Abstract
The goal of this study was to assess biomarkers of exposure to glyphosate and assess potential associations with renal function in children. Glyphosate is used ubiquitously in agriculture worldwide. While previous studies have indicated that glyphosate may have nephrotoxic effects, few have examined potential effects on kidney function in children. We leveraged three cohorts across different phases of child development and measured urinary levels of glyphosate. We evaluated associations of glyphosate with three biomarkers of kidney injury: albuminuria (ACR), neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury marker 1 (KIM-1). Multivariable regression analyses examined associations of glyphosate with kidney injury biomarkers controlling for covariates. We identified glyphosate in 11.1% of the total participants. The herbicide was particularly detected more frequently in the neonate population (30%). Multivariable regression models failed to identify significant associations of log-transformed glyphosate with any of the kidney injury biomarkers, controlling for covariates age, sex, and maternal education. While we confirm detectability of glyphosate in children’s urine at various ages and stages of life, there is no evidence in this study for renal injury in children exposed to low levels of glyphosate. Further studies of larger sample size are indicated to better understand putative deleterious effects of the herbicide after different levels of exposure.
Keywords: Glyphosate, nephrotoxicity, children, environmental exposures, renal biomarkers
Graphical Abstract
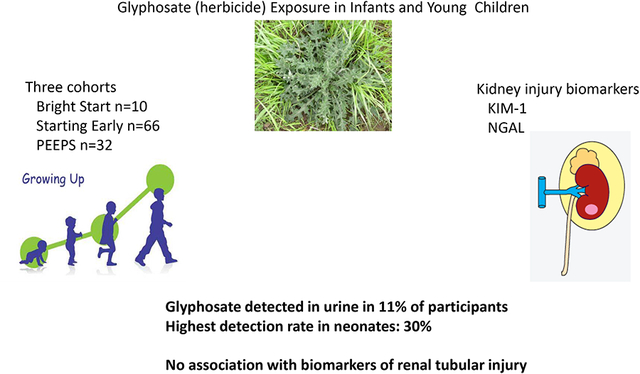
Capsule
Glyphosate is detectable is the urine of 11% of infants and young children (30% of neonates). However, there is no association with albuminuria or biomarkers of renal tubular injury.
1. Introduction
Genetically modified organism (GMO) crops now comprise 90% of corn and soybean planted in the US. While 2000 and 2004 National Academy of Sciences reviews identified no health hazards,(1) they focused primarily on genetic modifications in GMOs, and concerns were raised in both reports about potential health effects of herbicides such as glyphosate used to limit weeds.
Initial biomonitoring studies of glyphosate infrequently detected glyphosate in farmers and their families, or otherwise identified levels below the EPA reference dose which is subject to change over time.(2) There is limited information about glyphosate exposure in occupational settings and in the general population (3) In particular, there are little data on glyphosate exposure in pediatric patients, especially infants and toddlers (4, 5). These studies preceded the rapid increase in glyphosate that has occurred since 2000. More recent work has detected higher levels in food(6) and humans,(7). Average urinary glyphosate levels in occupationally exposed subjects range from 0.26 to 73.5 μg/L while urinary levels due to environmental exposure are considerably lower, 0.16 to 7.6 μg/L (3). Recent studies document effects in animals at environmentally relevant levels including oxidant stress,(8, 9) anti-androgenicity, and anti-estrogenicity,(10) and possible carcinogenicity.(11, 12) In particular, substantial bioaccumulation has been documented in kidney,(13). Nephrotoxicity has been shown to correlate with an altered metatranscriptomic profile, specifically with disturbances in the expression of genes associated with fibrosis, necrosis, phospholipidosis, mitochondrial membrane dysfunction and ischemia.(14)
Recent studies have identified the kidney as a susceptible organ to glyphosate. Previous studies have associated glyphosate exposure with changes in renal function, kidney injury, and chronic kidney disease of unknown etiology.(15–17) There is growing evidence linking glyphosate exposure with the epidemic of chronic kidney disease of unknown origin in farm workers in Central America, Sri Lanka and central India (18). However, the few available studies have yielded limited information with the majority being case-control studies, and none have examined children. Most of these studies were performed in occupational settings on agricultural workers. There is a paucity of studies on glyphosate exposures effects on kidney function in children.
Children are well known to be vulnerable to the effects of environmental contaminants, due to their greater consumption of food and water per unit body weight, as well as their developing organ systems. There are limited studies that have examined potential health effects of early life exposure to chemicals, especially pesticides. Therefore, we conducted the following study in which we leveraged three cohorts across different phases of child development (newborns, infants and younger children) to test the hypothesis that the prevalence and levels of glyphosate exposure are significant in infants and young children. Our second objective was to evaluate associations of glyphosate (as measured in urine) with three urinary biomarkers of kidney injury. In addition to albuminuria, a marker of glomerular injury,(19, 20) we measured concentrations of neutrophil gelatinase-associated lipocalin (NGAL),(21) and kidney injury marker 1 (KIM-1),(22) markers of distal and proximal tubular injury, respectively.
2. Methods
2.1. Study Populations
We briefly describe a longitudinal birth cohort of children (Starting Early), in whom urine samples were measured at 10–19 months of age, as well as two cross-sectional studies Preventing Environmental Exposures in Pregnancy (PEEPS), and Bright Start which represent older children (ages 3–8) and newborns (<30 days), respectively. The three cohorts are comprised of chronologically ordered categories of age in children as follows: Bright Start (newborns), Starting Early, and PEEPS. Inclusion in the present study was determined if sufficient urine was available for the experimental testing. All research was performed in accordance with relevant guidelines/regulations. In particular, informed consent was obtained from participants’ parents or their legal guardians for use of collected samples for use in future studies such as this one of glyphosate exposure.
The detailed descriptions of the three cohorts are provided in the Supplemental Materials
2.2. Urine collection and storage
Urine samples for each cohort were obtained at study visits using age-appropriate methods (urine bags and cotton balls in untrained babies and infants, freshly voided urine in trained children) and immediately transferred to sterile polyethylene cups. Within 2 hours after collection from the infants, urine was transferred to cryovials for storage at −80°C prior to laboratory analysis. We measured urinary markers of kidney injury NGAL, and KIM1 using Luminex xMAP technology (see below). Urine albumin and creatinine were measured using standard methods of quantitative spectrophotometry at the ARUP National Reference Laboratory (Salt Lake City, Utah) and the results were used to calculate ACR (Albumin-to-Creatinine Ratio [mg:mg])..
2.3. Analysis of urine samples for glyphosate
200 μL of urine sample was transferred into a 15-mL polypropylene (PP) tube and spiked with the labeled internal standard mixture (2-13C, 99%; 15N, 98+% Glyphosate), allowed to stand at room temperature for 30 min and then diluted to 1.0 mL (5-fold dilution) with 1% formic acid in water. The diluted sample was vortexed for 1 min, centrifuged and filtered through a nonsterile regenerated cellulose (RC) membrane filters (0.2 μm; Phenomenex, Inc., Torrance, CA, USA). The filtrate then transferred into an auto sampler vial for LC-MS/MS analysis. An Agilent 1260 Series HPLC system (Agilent Technologies Inc., Santa Clara, CA, USA) coupled with an ABSCIEX 4500 QTRAP mass spectrometer (Applied Biosystems, Foster City, CA, USA) running under negative mode electrospray ionization was used for the analysis. An anion-exchange column, Dionex IonPac AS 21 (2 mm × 250 mm, 7 μm) was employed for the separation of target chemicals under isocratic elution condition with a mobile phase consist of 1% formic acid in water and acetonitrile (95:5) mixture. Isotopic dilution mass spectrometry with MRM mode of analysis was used for selective quantification of the target chemicals. The QA/QC protocols included matrix spike (mean spike recoveries (n=5) for glyphosate was 109.5%) and procedural blanks (non-detected or <LOQ). Mid-point calibration standard and HPLC grade water were injected between every 20 samples analyzed to check the instrument detection linearity and carry-over effects, respectively. The limit of detection (LOD) and the limit of quantification (LOQ) were calculated as three and ten times of signal to noise ratio, respectively. LOD for glyphosate was 0.1 ng/mL, and LOQ was 0.33 ng/mL.
2.4. Analysis of kidney injury biomarkers
NGAL was measured in human urine using the Luminex performance human kidney Biomarker kit (FCSTM16-01, R&D Systems). Urine samples were thawed and diluted 1:10 in calibrator diluent and protocol followed manufacturer’s instructions. Data were captured on a Luminex MAGPIX instrument with xPonent 4.2 software. Standard curve R2 values were ≥0.98 and high and low quality controls were within expected range. KIM-1 was measured in human urine using the Milliplex MAP Human Kidney Injury Magnetic Bead Panel Kit (HKI1MAG-99K, EMD Millipore) Urine samples were thawed and diluted 1:2 in assay buffer and protocol followed manufacturer’s instructions. Data were captured on a Luminex MAGPIX instrument with xPonent 4.2 software. Standard curve R2 values were ≥0.97 and high and low quality controls were within the expected range.
2.5. Statistical Analysis
We first described the demographics of our study population (N=108), including age, sex, maternal education, race/ethnicity, BMI category, and intervention arm in the case of the Starting Early group. Body mass index (BMI), calculated as weight in kilograms divided by height in meters squared, was used to measure adiposity in the Starting Early group. We used standardized BMI z scores given that BMI varies widely by age and sex, following the Centers for Disease Control and Prevention year 2000 norms.(23) Overweight and obese were categorized as BMI z-score of 1.036 or greater (85th percentile for age and sex) and 1.64 or greater (95th percentile), respectively. We calculated median glyphosate, albuminuria, KIM-1, and NGAL levels, as well as interquartile ranges for all biomarkers and percent detected for glyphosate levels by study group. We also described mean and standard deviation in each one of the mentioned biomarkers of interest in the total population.
Univariate regressions examined log-transformed glyphosate concentrations and their detectability (using logistic regression) as dependent variables with covariates examined singly. We created dummy variables for maternal education with one category for those children whose mothers did not complete a high school education and another for those whose mothers at least completed high school, and created a dummy variable for missing values (N=34) that we used as reference. We also created an ordinal variable reflecting the age of the children in increasing order from youngest to oldest type as follows: Healthy Start, Starting Early, and PEEPS (N=108). We also performed univariate analyses by study arm in the Starting Early sample (N=66).
Multivariable analyses of continuous log-transformed glyphosate exposure examined age, sex, and maternal education as predictors (N=108). We assessed differences in detectability of glyphosate using multivariable logistic regressions with age, sex, and maternal education as predictors. All regressions examining predictors of log-transformed glyphosate were retransformed from the logarithmic scale.
We then examined associations of glyphosate with kidney injury. First, we calculated Pearson correlation coefficients for all log-transformed kidney injury markers KIM-1, NGAL, and ACR, as well as log-transformed glyphosate levels. For glyphosate levels below the limit of detection (LOD), we imputed values equal to LOD/square root of 2. We also performed univariate and multivariable regressions of urinary log-transformed glyphosate as predictor of log-transformed kidney injury markers KIM-1, NGAL, and ACR, controlling for age, sex and maternal education. Sensitivity analyses were also included; we performed univariate and multivariable regressions of creatinine-adjusted urinary log-transformed glyphosate as predictor of log-transformed kidney injury markers KIM-1, NGAL, and ACR. Statistical analysis was performed with Stata/IC Version 14 (StataCorp: College Station, TX).
3. Results
The population was largely Hispanic, and comprised roughly equal numbers of males and females (Table 1). Only 4% of our participants had a mother with a college degree but the majority of parents in our study population graduated from high school (62.1%). The mean urinary glyphosate concentration was 0.278±0.228 μg/mL, with a range of 0.105–2.125 μg/mL. We identified glyphosate in 11.1% of the participants. The herbicide was detected in 7.6–30% of the three cohorts: 30% of neonates had glyphosate exposure above the LOD, followed by PEEPS cohort (12.5%) and Starting Early (7.6%). We also observed a wide detection range in NGAL biomarkers. We also noted that 40% of children in the Starting Early cohort were overweight or obese.
Table 1.
Study Population (N=108)1
| Demographics | N | % | |
|---|---|---|---|
| Male (Sex) | 56 | 51.9 | |
| Study | Bright Start (Neonates) | 10 | 9.3 |
| Starting Early | 66 | 61.1 | |
| 39 | 59.1 | ||
| Control Arm | 27 | 40.9 | |
| PEEPS | 32 | 29.6 | |
| Maternal Education | None | 7 | 9.5 |
| Elementary/Middle | 21 | 28.4 | |
| High School/General Equivalency Diploma | 35 | 47.3 | |
| Some college | 7 | 9.5 | |
| College grad | 3 | 4.1 | |
| Other | 1 | 1.4 | |
| Race/Ethnicity | Hispanic | 67 | 90.5 |
| Non-Hispanic White | 5 | 6.8 | |
| Non-Hispanic Black | 1 | 1.4 | |
| Non-Hispanic Asian | 1 | 1.4 | |
| BMI Categories2 | Underweight | 2 | 3.0 |
| Normal | 40 | 60.6 | |
| Overweight | 12 | 18.2 | |
| Obese | 12 | 18.2 | |
| Distribution of Glyphosate and Kidney Injury Biomarkers | ||
|---|---|---|
| Mean (SD) | Detection Range (Min. & Max. Concentrations) | |
| Glyphosate (ng/mL) | 0.278 (0.228) | 0.105–2.125 |
| KIM-1 (ng/mL) | 80.17 (76.92) | 12.38–560.6 |
| NGAL (pg/mL) | 12,486 (21,934) | 530.7–131,196 |
| ACR3 (mg/g) | 26.23 (25.43) | 1.414–119.0 |
| Distribution of Glyphosate and Kidney Injury Biomarkers by Study Groups (N=108) | |||
|---|---|---|---|
| Study | Healthy Start (Neonates) | Starting Early | PEEPS |
| <LOD (<LOD-1.06) | <LOD (<LOD-LOD) | <LOD (<LOD-LOD) | |
| Percent Detected | 30.0% | 7.58 % | 12.5% |
| KIM-1 (ng/mL), Median (IQR) | 88.9 (71.0–114) | 47.1 (33.8–71.7) | 76.5 (40.3–140.2) |
| NGAL (pg/mL), Median (IQR) | 3,770 (2,520–7,200) | 9,080 (3,880–18,700) | 1,920 (1,240–5,770) |
| ACR3 (mg/g), Median (IQR) | 9.45 (6.00–34.3) | 28.0 (19.1–42.4) | 5.66 (3.54–9.00) |
Maternal Education 34 missing, Race/Ethnicity 34 missing, BMI 42 missing, KIM-1 9 missing, NGAL 13 missing, 480 and ACR 12 missing.
Underweight (Below −1.036 SD); Normal (Between −1.036 and +1.036 SD); Overweight (Between + 1.036 and +1.64 SD); Obese (Above+1.64 SD)
Albumin-to-cretinine ratio.
Younger children had higher urinary levels of glyphosate in both univariate (−0.04 ng/mL, 95% CI: −0.07, −0.01; Table 2) and multivariable (−0.16 ng/mL, 95% CI: −0.22, −0.09) regressions. Children with mothers without a high school diploma had slightly lower levels of urinary glyphosate (−0.17 ng/mL, 95% CI: −0.25, −0.08) relative to those with mothers who attained a high school education (−0.16 ng/mL, 95% CI: −0.24, −0.07) in our multivariable regression. However, we found no other significant associations of demographic covariates with glyphosate in univariate or multivariable models. Similarly, we found no statistically significant associations of demographic factors in relationship to detectability of urinary glyphosate in children.
Table 2.
Univariate and Multivariable Regressions for Demographic Predictors of Urinary Glyphosate.
| Univariate1 | Multivariable2 | |||
|---|---|---|---|---|
| Glyphosate | OR Glyphosate below LOD | Glyphosate | OR Glyphosate below LOD | |
| Age | −0.04 (−0.07, −0.01)* | 1.48 (0.53, 4.10) | −0.16 (−0.22, −0.09) * | 3.02 (0.84, 10.9) |
| Sex | −0.01 (−0.05, 0.03) | 1.30 (0.56, 7.10) | −0.01 (−0.05, 0.09) | 1.73 (0.44, 6.78) |
| Arm | 0.01 (−0.01, 0.03) | 0.34 (0.04, 3.20) | - | - |
| Education <HS | −0.02 (−0.06, 0.03) | 1.44 (0.31, 6.62) | −0.17 (−0.25, −0.08)* | 3.16 (0.48, 20.7) |
| Education >=HS | 0.005 (−0.04, 0.05) | 1.81 (0.45,7.32) | −0.16 (−0.24, −0.07)* | 6.23 (0.84, 46.2) |
| Z-BMI | 0.002 (−0.008, 0.01) | 1.11 (0.43, 2.86) | - | - |
P-value <0.05
Age N=108, Sex N=108, Arm N=66, Z-BMI N=66, and Maternal Education (N=108).
Note that Maternal Education had imputed missing observations as a reference category to be included in multivariable analyses with N=108.
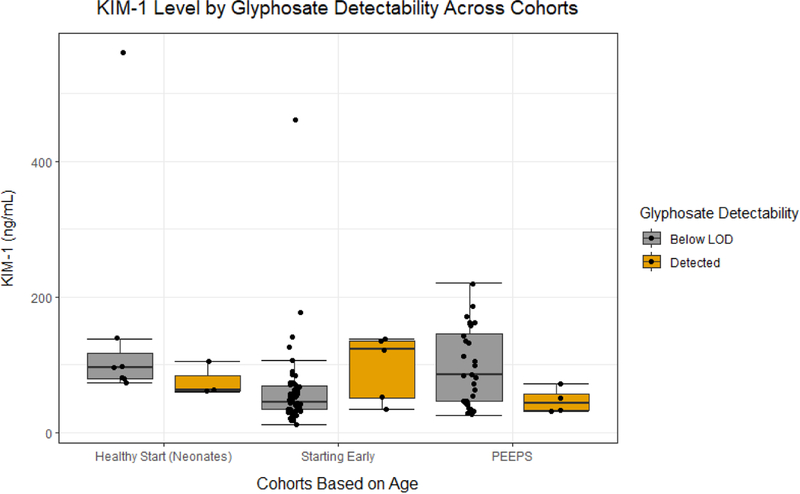
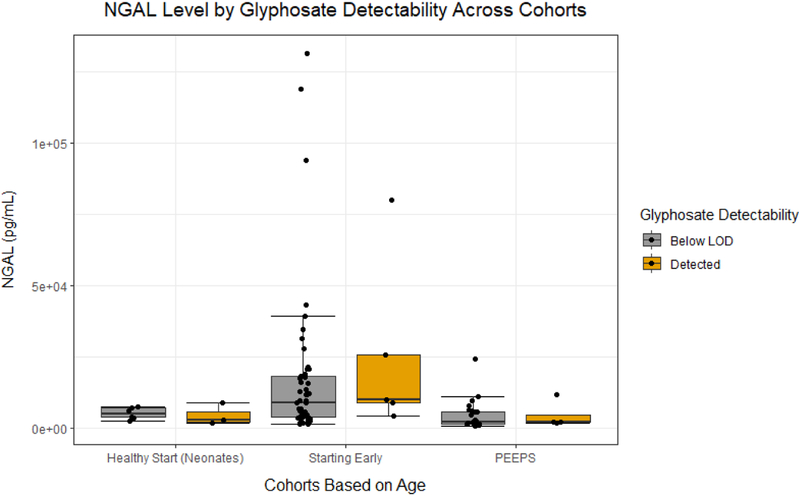
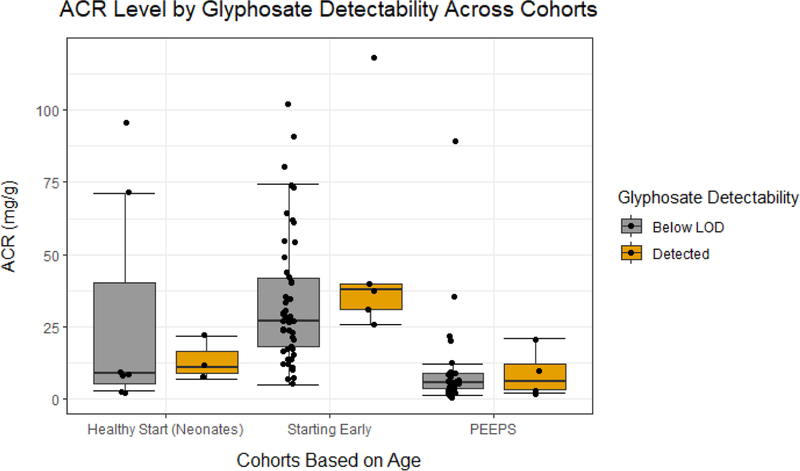
No univariate regressions with urinary glyphosate identified a statistically significant association with increased renal biomarker injury (KIM-1: 0.974 ng/mL, 95% CI: −17.92, 27.78; NGAL: −663.8 pg/mL, 95% CI: −2462, 2641; ACR: −0.287 mg/g, 95% CI: −6.773, 10.91; Figure 1A, 1B, and 1C respectively and Table 4). Multivariable regressions with urinary glyphosate failed to identify statistically significant associations of log-transformed glyphosate with KIM-1, NGAL, or ACR (KIM-1: −20.88 ng/mL, 95% CI: −54.89, 28.02; NGAL: −60.53 pg/mL, 95% CI: −291.2, 331.2; ACR: 0.3714 mg/g, 95% CI: −0.8725, 2.335; Table 4). In sensitivity analyses of univariate and multivariable regressions with creatinine-adjusted log-transformed glyphosate, there was also no statistically significant evidence for increased levels of markers for renal injury (Table 4). In additional sensitivity analyses without the newborns similar results were reported with no statistically significant associations of log-transformed glyphosate with kidney injury biomarkers (Table 4).
Figure 1A.
The dot plot illustrates the urinary KIM-1 level in participants enrolled in the different studies
Figure 1B.
The dot plot illustrates the urinary NGAL level in participants enrolled in the different studies
Figure 1C.
The dot plot illustrates the urinary albumin:creatine ratio (ACR) in participants enrolled in the different studies
Table 4.
Multivariable Regressions of Creatinine-adjusted Urinary Log-transformed Glyphosate as Predictor of Log-transformed KIM-1, NGAL, ACR Biomarkers1
| All ages N=108 § | |||
|---|---|---|---|
| Increment per one log unit increase | KIM-1 | NGAL | ACR |
| Glyphosate Univariate | 0.974 (−17.92, 27.78) | −663.8 (–2462, 2641) | −0.287 (−6.773, 10.91) |
| Glyphosate Multivariable | −1.55 (−14.93, 17.62) | −887.8 (−1800, 662.2) | −5.05 (−14.14, 9.85) |
| Creatinine-adjusted models ¶ | |||
| Glyphosate Univariate | −1.648 (−15.22, 17.47) | −640.5 (–3242, 4079) | 7.603 (−9.522, 32.88) |
| Glyphosate Multivariable | −3.70 (−13.47, 10.26) | −819.8 (−1688, 657.8) | 0.858 (−13.80, 22.43) |
| Non-neonates N=98 §§ | |||
| Increment per one log unit increase | KIM-1 | NGAL | ACR |
| Glyphosate Univariate | −5.351 (−24.13, 27.39) | 1489 (–4383, 17944) | 3.002 (−10.66, 35.99) |
| Glyphosate Multivariable | −3.568 (−14.90, 15.96) | 395.3 (–5128, 12782) | 12.05 (−37.98, 106.6) |
| Creatinine-adjusted models ¶¶ | |||
| Glyphosate Univariate | −4.89 (−20.66, 22.43) | 2083 (–5679, 23234) | 8.24 (−16.60, 54.17) |
| Glyphosate Multivariable | −3.73 (−14.45, 14.88) | 132.8 (–5165, 11668) | 10.37 (−25.33, 72.33) |
KIM-1 N=99; NGAL N=95; ACR N=96. Multivariable regression models controlling for demographic covariates age, sex, and maternal education, where maternal education missing observations were imputed as the majority category “Equal or Greater Than High School”.
Creatinine-adjusted regression models KIM-1 N=95; NGAL N=92; ACR N=96.
KIM-1 N=89; NGAL N=86; ACR N=86. Multivariable regression models controlling for demographic covariates age, sex, and maternal education, where maternal education missing observations were imputed
4. Discussion
The main findings of this study are the substantial and frequent prevalence of glyphosate in children across the first decade of life in three independently assembled samples. We identified glyphosate in 11.1% of the total participants, and the herbicide was detected more frequently in the neonate population (30%). However, urinary concentrations detected in the neonates, toddlers, and young school age children was significantly lower than levels documented in adults with occupational or environmental exposure (3). No association of urinary glyphosate was found with any of the three renal biomarkers (KIM-1, NGAL or ACR).
Urinary excretion of albumin and serum creatinine concentration are conventional biomarkers for renal dysfunction. Novel biomarkers such as urinary excretion of KIM-1 and NGAL have been developed and it has been that they have diagnostic utility for earlier detection of acute kidney injury (AKI) prior to significant loss of function (24). While their use is fairly well established in adult patients, there is ongoing work to establish diagnostic cut-off values in pediatric patients including neonates (25–27). Their use would be especially helpful in glyphosate-induced nephrotoxicity occurring predominantly in the human proximal tubules.(28, 29) Urinary microalbumin, and serum or plasma creatinine, have been utilized to evaluate chronic exposures to glyphosate herbicide in human and animal studies.(21, 30) KIM-1 also predicted renal damage from acute exposures in animal studies.(31) This is the first study to our knowledge examining kidney injury biomarkers in relationship to glyphosate exposure in children.
This study has a number of limitations. We leveraged convenience samples in this initial study. We were limited in interpretation within and across the three populations, given the small sample size and distinct differences in demographic and other factors of the three cohorts. A greater sample size perhaps could have given more accurate results. Recognizing the limited sample size, we were limited in our adjustment for covariates in our multivariable models. Thus we were unable to collect data about place of residence and the time of the year the samples were collected. We focused on known factors that may represent vulnerability to exposures and disease, such as age, gender, or maternal education. Age is associated with differential development to vulnerability stages, and younger populations tend to be more susceptible to exposures than their older counterparts through lactation.(32–35) Glyphosate effects have been also shown to be sex-dependent in animal studies (36–38). We used maternal education as a proxy for family educational attainment or socio-economic status (SES) which are well-known factors for different levels of chemical exposures in children or renal injury outcome.(39–43)
We emphasize that low sample size also limited us to perform analyses in the three cohorts in conjunction, in lieu of stratified analysis. We acknowledge that the strength of any conclusion about the frequency of detecting glyphosate in newborns is limited by the sample size. The source of the glyphosate in the newborns, which was detected more frequently than in the other groups but not at high levels, could be via lactation or infant formula. It is possible that immature or different metabolic pathways for glyphosate in neonates, kidney injury during delivery, or lactation may have affected the findings in this age group. However, the addition of neonates in our study evaluating the association of glyphosate and kidney injury biomarkers may have biased findings towards the null. Our results also suggest that glyphosate levels declined with increasing age. The differences by age may represent differences in dietary behavior and access to foods free of contamination or improved elimination of the herbicide, though further research is needed.
Previous larger scale case-control studies have shown in the past changes in renal function, kidney injury, or chronic kidney disease of unknown etiology (CKDu) upon glyphosate exposure, especially of adult occupational exposures(15–17, 44) There are limited prospective studies and the majority focus on highly exposed workers in the occupational setting. Further evaluation in children may enable more direct assessment of the renal toxicity of glyphosate. Longitudinal studies are suggested for further evaluation of the exposures from an early age. These could explore more in detail the mechanisms that underlie a potential association between glyphosate and renal biomarkers across different age groups.
Several strengths in this study are notable. Our study is the first to document the prevalence of glyphosate exposures in young children through age 8 years. Our frequent detection of glyphosate suggests the need for inclusion and biomonitoring of glyphosate exposures in national surveys or screenings in populations of interest, which could provide more information in regards to these exposures and outcomes. Glyphosate biomonitoring could be added into studies such as the National Health and Nutrition Examination Survey (NHANES). Our study is novel in that we examine specific renal biomarkers in relationship to glyphosate in a young population.
The lack of evident renal toxicity in association with glyphosate exposure in young children does not exclude a potential adverse impact of longer term exposure to the pesticide. Problems during labor and delivery can compromise renal perfusion leading to increased excretion of tubular injury biomarkers in the neonatal period.(45) This could interfere with the ability to detect an independent effect of glyphosate in this age group. In addition, the regenerative capacity of the renal tubular epithelium in infants and young children may mask an adverse effect of the pesticide on tubule integrity. Urinary biomarkers such as NGAL and KIM-1 have been demonstrated to be useful biomarkers if tubular injury even without reduced kidney function, highlighting their utility in detecting subclinical insults to the kidney.(46) Further research could explore more in detail the association between glyphosate and kidney injury biomarker types across different age groups in larger cohorts, and assess whether more prolonged exposure could have progressive deleterious effects.
5. Conclusions
In this study, we identify frequent prevalence of levels of glyphosate (11.1%), present across all three age ranges, and particularly present in the youngest population of neonates. There is no evidence for renal injury in young children exposed to low levels of glyphosate. Further studies of larger sample size are indicated to better understand the potential deleterious effects of the herbicide after different levels, routes, and duration of exposure.
Supplementary Material
Table 3.
Pearson Correlations across Kidney Injury Biomarkers, and Glyphosate1
| Glyphosate | KIM-1 | NGAL | ACR | |
|---|---|---|---|---|
| Glyphosate | X | 0.00880 | −0.0526 | −0.00690 |
| KIM-1 | 0.00880 | X | −0.137 | −0.263* |
| NGAL | −0.0526 | −0.137 | X | 0.512* |
| ACR | −0.00690 | −0.263* | 0.512* | X |
Glyphosate, KIM-1, NGAL, and ACR were log-transformed.
P-value <0.05
HIGHLIGHTS.
We leveraged three cohorts across different phases of child development and measured urinary levels of the herbicide, glyphosate, and evaluated associations with biomarkers of kidney injury.
We identified glyphosate in 11.1% of the participants. The herbicide was detected more frequently in neonates (30%). There were no significant associations of log-transformed glyphosate with any kidney injury biomarker, controlling for covariates age, sex, and maternal education.
Further studies of larger sample size are indicated to better understand putative deleterious effects of glyphosate after different levels of exposure
Footnotes
CONFLICT OF INTEREST
The authors have no financial conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Medicine Io, Council NR 2004. Safety of Genetically Engineered Foods: Approaches to Assessing Unintended Health Effects. The National Academies Press, Washington, DC, p 256. [PubMed] [Google Scholar]
- 2.Acquavella JF, Alexander BH, Mandel JS, Gustin C, Baker B, Chapman P, Bleeke M 2004. Glyphosate biomonitoring for farmers and their families: results from the Farm Family Exposure Study. Environ Health Perspect 112:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillezeau C, van Gerwen M, Shaffer RM, Rana I, Zhang L, Sheppard L, Taioli E 2019. The evidence of human exposure to glyphosate: a review. Environ Health 18:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sierra-Diaz E, Celis-de la Rosa Ad, Lozano-Kasten F, Trasande L, Peregrina-Lucano AA, Sandoval-Pinto E, Gonzalez-Chavez HJIjoer, health p 2019. Urinary pesticide levels in children and adolescents residing in two agricultural communities in Mexico. 16:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, Reynolds SJ, Alavanja MCJTAooh 2006. Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in Iowa. 51:53–65. [DOI] [PubMed] [Google Scholar]
- 6.Brausch JM, Smith PN 2007. Toxicity of three polyethoxylated tallowamine surfactant formulations to laboratory and field collected fairy shrimp, Thamnocephalus platyurus. Arch Environ Contam Toxicol 52:217–221. [DOI] [PubMed] [Google Scholar]
- 7.2013. RGM--- MLHB: Determination of Glyphosate residues in human urine samples from 18 European countries.
- 8.Uren Webster T, Santos E 2015. Global transcriptomic profiling demonstrates induction of oxidative stress and of compensatory cellular stress responses in brown trout exposed to glyphosate and Roundup. BMC Genomics 16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Shenawy NS 2009. Oxidative stress responses of rats exposed to Roundup and its active ingredient glyphosate. Environ Toxicol Pharmacol 28:379–385. [DOI] [PubMed] [Google Scholar]
- 10.Gasnier C, Dumont C, Benachour N, Clair E, Chagnon MC, Seralini GE 2009. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 262:184–191. [DOI] [PubMed] [Google Scholar]
- 11.Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. The Lancet Oncology 16:490–491. [DOI] [PubMed] [Google Scholar]
- 12.Loomis D, Guyton K, Grosse Y, El Ghissasi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. The Lancet Oncology 16:891–892. [DOI] [PubMed] [Google Scholar]
- 13.German Federal Agency BfR The BfR has finalised its draft report for the re-evaluation of glyphosate. 2014. Available at. http://www.bfr.bund.de/en/the_bfr_has_finalised_its_draft_report_for_the_re_evaluation_of_glyphosate-188632.html (Accessed 11 September 2015).
- 14.Mesnage R, Arno M, Costanzo M, Malatesta M, Seralini GE, Antoniou MN 2015. Transcriptome profile analysis reflects rat liver and kidney damage following chronic ultra-low dose Roundup exposure. Environ Health 14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayasumana C, Gunatilake S, Siribaddana S 2015. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol 16:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayasumana C, Paranagama P, Agampodi S, Wijewardane C, Gunatilake S, Siribaddana S 2015. Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ Health 14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F, Pan LP, Ding EM, Ge QJ, Zhang ZH, Xu JN, Zhang L, Zhu BL 2017. [Study of the effect of occupational exposure to glyphosate on hepatorenal function]. Zhonghua Yu Fang Yi Xue Za Zhi 51:615–620. [DOI] [PubMed] [Google Scholar]
- 18.Valcke M, Levasseur ME, Soares da Silva A, Wesseling C 2017. Pesticide exposures and chronic kidney disease of unknown etiology: an epidemiologic review. Environ Health 16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zappitelli M, Coca SG, Garg AX, Krawczeski CD, Thiessen Heather P, Sint K, Li S, Parikh CR, Devarajan P 2012. The association of albumin/creatinine ratio with postoperative AKI in children undergoing cardiac surgery. Clin J Am Soc Nephrol 7:1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Lu B, Ni H, Sheng X, Jin N 2013. Microalbuminuria can predict the development of acute kidney injury in critically ill septic patients. J Nephrol 26:724–730. [DOI] [PubMed] [Google Scholar]
- 21.Devarajan P 2008. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl 241:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV 2002. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62:237–244. [DOI] [PubMed] [Google Scholar]
- 23.Ogden CL, Carroll MD, Kit BK, Flegal KM 2012. PRevalence of obesity and trends in body mass index among us children and adolescents, 1999–2010. JAMA: The Journal of the American Medical Association 307:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menez S, Parikh CR JCoin, hypertension 2019. Assessing the health of the nephron in acute kidney injury: biomarkers of kidney function and injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenberg JH, Zappitelli M, Jia Y, Thiessen-Philbrook HR, De Fontnouvelle CA, Wilson FP, Coca S, Devarajan P, Parikh CR JJotASoN 2018. Biomarkers of AKI progression after pediatric cardiac surgery. 29:1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterling M, Al-Ismaili Z, McMahon KR, Piccioni M, Pizzi M, Mottes T, Lands LC, Abish S, Fleming AJ, Bennett MRJPb, cancer 2017. Urine biomarkers of acute kidney injury in noncritically ill, hospitalized children treated with chemotherapy. 64:e26538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sellmer A, Bech BH, Bjerre JV, Schmidt MR, Hjortdal VE, Esberg G, Rittig S, Henriksen TBJBp 2017. Urinary neutrophil gelatinase-associated lipocalin in the evaluation of patent ductus arteriosus and AKI in very preterm neonates: a cohort study. 17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin A, Columbia UoB, Stevens PE, Service NH, Bilous RW, Trust STHNF, Coresh J, Health BSoP, coresh@jhu.edu, Francisco ALMD, Valdecilla HUMd, Jong PED, Groningen Uo, Griffith KE, York Uo, Hemmelgarn BR, Calgary Uo, Iseki K, Ryukyus UHot, Lamb EJ, Columbia UoB, Levey AS, University T, Riella MC, Hospital EU, Shlipak MG, Francisco UoCaS, Wang H, University P, White CT, Columbia UoB, Winearls CG, Trust ORHN 2019. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements 3:1–150. [Google Scholar]
- 29.Mohamed F, Endre ZH, Pickering JW, Jayamanne S, Palangasinghe C, Shahmy S, Chathuranga U, Wijerathna T, Shihana F, Gawarammana I, Buckley NA 2016. Mechanism-specific injury biomarkers predict nephrotoxicity early following glyphosate surfactant herbicide (GPSH) poisoning. Toxicol Lett 258:1–10. [DOI] [PubMed] [Google Scholar]
- 30.Hamdaoui L, Naifar M, Mzid M, Ben Salem M, Chtourou A, Makni-Ayadi F, Sahnoun Z, Rebai T 2016. Nephrotoxicity of Kalach 360 SL: biochemical and histopathological findings. Toxicol Mech Methods 26:685–691. [DOI] [PubMed] [Google Scholar]
- 31.Wunnapuk K, Gobe G, Endre Z, Peake P, Grice JE, Roberts MS, Buckley NA, Liu X 2014. Use of a glyphosate-based herbicide-induced nephrotoxicity model to investigate a panel of kidney injury biomarkers. Toxicol Lett 225:192–200. [DOI] [PubMed] [Google Scholar]
- 32.Bruckner JV 2000. Differences in sensitivity of children and adults to chemical toxicity: the NAS panel report Regul Toxicol Pharmacol. Copyright 2000 Academic Press, Netherlands, pp 280–285. [DOI] [PubMed] [Google Scholar]
- 33.Pronczuk J, Akre J, Moy G, Vallenas C 2002. Global perspectives in breast milk contamination: infectious and toxic hazards. Environ Health Perspect 110:A349–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schug TT, Janesick A, Blumberg B, Heindel JJ 2011. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol 127:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seely JC 2017. A brief review of kidney development, maturation, developmental abnormalities, and drug toxicity: juvenile animal relevancy. J Toxicol Pathol 30:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lozano VL, Defarge N, Rocque LM, Mesnage R, Hennequin D, Cassier R, de Vendomois JS, Panoff JM, Seralini GE, Amiel C 2018. Sex-dependent impact of Roundup on the rat gut microbiome. Toxicol Rep 5:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dallegrave E, Mantese FD, Coelho RS, Pereira JD, Dalsenter PR, Langeloh A 2003. The teratogenic potential of the herbicide glyphosate-Roundup in Wistar rats. Toxicol Lett 142:45–52. [DOI] [PubMed] [Google Scholar]
- 38.Ren X, Li R, Liu J, Huang K, Wu S, Li Y, Li C 2018. Effects of glyphosate on the ovarian function of pregnant mice, the secretion of hormones and the sex ratio of their fetuses. Environ Pollut 243:833–841. [DOI] [PubMed] [Google Scholar]
- 39.Buekers J, Colles A, Cornelis C, Morrens B, Govarts E, Schoeters G 2018. Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status. Int J Environ Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crews DC, Pfaff T, Powe NR 2013. Socioeconomic factors and racial disparities in kidney disease outcomes. Semin Nephrol 33:468–475. [DOI] [PubMed] [Google Scholar]
- 41.Murray MP, Sharmin R 2015. Groundwater arsenic and education attainment in Bangladesh. J Health Popul Nutr 33:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norris KC, Agodoa LY 2005. Unraveling the racial disparities associated with kidney disease. Kidney Int 68:914–924. [DOI] [PubMed] [Google Scholar]
- 43.Ueker ME, Silva VM, Moi GP, Pignati WA, Mattos IE, Silva AMC 2016. Parenteral exposure to pesticides and occurence of congenital malformations: hospital-based case-control study. BMC Pediatr 16:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gunarathna S, Gunawardana B, Jayaweera M, Manatunge J, Zoysa K 2018. Glyphosate and AMPA of agricultural soil, surface water, groundwater and sediments in areas prevalent with chronic kidney disease of unknown etiology, Sri Lanka. J Environ Sci Health B 53:729–737. [DOI] [PubMed] [Google Scholar]
- 45.Kirkley MJ, Boohaker L, Griffin R, Soranno DE, Gien J, Askenazi D, Gist KM 2019. Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr Nephrol 34:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenberg JH, Parikh CR 2017. Biomarkers for Diagnosis and Prognosis of AKI in Children: One Size Does Not Fit All. Clin J Am Soc Nephrol 12:1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.