Abstract
Introduction:
Hepatocholangiocarcinoma (HCC-ICC) is a rare tumor presenting the histologic characteristics of both hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC). As there is no consensus on it management, the therapeutic strategy rests on the specific treatments for HCC or ICC. Programmed cell death 1 (PD-1) inhibitors showed encouraging results in the second line treatment of HCC after sorafenib but it efficacy in HCC-ICC has never been reported.
Methods and results:
We present the case of a 72-year-old male patient treated for metastatic HCC-ICC due to a viral hepatitis C cirrhosis in progression after two lines of treatment. Tumor was characterized by a PDL-1 status of 85%. Patient received pembrolizumab at doses of 200 mg every 21 days by intravenous infusion. After one injection he was presented an immediate clinical benefit, a partial response was observed after two months of treatment and a complete response two months later. This response was maintained over time along with toxicity-free tumor control after 18 months treatment.
Conclusion:
To our knowledge, we reported for the first time the efficacy of a PD1 inhibitor treatment in a patient presenting metastatic HCC-ICC due to viral cirrhosis and overexpressing PDL-1 after failure of two lines of treatment.
Keywords: hepatocholangiocarcinoma, immunotherapy, programmed death-ligand 1, pembrolizumab, positron emission tomography scanner
Introduction
Hepatocholangiocarcinoma (HCC-ICC) is a rare tumour presenting the histological characteristics of both hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC). The prevalence of these mixed tumours ranges from 1% to 5% of primary liver cancers. Associated cirrhosis is found in around 30% of cases. An estimated third of these tumours are diagnosed at a locally advanced stage.1–3 Due to the rarity of this orphan disease, there has been no randomised study assessing its specific management. The roles of surgery, chemoembolisation and systemic treatments have yet to be defined. In the absence of a standard treatment, the therapeutic strategy for HCC-ICC rests on the specific treatments for HCC or ICC and takes into account the possible existence of a predominant histological component, the evolution of tumour markers such as alpha-fetoprotein (AFP), CA 19-9 and the therapeutic response to previous lines.
Management of locally advanced or metastatic HCC consists of administering tyrosine kinase inhibitors targeting the vascular endothelial growth factor receptor (VEGF-R). Among these, sorafenib is the reference in first-line treatment with a median overall survival (OS) ranging from 6.5 months to 9.1 months.4,5 The standard first-line treatment of ICC in a metastatic setting is an association of a platinum salt and gemcitabine. A recent retrospective study of 30 patients assessed its efficacy in patients presenting an HCC-ICC and showed a response rate of 28.6%, progression-free survival (PFS) of 9 months and an OS of 16.2 months.6
For second-line treatment, the US Food and Drug Administration approved in September 2017 the use of nivolumab, a programmed cell death 1 (PD-1) inhibitor, in locally advanced or metastatic HCC after failure of sorafenib based on the results of the phase I–II CHECKMATE-040 trial.7 In the phase II KEYNOTE-224 trial, pembrolizumab, another PD-1 inhibitor, was also tested in second line after sorafenib versus placebo and showed very encouraging results.8 However, the initial results from the phase III KEYNOTE-240 trial presented at the last American Association of Clinical Oncology congress revealed only a nonsignificant improvement in its two main co-objectives PFS and OS.9 As marketing authorisation has not yet been granted in France, regorafenib remains the standard second-line treatment in selected patients with often a poor tolerance.10 Regarding advanced or metastatic ICC, the KEYNOTE-028 study evaluated pembrolizumab in patients who showed more than 1% programmed death–ligand 1 (PD-L1) tumour expression. In this study, the overall response rate (ORR) was 13% (all partial responses) and median duration of response was not reached. Median OS and PFS were respectively 6.2 months and 1.8 months.11
We report here the first case of a patient treated for metastatic HCC-ICC with pembrolizumab after failure of two lines of treatment.
Methods and results
We present the case of a 72-year-old man. The patient provided written informed consent for the publication of his medical information; ethics approval is not required for case reports at our institution. His medical history included a viral hepatitis C treated 18 months ago with 12 weeks of sofosbuvir, ribavirin and pegylated interferon currently presenting a nondetectable viral load, a stented cardiac ischaemia, an insulin-dependent diabetes and a benign prostate hyperplasia.
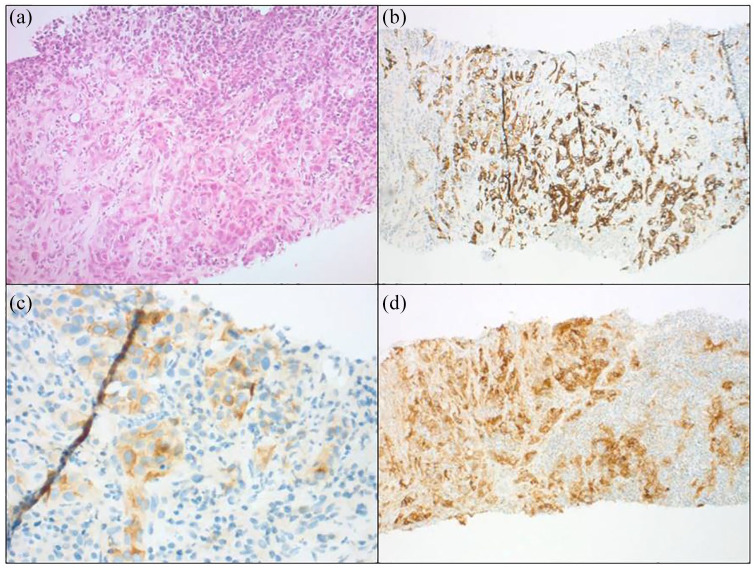
In June 2017, during monitoring of his hepatitis, a 5 cm mass at its longer axis was detected in his right liver lobe. Liver biopsy pointed to cell proliferation generating thick cords with focal glandular differentiation. These structures were composed of large, moderately pleomorphic cells with voluminous, irregular, hyperchromatic, nucleolated nuclei. Immunohistochemical analyses were negative for the antihepatocyte and cytokeratine 20 antibodies. The cytokeratine 7 antibody marked the glandular regions and cytokeratine 19 and glypican 3 markers were positive (Figure 1(a)–(c)). Hepatic biopsy of the healthy liver confirmed moderately active viral hepatitis C cirrhosis. Taken together, these findings pointed to an HCC-ICC secondary to viral hepatitis C cirrhosis. The staging work-up revealed the presence of isolated adenopathy of the hepatic hilum. Biologically, the cirrhosis Child–Pugh score was A. No cytopenia was found and kidney and liver functions were preserved. Given good performance status ((Eastern Cooperative Oncology Group; scale of performance status) ECOG 1) and the compensated cirrhosis, since July 2017, the patient has benefited from a treatment targeting the HCC component with sorafenib in association with local treatments: lipiodol chemoembolisation with doxorubicin 50 mg and liver radiofrequency ablation. Systemic treatment was quickly discontinued due to poor clinical and biological tolerance. Unfortunately, in February 2018, a reassessment positron emission tomography (PET) scan revealed lymph node progression with an increase in size of the hepatic hilar adenopathy and the presence of a 20 mm adenopathy in the anterior right paracardiac region associated with local tumour progression. Biopsy of the paracardiac adenopathy confirmed the presence of the previously mentioned immunohistochemical characteristics and, hence, the two tumoural components. Imaging reassessment in April 2018 showed continued tumour progression. An increased AFP at 125 µg/L was also noted compared with 68 µg/L in January 2018; CA 19-9 was stable at 88 U/ml. At that point, the patient was treated for a sepsis contraindicating administration of any antitumour treatment. Sepsis evolution was finally favourable in June 2018, allowing initiation of a second-line treatment. Given the maintained performance status (ECOG 1) and the isolated increase of AFP, treatment by regorafenib at lower doses was initiated. The first 2-month reassessment work-up revealed lymph node and liver tumour progression responsible for hepatalgia requiring the use of opioids. In the absence of a validated treatment option in a patient presenting tumour progression after two lines of treatment, a further complementary immunohistochemical evaluation of his PD-L1 status was requested, which showed 85% labelling of the tumour cells (Figure 1(d)). As DNA mismatch repair deficiency was not found, the patient tumour presented a microsatellite stability phenotype.
Figure 1.
Moderately differentiated tumour with focal glandular pattern, associated with a dense peritumoural lymphocyte-rich stroma (hemtoxylene eosin and safran × 100). (b) Diffuse expression of cytokeratine 7. (c) Focal positivity with glypican 3 antibody. (d) Strong and diffuse staining for programmed death-ligand 1.
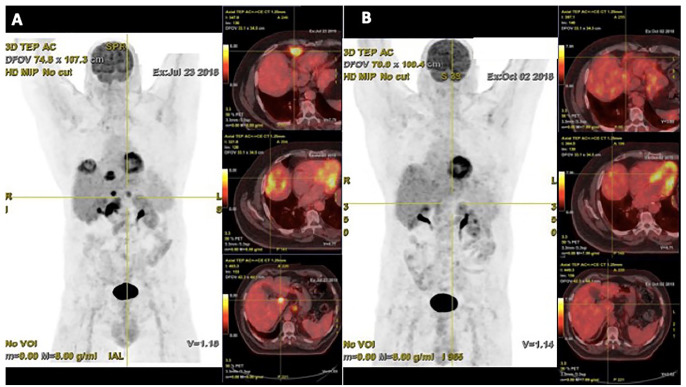
Pembrolizumab was administered in July 2018 at doses of 200 mg every 21 days by intravenous infusion. Following the first injection, the patient described complete disappearance of the hepatic pain, thus enabling analgesic withdrawal. The first PET scan reassessment performed in October 2018 after three injections of pembrolizumab revealed the normalisation of the voluminous liver and lymph nodes fixations with the exception of the pre-vena cava adenopathy, which showed a clear reduction of fixation (28.6 mm/SUV 3.6 (standardized uptake value) versus 47 mm/SUV 18) (Figure 2). A new reassessment work-up 2 months later showed the disappearance of the pathological fixation of the pre-vena cava adenopathy in favour of a complete remission. Currently, the patient is pursuing pembrolizumab. The latest PET scan in January 2020 indicated continuing tumour control and excellent clinical and biological tolerance (Figure 3).
Figure 2.
PET scan performed in July 2018 (baseline) showing liver and lymph-node involvement. (b) PET scan performed in October 2018 following four injections of pembrolizumab showing partial response at the supra- and sub-diaphragmatic targets.
PET, positron emission tomography.
Figure 3.
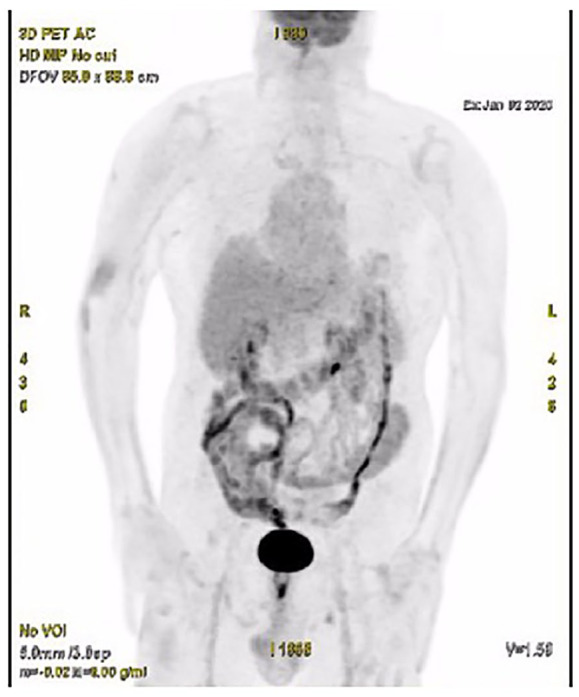
PET scan performed in January 2020 showing maintenance of complete tumour response after 18 months of treatment with pembrolizumab.
PET, positron emission tomography.
Discussion
We report here the case of a male patient presenting metastatic HCC-ICC in the context of Child–Pugh A cirrhosis due to viral hepatitis C. Following two lines of systemic treatment targeting the HCC component, the patient suffered major tumour progression. Regarding the overexpression of PD-L1 and the absence of an alternative treatment option, pembrolizumab was administered and showed an almost immediate clinical benefit and an excellent response rate at the first assessment. This response was maintained over time along with toxicity-free tumour control after 18 months treatment.
These results concur with the findings of Zhu et al., although the population studied by these authors suffered from advanced HCC unsuccessfully treated with sorafenib. Their objective response rate was 17% including one complete response with a non-reach median response time.8
Liver tumour microenvironment (TME) is immunosuppressive, it is composed of myeloid-derived suppressor cells, tumour-associated macrophages, tumour-infiltrating lymphocytes, regulatory T cells, natural killer cells, Kupffer cells, dendritic cells and an abundance of pro-inflammatory chemokines, cytokines and soluble factors like VEGF, transforming growth factor (TGF)-β and indoleamine 2,3-dioxygenase.12,13 In addition to the presence of cells with immune suppressive functions, the HCC TME is characterised by high expression of immune checkpoint molecules (PD-L1, PD1 and CTLA4).
In this context, restoring antitumour immunity by targeting immune checkpoints in HCC-ICC seems to be a promising approach. However, whether in the case of HCC14 or ICC,15 studies have shown that only a small fraction of patients respond to immune checkpoints inhibitors (ICIs). One of the ways to improve outcomes could be the combination of treatments based on synergies and mechanisms of resistance to ICIs.16 Among the potential leads in the treatment of HCC, combinations of ICIs with anti-angiogenic antibodies are about to change our practices. Recently the combination of bevacizumab plus atezolizumab, an anti PD-L1, showed an objective response of 33%, and a gain in survival (PFS and OS) and quality of life compared with sorafenib.17 Other combinations of ICIs with tyrosine kinase inhibitors are under investigation (NCT03713593; NCT01658878).
Given the highly heterogeneous nature of HCC-ICC, it is essential to identify the predictive biomarkers of immunomodulatory treatments in order to select patients who will benefit from this type of therapy. Our patient presented overexpression of PD-L1 in immunohistochemistry at 85%. This constitutes a predictive marker of therapeutic response to PD-1 inhibitors observed in many cancers, notably in non-small-cell lung carcinomas and stomach cancers.18,19 In the KEYNOTE-224 trial, the tumoural response rate was significantly correlated with a score combining PD-L1 expression by both immune and tumour cells.
In the case of our patient, it was unfortunately not possible to perform an additional molecular characterisation due to the lack of available tumour material. This could have oriented us towards a possible therapeutic target and represents a limitation to our case report.
Furthermore, treatment response assessment also entails the core issue of checkpoint inhibitors management. We know that tumours respond variously to immunotherapy compared with conventional therapies. This observation has recently given rise to changes in the radiological criteria for morphological evaluation of therapeutic response.20 More recently, the conclusions of the European Association of Nuclear Medicine (EANM) symposium have demonstrated that the PET scan is a very useful tool for the assessment of tumour response due to the contribution made by metabolic analysis.21 In our case, and in accord with EANM recommendations, a PET scan performed before the first pembrolizumab injection and early during the first reassessment enabled us to consolidate our therapeutic strategy.
Conclusion
To our knowledge, this is the first report on the efficacy of a PD1 inhibitor treatment in a patient presenting metastatic HCC-ICC due to viral cirrhosis and overexpressing PD-L1 after failure of two lines of treatment. These encouraging results support strongly the need for specific trials focusing on this cancer, which can be considered as an orphan disease.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: A. Saint  https://orcid.org/0000-0002-3963-6124
https://orcid.org/0000-0002-3963-6124
Contributor Information
Angélique Saint, Medical Oncology Department, Centre Antoine Lacassagne, 33 avenue de Valombrose, Nice, 06189, France.
Maxime Benchetrit, Laboratory of Anatomopathology, DIAG, Nice, France.
Sébastien Novellas, Institut Arnault Tzanck, Saint Laurent du Var, France.
Denis Ouzan, Institut Arnault Tzanck, Saint Laurent du Var, France.
Alexander Tuan Falk, Institut Arnault Tzanck, Saint Laurent du Var, France; Centre Azuréen de Cancérologie, Mougins, France.
Axel Leysalle, Radiotherapy Department, Centre Antoine Lacassagne, Nice, France; Medical Oncology Department, Polyclinique Saint Jean, Cagnes-sur-Mer, France.
Jérome Barriere, Medical Oncology Department, Centre Antoine Lacassagne, Nice, France; Medical Oncology Department, Polyclinique Saint Jean, Cagnes-sur-Mer, France.
References
- 1. Lee W-S, Lee K-W, Heo J-S, et al. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today 2006; 36: 892–897. [DOI] [PubMed] [Google Scholar]
- 2. Wachtel MS, Zhang Y, Xu T, et al. Combined hepatocellular cholangiocarcinomas; analysis of a large database. Clin Med Pathol 2008; 1: 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergquist JR, Groeschl RT, Ivanics T, et al. Mixed hepatocellular and cholangiocarcinoma: a rare tumor with a mix of parent phenotypic characteristics. HPB (Oxford) 2016; 18: 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 5. Cheng A-L, Kang Y-K, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 6. Salimon M, Prieux-Klotz C, Tougeron D, et al. Gemcitabine plus platinum-based chemotherapy for first-line treatment of hepatocholangiocarcinoma: an AGEO French multicentre retrospective study. Br J Cancer 2018; 118: 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 9. Finn RS, Ryoo B-Y, Merle P, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2019; 37(Suppl. 15): 4004. [Google Scholar]
- 10. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 11. Bang Y-J, Ueno M, Malka D, et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J Clin Oncol 2019; 37(Suppl. 15): 4079. [Google Scholar]
- 12. Lu C, Rong D, Zhang B, et al. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer 2019; 18: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu Y, Liu S, Zeng S, et al. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res 2019; 38: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Longo V, Brunetti O, Gnoni A, et al. Emerging role of immune checkpoint inhibitors in hepatocellular carcinoma. Medicina (Kaunas) 2019; 55: 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gou M, Zhang Y, Si H, et al. Efficacy and safety of nivolumab for metastatic biliary tract cancer. Onco Targets Ther 2019; 12: 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iñarrairaegui M, Melero I, Sangro B. Immunotherapy of hepatocellular carcinoma: facts and hopes. Clin Cancer Res 2018; 24: 1518–1524. [DOI] [PubMed] [Google Scholar]
- 17. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 18. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 19. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018; 4: e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18: e143–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aide N, Hicks RJ, Le Tourneau C, et al. FDG PET/CT for assessing tumour response to immunotherapy: report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging 2019; 46: 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]