Abstract
Regardless of one’s opinion of antimicrobial stewardship programs (ASPs), it is hardly possible to work in hospital care and not be exposed to the term or its practical effects. Despite the term being relatively new, the number of publications in the field is vast, including several excellent reviews of general and specific aspects. Work in antimicrobial stewardship is complex, and includes not only aspects of infectious disease and microbiology, but also of epidemiology, genetics, behavioural psychology, systems science, economics and ethics, to name a few. This review aims to take several of these aspects and the scientific evidence of antimicrobial stewardship studies and merge them into two questions: How should we design ASPs based on what we know today? And which are the most essential unanswered questions regarding antimicrobial stewardship on a broader scale?
This narrative review is written in two separate parts aiming to provide answers to the two questions. This first part is written as a step-wise approach to designing a stewardship intervention based on the pillars of unmet need, feasibility, scientific evidence and necessary core elements. It is written mainly as a guide to someone new to the field. It is sorted into five distinct steps: (a) focusing on designing aims; (b) assessing performance and local barriers to rational antimicrobial use; (c) deciding on intervention technique; (d) practical, tailored design including core element inclusion; and (e) evaluation and sustainability. The second part, published separately, formulates ten critical questions on controversies in the field of antimicrobial stewardship. It is aimed at clinicians and researchers with stewardship experience and strives to promote discussion, not to provide answers.
Keywords: antibiotics, antimicrobial stewardship, implementation science
Background and context
Antimicrobial resistance provides a formidable challenge to healthcare as we know it.1 A World Health Organisation (WHO) report presented to the secretary general of the United Nations in April 2019 stated that antimicrobial resistance may cause 10 million annual deaths worldwide by 2050 and cause economic damage comparable with the effects of the 2008–2009 economic crisis.2 Whereas most stakeholders, such as clinical doctors, politicians and researchers, agree on the threat of antimicrobial resistance, it is difficult to reach consensus regarding the most appropriate solutions. The complexity of the issue cannot be overstated. A variety of interacting drivers contribute to the generation of resistance and selection of resistant bacteria, including not only antibiotic misuse in human and animal health, but also correct use of antibiotics, transmission of resistant bacteria in hospitals and during travel and migration, as well as through foodstuffs and environmental contamination of antimicrobial residues.3 Efforts to estimate the relative contribution to resistance of these factors have been made, even though such research is, per definition, challenging and biased towards what has been studied more extensively.4 In this context, no single solution has the potential to be effective alone, and there is need for coordinated, synergistic strategies in order to successfully counteract resistance development.
ASPs in human medicine are thus only a part of the counter-measures needed.5,6 In the WHO action plan on antimicrobial resistance, five strategic objectives were defined. In addition to optimising the use of antimicrobial agents and reducing the incidence of infection, the WHO stresses the need for improving awareness, increasing knowledge as well as developing an economic case for sustainable investment in new antimicrobials, diagnostic procedures and vaccines.7 In the review on antimicrobial resistance chaired by Professor Jim O’Neill, 10 strategic objectives were stated.8 Most of them overlap with the WHO objectives, but more focus is given to antimicrobial use in agriculture and to human resources working in the infectious diseases area. Both reports stress global coalition and cooperation.
Even though antimicrobial overuse and misuse in human medicine is only a part of the problem, it is well-established that increased use of antibiotics is associated with increased risk of selection of microorganisms resistant to antibiotics in populations and individuals, even though the reporting of such associations need improvement.3,9–12 This particular issue is complex in its own right, and the impact between different antimicrobials and patterns of use likely differs.13 It is, however, clear that the use of antimicrobials varies substantially between different geographical regions, for reasons that are not solely motivated by differences in the incidence of infections where antimicrobials are needed. It is therefore reasonable to allocate resources to reduce the use of antibiotics where it does not directly promote health. Moreover, practical clinical problems pertaining to antimicrobial resistance are most evident in hospital care, and here the consequences are also potentially the most grave,14,15 even though resistance in hospital-acquired infections is not unequivocally associated with increased mortality.16,17 It is thus not surprising that most initial stewardship efforts have been initiated ‘bottom-up’ in hospital settings by infectious disease clinicians and researchers linked to hospitals. This is now changing quickly. ASPs are spreading to different contexts and to a variety of stakeholders with varying degrees of experience and knowledge. On 30 September 2019, it became a CMS (Centers for Medicare & Medicaid Services) requirement that all US acute-care hospitals develop and implement an ASP by 30 March 2020.18
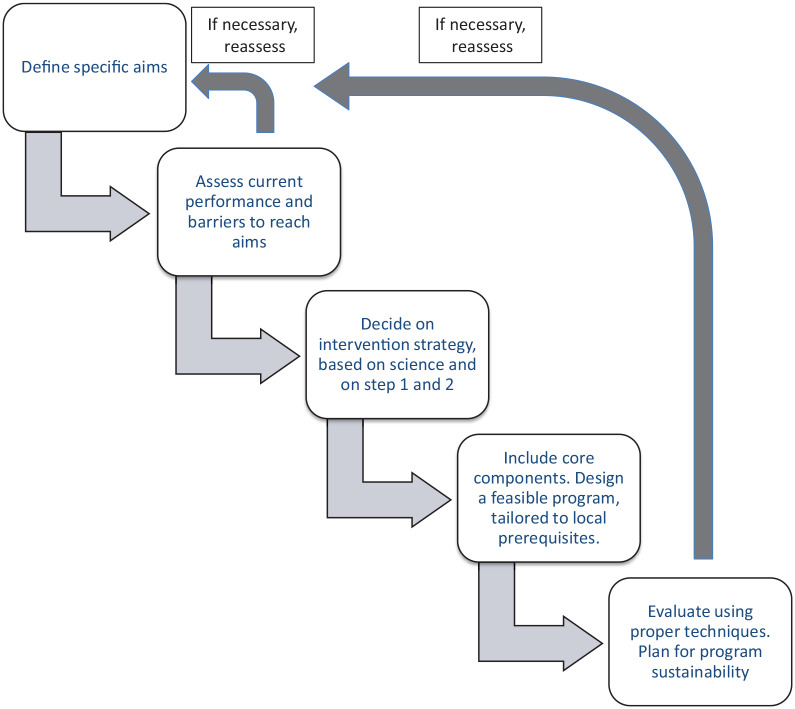
This first part aims to provide clarity to different aspects of designing an ASP from the start in five major steps (Figure 1) – the definition of clear aims, the assessment of current performance and of local barriers to rational antimicrobial use, the choice of methodology based on scientific evidence and results from steps 1 and 2, the practical design with inclusion of necessary core elements, tailored to local prerequisites and finally ensuring evaluation and sustainability. Each step is ended with a brief summary. Even though the steps are written in a logical order, the design process will in practice rarely be performed in simple, ordered steps. Findings in latter steps may, and should, prompt re-evaluation of prior steps. The scope of the review is thus very broad even though it is limited to ASPs addressing antibiotic misuse in human medicine, and the review does not aspire to be fully detailed in each aspect.
Figure 1.
The five steps of antimicrobial stewardship design.
A five-step guide to designing an antimicrobial stewardship intervention
Step 1: Prior to start – define specific aims of the ASP
Regardless of context, all ASPs should strive to have clearly defined aims. An overall aim of having an ASP is not specific enough, but potential adequate aims may vary. In this first step, the definition of antimicrobial stewardship is first presented, followed by a discussion on the universal objectives of ASPs. The section ends with a discussion on potential specific aims that are aligned with the overarching objectives.
Defining antimicrobial stewardship and responsible use of antibiotics
Antimicrobial stewardship is a term that is used liberally in a variety of contexts. Since many decision-makers recognise the need to counteract the development of antimicrobial resistance, and since ‘antimicrobial stewardship’ has been broadly (but sometimes vaguely) suggested to be a part of the solution, the term has an inherent value and there is a potential for misuse and confusion. According to the Oxford English dictionary, the contextual definition of a steward is ‘one who manages the affairs of an estate on behalf of his employer’.19 The general use of the term has a biblical origin, urging a good steward not to consume all he or she has been given, but to preserve some for others. The term ‘stewardship’ was first applied to antimicrobial use by McGowan and Gerding in 1996, even though coordinated work with the same objective had been carried out before the term was used.20 In the review by Dyer and colleagues,21 tracing the origin of the term, antimicrobial stewardship is def ined as: ‘a coherent set of actions which promote using antimicrobials responsibly’. This denotes a general strategy rather than a specific intervention. An ASP, in turn, defines the set of practical actions to be applied in this strategy and may vary. The definition leads to the question of how ‘responsible use of antimicrobials’ should be defined. A consensus definition of what responsible use is, including 22 different aspects – of which some are not generally associated with ASPs, such as waste disposal and local access – has been suggested by the DRIVE-AB initiative.22
What is the overarching objective of antimicrobial stewardship?
To define the main overarching objective of ASPs should be straightforward. After a read of key reviews on the subject, this is not necessarily the case. Simplified, there are two overlapping trains of thought on the primary objectives of ASPs: (a) using antibiotics in a way that aims to optimise healthcare outcomes while minimising unintended consequences of antibiotic use/the primary goal of hospital ASPs is to improve patient care23,24; and (b) using antibiotics in a way that aims to ensure sustainable access for all who need them.21,25 Depending on perspective, these objectives may be interpreted differently.26–28 The objectives are, while not mutually exclusive, subtly different in their basic premise. Whereas the first objective emphasises present patients and the reduction of unnecessary use of antibiotics primarily to reduce harm to the present patient, the latter objective to a greater degree implies balancing the need of present patients and potential future patients.
In an ideal situation, when all patients with infections have clearly defined diagnoses at the outset, including information on aetiology, and consensus documents on evidence-based therapeutic guidelines exist for all infections, the two interpretations are easily aligned. However, in real-life situations, where a substantial portion of antibiotic treatment in human medicine is given empirically, and where there is sometimes insufficient evidence to support consensus guidelines even when a diagnosis is clearly defined, a subjective assessment has to be made. This assessment will be affected by many of the factors which will later in this review be defined as potential barriers to rational use of antibiotics, including subject matter experience and knowledge, local treatment traditions and risk perception. The choice or interpretation of the primary objective of ASPs from the two above may also impact empirical treatment decisions, as a conflict between the two viewpoints may occur. It can be argued that, based on the evidence that unnecessary antibiotic treatment is one of the factors that leads to increased prevalence of resistance, we should strive to reduce unnecessary treatment even if this means that certain empirical choices entail a small, calculated risk to present patients, and then do our best to minimise this risk. On the contrary, it can also be fairly argued that such a level of risk, however small, is not acceptable to present patients since there is no way of being sure that this risk provides benefit to future patients. Both viewpoints strive to maximise total benefit, and the choice between the two is subjective, likely based on personality, experience and cultural context.
What are reasonable aims for stewardship programs?
In short, aims of ASPs should be (a) well-defined and preferably specific, (b) possible to measure and evaluate objectively and reliably, (c) related to the central objectives stated above, and (d) realistic and acceptable to all stakeholders. They do not necessarily have to be exactly quantitatively specified to be reasonable, but the more specifically stated they are, the easier they are to evaluate (Table 1). Potential general aims include improvement in patient outcomes, microbial outcomes or quality of use/process outcomes, as well as reductions in antimicrobial use or costs (including length-of-stay in hospitals). There is no absolute consensus on best practice, and stewardship programs can have an effect on a variety of outcomes.29–31 However, there seems to be a mismatch between the perception of the most important aims, and measures actually used. In a survey of physicians and pharmacists, most favoured aims related to patient outcomes and appropriateness of antibiotics, whereas performed studies had focussed mainly on quantitative antibiotic use and cost.26,32
Table 1.
Step 1 – Tips on defining aims of the planned intervention.
| Step 1 Define specific aims |
|---|
| Spend time and thought on this step Re-assess if necessary • Aims should always be: Well-defined Realistic Possible to measure effortlessly (automatically) and reliably over time Related to the general objectives of ASPs • Aims should preferably be: Specific and objective Ambitious Linked to a time-line Acceptable for all stake-holders Possible to benchmark and compare |
ASP, antimicrobial stewardship program.
All stewardship interventions should try to evaluate and report on objective patient outcomes, even if changes to patient outcomes are not the primary aim of the intervention.33 No effect on all-cause mortality from ASPs was seen in the latest Cochrane meta-analysis,30 so it is perhaps not realistic from scientific evidence that improvements in all-cause mortality would be generally expected, even though it has been demonstrated in some studies.29 All-cause mortality is objective, but has been criticised for not being causally related to stewardship. It is not recommended to define aims for a reduction of quantitative antibiotic use or cost without simultaneously assessing patient outcomes. However, the effect on treatment duration and quantitative antimicrobial use from the cited Cochrane review show high-certainty evidence that interventions can be effective in reducing duration of antibiotic treatment. Thus, a combined aim of reduced use with neutral effects on patient outcomes may be reasonable, and satisfies the objective of improving the use of antibiotics in a way that aims to optimize healthcare outcomes while minimising unintended consequences of antibiotic use.23
Aims that focus on improving antibiotic use quality/process measures, such as treatment guideline adherence, proportion of prescriptions where indications are documented in the medical record or increased appropriateness of prescribing are reasonable. Such outcomes have been criticised for measuring intermediates to general objectives rather than the objectives themselves.26,27 Also, as appropriateness has an inherent subjective component,34 and clinical treatment guidelines are not always as evidence-based as we assume,35 qualitative aims can be challenging to assess and interpret. Aims related to microbial outcomes (reduction of Clostridioides difficile or reduced carriage of resistant bacteria) may also be reasonable if they are combined with patient outcomes. A meta-analysis suggested potential effects on microbial outcomes from ASPs, especially when stewardship programs were implemented together with infection control measures.31 The Cochrane meta-analysis, which applies a more strict process in the selection of underlying studies, found microbial outcome results inconsistent.30 The merit of having defined aims, either regarding specific antimicrobials or overall quantitative use, has been demonstrated in several studies and programmes, from Scotland, Sweden and Denmark to name a few.20,36–38
Summary
Pre-defined aims should be a part of antimicrobial stewardship design. Aims should be specific, evaluable and in line with overall objectives of antimicrobial stewardship. The chosen aims may vary according to context and unmet need, but an assessment of objective patient outcomes should be included among them.
Step 2: Prior to start – choose metrics, assess current performance and barriers to rational and responsible antimicrobial use
Prior to a successful intervention, an assessment of current performance and an analysis of the most prominent barriers to reaching the proposed aim of the intervention (the unmet need) is essential. Unless the stewardship intervention is performed as a response to a particular problem (an outbreak or other), this step is not always performed in a formal and deliberate manner prior to the design of an intervention. Properly assessing performance prior to the start of change also facilitates evaluation considerably. Here, we describe the most common metrics related to antimicrobial stewardship and also describe the most common barriers to rational use of antimicrobials. If current performance and barriers suggest, the aims defined in step 1 can be re-defined.
By what metrics should current performance be assessed?
Several different metrics that reflect antimicrobial use are possible. They can be divided broadly into patient outcome measures, quantitative use measures, process/quality measures, microbial outcome measures and costs.39 Whatever metric is chosen, general rules apply. All metrics should preferably be objective, reliable (locally validated), easy to measure (now as well as later) and preferably directly related to aims and objectives. Even though objective comparison between healthcare settings requires the use of consensus metrics and common definitions of metrics, such are too seldom applied. Several reviews have specifically discussed the choice of appropriate metrics to assess the impact of ASPs.24,26,27,33,40–42 Here, available metrics are discussed, one group at a time (Table 2).
Table 2.
Step 2 – Potential metrics and their assessment.
| Step 2 Choose metrics, assess current performance and assess barriers to rational use | |
|---|---|
| Metrics to choose from | Assessment |
| Patient outcome metrics; All-cause mortality, infection-related re-occurrence or re-admission, number of unintended effects etc. |
Should be a part of an analysis. The more causally related to the intervention, the better patient outcome metric |
| Quantitative antibiotic use metrics; DDD, DOT or number of prescriptions per capita (outpatient care) |
Choose metric based on: What can be reliably measured, and What will allow benchmarking Adjust for case-mix or use SAAR |
| Strict process metrics; How is protocol followed, who attends rounds, how many changes to treatment courses are made, etc. |
Measures the reach, fidelity and usefulness of an
antimicrobial stewardship intervention Evaluates implementation quality and perceived usefulness |
| Qualitative metrics; Appropriateness of use, compliance to clinical guidelines, quality indicators |
Appealing in theory, but often challenging in
practice Quality indicators may be useful |
| Microbial outcome measures; Incidence of Clostridioides difficile infections, prevalence and infections caused by antibiotic-resistant organisms |
Effect affected by many factors outside of
ASPs Often slow changes over time |
| Cost measures; Costs directly related to antibiotics, costs related to length of stay in hospital, costs related to other outcomes. |
Often presented, Important in practice, but not directly related to the general objectives of ASPs |
ASP, antimicrobial stewardship program, DDD, defined daily dose; DOT, days of therapy; SAAR, standardized antimicrobial administration ratio.
Table 3.
Step 3 – Different intervention strategies and their assessment.
| Step 3 Decide on a general intervention strategy | |
|---|---|
| Intervention type | Assessment |
| Restriction intervention | Supported by scientific evidence May have low level of acceptance Flexible, but demanding Can be adjusted to barriers |
| Enablement intervention | Supported by scientific evidence Higher degree of acceptance Flexible, but demanding and sustained programs may be challenging Can be adjusted to barriers |
| Education and guidelines | Not supported by evidence as sole
measure, Complementary to restriction or intervention Can be adjusted to barriers |
| Antibiotic cycling | Not supported by scientific evidence Not very flexible Not very demanding |
| Behavourial change interventions | Promising Not enough studied in the context of antimicrobial stewardship |
| Mixed strategy | Appealing due to flexibility Better adjusted to actual barrier analysis Less generalizability |
The assessment of patient outcome measures is an important part of any ASP, regardless if it is the main outcome or not.43 A program that results in negative objective patient outcomes should be abandoned. Different patient outcome metrics are possible, depending on context: all-cause mortality, infection-related mortality, re-admissions to hospital and number of unintended consequences, to name a few. The main criticism against the most used patient outcome metrics is the lack of causal effect between the intervention and the outcome. The recent STEWARDS panel consensus statement did not consider a single patient outcome measure as ready for immediate use.24 However, until updated metrics on individual patient outcomes, such as the ranking of different combinations of efficacy and safety outcomes in a single patient according to desirability,44 are more widespread, it is still strongly recommended to pre-specify and report patient outcome metrics, mainly to make sure that the intervention is safe.33,41 Even though all-cause mortality is objective and should be considered, alternative outcome metrics, such as infection-related re-occurrence or readmission, disease/pathogen-specific rates of cure/treatment failure or on unintended effects, may be better purposed.26,40,41,45
Almost all stewardship programs include an assessment of quantitative antibiotic use. Quantitative use is only an intermediate in relation to the overarching objective, but with an assumed relation to it. The defined daily dose (DDD) or the days of therapy (DOT), per defined population or per admission (in hospitals), are most commonly applied. DDD is generally easier to access, whereas DOT is less affected by dosing differences between patients. The DDD measure is used by the European Surveillance of Antimicrobial Consumption Network (ESAC-NET)46 as well as by the WHO,47 whereas DOT is used by the US Centre for Disease Control and Prevention (CDC).48 In paediatric populations, DDD is not useful as it is not adjusted for dosing differences, and DOT is generally preferred.49 DOT is more intuitive to most clinicians and may correlate better with measures used in clinical trials.40 Unfortunately, the correlation between the two is not always good even when comparable dosing is used,50 possibly due to differences in data registration and extraction procedures. Most of the same metrics apply to quantitative use in outpatient care. A recent European interdisciplinary consensus document suggested a small range of outpatient use metrics; DDDs, prescriptions or courses per defined population or prescription/course per defined number of physician contacts.51 What metric to use for adults in the end depends on (a) what is locally feasible to measure reliably, (b) the need for comparison with other sources and (c) preference. It is worthwhile to consider what measures reflect; total population volume (DDD, DOT), or individual exposure (prescriptions), denominated by population or hospital admissions, since they reflect on different aspects of quantitative use.52 In order to better provide benchmarking for comparison, several ways of providing adjusted quantitative data for risk and setting have been proposed,53–56 of which the CDC’s SAAR (Standardized Antimicrobial Administration Ratio) metric has gained the most attention.
Process metrics that quantify the actions of a program or the adherence to a program, are important to document how resources are used, how often an indication for a prescription is recorded in the medical record and the level of adherence to stewardship program recommendations. Such measures are important features of assessing the success of the implementation process of a stewardship program and the impact of such a program,33 but have been criticised for measuring intermediates to the general objectives rather the objectives themselves.26,27
If qualitative process metrics, related to the appropriateness of use and compliance to clinical guidelines are included, however, they may be directly related to objectives. Appropriateness of use/adherence to guidelines is considered an attractive metric by most, including many reviews.26,57–60 Most also realize the inherent subjectiveness of appropriateness as a measure, including the difficulty of fitting clinical patients into clinical guidelines that in turn are not always evidence-based.24,34,40 Several attempts to define what appropriate really entails has been performed. Reviews have defined quality indicators in outpatient and inpatient antimicrobial use that reflect appropriateness. 57,58,60 Antimicrobial prescribing audit tools have also been designed.59 An assessment of quality indicators can very well be used as a metric, and improvement in them as an indication of improved quality, but it is recommended that they are accompanied by other outcome measures.
Microbial outcome metrics are related directly to the general objective of a stewardship program and are thus potentially important metrics. Metrics related to the incidence of C. difficile infection, to colonisation as well as infections with resistant bacteria are all potentially useful. Even though such metrics are recommended by the STEWARDS panel and other authors,24,33,41 the prevalence of resistant organisms are affected by screening processes, community transmissions as well as antimicrobial use, and often shifts over long periods of time. The potential effects in resistance levels are also dependent on starting levels.40,41 Such measures can thus be recommended as complementary, but rarely as primary aims of a stewardship program.
Cost metrics are secondary in the context of antimicrobial stewardship, even though they are often measured, and interventions are often associated with positive outcomes (lower costs).61 Cost measures are mainly secondary aims. It is important to note, however, that they are not secondary in the eyes of decision-makers, and cost-effectiveness analyses are needed for antimicrobial stewardship interventions, even though they are challenging. We will get back to cost-effectiveness analyses later in the review.
Benchmarking current performance
We suggest assessing current performance prior to the start of any antimicrobial stewardship intervention. Several studies have demonstrated that benchmarking can identify ‘outliers’ and problem areas with most urgent need for stewardship interventions.62,63 Whereas some measures, such as the proportion of appropriate prescriptions or adherence to guidelines, may intuitively tell us if the current performance is acceptable or not, most do not. The benchmarking of current assessment in turn requires the use of common metrics. This is the main reason for using the most commonly used metrics, especially for quantitative antimicrobial use. Yearly surveillance reports are published in several regions, but most prominently by the ECDC,46 that can be used for benchmarking. It is also often useful to assess local performance over time in an individual hospital or clinic, to adequately time and direct efforts. As earlier mentioned, recent publications have suggested metrics that adjust for case-mix and setting,53,63 which allows more fair comparisons. These should preferably be expanded.
Barriers to rational and responsible antimicrobial use
Why are antimicrobials not always given responsibly and rationally? Whereas the definition of what is responsible and rational is to a degree subjective, there is apparent unexplained differences in antibiotic use between different areas and settings, as evident by data from European countries in 2017.64 Even in the Netherlands, a country with consistent, low levels of antimicrobial use in human medicine,10,64 point-prevalence studies still suggest that 30% of initiated therapy is inappropriate.65 On a more general level, antimicrobial overuse has been applied to the context of the tragedy of the commons,66,67 where an exhaustible, common resource is used at the discretion of individuals and is subsequently depleted. Most experts believe that non-regulated antibiotic use has led to, or may lead to, a tragedy of the commons.68,69 In order to provide an adequate intervention to improve rational antimicrobial use, an assessment of the determinants of prescription behaviour is needed. This includes analysis of components of behaviour psychology, health-care structure and cultural context, and an addressing of those that lead to irrational use.70
Simplified, there are two levels on which determinants that affect prescriber behaviour and can be barriers to rational antimicrobial use exist, even though they often overlap. Internal barriers (barriers that affect subjective prescriber behaviour) and external or structural barriers, that in turn can occur in- and outside the healthcare system. The ‘internal barriers’ have in turn been separated into three different aspects in a recent review by Warreman et al.; behavioural beliefs (attitude towards behaviour, including their consequences, and responsibility towards patients, society and oneself), normative beliefs (the social team dynamics and relations to peer physicians) and control beliefs (including autonomy and hierarchy aspects that overlap with structural barriers).71
Some of the most commonly described internal barriers to rational use of antimicrobials include (a) insufficient knowledge regarding infectious diseases and clinical treatment guidelines,72–76 (b) a lack of trust in clinical treatment guidelines (indicating a discordance between guideline authors and prescribers),77–80 (c) a discounting of the effects of ones own prescription on resistance in general,75,81–83 (d) general uncertainty avoidance,72,75,84 (e) an unbalanced belief from anecdotal empirical experience,76,83 (f) an unbalanced wish to satisfy patient demands,85,86 (g) an ‘ownership’ of patients or a patient category that are viewed as exempt to general guidelines,87,88 (h) demand for autonomy79,80 and (i) a categorical perception of risk.89 Many of these factors co-exist, and some may be beneficial if properly balanced. For instance, some patient categories are more vulnerable to severe infections and a complete lack of uncertainty avoidance is not in the interest of patients. However, when guidelines are not followed, these are some of the prescriber behavioural factors that are often perceived as explanations.
External or structural barriers directly related to healthcare that may affect rational use of antimicrobials (excluding absence/presence of an antimicrobial stewardship intervention) include (a) insufficient available diagnostics,83 (b) stress/high work-load,73,90 (c) social team dynamics,73,76 (d) insufficient resources to provide a wait-and-see approach,83 (e) separate prescription pattern cultures in areas that deviate from guidelines,91 and (f) hierarchical structures that hinder decision sharing.86,88,92 External or structural barriers not related directly to health-care may include (a) lack of access to key antimicrobials,93 (b) access to over-the-counter antimicrobials,94 (c) low public awareness of antimicrobial resistance,95 and (d) reimbursement models or litigation processes that are discordant with rational use.86,96
How are barriers best assessed?
There are a number of ways to objectively assess what is going on when decisions regarding antimicrobial therapy are made and what determinants affect them. Excluding anecdotal reports, the most commonly used ways include structured surveys, semi-structured interviews, focus groups and direct observation. All of these methods have been used in assessment studies regarding barriers to rational antimicrobial use and they all have their respective merits and disadvantages.70 Whereas interviews and focus groups are costly, surveys are generally cheaper. All three mentioned methods are associated with response biases compared with direct observation. When conducting a survey, it is essential to acknowledge best practice techniques.97 It is possible to include an assessment of knowledge in addition to attitudes and behaviour in a survey context.74 It is recommended to use at least two complementary methods, ideally ones that do not have the same risks of bias, in order to provide a high-quality barrier assessment.98 It is suggested that one of these methods include an assessment not only of barriers but also of acceptability (and thus feasibility) of potential interventions, to improve detailed action planning and implementation techniques that is missing from most prior stewardship studies.99
Summary
Prior to initiating an ASP, the assessment of current performance as well as an assessment of barriers and facilitators to rational use of antibiotics, is recommended. This will not only provide an assessment of current performance, but also identify to the most pressing unmet needs, the best potential for intervention and a test of what metrics can be reliably assessed. This step may lead to a re-stating or adjustment of the aims defined in step 1.
Step 3: Prior to start – based on aims, current performance, barrier analysis and scientific evidence, decide on an intervention strategy
Whichever aims are stated, and whatever determinants are defined as the greatest barriers to reaching that aim, a specific intervention should always be designed with respect to the scientific evidence. In this section, we discuss different types of intervention methods and implementation techniques in antimicrobial stewardship.
Evidence-based stewardship design – a myth of fingerprints?
‘I’ve seen them all and man they’re all the same’ as Paul Simon sings in the song from his 1986 Graceland album. Does it matter what type of intervention is started, as long as people are truly engaged and agree on the aim? Scientific evidence suggests that it does. Antimicrobial stewardship interventions can be divided into four main types of strategies; restriction interventions, enablement interventions, educational interventions and antibiotic cycling, or a mix of several strategies (Table 3).
Restriction interventions can, for example, consist of formulary restrictions, the set-up of regulated lists of antibiotics, pre-authorization of use for selected antibiotics or that the choice of certain antimicrobial agents triggers an automatic infectious disease (ID) consultation. Restriction interventions have been demonstrated as independently associated with increased adherence to clinical guidelines and reducing antimicrobial use without negatively affecting patient outcomes.30 However, there is concern that restriction interventions, including need for pre-authorization, may lead to delays in appropriate treatment and that they generally do not foster a cooperative atmosphere regarding antimicrobial stewardship, which may lead to long-term negative effects.30,45 There are also publications questioning the feasibility of pre-authorization in settings with limited resources and expertise.100
Enablement interventions, which generally include audits or reviews of prescriptions and subsequent feedback, have also been demonstrated as independently associated with increased adherence to clinical guidelines and reduced antimicrobial use without evidence of patient harm.30 Moreover, they can enhance the effects of restriction interventions. It is clear from other types of healthcare interventions that monitoring (or self-monitoring) and feedback can be effective as behavioural change techniques.101,102 Such a strategy may thus directly affect barriers to rational use that are associated with physician behaviour (interval barriers in step 2). Unfortunately, enablement interventions in the area of antimicrobial stewardship are generally poorly specified and detailed in publications, perhaps because they are not viewed from the aspect of behavioural psychology.99 There is evidence that audit-feedback-based interventions are optimised if feedback is given using multimodal presentation, if feedback is repeated, if it is delivered by a colleague with sufficient mandate and is accompanied by clear targets and goals.103 If audit-feedback can be performed in the real-time patient care, it also borders on shared decision making, which is a related strategy that in turn has been suggested as beneficial.104,105 The downside of enablement interventions is that they are generally labour intensive.
Educational and guideline implementation strategies are intuitively attractive as they are comparatively simple and often feasible even with limited resources. Educational efforts and the spread of clinical guidelines also have a theoretical empowerment aspect. However, both the effects of ‘passive’ educational interventions and guideline publication on antimicrobial stewardship outcomes have been disappointing, generally explained by low guideline compliance without contextualised audit and feedback.42 Thus, educational efforts and guidelines are suggested as complementary aspects to restriction or enablement interventions in order to maximise effect. Antibiotic cycling is another strategy that has been suggested, but is supported neither by theory (mathematical modelling),106 nor clinical studies.107 Moreover, in studies that have been performed to test antibiotic cycling, adherence to protocols has been low.108
Choosing a strategy based on current performance and barrier assessments
It is thus clear from scientific evidence that certain types of interventions, mainly restriction and enablement interventions, have higher potential to change prescription behaviour. However, it is reasonable to assume that the efficacy of an intervention will reflect the current performance and the spectra of barriers with highest impact prior to the intervention.109 If current performance suggests high levels of use of wide-spectrum agents, combined with limitation in knowledge of clinical guidelines, a restriction intervention combined with an educational effort may be preferred. If the assessment shows that uncertainty avoidance and lack of clinical support are strong barriers to rational antimicrobial use, the effect of an enablement intervention with decision-sharing capacity will have the best potential to meet the unmet need. On the other hand, if external or structural factors, such as scheduling issues or reimbursement incentives promoting over-use, are found as essential barriers to rational use of antibiotics, efforts to change these should have higher priority. Thus, even though studies suggest that some general types of interventions have a better effect than others, which actual interventions to choose should be based on this evidence, but tailored to current performance and the state of the barriers to rational use.
How can barriers be overcome: evidence from behavioural science
As discussed in step 2, aspects of behavioural psychology often explain non-rational use of antimicrobials, even though structural factors within healthcare and outside of healthcare also contribute. Within behavioural science, specific taxonomy, methodology and implementation techniques have been designed to support behavioural change in other areas, such as smoking cessation and obesity.110,111 Until recently, very few studies or initiatives in the antimicrobial stewardship context had used or reported on the effects of such techniques.99,112,113 It is thus not clear to what extent such techniques are generalisable to the context of antimicrobial use. Recent reviews suggest using, and studies have used, proper behavioural science techniques,70,114,115 including intervention mapping and the behavioural change wheel in antimicrobial stewardship interventions, and the results are encouraging. However, since the issue of non-rational antimicrobial use is affected by a plethora of factors working on different levels and not only on individual behavioural psychology,116 more studies are needed to identify how to properly apply such techniques to the stewardship context.
Summary
Several different types of antimicrobial stewardship interventions may have a positive effect on outcomes, but the available scientific evidence favours programs based on either restriction or enablement interventions. The latter better promotes collegiality and is generally preferred. Complementary techniques may include educational activities and computer-based clinical support. Any chosen intervention technique should be tailored to the barriers that were identified in step 2.
Step 4: Start – Include core components, tailor to your particular setting and assure proper implementation
Following the definition of aims, the assessment of barriers to rational antimicrobial use and deciding on an antimicrobial stewardship intervention type, a practical stewardship program needs to be designed. This design should be guided by the three steps above, but needs to contain certain key core components and needs to be tailored to local prerequisites in order to succeed. The implementation phase of the program also has to be carefully planned in order to optimise chances of success. In this step, we discuss core components of stewardship programs, aspects of the stewardship team and aspects in certain patient populations or contexts. The section ends with a reflection on implementation techniques.
Core components of ASPs
The CDC, the European Commission, the Transatlantic Taskforce on Antimicrobial Resistance (TATFAR) and an international consensus group have all issued guidelines on core elements and components of ASPs.117–120 With the exception of the European Centre for Disease Prevention and Control (ECDC) document, which is structured differently, the core components of stewardship overlap in these documents and concern seven main aspects: (a) senior management commitment including resource allocation, (b) leadership including accountability, (c) expertise on infection management and antimicrobials, (d) action plans regarding rational antimicrobial use, (e) education and practical training, (f) monitoring use and resistance epidemiology and (g) reporting and feedback (Table 4). The TATFAR summary present 17 core indicators, whereas the CDC and international consensus group presents 29. The aspects of action plans regarding antimicrobial use and monitoring use and resistance epidemiology have been thoroughly covered in steps 2 and 3, and will not be discussed further here.
Table 4.
Step 4 – Core component and respective reflections.
| Step 4 Core components of antimicrobial stewardship programs | |
|---|---|
| Core component | Reflection |
| Senior management commitment | Essential for general support and resource
allocation. |
| Accountability and leadership | Essential for external accountability Define and document a team, respective responsibilities and plans for reporting Make sure the team and the proposed intervention is accepted and has mandate in the intervention target group |
| Subject matter expertise | A necessity in any expert-based intervention |
| Action plans for antimicrobial use improvement | Has been reflected on in steps 2 and 3 |
| Education and practical training | Of the stewardship team as well as of the target group
of the intervention Often overlooked |
| Monitoring use and disease epidemiology | A necessary part of any program, reflected upon in step 2 |
| Report, evaluate and give feedback | Will be reflected on in step 5 |
Senior management and resource allocation is a key core component of antimicrobial stewardship that is often not stressed enough. The effects of political leaders as well as hospital level management on enabling antimicrobial stewardship interventions cannot be over stated.121,122 This applies not only to proper resource allocation,123 but also to general attitudes towards prioritising the threat of antimicrobial resistance, which will affect chances of success. Thus, prior to starting a stewardship program, make sure that the initiative is supported by staff leaders and senior management, and that proper resources are allocated for its financing. As a second core component, the initiative should have a clear formal strategy with an identified leader, a defined multi-disciplinary team, documented roles of all participants, the collaborations between them and regular reporting. This is to ensure accountability of the stewardship program and its effect. In order to successfully drive such a program, the leader should have a clear mandate, not only formally but also informally among colleagues. Moreover, a continuous dialogue with prescribers and other target groups for the intervention, including involvement of local key opinion leaders, can increase adherence and acceptance and are potential factors for success.124,125
Providing expertise on infection management may sound self-evident, but providing that this includes proper diagnostic services as well as healthcare professionals trained in infectious disease management, not all hospitals or outpatient care facilities are self-sufficient. Recently, a consensus list of generic competencies in diagnostics, antimicrobial prescribing and stewardship was suggested.126 The fifth core component suggests that a stewardship program should contain an educational component on two levels: an education of staff working within an ASP as well as an education of prescribers targeted by the program, including easily accessed, updated facility-specific treatment guidelines. As mentioned, it has been established that a passive education of prescribers is not sufficient on its own, but has potential for effect when paired with other interventions. In some studies, active guideline dissemination has demonstrated positive effects on antimicrobial use.127 Finally, regular reporting on chosen objective outcomes, potentially including self-assessments, is a key core component of any healthcare intervention including antimicrobial stewardship, to provide a basis for sustainability and non-harm.
There are a number of practical guidelines based on evidence and core components above. The most cited include the Infectious Diseases Society of America (IDSA) guidelines from 2007 and 2016,23,107 and the ECDC guidelines,128 but several countries, including Germany, France, the UK and Australia, have published independent national guidelines that are compiled on the ECDC web page.129 On a more local level, several regions and hospitals have published their own guidelines tailored to respective prerequisites. The content and structure of these guidelines vary in scope and focus, but few of them suggest analysis of local barriers to rational use and adjusting interventions accordingly.
Adjusting to local prerequisites and settings
The stewardship team
In international literature, the stewardship team generally consists of infectious disease physicians, pharmacists, microbiologists and, possibly, administrators.42 However, the composition varies, as the role in clinical care for pharmacists, physicians and microbiologists varies between different settings. Not all countries have dedicated infectious disease physicians, who would normally take the team leader role to ensure that guidelines and policies are based on evidence and best practice. Even in countries where there are dedicated ID physicians, these may focus generally on infections in tertiary settings and not on stewardship. The roles of pharmacists vary between different settings, but, in settings where pharmacists process medication orders, they are essential to practical stewardship. They, however, have to be formally trained in antimicrobial use and stewardship. Pharmacist involvement has been demonstrated to lead to more appropriate therapy in studies.130 The clinical microbiologist should provide timely diagnostics as well as surveillance data. In addition, many teams have information technology experts and implementation scientists as essential components to provide adequate data, structure and follow-up.
The composition of a team will affect what types of intervention are feasible. Several publications, including the ECDC guidelines, suggest that nurses can contribute substantially to a stewardship team effort,131 and the same is suggested for infection control practitioners.128 The stewardship team components should be tailored to the aims and objectives of the program and the program design itself.
Populations and settings other than adult hospital care
Most ASPs focus on adults treated in hospital care. In reality, the majority of antibiotics are used in outpatient settings, in paediatrics and in long-term care facilities. Since paediatric hospital care, long-term care facilities and outpatient settings have distinct features that separate them from adult hospital settings, neither knowledge nor intervention types can be fully generalised to such settings.
In paediatrics, the use of multidisciplinary antimicrobial stewardship teams mimicking those for adults is encouraged, even though the evidence is limited compared with ASP in adults.49,132,133 Children are especially vulnerable to overuse (resistance and adverse events) as well as underuse of antibiotics, and treatment indications as well as dosing practice differ from adults. In addition, clinical and microbiological diagnostics is often challenging in children. This suggests that a multidisciplinary stewardship team in paediatrics should be separate from adult teams, with specific expertise tailored to paediatric care.134
With an increasing portion of the population reaching old age, the demand for healthcare facilities for the elderly has increased, and variants of long-term care facilities are increasingly common. However, the organisation of care in long-term care facilities differs substantially from that of hospital care. Elderly individuals cared for in long-term care facilities (LTCFs) are often frail and vulnerable to infections, and the diagnosis of infections is challenging due to atypical presentations and the reduced ability to describe complaints.135 There is vast variation of antimicrobial consumption in LTCFs and studies suggest a large proportion of inappropriate prescriptions.135,136 There is thus a need for stewardship interventions tailored to LTCF populations, but only moderate evidence of their effect, due mainly to study heterogeneity.137 Recently, reviews have suggested what specific components may facilitate successful stewardship interventions in LTCF settings (multidisciplinary education, review tools integrated in the workflow of prescribers and limiting unnecessary cultures from urine and wounds).115,138,139
Since the majority of antimicrobial use occurs in outpatients, and since studies suggest that a substantial proportion of this use is inappropriate or without documented indication,140,141 antimicrobial stewardship in outpatient settings has major potential in affecting antimicrobial use. Outpatient antimicrobial use primarily addresses primary care prescribing, but also includes antimicrobial use at discharge from the emergency department and from hospital care.142–144 Studies have suggested that individualised audit and feedback may have positive effects in this setting as well,145 but interventions have to be tailored to the outpatient setting. The CDC has identified core elements for outpatient stewardship that mirror those of hospital core elements, including leadership commitment, action plans for optimising use, surveillance/reporting and education/expertise.146 Different interventions have been tried in the outpatient setting, including feedback from a high-profile messenger resulting in a reduction in unnecessary use.147 Guidelines suggest that conditions where antimicrobials are rarely indicated should be targeted specifically (upper respiratory tract infections, pharyngitis and sinusitis).148 A systematic review on outpatient stewardship showed great heterogeneity in design and effect, and details of interventions were seldom reported.149
Considerations in low- and middle-income settings
The health-care system organisation, the availability of diagnostic testing and antibiotics in the AWaRe access category, public awareness and prescribing practices differs between high-income countries and low- and middle-income countries (LMIC).150 Cultural and contextual factors also vary widely between different settings, highlighting the need for contextualisation of interventions.151,152 A recent systematic review on hospital stewardship interventions in LMIC settings concluded that the majority of published studies reported positive effects, but heterogeneity of methodology and a lack of coverage of major parts of the world impaired generalisations.153 The WHO has issued a separate practical toolkit for ASPs in LMICs, based on its five-point action plan from 2015.7,154 A specific challenge is the surveillance and control of multi-drug resistant organisms in the LMIC setting, where prevalence is often high or unknown. There is optimism of increasing laboratory capacity in LMIC, in light of the successful rollout of point-of-care diagnostics for human immunodeficiency virus (HIV), tuberculosis (TB) and malaria. These may pave the way for similar point-of-care diagnostics for multi-resistant bacteria, increasing local surveillance and potentiating stewardship.155,156
Focusing on specific disease syndromes
In response to analysis of determinants of antimicrobial use, it may be reasonable and cost-effective to focus effort on specific disease syndromes, or even specific antibiotics. In addition to the most commonly targeted respiratory tract and urinary tract infections, efforts for antimicrobial stewardship have been suggested in urology, intensive care unit (ICU) infections, intra-abdominal infections and skin and soft tissue infection (SSTI), to name just a few.157–160 As further examples, specific interventions have also been proposed and performed to target fluoroquinolone and carbapenem use.161,162 The essential issue is to try to assess what interventions are needed and where they are needed prior to start, in order to optimise chances of effect.
Evidence-based implementation techniques of ASPs
In reality, the techniques of a healthcare intervention and the implementation of that intervention overlap substantially and are sometimes confused.163 The intervention part defines what type of intervention that is suggested to be clinically beneficial and should be implemented. The results can be evaluated using defined outcome measures as discussed above. The implementation part, in contrast, provides a structure on how this intervention should be spread to optimize adherence. This can be evaluated using process measures. However, if the results of process measures are poor, the outcome measures will be duly affected. Since interventions in clinical healthcare are complex, even if aspects of the first three steps in this review are considered carefully prior to starting a program, the program risks failure unless proper implementation technique is applied. A qualitative study from Virginia, assessing barriers to successful implementation of antimicrobial stewardship, highlights that the buy-in by target groups is an essential factor for success, and was in turn affected by communication style and conflict management.164 This emphasises a point from step 2, that an assessment of barriers to implementation to stewardship interventions is an important addition to assessment of barriers to rational antimicrobial use.99
In addition to context-dependent barriers to implementation, several considerations have to be made in the implementation phase of an intervention to ensure delivery according to the intended plan, with adequate coverage in spite of unexpected challenges.114 Implementation science is a scientific field in its own right, and suggests a step-wise process to implement complex interventions in reaction to new scientific information, guidelines or protocols. The steps include an initial proposal for change, an analysis of current performance and identification of targets for change, a problem analysis of the intended target group, a design of an evidence-based intervention strategy including factors of dissemination and maintenance, the testing and execution of intervention plans, the integration of the changes into sustainable routine, and the evaluation of intended and unintended consequences, with a readiness to go back and reassess at any step if necessary.165 This review has been written in the vein of implementation science, with the final three steps collapsed into one. Practical examples that may be used to ensure proper implementation include implementation mapping in parallel to intervention mapping and behaviour change techniques, such as the behavioural change wheel.166,167
Summary
Regardless of the prior steps, sustainable success of antimicrobial stewardship requires the establishment of core elements (such as commitment from leadership, proper resources, staff expertise and accountability). The ASP also needs to be tailored to local prerequisites and last, but not least, an intervention needs to heed proper implementation techniques in order for effects to be properly evaluated.
Step 5: After start – pilot, evaluate and either re-assess or integrate into clinical care
Three essential, but often neglected, parts of antimicrobial stewardship design are testing, evaluating and planning for sustainability or change. In this final step of the review, a reflection on the general quality of the scientific evaluations of ASPs in published research is made. Testing and evaluation techniques is then discussed, as well as attributes of programs that support sustainability and finally the aspect of cost-effectiveness of antimicrobial stewardship interventions.
What is the state of quality of research of antimicrobial stewardship interventions?
There is consensus that the reason for the relative shortage of scientific evidence regarding best practice of intervention and implementation strategies in antimicrobial stewardship is that many studies are of moderate quality and are heterogenous in design and outcome. This notion is supported by several reviews.29,109,168,169 Important aspects are often lacking or should have been performed differently. This includes elements of proper research design such as cluster randomisation or placebo-controlled interrupted time series,170,171 a lack of reporting on clinical and microbiological outcome data including unintended consequences,169 and a lack of details regarding the actual intervention program and process.99 These factors make the published data on stewardship intervention difficult to interpret and generalise.
However, antimicrobial stewardship interventions are complex healthcare interventions, and there is a need to contextualise. For most diseases subjected to intervention studies, the pathogenesis of the disease and the underlying objective is reasonably well known. This cannot be said for either the relationship between human antimicrobial use and resistance development in general or even in specific contexts, or for optimal objective assessment in the balance between use of antimicrobials today and sustainable access to all who need them. This does not defend using improper research designs. On the contrary, this emphasises the need for proper and rigorous research methodology and detailing of interventions, implementations of interventions and outcomes. However, even if this is properly performed, the generalisability of ASPs will still be unclear.
Practical testing, evaluation and reporting of stewardship interventions
A reasonable start to any complex intervention in a new setting is to perform a pilot intervention. Ideally, this should be a small-scale test of the plan on motivated persons, groups or institutions.165 This helps in preliminary evaluations of the validity of chosen outcome metrics and of intended and unintended outcomes, but also in assessments of process measures such as delivery, fidelity and acceptance of the intervention. If outcome metrics are not valid, they should be changed. If outcome measures do not meet expectations, first analyse process measures, and, if these perform poorly, the implementation method should probably be changed. If the process measures perform well, but preliminary outcome measures are poor, the reasons for this should be analysed and adjustments to aims, intervention strategy, core elements, target group or other factors should be adjusted. If process measures and outcome measures perform well, a plan for gradual expansion can be considered (Table 5).
Table 5.
Step 5 – A flowchart for initial evaluation.
| Step 5 Pilot, evaluate, re-assess or integrate into clinical care |
|---|
| Flow-chart |
| • Perform a small-scale pilot project on especially
motivated targets • Assess process measures (delivery, fidelity and acceptance) • Assess the validity and ease of applied metrics • If necessary, go back to step 2, 3 or 4 and adjust implementation technique and/or metric • If process measures are satisfactory and metrics valid, assess outcome metrics • If outcome metrics are satisfactory, plan for gradual expansion and normalisation • If outcome metrics are unsatisfactory, start over and go back to step 1 (Did we have realistic aims?), step 2 (Is there really room for improvement? Do we address the identified barriers?), step 3 (Is this intervention technique proper?) and 4 (Do we have core elements in place? Have we tailored the intervention properly?) |
How to evaluate change depends on the purpose of the evaluation, separated into to quality improvement assessment or research. Whereas projects planned for research publication should be stringently designed according to earlier discussion,170 interventions striving to increase quality of care can be evaluated differently. This ultimately goes back to the aims chosen in step 1 and the outcome and process metrics chosen in step 2. These aims should be precise and unambiguous as well as ambitious but realistic in order to evaluate success.165 These aims should also have a time-line or a deadline.
Reporting of results of interventions as well as on antibiotic use and resistance to all relevant stakeholders is a core element of stewardship guidelines. How results and evaluations should be reported depends on the context. However, it is crucial that regular reporting of process measures and outcomes measures in relation to objectives and goals are performed to ensure accountability. Such reporting can include feedback from audits and reviews. Several national and international antimicrobial stewardship guidelines mentioned earlier have useful tips on reporting facility-specific and appropriateness results as well as benchmarking results.118,172,173 Specific reports to providers including comparisons with comparable peers, may be an inspirational tool, and have been effective in improving antimicrobial use.174,175
How can we make effects sustainable?
One of the most challenging issues to antimicrobial stewardship interventions in addition to external validity has been the lack of sustainability. Old habits die hard, and the relapse of earlier behaviour and outcomes is often the case when a successful project is discontinued, which has been demonstrated for antimicrobial stewardship interventions from Maryland and Pennsylvania to name a few.114,176–178 Apart from securing long-term resources to a stewardship program that is successful, system analysis tells us that the best way to provide sustainable change is to integrate the planned improvement or intervention in normal clinical routines, a process referred to as normalisation.179 To achieve sustainable effect, a project should be planned from the outset based on the capacity to incorporate it into the normal work routine of the intended targets of the intervention. This suggests that interventions using intense resources and methods that depart substantially from clinical routines have slim chances of long-term success, however successful they may initially seem.
It is also important to consider what factors are most crucial to a sustained process or a sustained effect. Sustainability requires continuous involvement from all stakeholders (leadership, interventionists and target groups), continuous follow up, long-term budgets, the visibility of positive effects along the way, a dedicated champion/pioneer, continued good structure and adaptability.180 The UK National Health Service (NHS) has developed an instrument specifically dedicated to the sustainability of an intervention project, highlighting these factors and others such as credibility, vulnerability and infrastructure.181 It is recommended that this or similar guidelines are reviewed prior to the start of an antimicrobial stewardship intervention.
Evaluating costs and benefits
The issue of cost-effectiveness is essential in order to provide a rational basis for prioritising between different efforts to counter antimicrobial resistance. There are several ways to assess the costs of an ASP, including a cost-balance analysis, cost-effectiveness analyses and cost-benefit analyses. Whereas cost and cost-benefit analyses consider monetary units only (and consequences that are not strictly monetary are translated to monetary units), cost-effectiveness analyses consider health outcomes.182 Cost-effectiveness analysis, cost-utility analysis, cost-benefit analysis and direct cost analysis have all been used to evaluate ASPs.183
To perform a cost-effectiveness/cost-utility analysis (here batched for simplicity), a unit of health benefit and an incremental cost-effectiveness ratio (ICER) threshold first has to be defined. The chosen unit of health should lend itself to feasible, objective measurement. Quality-adjusted life year (QALY) is often used but alternatives can be reasonable, such as the number of infections prevented (vaccine studies) or years of life gained. An acceptable ICER threshold has to be pre-defined, and ultimately decides whether the intervention is cost-effective. Reviews suggest that restrictive or enablement antimicrobial stewardship interventions are likely cost-effective, but that the scientific evidence is limited and few studies have performed a full cost-effectiveness analysis.182,184,185 As in most research evaluating ASPs, studies are generally of moderate quality, and there is heterogeneity in design and outcomes.185
Cost-benefit analyses of stewardship programs imply a calculation of the total monetary costs of antimicrobial resistance, which is complicated. Different estimations have been performed to assess such costs, using costs of resistance compared with susceptible bacteria,14 costs per antibiotic consumed,186 total cost and total economic burden.187,188 However, due to the heterogeneity of studies and a lack of inclusion of multiple perspectives, failure to include marginal costs and to adjust for inflation, it is difficult to get a comprehensive view of the cost-benefits of ASPs.189
Summary
A necessary part of any intervention program is to have a plan for evaluation using robust evaluation techniques. Following proper evaluation, it is essential to adjust programs if they do not provide the effects hoped for. Move back to step 1 and re-assess aims, current performance and intervention strategies. If results are satisfactory, it is important to have a strategy for sustainability of a program, normally by incorporating it into the clinical routine.
Conclusion and future aspects
A successful antimicrobial stewardship intervention needs to be carefully planned and implemented in order to maximise chances of success. This five-step review offers a framework for doing so based on current scientific knowledge in the field of stewardship and on implementation science. However, antimicrobial stewardship is a comparatively young field in a complex context. There are still many unanswered questions and controversies. A selection of 10 such questions is raised in the second part of this review with the explicit purpose to stimulate discussion and discourse. Three recent, excellent reviews have also defined pressing research need within the field.45,178,190 It is likely that novel scientific findings, for instance regarding the complex dynamic between use and resistance,191–194 and the application of clinical support systems in treatment decisions based on artificial intelligence,195–197 will affect the way we perform antimicrobial stewardship in the not so distant future. It is clear that antimicrobial stewardship has come a long way since the start more than 20 years ago, but also clear that a combination of globally coordinated and locally adjusted efforts are needed to achieve even better impact.25,198
Footnotes
Author contributions: FR conceived, organised and wrote the review.
Conflict of interest statement: The author declare that there is no conflict of interest.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Swedish Government Fund for Clinical Research (ALF) and by PLATINEA (Plattform för Innovation av Existerande Antibiotika).
ORCID iD: Fredrik Resman  https://orcid.org/0000-0002-3433-512X
https://orcid.org/0000-0002-3433-512X
References
- 1. Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the infectious diseases society of America. Clin Infect Dis. 2009; 48: 1–12. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. No time to wait: securing the future from drug-resistant infections. Geneva, Switzerland: World Health Organization, http://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1 (2019, accessed 8 November 2019). [Google Scholar]
- 3. Barbosa TM, Levy SB. The impact of antibiotic use on resistance development and persistence. Drug Resist Updat 2000; 3: 303–311. [DOI] [PubMed] [Google Scholar]
- 4. Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387(10014): 176–187. [DOI] [PubMed] [Google Scholar]
- 5. Wilson J. A strategy for tackling antimicrobial resistance: it’s more than a prescribing problem. J Infect Prev 2019; 20: 64–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duerden B, Fry C, Johnson AP, et al. The control of methicillin-resistant staphylococcus aureus blood stream infections in England. Open Forum Infect Dis 2015; 2: ofv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. GLOBAL action plan on antimicrobial resistance. Geneva, Switzerland: World Health Organization, https://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ (2015) [DOI] [PubMed] [Google Scholar]
- 8. O’Neill JM. Tackling drug-resistant infections globally: final report and recommendations.Review on Antimicrobial Resistance, https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed 7 January 2020).
- 9. Bell BG, Schellevis F, Stobberingh E, et al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014; 14: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goossens H, Ferech M, Vander Stichele R, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005; 365: 579–587. [DOI] [PubMed] [Google Scholar]
- 11. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340: c2096. [DOI] [PubMed] [Google Scholar]
- 12. Tacconelli E, Cataldo MA, Paul M, et al. STROBE-AMS: recommendations to optimise reporting of epidemiological studies on antimicrobial resistance and informing improvement in antimicrobial stewardship. BMJ Open 2016; 6: e010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olesen SW, Barnett ML, MacFadden DR, et al. The distribution of antibiotic use and its association with antibiotic resistance. Elife 2018; 7: e39435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neidell MJ, Cohen B, Furuya Y, et al. Costs of healthcare- and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clin Infect Dis 2012; 55: 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Antimicrobial resistance: global report on surveillance. Geneva, Switzerland: World Health Organization, http://www.who.int/drugresistance/documents/surveillancereport/en/ (14 November 2014). [Google Scholar]
- 16. Klevens RM, Edwards JR, Gaynes RP; National Nosocomial Infections Surveillance System. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis 2008; 47: 927–930. [DOI] [PubMed] [Google Scholar]
- 17. Lambert ML, Suetens C, Savey A, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis 2011; 11: 30–38. [DOI] [PubMed] [Google Scholar]
- 18. Department of Health and Human Services. Medicare and medicaid programs; regulatory provisions to promote program efficiency, transparency, and burden reduction; fire safety requirements for certain dialysis facilities; hospital and critical access hospital (CAH) changes to promote innovation, flexibility, and improvement in patient care, http://www.govinfo.gov/content/pkg/FR-2019-09-30/pdf/2019-20736.pdf (2019, accessed 25 November 2019).
- 19. Oxford English Dictionary, https://www-oed-com.ludwig.lub.lu.se/view/Entry/190087?rskey=7BxByx&result=1&isAdvanced=false#eid (2019, accessed 9 October 2019).
- 20. Mölstad S, Erntell M, Hanberger H, et al. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish strama programme. Lancet Infect Dis 2008; 8: 125–132. [DOI] [PubMed] [Google Scholar]
- 21. Dyar OJ, Huttner B, Schouten J, et al. ; ESCMID Study Group for Antimicrobial stewardship. What is antimicrobial stewardship? Clin Microbiol Infect 2017; 23: 793–798. [DOI] [PubMed] [Google Scholar]
- 22. Monnier AA, Eisenstein BI, Hulscher ME, et al. ; DRIVE-AB WP1 group. Towards a global definition of responsible antibiotic use: results of an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73(Suppl. 6): vi3–vi16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dellit TH, Owens RC, McGowan JE, Jr, et al. ; Infectious Diseases Society of America, Society for Healthcare Epidemiology of America. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159–177. [DOI] [PubMed] [Google Scholar]
- 24. Moehring RW, Anderson DJ, Cochran RL, et al. ; Structured Taskforce of Experts Working at Reliable Standards for Stewardship (STEWARDS) Panel. Expert consensus on metrics to assess the impact of patient-level antimicrobial stewardship interventions in acute-care settings. Clin Infect Dis 2017; 64: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dyar OJ, Obua C, Chandy S, et al. Using antibiotics responsibly: are we there yet? Future Microbiol 2016; 11: 1057–1071. [DOI] [PubMed] [Google Scholar]
- 26. Dodds Ashley ES, Kaye KS, DePestel DD, et al. Antimicrobial stewardship: philosophy versus practice. Clin Infect Dis 2014; 59(Suppl. 3): S112–S121. [DOI] [PubMed] [Google Scholar]
- 27. McGowan JE. Antimicrobial stewardship–the state of the art in 2011: focus on outcome and methods. Infect Control Hosp Epidemiol 2012; 33: 331–337. [DOI] [PubMed] [Google Scholar]
- 28. Merrett GLB, Bloom G, Wilkinson A, et al. Towards the just and sustainable use of antibiotics. J Pharm Policy Pract 2016; 9: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schuts EC, Hulscher MEJL, Mouton JW, et al. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 847–856. [DOI] [PubMed] [Google Scholar]
- 30. Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017; 2: CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17: 990–1001. [DOI] [PubMed] [Google Scholar]
- 32. Bumpass JB, McDaneld PM, DePestel DD, et al. Outcomes and metrics for antimicrobial stewardship: survey of physicians and pharmacists. Clin Infect Dis 2014; 59(Suppl. 3): S108–S111. [DOI] [PubMed] [Google Scholar]
- 33. Schweitzer VA, van Werkhoven CH, Rodríguez Baño J, et al. ; Joint Programming Initiative on Antimicrobial Resistance Working Group on Design of Antimicrobial Stewardship Evaluations. Optimizing design of research to evaluate antibiotic stewardship interventions: consensus recommendations of a multinational working group. Clin Microbiol Infect 2020; 26: 41–50. [DOI] [PubMed] [Google Scholar]
- 34. DePestel DD, Eiland EH, III, Lusardi K, et al. Assessing appropriateness of antimicrobial therapy: in the eye of the interpreter. Clin Infect Dis 2014; 59(Suppl. 3): S154–S161. [DOI] [PubMed] [Google Scholar]
- 35. Lee DH, Vielemeyer O. Analysis of overall level of evidence behind infectious diseases society of America practice guidelines. Arch Intern Med 2011; 171: 18–22. [DOI] [PubMed] [Google Scholar]
- 36. Boel J, Andreasen V, Jarløv JO, et al. Impact of antibiotic restriction on resistance levels of escherichia coli: a controlled interrupted time series study of a hospital-wide antibiotic stewardship programme. J Antimicrob Chemother 2016; 71: 2047–2051. [DOI] [PubMed] [Google Scholar]
- 37. Huttner B, Harbarth S, Nathwani D; ESCMID Study Group for Antibiotic Policies. Success stories of implementation of antimicrobial stewardship: a narrative review. Clin Microbiol Infect 2014; 20: 954–962. [DOI] [PubMed] [Google Scholar]
- 38. Tängdén T, Eriksson BM, Melhus A, et al. Radical reduction of cephalosporin use at a tertiary hospital after educational antibiotic intervention during an outbreak of extended-spectrum beta-lactamase-producing klebsiella pneumoniae. J Antimicrob Chemother 2011; 66: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 39. Brotherton AL. Metrics of antimicrobial stewardship programs. Med Clin North Am 2018; 102: 965–976. [DOI] [PubMed] [Google Scholar]
- 40. Morris AM. Antimicrobial stewardship programs: appropriate measures and metrics to study their impact. Curr Treat Options Infect Dis 2014; 6: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dik JWH, Hendrix R, Poelman R, et al. Measuring the impact of antimicrobial stewardship programs. Expert Rev Anti Infect Ther 2016; 14: 569–575. [DOI] [PubMed] [Google Scholar]
- 42. MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev 2005; 18: 638–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gillespie D, Francis NA, Carrol ED, et al. Use of co-primary outcomes for trials of antimicrobial stewardship interventions. Lancet Infect Dis 2018; 18: 595–597. [DOI] [PubMed] [Google Scholar]
- 44. Evans SR, Rubin D, Follmann D, et al. Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis 2015; 61: 800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bassetti M, Giacobbe DR, Vena A, et al. Challenges and research priorities to progress the impact of antimicrobial stewardship. Drugs Context 2019; 8: 212600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. European Centre for Disease Prevention and Control. European Surveillance of Antimicrobial Consumption Network (ESAC-Net) - Data collection and analysis, http://www.ecdc.europa.eu/en/about-us/networks/disease-networks-and-laboratory-networks/esac-net-data (2019, accessed 30 September 2019).
- 47. World Health Organization. WHO methodology for a global programme on surveillance of antimicrobial consumption. World Health Organization, https://http://www.who.int/medicines/areas/rational_use/WHO_AMCsurveillance_1.0.pdf?ua=1 (2016, accessed 30 September 2019).
- 48. CDC. Antimicrobial use and resistance (AUR) module, http://www.cdc.gov/nhsn/pdfs/pscmanual/11pscaurcurrent.pdf (2019, accessed 30 September 2019).
- 49. Quaak CH, Cové E, Driessen GJ, et al. Trends in paediatric inpatient antibiotic therapy in a secondary care setting. Eur J Pediatr 2018; 177: 1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kallen MC, Natsch S, Opmeer BC, et al. How to measure quantitative antibiotic use in order to support antimicrobial stewardship in acute care hospitals: a retrospective observational study. Eur J Clin Microbiol Infect Dis 2019; 38: 347–355. [DOI] [PubMed] [Google Scholar]
- 51. Versporten A, Gyssens IC, Pulcini C, et al. ; DRIVE-AB WP1 Group. Metrics to assess the quantity of antibiotic use in the outpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73(Suppl. 6): vi59–vi66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Berrington A. Antimicrobial prescribing in hospitals: be careful what you measure. J Antimicrob Chemother 2010; 65: 163–168. [DOI] [PubMed] [Google Scholar]
- 53. van Santen KL, Edwards JR, Webb AK, et al. The standardized antimicrobial administration ratio: a new metric for measuring and comparing antibiotic use. Clin Infect Dis 2018; 67: 179–185. [DOI] [PubMed] [Google Scholar]
- 54. Brink AJ, Mendelson M. Be AWaRe: new metrics for paediatric antibiotic stewardship. Lancet Infect Dis 2019; 19: 6–7. [DOI] [PubMed] [Google Scholar]
- 55. Al-Hasan MN, Winders HR, Bookstaver PB, et al. Direct measurement of performance: a new era in antimicrobial stewardship. Antibiotics (Basel) 2019; 8: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Momattin H, Al-Ali AY, Mohammed K, et al. Benchmarking of antibiotic usage: an adjustment to reflect antibiotic stewardship program outcome in a hospital in Saudi Arabia. J Infect Public Health. 2018; 11: 310–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van den Bosch CMA, Hulscher MEJL, Natsch S, et al. Applicability of generic quality indicators for appropriate antibiotic use in daily hospital practice: a cross-sectional point-prevalence multicenter study. Clin Microbiol Infect 2016; 22: 888.e1–888.e9. [DOI] [PubMed] [Google Scholar]
- 58. Le Maréchal M, Tebano G, Monnier AA, et al. ; DRIVE-AB WP1 group. Quality indicators assessing antibiotic use in the outpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73(Suppl. 6): vi40–vi49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Spivak ES, Cosgrove SE, Srinivasan A. Measuring appropriate antimicrobial use: attempts at opening the black box. Clin Infect Dis 2016; 63: 1639–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Monnier AA, Schouten J, Le Maréchal M, et al. ; DRIVE-AB WP1 Group. Quality indicators for responsible antibiotic use in the inpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73(Suppl. 6): vi30–vi39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nathwani D, Varghese D, Stephens J, et al. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control 2019; 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vlahović-Palcevski V, Dumpis U, Mitt P, et al. Benchmarking antimicrobial drug use at university hospitals in five European countries. Clin Microbiol Infect 2007; 13: 277–283. [DOI] [PubMed] [Google Scholar]
- 63. Polk RE, Hohmann SF, Medvedev S, et al. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011; 53: 1100–1110. [DOI] [PubMed] [Google Scholar]
- 64. European Centre for Disease Prevention and Control. Consumption of antibacterials for systemic use (ATC group J01) in the community and hospital sector in Europe, reporting year 2018. Rates by country ECDC, http://www.ecdc.europa.eu/en/antimicrobial-consumption/database/rates-country (2018, accessed 14 September 2019).
- 65. Akhloufi H, Streefkerk RH, Melles DC, et al. Point prevalence of appropriate antimicrobial therapy in a Dutch university hospital. Eur J Clin Microbiol Infect Dis 2015; 34: 1631–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hardin G. The tragedy of the commons. The population problem has no technical solution; it requires a fundamental extension in morality. Science 1968; 162: 1243–1248. [PubMed] [Google Scholar]
- 67. Porco TC, Gao D, Scott JC, et al. When does overuse of antibiotics become a tragedy of the commons? PLoS One 2012; 7: e46505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. O’Brien KS, Blumberg S, Enanoria WTA, et al. Antibiotic use as a tragedy of the commons: a cross-sectional survey. Comput Math Methods Med 2014; 2014: 837929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carlet J, Collignon P, Goldmann D, et al. Society’s failure to protect a precious resource: antibiotics. Lancet 2011; 378: 369–371. [DOI] [PubMed] [Google Scholar]
- 70. Donisi V, Sibani M, Carrara E, et al. Emotional, cognitive and social factors of antimicrobial prescribing: can antimicrobial stewardship intervention be effective without addressing psycho-social factors? J Antimicrob Chemother 2019; 74: 2844–2847. [DOI] [PubMed] [Google Scholar]
- 71. Warreman EB, Lambregts MMC, Wouters RHP, et al. Determinants of in-hospital antibiotic prescription behaviour: a systematic review and formation of a comprehensive framework. Clin Microbiol Infect 2019; 25: 538–545. [DOI] [PubMed] [Google Scholar]
- 72. Avorn J, Solomon DH. Cultural and economic factors that (mis)shape antibiotic use: the nonpharmacologic basis of therapeutics. Ann Intern Med 2000; 133: 128–135. [DOI] [PubMed] [Google Scholar]
- 73. Schouten JA, Hulscher MEJL, Natsch S, et al. Barriers to optimal antibiotic use for community-acquired pneumonia at hospitals: a qualitative study. Qual Saf Health Care 2007; 16: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chaves NJ, Cheng AC, Runnegar N, et al. Analysis of knowledge and attitude surveys to identify barriers and enablers of appropriate antimicrobial prescribing in three Australian tertiary hospitals. Internal Med J 2014; 44: 568–574. [DOI] [PubMed] [Google Scholar]
- 75. Velasco E, Espelage W, Faber M, et al. A national cross-sectional study on socio-behavioural factors that influence physicians’ decisions to begin antimicrobial therapy. Infection 2011; 39: 289–297. [DOI] [PubMed] [Google Scholar]
- 76. Charani E, Castro-Sanchez E, Sevdalis N, et al. Understanding the determinants of antimicrobial prescribing within hospitals: the role of “prescribing etiquette”. Clin Infect Dis 2013; 57: 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Halm EA, Atlas SJ, Borowsky LH, et al. Understanding physician adherence with a pneumonia practice guideline: effects of patient, system, and physician factors. Arch Intern Med 2000; 160: 98–104. [DOI] [PubMed] [Google Scholar]
- 78. Majumdar SR, Simpson SH, Marrie TJ. Physician-perceived barriers to adopting a critical pathway for unity-acquired pneumonia. Jt Comm J Qual Saf 2004; 30: 387–395. [DOI] [PubMed] [Google Scholar]
- 79. Livorsi D, Comer AR, Matthias MS, et al. Barriers to guideline-concordant antibiotic use among inpatient physicians: a case vignette qualitative study. J Hosp Med 2016; 11: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Parker HM, Mattick K. The determinants of antimicrobial prescribing among hospital doctors in England: a framework to inform tailored stewardship interventions. Br J Clin Pharmacol 2016; 82: 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lv B, Zhou Z, Xu G, et al. Knowledge, attitudes and practices concerning self-medication with antibiotics among university students in Western China. Trop Med Int Health 2014; 19: 769–779. [DOI] [PubMed] [Google Scholar]
- 82. Metlay JP, Shea JA, Crossette LB, et al. Tensions in antibiotic prescribing: pitting social concerns against the interests of individual patients. J Gen Intern Med 2002; 17: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rodrigues AT, Roque F, Falcão A, et al. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents 2013; 41: 203–212. [DOI] [PubMed] [Google Scholar]
- 84. Borg MA. Prolonged perioperative surgical prophylaxis within European hospitals: an exercise in uncertainty avoidance? J Antimicrob Chemother 2014; 69: 1142–1144. [DOI] [PubMed] [Google Scholar]
- 85. Björkman I, Erntell M, Röing M, et al. Infectious disease management in primary care: perceptions of GPs. BMC Fam Pract 2011; 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Livorsi D, Comer A, Matthias MS, et al. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol 2015; 36: 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Broom J, Broom A, Kirby E, et al. How do hospital respiratory clinicians perceive antimicrobial stewardship (AMS)? A qualitative study highlighting barriers to AMS in respiratory medicine. J Hosp Infect 2017; 96: 316–322. [DOI] [PubMed] [Google Scholar]
- 88. Broom J, Broom A, Plage S, et al. Barriers to uptake of antimicrobial advice in a UK hospital: a qualitative study. J Hosp Infect 2016; 93: 418–422. [DOI] [PubMed] [Google Scholar]
- 89. Klein EY, Martinez EM, May L, et al. Categorical risk perception drives variability in antibiotic prescribing in the emergency department: a mixed methods observational study. J Gen Intern Med 2017; 32: 1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Barlow G, Nathwani D, Myers E, et al. Identifying barriers to the rapid administration of appropriate antibiotics in community-acquired pneumonia. J Antimicrob Chemother 2008; 61: 442–451. [DOI] [PubMed] [Google Scholar]
- 91. Shalit I, Low M, Levy E, et al. Antibiotic use in 26 departments of internal medicine in 6 general hospitals in Israel: variability and contributing factors. J Antimicrob Chemother 2008; 62: 196–204. [DOI] [PubMed] [Google Scholar]
- 92. Mol PGM, Rutten WJMJ, Gans ROB, et al. Adherence barriers to antimicrobial treatment guidelines in teaching hospital, the Netherlands. Emerg Infect Dis 2004; 10: 522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Malan L, Labuschagne Q, Brechtelsbauer E, et al. Sustainable access to antimicrobials; a missing component to antimicrobial stewardship - a tale of two countries. Front Public Health 2018; 6: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Auta A, Hadi MA, Oga E, et al. Global access to antibiotics without prescription in community pharmacies: a systematic review and meta-analysis. J Infect 2019; 78: 8–18. [DOI] [PubMed] [Google Scholar]
- 95. Grigoryan L, Burgerhof JGM, Degener JE, et al. Attitudes, beliefs and knowledge concerning antibiotic use and self-medication: a comparative European study. Pharmacoepidemiol Drug Saf 2007; 16: 1234–1243. [DOI] [PubMed] [Google Scholar]
- 96. Harbarth S, Samore MH. Antimicrobial resistance determinants and future control. Emerg Infect Dis 2005; 11: 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. McColl E, Jacoby A, Thomas L, et al. Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients. Health Technol Assess 2001; 5: 1–256. [DOI] [PubMed] [Google Scholar]
- 98. McKenna CK. Listening to stakeholders. In: Ziegenfuss J, Sassani J. (eds) Portable health administration. San Diego, CA: Elsevier Academic Press, 2004, pp. 173–184. [Google Scholar]
- 99. Davey P, Peden C, Charani E, et al. Time for action-improving the design and reporting of behaviour change interventions for antimicrobial stewardship in hospitals: early findings from a systematic review. Int J Antimicrob Agents 2015; 45: 203–212. [DOI] [PubMed] [Google Scholar]
- 100. Anderson DJ, Watson S, Moehring RW, et al. ; Antibacterial Resistance Leadership Group. Feasibility of core antimicrobial stewardship interventions in community hospitals. JAMA Netw Open 2019; 2: e199369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. de Vries H, Eggers SM, Bolman C. The role of action planning and plan enactment for smoking cessation. BMC Public Health 2013; 13: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dombrowski SU, Avenell A, Sniehott FF. Behavioural interventions for obese adults with additional risk factors for morbidity: systematic review of effects on behaviour, weight and disease risk factors. Obes Facts 2010; 3: 377–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ivers NM, Sales A, Colquhoun H, et al. No more ‘business as usual’ with audit and feedback interventions: towards an agenda for a reinvigorated intervention. Implement Sci 2014; 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Coxeter P, Del Mar CB, McGregor L, et al. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev 2015; 2015: CD010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. van Esch TEM, Brabers AEM, Hek K, et al. Does shared decision-making reduce antibiotic prescribing in primary care? J Antimicrob Chemother 2018; 73: 3199–3205. [DOI] [PubMed] [Google Scholar]
- 106. Bergstrom CT, Lo M, Lipsitch M. Ecological theory suggests that antimicrobial cycling will not reduce antimicrobial resistance in hospitals. Proc Natl Acad Sci U S A 2004; 101: 13285–13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the infectious diseases society of America and the society for healthcare epidemiology of America. Clin Infect Dis 2016; 62: e51–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Merz LR, Warren DK, Kollef MH, et al. Effects of an antibiotic cycling program on antibiotic prescribing practices in an intensive care unit. Antimicrob Agents Chemother 2004; 48: 2861–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hulscher MEJL, Prins JM. Antibiotic stewardship: does it work in hospital practice? A review of the evidence base. Clin Microbiol Infect 2017; 23: 799–805. [DOI] [PubMed] [Google Scholar]
- 110. Kok G, Gottlieb NH, Peters GJY, et al. A taxonomy of behaviour change methods: an intervention mapping approach. Health Psychol Rev 2016; 10: 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011; 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Charani E, Edwards R, Sevdalis N, et al. Behavior change strategies to influence antimicrobial prescribing in acute care: a systematic review. Clin Infect Dis 2011; 53: 651–662. [DOI] [PubMed] [Google Scholar]
- 113. Rawson TM, Moore LSP, Tivey AM, et al. Behaviour change interventions to influence antimicrobial prescribing: a cross-sectional analysis of reports from UK state-of-the-art scientific conferences. Antimicrob Resist Infect Control 2017; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lorencatto F, Charani E, Sevdalis N, et al. Driving sustainable change in antimicrobial prescribing practice: how can social and behavioural sciences help? J Antimicrob Chemother 2018; 73: 2613–2624. [DOI] [PubMed] [Google Scholar]
- 115. Chambers A, MacFarlane S, Zvonar R, et al. A recipe for antimicrobial stewardship success: using intervention mapping to develop a program to reduce antibiotic overuse in long-term care. Infect Control Hosp Epidemiol 2019; 40: 24–31. [DOI] [PubMed] [Google Scholar]
- 116. Hulscher MEJL, Grol RPTM, van der Meer JWM. Antibiotic prescribing in hospitals: a social and behavioural scientific approach. Lancet Infect Dis 2010; 10: 167–175. [DOI] [PubMed] [Google Scholar]
- 117. Pulcini C, Binda F, Lamkang AS, et al. Developing core elements and checklist items for global hospital antimicrobial stewardship programmes: a consensus approach. Clin Microbiol Infect 2019; 25: 20–25. [DOI] [PubMed] [Google Scholar]
- 118. Pollack LA, Srinivasan A. Core elements of hospital antibiotic stewardship programs from the centers for disease control and prevention. Clin Infect Dis 2014; 59(Suppl. 3): S97–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pollack LA, Plachouras D, Gruhler H, et al. Summary the modified Delphi process for common structure and process indicators for hospital antimicrobial stewardship programs. Transatlantic Taskforce on Antimicrobiol Resistance, https://www.cdc.gov/drugresistance/pdf/summary_of_tatfar_recommendation_1.pdf (2015, accessed 15 October 2019).
- 120. European Commission. EU Guidelines for the prudent use of antimicrobials in human health. European Commission, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52017XC0701(01)&from=EN (26 September 2019).
- 121. Van Katwyk SR, Grimshaw JM, Nkangu M, et al. Government policy interventions to reduce human antimicrobial use: a systematic review and evidence map. PLoS Med 2019; 16: e1002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Howard P, Pulcini C, Levy Hara G, et al. ; ESCMID Study Group for Antimicrobial Policies, ISC Group on Antimicrobial Stewardship. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother 2015; 70: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 123. Pulcini C, Morel CM, Tacconelli E, et al. Human resources estimates and funding for antibiotic stewardship teams are urgently needed. Clin Microbiol Infect 2017; 23: 785–787. [DOI] [PubMed] [Google Scholar]
- 124. Steinmann KE, Lehnick D, Buettcher M, et al. Impact of empowering leadership on antimicrobial stewardship: a single center study in a neonatal and pediatric intensive care unit and a literature review. Front Pediatr 2018; 6: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Drew RH. Antimicrobial stewardship programs: how to start and steer a successful program. J Manag Care Pharm 2009; 15(Suppl. 2): S18–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dyar OJ, Beovic B, Pulcini C, et al. ; ESCMID generic competencies working group. ESCMID generic competencies in antimicrobial prescribing and stewardship: towards a European consensus. Clin Microbiol Infect 2019; 25: 13–19. [DOI] [PubMed] [Google Scholar]
- 127. Chandy SJ, Naik GS, Charles R, et al. The impact of policy guidelines on hospital antibiotic use over a decade: a segmented time series analysis. PLoS One 2014; 9: e92206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. European Centre for Disease Prevention and Control. Proposals for EU guidelines on the prudent use of antimicrobials in human health. Stockholm, Sweden, http://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/EU-guidelines-prudent-use-antimicrobials.pdf (2017, accessed 5 January 2020).
- 129. European Centre for Disease Prevention and Control. Antimicrobial stewardship. European Centre for Disease Prevention and Control, http://www.ecdc.europa.eu/en/publications-data/directory-guidance-prevention-and-control/prudent-use-antibiotics/antimicrobial (2020, accessed 14 January 2020).
- 130. Weller TMA, Jamieson CE. The expanding role of the antibiotic pharmacist. J Antimicrob Chemother 2004; 54: 295–298. [DOI] [PubMed] [Google Scholar]
- 131. Gillespie E, Rodrigues A, Wright L, et al. Improving antibiotic stewardship by involving nurses. Am J Infect Control 2013; 41: 365–367. [DOI] [PubMed] [Google Scholar]
- 132. Principi N, Esposito S. Antimicrobial stewardship in paediatrics. BMC Infect Dis 2016; 16: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Smith MJ, Gerber JS, Hersh AL. Inpatient antimicrobial stewardship in pediatrics: a systematic review. J Pediatric Infect Dis Soc 2015; 4: e127–e135. [DOI] [PubMed] [Google Scholar]
- 134. Nichols K, Stoffella S, Meyers R, et al. ; Advocacy Committee for the Pediatric Pharmacy Advocacy Group. Pediatric antimicrobial stewardship programs. J Pediatr Pharmacol Ther 2017; 22: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Falcone M, Paul M, Yahav D, et al. ; Study Group for Infections in the Elderly. Antimicrobial consumption and impact of antimicrobial stewardship programmes in long-term care facilities. Clin Microbiol Infect 2019; 25: 562–569. [DOI] [PubMed] [Google Scholar]
- 136. Stuart RL, Wilson J, Bellaard-Smith E, et al. Antibiotic use and misuse in residential aged care facilities. Intern Med J 2012; 42: 1145–1149. [DOI] [PubMed] [Google Scholar]
- 137. Wu JHC, Langford BJ, Daneman N, et al. Antimicrobial stewardship programs in long-term care settings: a meta-analysis and systematic review. J Am Geriatr Soc 2019; 67: 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Katz MJ, Gurses AP, Tamma PD, et al. Implementing antimicrobial stewardship in long-term care settings: an integrative review using a human factors approach. Clin Infect Dis 2017; 65: 1943–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dyar OJ, Pagani L, Pulcini C. Strategies and challenges of antimicrobial stewardship in long-term care facilities. Clin Microbiol Infect 2015; 21: 10–19. [DOI] [PubMed] [Google Scholar]
- 140. Ray MJ, Tallman GB, Bearden DT, et al. Antibiotic prescribing without documented indication in ambulatory care clinics: national cross sectional study. BMJ 2019; 367: l6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016; 315: 1864–1873. [DOI] [PubMed] [Google Scholar]
- 142. Yogo N, Shihadeh K, Young H, et al. Intervention to reduce broad-spectrum antibiotics and treatment durations prescribed at the time of hospital discharge: a novel stewardship approach. Infect Control Hosp Epidemiol 2017; 38: 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Trinh TD, Klinker KP. Antimicrobial stewardship in the emergency department. Infect Dis Ther 2015; 4(Suppl. 1): 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bishop BM. Antimicrobial stewardship in the emergency department: challenges, opportunities, and a call to action for pharmacists. J Pharm Pract 2016; 29: 556–563. [DOI] [PubMed] [Google Scholar]
- 145. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA 2013; 309: 2345–2352. [DOI] [PubMed] [Google Scholar]
- 146. Sanchez GV, Fleming-Dutra KE, Roberts RM, et al. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016; 65: 1–12. [DOI] [PubMed] [Google Scholar]
- 147. Hallsworth M, Chadborn T, Sallis A, et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 2016; 387: 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Holubar M, Deresinski S. Antimicrobial stewrdship in outpatient settings. Wolters Kluwer, http://www.uptodate.com/contents/antimicrobial-stewardship-in-outpatient-settings (2020, accessed 24 January 2020).
- 149. Drekonja DM, Filice GA, Greer N, et al. Antimicrobial stewardship in outpatient settings: a systematic review. Infect Control Hosp Epidemiol 2015; 36: 142–152. [DOI] [PubMed] [Google Scholar]
- 150. Cox JA, Vlieghe E, Mendelson M, et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect 2017; 23: 812–818. [DOI] [PubMed] [Google Scholar]
- 151. Charani E, Smith I, Skodvin B, et al. Investigating the cultural and contextual determinants of antimicrobial stewardship programmes across low-, middle- and high-income countries-a qualitative study. PLoS One 2019; 14: e0209847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gebretekle GB, Haile Mariam D, Abebe W, et al. Opportunities and barriers to implementing antibiotic stewardship in low and middle-income countries: lessons from a mixed-methods study in a tertiary care hospital in Ethiopia. PLoS One 2018; 13: e0208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Van Dijck C, Vlieghe E, Cox JA. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: a systematic review. Bull World Health Organ 2018; 96: 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. World Health Organization. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries. World Health Organization, http://www.who.int (2019, accessed 2 December 2019). [DOI] [PMC free article] [PubMed]
- 155. Singh N, Manchanda V. Control of multidrug-resistant gram-negative bacteria in low- and middle-income countries-high impact interventions without much resources. Clin Microbiol Infect 2017; 23: 216–218. [DOI] [PubMed] [Google Scholar]
- 156. Jacobs J, Hardy L, Semret M, et al. Diagnostic bacteriology in district hospitals in Sub-Saharan Africa: at the forefront of the containment of antimicrobial resistance. Front Med (Lausanne) 2019; 6: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wagenlehner FME, Bartoletti R, Cek M, et al. Antibiotic stewardship: a call for action by the urologic community. Eur Urol 2013; 64: 358–360. [DOI] [PubMed] [Google Scholar]
- 158. Hoffmann C, Zak M, Avery L, et al. Treatment modalities and antimicrobial stewardship initiatives in the management of intra-abdominal infections. Antibiotics (Basel) 2016; 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Frattari A, Savini V, Polilli E, et al. Control of gram-negative multi-drug resistant microorganisms in an Italian ICU: rapid decline as a result of a multifaceted intervention, including conservative use of antibiotics. Int J Infect Dis 2019; 84: 153–162. [DOI] [PubMed] [Google Scholar]
- 160. Jenkins TC, Sabel AL, Sarcone EE, et al. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis 2010; 51: 895–903. [DOI] [PubMed] [Google Scholar]
- 161. Hecker MT, Son AH, Murphy NN, et al. Impact of syndrome-specific antimicrobial stewardship interventions on use of and resistance to fluoroquinolones: an interrupted time series analysis. Am J Infect Control 2019; 47: 869–875. [DOI] [PubMed] [Google Scholar]
- 162. Viale P, Tumietto F, Giannella M, et al. Impact of a hospital-wide multifaceted programme for reducing carbapenem-resistant enterobacteriaceae infections in a large teaching hospital in Northern Italy. Clin Microbiol Infect 2015; 21: 242–247. [DOI] [PubMed] [Google Scholar]
- 163. Eldh AC, Almost J, DeCorby-Watson K, et al. Clinical interventions, implementation interventions, and the potential greyness in between -a discussion paper. BMC Health Serv Res 2017; 17: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Pakyz AL, Moczygemba LR, VanderWielen LM, et al. Facilitators and barriers to implementing antimicrobial stewardship strategies: results from a qualitative study. Am J Infect Control 2014; 42(Suppl. 10): S257–S263. [DOI] [PubMed] [Google Scholar]
- 165. Grol R, Wensing M, Eccles M, et al. Improving patient care: the implementation of change in health care, 2nd ed. Chichester, West Sussex: John Wiley & Sons, 2013, p. 374. [Google Scholar]
- 166. Fernandez ME, Hoor GAT, van Lieshout S, et al. Implementation mapping: using intervention mapping to develop implementation strategies. Front Public Health 2019; 7: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Steinmo S, Fuller C, Stone SP, et al. Characterising an implementation intervention in terms of behaviour change techniques and theory: the ‘Sepsis Six’ clinical care bundle. Implement Sci 2015; 10: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ramsay C, Brown E, Hartman G, et al. Room for improvement: a systematic review of the quality of evaluations of interventions to improve hospital antibiotic prescribing. J Antimicrob Chemother 2003; 52: 764–771. [DOI] [PubMed] [Google Scholar]
- 169. Schweitzer VA, van Heijl I, van Werkhoven CH, et al. ; Consensus on Antimicrobial Stewardship Evaluations study group. The quality of studies evaluating antimicrobial stewardship interventions: a systematic review. Clin Microbiol Infect 2019; 25: 555–561. [DOI] [PubMed] [Google Scholar]
- 170. de Kraker MEA, Abbas M, Huttner B, et al. Good epidemiological practice: a narrative review of appropriate scientific methods to evaluate the impact of antimicrobial stewardship interventions. Clin Microbiol Infect 2017; 23: 819–825. [DOI] [PubMed] [Google Scholar]
- 171. Eccles M, Grimshaw J, Campbell M, et al. Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Saf Health Care 2003; 12: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Australian Commission on Safety and Quality in Health Care. Antimicrobial stewardship in Australian health care 6 measuring performance and evaluating antimicrobial stewardship programs, http://www.safetyandquality.gov.au (2018, accessed 3 January 2020).
- 173. Centers for Disease Control and Prevention. The core elements of hospital antibiotic stewardship programs. Atlanta, GA: US Department of Health and Human Services, 2019. [Google Scholar]
- 174. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315: 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Patel SJ, Saiman L, Duchon JM, et al. Development of an antimicrobial stewardship intervention using a model of actionable feedback. Interdiscip Perspect Infect Dis 2012; 2012: 150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Standiford HC, Chan S, Tripoli M, et al. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7-year program. Infect Control Hosp Epidemiol 2012; 33: 338–345. [DOI] [PubMed] [Google Scholar]
- 177. Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA 2014; 312: 2569–2570. [DOI] [PubMed] [Google Scholar]
- 178. Morris AM, Calderwood MS, Fridkin SK, et al. Research needs in antibiotic stewardship. Infect Control Hosp Epidemiol 2019; 40: 1334–1343. [DOI] [PubMed] [Google Scholar]
- 179. May CR, Mair F, Finch T, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009; 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Gruen RL, Elliott JH, Nolan ML, et al. Sustainability science: an integrated approach for health-programme planning. Lancet 2008; 372: 1579–1589. [DOI] [PubMed] [Google Scholar]
- 181. Maher L, Gustafson D, Evans A. Sustainability model and guide. NHS Institute for Innovation and Improvement, https://webarchive.nationalarchives.gov.uk/20160805122935/http://www.nhsiq.nhs.uk/media/2757778/nhs_sustainability_model_-_february_2010_1_.pdf (2010, accessed 24 January 2020). [Google Scholar]
- 182. Naylor NR, Zhu N, Hulscher M, et al. Is antimicrobial stewardship cost-effective? A narrative review of the evidence. Clin Microbiol Infect 2017; 23: 806–811. [DOI] [PubMed] [Google Scholar]
- 183. Dik JWH, Vemer P, Friedrich AW, et al. Financial evaluations of antibiotic stewardship programs - a systematic review. Front Microbiol 2015; 6: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wilton P, Smith R, Coast J, et al. Strategies to contain the emergence of antimicrobial resistance: a systematic review of effectiveness and cost-effectiveness. J Health Serv Res Policy 2002; 7: 111–117. [DOI] [PubMed] [Google Scholar]
- 185. Coulter S, Merollini K, Roberts JA, et al. The need for cost-effectiveness analyses of antimicrobial stewardship programmes: a structured review. Int J Antimicrob Agents 2015; 46: 140–149. [DOI] [PubMed] [Google Scholar]
- 186. Shrestha P, Cooper BS, Coast J, et al. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob Resist Infect Control 2018; 7: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wozniak TM, Barnsbee L, Lee XJ, et al. Using the best available data to estimate the cost of antimicrobial resistance: a systematic review. Antimicrob Resist Infect Control 2019; 8: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Naylor NR, Atun R, Zhu N, et al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control 2018; 7: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Gandra S, Barter DM, Laxminarayan R. Economic burden of antibiotic resistance: how much do we really know? Clin Microbiol Infect 2014; 20: 973–980. [DOI] [PubMed] [Google Scholar]
- 190. Rzewuska M, Charani E, Clarkson JE, et al. ; Joint Programming Initiative on Antimicrobial Resistance Working Group on Behavioural Approaches to Antibiotic Stewardship Programs. Prioritizing research areas for antibiotic stewardship programmes in hospitals: a behavioural perspective consensus paper. Clin Microbiol Infect 2019; 25: 163–168. [DOI] [PubMed] [Google Scholar]
- 191. Knight GM, Davies NG, Colijn C, et al. Mathematical modelling for antibiotic resistance control policy: do we know enough? BMC Infect Dis 2019; 19: 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Laws M, Shaaban A, Rahman KM. Antibiotic resistance breakers: current approaches and future directions. FEMS Microbiol Rev 2019; 43: 490–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Raymond B. Five rules for resistance management in the antibiotic apocalypse, a road map for integrated microbial management. Evol Appl 2019; 12: 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Pena-Miller R, Laehnemann D, Jansen G, et al. When the most potent combination of antibiotics selects for the greatest bacterial load: the smile-frown transition. PLoS Biol 2013; 11: e1001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Rodríguez-González A, Zanin M, Menasalvas-Ruiz E. Public health and epidemiology informatics: can artificial intelligence help future global challenges? An overview of antimicrobial resistance and impact of climate change in disease epidemiology. Yearb Med Inform 2019; 28: 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Rawson TM, Moore LSP, Hernandez B, et al. A systematic review of clinical decision support systems for antimicrobial management: are we failing to investigate these interventions appropriately? Clin Microbiol Infect 2017; 23: 524–532. [DOI] [PubMed] [Google Scholar]
- 197. Peiffer-Smadja N, Rawson TM, Ahmad R, et al. Machine learning for clinical decision support in infectious diseases: a narrative review of current applications. Clin Microbiol Infect. Epub ahead of print 17 September 2019. DOI: 10.1016/j.cmi.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 198. Charani E, Holmes A. Antibiotic stewardship - twenty years in the making. Antibiotics (Basel) 2019; 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]