Abstract
Background
CircMAN2B2 is a newly discovered circRNA that has been found to be an oncogene in lung cancer and glioma. The present study was designed to reveal the role of circMAN2B2 in gastric carcinoma (GC).
Methods
qRT‐PCR method was utilized to examine circMAN2B2 expression in GC tissues and paracancerous tissues. Next, circMAN2B2 expression in SNU‐16 and AGS cells was silenced by transfection. CCK‐8 assay, colony formation assay, flow cytometer, Transwell assay, and Western blot were conducted for testing cell phenotype changes. Further, the downstream genes and signaling were uncovered by qRT‐PCR and Western blot.
Results
As relative to paracancerous tissues, circMAN2B2 was high‐expressed in GC tissues. Silence of circMAN2B2 clearly declined SNU‐16 and AGS cells viability, survival, migration but enhanced apoptosis. Meanwhile, silence of circMAN2B2 induced the cleavage of caspases (−3 and −9), down‐regulation of MMPs (−2 and −9), and up‐regulation of miR‐145. The impacts of circMAN2B2 silence toward SNU‐16 and AGS cells were attenuated by miR‐145 silence. Moreover, circMAN2B2 silence deactivated PI3K, AKT while activated JNK through regulating miR‐145.
Conclusion
This work presented the oncogenic function of circMAN2B2 in GC cells growth and migration. CircMAN2B2 exerted its function possibly through regulating miR‐145 as well as PI3K/AKT and JNK pathways.
Keywords: AGS cell, apoptosis, JNK pathway, PI3K/AKT pathway, SNU‐16 cell
Highlights.
CircMAN2B2 is highly expressed in gastric cancer tissues;
CircMAN2B2 silence suppresses gastric cancer cells growth and migration;
CircMAN2B2 negatively regulates miR‐145;
CircMAN2B2 exerts its oncogenic function through miR‐145;
CircMAN2B2 regulates PI3K/AKT and JNK pathways through miR‐145.
1. INTRODUCTION
Gastric carcinoma (GC) is one of the top ten most common malignant tumors around the world. As estimated by Siegel and his colleagues, there are 28 000 cases and 10 960 GC‐related death occurred in United States at 2017.1 At present, surgery is still the main method to cure GC in the case of early diagnosed. But, most GC patients are diagnosed in the late stages since the symptoms of GC are generally vague, non‐specific and may not occur until late in disease progression.2 For those patients, unresectable therapeutic options like chemotherapy and radiation are always recommended, but unfortunately, this cancer is unlikely to be cured. What's worse, the high recurrence and metastasis of GC3, 4 make itself to be more intractable with lower overall survival. So, there is clinical significance to extensively investigate the deep pathogenesis of GC, which may be helpful for investigating novel treatment strategy.
Circular RNAs (circRNAs), formed by alternative splicing of pre‐mRNA transcripts, are stable and widespread non‐coding RNAs in human cells. Current studies have shown that circRNAs play important regulatory roles in a variety of diseases, such as cardiovascular diseases,5 nervous system diseases,6 hypertension,7 and preeclampsia.8 In addition, circRNAs are widely involved in the onset and progression of human cancers, including GC.9 As reported by Shao et al, 308 circRNAs were found to be differentially expressed in GC, among which 34.74% was up‐regulated and 65.26% was down‐regulated.10 More than that, circRNAs have clinical significance in treating GC. For the selected examples, silence of circCACTIN may suppress the growth, migration and invasion of GC cells.11 CircMAN2B2 (circRNA ID: hsa_circRNA_0069086) is a newly discovered circRNA that consists of two exons of 291 nucleotides in length. To date, the oncogenic function of circMAN2B2 toward lung cancer 12 and glioma 13 has been revealed. Nonetheless, the role of circMAN2B2 in GC is still unclear.
MicroRNAs (miRNAs) are recently discovered single‐strand non‐coding RNAs that are as small as 19‐22 nucleotides. In general, they are participated in regulating the expression of target genes at post‐transcriptional level. It is recently recognized that miRNAs play significant roles in the development of human cancers, as they acted as either oncogenes or tumor suppressors.14, 15 In GC, miR‐145 expression was found to be down‐expressed and was associated with poor survival rate.16 Moreover, miR‐145 acted as a tumor suppressor in GC through controlling tumor growth, chemo‐resistance, and metastasis.17, 18
In the present paper, in vitro experiments were carried out to reveal the role of circMAN2B2 in the growth and migration of GC cells. Besides that, the regulatory impact of circMAN2B2 on miR‐145 expression was studied to uncover the deep mechanisms of circMAN2B2’s function.
2. MATERIALS AND METHODS
2.1. Clinical specimens
Twenty‐five pairs of GC tissues and the paracancerous tissues were harvested from patients with GC which are attained from The Chinese People's Liberation Army Navy 971 Hospital (Qingdao, China). This experiment was approved by the Ethics Committee of The Chinese People's Liberation Army Navy 971 Hospital. These clinical specimens were harvested during tumor resection, before which none of them received any other therapies. Written informed consents were signed before the usage of the clinical specimens from each individual. Clinical specimens were washed with ice‐cold PBS and stored at −80°C until use.
2.2. Cells
Two GC cell lines (SNU‐16 and AGS) were brought from ATCC. SNU‐16 and AGS cells were respectively grown in RPMI‐1640 medium (ATCC) and F‐12K medium (ATCC), which are supplemented with 10% fetal bovine serum (Gibco). Cells were maintained at 37°C in 95% air and 5% CO2.
2.3. Cell transfection
siRNA specific against circMAN2B2 (si‐circMAN2B2) was designed and purchased from Biomics Biotechnologies. Non‐targeting sequence was used as siRNA control. MiR‐145 inhibitor and the negative control (NC inhibitor) were brought from GenePharma. Lipofectamine 2000 (Invitrogen) was utilized for transfection which was lasted for 48 hours. The sequence of si‐circMAN2B2 was displayed as follows: 5′‐TCGCAGGTCCAGCACCATGAT‐3′.
2.4. qRT‐PCR
Total RNAs were harvested from clinical specimens and the transfected cells through utilizing TRIzol reagent (Invitrogen). Then, the concentration of extracted RNA was detected through a NanoDrop Spectrophotometer (NanoDrop Technologies) at 260 nm/280 nm. For the test of circMAN2B2, PrimeScript™ RT reagent Kit and TB Green Premix Ex Taq II (Takara) were utilized for inverse transcription and quantitative PCR, respectively. For the test of miR‐145, Mir‐X™ miRNA First Strand Synthesis Kit and Mir‐X™ miRNA qRT‐PCR TB Green™ Kit were utilized (Takara). GAPDH and U6 served as reference controls for circMAN2B2 and miR‐145. The primer sequences used in the study were displayed as follows: circMAN2B2, forward 5′‐CCCAACATGAGTGAGCCTGT‐3′, reverse 5′‐GCACCGAGGCATTGAAGAAC‐3′; miR‐145, forward 5′‐ACACTCCAGCTGGGGTCCAGTTTTCCCAGGA‐3′, reverse 5′‐TGGTGTCGTGGAGTCG‐3′; GADPH, forward 5′‐GTCAACGGATTTGGTCTGTATT‐3′, reverse 5′‐AGTCTTCTGGGTGGCAGTGAT‐3′; U6, forward 5′‐CTCGCTTCGGCAGCACA‐3′, and reverse 5′‐AACGCTTCACGAATTTGCGT‐3′. Real‐time‐PCR reactions were performed by the ABI7300 cycler (Applied Biosystems) through ABI7300 software.
2.5. CCK‐8 assay
The transfected SNU‐16 and AGS cells in 96‐well plates (5000 cells/well) were cultured at 37°C for 48 hours. Then, the culture medium was replaced and 10 μL CCK‐8 solution (Dojindo Molecular Technologies) was added. Following incubation at 37°C for 4 hours, the samples were tested under a Microplate Reader (Bio‐Rad) at 450 nm.
2.6. Colony formation assay
The transfected SNU‐16 and AGS cells in 6‐well plates (500 cells/well) were cultured at 37°C for 2 weeks. Then, cells were fixed with 4% paraformaldehyde fix solution (Sangon Biotech) and stained with 0.5% crystal violet (Sangon Biotech). Survival fractions were calculated according to the following formula:
2.7. Apoptosis assay
The transfected SNU‐16 and AGS cells were harvested, and the apoptosis rate of 1 × 105 cells per sample was tested by utilizing Annexin V‐FITC/PI Apoptosis Detection Kit (Sangon Biotech) according to the manufacture's instruction. Flow cytometer detection was done in FACS Calibur (Becton Dickson) by counting FITC‐positive and PI‐negative cells through Cell Quest Research Software (Becton Dickinson).
2.8. Migration assay
Twenty four‐well Transwell chambers with 8 μm pore size (BD Biosciences) were used. The transfected SNU‐16 and AGS cells (1 × 105 cells/well) were suspended in serum‐free culture medium and placed on the upper side of the well. The lower side was filled with the complete culture medium. Following incubation at 37°C for 24 hours, the cells in the upper side were removed, and those in the lower side were stained by 0.5% crystal violet (Sangon Biotech) for 10 minutes. The migration rate was calculated by counting stained cells under IX73 inverted microscope (Olympus).
2.9. Protein immunoblot
Total proteins were harvested from the transfected SNU‐16 and AGS cells by utilizing RIPA buffer (Sangon Biotech) and quantified through BCA™ Protein Assay Kit (Pierce). Immunoblotting was done by using primary antibodies specific against cleaved‐caspase‐3 (orb126609, Biorbyt), cleaved‐caspase‐9 (orb227889), MMP‐2 (orb101824), MMP‐9 (orb315177), PI3K (orb395443), p‐PI3K (orb106105), AKT (orb29949), p‐AKT (orb344456), JNK (orb38050), p‐JNK (orb184488), c‐Jun (orb97352), p‐c‐Jun (orb184488), and β‐actin (orb86987). Target bands were developed by EasyBlot ECL kit (Sangon Biotech), and the gray level of the bands was analyzed by Image Lab™ Software (Bio‐Rad).
2.10. Statistics
Data were presented as mean ± SD. Statistics were done in SPSS 19.0 software. Comparison was done by utilizing one‐way ANOVA or Student t test. Statistical differences were set at P < .05 and indicated as asterisks in figures.
3. RESULTS
3.1. circMAN2B2 was highly expressed in GC tissues
qRT‐PCR analysis was utilized for testing the expression of circMAN2B2 in 25 pairs of GC tissues. As related to paracancerous tissues, level of circMAN2B2 in GC tissues was much higher (P < .05, Figure 1).
Figure 1.
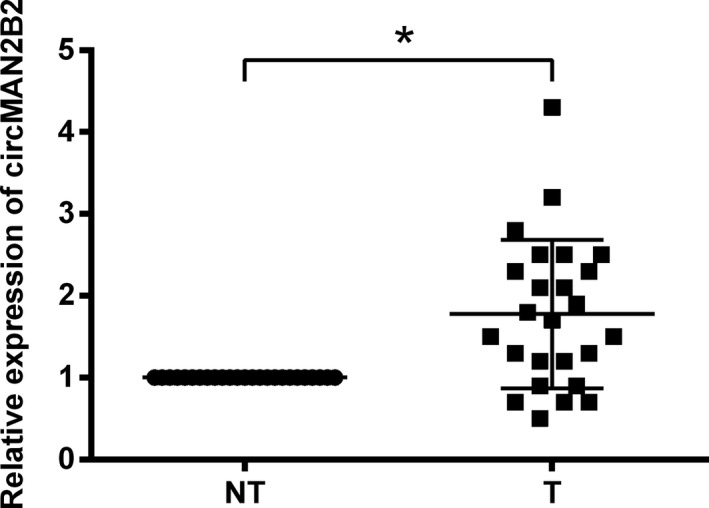
CircMAN2B2 was highly expressed in gastric carcinoma (GC) tissues. qRT‐PCR analysis was utilized for testing the expression of circMAN2B2 in 25 pairs of GC tissues (T) and paracancerous non‐tumor tissues (NT). *P < .05
3.2. Silence of circMAN2B2 suppressed the growth of GC cells
siRNA specific against circMAN2B2 was transfected into two GC cell lines (SNU‐16 and AGS) to see the effect of circMAN2B2 on the growth of GC cells. Data presented in Figure 2A showed that, circMAN2B2 expression was successfully repressed by siRNA transfection (P < .05). As compared with si‐NC transfection, transfection of cells with si‐circMAN2B2 significantly declined cell viability (P < .05, Figure 2B), survival fraction (P < .05, Figure 2C), but induced apoptosis (P < .05, Figure 2D). Meanwhile, the cleavage of caspase −3 and −9 was evoked by si‐circMAN2B2 transfection as relative to si‐NC transfection (P < .05, Figure 2E‐G).
Figure 2.
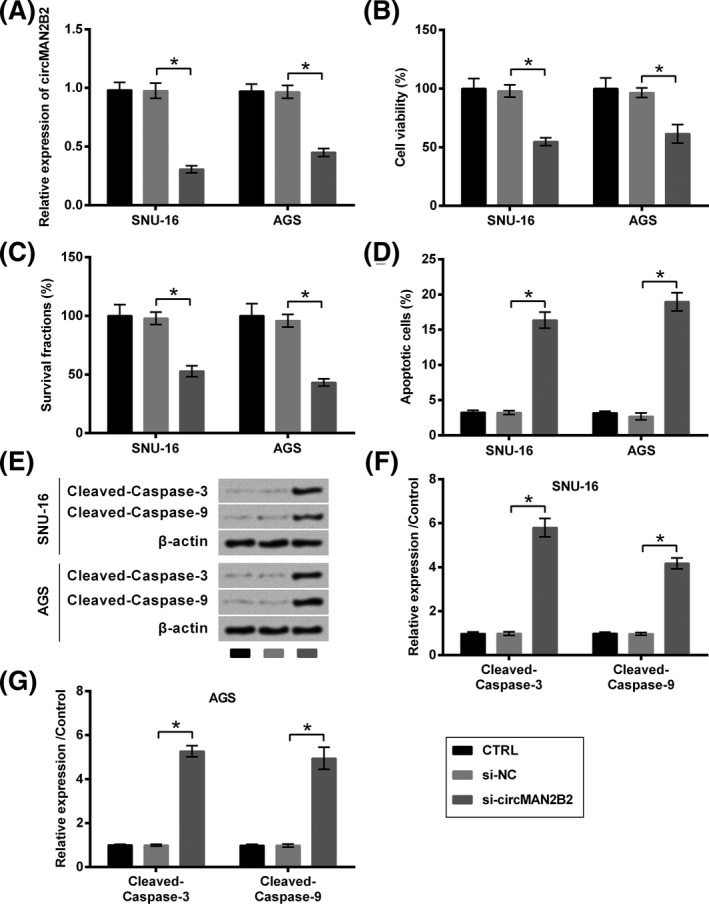
Silence of circMAN2B2 suppressed the growth of GC cells. SNU‐16 and AGS cells were transfected with nothing, si‐NC or si‐circMAN2B2. A, Transfection efficiency was verified by qRT‐PCR analysis which tested by expression of circMAN2B2. B, Cell viability, (C) survival, (D) apoptosis, and (E‐G) expression of caspases were respectively examined by CCK‐8 assay, colony formation assay, flow cytometry, and Western blot. *P < .05
3.3. Silence of circMAN2B2 suppressed the migration of GC cells
Also, the role of circMAN2B2 in the migration of GC cells was evaluated. As seen in Figure 3A, the migration of both SNU‐16 and AGS cells was repressed by transfection with si‐circMAN2B2, as relative to si‐NC (P < .05). Consistently, levels of migration‐related proteins (MMP‐2 and MMP‐9) were declined by transfection with si‐circMAN2B2, as relative to si‐NC (P < .05, Figure 3B‐D).
Figure 3.
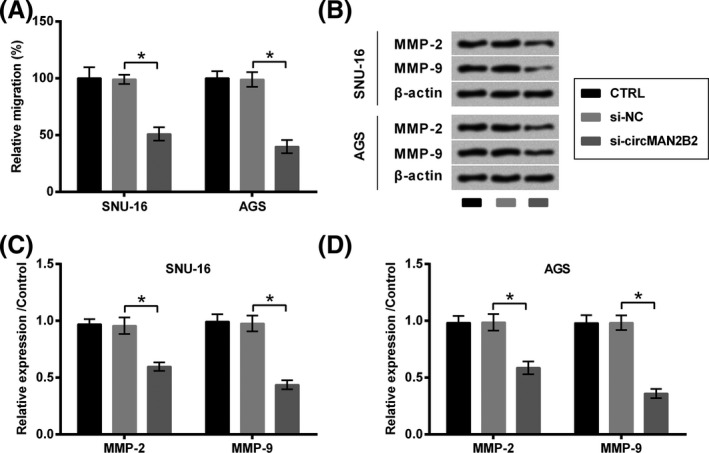
Silence of circMAN2B2 suppressed the migration of GC cells. SNU‐16 and AGS cells were transfected with nothing, si‐NC or si‐circMAN2B2. A, Cell migration and (B‐D) expression of MMPs were respectively examined by Transwell assay and Western blot. *P < .05
3.4. Silence of circMAN2B2 acted GC cells through regulating miR‐145
The expression change of miR‐145 in GC cells following transfection with si‐circMAN2B2 was tested. As qRT‐PCR data shown in Figure 4A, miR‐145 expression was significantly elevated by si‐circMAN2B2 as relative to si‐NC (P < .05). So, miR‐145 might be one of the downstream genes of circMAN2B2. To verify the authenticity of this hypothesis, miR‐145 expression in SNU‐16 and AGS cells was silenced by transfection with the specific inhibitor. Transfection efficiency shown in Figure 4B demonstrated that, miR‐145 expression was successfully declined by miR‐145 inhibitor as relative to NC inhibitor (P < .05).
Figure 4.
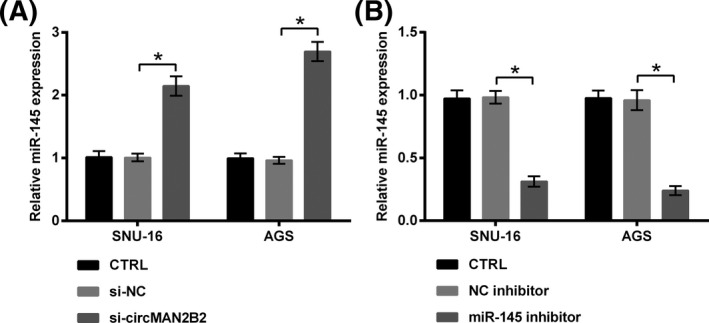
Silence of circMAN2B2 elevated miR‐145 expression. A, SNU‐16 and AGS cells were transfected with nothing, si‐NC or si‐circMAN2B2. B, SNU‐16 and AGS cells were transfected with nothing, NC inhibitor or miR‐145 inhibitor. miR‐145 expression was examined by qRT‐PCR. *P < .05
Following experiments found that, co‐transfection of cells with si‐circMAN2B2 and miR‐145 inhibitor significantly increased cell viability (P < .05, Figure 5A), survival fraction (P < .05, Figure 5B), while repressed apoptosis (P < .05, Figure 5C) and the cleavage of caspases (P < .05, Figure 5D‐F), as compared to co‐transfection with si‐circMAN2B2 plus NC inhibitor. Meanwhile, migration (P < .05, Figure 6A) and the expression of relative proteins (P < .05, Figure 6B‐D) were elevated by co‐transfection with si‐circMAN2B2 and miR‐145 inhibitor as compared to co‐transfection with si‐circMAN2B2 plus NC inhibitor.
Figure 5.
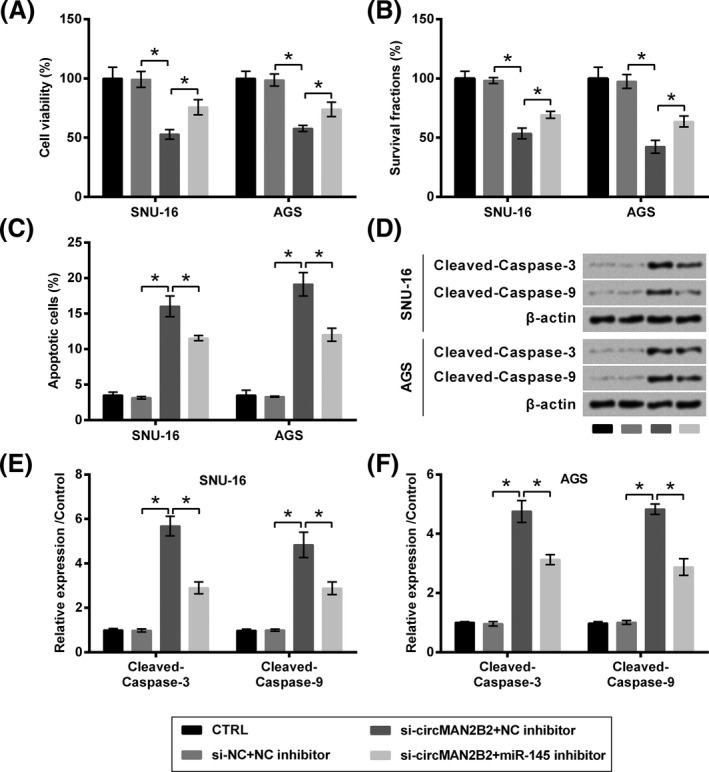
Silence of circMAN2B2 suppressed the growth of GC cells through regulating miR‐145. SNU‐16 and AGS cells were transfected with nothing, miR‐145 inhibitor, and/or si‐circMAN2B2. si‐NC and NC inhibitor were transfected as respective controls. A, Cell viability, (B) survival, (C) apoptosis, and (D‐F) expression of caspases were respectively examined by CCK‐8 assay, colony formation assay, flow cytometry, and Western blot. *P < .05
Figure 6.
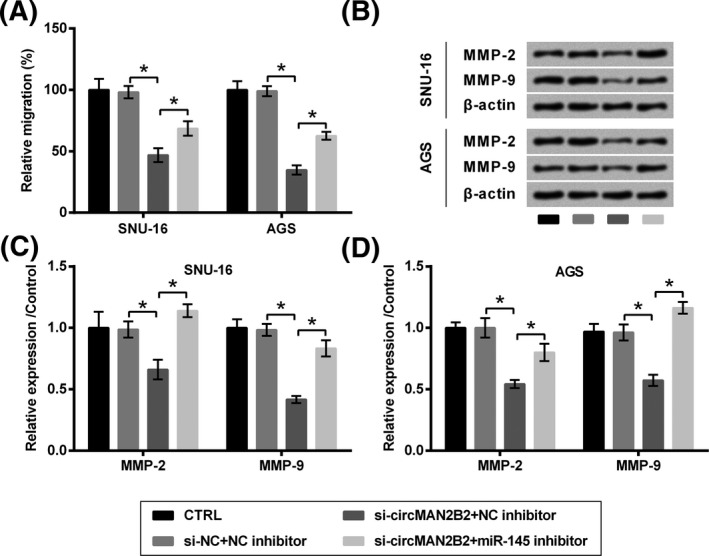
Silence of circMAN2B2 suppressed the migration of GC cells through regulating miR‐145. SNU‐16 and AGS cells were transfected with nothing, miR‐145 inhibitor, and/or si‐circMAN2B2. si‐NC and NC inhibitor were transfected as respective controls. A, Cell migration and (B‐D) expression of MMPs were respectively examined by Transwell assay and Western blot. *P < .05
3.5. Silence of circMAN2B2 regulated PI3K/AKT and JNK pathways through regulating miR‐145
To further decode the underlying mechanisms of which circMAN2B2 impacted GC cells, the expression of core factors in PI3K/AKT and JNK pathways was tested. As data presented in Figure 7A‐C, the expression of active (phospho) forms of PI3K and AKT was suppressed by transfection with si‐circMAN2B2 (P < .05). The impacts of si‐circMAN2B2 toward the expression of these two proteins were attenuated by miR‐145 inhibitor (P < .05). Of contrast, the expression of active form (phospho) of JNK and c‐Jun was elevated by si‐circMAN2B2 (P < .05), while was suppressed by miR‐145 inhibitor (P < .05, Figure 7D‐F).
Figure 7.
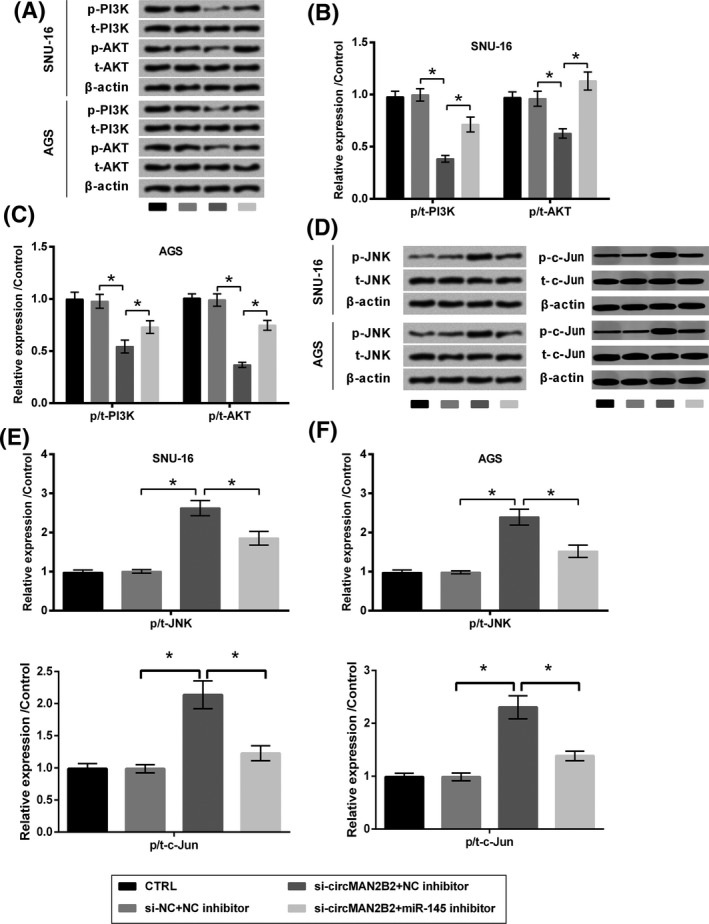
Silence of circMAN2B2 regulated PI3K/AKT and JNK pathways through regulating miR‐145. SNU‐16 and AGS cells were transfected with nothing, miR‐145 inhibitor, and/or si‐circMAN2B2. si‐NC and NC inhibitor were transfected as respective controls. Expression of (A‐C) PI3K, AKT, (D‐F) JNK, and c‐Jun was examined by Western blot. *P < .05
4. DISCUSSION
Over the past several years, an increasing amount of circRNAs were discovered to be involved in the progression of GC.9 For instance, hsa_circ_0008035,19 circCACTIN,11 and circ‐DCAF620 worked as oncogenic genes in GC, while circLMTK221 and hsa_circ_000136822 functioned as tumor suppressors. These previous findings linked circRNAs with GC, which provide a better understanding of GC and showed the promise of using circRNAs as novel therapeutic targets. From the current data, circMAN2B2 was found to be high‐expressed in GC tissues as relative to paracancerous non‐tumor tissues. Therefore, a possible mechanism that knockdown of circMAN2B2 might inhibit GC development was further explored.
It has been reported that circRNAs are participated in many cancer types and play diverse influences in gene expression, apoptosis, cell cycle, etc,23 GC is no exception. Multiple circRNAs were found to be specific difference between GC tissues and non‐tumor tissues10 and play important role in GC carcinogenesis, such as hsa_circ_0067582,24 hsa_circ_0005758,24 hsa_circ_0000467,25 hsa_circ_0000181 26 and hsa_circ_0003159.27 The present paper demonstrated that circMAN2B2 was highly expressed in GC tissues. Importantly, we verified our hypothesis that circMAN2B2 silence took an inhibitory role in GC development. Silence of circMAN2B2 significantly reduced the growth of SNU 16 and AGS cells, as evidenced by the viability loss, the repressed survival fraction and the induced apoptosis. Moreover, silence of circMAN2B2 was able to suppress the migration of GC cells. It is worth mentioning that, the impacts of circMAN2B2 silence on cell migration may due to the low expressed MMPs (MMP‐2 and MMP‐9), which are crucial in degrading extracellular matrix on cell surface and create a migration path.28 These findings were consistent with the oncogenic role of circMAN2B2 in other two cancers that were reported in previous studies, that is, lung cancer12 and glioma.13 For the first time, we illustrated the oncogenic function of circMAN2B2 in GC, which provided a promising biomarker for monitoring and treating GC in the future.
CircRNAs could work as miRNA sponge to modulate the genes regulation. MiRNAs are another sort of newly discovered RNAs that are crucially implicated in the pathogenesis of human cancer.14, 15 The expression of miRNAs is related to the biological processes of human cancers, such as cell growth, proliferation, differentiation, apoptosis, metastasis, and drug‐resistance.29, 30, 31, 32 MiR‐145 is one of such miRNAs and has been reported as a tumor inhibitor in various cancers, such as cervical carcinoma,33 esophageal squamous cell carcinoma (ESCC),34 colorectal cancer,35 prostate cancer,36 and GC.37 It was proved to inhibit GC cells growth, migration, and metastasis.18, 38 Based on the inhibitory role of circMAN2B2 silence and the reported role of miR‐145 in GC, it will be interesting to explore whether circMAN2B2 silence plays its roles in GC through regulating miR‐145. Our results showed that miR‐145 expression was up‐regulated by circMAN2B2 silence, suggesting a potential mechanism that the inhibition of miR‐145 might reverse the effects of circMAN2B2 silence on SNU 16 and AGS cells. As we expect, we found that the impact of circMAN2B2 silence was attenuated when miR‐145 was silenced simultaneously. These findings suggested that circMAN2B2 silence exerted anti‐GC impacts by up‐regulating miR‐145. Further work will be performed to explore the sponge relationship between circMAN2B2 and miR‐145 to further enrich this regulatory mechanism.
PI3K/AKT is one of the important signal transduction pathways that is greatly activated in a variety of human cancers, including GC.39 Its activation confers the common features of tumor cells, like excessive growth, repressed apoptosis death and metastasis.40 So, a series of inhibitors for this signaling have been developed and have entered into clinical trials.41 Similar to PI3K/AKT, JNK is another widely studied signaling in cancer research.42 JNK signaling can be activated by a number of stimuli such as cytokines, stress, toxins, and drugs.43, 44 Once activated, JNK alters the expression of multiple proteins like c‐Jun, c‐Fos, c‐Myc, and p53 to control cell proliferation, survival, death, intercellular adhesion, and migration.42, 45 It has been reported that miR‐145 played anti‐tumor roles through regulating PI3K/AKT and JNK pathways. For example, miR‐145 attenuated the growth and metastasis of thyroid cancer through PI3K/AKT pathway.46 MiR‐145 was reported to raise the sensitivity of ESCC to cisplatin (DPP) and promote DPP‐caused apoptosis through directly inhibiting PI3K/AKT pathway.47 Moreover, it could suppress epithelial‐mesenchymal transition in small cell lung cancer through regulating JNK pathway.48 Therefore, based on the above regulatory mechanism between circMAN2B2 and miR‐145, circMAN2B2 might act on these two pathways. As we expect, Western blot results in this study demonstrated that silence of circMAN2B2 deactivated PI3K and AKT while activated JNK, suggesting the regulatory role of circMAN2B2 in the activation of PI3K/AKT and JNK pathways. Additionally, the regulation of circMAN2B2 on these two pathways was also through regulating miR‐145.
To sum up, this work presented the oncogenic function of circMAN2B2 in the growth and migration of GC cells. CircMAN2B2 exerted its function possibly through regulating miR‐145 and PI3K/AKT and JNK pathways. Our study provided a potential goal for the treatment of GC.
AUTHORS' CONTRIBUTIONS
Bo Sun and Yue Lun: conceived and designed the experiments and wrote the manuscript; Bo Sun, Haiyuan Sun, Qunying Wang, Xinhong Wang, Jingzi Quan, Dongfang Dong, and Yue Lun: performed the experiments and analyzed the data; All authors: read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from the patient. The consent form is held by The Chinese People's Liberation Army Navy 971 Hospital.
CONSENT FOR PUBLICATION
All authors are informed and agree to publish.
Sun B, Sun H, Wang Q, et al. Circular RNA circMAN2B2 promotes growth and migration of gastric cancer cells by down‐regulation of miR‐145. J Clin Lab Anal. 2020;34:e23215 10.1002/jcla.23215
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Wang Y‐L, Ge X‐X, Wang Y, et al. The values of applying classification and counts of white blood cells to the prognostic evaluation of resectable gastric cancers. BMC Gastroenterol. 2018;18(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnston FM, Beckman M. Updates on management of gastric cancer. Curr Oncol Rep. 2019;21(8):67. [DOI] [PubMed] [Google Scholar]
- 3. Kanaji S, Suzuki S. Recent updates in perioperative chemotherapy and recurrence pattern of gastric cancer. Ann Gastroenterol Surg. 2018;2(6):400‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Youn GJ, Chung WC. Micrometastasis in gastric cancer. Korean J Gastroenterol. 2017;69(5):270‐277. [DOI] [PubMed] [Google Scholar]
- 5. Fan X, Weng X, Zhao Y, Chen W, Gan T, Xu D. Circular RNAs in cardiovascular disease: an overview. Biomed Res Int. 2017;2017:5135781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie L, Mao M, Xiong K, Jiang B. Circular RNAs: a novel player in development and disease of the central nervous system. Front Cell Neurosci. 2017;11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaiou M. Circular RNAs in hypertension: challenges and clinical promise. Hypertens Res. 2019;42(11):1653‐1663. [DOI] [PubMed] [Google Scholar]
- 8. Jia N, Li J. Role of circular RNAs in preeclampsia. Dis Markers. 2019;2019:7237495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang KW, Dong M. Role of circular RNAs in gastric cancer: Recent advances and prospects. World J Gastrointest Oncol. 2019;11(6):459‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shao Y, Li J, Lu R, et al. Global circular RNA expression profile of human gastric cancer and its clinical significance. Cancer Med. 2017;6(6):1173‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang L, Song X, Chen X, et al. Circular RNA CircCACTIN promotes gastric cancer progression by sponging MiR‐331‐3p and regulating TGFBR1 expression. Int J Biol Sci. 2019;15(5):1091‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma X, Yang X, Bao W, et al. Circular RNA circMAN2B2 facilitates lung cancer cell proliferation and invasion via miR‐1275/FOXK1 axis. Biochem Biophys Res Commun. 2018;498(4):1009‐1015. [DOI] [PubMed] [Google Scholar]
- 13. Xiong J, Wang T, Tang H, Lv Z, Liang P. Circular RNA circMAN2B2 facilitates glioma progression by regulating the miR‐1205/S100A8 axis. J Cell Physiol. 2019;234(12):22996‐23004. [DOI] [PubMed] [Google Scholar]
- 14. Vos PD, Leedman PJ. Modulation of miRNA function by natural and synthetic RNA‐binding proteins in cancer. Cell Mol Life Sci. 2019;76(19):3745‐3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang M, Yu F, Ding H, Wang Y, Li P, Wang K. Emerging function and clinical values of exosomal MicroRNAs in cancer. Mol Ther Nucleic Acids. 2019;16:791‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Wen X, Hu XL, Cheng LZ, Yu JY, Wei ZB. Downregulation of miR‐145‐5p correlates with poor prognosis in gastric cancer. Eur Rev Med Pharmacol Sci. 2016;20(14):3026‐3030. [PubMed] [Google Scholar]
- 17. Zeng JF, Ma XQ, Wang LP, Wang W. MicroRNA‐145 exerts tumor‐suppressive and chemo‐resistance lowering effects by targeting CD44 in gastric cancer. World J Gastroenterol. 2017;23(13):2337‐2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lei C, Du F, Sun L, et al. miR‐143 and miR‐145 inhibit gastric cancer cell migration and metastasis by suppressing MYO6. Cell Death Dis. 2017;8(10):e3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang S, Zhang X, Guan B, et al. A novel circular RNA hsa_circ_0008035 contributes to gastric cancer tumorigenesis through targeting the miR‐375/YBX1 axis. Am J Transl Res. 2019;11(4):2455‐2462. [PMC free article] [PubMed] [Google Scholar]
- 20. Wu L, Liu D, Yang Y. Enhanced expression of circular RNA circ‐DCAF6 predicts adverse prognosis and promotes cell progression via sponging miR‐1231 and miR‐1256 in gastric cancer. Exp Mol Pathol. 2019;110:104273. [DOI] [PubMed] [Google Scholar]
- 21. He J, Chen J, Ma B, Jiang L, Zhao G. CircLMTK2 acts as a novel tumor suppressor in gastric cancer. Biosci Rep. 2019;39(5):BSR20190363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu J, Zhang PY, Li P, et al. Circular RNA hsa_circ_0001368 suppresses the progression of gastric cancer by regulating miR‐6506‐5p/FOXO3 axis. Biochem Biophys Res Commun. 2019;512(1):29‐33. [DOI] [PubMed] [Google Scholar]
- 23. Zhao ZJ, Shen J. Circular RNA participates in the carcinogenesis and the malignant behavior of cancer. RNA Biol. 2017;14(5):514‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu R, Shao Y, Tao X, Ye G. Clinical significances of hsa_circ_0067582 and hsa_circ_0005758 in gastric cancer tissues. J Clin Lab Anal. 2019;33(9):e22984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu J, Zhang PY, Xie JW, et al. Hsa_circ_0000467 promotes cancer progression and serves as a diagnostic and prognostic biomarker for gastric cancer. J Clin Lab Anal. 2019;33(3):e22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao Q, Chen S, Li T, Xiao B. Clinical values of circular RNA 0000181 in the screening of gastric cancer. J Clin Lab Anal. 2018;32(4):e22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tian M, Chen R, Li T, Xiao B. Reduced expression of circRNA hsa_circ_0003159 in gastric cancer and its clinical significance. J Clin Lab Anal. 2018;32(3):e22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li WD, Li NP, Song DD, Rong JJ, Qian AM, Li XQ. Metformin inhibits endothelial progenitor cell migration by decreasing matrix metalloproteinases, MMP‐2 and MMP‐9, via the AMPK/mTOR/autophagy pathway. Int J Mol Med. 2017;39(5):1262‐1268. [DOI] [PubMed] [Google Scholar]
- 29. Maruyama S, Furuya S, Shiraishi K, et al. Inhibition of apoptosis by miR1225p in alpha‐fetoprotein‐producing gastric cancer. Oncol Rep. 2019;41(4):2595‐2600. [DOI] [PubMed] [Google Scholar]
- 30. Zhou T, Chen S, Mao X. miR‐145‐5p affects the differentiation of gastric cancer by targeting KLF5 directly. J Cell Physiol. 2019;234(5):7634‐7644. [DOI] [PubMed] [Google Scholar]
- 31. Cao Q, Liu F, Ji K, et al. MicroRNA‐381 inhibits the metastasis of gastric cancer by targeting TMEM16A expression. J Exp Clin Cancer Res. 2017;36(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang W, Ma J, Zhou W, et al. Molecular mechanisms and theranostic potential of miRNAs in drug resistance of gastric cancer. Expert Opin Ther Targets. 2017;21(11):1063‐1075. [DOI] [PubMed] [Google Scholar]
- 33. Ma L, Li LL. miR‐145 contributes to the progression of cervical carcinoma by directly regulating FSCN1. Cell Transplant. 2019;28(9–10):1299‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y, Zhang L, Wang R, Wang B, Hua P, Li J. LncRNA Erbb4‐IR promotes esophageal squamous cell carcinoma (ESCC) by downregulating miR‐145. J Cell Biochem. 2019;120(10):17566‐17572. [DOI] [PubMed] [Google Scholar]
- 35. Reimondez‐Troitino S, Gonzalez‐Aramundiz JV, Ruiz‐Banobre J, et al. Versatile protamine nanocapsules to restore miR‐145 levels and interfere tumor growth in colorectal cancer cells. Eur J Pharm Biopharm. 2019;142:449‐459. [DOI] [PubMed] [Google Scholar]
- 36. He JH, Han ZP, Zou MX, He ML, Li YG, Zheng L. CDX2/mir‐145‐5p/SENP1 pathways affect LNCaP cells invasion and migration. Front Oncol. 2019;9:477. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37. Obermannova R, Redova‐Lojova M, Vychytilova‐Faltejskova P, et al. Tumor expression of miR‐10b, miR‐21, miR‐143 and miR‐145 Is related to clinicopathological features of gastric cancer in a central European population. Anticancer Res. 2018;38(6):3719‐3724. [DOI] [PubMed] [Google Scholar]
- 38. Liu Z, Yan Y, Cao S, Chen Y. Long non‐coding RNA SNHG14 contributes to gastric cancer development through targeting miR‐145/SOX9 axis. J Cell Biochem. 2018;119(8):6905‐6913. [DOI] [PubMed] [Google Scholar]
- 39. Michl P, Downward J. Mechanisms of disease: PI3K/AKT signaling in gastrointestinal cancers. Z Gastroenterol. 2005;43(10):1133‐1139. [DOI] [PubMed] [Google Scholar]
- 40. Hu M, Zhu S, Xiong S, Xue X, Zhou X. MicroRNAs and the PTEN/PI3K/Akt pathway in gastric cancer (review). Oncol Rep. 2019;41(3):1439‐1454. [DOI] [PubMed] [Google Scholar]
- 41. Singh SS, Yap WN, Arfuso F, et al. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: a reality for personalized medicine? World J Gastroenterol. 2015;21(43):12261‐12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bubici C, Papa S. JNK signalling in cancer: in need of new, smarter therapeutic targets. Br J Pharmacol. 2014;171(1):24‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c‐Jun N‐terminal kinases. Microbiol Mol Biol Rev. 2006;70(4):1061‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143(2):307‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. You H, Lei P, Andreadis ST. JNK is a novel regulator of intercellular adhesion. Tissue Barriers. 2013;1(5):e26845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boufraqech M, Zhang L, Jain M, et al. miR‐145 suppresses thyroid cancer growth and metastasis and targets AKT3. Endocr Relat Cancer. 2014;21(4):517‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zheng TL, Li DP, He ZF, Zhao S. miR‐145 sensitizes esophageal squamous cell carcinoma to cisplatin through directly inhibiting PI3K/AKT signaling pathway. Cancer Cell Int. 2019;19(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang Y, Yan W, Sun C, Liu Q, Wang J, Wang M. miR‐145‐5p inhibits epithelial‐mesenchymal transition via the JNK signaling pathway by targeting MAP3K1 in non‐small cell lung cancer cells. Oncol Lett. 2017;14(6):6923‐6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


