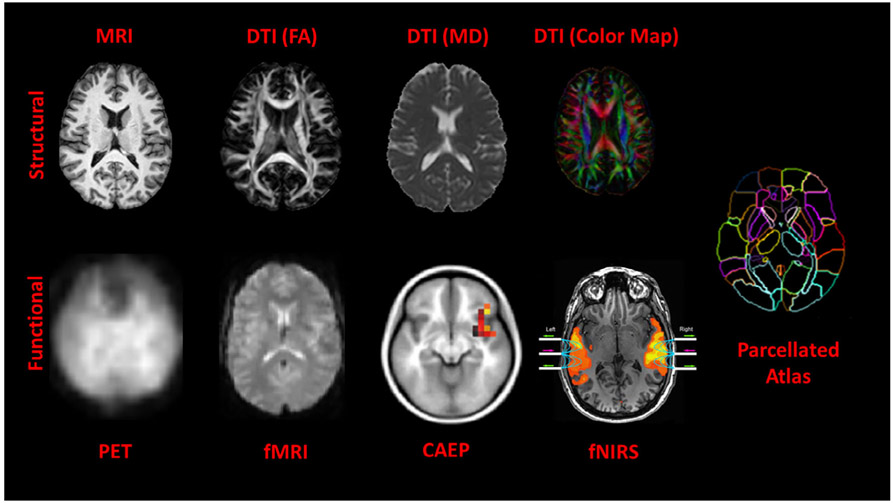
Figure 1 illustrates the different structural and functional neuroimaging modalities used to examine the brain. At the macroscopic scale, three types of brain tissues can be discerned in a 3D volume of about 250 × 250 × 200 (12.5 M) voxels of 1 mm3 resolution. These are gray matter, white matter, and cerebrospinal fluid. In general, gray matter is associated with cortical regions and subcortical structures while white matter is associated with connections between cortical regions and subcortical structures. The gray matter within the cortical region contains mostly neuronal cell bodies and unmyelinated fibers while subcortical regions contain deep gray nuclei and white matter contains axonal, usually myelinated, fibers.
FIGURE 1.
Different image modalities stratified into structural (top row) and functional (bottom row) imaging. The different contrasts at the macroscopic level of 1 mm3 provide information about three types of tissues: gray matter, white matter, and cerebrospinal fluid. An MRI scan provides a view of the highly folded cortex (shown in light grayscale) and the underlying white matter (shown in bright grayscale). The scalar modalities (FA, MD, and color map) derived from DTI scans provide different ways of looking at white matter structures. The red, green, and blue colors in the color map indicate orientation in the left–right, anterior–posterior, and superior–inferior directions, respectively. The PET and fMRI scans provide a view of responses to brain activity. CAEP and fNIRS brain activity are overlaid on MR scans for reference. Activity associated with the first positive peak in the CAEP waveform (i.e., P1) is located in the primary auditory cortex contained within the Heschl's gyrus. (Reprinted with permission from Sharma et al. (2016, fig. 2). Copyright 2016, Wolters Kluwer Health) Activity associated with speech is located in the superior temporal gyrus containing Heschl's gyrus and planum temporale (often called the primary and secondary auditory cortex) on both sides (Reprinted with permission from Figure 2b in Sevy et al. (2010). Copyright 2010, Elsevier). Mapping these scans to parcellated atlases provides an opportunity to perform quantitative analysis of structural and functional data in common coordinates (Miller, Faria, Oishi, & Mori, 2013; Miller, Younes, & Trouvé, 2014; Mori, Oishi, Faria, & Miller, 2013)
The different tissue contrasts provided by MRI scans and the scalar images of fractional anisotropy, mean diffusivity, axial and radial diffusivity derived from DTI scans make it possible to parcellate the brain into hundreds of regions (or groups of regions called lobes) by mapping to atlases. The DTI scan can also generate a color map to indicate the 3D orientation of the white matter connections. Finally, structural images can be registered in common coordinates with functional ones such as fMRI, PET, CAEP, and fNIRS (described in Section 8) so that brain activity in different parts of the brain in response to acoustic and visual stimuli can be studied.
With respect to the three magnetic resonance modalities that exploit the magnetic properties of water molecules in the brain, fMRI has been by far the most popular imaging modality with about a hundred studies of brain activity in the deafened brain in response to visual and acoustic stimuli. In contrast, there have been fewer but an increasing number of MRI and DTI studies examining structural properties of the deafened brain.
The review begins with a brief description of the deafened auditory pathway from the two cochleae to the brain. This is followed by summaries of structural MRI, DTI, and clinical studies of people with hearing loss. The focus will be on populations of pre- or peri- lingual hearing loss with congenital or early onset etiology and postlingual hearing loss in pre-to-late adolescence whose pathologies are distinct from those who acquired hearing loss in adulthood. Next, a discussion on the limited focus on volumetric measures suggests how additional measures of morphometry and connectivity widely used in other structural neuroimaging studies could contribute to a greater understanding of the effect of hearing loss on the brain. Then, an interpretation of the observed macroscopic structural differences is given. This is followed by a summary of how structural imaging can be combined with functional imaging to provide potential biomarkers for longitudinal tracking of amplification. The review concludes with a discussion of future directions and opportunities for expanding neuroimaging studies beyond those done so far.
2 ∣. THE DEAFENED AUDITORY PATHWAY
Figure 2 is a simplified schematic illustration of the transmission of acoustic information along the auditory pathway from the cochleae to the brain. Sensorineural hearing loss is attributed to missing or damaged hair cells in the cochlea in the inner ear (e.g., Ashmore et al., 2010; Fettiplace & Kim, 2014). The result is the diminished ability of the cochlear hair cells to transduce acoustic energy to electrical energy that is transmitted by nerves to the brain. Thus there is a cascade of atrophy resulting in degraded transmission of acoustic information (e.g., Kral, Hartmann, Tillein, Heid, & Klinke, 2000; Saada, Niparko, & Ryugo, 1996; Sanes & Kotak, 2011). Not even amplification provided by hearing aids is sufficient to provide neural activity levels for optimal transmission particularly at high frequencies (e.g., Takesian, Kotak, Sharma, & Sanes, 2013). However, it is clear that the high level stimulation rates provided by cochlear implants improve or restore integrity of neuroanatomical structures at different stages of the auditory pathway (e.g., Chen, Limb, & Ryugo, 2010; Muniak, Connelly, Tirko, O'Neil, & Ryugo, 2013; O'Neil, Connelly, Limb, & Ryugo, 2011; Ryugo & Limb, 2009). Thus, sensory deprivation causes plastic changes within the brain. These changes can be seen clearly at the microscopic scale albeit in post mortem human studies or animal models. There have been few post mortem studies of the auditory cortex and understandably none of babies with hearing loss (Huttenlocher & Dabholkar, 1997; Iyengar, 2012; Moore, 2002; Moore & Guan, 2001; Moore & Linthicum, 2007; Pundir et al., 2012). So, animal models have been used to understand the nature of atrophy at different stages of the auditory pathway (Butler & Lomber, 2013). Yet these microscopic studies have to be reconciled with structural neuroimaging studies at the macroscopic scale in humans which are reviewed in the next three sections.
FIGURE 2.
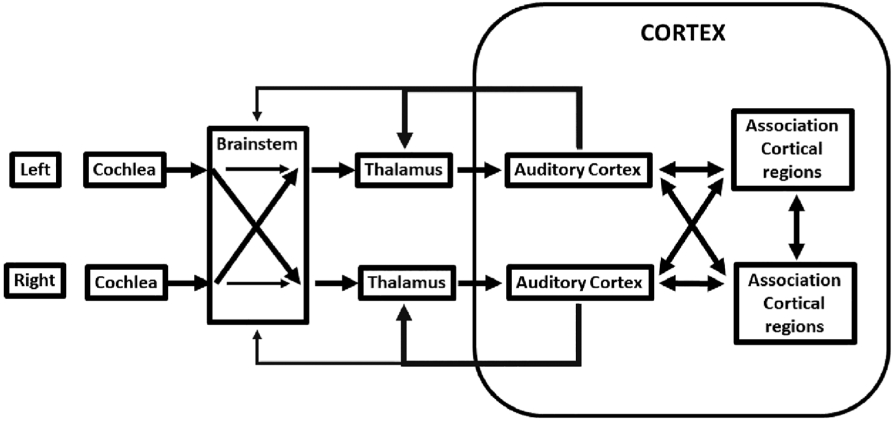
Simplified schematic illustration of transmission of acoustic information from the left and right cochleae to the brain. Note information crosses over in the brainstem as well as in the cortex. Please refer to Figures 3 and 4 for association cortical regions such as planum temporale. The white matter connections include those between primary and associated cortical regions and those that project back to other structures via the thalamus and brainstem
3 ∣. MRI ANALYSIS OF GRAY MATTER AND WHITE MATTER STRUCTURES IN PEOPLE WITH HEARING LOSS
MRI provides the opportunity to examine gray matter and white matter tissue in the brain at the macroscopic scale of 1 mm3. Here gray matter characterizes cellular contents of cortical and subcortical structures, while white matter characterizes connections between cortical and subcortical structures. The different biophysical properties of the water molecules in gray matter and white matter result in different responses to the magnetic field of the scanner. These differences provide the necessary contrasts between gray matter and white matter in 3D volumetric images of the brain (Figure 1). Thus it is possible to quantify morphometric properties of cortical and subcortical gray matter such as volume, surface area, and thickness.
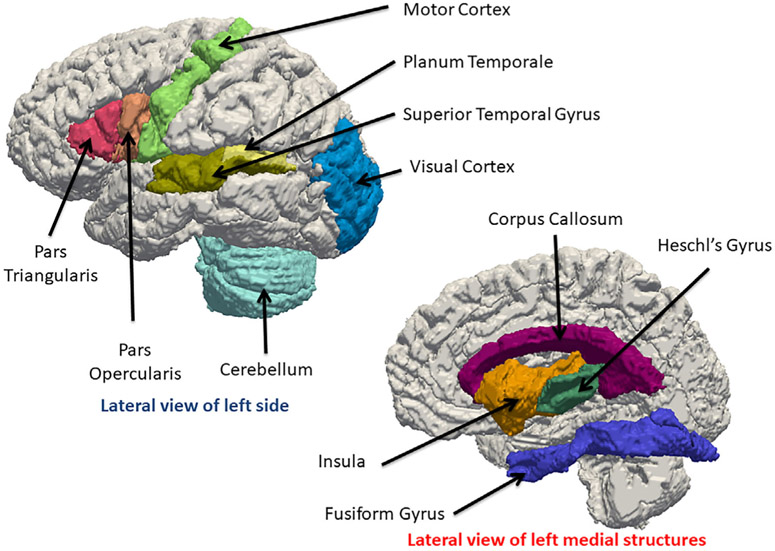
Table 1 indicates there have been 32 structural MRI studies comparing populations of people with and without hearing loss. Figure 3 provides a visualization of the location of the structures implicated in many of these studies. A few observations can be made. First, there is a wide variation in the sample size with larger groups associated with large population centers (Shibata, 2007). Second, these groups are by design homogeneous, that is, the subjects are native users of sign language and generally have not been using hearing aids since infancy. Third, there is also a wide variation in the age in these groups and only one study focused on babies who were being evaluated for cochlear implants (K. M. Smith et al., 2011). Fourth, while morphometry analysis focused on mostly volumes of gray matter and white matter structures, nine measured cortical thickness (Hribar et al., 2014; Kumar & Mishra, 2018; W. Li et al., 2013; J. Li et al., 2012; Pereira-Jorge et al., 2018; Ratnanather et al., 2019; Shiell et al., 2016; Shiohama et al., 2019; Smittenaar et al., 2016) and one measured surface area (Kara et al., 2006). Fifth, weak differences were observed in several structures. Prominent among these are Heschl's gyrus and planum temporale considered as primary and secondary auditory cortices, respectively, which both lie on the dorsal (upper) surface of the superior temporal gyrus (see also Figure 1). Other affected structures included motor cortex; frontal cortex including Broca's area; occipital cortex including early visual areas; corpus callosum; insula; fusiform; cerebellum. Sixth, some reported unilateral differences; others reported that asymmetry was mainly preserved in the temporal lobe specifically Heschl's gyrus, planum temporale, and superior temporal gyrus. The two gray matter connectivity studies (E. Kim et al., 2014; W. Li et al., 2015) suggested increased connectivity between auditory and visual areas as well as weaker connectivity between regions such as temporal and parietal (motor) ones.
TABLE 1.
Summary of magnetic resonance imaging studies of gray and white matter structures in people with hearing loss
| Studies | Groups | Age | Communication | Scanner strength |
Structure | Tissue | Measure | Analysis | Main result | Other results |
|---|---|---|---|---|---|---|---|---|---|---|
| Penhune, Cismaru, Dorsaint-Pierre, Petitto, and Zatorre (2003) | 12 DS vs. 10 | 29 | ASL or LSQ | 1.5 T | HG | GM and WM | Volume | ROI manual | No difference | Asymmetry preserved |
| PT | GM | Volume | ROI manual | No difference | Asymmetry preserved | |||||
| Left motor hand area | GM | Whole brain | VBM (SPM) | Larger density | Asymmetry preserved | |||||
| Emmorey, Allen, Bruss, Schenker, and Damasio (2003) | 25 DS vs. 25 | 23.8 ± 4.1 | ASL | 1.5 T | Temporal lobe | GM and WM | Volume | ROI manual | No difference in GM/WM ratio | |
| STG | GM and WM | Volume | ROI manual | Larger GM/WM ratio | ||||||
| HG | GM and WM | Volume | ROI manual | Larger GM/WM ratio | Asymmetry preserved | |||||
| PT | GM and WM | Volume | ROI manual | Left PT larger (GM) | ||||||
| Fine, Finney, Boynton, and Dobkins (2005) | 6 DS vs. 6 HS vs. 6 | 27 ± 5.7 | ASL | 1.5 T | Early visual areas | GM | Volume | Retinotopic fMRI | No difference | Only auditory area affected |
| Kara et al. (2006) | 18 DS vs. 18 | 41.2 ± 7.5 | TSL | 1.5 T | CC | WM | Length | ROI manual | No difference | |
| Width | ROI manual | No difference | ||||||||
| Area | ROI manual | No difference | ||||||||
| Shibata (2007) | 53 DS vs. 51 | 21 | ASL | 1.5 T | Left posterior STG | WM | Whole brain | VBM (SPM) | Focal deficit | |
| Posterior STG | WM | Whole brain | VBM (SPM) | Focal deficit in asymmetry | ||||||
| Frontal/temporal perisylvian | GM | Whole brain | VBM (SPM) | Asymmetry preserved | ||||||
| Meyer et al. (2007) | 6 DS vs. 6 | NR | DGS | 3 T | Left post-long uncinate fasisculus | WM | Whole brain | VBM (SPM) | Less | |
| Left post-inferior uncinate fasisculus | WM | Whole brain | VBM (SPM) | Less | ||||||
| Posterior Sylvian fissure | WM | Whole brain | VBM (SPM) | Steeper | ||||||
| Allen, Emmorey, Bruss, and Damasio (2008) | 25 DS vs 16 HS vs. 25 | 23.8 ± 4.1 | ASL | 1.5 T | Left posterior insula lobule | GM | Volume | ROI manual | Larger in DS | |
| Right insula | WM | Volume | ROI manual | Larger in DS and HS | ||||||
| Xia, Qi, and Li (2008) | 20 DS vs. 20 | 9–12 | CSL | 1.5 T | Left HG, right HG | GM/WM | Ratio | ROI manual | Larger in DS | |
| 20 DS vs. 20 | 19–22 | CSL | 1.5 T | Left HG and left STG | GM | Volume | ROI manual | Larger in DS | ||
| D. J. Kim, Park, Kim, Lee, and Park (2009) | 13 DS vs. 29 | 29.3 ± 6.8 | KSL | 3 T | STG | WM | Volume | VBM (SPM) | Smaller bilaterally | |
| Temporal sub-gyral | WM | Volume | VBM (SPM) | Smaller bilaterally | ||||||
| Left parietal | WM | Volume | VBM (SPM) | Smaller | ||||||
| Left superior frontal | WM | Volume | VBM (SPM) | Smaller | ||||||
| Left medial frontal | WM | Volume | VBM (SPM) | Smaller | ||||||
| Lepore et al. (2010) | 14 DS vs. 16 | 29.5 | LSQ | 1.5 T | Frontal lobe (Broca's) | WM | Volume | TBM (voxel-wise Jacobian) | Larger | |
| Adjacent: Motor | WM | Whole brain | TBM (voxel-wise Jacobian) | Larger | ||||||
| Adjacent: Language | WM | Volume | TBM (voxel-wise Jacobian) | Larger | ||||||
| CC (splenium: temporal/occipital) | WM | Manual | ROI manual | Difference | ||||||
| K. M. Smith et al., 2011 | 16 D vs. 26 | 12 ± 2.8 mo | NR | 3 T | HG | GM | Whole brain | VBM (SPM) | Increased | Asymmetry not preserved |
| WM | Whole brain | VBM (SPM) | Decreased | |||||||
| J. Li et al. (2012) | 16 DS vs. 16 | 14.56 ± 2.1 | CSL | 3 T | Left precentral gyrus | GM | Thickness | CIVET | Smaller | |
| Right postcentral gyrus | GM | Thickness | CIVET | Smaller | ||||||
| Left superior occipital gyrus | GM | Thickness | CIVET | Smaller | ||||||
| Left fusiform gyrus | GM | Thickness | CIVET | Smaller | ||||||
| Whole brain | GM | Thickness | CIVET | Smaller | ||||||
| Left middle frontal gyrus | WM | Volume | VBM (SPM) | Focal decrease | ||||||
| Right inferior occipital gyrus | WM | Volume | VBM (SPM) | Focal decrease | ||||||
| Allen, Emmorey, Bruss, and Damasio (2013) | 25 DS vs. 16 HS vs. 25 | 23.8 ± 4.1 | ASL | 1.5 T | Calcarine sulcus (VI) | GM | Volume | ROI manual | Larger in DS | Asymmetry in DS and HS |
| Pars Triangularis (Broca's) | GM | Volume | ROI manual | Larger in DS | ||||||
| Handknob (precentral gyrus) | GM | Volume | ROI manual | No difference | Asymmetry in DS and HS | |||||
| Boyen, Langers, de Kleine, and van Dijk (2013) | 16 HI vs. 24 | 63 ± 10 | Tinnitus stud | 3 T | STG, MTG | GM | Volume | VBM (SPM) | Increase | |
| SFG, occipital, hypothalamus | GM | Volume | VBM (SPM) | Decrease | ||||||
| Frontal | GM | Volume | ROI | Decrease | ||||||
| Limbic | GM | Volume | ROI | Increase | ||||||
| W. Li et al., 2013 | 16 DS vs. 16 | 14.56 ± 2.10 | CSL | 3 T | Cerebellum | GM | Volume | VBM (SPM) | Rightward asymmetrj | |
| Posterior cingulate gyrus | GM | Thickness | CIVET | Leftward asymmetry | ||||||
| Gyrus rectus | GM | Thickness | CIVET | Leftward asymmetry | Negatively correlated with HA | |||||
| Auditory cortex (HG) | GM | Thickness | CIVET | Asymmetry preserved | ||||||
| Penicaud et al. (2013). | 9 DS (birth) vs. 8 DS (early) vs. 6 DS (late) vs. 43 | 39.2 ± 12.3 | ASL | 1.5 T | Early visual areas | GM | Volume | VBM (SPM) | Later ASL: lower | Deaf not different from hearing |
| F. R. Lin et al. (2014) | 51 DS vs. 75 | 73.8 ± 7.3 | NR | 1.5 T | Right STG, right MTG, right ITG, right PHG | GM | Volume | RAVENS | Accelerated loss over time | |
| Hribar, Suput, Carvalho, Battelino, and Vovk (2014) | 14 DS vs. 14 | 35.4 ± 6 | SSL | 3 T | Left HG | WM | Volume | ROI manual | Less | |
| Middle medial left superior frontal gyrus | GM | Volume | FreeSurfer | Focal decrease | ||||||
| Left supramarginal gyrus | GM | Thickness | FreeSurfer | Focal decrease | ||||||
| Cerebellum | GM | Whole brain | VBM (FSL) | Increased volume | ||||||
| Olulade, Koo, LaSasso, and Eden (2014) | 15 DS vs. 15 HS vs. 15 DO | 23.4 ± 3.3 | ASL (DS/HS), oral/cued (DO) | 3 T | Early visual areas | GM | Volume | VBM (SPM) | Less in both DS and DO | |
| vs 15 | Left early auditory areas | GM | Volume | VBM (SPM) | Less in both DS and DO | |||||
| Left STG | WM | Volume | VBM (SPM) | Differences in DS | ||||||
| Left inferior frontal gyrus | WM | Volume | VBM (SPM) | Differences in DS | ||||||
| General | GM and WM | Volume | VBM (SPM) | DO less different than DS from controls | ||||||
| E. Kim et al. (2014) | 8 DS vs. 11 DO vs. 11 | 50.4 ± 6.1 | NR | 3 T | Primary auditory cortex | GM | Density | VBM (SPM) | No difference in DS | Decrease in DO |
| Primary auditory cortex | GM | Connectivity | Voxel-wise correlation | Correlated in DS | Increased bilateral temporal connectivity in DO | |||||
| Whole brain | GM | Connectivity | Brain connectivity toolbox | Connectivity measures increased in DS | Fronto-limbic and left temporal correlated and temporo-parietal weakly coupled in DS | |||||
| Tae (2015) | 8 DS vs. 9 | 15.6 | KSL | 1.5 T | Left anterior HG | GM | Volume | VBM | Decreased | |
| Left and right inferior colliculus | GM | Volume | VBM | Decreased | ||||||
| Lingual gyri, nucleus accumbens, thalamic reticular nucleus | GM | Volume | VBM | Decreased | ||||||
| Amaral et al. (2016) | 15 DS vs. 16 | 20.4 | CSL | 3 T | Thalamus | GM | Volume | Manual | Right > left | |
| Left geniculate nucleus | GM | Volume | Manual | Right > left | ||||||
| Inferior colliculus | GM | Volume | Manual | Right > left | ||||||
| Shiell, Champoux, and Zatorre (2016) | 11 DS vs. 11 | 28.2 | LSQ/ASL | 3 T | Right PT | GM | Thickness | FreeSurfer | Increased | |
| Smittenaar, MacSweeney, Sereno, and Schwarzkopf (2016) | 15 DS vs. 15 | 39 | BSL | 1.5 T | VI | GM | Thickness | FreeSurfer | Decreased | |
| Kumar and Mishra (2018) | 50 vs. 50 | 19.5 | Acquired ISL at age 10.8 years | 3 T | Bilateral STG, bilateral ITG, bilateral fusiform, bilateral MFG | WM/GM, GM, GM | Volume | VBM, SBM and PBCT | Decreased, increased, increased, increased | Increased thickness |
| Feng et al. (2018) | 37 vs. 40 | 17.9 mo | HA before CI | 3 T | Bilateral AC (middle part of STG) | GM/WM | Density | VBM and MVPS | Most significant, less significant—IFG, CG, occipital, hippocampus, precuneus | Regions unaffected by auditory deprivation provided good outcomes for CI |
| X. M. Xu et al. (2019a) | 35 vs. 54 | 56 | Postlingual | 3 T | Insula | GM | Density and connectivity | VBM | Insignificant differences | Reduced connectivity with other regions |
| X. M. Xu et al. (2019b) | 37 vs. 38 | 55.6 | Postlingual | 3 T | Thalamic subfield connectivity | GM | Relative volume | VBM | Decrease in right motor thalamus and somatosensory thalamus | |
| Pereira-Jorge et al. (2018) | 14 vs. 11 | 51.3 | Postlingual (before HA) | 1.5 T | PFC. precuneus. fusiform gyrus, and MTG; insula, supramarginal gyrus, medial temporal gyrus, occipital, posterior cingulate cortex, and claustrum | Thickness | FreeSurfer | Increase, decrease | After 1 year of HA, increase in multimodal integration regions | |
| Shiohama, McDavid, Levman, and Takahashi (2019) | 30 vs. 90 | 5.3–6.7 | NR | 3 T | Left middle occipital and left inferior occipital | GM | Thickness | CIVET | Smaller | |
| 35 vs. 23 | 39 ± 1.8 | NR | 3 T | GM | Volume | VBM (SPM) | Smaller | |||
| Qi, Su, Zou, Yang, and Zheng (2019) | Right fusiform and right middle occipital gyrus | |||||||||
| Ratnanather et al. (2019) | 5 DO vs. 5 | 31 | LSL and HA | 1.5 T | HG and PT | GM | Thickness | LCDM | Thicker | See also Dhir, Kutten, Li, Faria, and Ratnanather (2020) |
Note: Structures observed to be affected by hearing loss are shown in Figures 3 and 4. Gender information can be obtained from original papers and is not recorded here due to lack of correlation. Age is given as mean ± SD or mean.
Abbreviations: ACC, anterior cingulate cortex; ASL, American sign language; BSL, British sign language; CC, corpus callosum; CG, cingulate gyrus; CI, cochlear implant; CIVET, combined interoperability validation evaluation tool; CSL, Chinese sign language; D, deaf; DO, deaf oral; DS, deaf signing; DGS, German sign language; fMRI, functional MRI; FSL, FMRIB software library; GM, gray matter; HA, hearing aids; HG, Heschl's gyrus; HI, hearing impaired; HS, hearing signing; ISL, Indian sign language; ITG, inferior temporal gyrus; KSL, Korean sign language; LCDM, labeled cortical distance mapping; LSL, listening and spoken language; LSQ, langue signe quebecois; MFG, middle frontal gyrus; mo, month; MTG, middle temporal gyrus; MVPS, multivoxel pattern similarity; NR, not recorded; PBCT, projection-based cortical thickness; PFC, prefrontal cortex; PHG, parahippocampal gyrus; PT, planum temporale; RAVENS, regional analysis of volume examined in normal space; ROI, region of interest; SSL, Slovenian sign language; STG, superior temporal gyrus; SFG, superior frontal gyrus; SPM, statistical parametric mapping; TSL, Turkish sign language; TBM, tensor-based morphometry; VBM, voxel-based morphometry; WM, white matter.
FIGURE 3.
3D visualization of gray matter and white matter structures found to be different in people with hearing loss based on Table 1. Please refer to Figure 2 for the possible roles these structures play in the auditory pathway. Upper left shows the lateral view of the left side of the JHU-MNI-SS brain (Oishi et al., 2009); lower right shows the lateral view of the left medial structures adjacent to the mid-sagittal plane of the right hemi-brain. The cortical structures (Pars Triangularis, Pars Opercularis, Motor Cortex, Superior Temporal Gyrus, Planum Temporale, Visual Cortex and Cerebellum, Heschl's Gyrus, Insula, Fusiform Gyrus) and one white matter structure (corpus callosum) were obtained from the JHU-MNI-SS labels and triangulated. CAWorks (www.cis.jhu.edu/software/caworks) was used for visualization
Thus, MRI is potentially useful in providing quantitative differences in volumes of cortical regions that play an important role in speech, language and hearing networks. But none of these studies have provided a deeper understanding of the biological effects of hearing loss.
4 ∣. DTI ANALYSIS OF WHITE MATTER STRUCTURES IN PEOPLE WITH HEARING LOSS
DTI provides an opportunity to specifically examine white matter tissue in the brain at the resolution of 1 mm3. Here, white matter tissue is characterized by the orientation of neural connections between the cortical and subcortical gray matter structures. DTI is a variant of MRI based on the diffusion of water molecules in white matter structures and provides another noninvasive way of analyzing connections between brain structures. The 3 × 3 matrix representing the tensor model of water diffusion at each voxel in the DTI scan yields an ellipsoid representing the orientation of the neural fibers within the voxel from which eigenvalues are used to compute scalar quantities (Figure 1) such as fractional anisotropy, radial diffusivity, and mean diffusivity. These measures reflect the biophysical properties of the neurons passing through the voxel. For example, larger fractional anisotropy values indicate “dense axonal packing” (Feldman, Yeatman, Lee, Barde, & Gaman-Bean, 2010) while larger values of radial diffusivity indicate “axonal degeneration” and mean diffusivity is sensitive to “cellularity” (Tromp, 2016). The three corresponding eigenvectors are used to compute the color contrast map (Mori, Wakana, & Van Zijl, 2004).
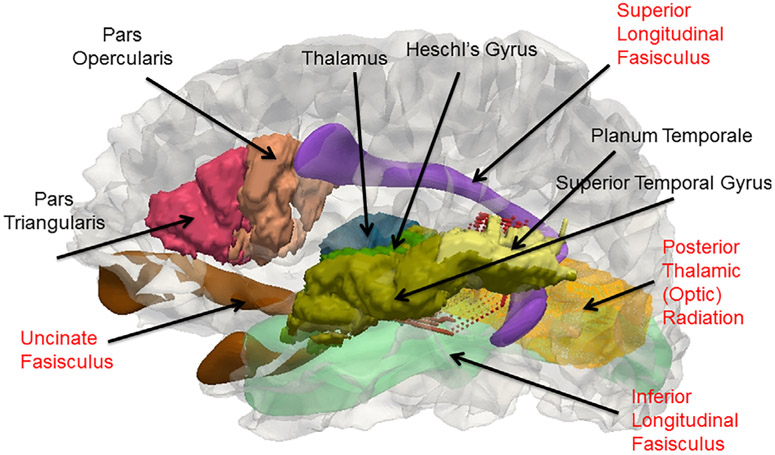
Table 2 indicates there have been 23 DTI studies comparing populations of people with and without hearing loss. Aside, Table 2 is similar to a summary table (Tarabichi et al., 2018). Figure 3 provides a visualization of the location of the white matter structures implicated in these studies. Again, a few observations can be made. First, with the exception of three studies all focused on homogeneous groups of hearing loss. One exceptional group consisted of adults who started using sign language in adolescence (Lyness et al., 2014), and two groups consisted of babies and young children prior to cochlear implantation (S. Wang et al., 2019; H. Wang et al., 2019). Second, there is again a wide variation in larger sample sizes from large population centers. Third, the analyses are mostly confirmatory in that differences in scalar measures, that is, fractional anisotropy (and sometimes radial diffusivity, mean diffusivity, and axial diffusivity) are seen in the temporal and occipital regions such as the acoustic radiation (or auditory tract), the optic radiation, superior temporal gyrus, corpus callosum, and with one exception (Cheng et al., 2019) the longitudinal fasisculi which connect the auditory and language cortical regions. Fourth, the two studies that focused on white matter connectivity were the ones at the scanner strength of 1.5 T, and one study not surprisingly revealed correlations in thalamo-cortical connections with temporal, parietal, motor, somatosensory, frontal and occipital lobes (Lyness et al., 2014).
TABLE 2.
Summary of diffusion tensor imaging studies of white matter structures in people with hearing loss
| Studies | Group | Age | Communication | Scanner strength |
Structure | Tissue | Measure | Analysis | Main result | Other results |
|---|---|---|---|---|---|---|---|---|---|---|
| Chang et al. (2004) | 10 D vs. 10 | 33.7 | NR but mild-to-severe HL | 3 T | SON, IC, TB, LL. AR | FA | Single brain analysis | Voxel-Wise | Abnormal | See also S. H. Lee, 2004 |
| Nath et al. (2007) | 14 D vs. 8 | 30 | NR | 1.5 T | LL | FA | Whole brain | ROI | Less | |
| IC | FA | Whole brain | ROI | Less | ||||||
| PCT | FA | Whole brain | ROI | Less | ||||||
| Y. Lin et al. (2008) | 37 D vs. 10 | 32.4 ± 11.9 | NR | 3 T | LL | FA and RD | Whole brain | DTIStudio | Less FA and more RD | |
| IC | FA and RD | Whole brain | DTIStudio | Less FA and more RD | ||||||
| Xia and Qi (2008) | 20 DS vs. 20 | 9–12 | CSL | 1.5 T | Right HG | ADC | Whole brain | ROI manual | Different | |
| 20 DS vs. 20 | 19–22 | CSL | 1.5 T | Bilateral HG | ADC | Whole brain | ROI manual | Different | ||
| S. Wang et al. (2009) | 6 CD vs. 6 | 20.8 | CSL (3 after HA usage at 4 years) | 3 T | Right STG | FA | Whole brain | Voxel-based analysis | Less | Asymmetry preserved |
| D. J. Kim et al. (2009) | 13 D vs. 29 | 29.3 ± 6.8 | No HA | 3 T | Superior temporal | FA | Whole brain | TBSS | Less | |
| Right internal capsule | FA | Whole brain | TBSS | Less | ||||||
| SLF | FA | Whole brain | TBSS | Less | ||||||
| Left inferior FOF | FA | Whole brain | TBSS | Less | ||||||
| Right forceps major | FA | Whole brain | TBSS | More | ||||||
| Left forceps major | FA | Whole brain | TBSS | More | ||||||
| Left STG | Volume | Whole brain | TBSS | Less WM | ||||||
| Right STG | Volume | Whole brain | TBSS | Less WM | ||||||
| Left parietal | Volume | Whole brain | TBSS | Less | ||||||
| Superior frontal | Volume | Whole brain | TBSS | Less | ||||||
| Medial frontal | Volume | Whole brain | TBSS | Less | ||||||
| Z.-H. Liu et al. (2010) | 15 D vs. 28 | 16 | CSL for more than 6 years and HA usage since 2 years old | 1.5 T | Left STG | FA | Whole brain | TBSS | Less | |
| Right STG | FA | Whole brain | TBSS | Less | ||||||
| Left IFG | FA | Whole brain | TBSS | Less | ||||||
| Right IFG | FA | Whole brain | TBSS | Less | ||||||
| Left post-medial MTG | FA | Whole brain | TBSS | Less | Reduction less in L than R | |||||
| Right post-medial MTG | FA | Whole brain | TBSS | Less | ||||||
| Right Int Cap | FA | Whole brain | TBSS | Less | ||||||
| Left Int Cap | FA | Whole brain | TBSS | Less | ||||||
| Right Ext Cap | FA | Whole brain | TBSS | Less | ||||||
| Left Ext Cap | FA | Whole brain | TBSS | Less | ||||||
| Left OR | FA | Whole brain | TBSS | Less | Reduction less in L than R | |||||
| Right OR | FA | Whole brain | TBSS | Less | ||||||
| Y. Li et al. (2012) | 60 CD vs. 36 AD vs. 38 | 21.1 ± 2.26 (CD), 21.5 ± 1.54 (AD) | CSL only | 3 T | Right STG | FA | Whole brain | TBSS | Less | Correlated with onset age |
| Left STG | FA | Whole brain | TBSS | Less | ||||||
| SCC | FA | Whole brain | TBSS | Less | ||||||
| Chang, Lee, Paik, Lee, and Lee (2012) | 18 D | 5.9 | NR | 3 T | Broca's | FA | Whole brain | Voxel-wise | Higher | Correlated with auditory scores after CI |
| Genu of CC | FA | Whole brain | Voxel-wise | Higher | Correlated with auditory scores after CI | |||||
| AR | FA | Whole brain | Voxel-wise | Higher | Correlated with auditory scores after CI | |||||
| MGN | FA | Whole brain | Voxel-wise | Correlated with auditory scores after CI | ||||||
| Miao et al. (2013) | 16 D vs. 16 | 14.56 ± 2.1 | CSL after late HA usage | 3 T | Left STG | FA and RD | Whole brain | TBSS | FA lower and RD higher | |
| Right STG | FA and RD | Whole brain | TBSS | FA lower and RD higher | RD correlated with CSL usage | |||||
| HG | FA and RD | Whole brain | TBSS | FA lower and RD higher | ||||||
| PP | FA and RD | Whole brain | TBSS | FA lower and RD higher | ||||||
| SCC | FA and RD | Whole brain | TBSS | FA lower and RD higher | ||||||
| Lyness, Alvarez, Sereno, and MacSweeney (2014) | 13 DS vs. 13 | 39.08 ± 11.08 | BSL after 10 years (11 used spoken language) | 1.5 T | Frontal and occipital | MD and RD | Thalamic Parcellation | FreeSurfer and FSL | Increased MD and RD | |
| Frontal | FA | Thalamo-cortical | FreeSurfer and FSL | Reduced FA | ||||||
| Motor | FA, MD and RD | Thalamo-cortical | FreeSurfer and FSL | Increased MD and RD | Reduced FA | |||||
| Somatosensory | FA and RD | Thalamo-cortical | FreeSurfer and FSL | Increased RD | Reduced FA | |||||
| Parietal | FA | Thalamo-cortical | FreeSurfer and FSL | Reduced FA | ||||||
| Occipital | FA | Thalamo-cortical | FreeSurfer and FSL | Reduced FA | ||||||
| C. X. Wu et al. (2014) | 92 (three groups) | 4.9 | 31 had CI | 1.5 T | STG | FA | Whole brain | DTIStudio | Less | |
| AR | FA | Whole brain | DTIStudio | Less | Correlated with CAP score in the 31 with CI | |||||
| Karns, Stevens, Dow, Schorr, and Neville (2017) | 23 DS vs. 26 | 28 ± 1.4 | ASL | 3 T | Bilateral HG, anterior STG, posterior STG | FA and volume | Whole brain | FSL | Less | More RD in bilateral HG; right posterior STG had larger differences between groups |
| Posterior CC | FA | Whole brain | FSL | Less | More RD | |||||
| J. Kim, Choi, Eo, and Park (2017) | 18 (10 postlingual) vs. 29 | KSL and HA | 3 T | Right Int Cap, right thalamus, SCC, right STG and left STG, right temporal lobe WM | Diffusion anisotropy | Whole brain | TBSS | Less | Prelingual less in R STG, bilateral WM, genu and anterior CC | |
| Maffei (2017) | 10 DS vs. 10 | 34 ± 6 | LIS | 4 T | Acoustic radiation | FA, RD and MD | Whole brain | FSL | Less, more | Atlas-based analysis, subset of Benetti et al. (2018) |
| Benetti et al. (2018) | 14 DS vs. 15 HS vs. 15 | 34 ± 6 | LIS | 4 T | Occipito-temporal, fusiform-temporal | FA and RD | Connectivity | FSL | Less FA and more RD | |
| Zou et al. (2018) | 80 vs. 78 | 41.7 | CSL for 10+ years | 3 T | Bilateral STG | GM and WM | Diffusion kurtosis | VBM (SPM) | Decreased | “Hypomyelination” of WM |
| Qi et al. (2019) | 35 vs. 23 | 39 ± 1.8 | NR | 3 T | Multiple WM structures | GM | FA | TBSS | Decreased | RD increased |
| M. Jiang et al. (2019) | 23 vs. 18 | 7.21 ± 2.67 | No HA usage | 3 T | Left ATR, right CST, CC | AD | Whole brain | TBSS | Increased | RD increased in several WM tracts |
| H. Wang et al. (2019) | 52 vs. 19 | 0.9–6 | Prior to CI | 3 T | Bilateral IC | FA | ROI | FSL, PickAtlas, SPM | Lower | Correlated with postop CAP scores |
| S. Wang et al. (2019) | 46 vs. 33 | 17.59 mo | Prior to CI | 3 T | Bilateral SLF, IFOF, ILF; right CST, PTR; left UF | FA | Whole brain | TBSS | Lower | Increased resting state functional connectivity between bilateral auditory cortices and right insula and STG |
| Cheng, Roth, Halgren, and Mayberry (2019) | 12 vs. 12 | 33.33 | ASL | 1.5 T | IFOF, AF, ILF, UF | FA | ROI | DTIStdio | No difference | |
| Dhir et al. (2020) | 5 DO vs. 10 | 31 ± 11 | LSL and HA | 1.5 T | AR | FA | Whole brain | MRICloud and DTIStudio | Less |
Note: Structures observed to be affected by hearing loss are shown in Figures 3 and 4. Gender information can be obtained from original papers and is not recorded here due to lack of correlation. Age is given as mean ± SD or mean.
Abbreviations: AD, acquired deaf; ADC, apparent diffusivity coefficient; AR/OR, acoustic/optic radiation; AF, arcuate fasciculus; ASL, American sign language; ATR, anterior thalamic radiation; BSL, British sign language; CAP, categories of auditory performance; CC, corpus callosum; CD, congenitally deaf; CI, cochlear implant; CSL, Chinese sign language; CST, corticospinal tract; D, deaf; DS, deaf signing; FA, fractional anisotropy; FOF, fronto-occiptal fasciculus; FOT, fronto-occipital tract; FSL, FMRIB software library; GM, gray matter; HA, hearing aid; HG, Heschl's gyrus; HL, hearing loss; HS, hearing signing; IC, inferior colliculus; IFG, inferior frontal gyrus; IFOF, inferior fronto-occiptal fasciculus; ILF, inferior longitudinal fasciculus; Int/Ext Cap, internal/external capsule; LL, lateral leminscus; LSL, listening and spoken language; LIS, lingua italiana dei segni; MD, mean diffusivity; MGN, medial geniculate nucleus; MTG, middle temporal gyrus; NR, not recorded; PCT, pontine crossing tract; PP, planum polare; PTR, posterior thalamic radiation; RD, radial diffusivity; ROI, region of interest; SCC, splenium of CC; SOF, superior occipial fasciculus; SLF, superior longitudinal fasciculus; SON, superior oliviary nucleus; SPM, statistical parametric mapping; STG, superior temporal gyrus; TB, trapezoid body; TBSS, tract-based spatial statistics; UF, uncinate fasciculus; WM, white matter.
Thus, DTI can be potentially useful in providing quantitative differences in connections between cortical and subcortical regions affected by hearing loss. Also combining MRI and DTI could be one way to uncover how people with hearing loss perform audio-visual integration tasks such as lipreading. To address this one would need to examine whether the long range white matter optic radiation tract connecting the occipital lobe and thalamus overlaps with the short range white matter tracts connecting the Heschl's gyrus and planum temporale (Figure 4).
FIGURE 4.
3D visualization of connectivity between cortical and subcortical structures found to be different in people with hearing loss based on Tables 2 and 3. Please refer to Figure 2 for the possible roles these structures play in the auditory pathway. The lateral view of the left side of the gray/white surface of the JHU-MNI-SS template (Oishi et al., 2009) generated by FreeSurfer and transferred to native space (Fischl, 2012) is shown. The cortical structures (Pars Triangularis, Pars Opercularis, Superior Temporal Gyrus, Planum Temporale, Heschl's Gyrus), one subcortical structure (Thalamus), and the white matter Posterior Thalamic Radiation tract which contains the optic radiation were obtained from the JHU-MNI-SS labels and triangulated. The other white matter fasisculi structures were obtained from the IXI template (Yushkevich, Zhang, Simon, & Gee, 2008) and transferred via diffeomorphic mapping (Ceritoglu et al., 2009) of the IXI fractional anisotropy image to the corresponding JHU-MNI-SS image. Short range fiber tracts from the Heschl's Gyrus to Planum Temporale generated by dynamic programming (M. Li, Ratnanather, Miller, & Mori, 2014; Ratnanather et al., 2013) are partially hidden. CAWorks (www.cis.jhu.edu/software/caworks) was used for visualization
5 ∣. CLINICAL MRI AND DTI SCANS OF PEOPLE WITH HEARING LOSS
The advent of cochlear implants has dramatically changed the landscape of auditory habilitation and rehabilitation for more than 350,000 children and adults with hearing loss worldwide (Zeng & Canlon, 2015). This achievement was recognized by the 2013 Lasker-DeBakey Clinical Research Award (Hampton, 2013; Holmes, 2013; Niparko, 2013; Roland & Tobey, 2013; Williams, 2013), the 2015 National Academy of Engineering Russ Prize (Clark, 2014; Hochmair, Hochmair, Nopp, Waller, & Jolly, 2014; Merzenich, 2015; Wilson, 2014), and the 2018 Shambough Prize for the developers of the multichannel cochlear implant.
Prior to surgery, patients have computer tomography or MRI scans of the temporal bones encasing the cochleae (Schwartz & Chen, 2014; Sweeney et al., 2014; Teschner, Polite, Lenarz, & Lustig, 2013; Young, Ryan, & Young, 2014). With respect to whole brain scans, Table 3 lists 15 reports of structural MRI and DTI studies. These studies are prospective and thus the sample sizes are larger than those reported in research studies. Not surprisingly many studies involved pediatric subjects if only to exclude the possibility of abnormalities in the central nervous system. In general, the observed white matter changes are linked with immature myelination possibly due to abnormal neuronal processes that occur in the developing embryo (Long, Wan, Roberts, & Corfas, 2018). But two studies correlated structural differences mainly in the connections from thalamus to the frontal and temporal cortical lobes with positive outcomes. Due to the risk of device displacement, MRI and DTI are contraindicated for people with cochlear implants. So it is imperative that quantitative analysis such as connectivity and topography be considered at baseline in future studies if reasonable imaging biomarkers for predicting positive outcomes with cochlear implants are to be developed.
TABLE 3.
Preclinical MRI and DTI whole brain imaging studies
| Studies | Size | Group | Age | Modality | Results |
|---|---|---|---|---|---|
| Lapointe, Viamonte, Morriss, and Manolidis (2006) | 40 | SNHL | Pediatric | T1 and T2 | Some changes in T2 but eight had abnormalities from myelination delays to migrational anomalies |
| Trimble, Blaser, James, and Papsin (2007) | 92 | Preop CI | Pediatric | FLAIR | 32% abnormalities in TB; some subcortical signal intensities discrepancies |
| Roche et al. (2010) | 118 | ANSD | Pediatric | MR | 40% had brain abnormalities; 28% had CN deficiencies |
| CT | 16% had cochlear dysplasia | ||||
| Hong, Jurkowski, and Carvalho (2010) | 57 | Preop CI | Pediatric | MR | 18% with white matter abnormalities (two had postop delays in performance), no serious CNS diseases |
| Chilosi et al. (2010) | 80 | SNHL | Pediatric | MR | 48% with additional disabilities (cognitive, behavioral–emotional and motor). 37 signal abnormalities—brain malformations (46%) and white matter abnormalities (54%) |
| Chang et al. (2012) | 18 | Preop CI | Pediatric | DT | FA in Broca's, genu CC, auditory tract and MGN correlated with auditory scores after CI |
| Mackeith, Joy, Robinson, and Hajioff (2012) | 158 | Preop CI | Pediatric and adults | MR | Detected abnormalities (n = 27.9%, missed by CT in 6.3%) of which only 12.7% considered significant |
| CT | 6.3% only noncritical abnormalities in CT | ||||
| Moon et al. (2012) | 177 | Preop CI | Pediatric | MR | Children with no lesions (n = 150) performed better than those with lesions |
| Proctor, Gawne-Cain, Eyles, Mitchell, and Batty (2013) | 51 | Preop CI | Pediatric and adults | MR | Five adults and 16 children—whole brain abnormalities; 36 had at least one CI. Of 15 who did not have CI, eight positive findings in whole brain MRI |
| Jonas et al. (2012) | 162 | Preop CI | Pediatric | MR | 30% had abnormalities mostly white matter changes related to pre-existing medical conditions |
| Z. Y. Jiang, Odiase, Isaacson, Roland, and Kutz (2014) | 188 | Preop CI | Adult | MR | 9% had cochlear pathway and white matter abnormalities; in others, 65% had normal MRI scans |
| X.-Q. Xu, Wu, Hu, Su, and Shen (2015) | 157 | Preop CI | Pediatric | MR and CT | White matter changes most common but effect on CI minimal |
| Huang et al. (2015) | 24 | Preop CI | Pediatric | DT | Lesser FA in TB, SON, IC, MGB, AR and WMHG in 16 with CAP <6 |
| Park, Chung, Kwon, and Lee (2018) | 1 | Preop CI | Pediatric | DT | Less FA in WMHG, IFOF, UF, SLF and forceps major only in age <4 years |
| Feng et al. (2018) | 37 | Preop CI | Pediatric | MR | Auditory association and cognitive brain regions which are unaffected by auditory deprivation provide positive outcomes |
Abbreviations: ANSD, auditory neuropathy spectrum disorder; AR, acoustic radiation; CAP, category of auditory performance; CC, corpus callosum; CI, cochlear implant; CN, cochlear nerve; CNS, central nervous system; CT, computed tomography; DT, diffusion tensor; FA, fractional anisotropy; FLAIR, fluid-attenuated inversion recovery; IC, inferior colliculus; IFOF, inferior fronto-occiptal fasciculus; MGB, medial geniculate body; MGN, medial geniculate nucleus; MR, magnetic resonance; Preop, pre-operative; SLF, superior longitudinal fasciculus; SNHL, sensorineural hearing loss; SON, superior oliviary nucleus; T1/T2, MR-weighted image; TB, temporal bone; UF, uncinate fasciculus; WMHG, white matter Heschl's gyrus.
6 ∣. MORPHOMETRY AND CONNECTIVITY OF STRUCTURES AFFECTED BY HEARING LOSS
A concern about MRI and DTI studies so far is the focus on volume. Volumes for closed structures such as subcortical ones can be interpreted. But that may be not the case for the cortical regions forming the highly folded ribbon that constitutes the cortex (Figure 1). This raises three points. First, the above studies were based on whole brain analyses which revealed inconclusive information about the effect of hearing loss on brain structures. In contrast, a region of interest approach based on networks of hypothesized structures maybe more meaningful and sensitive (e.g., Giuliani, Calhoun, Pearlson, Francis, & Buchanan, 2005) for generating biomarkers for positive outcomes for clinical procedures such as auditory training. This is where information from functional neuroimaging studies of language, speech and hearing may be helpful in focusing on structures hypothesized to be affected by hearing loss (see Section 8). Second, volume should be viewed as the product of two independent measures—surface area and thickness—to reflect the laminar structure of a cortical region (Dahnke & Gaser, 2018; Wagstyl & Lerch, 2018). This structure is brought about by the folding of the cortex to maximize cortical surface area in a confined space. Each cortical region is composed of fundamental units called cortical columns (Rakic, 1995, 1988) that traverse from the white matter to just beneath the skull. Also, each cortical region is composed of six layers which are stacked on top of each other such that thin layers in one part of the region are thicker in another part via the equivolumetric model of the cortex (Bok, 1929, 1959). Thus surface area and thickness may be associated with the distribution or density of cortical columns and the total thickness of the six layers, respectively. So, decreased or increased cortical volume may be misleading. In fact, decomposing volume into surface area and thickness was suggested for the primary auditory cortex in people with normal hearing by Meyer, Liem, Hirsiger, Jancke, and Hanggi (2014) who concluded that thickness and surface area should be quantified as separate measures. Also, given the possible effect of genetics on hearing loss (Dror & Avraham, 2009; R. J. H. Smith, Shearer, Hildebrand, & Van Camp, 1993) specifically in the development of cortical columns, it may be best to analyze thickness and surface area separately (Panizzon et al., 2009; Winkler et al., 2010). A more recent study suggested that cortical thickness may be an useful biomarker for identifying shape and location of the primary auditory cortex (Zoellner et al., 2019). In addition, more realistic measures of cortical thickness can be developed via sophisticated equivolumetric models for the cortex (Ratnanather et al., 2019; Younes, Kutten, & Ratnanather, in press). Third, in particular for subcortical structures which have not been examined in great detail, it may be helpful to perform shape analysis (Faria et al., 2015; Miller et al., 2013, 2014; Mori et al., 2013; Ratnanather, Liu, & Miller, 2020) given the role the thalamus plays in the auditory pathway (Figure 2). Here, shape biomarkers are determined from computations of deformations of the structure relative to a template. This allows for determining atrophied subregions of the structure. Such data could be useful in determining specific pathways of degeneration between structures.
Furthermore, thickness may be correlated to brain activity in auditory cortical areas. First, acoustic fMRI studies have shown increased neural activity in the primary auditory cortex (Patel et al., 2007; Tan et al., 2013). Second, given the importance of CAEP biomarkers in assessing neural activity with amplification via hearing aids or cochlear implants (J. D. Campbell, Cardon, & Sharma, 2011; Sharma, Dorman, & Spahr, 2002), Liem, Zaehle, Burkhard, Jancke, and Meyer (2012) showed that the first negative amplitude (N1) of CAEP waveform responses strongly correlated with cortical thickness of the superior temporal gyrus which encompasses both the Heschl's gyrus and planum temporale (see Figure 1). Third, following PET studies gray matter density (Duncan, Gravel, Wiebking, Reader, & Northoff, 2013) and thickness (la Fougere et al., 2011) were found to correlate with gamma-aminobutyric acid (GABA) binding within cortical regions. As GABA is the primary inhibitory neurotransmitter in the brain and plays a crucial role in regulating neuronal activity, different rates of neural activity from the thalamus to the auditory cortex may be attributed to differences in GABA density distribution (Takesian et al., 2013) and possibly thickness.
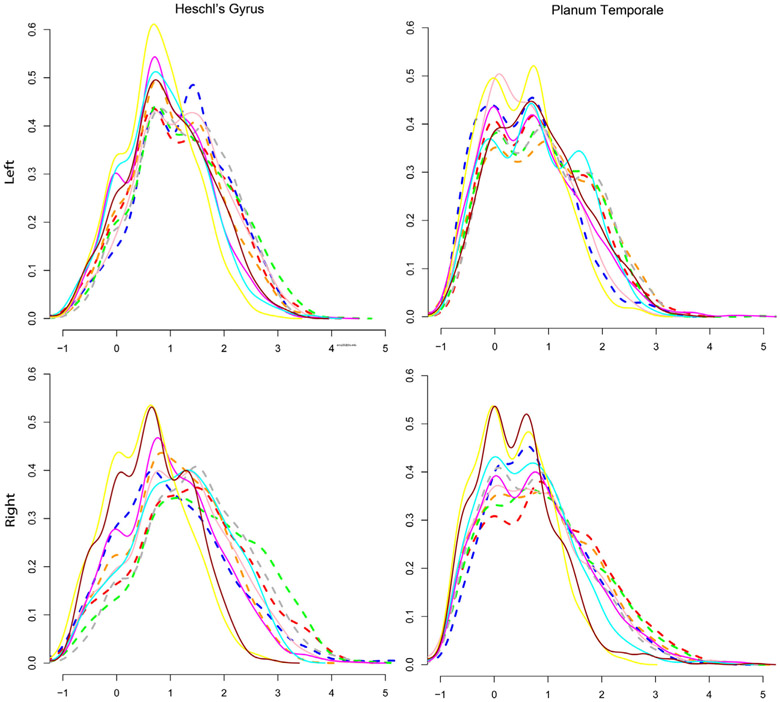
To illustrate the possible benefits of analyzing cortical thickness, consider the Labeled Cortical Distance Mapping (LCDM) technique (Miller et al., 2003; Miller, Massie, Ratnanather, Botteron, & Csernansky, 2000; Ratnanather et al., 2013, 2014). LCDM generates histograms of distances of gray matter voxels relative to the gray/white surface of the cortical region. In turn this gives rise to laminar thickness derived as the 95th percentile and the corresponding volume (as the area under the histogram). The shape of an individual LCDM for a cortical region is influenced by the folding of the region. A flat region yields a “top-hat” LCDM while a folded region with variable thickness yields a “skewed” LCDM; similar profiles have been observed for whole brains (Hutton, De Vita, Ashburner, Deichmann, & Turner, 2008). Together with the corresponding surface areas, LCDMs can be analyzed in different ways via statistical tools (Ceyhan et al., 2011, 2013). Figure 5 shows individual LCDMs for the left and right Heschl's gyrus and planum temporale in a pilot study of five adults with hearing loss and matched controls (Ratnanather et al., 2019). This study was challenging because more subjects could not be recruited having acquired a cochlear implant by the time they were contacted. Nonetheless, the statistical power of pooled (grouped) analysis (Ceyhan et al., 2011) can provide useful information with significant p-values from one-sided Kolmogorov–Smirnov tests (⪡.0001) for the pooled LCDM for the adults with hearing loss to be the left of that for the control subjects. As discussed in the next section, this suggests that in these auditory cortical areas there may be some similarities at smaller distances but differences at larger distances which have interesting interpretations at the microscopic level.
FIGURE 5.
Labeled cortical distance map (LCDM) histograms are normalized frequencies of distances of gray matter 1 mm3 voxels relative to gray/white cortical surfaces. Shown are individual LCDMs for the Heschl's gyrus and planum temporale in five adults with hearing loss (dashed) and five matched controls (solid lines). Horizontal and vertical scales are from −1 to 5 mm and 0.0 to 0.6, respectively. p-Values from one-sided Kolmogorov–Smirnov tests for the pooled cummulative distribution function (cdf) for the control subjects to be left of the pooled cdf for the subjects with hearing loss were significant for all four structures (⪡.0001). For pooled LCDMs, see Ratnanather et al. (2019)
It is worth noting that after using hearing aids since infancy, four of the five subjects with hearing loss now have cochlear implants with excellent speech comprehension in quiet situations. This suggests the structural benefit of providing auditory stimulus to the brain via hearing aids as soon as hearing loss is diagnosed. The difference in the shape of the LCDMs may reflect the delayed maturation of synaptic activity (Huttenlocher & Dabholkar, 1997) followed by synaptic pruning (Selemon, 2013) in the developing brain. The pooled distributions suggest little differences in the left Heschl's gyrus which is associated with temporal processing (Marie, Maingault, Crivello, Mazoyer, & Tzourio-Mazoyer, 2016) and some differences on the right Heschl's gyrus which is associated with spectral processing (Marie et al., 2016). The former may be attributed to auditory training used in listening and spoken language after early detection and intervention with hearing aids as infants while the latter may be attributed to high frequency hearing loss. By comparison, thicker visual cortical areas have been observed in people blinded since infancy (J. Jiang et al., 2009).
As for DTI, two studies combined with functional studies of hearing loss have yielded interesting correlations. First, brain waveform activity correlated with increases in fractional anisotropy measures of the brainstem specifically the inferior colliculus (Reiman et al., 2009). Second, aerobic exercising by children with hearing loss resulted in improved executive function associated with reshaping white matter integrity in several structures (Xiong et al., 2018). DTI also offers the potential to visualize the topography of structures such as the acoustic radiation and optic radiation (Figure 4) as well as other long range white matter tracts that play different but important roles in processing of speech and language (Friederici, 2012). Recently, Dhir et al. (2020) showed that it was possible to generate the acoustic radiation in clinical scans as opposed to research scans which require long scan times. For the same deaf adults studied in Figure 5, they confirmed the findings of Maffei (2017) who suggested that lower fractional anisotropy values may be associated with poor myelination in the acoustic radiation which may account for weaker neural transmission.
7 ∣. INTERPRETING STRUCTURAL MRI AND DTI CHANGES CAUSED BY HEARING LOSS
It would appear from MRI and DTI studies so far that subtle structural changes occur in the Heschl's gyrus and planum temporale. These two structures are the primary and secondary auditory cortical regions, respectively. Granted that other structures particularly association cortical regions in the speech and language network are also affected, an interpretation of these macroscopic changes with respect to the microscopic observations from animal models of hearing loss is now given.
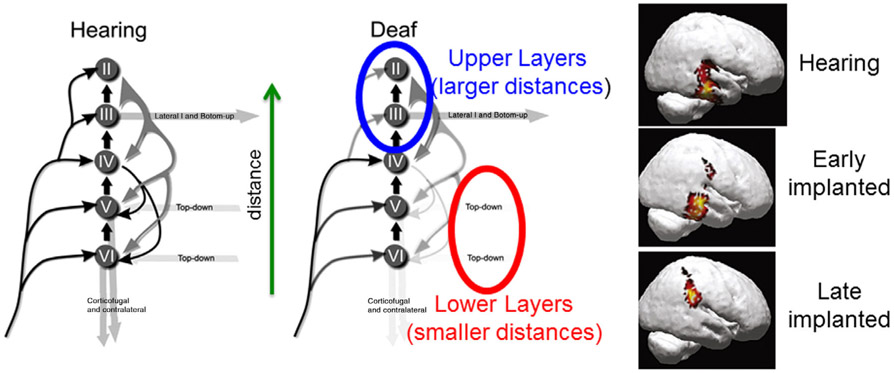
One of the most advanced and well developed animal model of cortical activity stemming from cochlear implants has been the cat (Kral, 2013; Raggio & Schreiner, 1994, 1999, 2003; Ryugo & Menotti-Raymond, 2012; Schreiner & Raggio, 1996). Specifically, electrophysiological measurements across the six layers of the primary and secondary auditory cortices have revealed the effect of the absence of neural activity in the sensitive period of development (Eggermont & Ponton, 2003; Kral, 2013; Kral & Tillein, 2006; Kral & Eggermont, 2007; Kral, Tillein, Heid, Hartmann, & Klinke, 2005; Kral, Tillein, Heid, Klinke, & Hartmann, 2006). Specifically, there is a delay in the synaptic activation of the upper (supragranular) layers and virtual absence of activity in the lower/deep (infragranular) layers. The absence of activity in the lower/deep layers may be attributed to incomplete development and alteration of information flow to and within the primary auditory cortex. While neurons project from the upper layers of the primary area to the secondary areas, some project back to lower/deep layers of the primary area. Thus the absence of activity in the lower/deep layers suggests that the primary auditory area is decoupled from the secondary area, and the feedback loop is weakened. In this decoupling hypothesis (Kral et al., 2005) illustrated in Figure 6, the secondary area is no longer able to provide “top-down” cognitive processing which is helpful for comprehension of spoken language (Kral & Eggermont, 2007). At the same time, the upper layers are unable to perform “bottom-up” processing which is helpful for discerning phonemes that are the basic elements of spoken language.
FIGURE 6.
Interpretation of Kral's decoupling hypothesis (Adapted from Kral and Eggermont (2007, fig. 3). Copyright 2007 Elsevier) based on LCDM analysis in Figure 5. Similarities at smaller distances, that is, lower cortical layers may facilitate top-down processing, that is, contextual or linguistic comprehension. This may be due to priming of the auditory pathway in childhood via amplification with hearing aids albeit at a lower rate than with cochlear implants. However, this might be compromised by larger differences at larger distances, that is, upper cortical layers which may be attributed to weaker thalamic inputs and make bottom-down processing, that is, comprehension of phonemes comprehension difficult and complex. In turn, the inputs to the lower layers and thence the other cortical areas are weakened. Additional evidence of weaker thalamic connections may manifest in those to other cortical areas such as the parietal cortex as might be in the case in the visualization of current density reconstruction in late implanted children (lower right panel from fig. 3 in Gilley, Sharma, & Dorman (2008). Copyright 2008, Elsevier). This suggests that hearing loss results in two-speed thalamic inputs (Takesian et al., 2013). One conjectures that amplification provided by hearing aids is weaker than that provided by cochlear implants and further that the thalamo-parietal pathway cannot tolerate the high activity levels stemming almost immediately after activation of the cochlear implant, thus forcing the neural activity to traverse along the acoustic radiation to the Heschl's gyrus (top and middle right panels from fig. 2 in Gilley et al. (2008))
The LCDMs of adults with hearing loss (Figure 5) who had been using listening and spoken language via hearing aids since infancy suggest that the decoupling mechanism can be averted with consistent use of amplification. Indeed, the similarities (with small variance) at smaller LCDM distances corresponding to the lower/deep layers suggest that sufficiently aided adults with hearing loss can develop linguistic understanding and the larger differences (with larger variances) at LCDM distances corresponding to the upper layers suggest that these adults may be able to understand speech only in quiet. Thus the shapes of LCDMs may reveal a little more information about the upper and lower layers than just the overall laminar thickness that is computed from the distance between the gray/white and gray/inner surfaces. But for morphometry of the layers one may need to consider equivolumetric models of cortical folding (Ratnanather et al., 2019; Younes et al., in press).
However, the weaker input from the thalamus to the auditory cortex may manifest in diverted inputs to other cortical areas such as the parietal (motor) cortex as observed in 3D reconstruction of CAEP activity in late implanted children (lower right panel in Figure 6 [fig. 3 of Gilley et al., 2008]). This suggests that hearing loss results in two-speed thalamic transmission (Takesian et al., 2013). One conjectures that the demyelinated thalamo-parietal pathway cannot tolerate the high activity levels stemming almost immediately after activation of the cochlear implant, thus enforcing the neural transmission along the acoustic radiation to the Heschl's gyrus (top and middle right panels in Figure 6 [fig. 2 of Gilley et al., 2008]).
However, more substantial quantitative analysis of morphometry and connectivity is needed to provide a more complete model of the structure and functional relationship between cortical and subcortical structures in the deafened brain.
8 ∣. COMBINING STRUCTURAL AND FUNCTIONAL IMAGING FOR LONGITUDINAL TRACKING OF AMPLIFICATION
It is constructive to see how recent functional neuroimaging technologies could be combined with structural imaging to shed light on the benefits of amplification on the deafened brain. The importance of functional changes accruing from amplification cannot be understated (J. D. Campbell et al., 2011; Cardin et al., 2013; Shiell, Champoux, & Zatorre, 2015). So changes in the brain due to amplification should correlate morphometric and connectivity measures with data derived from CAEP (Gilley et al., 2008; Liem et al., 2012), PET (Barone, Lacassagne, & Kral, 2013; Lazard, Lee, Truy, & Giraud, 2013; Liem, Hurschler, Jancke, & Meyer, 2014; Strelnikov et al., 2014), fMRI (Patel et al., 2007; Tan et al., 2013), and fNIRS (Lawler, Wiggins, Dewey, & Hartley, 2015; Sevy et al., 2010).
In particular, functional neuroimaging should be combined with structural neuroimaging in longitudinal studies of amplification or auditory training (Boothroyd, 2010). This could be achieved via MRI (Teschner et al., 2013) as well as DTI and resting state fMRI (Z. Li et al., 2015; B. Liu et al., 2015; Zhang et al., 2015) followed by one of PET, CAEP, or fNIRS. In the case of cochlear implants, MRI, DTI and fMRI can only be done at baseline prior to surgery. A possible translational study would be to find regional or subregional biomarkers in the superior temporal gyrus that correlate with phonetic processing of speech with amplification (Boatman, 2004; Crinion, Lambon-Ralph, Warburton, Howard, & Wise, 2003; Mesgarani, David, Fritz, & Shamma, 2014; Nourski & Howard 3rd, 2015).
The notion of a “sensitive” period in sensory neurodevelopment (Knudsen, 2004) alluded to in the previous section is supported by CAEPs which are noninvasive electroencephalography measurements that track the maturation of the central auditory system via changes in latency and amplitude (Steinschneider, Nourski, & Fishman, 2013). The first positive peak (P1), which is a summation of synaptic activities and neuronal conduction times as the signal travels from the ear to the primary auditory cortex, decreases with age in children with normal hearing (Eggermont & Ponton, 2003). Prompted by the seminal work by Ponton et al. (1996), Sharma et al. (2002) performed what is now a landmark study of children with cochlear implants. They observed that children with shortest period of deprivation (i.e., absence of auditory stimuli) of 3.5 years or less had P1 latencies fall into the normal range about 6 months after implantation while those with deprivation periods of 7 years or more had abnormal CAEPs. Similar observations were seen in children who used hearing aids consistently even before getting a CI (J. D. Campbell et al., 2011). Also, the first negative peak (N1) which manifests itself post-adolescence in normal hearing also occurs in people who had been using amplification since infancy (Sharma, Campbell, & Cardon, 2015).
Evidence suggests that P1 and N1 latencies reflect neural generators from thalamo-cortical projections to the primary auditory cortex in the Heschl's gyrus and the secondary auditory cortex in the planum temporale, respectively (Liegeois-Chauvel, Musolino, Badier, Marquis, & Chauvel, 1994) together with second order processing via a feedback loop between the primary and secondary auditory cortices mentioned earlier (Kral & Eggermont, 2007). Gilley et al. (2008) observed bilateral activation of the auditory cortical areas (superior temporal gyrus and inferior temporal gyrus) in normal hearing children. Children who received cochlear implants at an early age showed activation in the auditory cortical areas (contralateral to the implant) which were similar to those in normal hearing children while activation in late-implanted children was severely compromised. This led to the decoupling hypothesis (Kral et al., 2005) which may be the basis of cross-modal plasticity via increased activity in occipital and motor lobes. This notion of visual dominance in audio-visual integration and/or takeover of the auditory areas by visual stimuli was suggested by Bavelier and Neville (2002). It is worth noting that Shiell et al. (2015) observed that consistent use of hearing aids (i.e., amplification) resulted in reduced visual fMRI activity in contrast with those who did not use hearing aids. These differences have also been observed in a recent fMRI study of different groups of people using hearing aids or sign language (Cardin et al., 2013).
For people with hearing loss, fMRI is challenging because it is uncertain whether the subject would be able to comprehend speech especially if the degree of hearing loss is profound given the noisy environment of the scanner. fMRI measures brain activity detecting changes associated with increased blood flow into a cortical region that is responding to stimuli such as speech. A few laboratories have been able to provide acoustic stimuli through tubephones and headphones customized to deliver sound levels up to 130 db with low distortion, flat frequency response, reliable phase and noise cancellation (Hall & Paltoglou, 2009). While there have been no reported studies of people with hearing loss with these customized devices, another group has examined the use of fMRI in sedated babies prior to cochlear implantation (DiFrancesco, Robertson, Karunanayaka, & Holland, 2013; Patel et al., 2007; Schmithorst et al., 2005). They demonstrated that levels of brain activity as a reflection of hearing levels in the primary auditory cortex correlated strongly with the improvement in hearing after getting a cochlear implant. More recently, the same group applied pattern classification methods to results of MRI and fMRI data to make some predictions about speech and language outcomes in babies who then received a cochlear implant (Deshpande, Tan, Lu, Altaye, & Holland, 2016; Tan et al., 2013). Others have observed brain activity at low frequencies in the auditory cortex of people with partial hearing loss (Skarzynski et al., 2013) and positive changes in activation of the auditory cortex after a period of using hearing aids (Hwang, Wu, Chen, & Liu, 2006). So it ought to be possible to adapt tubephones or headphones to create a hearing aid-like transfer function rather than a flat one (e.g., Palmer, Bullock, & Chambers, 1998) to examine how the brain functions with hearing aids.
Given the contraindication of ferromagnetic properties of cochlear implants with MRI scanners, PET has emerged as a tool for longitudinal tracking of cochlear implants. PET measures metabolic processes in the brain so cortical regions that are actively responding to stimuli such as speech have increased metabolism (D. S. Lee et al., 2001). Significant brain reorganization in the first few months after cochlear implantation has been observed mainly in the left superior temporal gyrus and Broca's area in the frontal cortex of subjects with postlingual hearing loss but not those with prelingual hearing loss (Petersen, Gjedde, Wallentin, & Vuust, 2013). This suggests that prior experience of language which is the case in the former group is a good indicator of positive outcomes. Further, visual cues may have a positive effect on auditory perception (Strelnikov et al., 2014) which suggests audio-visual integration plays an important role in brain plasticity (R. Campbell, MacSweeney, & Woll, 2014). Earlier studies reviewed by Giraud and Lee (2007) suggested that resting metabolism can be a good measure of speech performance after cochlear implantation and changes in PET activity reflect adaptation in higher order cognitive processes.
A major limitation of PET is the use of radioactive tracers which limits the ability to perform longitudinal analysis over a short period especially when plasticity changes are significant. This could be overcome by fNIRS which has just emerged in the past decade as a potentially useful tool (Sevy et al., 2010). Here as in fMRI, neuronal activity results in changes in levels of oxygen in blood but with near-infrared light passing through brain tissue. It is now possible to assess activity in the auditory cortex in response to speech (Lawler et al., 2015), differentiate from scrambled speech as a measure of outcome with amplification (Pollonini et al., 2014), lipreading before and after cochlear implantation (Anderson, Lazard, & Hartley, 2017; Anderson, Wiggins, Kitterick, & Hartley, 2017) and speech and language processing (Bortfeld, 2019; McKay et al., 2016; Zhou et al., 2018). Limitations such as sensitivity and accuracy of quantification of brain activity in deeper cortical regions (e.g., the Heschl’s Gyrus) may be resolved by newer optical measurements (Hasnain, Mehta, Zhou, Li, & Chen, 2018; Mehta et al., 2017).
9 ∣. FUTURE DIRECTIONS AND OPPORTUNITIES
This review has revealed limitations that make it difficult to make inferences about plastic changes in the brain caused by hearing loss regardless of whether amplification was used or not. Several suggestions are offered that could increase the impact of structural neuroimaging as a biomarker to aid the development of speech, language, and hearing.
Future studies should extend beyond homogeneous groups that in fact represent a very small segment of the spectrum of people with hearing loss. In fact, the World Health Organization estimated that 15% of the world's population has a hearing loss of which a third, that is, 360 million, have a disabling hearing loss (World Health Organization, 2013) ranging from partial to profound. Further, the World Federation of the Deaf estimates that 70 million use sign language (http://wfdeaf.org/faq). This means that these homogeneous groups characterize just 6% of people with hearing loss. Only one neuroimaging study considered this limitation and attempted to provide new answers (Olulade et al., 2014).
Studies should go beyond the structures other than the ones known to play an important role in speech, language, and hearing. Granted that hearing loss has broad consequences for the developing and maturing brain, it is important to discern the different forms of plasticity in the brain. Use of more quantitative and sophisticated analyses of morphometry and connectivity measures could go a long way to deepen understanding of the biological substrates of plasticity. Further, these methods could be useful for analysis of structural neuroimaging of other types of hearing loss such as aged-induced hearing loss (Eckert et al., 2013; Eckert, Cute, Vaden, Kuchinsky, & Dubno, 2012; F. R. Lin et al., 2014; Peelle, Troiani, Grossman, & Wingfield, 2011; Vaden, Kuchinsky, Ahlstrom, Dubno, & Eckert, 2015), unilateral hearing loss (Rachakonda, Shimony, Coalson, & Lieu, 2014; C. M. Wu, Ng, & Liu, 2009; Yang et al., 2014) and tinnitus (Husain et al., 2011).
Studies should also consider federating neuroimaging datasets by archiving data from all over the world. The sample sizes given in Tables 1 and 2 are relatively small compared to those in recorded in other structural neuroimaging studies. On the other hand, the wider spectrum of people with hearing loss calls for alternative and more sophisticated statistical tests to deal with sizes and heterogeneity of these samples; for example, snowball sampling (Cardin et al., 2013) or pooling (Ceyhan et al., 2011). Federation is becoming common in neuroimaging projects such as schizophrenia (Alpert, Kogan, Parrish, Marcus, & Wang, 2015) and the ENIGMA project for many neurodegeneration and neurodevelopmental diseases (Thompson et al., 2014). This is where “Big Data” analytical tools such as data mining (Ramos-Miguel, Perez-Zaballos, Perez, Falconb, & Ramosb, 2014; Tan et al., 2013) could be used to uncover potential biomarkers for positive outcomes for amplification. Combining such data will require techniques such as diffeomorphometry (Miller et al., 2014; Ratnanather et al., 2020) to map imaging data to common coordinates for analysis and comparison. Personalized inference of clinical and behavioral data could then be achieved (Faria et al., 2015; Miller et al., 2013; Mori et al., 2013). A significant step in that direction was taken by Feng et al. (2018) who used machine learning methods to find that neural structures unaffected by auditory deprivation were best predictors for outcomes with cochlear implants in young children.
10 ∣. CONCLUSION
Plastic changes in the brain due to pre- or peri-lingual pediatric hearing loss with congenital or early onset etiology and post-lingual hearing loss in pre-to-late adolescence are seen to be multifocal rather than diffuse. Differences are consistent across most of the regions implicated in the hearing, speech and language networks in the brain (Friederici, 2012) regardless of modes of communication and amplification, be these via listening and spoken language or sign language. To a lesser extent, structures in networks that play an important role in cognition are affected (X. M. Xu et al., 2019b). Quantitatively differences are subtle for some structures and variable for other structures. That said, it is remarkable that the asymmetry properties of the structures in the hearing, speech and language pathways are mostly preserved. Yet, little is known about the deeper underlying biological effects of hearing loss on the brain. For example, one asks what are the structural consequences of limited acoustic stimuli that belies demyelination (Long et al., 2018) and increasing fatigue and effort associated with listening (Willis, 2018). If the classic tensegrity model of brain connectivity by Van Essen (1997) holds, then one may expect to see weaker tension in the white matter fibers connecting cortical regions responsible for auditory function. In turn, the weaker tension could result in abnormal cortical folding with weaker mechanical forces upon the thicker and shallower sulcal fundi (cortical folds or valleys). This will have mechanical and morphological effect on the deep layers that have been observed to be inactive in animal models of auditory deprivation. Such an interpretation remains to be tested at the macroscopic level. However, new methods that are capable of analyzing properties of the acoustic radiation, optic radiation, thalamo-cortical, and cortico-cortical connections may contribute to a greater understanding of the anatomical pathologies of hearing loss in the brain. Thus there is a need for clinical neuroimaging to uncover biomarkers for longitudinal tracking and monitoring of progress with amplification provided by either cochlear implants or hearing aids.
ACKNOWLEDGMENTS
The author wishes to thank Timothy Brown for generating Figures 3 and 4. He also thanks Dr Andreia Faria, Dr Peter Hubka and Dr Anu Sharma for their assistance with Figures 1 and 2. Finally, the author would like to thank one reviewer and the Associate Editor whose comments helped to improve the review.
Funding information
National Institute of Biomedical Imaging and Bioengineering, Grant/Award Number: P41 EB015909; National Institute on Deafness and Other Communication Disorders, Grant/Award Number: R01 DC016784; National Organization for Hearing Research Foundation
Footnotes
CONFLICT OF INTEREST
The author has declared no conflicts of interest for this article.
REFERENCES
- Allen JS, Emmorey K, Bruss J, & Damasio H (2008). Morphology of the insula in relation to hearing status and sign language experience. The Journal of Neuroscience, 28, 11900–11905. 10.1523/JNEUROSCI.3141-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JS, Emmorey K, Bruss J, & Damasio H (2013). Neuroanatomical differences in visual, motor, and language cortices between congenitally deaf signers, hearing signers, and hearing non-signers. Frontiers in Neuroanatomy, 7, 26 10.3389/fnana.2013.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert K, Kogan A, Parrish T, Marcus D, & Wang L (2015). The Northwestern University Neuroimaging Data Archive (NUNDA). NeuroImage, 124, 1131–1136. 10.1016/j.neuroimage.2015.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral L, Ganho-Avila A, Osorio A, Soares MJ, He D, Chen Q, … Almeida J (2016). Hemispheric asymmetries in subcortical visual and auditory relay structures in congenital deafness. The European Journal of Neuroscience, 44, 2334–2339. 10.1111/ejn.13340 [DOI] [PubMed] [Google Scholar]
- Anderson CA, Lazard DS, & Hartley DE (2017). Plasticity in bilateral superior temporal cortex: Effects of deafness and cochlear implantation on auditory and visual speech processing. Hearing Research, 343, 138–149. https://doi.org/10.1016Zj.heares.2016.07.013 [DOI] [PubMed] [Google Scholar]
- Anderson CA, Wiggins IM, Kitterick PT, & Hartley DEH (2017). Adaptive benefit of cross-modal plasticity following cochlear implantation in deaf adults. Proceedings of the National Academy of Sciences of the United States of America, 114, 10256–10261. 10.1073/pnas.1704785114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J, Avan P, Brownell WE, Dallos P, Dierkes K, Fettiplace R, … Canlon B (2010). The remarkable cochlear amplifier. Hearing Research, 266, 1–17. 10.1016/j.heares.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P, Lacassagne L, & Kral A (2013). Reorganization of the connectivity of cortical field DZ in congenitally deaf cat. PLoS One, 8, e60093 10.1371/journal.pone.0060093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, & Neville HJ (2002). Cross-modal plasticity: Where and how? Nature Reviews. Neuroscience, 3, 443–452. 10.1038/nrn848 [DOI] [PubMed] [Google Scholar]
- Benetti S, Novello L, Maffei C, Rabini G, Jovicich J, & Collignon O (2018). White matter connectivity between occipital and temporal regions involved in face and voice processing in hearing and early deaf individuals. NeuroImage, 179, 263–274. 10.1016/j.neuroimage.2018.06.044 [DOI] [PubMed] [Google Scholar]
- Boatman D (2004). Cortical bases of speech perception: Evidence from functional lesion studies. Cognition, 92, 47–65. 10.1016/j.cognition.2003.09.010 [DOI] [PubMed] [Google Scholar]
- Bok ST (1929). The effect of the flexion of the furrows and convolutions of the cerebral cortex on the cortical structure. Zeitschrift Fur Die Gesamte Neurologie Und Psychiatrie, 121, 682–750. 10.1007/Bf02864437 [DOI] [Google Scholar]
- Bok ST (1959). Histonomy of the cerebral cortex. Amsterdam, New York: Elsevier. [Google Scholar]
- Boothroyd A (2010). Adapting to changed hearing: The potential role of formal training. Journal of the American Academy of Audiology, 21, 601–611. 10.3766/jaaa.21.9.6 [DOI] [PubMed] [Google Scholar]
- Bortfeld H (2019). Functional near-infrared spectroscopy as a tool for assessing speech and spoken language processing in pediatric and adult cochlear implant users. Developmental Psychobiology, 61, 430–443. 10.1002/dev.21818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyen K, Langers DR, de Kleine E, & van Dijk P (2013). Gray matter in the brain: Differences associated with tinnitus and hearing loss. Hearing Research, 295, 67–78. 10.1016/j.heares.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Butler BE, & Lomber SG (2013). Functional and structural changes throughout the auditory system following congenital and early-onset deafness: Implications for hearing restoration. Frontiers in Systems Neuroscience, 7, 92 10.3389/fnsys.2013.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JD, Cardon G, & Sharma A (2011). Clinical application of the P1 cortical auditory evoked potential biomarker in children with sensorineural hearing loss and auditory neuropathy spectrum disorder. Seminars in Hearing, 32, 147–155. 10.1055/s-0031-1277236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R, MacSweeney M, & Woll B (2014). Cochlear implantation (CI) for prelingual deafness: The relevance of studies of brain organization and the role of first language acquisition in considering outcome success. Frontiers in Human Neuroscience, 8, 834 10.3389/fnhum.2014.00834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin V, Orfanidou E, Ronnberg J, Capek CM, Rudner M, & Woll B (2013). Dissociating cognitive and sensory neural plasticity in human superior temporal cortex. Nature Communications, 4, 1473 10.1038/ncomms2463 [DOI] [PubMed] [Google Scholar]
- Ceritoglu C, Oishi K, Li X, Chou MC, Younes L, Albert M, … Mori S (2009). Multi-contrast large deformation diffeomorphic metric mapping for diffusion tensor imaging. NeuroImage, 47, 618–627. 10.1016/j.neuroimage.2009.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceyhan E, Hosakere M, Nishino T, Alexopoulos J, Todd RD, Botteron KN, … Ratnanather JT (2011). Statistical analysis of cortical morphometrics using pooled distances based on labeled cortical distance maps. Journal of Mathematical Imaging and Vision, 40, 20–35. 10.1007/s10851-010-0240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceyhan E, Nishino T, Alexopolous D, Todd RD, Botteron KN, Miller MI, & Ratnanather JT (2013). Censoring distances based on labeled cortical distance maps in cortical morphometry. Frontiers in Neurology, 4, 155 10.3389/fneur.2013.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Lee HR, Paik JS, Lee KY, & Lee SH (2012). Voxel-wise analysis of diffusion tensor imaging for clinical outcome of cochlear implantation: Retrospective study. Clinical and Experimental Otorhinolaryngology, 5(Suppl. 1), S37–S42. 10.3342/ceo.2012.5.S1.S37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Lee SH, Lee YJ, Hwang MJ, Bae SJ, Kim MN, … Kang DS (2004). Auditory neural pathway evaluation on sensorineural hearing loss using diffusion tensor imaging. Neuroreport, 15, 1699–1703. [DOI] [PubMed] [Google Scholar]
- Chen I, Limb CJ, & Ryugo DK (2010). The effect of cochlear-implant-mediated electrical stimulation on spiral ganglion cells in congenitally deaf white cats. Journal of the Association for Research in Otolaryngology, 11, 587–603. 10.1007/s10162-010-0234-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Roth A, Halgren E, & Mayberry RI (2019). Effects of early language deprivation on brain connectivity: Language pathways in deaf native and late first-language learners of American sign language. Frontiers in Human Neuroscience, 13, 320 10.3389/fnhum.2019.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi AM, Comparini A, Scusa MF, Berrettini S, Forli F, Battini R, … Cioni G (2010). Neurodevelopmental disorders in children with severe to profound sensorineural hearing loss: A clinical study. Developmental Medicine and Child Neurology, 52, 856–862. 10.1111/j.1469-8749.2010.03621.x [DOI] [PubMed] [Google Scholar]
- Clark GM (2014). The multi-channel cochlear implant: Multi-disciplinary development of electrical stimulation of the cochlea and the resulting clinical benefit. Hearing Research, 322, 4–13. 10.1016/j.heares.2014.08.002 [DOI] [PubMed] [Google Scholar]
- Crinion JT, Lambon-Ralph MA, Warburton EA, Howard D, & Wise RJ (2003). Temporal lobe regions engaged during normal speech comprehension. Brain, 126, 1193–1201. [DOI] [PubMed] [Google Scholar]
- Dahnke R, & Gaser C (2018). Surface and shape analysis In Spalletta G, Piras F, & Gili T (Eds.), Brain morphometry (pp. 51–73). New York, NY: Springer. [Google Scholar]
- Deshpande AK, Tan L, Lu LJ, Altaye M, & Holland SK (2016). fMRI as a preimplant objective tool to predict postimplant oral language outcomes in children with cochlear implants. Ear and Hearing, 37, e263–e272. 10.1097/AUD.0000000000000259 [DOI] [PubMed] [Google Scholar]
- Dhir SB, Kutten KS, Li M, Faria AV, & Ratnanather JT (2020). Topographic visualization of the acoustic radiation in subjects with normal hearing and hearing loss via semi-automated tractography. (submitted) [Google Scholar]
- DiFrancesco MW, Robertson SA, Karunanayaka P, & Holland SK (2013). BOLD fMRI in infants under sedation: Comparing the impact of pentobarbital and propofol on auditory and language activation. Journal of Magnetic Resonance Imaging, 38, 1184–1195. 10.1002/Jmri.24082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror AA, & Avraham KB (2009). Hearing loss: Mechanisms revealed by genetics and cell biology. Annual Review of Genetics, 43, 411–437. 10.1146/annurev-genet-102108-134135 [DOI] [PubMed] [Google Scholar]
- Duncan NW, Gravel P, Wiebking C, Reader AJ, & Northoff G (2013). Grey matter density and GABAA binding potential show a positive linear relationship across cortical regions. Neuroscience, 235, 226–231. 10.1016/j.neuroscience.2012.12.075 [DOI] [PubMed] [Google Scholar]
- Eckert MA, Cute SL, Vaden KI Jr., Kuchinsky SE, & Dubno JR (2012). Auditory cortex signs of age-related hearing loss. Journal of the Association for Research in Otolaryngology, 13, 703–713. 10.1007/s10162-012-0332-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Kuchinsky SE, Vaden KI, Cute SL, Spampinato MV, & Dubno JR (2013). White matter hyperintensities predict low frequency hearing in older adults. Journal of the Association for Research in Otolaryngology, 14, 425–433. 10.1007/s10162-013-0381-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, & Ponton CW (2003). Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: Correlations with changes in structure and speech perception. Acta Oto-Laryngologica, 123, 249–252. [DOI] [PubMed] [Google Scholar]
- Emmorey K, Allen JS, Bruss J, Schenker N, & Damasio H (2003). A morphometric analysis of auditory brain regions in congenitally deaf adults. Proceedings of the National Academy of Sciences of the United States of America, 100, 10049–10054. 10.1073/pnas.1730169100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria AV, Oishi K, Yoshida S, Hillis A, Miller MI, & Mori S (2015). Content-based image retrieval for brain MRI: An image-searching engine and population-based analysis to utilize past clinical data for future diagnosis. NeuroImage, 7, 367–376. 10.1016/j.nicl.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HM, Yeatman JD, Lee ES, Barde LHF, & Gaman-Bean S (2010). Diffusion tensor imaging: A review for pediatric researchers and clinicians. Journal of Developmental & Behavioral Pediatrics, 31, 346–356. 10.1097/DBP.0b013e3181dcaa8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Ingvalson EM, Grieco-Calub TM, Roberts MY, Ryan ME, Birmingham P, … Wong PCM (2018). Neural preservation underlies speech improvement from auditory deprivation in young cochlear implant recipients. Proceedings of the National Academy of Sciences of the United States of America, 115, E1022–E1031. 10.1073/pnas.1717603115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R, & Kim KX (2014). The physiology of mechanoelectrical transduction channels in hearing. Physiological Reviews, 94, 951–986. 10.1152/physrev.00038.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine I, Finney EM, Boynton GM, & Dobkins KR (2005). Comparing the effects of auditory deprivation and sign language within the auditory and visual cortex. Journal of Cognitive Neuroscience, 17, 1621–1637. 10.1162/089892905774597173 [DOI] [PubMed] [Google Scholar]
- Fischl B (2012). FreeSurfer. NeuroImage, 62, 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2012). The cortical language circuit: From auditory perception to sentence comprehension. Trends in Cognitive Sciences, 16, 262–268. 10.1016/j.tics.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, & Dorman MF (2008). Cortical reorganization in children with cochlear implants. Brain Research, 1239, 56–65. 10.1016/j.brainres.2008.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, & Lee HJ (2007). Predicting cochlear implant outcome from brain organisation in the deaf. Restorative Neurology and Neuroscience, 25, 381–390. [PubMed] [Google Scholar]
- Giuliani NR, Calhoun VD, Pearlson GD, Francis A, & Buchanan RW (2005). Voxel-based morphometry versus region of interest: A comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophrenia Research, 74, 135–147. 10.1016/j.schres.2004.08.019 [DOI] [PubMed] [Google Scholar]
- Hall D, & Paltoglou A (2009). fMRI of the central auditory system. NeuroMethods, 41, 537–573. [Google Scholar]
- Hampton T (2013). 2013 Lasker awards honor biomedical researchers and champions of public service. JAMA, 310, 1011–1013. 10.1001/jama.2013.276942 [DOI] [PubMed] [Google Scholar]
- Hasnain A, Mehta K, Zhou X, Li H, & Chen N (2018). Laplace-domain diffuse optical measurement. Scientific Reports, 8, 12134 10.1038/s41598-018-30353-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmair I, Hochmair E, Nopp P, Waller M, & Jolly C (2014). Deep electrode insertion and sound coding in cochlear implants. Hearing Research, 322, 14–23. 10.1016/j.heares.2014.10.006 [DOI] [PubMed] [Google Scholar]
- Holmes D (2013). Lasker Foundation honours cochlear-implant pioneers. Lancet, 382, 926. [DOI] [PubMed] [Google Scholar]
- Hong P, Jurkowski ZC, & Carvalho DS (2010). Preoperative cerebral magnetic resonance imaging and white matter changes in pediatric cochlear implant recipients. International Journal of Pediatric Otorhinolaryngology, 74, 658–660. 10.1016/j.ijporl.2010.03.014 [DOI] [PubMed] [Google Scholar]
- Hribar M, Suput D, Carvalho AA, Battelino S, & Vovk A (2014). Structural alterations of brain grey and white matter in early deaf adults. Hearing Research, 318, 1–10. 10.1016/j.heares.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Huang L, Zheng W, Wu C, Wei X, Wu X, Wang Y, & Zheng H (2015). Diffusion tensor imaging of the auditory neural pathway for clinical outcome of cochlear implantation in pediatric congenital sensorineural hearing loss patients. PLoS One, 10, e0140643 10.1371/journal.pone.0140643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain FT, Medina RE, Davis CW, Szymko-Bennett Y, Simonyan K, Pajor NM, & Horwitz B (2011). Neuroanatomical changes due to hearing loss and chronic tinnitus: A combined VBM and DTI study. Brain Research, 1369, 74–88. 10.1016/j.brainres.2010.10.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, & Dabholkar AS (1997). Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology, 387, 167–178. [DOI] [PubMed] [Google Scholar]
- Hutton C, De Vita E, Ashburner J, Deichmann R, & Turner R (2008). Voxel-based cortical thickness measurements in MRI. NeuroImage, 40, 1701–1710. 10.1016/j.neuroimage.2008.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Wu CW, Chen JH, & Liu TC (2006). Changes in activation of the auditory cortex following long-term amplification: An fMRI study. Acta Oto-Laryngologica, 126, 1275–1280. 10.1080/00016480600794503 [DOI] [PubMed] [Google Scholar]
- Iyengar S (2012). Development of the human auditory system. Journal of the Indian Institute of Science, 92, 427–440. [Google Scholar]
- Jiang J, Zhu W, Shi F, Liu Y, Li J, Qin W, … Jiang T (2009). Thick visual cortex in the early blind. The Journal of Neuroscience, 29, 2205–2211. 10.1523/JNEUROSCI.5451-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Wen Z, Long L, Wong CW, Ye N, Zee C, & Chen BT (2019). Assessing cerebral white matter microstructure in children with congenital sensorineural hearing loss: A tract-based spatial statistics study. Frontiers in Neuroscience, 13, 597 10.3389/fnins.2019.00597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZY, Odiase E, Isaacson B, Roland PS, & Kutz JW Jr. (2014). Utility of MRIs in adult cochlear implant evaluations. Otology & Neurotology, 35, 1533–1535. 10.1097/MAO.0000000000000453 [DOI] [PubMed] [Google Scholar]
- Jonas NE, Ahmed J, Grainger J, Jephson CG, Wyatt ME, Hartley BE, … Cochrane LA (2012). MRI brain abnormalities in cochlear implant candidates: How common and how important are they? International Journal of Pediatric Otorhinolaryngology, 76, 927–929. 10.1016/j.ijporl.2012.02.070 [DOI] [PubMed] [Google Scholar]
- Kara A, Hakan Ozturk A, Kurtoglu Z, Umit Talas D, Aktekin M, Saygili M, & Kanik A (2006). Morphometric comparison of the human corpus callosum in deaf and hearing subjects: An MRI study. Journal of Neuroradiology, 33, 158–163. [DOI] [PubMed] [Google Scholar]
- Karns CM, Stevens C, Dow MW, Schorr EM, & Neville HJ (2017). Atypical white-matter microstructure in congenitally deaf adults: A region of interest and tractography study using diffusion-tensor imaging. Hearing Research, 343, 72–82. 10.1016/j.heares.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Park SY, Kim J, Lee DH, & Park HJ (2009). Alterations of white matter diffusion anisotropy in early deafness. Neuroreport, 20, 1032–1036. 10.1097/WNR.0b013e32832e0cdd [DOI] [PubMed] [Google Scholar]
- Kim E, Kang H, Lee H, Lee HJ, Suh MW, Song JJ, … Lee DS (2014). Morphological brain network assessed using graph theory and network filtration in deaf adults. Hearing Research, 315, 88–98. 10.1016/j.heares.2014.06.007 [DOI] [PubMed] [Google Scholar]
- Kim J, Choi JY, Eo J, & Park HJ (2017). Comparative evaluation of the white matter fiber integrity in patients with prelingual and post-lingual deafness. Neuroreport, 28, 1103–1107. 10.1097/WNR.0000000000000894 [DOI] [PubMed] [Google Scholar]
- Knudsen EI (2004). Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience, 16, 1412–1425. 10.1162/0898929042304796 [DOI] [PubMed] [Google Scholar]
- Kral A (2013). Auditory critical periods: A review from system's perspective. Neuroscience, 247, 117–133. 10.1016/j.neuroscience.2013.05.021 [DOI] [PubMed] [Google Scholar]
- Kral A, & Eggermont JJ (2007). What's to lose and what's to learn: Development under auditory deprivation, cochlear implants and limits of cortical plasticity. Brain Research Reviews, 56, 259–269. 10.1016/j.brainresrev.2007.07.021 [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, & Klinke R (2000). Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer-specific manner. Cerebral Cortex, 10, 714–726. [DOI] [PubMed] [Google Scholar]
- Kral A, & Tillein J (2006). Brain plasticity under cochlear implant stimulation. Advances in Oto-Rhino-Laryngology, 64, 89–108. 10.1159/000094647 [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Heid S, Hartmann R, & Klinke R (2005). Postnatal cortical development in congenital auditory deprivation. Cerebral Cortex, 15, 552–562. 10.1093/cercor/bhh156 [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Heid S, Klinke R, & Hartmann R (2006). Cochlear implants: Cortical plasticity in congenital deprivation. Progress in Brain Research, 157, 283–313. [DOI] [PubMed] [Google Scholar]
- Kumar U, & Mishra M (2018). Pattern of neural divergence in adults with prelingual deafness: Based on structural brain analysis. Brain Research, 1701, 58–63. 10.1016/j.brainres.2018.07.021 [DOI] [PubMed] [Google Scholar]
- la Fougere C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, … Thiel A (2011). Where in-vivo imaging meets cytoarchitectonics: The relationship between cortical thickness and neuronal density measured with high-resolution [18F]flumazenil-PET. NeuroImage, 56, 951–960. 10.1016/j.neuroimage.2010.11.015 [DOI] [PubMed] [Google Scholar]
- Lapointe A, Viamonte C, Morriss MC, & Manolidis S (2006). Central nervous system findings by magnetic resonance in children with profound sensorineural hearing loss. International Journal of Pediatric Otorhinolaryngology, 70, 863–868. 10.1016/j.ijporl.2005.09.022 [DOI] [PubMed] [Google Scholar]
- Lawler CA, Wiggins IM, Dewey RS, & Hartley DE (2015). The use of functional near-infrared spectroscopy for measuring cortical reorganisation in cochlear implant users: A possible predictor of variable speech outcomes? Cochlear Implants International, 16(Suppl. 1), S30–S32. 10.1179/1467010014Z.000000000230 [DOI] [PubMed] [Google Scholar]
- Lazard DS, Lee HJ, Truy E, & Giraud AL (2013). Bilateral reorganization of posterior temporal cortices in post-lingual deafness and its relation to cochlear implant outcome. Human Brain Mapping, 34, 1208–1219. 10.1002/hbm.21504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, … Kim CS (2001). Cross-modal plasticity and cochlear implants. Nature, 409, 149–150. 10.1038/35051653 [DOI] [PubMed] [Google Scholar]
- Lee SH, Chang Y, Lee JE, & Cho JH (2004). The values of diffusion tensor imaging and functional MRI in evaluating profound sensorineural hearing loss. Cochlear Implants International, 5(Suppl. 1), 149–152. 10.1179/cim.2004.5.Supplement-1.149 [DOI] [PubMed] [Google Scholar]
- Lepore N, Vachon P, Lepore F, Chou YY, Voss P, Brun CC, … Thompson PM (2010). 3D mapping of brain differences in native signing congenitally and prelingually deaf subjects. Human Brain Mapping, 31, 970–978. 10.1002/hbm.20910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li W, Xian J, Li Y, Liu Z, Liu S, … He H (2012). Cortical thickness analysis and optimized voxel-based morphometry in children and adolescents with prelingually profound sensorineural hearing loss. Brain Research, 1430, 35–42. 10.1016/j.brainres.2011.09.057 [DOI] [PubMed] [Google Scholar]
- Li M, Ratnanather JT, Miller MI, & Mori S (2014). Knowledge-based automated reconstruction of human brain white matter tracts using a path-finding approach with dynamic programming. NeuroImage, 88, 271–281. 10.1016/j.neuroimage.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li J, Wang Z, Li Y, Liu Z, Yan F, … He H (2015). Grey matter connectivity within and between auditory, language and visual systems in prelingually deaf adolescents. Restorative Neurology and Neuroscience, 33, 279–290. 10.3233/RNN-140437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li J, Xian J, Lv B, Li M, Wang C, … Sabel BA (2013). Alterations of grey matter asymmetries in adolescents with prelingual deafness: A combined VBM and cortical thickness analysis. Restorative Neurology and Neuroscience, 31, 1–17. 10.3233/RNN-2012-120269 [DOI] [PubMed] [Google Scholar]
- Li Y, Ding G, Booth JR, Huang R, Lv Y, Zang Y, … Peng D (2012). Sensitive period for white-matter connectivity of superior temporal cortex in deaf people. Human Brain Mapping, 33, 349–359. 10.1002/hbm.21215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhu Q, Geng Z, Song Z, Wang L, & Wang Y (2015). Study of functional connectivity in patients with sensorineural hearing loss by using resting-state fMRI. International Journal of Clinical and Experimental Medicine, 8, 569–578. [PMC free article] [PubMed] [Google Scholar]
- Liegeois-Chauvel C, Musolino A, Badier JM, Marquis P, & Chauvel P (1994). Evoked potentials recorded from the auditory cortex in man: Evaluation and topography of the middle latency components. Electroencephalography and Clinical Neurophysiology, 92, 204–214. [DOI] [PubMed] [Google Scholar]
- Liem F, Hurschler MA, Jancke L, & Meyer M (2014). On the planum temporale lateralization in suprasegmental speech perception: Evidence from a study investigating behavior, structure, and function. Human Brain Mapping, 35, 1779–1789. 10.1002/hbm.22291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem F, Zaehle T, Burkhard A, Jancke L, & Meyer M (2012). Cortical thickness of supratemporal plane predicts auditory N1 amplitude. Neuroreport, 23, 1026–1030. 10.1097/WNR.0b013e32835abc5c [DOI] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, An Y, Goh JO, Doshi J, Metter EJ, … Resnick SM (2014). Association of hearing impairment with brain volume changes in older adults. NeuroImage, 90, 84–92. 10.1016/j.neuroimage.2013.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wang J, Wu C, Wai Y, Yu J, & Ng S (2008). Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: Changes in radial diffusivity and diffusion anisotropy. Journal of Magnetic Resonance Imaging, 28, 598–603. 10.1002/jmri.21464 [DOI] [PubMed] [Google Scholar]
- Liu B, Feng Y, Yang M, Chen JY, Li J, Huang ZC, & Zhang LL (2015). Functional connectivity in patients with sensorineural hearing loss using resting-state MRI. American Journal of Audiology, 24, 145–152. 10.1044/2015_AJA-13-0068 [DOI] [PubMed] [Google Scholar]
- Liu Z-H, Li M, Xian J-F, He H-G, Wang J-C, Li Y, … Liu S (2010). Investigation of the white matter with tract based spatial statistics in congenitally deaf patients. Chinese Journal of Medical Imaging Technology, 26, 1226–1229. [Google Scholar]
- Long P, Wan G, Roberts MT, & Corfas G (2018). Myelin development, plasticity, and pathology in the auditory system. Developmental Neurobiology, 78, 80–92. 10.1002/dneu.22538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyness RC, Alvarez I, Sereno MI, & MacSweeney M (2014). Microstructural differences in the thalamus and thalamic radiations in the congenitally deaf. NeuroImage, 100, 347–357. 10.1016/j.neuroimage.2014.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackeith S, Joy R, Robinson P, & Hajioff D (2012). Pre-operative imaging for cochlear implantation: Magnetic resonance imaging, computed tomography, or both? Cochlear Implants International, 13, 133–136. 10.1179/1754762811Y.0000000002 [DOI] [PubMed] [Google Scholar]
- Maffei C (2017). Finding the missing connection: Diffusion-based tractography reconstruction of the acoustic radiation and other applications. (PhD thesis). University of Trento. [Google Scholar]
- Marie D, Maingault S, Crivello F, Mazoyer B, & Tzourio-Mazoyer N (2016). Surface-based morphometry of cortical thickness and surface area associated with Heschl’s Gyri duplications in 430 healthy volunteers. Frontiers in Human Neuroscience, 10, 69 10.3389/fnhum.2016.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay CM, Shah A, Seghouane AK, Zhou X, Cross W, & Litovsky R (2016). Connectivity in language areas of the brain in cochlear implant users as revealed by fNIRS. Advances in Experimental Medicine and Biology, 894, 327–335. 10.1007/978-3-319-25474-6_34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta K, Hasnain A, Zhou X, Luo J, Penney TB, & Chen N (2017). Spread spectrum time-resolved diffuse optical measurement system for enhanced sensitivity in detecting human brain activity. Journal of Biomedical Optics, 22, 45005 10.1117/1.JBO.22.4.045005 [DOI] [PubMed] [Google Scholar]
- Merzenich MM (2015). Early UCSF contributions to the development of multiple-channel cochlear implants. Hearing Research, 322, 39–46. 10.1016/j.heares.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Mesgarani N, David SV, Fritz JB, & Shamma SA (2014). Mechanisms of noise robust representation of speech in primary auditory cortex. Proceedings of the National Academy of Sciences of the United States of America, 111, 6792–6797. 10.1073/pnas.1318017111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Liem F, Hirsiger S, Jancke L, & Hanggi J (2014). Cortical surface area and cortical thickness demonstrate differential structural asymmetry in auditory-related areas of the human cortex. Cerebral Cortex, 24, 2541–2552. 10.1093/cercor/bht094 [DOI] [PubMed] [Google Scholar]
- Meyer M, Toepel U, Keller J, Nussbaumer D, Zysset S, & Friederici AD (2007). Neuroplasticity of sign language: Implications from structural and functional brain imaging. Restorative Neurology and Neuroscience, 25, 335–351. [PubMed] [Google Scholar]
- Miao W, Li J, Tang M, Xian J, Li W, Liu Z, … He, H. (2013). Altered white matter integrity in adolescents with prelingual deafness: A high-resolution tract-based spatial statistics imaging study. AJNR. American Journal of Neuroradiology, 34, 1264–1270. 10.3174/ajnr.A3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Faria AV, Oishi K, & Mori S (2013). High-throughput neuro-imaging informatics. Frontiers in Neuroinformatics, 7, 31 10.3389/fninf.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Hosakere M, Barker AR, Priebe CE, Lee N, Ratnanather JT, … Csernansky JG (2003). Labeled cortical mantle distance maps of the cingulate quantify differences between dementia of the Alzheimer type and healthy aging. Proceedings of the National Academy of Sciences of the United States of America, 100, 15172–15177. 10.1073/pnas.2136624100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Massie AB, Ratnanather JT, Botteron KN, & Csernansky JG (2000). Bayesian construction of geometrically based cortical thickness metrics. NeuroImage, 12, 676–687. 10.1006/nimg.2000.0666 [DOI] [PubMed] [Google Scholar]
- Miller MI, Younes L, & Trouvé A (2014). Diffeomorphometry and geodesic positioning systems for human anatomy. Technology, 2, 36–43. 10.1142/S2339547814500010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon IJ, Kim EY, Park GY, Jang MS, Kim JH, Lee J, … Hong SH (2012). The clinical significance of preoperative brain magnetic resonance imaging in pediatric cochlear implant recipients. Audiology & Neuro-Otology, 17, 373–380. 10.1159/000341818 [DOI] [PubMed] [Google Scholar]
- Moore JK (2002). Maturation of human auditory cortex: Implications for speech perception. The Annals of Otology, Rhinology, and Laryngology, 111(Suppl. 189), 7–10. [DOI] [PubMed] [Google Scholar]
- Moore JK, & Guan YL (2001). Cytoarchitectural and axonal maturation in human auditory cortex. Journal of the Association for Research in Otolaryngology, 2, 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JK, & Linthicum FH Jr. (2007). The human auditory system: A timeline of development. International Journal of Audiology, 46, 460–478. 10.1080/14992020701383019 [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Faria AV, & Miller MI (2013). Atlas-based neuroinformatics via MRI: Harnessing information from past clinical cases and quantitative image analysis for patient care. Annual Review of Biomedical Engineering, 15, 71–92. 10.1146/annurev-bioeng-071812-152335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, & Van Zijl PCM (2004). MRI atlas of human white matter. Amsterdam, The Netherlands/San Diego, CA: Elsevier. [Google Scholar]
- Muniak MA, Connelly CJ, Tirko NN, O'Neil JN, & Ryugo DK (2013). Synaptic organization and plasticity in the auditory system of the deaf white cat In Kral A, et al. (Eds.), Deafness (Vol. 47, pp. 83–128). New York: Springer. [Google Scholar]
- Nath K, Syal R, Haris M, Goyal A, Purwar A, Rathore DK, … Gupta RK (2007). Diffusion tensor imaging of auditory neural pathway in patients with sensori-neural hearing loss. Proceedings of the International Society for Magnetic Resonance in Medicine, 15, 3513. [Google Scholar]
- Niparko JK (2013). The significance of cochlear implant history. JAMA Otolaryngology. Head & Neck Surgery, 139, 454 10.1001/jamaoto.2013.304 [DOI] [PubMed] [Google Scholar]
- Nourski KV, & Howard MA 3rd. (2015). Invasive recordings in the human auditory cortex. Handbook of Clinical Neurology, 129, 225–244. 10.1016/B978-0-444-62630-1.00013-5 [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, … Mori S (2009). Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: Application to normal elderly and Alzheimer’s disease participants. NeuroImage, 46, 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olulade OA, Koo DS, LaSasso CJ, & Eden GF (2014). Neuroanatomical profiles of deafness in the context of native language experience. The Journal of Neuroscience, 34, 5613–5620. 10.1523/INEUROSCI.3700-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil JN, Connelly CJ, Limb CJ, & Ryugo DK (2011). Synaptic morphology and the influence of auditory experience. Hearing Research, 279, 118–130. 10.1016/j.heares.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AR, Bullock DC, & Chambers JD (1998). A high-output, high-quality sound system for use in auditory fMRI. NeuroImage, 7, S357. [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, … Kremen WS (2009). Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex, 19, 2728–2735. 10.1093/cercor/bhp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Chung WH, Kwon H, & Lee JM (2018). Evaluation of cerebral white matter in prelingually deaf children using diffusion tensor imaging. BioMed Research International, 2018, 1–7. 10.1155/2018/6795397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AM, Cahill LD, Ret J, Schmithorst V, Choo D, & Holland S (2007). Functional magnetic resonance imaging of hearing-impaired children under sedation before cochlear implantation. Archives of Otolaryngology – Head & Neck Surgery, 133, 677–683. 10.1001/archotol.133.7.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Grossman M, & Wingfield A (2011). Hearing loss in older adults affects neural systems supporting speech comprehension. The Journal of Neuroscience, 31, 12638–12643. 10.1523/FNEUROSCI.2559-n.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB, Cismaru R, Dorsaint-Pierre R, Petitto LA, & Zatorre RJ (2003). The morphometry of auditory cortex in the congenitally deaf measured using MRI. NeuroImage, 20, 1215–1225. 10.1016/S1053-8119(03)00373-2 [DOI] [PubMed] [Google Scholar]
- Penicaud S, Klein D, Zatorre RJ, Chen JK, Witcher P, Hyde K, & Mayberry RI (2013). Structural brain changes linked to delayed first language acquisition in congenitally deaf individuals. NeuroImage, 66, 42–49. 10.1016/j.neuroimage.2012.09.076 [DOI] [PubMed] [Google Scholar]
- Pereira-Jorge MR, Andrade KC, Palhano-Fontes FX, Diniz PRB, Sturzbecher M, Santos AC, & Araujo DB (2018). Anatomical and functional MRI changes after one year of auditory rehabilitation with hearing aids. Neural Plasticity, 2018, 1–13. 10.1155/2018/9303674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B, Gjedde A, Wallentin M, & Vuust P (2013). Cortical plasticity after cochlear implantation. Neural Plasticity, 2013, 1–11. 10.1155/2013/318521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollonini L, Olds C, Abaya H, Bortfeld H, Beauchamp MS, & Oghalai JS (2014). Auditory cortex activation to natural speech and simulated cochlear implant speech measured with functional near-infrared spectroscopy. Hearing Research, 309, 84–93. 10.1016/j.heares.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton CW, Don M, Eggermont JJ, Waring MD, Kwong B, & Masuda A (1996). Auditory system plasticity in children after long periods of complete deafness. Neuroreport, 8, 61–65. [DOI] [PubMed] [Google Scholar]
- Proctor RD, Gawne-Cain ML, Eyles J, Mitchell TE, & Batty VB (2013). MRI during cochlear implant assessment: Should we image the whole brain? Cochlear Implants International, 14, 2–6. 10.1179/1754762811Y.0000000029 [DOI] [PubMed] [Google Scholar]
- Pundir AS, Hameed LS, Dikshit PC, Kumar P, Mohan S, Radotra B, … Iyengar S (2012). Expression of medium and heavy chain neurofilaments in the developing human auditory cortex. Brain Structure & Function, 217, 303–321. 10.1007/s00429-011-0352-7 [DOI] [PubMed] [Google Scholar]
- Qi R, Su L, Zou L, Yang J, & Zheng S (2019). Altered gray matter volume and white matter integrity in sensorineural hearing loss patients: A VBM and TBSS study. Otology & Neurotology, 40, e569–e574. 10.1097/MA0.0000000000002273 [DOI] [PubMed] [Google Scholar]
- Rachakonda T, Shimony JS, Coalson RS, & Lieu JE (2014). Diffusion tensor imaging in children with unilateral hearing loss: A pilot study. Frontiers in Systems Neuroscience, 8, 87 10.3389/fnsys.2014.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggio MW, & Schreiner CE (1994). Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation. I. Intensity dependence of firing rate and response latency. Journal of Neurophysiology, 72, 2334–2359. [DOI] [PubMed] [Google Scholar]
- Raggio MW, & Schreiner CE (1999). Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation. III. Activation patterns in short- and long-term deafness. Journal of Neurophysiology, 82, 3506–3526. [DOI] [PubMed] [Google Scholar]
- Raggio MW, & Schreiner CE (2003). Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation: IV. Activation pattern for sinusoidal stimulation. Journal of Neurophysiology, 89, 3190–3204. 10.1152/jn.00341.2002 [DOI] [PubMed] [Google Scholar]
- Rakic P (1988). Specification of cerebral cortical areas. Science, 241, 170–176. [DOI] [PubMed] [Google Scholar]
- Rakic P (1995). A small step for the cell, a giant leap for mankind: A hypothesis of neocortical expansion during evolution. Trends in Neurosciences, 18, 383–388. [DOI] [PubMed] [Google Scholar]
- Ramos-Miguel A, Perez-Zaballos T, Perez D, Falconb JC, & Ramosb A (2014). Use of data mining to predict significant factors and benefits of bilateral cochlear implantation. European Archives of Oto-Rhino-Laryngology, 272, 3157–3162. 10.1007/s00405-014-3337-3 [DOI] [PubMed] [Google Scholar]
- Ratnanather JT, Arguillere S, Kutten KS, Hubka P, Kral A, & Younes L (2019). 3D normal coordinate systems for cortical areas In Kushnarev S, et al. (Eds.), Mathematics of shapes and applications (Vol. 37, pp. 167–179). Singapore: World Scientific. [Google Scholar]
- Ratnanather JT, Cebron S, Ceyhan E, Postell E, Pisano DV, Poynton CB, … Barta PE (2014). Morphometric differences in planum temporale in schizophrenia and bipolar disorder revealed by statistical analysis of labeled cortical depth maps. Frontiers in Psychiatry, 5, 94 10.3389/fpsyt.2014.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnanather JT, Lal RM, An M, Poynton CB, Li M, Jiang H, … Miller MI (2013). Cortico-cortical, cortico-striatal, and corticothalamic white matter fiber tracts generated in the macaque brain via dynamic programming. Brain Connectivity, 3, 475–490. 10.1089/brain.2013.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnanather JT, Liu C-F, & Miller MI (2020). Shape diffeomorphometry of brain structures in degeneration and development diseases In Thakor N (Ed.), Handbook of neuroengineering. New York: Springer. [Google Scholar]
- Ratnanather JT, Poynton CB, Pisano DV, Crocker B, Postell E, Cebron S, … Barta PE (2013). Morphometry of superior temporal gyrus and planum temporale in schizophrenia and psychotic bipolar disorder. Schizophrenia Research, 150, 476–483. https://doi.org/10.1016Zj.schres.2013.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman M, Parkkola R, Johansson R, Jaaskelainen SK, Kujari H, Lehtonen L, … PIPARI Study Group. (2009). Diffusion tensor imaging of the inferior colliculus and brainstem auditory-evoked potentials in preterm infants. Pediatric Radiology, 39, 804–809. 10.1007/s00247-009-1278-6 [DOI] [PubMed] [Google Scholar]
- Roche JP, Huang BY, Castillo M, Bassim MK, Adunka OF, & Buchman CA (2010). Imaging characteristics of children with auditory neuropathy spectrum disorder. Otology & Neurotology, 31, 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PS, & Tobey E (2013). A tribute to a remarkably sound solution. Cell, 154, 1175–1177. 10.1016/jxell.2013.08.047 [DOI] [PubMed] [Google Scholar]
- Ryugo DK, & Limb CJ (2009). Brain plasticity: The impact of the environment on the brain as it relates to hearing and deafness In Niparko JK (Ed.), Cochlear implants: Principles and practices (pp. 19–37). Philadelphia, PA: Lippincott, Williams & Wilkins. [Google Scholar]
- Ryugo DK, & Menotti-Raymond M (2012). Feline deafness. The Veterinary Clinics of North America. Small Animal Practice, 42, 1179–1207. 10.1016/j.cvsm.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saada AA, Niparko JK, & Ryugo DK (1996). Morphological changes in the cochlear nucleus of congenitally deaf white cats. Brain Research, 736, 315–328. [DOI] [PubMed] [Google Scholar]
- Sanes DH, & Kotak VC (2011). Developmental plasticity of auditory cortical inhibitory synapses. Hearing Research, 279, 140–148. 10.1016/j.heares.2011.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Ret J, Duggins A, Aijmand E, & Greinwald J (2005). Cortical reorganization in children with unilateral sensorineural hearing loss. Neuroreport, 16, 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner CE, & Raggio MW (1996). Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation. II. Repetition rate coding. Journal of Neurophysiology, 75, 1283–1300. [DOI] [PubMed] [Google Scholar]
- Schwartz SR, & Chen BS (2014). The role of preoperative imaging for cochlear implantation in postlingually deafened adults. Otology & Neurotology, 35, 1536–1540. 10.1097/MAO.0000000000000499 [DOI] [PubMed] [Google Scholar]
- Selemon LD (2013). A role for synaptic plasticity in the adolescent development of executive function. Translational Psychiatry, 3, e238 10.1038/tp.2013.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevy AB, Bortfeld H, Huppert TJ, Beauchamp MS, Tonini RE, & Oghalai JS (2010). Neuroimaging with near-infrared spectroscopy demonstrates speech-evoked activity in the auditory cortex of deaf children following cochlear implantation. Hearing Research, 270, 39–47. 10.1016/j.heares.2010.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Campbell J, & Cardon G (2015). Developmental and cross-modal plasticity in deafness: Evidence from the P1 and N1 event related potentials in cochlear implanted children. International Journal of Psychophysiology, 95, 135–144. 10.1016/j.ijpsycho.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, & Spahr AJ (2002). A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear and Hearing, 23, 532–539. 10.1097/01.AUD.0000042223.62381.01 [DOI] [PubMed] [Google Scholar]
- Sharma A, Glick H, Campbell J, Torres J, Dorman M, & Zeitler DM (2016). Cortical plasticity and reorganization in pediatric single-sided deafness pre- and postcochlear implantation: A case study. Otology & Neurotology, 37, e26–e34. 10.1097/MAO.0000000000000904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata DK (2007). Differences in brain structure in deaf persons on MR imaging studied with voxel-based morphometry. AJNR. American Journal of Neuroradiology, 28, 243–249. [PMC free article] [PubMed] [Google Scholar]
- Shiell MM, Champoux F, & Zatorre RJ (2015). Reorganization of auditory cortex in early-deaf people: Functional connectivity and relationship to hearing aid use. Journal of Cognitive Neuroscience, 27, 150–163. 10.1162/jocn_a_00683 [DOI] [PubMed] [Google Scholar]
- Shiell MM, Champoux F, & Zatorre RJ (2016). The right hemisphere planum temporale supports enhanced visual motion detection ability in deaf people: Evidence from cortical thickness. Neural Plasticity, 2016, 1–9. 10.1155/2016/7217630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiohama T, McDavid J, Levman J, & Takahashi E (2019). The left lateral occipital cortex exhibits decreased thickness in children with sensorineural hearing loss. International Journal of Developmental Neuroscience, 76, 34–40. 10.1016/j.ijdevneu.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarzynski PH, Wolak T, Skarzynski H, Lorens A, Sliwa L, Rusiniak M, … Olszewski L (2013). Application of the functional magnetic resonance imaging (fMRI) for the assessment of the primary auditory cortex function in partial deafness patients—A preliminary study. Journal of International Advanced Otology, 9, 153–160. [Google Scholar]
- Smith KM, Mecoli MD, Altaye M, Komlos M, Maitra R, Eaton KP, … Holland SK (2011). Morphometric differences in the Heschl's gyrus of hearing impaired and normal hearing infants. Cerebral Cortex, 21, 991–998. 10.1093/cercor/bhq164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJH, Shearer AE, Hildebrand MS, & Van Camp G (1993). Deafness and hereditary hearing loss overview In Pagon RA, et al. (Eds.), GeneReviews®. Seattle, WA: University of Washington. [Google Scholar]
- Smittenaar CR, MacSweeney M, Sereno MI, & Schwarzkopf DS (2016). Does congenital deafness affect the structural and functional architecture of primary visual cortex? Open Neuroimaging Journal, 10, 1–19. 10.2174/1874440001610010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinschneider M, Nourski KV, & Fishman YI (2013). Representation of speech in human auditory cortex: Is it special? Hearing Research, 305, 57–73. 10.1016/j.heares.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelnikov K, Marx M, Lagleyre S, Fraysse B, Deguine O, & Barone P (2014). PET-imaging of brain plasticity after cochlear implantation. Hearing Research, 322, 180–187. 10.1016/j.heares.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Sweeney AD, Carlson ML, Rivas A, Bennett ML, Haynes DS, & Wanna GB (2014). The limitations of computed tomography in adult cochlear implant evaluation. American Journal of Otolaryngology, 35, 396–399. 10.1016/j.amjoto.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Tae W-S (2015). Reduced gray matter volume of auditory cortical and subcortical areas in congenitally deaf adolescents: A voxel-based morphometric study. Investigative Magnetic Resonance Imaging, 19, 1–9. [Google Scholar]
- Takesian AE, Kotak VC, Sharma N, & Sanes DH (2013). Hearing loss differentially affects thalamic drive to two cortical interneuron subtypes. Journal of Neurophysiology, 110, 999–1008. 10.1152/jn.00182.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Chen Y, Maloney TC, Care MM, Holland SK, & Lu LJ (2013). Combined analysis of sMRI and fMRI imaging data provides accurate disease markers for hearing impairment. NeuroImage, 3, 416–428. 10.1016/j.nicl.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabichi O, Kozin ED, Kanumuri VV, Barber S, Ghosh S, Sitek KR, … Lee DJ (2018). Diffusion tensor imaging of central auditory pathways in patients with sensorineural hearing loss: A systematic review. Otolaryngology and Head and Neck Surgery, 158, 432–442. 10.1177/0194599817739838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschner M, Polite C, Lenarz T, & Lustig L (2013). Cochlear implantation in different health-care systems: Disparities between Germany and the United States. Otology & Neurotology, 34, 66–74. 10.1097/MAO.0b013e318278bf58 [DOI] [PubMed] [Google Scholar]
- Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, … Saguenay Youth Study (SYS) Group. (2014). The ENIGMA Consortium: Large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging and Behavior, 8, 153–182. 10.1007/s11682-013-9269-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble K, Blaser S, James AL, & Papsin BC (2007). Computed tomography and/or magnetic resonance imaging before pediatric cochlear implantation? Developing an investigative strategy. Otology & Neurotology, 28, 317–324. 10.1097/01.mao.0000253285.40995.91 [DOI] [PubMed] [Google Scholar]
- Tromp D (2016). DTI Scalars (FA, MD, AD, RD)—How do they relate to brain structure? The Winnower, 6, e146119.94778 10.15200/winn.146119.94778 [DOI] [Google Scholar]
- Vaden KI Jr., Kuchinsky SE, Ahlstrom JB, Dubno JR, & Eckert MA (2015). Cortical activity predicts which older adults recognize speech in noise and when. The Journal of Neuroscience, 35, 3929–3937. 10.1523/JNEUROSCI.2908-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC (1997). A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature, 385, 313–318. 10.1038/385313a0 [DOI] [PubMed] [Google Scholar]
- Wagstyl K, & Lerch JP (2018). Cortical thickness In Spalletta G, et al. (Eds.), Brain morphometry (pp. 35–49). New York, NY: Springer. [Google Scholar]
- Wang H, Liang Y, Fan W, Zhou X, Huang M, Shi G, … Shen G (2019). DTI study on rehabilitation of the congenital deafness auditory pathway and speech center by cochlear implantation. European Archives of Oto-Rhino-Laryngology, 276, 2411–2417. 10.1007/s00405-019-05477-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chen B, Yu Y, Yang H, Cui W, Li J, & Fan GG (2019). Alterations of structural and functional connectivity in profound sensorineural hearing loss infants within an early sensitive period: A combined DTI and fMRI study. Developmental Cognitive Neuroscience, 38, 100654 10.1016/j.dcn.2019.100654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Li Y-H, Zhou Y, Yu C-S, Xu C-L, Qin W, … Jiang T-Z (2009). Diffusion tensor imaging observation of brain white matter in congenitally deaf. Chinese Journal of Medical Imaging Technology, 25, 585–587. [Google Scholar]
- Williams C (2013). Hearing restoration: Graeme Clark, Ingeborg Hochmair, and Blake Wilson receive the 2013 Lasker-DeBakey Clinical Medical Research Award. The Journal of Clinical Investigation, 123, 4102–4106. 10.1172/JCI72707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis H (2018). The feasibility of the dual-task paradigm as a framework for a clinical test of listening effort in cochlear implant users. (PhD thesis). University College London. [Google Scholar]
- Wilson BS (2014). Getting a decent (but sparse) signal to the brain for users of cochlear implants. Hearing Research, 322, 24–38. 10.1016/j.heares.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, … Glahn DC (2010). Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage, 53, 1135–1146. 10.1016/j.neuroimage.2009.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2013). Millions of people in the world have hearing loss that can be treated or prevented.
- Wu CM, Ng SH, & Liu TC (2009). Diffusion tensor imaging of the subcortical auditory tract in subjects with long-term unilateral sensorineural hearing loss. Audiology & Neuro-Otology, 14, 248–253. 10.1159/000191282 [DOI] [PubMed] [Google Scholar]
- Wu CX, Huang LX, Tan H, Wang YT, Zheng HY, Kong LM, & Zheng WB (2014). Diffusion tensor imaging and MR spectroscopy of microstructural alterations and metabolite concentration changes in the auditory neural pathway of pediatric congenital sensorineural hearing loss patients. Brain Research, 1639, 228–234. 10.1016/j.brainres.2014.12.025 [DOI] [PubMed] [Google Scholar]
- Xia S, & Qi J (2008). The study of diffusion weighted imaging and MR spectroscopy in auditory cortex and related area of prelingual hearing-loss patients. Chinese Journal of Radiology, 42, 702–705. [Google Scholar]
- Xia S, Qi J, & Li Q (2008). High-resolution MR study of auditory cortex in prelingual sensorineural hearing loss. Chinese Journal of Medical Imaging Technology, 24, 1705–1707. [Google Scholar]
- Xiong X, Zhu LN, Dong XX, Wang W, Yan J, & Chen AG (2018). Aerobic exercise intervention alters executive function and white matter integrity in deaf children: A randomized controlled study. Neural Plasticity, 2018, 1–8. 10.1155/2018/3735208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Jiao Y, Tang TY, Zhang J, Lu CQ, Salvi R, & Teng GJ (2019b). Sensorineural hearing loss and cognitive impairments: Contributions of thalamus using multiparametric MRI. Journal of Magnetic Resonance Imaging, 50, 787–797. 10.1002/jmri.26665 [DOI] [PubMed] [Google Scholar]
- Xu XM, Jiao Y, Tang TY, Zhang J, Salvi R, & Teng GJ (2019a). Inefficient involvement of insula in sensorineural hearing loss. Frontiers in Neuroscience, 13, 133 10.3389/fnins.2019.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-Q, Wu F-Y, Hu H, Su G-Y, & Shen J (2015). Incidence of brain abnormalities detected on preoperative brain MR imaging and their effect on the outcome of cochlear implantation in children with sensorineural hearing loss. International Journal of Biomedical Imaging, 2015, 6–6. 10.1155/2015/275786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chen HJ, Liu B, Huang ZC, Feng Y, Li J, … Teng GJ (2014). Brain structural and functional alterations in patients with unilateral hearing loss. Hearing Research, 316, 37–43. 10.1016/j.heares.2014.07.006 [DOI] [PubMed] [Google Scholar]
- Younes L, Kutten KS, & Ratnanather JT (In press). Normal and equivolumetric coordinate systems for cortical areas In Breup M, Bruckstein AM, Kiselman CO, & Maragos P (Eds.), Shape Analysis: Euclidean, discrete and algebraic geometric methods. New York, NY: Springer; Available from https://arxiv.org/pdf/1911.07999.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JY, Ryan ME, & Young NM (2014). Preoperative imaging of sensorineural hearing loss in pediatric candidates for cochlear implantation. Radiographics, 34, E133–E149. 10.1148/rg.345130083 [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Zhang H, Simon TJ, & Gee JC (2008). Structure-specific statistical mapping of white matter tracts. NeuroImage, 41, 448–461. 10.1016/j.neuroimage.2008.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, & Canlon B (2015). Recognizing the journey and celebrating the achievement of cochlear implants. Hearing Research, 322, 1–3. 10.1016/j.heares.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Zhang GY, Yang M, Liu B, Huang ZC, Chen H, Zhang PP, … Teng GJ (2015). Changes in the default mode networks of individuals with long-term unilateral sensorineural hearing loss. Neuroscience, 285, 333–342. 10.1016/j.neuroscience.2014.11.034 [DOI] [PubMed] [Google Scholar]
- Zhou X, Seghouane AK, Shah A, Innes-Brown H, Cross W, Litovsky R, & McKay CM (2018). Cortical speech processing in postlingually deaf adult cochlear implant users, as revealed by functional near-infrared spectroscopy. Trends in Hearing, 22, 2331216518786850 10.1177/2331216518786850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoellner S, Benner J, Zeidler B, Seither-Preisler A, Christiner M, Seitz A, … Schneider P (2019). Reduced cortical thickness in Heschl's gyrus as an in vivo marker for human primary auditory cortex. Human Brain Mapping, 40, 1139–1154. 10.1002/hbm.24434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Yang Y, Fan W, Yu Q, Wang M, Han P, & Ma H (2018). Microstructural alterations in the brains of adults with prelingual sensori-neural hearing loss: A diffusion kurtosis imaging study. Otology & Neurotology, 39, e936–e943. 10.1097/MAO.0000000000002000 [DOI] [PubMed] [Google Scholar]