Abstract
NMDAR-mediated excitotoxicity has been implicated in some of the impairments following fetal ethanol exposure. Previous studies suggest that both neuronal cell death and some of the behavioral deficits can be reduced by NMDAR antagonism during withdrawal, including antagonism of a subpopulation of receptors containing NR2B subunits. To further investigate NR2B involvement, we selected a compound, CP-101,606 (CP) which binds selectively to NR2B/2B stoichiometries, for both in vitro and in vivo analyses. For the in vitro study, hippocampal explants were exposed to ethanol for 10 days and then 24 h following removal of ethanol, cellular damage was quantified via propidium iodide fluorescence. In vitro ethanol withdrawal-associated neurotoxicity was prevented by CP (10 and 25 nM). In vivo ethanol exposure was administered on PNDs 1–7 with CP administered 21 h following cessation. Activity (PND20–21), motor skills (PND31–33), and maze navigation (PND43–44) were all susceptible to ethanol insult; treatment with CP (15 mg/kg) rescued these deficits. Our findings show that CP-101,606, a drug that blocks the NR2B/2B receptor, can reduce some of the damaging effects of “3rd trimester” alcohol exposure in our rodent model. Further work is clearly warranted on the neuroprotective potential of this drug in the developing brain.
Keywords: prenatal alcohol, hippocampal slices, glutamate antagonist, traxoprodil, behavioral teratology, neuroprotection
1. Introduction
Ethanol exposure during pregnancy can result in a variety of behavioral and physiological impairments in the offspring that often persist into adulthood (Hannigan et al., 2000). These effects have been referred to as Fetal Alcohol Spectrum Disorder (FASD), reported in approximately 9/1000 live births (Sampson et al., 1997). Some of the behavioral dysfunctions described among FASD populations include learning and memory deficits, hyperactivity, and attention problems (Hamilton et al., 2003; Mattson et al., 1996; 1997; Mattson and Riley 2000; Roebuck et al., 1999). Deficits in motor skills are common, including delayed motor development, poor eye-hand coordination, fine motor dysfunctions and problems with balance (Kyllerman et al., 1985; Streissguth et al., 1980). A number of mechanisms have been proposed to explain how prenatal ethanol (ETOH) exposure affects the developing CNS (for reviews see Goodlett et al., 2005; Riley et al., 2001).
Glutamatergic excitotoxicity, and more specifically N-methyl-D-aspartate receptor (NMDAR) overactivity, during withdrawal from ETOH appears to be one important mechanism. Acutely, ETOH inhibits NMDAR activity (Lovinger et al., 1989; Simson et al., 1991), reducing calcium influx (Hoffman, 1989). Chronically, this inhibition results in an upregulation of genes encoding the receptor, an increase in receptor trafficking to the cell surface, and substitution of receptor subunits to restore a basal level of cellular glutamate sensitivity (Chandler et al., 1999; Hu and Ticku 1995; Kalluri et al., 1998). These homeostatic mechanisms serve to maintain function while ETOH remains in the system, but become pathological during the withdrawal phase. In the absence of ETOH’s inhibitory effects, the homeostatic changes result in a hypersensitivity to glutamate (Davidson et al., 1995), which, coupled with known increases in extracellular glutamate during withdrawal (Dahchour and De Witte, 2000) produce a toxic level of intracellular calcium resulting in necrotic and apoptotic events (Gibson et al., 2003; Hoffman et al., 1995; Hoffman and Tabakoff, 1994; Iorio et al., 1993; Michaelis et al., 1980).
Reducing NMDAR activity during withdrawal has become a major pharmacotherapeutic strategy for reducing ETOH-associated damage. NMDARs are tetrameric proteins, composed of at least two NR1 and two NR2 subunits (McBain and Mayer, 1994; Schorge and Colquhoun 2003). Four NR2 transcripts have been identified (NR2A-D), and are implicated in the pharmacologic specificity of the receptor, such that different subunit combinations can vary widely in their pharmacology (Wafford et al., 1993; for reviews see Cull-Candy et al., 2001; Monaghan et al., 1998; Scheetz and Constantine-Paton, 1994). Those containing NR2A or NR2B subunits display twice the sensitivity to ETOH as those containing NR2C or NR2D subunits (Kuner et al., 1993; Masood et al., 1994; Mirshahi and Woodward, 1995; for reviews see Allgaier, 2002; Sucher et al., 1996). In addition to being highly sensitive to ETOH, the NR2B subunit may be a promising target for selective antagonism due to its interaction with polyamines (Williams et al., 1994), low molecular weight cations which are ubiquitous and highly expressed during development (for review, see Kusano et al., 2008; Slotkin and Bartolome, 1986). Polyamines have direct effects on several ion channels and receptors, including NMDARs, which are enhanced by polyamine stimulation (Williams et al., 1994; 1991). Examined in an in vitro developmental model, polyamines potentiate ETOH withdrawal-induced cell death, while polyamine blockade reduces excitotoxic damage (Barron et al., 2008; Gibson et al., 2003; Mayer et al., 2002). In vivo, disrupting polyamine synthesis prior to ETOH withdrawal improves behavioral outcome (Rubin et al., 2009), as does disrupting polyamine binding during ETOH withdrawal (Lewis et al., 2007; Thomas et al., 2004). NR2B-containing receptors display the greatest sensitivity to polyamine stimulation, due to their greater number of polyamine binding sites (Littleton et al., 2001). This suggests that NMDARs containing NR2B subunits are particularly sensitive to both ETOH and to polyamine stimulation during withdrawal.
Known increases in NR2B subunits and polyamine concentrations during development may create a particularly vulnerable system for ETOH-associated excitotoxic damage. For this reason, we have selected an NR2B-specific polyamine modulator, CP-101,606 (CP; Traxoprodil; Pfizer) as a pharmacological intervention in the current study. CP’s unique binding profile, characterized by selectivity for NR2B/2B composition (Chazot et al., 2002) make its investigation useful as both as a tool to further understand excitotoxic mechanisms and as a potential pharmacotherapy.
The current project investigated the ability of CP to reduce behavioral deficits associated hippocampal slice culture (OHSC) model. This CNS structure was selected with developmental ETOH exposure, as well as block cell death in a developmental organotypic due to both the disproportional number of behavioral deficits associated with prenatal ETOH exposure that are also linked to hippocampal dysfunction and to prior studies showing the sensitivity of the hippocampus to ETOH effects during development, suggesting it represents a valuable target for both prevention and recovery of structural and functional deficits. For the behavioral experiments, ETOH was administered to neonatal rats during the first week ex utero, as a model to study its effects during a period of CNS development that overlaps the human “brain growth spurt”. While rat and human development occurs at different speeds and developmental ages, similar trajectories are noted, however “mapping” brain development across species depends largely on brain area of interest and selection of developmental markers. For instance, Dobbing and Sands (1979) suggest that by examining the rapidity of brain growth across species, the first postnatal week in the rat mimics the ‘brain growth spurt’ in the human 3rd trimester. However, more recent examinations using neurogenic endpoints suggest that in hippocampus (for example), the first neonatal week (PND1–7) in the rat may be more analogous to a period overlapping the second and early third trimesters (human gestational weeks 19–28; Bayer et al., 1993; for review see Rice & Barone, 2000).
Behavioral endpoints included locomotor activity in an open field, motor coordination in a dowel-rod balance task, and learning and memory in a water maze. These behaviors were chosen as markers of ETOH-associated dysfunction based on the prevalence of similar dysfunctions in clinical reports, and their known sensitivity to developmental ETOH exposure.
2. MATERIALS AND METHODS
All protocols were approved by the Institutional Animal Care and Use Committee at the University of Kentucky and are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
ORGANOTYPIC HIPPOCAMPAL SLICES
2.1. Subjects
Hippocampal slices were derived from Sprague Dawley rat pups born in our breeding facility in the University of Kentucky Psychology Department. Parent animals were obtained from Harlan Labs (Indianapolis, IN). Following the observation of a seminal plug, pregnant females were individually housed in plastic cages and brought into a temperature and humidity controlled nursery (70°+/−2 F) set on a 12:12 h light-dark cycle, with food and water provided ad libitum. On the day following birth, postnatal day 1 (PND 1), litters were culled to 10 animals, maintaining a 1:1 sex ratio whenever possible.
2.2. Hippocampal Slice Preparation
The organotypic slice culture procedures used in our laboratory are a modification of those developed by Stoppini and colleagues (1991). For each replicate, six PND 8 pups (3/sex) were sacrificed, their brains aseptically removed and transferred to ice-cold dissecting media [Minimum Essential Medium (MEM; Gibco BRL, Gaithersburg, MD), 25 mM HEPES (ATCC, Manassas, VA), 200 mM L-glutamine (Invitrogen, Carlsbad, CA), 50 μM streptomycin/penicillin (ATCC, Manassas, VA)]. Hippocampi were dissected out, sectioned coronally (200 μm) (McIllwain Tissue Chopper; Campden Instruments Ltd., Lafayette, ID), plated in triplicate onto 0.4 μm Biopore membranes (Millipore, Marlborough, MA), and suspended in 1 ml culture media [dissecting media, 36 mM glucose, 25% Hanks’ balanced salt solution (HBSS; Gibco BRL, Gaithersburg, MD), 25% heat-inactivated horse serum (HIHS; Sigma, St. Louis, MO)] using six-well plates. Approximately 24 slices were obtained from each animal (12/hippocampus), that were equally distributed among treatment groups. Plates were incubated at 37 °C in a 5% CO2/21% O2/74% N2 gas composition for 5 days in vitro (DIV) prior to any treatment, to allow the slice to affix to the membrane.
2.3. ETOH Exposure and Drug Treatment
Beginning on DIV 5, half of the wells were exposed to ETOH (100 mM; Sigma; St. Louis, MO) in the media for 10 days. ETOH-treated cultures were surrounded with sterilized distilled water containing 100 mM ETOH in topless polypropylene containers. The culture plates were then enclosed in a plastic bag filled to capacity with incubator-equivalent air mixture to minimize ETOH evaporation during incubation. 5-day reductions in ETOH concentration to approximately 50 mM have been noted (Prendergast et al., 2004), thus slices were transferred to fresh media on DIV 10. On DIV 15, ETOH was removed, and all slices were given fresh media containing 5 μM NMDA (Sigma, St. Louis, MO; excepting the 20 μM NMDA positive control slices) and the fluorescent nucleic acid stain propidium iodide (PI; 3.74 μM, Molecular Probes). Groups included untreated controls, a CP-101,606 (5, 10, 25, 50 or 75 nM) treatment group, an MK-801 (20 μM) negative control and a 20 μM NMDA positive control, each of which were represented in both ETOH-positive and naïve conditions. The addition of 5 μM NMDA, a non-toxic concentration, was used to model hippocampal glutamate increases that normally occurs in vivo during ETOH withdrawal (Dahchour and De Witte, 1999). At least 3 slices from each animal were exposed to all possible treatment conditions. Uptake of PI allowed visualization and quantification of compromised cell membranes using densitometry 24 h after the removal of ETOH (DIV 16).
2.4. Fluorescent Microscopy and Data Analysis
PI binds to DNA, entering cells via compromised cell membranes, and produces a red fluorescence at 630 nm (Zimmer et al., 2000), providing an index of cell damage. Indices of damage based on PI fluorescence have been validated by several other markers including neuronal nuclear protein and calbindin D28k (Wilkins et al., 2006). Slices were visualized at 5x objective using a Leica DMBIRM microscope (W. Nuhsbaum Inc.; McHenry, IL) fitted for fluorescent detection (Mercury-arc lamp), and imaging software (SPOT Advanced, version 4.0.2, W. Nuhsbaum Inc.; McHenry, IL). Densitometry was conducted using Image J software, v1.29x (National Institutes of Health, Bethesda, MD) to quantify cell death in the primary neuronal layers of the CA1 and CA3 regions, and the dentate gyrus (pyramidal and granule cell layers, respectively). Non-specific background fluorescence was subtracted from each area. Variability in PI uptake was minimized between replications by converting fluorescence to percent control prior to analysis. An initial two-way ANOVA was conducted (TREATMENT × SEX), for each region. If no main effect of sex was noted, data were collapsed in subsequent analyses. When appropriate, post hoc tests (Fisher’s Least Significant Difference; LSD) were conducted. The significance level was set at p<.05.
3. HIPPOCAMPAL CULTURE: RESULTS
CA1:
ETOH exposure resulted in an increase in PI uptake in the CA1 pyramidal cell layer. Both 10 and 25 nM CP treatment successfully reduced this damage (see Figure 1). The ANOVA revealed a main effect of TREATMENT, F(14, 155)=21.338, p<.001. Fisher’s post hoc tests revealed decreased PI uptake in slices treated with 10 nM CP (p<.001), 25 nM CP (p<.001) and MK-801 (p<.001), relative to ETOH controls. Increased PI uptake was observed in the highest dose of CP (75 nM CP; p<.05), and (as expected) the 20 μM NMDA control (p<.001). In order to examine whether these treatments had effects on non-alcohol exposed control slices, we used an additional statistical approach in which separate ANOVAs were conducted for slices in each control replication. This was necessary because the standard ANOVAs (as described above), use a conversion to % control and thus precludes the comparison of ETOH-naïve cultures in the combined data set. Analyses of PI uptake in ETOH-naïve slices revealed that neither the 10 or 25 nM CP dose had any impact on PI uptake although there was an increase in PI uptake with 75 nM CP (p<.001). Of the three replicates exposed to 50 nM CP, increased PI uptake was observed in only one (p<.01) suggesting this might be a threshold dose for increased toxicity.
Figure 1.
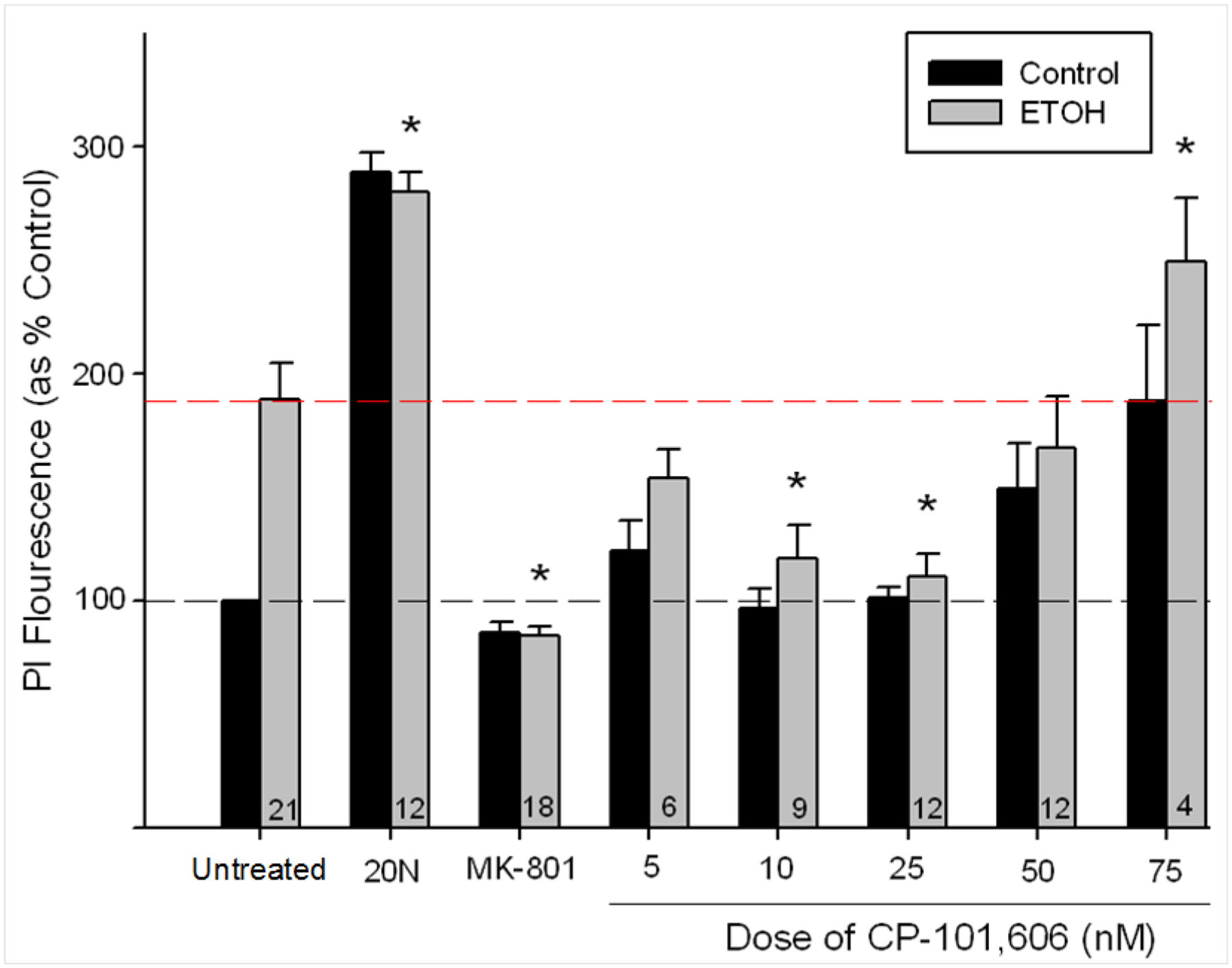
Mean (± SEM) PI uptake in CA1, expressed as percent control as a function of treatment conditions (n of subjects represented expressed in the bars). 20 μM MK-801, 10 nM and 25 nM CP all reduced PI florescence compared with the ETOH-exposed control (5 μM NMDA) group. Broken black line denotes flourescence in ETOH-naïve controls, red indicates mean fluorescence in ETOH-treated controls. * denotes p<.05.
CA3 and DG
Ethanol did not increase PI uptake in either the CA3 or DG (see Figure 2). While there was a main effect of TREATMENT for both regions, this was due to the increased PI uptake in the 20 uM NMDA control group, F(14,155)=8.864, p <.001, and F(14,155)=8.290, p <.001, in the CA3 and DG, respectively.
Figure 2.
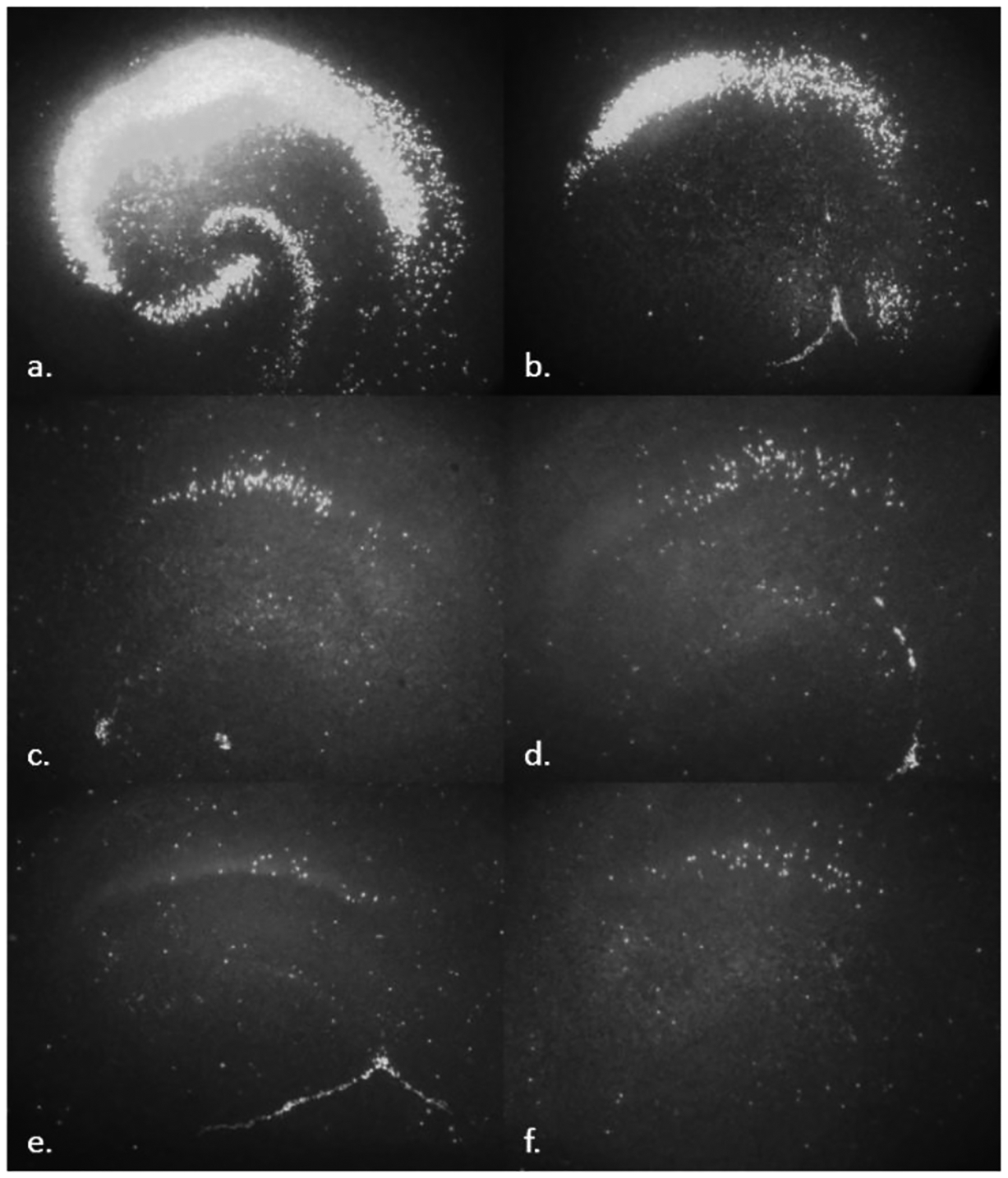
Representative images of PI flourescence in hippocampus. a) 20 μM NMDA b) ETOH c) ETOH + 10 nM CP d) ETOH + 25 nM CP e) ETOH + 20 μM MK-801 f) untreated controls.
4. MATERIALS AND METHODS
IN VIVO NEONATAL EXPOSURE MODEL
CP’s ability to reduce excitotoxicity resulting from ETOH withdrawal in vitro suggested its ability to mediate withdrawal-associated damage in vivo. We examined this hypothesis using three behavioral paradigms considered sensitive to ETOH-associated developmental insult.
4.1. Neonatal Drug Administration and Housing
Subjects were derived and housed in an identical manner to those used for in vitro experiments. 24 hr after parturition, litters were culled to 10, maintaining a 1:1 sex ratio when possible. The litters were then randomly divided into five treatment conditions: 6 g/kg/day ethanol (ETOH), 15 mg/kg CP-101,606 (CP), ethanol and 15 mg/kg CP-101,606 (ETOH/CP), a milk control (MILK), and a non-treated control (NTC). This dose of CP was selected based on pilot experiments (unpublished data) and doses used in adult rodent literature (e.g., Kundrotiene et al. 2004, Yang et al. 2003). No more than one animal per litter, per sex, was assigned to any treatment condition to preclude potential litter effects (Abbey and Howard, 1973). ETOH was administered via gastric intubation (.0278 ml/g body weight) in a solution developed to nutritionally mimic rat milk (West et al., 1984). Intubations were conducted twice daily (1000 and 1400 h) for seven days (PND 1–7). Exposure during this first postnatal week is used as a model to study a period of brain development which overlaps the 3rd trimester of human pregnancy. Animals were removed from the dam for approximately 5 min during each intubation session. Heating pads placed under the home cage helped maintain pup body temperature. Neonates received ETOH or MILK via intubation using a syringe connected to PE-50 and PE-10 polyethylene tubing (Clay Adams) coated with corn oil to ease esophageal passage. CP was administered subcutaneously on PND 8, 21 h following the final intubation (a time course during which similar interventions have demonstrated efficacy; Thomas et al., 2001). Litters were weaned upon completion of locomotor activity testing into standard polycarbonate cages with 1–2 same-sex conspecifics. Subjects were tested in all behavioral experiments. N = 98 (8–11/group) for locomotor and balance tests, with a subset (N = 89; 7–10/group) tested in the water maze.
4.2. Locomotor activity
Open field activity was measured prior to weaning on PND 20 and 21. The apparatus was a circular chamber (36 cm height; 58 cm diameter) designed to prevent thigmotaxis or excessive time in corners. Each day, subjects were brought into the darkened test room and habituated for 10 min. Each subject was then individually placed in the open-field and activity was recorded for 30 min using a video tracking system (SMART program; Panlab, S.L.). For analysis, the open field was divided into center and outer zones, comprising 25% and 75% of the total area, respectively. The dependent variables included distance traveled (in 5 min blocks) and the ratio of activity in the center vs. outer zone of the field, a potential marker of motor impulsivity and/or anxiety (Royce, 1977).
4.3. Balance Performance
Subjects were tested on PND 31–33. The balance apparatus consisted of a single elevated dowel rod (114 cm long, 1.85 cm diameter) raised 75 cm above the ground (which was padded in case of falls), with a darkened escape box (21 × 10 × 17 cm) on one end. Each animal was brought into the test room individually and habituated to the test room, then escape box, for 1 min each. For the first trial, the rat was placed on the rod 10 cm from the escape box. A successful trial was defined as running to the escape box without falling or swinging from the rod. After successful completion of a trial, the distance required on the following trial was increased by 13 cm. On a failed trial, the subject was retrieved and placed in the escape box. Animals were allowed to remain in the escape box for 10 sec, and then returned to the home cage for a 30 sec intertrial interval (ITI). Animals were tested over 3 days (3 trials/day) to avoid fatigue. The greatest distance achieved was the dependent variable.
4.4. Water Maze Performance
Animals were tested in a water maze learning task on PND 43 and 44. The apparatus was a 130 × 90 × 40 cm black Plexiglas chamber, divided such that several divergent paths (depicted in Figure 3), each 18 cm wide, branched off from the central start area. The apparatus and methodology were derived from Von Euler and colleagues (2006). Water temperature was maintained at 76° ±2 F. Animals were required to make three successive right/left choices to reach a hidden, submerged escape platform. The water was made black with the addition of non-toxic black tempura paint to obscure the submerged platform. A plastic sheet surrounded the maze, reducing extra-maze cues, including the experimenter. A major advantage of this maze is that control animals can learn the maze in a single day. Movement in the maze was recorded using a video tracking system (SMART program; Panlab, S.L.).
Figure 3.
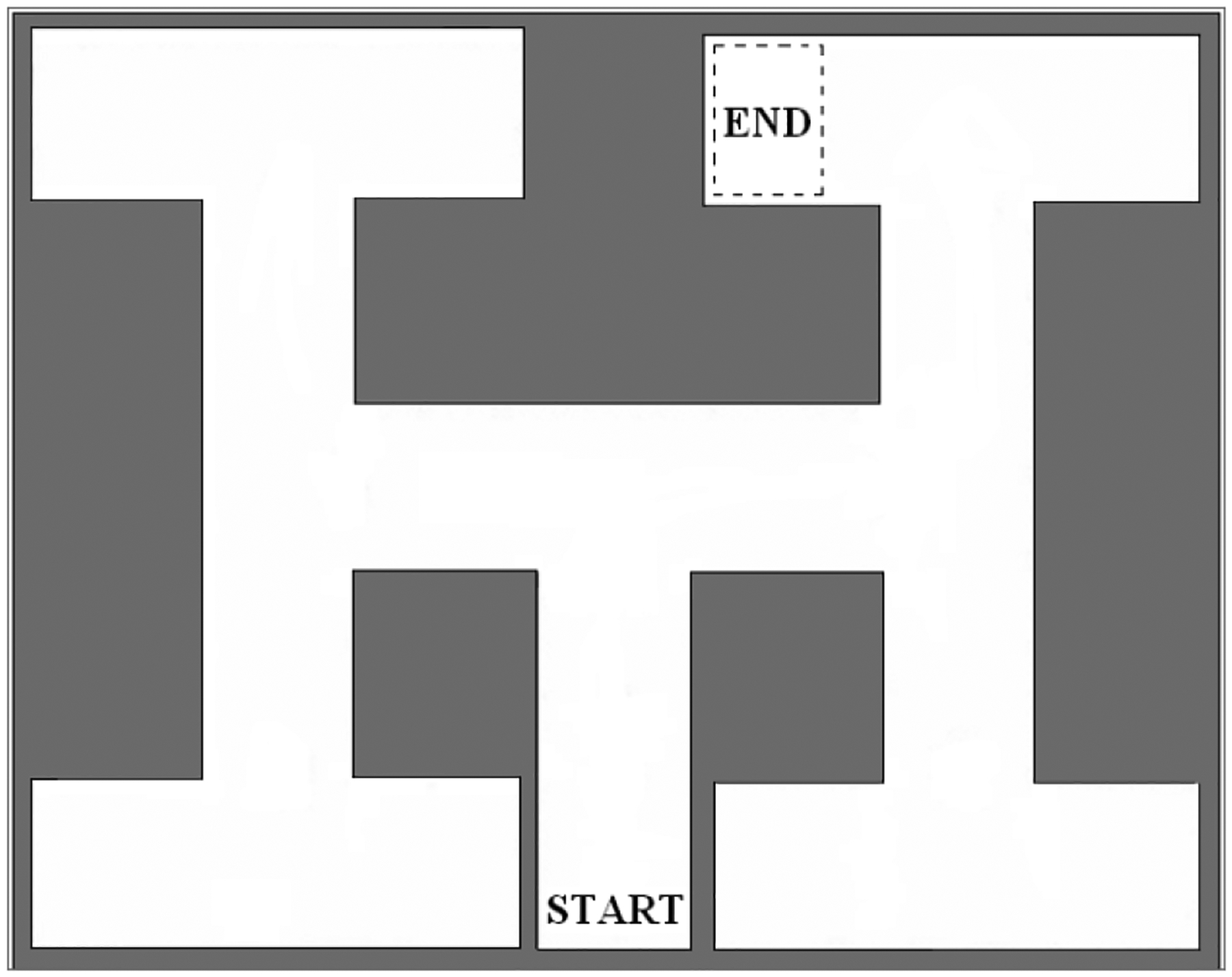
Water Maze, adapted from von Euler et al., 2006.
Each subject was habituated to the test room for 1 min, then placed in the water maze and allowed to swim to the platform. The latency to reach the platform was recorded with a ceiling of 2 min/trial. If the animal did not find the platform within this 2 min ceiling, the animal was gently guided through the maze to the escape platform. Animals were allowed to remain on the platform for 5 sec, and were then toweled off and placed in a cage warmed by a heat lamp (25 watts) for a 1 min ITI.
The complexity of the task requires three correct right/left choices, making success by chance 12.5% (1/8). The criterion for acquisition of the task was three consecutive errorless trials. The only exception was if there were no errors on the first trial, it did not count toward the acquisition criterion since it reflected chance occurrence rather than learning per se. The ceiling for the number of trials to reach acquisition was 20. Only those subjects that reached acquisition on the first day of testing were tested for retention, however all subjects were included in an initial Day 1 performance analysis (see below). Retention of the task was examined 24 hr following acquisition. The criterion for retention was 2 consecutive errorless trials.
4.5. Behavioral Data Analysis
The data were analyzed with analysis of variance (ANOVA). Prior to each analysis, the MILK and NTC control groups were compared. If these groups did not differ, they were combined into a single control group, facilitating a 2 × 2 ANOVA with ETOH and CP as between-subject variables with two levels each. This then allowed a direct examination of any ETOH x CP interaction. If a difference between MILK and NTC groups was detected, neonatal treatment was examined as the between subjects variable with 5 levels (ETOH, CP, ETOH/CP, MILK, and NTC). Body weights were also analyzed in this manner. In all cases, sex was included as a between subjects variable and if there was no main effect or interaction with sex, the data were collapsed in subsequent analyses. Additionally, due to the concern that body weights might influence open field or balance performance, correlation analyses between body weight and performance were conducted within each group as well as overall.
5. NEONATAL EXPOSURE MODEL: RESULTS
5.1. Locomotor Activity
An initial (2 × 2[6]; GROUP × DAY[BLOCK]) mixed ANOVA between MILK and NTC controls revealed that the NTC animals displayed lower activity than the MILK control, F(1,37)=7.024 p<.02, precluding their combination in an overall (2 × 2; ETOH × CP) analysis. Rather, the ANOVA was conducted with neonatal treatment and sex as grouping variables and 5 min trials nested within day as the within group repeated measure (5 × 2 × 2[6]; GROUP × SEX × DAY[TRIAL]). This ANOVA yielded main effects of GROUP, F(1,88)=8.510 p<.001, and SEX F(1,88)=5.664, p<.02. The main effect of sex was due to females being more active than males. Post hoc (LSD) analyses of the neonatal treatment groups revealed that the ETOH exposed animals were hyperactive compared with CP (p<0.005), ETOH/CP (p<.05), MILK (p<.005) and NTC (p<.001) groups (see Figure 4). Of particular note, the addition of CP reduced the ETOH-induced hyperactivity. other groups (In addition, the NTC animals were hypoactive compared to all p’s < .01). No differences between the CP, ETOH/CP and MILK groups were detected. There were no differences in the distance traveled in the center zone as a function of neonatal treatment or sex.
Figure 4.
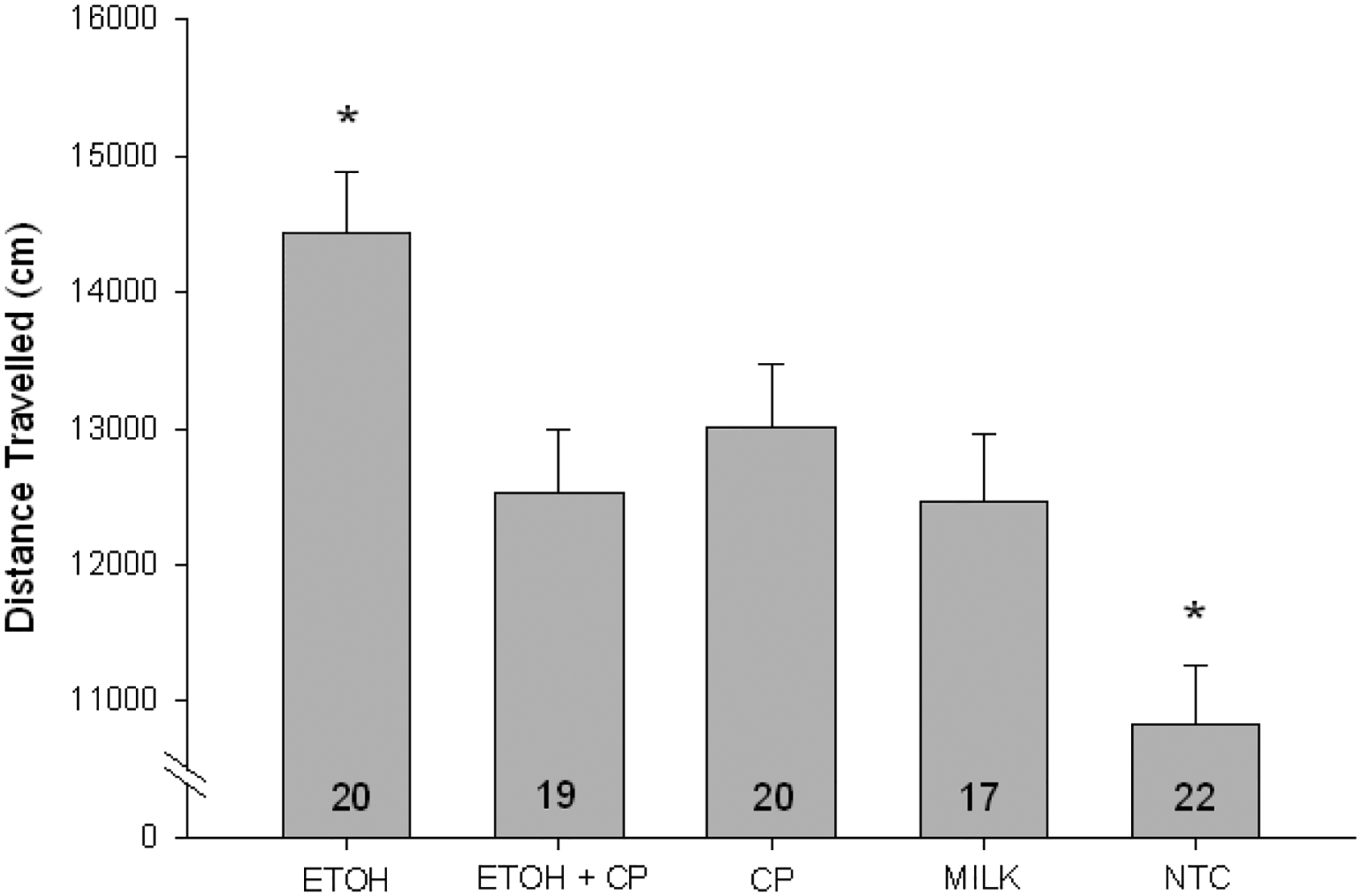
Mean (± SEM) distance travelled in the open field, collapsed across days. Compared with all other groups, ETOH exposed animals were hyperactive. Compared with all other groups, NTC animals were hypoactive. * denotes p<.05
Body weights were recorded on PND 21 following open field testing (see Table 1) and analyzed using a 2 × 2 × 2 (ETOH x CP x SEX) ANOVA. Animals treated with ETOH weighed less than ETOH-naïve animals F(1, 97)=10.038, p<.005, an effect which was not reversed by CP. Males outweighed females F(1, 97)=12.018, p=.001. No significant correlations were detected. Weights are presented in Table 1, below.
Table 1.
Body Weights (in g)
| Maze | Drug Tx** (PND 8) |
Open Field* PND 21) |
Balance* (PND 33) |
Water (PND 44) |
|---|---|---|---|---|
| Group | M± SEM | M± SEM | M± SEM | M± SEM |
| ETOH | ||||
| Males | 14.0± .8 | 49.8± 3.9 | 102.6± 4.0 | 169.7± 6.2 |
| Females | 12.3± .9 | 47.0± 3.2 | 97.9± 3.2 | 150.9± 4.6 |
| CP-101,606 | ||||
| Males | 19.7± .6 | 60.7± 3.8 | 116.0± 4.8 | 182.6± 5.2 |
| Females | 17.6± .8 | 48.7± 2.1 | 104.1± 2.8 | 153.6± 3.0 |
| ETOH + CP | ||||
| Males | 14.2± .8 | 53.7± 4.3 | 108.2± 5.1 | 187.1± 7.9 |
| Females | 12.7± 0 | 43.4± 2.3 | 95.6± 2.6 | 148.7± 9.4 |
| CONT | ||||
| Males | 19.0± .0 | 57.5± 2.5 | 111.3± 3.4 | 189.6± 6.2 |
| Females | 18.9± .6‵ | 53.6± 1.8 | 107.6± 2.1 | 159.3± 4.2 |
Denotes main effect of ETOH (p<.001)
Denotes main effect of ETOH (p<.01)
5.2. Balance Performance
There were no differences in performance across the two control groups and so the data were examined using a 2 × 2 × 2 × 3[3] mixed ANOVA (ETOH x CP x SEX x DAY[TRIAL]). The ANOVA yielded an ETOH × CP interaction, F(1,88)=4.969, p<.05. While the ETOH exposed offspring showed deficits in balance, the addition of CP improved performance (see Figure 5). Additionally, a main effect of sex was observed, F(1,88)=6.732, p<.05 with females outperforming males (although sex failed to interact with either ETOH or CP), as well as an ETOH x CP x DAY[TRIAL] interaction, F(1,88)=5.426, p<.05. In order to probe this latter interaction, ANOVAs (ETOH x CP) were conducted for trials 1–9 (see below for trial 1 analysis). Significant interactions were noted on TRIAL 1, F(1,97)=10.430, p<.005; TRIAL 4, F(1,97)=4.318, p<.05; TRIAL 6, F(1,97)=4.558, p<.05; TRIAL 8, F(1,97)=4.521, p<.05; and TRIAL 9, F(1,97)=6.296, p<.02. These interactions were driven by balance improvements in the ETOH/CP group.
Figure 5.
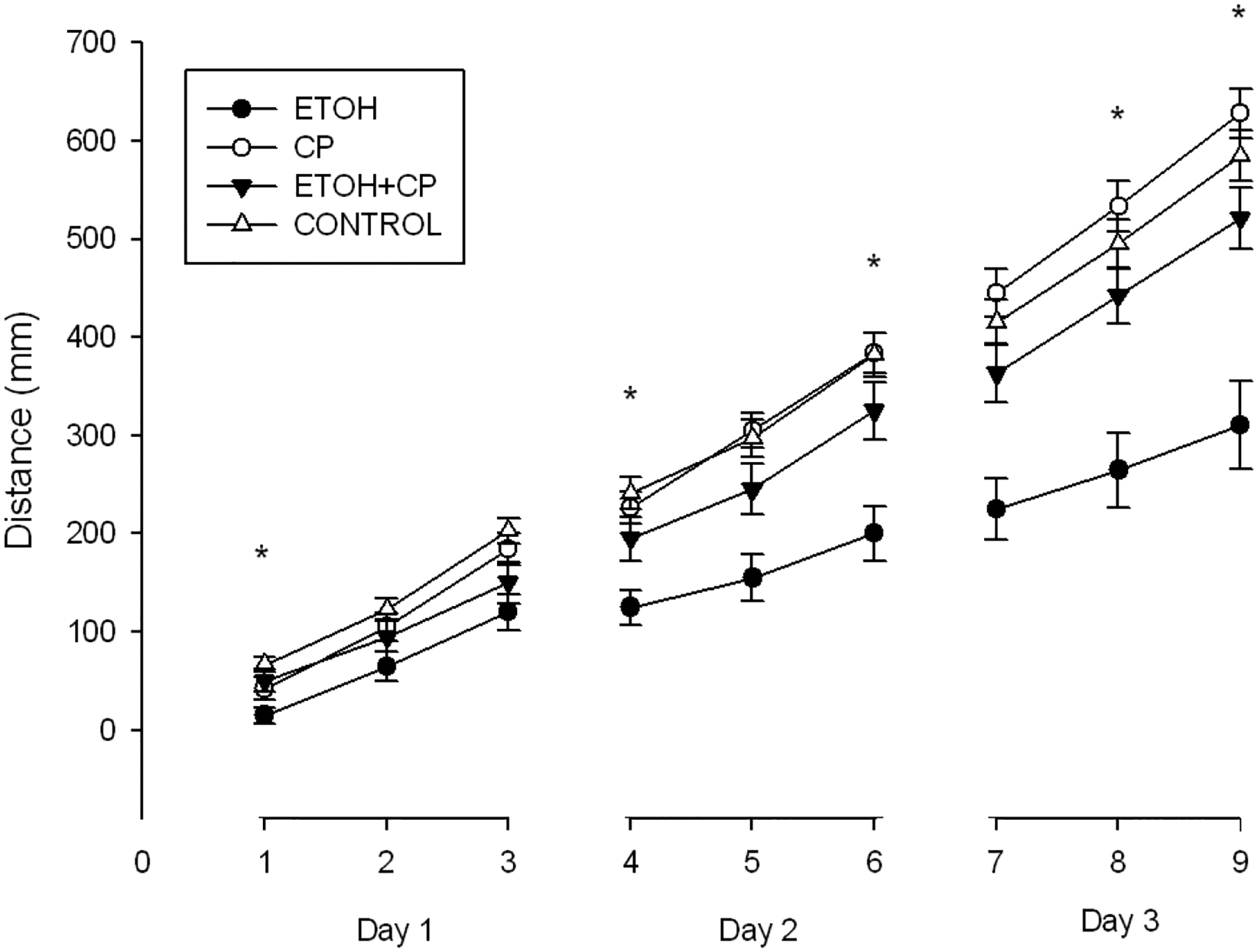
Mean (± SEM) distance achieved across three days of balance testing. A main effect of sex was observed, with females outperforming males. Significant interactions between ETOH and CP were observed during trials 4, 6, 8 and 9. * denotes p<.05
TRIAL 1 represented a unique behavioral measure relative to subsequent trials, in that performance was based solely on motor coordination since there had been no previous exposure to the balance paradigm, whereas performance in subsequent trials relied on both coordination and motor learning acquired in previous trials. Additionally, the TRIAL 1 dependent variable was dichotomous, indicating only failure or success, whereas performance on subsequent trials varied based on previous performance. For these reasons, TRIAL 1 was also analyzed using the non-parametric Kruskal-Wallis test. 15% of ETOH animals were successful on the first trial, versus 42% of CP, 50% of ETOH/CP and 67% of CONTROLS. A significant difference was noted between groups, chi-square (3, N=89)=14.320, p <.005. To further examine this difference, pairwise comparisons (Mann-Whitney U tests) were conducted between ETOH and all other groups, given the a priori hypothesis of ETOH-associated balance deficits. ETOH animals performed more poorly than the ETOH/CP and CONTROLS (p<.05 and p<.001, respectively), but not CP animals (p=.063).
Body weights were recorded on PND 33 following the final trial (see Table 1) and analyzed with a 2 × 2 × 2 (ETOH x CP x SEX) ANOVA. Animals treated with ETOH weighed less than ETOH-naïve animals F(1, 97)=14.681, p<.001, an effect which was not reversed by CP. Males outweighed females F(1, 97)=12.819, p=.001. No significant correlations were detected.
5.3. Water Maze
A preliminary ANOVA was conducted for platform acquisition speed during the first trial to ensure that there was no bias due to gross differences in swim speed. Subsequently, trials to acquisition between MILK and NTC groups were compared; no differences were detected, therefore the controls were combined. The first ANOVA on water maze examined Day 1 performance, with all subjects included, regardless of their ability to meet criterion learning on the task (subjects who failed to learn the navigation were assigned the ceiling score of 20). The ETOH x CP x SEX (2 × 2 × 2) analysis indicated no main effects or interactions during Day 1, suggesting neonatal ethanol exposure did not affect acquisition of the water maze task. A chi-square analysis was conducted to compare the freqencies of learners vs. non-learners, by group. This analysis was conducted to allay two concerns: firstly, that while no acquisition differences were noted, more animals in one group (e.g., ETOH) may have failed to reach criterion learning. Relatedly, if differences in frequencies of learners/non-learners varied by group, removing non-learners from the subsequent analysis of Day 2 performance (retention) might have biased the data. The analysis revealed no differences in frequencies of animals failing to reach criterion (42% of ETOH, 27% of ETOH/CP, 12% of CP, and 18% of CONTROL), chi-square (3, N=89)=5.357, p =.147.
In order to explore retention on the task, animals that failed to reach criteria were removed from the dataset. A mixed ANOVA examining performance across days (acquisition vs. retention) was conducted using a (ETOH x CP x SEX x DAY; 2 × 2 × 2 × 2) analysis. The mixed ANOVA including only “learners” (see Figure 6 for remaining n’s/group) revealed a CP x ETOH interaction, F(1,61)=5.877, p<.02, such that CP improved the performance of ETOH-treated animals (see Figure 6). Post hoc analyses (LSD) conducted separately for each day again indicated no difference during acquisition, but detected differences during retention between the ETOH group and all others (which were indistinguishable from one another), including ETOH+CP, p<.05; CP, p<.01; and CONTROL, p=.005.
Figure 6.
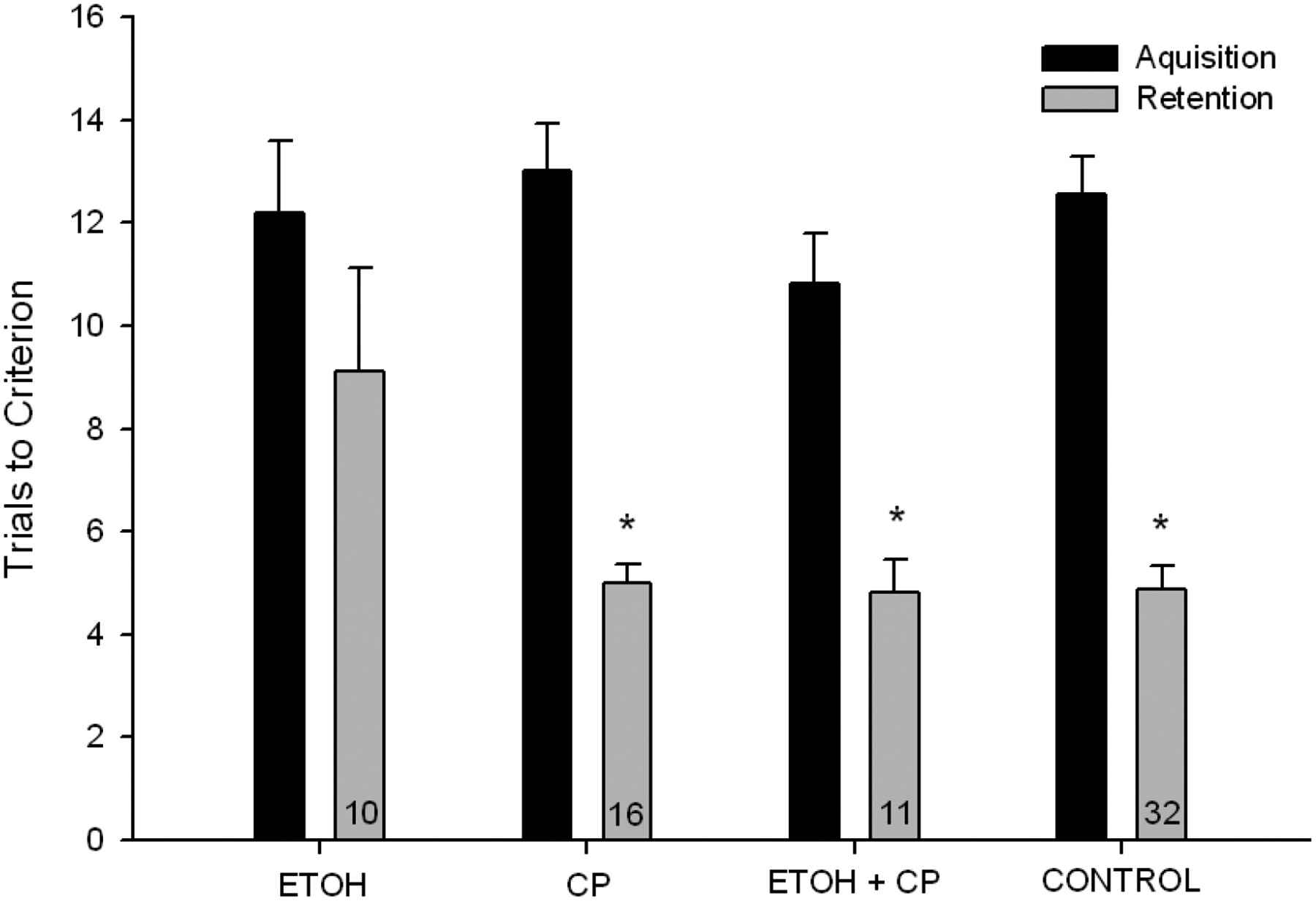
Acquisition and retention in water maze, represented by number of trials to reach performance criterion. Includes only animals meeting learning criterion for Day 1. ETOH-exposed animals took more trials to reach criterion during retention than all other groups. * denotes p<.05
Body weights were recorded on PND 44 (see Table 1). No effects of ETOH were detected on body weight. Only sex differences were noted, with males outweighing females F(1, 89)=34.269, p<.001.
5.4. PND 8 Body Weights
Body weights on PND 8 (see Table 1) were analyzed with a 2 × 2 (ETOH x SEX) ANOVA, since CP could not have affected body weight at this time. Main effects of both variables were detected, with males outweighing females, F(1, 97)=4.736, p<.05, and ETOH-naïve animals outweighing exposed animals, F(1, 97)=104.122, p<.001.
Body weights were collected throughout the intubation period, as well as following each behavioral experiment; weights across all periods are presented in Table 1.
6. DISCUSSION
This study was designed to examine whether CP-101,606, an NR2B-specific NMDAR antagonist, could reduce neurotoxicity and behavioral deficits following neonatal ETOH exposure and withdrawal. The results from this study demonstrate that following chronic, binge-like ETOH exposure, a single administration of CP during withdrawal consistently reduced or eliminated ETOH’s damaging effects, both in an in vitro organotypic hippocampal slice model and an in vivo neonatal “3rd trimester” exposure model.
6.1. Hippocampal Culture
The hippocampal slice model was utilized because it preserves structural and functional integrity and because it maintains trisynaptic circuitry despite a lack of input to the DG thus serving as a relatively complex in vitro model of hippocampal function (Gutierrez and Heinemann, 1999). Chronic ETOH exposure was associated with an uptake of PI approximating a 90% increase, relative to controls, in the CA1 region 24 h following ETOH cessation. Ten and 25 nM doses of CP-101,606 reduced this damage.
Given the hypothesis that NMDAR-mediated excitotoxicity underlies ETOH-associated damage, hippocampal vulnerability to developmental ETOH insult is not surprising, given its rich expression of NMDARs. The CA1 region is known to be particularly sensitive to ETOH (e.g., Harris et al., 2003; Prendergast et al., 2000), in part, because of the density of neurons expressing NR2B subunits in this area (Butler et al., 2010). NR2B-specific antagonists are known to attenuate ETOH-associated hippocampal cell death (e.g., Barron et al., 2008; Butler et al., 2010; Harris et al., 2003), however little is known about which sub-populations of NR2B-containing receptors are critical. Since CP is a relativity specific NR2B/NR2B antagonist, our results suggest that this receptor at the very least, contributes to ETOH’s detrimental effects in the developing CNS. CP is known to protect hippocampal neurons from glutamate toxicity (Menniti et al., 1997) and has been shown to reduce ETOH withdrawal-associated excitotoxicity in cortical neurons (Nagy et al., 2004). Both findings are consistent with our data and with the hypothesis of NR2B-mediated vulnerability to excitotoxic damage, however the current project is the first to examine attenuation of ETOH-associated hippocampal damage in the developing brain.
6.2. In Vivo Neonatal Exposure Experiments
In this study, we also employed an open field paradigm, a dowel-rod balance paradigm, and a water-maze navigation task to examine some of the behavioral consequences associated with neonatal ETOH exposure. For each, ETOH was associated with behavioral deficits that were abolished or reduced by the addition of CP treatment. It is also important to note that CP did not exert any effect on its own in any of the control groups.
Neonatal ETOH exposure was associated with hyperactivity in the open field and this was reduced by CP treatment. Additionally, the NTC showed unusually low levels of activity relative to all other groups. While the mildly stressful intubation procedure may have contributed to the differences in activity between the intubated and non-intubated controls, we have not observed this phenomenon in other studies in our laboratory (e.g., Rubin et al., 2009; Wellmann et al., in press). Distance travelled in the center zone did not differ among groups, which suggests that anxiety-like behaviors (avoiding the center) and/or impulsive behaviors (entering the center) did not differ as a function of treatment in this paradigm. Hyperactivity is one of the most consistent findings observed in children and animal models following a history of prenatal alcohol exposure (for reviews see Abel, 1984; Appelbaum et al., 1995; Danis et al., 1981). Studies in animal models have demonstrated that NMDAR antagonists reduce this hyperactivity (Thomas et al., 2002; Wellmann et al., in press), consistent with our findings, although our use of an NR2B/2B selective compound is novel for this behavior.
Neonatal ETOH exposure also produced deficits in balance, which were eliminated by treatment with CP. While the balance procedure clearly tests motor learning, it can also be considered a test of baseline motor coordination (Trial 1). It should be noted that Trial 1 was the only trial not influenced by practice effects and that ETOH exposed animals showed impairment, relative to the other groups. The remaining trials (Trials 2–9) required both motor skills and learning. Performance deficits in the ETOH-exposed animals were amplified across the trials relative to other groups, suggesting a possible role for both baseline differences in motor ability as well as differences in motor learning. Further research could better clarify this. There was also a main effect of sex, with females from all neonatal treatment groups displaying better performance on the dowel rod. We have previously observed this sex difference (Lewis et al., 2007; Wellmann et al., in press), which may be due to sex differences in size at this age. The female rats were smaller, perhaps contributing to their ease of traversing the rod relative to the larger males. Alternatively, female mammals mature physically and neurobiologically more quickly (for reviews see Pinyerd & Zipf, 2005; Blakemore et al., 2010) than males and thus subtle differences in development may have advantaged female performance.
In our water-maze navigation task, no acquisition differences were detected between groups. Differences in retention were noted, such that ETOH exposed animals took nearly twice as many trials to reach Day 2 criteria as controls, despite a lack of difference in task acquisition. This retention deficit was attenuated by CP treatment. Our findings expand on those of other navigational studies, most of which used the Morris Water Maze (Morris, 1984), employing prenatal (e.g., Dursun et al., 2006; Kim et al., 1997; Blanchard et al., 1987; Iqbal et al., 2004; Toso et al., 2006) and/or early postnatal ETOH exposure paradigms (e.g., Goodlett et al., 1997; Marino et al., 2004; Girard et al., 2000; Cronise et al., 2001; Johnson & Goodlett, 2002; Pauli et al., 1995; Goodlett & Peterson, 1995; Tomlinson et al., 1998). Retention deficits in Morris Maze following ETOH exposure, despite a lack of acquisition differences, have also been noted (Savage et al., 2010). A unique feature of the current paradigm is its ability to assess spatial working memory acquired in a single day of testing, which precludes interpretational issues regarding deficits in retention impairing acquisition, which arise when multiple days are required to learn a navigational task. While CP-101,606 has been shown to attenuate navigational deficits in a rodent model of traumatic brain injury (Okiyama et al., 1997), ours is the first demonstration of such attenuation in a developmental model following chronic ETOH. While retention deficits were apparent, it is not clear whether this was due to dysfunction in memory consolidation or retrieval of Day 1 learning (or perhaps both), a nuance that certainly warrants further investigation.
Hippocampal function is critical for spatial learning & memory and there is a significant literature documenting that developmental ETOH exposure affects hippocampal structure and function. The findings from the current study, utilizing both this in vivo spatial paradigm as well as our in vitro hippocampal slice model, provide further support for the hypothesis that ETOH- associated behavioral impairments are mediated, at least in part, by effects on hippocampal structure and/or function (for review see Berman & Hannigan, 2000). It should be noted, however, that criterion performance on this maze is also possible via striatum-based response strategies; the striatum, which has a recognized importance in navigation is also sensitive to developmental ETOH exposure (e.g., Fryer et al., 2007). However, it is suggested that spatial learning initially relies more on hippocampal function and that activation of striatum-dependent motor learning occurs more with repetition of successful navigation (Chang & Gold, 2003; Packard & McGaugh, 1996). The paradigm used in the current study did not involve overtraining, thus it is likely reflective of hippocampal function, although further examination of specific learning and memory strategies are certainly warranted, for example with the Morris Water Maze.
6.3. CP-101,606
NR2B-specific antagonists, most notably eliprodil and ifenprodil, have been investigated as neuroprotective compounds for a variety of disorders. They have shown efficacy in a number of traumatic brain injury and ischemia models (Bath et al., 1996; Dogan et al., 1997; Toulmond et al., 1993) and appear to attenuate excitotoxic hippocampal cell death (Mayer et al., 2002; Reyes et al., 1998; Tamura et al., 1993). They appear effective developmentally as well, preventing hippocampal-dependent learning deficits following neonatal ETOH exposure (Thomas et al., 2004). However, their clinical development has been slowed due to interactions with additional transmitter systems; CP appears to lack significant interaction with α1-adrenoceptors, sigma receptors or calcium channels (Chenard et al., 1995; Menniti, 1997), and is particularly noted for its selectivity for NR2B/2B receptor compositions. While the current study is one of but a few to examine CP in ETOH-associated toxicity, and the first to examine it in a developmental model, CP has been used in several clinical studies, where it appeared well-tolerated (Johnson et al., 2003; Merchant et al., 1999). Taken together with our results, CP has potential as a neuroprotective agent for ETOH withdrawal-associated CNS damage, however while exciting, further preclinical examinations of CP are warranted.
6.4. ETOH and Drug Administration Models
Twice daily intubations of 3g/kg ETOH were administered, achieving a pattern of binge-withdrawal cycles involving high peak blood ethanol content (BEC) typical of a pattern of maternal consumption particularly damaging to developing organisms (West et al., 1989). Previous studies have shown that the BEC achieved using this exposure regimen typically peaks at ~230mg/dl, falling to 0 between 8 and 10 h following the last intubation (Lewis et al., 2007). Importantly, while this peak would be considered clinically high, it is well within the range displayed by human binge drinkers (Cherpitel, 2007; Urso et al., 1981). CP was not administered until 21h after the final ETOH intubation, at which time BEC was neglible, thus could not have affected ETOH pharmacokinetics.
In addition to producing relevant ETOH concentrations, our drug administration paradigm was selected to model potential clinical intervention in prenatally-exposed neonates. Prenatal treatment for chronic ETOH exposure is difficult for a variety of reasons, including compliance issues and the unique challenges that fetal drug delivery present. However, while perinatal treatment appears the easiest avenue for intervention, it may also be the most promising; birth constitutes a period of prolonged withdrawal, potentially compounded by stress and hypoxia, and as such may constitute the single most dangerous period for excitotoxic damage, and a The ability of promising window for intervention. a single intervention, given prior to a final period of prolonged withdrawal, to attenuate ETOH effects is supported in the preclinical literature. Even when neonates are subjected to daily binge-like doses of ETOH, a single intervention during the final withdrawal has been shown to attenuate behavioral deficits (e.g., Lewis et al., 2007; Rubin et al., 2009; Thomas et al., 2004; Wellmann et al., in press). Furthermore, Thomas and colleagues (2001) have reported that behavioral deficits may be attenuated even when the intervention follows ETOH cessation by up to 33 hours, highlighting both the importance of prolonged periods of withdrawal, and in particular, the potential utility of perinatal interventions.
6.5. Conclusion
Developmental ETOH exposure was associated with persistent behavioral deficits. Our findings implicate NR2B-dependent excitotoxicity in the pathogenesis of such deficits, and demonstrate that they may be attenuated with a single administration of an antagonist selective for NR2B/2B-containing receptors during withdrawal. Further elucidation of involved mechanisms, as well as continued development and investigation of NR2B-specific compounds are necessary. However, the demonstration that a compound which has safely completed multiple phase II clinical trials can prevent behavioral dysfunction and cellular damage following ETOH exposure is exciting, particularly because such effects are noted following a single administration, in a manner modeling perinatal intervention.
Highlights.
This study used a rodent model to study “3rd trimester” effects of ethanol on offspring outcome.
CP-101,606, an NMDAR NR2B antagonist, was administered during ethanol withdrawal to reduce some of ethanol’s effects on the developing brain.
CP-101,606 reduced cell death in hippocampal slices during ethanol withdrawal.
CP-101,606 also reduced behavioral deficits associated with 3rd trimester ethanol exposure.
These finding suggest that NMDAr and more specifically NR2B activity during ethanol withdrawal appears to play a role in the damaging effects of ethanol.
Acknowledgements
This work was supported, in part, by NIAAA14032 to SB. We thank Dr. Prendergast and his laboratory for their assistance with the cell culture experiment, Pfizer for CP-101,606 and Purina Protein for their protein mixture.
Abbreviations:
- BEC
Blood Ethanol Content
- CA1/CA3
Cornu Ammonis Region 1/3
- CP
CP-101,606
- Traxoprodil
- DG
Dentate Gyrus
- DIV
Days in Vitro
- FASD
Fetal Alcohol Spectrum Disorder
- HBSS
Hank’s Balanced Salt Solution
- HIHS
Heat Inactivated Horse Serum ITI - Intertrial Interval
- LSD
Fisher’s Least Significant Difference post hoc test
- NMDAr
n-methyl-d-aspartate receptor
- MEM
Minimum Essential Media
- NTC
Nontreated Control
- OHSC
Organotypic Hippocampal Slice Culture
- PND
Postnatal Day
- PI
Propidium Iodide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbey H, Howard E. Statistical procedure in developmental studies on species with multiple offspring. Dev Psychobiol. 1973;6:329–35. [DOI] [PubMed] [Google Scholar]
- Abel EL. Prenatal effects of alcohol. Drug Alcohol Depend. 1984;14:1–10. [DOI] [PubMed] [Google Scholar]
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–82. [DOI] [PubMed] [Google Scholar]
- Appelbaum MG. Fetal alcohol syndrome: diagnosis, management, and prevention. Nurse Pract. 1995;20:24–37. [PubMed] [Google Scholar]
- Barron S, Mulholland PJ, Littleton JM, Prendergast MA. Age and gender differences in response to neonatal ethanol withdrawal and polyamine challenge in organotypic hippocampal cultures. Alcohol Clin Exp Res. 2008;32:929–36. [DOI] [PubMed] [Google Scholar]
- Bath CP, Farrell LN, Gilmore J, Ward MA, Hicks CA, O’Neill MJ, et al. The effects of ifenprodil and eliprodil on voltage-dependent Ca2+ channels and in gerbil global cerebral ischaemia. Eur J Pharmacol. 1996;299:103–12. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31:926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Riley EP, Hannigan JH. Deficits on a spatial navigation task following prenatal exposure to ethanol. Neurotoxicol Teratol. 1987;9:253–8. [DOI] [PubMed] [Google Scholar]
- Butler TR, Self RL, Smith KJ, Sharrett-Field LJ, Berry JN, Littleton JM, et al. Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-D-asparate-type glutamate receptors. Neuroscience. 2010;165:525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol Clin Exp Res. 1999;23:363–70. [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003;23:3001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazot PL, Lawrence S, Thompson CL. Studies on the subtype selectivity of CP-101,606: evidence for two classes of NR2B-selective NMDA receptor antagonists. Neuropharmacology. 2002;42:319–24. [DOI] [PubMed] [Google Scholar]
- Chenard BL, Bordner J, Butler TW, Chambers LK, Collins MA, De Costa DL, et al. (1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-D-aspartate responses. J Med Chem. 1995;38:3138–45. [DOI] [PubMed] [Google Scholar]
- Cherpitel CJ. Alcohol and injuries: a review of international emergency room studies since 1995. Drug Alcohol Rev. 2007;26:201–14. [DOI] [PubMed] [Google Scholar]
- Cronise K, Marino MD, Tran TD, Kelly SJ. Critical periods for the effects of alcohol exposure on learning in rats. Behav Neurosci. 2001;115:138–45. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–35. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clin Exp Res. 1999;23:1698–703. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Ethanol and amino acids in the central nervous system: assessment of the pharmacological actions of acamprosate. Prog Neurobiol. 2000;60:343–62. [DOI] [PubMed] [Google Scholar]
- Danis RP, Newton N, Keith L. Pregnancy and alcohol. Curr Probl Obstet Gynecol. 1981;4:2–48. [PubMed] [Google Scholar]
- Davidson M, Shanley B, Wilce P. Increased NMDA-induced excitability during ethanol withdrawal: a behavioural and histological study. Brain Res. 1995;674:91–6. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. [DOI] [PubMed] [Google Scholar]
- Dogan A, Rao AM, Baskaya MK, Rao VL, Rastl J, Donaldson D, et al. Effects of ifenprodil, a polyamine site NMDA receptor antagonist, on reperfusion injury after transient focal cerebral ischemia. J Neurosurg. 1997;87:921–6. [DOI] [PubMed] [Google Scholar]
- Dursun I, Jakubowska-Dogru E, & Uzbay T. Effects of prenatal exposure to alcohol on activity, anxiety, motor coordination, and memory in young adult Wistar rats. Pharmacol Biochem Behav. 2006;85:345–55. [DOI] [PubMed] [Google Scholar]
- Fryer SL TS, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007;31:1415–24. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Harris BR, Prendergast MA, Hart SR, Blanchard JA 2nd, Holley RC, et al. Polyamines contribute to ethanol withdrawal-induced neurotoxicity in rat hippocampal slice cultures through interactions with the NMDA receptor. Alcohol Clin Exp Res. 2003;27:1099–106. [DOI] [PubMed] [Google Scholar]
- Girard TA, Xing HC, Ward GR, Wainwright PE. Early postnatal ethanol exposure has long-term effects on the performance of male rats in a delayed matching-to-place task in the Morris water maze. Alcohol Clin Exp Res. 2000;24:300–6. [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood). 2005;230:394–406. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–46. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiol Learn Mem. 1995;64:265–75. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Heinemann U. Synaptic reorganization in explanted cultures of rat hippocampus. Brain Res. 1999;815:304–16. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res. 2003;143:85–94. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Armant DR. Alcohol in pregnancy and neonatal outcome. Semin Neonatol. 2000;5:243–54. [DOI] [PubMed] [Google Scholar]
- Harris BR, Gibson DA, Prendergast MA, Blanchard JA, Holley RC, Hart SR, et al. The neurotoxicity induced by ethanol withdrawal in mature organotypic hippocampal slices might involve cross-talk between metabotropic glutamate type 5 receptors and N-methyl-D-aspartate receptors. Alcohol Clin Exp Res. 2003;27:1724–35. [DOI] [PubMed] [Google Scholar]
- Hoffman PL. Glutamate receptors in alcohol withdrawal-induced neurotoxicity. Metab Brain Dis. 1995;10:73–9. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Moses F, Tabakoff B. N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J Neurochem. 1989;52:1937–40. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Tabakoff B. The role of the NMDA receptor in ethanol withdrawal. EXS. 1994;71:61–70. [DOI] [PubMed] [Google Scholar]
- Hu XJ, Ticku MK. Chronic ethanol treatment upregulates the NMDA receptor function and binding in mammalian cortical neurons. Mol Brain Res. 1995;30:347–56. [DOI] [PubMed] [Google Scholar]
- Iorio KR, Tabakoff B, Hoffman PL. Glutamate-induced neurotoxicity is increased in cerebellar granule cells exposed chronically to ethanol. Eur J Pharmacol. 1993;248:209–12. [DOI] [PubMed] [Google Scholar]
- Iqbal U, Dringenberg HC, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure alters hippocampal GABA(A) receptors and impairs spatial learning in the guinea pig. Behav Brain Res. 2004;150:117–25. [DOI] [PubMed] [Google Scholar]
- Johnson K, Shah A, Jaw-Tsai S, Baxter J, Prakash C. Metabolism, pharmacokinetics, and excretion of a highly selective N-methyl-D-aspartate receptor antagonist, traxoprodil, in human cytochrome P450 2D6 extensive and poor metabolizers. Drug Metab Dispos. 2003;31:76–87. [DOI] [PubMed] [Google Scholar]
- Johnson TB, Goodlett CR. Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposure in male rats. Alcohol Clin Exp Res. 2002;26:83–93. [PubMed] [Google Scholar]
- Kalluri HS, Mehta AK, Ticku MK. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Mol Brain Res. 1998;58:221–4. [DOI] [PubMed] [Google Scholar]
- Kim CK, Kalynchuk LE, Kornecook TJ, Mumby DG, Dadgar NA, Pinel JP, et al. Object-recognition and spatial learning and memory in rats prenatally exposed to ethanol. Behav Neurosci. 1997;111:985–95. [DOI] [PubMed] [Google Scholar]
- Kundrotiene J, Cebers G, Wagner A, Liljequist S. The NMDA NR2B subunit-selective receptor antagonist, CP-101,606, enhances the functional recovery the NMDA NR2B subunit-selective receptor and reduces brain damage after cortical compression-induced brain ischemia. J Neurotrauma. 2004;21:83–93. [DOI] [PubMed] [Google Scholar]
- Kuner T, Schoepfer R, Korpi ER. Ethanol inhibits glutamate-induced currents in heteromeric NMDA receptor subtypes. Neuroreport. 1993;5:297–300. [DOI] [PubMed] [Google Scholar]
- Kusano T, Berberich T, Tateda C, Takahashi Y. Polyamines: essential factors for growth and survival. Planta. 2008;228:367–81. [DOI] [PubMed] [Google Scholar]
- Kyllerman M, Aronson M, Sabel KG, Karlberg E, Sandin B, Olegard R. Children of alcoholic mothers. Growth and motor performance compared to matched controls. Acta Paediatr Scand. 1985;74:20–6. [DOI] [PubMed] [Google Scholar]
- Lewis B, Wellmann KA, Barron S. Agmatine reduces balance deficits in a rat model of third trimester binge-like ethanol exposure. Pharmacol Biochem Behav. 2007;88:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JM, Lovinger D, Liljequist S, Ticku R, Matsumoto I, Barron S. Role of polyamines and NMDA receptors in ethanol dependence and withdrawal. Alcohol Clin Exp Res. 2001;25:132S–6S. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–4. [DOI] [PubMed] [Google Scholar]
- Marino MD, Aksenov MY, Kelly SJ. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. Int J Dev Neurosci. 2004;22:363–77. [DOI] [PubMed] [Google Scholar]
- Masood K, Wu C, Brauneis U, Weight FF. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol Pharmacol. 1994;45:324–9. [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcohol Clin Exp Res. 2000;24:226–31. [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:810–6. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–21. [DOI] [PubMed] [Google Scholar]
- Mayer S, Harris B, Gibson DA, Blanchard J, Prendergast MA, Holley RC, et al. Acamprosate has no effect on NMDA-induced toxicity but reduces toxicity induced by spermidine or by changing the medium in organotypic hippocampal slice cultures from rat. Alcohol Clin Exp Res. 2002;26:655–62. [PubMed] [Google Scholar]
- Mayer S, Harris BR, Gibson DA, Blanchard JA, Prendergast MA, Holley RC, et al. Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus. Alcohol Clin Exp Res. 2002;26:1468–78. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–60. [DOI] [PubMed] [Google Scholar]
- Menniti F, Chenard B, Collins M, Ducat M, Shalaby I, White F. CP-101,606, a potent neuroprotectant selective for forebrain neurons. Eur J Pharmacol. 1997;331:117–26. [DOI] [PubMed] [Google Scholar]
- Merchant RE, Bullock MR, Carmack CA, Shah AK, Wilner KD, Ko G, et al. A double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of CP-101,606 in patients with a mild or moderate traumatic brain injury. Ann N Y Acad Sci. 1999;890:42–50. [DOI] [PubMed] [Google Scholar]
- Michaelis EK, Michaelis ML, Freed WJ. Chronic ethanol intake and synaptosomal glutamate binding activity. Adv Exp Med Biol. 1980;126:43–56. [DOI] [PubMed] [Google Scholar]
- Mirshahi T, Woodward JJ. Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NMDAR1 Mg(2+)-insensitive mutants. Neuropharmacology. 1995;34:347–55. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Andaloro VJ, Skifter DA. Molecular determinants of NMDA receptor pharmacological diversity. Prog Brain Res. 1998;116:171–90. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. [DOI] [PubMed] [Google Scholar]
- Nagy J. The NR2B subtype of NMDA receptor: a potential target for the treatment of alcohol dependence. Curr Drug Targets CNS Neurol Disord. 2004;3:169–79. [DOI] [PubMed] [Google Scholar]
- Okiyama K, Smith DH, White WF, Richter K, McIntosh TK. Effects of the novel NMDA antagonists CP-98,113, CP-101,581 and CP-101,606 on cognitive function and regional cerebral edema following experimental brain injury in the rat. J Neurotrauma. 1997;14:211–22. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. [DOI] [PubMed] [Google Scholar]
- Pauli J, Wilce P, Bedi KS. Spatial learning ability of rats following acute exposure to alcohol during early postnatal life. Physiol Behav. 1995;58:1013–20. [DOI] [PubMed] [Google Scholar]
- Pinyerd B, Zipf WB. Puberty-timing is everything! J Pediatr Nurs. 2005;20:75–82. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Blanchard JA 2nd, Mayer S, Gibson DA, Littleton JM. In vitro effects of ethanol withdrawal and spermidine on viability of hippocampus from male and female rat. Alcohol Clin Exp Res. 2000;24:1855–61. [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA 2nd, Gibson DA, Holley RC, et al. Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-D-aspartate receptors. Neuroscience. 2004;124:869–77. [DOI] [PubMed] [Google Scholar]
- Reyes M, Reyes A, Opitz T, Kapin MA, Stanton PK. Eliprodil, a non-competitive, NR2B-selective NMDA antagonist, protects pyramidal neurons in hippocampal slices from hypoxic/ischemic damage. Brain Res. 1998;782:212–8. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 Suppl 3:511–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Thomas JD, Goodlett CR, Klintsova AY, Greenough WT, Hungund BL, et al. Fetal alcohol effects: mechanisms and treatment. Alcohol Clin Exp Res. 2001;25:110S–6S. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Behavioral and psychosocial profiles of alcohol-exposed children. Alcohol Clin Exp Res. 1999;23:1070–6. [PubMed] [Google Scholar]
- Royce JR. On the construct validity of open-field measures. Psychology Bulletin. 1977;84:1098–106. [Google Scholar]
- Rubin MA, Wellmann KA, Lewis B, Overgaauw BJ, Littleton JM, Barron S. Difluoromethylornithine (DFMO) reduces deficits in isolation-induced ultrasonic vocalizations and balance following neonatal ethanol exposure in rats. Pharmacol Biochem Behav. 2009;92:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, et al. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–26. [DOI] [PubMed] [Google Scholar]
- Savage DD, Rosenberg MJ, Wolff CR, Akers KG, El-Emawy A, Staples MC, et al. Effects of a novel cognition-enhancing agent on fetal ethanol-induced learning deficits. Alcohol Clin Exp Res. 2010;34:1793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz AJ, Constantine-Paton M. Modulation of NMDA receptor function: implications for vertebrate neural development. FASEB J. 1994;8:745–52. [DOI] [PubMed] [Google Scholar]
- Schorge S, Colquhoun D. Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci. 2003;23:1151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simson PE, Criswell HE, Johnson KB, Hicks RE, Breese GR. Ethanol inhibits NMDA-evoked electrophysiological activity in vivo. J Pharmacol Exp Ther. 1991;257:225–31. [PubMed] [Google Scholar]
- Slotkin TA, Bartolome J. Role of ornithine decarboxylase and the polyamines in nervous system development: a review. Brain Res Bull. 1986;17:307–20. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–82. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Landesman-Dwyer S, Martin JC, Smith DW. Teratogenic effects of alcohol in humans and laboratory animals. Science. 1980;209:353–61. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Awobuluyi M, Choi YB, Lipton SA. NMDA receptors: from genes to channels. Trends Pharmacol Sci. 1996;17:348–55. [PubMed] [Google Scholar]
- Tamura Y, Sato Y, Yokota T, Akaike A, Sasa M, Takaori S. Ifenprodil prevents glutamate cytotoxicity via polyamine modulatory sites of N-methyl-D-aspartate receptors in cultured cortical neurons. J Pharmacol Exp Ther. 1993;265:1017–25. [PubMed] [Google Scholar]
- Thomas JD, Fleming Sl, Riley EP. MK-801 can exacerbate or attenuate behavioral alterations associated with neonatal alcohol exposure in the rat, depending on the timing of administration. Alcohol Clin Exp Res. 2001;25:764–73. [PubMed] [Google Scholar]
- Thomas JD, Fleming SL, Riley EP. Administration of low doses of MK-801 during ethanol withdrawal in the developing rat pup attenuates alcohol’s teratogenic effects. Alcohol Clin Exp Res. 2002;26:1307–13. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garcia GG, Dominguez HD, Riley EP. Administration of eliprodil during ethanol withdrawal in the neonatal rat attenuates ethanol-induced learning deficits. Psychopharmacology (Berl). 2004;175:189–95. [DOI] [PubMed] [Google Scholar]
- Tomlinson D, Wilce P, Bedi KS. Spatial learning ability of rats following differing levels of exposure to alcohol during early postnatal life. Physiol Behav. 1998;63:205–11. [DOI] [PubMed] [Google Scholar]
- Toso L, Endres M, Vink J, Abebe DT, Brenneman DE, Spong CY. Learning enhancement with neuropeptides. Am J Obstet Gynecol. 2006;194:1153–8. [DOI] [PubMed] [Google Scholar]
- Toulmond S, Serrano A, Benavides J, Scatton B. Prevention by eliprodil (SL 82.0715) of traumatic brain damage in the rat. Existence of a large (18 h) therapeutic window. Brain Res. 1993;620:32–41. [DOI] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci. 1981;28:1053–6. [DOI] [PubMed] [Google Scholar]
- von Euler M, Bendel O, Bueters T, Sandin J, von Euler G. Profound but transient deficits in learning and memory after global ischemia using a novel water maze test. Behav Brain Res. 2006;166:204–10. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Le Bourdelles B, Whiting PJ, Kemp JA. Preferential co-assembly of recombinant NMDA receptors composed of three different subunits. Neuroreport. 1993;4:1347–9. [DOI] [PubMed] [Google Scholar]
- West JR, Goodlett CR, Bonthius DJ, Pierce DR. Manipulating peak blood alcohol concentrations in neonatal rats: review of an animal model for alcohol-related developmental effects. Neurotoxicology. 1989;10:347–65. [PubMed] [Google Scholar]
- West JR, Hamre KM, Pierce DR. Delay in brain growth induced by alcohol in artificially reared rat pups. Alcohol. 1984;1:213–22. [DOI] [PubMed] [Google Scholar]
- Wilkins LH Jr., Prendergast MA, Blanchard J, Holley RC, Chambers ER, Littleton JM. Potential value of changes in cell markers in organotypic hippocampal cultures associated with chronic EtOH exposure and withdrawal: comparison with NMDA-induced changes. Alcohol Clin Exp Res. 2006;30:1768–80. [DOI] [PubMed] [Google Scholar]
- Williams K, Romano C, Dichter MA, Molinoff PB. Modulation of the NMDA receptor by polyamines. Life Sci. 1991;48:469–98. [DOI] [PubMed] [Google Scholar]
- Williams K, Zappia AM, Pritchett DB, Shen YM, Molinoff PB. Sensitivity of the N-methyl-D-aspartate receptor to polyamines is controlled by NR2 subunits. Mol Pharmacol. 1994;45:803–9. [PubMed] [Google Scholar]
- Yang Y, Li Q, Yang T, Hussain M, Shuaib A. Reduced brain infarct volume and improved neurological outcome by inhibition of the NR2B subunit of NMDA receptors by using CP101,606–27 alone and in combination with rt-PA in a thromboembolic stroke model in rats. J Neurosurg. 2003;98:397–403. [DOI] [PubMed] [Google Scholar]
- Zimmer J, Kristensen BW, Jakobsen B, Noraberg J. Excitatory amino acid neurotoxicity and modulation of glutamate receptor expression in organotypic brain slice cultures. Amino Acids. 2000;19:7–21. [DOI] [PubMed] [Google Scholar]


