Abstract
BRAF-V600 mutations occur in approximately 50% of patients with metastatic melanoma. Immune-checkpoint inhibitors and targeted therapies are both active as first-line treatments in these patients regardless of their mechanisms of action and toxicities. However, an upfront therapeutic strategy is still controversial. In fact, waiting for results of ongoing clinical trials and for new biomarkers, clinicians should base their decision on the clinical characteristics of the patient and on the biological aspects of the tumor. This review provides an overview on BRAF-V600 mutations in melanoma and will discuss their prognostic and clinical significance. Moreover, it will suggest a therapeutic algorithm that can drive therapeutic choice in a first-line setting for BRAF-V600 mutant melanoma patients.
Keywords: BRAF-V600 mutations, immunotherapy, metastatic melanoma, prognostic biomarker, targeted therapy
Epidemiology and biology of BRAF-V600 mutant melanoma
Malignant melanoma is one of the most lethal types of skin cancer with a dramatic increase of its incidence in the last 50 years.1 In a case study of patients diagnosed with melanoma between 2009 and 2015, only one in four patients (25%) with metastatic disease was still alive after 5 years from diagnosis.2 Recent update from two clinical trials of metastatic melanoma patients treated with first-line immunotherapy, KEYNOTE-006 and Checkmate-067, have shown a 5-year survival of 38% and 52%, respectively.3,4 Approximately 50% of patients with cutaneous melanoma have mutations in BRAF, a proto-oncogene belonging to the RAF family of serine/threonine protein kinases.5 Activating mutations in BRAF kinase is involved in mitogen-activated protein kinase (MAPK) pathways, linking extracellular signals to intracellular processes such as growth, differentiation, proliferation, migration and apoptosis.6 The most common mutation in the BRAF gene in melanoma is localized in exon 15 – codon 600 – of the BRAF gene, with a substitution of amino acid glutamic acid for valine at position 600 (V600E), accounting for 70–88% of all BRAF mutations. The second most common mutation accounting for 10–20% of all BRAF mutations is V600K. Less frequent mutations are V600R, V600D and V600M, comprising 2–5%, 1–4% and <1% of all BRAF mutations, respectively.7 These mutations seem to confer the same sensitivity to drugs of the V600E and V600K mutations, but to date clinical data on them are not significative.8–10 Non-V600 BRAF mutations such as L597P/Q/R/S and K601E, occurring in less than 5% of all melanoma patients, have also been described, even if, at the moment, more data are needed to elucidate their predictive and prognostic role.11 V600 mutations involve the kinase domain of the serine/threonine protein kinase BRAF, with constitutive activation of the MAPK pathway leading to an uncontrolled cell proliferation and survival.12 Differences in biological and clinical features have been demonstrated between BRAF-V600E and V600K melanomas. In fact, comparison between BRAF-V600E and V600K cutaneous melanoma samples from the Cancer Genome Atlas showed that energy-metabolism and protein-translation pathways were upregulated in V600K tumors compared with V600E tumors, while proapoptotic pathways were downregulated.13 A higher degree of cumulative sun-induced damage has been observed in V600K melanoma rather than in V600E melanomas, as well as a higher mutational burden. In fact, V600K mutations increase with increasing age.14,15 Moreover, metastatic melanoma patients harboring BRAF-V600K mutation showed a shorter disease-free interval from diagnosis of primary melanoma to the occurrence of first distant metastasis compared with V600E mutant melanomas, even if no difference in overall survival (OS) has been demonstrated between the two groups.14
Targeting BRAF-V600 mutation in melanoma treatment
BRAF inhibitors
In the early 2000s, the discovery of BRAF-V600 mutations’ role in cutaneous melanoma pathogenesis led researchers to develop new drugs able to block members of the MAPK pathway.16 The first targeted agent tested against BRAF-V600 mutant melanoma patients, sorafenib, despite preclinical evidences of efficacy,17 unfortunately failed to demonstrate any clinical activity, as it did not improve progression-free survival (PFS) in phase II–III clinical trials.18,19 Subsequently, a more selective and potent BRAF inhibitor, vemurafenib, formerly known as PLX4032, showed antitumor activity in both preclinical models and the early clinical setting.20,21 In fact, in the phase III BRIM-3 trial, 675 patients with BRAF-V600 mutant unresectable or metastatic melanoma were randomly assigned to receive either vemurafenib, at a dose of 960 mg twice daily orally (p.o.) or dacarbazine by intravenous infusion (i.v.).22 In the vemurafenib and in the dacarbazine groups, PFS was 5.3 months and 1.6 months with a response rate of 48% and 5%, respectively, leading to the approval of vemurafenib by Food and Drug Administration (FDA) and European Medicine Agency (EMA) in this clinical scenario. Common adverse events (AEs) in patients treated with vemurafenib were mainly cutaneous events such as erythematous rash, photosensitivity and cutaneous squamous cell carcinomas, but also non-cutaneous events such as arthralgia and fatigue. Shortly thereafter, another second-generation BRAF inhibitor, dabrafenib, was approved for treatment of BRAF-V600 mutant unresectable or metastatic melanoma patients, based on the results of the phase III BREAK-3 trial.23 Dabrafenib showed similar activity compared with vemurafenib, although with a different toxicity profile including fewer photosensitivity, less frequent cutaneous squamous cell carcinoma and stronger pyrexia. Although dabrafenib and vemurafenib showed a strong activity in BRAF-V600 mutant metastatic melanoma patients, their efficacy is limited by adverse events and by the emergence of acquired resistance mechanisms. These observations led to the design and development of new therapeutic agents. Encorafenib, formerly known as LGX-818, is the newest second-generation BRAF inhibitor with a higher affinity to BRAF and extended binding time. In fact, it has a peculiar pharmacodynamic profile, with a dissociation half-life of 30 hours compared with 2 and 0.5 hours of dabrafenib and vemurafenib, respectively. This prolonged effect causes a longer inhibition of its target compared with the old generations of BRAF inhibitors with a decrease of off-target effects responsible for adverse events, such as cutaneous squamous cell carcinoma.24,25 A phase I dose escalation and expansion study of single agent encorafenib in metastatic BRAF-V600 mutant melanoma showed a good clinical profile with no significant toxicity. The most common related adverse events were hand-foot syndrome and alopecia; other toxicities included vomiting and myopathy.26,27
Dual inhibition of BRAF and MEK
In clinical setting, the use of BRAF inhibitors pointed out two different concerns: a limited efficacy, due to the rapid onset of acquired resistance mechanisms, and an increased incidence of cutaneous squamous cell carcinomas.28–30 Acquired resistance to BRAF inhibitors could be caused by several mechanisms and how to overcome it has become a relevant clinical need.31–33 Multiple mechanisms of resistance have been identified. In particular, preclinical studies showed a key role of MEK, a serine/threonine protein kinase activated by BRAF kinase that through phosphorylation of ERK kinase, the last effector of the MAPK pathway, is physiologically involved in proliferation, differentiation, motility and survival in response to extracellular stimuli.34,35 ERK kinases accumulate in nucleus, dimerize and activate substrates such as SRC-1, c-Fos, c-Myc, and STAT3.36 Even though MEK is mainly activated by BRAF, mechanisms of RAF-independent MEK activation have been described.37,38
Paradoxical activation of MAPK signaling using BRAF inhibitors consists of heterodimerization between impaired BRAF mutant kinases and non-mutant RAF isoforms, resulting in downstream MAPK pathway activation. Paradoxical activation depends on the upstream activity from an increased receptor tyrosine kinase (RTK) signaling or as a result of activating RAS mutations, thus explaining the higher incidence of cutaneous squamous cell carcinoma observed with single-agent BRAF inhibitors.39,40
The evidence that combined BRAF/MEK inhibition could delay the onset of resistance and avoid the paradoxical activation of the MAPK pathway provided the rationale for testing the association of BRAF and MEK inhibitors (trametinib, cobimetinib and binimetinib) in this clinical scenario.41,42 Clinical trials investigating the combination of BRAF and MEK inhibitors in BRAF-V600 mutant melanoma patients reported a prolongation in both PFS and OS compared with single agent BRAF inhibitor.43–47 In particular, coBRIM, a phase III clinical trial evaluating the addition of cobimetinib (MEK inhibitor) to vemurafenib (BRAF inhibitor) in untreated BRAF-V600 metastatic melanoma patients, demonstrated better PFS, 9.9 versus 6.2 months, and overall response rate (ORR), 67 versus 44%, for doublet versus single agent drug.43 COMBI-d and COMBI-v are two phase III clinical trials evaluating the addition of trametinib (MEK inhibitor) to dabrafenib (BRAF inhibitor) in untreated BRAF-V600E/K metastatic melanoma patients. The two trials differed in the single agent arm, using dabrafenib in COMBI-d and vemurafenib in COMBI-v, respectively. However, a statistically significant improvement in OS was demonstrated in both trials.44,45 Five-year analysis of pooled extended-survival data from COMBI-d and COMBI-v showed PFS and OS rates of 19% and 34%, respectively. Interestingly, a multivariate analysis highlighted a 5-year OS of 55% in patients with normal baseline lactate dehydrogenase (LDH) levels and less than three metastatic sites and, in addition, 71% of patients who achieved a complete response (CR) were alive after 5 years.46
Recently, COLUMBUS, a two-part randomized phase III trial, evaluated the newest doublet encorafenib (BRAF inhibitor) plus binimetinib (MEK inhibitor) in untreated BRAF-V600 mutant melanoma patients. In part 1, patients were randomly allocated to receive encorafenib 450 mg (p.o., once daily) plus binimetinib 45 mg (p.o., twice daily) or encorafenib 300 mg (p.o., once daily) or vemurafenib 960 mg (p.o., twice daily). This trial showed an improvement of PFS from 7 to 15 months and OS from 16.9 to 33.6 months in the combined treatment arm compared with single agent vemurafenib. Results of part 2, evaluating the effect of encorafenib plus binimetinib versus encorafenib alone, have not yet been published.47 Interestingly, all above-mentioned clinical trials showed that double inhibition of BRAF and MEK results in better clinical outcomes and it is known to reduce adverse events affecting patients treated with BRAF inhibitors alone, such as cutaneous squamous cell carcinoma associated to vemurafenib and dabrafenib and hand-foot syndrome associated to dabrafenib and encorafenib.43–47
Recent data showed that BRAF-V600E and V600K mutant melanoma patients have distinct molecular profiles. In particular, molecular pathway analysis showed that BRAF-V600K mutant melanomas have lower expression of ERK and higher expression of PI3K-AKT genes than V600E mutant melanoma and this could in part explain the lower sensitivity of BRAF-V600K mutant melanomas to double BRAF and MEK inhibition.15 PI3K-AKT pathway signaling, however, seems to play a complex role in resistance to double BRAF and MEK inhibition, because it seems to favor the selection and expansion of resistant tumor subclones with MAPK-reactivating mutations rather than being a molecular driver of resistance itself.48
Immunotherapy and BRAF-V600 mutant melanoma
The introduction of immune-checkpoint inhibitors (ICIs) has made the greatest impact in the management of melanoma treatment. Ipilimumab, a monoclonal antibody that activates the immune system by inhibiting CTLA-4 (cytotoxic T-lymphocyte associated protein 4), has been the first ICI to improve survival of advanced/metastatic melanoma patients.49 Nivolumab and pembrolizumab, two monoclonal antibodies against PD-1 (programmed cell death protein 1), demonstrated better PFS and OS than ipilimumab, with a more favorable toxicity profile.50,51 Nivolumab and ipilimumab combined treatment showed an increase in both PFS and OS, with a remarkable and significant increase in ORR, PFS and OS compared with ipilimumab as first-line treatment in patients without brain metastases, although at the cost of more grade 3–4 toxicities, which occurred in 59% of patients treated in the combination arm.4,52
Data regarding the efficacy of ICIs in BRAF-V600 mutant melanoma have been retrospectively extrapolated from subgroup analysis of the main phase III clinical trials which led to the approval of ipilimumab, pembrolizumab and nivolumab in advanced/metastatic melanoma patients and included different percentages of BRAF mutants. In particular, KEYNOTE-006 was an open-label, multicenter controlled, phase III clinical study evaluating the role of pembrolizumab (10 mg/kg) every 2 or 3 weeks versus four doses of ipilimumab (3 mg/kg) every 3 weeks in ipilimumab-naive histologically confirmed advanced melanoma patients with known BRAF-V600 status and up to one previous systemic therapy. The estimated 6-month PFS rate was 47.3% for pembrolizumab arm every 2 weeks, 46.4% for pembrolizumab arm every 3 weeks and 26.5% for the ipilimumab arm. Interestingly, 36.2% of enrolled patients were BRAF-V600 mutant and approximately 50% of them had received BRAF inhibitor treatment. Most patients pretreated with BRAF inhibitors had elevated baseline LDH levels or symptomatic and rapidly progressive disease at diagnosis. Furthermore, the hazard ratio (HR) for PFS for both untreated and previously treated BRAF-V600 patients was in a range between 0.44 and 0.87 in favor of pembrolizumab (both 2 and 3-week schedules), while HR for OS was in a range between 0.67 and 0.84, with a not statistically significant benefit in OS for both pembrolizumab arms. However, it must be noted that patients were not stratified according to BRAF status in this clinical trial.51 The 5-year update of the study showed a median OS of 20.4 months in BRAF-V600 patients.4
Although in the phase III clinical trial, CheckMate 066, evaluating the efficacy of nivolumab versus dacarbazine, no BRAF-V600 mutant patients were enrolled,50 efficacy data of nivolumab in BRAF-V600 mutant patients can be extrapolated from CheckMate 067, a phase III clinical trial evaluating the activity of nivolumab in combination with ipilimumab versus nivolumab alone versus ipilimumab alone as first-line metastatic melanoma treatment.52 In this trial, approximately 31% of patients were BRAF-V600 mutant and the mutational status of BRAF was also a stratification factor for randomization. In the combination arm, PFS of BRAF-V600 mutant and BRAF wild-type melanoma patients was similar, 11.7 and 11.2 months, respectively. However, a recent update of this clinical trial has shown that 5-year OS for patients with BRAF-V600 mutant tumors reached 60% in the combination group, 46% in the nivolumab group and 30% in the ipilimumab group, whereas these results for BRAF wild-type patients were 48%, 43% and 25%, respectively.4 In a descriptive comparison between treatments in BRAF-V600 mutant patients, the combination treatment seemed to provide more benefit than nivolumab monotherapy, with a HR of 0.70 for OS.53 In addition, a prespecified subgroup analysis revealed the possible predictive role of PD-L1 expression. In fact, median PFS was 14 months in patients with a positive PD-L1 tumor (defined as at least 5% of tumor cells showing PD-L1 staining) treated either with nivolumab or nivolumab plus ipilimumab, whereas median PFS in patients with a negative PD-L1 tumor was 5.3 and 11.2 months in the nivolumab and combination arm, respectively.52
Although PD-L1 positive melanoma patients could potentially benefit more than PD-L1 negative patients from immune-based therapies,52,54 anti-PD-1 antibodies are currently used regardless of PD-L1 expression status because efficacy data have been observed also in the PD-L1 negative population. Moreover, new biomarkers that could be used to differentiate between patients who will derive the most benefit from treatment are needed. Tumor mutational burden (TMB) is a measurement of the number of mutations carried by tumor cells, that has recently been used to predict a favorable outcome to PD-1/PD-L1 blockade across different metastatic tumors, including melanoma.55 A retrospective study evaluating TMB differences in BRAF-V600 mutant versus BRAF wild-type melanomas showed a significant difference in TMB between these two groups.56,57 However, the impact of TMB as a biomarker of response to immunotherapy in BRAF-V600 mutant melanoma patients remains unknown.
Prognostic and clinical factors in BRAF-V600 mutant melanoma for optimal treatment strategies
Currently, we do not yet have results of ongoing clinical trials comparing targeted therapy and immunotherapy in first-line treatment BRAF-V600 mutant metastatic melanoma patients. For naive BRAF-V600 mutant melanoma patients, the choice between these two treatments should be made by considering different aspects such as prognostic factors, the different mechanism of action of therapeutic agents used and the inter-tumor variability among melanoma patients. Prognostic factors that have been studied until now in BRAF-V600 mutant melanoma patients include serum LDH, site/number of metastasis and clinical factors (Eastern Cooperative Oncology Group performance status [ECOG PS]) (Table 1).58–61 Other prognostic factors are currently under investigation.
Table 1.
Prognostic and clinical factors and their influence on therapeutic response in metastatic melanoma patients.
| Prognostic factor | Value | Immunotherapy*58,59 | BRAF + MEK inhibitors60,61 |
|---|---|---|---|
| LDH | Normal | 1-year OS: 72% | 2-year PFS: 39–40% |
| >ULN | 1-year OS: 44% | 2-year PFS: 14% | |
| >2× ULN | 2-year PFS: 6% | ||
| 3-year PFS: 0% | |||
| Sum of lesion diameters (SLD) | ≤44 mm | 3-year PFS: 52% | |
| >44 mm | 3-year PFS: 0% | ||
| <66 mm | 3-year PFS: 43% | ||
| ≥66 mm | 3-year PFS: 27% | ||
| 102 mm | ORR: 42% | ||
| ≥102 mm | ORR: 24% |
ULN, upper limit of normal; OS, overall survival; PFS, progression-free survival; ORR, overall response rate.
Immunotherapy data include both BRAF wild-type and V600-mutant patients.
LDH
Elevated serum LDH is one of the strongest independent prognostic factors in metastatic melanoma patients, regardless of the number and site of metastases.62,63 LDH is not just a secreted enzyme. In fact, its elevated serum levels reflect the switch to a glycolytic phenotype in hypoxic conditions by melanoma cells.63 The evidence of the prognostic role of LDH in metastatic melanoma patients had led researchers to include its levels in the penultimate American Joint Committee on Cancer (AJCC) melanoma staging system edition (VII edition, 2009). In fact, patients with elevated LDH levels were assigned to M1c category regardless of distant metastatic sites. Recently, in the ultimate AJCC melanoma staging system (VIII edition, 2018), levels of LDH are no longer used as a stratification criterion for assigning patients to M1 subgroups, even if each subgroup has the number (0) or (1) to indicate normal or elevated LDH, respectively.64
The role of baseline LDH levels as a prognostic marker has been evaluated in patients with advanced/metastatic melanoma treated with nivolumab and pembrolizumab in a single institution trial. Patients with an elevated baseline LDH level had a significantly shorter OS compared with patients with a normal baseline LDH level and, interestingly, a change in LDH levels during treatment was predictive of response and OS. However, in this retrospective analysis, there was no selection criterion for the line of treatment and the number of BRAF-V600 mutant patients was not balanced between the two groups.58
A recent pooled analysis of four randomized clinical trials including BRAF-V600 mutant metastatic melanoma patients treated with vemurafenib ± cobimetinib showed clearly the influence on PFS by baseline LDH level. In fact, in patients treated with doublet therapy, 2-year PFS decreased dramatically from 40% for normal baseline LDH levels to less than 6% for baseline LDH > 2× upper limit of normal (ULN).60 A second pooled analysis of two-phase III clinical trials in BRAF-V600 mutant metastatic melanoma patients treated with dabrafenib and trametinib showed similar results, with 2-year PFS of 39% for normal baseline LDH levels compared with 14% for elevated baseline LDH levels,61 confirming the results of a previous pooled analysis of a pivotal phase II and two phase III trials with the same drugs.65 Noteworthy, in both vemurafenib plus cobimetinib and dabrafenib plus trametinib pooled analysis, 3-year PFS in patients with baseline LDH > 2× ULN was 0%.60,61
Site, number and size of metastases
Melanoma cells could spread through lymphatic or hematic circulation, affecting subcutaneous tissues and regional/distant lymph nodes or vital organs such as lungs, liver and/or brain.66 Sites of metastases have been pointed out as strong prognostic factors in melanoma patients. In particular, central nervous system (CNS) invasion represented the worst prognostic factor, with median OS for patients with brain metastasis of 17–22 weeks.67 The VII edition the AJCC melanoma staging system divided stage IV into three different prognostic categories based on metastatic sites and LDH levels: M1a included patients with distant metastasis in the skin, subcutaneous tissue, or distant lymph nodes and a normal LDH level (good prognosis); M1b included patients with lung metastasis and a normal LDH level (intermediate prognosis); and, finally, M1c patients with other visceral metastatic sites or with elevated LDH level independently from metastatic sites (poor prognosis).68 The VIII edition the AJCC melanoma staging system revised M classification. In fact, elevated LDH does not identify a specific subgroup and a new category, M1d, identifies patients with CNS metastases (irrespective of the presence of metastatic disease at other sites).64
In addition, baseline disease burden has been shown to be associated with therapeutic responses and outcomes across multiple cancer types, including metastatic melanoma.68 Although tumor burden can be measured and reported in different ways, patients who have smaller and fewer metastases have improved clinical and pathological responses and survival compared with patients with greater baseline tumor burden.68 A pooled analysis of 581 patients treated with pembrolizumab and with measurable disease showed that patients with baseline tumor size less than the median (10.2 cm) had an ORR of 42%, whereas patients with tumor burden at or above the median had an ORR of 24%.59 The role of tumor burden as a negative prognostic biomarker has been evaluated in a pooled analysis of advanced/metastatic melanoma patients treated with BRAF and MEK inhibitors. The analysis showed that metastatic burden has a negative effect on PFS. In particular, when ECOG PS is 0 and baseline LDH level is <2× ULN, patients treated with vemurafenib plus cobimetinib had a 3-year PFS of 51.8% and 0% if baseline sum of lesion diameters (SLD) was ≤44 mm or >44 mm, respectively.60 In the case of normal baseline LDH level, in patients treated with dabrafenib plus trametinib the 3-year PFS was 43% and 27% if SLD was <66 mm or ≥66 mm, respectively.61
Clinical factors
Performance status (PS) deterioration due to rapid growth of metastases is not uncommon in melanoma patients, especially in BRAF-V600 mutant patients. In first-line metastatic melanoma phase III trials, patients with ECOG PS ⩾1 were represented within a variable range of 26–35%.43–47,50–52 Pooled analysis showed that baseline PS could play a key role for survival outcome in BRAF-V600 mutant metastatic melanoma patients treated with BRAF and/or MEK inhibitors. In fact, in the good prognosis patients’ group (LDH ⩽ 2× ULN), the subgroup with ECOG PS = 0 had a 1-year PFS of 47% whereas patients with ECOG PS = 1 had a 1-year PFS of 11%.60 Moreover, in metastatic melanoma patients treated with dabrafenib plus trametinib as first-line therapy, PFS is strongly affected by performance status, with a HR between PS 0 and PS ⩾ 1 of 0.585 (p < 0.0001). A meta-analysis of 18 randomized trials was performed to investigate the predictive role of PS towards treatment with ICIs. The treatment with checkpoint inhibitors improves survival regardless of patients’ PS. In particular, four clinical trials including advanced melanoma patients were incorporated in this meta-analysis and demonstrated that patients with ECOG PS = 1 benefited from immunotherapy (HR 0.82, range: 0.72–0.93).69 However, most patients with poor performance status (PS ⩾ 2) have been excluded from pivotal phase III immunotherapy trials.70
New prognostic and predictive factors
Analysis of circulating tumor DNA (ctDNA) is a non-invasive method that could be used for many clinical purposes, from early detection of cancer to monitoring anti-cancer treatment efficacy.71 Tumor DNA can be released by cancer cells either through a passive mechanism by apoptotic and necrotic cancer cells and/or through active mechanism of spontaneous release of DNA fragments into the circulation.72 Detection and quantification of ctDNA is mostly performed with digital polymerase chain reaction (dPCR) and next generation sequencing (NGS). To assess whether ctDNA levels could be used as an early indicator of changes in tumor burden in melanoma patients with advanced disease and treated with checkpoints inhibitors, plasma samples have been collected serially from 12 patients. Levels of ctDNA correlated with clinical and radiological outcomes.73 Moreover, basal ctDNA levels could predict response to treatment in naive metastatic melanoma patients. In fact, in a cohort of 48 Australian subjects, 35 of them had detectable ctDNA in the plasma at baseline, and lower levels were significantly associated with response to treatment and prolonged PFS, regardless of the therapeutic approach adopted. In addition, ctDNA levels were shown to predict resistance to treatment earlier than radiological detection of progressive disease.74 To investigate the possible role of ctDNA levels as prognostic biomarker in BRAF-V600 mutant advanced melanoma a retrospective analysis of four clinical trials (BREAK-2, BREAK-3, BREAK-MB and METRIC) has been performed. In particular, BRAF ctDNA mutations were detectable in 76% and 81% of patients harboring BRAF-V600E and V600K mutations, respectively. However, BRAF-V600E and V600K mutant positive tissue patients that were negative for BRAF ctDNA mutations in plasma had longer PFS and OS compared with patients with detectable BRAF ctDNA mutations, suggesting the role of BRAF-V600 mutant ctDNA as an independent prognostic factor for PFS.75 Waiting for further studies to validate these interesting results, ctDNA is now in the spotlight as a new feasible prognostic marker in BRAF-V600 mutant metastatic melanoma. However, how these results will change the treatment paradigm is still unknown.
Recently, gene expression signatures have been proposed as a prognostic and/or predictive tool in cancer treatment.76 Over the past 10 years, BRAF mutation-associated gene expression signature has been identified including different genes involved in melanoma immune response such as MAGE-D2, CD63 and HSP70.77,78 These immune gene expression signatures could be useful to characterize tumor immunity for predicting response to immunotherapy. Recently, interferon gamma (IFN-γ) signature has been evaluated as a marker of response to immunotherapy with significant results in terms of prediction of response in both non-small cell lung cancer and melanoma.79 Interestingly, in patients with resected BRAF-V600 mutant stage III melanoma from the COMBI-AD trial (combination of dabrafenib and trametinib versus placebo as adjuvant treatment), IFN-γ signature played a crucial role as a prognostic factor in both arms, identifying patients with longer relapse-free survival (RFS) independently of TMB status.80,81 Thus, IFN-γ signature could be a potential prognostic and predictive factor in BRAF-V600 mutant melanoma. However, gene expression signature could not be used in clinical practice yet due to paucity of data.
Treatment of brain metastases in BRAF-V600 mutant melanoma
Melanoma is one of the malignant tumors with the highest probability to develop brain metastases.82 Concerning BRAF-V600 mutant melanoma, genetic concordance between paired tissue samples from primary tumors and brain metastases is variable from 80–100%, with cases of BRAF-V600 positive brain metastases and paired negative melanoma primaries.83,84 Different strategies could be used against melanoma brain metastases such as surgery (metastasectomy), radiotherapy (stereotactic radiosurgery: SRS), medical therapy (immunotherapy, targeted therapy) and/or combinations of them. The number, size, localization and symptoms should guide clinicians to choose the right approach for every single patient with melanoma brain metastases.
Targeted therapy
Preclinical studies have demonstrated that concentrations of targeted drugs such as vemurafenib, dabrafenib and trametinib in the brain of murine models without CNS metastases were very low.82 However, it should be assumed that the presence of brain metastases (>0.25 mm²) alters the blood–brain barrier (BBB), allowing penetration of these drugs in metastatic sites.85 Dabrafenib and vemurafenib monotherapy showed variable intracranial ORR ranging from 15–35%, with lower responses in BRAF-V600K mutant patients.86,87
COMBI-MB was a phase II clinical trial evaluating the role of dabrafenib plus trametinib in BRAF-V600 mutant melanoma patients with brain metastases. Patients were divided into four cohorts: cohort A including patients harboring BRAF-V600E mutation, with asymptomatic brain metastases, no previous local brain therapy and an ECOG PS of 0 or 1; cohort B including patients harboring BRAF-V600E mutation, with asymptomatic brain metastases, with previous local brain therapy and an ECOG PS of 0 or 1; cohort C including patients harboring BRAF-V600D/K/R mutations, asymptomatic brain metastases, with or without previous local brain therapy and an ECOG PS of 0 or 1; cohort D including patients harboring BRAF-V600D/E/K/R mutations, symptomatic brain metastases, with or without previous local brain therapy and an ECOG PS of 0, 1, or 2. Results of this trial showed that overall intracranial responses (complete and partial responses, CR + PR) were high (56–59%) in cohorts A, B and D, but not in cohort C (44%). Median PFS and OS were longer in cohort B. However, the small sample size and the non-randomized design limit the external validity of these results.88
Immunotherapy
Even if in physiological condition BBB limits lymphocytes trafficking, mechanisms such as cytokines, cell adhesion molecules and matrix metalloproteinases could allow T cells to access the CNS parenchyma.82 Clinical trials evaluating immunotherapy in melanoma patients often did not include those with CNS metastases, especially patients who were symptomatic and/or requiring steroids. Nevertheless, few data demonstrating the efficacy and safety of immunotherapy in melanoma patients with brain metastases are available. In particular, in a phase II clinical trial it has been shown that ipilimumab could prolong survival in asymptomatic patients with brain metastases, with a median OS of 7 months even if CNS objective responses, the primary endpoint of this trial, were 16% in asymptomatic patients and only 5% in symptomatic patients.89 The combination of ipilimumab and nivolumab increased response rate in patients with untreated brain metastases from cutaneous melanoma, with a rate of intracranial clinical benefit of 57%, and reaching a CR rate of 26%, a partial response (PR) rate of 30% and a 81.5% estimated rate of OS at 12 months. In this trial, 57.4% of patients had BRAF-V600 mutant metastatic melanoma and, interestingly, this patient subgroup had the higher objective response rate (57% versus 40% of BRAF wild-type).90 The Anti-PD-1 Brain Collaboration trial evaluated the efficacy of nivolumab alone or in combination with ipilimumab in patients with active melanoma brain metastases, including BRAF-V600 mutant tumors, either treated or untreated with BRAF and MEK inhibitors. The higher intracranial response rate (56%) was registered in the cohort of patients with asymptomatic melanoma brain metastases who had no previous local brain therapy and were treated with ipilimumab plus nivolumab. Notably, intracranial response, PFS and OS were low after progression on BRAF and MEK inhibitors, according to translational evidence of immune-resistance phenotype developing progression on BRAF inhibitor-based therapy.91
Combination strategies
Combining immunotherapy and targeted therapy for BRAF-V600 mutant metastatic brain melanoma (MBM) patients could be a more effective treatment compared with single modalities. Ongoing clinical trials are evaluating this possibility.82 Radiation therapy and immunotherapy combined strategy for MBM has never been investigated prospectively. Retrospective studies showed a significant improvement in OS in patients treated with ipilimumab and SRS (≈20 months).92,93 Also, radiosurgery has been investigated retrospectively, with better outcomes observed in patients that started ICIs within 8 weeks after undergoing radiosurgery.94 Targeted therapy in combination with radiotherapy is currently being investigated in ongoing clinical trials, but at the cost of potential increased toxicities (mostly dermatological, pulmonary and neurological) from the combination of BRAF inhibitors and radiotherapy.95–98
Current scenario and future direction in BRAF-V600 mutant melanoma treatment
First-line and second-line therapy
At present, we do not have any data from randomized trials regarding the best first-line treatment for BRAF-V600 mutant metastatic melanoma patients. In fact, immunotherapy and targeted therapy could both be used as first-line treatment for these patients, even if prognostic and clinical factors suggest important differences between the two therapeutic approaches. The main differences between immunotherapy and targeted therapy are the following:
1) Onset of clinical response, generally rapid for targeted therapy and slow for immunotherapy;
2) Duration and deepness of response, generally longer and more durable with immunotherapy than targeted agents. On the other hand, a higher response rate is observed with targeted therapy even though resistance could develop in a relevant percentage of cases;
3) Patterns of progression. In particular, clinical data showed that rapid disease progression and poor performance status after targeted therapies could significantly reduce the proportion of patients receiving immunotherapy as second-line treatment.99
4) Patients’ medical history and comorbidities. ICIs should be avoided in patients with autoimmune diseases, especially if requiring high-dose corticosteroids or immunosuppressive drugs. Targeted therapies could worsen heart failure, arrhythmias or retinal pathologies. Risk–benefit ratio must be considered for each patient before starting any of these drugs.100
In the absence of randomized trials comparing, head to head, the efficacy of immunotherapy versus targeted therapy, clinical and prognostic factors, such as baseline LDH, site and number of metastases and ECOG PS, should be addressed by clinicians in the treatment of BRAF-V600 mutant melanoma patients.
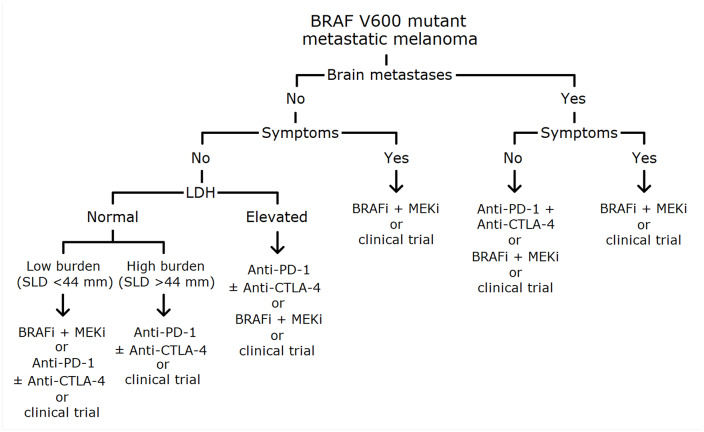
Based on available data from phase III clinical trials and meta-analysis, some considerations are needed. In particular, in advanced melanoma patients with normal baseline LDH levels, targeted therapy should be preferred in the case of low metastatic burden or in the case of aggressive and/or symptomatic disease, even if a short-term involvement of vital organs is expected, whereas immunotherapy could be considered as first-line treatment in patients with high metastatic burden but slowly progressive and without symptoms, and also in the case of low metastatic burden in the light of long-term responses.60,61 The combination of nivolumab and ipilimumab could be preferred to nivolumab single agent, especially in case of PD-L1 expression <5% as discussed before, even if, regardless of PD-L1 expression, in BRAF V600 patients the difference in 5-year OS between the combination and nivolumab is not statistically significant (HR 0.70, range 0.41–1.05)53 and the rate of G3-4 AEs is more than doubled (59% versus 23%, respectively).4,52 In the case of advanced melanoma patients with elevated baseline LDH levels even if PFS and OS data are in favor of first-line treatment with immunotherapy, the goal of rapid response in the case of symptomatic disease should suggest clinicians to use targeted therapy. For untreated patients with brain metastases, even in the absence of direct comparison, targeted therapy achieves better results than immunotherapy, especially in symptomatic patients, even if nivolumab plus ipilimumab could be a good option for asymptomatic patients as described before88–90 (Figure 1). Second-line treatment depends on which therapy that patient has previously received (immunotherapy after targeted therapy and vice versa).
Figure 1.
Proposed algorithm for first-line treatment choice in untreated BRAF-V600 mutant metastatic melanoma patients.
BRAFi, BRAF inhibitor; LDH, serum lactate dehydrogenase; MEKi, MEK inhibitor; SLD, sum of lesion diameters.
Future of BRAF-V600 mutant melanoma treatment
Several trials are evaluating the association between immunotherapy and targeted therapy as first-line treatment in BRAF-V600 mutant melanoma patients (NCT02902042, NCT02908672, NCT02910700, NCT01940809). The rationale of combining immune and targeted therapy is to obtain maximal response rates and durable responses, with an acceptable safety profile. Results of the phase II KEYNOTE-022 trial, in which BRAF-V600 metastatic melanoma patients were randomly assigned to receive pembrolizumab or placebo in addition to dabrafenib and trametinib as first-line treatment, have recently been published. PFS, the primary endpoint of this study, showed an increase from 10.3 months for the dabrafenib and trametinib combined treatment group to 16 months for the addition of pembrolizumab to the doublet treatment group. However, this improvement of PFS did not reach the planned benefit for a statistically significant improvement. Moreover, a higher incidence of grade 3–4 toxicities in the combination arm compared with doublet (58.3 versus 26.7%) has been described.101 Despite these results, ongoing phase III clinical trials (NCT02908672, NCT02967692) will better elucidate the role of association between immunotherapy and targeted therapy in these patients.
Another strategy under investigation is to find the optimal sequence for BRAF mutant melanoma patients’ treatment. Data from ongoing clinical trials, mainly NCT02224781, evaluating dabrafenib plus trametinib followed by nivolumab plus ipilimumab or vice versa, and NCT02631447, also known as SECOMBIT, are still immature, but they will definitely answer important clinical questions about the right treatment approach in BRAF-V600 mutant patients. The phase II SECOMBIT trial, evaluating the best sequencing approach with the combination of encorafenib plus binimetinib and the combination of nivolumab plus ipilimumab, will also add interesting data about patients treated with an induction of targeted therapy followed immediately by immunotherapy and, in the case of progression, rechallenge with targeted therapy (‘sandwich’ strategy). A phase II clinical trial has evaluated the role of rechallenge treatment with BRAF and MEK inhibitors in BRAF V600E/K mutant patients who had previously progressed on first-line BRAF inhibitors (with or without MEK inhibitors) and who had progressed also on second-line to ICIs, with an interval between recruitment and the last administration of a BRAF inhibitor of more than 12 weeks.102 An interesting disease control rate (DCR) of 72% was achieved, corroborating the use of BRAF and MEK inhibitors in this setting, but only data from future phase III clinical trials could clarify the role of rechallenge with these drugs.
Currently, data from clinical practice have not shown any difference in sequencing immune and targeted therapy,103 but a simulation model developed to estimate the cost-effectiveness of treatment sequences in BRAF-V600 mutant metastatic melanoma suggests that starting treatment with anti-PD-1 plus anti-CTLA-4 is more cost-effective than starting with anti-PD-1 monotherapy or targeted therapy.104
Conclusion
New therapies such as targeted agents and ICIs have changed outcomes of BRAF-V600 mutant metastatic melanoma patients. These two classes of drugs have substantial differences regarding spectrum of activity, safety profiles and, nevertheless, costs. Currently, prognostic and clinical factors should guide the choice between ICIs and targeted therapies, waiting for the results of ongoing clinical trials evaluating which one could be the best upfront approach. The combination of immune and targeted therapies could represent a better choice, but probably not for all patients and, almost definitely, at the cost of more toxicities. The optimization of treatment strategies with current drugs, the validation of promising predictive factors and the discovery of new pharmacological agents will meaningfully improve, in the near future, the clinical management of these patients.
Footnotes
Conflict of interest statement: Teresa Troiani: Institutional research grants: Roche, Merck, Sanofi, Servier, Novartis, Bayer. Fortunato Ciardiello: Advisory boards: Roche, Amgen, Merck, Pfizer, Sanofi, Bayer, Servier, BMS, Cellgene Lilly. Institutional research grants: Bayer, Roche, Merck, Amgen, AstraZeneca, Takeda. The remaining authors have no conflicts of interest to declare
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Emilio Francesco Giunta  https://orcid.org/0000-0002-5383-4469
https://orcid.org/0000-0002-5383-4469
Contributor Information
Emilio Francesco Giunta, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, Italy.
Vincenzo De Falco, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, Italy.
Stefania Napolitano, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, Italy; Department of Gastrointestinal Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Giuseppe Argenziano, Dermatology Unit, Department of Mental and Physical Health and Preventive Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, Italy.
Gabriella Brancaccio, Dermatology Unit, Department of Mental and Physical Health and Preventive Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, Italy.
Elvira Moscarella, Dermatology Unit, Department of Mental and Physical Health and Preventive Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, Italy.
Davide Ciardiello, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, Italy.
Fortunato Ciardiello, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, Italy.
Teresa Troiani, Dipartimento di Medicina di Precisione, Università degli Studi della Campania “Luigi Vanvitelli”, Via S Pansini 5, Naples 80131, Italy.
References
- 1. Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of melanoma – cutaneous melanoma: etiology and therapy [Internet]. Brisbane: Codon Publications, 2017. Chapter 1, 10.15586/codon.cutaneousmelanoma.2017.ch. [DOI] [Google Scholar]
- 2. American Cancer Society. Survival rates for melanoma skin cancer. https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/survival-rates-for-melanoma-skin-cancer-by-stage.html (accessed 18 February 2020).
- 3. Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019; 20: 1239–1251. [DOI] [PubMed] [Google Scholar]
- 4. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019; 381: 1535–1546. [DOI] [PubMed] [Google Scholar]
- 5. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 2011; 29: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 6. Dhillon AS, Hagan S, Rath O, et al. MAP kinase signalling pathways in cancer. Oncogene 2007; 26: 3279–3290. [DOI] [PubMed] [Google Scholar]
- 7. Bradish JR, Cheng L. Molecular pathology of malignant melanoma: changing the clinical practice paradigm toward a personalized approach. Hum Pathol 2014; 45: 1315–1326. [DOI] [PubMed] [Google Scholar]
- 8. Ponti G, Pellacani G, Tomasi A, et al. The somatic affairs of BRAF: tailored therapies for advanced malignant melanoma and orphan non-V600E (V600R-M) mutations. J Clin Pathol 2013; 66: 441–445. [DOI] [PubMed] [Google Scholar]
- 9. Malkhasyan KA, Rooney SL, Snow AN, et al. The clinical characteristics of melanoma with BRAF V600R mutation: a case series study. Melanoma Res 2020; 30: 107–112. [DOI] [PubMed] [Google Scholar]
- 10. Popescu A, Haidar A, Anghel RM. Treating malignant melanoma when a rare BRAF V600M mutation is present: case report and literature review. Rom J Intern Med 2018; 56: 122–126. [DOI] [PubMed] [Google Scholar]
- 11. Menzer C, Menzies AM, Carlino MS, et al. Targeted therapy in advanced melanoma with rare BRAF mutations. J Clin Oncol 2019; 37: 3142–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417: 949–954. [DOI] [PubMed] [Google Scholar]
- 13. Li Y, Umbach DM, Li L. Putative genomic characteristics of BRAF V600K versus V600E cutaneous melanoma. Melanoma Res 2017; 27: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Menzies AM, Haydu LE, Visintin L, et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res 2012; 18: 3242–3249. [DOI] [PubMed] [Google Scholar]
- 15. Pires da Silva I, Wang KYX, Wilmott JS, et al. Distinct molecular profiles and immunotherapy treatment outcomes of V600E and V600K BRAF-mutant melanoma. Clin Cancer Res 2019; 25: 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rebecca VW, Sondak VK, Smalley KS. A brief history of melanoma: from mummies to mutations. Melanoma Res 2012; 22: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharma A, Trivedi NR, Zimmerman MA, et al. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res 2005; 65: 2412–2421. [DOI] [PubMed] [Google Scholar]
- 18. Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 2009; 27: 2823–2830. [DOI] [PubMed] [Google Scholar]
- 19. Ott PA, Hamilton A, Min C, et al. A phase II trial of sorafenib in metastatic melanoma with tissue correlates. PLoS One 2010; 5: e15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang H, Higgins B, Kolinsky K, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res 2010; 70: 5518–5527. [DOI] [PubMed] [Google Scholar]
- 21. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010; 363: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380: 358–365. [DOI] [PubMed] [Google Scholar]
- 24. Sun J, Zager JS, Eroglu Z. Encorafenib/binimetinib for the treatment of BRAF-mutant advanced, unresectable, or metastatic melanoma: design, development, and potential place in therapy. Onco Targets Ther 2018; 11: 9081–9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koelblinger P, Thuerigen O, Dummer R. Development of encorafenib for BRAF-mutated advanced melanoma. Curr Opin Oncol 2018; 30: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delord JP, Robert C, Nyakas M, et al. Phase I dose-escalation and -expansion study of the BRAF inhibitor encorafenib (LGX818) in metastatic BRAF-mutant melanoma. Clin Cancer Res 2017; 23: 5339–5348. [DOI] [PubMed] [Google Scholar]
- 27. Gogas HJ, Flaherty KT, Dummer R, et al. Adverse events associated with encorafenib plus binimetinib in the COLUMBUS study: incidence, course and management. Eur J Cancer 2019; 119: 97–106. [DOI] [PubMed] [Google Scholar]
- 28. Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010; 464: 431–435. [DOI] [PubMed] [Google Scholar]
- 29. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 2012; 366: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Menzies AM, Kefford R, Long GV. Paradoxical oncogenesis: are all BRAF inhibitors equal? Pigment Cell Melanoma Res 2013; 26: 611–615. [DOI] [PubMed] [Google Scholar]
- 31. Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014; 4: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amaral T, Sinnberg T, Meier F, et al. The mitogen-activated protein kinase pathway in melanoma part I – activation and primary resistance mechanisms to BRAF inhibition. Eur J Cancer 2017; 73: 85–92. [DOI] [PubMed] [Google Scholar]
- 33. Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF (V600E) inhibition by RTK or N-RAS upregulation. Nature 2010; 468: 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004; 116: 855–867. [DOI] [PubMed] [Google Scholar]
- 35. Solus JF, Kraft S. Ras, Raf, and MAP kinase in melanoma. Adv Anat Pathol 2013; 20: 217–226. [DOI] [PubMed] [Google Scholar]
- 36. Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 2004; 68: 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park ER, Eblen ST, Catling AD. MEK1 activation by PAK: a novel mechanism. Cell Signal 2007; 19: 1488–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu S, Khoo S, Dang A, et al. Differential regulation of mitogen-activated protein/ERK kinase (MEK)1 and MEK2 and activation by a Ras-independent mechanism. Mol Endocrinol 1997; 11: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 39. Gibney GT, Messina JL, Fedorenko IV, et al. Paradoxical oncogenesis and the long term consequences of BRAF inhibition in melanoma. Nat Rev Clin Oncol 2013; 10: 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010; 464: 427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 2010; 18: 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paraiso KH, Fedorenko IV, Cantini LP, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer 2010; 102: 1724–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 2016; 17: 1248–1260. [DOI] [PubMed] [Google Scholar]
- 44. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372: 30–39. [DOI] [PubMed] [Google Scholar]
- 45. Long GV, Stroyakovskiy D, Gogas HJ, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015; 386: 444–451. [DOI] [PubMed] [Google Scholar]
- 46. Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019; 381: 626–636. [DOI] [PubMed] [Google Scholar]
- 47. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2018; 19: 1315–1327. [DOI] [PubMed] [Google Scholar]
- 48. Irvine M, Stewart A, Pedersen B, et al. Oncogenic PI3K/AKT promotes the stepwise evolution of combination BRAF/MEK inhibitor resistance in melanoma. Oncogenesis 2018; 7: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–330. [DOI] [PubMed] [Google Scholar]
- 51. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 52. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. 5-Year survival outcomes of the CheckMate 067 phase 3 trial of nivolumab plus ipilimumab (NIVO+IPI) combination therapy in advanced melanoma. Ann Oncol 2019; 30 (Suppl. 5): v851–v934. [Google Scholar]
- 54. Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol 2016; 34: 4102–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017; 16: 2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mar VJ, Wong SQ, Li J, et al. BRAF/NRAS wild-type melanomas have a high mutation load correlating with histological and molecular signatures of UV damage. Clin Cancer Res 2013; 19: 4589–4598. [DOI] [PubMed] [Google Scholar]
- 57. Gibney GT, Tang S, Poorman K. Associations of age, PD-L1 status, BRAF mutation and tumor mutational burden (TMB) in advanced melanoma. J Clin Oncol 2018; 36 (Suppl. 15): e21609–e21609. [Google Scholar]
- 58. Diem S, Kasenda B, Spain L, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer 2016; 114: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Poklepovic AS, Carvajal RD. Prognostic value of low tumor burden in patients with melanoma. Oncology (Williston Park) 2018; 32: e90–e96. [PubMed] [Google Scholar]
- 60. Hauschild A, Larkin J, Ribas A, et al. Modeled prognostic subgroups for survival and treatment outcomes in BRAF V600-mutated metastatic melanoma: pooled analysis of 4 randomized clinical trials. JAMA Oncol 2018; 4: 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schadendorf D, Long GV, Stroiakovski D, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer 2017; 82: 45–55. [DOI] [PubMed] [Google Scholar]
- 62. Markovic SN, Erickson LA, Rao RD, et al. Malignant melanoma in the 21st century, part 2: staging, prognosis, and treatment. Mayo Clin Proc 2007; 82: 490–513. [DOI] [PubMed] [Google Scholar]
- 63. Palmer SR, Erickson LA, Ichetovkin I, et al. Circulating serologic and molecular biomarkers in malignant melanoma. Mayo Clin Proc 2011; 86: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017; 67: 472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol 2016; 17: 1743–1754. [DOI] [PubMed] [Google Scholar]
- 66. Tas F. Metastatic behavior in melanoma: timing, pattern, survival, and influencing factors. J Oncol 2012; 2012: 647684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vosoughi E, Lee JM, Miller JR, et al. Survival and clinical outcomes of patients with melanoma brain metastasis in the era of checkpoint inhibitors and targeted therapies. BMC Cancer 2018; 18: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27: 6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bersanelli M, Brighenti M, Buti S, et al. Patient performance status and cancer immunotherapy efficacy: a meta-analysis. Med Oncol 2018; 35: 132. [DOI] [PubMed] [Google Scholar]
- 70. Donia M, Kimper-Karl ML, Høyer KL, et al. The majority of patients with metastatic melanoma are not represented in pivotal phase III immunotherapy trials. Eur J Cancer 2017; 74: 89–95. [DOI] [PubMed] [Google Scholar]
- 71. Yang M, Forbes ME, Bitting RL, et al. Incorporating blood-based liquid biopsy information into cancer staging: time for a TNMB system? Ann Oncol 2018; 29: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol 2013; 10: 472–484. [DOI] [PubMed] [Google Scholar]
- 73. Lipson EJ, Velculescu VE, Pritchard TS, et al. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J Immunother Cancer 2014; 2: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gray ES, Rizos H, Reid AL, et al. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget 2015; 6: 42008–42018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Santiago-Walker A, Gagnon R, Mazumdar J, et al. Correlation of BRAF mutation status in circulating-free DNA and tumor and association with clinical outcome across four BRAFi and MEKi clinical trials. Clin Cancer Res 2016; 22: 567–574. [DOI] [PubMed] [Google Scholar]
- 76. Chibon F. Cancer gene expression signatures – the rise and fall? Eur J Cancer 2013; 49: 2000–2009. [DOI] [PubMed] [Google Scholar]
- 77. Johansson P, Pavey S, Hayward N. Confirmation of a BRAF mutation-associated gene expression signature in melanoma. Pigment Cell Res 2007; 20: 216–221. [DOI] [PubMed] [Google Scholar]
- 78. Kannengiesser C, Spatz A, Michiels S, et al. Gene expression signature associated with BRAF mutations in human primary cutaneous melanomas. Mol Oncol 2008; 1: 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Karachaliou N, Gonzalez-Cao M, Crespo G, et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther Adv Med Oncol 2018; 10: 1758834017749748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 2017; 377: 1813–1823. [DOI] [PubMed] [Google Scholar]
- 81. Long GV, Hauschild A, Santinami M, et al. Updated relapse-free survival (RFS) and biomarker analysis in the COMBI-AD trial of adjuvant dabrafenib + trametinib (D + T) in patients (pts) with resected BRAF V600-mutant stage III melanoma. Ann Oncol 2018; 29 (Suppl. 8): mdy424.053. [Google Scholar]
- 82. Glitza Oliva IC, Schvartsman G, Tawbi H. Advances in the systemic treatment of melanoma brain metastases. Ann Oncol 2018; 29: 1509–1520. [DOI] [PubMed] [Google Scholar]
- 83. Colombino M, Capone M, Lissia A, et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J Clin Oncol 2012; 30: 2522–2529. [DOI] [PubMed] [Google Scholar]
- 84. Chen G, Chakravarti N, Aardalen K, et al. Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clin Cancer Res 2014; 20: 5537–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fidler IJ. The biology of brain metastasis: challenges for therapy. Cancer J 2015; 21: 284–293. [DOI] [PubMed] [Google Scholar]
- 86. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 87. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann Oncol 2017; 28: 634–641. [DOI] [PubMed] [Google Scholar]
- 88. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAF V600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol 2017; 18: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012; 13: 459–465. [DOI] [PubMed] [Google Scholar]
- 90. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 2018; 379: 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 2018; 19: 672–681. [DOI] [PubMed] [Google Scholar]
- 92. Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013; 2: 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 2012; 117: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Robin TP, Breeze RE, Smith DE, et al. Immune checkpoint inhibitors and radiosurgery for newly diagnosed melanoma brain metastases. Neurooncol 2018; 140: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Anker CJ, Ribas A, Grossmann AH, et al. Severe liver and skin toxicity after radiation and vemurafenib in metastatic melanoma. J Clin Oncol 2013; 31: e283–e287. [DOI] [PubMed] [Google Scholar]
- 96. Forschner A, Zips D, Schraml C, et al. Radiation recall dermatitis and radiation pneumonitis during treatment with vemurafenib. Melanoma Res 2014; 24: 512–516. [DOI] [PubMed] [Google Scholar]
- 97. Liebner DA, Walston SA, Cavaliere R, et al. Radiation necrosis mimicking rapid intracranial progression of melanoma metastasis in two patients treated with vemurafenib. Melanoma Res 2014; 24: 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys 2016; 95: 632–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mason R, Heng S, Atkinson V. Outcomes following progression on BRAF/MEK inhibition in metastatic melanoma. J Clin Oncol 2017; 35 (Suppl. 15): Abstract 9537. [Google Scholar]
- 100. Kennedy LC, Bhatia S, Thompson JA, et al. Preexisting autoimmune disease: implications for immune checkpoint inhibitor therapy in solid tumors. J Natl Compr Canc Netw 2019; 17: 750–757. [DOI] [PubMed] [Google Scholar]
- 101. Ascierto PA, Ferrucci PF, Stephens R, et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med 2019; 25: 941–946. [DOI] [PubMed] [Google Scholar]
- 102. Schreuer M, Jansen Y, Planken S, et al. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol 2017; 18: 464–472. [DOI] [PubMed] [Google Scholar]
- 103. Aguilera JV, Paludo J, Tschautscher M, et al. A clinical insight into therapeutic sequence in advanced melanoma. J Clin Oncol 2018; 36 (Suppl. 5): Abstract 186. [Google Scholar]
- 104. Tarhini A, McDermott D, Ambavane A, et al. Clinical and economic outcomes associated with treatment sequences in patients with BRAF-mutant advanced melanoma. Immunotherapy 2019; 11: 283–295. [DOI] [PubMed] [Google Scholar]